Open Access Article
Open Access ArticleA comprehensive review on isochoric freezing: a recent technology for preservation of food and non-food items
Dhanya
R.
,
Abhirami
Panoth
and
N.
Venkatachalapathy
 *
*
Department of Food Engineering, NIFTEM-T, Thanjavur, TamilNadu, India. E-mail: venkat@iifpt.edu.in
First published on 12th October 2023
Abstract
Isochoric freezing, a cutting-edge preservation technique, has gained significant attention in recent years due to its potential to revolutionize various industries, including food preservation, biotechnology, and cryopreservation. It effectively increases the shelf life of farm-fresh produce while reducing browning reactions. This review focuses on presenting the state-of-the-art isochoric freezing of foods, emphasizing the fundamental principles that make it special and comprehending its impact on food quality and food and non-food applications, taking papers published in the last ten years into account. In contrast to conventional nonthermal activities, isochoric freezing can be achieved without using additional apparatus such as elevated pressure equipment or pulsed electric fields using simple, inexpensive, rigid closed-volume containers like house refrigerators or commercial cold storage facilities. Recent break-throughs in the field, such as food applications of isochoric freezing in grape tomatoes, spinach, potatoes, sweet cherries, and pomegranates, and non-food applications in mammalian cells, pancreatic islets, Escherichia coli, nematodes, and rat hearts, are highlighted. Hence isochoric freezing is a value-added process that helps to enhance food safety, and therefore on-going research and development in this area can make it an easily accessible preservation method in the near future.
Sustainability spotlightIsochoric freezing is a novel technique, which maintains food items at subfreezing temperatures without causing damage from ice crystal formation inside the product. It has relatively low energy needs. Food items preserved using isochoric freezing have a higher quality than those preserved through traditional freezing and use less energy overall. This review article enables understanding of various benefits of isochoric freezing in the food sector and also expands its application to various food products. Therefore, this review article is expected to contribute to Goal 2 “zero hunger” to achieve food security by minimising the post-harvest loss of fruits and vegetables and Goal 7 “affordable and clean energy” to achieve less energy utilization for the process under the SDGs. |
1 Introduction
Freezing of foods is considered one of the oldest methods of food preservation. Generally, the traditional freezing method entails bringing the meal's temperature to or below −18 °C. Isochoric preservation is an alternative to conventional cryopreservation methods since it requires less cryoprotectant and is simpler to apply. The temperature of the meal decreases when it is in contact with the freezing medium due to heat transfer. Food quality is influenced by several factors, including designs of systems, crystallization of ice, phase changes, freezing times, and others.1 Food is preserved through freezing, a technique that involves lowering the product's temperature to a point where ice crystals start to form inside the product's structure. To lower reaction rates that lead to product quality deterioration, the process's goal is to lower the product's temperature as much as is economically practical.2 Freezing can keep food at a low temperature, reducing water activity and trying to prevent the growth of microorganisms. However, permanent food damage can occur when ice crystals are large, abundant, and irregularly distributed. As a result, ice development is a critical parameter that must be monitored throughout frozen food manufacturing and storage.3,47 The physicochemical and biological mechanisms that control food deterioration are slowed down but not stopped by freezing. Below −18 °C, microbial growth is halted, and during frozen storage, non-enzymatic and enzymatic changes proceed at substantially slower rates.4,49 Different freezing methods and novel food freezing technologies, including microwave-assisted freezing, high-pressure freezing, cryogenic freezing, and vacuum freezing, can preserve food by freezing. Cold storage is an effective strategy for preserving the integrity of perishable commodities from manufacturing to consumer consumption.According to the FAO, 1.3 billion tonnes of food are lost yearly, accounting for 33% of total production. Food demand steadily increases and may reach 150–170% of current levels by 2050.5 In comparison, the loss rate of fruits and vegetables (FV) is higher than that of all other foods, such as meat, oil seeds, milk, cereals, fish, and seafood. The loss of FVs during the storage stage is 10%, which is higher than the loss of FVs during the harvesting, storage, processing, and distribution stages of the postharvest life cycle.6 Traditional freezing processes use an isobaric concept wherein volume and temperature variations are synched. The isobaric system freezes an unbounded quantity of the fluid in the food. The development of ice crystals inside food damages the cell structure of the food items. Isochoric freezing can prevent cell integrity losses by circumventing such constraints. Throughout isochoric freezing, the system's volume remains constant, while other elements, like temperature and pressure, change simultaneously.1 The use of technology in isochoric freezing is crucial. An airtight container positioned within a freezer serves as an isochoric freezing system. It is an easy-to-use, dependable device that doesn't require maintenance. Contrasting an isochoric system to a hyperbaric system, the technology is fairly simple. Unlike a hyperbaric freezing procedure, an isochoric system has no moving components, doesn't need the energy to run continuously, and has no danger of closure or movable component deterioration.6 Isochoric freezing is one of the preservation methods that has gained increasing attention because it has the potential to maintain the quality of vegetables and fruits that have been harvested without dramatically altering their physico-chemical and nutritional characteristics.7
2 Isochoric process principle
Rubinsky, Perez and Carlson, 2005 (ref. 8), originally studied the thermodynamic foundations of isochoric preservation. As an alternative method to decrease the negative impacts of freezing, such as structural destruction brought about by ice crystal formation, the thermodynamic advantages of freezing under a constant volume as opposed to constant pressure (isobaric) circumstances were introduced.8 The unique concept of “isochoric freezing” for preserving food below-freezing temperatures was developed based on fundamental thermodynamic ideas. Isochoric freezing can be seen as a unique method used under high-pressure conditions. Unlike conventional freezing methods, isochoric freezing increases pressure by lowering the temperature while keeping the volume constant. This means that pressure and temperature in this process are closely linked and remain in thermodynamic equilibrium throughout the phase transition due to the control of volume.9 The current industry practices for cold storage, which take place under constant volume (isochoric) instead of constant atmospheric pressure (isobaric) conditions, are intended to be replaced by this storage approach.10 An isochoric vessel remains closed to the environment, giving the perception that there is no difference between the two systems; however, when an isobaric vessel is exposed to the outside environment, it acts like a pressure reservoir. However, the studies based on thermodynamic properties show that the isochoric technique for cold storage differs greatly from its isobaric equivalent, which results in a large increase in the structural integrity of preserved food.11,12While freezing associated with high pressure has long been acknowledged as a potential way to reduce ice nucleation, hyperbaric (high-pressure) methods were previously primarily used in the food and medical industries and for cryomicroscopy fixation.13 Researchers first proposed pressure-freezing in 1968 while employing mechanically produced pressure. This method of hyperbaric cryopreservation kept the system's temperature well below 0 °C, while maintaining a high pressure, and it was followed by an abrupt pressure release that triggered rapid freezing.14 This resulted in the preserved material's structure being more like the original non-cryopreserved cell or tissue. The technique uses Le Chatelier's theory, which describes how the tremendous pressure created when water expands during freezing prevents ice from crystallizing. The current aqueous solution's phase transition temperature is dropping, the high pressure's most noticeable immediate consequence. The freezing temperature of the system can be lowered by 2100 bars, equivalent to about 20% glycerol concentration, and the procedure typically minimizes the number of chemical concentrations required at sub 0 °C.15
Supercooling refers to reducing the temperature of food beyond its freezing point without the development of ice crystals in the interior portion. Food processing industries have not embraced supercooling despite its evident advantages because freezing can occur in response to various kinetic stimuli and unpredictably changing thermal conditions. Another thermodynamically metastable state is supercooling. But recent studies have illustrated that isochoric circumstances considerably elevate metastable supercooled systems' reliability and reduce the frequency of the formation of ice crystals to nearly zero under moderate supercooled conditions.16,32Table 1 demonstrates different thermodynamic freezing storage modes and their operating temperatures.
