Open Access Article
Open Access ArticleUnlocking a nutritional treasure: health benefits and sustainable applications of spent coconut meal
Heeba
Shakeela
ab,
Kavya
Mohan
ab and
Nisha
P
 *ab
*ab
aAgro Processing and Technology Division, CSIR-National Institute for Interdisciplinary Science and Technology (CSIR-NIIST), Thiruvananthapuram, 695019, Kerala, India. E-mail: pnisha@niist.res.in; bp.nisha@yahoo.com; Fax: +91 471 249505; Tel: +91 471 2515348
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad, 201002, India
First published on 25th January 2024
Abstract
This paper investigates the previously overlooked potential of spent coconut meal, a byproduct resulting from the extraction of virgin coconut oil with a residual oil content of 25–54%. Traditionally considered as waste, spent coconut meal (SCM) is now recognized as a nutritional powerhouse with multifaceted health advantages. It is abundant in dietary fiber (45–55%), protein (14–25%), and essential nutrients, and these byproducts present a sustainable avenue for bolstering food security while concurrently mitigating environmental impact. This review thoroughly examines the extraction techniques employed for obtaining spent coconut flour (SCF) obtained from further processing of SCM, scrutinizes its nutritional profile, and highlights its diverse health benefits. Beyond its nutritional richness, the study underscores the applicability of both SCM and SCF in the realm of functional foods. The paper advocates for a paradigm shift in perceiving SCM as not merely waste but as a valuable resource contributing to both nutritional well-being and ecological sustainability.
Sustainability spotlightThe value addition of spent coconut meal in food applications contributes to several United Nations Sustainable Development Goals (SDGs). Goal 1—no poverty, sustainable practice of value addition of spent coconut creates new opportunities for income and employment, particularly for communities involved in coconut cultivation and processing. Additionally, Goal 3, focused on promoting good health and well-being, is supported by the nutritional value that spent coconut flour can be used as a value added or functional ingredient in food products. By incorporating the spent coconut flour into diets, there is potential for positive impacts on public health, contributing to the broader goal of ensuring well-being for all. This practice also supports SDG 2 (zero hunger) by exploring innovative ways to enhance food security and nutrition. The incorporation of spent coconut flour into the food supply chain reflects a commitment to sustainable practices and a circular economy approach, contributing to broader global efforts toward achieving the SDGs. In addition to this, it also aligns with SDG 12 (responsible consumption and production) by minimizing waste and promoting the efficient use of resources in the food industry. |
1. Introduction
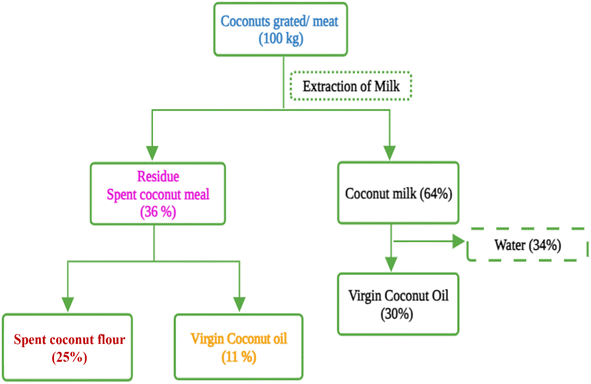
Industrialization and modernization have transformed the food processing sector with a significant increase in producing enormous volumes of agro-industrial spent material. Approximately 1.3 billion tons of food, or one-third of the world's produce, are wasted annually, according to estimates from the Food and Agriculture Organization (FAO).1 Worldwide, a variety of agro-industrial spent and agricultural wastes are produced yearly in addition to this food waste. The majority of this plant-based waste is either incinerated with other combustible materials or landfilled or rarely used as animal feed. Because of its nutritional profile and functional properties, this food industrial spent material can be further value-added for food/feed and functional ingredients. Recently, the food industry has been investigating zero waste methods to create food and nutritional supplements in concern for food safety, environmental resilience, and the sustainability of agriculture and food. With an estimated of 10 billion people on Earth by 2050, there could be a more than 50% rise in the demand for foods.2–4 A sustainable approach in the food processing sector is the need of the hour in order to ensure food security.The virgin oil industry makes a substantial contribution to the current food processing sector, playing a pivotal role in both culinary practices and consumer preferences. Virgin oils, such as coconut oil, olive oil, and avocado oil, are key components in a functional food formulation due to their essential fatty acid composition and antioxidant properties. The current market has seen a remarkable rise in the importance of virgin coconut oil (VCO) due to the increasing global interest in health and wellness.5 The market size for VCO has a compound annual growth rate (CAGR) of 8.6% predicted between 2023 and 2030, as stated in a report by Grand View Research.6 This expansion is linked to the growing popularity of natural and organic products, increasing awareness of their health advantages, and the increased use of VCO in the food, pharmaceutical, and cosmetics sectors. As a result of the increased production of VCO, there is a substantial amount of spent coconut meal generated, prompting the coconut industry to explore ways to minimize environmental impact and generate additional revenue streams from this spent material.7 The industrial production of VCO through methods such as cold pressing or mechanical expeller not only yields the sought-after oil but also generates a significant amount of byproduct known as spent coconut meal (SCM). SCM is also referred to as coconut residue, coconut cake, virgin coconut meal, partially defatted coconut residue, and deoiled coconut residue. However, the terms such as coconut residue, coconut cake, partially defatted coconut residue, and deoiled coconut residue can be misleading as they can also represent the spent material generated from any coconut oil industries other than VCO industries. To be more specific, the term SCM is used to denote the spent material from VCO industries in this article. It comprises 40–60% of the initial coconut material, and this spent material contains approximately 25–54% virgin oil and is enriched with significant amounts of protein and dietary fiber. The value addition of the spent material after the extraction of remaining virgin oil is very important for the related industries for sustainable development and from a circular economy point of view. This spent material is further processed and pulverized into a soft, fine flour named spent coconut flour (SCF) also known as defatted coconut residue. The nutritional composition of SCF varies depending upon the type and method of processing, and mostly due to the fat content (0.5–20%). It is a protein enriched, gluten-free, fat and fiber replacement to standard grain-based flours, with fiber accounting for about 75% of total carbohydrates.7,8Fig. 1 represents the mass balance of SCM and SCF generated from fresh coconut.
