Polycyclic aromatic hydrocarbons in dust from rural communities around gas flaring points in the Niger Delta of Nigeria: an exploration of spatial patterns, sources and possible risk†
Eze W.
Odali
a,
Chukwujindu M. A.
Iwegbue
 *a,
Francis E.
Egobueze
b,
Godwin E.
Nwajei
a and
Bice S.
Martincigh
*a,
Francis E.
Egobueze
b,
Godwin E.
Nwajei
a and
Bice S.
Martincigh
 c
c
aDepartment of Chemistry, Delta State University, P.M.B. 1, Abraka, Nigeria. E-mail: cmaiwegbue@delsu.edu.ng; maxipriestley@yahoo.com
bNigerian Agip Oil Company, Rumueme, Port Harcourt, Nigeria
cSchool of Chemistry and Physics, University of KwaZulu-Natal, Westville Campus, Private Bag X54001, Durban 4000, South Africa
First published on 16th May 2023
Abstract
Indoor and outdoor dust from three rural communities (Emu-Ebendo, EME, Otu-Jeremi, OTJ, and Ebedei, EBD) around gas flaring points, and a rural community (Ugono Abraka, UGA) without gas flare points, in the Niger Delta of Nigeria, was analysed for the concentrations and distribution of polycyclic aromatic hydrocarbons (PAHs), their sources, and possible health risk resulting from human exposure to PAHs in dust from these rural communities. The PAHs were extracted from the dust with a mixture of dichloromethane/n-hexane by ultrasonication, and purified on a silica gel/alumina packed column. Gas chromatography-mass spectrometry was employed to determine the identity and concentrations of PAHs in the cleaned extracts. The Σ16PAH concentrations in the indoor dust ranged from 558 to 167![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 6580 to 413
000, 6580 to 413![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and 2350–37
000, and 2350–37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 μg kg−1 for EME, OTJ and EBD respectively, while those of their outdoor counterparts varied from 347 to 19
500 μg kg−1 for EME, OTJ and EBD respectively, while those of their outdoor counterparts varied from 347 to 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700, 15
700, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 130
000 to 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and 1780 to 46
000, and 1780 to 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 μg kg−1 for EME, OTJ and EBD respectively. On the other hand, the UGA community without gas flare points had Σ16PAH concentrations in the range of 444–5260 μg kg−1 for indoor dust, and 154–7000 μg kg−1 for outdoor dust. The lifetime cancer risk values for PAHs in these matrices surpassed the acceptable limit of 10−6 suggesting a potential carcinogenic risk resulting from human exposure to PAHs in indoor and outdoor dust from these rural communities. Principal component analysis suggested that PAH contamination of dust from these communities arises principally from gas flaring, combustion of wood/biomass, and vehicular emissions.
300 μg kg−1 for EME, OTJ and EBD respectively. On the other hand, the UGA community without gas flare points had Σ16PAH concentrations in the range of 444–5260 μg kg−1 for indoor dust, and 154–7000 μg kg−1 for outdoor dust. The lifetime cancer risk values for PAHs in these matrices surpassed the acceptable limit of 10−6 suggesting a potential carcinogenic risk resulting from human exposure to PAHs in indoor and outdoor dust from these rural communities. Principal component analysis suggested that PAH contamination of dust from these communities arises principally from gas flaring, combustion of wood/biomass, and vehicular emissions.
Environmental significanceDust is an important repository for various organic contaminants, and is a gateway for human exposure to them. It can be used as a proxy for evaluating the contamination status of an area, and the consequent possible risk to the ecosystem and humans. This study evaluated the relationships between the concentrations, compositions, sources, and risk of PAHs in indoor and outdoor dust from rural communities around gas flare points in the Niger Delta. Our findings suggest that indoor and outdoor dust from these rural communities around gas flare points were highly contaminated with PAHs and require clean up, remedial actions, and the implementation of stringent pollution control measures with a view to reducing the adverse consequences of PAHs on the ecosystem and humans. |
Introduction
Besides oil spillages, gas flaring is a major source of environmental pollution associated with the activities of oil and gas industries in the Niger Delta. Despite the existence of the Nigerian “Associated Gas Reinjection Act of 1979”, which prohibits gas flaring by oil industries beyond January 1984, gas flaring has continued unabated as a cheap and safe way of disposing of gases produced alongside crude oil due to the lack of capacity to (i) enforce this regulation, and (ii) utilize these gases for other purposes.1,2In Nigeria, over 70% of the natural gas produced by oil companies is flared.3 Historically, gas flaring has been practiced in Nigeria for over six decades. Apart from the loss of a potential energy source, gas flaring is a typical incomplete combustion process which produces a wide variety of pollutants including polycyclic aromatic hydrocarbons (PAHs).
PAHs constitute a large group of semi-volatile and persistent organic compounds with two or more fused aromatic rings arranged in either an angular, cluster or linear form.4 PAHs are released into the environment mainly from anthropogenic processes such as coke production, oil spillages, and incomplete burning of fossil fuels, among others. Natural events, such as oil seeps, volcanic eruptions, and forest fires, also contribute to the PAH load in the environment, but to a lesser extent than anthropogenic inputs.5,6
PAHs are priority environmental pollutants because of their toxicity, persistent nature, propensity for bioaccumulation, endocrine and immune system disruptions, and capacity to induce carcinogenic and mutagenic effects.7
Dust is an indispensable component of the environment and can serve as an effective sink for PAHs and other contaminants. It forms a gateway for human exposure to contaminants in it through inhalation, skin contact, unconscious ingestion, and consumption of foods contaminated with dust.6,8
The occurrence of significant concentrations of PAHs in dust is a source of concern because dust particles are mobile, and have relatively small particle sizes that can easily be inhaled, unconsciously ingested, or adhere to human skin. The widespread nature of dust makes it possible for humans to be constantly exposed to contaminants in dust particles.
Dust plays a significant role in the geochemical cycling of pollutants and can be used as a proxy for monitoring the contamination status of a site and the possible risk to the ecosystem and humans. The contamination status of indoor dust is of concern because of the long period of time people spend in an indoor environment per day. It has been estimated that people stay in one indoor environment or the other for more than 80% of the day. Infants and toddlers are at higher risk of exposure to contaminants in indoor dust than their adult counterparts because they play close to the floor, through their hand-to-mouth habits, licking of toys, and touching and putting other dust-contaminated household objects into their mouths.9,10
Outdoor dust has the capacity to exchange pollutants with air and water depending on the weather conditions. For example, during dry weather periods, it interacts with atmospheric aerosols via desorption and re-suspension by turbulence,11 while in wet weather periods dust can cause an upsurge in the pollution level of surface runoff through adsorption and desorption processes caused by washing away of adsorbed contaminants.12–14 Therefore, a knowledge of the distribution patterns, fate and sources of PAHs in dust is necessary for improving environmental quality, source control, and reducing the adverse impacts on humans and the ecosystem.
There are dozens of reports concerning the distribution of PAHs in indoor and outdoor dust.15–27 In Nigeria, like other regions, most of the studies were focused on urban areas with high vehicular emissions, and intense commercial and industrial activities.6,28–36 Most considered outdoor or indoor dust and only a few explored their relationship.
Most gas flare points in the Niger Delta are located in rural communities, and the resultant emissions from this process can potentially contribute to adverse environmental and public health effects on humans residing in these rural areas. Despite extensive studies on the impacts of oil production activities on the environment of the Niger Delta, there is no report on the concentrations of PAHs in dust, indoor or outdoor, from rural communities around gas flare points in the Niger Delta. This study evaluated the concentrations, sources, and associated risk of PAHs in indoor and outdoor dust from rural communities around gas flare points. Such information is necessary for developing pollution abatement programs and risk and environmental quality management.
Materials and methods
The study areas
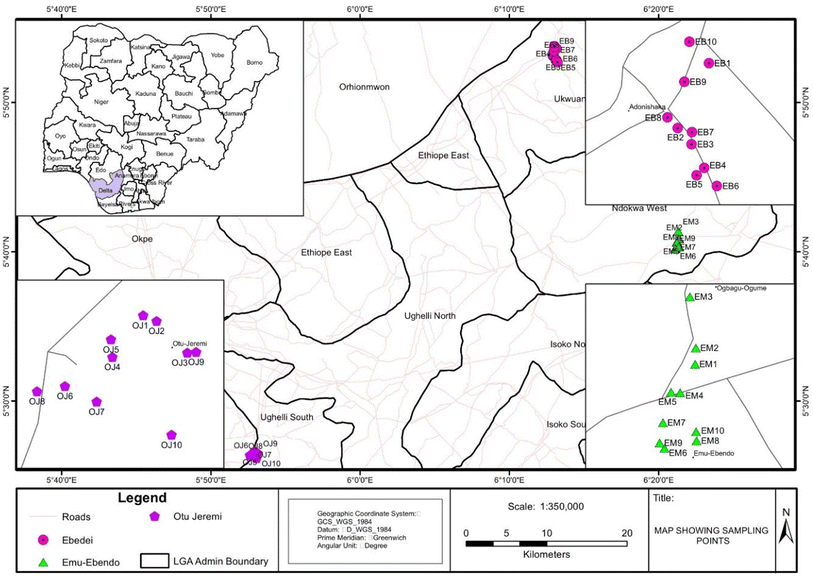
The study areas included Ebedei (EBD) (latitude 5.8324° N and 6.2482° E), Emu-Ebendo (EME) (latitude 5.67084° N and longitude 6.35168° E), Otu-Jeremi (OTJ) (latitude 5.43818° N and longitude 5.87829° E) and Ugono-Abraka (UGA) (control site) in Delta State, Nigeria (Fig. 1). The estimated population of these communities ranged from one to five thousand. The study areas experience a tropical equatorial climate with a distinct dry season (November to April) and wet (rainy) season (May to October). The mean annual temperature and rainfall of the study areas are 32.8 °C and 2673.8 mm respectively.Sample collection
Dust samples (n = 60) comprising ten samples each were collected from in- and outdoor environments of the three communities at 700 m to 1.5 km from gas flare points in Delta State, Nigeria. An additional 10 dust samples comprising five each from in- and outdoor environments of Ugono-Abraka with no history of gas flaring were employed as the control. The relative distances of the sampling sites from the control site are 23.1, 45.5, and 55.8 km for EBD, EME and OTJ respectively. The prevailing wind direction in the study areas is south-west. A pan and brush method was employed for collection of dust samples.29,30Indoor dust samples were collected from living rooms, while outdoor dust samples were collected within 50 m2 from where the indoor dust samples were obtained. The indoor samples were collected from houses made of cement blocks and mud. Some of them had no cemented floors and walls, ceilings and paint. The windows in these buildings were predominantly wooden, while a few had glass louvers or sliding aluminium-framed glass windows. Electricity supply in these rural communities is episodic. Very few homes use electricity generators, while kerosene-powered lamps are the predominant source of light in these homes. The indoor dust samples were collected from floor areas, ceiling fans, cabinetry, wooden beams, and other surfaces by gentle sweeping into the dust pan.
