Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Does green mean clean? Volatile organic emissions from regular versus green cleaning products†
Ellen
Harding-Smith
 *ab,
David R.
Shaw
*ab,
David R.
Shaw
 a,
Marvin
Shaw
c,
Terry J.
Dillon
b and
Nicola
Carslaw
a,
Marvin
Shaw
c,
Terry J.
Dillon
b and
Nicola
Carslaw
 *a
*a
aDepartment of Environment and Geography, University of York, UK. E-mail: nicola.carslaw@york.ac.uk
bDepartment of Chemistry, Wolfson Atmospheric Chemistry Laboratory, University of York, UK
cNational Centre for Atmospheric Science, University of York, York, UK
First published on 23rd January 2024
Abstract
Cleaning products emit a range of volatile organic compounds (VOCs), including some which are hazardous or can undergo chemical transformations to generate harmful secondary pollutants. In recent years, “green” cleaners have become increasingly popular, with an implicit assumption that these are better for our health and/or the environment. However, there is no strong evidence to suggest that they are better for indoor air quality compared to regular products. In this study, the VOC composition of 10 regular and 13 green cleaners was examined by headspace analysis. Monoterpenes were the most prevalent VOCs, with average total monoterpene concentrations of 8.6 and 25.0 mg L−1 for regular and green cleaners, respectively. Speciated monoterpene emissions were applied to a detailed chemical model to investigate the indoor air chemistry following a typical cleaning event. Green cleaners generally emitted more monoterpenes than regular cleaners, resulting in larger increases in harmful secondary pollutant concentrations following use, such as formaldehyde (up to 7%) and PAN species (up to 6%). However, emissions of the most reactive monoterpenes (α-terpinene, terpinolene and α-phellandrene), were observed more frequently from regular cleaners, resulting in a disproportionately large impact on the concentrations of radical species and secondary pollutants that were formed after cleaning occurred.
Environmental significanceIncreased consumer interest in cleaning products which are safer for our health/the environment has resulted in the advent of products marketed as “green”. Cleaning products are large sources of volatile organic compounds (VOCs) indoors, including species which react with oxidants to form hazardous secondary pollutants. Here, the VOC emissions from regular and green cleaners are determined experimentally, and the secondary pollutant formation is estimated using an indoor air chemistry model. This work reveals that green cleaners are larger emitters of monoterpenes, though some regular cleaners are larger sources of very reactive monoterpenes, impacting the formation of hazardous secondary pollutants. Our results will provide pointers to influence how fragranced products can be formulated to improve indoor air quality. |
1 Introduction
In developed countries, it is estimated that we spend approximately 90% of our time indoors. As a result, a large proportion of our personal exposure to air pollutants occurs in indoor environments. There are many sources of gaseous and particulate air pollutants indoors, including building materials and furnishings (wood, plastics, floorings, etc.1–3), personal care products,4 household appliances (stoves, photocopiers, fires, etc.3,5) and occupant activities such as cooking and cleaning.6–8Household cleaning products are widely used in the built environment to promote cleanliness and hygiene.9 Cleaning products generally constitute complex mixtures of chemicals including water, solvents, surfactants, preservatives and fragrances. Depending on the usage purpose, other compounds can be included such as disinfectants, acids, bases, bleaching agents, abrasives, or enzymes.10 Many of the components of cleaning products are volatile, and therefore cleaning products can be a major source of volatile organic compounds (VOCs) in indoor environments.
The fragrance component of household cleaners is a key selling point to consumers, promoting the perception of a clean environment through the concealing of malodours.11 Natural and synthetic fragrance ingredients used in scented products are chemically complex mixtures containing terpene and terpenoid compounds. Consequently, cleaning products have been identified as one of the largest sources of terpenes indoors.12 In a study of 25 UK homes, highly variable indoor concentrations of limonene and α-pinene were measured at much higher concentrations than outdoors (mean indoor/outdoor ratios of 8 and 6, respectively), following the intermittent use of fragranced products such as household cleaners indoors.12
Many terpenoid species are susceptible to oxidation by oxidants present indoors such as ozone (O3), and hydroxyl (OH) and nitrate (NO3) radicals. Such chemistry results in the production of a wide range of secondary pollutants, such as organic nitrates, carbonyls (such as formaldehyde), peroxyacetyl nitrates (RCO3NO2, henceforth PAN) and particulate matter (PM).13 Some secondary pollutants from terpenoid oxidation have been associated with adverse health effects,14–18 although the toxicology of many secondary pollutants remains poorly characterised. Evidence suggests that occupant exposure to pollutants from cleaning products may cause adverse respiratory effects and asthma prevalence in cleaning staff.19 Some secondary pollutants are more detrimental to health than the parent VOC,20 hence it is important to study both the primary VOC emissions from cleaning and the chemical transformations that follow to improve indoor air quality and reduce occupant health risks.
The chemical composition of cleaning product formulations is often unclear from the product labels, as manufacturers are not required to disclose all formulation ingredients. This was illustrated in a study of 134 common consumer products, where fewer than 4% of the identified VOCs were listed as product ingredients.21 The fragrance component of consumer products is often listed as “parfum”, or an equivalent term, with no chemical detail about the fragrance components. Under regulation (EC) 648/2004, disclosure of specific fragrance compounds is only required if they are allergenic and at a concentration exceeding 0.01%. As such, there is large variability and uncertainty in the current knowledge of primary VOC emissions and secondary pollutants from indoor cleaning activities.
An increasing awareness surrounding the environmental and health impacts of household products has driven a recent shift in consumer choice towards “green” products, with the assumption that they are less polluting and therefore less harmful than their regular counterparts.22 However, owing to the ambiguity surrounding the chemical composition of cleaning products, it is not possible to substantiate these consumer perceptions with relation to indoor air pollution. Additionally, there is no official designation of “green” and no standard certification to ensure that products marketed as “green” have lower concentrations of chemicals of concern.23 Research comparing the VOC emissions from regular and green cleaners remains limited. Several studies suggest that there is no significant difference between regular and green cleaners,21,24–26 however other studies have observed reduced air concentrations of hazardous VOCs from green cleaners.23,27,28 To our knowledge, there currently exists no studies investigating the secondary pollution from fragranced regular and green cleaners.
Therefore, the aim of this study was to investigate the primary VOC emissions and resultant secondary pollutant formation from 10 regular and 13 green cleaning products. The VOC composition of the cleaners was determined by headspace analysis techniques, and results were used to estimate VOC emission rates during a typical cleaning event on a realistic scale. The chemical transformations of reactive monoterpene emissions were investigated using an indoor chemical model, and the resulting key harmful secondary pollutants were identified. This is the first study to investigate the chemical processing of complex mixtures of reactive terpene emissions relevant to commercially available products, including those marketed as “green”.
2 Methods
2.1 Cleaning products
Twenty-three commercially available household cleaning products were selected for comparison (Table 1). Four product categories (surface cleaner, bathroom cleaner, floor cleaner and dishwashing detergent) were identified as the most frequently used household cleaners based on results from a European household survey on the use of domestic products.29 Within each product category, multiple “regular” products (those which do not make a claim to be “green” in any way) and “green” products (those which make a claim such as “green”, “environmentally friendly”, “natural”, “plant-based”, “nontoxic” etc.) were selected. The products included market leading brands selected from market size data (Household Cleaners UK Generated by Mintel Market Sizes, 2019), budget brands and upmarket brands.| ID | Class | Regular | Green | Scented |
|---|---|---|---|---|
| SR1 | Surface cleaner | ✓ | ✓ | |
| SR2 | Surface cleaner | ✓ | ✓ | |
| SR3 | Surface cleaner | ✓ | ✓ | |
| SR4 | Surface cleaner | ✓ | ✓ | |
| SG1 | Surface cleaner | ✓ | ✓ | |
| SG2 | Surface cleaner | ✓ | ✓ | |
| SG3 | Surface cleaner | ✓ | ✓ | |
| SG4 | Surface cleaner | ✓ | ||
| BR1 | Bathroom cleaner | ✓ | ✓ | |
| BR2 | Bathroom cleaner | ✓ | ✓ | |
| BG1 | Bathroom cleaner | ✓ | ✓ | |
| BG2 | Bathroom cleaner | ✓ | ✓ | |
| BG3 | Bathroom cleaner | ✓ | ||
| FR1 | Floor detergent | ✓ | ✓ | |
| FR2 | Floor detergent | ✓ | ✓ | |
| FG1 | Floor detergent | ✓ | ✓ | |
| FG2 | Floor detergent | ✓ | ✓ | |
| FG3 | Floor detergent | ✓ | ✓ | |
| FG4 | Floor detergent | ✓ | ✓ | |
| DR1 | Dishwashing detergent | ✓ | ✓ | |
| DR2 | Dishwashing detergent | ✓ | ✓ | |
| DG1 | Dishwashing detergent | ✓ | ||
| DG2 | Dishwashing detergent | ✓ | ✓ |
2.2 Experimental
Each sample was heated to 50 °C and intermittently agitated at 250 rpm for 5 minutes in the pre-heating module to allow equilibration of the headspace. Equilibration temperatures of 40, 50, 60 and 70 °C and times of 1, 3, 5, 7, 9 and 15 minutes were tested to optimise sensitivity of the qualitative headspace analysis (Fig. S1†). Following the equilibration period, 250 μL of gaseous headspace was injected into the GC-MS system with a split ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and an inlet temperature of 290 °C. A BPX5 column (50 m × 320 μm × 1 μm) was used for chromatographic separation, with a helium carrier gas at flow rate 1.5 mL min−1. The duration of the method was 34 minutes, with the following oven temperature program: 40 °C (2 min), 10 °C min−1 to 125 °C (3 min), 10 °C min−1 to 300 °C (3 min). The detector temperature was 310 °C.