Recognizing the potential value embedded in this byproduct, the recovery of virgin oil from the spent material emerges as a critical aspect of sustainable coconut processing and from a circular economy point of view. Beyond oil recovery, the importance of value addition to the fiber component of the residue cannot be overstated. The protein and fiber-rich nature of the spent coconut meal makes it an attractive raw material for functional food applications presenting opportunities for innovative and sustainable practices in the food industry.8 By integrating efficient technologies for oil recovery and exploring creative ways to incorporate the protein and fiber content into food products, the coconut industry can achieve a more circular and resource-efficient model, contributing to the overall sustainable development of related industries. The spent coconut flour industry witnessed steady growth during the historical period 2018 to 2022 with a CAGR of 7.2% and it is projected to be US$ 6325.6 million in 2033.9 The industrial growth was attributed to the increasing demand for gluten-free and low-carb baked goods and confectionery products, as well as positive awareness about the health benefits of coconut meal. A literature survey reveals a review on coconut meal in ref. 7 that is primarily concerned with nutritional aspects and applications. However, there are many recent reports available in the last 5 years, which report the advanced extraction, further application and health benefits which form the subject matter of the current review. This current review concentrated on the advanced extraction method, macromolecular composition, functional properties, health benefits, and application of spent coconut meal in the development of functional foods, vegan foods, etc. Since SCM is reported to be a good source of protein and fibre, the prospects of this unique combination are also addressed here.
2. Processes of VCO production wherein SCM is generated as a by-product
Traditional methods entail grinding the coconut, extracting the coconut milk, and then drying or pressing the spent material into cake. Solvent extraction is the process of extracting oil with solvents, leaving behind material that can be dried into powder. For the past few years, the common approaches for extracting VCO were through dry extraction and wet extraction methods. In dry processing, freshly picked mature coconuts undergo dehusking and shelling before being shredded and dried at around 70 °C in a vacuum or tray drier. The dried pieces of coconuts are then cold pressed to obtain virgin coconut oil, while the remaining fat-less spent material (SCM) is further processed and ground to form SCF. On the other hand, wet processing involves extracting coconut milk from fresh kernels, which are then naturally fermented for 16–24 hours at around 30 to 40 °C to obtain VCO from coconut milk curdling via phase splitting. Centrifuge extraction separates coconut milk and SCM using centrifugal force.10,11 Enzymatic extraction involves treating the grated coconut with enzymes such as cellulase, polygalacturonase, α-amylase and protease, which break the cell wall and release the coconut milk and this SCM is further processed into SCF. The higher yield of virgin oil extraction using a combination of enzymes can be attributed to the cell wall components exhibiting a rapid hydrolysis rate. The water–oil emulsion is broken using a chill, freeze, and thaw method. Coconut milk is centrifuged for 10 minutes, and the supernatant is removed. The cream is then thawed at 40 °C until it reaches ambient temperature. The thawing process forms oil droplets, resulting in a 69% oil extraction yield with remaining oil in the SCM.12 Recent research found that utilizing supercritical carbon dioxide (SC-CO2) as the extraction substance could yield 100% of the oil with high quality SCF in a shorter duration.13 Generally, the extraction process is determined by criteria such as desired end products, available equipment, and production size. Co-extraction of dried coconut shreds and turmeric (Curcuma longa L.) by supercritical fluid extraction (SFE) recently demonstrated the importance of extracting bioactive enhanced residue and oil.14 Similarly, enhanced functional food compositions from co-extraction of dried coconut shreds (Cocos nucifera L.) and marigold flowers (Tagetes erecta L.) using SFE were noticed by researchers.15 The utilization of SCM for microwave-assisted extraction and ultrasonic-assisted extraction of virgin coconut oil and the subsequent analysis of the virgin oil properties have been the focus of recent research.16,17 However, a dearth of data pertaining to the characteristics of SCF following such extraction methodologies necessitates further exploration in subsequent studies for sustainability and from a circular economy point of view.3. Nutritional profile of SCM and SCF
Plant proteins are gaining popularity as a low-cost alternative to animal proteins due to increased consumer demand stemming from health concerns, religious restrictions, and vegetarianism trends.18 Particularly from oil processing, a large number of plant spent material from the food industry makes excellent candidates for low-cost sources of plant proteins. SCM contains a lot of desirable protein which can be recovered and it can be used as an essential component for creating plant-based substitutes.19 The composition of spent coconut meal is mostly determined by the maturity of the coconut, the kinds used, and the process used to extract virgin coconut oil. According to Khan,20 SCM contains 14.3% protein, 1.55% ash, 20.50% fiber, 54.0% fat, 6.7% moisture, and 23.40% carbohydrate. Similarly, Ramya and Anitha21 compared the nutritional profiles of wheat flour (11% protein and 0.3% fiber) and SCM, which indicate 20.92% fiber and 10.77% protein. The albumin and globulin fractions account for 21% and 40% of the total protein in SCM protein powder, respectively. The globulin fraction of SCM protein was made up of one 53 kDa main polypeptide and five subunits (MW 27, 22, 34, 25, and 20 kDa). In the albumin fraction, two main polypeptides (18 and 25 kDa) are found. The glutelin-1 acid-soluble component accounts for 14.4%. Prolamin and glutelin-2 fractions were reported to be less than 5% of total protein (3.3% and 4.8%, respectively).22 Numerous studies reported the nutritional profile of SCF also. The author reported a protein content of 19.11% and a crude fiber level of 11.16% in SCF and its application in cake.23 Likewise, Igbabul24 studied the nutritional parameters of SCF derived from fermented coconut pieces deoiled by a dry extraction technique. As a result, the obtained SCF has 5.27% moisture, 2.76% ash, 12.31% protein, 0.48% fat, 11.81% fiber, and 67.37% carbohydrate. Dry-processed SCF is high in protein content, and wet-processed powder is high in fiber content. Another study25 reported that SCF contains only 5.0% protein content, which could be attributed to the thermal extraction procedures used.Dietary fibers, which provide plant cell walls their structural rigidity, are carbohydrate polymers like cellulose, hemicellulose, lignin, and pectin. Dietary fibers are categorised into two categories: insoluble dietary fiber (IDF) and soluble dietary fiber (SDF), based on their water solubility.26 Current research provides information on the different types and origins of dietary fiber derived from spent material in agri-foods.27 Consuming dietary fiber encourages a wide range of metabolic interactions between the different species of bacteria that comprise the microbial community in the gastrointestinal tract. Increased dietary fiber consumption lowers triglycerides, cholesterol, and gastrointestinal issues, which in turn helps prevent and treat cardiovascular disease. For healthy adults, consuming 20–35 grams of dietary fiber per day is advised.27,28 According to USDA data,29 SCM has 40% dietary fiber and 13.3% protein. Trinidad8 estimated that SCF has a dietary fiber content of 60.0/100 g sample, with 56% insoluble and 4% soluble fiber. Similarly, Salama30 reported 50.50% fiber content in SCF. The Vansoest method yielded 38.3 and 24.2% neutral detergent fiber and acid detergent fiber, respectively, with 14.1% hemicelluloses, which is the predominant acid soluble carbohydrate, in SCF. SCF has 10.3% cellulose, the most insoluble fiber. These findings suggested that coconut flour contains a variety of edible fibers.31
Coconut plants are typically cultivated in soil that is abundant in minerals, resulting in the formation of trace minerals like calcium (57 mg/100 g), iron (18 mg/100 g) and sodium (200 mg/100 g). The absence of phytic acid in coconuts allows for easy absorption of these minerals, unlike in various cereal products where phytic acid hinders the bioavailability of trace elements.32 The nutritional profiles of SCM and SCF are shown in Table 1.