More generally, some distinctive characteristics distinguish the freezing process in an isochoric system from that in an isobaric system, as in Table 2. The temperature at the ice–water interface that changes the phases of an isobaric set-up is consistent, independent of the interface placement and also the thermal records while freezing. On the other hand, in an isochoric system, the thermal records and placement of the interface affect the temperature at the contact. The pressure of an isochoric system changes whenever the transition of the phase interface, which initiates freezing, moves slightly. Simple explanations link the fluctuations to the difference in the densities of water and ice. When a specific volume of water freezes, a closed-volume system experiences elevated pressure resulting from the reduction in density causes further freezing. During solidification, we anticipate that this pressure change will be swift and steady along the whole isochoric system. The ice/water contact temperature will equalize at the new pressure due to the pressure change, which will immediately modify the temperature at the interface where the phase change occurs. The temperature of the phase transition contact is known and remains constant when solidification at the isobaric state occurs. The exact location of the interface is unknown; however, there must be a solution because it is unclear where and at what temperature isochoric solidification occurs.17
| Specification | Isobaric freezing system | Isochoric freezing system | Reference |
|---|---|---|---|
| Thermodynamic principle | Pressure is constant | Constant volume | 8 and 18 |
| Helmholtz free energy | Gibbs free energy | ||
| Nucleation of ice | Non-uniform nucleation | Homogeneous nucleation | 18 |
| Design | Open vessel | Airtight container | 19 |
| Energy | Energy consumption is high | 70% less energy than the conventional method | 18 |
| Price | Very expensive | Less cost | 18 |
3 Isochoric system
The first isochoric system was created to endure temperatures as moderate as −15 °C and pressures as 75.8 MPa. The system was equipped with tools for measuring temperature and pressure. The vitrification of the aqueous phase in an isochoric system was the subject of Szobota's second investigation, which was theoretical in nature.2,18 Preciado15 invented a new isochoric device that could endure pressures of 275 MPa and temperatures as low as 20 °C. The apparatus allowed for an increase in experimental temperature and pressure readings from −12 °C to −20 °C in an isochoric aqueous phase system. The suggested method was recently used to demonstrate that the worm C. elegans could survive in an isochoric system at 5 °C and 60 MPa.20 A cylinder-shaped, double-walled, jacketed stainless steel cylinder with carbon fiber composites, potent thermosets, and pressure transducers makes up the isochoric unit.21In isochoric operations, rupture discs are used, which are based on the pressure and temperature of the system. Furthermore, salt/sugar solutions work similarly to hurdle technology regarding preservation. After that, ice crystals form there and serve as a nucleation site. A nucleator is needed to stop ice crystals from accumulating while the food constituents remain in their aqueous phase.1 The ice and solution inside the chamber will remain in equilibrium if the extrinsic component is constant. The chambers have the following measurements: an inner diameter of 1 inch with a length of 3 inches and an exterior diameter of 2.13 inches. They are manufactured from stainless steel. A screw, a metal seal, and an extra electronic pressure transducer with a ruptured disc of 60 MPa are used to close and seal the chamber. In order to control the temperature, the device requires a water bath.22,48
4 Energy requirements
The current global cold chain uses much energy, and it's estimated that only domestic food cold storage uses close to 4% of the total annual electricity used worldwide, emitting 6.54 × 108 metric tons of emitted carbon dioxide, which costs more than 120 billion dollars. There are structural differences in food availability between high- and low-income countries due to the high cost of essential cold storage infrastructure, which is determined by economic and climatological factors.23 The stability of the world's expanding population depends on an effective cold food supply system; by 2027, the global market for frozen foods is anticipated to reach $404.8 billion. However, under typical industry-standard isobaric settings, frozen meals are frequently kept below 19 degrees Fahrenheit, which can harm the food's nutritional content and textural quality and result in high energy and carbon emissions.11,245 Advantages and disadvantages of the isochoric process
In the food industry, freezing under atmospheric pressure is the main method for extending the shelf life of the food product. The interactions that cause deterioration are hindered by crystallization and low temperatures of water while freezing, which also stops the growth of pathogens and spoilage germs. But as food is frozen, ice forms, which brings down the quality of the thawed food and hinders the customer acceptability of these frozen items. Modern freezing technology, known as isochoric freezing, can greatly enhance the texture and flavor of frozen foods. The thermodynamic circumstances of freezing are very important. Research on potatoes, tomatoes and cherries has indicated that isochoric freezing increases the nutritional value of food stored below the freezing point. According to this research, isochoric freezing greatly improved the nutritional, chemical, and physical quality of foods stored compared to traditional techniques. The sample has no ice crystal formation, and the technique was carried out at low pressures and temperatures; thus, the quality is quite good.25In addition, the current research has demonstrated that isochoric freezing can obliterate potentially hazardous germs. According to experiments, bacteria can be killed by a synergistic mixture of moderately high pressure (135 MPa) and moderately low temperature (−15 °C) and freezing in an isochoric system requires less energy than freezing in a conventional freezer. Basic thermodynamic calculations have mentioned that freezing in an isochoric system can save up to 70% of energy over conventional freezing. The reason for this is that as the temperature rises, the whole frozen mass decreases, and also, a declining trend is observed in the latent heat of fusion.
