A deformable complementary moisture and tribo energy harvester†
Gwanho
Kim
a,
Jae Won
Lee
 b,
Kaiying
Zhao
a,
Taebin
Kim
a,
Woojoong
Kim
a,
Jin Woo
Oh
a,
Kyuho
Lee
a,
Jihye
Jang
a,
Guangtao
Zan
a,
Jong Woong
Park
b,
Kaiying
Zhao
a,
Taebin
Kim
a,
Woojoong
Kim
a,
Jin Woo
Oh
a,
Kyuho
Lee
a,
Jihye
Jang
a,
Guangtao
Zan
a,
Jong Woong
Park
 a,
Seokyeong
Lee
a,
Yeonji
Kim
a,
Wei
Jiang
a,
Shengyou
Li
a and
Cheolmin
Park
a,
Seokyeong
Lee
a,
Yeonji
Kim
a,
Wei
Jiang
a,
Shengyou
Li
a and
Cheolmin
Park
 *a
*a
aDepartment of Materials Science and Engineering, Yonsei University, Seoul 03722, Republic of Korea. E-mail: cmpark@yonsei.ac.kr
bDepartment of Materials Science and Engineering, Kangwon National University, Samcheok 25913, Republic of Korea
First published on 10th November 2023
Abstract
Although energy harvesting based on moisture-induced electric generators (MEGs) has become popular with the development of numerous moisture and ion-selective materials, the single-cell combination of an MEG with another energy harvester for further boosting the power efficiency has seldom been demonstrated. Herein, we present a single-cell complementary energy harvester capable of simultaneously generating moisture-induced as well as triboelectric power. Our harvester is based on a highly resilient and deformable three-dimensional melamine foam coated with two-dimensional conductive MXene (Ti3C2Tx) nanosheets. One fifth of the MXene-coated foam is additionally covered with an organo-ionic hydrogel as an asymmetric moisture and ion source for the MXene over a broad range of humidity and temperature, producing an MEG operated under a variety of mechanical deformations. Our resilient MXene/organo-ionic hydrogel foam is sufficiently tolerant to repetitive and harsh triboelectric contacts and can be used in a triboelectric nanogenerator (TENG). Our single-cell MXene/organo-ionic hydrogel foam device exhibits a maximum voltage and current of 55 V and 102 μA, respectively, and a high electric power of approximately 83 μW cm−2 with excellent stretchability and compression strength of approximately 30% and 2.1 MPa, respectively. Moreover, based on the unique DC and AC outputs from the complementary MEG and TENG with fast capacitor charging capability, respectively, a novel emergency alarm and guidance system is demonstrated, wherein a constant light emitted from an alarm sensor powered by the MEG is amplified by the self-powered TENG driven by transient human walking motions in an emergency, effectively guiding people to an exit.
Broader contextThe great demand for renewable and sustainable energy has made moisture-induced electric generators (MEGs) as well as triboelectric nanogenerators (TENGs) significantly important. Their practical applications are, however, often limited due to the difficulty in adequately combining the two energy harvesters with minimal environmental constraints. Our work addresses these issues by introducing a complementary, deformable energy harvester of MEG and TENG technologies into a single cell, based on a mechanically resilient three-dimensional melamine foam coated with two-dimensional conductive MXene (Ti3C2Tx) nanosheets. With excellent stretchability and compression strength of approximately 30% and 2.1 MPa, respectively, our harvester provides a maximum voltage and current of 55 V and 102 μA, respectively, and a high electric power of approximately 83 μW cm−2, outperforming the harvesters in the literature. Moreover, the unique direct current (DC) and alternating current (AC) outputs from the complementary MEG and TENG with fast capacitor charging capability, respectively, allowed for developing a novel emergency alarm and guidance system, wherein a constant light emitted from an alarm sensor powered by the MEG is amplified by the self-powered TENG driven by transient human walking motions in an emergency, effectively guiding people to an exit. Our research sets a new standard for energy harvesting and opens new paths for practical applications in self-powered electronics and emergency systems. It serves as a blueprint for future work aimed at creating versatile, environment-agnostic energy solutions. |
Introduction
Energy harvesting using moisture-induced electric generators (MEGs) is of great interest because water is ubiquitous, covering two-thirds of the Earth's surface, and approximately 10% of freshwater exists in the atmosphere in the form of clouds and fog.1–5 These blue energy harvesters convert chemical energy into electrical energy by utilizing the gradient in the ion concentration, which arises when free charged ions are released upon the spontaneous adsorption of water molecules on hygroscopic functional groups (such as –OH, –COOH, etc.) within an asymmetric moisturizing regulation or chemical-gradient structure.6–9 Several studies have attempted to realize high-performance MEGs using various materials such as surface-modified carbon,10,11 graphene oxide,12,13 cellulose-based materials,14–16 protein-based biomaterials,17,18 polymers,19 metal–organic frameworks,20,21 and metal oxides.22 Nevertheless, the current densities of the device units have been unsatisfactory and some devices require water immersion, which makes them unsuitable for application in portable and wearable electronics.Recently, significant advancements have been witnessed in the development of MEGs with ionic hydrogels that possess exceptional water-capturing and fast ion-transport capabilities through their three-dimensional (3D) porous structure.23,24 The MEGs with ionic hydrogels efficiently overcome the issue of insufficient total ions in ionized water, thereby enhancing the output currents of the devices. Furthermore, owing to their distinctive interactions with water molecules, ionic hydrogels can be employed as water reservoirs that continuously supply hydrated ions over an extended period. To further improve the harvesting performance, MEGs can be effectively integrated with other energy harvesters.25–27 For instance, a high-performance MEG system was developed by incorporating a photosensitive phytochrome into a hydrophilic polyelectrolyte.25 However, although the hybrid MEG system successfully harvested both moisture and sunlight synchronously, the power still needs to be improved, requiring the arrays of the hybrid MEGs to operate conventional electronic devices due to their output voltage typically in the range of hundreds of millivolts to approximately 1.0 V. We envisioned that harvesters based on mechanical-energy conversions such as piezo- and triboelectricity could be good candidates for high-performance hybrid MEGs because of their capability for readily generating high output voltages.28–30 An MEG with a unique ion-selective surface could also be suitable for triboelectrification, particularly, involving repetitive mechanical contact electrification, giving rise to a high-power hybrid MEG with a triboelectric nanogenerator (TENG).31,32 However, to develop such a hybrid MEG, several issues should be resolved. First, energy harvesting at high humidities often requires MEG operation to be avoided because of a substantial decrease in the power efficiency of the TENG at high humidities.33 More importantly, the mechanical resilience and robustness of the hybrid MEG should be guaranteed, making the device tolerant to harsh deformation and repetitive contact.34
Herein, we present a deformable complementary moisture and triboelectric energy harvester with positive-ion-selective two-dimensional (2D) MXene (Ti3C2Tx) flakes deposited on a resilient 3D melamine foam. To ensure high performance of the MEG at low humidities, one fifth of the MXene foam is coated with an organo-ionic hydrogel that continuously supplies water and salt ions to the upper uncoated MXene surface. The hybrid harvester exhibits its MEG performance under various mechanical deformations with a maximum output open-circuit voltage (VOC), short-circuit current density (JSC), and power density of approximately 310 mV, 877 μA cm−2, and 9.15 μW cm−2, respectively. Our resilient MXene/organo-ionic hydrogel foam is sufficiently tolerant to more than 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 repetitive triboelectric contacts, leading to reliable TENG performance with an alternating current (AC) voltage of approximately 80 V. Complementary energy harvesting is achieved in our single MXene/organo-ionic hydrogel foam (MOHF) device, resulting in a maximum voltage and current of 55 V and 102 μA, respectively, and a high electric power of approximately 83 μW cm−2 with excellent stretchability and compression strength of approximately 30% and 2.1 MPa, respectively. Moreover, a novel optical emergency alarm and guidance system is demonstrated, powered by our complementary energy harvester with fast capacitor charging capability. The DC light emission of an alarming sensor powered in the MEG mode is amplified by the AC power in the TENG mode harvested from transient human walking motions in an emergency, effectively guiding people to an exit.
000 repetitive triboelectric contacts, leading to reliable TENG performance with an alternating current (AC) voltage of approximately 80 V. Complementary energy harvesting is achieved in our single MXene/organo-ionic hydrogel foam (MOHF) device, resulting in a maximum voltage and current of 55 V and 102 μA, respectively, and a high electric power of approximately 83 μW cm−2 with excellent stretchability and compression strength of approximately 30% and 2.1 MPa, respectively. Moreover, a novel optical emergency alarm and guidance system is demonstrated, powered by our complementary energy harvester with fast capacitor charging capability. The DC light emission of an alarming sensor powered in the MEG mode is amplified by the AC power in the TENG mode harvested from transient human walking motions in an emergency, effectively guiding people to an exit.
