The reciprocity principle in mulch film deterioration and microplastic generation
Runhao
Bai†
 ab,
Zhen
Li†
ab,
Qin
Liu
ab,
Qi
Liu
ab,
Jixiao
Cui
*abc and
Wenqing
He
*abc
ab,
Zhen
Li†
ab,
Qin
Liu
ab,
Qi
Liu
ab,
Jixiao
Cui
*abc and
Wenqing
He
*abc
aInstitute of Environment and Sustainable Development in Agriculture, Chinese Academy of Agricultural Sciences, Beijing 100081, PR China. E-mail: hewenqing@caas.cn; cuijixiao@caas.cn
bKey Laboratory of Prevention and Control of Residual Pollution in Agricultural Film, Ministry of Agriculture and Rural Affairs, Beijing 100081, PR China
cInstitute of Western Agricultural, Chinese Academy of Agricultural Sciences, Changji 831100, China
First published on 5th December 2023
Abstract
Plastic film mulching stands as a globally employed agricultural technology pivotal to agricultural progress. Nevertheless, the environmental degradation of plastic mulch films underscores their role as a major source of secondary plastic pollutants, particularly microplastics. While a growing body of research has drawn attention to the rising issue of microplastic pollution and its environmental implications stemming from the use of plastic mulch films, there remains a significant knowledge gap regarding the kinetics and rate-limiting mechanisms governing the generation of microplastics during processes driven by plastic photodegradation. Moreover, a comprehensive quantification of the connection between mulch deterioration and the behavior of microplastic release and accumulation has yet to be fully realized. In this study, a kinetic equation was formulated to characterize the degradation of plastic mulch films and the subsequent release and accumulation of microplastics under light exposure. The results demonstrate that with increasing irradiation time, the change in the release rate exhibits a bell-shaped Gaussian probability distribution, while the cumulative alteration of microplastics follows a Gaussian distribution. Remarkably, once the exposure time reaches μ + 3σ, the accumulation plateaus at 99.7%. This research establishes a theoretical framework for the prospective assessment of plastic mulch lifespan and its environmental repercussions. Moreover, the findings provide valuable insights for optimizing plastic mulch design and devising strategies to mitigate microplastic pollution.
Environmental significancePlastic mulch films are widely used in agricultural practices, driving agricultural progress. However, their environmental degradation poses a significant threat to agricultural ecosystems and sustainability. This study aims to comprehensively understand the kinetics and rate-limiting processes of microplastic generation during plastic mulch film degradation. By developing a generalized empirical model that connects degradation performance with the molecular structure of plastic mulch films under solar exposure, this research establishes a theoretical framework to assess the longevity and environmental impact of these films. The findings provide valuable insights for the design and management of plastic mulch films, contributing to mitigating their environmental consequences. |
Plastic film mulching stands as one of the most widely employed agricultural technologies globally. This technique exhibits outstanding performance,1,2 capable of augmenting crop yields by approximately 30% to 50%, enhancing water use efficiency by 20% to 30%, and significantly reducing the need for chemical fertilizers and weed control agents.3–5 The annual worldwide application of plastic film exceeds 1.6 million tons, covering more than 20 million hectares of farmland, and continues to experience steady growth.6 However, when introduced into the environment, plastic mulches undergo a rigorous weathering process, leading to the deterioration and fragmentation of mulch residues, including microplastics.7 Microplastics are characterized as minute plastic particles, with a maximum dimension of less than 5 mm, encompassing diverse forms such as fragments, particles, films, or fibers.8 Existing studies further underscore that the persistent presence of microplastics in farmland soil can alter the soil's physical, chemical, and biological attributes, thereby posing potential ecological risks and undermining soil health. In the current context, microplastics have emerged as a significant and prevalent global pollutant.