Furthermore, food processed through isochoric processes can prevent potential variations in temperature during transportation and storage since temperature variations induce phase shifts compared to sensible temperature changes. As a result, rather than the food product heating up at a high temperature, the ice inside the chamber begins to melt. One major downside of isochoric freezing is the development of high pressure within the processing compartment. Hydrostatic pressure can cause tissue breakdown in biological food matter, reducing quality. However, the processing temperature can be changed because it is connected to control the maximum processing pressure.26
6 Food application of the isochoric process
6.1 Grape tomatoes
An isochoric freezing study was conducted on tomatoes. High vitamin C, phenols, and carotenes (particularly lycopene) contribute significantly to human nutrition in tomatoes. Tomatoes, especially grape tomatoes, should be maintained at 10 °C or above after harvesting to prevent chilling damage.27 Tomatoes are unsuitable for conventional freezing because of severe texture degradation, color change, and nutritional degradation throughout and after frozen storage. An OC-9 pressure chamber constructed from 316 stainless steel that is sealed and coupled to an electronic pressure transducer makes up the isochoric system. The equipment was cooled using a water and ethylene glycol bath with a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 composition. This system operates at a temperature of −2.5 °C. Compared to conventional techniques like cold storage and IQF, isochoric preservation produced tomatoes with more consistent quality. Tomatoes kept in an isochoric system at −2.5 °C showed the best mass, morphology, color, and textural features. Furthermore, the ascorbic acid content, lycopene level, antioxidant qualities and phenolic compounds of tomatoes were all preserved because the segregation of the cells was preserved after isochoric freezing. Because isochoric freezing avoids the development of ice crystals and only slightly damages tomato tissues, as cryo-SEM shows, it preserves tomatoes' nutritional content.28
50 composition. This system operates at a temperature of −2.5 °C. Compared to conventional techniques like cold storage and IQF, isochoric preservation produced tomatoes with more consistent quality. Tomatoes kept in an isochoric system at −2.5 °C showed the best mass, morphology, color, and textural features. Furthermore, the ascorbic acid content, lycopene level, antioxidant qualities and phenolic compounds of tomatoes were all preserved because the segregation of the cells was preserved after isochoric freezing. Because isochoric freezing avoids the development of ice crystals and only slightly damages tomato tissues, as cryo-SEM shows, it preserves tomatoes' nutritional content.28
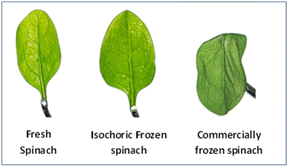
6.2 Spinach
The High Pressure Equipment Company supplied an alloy steel pressure chamber for the isochoric system (Erie, PA, USA). The capacity of the entire volume was 66 mL. The pressure chamber was cooled in a recirculation bath and connected to an electronic pressure transducer. The effects of three different methods of spinach preservation were compared to those of fresh and industrially frozen spinach. In the first method, spinach leaves were immersed in an isochoric container with a concentration of 10° Brix sucrose. The chamber was filled with the 10° Brix sucrose solutions and then tightly closed. Then, it was put in a recirculating chilling bath set at −4.0 ± 0.9 °C. The pressure inside the chamber rose to 29.7 ± 0.2 MPa. Between 1 and 7 days, spinach leaves were analysed. The second method contained spinach leaves in an isobaric system with a plastic bag holding a 10° Brix sucrose solution. The bag was left in a circulating water bath at −4.0 ± 0.9 °C for up to 7 days.In the third approach, spinach leaves were vacuum-packed and then submerged in a recirculating bath for up to seven days at −4.0 ± 0.9 °C. Samples were defrosted following each treatment for 30 minutes at room temperature before being analyzed. Isochoric freezing of spinach preserves qualitative attributes and keeps the texture of the leaves better than isobaric freezing. Furthermore, compared to commercially frozen isochoric samples, those stored for a week showed improved features. Isochoric freezing has been associated with decreased cell damage since there are no ice crystals in the spinach leaves during freezing.22,29
(Source: Bilbao et al.,22 2020).
6.3 Potatoes
Simple isochoric freezing mechanisms were used for the preservation of potatoes. A constant volume chamber is required to resist the system's developing pressures with little distortion. They need a pressure transducer for control. An OC-1 pressure vessel made specifically for the isochoric chamber has an O-ring of 316 stainless steel, an inner capacity of 125 mL, a working pressure of 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 psi, and a test pressure of 20
800 psi, and a test pressure of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 psi. A pressure gauge and an NI, my DAQ connector, are linked to the constant volume cabinet, which is fastened with a screw and metal seal. LabVIEW is used to record and display the data from the experiment. A rupture disc restricted the pressure to 60 MPa for safety. A bath of water and ethylene glycol (50/50) was used to cool the isochoric chamber.
000 psi. A pressure gauge and an NI, my DAQ connector, are linked to the constant volume cabinet, which is fastened with a screw and metal seal. LabVIEW is used to record and display the data from the experiment. A rupture disc restricted the pressure to 60 MPa for safety. A bath of water and ethylene glycol (50/50) was used to cool the isochoric chamber.
The samples underwent three treatments: isochoric therapy, isobaric treatment, and untreated. The isochoric chamber was carefully shut after being filled with an isotonic sucrose solution to prevent air bubbles from getting trapped. It is crucial to stress that caution must be taken when removing air from the system. The outcomes may be impacted by undissolved air.30 The chamber was then chilled to −5 °C and submerged entirely in the cooling fluid. Warmth was applied to the isochoric chamber until atmospheric pressure was achieved. Then, the cabinet was evacuated to examine the sample. The isobaric therapy followed identical procedures to the isochoric therapy, except that the cabinet was exposed to atmospheric pressure.