The samples were packed in amber-coloured glass bottles previously cleaned with acetone, and carried in a cooler with ice packs to the laboratory. The samples were sieved through a <63 μm sieve and kept in a refrigerator at −20 °C prior to analysis. The pH, total organic carbon (TOC), and electrical conductivity (EC) of the dust were determined following standard methods.37
Chemicals
The solvents for extraction, namely, dichloromethane (DCM, 99%) and n-hexane (96.0%) were purchased from Merck (Darmstadt, Germany), while alumina (70–230 mesh), silica gel (>632 mesh) and anhydrous sodium sulfate were products of Honeywell Fluka (Seelze, Germany). A US EPA 16 PAH (2000 μg mL−1) mix supplied by AccuStandard Inc. (New Haven, CT, USA) was used for the preparation of calibration standards. A mixed standard containing six labelled 13C12-PAHs was obtained from Cambridge Isotope Laboratories, Inc. (Tewksbury, MA, USA).PAH extraction and instrumental analysis
The PAHs were extracted from the dust samples by employing the US EPA-3550C-ultrasonic extraction method.38 Briefly, 10.0 g of dust was spiked with 200 ng of 13C12-labelled PAHs and homogenised with 10 g of Na2SO4. The PAHs were extracted from the homogenate with a 50 mL mixture of dichloromethane/n-hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) by sonication at 30 °C for 30 min. The extract was filtered, and the extraction cycle was carried out two more times on the residue with fresh portions of the mixed solvent mixture. The extracts were combined and reduced to 2 mL by rotary evaporation; followed by purification on an aluminium oxide/silica gel column (1
1 v/v) by sonication at 30 °C for 30 min. The extract was filtered, and the extraction cycle was carried out two more times on the residue with fresh portions of the mixed solvent mixture. The extracts were combined and reduced to 2 mL by rotary evaporation; followed by purification on an aluminium oxide/silica gel column (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v). The elution of PAHs from the column was achieved with a 30 mL mixture of n-hexane/dichloromethane (3
2 v/v). The elution of PAHs from the column was achieved with a 30 mL mixture of n-hexane/dichloromethane (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) and subsequently reduced to approximately 2 mL under a mild stream of pure nitrogen gas.
1 v/v) and subsequently reduced to approximately 2 mL under a mild stream of pure nitrogen gas.
The quantification of individual PAHs in the extracts was achieved by using an Agilent 7890A gas chromatograph coupled to a 5975C mass selective detector operating in selected ion monitoring mode (SIM). A DB-5 column of 30 m length with 0.25 mm internal diameter and 0.25 μm film thickness was used for the separation of the PAHs (Agilent J &W, Folsom, CA). The mobile phase was high purity helium gas with a flow rate of 1 mL s−1. The initial temperature of the column was 50 °C held for 3 min, thereafter increased to 180 °C at 10 °C min−1, and further increased to 250 °C at 5 °C min−1 and finally to 300 °C at 2 °C min−1. The injector port temperature was fixed at 250 °C and 2.0 μL of sample was injected in a splitless mode.
Quality assurance/quality control
Analyses of method blanks, field blanks, sample matrix spikes, and replicates were applied to validate the analytical procedure. The sample matrix spikes were achieved by introducing known concentrations of PAHs (at 3 concentration levels viz 5, 30 and 100 μg kg−1) into fresh portions of already analyzed samples and subjecting them to the entire analysis cycle from extraction to GC-MS analysis. The average recoveries of 13C12-labelled PAHs were 78–100% while those of the sample matrix spike were 74 to 101%. None of the target compounds were found in the procedural blanks (n = 5). All samples were analyzed in triplicate with relative standard deviations (RSD) between 1.5 and 8.9%. The PAH concentrations were obtained by an external calibration method and R2 of the calibration curves for the PAH compounds ranged from 0.9992 to 0.9998. The method detection limits for the PAHs ranged between 0.01 and 0.1 μg kg−1.Statistical analysis
The Kruskal Wallis test was used to establish the difference in concentrations and composition of PAHs within the study sites, whereas the Shapiro–Wilk test was employed to evaluate the normality of the data. The Student's t-test was used to evaluate the differences between the PAH concentrations in the indoor and outdoor dust from the same location, while the Duncan multiple range test was used for comparison of mean concentrations of PAHs in dust from the three locations. Principal component analysis (PCA) and regression analysis were employed to establish the relationships between the test compounds. SPSS version 20.0 software was used for the statistical analyses at p = 0.05.Evaluation of ecological risk of PAHs
The ecological risk of PAHs in these matrices was assessed by utilizing the risk quotient (RQ) method described by Kalf et al.39 The detailed equations for the RQ method are given in ESI S1.† The interpretation of RQ values is given as follows. The values of RQ(NCs) and RQ(MPCs) represent a measure of the level of risk of PAHs to the ecosystem. When RQNCs < 1.0 it suggests that there is negligible risk associated with exposure to the individual PAHs, while RQMPCs ≥ 1 indicates that exposure to the individual PAHs could cause severe risk to the ecosystem. In such a circumstance, source control and remedial actions are urgently needed to minimize the impact. RQNCs ≥ 1.0 and RQMPCs < 1 suggest a moderate risk for the individual PAHs; therefore, some control and remedial actions are needed to reduce the impact on the ecosystem. RQ∑PAHs(NCs) ≥ 800 and RQ∑PAHs(MPCS) = 0 suggest moderate-risk1. RQ∑PAHs(NCs) < 800 and RQ∑PAHs(MPCs) ≥1 suggest that the ΣPAHs can be of moderate-risk2 while RQ∑PAHs(NCs) ≥ 800 and RQ∑PAHs(MPCs) ≥ 1 suggest that the ΣPAH concentrations can cause a high-risk to the ecosystem (ESI Table S2†).Health risk assessment
| BaPTEQ = ∑Ci × BaPTEF | (1) |
| BaPMEQ = ∑Ci × BaPMEF | (2) |
Results and discussion
PAH concentrations and composition of dust around gas flare points
The Σ16 PAH concentrations in indoor dust from EME, OTJ and EBD ranged from 558 to 167![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 6580 to 413
000, 6580 to 413![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and 2350 to 37
000, and 2350 to 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 μg kg−1 respectively, while those of outdoor dust ranged from 347 to 19
500 μg kg−1 respectively, while those of outdoor dust ranged from 347 to 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700, 15
700, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 130
000 to 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and 1780 to 46
000, and 1780 to 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 μg kg−1 for EME, OTJ and EBD respectively (Tables 1 and ESI S5†). The Σ16 PAH concentrations in the indoor and outdoor dust from these communities with gas flare points were significantly (p < 0.05) higher than those of the control site (UGA: 154 to 7000 μg kg−1). This suggests discernible impacts of gas flaring activities on the PAH contamination status of these areas.
300 μg kg−1 for EME, OTJ and EBD respectively (Tables 1 and ESI S5†). The Σ16 PAH concentrations in the indoor and outdoor dust from these communities with gas flare points were significantly (p < 0.05) higher than those of the control site (UGA: 154 to 7000 μg kg−1). This suggests discernible impacts of gas flaring activities on the PAH contamination status of these areas.
| Emu-Ebendo (EME) | Otu-Jeremi (OTJ) | Ebedei (EBD) | Ugono-Abraka [UGA] (control site) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | SD | Min | Max | Mean | Median | SD | Min | Max | Mean | Median | SD | Min | Max | Mean | Median | SD | Min | Max | ||
| Indoor | pH | 5.69 | 0.79 | 5.75 | 4.30 | 6.70 | 5.66 | 0.73 | 5.70 | 4.40 | 6.70 | 5.69 | 1.09 | 5.60 | 4.20 | 7.40 | 6.22 | 6.64 | 1.20 | 4.62 | 7.38 |
| EC (μS cm−1) | 864 | 850 | 462 | 132.0 | 2680 | 1135 | 1048 | 695 | 320 | 3190 | 252 | 96.6 | 213 | 153 | 448 | 0.41 | 0.17 | 0.59 | 0.07 | 1.45 | |
| TOC (%) | 1.27 | 0.37 | 1.32 | 0.36 | 1.69 | 1.43 | 0.65 | 1.48 | 0.41 | 2.49 | 1.56 | 0.42 | 1.54 | 0.98 | 2.31 | 34.5 | 38.4 | 9.12 | 22.1 | 43.4 | |
| Nap | 255 | 1.18 | 643 | <LOQ | 2040 | 8.5 | 4.6 | 13 | <LOQ | 43.7 | 28.4 | 1.56 | 63.3 | 0.28 | 190 | 174 | 58 | 200 | 8.00 | 484 | |
| Ace | 1320 | 3.61 | 4040 | <LOQ | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
26.7 | 15.7 | 35.4 | 2.64 | 123 | 490 | 2.43 | 1180 | 1.02 | 3380 | 73.6 | 44 | 71.2 | 8.00 | 94 | |
| Acy | 2900 | 7.59 | 9070 | <LOQ | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
5.18 | 4.32 | 3.64 | 0.4 | 11.2 | 280 | 3.93 | 456 | 0.54 | 1050 | 96.8 | 48 | 100 | 8.00 | 174 | |
| Flu | 4380 | 2.71 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
<LOQ | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
8.3 | 3.08 | 11.9 | 0.84 | 37.6 | 392 | 12.5 | 623 | 1.30 | 1650 | 109 | 56 | 115 | 4.00 | 198 | |
| Phen | 2690 | 3.85 | 5340 | <LOQ | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
7.77 | 7.38 | 4.73 | 0.3 | 16.0 | 469 | 11.8 | 782 | 0.56 | 2160 | 96 | 76 | 108 | 14.0 | 94 | |
| Ant | 2670 | 11.5 | 6040 | <LOQ | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
3.17 | 3.15 | 1.97 | 0.94 | 7.70 | 651 | 49.2 | 1030 | 0.80 | 2450 | 64 | 66 | 54 | 4.00 | 98 | |
| Flt | 944 | 10 | 2790 | 0.24 | 8880 | 3.81 | 3.37 | 2.65 | 0.36 | 9.10 | 191 | 111 | 221 | 1.12 | 577 | 190 | 120 | 255 | 6.00 | 172 | |
| Pyr | 406 | 29 | 1050 | 0.24 | 3380 | 3.86 | 3.48 | 2.74 | <LOQ | 9.58 | 624 | 482 | 695 | 0.68 | 1710 | 94 | 98 | 85.4 | 10.0 | 102 | |
| BaA | 274 | 13 | 752 | 0.62 | 2410 | 25.1 | 2.22 | 60.1 | <LOQ | 194 | 737 | 270 | 924 | 2.26 | 2420 | 285 | 140 | 320 | 24.0 | 760 | |
| Chry | 608 | 80 | 1270 | 9.82 | 4160 | 26.4 | <LOQ | 62.9 | <LOQ | 192 | 728 | 9.05 | 1190 | 1.98 | 2860 | 191 | 242 | 132 | 24.0 | 306 | |