10 and an inlet temperature of 290 °C. A BPX5 column (50 m × 320 μm × 1 μm) was used for chromatographic separation, with a helium carrier gas at flow rate 1.5 mL min−1. The duration of the method was 34 minutes, with the following oven temperature program: 40 °C (2 min), 10 °C min−1 to 125 °C (3 min), 10 °C min−1 to 300 °C (3 min). The detector temperature was 310 °C.
Visualisation and processing of the GC-MS data were performed using MassHunter Workstation Software (Version 7.0 Qualitative Analysis, Agilent Technologies). The background-subtracted mass spectra of the peaks were extracted, and compounds were tentatively identified by spectra library matching using the National Institute of Standards and Technology (NIST) Mass Spectral Search Program (version 2.3, NIST) and an R match factor of >700. Inter-comparison of peak identification results relative to retention time was performed to improve confidence in identification.
The SIFT-MS was operated in selected ion monitoring mode (SIM), dynamically measuring 15 VOCs with a time resolution of 6 seconds (54 masses scanned, 0.1 second ion dwell time). The compounds measured by SIFT-MS and the corresponding reagent ions, molecular masses and product ion molecular formulae are given in Table S1.† Due to the low mass resolution of SIFT-MS, it was not possible to differentiate between the product ions of isobaric species such as monoterpenes (m/z 136) and sesquiterpenes (m/z 205). Therefore, these are reported as total monoterpenes and total sesquiterpenes, respectively.
A 50 cm3 gas-tight vessel was used as a headspace sampling chamber, which comprised of a stainless-steel screw-down lid and Viton O-ring seal and two 1/16 in stainless steel Swagelok bulkhead connectors to provide an inlet and outlet.4 VOC-free N2 diluent gas was supplied to the chamber from a Teflon bag connected to the inlet. The SIFT-MS was connected to the chamber outlet and was supplied with a 5 mL min−1 flow of sample gas via a mass flow controller. The sampling chamber was thermostatically controlled at 25 °C to achieve a stable ambient temperature throughout the experiment.
For each sample, background measurements of the headspace chamber were acquired for about 10 minutes prior to sample introduction. 1 μL of sample was then decanted onto a small open vial and placed into the sampling chamber immediately. The headspace gas was then measured for a further 60 minutes, or until VOC concentrations stabilised at background concentrations.
Background VOC concentrations, defined as the mean concentration of a 2 minutes period immediately prior to sample introduction, were subtracted from the data. Data were calibrated for acetaldehyde, benzene, ethanol, methanol, and total monoterpenes using calibration factors determined from gas standards (1 ppm in nitrogen, National Physical Laboratories) (Fig. S3†). For all other species, concentrations were determined using literature compound specific rate constants and ion transmission data, which was obtained weekly. For details regarding the uncertainty in SIFT-MS measurements, see ESI, Table S3.†
The concentration (C) of species i in the cleaning product formulation (μg μL−1) was calculated from the integral of the calibrated, background subtracted SIFT-MS data using eqn (1). The concentration profiles showed a peak shortly after sample introduction followed by a decline back to baseline concentrations in most cases, indicating that the VOC source was depleted within the duration of the sampling time. The integral of the VOC peak was therefore assumed to be equivalent to the total amount of that VOC emitted from the sample under the given conditions. VOC concentration profiles which showed no or insignificant peaks were identified by visual inspection of the data and were discounted from subsequent analysis.
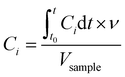 | (1) |
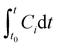 is the integral of Ci with respect to time, t0 and t are the times at which the sample was introduced to the headspace chamber and the end of the sampling period, respectively, ν is the sample flow rate (8.3 × 10−7 m3 s−1), and Vsample is the sample volume (1 μL).
is the integral of Ci with respect to time, t0 and t are the times at which the sample was introduced to the headspace chamber and the end of the sampling period, respectively, ν is the sample flow rate (8.3 × 10−7 m3 s−1), and Vsample is the sample volume (1 μL).
The VOC concentrations were used to estimate emission rates during a typical cleaning event in an indoor environment. The following assumptions were made:
• Product volume: the volume of cleaning product used during a realistic cleaning event was assumed to be 10 mL for surface and bathroom cleaners (based on semi-realistic cleaning experiments), 107 mL for floor cleaners (based on an assumed floor surface area of 8.4 m2 and a floor solution application of 12.82 mL m−2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32,33), and 50 mL for dishwashing detergent.
32,33), and 50 mL for dishwashing detergent.
• Dilution factor: manufacturer guidance was used to calculate a dilution factor where possible. Otherwise, dishwashing detergents were assumed to have a dilution factor of 0.001 (0.1% v/v).
• Room volume: an average kitchen volume of 25 m3 was assumed based on a detailed study of the surface areas of 9 kitchens.32
• Emission period: an emission period of 3 minutes was assumed, based on previous cleaning activity experiments.
Using these assumptions, VOC emission rates (kEm(i), molecule cm−3 s−1) were determined using eqn (2):
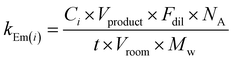 | (2) |
Estimating realistic-scale VOC emissions in this way has limitations. Emission rates were calculated based on the assumption that all VOCs were emitted from the cleaning product during a cleaning activity period of 3 minutes. However, there is evidence to suggest that indoor emission sources such as cleaning products can emit VOCs for a period following the activity due to reversible surface partitioning and emissions from product residues.8,34 Complex emission dynamics including multiphase interactions (i.e., partitioning of VOCs to organic or aqueous surface films) and the effects of different product application modes are not taken into consideration here. Finally, it is acknowledged that the VOC species targeted for this analysis do not account for all volatile components of the cleaning products, and some VOC emissions are not accounted for. However, our results do provide a comparative study between the different cleaners and importantly, between the green and regular products. The approach used in this study to estimate VOC emission rates from product formulation compositional data was evaluated by applying it to data reported in a previous study,33 see ESI.†
The monoterpene fraction of the cleaning products was quantified using calibration standards to account for different sensitivities of the monoterpene compounds. A standard solution of α-pinene, camphene, β-myrcene, α-phellandrene, D-limonene, γ-terpinene and terpinolene in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 H2O
50 H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (2 μg L−1) was prepared. Calibration standards were analysed in the range 125–1000 ng L−1 with the addition of internal standard. The concentrations of monoterpenes in the samples were quantified using the resultant calibration curve (Fig. S2†). For monoterpene species which were not present in the analytical standard, an average of the instrument response to all monoterpenes in the standard was assumed. For β-pinene and α-terpinene, the instrument response was assumed to be equivalent to that of their isomers, α-pinene and γ-terpinene, respectively.
methanol (2 μg L−1) was prepared. Calibration standards were analysed in the range 125–1000 ng L−1 with the addition of internal standard. The concentrations of monoterpenes in the samples were quantified using the resultant calibration curve (Fig. S2†). For monoterpene species which were not present in the analytical standard, an average of the instrument response to all monoterpenes in the standard was assumed. For β-pinene and α-terpinene, the instrument response was assumed to be equivalent to that of their isomers, α-pinene and γ-terpinene, respectively.
The quantified monoterpene fraction was used to calculate the relative abundance ratios of monoterpenes in each cleaning product, which were applied to the total monoterpene emission rates determined from SIFT-MS to calculate individual monoterpene emission rates for each cleaner.
2.3 Model simulations
INCHEM-Py v1.2 was used to model the indoor air chemistry following the emission of VOCs from cleaning. INCHEM-Py is an open-source box-model that has been re-factored from the indoor detailed chemical model (INDCM).35,36 The model creates and solves a series of ordinary differential equations (ODEs) to estimate indoor species concentrations over time, assuming a well-mixed environment. The model utilises the near-explicit Master Chemical Mechanism (MCM)37,38 which describes the gas-phase chemical degradation of 142 non-methane VOCs to H2O and CO2 end-products. Additional reaction mechanisms for species unique to the indoor environment, and therefore not included in the MCM, have been (and continue to be) developed and included in the model.13,36,39–41The general equation for the ODEs created and solved by INCHEM-Py to calculate the concentration C of species i through time is as follows:
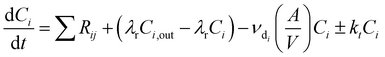 | (3) |
The irreversible deposition of species onto indoor surfaces is described in INCHEM-Py for 3371 species by species-specific deposition velocities. The rate of irreversible loss to indoor surfaces is independent of the surface material and does not consider subsequent emission of secondary pollutants from surface chemistry. However, for key species O3 and H2O2 deposition mechanisms have been developed which include the deposition and secondary pollutant emissions from specific indoor surface materials.42 Loss rates of O3 and H2O2 to indoor surfaces and subsequent emission of aldehydes is calculated from the specific deposition velocities and SAVs of the following materials: metal, glass, wood, plastic, linoleum, paint, paper, concrete, soft furnishings, and skin.