Results and discussion
Design and characteristics of a complementary harvester with MOHF
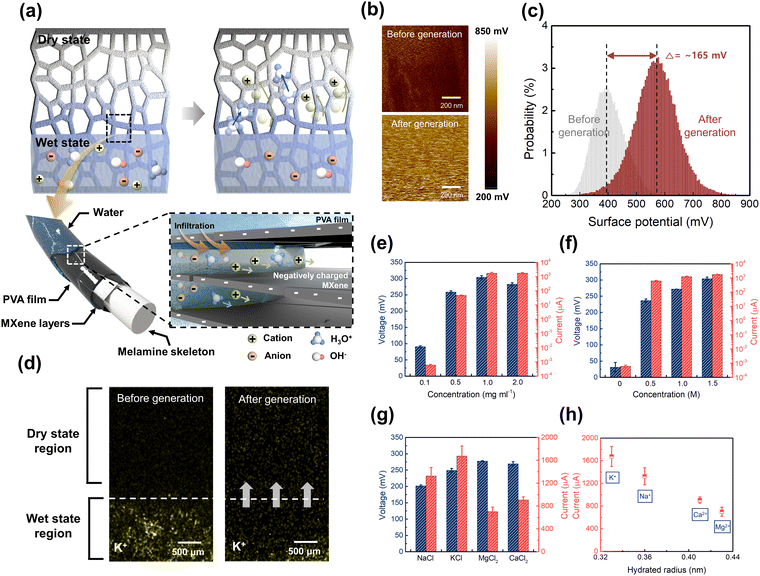
A MOHF was fabricated through a series of dip-coating processes, followed by the partial infusion of an organo-ionic hydrogel into a dip-coated melamine foam, as shown schematically in Fig. 1a (Fig. S1, ESI†). MXene flakes were dip-coated on the surface of the melamine foam. Not only to ensure adhesion with an organo-ionic hydrogel but also to facilitate transport of both ionized water and ions from the salts dissolved in the organo-ionic hydrogel, a thin poly(vinyl alcohol) (PVA) film was subsequently coated on the MXene treated melamine foam. The organo-ionic hydrogel based on polyacrylamide (PAM), containing glycerol and an ionic salt (KCl), was partially coated on the PVA-treated MXene/melamine foam, producing an MOHF. The melamine foam was selected as a structural framework providing excellent resilience upon various mechanical deformations associated with triboelectrification.35 The thin MXene film coated on the melamine foam ensured high-performance moisture energy generation owing to its large specific surface area as well as high electrical conductivity.31,36 The hydrophilic PVA coated on MXene facilitated the movement of ionized water and ions, improving the MEG performance.10 Finally, the organo-ionic hydrogel with a unique 3D cross-linked hygroscopic polymer network served as a water and ion reservoir, continuously supplying hydration ions and salt ions to the non-coated PVA/MXene-melamine foam,37 as schematically shown in Fig. 1a.The MOHF was approximately 10 mm in height, and the bottom 2 mm were coated with the organo-ionic hydrogel, as shown in the photograph in Fig. 1b. The thin MXene film coated on the melamine foam was examined with the scanning electron microscope (SEM), and the results in Fig. 1c (Fig. S2, ESI†) show that the MXene flakes completely covered the melamine foam, producing the characteristic rough structure arising from the variation in the number of MXene flakes on the surface (see the inset of Fig. 1c). The MXene flakes coated the melamine foam well, owing to the various terminal groups (Tx, = –OH, –O, –F) on the surface of MXene, which facilitated strong electrostatic interactions with the terminal amine groups on the melamine foam.38 The coating of MXene multilayers on the melamine foam was confirmed by the transmission electron microscopy (TEM) and energy-dispersive X-ray spectroscopy (EDS) images of a cross-section of a single PVA/MXene-melamine skeleton (see Fig. S3 and S4, ESI†).
The PVA/MXene-melamine foam was further examined using high-resolution X-ray diffraction (HR-XRD) and high-resolution X-ray photoelectron spectroscopy (HR-XPS), and the results are shown in Fig. 1d and e, respectively. The HR-XRD results show a broad peak at 22.5°, indicating the amorphous structure of the melamine foam, and a sharp peak at 5.8° corresponding to the (002) reflection of the basal plane of Ti3C2.38,39 It should be noted that the amorphous characteristics of PVA are hardly recognized in the HR-XRD results, most probably because of the low concentration of PVA. The C 1s HR-XPS results in Fig. 1e display notable peaks at 281.5 eV (C–Ti–Tx) and 284.5 eV (C–C) associated with MXene, 286.1 eV associated with the C–O bond of PVA, and 288.7 eV corresponding to the C–N energy of melamine.40–42 The O 1s and N 1s HR-XPS results further confirm the development of thin MXene as well as PVA layers on the melamine foam (Fig. S5 and S6, ESI†).
The excellent water retention of our organo-ionic hydrogel was evaluated, and the results are presented in Fig. 1f. The weight variation of the hydrogel with time was monitored at a relative humidity (RH) of 20%. An organo-ionic hydrogel exhibited that its water content was rapidly decreased in a day of exposure, but the reduced water content was maintained even after seven days because of the strong hydrogen bonding interactions between glycerol and the adjacent water molecules.43 In contrast, a conventional ionic hydrogel without glycerol exhibited a rapid decrease and reach to nearly 0% in its water content within 6 hours of exposure because of the fast evaporation of water from the ionic hydrogel (Fig. 1f). The ionic hydrogel became brittle with a substantial reduction in its dimensions, owing to the water loss, after the seven-day exposure, whereas the organo-ionic hydrogel exhibited initial mechanical flexibility (Fig. S7, ESI†). Furthermore, the organo-ionic hydrogel in our MOHF readily adhered to various materials such as plastic, glass, metal, rubber, wood, and fabric, owing to its excellent moisture retention (Fig. S8, ESI†). The MOHF was mechanically resilient and susceptible to diverse mechanical deformations such as stretching, compression, bending, and twisting, as shown in the photographs in Fig. 1g. The details of the mechanical properties of the MOHF are presented in Fig. 1h and i. The stress–strain curve of the MOHF in the tensile state exhibits a maximum tensile stress of 173 kPa at a strain of 32%. Because the organo-ionic hydrogel in our MOHF is strongly bonded to PVA/MXene-melamine foam through hydrogen bonds,34 the tensile stress of the MOHF is higher than that of both the PVA/MXene-melamine foam and melamine foam. In the compressive tests, our MOHF exhibited a maximum stress of ∼2.1 MPa at a strain of 95%, and its excellent resilience was demonstrated with nearly identical stress–strain hysteresis loops even after 30 pressing/depressing cycles.
MEG performance of a MOHF
A MEG with our MOHF (MEG–MOHF) was developed by placing the MOHF on a silver-paste-coated glass substrate and inserting a silver-paste-coated polyethylene terephthalate (PET) substrate into the middle part of the PVA/MXene-melamine foam of MOHF not covered with the organo-ionic hydrogel, as shown schematically in Fig. 2a. The electrical performance of our MEG–MOHF was evaluated, and the results are presented in Fig. 2b. Under ambient conditions with low relative humidity (RH 20%), the MEG–MOHF demonstrated a stable VOC of approximately 300 mV and short-circuit current (ISC) ranging from approximately 1800 to 100 μA over a 30 min period. The power generation with reliable VOC and high ISC was ascribed to the asymmetrical moisture absorption characteristics of the MOHF, giving rise to an excellent moisture gradient from the bottom to the top of the MOHF.24 To assess the electrical performance of the MEG influenced by the hygroscopic and ion diffusion properties of our MOHF, we carefully examined the initial stage of energy harvesting of a nearly dried MEG–MOHF. When a MEG–MOHF dried at 60 °C was exposed to air, the device exhibited a gradual increase in voltage with time, reflecting the moisture absorption to the hygroscopic organo-ionic hydrogel and subsequent diffusion of the moisture and ions toward the upper-layer electrode. The voltage was saturated after approximately 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s exposure at which a constant moisture and ion gradient was developed in the device by balancing the moisture and ion diffusion rate with the evaporation rate at the top electrode (Fig. S9, ESI†). In contrast, MEG devices with a symmetrical moisture-absorption structure, such as silver/PVA/MXene-melamine foam/silver or silver/organo-ionic hydrogel/silver rarely showed noticeable VOC or ISC, owing to the negligible moisture gradient (Fig. S10, ESI†).1,20
000 s exposure at which a constant moisture and ion gradient was developed in the device by balancing the moisture and ion diffusion rate with the evaporation rate at the top electrode (Fig. S9, ESI†). In contrast, MEG devices with a symmetrical moisture-absorption structure, such as silver/PVA/MXene-melamine foam/silver or silver/organo-ionic hydrogel/silver rarely showed noticeable VOC or ISC, owing to the negligible moisture gradient (Fig. S10, ESI†).1,20
Fig. 2c presents the electrical performance of the MEG–MOHF, according to the PVA concentration. When a low concentration (0.1 wt%) of PVA was coated on the MXene/melamine foam, the resulting MEG–MOHF produced a VOC of approximately 300 mV and maximum ISC of approximately 1600 μA. However, when the PVA concentration was increased to 1.0 wt%, a significant reduction in the maximum ISC, to approximately 34.6 μA, was observed. This significant reduction in ISC could be attributed to the electrically insulating characteristics of PVA (Fig. S11, ESI†), which hindered the charge transport from the conductive MXene to an external electrode.44 The slight decrease in VOC can be also explained by the decrease ionic gradient arising from the abundant moisture in the PVA. It should, however, be noted that PVA coating is essential because, in the absence of PVA treatment, the generated voltage gradually decreased over time because of the insufficient movement of water required to induce an ionic potential in the MOHF (Fig. S12, ESI†). An optimal PVA concentration of 0.1 wt% was thus chosen for the subsequent experiments.