| BbF | 2890 | 68 | 8340 | <LOQ | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
972 | 747 | 886 | <LOQ | 2560 | 604 | 47.6 | 1030 | 4.62 | 3020 | 176 | 234 | 150 | 18.0 | 352 | |
| BkF | 4520 | 184 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
<LOQ | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1640 | <LOQ | 2200 | <LOQ | 5400 | 1300 | 235 | 1940 | 19.3 | 5130 | 461 | 96 | 705 | 32 | 408 | |
| BaP | 1470 | 558 | 2030 | <LOQ | 6300 | 1440 | <LOQ | 4550 | <LOQ | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
3000 | 1280 | 3750 | 515 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
548 | 680 | 513 | 6 | 860 | |
| DahA | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
878 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
<LOQ | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
<LOQ | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1920 | 466 | 3300 | 137 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
311 | 114 | 442 | 10 | 318 | |
| IndP | 3780 | 830 | 5350 | 100 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
7700 | 141![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2150 | 405![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
906 | 258 | 1420 | 0.58 | 4130 | 429 | 104 | 506 | 22 | 980 | |
| BghiP | 1490 | 1200 | 1670 | 60 | 5280 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
6290 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
279 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1130 | 401 | 1580 | 117 | 4080 | 357 | 64 | 497 | 10 | 476 | |
| Total |
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
558 |
167![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
119![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
6580 |
413![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
9410 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
2350 |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
3650 | 2710 | 3730 | 444 | 5260 | |
| 2R | 364 | 16 | 758 | <LOQ | 2040 | 8.51 | 3.54 | 13.7 | <LOQ | 43.7 | 28.4 | 1.56 | 63.2 | 0.3 | 190 | 174 | 58 | 200 | 8 | 484 | |
| 3R | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
27 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
<LOQ | 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
51.1 | 39.9 | 47.8 | 18.1 | 182 | 2280 | 92.4 | 3750 | 6.4 | 8940 | 439 | 290 | 438 | 42 | 658 | |
| 4R | 2230 | 276 | 4760 | 12.5 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
56.5 | 18.6 | 84.4 | 6.08 | 233 | 2280 | 1250 | 2640 | 10.7 | 6390 | 759 | 720 | 611 | 100 | 1050 | |
| 5R | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1930 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
<LOQ | 152![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
<LOQ | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
6830 | 2200 | 9110 | 803 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1500 | 1150 | 1680 | 68 | 1940 | |
| 6R | 5270 | 2040 | 6770 | 168 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
125![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
6540 | 413![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2030 | 454 | 2680 | 262 | 8210 | 786 | 168 | 969 | 32 | 1460 | |
| Outdoor | pH | 6.35 | 0.69 | 6.25 | 5.4 | 7.4 | 6.43 | 0.8 | 6.35 | 5.40 | 7.70 | 6.39 | 0.87 | 6.50 | 5.10 | 7.70 | 6.08 | 6.24 | 0.74 | 5.08 | 7.06 |
| EC (μS cm−1) | 123 | 70.2 | 104 | 28 | 274 | 268 | 138 | 248 | 78.0 | 521 | 217 | 161 | 153 | 66.0 | 512 | 64.2 | 11.6 | 62.4 | 49.5 | 80.6 | |
| TOC (%) | 0.41 | 0.18 | 0.34 | 0.18 | 0.68 | 0.72 | 0.37 | 0.72 | 0.21 | 1.42 | 0.87 | 0.45 | 0.93 | 0.3 | 1.6 | 0.10 | 0.06 | 0.10 | 0.02 | 0.16 | |
| Nap | 152 | 0.65 | 480 | <LOQ | 1520 | 7.7 | 2.1 | 11.4 | 0.64 | 29.8 | 56.1 | 4.52 | 147 | 0.4 | 449 | 186 | 12 | 368 | <LOQ | 842 | |
| Ace | 130 | 0.63 | 408 | <LOQ | 1290 | 50.2 | 8.3 | 90.5 | 3.86 | 297 | 547 | 2.64 | 1720 | 1.16 | 5440 | 20.4 | 10 | 25 | <LOQ | 62 | |
| Acy | 253 | 0.69 | 799 | <LOQ | 2530 | 21.4 | 3.9 | 38.9 | 0.16 | 126 | 859 | 4.79 | 1800 | 0.5 | 4500 | 70.8 | 32 | 100 | <LOQ | 244 | |
| Flu | 329 | 0.56 | 1040 | <LOQ | 3280 | 13.1 | 2.3 | 33.4 | 0.56 | 108 | 674 | 6.87 | 1200 | 0.84 | 3400 | 63.6 | 12 | 116 | <LOQ | 270 | |
| Phen | 99 | 0.94 | 301 | 0.34 | 954 | 162 | 12.2 | 457 | 2.04 | 1460 | 1070 | 18.9 | 1900 | 0.66 | 5500 | 110 | 10 | 206 | <LOQ | 476 | |
| Ant | 225 | 1.4 | 654 | 0.16 | 2080 | 144 | 12.9 | 412 | 1.16 | 1320 | 598 | 15.6 | 1030 | 0.84 | 2700 | 261 | 20 | 528 | <LOQ | 1200 | |
| Flt | 113 | 0.79 | 355 | 0.18 | 1120 | 108 | 2.60 | 233 | 0.7 | 674 | 410 | 7.88 | 680 | 0.4 | 1700 | 37.2 | 12 | 63 | <LOQ | 148 | |
| Pyr | 369 | 0.62 | 1160 | 0.2 | 3680 | 75.5 | 4.00 | 223 | 0.96 | 710 | 1120 | 540 | 1720 | 3.54 | 5590 | 12.8 | 10 | 11 | <LOQ | 26 | |
| BaA | 281 | 2.13 | 700 | 1.2.0 | 2200 | 144 | 6.2.0 | 339 | 1.1 | 1030 | 650 | 19.7 | 851 | 1.96 | 2090 | 13.2 | 10 | 12 | <LOQ | 28 | |
| Chry | 52 | 6.94 | 130 | 1.6 | 420 | 238 | 137 | 253 | 3.26 | 627 | 1190 | 20.8 | 2610 | 5.02 | 8340 | 152 | 32 | 290 | 4 | 670 | |
| BbF | 69 | 18.5 | 144 | 8 | 475 | 2130 | 906 | 2200 | 218 | 6280 | 2460 | 1100 | 4380 | 7.66 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
228 | 22 | 464 | 6 | 1060 | |
| BkF | 128 | 74 | 140 | 24.6 | 454 | 6040 | 5160 | 5850 | 1050 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1610 | 264 | 3100 | 5.36 | 9250 | 142 | 34 | 266 | 6 | 618 | |
| BaP | 228 | 118 | 352 | 38.1 | 1210 | 8320 | 7680 | 6800 | 2000 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 940 940 |
1570 | 1760 | 1260 | 76.3 | 3800 | 352 | 34 | 726 | 6 | 1650 | |
| DahA | 131 | 103 | 81.0 | 20.0 | 290 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2470 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1810 | 499 | 2440 | 80.8 | 6100 | 214 | 26 | 399 | 6 | 926 | |
| IndP | 81.0 | 95.0 | 43.0 | 0.70 | 126 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
6390 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
622 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1140 | 233 | 1870 | 10.8 | 5800 | 270 | 30 | 521 | 22 | 1202 | |
| BghiP | 81.0 | 82.0 | 36.0 | 9.2 | 138 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
758 | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
763 | 170 | 1130 | 3.66 | 3320 | 211 | 54 | 375 | 18 | 880 | |
| Total | 2720 | 633 | 6010 | 347 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1780 |
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
2340 | 1310 | 2800 | 154 | 7000 | |
| 2R | 152 | 0.65 | 480 | <LOQ | 1520 | 7.66 | 2.09 | 11.4 | 0.6 | 29.8 | 50.5 | 3.52 | 140 | <LOQ | 449 | 186 | 12 | 368 | <LOQ | 842 | |
| 3R | 1040 | 5.82 | 3200 | 1.88 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
391 | 54.8 | 947 | 17 | 3060 | 3740 | 53.1 | 6730 | 7.82 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
525 | 100 | 941 | <LOQ | 2200 | |
| 4R | 815 | 10.1 | 1890 | 3.84 | 5810 | 480 | 103 | 914 | 7.81 | 2930 | 3370 | 2190 | 3890 | 18 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
215 | 88 | 265 | 24 | 670 | |
| 5R | 556 | 349 | 607 | 123 | 2240 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
<LOQ | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
7450 | 3800 | 9120 | 177 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
936 | 112 | 1860 | 24 | 4250 | |
| 6R | 162 | 175 | 57 | 9.9 | 210 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
2470 | 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2000 | 455 | 2660 | 14.4 | 7770 | 481 | 84 | 896 | 44 | 2080 | |
On average, the Σ16 PAH concentrations in the indoor dust from these sites were significantly higher (p < 0.05) than those of their outdoor counterparts except for EBD. The high concentrations of PAHs in the indoor dust relative to those of the outdoor dust may be related to the fact that outdoor dust suffers dilution effects from the circulating air, photodegradation arising from high intensity sunlight, and leaching actions of rain. In addition, indoor dust receives additional inputs of PAHs from indoor activities such as the use of kerosene- and diesel-powered lamps, smoking, coking, and burning of incense and mosquito coils. Furthermore, the smaller particle size and higher organic content of indoor dust could aid increased sorption of PAHs compared with outdoor dust.21,30,42,43
The concentrations of PAHs in the indoor and outdoor dust from OTJ were significantly (p < 0.05) higher than those of EBD and EME which may be related to the intensity of gas flaring, age of gas flaring activities in this community, number of gas flare points, and nature of the flare stack (down or up). For example, gas flaring activities started at OTJ in 1999, while those of EME and EBD commenced in 2009. In addition, OTJ and EME have downward flare stacks, while EBD has upward flare stacks.
The site to site concentrations of PAHs in the indoor and outdoor dust within each of these locations showed significant spatial discrepancies (p < 0.05). The difference in PAH concentrations of the indoor dust is related to diversity in house-keeping habits, and nature and intensity of indoor and outdoor anthropogenic activities within the area. For example, the dominant building patterns in these rural communities are the room and parlour systems with the kitchen detached from the living rooms. Samples collected from buildings with the kitchens closer to the living rooms and those with outdoor frying activities or closer to cassava processing engines showed higher PAH concentrations. Also, the concentrations of PAHs in indoor dust from buildings without cement floors were higher than those with cemented floors and tiles. In addition, indoor dust collected from homes with wooden windows and without ceilings showed lower PAH concentrations than those with ceilings and sliding glass windows. This is because wooden windows are wide open without restrictions, as compared to those with louvres and sliding aluminum framed glass, which enhances the circulation and exchange of air between the indoor and outdoor environments. The dilution effects of the circulating air are more pronounced in buildings with wooden windows and without ceilings.