Samples were placed in a cooling water bath at a temperature of −5 °C for 2 hours. There is no discernible variation in weight when kept at −5 °C under isochoric conditions vs. room temperature storage. Moreover, despite isochoric refrigeration, no weight loss is observed after isochoric storage at temperatures below 0 °C. The considerable decrease in browning under isochoric preservation compared to isobaric freezing at the same temperature may be another important aspect of isochoric chilling. The integrity of cellular membranes and the isosmotic characteristics of the intracellular and extracellular environment prevent weight loss or severe browning when isochoric refrigeration is carried out to a temperature of −5 °C.31
Yuanheng Zhao32 and his team experimented with freezing potato cylinders within an isochoric system. They explored a comprehensive set of factors, including three processing methods (submersion in water, vacuum packaging, and submersion in an ascorbic acid solution), four different freezing temperature/pressure conditions (−3 °C/37 MPa, −6 °C/71 MPa, −9 °C/101 MPa, and −15 °C/156 MPa), and two average compression rates (below 0.02 and above 0.16 MPa s−1), which focused on understanding how these variables affected the critical quality aspects of frozen potatoes, such as changes in the mass, volume, water retention capacity, color, and texture after thawing.
The analysis revealed that the processing method and freezing temperature/pressure significantly impacted the results, whereas the compression rate had a comparatively lower influence. Among the processing methods, immersing the potatoes in a 5% ascorbic acid solution was the most effective in preserving quality attributes. At the highest pressure level of 156 MPa and a low compression rate of 0.02 MPa s−1, potato samples treated with ascorbic acid retained their color, 98.5% of their mass, and an 84% elasticity modulus value. These samples also exhibited a 1% increase in volume and a 13% increase in maximum stress, attributed to pressure-induced hardening.
6.4 Sweet cherries
Before processing the samples, fresh cherries (6–7 g) were stored at 5 °C after being purchased from a nearby supermarket and local growers. The R1 pressure chamber, composed of 4340 alloy steel, was the main component of the isochoric system. It possessed an internal width of 1 inch, an external width of 3.1/5 inches, and an internal length of 6 inches, with a combined volume capacity of 66 mL. A screw and metal seal were used to seal the constant volume compartment. A laptop and an electronic pressure transducer were attached to the pressure chamber. Data recording and the graphical software Additel were used to record and show the data. The equipment was cooled using a bath of ethylene glycol and water (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) solutions. Three techniques were utilized to preserve fresh cherries: individual fast freezing (IQF), preservation for a full day in the range of −4 °C to −7 °C in an isochoric system, and preservation for 24-hours at −4 °C or −7 °C in an isobaric system. Sweet cherries had precisely the same nutritional and qualitative characteristics as fresh cherries when they were isochronally frozen at −4 °C. At −4 °C, isochoric freezing preserved the texture of the cherry and decreased drip loss. As examined under cryo-SEM, the effectiveness of isochoric freezing, which lessened cellular damage in cherry tissue, was correlated with the absence of ice crystals in the sweet cherries throughout freezing. The cherries had the closest color to fresh cherries despite being translucent due to isochoric freezing. Additionally, isochoric freezing at −4 °C retained the antioxidant activity, phenolic compounds, and ascorbic acid of cherries because cell fragmentation was preserved throughout freezing.6
50) solutions. Three techniques were utilized to preserve fresh cherries: individual fast freezing (IQF), preservation for a full day in the range of −4 °C to −7 °C in an isochoric system, and preservation for 24-hours at −4 °C or −7 °C in an isobaric system. Sweet cherries had precisely the same nutritional and qualitative characteristics as fresh cherries when they were isochronally frozen at −4 °C. At −4 °C, isochoric freezing preserved the texture of the cherry and decreased drip loss. As examined under cryo-SEM, the effectiveness of isochoric freezing, which lessened cellular damage in cherry tissue, was correlated with the absence of ice crystals in the sweet cherries throughout freezing. The cherries had the closest color to fresh cherries despite being translucent due to isochoric freezing. Additionally, isochoric freezing at −4 °C retained the antioxidant activity, phenolic compounds, and ascorbic acid of cherries because cell fragmentation was preserved throughout freezing.6
6.5 Pomegranate
In order to bring down the initial microbial load, 200 liters of sodium hypochlorite solution were used to wash pomegranates. Husks from pomegranate fruits have undergone physical processing to separate the arils. Pomegranate fruits as whole and freshly cut arils were stored for one month using four different techniques: isochoric freezing at 2.5 °C and 12 MPa, kept in cold storage with a temperature of 5 °C and 95% RH, isochoric super cooling at a temperature of 2.5 °C and 0.1 MPa and isobaric freezing at 2.5 °C and 0.1 MPa. The color, antioxidant activity, texture and ascorbic acid content increased when the whole pomegranate was isochoric supercooled. Isochoric super cooling at a temperature of −2.5 °C was among the effective preservation techniques for newly cut arils. Isochoric super cooling improved color and texture maintenance, ascorbic acid content, and microbial growth. Cold storage resulted in observable microbiological damage and quality loss in the volume, color, texture, and phytochemical content. Isobaric freezing, which also dramatically changed the color and texture of the arils, was the cause of the bulk of the mass loss. Isochoric freezing at a temperature of 2.5 °C hindered the proliferation of microbes compared to isochoric supercooling; however, it negatively damaged the color of the pomegranate aril.336.6 Meat
Tsekwi Gracious Rinwi and coworkers34 conducted isochoric freezing experiments on beef for the first time. The effects of process factors like pressure, temperature, and solution concentration were examined on the sample's quality traits, such as color, weight loss, texture, microstructure, water holding capacity, and water mobility. At 4 and 8 °C, chicken breast pieces were submerged in isochoric sodium chloride solutions with concentrations ranging from 0, 1.5, and 2.5%. The findings of the study showed that increasing the concentration of sodium chloride decreased the pressure and freezing point, and samples infused with pure water and 1.5% NaCl solution at 4 and 8 °C showed a notable reduction (P ≤ 0.05) in the quality factors, while samples treated with 2.5% solution at 4 and 8 °C showed no significant difference from the control sample. This study suggested enhancing the meat quality stored in isochoric systems.34The fundamental mechanisms governing moisture movement in three distinct isochoric treatment scenarios: direct immersion freezing (DIF), vacuum pack freezing (VPF), and vacuum immersion pack freezing (VIPF) and their molecular interactions and their impact on the overall quality of chicken breast meat were studied, utilizing a 2.5 g dL−1 sodium chloride solution at a temperature of −4 °C. Various moisture transport processes, including diffusion and infusion, led to alterations in water distribution and mobility, which had a significant (P < 0.05) influence on several characteristics such as color, water holding capacity (WHC), pH, cooking loss, total volatile basic nitrogen, thiobarbituric acid reactive substances, solubility, and microstructural integrity of the DIF-treated samples. In contrast, VIPF-treated samples displayed minor changes, except for pH and WHC, while VPF-treated samples did not significantly differ from fresh samples (P > 0.05). This study suggests that the choice of the isochoric freezings can be tailored depending on the nature of the sample to achieve intended results.35
6.7 Fish
As isochoric freezing employs extremely low temperatures and prevents ice production inside the fillets, it is a potential approach for preserving tilapia. Freezing, super chilling and chilling were contrasted with isochoric freezing. Muscle color changes caused by isochoric freezing were comparable to those caused by other preservation techniques.The fish fillets became softer after all preservation methods, with the isochoric frozen fish fillet exhibiting a texture that resembles the fresh sample. The thiobarbituric acid reactive components of isochoric samples were comparable to those of fresh samples. TBARS increased by 53%, 55%, and 34% for chilled, super-chilled, and frozen samples, respectively. The whole volatile nitrogen level for isochoric samples was 1.4 times more than for fresh samples. Isochoric freezing delayed the post-mortem deterioration processes naturally without having the negative impacts of ice formation that come with subfreezing temperature storage techniques. Therefore, microbial spoilage, lipid oxidation, and muscle damage were decreased compared to those processed under frozen, chilled, and super-chilled storage settings. However, due to tissue distortions brought about by pressure under isochoric conditions, isochoric freezing retained the firmness of the fish fillet more effectively than standard preservation methods.29 Năstase and team45 compared isochoric and isobaric freezing of fish muscle and observed that the cellular dehydration is absent in isochoric freezing, which consequently ensures that the frozen tissue's structure remains unchanged. Table 3 summarises the food applications of isochoric freezing.