| Samples | Components | Reference |
|---|---|---|
| Spent coconut meal (SCM) | Moisture 6.7%, protein 14.3%, crude fiber 2.50%, fat 54.0%, ash content 1.55% and total carbohydrates 23.40% | 20 |
| Moisture 7.20%, ash 1.15%, fat 25.73%, protein 10.77%, fiber 20.92%, iron 0.10 mg, carbohydrate 55.15 g, calcium 0.31 mg, phosphorus 0.41 mg | 21 | |
| Spent coconut flour (SCF) | Moisture 4.50%, ash 4.10%, fat 10.70%, protein 19.11%, fiber 11.16%, carbohydrate 49.80 g, iron 0.10 mg g−1, calcium 0.31 mg g−1, phosphorus 0.41 mg g−1, magnesium 0.04 mg g−1, potassium 0.40 mg g−1 | 23 |
| Moisture 5.27%, protein 12.31%, crude fiber 11.81%, fat 0.48%, ash content 2.76% and total carbohydrates 67.37% | 24 | |
| Protein 5%, crude fiber 10%, fat 4%, and total carbohydrates 18% | 25 | |
| Total dietary fiber 60%, insoluble dietary fiber 56% and soluble dietary fiber 4% | 8 |
4. Functional properties of SCF
Functional characteristics are a cornerstone for successful food product development. Oil holding capacity, swelling capacity and water holding capacity are important functional qualities of flour that have a large impact on the formulation of food products. These characteristics are crucial in defining the texture, stability, and overall quality of a wide variety of food products. As fermentation time increased, the author found an increase in water absorption capacity and a reduction in oil absorption capacity.24 The grinding process reduces the size of particles from 1127 to 550 μm, enhancing hydration properties. However, below 550 μm the fiber matrix is damaged and the pores are collapsed, lowering the hydration properties.33 Supporting previous research, the author evaluated the functional qualities of SCF with varied particle sizes, demonstrating that water absorption capacity and swelling capacity improved with change in particle size between 0.20 and 0.25 mm.34 According to Sangnark,35 decreasing particle sizes alters the structure of carbohydrate matrices, allowing particles to absorb less water. As a result, the hydration properties changed. Raghavarao36 examined the functional qualities of coconut fiber, which has the highest capacity to swell when compared to other fibers, with a water retaining capacity of (7.1 g g−1) and a swelling capacity of (20 mL g−1 of SCF). SCF is high in protein and contains both hydrophilic and hydrophobic components. Proteins possess a hydrophilic component that enables them to interact with water. Additionally, SCF is rich in soluble fiber, which can enhance water absorption. The study indicates that the SCF's high water absorption capacity makes it suitable for high viscosity food formulations. This increase in absorption may be due to an increase in the exposure of non-polar amino acid groups to fat as well as the higher fiber content in the SCF samples, which causes the flour to absorb more oil. SCF was reported to have an oil absorption capacity of 3.93 g g−1. The capacity of food products to absorb oil contributes to the enhancement of mouth-feel and flavor retention.34 Proteins are important foaming agents in various food formulation. Flexible proteins are known to have a higher capacity for foaming than spherical proteins.37–39 Proteins are one of the most commonly used emulsifiers in the stabilization of oil in water (O/W) food emulsions, preventing coalescence via adsorption onto the oil droplet surfaces. An advantage of proteins as amphiphilic surfactants that can act as a barrier to separate the oil droplets is that they are partially soluble in both aqueous and oil phases. According to Chambal 19, the crude freeze-dried alkaline protein extract from SCM demonstrated a good ability to form oil-in-water emulsions beyond the pH range of low solubility.The amphiphilic nature of protein and polysaccharides in SCF is depicted in Fig. 2, and it could be a suitable candidate for the development of O/W or W/O emulsions. Interfacial stabilization is a key mechanism in emulsion formation, and it is closely related to the amphiphilic nature of molecules involved. Emulsions come into existence through the alignment of amphiphilic molecules, at the interface of two immiscible liquids. The hydrophilic heads of these molecules position themselves towards the continuous phase, establishing interactions with water molecules. Simultaneously, the hydrophobic tails extend into the dispersed phase, forming interactions with the immiscible liquid. This particular configuration establishes a stable interface, effectively inhibiting the coalescence of dispersed droplets and ensuring the enduring stability of the emulsion.40,41 These characteristics show the emulsion formation possibilities of SCF and this SCF based emulsion can be incorporated into food systems for an enriched nutritional profile and product stability. However, more research is needed to explore its emulsifying capabilities, including factors such as concentration, processing methods, and compatibility with different phases.