The target and intervention values for PAHs in soils established by the Nigerian regulatory authority are 1000 and 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg kg−1 respectively.44 PAH concentrations in these matrices exceeded the target values, while 70% of the indoor and outdoor dust from EME and OTJ exceeded the intervention value. It implies that the majority of sites from EME and OTJ require clean up and remedial action. However, sustainable environmental management is required for sites from EBD since the PAH concentrations were above the target values but below the intervention value.
000 μg kg−1 respectively.44 PAH concentrations in these matrices exceeded the target values, while 70% of the indoor and outdoor dust from EME and OTJ exceeded the intervention value. It implies that the majority of sites from EME and OTJ require clean up and remedial action. However, sustainable environmental management is required for sites from EBD since the PAH concentrations were above the target values but below the intervention value.
Table 2 provides a comparison of PAH concentrations in dust from these rural communities around gas flare points with those of other regions with diverse anthropogenic pressures. Despite the differences in the analytical approach, number of PAHs analyzed, and climatic conditions, such a comparison provides information on global concentration trends. The PAH concentrations in the indoor dust from these areas were higher than those reported for indoor dust from rural, semi-urban and urban areas in Nigeria28,30,33,34 and other regions,22,24,27,45 but were comparable to those found for indoor dust from Warri, Nigeria30 and Changchun, China.22 The PAH concentrations in the outdoor dust from these rural communities were higher than those previously reported for similar matrices from the Niger Delta and other parts of Nigeria6,31,32,34–36 and some cities with diverse anthropogenic pressures in other regions,20,23,25,26,47–49 but were lower than those of outdoor dust from Ulsan, Korea.17 Kong et al.50 reported PAH concentrations of 1410 to 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 780 μg kg−1 in dust from the Dongying oilfield in China, which were lower than the PAH concentrations in dust from OTJ but were higher than those of EBD and EME.
780 μg kg−1 in dust from the Dongying oilfield in China, which were lower than the PAH concentrations in dust from OTJ but were higher than those of EBD and EME.
| Location | Environmental media | No. of PAHs | Concentration range | Mean | Median | Reference |
|---|---|---|---|---|---|---|
| Emu-Ebendo, Nigeria | Indoor dust (rural) | 16 | 558–167![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
This study |
| Otu-Jeremi, Nigeria | Indoor dust (rural) | 16 | 6580–413![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
46 500 | This study |
| Ebedei, Nigeria | Indoor dust (rural) | 16 | 2350–37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
9410 | This study |
| Warri, Nigeria | Indoor dust (urban) | 15 | 4531–111![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 914 914 |
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 117 117 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 604 604 |
30 |
| Abraka, Nigeria | Indoor dust (semi-urban) | 16 | 124–2131 | 1121 | 1263 | 30 |
| Emu-Uno, Nigeia | Indoor dust (rural) | 16 | 60–1413 | 828 | 870 | 30 |
| Port Harcourt, Nigeria | Indoor dust (urban) | 17 | 276–9130 | 2590 | 1598 | 34 |
| Ilorin, Nigeria | Indoor dust (urban) | 16 | 3950–8700 | 6090 | 33 | |
| 23 Cities, China | Indoor dust (urban) | 16 | 1000–470![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
17 | ||
| Cape Coast, Ghana | Indoor dust (urban) | 15 | nd – 3240 | 18 | ||
| Changchun, China | Indoor dust (urban) | 16 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800–329 800–329![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
22 | ||
| Lagos, Nigeria | Indoor dust (urban) | 16 | 304–7677 | 28 | ||
| Jeddah, Saudi Arabia | Indoor dust (urban) | 16 | 22–9150 | 22 | ||
| Cities of Nepal | Indoor dust (urban) | 16 | 747–4910 | 1320 | 24 | |
| Vojvodina province, Serbia | Indoor dust (urban) | 16 | 140–8265 | 1825 | 1404 | 27 |
| Amman, Jordan | Indoor dust (urban) | 16 | 641–65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 422 422 |
45 | ||
| Emu-Ebendo, Nigeria | Outdoor dust (urban) | 16 | 347–19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
2720 | 633 | This study |
| Otu-Jeremi, Nigeria | Outdoor dust (rural) | 16 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–130 000–130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
This study |
| Ebedei, Nigeria | Outdoor dust (rural) | 16 | 1780–46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
This study |
| Lagos, Nigeria | Outdoor dust (urban) | 16 | 289–17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 343 343 |
3162 | 1047 | 31 |
| Benin City, Nigeria | Outdoor dust (urban) | 16 | 153–2303 | 32 | ||
| Port Harcourt, Nigeria | Outdoor dust (urban) | 17 | 44–13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
2488 | 661 | 34 |
| Warri, Nigeria | Outdoor dust (urban) | 16 | 165–1012 | 6 | ||
| Delta State, Nigeria | Outdoor dust (informal trade site) | 16 | 120–8790 | 36 | ||
| Ibadan, Nigeria | Outdoor dust (urban) | 16 | 365![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100–430 100–430![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
35 | ||
| Babylon, Iraq | Outdoor dust (urban) | 16 | 556–1890 | 1060 | 26 | |
| Dongying, China | Outdoor dust (oilfield) | 17 | 1410–54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 780 780 |
50 | ||
| Bushehr city, Iran | Outdoor dust (urban) | 17 | 736–5491 | 51 | ||
| Rio de Janeiro, Brazil | Outdoor dust (urban) | 16 | 108–8570 | 20 | ||
| Jeddah, Saudi Arabia | Outdoor dust (urban) | 16 | 1660–4980 | 47 | ||
| Newcastle upon Tyne, UK | Outdoor dust (urban) | 16 | 550–46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
52 | ||
| Ulsan, Korea | Outdoor dust (urban) | 16 | 118![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–245 000–245![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
17 | ||
| Greater Cairo, Egypt | Outdoor dust (urban) | 16 | 45–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
53 | ||
| Mashhad, Iran | Outdoor dust (urban) | 16 | 764–8990 | 2183 | 1891 | 48 |
| Bangalore, India | Outdoor dust (urban) | 22 | 670–1800 | 1100 | 46 | |
| Dresden, Germany | Outdoor dust (urban) | 16 | 950–270 | 25 | ||
| Tokyo, Japan | Outdoor dust (urban) | 16 | 250–5260 | 23 | ||
| Hanoi, Vietnam | Outdoor dust (urban) | 22 | 530–4700 | 46 | ||
| Birmingham, UK | Outdoor dust (urban) | 16 | 200–99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
15 | ||
| Nova Sad, Serbia | Outdoor dust (urban) | 16 | 35–2422 | 49 | ||
| Bangkok, Thailand | Outdoor dust (urban) | 16 | 300–1100 | 16 |
The PAH homologue profiles in indoor and outdoor dust from these study areas showed variations. The order of average concentrations of PAH homologues in indoor dust from EME was 5 > 3 > 6 > 4 > 2-ring PAHs and those of outdoor dust followed the sequence of 3 > 4 > 5 > 6 > 2-ring PAHs. In OTJ, the sequence of average concentrations of PAH homologues in the in- and outdoor dust was 6 > 5 > 4 > 3 > 2-ring PAHs. In the case of EBD, the PAH homologue profile in indoor dust was in the order of 5 > 4 = 3 > 6 > 2-rings, while those of outdoor dust followed the order of 5 > 3 > 4 > 6 > 2-ring PAHs (Fig. 2a and b). The homologue distribution patterns in the study areas contrast those obtained in the control site (i.e. 5 > 6 > 4 > 3 > 2-ring PAHs and 5 > 3 > 6 > 4 > 2-ring PAHs for indoor and outdoor dust respectively). The similarity in the compositional patterns of PAHs in the indoor and outdoor dust from OTJ and EBD suggest a common source, while the differences observed in the case of EME reflect dissimilarities in their fate and sources.
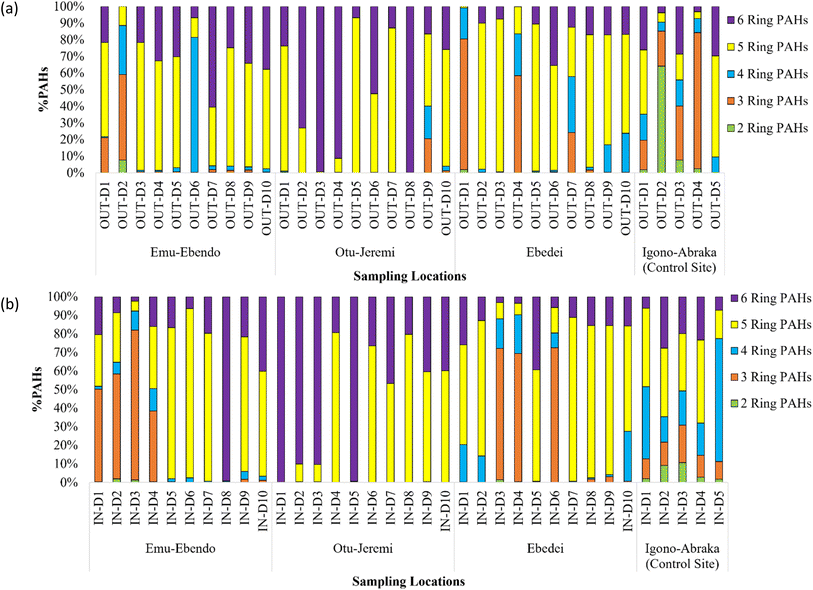 | ||
| Fig. 2 PAH composition of (a) outdoor dust and (b) indoor dust from rural communities around gas flare points. | ||
There was poor correlation between the ∑16 PAHs in the indoor dust with that of outdoor dust from these areas (ESI Fig. S1†) which suggests differences in the strength of their input sources, transport processes and other environmental variables that affect the fate of PAHs. Similarly, there was poor correlation between the physicochemical properties of dust, such as pH, TOC, and EC, and ∑16 PAHs (ESI Fig. S2–S4†). This implies that the PAH contamination of the indoor and outdoor dust from these areas may have come from recent and continuous contamination events which can induce distortion in the adsorption equilibrium between TOC and PAHs in these matrices. In addition, it indicates that TOC plays little or no discernible role in the fate of PAHs in these matrices.31
The high molecular weight (HMW) PAHs represented 76.3 to 99.9% of the ∑16 PAHs in the indoor and outdoor dust from these areas. The dominance of HMW PAHs over LMW PAHs may be due to the lipophilic nature of HMW PAHs which enables them to be preferentially associated with the particulate phase, while the LMW PAHs are linked with gas phase partitioning which makes them more volatile and mobile. In addition, LMW PAHs exhibit multi-hop transport characteristics and are prone to leaching by both microbial and photo-degradation as compared with their HMW counterparts. Again, HMW PAHs are associated with high temperature combustion processes including gas flaring.31,54
The 5-ring PAHs were the dominant homologues in the indoor and outdoor dust from EBD with significant contributions from BkF and BaP respectively. In the case of EME, 5-ring PAHs were the dominant species in the indoor dust with a significant contribution from DahA, while 3-ring PAHs were the dominant species in the outdoor dust with a significant input from Flu. The 6-ring PAHs were the dominant species in the indoor and outdoor dust from OTJ, with major contributions from IndP and BghiP respectively. The 3-ring PAHs accounted for <25% of the ∑16 PAHs in dust from the study areas except for those from EME. The low concentrations of 3-ring PAHs recorded in this study affirmed the fact that volatilization controls the fate of LMW PAHs.