| Food item | Isochoric conditions | Remarks | References |
|---|---|---|---|
| Grape tomatoes | −2.5 °C and 25 MPa | Preserves nutritional value and maintains physicochemical | 28 |
| Spinach | −4 °C and 29.7 MPa | Better preserved the quality characteristics, kept cells intact and prevented cellular dehydration | 22 |
| Potatoes | −5 °C and −3 °C (30 MPa) | No weight loss or significant browning, maintained cell integrity | 31 |
| Sweet cherries | −4 °C or −7 °C, 29.5 MPa, 24 h | Reduced cellular damage, preserved the nutritional value | 6 |
| Pomegranates | −2.5 °C or 12 MPa | Color and texture retention reduced microbial | 33 |
| Meat | −4 and −8 °C | Maintained the quality | 34 |
| Fish (tilapia) | −5 °C, 3 h | Retained the texture, reduced the microbial load | 29 |
7 Non-food application of isochoric freezing preservation
7.1 Escherichia coli
Escherichia coli cultures were used to study how the isochoric freezing method affected them. The cultures were housed in stainless steel containers to withstand the internal pressures created during cooling in isochoric settings. Unlike high hydrostatic pressure (HHP), this method needs cooling, which requires an external mechanism to provide a load and increase pressure. The volume remains unchanged when the sample is cooled below the freezing point. According to the water phase diagram, this causes a significant increase in internal pressure.6 This study combines spectrophotometric survival curves and atomic force microscope pictures. The findings demonstrate that Escherichia coli populations are partially destroyed by cooling at −20 and −30 °C.367.2 Salmonella typhimurium and Listeria monocytogenes
The application of isochoric freezing to reduce bacterial load is particularly appealing due to its ease of engineering. Unlike traditional pasteurization techniques, isochoric freezing does not involve heat, so it can be applied to foods that cannot tolerate heating. The simple isochoric system was made up of steel alloy and had an inner diameter of 2.54 cm with a total capacity of 66 mL. Customers have another way to sterilize food at home besides boiling it by using it in their home freezers. Because it is a simple rigid, closed-volume device, the isochoric system can be used for preservation and microbial reduction in standard industrial chilling and freezing infrastructure without altering the present facilities.387.3 Pancreatic islets
The basic, rigid, constant-volume enclosure used to conserve the islets is maintained at a steady subzero temperature. The pressure chamber is an OC-1 cylindrical 316 stainless steel pressure chamber specially made for the job. It has one standard 14′′ OD port and a volume of 125 mL with a working pressure of 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 psi. Additionally, a 29
800 psi. Additionally, a 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 psi rupture disc was deployed as a safeguard. Data were collected using a data collecting device, LabVIEW 2016, and a digital pressure gauge (Ashcroft 2174) to determine the chamber's internal pressure. With ethylene glycol and water mixed 50/50 as the liquid medium, a refrigerated bath/circulator was used to chill and maintain the temperature of the isochoric chamber. Simple mechanical agitation cannot cause islets to de-cluster because of the restricted and pressured liquid volume in which they are kept during isochoric freezing. Because of these innate qualities, isochoric freezing techniques are excellent for islet preservation.10
000 psi rupture disc was deployed as a safeguard. Data were collected using a data collecting device, LabVIEW 2016, and a digital pressure gauge (Ashcroft 2174) to determine the chamber's internal pressure. With ethylene glycol and water mixed 50/50 as the liquid medium, a refrigerated bath/circulator was used to chill and maintain the temperature of the isochoric chamber. Simple mechanical agitation cannot cause islets to de-cluster because of the restricted and pressured liquid volume in which they are kept during isochoric freezing. Because of these innate qualities, isochoric freezing techniques are excellent for islet preservation.10
7.4 Mammalian cells
Preserving cells and organs by isochoric freezing has recently been proposed as a new technique. To investigate the effects of isochoric freezing on mammalian cells, Madin–Darby canine kidney epithelial cells (MDCK) were kept in an isochoric system and frozen, in a simple extracellular phosphate buffered solution, at −10 °C, −15 °C, and −20 °C, for 60 and 120 minutes, respectively. The structural integrity of the cell membranes and metabolism were examined using a live/dead viable cell assay and cell imaging. Cell survival declines as the exposure time and pressure rise (lowering temperatures). For instance, just 18% of cells survived 120 minutes at – 10 °C, while 60% did so for 60 minutes. At – 20 °C, negligible survival was seen. This study may provide a starting point for future investigations into methods for enhancing the impact of isochoric freezing on human biological matter.397.5 Rat hearts
Rat hearts were kept in intracellular solution for an hour at isochoric pressures of 0 °C (0.1 MPa), −4 °C (41 MPa), −6 °C (60 MPa), and −8 °C (78 MPa). Histological analysis and Langendorff perfusion were used to gauge the heart's vitality. Regarding their physiological function, hearts preserved on ice at atmospheric pressure and those stored at 4 °C (41 MPa) showed no appreciable variation in the histological injury score. Hearts at −4 °C had much less interstitial edema, indicating protection from increasing vascular permeability during storage. Cell swelling and considerable edema destruction occurred in hearts held at −6 °C (60 MPa), while major morphological deformation occurred at −8 °C (78 MPa). Importantly, isochoric preservation increases pressure and causes tissue damage at lower temperatures (−6 °C or lower), which could be dangerous. Techniques for isochoric preservation should be improved to prevent the negative effects of high pressure while keeping the benefits of low temperatures and slowed metabolism.407.6 Nematode – Caenorhabditis elegans
The study was conducted on whether the nematodes survive under isochoric freezing conditions. The experimental setup comprises a cryogenic vial containing the nematodes, an instrumented isochoric chamber (O-ring, 316 SS, inner volume 100 mL, maximal pressure 241.3 MPa) and a chilling bath. The pressure formed inside the isochoric chamber was recorded by using an Ashcroft 4–20 mA Loop-Powered 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 psi pressure gauge (made by National Instruments), and LabVIEW was used to record and display the data. In a cooling bath, the isochoric container is cooled. The results show that the longer exposure unaffected the worm's 100% survival rate. When immersed in a simple isotonic medium without cryoprotectants, the nematode Caenorhabditis elegans may withstand subfreezing temperatures inside an isochoric system. In an isochoric system lacking cryoprotective chemicals, biological material may be able to endure temperatures below freezing. By confirming the theoretical hypotheses, this study offers a novel technique for the subfreezing preservation of tissue, organs, and organisms that may be useful for biotechnology and medicine as well as new potential techniques of live creature survival in nature.41 In a nutshell, various non-food applications of isochoric freezing have been listed in Table 4.