Recent investigations reveal that mulch can release microplastics resulting from mechanical action, photothermal aging, and biodegradation. Given their relatively stable plastic polymer composition, secondary microplastics released into the environment exhibit slow degradation. Additionally, their small size, extensive specific surface area, and active surface functional groups make them prone to interacting with soil particles, plants, and biological organisms. Consequently, these microplastics can serve as a conducive habitat for heavy metals, antibiotics, and other pollutants, thereby posing significant potential environmental risks. Nowadays, mulch-derived microplastics are increasingly acknowledged as a grave threat to agricultural systems.9 Nonetheless, current research often lacks the quantitative and kinetic analysis of microplastics released by the mulch film. The underlying molecular structure and key environmental aging factors governing the microplastic release process determine the fundamental parameters associated with mulch film degradation and microplastic fragmentation.10
Low-density polyethylene (LDPE) represents a polymer material frequently employed in the manufacturing of plastic mulch. Plastic mulch crafted from LDPE exhibits outstanding properties, including excellent light transmission, exceptional thermal insulation performance, commendable waterproof characteristics, and high durability. In traditional plastic mulch, polyethylene typically constitutes 95% to 98% of its total composition. Microscopically, polyethylene is a linear polymer formed through the homopolymerization of hundreds to thousands of ethylene molecules or copolymerization with a small quantity of 1-olefin. The resulting molecular groups are held together by robust and stable covalent chemical bonds, endowing polyethylene with unique inertness and hydrophobicity. The structural stability of its chemical bonds renders polyethylene's structural units highly resistant to various weathering phenomena, including water hydrolysis, thermal oxidation, and biological processes. Existing research demonstrates that PE undergoes very slow degradation in natural environments. Albertsson et al. (1988) observed that the degradation rate of polyethylene film buried in soil for a decade ranged from only 0.2% to 0.5%.11 Ohtake et al. (1998) estimated that approximately 300 years would be required for a 60 μm-thick polyethylene film to completely degrade under typical field soil landfill conditions.12 Natural microbial degradation in the soil environment occurs at a sluggish pace, and the intricate degradation processes defy easy modeling. Therefore, research predominantly fixates on isolated environmental factors to conduct simulations pertaining to microplastic release. For instance, Ouyang et al. (2023) simulated the influence of soil particle abrasion on the crushing and degradation of PE films, leading to the derivation of a relatively straightforward kinetic equation for microplastic release.13 Some studies have also ventured into deducing the release of microplastics theoretically during alterations in the mechanical properties of plastics due to impacts, fractures, and wear, thereby constructing corresponding kinetic models.14 These investigations serve to address the limitations of mechanical models for microplastic release. However, it is important to note that the incessant high-frequency friction represented in these studies may not replicate natural conditions faithfully.
In the context of natural environments, the degradation of plastic coverings and the subsequent release of microplastics entail the rupture of covalent bonds, a process necessitating the influence of high-energy environmental factors, particularly light and heat. Of these factors, ultraviolet (UV) exposure, primarily originating from sunlight, is renowned for its potency in breaking molecular bonds. Thus, it is widely acknowledged as a pivotal catalyst in the degradation of plastic mulch films utilized in agricultural applications, leading to the formation of microplastics17 (Fig. 1). Current research often employs xenon lamps or ultraviolet lamps that emit high-intensity radiation to simulate rapid weathering of membrane materials indoors. Briassoulis et al. (2015) investigated the accelerated degradation of PE under high ultraviolet radiation exposure using an intense ultraviolet aging acceleration system. They discovered that after 800 hours of high ultraviolet radiation treatment, the polyethylene residual film could completely decompose into microplastics when reintroduced into the soil over several years.18 Expanding on previous research, Xie et al. (2022) developed a predictive methodology to estimate the practical lifespan of mulch under field conditions. Building upon this foundation, Xie et al. (2022) devised a predictive method for estimating the actual lifespan of field coverings, drawing from both laboratory-simulated light aging experiments and real-world aging trials.19 Yang et al. (2022) research marked the pioneering effort in establishing and provisionally validating a kinetic model for microplastic release in response to light-induced mulch deterioration, employing quantum theory and Schwartz's law. Nevertheless, this study primarily focused on characterizing the material's chemical structure and did not establish a direct link between microplastic release and changes in material properties. The development of kinetic equations yielded satisfactory results only at the verification stage.15