On average, the prominent 3, 4, 5 and 6 ring PAHs in indoor dust from EME were Flu, Flt, DahA and IndP respectively, while those of the outdoor counterparts were Flu, Pyr, BaP and IndP for 3, 4, 5 and 6-ring PAHs respectively. In OTJ, the dominant 3, 4, 5 and 6 ring PAHs in the indoor dust were Ace, Chry, DahA and IndP respectively, while Phen, Chry, DahA and BghiP were the dominant 3, 4, 5 and 6-ring PAHs in the outdoor dust from OTJ. In the case of indoor dust from EBD, Ace, BaA, BaP and BghiP were the dominant 3, 4, 5 and 6-ring PAHs respectively, while Phen, Chry, BbF and IndP were the respective dominant 3, 4, 5 and 6-ring PAHs in the outdoor dust. This suggests transformation of PAHs arising from changes in the environmental characteristics.
Ecological risk of PAHs
The computed risk quotient (RQ) for both the negligible concentrations (RQ(NCs)) and maximum permissible concentrations (RQ(MPCs)) of PAHs in dust are shown in ESI Tables S6 and S7† respectively. The computed RQ(NCs) values for indoor and outdoor dust varied from 116–98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 and 43.5–28 400 respectively, while those for ΣRQ(MPCs) ranged from 0.00–981 and 0.00–283 for indoor and outdoor dust respectively.
200 and 43.5–28 400 respectively, while those for ΣRQ(MPCs) ranged from 0.00–981 and 0.00–283 for indoor and outdoor dust respectively.
The ΣRQ(NCs) and ΣRQ(MPCs) values of PAHs were above 800 and 1 in the majority of the indoor and outdoor dust samples investigated. This suggests a high ecological risk associated with exposure to PAHs from these areas. However, of the ten outdoor dust samples from EME, only two sites had ΣRQ(NCs) values above 800, while four samples of the outdoor dust had ΣRQ(MPCs) values above 1. This indicates that exposure to PAHs in dust in the majority of sites from EME is of negligible ecological risk. The RQ(NCs) and RQ(MPCs) recorded in this study signify the need for remedial actions in order to reduce the ecological risks of PAHs in indoor and outdoor dust of these studied areas. BaP, DahA, IndP and BghiP were among the PAHs in the dust that contributed to the ecological risk of PAHs in the study areas.
Human health risk
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 and 103–73
900 and 103–73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 μg kg−1 for indoor and outdoor dust respectively, while the BaPMEQ concentrations varied from 194–126
500 μg kg−1 for indoor and outdoor dust respectively, while the BaPMEQ concentrations varied from 194–126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 117–21
000 and 117–21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 μg kg−1 for indoor and outdoor dust respectively.
300 μg kg−1 for indoor and outdoor dust respectively.
On average the BaPTEQ and BaPMEQ concentrations of PAHs in dust (in- and outdoor) followed the sequence of OTJ > EBD > EME. The ∑BaPTEQ concentrations recorded in this study were higher than those reported in previous studies.6,31,34,55 The BaPTEQ in these matrices surpassed the Dutch target value of 33 μg kg−1 (ref. 56) and Method B cleanup level of 137 μg kg−1 for soils.57 BaP, DahA and IndP were the major compounds influencing the BaPTEQ and BaPMEQ concentrations of PAHs in these matrices.
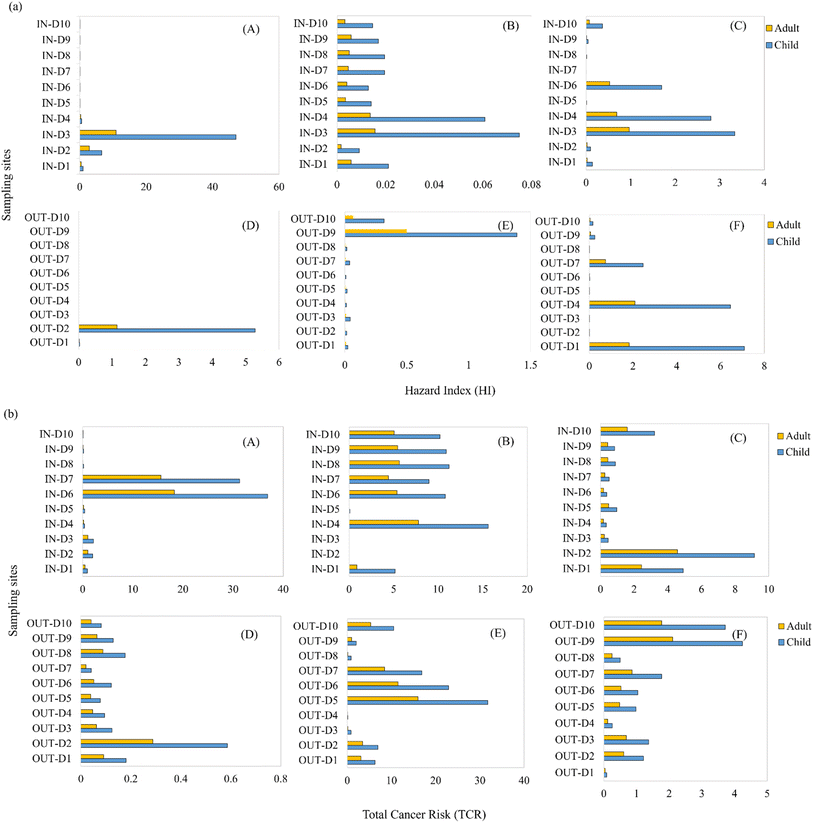
The HI values arising from exposure to outdoor dust were greater than those of the indoor dust in OTJ and EBD, whereas the HI values for exposure to indoor dust from EME were higher than those of outdoor dust. The HI values related to exposure to both indoor and outdoor dust from these areas were majorly <1 for adults and children, which indicate no considerable non-cancer risk resulting from human exposure to PAHs in dust from this area. However, there are a few sites in EME (IN-D1, IN-D2, IN-D3 and OUT-D2) with HI values for adults' and children' exposure greater than 1. Also, the HI values for children's exposure to PAHs in dust from sites IN-D3, IN-D4, IN-D6 and OUT-D1, OUT-D4, OUT-D7 of EBD exceeded 1 (ESI Table S9†). This suggests there is a probable adverse non-cancer risk for children at these sites in EBD and EME.
The ILCRderm values were higher than those of ILCRing and ILCRinh for both adults and children in the studied areas. This implies that human exposure via non-dietary ingestion contributed more to the total cancer risk. The total cancer risk values for children were higher than those of adults. This may be due to the smaller body weight of children. The total cancer risk values and those related to exposure through unconscious oral ingestion and dermal contact with PAHs in dust by both adults and children in these areas were higher than the acceptable limit of 10−6. This implies a high potential cancer risk resulting from human exposure to dust from these areas.
The total cancer risk values recorded in this study were higher than those previously reported from human exposure to PAHs in dust from urban and rural areas of Delta State in Nigeria.6,25 The ILCR results recorded in this study imply that there is an urgent need for remedial actions and source control measures in order to reduce the risk resulting from human exposure to PAHs in these environments.
PAH source analysis
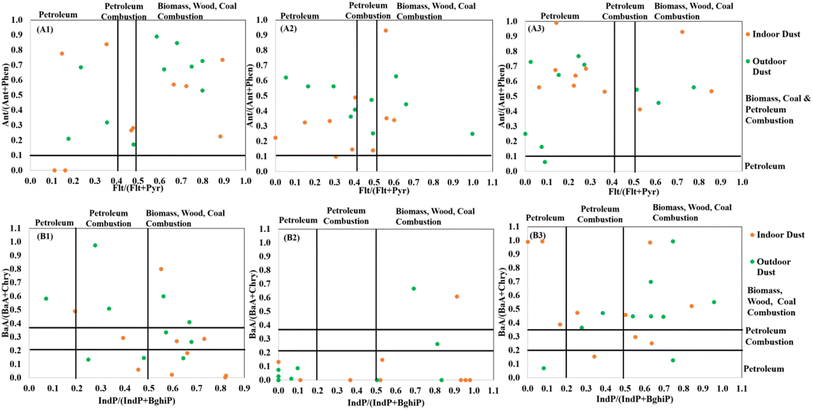
The ratios of Ant/(Ant + Phen) ranged from 0.0 to 0.93 and 0.06 to 0.89 for indoor and outdoor dust respectively. An Ant/(Ant + Phen) ratio < 0.1 is related to petroleum sources, while an Ant/(Ant + Phen) ratio > 0.1 is associated with combustion processes. The ratio values of Ant/(Ant + Phen) in the indoor and outdoor dust from the studied areas advocate the prevalence of PAHs originating from combustion processes. The ratio values of Flt/(Flt + Pyr) in the indoor and outdoor dust from these areas ranged from 0.0 to 0.89 and 0.0 to 1.00 respectively. Flt/(Flt + Pyr) values between 0.4 and 0.5 describe sources linked to burning of liquid petroleum, and values > 0.5 are associated with burning of biomass, wood and coal.52 The ratio values of Flt/(Flt + Pyr) were greater than 0.4 in 60 to 70%, 50 to 60% and 30% of the indoor and outdoor dust from EME, OTJ and EBD respectively. The Flt/(Flt + Pyr) values advocate the prevalence of PAHs originating from burning of liquid petroleum and wood in dust from EME and OTJ, and contamination with petroleum-derived PAHs for dust from EBD.Ratios of BaA/(BaA + Chry) < 0.2 and IndP/(IndP + BghiP) < 0.2 indicate petrogenic sources, while ratios of BaA/(BaA + Chry) between 0.2 and 0.35, and IndP/(IndP + BghiP) ratios between 0.2 and 0.5 describe sources related to the combustion of petroleum such as liquid fossil fuels, and vehicles and crude oil. Ratios of BaA/(BaA + Chry) > 0.35 and IndP/(IndP + BghiP) > 0.5 suggest inputs from biomass and coal combustion.52,58 The ratios of BaA(BaA + Chry) and IndP/(IndP + BghiP) in indoor and outdoor dust ranged from 0.00–0.99 (Fig. 4 and ESI Table S11†), indicating multiple sources of PAHs which include petrogenic sources, combustion of wood, liquid fossil fuels, crude oil and vehicles. The IndP/(IndP + BghiP) ratios in 40 to 70% of the in- and outdoor dust from EBD, EME and OTJ were greater than 0.5 which advocates the prevalence of PAHs originating from burning of biomass and wood.