000 psi pressure gauge (made by National Instruments), and LabVIEW was used to record and display the data. In a cooling bath, the isochoric container is cooled. The results show that the longer exposure unaffected the worm's 100% survival rate. When immersed in a simple isotonic medium without cryoprotectants, the nematode Caenorhabditis elegans may withstand subfreezing temperatures inside an isochoric system. In an isochoric system lacking cryoprotective chemicals, biological material may be able to endure temperatures below freezing. By confirming the theoretical hypotheses, this study offers a novel technique for the subfreezing preservation of tissue, organs, and organisms that may be useful for biotechnology and medicine as well as new potential techniques of live creature survival in nature.41 In a nutshell, various non-food applications of isochoric freezing have been listed in Table 4.
| Sample/item | Isochoric conditions | Remarks | References |
|---|---|---|---|
| Mammalian cells | −10 °C, −15 °C, and −20 °C for 60 and 120 minutes, respectively | 60% of cells are alive at 10 °C for 60 minutes | 39 |
| Pancreatic islet | −3 °C | 72 hour survival, resilient morphological integrity | 10 |
| Salmonella typhimurium and Listeria monocytogenes | −15 °C, 24 h | Living in an isochoric environment | 38 |
| Escherichia coli | −15 °C | All bacteria are eliminated | 36 and 37 |
| Rat hearts | −4 °C | Decrease in interstitial edema | 40 |
| Nematode | −5 °C | Alive in isochoric freezing | 41 |
8 Industrial applications
Nearly all biological substances contain substantial amounts of moisture, and reducing the temperature to the freezing point of water can lead to physical harm. The formation of ice inside and outside of cells can substantially impact the overall quality of preserved materials. Isochoric preservation, which maintains a constant volume during freezing, is being investigated in the food industry to reduce food waste and retain the nutritional and sensory characteristics of perishable products while extending their shelf life. This breakthrough opens up opportunities to enhance the marketability of food products that, under traditional freezing methods, suffer from quality deterioration during freezing and thawing processes, examples being tomatoes, berries, and leafy greens. Furthermore, this innovative technology could also find practical applications in sterilizing and preserving beverages like milk, tea, and juices.42,44 Isochoric freezing can significantly prolong the shelf life of these products by reducing microbial contamination, while retaining most of their sensory, nutritional, and functional attributes.Additionally, isochoric freezing introduces a fresh perspective on incorporating solutes into food products by immersing them in hypertonic solutions. These solutions can be enriched with micronutrients and bioactive components, resulting in novel products endowed with innovative sensory properties and improved nutritional profiles. Isochoric freezing can be an advantageous addition to the food cold chain. Nonetheless, there remains a requirement for developing and producing large-scale isochoric freezing chambers that can efficiently and economically cater to industrial demands.43,46
9 Conclusion and future scope
The food industry uses various preservation techniques to preserve food quality while minimizing storage losses. Because food preservation affects several nutritional factors, a creative, cost-effective strategy is now required, and some researchers have stated that isochoric freezing can improve preservation outcomes to obtain better results. One of the most advantageous aspects of the isochoric freezing process is the ripening delay without losing nutritional characteristics. Compared to other standard freezing procedures, isochoric treatment significantly improves the preservation of favorable storage conditions without affecting the water activity of foods. Isochoric freezing doesn't require changing the existing refrigeration system because isochoric compartments can be stored in any industrial freezer.Consequently, isochoric freezing might be a technology with added value in the cold supply chain. Industrial-scale design and production of isochoric freezing chambers are still required to meet industrial needs. Isochoric freezing is more effective in lowering microbial loads than conventional (isobaric) procedures. The circumstances that result in this antibacterial action can easily be exploited under frozen-storage conditions for pasteurization and boosting food safety, even if it is unknown how isochoric freezing eliminates bacteria. Isochoric freezing considerably raises food quality, while extending the shelf life of newly harvested and other perishable food items. It is used in fields as diverse as medicine, biology, and pharmaceuticals for inter-biopreservation.
Author contributions
Dhanya R.: conceptualization, methodology, and writing – original draft; Panoth Abhirami: writing – original draft and review & editing; N. Venkatachalapathy: review & editing and supervision.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors thank the National Institute of Food Technology, Entrepreneurship and Management – Thanjavur, Ministry of Food Processing Industries, Government of India, for all the support.References
- S. Nida, J. A. Moses and C. Anandharamakrishnan, Isochoric freezing and its emerging applications in food preservation, Food Eng. Rev., 2021, 1–10, DOI:10.1007/s12393-021-09284-x.
- D. R. Heldman, Food freezing, in Handbook of food engineering, CRC Press, 2006, pp. 439–482 Search PubMed.