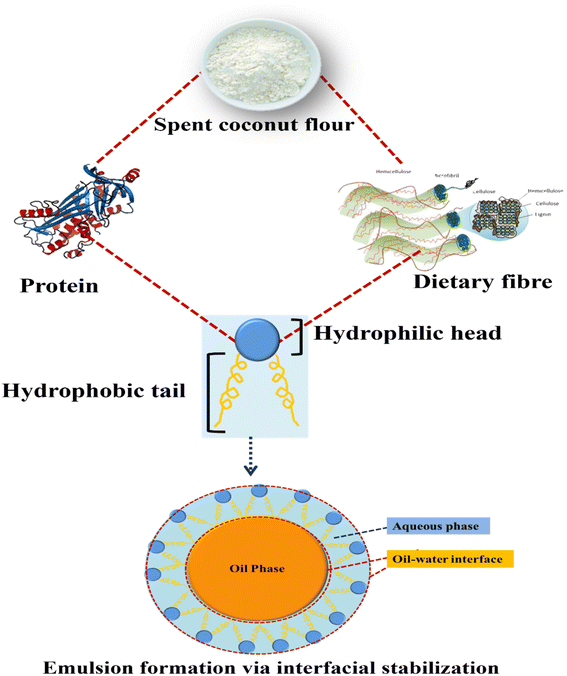 | ||
| Fig. 2 Schematic representation of the amphiphilic nature and emulsification capacity of spent coconut flour. | ||
5. Health effects of SCM and SCF
Numerous studies, both in vitro and in vivo, have highlighted various health benefits associated with consumption of SCM and SCF. High protein and fibre content makes it a suitable alternative for gluten-free products and processed foods. Coconut dietary fiber and coconut kernel proteins possess beneficial properties for individuals with diabetes. The presence of L-arginine in coconut aids in maintaining glucose balance through the nitric oxide synthase pathway, leading to the regeneration of pancreatic beta cells.42 This results in lowered blood glucose levels and improved insulin sensitivity in both non-diabetic and diabetic individuals. In a study conducted by Fraser,43 it was suggested that SCM albumin may contain bioactive peptides with ACE-inhibitory and antioxidant properties. Additionally, two peptides derived from SCM protein isolates, namely Pro-Gln-Phe-Tyr-Trp and Arg-Pro-Glu-Ile-Val, exhibited significant free radical scavenging activities with IC50 values of 4.28 and 7.65 g mL−1, respectively.44 In terms of lysine content, wheat flour was found to have a lower range of 1.7–1.9 g/100 g of protein, compared to the SCF reported range of 3.1–4.7 g/100 g of protein by Kwon45 and Rasyid.46 To support this, SCF has been shown to reduce blood cholesterol, triglyceride, and LDL cholesterol levels, consequently reducing the risk of cardiovascular disease. The high content of L-arginine in coconut proteins also contributes to their hypolipidemic impact.47 Nitric oxide, a naturally occurring vasodilator, has been found to have additional effects on platelet aggregation and adhesion. Initially identified as an endothelium-derived relaxing factor (EDRF), nitric oxide plays a crucial role in maintaining vascular health. In the context of hypercholesterolemia, dietary arginine has been shown to compensate for low EDRF levels, thereby reducing cholesterol levels.43Dietary fibers play a crucial role as prebiotics in supporting the intestinal microflora. The enzymatic hydrolysis of polysaccharide compounds found in SCF results in the production of oligosaccharides, which possess potent prebiotic properties for humans.48 These non-digestible oligosaccharides promote the growth of beneficial bacteria such as Lactobacilli, Bifidobacteria, and other microorganisms that contribute to the immune responses within the intestines.49 Through prebiotic oligosaccharide fermentation, these bacteria produce short-chain fatty acids. One such disaccharide, β-1,4-mannobiose, derived from SCF, not only shows immune-modulating effects but also acts as a prebiotic. It enhances the formation of antibody IgA, which aids in preventing the production of pathogenic bacteria by promoting the phagocytic activity.50 Dietary fiber has been found to provide significant health benefits in the prevention of chronic illnesses such as cardiovascular disease, cancer, and type 2 diabetes. The ingestion of propionate has been reported to suppress the activity of HMG CoA reductase, the enzyme that limits cholesterol production. Furthermore, butyrate has been found to promote cell differentiation, which can reduce the growth of colon tumors. Moreover, it promotes water absorption in the intestines, which can prevent constipation.7,51
In a specific in vivo study, the inclusion of SCF in a hypoenergetic diet resulted in reduced glucose and cholesterol levels, aiding in the management of obesity.52 Trinidad53 suggested that the fibrous nature of SCF played a crucial role in binding bile acids, preventing their reabsorption in the liver and promoting their elimination. Additionally, the high dietary fiber content in SCF contributes to slower glycaemic responses in foods, which can be particularly advantageous for individuals with elevated blood glucose levels.32 Moreover, the consumption of fiber-rich foods contributes to improved heart health by reducing blood pressure and serum cholesterol levels, thereby decreasing the risk of hypertension and stroke. Smith54 demonstrated that SCF extracts have the ability to disrupt the Caco-2 cancer cell integrity inside the colon, as evidenced by the release of lactate dehydrogenase in cytotoxicity experiments. Additionally, these extracts were found to enhance the activity of catalase, an enzyme responsible for neutralizing hydrogen peroxide, thus promoting detoxification processes.