To investigate the production of secondary species from cleaning activities in a realistic environment, the model was parameterised using a ‘typical’ kitchen setting. Based on Manuja et al.,32 a room volume of 25 m3 and a total surface area of 63.27 m2 was assumed. The surface area to volume ratios for each of the materials considered in the model were as follows, assuming one adult (2 m2) is in the room: soft furnishings = 0.081 m−1; paint = 0.992 m−1; wood = 0.665 m−1; metal = 0.311 m−1; concrete = 0.048 m−1; paper = 0.008 m−1; plastic = 0.220 m−1; linoleum = 0.070 m−1; glass = 0.058 m−1; and skin = 0.080 m−1. The temperature and relative humidity of the room were assumed to be 20 °C and 53.5%, respectively.
The background concentrations of VOCs indoors were determined in part from the indoor–outdoor exchange of species, controlled by the outdoor concentrations and the ACR. The ACR was assumed to be 0.5 h−1 based on a review of residential dwelling ventilation.43 Outdoor concentrations of 110 VOCs were defined as static concentrations sourced from published literature and measurement databases, while outdoor O3, NO and NO2 were defined as diurnal concentrations. The diurnal concentrations of O3, NO and NO2 were provided by the ‘London suburban’ profile, which is calculated from hourly average concentrations measured over a 3 months period (July, August, September) by a monitoring station in a suburban London location (data provided by the European Air Quality Database). Additional background concentrations of acetone, ethanol, methanol, isopropanol and isoprene were contributed to by constant indoor emissions from the breath of one adult occupant.42
The total indoor photolysis conditions were determined from a combination of attenuated light and artificial indoor light. Attenuated light was defined using a latitude of 51.45 °N, a date of 21/06/2020, and low emissivity glass (transmittance wavelength range 330–800 nm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44). Artificial indoor lighting was assumed to be incandescent and was on between 07
44). Artificial indoor lighting was assumed to be incandescent and was on between 07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 and 19
00 and 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00.
00.
To simulate a cleaning activity, the speciated monoterpene emission rates were applied to the model per cleaner as timed emissions at midday for an assumed cleaning period of 3 minutes. All other VOC emission rates determined from SIFT-MS analysis were not included in these simulations, because the VOCs were either not available in the model, or they exhibit low reactivity and therefore were not considered important drivers of indoor air chemistry. Cleaners with no observed monoterpene emission (SG4, BR2, BG3, DG1) were discounted from all subsequent analyses.
The chemical degradation of nine monoterpene species were represented in the model, see Fig. S4† for their chemical structures. Species α-pinene, β-pinene, and D-limonene were included as fully explicit reaction schemes, provided by the MCM.37,38,45 Proxy-schemes for camphene, carene and γ-terpinene, developed by Carslaw et al.,36 were utilised which use the rate coefficients for the preliminary oxidation steps using data from the literature and then mapping oxidation products onto existing MCM species. For the purpose of this study the same approach was used to develop degradation schemes for α-phellandrene, α-terpinene, and terpinolene. Inclusion of these nine monoterpenes accounted for over 95% of the total amount of monoterpenes identified from GC-MS analysis. Other monoterpenes identified (tricyclene, cyclofenchene, allocimene, α-thujene, α-fenchene, β-myrcene, sabinene, β-ocimene, β-phellandrene) were not included in the model because either their oxidation rate coefficients were not available in the literature, or they were not present in significant abundance.
3 Results and discussion
3.1 Characterisation of VOCs
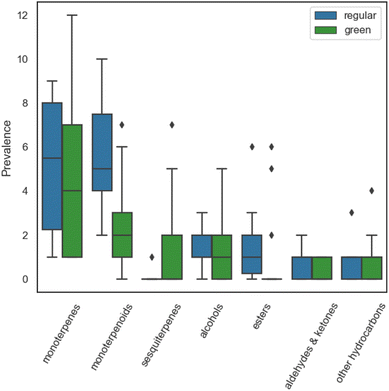
High resolution gas chromatography mass spectrometry (GC-MS) was used to analyse the headspace composition of the cleaning products selected for this study. A total of 317 VOCs occurrences were observed, representing 97 VOC identities emitted from 23 cleaning products. Of the 317 VOCs emitted from the cleaners, 44 VOCs were detected just once, 18 VOCs were detected twice, and 36 VOCs were detected in three or more cleaners. The green cleaners exhibited a 6% greater number of VOC occurrences and a 36% greater number of VOC identities compared to the regular cleaners, demonstrating the variety in VOC composition of the green product formulations. The identified VOCs included 18 monoterpenes, 23 monoterpenoids, 8 sesquiterpenes, 17 alcohols, 17 esters, 6 aldehyde/ketone species and 8 other hydrocarbons (aromatics, alkanes, alkenes). The prevalence of the main chemical classes identified from regular and green cleaners is shown in Fig. 1.Monoterpenes and monoterpenoids were the most commonly identified species in both regular and green cleaners, with five monoterpenes/monoterpenoids being identified in over 50% of the cleaners tested: limonene, eucalyptol, β-pinene, 3-carene and linalool. Limonene was the most prevalent VOC identified in regular and green cleaners, which is consistent with other studies of fragranced consumer products.25,27 The detected monoterpenoid species included 8 alcohols, 8 esters, 4 ethers, 2 ketones and 1 aldehyde. The monoterpene alcohols were common in both regular and green cleaners, while monoterpene esters were twice as prevalent in the regular cleaners. The median number of monoterpenes and monoterpenoids was greater for the regular cleaners compared to the green cleaners, although the spread of monoterpenes was greater for green cleaners. This can be explained by the inclusion of 3 non-fragranced green cleaners in the analysis, which contained only 1 monoterpene, D-limonene. The monoterpenes α-thujene, β-ocimene, β-myrcene and allocimene were identified in green cleaners only, while α-fenchene was only identified in a regular cleaner (“ocean” scented). The greater variety of monoterpene compounds in green cleaners could be an indication that naturally derived fragrance ingredients (such as essential oils, which usually contain more than 100 different chemical substances46) are more commonly used in the formulation of green cleaners.
A greater number of green cleaners contained sesquiterpenes compared to regular cleaners, while esters, aldehydes and ketones were more prevalent in the regular cleaners. A possible explanation for this difference could be that green cleaners typically use natural fragrances such as essential oils or plant extracts which consist largely of biologically synthesised terpene compounds (including monoterpenes and sesquiterpenes) and oxygenated terpene derivatives.47,48 Conversely, regular products typically incorporate synthetically derived fragrance mixtures, which utilise synthetic aroma chemicals such as esters and other carbonyls to replicate a “natural-identical” scent (although the exact chemical composition of synthetic fragrances is often proprietary information).46
3.2 Targeted quantification of VOCs
SIFT-MS was used with dynamic headspace sampling to directly quantify a targeted subset of VOCs in the cleaning product formulations. The compounds targeted in this analysis were selected based on the information obtained from the product ingredient lists, results from GC-MS analysis, and common VOCs reported in the literature regarding cleaning product emissions.The headspace VOC concentrations increased immediately after the sample was introduced into the sample chamber, as the VOCs partitioned from the liquid to the gas phase. Over the duration of the 60 minutes measurement period the VOC concentrations peaked and then declined as the emission source was depleted, finally returning to background concentrations. An example of the VOC concentration profiles measured from a green surface cleaner (SG2) is shown in Fig. 2. This characteristic VOC concentration profile supported the assumption that the total amount of each VOC in the product formulation was released within the measurement period.
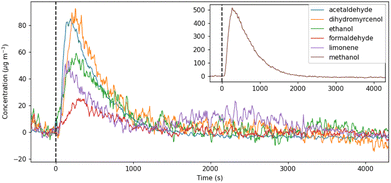 | ||
| Fig. 2 The concentration profile of VOCs measured by SIFT-MS with dynamic headspace sampling of cleaner SG2. t0 = time when sample was introduced to the headspace chamber (black dashed line). | ||
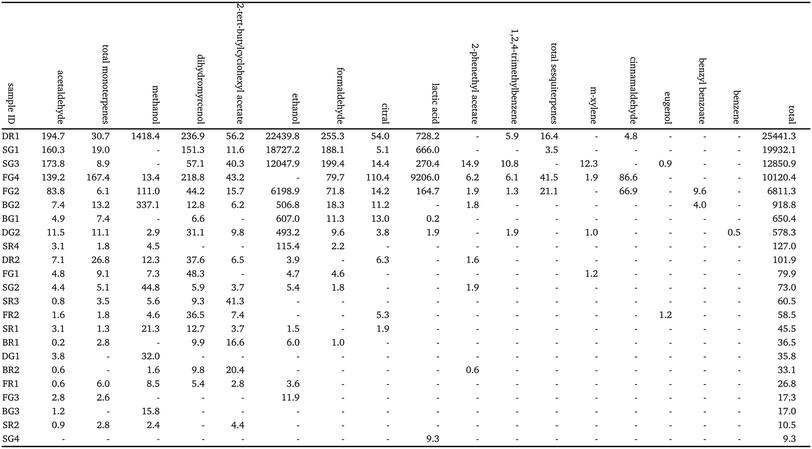
The mass concentrations of VOCs in the cleaning product formulations are reported in Table 2. The total VOC mass concentrations measured in this study ranged from 9.3 to 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 441 mg L−1, which is comparable to a study by Temkin et al., who reported mass concentrations ranging from 0.97 to 38
441 mg L−1, which is comparable to a study by Temkin et al., who reported mass concentrations ranging from 0.97 to 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 035 mg L−1 (μg g−1) from 30 regular and green cleaning products.27 DR1, SG1, and SG3 contained the largest total mass concentration of VOCs, with the measured compounds accounting for 2.5%, 2.0% and 1.3% of the total sample (w
035 mg L−1 (μg g−1) from 30 regular and green cleaning products.27 DR1, SG1, and SG3 contained the largest total mass concentration of VOCs, with the measured compounds accounting for 2.5%, 2.0% and 1.3% of the total sample (w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v%). Ethanol was the greatest contributor to total VOC concentration for these cleaners, and was identified in 15 of the 23 samples, suggesting that it may be used as a common solvent in cleaning product formulations. Ethanol was explicitly listed as an ingredient in only one product (FG2), although a further 8 included ‘alcohol’ in the ingredient list. Methanol was also identified as a component of most of the cleaning product formulations, with quantifiable measurements made from 17 of the 23 cleaners ranging from 1418 to <10 mg L−1.