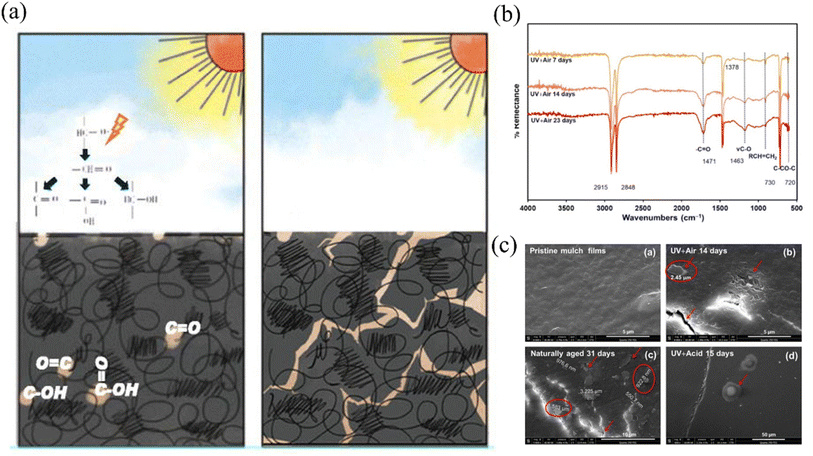 | ||
| Fig. 1 (a) Schema of photodegradation of plastic film covered in the field;15 (b) the infrared spectra of PE film before and after UV light irradiation;16 (c) microplastics detected on the surface of PE film after photoaging.16 | ||
To achieve a more precise quantification of the relationship between structural alterations in mulch material and the production of microplastics during photodegradation, a more robust reliance on quantum theory, grounded in the fundamental properties of LDPE, is imperative for constructing a dynamic model based on Schwartz's law. In accordance with quantum theory, photodegradation can be understood as an unimolecular reaction that commences when high-energy photons interact with polymer chains within the plastic mulch, resulting in the breakage of chemical bonds and the subsequent formation of free radicals. These free radicals then undergo reactions with oxygen, initiating a complex sequence of free radical reactions that ultimately culminate in chain scission. The efficiency of photodegradation can be quantified as the probability that an absorbed photon triggers the cleavage of molecular bonds, a parameter influenced by the intensity of UV radiation and the abundance of photosensitive species present within the plastic mulch. It is postulated that the presence of unsaturated chromophores, external impurities, and structural anomalies formed during the processing of plastic mulch contributes to the absorption of light energy, thereby facilitating the degradation process. Significantly, all of these structural irregularities are predominantly distributed within amorphous regions.20
LDPE is typically synthesized under conditions of high temperature and high-pressure reactions, resulting in a substantial number of molecular chain branches, a loose molecular arrangement, and relatively low crystallinity. Plastic mulch is based on the semi-crystalline configuration of LDPE molecules, possessing a heterogeneous three-dimensional porous structure.21 Crystalline regions are the parts of polymer molecules with atoms densely situated in a repeating and periodic array and, thus are compact and rigid in nature.22 On the contrary, amorphous regions that medium around crystalline are of much less regular disposition, but instead largely increased in flexibility and permeability, therefore allowing permeation of oxygen and thus accessible to oxidized degradation upon photo-irradiation.23 As a consequence, chain scissions at photo exposures specifically take place in the amorphous region. Along with photodegradation, chain scissions at the amorphous regions cause increased local pressures, which when exceeded by the strength of the polymer matrix, result in micron cracks, pits, and polymer fragmentations.23,24 Small fragments from amorphous regions with molecular weight less than 500 kDa can be quickly assimilated by microorganisms to form CO2, H2O, and biomass. However, fragments of higher molecular weight or high crystalline ratio are most likely to persist in the environment.25 From this point of view, the deterioration of plastic mulch film and the formation of microplastics can be expressed as the degradation of amorphous areas to separate from the crystalline regions. Of which the kinetic rate of the degradation process is largely determined by the accessibility and susceptibility of the amorphous areas, while the number and size of microplastics are generally encoded in the original crystalline morphology.26
To gain deeper insights into the microplastic characteristics entailed in the amorphous regions' encoding during the quantitative material degradation, the utilization of kinetic modeling becomes imperative. This robust tool enables the elucidation of these properties without necessitating an intricate dive into complex chemical reactions.27 In the context of numerous chemical reactions, including photo-oxidation, the involved steps often display considerable magnitudes that exceed experimental feasibility in terms of parameter determination. When considering environmental processes where photo-irradiation wields a crucial influence, alterations in material properties predominantly hinge on the cumulative absorbed energy of photo-irradiation. Accordingly, an anticipatable dose-dependent relationship with photo irradiance emerges, commonly expressed via the reciprocity principle or the broader the Schwarzschild's principle, as depicted in eqn (1):
| Ip·t = constant | (1) |
Deep investigation on base of the quantum theory and Schwardzchild kinetic principle may provide a time-dependent profile for the formation of microplastics during mulch film deterioration, may hopefully bridge the knowledge gap between molecule morphology and deterioration properties of plastic mulch film, also pull an alarm on the overall quantity of microplastic fragments that could be generated and the critical time length when that microplastic generation reach a peak concentration.