6. Application of SCM and SCF in food formulation
Numerous research studies have documented that incorporating SCM and SCF into food products enhanced their functional properties, thereby showing the possibilities of prevention and management of lifestyle associated diseases (Table 2). As SCM and SCF are rich in insoluble dietary fibre, they are not palatable when consumed directly and are always combined with other ingredients to make them more palatable. Srivastava55 prepared ladoo, a traditional Indian sweet meat, from SCM and examined the product's shelf life under various circumstances at room temperature, which ranged from 15 to 35 °C. The author came up with the idea of development of tablets with four cold-pressed cakes—coconut (SCM), flax, sunflower, and pumpkin and observed enhanced hardness and resistance to cutting.56 In order to improve the fiber, protein and amino acid profile in refined wheat flour bread, Gunathilake and Abeyrathne57 used SCF in varying proportions (10–30%). According to the study's findings, bread can be produced with an acceptable quality by substituting 20% of the SCF with wheat flour. In another study, bio-yogurt drinks with varying SCF ratios demonstrated better qualities, especially viscosity, and were well-received by the sensory panel.58 Similarly, the author observed high fiber content and comparable sensory properties, with SCF (4.8%) and cassava flour in place of wheat flour as fillers in chicken sausages.59 With the addition of SCF, Mihirani60 enhanced the fiber, protein, and mineral content of snack crackers. In a nutshell, they discovered that adding 20% SCF to wheat flour did not affect the flour's sensory qualities. Past studies show that the cupcake volume and crumb structure were influenced by the size of the SCF particles; smaller flour particles produced higher volumes and lower crumb densities.61 The research group found that up to a 20% addition of SCF did not significantly alter the sensory qualities of noodles compared to that with wheat flour. However, a 30% substitution has a negative impact on the product's appearance, texture, and general acceptability.62 Recent studies show that biscuits with the highest ratings for flavor, odor, crumbliness, hardness, and general acceptance were those made with up to 40% SCF.63| Food products | SCM/SCF addition | Observation | Reference |
|---|---|---|---|
| Biscuit | Up to 40% | Maximum score has been attained for the flavor, odour, crumbly consistency, firmness, and overall approval of the biscuit | 63 |
| Wheat flour (Triticum aestivum)-based tortillas | 20% | WF tortilla exhibited a lower dietary fiber and protein content compared to the tortilla with higher coconut powder content | 67 |
| Additionally, the latter showed a slight decrease in extensibility | |||
| Rice noodles | 10% | The control sample exhibited lower levels of crude fibre, protein, antioxidants, polyphenols, flavonoids, and minerals compared to the optimized formulations | 62 |
| The shelf life of optimized noodle samples is 351 days when stored at a temperature of 29 °C | |||
| Crispy waffles | Higher than 50% | Enhance the nutritional profile of crispy waffles by increasing their protein, fat, and dietary fiber content, while taking into account the potential impact on their texture, color, size, thickness, and spread ability | 69 |
| Baked snack | 55.3% | SCF addition exhibits a higher concentration of protein, fat, and ash when compared to the nixtamalized corn flour | 70 |
| Cookies | 50% | Cookies produced using a combination of 60% sago flour and 40% SCM were highly favoured due to their appealing texture and color | 71 |
| Conversely, when it came to taste and aroma, the pastry products made from an equal blend of 50% coconut meal flour and 50% sago flour were relatively more preferred | |||
| Probiotic frozen yoghurt | 50% | Viability of Bifidobacterium breve, Bifidobacterium bifidum, Streptococci, Lactobacilli, and the total bacterial count in frozen yoghurt were significantly improved during the frozen storage phase | 30 |
| Rusk | 20% | Composition of the product included 66.2% medium chain fatty acids, with lauric acid being the dominant fatty acid, and it was completely free from trans fatty acids | 64 |
| Baked wheat based bread | 15% of coconut and chestnut flour | Optimized formulation exhibited a higher quality when compared to the wheat bread, and its fiber content was significantly elevated | 72 |
| Pasta | Up to 15% | Superior levels of dietary fiber, protein, and lipids in comparison to the control group, indicating high quality and content | 73 |
| Muffins | 20% | Sensory and physicochemical attributes of the muffin samples were enhanced | 74 |
In place of fat in the rusk formulation, SCF, a source of medium chain fatty acids, was chosen. The study examined the impact of substituting 10%, 20%, and 30% of wheat flour with partially defatted SCF on the quality attributes of rusk. Lauric acid was the main noted medium-chain fatty acid in the SCF which reduced rusk's fat, with zero trans fatty acids.64 Studies have shown that the glycaemic index of confectionery foods can be considerably lowered by incorporating SCF, which is high in dietary fiber.65 Similarly, the study analyzed flour blends made with nixtamalized maize and defatted SCF, focusing on dietary fiber, functional properties, pasting, and antioxidant properties. Results showed increased protein, fat, fiber, water and oil absorption capacity, and sensory acceptability, with 10% SCF incorporation being preferred.66 By adding 25% SCF to the batter, the muffin samples' physico-chemical and sensory qualities were enhanced, and as a result, their nutritional value was raised.21 One of the most popular flatbreads is made with wheat flour is tortillas. A blend of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 wheat flour and SCF exhibits the highest firmness (7.9 N), while the 90
20 wheat flour and SCF exhibits the highest firmness (7.9 N), while the 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 blended tortillas and the control group did not differ in firmness (p > 0.05). The 20% SCF-containing tortilla was found to have higher dietary fiber and protein contents than the wheat flour tortilla, as well as a slight reduction in extensibility when compared to the wheat flour tortilla, according to the physicochemical properties of the tortillas.67 Yalegama68 developed cookies with SCF fiber extracts, removing fats, sugar and protein. The 10% SCF fibre powder replacement in cookies showed good overall acceptability. Incorporating this functional ingredient into food products enhances nutritional value by providing a gluten-free alternative rich in fiber, healthy fats, and essential nutrients. From baked goods to savory dishes, SCF lends a unique texture and nutritional boost, catering to a diverse range of dietary preferences. Several other applications of SCF in food products like crispy waffles, pasta, biscuits, muffins, and cookies have been reported in the past three years as reported in Table 1.69–74 Nowadays, the application of nanotechnology in food processing holds tremendous potential.75 The author reported that during frozen storage of yogurt, the total bacterial count and the viability of Bifidobacterium bifidum, Bifidobacterium breve, Streptococci and Lactobacilli were enhanced by the addition of SCF nanoparticles with sizes ranging from 81.96 nm to 83.53 nm. In addition to the microbial finding, the SCF nanoparticle enhanced the chemical, physicochemical and sensory properties of yogurt.30