v%). Ethanol was the greatest contributor to total VOC concentration for these cleaners, and was identified in 15 of the 23 samples, suggesting that it may be used as a common solvent in cleaning product formulations. Ethanol was explicitly listed as an ingredient in only one product (FG2), although a further 8 included ‘alcohol’ in the ingredient list. Methanol was also identified as a component of most of the cleaning product formulations, with quantifiable measurements made from 17 of the 23 cleaners ranging from 1418 to <10 mg L−1.
Formaldehyde (methanal, HCHO) and acetaldehyde (ethanal, CH3CHO) were also emitted from most of the cleaning products at mass concentrations as high as 255.3 mg L−1 and 194.7 mg L−1, respectively. These results are consistent with Temkin et al., who observed formaldehyde and acetaldehyde emissions from over 30% of a range of regular and green cleaning products in the U.S. market.27 Short chain aldehydes such as HCHO and CH3CHO are pollutants of concern in the indoor environment due to their known or suspected toxicity, carcinogenicity and mutagenicity.14,49 These results suggest that while they were not listed ingredients for any of the formulations, some cleaning products may be primary sources of HCHO and CH3CHO in the built environment.
Lactic acid was identified as a component of 7 green cleaners and 1 regular cleaner. This compound was included in the ingredient list of 5 of the green cleaners including SG1 and SG3, for which relatively large mass concentrations were measured (660.0 and 270.4 mg L−1, respectively). However, the largest mass concentrations of lactic acid were measured from FG4 (9206.0 mg L−1) and DR1 (728.2 mg L−1), which did not state lactic acid as an ingredient. Lactic acid is used in the green cleaning industry as a descaling and antimicrobial agent, which is produced by fermentation based on natural and renewable resources, and is an alternative to synthetic agents such as inorganic acids.50
Of the 23 cleaners studied, 20 of them listed fragrance components (usually non-specific e.g., ‘parfum’) in their ingredients, with 10 explicitly listing limonene. The results from SIFT-MS were in good agreement with this observation, as monoterpenes were measured in 19 of the fragranced cleaners. Monoterpenes were undetected in the 3 unfragranced cleaners (DG1, BG3, SG4), as well as fragranced cleaner BR2. Of the fragranced cleaners, the average mass concentration of total monoterpenes was 8.6 and 25.0 mg L−1, respectively. Although there is limited information available regarding liquid composition of cleaning products, this value is low compared to those reported in other studies. Singer et al., reported a mass concentration of 44850 mg L−1 (44.85 mg mL−1) of monoterpenes in a pine oil-based general-purpose cleaner,33 while Angulo Milhem et al., reported mass concentrations ranging from 15.0 to 992.6 mg L−1 (μg g−1) of monoterpenes from 6 essential oil-based cleaners.51 However, it is worth noting that the chemical formulation of cleaning products is likely to vary widely depending on the type of cleaning agent, the manufacturer, and regional regulations and policies regarding product formulation (with the latter likely changing over time, making it particularly hard to compare between studies separated by significant time periods). Additionally, both of these past studies focussed on cleaning products which were essential oil-based (i.e., an essential oil was listed as an ingredient of the cleaner), whereas this was not a requirement for product selection in this study. Essential oils are mainly composed of terpenes and terpenoids:52 formulations containing these ingredients are likely to have a larger concentration of monoterpenes compared to other products.
Other fragrance compounds identified in the product formulations included dihydromyrcenol (C10H20O, found in 17 cleaners), citral (C10H16O, found in 11 cleaners), sesquiterpenes (C15H24, found in 4 cleaners), cinnamaldehyde (C9H8O, found in 3 cleaners) and eugenol (C10H12O2, found in 2 cleaners). It is worth nothing that monoterpene alcohols (C10H18O) were not measured by SIFT-MS, and therefore concentrations of species such as linalool and eucalyptol, which were identified in the formulations using GC-MS, were not quantified.
Emission rates of the measured VOC species from a typical cleaning activity on a realistic scale were estimated using the approach outlined in the Methods section. The emission rates were used to initialise an indoor air chemistry model to gain a better understanding of the chemical fate of VOC emissions following cleaning, and the potential for harmful secondary pollutant formation.
3.3 Monoterpene emissions and implications for indoor air chemistry
The presence of monoterpenes in indoor environments is potentially important, because these compounds are chemically reactive towards indoor oxidants, such as O3, and OH and NO3 radicals.36,38 Through multiple oxidation steps, monoterpenes can produce a range of secondary pollutants, including carbonyls, organic acids, peroxide species, organic nitrates and particulate matter.13 There is evidence to suggest that the secondary pollutants from terpene oxidation chemistry are more hazardous to human health than the terpenes themselves. For example, Wolkoff et al. found that exposure of mice to an O3/limonene mixture resulted in sensory irritation of the upper airways at concentrations below the no-observed-effect-levels (NOELs) of the parent compounds.53 Additionally, the detrimental health effects of some terpene/O3 reaction products are well documented such as HCHO, which is accepted to be a sensory irritant and human carcinogen.14To investigate the production of secondary pollutants from terpene oxidation chemistry following cleaning, the measured monoterpene emission rates were used to drive the indoor air chemistry model, INCHEM-Py.35 It was anticipated that the different combinations of monoterpene emissions in the cleaning simulations would give rise to different concentrations of secondary pollutants. This was observed by Carslaw et al., who found that emissions of a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mix of limonene and α-pinene resulted in more efficient production of formaldehyde than the same concentration of the individual terpenes, while emissions of each monoterpene individually resulted in more efficient production of radical species and particulate matter.54 Therefore, the secondary chemistry resulting from the complex terpene mixtures contained within fragranced cleaning products is likely to depend not only on the chemical reactivity of the individual species towards indoor oxidants, but also on the interplay between the chemical transformations and relative concentrations of each compound emitted from the cleaner.
50 mix of limonene and α-pinene resulted in more efficient production of formaldehyde than the same concentration of the individual terpenes, while emissions of each monoterpene individually resulted in more efficient production of radical species and particulate matter.54 Therefore, the secondary chemistry resulting from the complex terpene mixtures contained within fragranced cleaning products is likely to depend not only on the chemical reactivity of the individual species towards indoor oxidants, but also on the interplay between the chemical transformations and relative concentrations of each compound emitted from the cleaner.
The chemical reactivity of the nine monoterpenes included in the model simulations is described in Table 3. For each monoterpene the rate of reaction is generally fastest with OH, followed by NO3. The rate of reaction with O3 is much slower, with rate coefficients of the order of 10−14 to 10−19. However, due to the high reactivity and instability of radical species, OH and NO3 are short lived and are present in indoor environments at much lower concentrations compared to O3, which has a longer lifetime and originates mostly from outdoor environments.55 Therefore, initial oxidation of monoterpenes is generally more likely to occur via ozonolysis. The most reactive monoterpene towards O3 is α-terpinene, while the least reactive is camphene.
| Monoterpene | Rate coefficient (cm3 molecule−1 s−1) | OH yield(%) | OH production/loss | ||
|---|---|---|---|---|---|
| k(OH) | k(NO3) | k(O3) | |||
a The final column shows an estimate of the ratio of OH production: loss calculated as k(O3)[O3] × OH yield/k(OH)[OH], where [O3] = 1.06 × 1011 molecule cm−3 and [OH] = 1.23 × 106 molecule cm−3 (simulated indoor concentrations at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00).
b
Atmos. Chem. Phys. 2021, 21, 12665–12685, DOI: https://doi.org/10.5194/acp-21-12665-2021.
c
Atmospheric Environment, Part A: General Topics, 1990, 24(10), 2647–2654. 00).
b
Atmos. Chem. Phys. 2021, 21, 12665–12685, DOI: https://doi.org/10.5194/acp-21-12665-2021.