Kinetic modeling for microplastic generation
Changes in material properties as the outcome of photodegradation depend dominantly on the total absorbed energy of photo-irradiation. The value of radiant intensity, I, and coefficient factor, p, can be assumed essentially constant for indicated material and exposure, especially for the exposure longer than diurnal. Therefore, as degradation proceeds, the alteration in surface area and thus population size of susceptible species would be the only variant parameter that determines the kinetic pattern.28Regarding plastic mulch films, the area concentration of the susceptible population is decided by the amorphous regions, as is expressed in eqn (2):
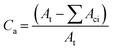 | (2) |
Similar to previous studies, at the initial moment of photodegradation, we assumed that light irradiation would immediately and uniformly affect the entire mulch sample.28,29 With the increase of exposure time, molecules on the mulch surface break and are released into the environment. This process creates pits and cracks on the mulch surface, subsequently increasing the surface area exposed to light and enlarging the susceptible population during the initial degradation phase. As exposure time lengthens, more pronounced degradation occurs in the amorphous region, causing the proportion of non-degraded surface area in the amorphous phase to gradually diminish to zero.30 Given the underlying assumption of random material degradation and the stochastic nature of photodamage, alterations in the accessible surface area within the amorphous region or the population size of susceptible species follow a normalized Gaussian distribution, as depicted in eqn (3). The cumulative Gaussian distribution for the response population is represented in eqn (4):
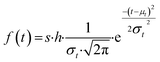 | (3) |
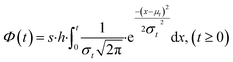 | (4) |
| μt = Ip·s·h·k | (5) |
The cumulative quantity of microplastic fragmentation to photo exposure in terms of the Schwarzschild's principle can be calculated as eqn (6):
| Qt = k·Ip·t·Ca·Φ(t) | (6) |
The total quantity of microplastics that could potentially originate from plastic mulch films can be easily estimated based on their fundamental characteristics, as demonstrated by eqn (7):
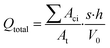 | (7) |
By visualizing the exposure time, the long-term performance of plastic mulches, and the generation of microplastics can be predicted quantitatively. Both the release rate and cumulative quantity of microplastic fragments can serve as indicators of aging evolution. They are represented by a normalized distribution curve (Fig. 2a) and a cumulative Gaussian distribution curve (Fig. 2b), respectively.
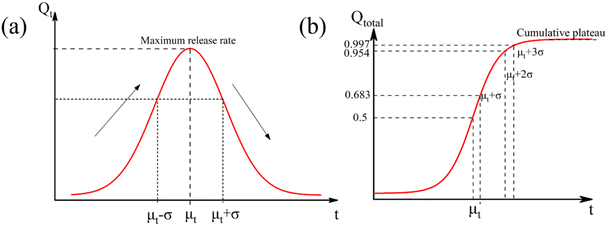 | ||
| Fig. 2 (a) Schematic diagram of release rate of microplastics with exposure time; (b) schematic diagram of cumulative release of microplastics with exposure time. | ||
The normalized Gaussian distribution curve, characterized by its classic bell shape, illustrates the variation in microplastic release rate with prolonged light exposure time. Before reaching time μT, the rate of microplastic formation continues to rise due to the rapid expansion of the susceptible surface area. However, this growth rate gradually diminishes. At μT, the release rate of microplastics peaks. Beyond μT, the rate of microplastic production steadily declines as the population size of susceptible species decreases, undegraded surface area diminishes, and internal degradation occurs. This bell curve offers a quantitative means to monitor the aging process of plastic mulches and can inform decisions related to microplastic risk assessment and mulch recycling.
The accumulation of microplastics follows a Gaussian accumulation distribution pattern. According to the Gaussian distribution probability function, the cumulative quantity of ruptured microplastics exhibits continuous growth with the extension of light exposure time and changes in film material properties. Eventually, it reaches a cumulative plateau when the susceptible area approaches zero due to extensive degradation. Specifically, the release rate of microplastics changes, and the cumulative acceleration of microplastics increases initially, followed by a decrease before and after μT. According to the Gaussian distribution probability function properties, 68.3%, 95.5%, and 99.7% of the total microplastics are released and accumulated during the time intervals of μ + σ, μ + 2σ, and μ + 3σ, respectively. After μ + 3σ, the release kinetics of microplastics gradually diminish to zero, and the cumulative emission of microplastics reaches its final plateau.