To evaluate the influence of various environmental conditions, first, we measured the electrical performance of the MEG–MOHF under a wide range of RHs from 20 to 90% for 30 min, and the results are presented in Fig. 2d. At 20% RH, the MEG–MOHF after 30 min operation exhibited a VOC and ISC of approximately 285 mV and 124 μA, respectively. A slight decrease in VOC was observed upon increasing the RH, but this decrease was marginal. On the other hand, the ISC of the device after 30 min of operation increased slightly with the RH, exhibiting values of approximately 183, 198, 221, and 245 μA for 40, 60, 80, and 90%, respectively. In the initial state, a moisture and ionic gradient was developed in our MOHF from bottom to top because of the partially coated organo-ionic hydrogel in the MOHF. When the RH increased, both the moisture absorption and ion dissociation were enhanced, with many ions into regions not coated with the organo-ionic hydrogel, producing a gradual decrease in the ionic gradient. We speculate that the decrease and increase in VOC and ISC with RH, respectively, can be attributed to the reduction in the ionic gradient with the RH.21
Second, we investigated the output VOC of the MEG–MOHF across a range of temperatures from −20 to 60 °C, as shown in Fig. 2e. The highest VOC of approximately 300 mV was obtained at 20 °C. Although water could flow to the coated PVA/MXene layer in a MOHF at temperatures below the freezing point of water, because of the excellent anti-freezing ability of the glycerol and ionic salts in the organo-ionic hydrogel,12,45 the ion mobility within the MOHF decreased. This reduced mobility led to a decrease in the ionic gradient, thereby decreasing the VOC. At elevated temperatures, the upper part of a device can become drier at higher temperatures due to the faster water evaporation, making an ionic gradient larger with temperature. At the same time, the ion mobility is enhanced at elevated temperatures, leading to a reduction in the ionic gradient. We believe that these two forces are competitive in our MEG–MOHF. In the temperature range we examined from 20 to 60 °C, the reduction in the ionic gradient due to the facilitated ion mobility might be dominant, giving rise to the degradation of the device performance. Therefore, the balanced water-molecule absorption and ion transport at an appropriate temperature is significant for obtaining a stable electrical output from the MEG–MOHF. Our single MEG–MOHF can deliver a stable VOC with a maximum value of 310 mV for over an entire day under an open ambient environment with slightly fluctuating RH (23 to 30%) and temperature (17 to 20 °C) (Fig. 2f). At the initial stage of energy harvesting, the voltage was gradually increased due to the moisture uptake from a hygroscopic organo-ionic hydrogel, followed by the diffusion of moisture and ions to the top electrode, as schematically shown in Fig. S13a (ESI†). The voltage was saturated and stable after a certain time (Fig. S9 and S13b, ESI†). The stable VOC of the MEG–MOHF, attributed to the long-term sustained ionic gradient within the MOHF, was further validated through COMSOL simulations (Fig. S14, ESI†). Moreover, we examined the variation of the electrical resistance of a MOHF with time, and the results are shown in Fig. S15 (ESI†). The resistance was slightly increased from approximately 28.3 to 36.6 kΩ after 1 day and became approximately to 115.4 kΩ after air exposure for 7 days possibly due to the oxidation of MXene facilitated by an organo-ionic hydrogel. A PVA coated MXene-melamine foam exhibited its electrical resistance rarely altered upon air exposure for 7 days (Fig. S15, ESI†). Considering that our MEG–MOHF generated a reliable voltage of approximately 300 mV for more than 24 hours, the oxidation of MXene after 1 day might rarely affect the harvesting performance.
The stability of the charge–discharge cycle was examined, and the results are shown in Fig. S16 (ESI†). When the MEG device was connected to an external electronic device, the current was gradually decreased with time, leading to a decline in electrical output. The voltage was gradually increased in an open circuit, reaching a value similar to the initial one over time. The power densities of the MEG–MOHF were obtained by measuring the VOC and JSC as functions of the external loads from 10 Ω to 10 MΩ, as presented in Fig. 2g. The VOC significantly increased with increasing resistance whereas the output JSC decreased. A maximum power density of 9.15 μW cm−2 was obtained at 50 kΩ (Fig. 2h).
Working principle of MEG–MOHF
The recent MEG works often demonstrated the instances where electric power was generated by ambient moisture. When ambient moisture is in contact with a surface possessing hydrophilic functional groups such as –OH, –COOH and –SO3H, it produces hydrogen ions due to hydrolysis. When the density of the functional groups on a substrate is spatially controlled by asymmetric geometries of the MEGs and area-dependent surface chemical modification, a voltage is developed due to the difference in the spatial concentration of the hydrogen ions,23,24 followed by the generation of electrical current.6,7 The detailed mechanism of electricity generation in an MEG–MOHF is schematically presented in Fig. 3a. The PVA/MXene layers in an MOHF contain various functional groups such as oxygen (–O), hydroxyl (–OH), and fluorine (–F), which render the surface of the MOHF hydrophilic with a negative zeta potential (Fig. S17, ESI†).46 When water infiltrates the thinly coated PVA film and moves through the nanosized MXene channels, the MXene channels become negatively charged because of water hydrolysis. The negatively charged MXene nanochannels hinder the movement of the negatively charged ions (OH− and Cl−) while they allow the passage of positively charged ions (H3O+ and K+) in the organo-ionic hydrogel of the MOHF.2,47 This preferential cation transport promotes a streaming potential, thereby establishing an electric field and a directional diffusion current is initiated between the bottom and top electrodes (Fig. S18, ESI†).48,49 In the absence of an external circuit, the generated electric field within the MXene nanochannels initiates a counteractive drift current, which exists in equilibrium with the internal resistance of the MOHF, thus offsetting the diffusion current. When an external circuit is connected, the equilibrium drift current within the MOHF proportionately diminishes. The residual diffusion current is thus balanced via the external circuit, resulting in the generation of a drift current through the external resistor.31A Kelvin probe force microscope (KPFM) validated our proposed mechanism, and the results are presented in Fig. 3b and c. By scanning the surface of the top electrode section of a MEG–MOHF, we observed an increase in the potential, from 398 to 563 mV, after power generation of the MEG–MOHF (Fig. 3b and c). The results implied the preferential accumulation of positive charge carriers through the coated PVA/MXene layers. Furthermore, in the energy-dispersive X-ray spectroscopy (EDS) image of a cross-sectional MOHF, as demonstrated in Fig. 3d, K+ ions were preferentially diffused into the dry part of MOHF, leaving Cl− ions behind (Fig. S19, ESI†), giving rise to the output voltage. The preferential diffusion of K+ ions continuously occurred, giving rise to the reliable output voltage generation for more than 24 hours in our MEG–MOHF (see Fig. 2f). When the volume of the PVA/MXene not coated with the organo-ionic hydrogel increased, the VOC increased slightly, because of the enhanced ionic gradient in the enlarged PVA/MXene. On the other hand, the ISC decreased because of the increased internal resistance (Fig. S20, ESI†), which corroborated our proposed mechanism based on preferential ion separation and transport in the PVA/MXene layers.