c
Atmospheric Environment, Part A: General Topics, 1990, 24(10), 2647–2654.
|
|||||
| α-Terpinene | 3.5 × 10−10 | 1.8 × 10−10 | 1.9 × 10−14 | 0.38 | 1.77 |
| Terpinolene | 2.2 × 10−10 | 9.7 × 10−11 | 1.6 × 10−15 | 0.70 | 0.44 |
| α-Phellandrene | 3.2 × 10−10 | 7.3 × 10−11 | 2.9 × 10−15 | 0.32 | 0.25 |
| α-Pinene | 5.3 × 10−11 | 6.2 × 10−12 | 9.4 × 10−17 | 0.80 | 0.12 |
| D-Limonene | 1.6 × 10−10 | 1.2 × 10−11 | 2.1 × 10−16 | 0.87 | 0.10 |
| γ-Terpinene | 1.7 × 10−10 | 2.9 × 10−11 | 1.6 × 10−16 | 0.81 | 0.07 |
| 3-Carene | 8.8 × 10−11b | 9.1 × 10−12 | 4.9 × 10−17 | 0.86 | 0.04 |
| β-Pinene | 7.9 × 10−11 | 2.5 × 10−12 | 1.9 × 10−17 | 0.35 | 0.01 |
| Camphene | 5.3 × 10−11c | 6.6 × 10−13 | 5.0 × 10−19 | 0.18 | 0.00 |
Oxidation of a monoterpene can lead to net OH production or loss, depending on the balance between OH formation through ozonolysis, versus OH loss through reaction with the monoterpene. At any time, this balance depends on the ratio of the rate coefficients for reactions with OH and O3 and the OH yield following ozonolysis for a particular monoterpene, and the OH and O3 concentrations. Table 3 shows a proxy for this metric in the final column, which represents the balance between formation and loss of OH for each monoterpene using the O3 and OH concentrations estimated by the model at midday. Under these conditions, α-terpinene will contribute most to OH production indoors, due to its large O3 rate coefficient compared to the OH rate coefficient. Camphene is the least important for OH production due to its very low O3 rate coefficient. Depending on the mixtures of these monoterpene species indoors, one might expect quite different oxidation chemistry and hence secondary pollutant formation indoors.
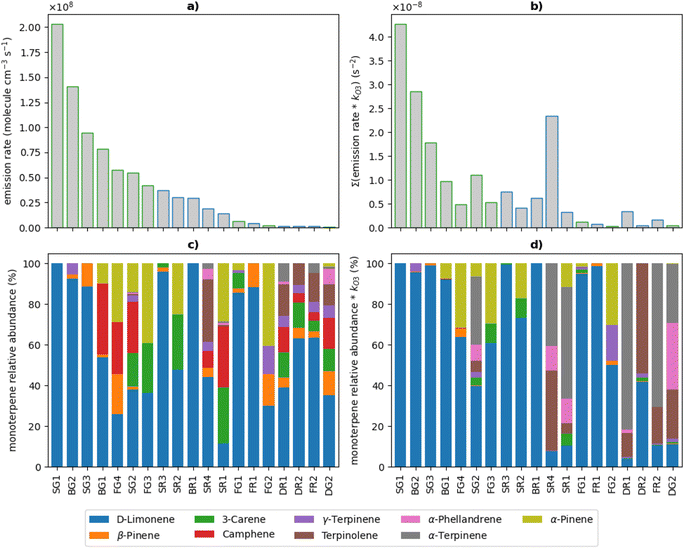
The total monoterpene emission rates applied to the model are shown in Fig. 3A. Emission rates ranged from 5.9 × 105 to 2.0 × 108 molecule cm−3 s−1 (equivalent to 85 ppt h−1 and 29 ppb h−1, respectively) with the top 7 emitters being green cleaners. Dishwashing liquids were the lowest emitters of monoterpenes, likely owing to the large dilution upon use of these products. The relative abundance ratios of monoterpene isomers used to speciate the total monoterpene emissions are shown in Fig. 3B. Limonene was the most abundant monoterpene in most cleaners, with 2 cleaners emitting 100% limonene. Other cleaners consisted of unique monoterpene profiles relating to the specific formulation ingredients of each product.
To illustrate the differences in reactivity towards indoor oxidants, the speciated monoterpene emission rates and relative abundance ratios for each cleaner were scaled to their corresponding O3 rate coefficients (Table 3) in Fig. 3C and D, respectively. A similar approach has been taken to evaluate the differences in reactivity of the speciated monoterpene emissions from each cleaner towards OH radicals, shown in Fig. S5.† This process is illustrative given that monoterpenes react readily with O3 and OH indoors.
The relative magnitude of the kO3-scaled emission rates increased or decreased in comparison to the total monoterpene emission rates per cleaning product, depending on the concentration and chemical reactivity of the different mixtures of monoterpene species emitted. SG1 had the highest monoterpene emission rate of all the cleaning products, consisting entirely of limonene, and also gave the largest value when scaled to kO3. Cleaning products which contained the most reactive monoterpenes α-terpinene, α-phellandrene and terpinolene typically resulted in an increase in the relative magnitude of the kO3-scaled emission rate. This was particularly observed for cleaners SG2, SR4, DR1, and FR2, which were the 4 largest emitters of these compounds. Interestingly, the cleaners containing the most reactive monoterpenes were mainly regular products. Conversely, when there were greater contributions to the total monoterpene emission rate from less reactive species such as camphene, β-pinene and 3-carene, a decrease in the relative magnitude of the kO3-scaled emission rate was observed. This was the case for cleaners FG4, FG3 and SR2, which were among the highest emitters of these relatively low-reactivity monoterpenes.
The total monoterpene emission rates of the regular surface cleaners SR4 and SR1 were of similar magnitude (1.87 × 107 molecule cm−3 s−1 and 1.43 × 107 molecule cm−3 s−1, respectively), however the magnitude of the kO3-scaled emission rates were considerably different for these two cleaners. The monoterpene emissions from SR1 consisted of 2% the most reactive monoterpenes and 58% of the less reactive monoterpenes, while those from SR4 consisted of 38% of reactive monoterpenes and 13% of low-reactivity monoterpenes. When scaled to the O3 rate coefficients, the reactive monoterpene emissions contributed 72% and 91% for SR1 and SR4, respectively, despite these species only making up 2% of the overall monoterpene emission rate for SR1. This demonstrates that the more reactive species will dominate the chemistry and sequester the most O3 from the indoor atmosphere, thus limiting the reactions of other monoterpenes with O3. Therefore, reactive species such as α-terpinene, α-phellandrene and terpinolene will have a greater impact on the indoor air chemistry and resulting secondary pollutant concentrations, compared to less reactive species such as camphene, β-pinene and 3-carene.
In the simulated cleaning events, the peak total monoterpene mixing ratio following the timed emissions ranged from <0.2 to 4.8 ppb. These concentrations are low compared to a study performed by Singer et al., who reported an average concentration of 2857 μg m−3 (513 ppb) monoterpenes in the first 60 minutes after mopping the floors of a 50 cm3 chamber with a pine oil-based general-purpose cleaner.33 By contrast, in the House Observations of Microbial and Environmental Chemistry (HOMEChem) campaign, an increase in limonene concentration of roughly 3 ppb was reported when mopping the floors of an experimental house with a terpene cleaner, much closer to our observed results.58 The results reported in the literature demonstrate the large variability in terpene emissions from cleaning, likely arising from differences in product composition, dependencies on behavioural factors such as how the product is used/applied and how long for, and environmental factors such as ventilation conditions. These dependencies result in wide variations in experimental methodologies used to assess the VOC emissions from cleaning activities, thus limiting the ability to make meaningful comparisons of the reported results.51
The effect of monoterpene emissions on oxidant and radical species concentrations were investigated in greater detail to understand the chemical transformations that take place following a cleaning event. Fig. 4 shows the relative change in concentration of various species compared to a baseline simulation for the regular and green surface cleaners only, for simplicity. Following cleaning, OH concentrations undergo a rapid decrease as they react with the monoterpenes introduced to the system. The exception is SR4, which shows an increase in OH radicals following cleaning. Fig. 3 shows that this product contains α-terpinene, which is very effective at producing OH (see Table 3). SG1 shows the biggest decrease in OH concentration, which is composed entirely of limonene. Under these conditions, limonene effectively removes the OH.