It's noteworthy that in eqn (7), Aci, At, s, h, and V0 are fundamental physical properties that can be directly measured. For a given material and exposure condition, the values of p and k may remain constant. Since changes in performance properties can be translated into susceptible surface area, only early data on the release rate of microplastics per unit area of plastic mulch are necessary. The values of μ and σ can be computed based on the fundamental physical properties of the mulch and simple short-term aging experiments.
Utilizing the microplastic accumulation equation we derived, it is possible to make a rough estimate of microplastic accumulation following the photoaging of mulch films in real-world conditions, based on parameters available in the literature. However, it's important to acknowledge that the parameters obtained from the literature pertain to idealized scenarios and may not align perfectly with real-world conditions.31 For instance, the average UV radiation intensity in northern China typically ranges from 300 to 900 W m−2 during spring and summer. In practice, standard PE mulch films have a thickness of 10−5 m, and the period from mulch film application to recycling is approximately 80 days. These parameters and others can be found in Yang et al.'s research (2022).15 Given an average daily irradiance of 500 W m−2, the cumulative theoretical release of microplastics under photodegradation amounts to 7.86 × 105 items per m2. As indicated by the derived equation, the irradiance value serves as the primary limiting factor in this estimation.
Plastic additives upon photodegradation
Various types of chemical additives can be incorporated into plastic mulch films to achieve specific functional properties. However, it's important to note that these plastic additives can significantly influence the deterioration properties of plastic mulch films.Typically, to mitigate the impact of photothermal oxidation reactions from atmospheric oxygen and solar ultraviolet radiation on mulch materials, thereby preserving mechanical integrity and water retention capacity throughout crop growth, researchers incorporate additives into mulch materials, including UV-absorbing agents. Anti-ultraviolet absorbers play a pivotal role by acting as optical shields, absorbing, and transferring light energy, as well as quenching or capturing free radicals. These absorbers can be categorized into four groups based on their mechanisms of action: light shielding agents, ultraviolet absorbers, quenchers, and hindered amine light stabilizers.
Both quenchers and ultraviolet absorbers effectively enhance material light stability and diminish the degradation rate of plastic mulch through light energy transfer.32 Light shielding agents, such as carbon black predominantly employed in colored mulch films, exhibit robust light absorbance, effectively preventing photon-induced bond cleavage within polymer chains, resulting in UV stabilization. Hindered amine light stabilizers, a subset of organic amine compounds characterized by steric hindrance, demonstrate the ability to decompose hydroperoxides, quench excited oxygen, and capture free radicals. This group serves as highly efficient light stabilizers and antioxidants.33
It is imperative to account for the initial retarding effect on photodegradation processes attributed to these additives in plastic mulches. Given that the types, concentrations, proportions, and homogeneity of various stabilizers within mulch films impact their performance, determining the distribution function relationship for initial delayed action necessitates empirical degradation and weathering experiments. In the presence of degradation-delaying additives, photodamage within the amorphous region occurs randomly. However, variations in accessible surface area or susceptible species population size within the amorphous region follow a convolutional Gaussian distribution, as expressed in eqn (8):
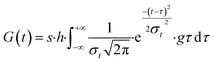 | (8) |
In these circumstances, plotting of release kinetic of microplastic versus aging time presents a negatively skewed curve, with a corresponding intercept on the aging time axis quantifying the durability of the plastic stabilizer.
There are other types of plastic additives that do not react to the degradation procedure of plastic mulch film, however, their emission during mulch film deterioration may cause serious environmental contamination. Among these, phthalates (PAEs) are frequently employed as plastic additives in mulch films, serving as plasticizers to enhance film flexibility and ductility. The leakage of this substance into the environment poses significant environmental risks and carries substantial health hazards for humans. PAEs have been designated as priority pollutants due to their carcinogenic and endocrine-disrupting potential.34 PAEs, characterized as a class of small chemical molecules, are introduced during the synthetic process of mulch film production. They form physical, rather than chemical, bonds with the mulch film, typically through weak hydrogen bonds or van der Waals forces, and are loosely dispersed among the polymer chains within the mulch film's porous structure. As a result, PAEs can be readily leached and released into the environment during mulch application and degradation. The release of PAEs molecules primarily occurs through diffusion within the porous structure of the plastic mulch. This release rate is influenced by the physical and chemical properties of the PAEs themselves as well as the characteristics of the plastic mulch. In comparison to high molecular weight PAEs like DEHP, low molecular weight PAEs such as DMP are more hydrophilic, making them more prone to migrate from the polymer matrix and potentially causing environmental exposure.35,36 The release rate of PAEs molecules, even of the same type, exhibits a strong correlation with the deterioration status of plastic mulch films. This correlation arises because the breakdown of molecular chains within the plastic enlarges the porous volumes and creates larger surface areas, which in turn facilitate the release of smaller chemicals.37 Therefore, the release rate of PAEs molecules may as well follow the normalized Gaussian distribution as expressed in eqn (3), with parameters valued according to the additive's performance.