The ΣCOMB/TPAH ratio describes the relationship between the combustion profile of typical organics and their origins.16 A ΣCOMB/TPAH ratio < 0.3 indicates a contribution from petrogenic sources, while values ranging from 0.3 to 0.7 indicate a contribution from mixed sources, and values > 0.7 indicate PAH contributions from combustion processes at high temperature. The ΣCOMB/TPAH values in these matrices varied from 0.07–1.00. The ΣCOMB/TPAH ratio suggests dominance of PAHs originating from high temperature combustion sources in the studied areas.
The total index values for indoor and outdoor dust ranged from 1.75–13.7 and 4.13–13.6 respectively, with the majority of the samples having total index values greater than 4, which indicates that PAH concentrations in indoor and outdoor dust of these areas arise from high temperature combustion processes such as gas flaring.
PCA like other receptor models such as positive matrix factorization (PMF) and chemical mass balance (CMB) has its strengths and weaknesses. For instance, CMB might misspecify sources and have a collinearity problem which would give rise to negative source contributions. PCA and PMF assume that all sources have been identified and might fail to separate out some factors (sources) because of the high correlation coefficient in these source contributors and the similarity in their source profiles. Therefore, no single model is sufficient enough to give feasible results for sources,59,60 but a combination of these models gives better output for source apportionment. However, PCA is easier to use, gives qualitative information about the sources, and provides a good visual output of the results.
The PCA of PAHs in indoor and outdoor dust of EME was resolved into three components which represented 92.9% and 95.6% of the variance respectively (ESI Tables S12 and S13†). For the indoor dust, Factor 1 captured 55.5% of the total variance and was dominated by Nap, Ace, Acy, Flu, Phen, Ant, Flt, Pyr, and BaA. Apart from Nap, Factor 1 was dominated by 3- and 4-ring PAHs. Nap is a marker for incomplete combustion processes,16,31,61 while Chry, BaA, Pyr and Phen are considered as markers for natural gas combustion.62,63 Acy is believed to come from liquefied petroleum gas, natural gas and coal combustion.64,65 Factor 2 contributed 23.1% of the total variance with Chry, BbF, BkF and DahA as dominant PAH compounds. Chry, BkF and DahA are characteristic compounds for diesel emissions,30,66 while BbF is a tracer of vehicular emissions.30,67 Factor 3 was dominated by BaP, IndP and BghiP and contributed 14.4% of the total variance. These are tracers of gasoline/gas engine emissions and combustion of heavy oil.68,69
For outdoor dust from Emu-Ebendo, Factor 1 captured 77.7% of the total variance and consisted of NaP, Ace, Acy, Flu, Phen, Ant, Flt, Pyr, Chry, BbF, BkF and BaP. NaP is a marker for incomplete combustion.16,30,61 As stated earlier, Chry, Pyr, and Phen are tracers for natural gas combustion.62,63 Acy is associated with liquefied petroleum gas, natural gas and coal combustion,64,65 while Ace, Acy, Flu, Phen, Ant, Flt and Pyr are associated with burning of biomass, wood and liquid petroleum. Chry and BkF are indicators of diesel emission related sources,30 while BaP and BbF are indicators of automobile emission related sources.30,67 Therefore, the PAHs from Factor 1 came from mixed sources including combustion of natural gas (gas flaring), wood and vehicular emissions. Factor 2 accounted for 10.3% and contained BaA and BghiP as the dominant PAHs. These are indicators of gasoline emission related sources. Component 3 contributed 7.6% of the total variance with DahA as the only dominant compound. DahA is an indicator of diesel emission sources.64
The PCA for PAHs in indoor and outdoor dust of OTJ was resolved into 5 components each, explaining 92.3% and 92.7% of the total variance for indoor and outdoor dust respectively. In indoor dust, Factor 1 accounted for 23.5% of the total variance with Ant, BaA and BaP as dominant compounds. These are indicators of coal combustion and vehicular emissions.30,70 Factor 2 was dominated by Ace, Pyr, BkF and BghiP and accounted for 23.3% of the total variance. Pyr and Ace are indicators of low temperature combustion (wood/biomass combustion) related sources,68,69 while BkF and BghiP are tracers of diesel combustion (diesel vehicle emissions).30 Factor 3 contributed 19.2% of the total variance and was dominated by Flu, Flt and IndP. Flu and Flt are characteristic of wood/biomass and fossil fuel combustion,68,69 while IndP is an indicator of gasoline emissions. Component 4 accounted for 13.3% of the total variance and was dominated by Phen. This is related to emissions from fossil fuel combustion. Factor 5 was dominated by NaP and Acy. NaP is a marker for incomplete combustion, while Acy is derived from liquefied petroleum gas, natural gas and coal combustion.64,65
For the outdoor dust from OTJ, Factor 1 accounted for 46.9% of the total variance with NaP, Acy, Flu, Phen, Ant, Flt, Pyr and BaA as the dominant PAHs. NaP as earlier stated is an indicator for incomplete combustion, and BaA, Pyr and Phen are typical markers for natural gas combustion,62,63 while Acy, Flu, Phen, Ant, Flt, Pyr and BaA are typical markers for wood/biomass and fossil fuel combustion.30,71 Therefore, Factor 1 consists of PAHs arising from gas flaring and biomass combustion. Factor 2 was dominated by Ace, Chry and BaP and contributed 15.5% of the total variance. These are compounds from automobile emission related sources.16,72 Factor 3 was dominated by BbF and accounted for 10.9% of the total variance. BbF is a tracer for vehicular emissions.67 Factor 4 contributed 10.1% of the total variance and was dominated by IndP which is an indicator of gasoline emission related sources. Factor 5 accounted for 9.4% of the total variance with BkF as the dominant compound. This is an indicator of diesel emission.30
The PCA for PAHs in EBD was resolved into 3 components for indoor dust and 4 components for outdoor dust, explaining a total variance of 89.0% and 92.7% for indoor and outdoor dust respectively. In indoor dust, Factor 1 accounted for 37.7% of the total variance and was dominated by NaP, Ace, Acy, Flu, Phen, Ant, and Flt. NaP is an indicator for incomplete combustion, while the rest of the PAH compounds are markers of burning of biomass, wood and liquid petroleum. Factor 2 contributed 31% of the total variance with Chry, BkF, BaP, DahA and BghiP as dominant PAH compounds. BaP and BghiP are tracers of automobile emissions,16,72 while BkF, Chry and DahA are compounds of diesel emissions.67 Factor 3 was dominated by Pyr, BaA, BbF and IndP and accounted for 20.2% of the total variance. These compounds are markers for coal combustion and vehicular emissions.70 BaA and Pyr are tracers for natural gas combustion.62,63
In outdoor dust, Factor 1 accounted for 26.4% of the total variance with Chry, BbF, DahA and IndP as dominant compounds. BbF and IndP are compounds of emissions from vehicular transportation,30,55 while Chry and DahA are markers for diesel emissions as stated earlier. Factor 2 contained NaP, Ace, Ant, Flt and BaA as dominant compounds and contributed 26.0% of the total variance. NaP as earlier stated is a marker for incomplete combustion. Factor 3 accounted for 23.1% of the total variance and was dominated by Acy, Flu, Phen and Pyr. These compounds are markers for wood/biomass and fossil fuel combustion.68,69 Factor 4 contained BkF, BaP and BghiP as dominant compounds and accounted for 17.2% of the total variance. These compounds are tracers of automobile emissions. The PCA results showed that the incomplete combustion resulting from gas flaring was one of the major sources of PAHs in the indoor and outdoor dust of the studied locations.
Conclusions
The results from this study revealed that there was a discernible impact of gas flaring on the PAH concentrations in indoor and outdoor dust from the study areas. The PAH homologue distribution in these media showed dominance of 5- and 6-ring PAHs which are associated with high temperature combustion processes. PAH source analysis suggests that the PAH contamination of dust from these rural communities arises from gas flaring, combustion of wood/biomass, liquid fossil fuels and vehicular emissions. Exposure to PAHs in these matrices is of potential risk for both humans and the ecosystem. The PAH contamination status of indoor and outdoor dust from these areas was in the sequence of OTJ > EME > EBD. The PAH concentrations in dust from the majority of sites from OTJ and EME exceeded intervention values and therefore, clean up, remedial actions and stringent pollution control measures are necessary for these areas in order to ameliorate the adverse consequences of exposure to PAHs.Conflicts of interest
There are no conflicts of interest to declare.References
- C. D. Elvidge, D. Ziskin, K. E. Baugh, B. T. Tuttle, T. Ghosh, D. W. Pack, E. H. Erwin and M. Zhizhin, A fifteen year record of global natural gas flaring derived from satellite data, Energies, 2009, 2, 595–622, DOI:10.3390/en20300595.
- O. Anomohanran, Determination of greenhouse gas emission resulting from gas flaring activities in Nigeria, Energy Policy, 2012, 45, 666–670, DOI:10.1016/j.enpol.2012.03.018.
- C. N. Nwankwo and D. O. Ogagarue, Effects of gas flaring on surface and ground waters in Delta State Nigeria, J. Geol. Min. Res., 2011, 3(5), 131–136 Search PubMed.
- G. O. Tesi, C. M. A. Iwegbue, F. N. Emuh and G. E. Nwajei, Ladgo dam flood disaster of 2012: an assessment of the concentrations, sources, and risks of PAHs in floodplain soils of the lower reaches of River Niger, Nigeria, J. Environ. Qual., 2016, 45, 305–314, DOI:10.2134/jeq2015.02.0116.
- Q. Yang, H. Chen and B. Li, Polycyclic aromatic hydrocarbons (PAHs) in indoor dusts of Guizhou, southwest of China: status, sources and potential human health risk, PLoS One, 2015, 10(2), e0118141, DOI:10.1371/journal.pone.0118141.
- C. M. A. Iwegbue and G. Obi, Distribution, sources, and health risk assessment of polycyclic aromatic hydrocarbons in dust from urban environment in the Niger Delta, Nigeria, Hum. Ecol. Risk Assess., 2016, 22, 623–638, DOI:10.1080/10807039.2015.1100157.
- X. He, Y. Pang, X. Song, B. Chen, Z. Feng and Y. Ma, Distribution, sources and ecological risk assessment of PAHs in surface sediments from Guan River Estuary, China, Mar. Pollut. Bull., 2014, 80, 52–58, DOI:10.1016/j.marpolbul.2014.01.051.
- E. Kaya, Y. Dumanoglu, M. Kara, H. Altiok, A. Bayram, T. Elbir and M. Odabasi, Spatial and temporal variation and air-soil exchange of atmospheric PAHs and PCBs in an industrial region, Atmos. Pollut. Res., 2012, 3(4), 435–449, DOI:10.5094/APR.2012.050.