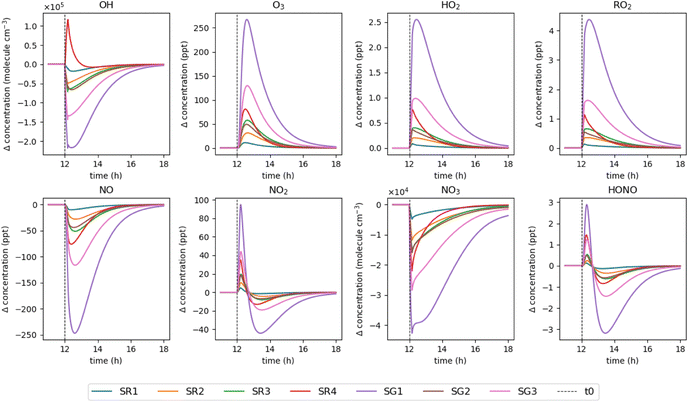 | ||
| Fig. 4 Relative change in concentration of key indoor species from 11–18 h for each surface cleaner simulation, compared to a baseline simulation (no emissions). | ||
For all cleaning simulations, the concentration of O3 increases compared to the baseline simulation despite the occurrence of monoterpene-O3 reactions. This is due to the production of peroxy (RO2) and hydroxy (HO2) radicals from monoterpene oxidation chemistry which react with nitric oxide (NO) to generate nitrogen dioxide (NO2), as evidenced by the changes in these species' concentrations shown in Fig. 4. NO2 undergoes photolysis to generate an oxygen atom, which then rapidly reacts with O2 to generate O3. Additionally, the sequestration of NO by reaction with RO2 and HO2 limits O3 loss via reaction with NO, thus enhancing O3 concentrations in the system compared to baseline. The greatest increase in O3 concentration is observed for SG1 and SG3 which are the two largest monoterpene emitters, both consisting of over 85% limonene. SR4 causes the next largest increase in O3, despite having the second smallest monoterpene emission of the surface cleaners. Fig. 3C shows that this product contains highly reactive monoterpenes (30% terpinolene, 3% α-terpinene and 5% α-phellandrene) resulting in efficient HO2 and RO2 production, hence more efficient NO2 production and NO removal, favouring an overall increase in O3 concentration.
The concentrations of NO2 and nitrous acid (HONO) both show an initial increase following cleaning, followed by a rapid decrease to concentrations lower than the baseline simulation a few hours after cleaning. The main pathway of NO2 production in the model is via the NO + O3 reaction, therefore the concentration of NO2 will depend critically on the concentrations of NO and O3. Initially after cleaning, the NO and O3 concentrations are sufficient for efficient production of NO2. However, as time proceeds NO concentrations decline due to the increasing concentrations of RO2 and HO2 radicals, thus NO concentration becomes the limiting factor for NO2 production, causing a decline in NO2 concentrations relative to the baseline simulation. The main production pathway for HONO in the model is via heterogeneous chemistry of NO2 on indoor surfaces at a rate of (2.9 ± 1.8) × 10−3 m min−1,59,60 hence the concentration of HONO is strongly coupled to the concentration of NO2 under these conditions.
The concentration of NO3 radicals was low and decreased in all simulations owing to its high reactivity towards VOCs, particularly unsaturated compounds such as monoterpenes.45 This chemistry further contributes to the production of RO2 and nitrooxy-substituted RO2 radicals and organic nitrates, which are an important precursor for secondary organic aerosol (SOA).39
The impact of the chemistry following cleaning on the production of secondary pollutants was evaluated for each cleaner. The percentage change in concentration of HCHO, PAN species and organic nitrates (RNO3) are shown in Fig. 6. These species have been selected based on their known or suspected health impacts. HCHO is well understood to be a carcinogen14,15 and is produced via the reaction of methoxy radicals (CH3O) with ambient oxygen (Fig. 5, pathway 2). There is less evidence regarding the detrimental health effects of PAN species and organic nitrates, although they are both suspected of being irritants.16–18,61 PAN species are formed by reaction of peroxyacetyl radicals (RCO3) with NO2 (Fig. 5, pathway 3), while organic nitrates are formed as minor reaction products of RO2–NO chemistry (Fig. 5, pathway 1).
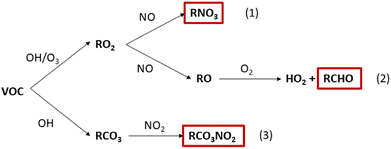 | ||
| Fig. 5 The general VOC oxidation chemistry leading to the formation of key secondary pollutants: organic nitrates (RNO3), formaldehyde (HCHO) and PAN species (RCO3NO2). | ||
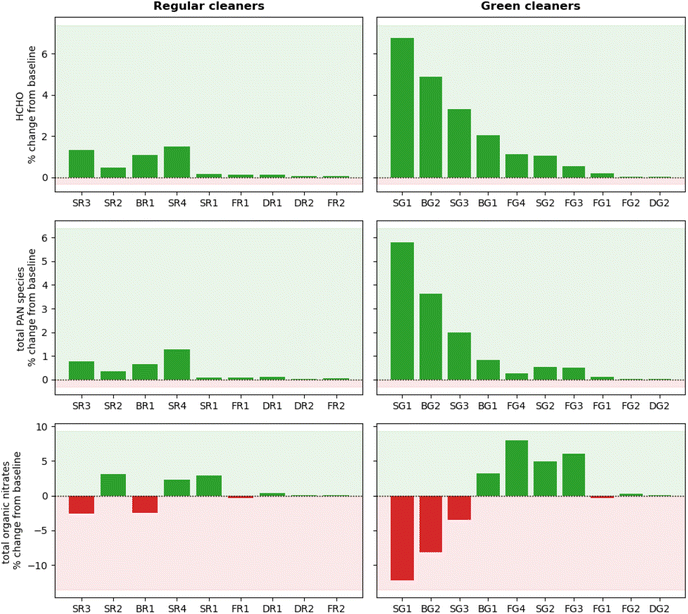
HCHO was produced in all cleaning simulations as a product of monoterpene oxidation. The extent of HCHO production varied from <0.1 to 6.8% relative to the baseline simulation over an average of 3 hours following cleaning. This relates to absolute HCHO mixing ratios of <2 ppb for all simulations, which is well below the safe exposure limits.14 However, it is worth noting that while HCHO concentrations fall well below the recommended exposure limit of 0.1 ppm under these conditions,62 larger concentrations are likely to arise from cleaning events involving multiple products and/or for longer cleaning periods. This is a particularly important consideration when evaluating occupational exposure to hazardous pollutants from cleaning, as professional cleaners will likely be exposed to higher concentrations of pollutants and for a much longer time than considered in this study.
Increases in HCHO concentration remained below 2% for all regular cleaners, while larger changes were observed for some green surface and bathroom cleaners (SG1, BG2, SG3, BG1). The increase in HCHO concentration correlates well with the magnitude of total monoterpene emissions. The exception is SR4, which produced a similar increase in HCHO concentration as FG3 (1.5% and 1.1% increase in HCHO, respectively), despite SR4 having a total monoterpene emission rate of less than half that of than FG3 (1.9 × 107 molecule cm−3 s−1 and 4.2 × 107 molecule cm−3 s−1, respectively). Again, this can be attributed to the high reactivity of the monoterpenes emitted from SR4 towards indoor oxidants, leading to efficient RO2 formation. When the RO2 radical formed is CH3O2, HCHO is produced via reaction pathway 2 in Fig. 5.
PAN species were produced in all cleaning simulations, owing to the oxidation of emitted monoterpenes by OH to produce RCO3 species which further react via pathway 3 in Fig. 5. The formation of PAN species following cleaning was small for most cleaners, with only three cleaners resulting in a 3 hours average change of >1% from the baseline simulation. SG1 was the largest producer of PAN species, resulting in a 3 hours average absolute mixing ratio of 25 ppt. SG1 was the largest emitter of total monoterpenes, consisting consisted entirely of limonene which has a relatively high OH rate coefficient (Table 3). The formation of PAN species was dependant on the magnitude of monoterpene emissions from the cleaning event and the reactivity of the emitted monoterpenes towards OH.
Finally, the concentration of organic nitrate compounds showed positive and negative changes in the simulations of both regular and green cleaning emissions. The formation of organic nitrates is dependent on the branching ratio of RO2 + NO reactions to form RO (≥80%) or organic nitrate species (≤20%).7 Hence, the specific RO2 species formed by initial oxidation of monoterpene species determines the yield of organic nitrate via pathway 1 in Fig. 5. This is further evidence of how the complex mixture of different VOCs in cleaning products can influence the indoor air chemistry and the concentrations of potentially hazardous air pollutants.
4 Conclusions
Mass spectrometric techniques coupled with headspace sampling have been implemented to characterise and quantify the VOCs present in a range of regular and green household cleaning products. While the composition of each product formulation was unique, it was found that both regular and green cleaners contained VOCs pertaining to the broad chemical classes of monoterpenes, monoterpenoids, sesquiterpenes, alcohols, esters, carbonyls and other hydrocarbons. Monoterpenes and monoterpenoids were the most common compounds identified in the formulations of fragranced cleaners.Targeted quantitative analysis of each formulation showed that there was large variety in the concentrations of VOCs in the product formulations. A comparison of the compounds detected versus those disclosed by manufacturers on the product labels supported evidence of ambiguity regarding cleaning product compositional information, highlighted in previous studies.24,25 Alcohols (ethanol and methanol) were measured in high concentrations from some regular and green cleaners, while lactic acid was observed in predominantly green cleaners. These observations highlight potential compositional differences in the formulations of regular and green cleaners, for which there is currently very little information on in the available literature.
The implications of reactive monoterpene emissions from each cleaner on the indoor air chemistry was investigated using a detailed chemical model. The results of the model simulations highlighted the significance of both the quantity and the chemical reactivity of monoterpene emissions on the concentrations of oxidants, radicals and secondary pollutants indoors. In the present study, green cleaners were generally larger sources of monoterpene emissions compared to regular cleaners, resulting in larger increases in harmful secondary pollutants such as HCHO and PAN species. However, emissions of highly reactive monoterpenes such as α-terpinene, terpinolene and α-phellandrene were observed from more regular cleaners than green cleaners, resulting in a disproportionately large impact on the concentrations of radical species and the production of HCHO.