Quick analytical method on photostability
It is of paramount importance to obtain a comprehensive assessment of the property performance of plastic mulch films and the fragmentation characteristics of microplastics throughout the entire process. Reliable and practical accelerated aging techniques are essential for expediting the evaluation of material degradation. Photodegradation experiments involving materials have employed various light sources, including ultraviolet lamps, ultraviolet LEDs, and xenon lamps. Among these options, xenon lamps are widely preferred due to their ability to closely replicate natural sunlight, establishing them as the primary tool for assessing degradation and material property alterations resulting from light exposure. Xenon lamps utilize xenon gas to generate intense broadband light emissions spanning the ultraviolet, visible, and near-infrared spectra. This broad-spectrum irradiation closely resembles natural sunlight, enabling researchers to replicate outdoor exposure conditions. Additionally, these lamps can be equipped with filters to tailor the irradiance to specific wavelength requirements. In photoaging experiments involving plastic film materials, the irradiance of the xenon lamp (W m−2) serves as a crucial control parameter. The xenon lamp aging test chambers currently available in the market excel in precisely controlling this parameter. By calculating the cumulative xenon lamp irradiance alongside natural sunlight irradiance, researchers can effectively simulate the coefficient of accelerated aging to a certain extent. It is important to note that the operation of xenon lamps generates a significant amount of heat. For this reason, the influence of heat on degradation must be taken into account when conducting mulch aging tests. To address this issue, xenon lamp testing equipment often incorporates cooling fans to enhance heat dissipation. However, in aging experiments involving plastic film intended to explore the formation and release of microplastics, careful adjustments to parameters such as wind speed and wind direction are necessary to prevent the film from being displaced by the cooling airflow.Following the simulation of rapid material photodegradation, various analytical techniques can be applied to characterize and detect physical or chemical changes occurring during plastic degradation (Fig. 3). These techniques encompass nuclear magnetic resonance (NMR), high-performance liquid chromatography (HPLC), electrospray mass spectrometry (EMI-MS), and gas chromatography-mass spectrometry (GC-MS) for investigating the chemical modifications of the polymer matrix. Moreover, techniques such as scanning electron microscopy (SEM), atomic force microscopy (AFM), and laser scanning confocal microscopy (LSCM) are not only valuable for assessing alterations in material surface morphology but can also serve as powerful tools for the quantitative identification of microplastics. Additionally, monitoring CO2 evolution or O2 consumption offers insights into the extent of polymer degradation. These multifaceted analytical tools collectively contribute to a comprehensive understanding of plastic degradation and microplastic fragmentation characteristics. Furthermore, there is an immediate imperative to investigate the establishment of corresponding relationship models between frequently employed macroscopic characterizations of plastic mulches, including mechanical strength, surface haze, and water vapor transmission rate, and the aforementioned microscopic characterizations.
In addition, recently reported techniques for statistical study of polymer aging at microscopic scales include: a three-dimensional monitor technique, established by combining the use of confocal laser scanning microscopy with fluorescent-bonded boronic acids, which may specifically target aging-induced hydroxyl groups and give a three-dimensional visualization on the degradation processes of aging polymers, therefore helps in quantitative evaluation on the dimensional-dependent surface areas of susceptible populations during photodegradation process.38 Moreover, a micro-FT-IR spectroscopy equipment by in situ aging that may simulate photodegradation under complex environmental circumstances, and quantitatively monitor the spectral changes of plastic polymers to provide statistical information on degradation rate and constant. Most importantly, as is reported, this technique may accomplish the examination of photostability of aging material in a few couples of hours.39
The latest techniques hold great promise for advancing research in the field of polymer degradation and microplastic release, offering opportunities to enhance our understanding of these processes and develop strategies for mitigating their environmental impact.