10 blended tortillas and the control group did not differ in firmness (p > 0.05). The 20% SCF-containing tortilla was found to have higher dietary fiber and protein contents than the wheat flour tortilla, as well as a slight reduction in extensibility when compared to the wheat flour tortilla, according to the physicochemical properties of the tortillas.67 Yalegama68 developed cookies with SCF fiber extracts, removing fats, sugar and protein. The 10% SCF fibre powder replacement in cookies showed good overall acceptability. Incorporating this functional ingredient into food products enhances nutritional value by providing a gluten-free alternative rich in fiber, healthy fats, and essential nutrients. From baked goods to savory dishes, SCF lends a unique texture and nutritional boost, catering to a diverse range of dietary preferences. Several other applications of SCF in food products like crispy waffles, pasta, biscuits, muffins, and cookies have been reported in the past three years as reported in Table 1.69–74 Nowadays, the application of nanotechnology in food processing holds tremendous potential.75 The author reported that during frozen storage of yogurt, the total bacterial count and the viability of Bifidobacterium bifidum, Bifidobacterium breve, Streptococci and Lactobacilli were enhanced by the addition of SCF nanoparticles with sizes ranging from 81.96 nm to 83.53 nm. In addition to the microbial finding, the SCF nanoparticle enhanced the chemical, physicochemical and sensory properties of yogurt.30
Recent trends indicate the demand for plant based alternatives to animal-based foods, including meat, fish, eggs, milk, and related products. This shift is expected to be more seamless with increased accessibility to affordable, convenient, sustainable, nutritious, and flavourful plant-based food options. Currently, the food industry is demonstrating notable success in creating top-notch imitations of minced meat and dairy products, such as burgers, sausages, nuggets, and cheese, employing functional ingredients to replicate their textures and structures.76,77 In a recent study, with SCF as the primary ingredient, the author formulated a vegan sausage that is acceptable in sensory, proximate, and microbiological studies. Additionally, the total CO2 emission per kg of the vegan sausage is reduced from 0.678 kg CO2 per kg to 0.477 kg CO2 per kg.78
7. Shelf life and preservation of SCM and SCF
The shelf life of SCM can vary widely, spanning from a few weeks to 140 days. This variation is influenced by factors such as fat content, moisture levels, exposure to air, method of drying, temperature, storage conditions, vacuum packaging, and modified atmospheric packaging.79 SCF typically has a longer shelf life, ranging between 9 and 26 months. Bawalan (2000) reported in his study that SCF is shelf stable, in an airtight container, for 26, 14 and 9 months when stored at 20, 30, and 40 °C, respectively.808. Challenges, constraints and future of spent coconut meal
The effective utilization of SCM and SCF confronts various obstacles. These byproducts, residues remaining after milk extraction, often present challenges due to their high fiber content, impeding their application in certain food products seeking a smoother texture. The variability in nutrient composition, moisture levels, and the presence of other miscellaneous factors further complicate efforts to standardize their use. The residual lipid content may restrict their suitability for applications requiring low-fat ingredients, while processing costs, encompassing drying and storage, add economic considerations. Limited research and development, along with market demand and consumer perceptions, compound the difficulties in establishing economically viable and widely accepted uses for SCF. Addressing these diverse challenges necessitates a holistic approach involving agricultural, food technology, and economic considerations. Future application of SCF focuses on printable food formulation using 3D food printing, nanotechnology as a biocompatible material for bioactive delivery, as a natural emulsifier, oleogelators, and edible films for food packaging. The high fiber and protein content, in particular, makes it a valuable addition to functional foods, promoting digestive health and potentially aiding in weight management. Detailed studies are warranted to establish the health benefits of SCF in gut modulation and associated health benefits, including management of obesity.9. Conclusion
The nutritional composition of SCF reveals a rich profile of dietary fiber, protein, vitamins and minerals. The presence of essential nutrients, such as proteins and micronutrients, further enhances the nutritional value of SCF, making it a wholesome ingredient for diverse applications. The health benefits associated with SCF are noteworthy. The functional properties of SCF, such as its ability to improve texture, moisture and oil retention, make it an attractive option for innovative and health-promoting food formulation e.g. vegan food applications. The diverse extraction techniques, including cold and supercritical fluid extraction, offer various options for obtaining SCF, each with its unique set of advantages. These methods allow the recovery of valuable components from coconut by-products, minimizing waste and contributing to sustainable food production practices. However, it is crucial to conduct further research and development to optimize extraction processes, explore novel applications, and ensure the seamless integration of the products into a variety of food/functional food products. SCF thus offers great potential as a source of nutritionally important biopolymers and active molecules, which needs to be explored more effectively for the sustainable development of the sector and also from the food security point of view. As most of the phytochemicals are bound to the fibre, SCF could be further explored to evaluate the left over polyphenols that may present in SCF. This dynamic approach will not only enhance the versatility of SCF but also contribute to circular economy leading to a more sustainable process.Conflicts of interest
The author asserts that there are no conflicts of interest to disclose.Acknowledgements
Heeba S, Kavya Mohan and Dr P Nisha acknowledge CSIR, India for the facilities.References
- Food Wastage: Key Facts and Figures, Food and Agriculture Organization (FAO) of the United Nations, https://www.fao.org/news/story/en/item/196402/icode/, accessed on 29 November 2023 Search PubMed.
- N. Ramankutty, Z. Mehrabi, K. Waha, L. Jarvis, C. Kremen, M. Herrero and L. H. Rieseberg, Annu. Rev. Plant Biol., 2018, 69, 789–815 CrossRef CAS PubMed.
- F. M. de Matos, G. B. Rasera and R. J. S. de Castro, Sustainable Food Technol., 2024, 2, 19–31 RSC.
- P. Singh and K. Krishnaswamy, Trends Food Sci. Technol., 2022, 128, 331–344 CrossRef CAS.
- Y.-Q. Zeng, J.-T. He, B.-Y. Hu, W. Li, J. Deng, Q.-L. Lin and Y. Fang, Crit. Rev. Food Sci. Nutr., 2022, 1–24 Search PubMed.
- Grand View Research Report, Virgin Coconut Oil Market Size & Trends Report, 2023–2030, 2022 Search PubMed.
- K. Kaur, N. Chhikara, P. Sharma, M. K. Garg and A. Panghal, Foods Raw Mater., 2019, 419–427 CAS.