The production of secondary pollutants from cleaning emissions reported in this study are unlikely to cause detrimental health effects to occupants. However, it is important to note that there is large variability in product formulations and occupant/environmental factors that would influence the VOC emissions from cleaning and subsequent chemical processing. Therefore, it is necessary to investigate a broader range of products and study VOC emissions from cleaning on a more realistic scale, to get a better understanding of how cleaning can contribute to indoor air pollution.
As sales of green cleaning products are increasing there exists a greater need for better regulation of these products. More transparent disclosure of cleaning product formulation ingredients is required to better inform consumers about potential exposure risks. Also, more careful consideration is required for the potential exposure to secondary pollutants resulting from chemical processing of the mixtures of reactive primary VOC emissions from cleaning. The quantity and chemical reactivity of monoterpene compounds used to provide fragrance for cleaning products should be carefully considered in the formulation development stage of product manufacture, and the potential implications on indoor air pollution assessed. These findings are also applicable to other fragranced household products, such as personal care products and laundry products.
Author contributions
E. H. S. conceptualisation, methodology, data curation, formal analysis, validation, visualisation, writing – original draft, writing – review & editing. D. S. formal analysis, methodology, writing – review & editing. M. S. methodology, data curation, writing – review & editing. T. D. conceptualisation, writing – review & editing, supervision. N. C. conceptualisation, writing – review & editing, supervision, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge EPSRC who funded this work under grant numbers EP/T014474/1 and EP/T518025/1. The development of INCHEM-Py has been funded by grant from the Alfred P. Sloan Foundation COMMODIAC (G-2018-10083). Conclusions reached or positions taken by researchers or other grantees represent the views of the grantees themselves and not those of the Alfred P. Sloan Foundation or its trustees, officers, or staff. The authors acknowledge the provision of cleaning products from Tincture London and Which? Ltd, the latter under the contractual agreement ”Natural Cleaning Products/27471”. The authors thank Amber Yeoman for their help with interpreting SIFT-MS data, and Martyn Ward for their technical help with GC-TOF-MS.Notes and references
- Y. Liu, P. K. Misztal, J. Xiong, Y. Tian, C. Arata, R. J. Weber, W. W. Nazaroff and A. H. Goldstein, Characterizing sources and emissions of volatile organic compounds in a northern california residence using space- and time-resolved measurements, Indoor Air, 2019, 29, 630–644 CAS.
- H. Wang, J. Xiong and W. Wei, Measurement methods and impact factors for the key parameters of voc/svoc emissions from materials in indoor and vehicular environments: A review, Environ. Int., 2022, 168, 107451 CrossRef CAS PubMed.
- J. Charles, Weschler. Changes in indoor pollutants since the 1950s, Atmos. Environ., 2009, 43, 153–169 CrossRef.
- A. M. Yeoman, M. Shaw, N. Carslaw, T. Murrells, N. Passant and A. C. Lewis, Simplified speciation and atmospheric volatile organic compound emission rates from non-aerosol personal care products, Indoor Air, 2020, 30(3), 459–472 CrossRef CAS PubMed.
- H. Destaillats, R. L. Maddalena, B. C. Singer, A. T. Hodgson and T. E. McKone, Indoor pollutants emitted by office equipment: A review of reported data and information needs, Atmos. Environ., 2008, 42, 1371–1388 CrossRef CAS.
- K. L. Abdullahi, J. M. D. Saborit and R. M. Harrison, Emissions and indoor concentrations of particulate matter and its specific chemical components from cooking: A review, Atmos. Environ., 2013, 71, 260–294 CrossRef CAS.
- H. L. Davies, C. O'Leary, D. Terry, D. R. Shaw, M. Shaw, A. Mehra, G. Phillips and N. Carslaw, A measurement and modelling investigation of the indoor air chemistry following cooking activities, Environ. Sci.: Processes Impacts, 2023, 25, 1532–1548 RSC.
- W. W. Nazaroff and J. W. Charles, Cleaning products and air fresheners: Exposure to primary and secondary air pollutants, Atmos. Environ., 2004, 38(18), 2841–2865 CrossRef CAS.
- P. Wolkoff, T. Schneider, K. Jan, R. Degerth, M. Jaroszewski and H. Schunk, Risk in cleaning: chemical and physical exposure, Sci. Total Environ., 1998, 215, 135–156 CrossRef CAS PubMed.
- D. Missia, T. Kopanidis, J. Bartzis, G. Ventura Silvia, E. De Oliveira Fernandes, P. Carrer, P. Wolkoff, M. Stranger, and E. Goelen. WP4 Literature Review on Product Composition, Emitted Compounds and Emissions Rates and Health End Points from Consumer Products. Technical Report, 2012 Search PubMed.
- R. S. Herz, M. Larsson, R. Trujillo, M. C. Casola, F. K. Ahmed, S. Lipe and M. E. Brashear, A three-factor benefits framework for understanding consumer preference for scented household products: psychological interactions and implications for future development, Cogn. Res.: Princ. Implic., 2022, 7(1), 28 CrossRef PubMed.
- C. M. Wang, B. Barratt, N. Carslaw, A. Doutsi, R. E. Dunmore, M. W. Ward and A. C. Lewis, Unexpectedly high concentrations of monoterpenes in a study of UK homes, Environ. Sci.: Processes Impacts, 2017, 19(4), 528–537 RSC.
- N. Carslaw, A mechanistic study of limonene oxidation products and pathways following cleaning activities, Atmos. Environ., 2013, 80, 507–513 CrossRef CAS.
- World Health Organization, WHO, Guidelines for Indoor Air Quality: Selected Pollutants, World Health Organization. Regional Office for Europe, 2010 Search PubMed.
- G. D. Nielsen and P. Wolkoff, Cancer effects of formaldehyde: a proposal for an indoor air guideline value, Arch. Toxicol., 2010, 84(6), 423–446 CrossRef CAS PubMed.
- J. Q. Koenig, D. S. Covert and W. E. Pierson, Effects of Inhalation of Acidic Compounds on Pulmonary Function in Allergic Adolescent Subjects, Environ. Health Perspect., 1989, 79, 173–178 CrossRef CAS PubMed.
- T. Berkemeier, M. Ammann, T. F. Mentel, P. Ulrich and M. Shiraiwa, Organic nitrate contribution to new particle formation and growth in secondary organic aerosols from α-pinene ozonolysis, Environ. Sci. Technol., 2016, 50, 6334–6342 CrossRef CAS PubMed.
- G. Zhang, Y. Mu, L. Zhou, C. Zhang, Y. Zhang, J. Liu, S. Fang and B. Yao, Summertime distributions of peroxyacetyl nitrate (pan) and peroxypropionyl nitrate (ppn) in beijing: Understanding the sources and major sink of pan, Atmos. Environ., 2015, 103, 289–296 CrossRef CAS.
- E. M. C. Mendonça, E. Algranti, J. B. P. Freitas, E. A. Rosa, J. A. D. S. Freire, U. P. Santos, J. Pinto and M. A. Bussacos, Occupational asthma in the City of São Paulo, 1995-2000, with special reference to gender analysis, Am. J. Ind. Med., 2003, 43(6), 611–617 CrossRef PubMed.
- I. S. H. Buchanan, M. J. Mendell, A. G. Mirer and M. G. Apte, Air filter materials, outdoor ozone and building-related symptoms in the BASE study, Indoor Air, 2008, 18(2), 144–155 CrossRef CAS PubMed.
- N. Neda, S. D. Kolev and A. Steinemann, Volatile chemical emissions from 134 common consumer products, Air Qual., Atmos. Health, 2019, 12, 1259–1265 CrossRef.
- A. Bearth, L. Miesler and M. Siegrist, Consumers' Risk Perception of Household Cleaning and Washing Products, Risk Anal., 2017, 37(4), 647–660 CrossRef PubMed.
- L. Calderon, R. Maddalena, M. Russell, S. Chen, J. E. S. Nolan, A. Bradman and K. G. Harley, Air concentrations of volatile organic compounds associated with conventional and “green” cleaning products in real-world and laboratory settings, Indoor Air, 2022, 32(11), e13162 CrossRef CAS PubMed.
- A. C. Steinemann, Fragranced consumer products and undisclosed ingredients, Environ. Impact Assess. Rev., 2009, 29(1), 32–38 CrossRef.
- A. Steinemann, Volatile emissions from common consumer products, Air Qual., Atmos. Health, 2015, 8(3), 273–281 CrossRef CAS.
- A. Steinemann, N. Neda, B. Rismanchi, N. Goodman and S. D. Kolev, Pandemic products and volatile chemical emissions, Air Qual., Atmos. Health, 2021, 14, 47–53 CrossRef CAS PubMed.
- A. M. Temkin, S. L. Geller, S. A. Swanson, N. S. Leiba, O. V. Naidenko and D. Q. Andrews, Volatile organic compounds emitted by conventional and “green” cleaning products in the u.s. market, Chemosphere, 2023, 139570 CrossRef CAS PubMed.