Conclusion and outlook
This study is based on Schwartz's law, starting from the degradation and degradation of polyethylene film that is susceptible to light exposure, and deduces the kinetic equations for material degradation and the generation and accumulation of microplastics. The results demonstrate that with increasing irradiation time, the change in the release rate exhibits a bell-shaped Gaussian probability distribution, while the cumulative alteration of microplastics follows a Gaussian distribution. Remarkably, once the exposure time reaches μ + 3σ, the accumulation plateaus at 99.7%. According to this equation, during a single mulching growth period in the mulching area in northern China, the mulch film per unit area (m2) will cumulatively release tens of thousands of microplastics. Existing aging equipment and advanced material structure characterization instruments will assist in this assessment process. In addition, there is an urgent need to study the correspondence model between commonly used macroscopic characteristics of plastic coverings (including mechanical strength, surface haze, and water vapor transmission rate) and the microscopic characteristics mentioned above. The results of this study will enable rapid assessment of the formation and release of microplastics and provide a way to more effectively predict the environmental risks associated with microplastics.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant number 42007394), and Fundamental Research Funds of IEDA, CAAS (Grant number BSRF202207).References
- S. Kasirajan and M. Ngouajio, Agron. Sustainable Dev., 2012, 32, 501–529 CrossRef CAS.
- D. Sun, H. Li, E. Wang, W. He, W. Hao, C. Yan, Y. Li, X. Mei, Y. Zhang, Z. Sun, Z. Jia, H. Zhou, T. Fan, X. Zhang, Q. Liu, F. Wang, C. Zhang, J. Shen, Q. Wang and F. Zhang, Natl. Sci. Rev., 2020, 7, 1523–1526 CrossRef CAS.
- H. Gao, C. Yan, Q. Liu, Z. Li, X. Yang and R. Qi, Agric. Water Manage., 2019, 225, 105741 CrossRef.
- L. Zhu, S. Xu, R. Chen, L. Li and T. Liu, Nutr. Cycling Agroecosyst., 2022, 122, 31–39 CrossRef CAS.
- W. Zhang, A. Dong, F. Liu, W. Niu and K. H. M. Siddique, Soil Tillage Res., 2022, 221, 105392 CrossRef.
- K. Koskei, A. N. Munyasya, Y.-B. Wang, Z.-Y. Zhao, R. Zhou, S. N. Indoshi, W. Wang, W. K. Cheruiyot, D. M. Mburu, A. B. Nyende and Y.-C. Xiong, J. Hazard. Mater., 2021, 414, 125521 CrossRef CAS PubMed.
- Y. Huang, Q. Liu, W. Jia, C. Yan and J. Wang, Environ. Pollut., 2020, 260, 114096 CrossRef CAS PubMed.
- N. Khalid, M. Aqeel, A. Noman and Z. Fatima Rizvi, J. Hazard. Mater., 2023, 445, 130455 CrossRef CAS PubMed.
- H. Gao, Q. Liu, C. Yan, K. Mancl, D. Gong, J. He and X. Mei, Resour., Conserv. Recycl., 2022, 186, 106549 CrossRef CAS.
- N. Khalid, M. Aqeel, A. Noman and Z. Fatima Rizvi, J. Hazard. Mater., 2023, 445, 130455 CrossRef CAS PubMed.
- A.-C. Albertsson and S. Karlsson, J. Appl. Polym. Sci., 1988, 35, 1289–1302 CrossRef CAS.
- Y. Ohtake, T. Kobayashi, H. Asabe and N. Murakami, Polym. Degrad. Stab., 1998, 60, 79–84 CrossRef CAS.
- D. Ouyang, Y. Peng, B. Li, F. Shao, K. Li, Y. Cai, T. Guo and H. Zhang, Sci. Total Environ., 2023, 893, 164821 CrossRef CAS PubMed.
- A. Boersma, K. Grigoriadi, M. G. A. Nooijens, S. Henke, I. M. Kooter, L. A. Parker, A. Dortmans and J. H. Urbanus, Polymers, 2023, 15, 2185 CrossRef CAS PubMed.
- Y. Yang, Z. Li, C. Yan, D. Chadwick, D. L. Jones, E. Liu, Q. Liu, R. Bai and W. He, Sci. Total Environ., 2022, 814, 152572 CrossRef CAS PubMed.
- T. Wang, Y. Ma and R. Ji, Bull. Environ. Contam. Toxicol., 2021, 107, 736–740 CrossRef CAS PubMed.
- F. Portillo, O. Yashchuk and É. Hermida, Polym. Test., 2016, 53, 58–69 CrossRef CAS.