- C. M. A. Iwegbue, E. C. Oliseyenum and B. S. Martincigh, Spatio-temporal distribution of metals in household dust from rural, semi-urban and urban environments in the Niger Delta, Nigeria, Environ. Sci. Pollut. Res., 2017, 24, 14040–14059, DOI:10.1007/s11356-017-8609-1.
- N. Ali, Polycyclic aromatic hydrocarbons (PAHs) in indoor air and dust samples of different Saudi microenvironments; health and carcinogenic risk assessment for the general population, Sci. Total Environ., 2019, 696, 133995, DOI:10.1016/j.scitotenv.2019.133995.
- S. Dong, R. Ochoa Gonzalez, R. M. Harrison, D. Green, R. North, G. Fowler and D. Weiss, Isotopic signatures suggest important contributions from recycled gasoline, road dust and non-exhaust traffic sources for copper, zinc and lead in PM10 in London, United Kingdom, Atmos. Environ., 2017, 165, 88–98, DOI:10.1016/j.atmosenv.2017.06.020.
- P. Loganathan, S. Vigneswaran and J. Kandasamy, Road-deposited sediment pollutants: a critical review of their characteristics, source apportionment, and management, Crit. Rev. Environ. Sci. Technol., 2013, 43, 1315–1348, DOI:10.1080/10643389.2011.644222.
- Z. Xu, J. Wu, H. Li, Y. Chen, J. Xu, L. Xiong and J. Zhang, Characterizing heavy metals in combined sewer overflows and its influence on microbial diversity, Sci. Total Environ., 2018, 625, 1272–1282, DOI:10.1016/j.scitotenv.2017.12.338.
- J. Zhang, R. Li, X. Zhang, C. Ding and P. Hua, Traffic contribution to polycyclic aromatic hydrocarbons in road dust: a source apportionment analysis under different antecedent dry-weather periods, Sci. Total Environ., 2019, 658, 996–1005, DOI:10.1016/j.scitotenv.2018.12.281.
- D. J. T. Smith, E. C. Edelhauser and R. M. Harrison, Polynuclear aromatic hydrocarbon concentrations in road dust and soil samples collected in the United Kingdom and Pakistan, Environ. Technol., 1995, 16, 45–53, DOI:10.1080/09593331608616244.
- R. Boonyatumanond, M. Murakami, G. Wattayakorn, A. Togo and H. Takada, Sources of polycyclic aromatic hydrocarbons (PAHs) in street dust in a tropical Asian mega-city, Bangkok, Thailand, Sci. Total Environ., 2007, 384, 420–432, DOI:10.1016/j.scitotenv.2007.06.046.
- T. T. T. Dong and B.-K. Lee, Characteristics, toxicity, and source apportionment of polycylic aromatic hydrocarbons (PAHs) in road dust of Ulsan, Korea, Chemosphere, 2009, 74, 1245–1253, DOI:10.1016/j.chemosphere.2008.11.035.
- H. Qi, W.-L. Li, N.-Z. Zhu, W.-L. Ma, L.-Y. Liu, F. Zhang and Y.-F. Li, Concentrations and sources of polycyclic aromatic hydrocarbons in indoor dust in China, Sci. Total Environ., 2014, 491–492, 100–107, DOI:10.1016/j.scitotenv.2014.01.119.
- D. K. Essumang, J. Ofori, D. K. Dodoo and J. K. Adjei, Polycyclic aromatic hydrocarbons in settled dust particles in selected Ghanaian environments: levels, source characterization, and assessment of inhalational health risks, Indoor Built Environ., 2016, 25, 242–253, DOI:10.1177/1420326X14544530.
- B. A. M. Bandowe and M. A. Nkansah, Occurrence, distribution and health risk from polycyclic aromatic compounds (PAHs, oxygenated-PAHs and azaarenes) in street dust from a major West African Metropolis, Sci. Total Environ., 2016, 553, 439–449, DOI:10.1016/j.scitotenv.2016.02.142.
- C. F. J. Franco, M. F. de Resende, L. de Almeida Furtado, T. F. Brasil, M. N. Eberlin and A. D. P. Netto, Polycyclic aromatic hydrocarbons (PAHs) in street dust of Rio de Janeiro and Niterói, Brazil: particle size distribution, sources and cancer risk assessment, Sci. Total Environ., 2017, 599–600, 305–313, DOI:10.1016/j.scitotenv.2017.04.060.
- Z. Wang, S. Wang, J. Nie, Y. Wang and Y. Liu, Assessment of polycyclic aromatic hydrocarbons in indoor dust from varying categories of rooms in Changchun city, northeast China, Environ. Geochem. Health, 2017, 39, 15–27, DOI:10.1007/s10653-016-9802-8.
- M. W. Kadi, N. Ali and H. M. S. A. Albar, Phthalates and polycyclic aromatic hydrocarbons (PAHs) in the indoor settled carpet dust of mosques, health risk assessment for public, Sci. Total Environ., 2018, 627, 134–140, DOI:10.1016/j.scitotenv.2018.01.146.
- R. Khanal, H. Furumai, F. Nakajima and C. Yoshimura, Carcinogenic profile, toxicity and source apportionment of polycyclic aromatic hydrocarbons accumulated from urban road dust in Tokyo, Japan, Ecotoxicol. Environ. Saf., 2018, 165, 440–449, DOI:10.1016/j.ecoenv.2018.08.095.
- I. C. Yadav, N. L. Devi, J. Li and G. Zhang, Polycyclic aromatic hydrocarbons in house dust and surface soil in major urban regions of Nepal: Implication on source apportionment and toxicological effect, Sci. Total Environ., 2018, 616–617, 223–235, DOI:10.1016/j.scitotenv.2017.10.313.
- R. A. Grmasha, O. J. Al-sareji, J. M. Salman and K. S. Hashim, Polycyclic aromatic hydrocarbons (PAHs) in urban street dust within three land-uses of Babylon governorate, Iraq: distribution, sources, and health risk assessment, J. King Saud Univ., Eng. Sci., 2022, 34, 231–239, DOI:10.1016/j.jksues.2020.11.002.
- J. Živančev, I. Antić, M. Buljovčić and N. Đuriŝić-Mladenović, A case study on the occurrence of polycyclic aromatic hydrocarbons in indoor dust of Serbian households: distribution, source apportionment and health risk assessment, Chemosphere, 2022, 295, 133856, DOI:10.1016/j.chemosphere.2022.133856.
- T. O. Oluseyi, O. T. Adetunde, A. O. Oyeyiola, S. J. Harrad and Y. Ma, Distributions and health risks of polycyclic aromatic hydrocarbons (PAHs) in dust from cars, homes and offices in Nigeria: implications for human exposure, in International Chemistry Conference: Theme: Chemical Sciences Solutions to Global Challenges, held on 15th–17th February, 2016 at Julius Berger Lecture Theatre, University of Lagos, Akoka, Lagos, Nigeria, 2016 Search PubMed.
- C. M. A. Iwegbue, G. Obi, S. A. Uzoekwe, F. E. Egobueze, E. W. Odali, G. O. Tesi, G. E. Nwajei and B. S. Martincigh, Distribution, sources and risk of exposure to polycyclic aromatic hydrocarbons in indoor dusts from electronic repair workshops in southern Nigeria, Emerging Contam., 2019, 5, 23–30, DOI:10.1016/j.emcon.2018.12.003.
- C. M. A. Iwegbue, E. C. Iteku-Atata, E. W. Odali, F. E. Egobueze, G. O. Tesi, G. E. Nwajei and B. S. Martincigh, Distribution, sources and health risks of polycyclic aromatic hydrocarbons (PAHs) in household dusts from rural, semi-urban and urban areas in the Niger Delta, Nigeria, Exposure Health, 2019, 11(3), 209–225, DOI:10.1007/s12403-018-0276-z.
- C. M. A. Iwegbue, M. J. Ehigbor, G. O. Tesi, O. I. Eguavoen and B. S. Martincigh, Occurrence, sources and exposure risk of polycyclic aromatic hydrocarbons (PAHs) in street dusts from the Nigerian megacity, Lagos, Polycyclic Aromat. Compd., 2022, 42, 49–69, DOI:10.1080/10406638.2020.1716027.
- C. M. A. Iwegbue, E. F. Kekeke, G. O. Tesi, C. Olisah, F. E. Egobueze, E. Chukwu-Madu and B. S. Martincigh, Impact of land-use types on the distribution and exposure risk of polycyclic aromatic hydrocarbons in dusts from Benin City, Nigeria, Arch. Environ. Contam. Toxicol., 2021, 81, 210–226, DOI:10.1007/s00244-021-00861-z.
- J. A. Adeniran, M. O. Abdulraheem, H. A. Ameen, E. T. Odediran and M.-N. O. Yusuf, Source identification and health risk assessments of polycyclic aromatic hydrocarbons in settled dusts from different population density areas of Ilorin, Nigeria, Environ. Monit. Assess., 2021, 193, 777, DOI:10.1007/s10661-021-09566-1.
- C. J. Ossai, C. M. A. Iwegbue, G. O. Tesi, C. Olisah, F. E. Egobueze, G. E. Nwajei and B. S. Martincigh, Distribution, sources and exposure risk of polycyclic aromatic hydrocarbons in soils, and indoor and outdoor dust from Port Harcourt city, Nigeria, Environ. Sci.: Processes Impacts, 2021, 23, 1328–1350, 10.1039/d1em00094b.
- R. O. Yusuf, E. T. Odediran, J. A. Adeniran and O. A. Adesina, Polycyclic aromatic hydrocarbons in road dusts of a densely populated African city: spatial and seasonal distribution, source, and risk assessment, Environ. Sci. Pollut. Res., 2022, 29, 44970–44985, DOI:10.1007/s11356-022-18943-3.
- C. M. A. Iwegbue, A. A. Ogbuta, G. O. Tesi, C. J. Ossai, C. Olisah, G. E. Nwajei and B. S. Martincigh, Spatial distribution of polycyclic aromatic hydrocarbons in dust and soils from informal trade sites in southern Nigeria: implications for risk and source analysis, Chemosphere, 2023, 315, 137624, DOI:10.1016/j.chemosphere.2022.137624.
- M. Radojević and V. N. Bashkin, Practical Environmental Analysis, Royal Society of Chemistry, Cambridge, United Kingdom, 1999, 466 Search PubMed.
- US EPA, Testing Methods for Evaluating Solid Waste, Physical and Chemical Methods, Method 3550C; Ultra-sonication Extraction, USEPA, Washington, DC. 2007, Retrieved from http://www.epa.gov/solidwaste/hazard/testmethods/sw846/pdfs/3550c.pdf on January 30, 2020 Search PubMed.
- D. F. Kalf, T. Crommentuijn and E. J. van de Plassche, Environmental quality objectives for 10 polycyclic aromatic hydrocarbons (PAHs), Ecotoxicol. Environ. Saf., 1997, 36, 89–97, DOI:10.1006/eesa.1996.1495.