- T. P. Trinidad, A. C. Mallillin, D. H. Valdez, A. S. Loyola, F. C. Askali-Mercado, J. C. Castillo, R. R. Encabo, D. B. Masa, A. S. Maglaya and M. T. Chua, Innovative Food Sci. Emerging Technol., 2006, 7, 309–317 CrossRef CAS.
- Future Market Insights Global and Consulting Pvt. Ltd., 2023, https://www.futuremarketinsights.com/reports/coconut-flour-market, accessed on 29 November 2023.
- R. K. Agarwal, MOJ Food Process. Technol., 2017, 4, 00087 Search PubMed.
- C. Jayasekara and K. D. P. P. Gunathilake, Proceedings of International Cococnut Summit, 2007, pp. 7–11 Search PubMed.
- S. Sundrasegaran and S. H. Mah, eFood, 2020, 1, 381–391 CrossRef.
- E. Aytaç, Sep. Sci. Technol., 2022, 57, 426–432 CrossRef.
- A. Sharma, A. Ray and R. S. Singhal, J. Cleaner Prod., 2023, 382, 135313 CrossRef.
- A. A. Shaikh, A. Ray and R. S. Singhal, Food Chem. Adv., 2023, 2, 100189 CrossRef.
- M. H. Hasni, S. Sulaiman, D. N. Jimat and A. Amid, Chem. Eng. Commun., 2023, 210, 330–347 CrossRef CAS.
- A. Syahir, S. Sulaiman, M. Mel and H. Veny, Biomass Convers. Biorefin., 2023, 1–11 Search PubMed.
- L. Y. Aydemir and A. Yemenicioğlu, LWT–Food Sci. Technol., 2013, 50, 686–694 CrossRef CAS.
- B. Chambal, B. Bergenståhl and P. Dejmek, Food Nutr. Sci., 2013, 04, 29–37 CAS.
- M. A. Khan, C. Mahesh, A. D. Semwal and G. K. Sharma, Int. J. Adv. Res., 2015, 3, 717–725 CAS.
- H. N. Ramya and S. Anitha, Int. J. Curr. Microbiol. Appl. Sci., 2020, 9, 2231–2240 CrossRef CAS.
- Y. Li, Y. Zheng, Y. Zhang, J. Xu and G. Gao, Molecules, 2018, 23, 707 CrossRef PubMed.
- N. A. Afoakwah, J. Owusu and V. Owusu, Asian Food. Sci. J., 2019, 1–11 Search PubMed.
- B. D. Igbabul, F. A. Bello and E. C. Ani, Sky J. Food Sci., 2014, 3, 34–40 Search PubMed.
- J. Khyat, S. Gayathri and D. Madhavi, Development and standardization of high fibre and gluten free cococnut four cookie icecream sandwich, ISSN:2278-4632, 2020, https://junikhyatjournal.com/no_6_jun_20/9.pdf.
- M. Elleuch, D. Bedigian, O. Roiseux, S. Besbes, C. Blecker and H. Attia, Food Chem., 2011, 124, 411–421 CrossRef CAS.
- C. Pop, R. Suharoschi and O. L. Pop, Sustainability, 2021, 13, 7219 CrossRef CAS.
- A. Ahmed, H. Muhammad Rizwan Abid, A. Ahmad, N. Khalid and S. S. Ahmed, Asian J. Agric. Biol., 2020, 8, 1–13 CrossRef.
- USDA Data Coconut Products – Food Composition Database: United States Department of Agriculture, Agriculture research service, 2018 Search PubMed.
- H. H. Salama, S. M. Abdelhamid and N. S. Abd-Rabou, Curr. Bioact. Compd., 2019, 16, 661–670 CrossRef.
- K. D. P. P. Gunathilake, C. Yalegama and A. A. N. Kumara, Asian J. Food Agro-Ind., 2009, 2, 382–391 Search PubMed.
- L. Ramaswamy, Int. J. Ayurvedic Herb. Med., 2014, 3, 1426–1436 Search PubMed.
- S. N. Raghavendra, S. R. Ramachandra Swamy, N. K. Rastogi, K. S. M. S. Raghavarao, S. Kumar and R. N. Tharanathan, J. Food Eng., 2006, 72, 281–286 CrossRef.
- L. Q. Dat, Vietnam J. Sci. Technol., 2018, 55, 100 CrossRef.
- A. Sangnark and A. Noomhorm, Food Chem., 2003, 80, 221–229 CrossRef CAS.
- K. S. M. S. Raghava Rao, S. N. Raghavendra and N. K. Rastogi, Coconut J., 2008, 51, 2–7 Search PubMed.
- K. S. M. S. Raghava Rao, S. N. Raghavendra and N. K. Ratogi, Int. J. Res. Granthaalayah, 2018, 6, 172–183 Search PubMed.
- G. Okafor and G. Usman, Agro-Sci., 2015, 13, 7 CrossRef.
- S. Jitngarmkusol, J. Hongsuwankul and K. Tananuwong, Food Chem., 2008, 110, 23–30 CrossRef CAS PubMed.
- C. C. Berton-Carabin and K. Schroën, Annu. Rev. Food Sci. Technol., 2015, 6, 263–297 CrossRef CAS PubMed.
- D. J. McClements, Food Emulsions, CRC Press, 2004 Search PubMed.
- G. Salil, K. G. Nevin and T. Rajamohan, J. Sci. Food Agric., 2012, 92, 1903–1908 CrossRef CAS PubMed.
- G. Fraser, Am. J. Clin. Nutr., 1994, 59, 1117S–1123S CrossRef CAS PubMed.
- Y. Zheng, Y. Li, Y. Zhang and S. Zhao, RSC Adv., 2016, 6, 54346–54356 RSC.
- K. Kwon, K. H. Park and K. C. Rhee, J. Agric. Food Chem., 1996, 44, 1741–1745 CrossRef CAS.
- F. Rasyid, M. Manullang and P. M. T. Hansen, Food Hydrocolloids, 1992, 6, 301–314 CrossRef CAS.
- T. Rajmohan and S. Mini, Indian J. Exp. Biol., 2004, 42, 53–57 Search PubMed.
- P. Khuwijitjaru, K. Watsanit and S. Adachi, J. Ind. Eng. Chem., 2012, 18, 225–229 CrossRef CAS.