- D. Briassoulis, E. Babou, M. Hiskakis and I. Kyrikou, J. Appl. Polym. Sci., 2015, 132(30), 42289 CrossRef.
- J. Xie, Y. Yan, S. Fan, X. Min, L. Wang, X. You, X. Jia, G. I. N. Waterhouse, J. Wang and J. Xu, Environ. Sci. Technol., 2021, 56(12), 9041–9051 CrossRef PubMed.
- B. Gewert, M. M. Plassmann and M. MacLeod, Environ. Sci.: Processes Impacts, 2015, 17, 1513–1521 RSC.
- A. A. Basfar and K. M. Idriss Ali, Polym. Degrad. Stab., 2006, 91, 437–443 CrossRef CAS.
- T. Menzel, N. Meides, A. Mauel, U. Mansfeld, W. Kretschmer, M. Kuhn, E. M. Herzig, V. Altstädt, P. Strohriegl, J. Senker and H. Ruckdäschel, Sci. Total Environ., 2022, 826, 154035 CrossRef CAS PubMed.
- A. Ammala, S. Bateman, K. Dean, E. Petinakis, P. Sangwan, S. Wong, Q. Yuan, L. Yu, C. Patrick and K. H. Leong, Prog. Polym. Sci., 2011, 36, 1015–1049 CrossRef CAS.
- V. M. Aslanian, V. I. Vardanian, M. H. Avetisian, S. S. Felekian and S. R. Ayvasian, Polymer, 1987, 28, 755–757 CrossRef CAS.
- B. Thakur, J. Singh, J. Singh, D. Angmo and A. P. Vig, Sci. Total Environ., 2023, 877, 162912 CrossRef CAS.
- S. Kumar Sen and S. Raut, J. Environ. Chem. Eng., 2015, 3, 462–473 CrossRef CAS.
- J. W. Martin, J. W. Chin and T. Nguyen, Prog. Org. Coat., 2003, 47, 292–311 CrossRef CAS.
- S. G. Croll, Prog. Org. Coat., 2019, 127, 140–150 CrossRef CAS.
- S. G. Croll, B. R. Hinderliter and S. Liu, Prog. Org. Coat., 2006, 55, 75–87 CrossRef CAS.
- F. Julienne, F. Lagarde and N. Delorme, Polym. Degrad. Stab., 2019, 170, 109012 CrossRef.
- L. Ding, Z. Ouyang, P. Liu, T. Wang, H. Jia and X. Guo, Sci. Total Environ., 2022, 802, 149840 CrossRef CAS PubMed.
- C. W. Klampfl and M. Himmelsbach, Anal. Chim. Acta, 2016, 933, 10–22 CrossRef CAS PubMed.
- X. Wang, H. Pan, S. Jia, Z. Lu, L. Han and H. Zhang, Polym. Bull., 2023, 80, 2485–2501 CrossRef CAS.
- Q.-Q. Zhang, Z.-R. Ma, Y.-Y. Cai, H.-R. Li and G.-G. Ying, Environ. Sci. Technol., 2021, 55, 12459–12470 CrossRef CAS PubMed.
- Y. Wang, F. Wang, L. Xiang, C. Gu, M. Redmile-Gordon, H. Sheng, Z. Wang, Y. Fu, Y. Bian and X. Jiang, Environ. Sci. Technol., 2021, 55, 3676–3685 CrossRef CAS PubMed.
- G. E. De-la-Torre, D. C. Dioses-Salinas, S. Dobaradaran, J. Spitz, I. Nabipour, M. Keshtkar, R. Akhbarizadeh, M. Tangestani, D. Abedi and F. Javanfekr, Environ. Res., 2022, 215, 114337 CrossRef CAS.
- A. G. Uzamurera, P.-Y. Wang, Z.-Y. Zhao, X.-P. Tao, R. Zhou, W.-Y. Wang, X.-B. Xiong, S. Wang, K. Wesly, H.-Y. Tao and Y.-C. Xiong, J. Hazard. Mater., 2023, 448, 130897 CrossRef CAS.
- Z. Zhang, R. Tian, P. Zhang, C. Lu and X. Duan, ACS Cent. Sci., 2020, 6, 771–778 CrossRef CAS.
- Z. An, Z. Xu, Y. Ye and R. Yang, Anal. Chim. Acta, 2021, 1169, 338632 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this article. |
| This journal is © The Royal Society of Chemistry 2024 |