- USEPA (United States Environmental Protection Agency), Risk Assessment Guidance for Superfund, Human Health Evaluation Manual EPA/se0/1-89/002, Office of Solid Waste & Emergency Response, 1989, vol. 1 Search PubMed.
- USEPA (United States Environmental Protection Agency), Risk Assessment Guidance for Superfund, Human Health Evaluation Manual (F, Supplemental Guidance for Inhalation Risk Assessment) EPA/540/R/070/002, Office of Superfund Remediation and Technology Innovation, 2009, vol. 1 Search PubMed.
- L. Mølhave, T. Schneider, S. K. Kjærgaard, L. Larsen, S. Norn and O. Jørgensen, House dust in seven Danish offices, Atmos. Environ., 2000, 34, 4767–4779, DOI:10.1016/S1352-2310(00)00104-7.
- S. P. Wu, S. Tao, F. L. Xu, R. Dawson, T. Lan, B. G. Li and J. Cao, Polycyclic aromatic hydrocarbons in dustfall in Tianjin, China, Sci. Total Environ., 2005, 345, 115–126, DOI:10.1016/j.scitotenv.2004.11.003.
- Department of Petroleum Resources (DPR), Environmental Guidelines and Standards for the Petroleum Industry in Nigeria (Revised Edition), Department of Petroleum Resources of Nigeria, Ministry of Petroleum and Mineral Resources, Abuja, Nigeria, 2012 Search PubMed.
- A. Maragkidou, Y. Ma, O. Jaghbeir, D. Faouri, S. Harrad, A. Al-Hunaiti, S. Arar, K. Hameri and T. Hussein, PAHs in household floor dust collected in Amman, Jordan, J. Chem. Eng. Process Technol., 2016, 7, 292, DOI:10.4172/2157-7048.1000292.
- L. H. Tuyen, N. M. Tue, S. Takahashi, G. Suzuki, P. H. Viet, A. Subramanian, K. A. Bulbule, P. Parthasarathy, A. Ramanathan and S. Tanabe, Methylated and unsubstituted polycyclic aromatic hydrocarbons in street dust from Vietnam and India: occurrence, distribution and in vitro toxicity evaluation, Environ. Pollut., 2014, 194, 272–280, DOI:10.1016/j.envpol.2014.07.029.
- I. I. Shabbaj, M. A. Alghamdi and M. I. Khoder, Street dust—bound polycyclic aromatic hydrocarbons in a Saudi coastal city: status, profile, sources, and human health risk assessment, Int. J. Environ. Res. Public Health, 2018, 15, 2397, DOI:10.3390/ijerph15112397.
- A. Najmeddin, F. Moore, B. Keshavarzi and Z. Sadegh, Pollution, source apportionment and health risk of potentially toxic elements (PTEs) and polycyclic aromatic hydrocarbons (PAHs) in urban street dust of Mashhad, the second largest city of Iran, J. Geochem. Explor., 2018, 190, 154–169, DOI:10.1016/j.gexplo.2018.03.004.
- B. Škrbic, N. Đurišić-Mladenović, J. Živančev and Đ. Tadić, Seasonal occurrence and cancer risk assessment of polycyclic aromatic hydrocarbons in street dust from the Novi Sad city, Serbia, Sci. Total Environ., 2019, 647, 191–203, DOI:10.1016/j.scitotenv.2018.07.442.
- S. Kong, B. Lu, Y. Ji, Z. Bai, Y. Xu, Y. Liu and H. Jiang, Distribution and sources of polycyclic aromatic hydrocarbons in size-differentiated re-suspended dust on building surfaces in an oilfield city, China, Atmos. Environ., 2012, 55, 7–16, DOI:10.1016/j.atmosenv.2012.03.044.
- B. Keshavarzi, H. Sajjad Abbasi, F. Moore, H. Delshab and N. Soltani, Polycyclic aromatic hydrocarbons in street dust of Bushehr city, Iran: status, source, and human health risk assessment, Polycyclic Aromat. Compd., 2020, 40, 61–75, DOI:10.1080/10406638.2017.1354897.
- D. Lorenzi, J. A. Entwistle, M. Cave and J. R. Dean, Determination of polycyclic aromatic hydrocarbons in urban street dust: Implications for human health, Chemosphere, 2011, 83, 970–977, DOI:10.1016/j.chemosphere.2011.02.020.
- M. A. Hassanien and N. M. Abdel-Latif, Polycyclic aromatic hydrocarbons in road dust over Greater Cairo, Egypt, J. Hazard. Mater., 2008, 151, 247–254, DOI:10.1016/j.jhazmat.2007.05.079.
- W.-L. Ma, D.-Z. Sun, W.-G. Shen, M. Yang, H. Qi, L.-Y. Liu, J.-M. Shen and Y.-F. Li, Atmospheric concentrations, sources and gas-particle partitioning of PAHs in Beijing after the 29th Olympic Games, Environ. Pollut., 2011, 159, 1794–1801, DOI:10.1016/j.envpol.2011.03.025.
- B. Yang, N. Xue, L. Zhou, F. Li, X. Cong, B. Han, H. Li, Y. Yan and B. Liu, Risk assessment and sources of polycyclic aromatic hydrocarbons in agricultural soils of Huanghuai plain, China, Ecotoxicol. Environ. Saf., 2012, 84, 304–310, DOI:10.1016/j.ecoenv.2012.07.027.
- Netherlands Ministry of Housing and Environment, Environmental Quality Objectives in the Netherlands: A Review of Environmental Quality Objectives and Their Policy Framework in the Netherlands. Risk Assessment and Environmental Quality Division. Directorate for Chemicals, External Safety and Radiation Protection, Ministry of Housing, Spatial Planning and the Environment, Netherlands, 1994 Search PubMed.
- Washington State Department of Ecology, Evaluating the Toxicity and Assessing the Carcinogenic Risk of Environmental Mixtures Using Toxicity Equivalency Factors, 2007, Retrieved on 20 January, 2020 from http://fortress.wa.gov/ecy/clarc/FocusSheets/tef.pdf Search PubMed.
- M. B. Yunker, R. W. Macdonald, R. Vingarzan, R. H. Mitchell, D. Goyette and S. Sylvestre, PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition, Org. Geochem., 2002, 33, 489–515, DOI:10.1016/S0146-6380(02)00002-5.
- G. Brinkman, G. Vance, M. P. Hannigan and J. B. Milford, Use of synthetic data to evaluate positive matrix factorization as a source apportionment tool for PM2.5 exposure data, Environ. Sci. Technol., 2006, 40, 1892–1901 CrossRef CAS PubMed.
- G.-L. Shi, X. Li, Y.-C. Feng, Y.-Q. Wang, J.-H. Wu, J. Li and T. Zhu, Combined source apportionment, using positive matrix factorization–chemical mass balance and principal component analysis/multiple linear regression–chemical mass balance models, Atmos. Environ., 2009, 43, 2929–2937, DOI:10.1016/j.atmosenv.2009.02.054.
- W. Wang, M.-J. Huang, Y. Kang, H.-S. Wang, A. O. W. Leung, K. C. Cheung and M. H. Wong, Polycyclic aromatic hydrocarbons (PAHs) in urban surface dust of Guangzhou, China: status, sources and human health risk assessment, Sci. Total Environ., 2011, 409, 4519–4527, DOI:10.1016/j.scitotenv.2011.07.030.
- M. F. Simcik, S. J. Eisenreich and P. J. Lioy, Source apportionment and source/sink relationships of PAHs in the costal atmosphere of Chicago and Lake Michigan, Atmos. Environ., 1999, 33, 5071–5079, DOI:10.1016/S1352-2310(99)00233-2.
- A. Qishlaqi and F. Beiramali, Potential sources and health risk assessment of polycyclic aromatic hydrocarbons in street dusts of Karaj urban area, northern Iran, J. Environ. Health Sci. Eng., 2019, 17, 1029–1044, DOI:10.1007/s40201-019-00417-3.
- N. Soltani, B. Keshavarzi, F. Moore, T. Tavakol, A. R. Lahijanzadeh, N. Jaafarzadeh and M. Kermani, Ecological and human health hazards of heavy metals and polycyclic aromatic hydrocarbons (PAHs) in road dust of Isfahan metropolis, Iran, Sci. Total Environ., 2015, 505, 712–723, DOI:10.1016/j.scitotenv.2014.09.097.
- X. L. Zhen, G. Liu, J. H. Li, H. Xu and D. Wu, PAHs in road dust of Nanjing Chemical Industry Park, China: chemical composition, sources, and risk assessment, J. Environ. Sci. Health, Part A, 2020, 55, 33–43, DOI:10.1080/10934529.2019.1667166.
- L. C. Marr, T. W. Kirchstetter, R. A. Harley, A. H. Miguel, S. V. Hering and S. K. Hammond, Characterization of polycyclic aromatic hydrocarbons in motor vehicle fuels and exhaust emissions, Environ. Sci. Technol., 1999, 33, 3091–3099, DOI:10.1021/es981227l.
- F. Esen, Y. Tasdemir and N. Vardar, Atmospheric concentrations of PAHs, their possible sources and gas-to-particle partitioning at a residential site of Bursa, Turkey, Atmos. Res., 2008, 88, 243–255, DOI:10.1016/j.atmosres.2007.11.022.
- N. R. Khalili, P. A. Scheff and T. M. Holsen, PAH source fingerprints for coke ovens, diesel and, gasoline engines, highway tunnels, and wood combustion emissions, Atmos. Environ., 1995, 29, 533–542, DOI:10.1016/1352-2310(94)00275-P.
- H.-O. Kwon and S.-D. Choi, Polycyclic aromatic hydrocarbons (PAHs) in soils from a multi-industrial city, South Korea, Sci. Total Environ., 2014, 470–471, 1494–1501, DOI:10.1016/j.scitotenv.2013.08.031.
- J. Hu, C. Liu, Q. Guo, J. Yang, C. P. Okoli, Y. Lang, Z. Zhao, S. Li, B. Liu and G. Song, Characteristics, source, and potential ecological risk assessment of polycyclic aromatic hydrocarbons (PAHs) in the Songhua River Basin, Northeast China, Environ. Sci. Pollut. Res., 2017, 24, 17090–17102, DOI:10.1007/s11356-017-9057-7.
- R. K. Larsen III and J. E. Baker, Source apportionment of polycyclic aromatic hydrocarbons in the urban atmosphere: a comparison of three methods, Environ. Sci. Technol., 2003, 37, 1873–1881, DOI:10.1021/es0206184.
- R. M. Harrison, D. J. T. Smith and L. Luhana, Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Birmingham, UK, Environ. Sci. Technol., 1996, 30, 825–832, DOI:10.1021/es950252d.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3em00048f |
| This journal is © The Royal Society of Chemistry 2024 |