- G. Jia, Y. Chen, A. Sun and V. Orlien, Control of ice crystal nucleation and growth during the food freezing process, Compr. Rev. Food Sci. Food Saf., 2022, 21(3), 2433–2454, DOI:10.1111/1541-4337.12950.
- M. S. Rahman and J. F. Velez-Ruiz, Food preservation by freezing, in Handbook of food engineering, CRC Press, 2007, pp. 653–684 Search PubMed.
- A. M. Elgarahy, M. G. Eloffy, A. Alengebawy, D. M. El-Sherif, M. S. Gaballah, K. Z. Elwakeel and M. El-Qelish, Sustainable management of food waste; pre-treatment strategies, techno-economic assessment, bibliometric analysis, and potential utilisations: a systematic review, Environ. Res., 2023, 115558, DOI:10.1016/j.envres.2023.115558.
- C. Bilbao-Sainz, A. Sinrod, M. J. Powell-Palm, L. Dao, G. Takeoka, T. Williams and T. McHugh, Preservation of sweet cherry by isochoric (constant volume) freezing, Innovative Food Sci. Emerging Technol., 2019, 52, 108–115, DOI:10.1016/j.ifset.2018.10.016.
- H. Afreen and I. S. Bajwa, An IoT-based real-time intelligent monitoring and notification system of cold storage, IEEE Access, 2021, 9, 38236–38253, DOI:10.1109/ACCESS.2021.3056672.
- B. Rubinsky, P. A. Perez and M. E. Carlson, The thermodynamic principles of isochoric cryopreservation, Cryobiology, 2005, 50, 121–138, DOI:10.1016/j.cryobiol.2004.12.002.
- Z. Zhu, T. Li and D. W. Sun, Pressure-related cooling and freezing techniques for the food industry: Fundamentals and applications, Crit. Rev. Food Sci. Nutr., 2021, 61(17), 2793–2808, DOI:10.1080/10408398.2020.1841729.
- M. J. Powell-Palm, Y. Zhang, J. Aruda and B. Rubinsky, Isochoric conditions enable high subfreezing temperature pancreatic islet preservation without osmotic cryoprotective agents, Cryobiology, 2019, 86, 130–133, DOI:10.1016/j.cryobiol.2019.01.003.
- Y. Zhao, M. J. Powell-Palm, J. Wang, C. Bilbao-Sainz, T. McHugh and B. Rubinsky, Analysis of global energy savings in the frozen food industry made possible by transitioning from conventional isobaric freezing to isochoric freezing, Renewable Sustainable Energy Rev., 2021, 151, 111621, DOI:10.1016/j.rser.2021.111621.
- P. K. Solanki and Y. Rabin, Thermo-mechanics aspects of isochoric cryopreservation: A new modeling approach and comparison with experimental data, PLoS One, 2022, 17(4), e0267852, DOI:10.1371/journal.pone.0267852.
- L. Cheng, D. W. Sun, Z. Zhu and Z. Zhang, Emerging techniques for assisting and accelerating food freezing processes: A review of recent research progresses, Crit. Rev. Food Sci. Nutr., 2017, 57(4), 769–781, DOI:10.1080/10408398.2015.1004569.
- D. Li, Z. Zhu and D. W. Sun, Effects of freezing on cell structure of fresh cellular food materials: A review, Trends Food Sci. Technol., 2018, 75, 46–55, DOI:10.1016/j.tifs.2018.02.019.
- J. A. Preciado and B. Rubinsky, Isochoric preservation: a novel characterization method, Cryobiology, 2010, 60(1), 23–29, DOI:10.1016/j.cryobiol.2009.06.010.
- J. M. Powell-Palm, B. Rubinsky and W. Sun, a. Freezing water at constant volume and under confinement, Commun. Phys., 2020, 3, 39, DOI:10.1038/s42005-020-0303-9.
- C. James, G. Purnell and S. J. James, A review of novel and innovative food freezing technologies, Food Bioprocess Technol., 2015, 8, 1616–1634, DOI:10.1007/s11947-015-1542-8.
- S. A. Szobota and B. Rubinsky, Analysis of isochoric subcooling, Cryobiology, 2006, 53(1), 139–142, DOI:10.1016/j.cryobiol.2006.04.001.
- J. A. Preciado and B. Rubinsky, Isochoric preservation: a novel characterization method, Cryobiology, 2010, 60(1), 23–29, DOI:10.1016/j.cryobiol.2009.06.010.
- H. Mikus, A. Miller, G. Nastase, A. Serban, M. Shapira and B. Rubinsky, The nematode Caenorhabditis elegans survives subfreezing temperatures in an isochoric system, Biochem. Biophys. Res. Commun., 2016, 477(3), 401–405, DOI:10.1016/j.bbrc.2016.06.089.
- S. Thakur, B. Jha, N. Bhardwaj, A. Singh, P. D. Sawale and A. Kumar, Isochoric freezing of foods: A review of instrumentation, mechanism, physicochemical influence, and applications, J. Food Process. Preserv., 2022, e17113, DOI:10.1111/jfpp.17113.
- C. Bilbao-Sainz, A. G. Sinrod, L. Dao, G. Takeoka, T. Williams, D. Wood and T. McHugh, Preservation of spinach by isochoric (constant volume) freezing, Int. J. Food Sci. Technol., 2020, 55(5), 2141–2151, DOI:10.1111/ijfs.14463.
- J. L. Dupont, P. Domanski, P. Lebrun and F. Ziegler, The Role of Refrigeration in the Global Economy-38, Informatory Note on Refrigeration Technologies, 2019 Search PubMed.
- A. Kumari, A. K. Chauhan and P. Tyagi, Isochoric freezing: An innovative and emerging technology for retention of food quality characteristics, J. Food Process. Preserv., 2022, 46(8), e16704, DOI:10.1111/jfpp.16704.
- G. Năstase, F. Botea, G. A. Beşchea, Ş. I. Câmpean, A. Barcu, I. Neacşu and A. Şerban, Isochoric Supercooling Organ Preservation System, Bioengineering, 2023, 10(8), 934, DOI:10.3390/bioengineering10080934.
- T. McHugh, Isochoric freezing: A new technology for food preservation, Food Technol., 2019, 73(11), 66–68 Search PubMed.
- M. Cantwell, X. Nie and G. Hong, Impact of storage conditions on grape tomato quality, in 6th ISHS postharvest symposium, Antalya, Turkey, 2009, vol. 8 Search PubMed.