- K. G. Harley, L. Calderon, J. E. S. Nolan, R. Maddalena, M. Russell, K. Roman and S. Mayo-Burgos, Jessica Cabrera, Norma Morga, and Asa Bradman. Changes in latina women’s exposure to cleaning chemicals associated with switching from conventional to “green” household cleaning products: The lucir intervention study, Environ. Health Perspect., 2021, 129(9), 97001 CrossRef CAS PubMed.
- C. Dimitroulopoulou, E. Lucica, A. Johnson, M. R. Ashmore, I. Sakellaris, M. Stranger and E. Goelen, EPHECT I: European household survey on domestic use of consumer products and development of worst-case scenarios for daily use, Sci. Total Environ., 2015, 536, 880–889 CrossRef CAS PubMed.
- P. Spanel and D. Smith, Selected ion flow tube: a technique for quantitative trace gas analysis of air and breath, Med. Biol. Eng. Comput., 1996, 34, 409–419 CrossRef CAS PubMed.
- D. Smith and P. Španěl, Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis, Mass Spectrom. Rev., 2005, 24(5), 661–700 CrossRef CAS PubMed.
- A. Manuja, J. Ritchie, K. Buch, Y. Wu, C. M. A. Eichler, J. C. Little and L. C. Marr, Total surface area in indoor environments, Environ. Sci.: Processes Impacts, 2019, 21(8), 1384–1392 RSC.
- B. C. Singer, H. Destaillats, A. T. Hodgson and W. W. Nazaroff, Cleaning products and air fresheners: Emissions and resulting concentrations of glycol ethers and terpenoids, Indoor Air, 2006, 16(3), 179–191 CrossRef CAS PubMed.
- R. Meininghaus, T. Salthammer and H. Knöppel, Interaction of volatile organic compounds with indoor materials—a small-scale screening method, Atmos. Environ., 1999, 33(15), 2395–2401 CrossRef CAS.
- D. R. Shaw, T. J. Carter, H. L. Davies, E. Harding-Smith, E. C. Crocker, G. Beel, Z. Wang and N. Carslaw, INCHEM-Py v1.2: A community box model for indoor air chemistry, EGUsphere, 2023, 1–32, 2023 Search PubMed.
- N. Carslaw, A new detailed chemical model for indoor air pollution, Atmos. Environ., 2007, 41(6), 1164–1179 CrossRef CAS.
- M. E. Jenkin, S. M. Saunders and M. J. Pilling, The tropospheric degradation of volatile organic compounds: A protocol for mechanism development, Atmos. Environ., 1997, 31(1), 81–104 CrossRef CAS.
- S. M. Saunders, M. E. Jenkin, R. G. Derwent and M. J. Pilling, Protocol for the development of the Master Chemical Mechanism, MCM v3 (Part A): Tropospheric degradation of non-aromatic volatile organic compounds, Atmos. Chem. Phys., 2003, 3(1), 161–180 CrossRef CAS.
- N. Carslaw, T. Mota, M. E. Jenkin, M. H. Barley and G. McFiggans, A Significant role for nitrate and peroxide groups on indoor secondary organic aerosol, Environ. Sci. Technol., 2012, 46(17), 9290–9298 CrossRef CAS PubMed.
- N. Carslaw, L. Fletcher, D. Heard, T. Ingham and H. Walker, Significant OH production under surface cleaning and air cleaning conditions: Impact on indoor air quality, Indoor Air, 2017, 27(6), 1091–1100 CrossRef CAS PubMed.
- A. C. Terry, N. Carslaw, M. Ashmore, S. Dimitroulopoulou and D. C. Carslaw, Occupant exposure to indoor air pollutants in modern European offices: An integrated modelling approach, Atmos. Environ., 2014, 82, 9–16 CrossRef CAS.
- T. J. Carter, D. G. Poppendieck, D. Shaw and N. Carslaw, A Modelling Study of Indoor Air Chemistry: The Surface Interactions of Ozone and Hydrogen Peroxide, Atmos. Environ., 2023, 297, 119598 CrossRef CAS.
- W. W. Nazaroff, Residential air-change rates: A critical review, Indoor Air, 2021, 31(2), 282–313 CrossRef PubMed.
- M. Blocquet, F. Guo, M. Mendez, M. Ward, S. Coudert, S. Batut, C. Hecquet, N. Blond, C. Fittschen and C. Schoemaecker, Impact of the spectral and spatial properties of natural light on indoor gas-phase chemistry: Experimental and modeling study, Indoor Air, 2018, 28(3), 426–440 CrossRef CAS PubMed.
- R. Atkinson and J. Arey, Gas-phase tropospheric chemistry of biogenic volatile organic compounds: A review, Atmos. Environ., 2003, 37(2), 197–219 CrossRef.
- C. Lassen, S. Havelund, S. Mikkelsen, I. Bondgaard, and M. Silberschmidt, Survey and Health Assessment of Chemical Substances in Essential Oils and Fragrance Oils. Technical Report, Danish Environmental Protection Agency, 2008 Search PubMed.
- B. Teixeira, A. Marques, C. Ramos, N. R. Neng, J. M. F. Nogueira, J. Alexandre Saraiva and M. L. Nunes, Chemical composition and antibacterial and antioxidant properties of commercial essential oils, Ind. Crops Prod., 2013, 43(1), 587–595 CrossRef CAS.
- S. Angulo Milhem, M. Verriele, M. Nicolas and F. Thevenet, Does the ubiquitous use of essential oil-based products promote indoor air quality? A critical literature review, Environ. Sci. Pollut. Res., 2020, 27(13), 14365–14411 CrossRef CAS PubMed.
- European Chemicals Agency, ECHA, Date Accessed: 20-09-2023.
- J. H. Hwang, S. Lee, H. G. Lee, D. Choi and K. M. Lim, Evaluation of Skin Irritation of Acids Commonly Used in Cleaners in 3D-Reconstructed Human Epidermis Model, KeraSkinTM, Toxics, 2022, 10, 558 CrossRef CAS PubMed.
- S. Angulo Milhem, M. Verriele, M. Nicolas and F. Thevenet, Indoor use of essential oil-based cleaning products: Emission rate and indoor air quality impact assessment based on a realistic application methodology, Atmos. Environ., 2021, 246, 118060 CrossRef CAS.
- W. Dhifi, S. Bellili, S. Jazi, B. Nada and W. Mnif, Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review, Medicines, 2016, 3(4), 25 CrossRef PubMed.
- P. Wolkoff, P. A. Clausen, C. K. Wilkins and G. D. Nielsen, Formation of Strong Airway Irritants in Terpene/Ozone Mixtures, Indoor Air, 2000, 10(2), 82–91 CrossRef CAS PubMed.
- N. Carslaw and D. Shaw, Modification of cleaning product formulations could improve indoor air quality, Indoor Air, 2022, 32(3), e13021 CrossRef CAS PubMed.
- W. W. Nazaroff and C. J. Weschler, Indoor ozone: Concentrations and influencing factors, Indoor Air, 2022, 32(1), e12942 CAS.
- Master Chemical Mechanism, MCM, Date Accessed: 20-09-2023.
- International Union of Pure and Applied Chemistry, IUPAC, Task Group on Atmospheric Kinetic Data Evaluation, Date Accessed: 20-09-2023 Search PubMed.
- D. K. Farmer, M. E. Vance, J. P. D. Abbatt, A. Abeleira, M. R. Alves, C. Arata, E. Boedicker, S. Bourne, F. Cardoso-Saldaña, R. Corsi, P. F. DeCarlo, A. H. Goldstein, V. H. Grassian, L. H. Ruiz, J. L. Jimenez, T. F. Kahan, E. F. Katz, J. M. Mattila, W. W. Nazaroff, A. Novoselac, R. E. O'Brien, V. W. Or, S. Patel, S. Sankhyan, P. S. Stevens, Y. Tian, M. Wade, C. Wang, S. Zhou and Y. Zhou, Overview of HOMEChem: House Observations of Microbial and Environmental Chemistry, Environ. Sci.: Processes Impacts, 2019, 21(8), 1280–1300 RSC.
- T. Wainman, C. J. Weschler, P. J. Lioy and J. Zhang, Effects of Surface Type and Relative Humidity on the Production and Concentration of Nitrous Acid in a Model Indoor Environment, Environ. Sci. Technol., 2001, 35(11), 2200–2206 CrossRef CAS PubMed.
- R. Kurtenbach, K. H. Becker, J. A. G. Gomes, J. Kleffmann, J. C. Lörzer, M. Spittler, P. Wiesen, R. Ackermann, A. Geyer and U. Platt, Investigations of emissions and heterogeneous formation of HONO in a road traffic tunnel, Atmos. Environ., 2001, 35(20), 3385–3394 CrossRef CAS.
- A. P. Altshuller, Assessment of the contribution of chemical species to the eye irritation potential of photochemical smog, J. Air Pollut. Control Assoc., 1978, 28(6), 594–598 CrossRef CAS PubMed.
- R. Golden, Identifying an indoor air exposure limit for formaldehyde considering both irritation and cancer hazards, Crit. Rev. Toxicol., 2011, 41, 672–721 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3em00439b |
| This journal is © The Royal Society of Chemistry 2024 |