We further investigated how the various factors affected the electrical characteristics of the MEG–MOHF. Fig. 3e shows the VOC and ISC of the MEG–MOHF as a function of the MXene concentration, in which both the VOC and ISC were substantially enhanced with an increase in the MXene concentration, reaching a maximum VOC of 305 mV and ISC of ∼1700 μA at a concentration of 1.0 mg ml−1. Above the concentration of 1.0 mg ml−1, however, no significant changes were observed. The improved performance was attributed to the enlarged specific surface area as well as the enhanced electrical conductivity of the MOHF with increased MXene concentration (Fig. S21, ESI†). When MXene was increased on the melamine scaffold, we believe that in addition to the enhanced conductivity, the number of MXene channels were increased. In this situation, VOC was enhanced due to the increased cation (K+)-selective MXene channels at high MXene concentration. The diffusion of ion solution along with a charged surface creates a region in which the potential decays exponentially. A characteristic length is defined as the Debye length (λD), which can be extracted by the following equation: 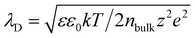 where ε is the dielectric constant of water, ε0 is the permittivity of vacuum, nbulk represents the bulk ion concentration, z is the valence of the ions, and e is the charge of an electron. When the charged channel is narrower than the Debye length, the channel would show high selectivity to the ions opposite to the surface charge owing to the Debye screening effect.2 In our MOHF, the Debye length of ions with the concentration of 1.5 M in the organo-ionic hydrogel is approximately 1 nm. Since the diameter of the MXene channel is approximately 1–2 nm, our MXene channels in a MOHF can ensure the cation selectivity. We also investigated the effect of KCl concentration on the VOC and ISC of MEG–MOHF, and the results are presented in Fig. 3f. The use of an organo-hydrogel without KCl resulted in a VOC and ISC of approximately 31 mV and 0.6 nA, respectively. Substantial enhancements in both VOC and ISC were evident, supporting our claim of salt-associated electricity generation. The performance of a MEG–MOHF might be, however, degraded with a more concentrated solution due to the reduced cation selectivity in the regions of macropores in our melamine scaffold ranging from 30 to 150 μm in diameter. In our MEG–MOHF, the performance degradation was not observed with a KCl concentration of 1.5 M which was the maximum solubility in an organo-ionic hydrogel.
where ε is the dielectric constant of water, ε0 is the permittivity of vacuum, nbulk represents the bulk ion concentration, z is the valence of the ions, and e is the charge of an electron. When the charged channel is narrower than the Debye length, the channel would show high selectivity to the ions opposite to the surface charge owing to the Debye screening effect.2 In our MOHF, the Debye length of ions with the concentration of 1.5 M in the organo-ionic hydrogel is approximately 1 nm. Since the diameter of the MXene channel is approximately 1–2 nm, our MXene channels in a MOHF can ensure the cation selectivity. We also investigated the effect of KCl concentration on the VOC and ISC of MEG–MOHF, and the results are presented in Fig. 3f. The use of an organo-hydrogel without KCl resulted in a VOC and ISC of approximately 31 mV and 0.6 nA, respectively. Substantial enhancements in both VOC and ISC were evident, supporting our claim of salt-associated electricity generation. The performance of a MEG–MOHF might be, however, degraded with a more concentrated solution due to the reduced cation selectivity in the regions of macropores in our melamine scaffold ranging from 30 to 150 μm in diameter. In our MEG–MOHF, the performance degradation was not observed with a KCl concentration of 1.5 M which was the maximum solubility in an organo-ionic hydrogel.
We also examined the effects of various desiccant chlorides on the energy-harvesting performance of an MEG–MOHF, and the results are presented in Fig. 3g and h. Four commonly used desiccant chlorides, namely, NaCl, KCl, CaCl2, and MgCl2, were examined at equimolar concentrations of 1.0 M. The salts with monovalent ions exhibited higher ISC but lower VOC than that with divalent ions. This behavior could be attributed to the difference in the charge density and mobility of the ions.50 Divalent ions have a high charge density, contributing to an increased output voltage because of the increased potential difference in an MOHF. In contrast, monovalent ions, with their smaller hydrated radii, experience less hindrance in their movement across the PVA/MXene layers, leading to a higher current generation than that of the divalent ions. Therefore, the K+ ion with the smallest hydrated radius exhibits the highest ISC, as shown in Fig. 3h.
Mechanically deformable MEG–MOHF
The MEG performance of our mechanically deformable MOHFs was explicitly examined, and the results are presented in Fig. 4. First, we measured the VOC and ISC under conditions of stretching, pressing, twisting, and bending, and the results are presented in Fig. 4a, b, c, d, respectively. For the electrical measurement under stretching, twisting, and bending, an MEG–MOHF with a volume of 40 × 10 × 4 mm3 was examined, while the sample subjected to pressing had a volume of 20 × 20 × 10 mm3. In all cases, silver-paste-coated PET electrodes were used. Additionally, given that the ISC decreases over time, measurements were conducted after the ISC was sufficiently stabilized, with a value of approximately 20 μA (see Fig. 2b). Fig. S22a (ESI†) demonstrates that the electrodes remain intact during and after various mechanical deformations such as stretching, bending, twisting, and pressing. SEM images of the devices after the deformations showed the electrodes firmly adhering to the MOHF, as shown in Fig. S22b (ESI†), corroborating the MEG performance under the various mechanical deformations (Fig. 4a–d). The results showed that the VOC values were rarely altered even after various deformations, in all samples. The results suggested that the reduced pore size in the MOHF, arising from the compression, stretching, twisting, and bending, rarely altered the ionic gradient within the MOHF.In contrast, the ISC increased when the MEG–MOHF was deformed under various mechanical forces. The increase in ISC could be explained with the resistor network model of the conductive framework,51 as schematically shown in Fig. 4e. The electrical resistance of the composite material (R) could be expressed as the sum of resistances of the outer layers on both sides (RO1 and RO2) and the resistance of the central layer (RC). As the layer coated with the organo-ionic hydrogel exhibited a higher compressive modulus than the uncoated regions (Fig. S23, ESI†), the mechanical deformation was assumed to dominantly occur in the uncoated part. At low strain regimes, the cells in the upper outer layers were flattened first, leading to the formation of conductive connections within those layers, as shown in the middle of Fig. 4e (Fig. S24, ESI†). The electrical resistance in the upper outer layer comprised the initial resistance RO1 and resistance RN associated with the new conductive pathways in parallel. Meanwhile, the resistances of the central layer (RC) and another outer layer (RO2) remained unchanged. In this situation, the total resistance could be calculated as R = RO1//RN + RC + RO2. As the strain increased, both the upper outer and central cells flattened, resulting in the formation of many conductive connections (Fig. S24, ESI†). In this case, the total resistance could be expressed as R = RC//RN + RO2. We believe that the formation of conductive paths upon mechanical deformation is responsible for the increase in ISC.
Further experiments were carried out on our MEG–MOHF to assess its energy generation under more severe deformation. The superior flexibility and durability of the MEG–MOHF was showcased by knotting, rolling, and folding a large piece, and the MEG–MOHF concurrently maintained a stable VOC of ∼280 mV, as shown in Fig. 4f. For the extreme pressing test, we drove a car over the MEG–MOHF and monitored the VOC values when the MEG-MOHF was compressed under a moving tire of a commercial car (Fig. S25, ESI†); the result is presented in Fig. 4g. The VOC generated in a stable state (stage 1 of Fig. 4g) abruptly decreased to ∼25 mV when the car passes over the MEG–MOHF (stage 2 of Fig. 4g). However, after the car passed, the VOC of the MEG–MOHF recovered to approximately 200 mV, exhibiting the excellent resilience of our MEG–MOHF under mechanical deformation as well as its capability for moisture energy generation.
TENG performance of a MOHF
Our mechanically resilient MOHF serves as an excellent triboelectric layer in a TENG for harvesting mechanical energy via repetitive vertical contact in addition to the energy harvesting from moisture. A TENG with our MOHF (TENG–MOHF) was implemented by establishing direct contact between a perfluoroalkoxy (PFA) film attached to a Cu electrode and the MOHF incorporating a silver-coated PET substrate, as schematically illustrated in Fig. 5a. The working principle of the TENG–MOHF in the contact-separation mode is schematically shown in Fig. 5b.52,53 When the PFA film encounters the MOHF, triboelectrification occurs, generating static charges with opposite signs on the two triboelectric surfaces due to the differences in their work functions. The PFA film acquires a negative charge owing to its high affinity for electrons. Meanwhile, the surface of MOHF becomes positively charged, thereby inducing a potential difference between the two electrodes. As the PFA film begins to separate from the MOHF, electrons flow from the Cu electrode to the silver electrode via an external circuit, for maintaining charge equilibrium. This flow of electrons generates an electrical output current and continues until all the negative charges accumulated on the surface of the PFA are completely compensated. When the PFA film subsequently approaches the MOHF again, the direction of electron movement reverses from the silver electrode back to the Cu electrode, for maintaining the charge balance, thereby producing an electrical output signal of the opposite polarity (Fig. 5b).The triboelectric output voltage and current of a TENG–MOHF were examined as functions of the relative humidity, and the results are presented in Fig. 5c and d, respectively. The humidity-dependent triboelectric performance of our device is of importance to develop a complementary energy generator integrating MEG and TENG with our MOHF (complementary MEG–TENG–MOHF), as will be shown later. The surface area of the PFA film was adjusted to 2 × 2 cm2 to match the top surface area of the MOHF, prior to the measurement. At 20% RH, the TENG–MOHF exhibited a maximum VOC of 80 V and ISC of 3.5 μA. These values surpassed the electrical performances of a TENG with either the bare melamine foam or the thin MXene film coated on melamine foam (Fig. S26, ESI†). However, a gradual decrease in both VOC and ISC was observed with the RH increase. A VOC of 30 V and ISC of 0.65 μA were harvested at 80% RH. Because moisture in the air could induce a current between the charged surfaces, the static electricity arising from the TENG–MOHF was readily dispersed at high humidities, leading to a decreased power output, consistent with the previous results.33,54,55
Since continuous supply of moisture from an organo-ionic hydrogel would degrade the TENG performance after long term operation, we examined the TENG performance of PVA/MXene-melamine foam without organo-ionic hydrogel at a relative humidity of 20%, and the results are shown in Fig. S27 (ESI†). The device exhibited VOC and ISC of 80 V and ∼3.8 μA, respectively, comparable to that of TENG–MOHF. The results indicate that the organo-ionic hydrogel in MOHF rarely affected the TENG performance. To validate our argument, we employed a MOHF with organo-ionic hydrogel containing fluorescent rhodamine B allowing the diffusion of moisture from the organo-ionic hydrogel under UV exposure. The results in Fig. S28 (ESI†) show that the diffusion of the moisture for 48 hours evidenced by the fluorescence of Rhodamine B was limited to approximately half of the MOHF, making the top surface of the MOHF intact. The results clearly show that the TENG performance was dominantly affected by the external humidity rather than moisture absorption from an organo-ionic hydrogel. The results anticipate that a complementary MEG–TENG–MOHF could allow the generation of high power under low humidity conditions under which most of the MEGs exhibit low harvesting performance owing to the lack of moisture.
Because of the excellent mechanical flexibility and resilience of our MOHF, a TENG–MOHF can harvest energy under various compression conditions, and the results are presented in Fig. 5e. The VOC of the TENG–MOHF increased from 50 to 110 V and the ISC increased from 2.5 to 7.6 μA as the compression pressure increased from 7.8 to 28.3 kPa. The various pressures were applied to result in the compression of the device from 10 to 52%. Furthermore, the TENG–MOHF exhibited a substantial increase in both VOC and ISC with the increase in the operation frequency from 0.5 to 10 Hz, as shown in Fig. 5f. The enhancement of the device performance at high frequencies was due to the low dispersion of the induced charges in the PFA film and MOHF. Fig. 5g demonstrates the mechanical robustness of our MOHF, which allows us to harvest the triboelectric energy of a TENG–MOHF patched on a shoe, arising from various human motions such as walking, jumping, and running. The mechanical durability of the TENG–MOHF was further confirmed by its reliable TENG performance, even after more than 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 consecutive triboelectric contacts, as shown in Fig. 5h. The power densities of the TENG–MOHF were determined by measuring the VOC and JSC across various external loads ranging from 30 kΩ to 70 MΩ, as depicted in Fig. 5i. These results show that a maximum power density of approximately 30.1 μW cm−2 can be achieved at 30 MΩ (Fig. 5j).
000 consecutive triboelectric contacts, as shown in Fig. 5h. The power densities of the TENG–MOHF were determined by measuring the VOC and JSC across various external loads ranging from 30 kΩ to 70 MΩ, as depicted in Fig. 5i. These results show that a maximum power density of approximately 30.1 μW cm−2 can be achieved at 30 MΩ (Fig. 5j).
Deformable complementary MEG–TENG–MOHF
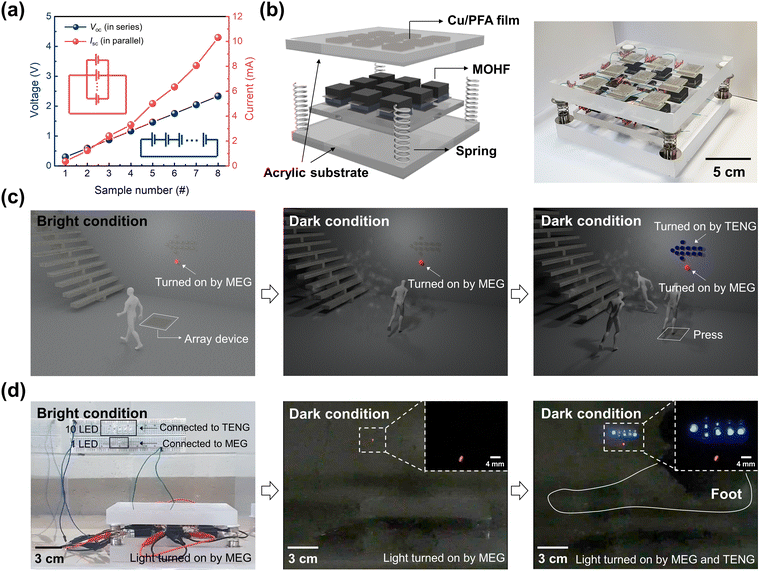
By utilizing the capability of our MOHF for harvesting energy from ambient moisture as well as tribo-contact force, we developed a complementary MEG–TENG–MOHF where both MEG and TENG were integrated in a single device platform with a shared MOHF, as schematically illustrated in Fig. 6a. The silver-coated PET electrode was inserted into the middle of the PVA/MXene-melamine foam within the MOHF. The inserted electrode served as a mutual electrode, functioning as the top electrode for the MEG–MOHF and bottom electrode for the TENG–MOHF. The Cu/PFA tribo-contact electrode (the top electrode of the TENG–MOHF) and the inserted electrode (the bottom electrode of the TENG–MOHF) were connected to two terminals of a bridge rectifier to convert alternating current (AC) into direct current (DC), as schematically shown in Fig. 6b. The remaining two terminals of the bridge rectifier were connected to the top and bottom electrodes of the MEG–MOHF (Fig. 6b). Furthermore, a diode was connected to the bottom electrode of the MEG–MOHF to inhibit the electron leakage during energy generation.56 A complementary MEG–TENG–MOHF was successfully fabricated with the designed circuits, as shown in the photograph in Fig. 6b (Fig. S29, ESI†). | ||
| Fig. 6 Electrical performance of a complementary MEG–TENG–MOHF. (a) Schematic illustrating the device architecture of the complementary MEG–TENG–MOHF. (b) Circuit diagram and photograph of the complementary MEG–TENG–MOHF used for powering the load device. Comparison of (c) VOC and (d) ISC for the MEG–MOHF, TENG–MOHF, and complementary MEG–TENG–MOHF. (e) Dependence of the VOC and JSC of complementary MEG–TENG–MOHF on the load resistances at 20% RH. (f) Power density calculated from the results in (e). (g) Radial plot of the voltage, current, power density, tensile strength, and compressive strength for our complementary MEG–TENG–MOHF (see Table S1, ESI†). (h) Capacitor charging characteristics of each energy generator using a 4.7 μF capacitor. | ||
The electrical performance of our complementary MEG–TENG–MOHF was evaluated at an RH of 20%, and the results are shown in Fig. 6c and d. The complementary MEG–TENG–MOHF exhibited a VOC and ISC of approximately 55 V and 100 μA, respectively, accompanied by intermittent peaks at regular intervals. As noted from the electrical performances of the MEG–MOHF and TENG–MOHF obtained at the same time for comparison, the high VOC and ISC in the complementary MEG–TENG–MOHF could be ascribed to the high output voltage of the TENG–MOHF and high current of the MEG–MOHF, respectively. Bright light was emitted from two commercial LEDs connected in series with our complementary MEG–TENG–MOHF, while no or poor light emission was observed from the MEG–MOHF and TENG–MOHF (Fig. S30, ESI†). In general, the power density values of both MEG and TENG were determined when the external resistance was matched with that of each device. For most TENGs, insulating layers are often employed for boosting their outputs, making their resistance greater than the tens of MΩ, as shown in the plot of Fig. S31 (ESI†). On the other hand, the resistance values of most MEGs are less than 1 MΩ due to the conductive components in the devices for enhancing electrical current (Fig. S31, ESI†). For our MEG–MOHF and TENG–MOHF, the maximum power densities are achieved at 50 kΩ and 30 MΩ, respectively. The power densities of the complementary MEG–TENG–MOHF were evaluated from the load-resistance-dependent output voltages and current densities of the device, and the results are presented in Fig. 6g and f. Across a wide range of load resistances from 1 kΩ to 70 MΩ, the average power density of our complementary MEG–TENG–MOHF was determined to be approximately 33.6 μW cm−2. The maximum power density achieved was approximately 83 μW cm−2 at 700 kΩ, as shown in Fig. 6f.
We also measured the power density values of our MEG–TENG–MOHF as a function of the relative humidity, and the results are shown in Fig. S32 (ESI†). The power density of 83 μW cm−2 at 20% humidity (see Fig. 6f) was slightly decreased to approximately 70.2 μW cm−2 at 40% humidity. The power density values were further decreased to approximately 50.4 μW cm−2 and 34.5 μW cm−2, at the humidity of 60 and 80%, respectively. We believe that the decrease in power density with humidity was mainly ascribed to the degradation of the TENG performance with humidity. It should be noted that the power density value of the MEG–TENG–MOHF at the humidity of 80% (34.5 μW cm−2) was greater than that of the TENG obtained at the humidity of 20% (a maximum power density of approximately 30.1 μW cm−2). It is also worth noting that the maximum power density and mechanical performance of our complementary device is superior to the performances of the recent MEGs (Fig. 6g and Table S1, ESI†). The power density is comparable with that obtained from solar and other MEG hybrid devices (Table S1, ESI†). Moreover, our complementary MEG–TENG–MOHF with excellent mechanical flexibility and resilience is suitable for applications associated with large mechanical force, as will be shown later.
To further explore the synergy of energy-storage capabilities of MEG–MOHF and TENG in our complementary MEG–TENG–MOHF device, we performed charging experiments with a 4.7 μF capacitor, and the results are shown in Fig. 6h. In the capacitor charging curve of the complementary device, the initial voltage of the capacitor rapidly reached approximately 0.25 V within 1–2 s. Subsequently, the voltage increased almost linearly over time, reaching approximately 0.9 V after 80 s. The charging curve of the MEG–MOHF showed a rapid increase in the charge voltage to 0.25 V in a few seconds. The charging from the TENG–MOHF occurred almost linearly with time, reaching the charging voltage of approximately 0.8 V after 80 s (Fig. 6h). The high capacitor-charging performance of the complementary MEG–TENG–MOHF was explained by the rapid initial voltage increase of the capacitor, a result of the DC characteristics of the MEG–MOHF, followed by the subsequent charging of the device, facilitated by the TENG–MOHF part. The high charging performance of our complementary harvester was facilitated in capacitors with higher capacities (Fig. S33, ESI†). In addition, the reliable charging and discharging characteristics of a complementary MEG–TENG–MOHF were confirmed (Fig. S34, ESI†).
Self-powered emergency-exit guidance system with deformable complementary MEG–TENG–MOHF
For practical applications suitable with our deformable complementary MEG–TENG–MOHF, it is necessary to scale up the power output of the system. Fig. 7a shows the connection of multiple MEG–TENG–MOHFs (up to eight devices) in series or parallel to amplify the output voltage and current, respectively, in MEG mode, thereby enabling the operation of diverse commercial electronic products (Fig. S35a–c, ESI†). Based on the long-term energy harvesting performance of our MEG–MOHF, we also demonstrated the long-term operational capability of a calculator connected to eight MEG–MOHF units in series. The calculator was turned on for 15 minutes after which the device was turned off due to the discharge of the power. After charging, the calculator was turned on again (Fig. S35d, ESI†). To capitalize on the mechanical resilience of our complementary MEG–TENG–MOHF, which can withstand human weight, we propose a novel self-powered emergency-exit guidance system, which can visually guide people to an emergency exit when needed. 3 × 3 arrays of complementary MEG–TENG–MOHFs were fabricated on an acrylate substrate with Cu/PFA films separated from the arrays of MOHFs with four springs, as shown in the schematic and photograph in Fig. 7b. In the array system, nine MEG–MOHF units were connected in series, producing continuous moisture-driven DC output power, while the TENG–MOHF units were connected in parallel, producing high-power AC output from the transient human walking motion.Fig. 7c demonstrates the scenario of the implementation of our self-powered emergency-exit guidance system. Under normal conditions, an LED sign near the exit is always ON, driven by the continuous DC power from the arrays of MEG–MOHFs; however, this sign is not sufficiently bright owing to the relatively low output power of the MEG. In emergency situations such as fires, where indoor lighting is limited, the MEG-driven light can serve as an initial guide toward the emergency exit, allowing nearby individuals to follow the light and escape. When the individuals follow the sign light and step on our array system located near the exit while evacuating, the arrays of TENG–MOHFs begin to generate additional output power, from the repetitive contacts of the Cu/PFA plates on MOHFs, giving rise to additional visual guidance toward the exit, as schematically shown in Fig. 7c. When more individuals move toward the exit, more power is generated from the array system with more vivid exit guidance, allowing for development of a self-powered and self-perpetuating system for effective evacuation.
A proof-of-the-concept of the self-powered emergency exit guidance system was developed using arrays of our complementary MEG–TENG–MOHFs, and the results are presented in Fig. 7d. A single red LED is connected to the circuit of arrays of MEG–MOHF units while 10 blue LEDs arranged in the shape of an arrow are connected to the circuit of arrays of the TENG–MOHF units. Under bright conditions, the red light emitted by the MEG–MOHF array is hardly visible, as shown in the left image of Fig. 7d; however, it becomes easily noticeable in the simulated emergency with the surrounding lights turned off. Subsequently, when the array system is repetitively stepped on by foot, the arrow shaped blue LEDs are turned on by the output power produced by the arrays of TENG–MOHFs, significantly enhancing the perception of lights, as shown in the right photograph in Fig. 7d. The entire simulation process is demonstrated in detail in Movie S1 (ESI†).
Conclusions
We demonstrated a highly deformable energy harvester capable of harvesting both moisture-induced electricity and triboelectricity in a single cell. The complementary moisture-induced and triboelectric energy harvesting was accomplished with MXene/organo-ionic hydrogel foam (MOHF) in which positive-ion-selective 2D MXene flakes were deposited on 3D melamine foam, followed by the partial coating of an organo-ionic hydrogel on the MXene-melamine foam. The energy-harvesting capability of our MOHFs was systematically investigated in the MEG mode, particularly, under various severe mechanical deformations including knotting, bending, and folding. In addition, the mechanically robust MOHFs allowed for the development of high-performance TENGs with MOHFs as tribo-contact layers. By capitalizing on the energy harvesting in both MEG and TENG enabled with our MOHFs, a complementary MEG–TENG device gave rise to its performance of a maximum voltage and current of 55 V and 102 μA, respectively, and a high electric power of approximately 83 μW cm−2 with a fast capacitor charging capability. The harvesting performance was facilitated with the excellent mechanical properties of stretchability and compression strength of approximately 30% and 2.1 MPa, respectively. Moreover, the arrays of deformable complementary MEG–TENG harvesters were employed as a novel optical emergency-exit guidance system in which a DC light of an alarm sensor powered in the MEG mode was readily combined with an additional AC-powered light turned on by the walking motions of humans evacuating in an emergency, providing effective guidance for the people to an exit. This work offers an efficient route for harvesting energy from multiple sources, making our complementary harvester potentially suitable for a variety of applications in self-powered high mechanic electronics.Experimental section
Materials
The melamine foam used was a commercially available product in Korea. Acrylamide (≥99%), N,N′-methylenebis(acrylamide) (MBAA, 98%), ammonium persulfate (APS, 98%), glycerol (≥99.5%), KCl, NaCl, CaCl2, MgCl2, and poly(vinyl alcohol) (PVA, Mw = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–70
000–70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) were purchased from Sigma-Aldrich, Korea. The MXene suspension (10 mg ml−1) was purchased from XinXi Technology Co., Ltd (Foshan, China). Deionized (DI) water (18.3 MΩ cm) was prepared using a reverse-osmosis water system (Human Corporation, Korea). The 25 μm thick perfluoroalkoxy alkane (PFA) was purchased from Alphaflon, Korea.
000) were purchased from Sigma-Aldrich, Korea. The MXene suspension (10 mg ml−1) was purchased from XinXi Technology Co., Ltd (Foshan, China). Deionized (DI) water (18.3 MΩ cm) was prepared using a reverse-osmosis water system (Human Corporation, Korea). The 25 μm thick perfluoroalkoxy alkane (PFA) was purchased from Alphaflon, Korea.
Preparation of melamine-foam coated with MXene and PVA (PVA/MXene-melamine foam)
The preparation of the PVA/MXene-melamine foam involved a dip-coating method. Initially, the melamine foam was cut into cubes of dimension 20 × 20 × 10 mm3. The foam was then immersed in MXene solution diluted in DI water (1.0 mg ml−1) for 10 min and was squeezed several times to ensure that the solution permeated well into the foam. The wet melamine foam was dried overnight at 60 °C in an oven. Subsequently, the dried sample was dipped in a PVA solution (0.1 wt%) for 10 min. The final product, PVA/MXene-melamine foam, was obtained after completely drying overnight in an oven at 60 °C.Preparation of MXene/organo-ionic hydrogel foam (MOHF)
Acrylamide monomers (1.54 g), MBAA cross-linker (0.006 g), and APS initiator (0.016 g) were dissolved in a solvent containing a mixture of 10 ml glycerol and DI water in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 volume ratio. The solution was stirred magnetically for 30 min, and KCl was added to the solution to obtain various concentrations. Then, 1 ml of the solution was poured into a custom-designed mold, and the prepared PVA/MXene-melamine foam was placed on top to allow infusion of the organo-ionic hydrogel solution into the lower part through capillary action. Finally, the solution was thermally treated on a hot plate at 85 °C for 10 minutes until the viscous solution gradually solidified, forming the MOHF.
2 volume ratio. The solution was stirred magnetically for 30 min, and KCl was added to the solution to obtain various concentrations. Then, 1 ml of the solution was poured into a custom-designed mold, and the prepared PVA/MXene-melamine foam was placed on top to allow infusion of the organo-ionic hydrogel solution into the lower part through capillary action. Finally, the solution was thermally treated on a hot plate at 85 °C for 10 minutes until the viscous solution gradually solidified, forming the MOHF.
Characterization & measurement
The surface morphology and crystallinity of the melamine foam and PVA/MXene-melamine foam were examined using field-emission scanning electron microscopy (FE-SEM) at an acceleration voltage of 10.0 kV (JEOL, JSM-7001F) and high-resolution X-ray diffraction (HR-XRD, Rigaku, SmartLab). The coated layer of MXene and PVA on the melamine foam was confirmed through transmission electron microscopy (TEM, JEOL, JEM-ARM 200F). The chemical compositions of the PVA/MXene-melamine foam and its components were studied using X-ray photoelectron spectroscopy (XPS, Thermo U. K., K-alpha). The zeta potential of the PVA/MXene-melamine foam was measured using a zeta potential analyzer (Otsuka Electronics, ELS-1000ZS). Numerical simulations of the concentration distribution of K+ over time in the MOHF were conducted using finite element analysis (FEA) software (COMSOL, Inc., COMSOL Multiphysics 5.6). The mechanical properties were evaluated on a universal testing machine (UTM, Instron, Instron 3366) with a 50 N load cell. Strip-shaped samples sized 40 × 10 × 4 mm3 were axially stretched at a loading rate of 10 mm min−1 with a gauge length of 20 mm between the clamps. Additionally, a sample of size 20 × 20 × 10 mm3 was vertically compressed at a loading rate of 10 mm min−1 for compressive testing. The VOC and ISC of the MEG–MOHF, TENG–MOHF, and complementary MEG–TENG–MOHF were obtained using a commercial multimeter, Keithley 6514 electrometer, and Keithley 6485 picoammeter. The RH within a controlled environment was maintained in the homemade acrylic humidity chamber. To establish a controlled temperature range below freezing (−20 °C) to 60 °C, a refrigerator and hot plate were utilized in the experimental setup. The transport of ions and changes in the surface potential following the generation from the MEG–MOHF were analyzed using energy-dispersive spectrometry (EDS, JEOL, JSM-7610F-Plus) and Kelvin probe force microscope (KPFM) characterization (Park Systems, NX-10).Author contributions
G. K. conceived the idea and designed experiments. G. K. conducted the overall experiment and characterization and analyzed the results. J. W. L. designed the circuit for electrical output. K. Z. and T. K. gave advice on electrical measurements. W. K. conducted mechanical characterization and performed analysis. J. W. O., K. L. and J. J. contributed to development of custom-built system for applications. G. Z. and J. W. P. helped with interpreting the electrical properties based on the types of ions. S. L. and Y. K. carried out analysis of Ti3C2Tx MXene characterization. W. J. and S. L. were primarily responsible for the experiments and material preparation. C. P. supervised the entire research and wrote the first draft of the paper, and all authors discussed the results and contributed to the draft and revision of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the Creative Materials Discovery Program and the Pioneer Research Center Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2018M3D1A1058536 and NRF-2022M3C1A3081211). This study was also supported by a grant from the National Research Foundation of Korea (NRF) funded by the Korean government (MEST) (No. RS-2023-00208577). This work was partially supported by the National R&D Program through the National Research Foundation of Korea funded by the Ministry of Science and ICT (2021M3H4A1A03047331). This work was also supported by the Open Resource Research Program of the Korea Institute of Science and Technology (2E31551). This research was also supported by “Regional Innovation Strategy (RIS)” through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE) (2022RIS-005).Notes and references
- G. Xue, Y. Xu, T. Ding, J. Li, J. Yin, W. Fei, Y. Cao, J. Yu, L. Yuan, L. Gong, J. Chen, S. Deng, J. Zhou and W. Guo, Nat. Nanotechnol., 2017, 12, 317–321 CrossRef CAS PubMed.
- Y. Qin, Y. Wang, X. Sun, Y. Li, H. Xu, Y. Tan, Y. Li, T. Song and B. Sun, Angew. Chem., Int. Ed., 2020, 59, 10619–10625 CrossRef CAS PubMed.
- D. Shen, W. W. Duley, P. Peng, M. Xiao, J. Feng, L. Liu, G. Zou and Y. N. Zhou, Adv. Mater., 2020, 32, 2003722 CrossRef PubMed.
- H. Wang, T. He, X. Hao, Y. Huang, H. Yao, F. Liu, H. Cheng and L. Qu, Nat. Commun., 2022, 13, 2524 CrossRef CAS PubMed.
- X. Zhang, M. Wang, Y. Wu, X. Chen, K. Wu, Q. Fu and H. Deng, Adv. Funct. Mater., 2023, 33, 2210027 CrossRef CAS.
- H. Cheng, Y. Huang, F. Zhao, C. Yang, P. Zhang, L. Jiang, G. Shi and L. Qu, Energy Environ. Sci., 2018, 11, 2839–2845 RSC.
- Z. Sun, L. Feng, X. Wen, L. Wang, X. Qin and J. Yu, Mater. Horiz., 2021, 8, 2303–2309 RSC.
- C. Liu, S. Wang, X. Wang, J. Mao, Y. Chen, N. X. Fang and S. P. Feng, Energy Environ. Sci., 2022, 15, 2489–2498 RSC.
- H. Wang, Y. Sun, T. He, Y. Huang, H. Cheng, C. Li, D. Xie, P. Yang, Y. Zhang and L. Qu, Nat. Nanotechnol., 2021, 16, 811–819 CrossRef CAS PubMed.
- L. Li, M. Hao, X. Yang, F. Sun, Y. Bai, H. Ding, S. Wang and T. Zhang, Nano Energy, 2020, 72, 104663 CrossRef CAS.
- K. Liu, P. Yang, S. Li, J. Li, T. Ding, G. Xue, Q. Chen, G. Feng and J. Zhou, Angew. Chem., Int. Ed., 2016, 128, 8135–8139 CrossRef.
- Y. Huang, H. Cheng, C. Yang, H. Yao, C. Li and L. Qu, Energy Environ. Sci., 2019, 12, 1848–1856 RSC.
- Y. Huang, H. Cheng, C. Yang, P. Zhang, Q. Liao, H. Yao, G. Shi and L. Qu, Nat. Commun., 2018, 9, 4166 CrossRef PubMed.
- J. Garemark, F. Ram, L. Liu, I. Sapouna, M. F. Cortes Ruiz, P. T. Larsson and Y. Li, Adv. Funct. Mater., 2023, 33, 2208933 CrossRef CAS.
- B. Shao, Z. Song, X. Chen, Y. Wu, Y. Li, C. Song, F. Yang, T. Song, Y. Wang, S. T. Lee and B. Sun, ACS Nano, 2021, 15, 7472–7481 CrossRef CAS PubMed.
- S. Lee, H. Jang, H. Lee, D. Yoon and S. Jeon, ACS Appl. Mater. Interfaces, 2019, 11, 26970–26975 CrossRef CAS PubMed.
- X. Liu, H. Gao, J. E. Ward, X. Liu, B. Yin, T. Fu, J. Chen, D. R. Lovley and J. Yao, Nature, 2020, 578, 550–554 CrossRef CAS PubMed.
- J. Liu, L. Huang, W. He, X. Cai, Y. Wang, L. Zhou and Y. Yuan, Nano Energy, 2022, 102, 107709 CrossRef CAS.
- T. Xu, X. Ding, Y. Huang, C. Shao, L. Song, X. Gao, Z. Zhang and L. Qu, Energy Environ. Sci., 2019, 12, 972–978 RSC.
- Q. Ma, Q. He, P. Yin, H. Cheng, X. Cui, Q. Yun and H. Zhang, Adv. Mater., 2020, 32, 2003720 CrossRef CAS PubMed.
- D. Lv, S. Zheng, C. Cao, K. Li, L. Ai, X. Li, Z. Yang, Z. Xu and X. Yao, Energy Environ. Sci., 2022, 15, 2601–2609 RSC.
- C. Shao, B. Ji, T. Xu, J. Gao, X. Gao, Y. Xiao, Y. Zhao, N. Chen, L. Jiang and L. Qu, ACS Appl. Mater. Interfaces, 2019, 11, 30927–30935 CrossRef CAS PubMed.
- S. Yang, X. Tao, W. Chen, J. Mao, H. Luo, S. Lin, L. Zhang and J. Hao, Adv. Mater., 2022, 34, 2200693 CrossRef CAS PubMed.
- Y. Zhang, S. Guo, Z. G. Yu, H. Qu, W. Sun, J. Yang, L. Suresh, X. Zhang, J. J. Koh and S. C. Tan, Adv. Mater., 2022, 34, 2201228 CrossRef CAS PubMed.
- J. Bai, Y. Huang, H. Wang, T. Guang, Q. Liao, H. Cheng, S. Deng, Q. Li, Z. Shuai and L. Qu, Adv. Mater., 2022, 34, 2103897 CrossRef CAS PubMed.
- J. Bai, Q. Liao, H. Yao, T. Guang, T. He, H. Cheng and L. Qu, Energy Environ. Sci., 2023, 16, 3088–3097 RSC.
- Z. Li, X. Ma, D. Chen, X. Wan, X. Wang, Z. Fang and X. Peng, Adv. Sci., 2021, 8, 2004552 CrossRef CAS PubMed.
- C. Park, M. Koo, G. Song, S. M. Cho, H. S. Kang, T. H. Park, E. H. Kim and C. Park, ACS Nano, 2020, 14, 755–766 CrossRef CAS PubMed.
- R. Zhang, C. Dahlström, H. Zou, J. Jonzon, M. Hummelgård, J. Örtegren, N. Blomquist, Y. Yang, H. Andersson, M. Olsen, M. Norgren, H. Olin and Z. L. Wang, Adv. Mater., 2020, 32, 2002824 CrossRef CAS PubMed.
- S. Bairagi, S. Islam, M. Shahadat, D. M. Mulvihill and W. Ali, Nano Energy, 2023, 111, 108414 CrossRef CAS.
- K. Zhao, J. W. Lee, Z. G. Yu, W. Jiang, J. W. Oh, G. Kim, H. Han, Y. Kim, K. Lee, S. Lee, H. Y. Kim, T. Kim, C. E. Lee, H. Lee, J. Jang, J. W. Park, Y. W. Zhang and C. Park, ACS Nano, 2023, 17, 5472–5485 CrossRef CAS PubMed.
- W. Zhang, X. Chen, J. Zhao, X. Wang, X. Li, T. Liu, B. Luo, Y. Qin, S. Zhang, M. Chi, S. Wang and S. Nie, Nano Energy, 2023, 108, 108196 CrossRef CAS.
- Y. T. Jao, P. K. Yang, C. M. Chiu, Y. J. Lin, S. W. Chen, D. Choi and Z. H. Lin, Nano Energy, 2018, 50, 513–520 CrossRef CAS.
- Y. Long, Z. Wang, F. Xu, B. Jiang, J. Xiao, J. Yang, Z. L. Wang and W. Hu, Small, 2022, 18, 2203956 CrossRef CAS PubMed.
- Z. Wang, R. Jiang, G. Li, Y. Chen, Z. Tang, Y. Wang, Z. Liu, H. Jiang and C. Zhi, ACS Appl. Mater. Interfaces, 2017, 9, 22685–22693 CrossRef CAS PubMed.
- S. Lee, E. H. Kim, S. Yu, H. Kim, C. Park, T. H. Park, H. Han, S. W. Lee, S. Baek, W. Jin, C. M. Koo and C. Park, Adv. Funct. Mater., 2020, 30, 2001224 CrossRef CAS.
- X. Zhu, F. Zhang, L. Zhang, L. Zhang, Y. Song, T. Jiang, S. Sayed, C. Lu, X. Wang, J. Sun and Z. Liu, Adv. Funct. Mater., 2018, 28, 17050515 Search PubMed.
- S. Lee, E. H. Kim, S. Yu, H. Kim, C. Park, S. W. Lee, H. Han, W. Jin, K. Lee, C. E. Lee, J. Jang, C. M. Koo and C. Park, ACS Nano, 2021, 15, 8940–8952 CrossRef CAS PubMed.
- J. Xue, L. Zhu, X. Zhu, H. Li, C. Ma, S. Yu, D. Sun, F. Xia and Q. Xue, Sep. Purif. Technol., 2021, 259, 1181606 CrossRef.
- Y. Cao, Q. Deng, Z. Liu, D. Shen, T. Wang, Q. Huang, S. Du, N. Jiang, C. Te Lin and J. Yu, RSC Adv., 2017, 7, 20494–20501 RSC.
- B. Zuo, Y. Hu, X. Lu, S. Zhang, H. Fan and X. Wang, J. Phys. Chem. C, 2013, 117, 3396–3406 CrossRef CAS.
- A. M. Bakry, F. S. Awad, J. A. Bobb, A. A. Ibrahim and M. S. El-Shall, RSC Adv., 2020, 10, 37883–37897 RSC.
- H. Liao, X. Guo, P. Wan and G. Yu, Adv. Funct. Mater., 2019, 29, 1904507 CrossRef.
- A. Nawaz, C. De Col and I. A. Hömmelgen, Mater. Res., 2016, 19, 1201–1206 CrossRef CAS.
- P. He, J. Wu, X. Pan, L. Chen, K. Liu, H. Gao, H. Wu, S. Cao, L. Huang and Y. Ni, J. Mater. Chem. A, 2020, 8, 3109–3118 RSC.
- A. K. Fard, G. Mckay, R. Chamoun, T. Rhadfi, H. Preud’Homme and M. A. Atieh, Chem. Eng. J., 2017, 317, 331–342 CrossRef CAS.
- B. Shao, Y. Wu, Z. Song, H. Yang, X. Chen, Y. Zou, J. Zang, F. Yang, T. Song, Y. Wang, M. Shao and B. Sun, Nano Energy, 2022, 94, 106917 CrossRef CAS.
- L. Li, S. Feng, Y. Bai, X. Yang, M. Liu, M. Hao, S. Wang, Y. Wu, F. Sun, Z. Liu and T. Zhang, Nat. Commun., 2022, 13, 1043 CrossRef CAS PubMed.
- F. H. J. Van Der Heyden, D. J. Bonthuis, D. Stein, C. Meyer and C. Dekker, Nano Lett., 2006, 6, 2232–2237 CrossRef CAS PubMed.
- J. Bae, M. S. Kim, T. Oh, B. L. Suh, T. G. Yun, S. Lee, K. Hur, Y. Gogotsi, C. M. Koo and I. D. Kim, Energy Environ. Sci., 2022, 15, 123–135 RSC.
- B. X. Zhang, Z. L. Hou, W. Yan, Q. L. Zhao and K. T. Zhan, Carbon, 2017, 125, 199–206 CrossRef CAS.
- V. U. Somkuwar, A. Pragya and B. Kumar, J. Mater. Sci., 2020, 55, 5177–5189 CrossRef CAS.
- Y. Liu, Y. Zheng, Z. Wu, L. Zhang, W. Sun, T. Li, D. Wang and F. Zhou, Nano Energy, 2021, 79, 105422 CrossRef CAS.
- J. H. Zhang, Y. Li, J. Du, X. Hao and Q. Wang, Nano Energy, 2019, 61, 486–495 CrossRef CAS.
- V. Vivekananthan, N. R. Alluri, Y. Purusothaman, A. Chandrasekhar, S. Selvarajan and S. J. Kim, ACS Appl. Mater. Interfaces, 2018, 10, 18650–18656 CrossRef CAS PubMed.
- Z. Wen, M.-H. Yeh, H. Guo, J. Wang, Y. Zi, W. Xu, J. Deng, L. Zhu, X. Wang, C. Hu, L. Zhu, X. Sun and Z. L. Wang, Sci. Adv., 2016, 2, e1600097 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ee03052k |
| This journal is © The Royal Society of Chemistry 2024 |