- C. Bilbao-Sainz, A. J. Sinrod, L. Dao, G. Takeoka, T. Williams, D. Wood and T. McHugh, Preservation of grape tomato by isochoric freezing, Food Res. Int., 2021, 143, 110228, DOI:10.1016/j.foodres.2021.110228.
- C. Bilbao-Sainz, A. J. Sinrod, T. Williams, D. Wood, B. S. Chiou, D. F. Bridges and T. McHugh, Preservation of tilapia (Oreochromis aureus) fillet by isochoric (constant volume) freezing, J. Aquat. Food Prod. Technol., 2020, 29(7), 629–640, DOI:10.1080/10498850.2020.1785602.
- P. A. Perez, J. Preciado, G. Carlson, R. DeLonzor and B. Rubinsky, The effect of undissolved air on isochoric freezing, Cryobiology, 2016, 72(3), 225–231, DOI:10.1016/j.cryobiol.2016.04.002.
- C. Lyu, G. Nastase, G. Ukpai, A. Serban and B. Rubinsky, Isochoric refrigeration of food products (No. e2740v1), PeerJ Prepr., 2017 DOI:10.7287/peerj.preprints.2740v1.
- Y. Zhao, C. Bilbao-Sainz, D. Wood, B. S. Chiou, M. J. Powell-Palm, L. Chen and B. Rubinsky, Effects of isochoric freezing conditions on cut potato quality, Foods, 2021, 10(5), 974, DOI:10.3390/foods10050974.
- C. Bilbao-Sainz, B. S. Chiou, G. Takeoka, T. Williams, D. Wood, M. J. Powell-Palm and T. McHugh, Isochoric freezing and isochoric supercooling as innovative postharvest technologies for pomegranate preservation, Postharvest Biol. Technol., 2022, 194, 112072, DOI:10.1016/j.postharvbio.2022.112072.
- T. G. Rinwi, D. W. Sun, J. Ma and Q. J. Wang, Effects of isochoric freezing on freezing process and quality attributes of chicken breast meat, Food Chem., 2023, 405, 134732, DOI:10.1016/j.foodchem.2022.134732.
- T. G. Rinwi, D. W. Sun, J. Ma and Q. J. Wang, Underlying mechanisms and molecular interactions of isochoric freezing on enhancing the freshness of chicken breast meat, LWT, 2023, 115305, DOI:10.1016/j.lwt.2023.115305.
- S. Salinas-Almaguer, A. Angulo-Sherman, F. J. Sierra-Valdez and H. Mercado-Uribe, Sterilization by cooling in isochoric conditions: the case of Escherichia coli, PLoS One, 2015, 10(10), e0140882, DOI:10.1371/journal.pone.0140882.
- M. J. Powell-Palm, J. Preciado, C. Lyu and B. Rubinsky, Escherichia coli viability in an isochoric system at subfreezing temperatures, Cryobiology, 2018, 85, 17–24, DOI:10.1016/j.cryobiol.2018.10.262.
- D. F. Bridges, C. Bilbao-Sainz, M. J. Powell-Palm, T. Williams, D. Wood, A. J. Sinrod and V. C. Wu, Viability of Listeria monocytogenes and Salmonella Typhimurium after isochoric freezing, J. Food Saf., 2020, 40(5), e12840, DOI:10.1111/jfs.12840.
- J. Preciado and B. Rubinsky, The effect of isochoric freezing on mammalian cells in an extracellular phosphate buffered solution, Cryobiology, 2018, 82, 155–158, DOI:10.1016/j.cryobiol.2018.04.004.
- L. Wan, M. J. Powell-Palm, C. Lee, A. Gupta, B. P. Weegman, M. G. Clemens and B. Rubinsky, Preservation of rat hearts in subfreezing temperature isochoric conditions to–8 C and 78 MPa, Biochem. Biophys. Res. Commun., 2018, 496(3), 852–857, DOI:10.1016/j.bbrc.2018.01.140.
- H. Mikus, A. Miller, G. Nastase, A. Serban, M. Shapira and B. Rubinsky, The nematode Caenorhabditis elegans survives subfreezing temperatures in an isochoric system, Biochem. Biophys. Res. Commun., 2016, 477(3), 401–405, DOI:10.1016/j.bbrc.2016.06.089.
- M. J. Powell-Palm, J. Aruda and B. Rubinsky, Thermodynamic theory and experimental validation of a multiphase isochoric freezing process, J. Biomech. Eng., 2019, 141(8), 081011, DOI:10.1115/1.4043521.
- Z. Zhu, T. Li and D. W. Sun, Pressure-related cooling and freezing techniques for the food industry: Fundamentals and applications, Crit. Rev. Food Sci. Nutr., 2021, 61(17), 2793–2808, DOI:10.1080/10408398.2020.1841729.
- M. J. Powell-Palm and B. Rubinsky, A shift from the isobaric to the isochoric thermodynamic state can reduce energy consumption and augment temperature stability in frozen food storage, J. Food Eng., 2019, 251, 1–10, DOI:10.1016/j.jfoodeng.2019.02.001.
- G. Năstase, C. Lyu, G. Ukpai, A. Şerban and B. Rubinsky, Isochoric and isobaric freezing of fish muscle, Biochem. Biophys. Res. Commun., 2017, 485(2), 279–283, DOI:10.1016/j.bbrc.2017.02.091.
- G. Năstase, P. A. Perez, A. Şerban, A. Dobrovicescu, M. F. Ştefănescu and B. Rubinsky, Advantages of isochoric freezing for food preservation: A preliminary analysis, Int. Commun. Heat Mass Transfer, 2016, 78, 95–100, DOI:10.1016/j.icheatmasstransfer.2016.08.026.
- J. A. Preciado and B. Rubinsky, Isochoric preservation: a novel characterization method, Cryobiology, 2010, 60(1), 23–29, DOI:10.1016/j.cryobiol.2009.06.010.
- G. Ukpai, G. Năstase, A. Şerban and B. Rubinsky, Pressure in isochoric systems containing aqueous solutions at subzero Centigrade temperatures, PLoS One, 2017, 12(8), e0183353, DOI:10.1371/journal.pone.0183353.
- M. J. Powell-Palm and B. Rubinsky, Response to “‘Isochoric freezing’: Ambitions and reality”, J. Food Eng., 2023, 349, 111461, DOI:10.1016/j.jfoodeng.2023.111461.
| This journal is © The Royal Society of Chemistry 2024 |