- A. Panghal, S. Janghu, K. Virkar, Y. Gat, V. Kumar and N. Chhikara, Food Biosci., 2018, 21, 80–89 CrossRef CAS.
- J. Kovacs-Nolan, H. Kanatani, A. Nakamura, M. Ibuki and Y. Mine, J. Nutr., 2013, 143, 384–391 CrossRef CAS PubMed.
- H. M. Hamer, D. Jonkers, K. Venema, S. Vanhoutvin, F. J. Troost and R. -J. Brummer, Aliment. Pharmacol. Ther., 2008, 27, 104–119 CrossRef CAS PubMed.
- E. de Paula Franco, G. M. M. de Oliveira, R. R. Luiz and G. Rosa, Nutr. Hosp., 2015, 32 Search PubMed.
- T. P. Trinidad, A. S. Loyola, A. C. Mallillin, D. H. Valdez, F. C. Askali, J. C. Castillo, R. L. Resaba and D. B. Masa, J. Med. Food, 2004, 7, 136–140 CrossRef CAS PubMed.
- L. F. Smith, J. Patterson, L. T. Walker and M. Verghese, Int. J. Cancer Res., 2015, 12, 29–39 CrossRef.
- Y. Srivastava, A. D. Semwal, G. K. Sharma and A. S. Bawa, Food Nutr. Sci., 2011, 02, 214–221 CAS.
- P. Sobczak, K. Zawiślak, A. Starek, W. Żukiewicz-Sobczak, A. Sagan, B. Zdybel and D. Andrejko, Sustainability, 2020, 12, 1567 CrossRef CAS.
- K. D. P. P. Gunathilake and Y. M. R. K. Abeyrathne, J. Food Process. Preserv., 2008, 32(1), 133–142 CrossRef CAS.
- H. H. Salama, S. M. Abdelhamid and R. M. K. Dairouty, Pak. J. Biol. Sci., 2019, 22, 527–536 CrossRef CAS PubMed.
- D. O. Ayandipe, A. A. Adebowale, O. Obadina, K. Sanwo, S. B. Kosoko and C. I. Omohimi, J. Culin. Sci. Technol., 2022, 20, 1–32 CrossRef.
- M. K. S. Mihiranie, J. M. M. A. Jayasundera, P. M. H. D. Pathiraje and O. D. A. N. Parera, Physico-Chemical and Organoleptic Properties of Snack Crackers Incorporated with Defatted Coconut Flour, Proceedings of the Peradeniya University, International Research Sessions, Sri Lanka, 2014 Search PubMed.
- L. Hopkin, H. Broadbent and G. J. Ahlborn, Food Chem.: X, 2022, 13, 100182 CAS.
- T. Sundaresan, S. Subramaniam, V. Chinnapa and B. Rajoo, Int. J. Food Sci. Technol., 2023, 58, 5077–5088 CrossRef CAS.
- R. Jiamjariyatam, P. Roskhrua and S. Attiwittayaporn, J. Culin. Sci. Technol., 2022, 20, 278–292 CrossRef.
- S. Chandrashekar, J. Thangaraj and I. Dasappa, Int. J. Food Sci. Technol., 2019, 54, 1769–1776 CrossRef CAS.
- T. P. Trinidad, D. H. Valdez, A. C. Mallillin, F. C. Askali, A. S. Maglaya, M. T. Chua and D. B. Masa, Indian Coconut J., 2001, 6, 9–13 Search PubMed.
- J. B. Adeloye, H. Osho and L. O. Idris, J. Agric. Food Res., 2020, 2, 100042 Search PubMed.
- A. R. Islas-Rubio, F. Laborin-Escalante, F. Vásquez-Lara, L. C. Montoya-Ballesteros, G. Ramos-Clamont Montfort, A. M. Calderón de la Barca and N. G. Heredia-Sandoval, Plant Foods Hum. Nutr., 2023, 78, 314–319 CrossRef CAS PubMed.
- L. L. W. C. Yalegama, D. N. Karunaratne, R. Sivakanesan and C. Jayasekara, Food Chem., 2013, 141, 124–130 CrossRef CAS PubMed.
- T. Asavarujanon, S. Ratanasumawong and P. Rumpagaporn, J. Food Sci. Agri. Technol., 2022, 6, 72–77 Search PubMed.
- V. Fonseca-Bustos, T. J. Madera-Santana, M. Valenzuela-Melendres, A. R. Islas-Rubio and L. d C. Montoya-Ballesteros, J. Food Process. Preserv., 2023, 2023, 1–13 CrossRef.
- S. H. Tayang, M. M. Tahir and A. Syarifuddin, AIP conference proceedings, 2023, p. 040033 Search PubMed.
- M. Raczyk, B. Kruszewski and D. Michałowska, Molecules, 2021, 26, 4641 CrossRef CAS PubMed.
- E. Sykut-Domańska, P. Zarzycki, A. Sobota, D. Teterycz, A. Wirkijowska, A. Blicharz-Kania, D. Andrejko and J. Mazurkiewicz, J. Food Process. Preserv., 2020, 44(7), 14490 Search PubMed.
- N. Roshiya, S. Peter, S. S. Singh and K. K. Patel, Pharma Innovation, 2022, 11, 2029–2032 CrossRef CAS.
- S. H. Nile, V. Baskar, D. Selvaraj, A. Nile, J. Xiao and G. Kai, Nano-Micro Lett., 2020, 12, 45 CrossRef CAS PubMed.
- L. Grossmann and D. J. McClements, Trends Food Sci. Technol., 2021, 118, 207–229 CrossRef CAS.
- D. J. McClements and L. Grossmann, NPJ Sci. Food, 2021, 5, 17 CrossRef PubMed.
- I. Paranagama, I. Wickramasinghe, D. Somendrika and K. Benaragama, J. Microbiol., Biotechnol. Food Sci., 2022, 11, e4029 CrossRef CAS.
- J. S. Jongyingcharoen, P. Wuttigarn and R. Assawarachan, IOP Conf. Ser. Earth Environ. Sci., 2019, 301, 012033 CrossRef.
- D. D. Bawalan, Center for Occupational Research and Development, 2000, vol. 16, 01, pp. 34–34 Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |