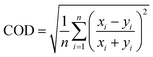Open Access Article
Open Access ArticleUrban and Remote cheMistry modELLing with the new chemical mechanism URMELL: part I gas-phase mechanism development†
Marie Luise
Luttkus
 *a,
Erik Hans
Hoffmann
*a,
Erik Hans
Hoffmann
 b,
Andreas
Tilgner
b,
Andreas
Tilgner
 b,
Ralf
Wolke
b,
Ralf
Wolke
 a,
Hartmut
Herrmann
a,
Hartmut
Herrmann
 b and
Ina
Tegen
b and
Ina
Tegen
 a
a
aDepartment Modeling of Atmospheric Processes, Leibniz Institute for Tropospheric Research (TROPOS), Permoserstr. 15, 04318 Leipzig, Germany. E-mail: luttkus@tropos.de
bAtmospheric Chemistry Department (ACD), Leibniz Institute for Tropospheric Research (TROPOS), Permoserstr. 15, 04318 Leipzig, Germany
First published on 3rd January 2024
Abstract
Air quality is a globally pressing issue as it poses a major threat for human health and ecosystems. Non-methane volatile organic compounds (NMVOCs) are highly reactive substances and known for their impact on O3, HOx (OH + HO2) and NOx (NO + NO2) concentrations. NMVOCs comprise a variety of anthropogenic and biogenic compounds with highly complex and entangled relations. Therefore, it is key to capture these interdependencies for any air quality assessment through modeling. Unfortunately, chemical mechanisms used for air quality modeling are often too simplified and partly outdated. Here, we present the development of the chemical mechanism URMELL (Urban and Remote cheMistry modELLing) comprising an extended chemical treatment of major anthropogenic and biogenic NMVOCs based on current knowledge. Box model simulations of standardized urban and remote conditions were performed with URMELL and other mechanisms, and the obtained concentration time profiles of key compounds were compared. High correlations (>0.9) with the benchmark mechanism MCMv3.3.1 are found for all urban conditions. For remote conditions, the simulations using URMELL have much higher oxidant concentrations, especially for OH reaching concentrations ∼106 molecules per cm3 which is in the same range of measured ambient OH concentrations at remote isoprene-dominated sites. For further evaluation, URMELL was applied in the chemical transport model COSMO-MUSCAT and simulations for Germany in May 2014 were performed. Modeled O3, NO and NO2 concentrations were compared with 57 measurement sites indicating improved ozone correlations for urban as well as remote isoprene-influenced sites than the currently applied mechanism.
Environmental significanceThe chemical degradation of anthropogenic and biogenic NMVOCs differs significantly implying individual impacts on air pollutants. Future emission regulations will lead to a decline of anthropogenic sources whereas biogenic sources will increase, due to climate warming and climate mitigation strategies such as tree planting programs. Therefore, the importance of biogenic NMVOCs such as isoprene with respect to air quality measures will increase and will require a more comprehensive treatment in chemical transport models than is currently the case. To this aim, a gas-phase chemistry mechanism for regional CTMs was developed with extended treatment for key biogenic and anthropogenic NMVOCs. This study highlights specific important adjustments through sensitivity studies and quantifies the significant increases in isoprene-dominated remote OH and O3 concentrations. |
1 Introduction
Non-methane volatile organic compounds (NMVOCs) are emitted into the troposphere by various anthropogenic (AVOC) and biogenic (BVOC) sources. Once emitted, they initiate complex chemical and photochemical degradation chains impacting tropospheric chemistry and thus the gas- as well as particle-phase composition. The multistep oxidation of NMVOCs therefore, impacts regional air quality1–4 on a short term and the climate5,6 on a larger timescale. NMVOC oxidation is coupled with the NOx (NOx = NO + NO2) and HOx (HOx = OH + HO2) budget and thus affects both directly and/or indirectly O3 as well as secondary organic aerosol (SOA) formation.4,7–10 As SOA formation can significantly impact the aerosol particle number, mass concentration and chemical composition, NMVOC oxidation affects the physico-chemical properties of clouds and thus cloud and precipitation formation processes.6,11–17Moreover, the oxidation of NMVOCs impacts the atmospheric oxidizing capacity, especially the OH concentration. This has major effects on the lifetime of numerous other trace gases including SO2, CO and the greenhouse gas methane which all favor OH as reaction partner. However, the extent of NMVOC chemistry induced perturbations depend on the atmospheric environment in which the oxidation occurs. Accordingly, different impacts have to be expected for clean rural and polluted urban/industrialized areas.18–21 Progressing NMVOC oxidation leads to the production of highly oxidized products with multifunctional groups: under high NOx conditions, organic nitrates can form,22,23 while in cleaner environments, products with multiple hydroperoxy (OOH), carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and/or hydroxy (OH) groups form.24,25 The formation of organic nitrates, in particular PAN, can be an important NOx reservoir on the one hand decreasing NOx concentrations in a certain area. On the other hand, due to atmospheric transport and decomposition processes, PANs can increase NOx concentrations in other less polluted areas. Under low NOx conditions, the same holds for highly oxidized organic molecules (HOMs) which affect the HOx budget equally. But, in contrast to PANs, HOMs are important SOA sources.
O) and/or hydroxy (OH) groups form.24,25 The formation of organic nitrates, in particular PAN, can be an important NOx reservoir on the one hand decreasing NOx concentrations in a certain area. On the other hand, due to atmospheric transport and decomposition processes, PANs can increase NOx concentrations in other less polluted areas. Under low NOx conditions, the same holds for highly oxidized organic molecules (HOMs) which affect the HOx budget equally. But, in contrast to PANs, HOMs are important SOA sources.
The majority of NMVOCs are BVOCs emitted from plants.6,26 Globally, the most abundant BVOC is isoprene (C5H8) with estimated 535 Tg per year.26 Therefore, CTMs have to describe its chemistry accurately, even if only treating parameterized reaction sequences. Otherwise the CTMs can fail to predict concentrations of adjunct chemical systems. During the last decades, a discrepancy between modeled and measured HOx concentration was identified in remote isoprene-rich environments.9,10,28 Thus, a lot of efforts have been made to improve our knowledge of isoprene chemistry and to adapt the applied gas-phase chemistry mechanisms.9,10,27–30
To capture BVOC effects on the gas- and particle-composition of the troposphere also mono- and sesquiterpene chemistry have to be described adequately, too. Sesquiterpenes account for about 3% (29 Tg per year) of total BVOC emissions and therefore only play a minor role.26 For β-caryophyllene, the most abundant sesquiterpene, a reduced mechanism has been published by Khan et al.31 including the determination of SOA precursor substances. Monoterpenes account for about 20% (162 Tg per year) of global BVOC emissions,26 but are highly plant species-specific and thus depend on vegetation cover. Their influence on atmospheric chemistry intensifies poleward, as coniferous forests emit less isoprene but higher quantities of monoterpenes. The principal NOx/O3 interrelations are similar to isoprene while the final ozone forming potentials differ.32–34 Moreover, monoterpenes have a higher SOA formation potential and dominate the SOA composition in coniferous forests.12,35,36 So far, only a few lumped monoterpene species are currently included in chemical mechanisms of CTMs.37–43 Recent research suggests, that even within these lumped clusters the SOA forming potential can vary significantly between different monoterpenes.24,25,44–48 However, as this is still an open research field and new findings emerge frequently, the present study mainly remains focused on the most abundant BVOC isoprene.
Considering the fact that most people live in cities and the number of city dwellers is still climbing, anthropogenic emission sources are also of key importance, especially in terms of urban air quality. Cities face new challenges in the near future of yet unknown consequences, due to air quality measures, climate change and mitigation strategies. In urban areas, air quality measures such as changes in the car fleet will decrease NOx and AVOC emissions in the future. At the same time an increase in urban green infrastructure is favored by urban development planning due to climate mitigation measures. Thus, urban environments will likely undergo a shift from AVOC to BVOC emissions. Thereby, urban green infrastructure strategies rest on planting trees. Importantly, the tree species-specific BVOC emissions are additionally impacted by stressors such as heat and drought.49–53 Overall, cities will undergo a change in the near future making it more challenging to determine the sources of air pollutants such as O3 and particular matter (PM), because of growing NMVOC diversity. Hence, as O3, NOx and PM impact the human health and the ecosystem it is of utmost importance to cover a wide variety of VOCs along with their HOx/NOx/O3/SOA interdependencies in CTMs typically used for air quality assessments.
Numerous chemical gas-phase mechanisms with varying complexity are currently available. The most detailed one is the MCM3.3.1,54–59 but due to its high complexity it is not directly applicable in CTMs. Nevertheless, the MCM is often used as reference mechanism when mechanisms with reduced complexity are developed. In CTMs, these condensed mechanisms are favored due to their smaller amount of substances and reactions while remaining a sufficient accuracy and minimizing the computation time. Commonly used CTM mechanisms are: RACM,37 RACM2,38 JAM,39 MOZART,40 the carbon bond mechanism41 and SAPRC.42,43 While all these mechanisms focus on NOx/O3 predictability, the formation of higher oxidized molecules is not described sufficiently enough to enable the inclusion of direct SOA formation. Instead gas-phase chemistry and SOA formation are treated separately, if considered at all.
Next to the chemical mechanism, an accurate description of emission fields is mandatory including vegetative/natural and manmade emissions. This will ensure adequate anthropogenic and biogenic interactions from the origin, processing throughout the transport to the deposition.
To date, the currently applied mechanisms in and emission setups of CTMs are not capable to project these problems sufficiently, because of issues related to complexity of chemistry mechanisms and deposition schemes, as well as adequate input data such as the description of emission and meteorological fields etc. In order to address these issues, we developed a more complex biogenic emission scheme to capture tree species-specific BVOC emissions within the CTM COSMO-MUSCAT in a previous study.4 As a next step, we developed and applied the new chemistry mechanism URMELL (Urban and Remote cheMistry modELLing), which is presented in this study. URMELL combines recent knowledge of both anthropogenic (e.g. aromatic chemistry) and biogenic (e.g. isoprene chemistry) organic compounds. We present multiple comparisons of various sensitivity box model and CTM simulations that reveal the appropriateness of URMELL for air quality modelling.
2 Mechanism developments
The basis for the URMELL mechanism development is the chemical mechanism JAM version 002b39 applied in the global chemistry-climate model ECHAM6.3-HAM2.3-MOZ1.0. This mechanism is further denoted JAMv2b. JAMv2b is based on the Model of Ozone and Related Tracers (MOZART) v.4 for tropospheric and the Whole Atmosphere Chemistry Climate Model (WACCM) for stratospheric chemistry.40,60 Additionally, Jamv2b also includes a more detailed representation of isoprene by integrating the Mainz Isoprene Mechanisms 2 (MIM-2),29 1,6 H-shift reactions,27 epoxide formation61 and HPALD photolysis.62 Further information about JAMv2b39 can be found in the corresponding publication. Compared to other mechanisms available at the time of publication and initial URMELL mechanism development, JAMv2b is more detailed and treats a larger number of species explicitly whereby reducing the number of lumped species and featuring newer measurement results.So far, RACM-MIM2-ext4,37,63 had been used for air quality assessments in the model framework COSMO-MUSCAT at TROPOS.64–66 Advantageously, the JAMv2b mechanism already contains a more up-to-date chemistry implementation compared to RACM.37,39 However, within the last five years since JAMv2b was published, new findings from laboratory experiments emerged. In order to take them into account, the reactions within this mechanism have been first screened to include the latest recommendations of kinetic reaction rate constants and oxidation products (see Sect. 2.1–2.5 for further details).
However, the major focus of the mechanism development described here has been put on the integration of possible SOA precursor compounds to enable a direct and more explicit SOA approach. Therefore, already existing reaction pathways have been investigated to identify possible reaction chains leading to highly functionalized products with low volatility that are currently not included in JAMv2b. As URMELL is intended to be applied for air quality assessments considering both anthropogenic and biogenic sources for air pollutants, these SOA extensions are considered for their main contributors: aromatics (Sect. 2.4) and isoprene (Sect. 2.5). Here, the focus is on aromatics and isoprene, as a lot of effort has already been made to determine their chemical degradation schemes.
All in all, this study focuses on aromatics and isoprene chemistry updates and extensions leading to an advanced gas-phase mechanism. Finally, this mechanism development aims at a more sophisticated Urban and Remote cheMistry modELLing, from which the designation of the new mechanism URMELL was derived. Species number and complexity was increased substantially by maintaining possible gas-phase SOA compounds in order to enable an explicit SOA formation implementation. Overall, URMELL contains 313 species (listed in Table S1-1†), and 916 reactions (summarized in Tables S1-2 and S1-3†). In Table S1-1,† new species are presented in bold font and new and/or changed reactions in Tables S1-2 and S1-3† are marked in a separate column. The most important changes to JAMv2b are summarized in the following subsections (2.1–2.5). Note while all the described changes apply to JAMv2b, some of the updates might already be considered in other mechanisms to some extent. After a positive follow-up feasibility study in terms of explicit SOA modeling, URMELL will be extended to monoterpenes where feasible, too.
2.1 Important general modifications in URMELL
In the following, a brief description of the most important general modifications in URMELL is provided. All reactions of JAMv2b were first screened to include more recent recommendations of kinetic reaction rate constants as well as oxidation products and photolysis rates, e.g. by using either the database of the IUPAC task group on atmospheric chemical kinetic data evaluation (https://iupac.aeris-data.fr/en/home/)67–74 or the near explicit master chemical mechanism (MCM3.3.1; http://mcm.york.ac.uk/).54–59 These updates already affect the simple Ox, HOx and NOx chemistry, as well as smaller organic compounds such as glyoxal (GLY), methylglyoxal (MGLY) or methyl ethyl ketone (MEK).2.2 Ozonolysis implementation updates
Besides the OH-radical oxidations, the ozonolysis reaction schemes of ethene and propene are updated using the recent IUPAC task group recommendations by Cox et al.73 The ozonolysis of ethene (R-1) and (R-2) yields formaldehyde and the exited Criegee intermediate [CH2OO]* which yields the stable Criegee intermediate CH2OO. Further details on CH2OO chemistry are given in the next section (Sect. 2.3). As for the MCM3.3.1, in URMELL the reaction products HCO and H of (R-2) are considered to immediately react with O2 and turn into CO + HO2 (JPL) and HO2, respectively.| C2H4 + O3 → HCHO + [CH2OO]* | (R-1) |
| [CH2OO]* → 0.17(OH + HCO) + 0.18(CO + H2O) + 0.23CO2 + 0.18H2 + 0.1H + 0.42CH2OO | (R-2) |
The ozonolysis of propene (R-3) yields acetaldehyde (CH3CHO), [CH2OO]*, formaldehyde and two excited Criegee intermediates [E-CH3CHOO]* and [Z-CH3CHOO]* which both can be transferred into stabilized Criegee intermediates E-CH3CHOO and Z-CH3CHOO. For the latter one, thermal decomposition is expected to be the dominating pathway releasing OH and CH2CHO, which oxidation by O2 leads to OOCH2CHO. Following the main reaction pathway of HCOCH2O2 (MCM3.3.1), OOCH2CHO becomes HCHO + HO2 + CO. About 60% of [E-CH3CHOO]* decomposes yielding CH4, CO2, CH3OH, CO, CH2CO and H2O. Subsequent oxidation of CH2CO by OH and O2 (IUPAC) produces CO + HCHO + HO2.77 The remaining 40% form the stabilized Criegee intermediate E-CH3CHOO.
| C3H6 + O3 → 0.38CH3CHO + 0.38[CH2OO]* + 0.62HCHO + 0.32[E-CH3CHOO]* + 0.3[Z-CH3CHOO]* | (R-3) |
Within the next section, the chemistry of the stabilized Criegee intermediates CH2OO and E-CH3CHOO are addressed further. Note, that in Jamv2b the reaction of C3H6 with O3 has a direct CH3O2 production channel and the MCM3.3.1 also produces CH3O2 through the exited Criegee intermediate (CH3CHOOA) channel.
Based on the IUPAC recommendations, the chemical degradation of CH2OO under tropospheric conditions is dominated by the reaction with the water dimer.73 Unfortunately, the two laboratory studies from Sheps et al.78 (ratios presented in bold) and Nguyen et al.79 (ratios given in brackets) provide inconsistent results regarding the branching ratios of the reaction pathways (R-4):
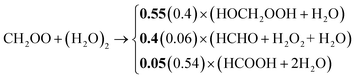 | (R-4) |
Both studies report comparable branching ratios for (R-4) of 0.55 and 0.4, respectively. However, the contributions to (R-4) (0.4 and 0.06) and (R-4) (0.05 and 0.54) are very different. This generates uneven production strengths for formaldehyde (HCHO) and formic acid (HCOOH). Recent studies revealed that potential chemical sources of HCOOH are missing in up-to-date mechanisms.80–82 Therefore, the formation pathways of HCOOH are in the focus of current research, because of its importance for future evolvement of atmospheric acidity. But no recommendations have been done so far for (R-4).73 Therefore, after the main mechanism development was finished, first evaluation simulations were performed (see S2.1 for details). In these sensitivity simulations, either the yields of Nguyen et al.79 or the yields from Sheps et al.78 are applied to examine their effect on the gas-phase compositions predicted by URMELL (see Fig. 2). The sensitivity studies reveal a significant impact on gas-phase HCOOH production. Therefore, it is important to further clarify the branching ratios for this reaction. This would also help to quantify the discrepancies between measured and modeled values.81,82 Finally, the newer ratios from Sheps et al.78 are implemented in URMELL representing the lower limit of HCOOH production from Criegee degradation.
For the newly included oxidation product HOCH2OOH, reactions with OH (R-5)83 and photolysis ((R-6); IUPAC) are implemented. The IUPAC photolysis parameters are transformed into photolysis rate formula parameters as used in URMELL (see Tables S1-3 and S1-4†). The photolysis of HOCH2OOH produces OH and the HOCH2O radical. The reaction of HOCH2OOH with OH generates a hydroxy hydroperoxide radical HOCH2OO, for which several channels are possible (R-7) till (R-10) and included in URMELL following the IUPAC recommendation. For the reaction of HOCH2OO with HO2(R-9) the IUPAC recommendations are uncertain as for one reaction channel two possible formulations exist. Here, we use the recommendation from Jenkin et al.84 including the HOCH2O radical formation rather than the direct production of HCOOH and HO2. For the self-reaction (R-10), only the dominant branch is considered. The most likely degradation channel for the reaction product HOCH2O is reaction with O2 forming HCOOH with HO2(R-11).83
| HOCH2OOH + OH → HOCH2OO, | (R-5) |
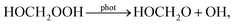 | (R-6) |
| HOCH2OO → HCHO + HO2, | (R-7) |
| HOCH2OO + NO → HOCH2O + NO2, | (R-8) |
| HOCH2OO + HO2 → 0.5HOCH2OOH + 0.3(HCOOH + H2O) + 0.2(OH + HOCH2O) + O2, | (R-9) |
| HOCH2OO + HOCH2OO → 2HOCH2O + O2, | (R-10) |
| HOCH2O + O2 → HCOOH + HO2. | (R-11) |
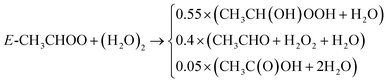 | (R-12) |
Except for the reaction rate constant of CH3CH(OH)OOH with OH (R-13), no separate data was available. Therefore, CH3CH(OH)OOH chemistry (R-14)–(R-19) was adapted analog to HOCH2OOH. According to Chen et al.83 and the IUPAC recommendation, the most likely degradation channel for CH3CH(OH)O is C1–C2 bond separation producing HCOOH and CH3 which reacts with O2 and produces CH3O2(R-19).
| CH3CH(OH)OOH + OH → CH3CH(OH)OO, | (R-13) |
 | (R-14) |
| CH3CH(OH)OO → CH3CHO + HO2, | (R-15) |
| CH3CH(OH)OO + NO → CH3CH(OH)O + NO2, | (R-16) |
| CH3CH(OH)OO + HO2 → 0.5CH3CH(OH)OOH + 0.3(CH3C(O)OH + H2O) + 0.2(OH + CH3CH(OH)O) + O2, | (R-17) |
| CH3CH(OH)OO + CH3CH(OH)OO → 2HOCH2O + O2, | (R-18) |
| CH3CH(OH)O → HCOOH + CH3. | (R-19) |
2.3 Organic acyl peroxy radicals
Further updates relate to branching ratios and rate coefficients of organic acyl peroxy radicals (RC(O)O2) based on recent recommendations from Jenkin et al.85 Accordingly, the reaction of CH3C(O)O2 with HO2(R-20) was adjusted as follows (in bold new ratios in brackets old ratios):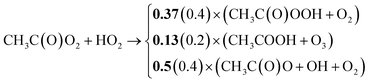 | (R-20) |
The reaction product CH3C(O)O decomposes into CO2 and CH3 and subsequent O2 reaction produces CH3O2. Furthermore, the reaction rate constant changed from 5.2 × 10−13 × exp(980/T) (MCM3.3.1) to 1.73 × 10−12 × exp(730/T). This results in a faster reaction and a higher OH and CH3O2 production (see Fig. 1a). Note Wennberg et al.30 uses the previous IUPAC recommendation before the latest change in preferred values in 2019 occurred resulting in slightly higher kHO2 values than the actual one (see black line in Fig. 1a). The recommendations of Jenkin et al.85 have further been applied to CH3C(O)O2-radical equivalent reactions of RC(O)O2 with HO2 using the Jenkin et al.85 formula kAPHO2. kAPHO2 is visualized in Fig. 1a for 298 K as a function of carbon, nitrogen and oxygen atom number nCON (excluding the peroxy radical oxygen atoms) in gray. With increasing atom number the HO2 reaction rate coefficient increases. For nCON = 3 excellent agreement with the IUPAC recommendation for CH3C(O)O2 is reached (gray dashed line in Fig. 1a).
| RC(O)O2 + CH3O2 → O2 + 0.9CH3O + 0.9RC(O)O + 0.1RC(O)OH + 0.1HCHO | (R-21) |
| RC(O)O2 + CH3C(O)O2 → RC(O)O + CH3C(O)O + O2 | (R-22) |
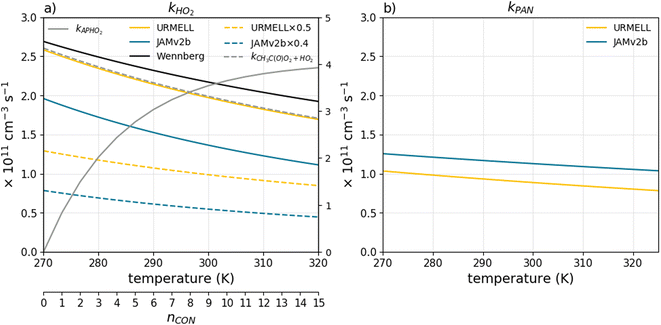 | ||
| Fig. 1 Temperature dependent reaction rate coefficient for (a) the reaction of CH3C(O)O2 with HO2kHO2, as well as the product of kHO2 and the ratio for the third channel producing OH and CH3O2 together with kAPHO2 as a function of nCON and kAPHO2 for CH3C(O)O2; (b) the reaction of CH3C(O)O2 with NO2kNO2 leading to PAN. Moreover, the branching ratios for the reactions of RC(O)O2 with CH3O2(R-21) and CH3C(O)O2(R-22) are adapted based on the IUPAC recommendations for CH3C(O)O2. | ||
CH3O forms HCHO and HO2 after oxidation by O2 (MCM3.3.1). The reaction rate constants of RC(O)O2 with CH3O2, CH3C(O)O2, NO, NO3 and NO2 are also based on IUPAC preferred values for CH3C(O)O2 if not stated otherwise in Table S1-2.†
The reaction of RC(O)O2 radicals with NO2 leads to the formation of PANs. Changes to the reaction rate constant describing PAN formation or decomposition can impact the NOx as well as the O3 budget, as they are closely linked. In URMELL, PAN formation and decomposition follow the IUPAC recommendations, but the rate coefficient for PAN formation ktroe(3.28 × 10−28, −6.87, 0, 1.125 × 10−11, −1.105, 0, 0.3) differs from the one applied in JAMv2b ktroe(2.7 × 10−28, −7.1, 0, 1.2 × 10−11, −0.9, 0, 0.6). For calculation specifications see ESI S1.† Both functions are shown in Fig. 1b for 1 atm (molecular density M = 7.34 × 1021/T(K)) and varying temperature values. A sensitivity study was carried out to investigate the impact of the updated reaction rate constant. An additional sensitivity study was performed addressing the overall change on RC(O)O2 chemistry. For the sake of clarity, the results are discussed in more detail in the ESI S2.2.1.† Both sensitivity studies indicate an increase in O3, OH, HO2, NO, NO2, NO3 and HNO3 peak concentrations for the updated RC(O)O2 reaction pathways.
2.4 Advanced oxidation scheme for aromatic compounds
As outlined before, the present study aims at an improved air quality modeling in urban environments, thus the treatment of anthropogenic SOA sources, e.g. from aromatic compounds was revised and extended. The original JAMv2b mechanism describes the oxidation of benzene, toluene and lumped xylenes as well as their corresponding phenols (PHENOL, CRESOL, XYLOL). The oxidation with OH of these monohydroxy phenols form ring-opening products (peroxyl radicals; BENZO2, TOLO2, XYLO2), lumped aromatic 1,2-diols (CATECHOL) and lumped phenoxy radicals (C6H5O). The latter are currently implemented to yield nitrophenols when reacting with NO2. Nitrophenols are not very reactive towards OH radicals and are thus determined as sticky nitrate compounds.39 Therefore, in the previous studies using JAMv2b, their fate was determined by deposition rather than oxidation processes. However, aromatic compounds with an added hydroxyl group are sensitive towards oxidation by NO3 radicals (IUPAC, MCM3.3.1). Therefore, oxidation by NO3 radicals might determine their oxidative fate in urban polluted environments, where the conditions of high NOx and ozone concentrations enable stronger NO3-radical formation. As the mechanism URMELL is projected to be applied for such environments, the formation of nitro-hydroxy compounds and its further oxidation by OH and NO3 radicals have been included. The same applies for the formation of aromatic nitro-dihydroxy compounds, reaction products of CATECHOL. The further oxidation of these nitro-hydroxy and -dihydroxy products leads to highly oxidized molecules with extremely low vapor pressures.Furthermore, the oxidation of monohydroxy phenols with NO3 form lumped phenoxy radicals (C6H5O), ring-opening products without (PHENO2, CRESO2) and with an ON(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)
O)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group (NPHENOLO2, NCRESO2). The formation of the latter products is handled very differently between different mechanisms and are in general not treated explicitly in reduced mechanisms.37,39,43,86 A sensitivity study, considering three different approaches for the handling of the phenol + NO3 reaction channels can be found in the ESI Sect. S2.2.† In JAMv2b NPHENOLO2 and NCRESO2 are approximated with PHENO2. While this has no significant impact for warmer temperatures and longer solar radiation periods (see Sec. 4.1 summer cases) it gains in importance for colder temperatures and longer nights. Then significant amounts of NO3 can form which facilitate NO3 degradation channels. When including NPHENOLO2 and NCRESO2 their further oxidation by NO, NO3, CH3O2 and CH3C(O)O2 produces NO2 while when using PHENO2 HO2 is released. This has major impacts on the entire oxidizing capacity especially when reducing the solar radiation impacts (see Sect. 4.3 and ESI S2† winter_05 case). The oxidation of NPHENOLO2 and NCRESO2 with HO2 leads to NPHENOLOOH and NCRESOOH, two additional SOA precursor substances. Further possible SOA branches are included by integrating nitrate formation (BENZN, TOLN, XYLN) from bicyclic peroxy radical (BENZO2, TOLO2, XYLO2) reactions with NO and their OH reaction products (BENZ
O group (NPHENOLO2, NCRESO2). The formation of the latter products is handled very differently between different mechanisms and are in general not treated explicitly in reduced mechanisms.37,39,43,86 A sensitivity study, considering three different approaches for the handling of the phenol + NO3 reaction channels can be found in the ESI Sect. S2.2.† In JAMv2b NPHENOLO2 and NCRESO2 are approximated with PHENO2. While this has no significant impact for warmer temperatures and longer solar radiation periods (see Sec. 4.1 summer cases) it gains in importance for colder temperatures and longer nights. Then significant amounts of NO3 can form which facilitate NO3 degradation channels. When including NPHENOLO2 and NCRESO2 their further oxidation by NO, NO3, CH3O2 and CH3C(O)O2 produces NO2 while when using PHENO2 HO2 is released. This has major impacts on the entire oxidizing capacity especially when reducing the solar radiation impacts (see Sect. 4.3 and ESI S2† winter_05 case). The oxidation of NPHENOLO2 and NCRESO2 with HO2 leads to NPHENOLOOH and NCRESOOH, two additional SOA precursor substances. Further possible SOA branches are included by integrating nitrate formation (BENZN, TOLN, XYLN) from bicyclic peroxy radical (BENZO2, TOLO2, XYLO2) reactions with NO and their OH reaction products (BENZ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, TOL
O, TOL![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
Additionally, the reaction pathways of aromatic RO2 radicals are extended by using the product yields given in MCM3.3.1. So far, only the reaction products glyoxal, methylglyoxal and the lumped species for unsaturated oxidized aldehydes (BIGALD1, BIGALD2, BIGALD3, BIGALD4) are considered in JAMv2b. For the unsaturated oxidized aldehydes, only photolysis processes are considered in JAMv2b. In URMELL, also reactions with OH, NO3 and ozone are added following MCM3.3.1. Other products of aromatic RO2 radicals such as unsaturated organic acids, furanones, quinones and anhydrides as described in the MCM3.3.1 are missing in JAMv2b. As a consequence, the carbon balance is not closed and possible important SOA pathways are neglected. To overcome this gap, these potential SOA precursor species are incorporated into URMELL. The unsaturated oxidized organic mono acids are represented by the lumped species BIGACID1, BIGACID2 and BIGACID3 in accordance to BIGALD compounds for unsaturated oxidized aldehydes. Furanones, quinones as well as anhydrides are represented by the lumped species BZFUONE, FUONE, BZQONE and MALANHY, respectively (Table S1-1†). Also, the photolysis products of BEPOMUC and TEPOMUC are adjusted to include the SOA products C5DIALOOH and C615CO2OOH.
2.5 Advanced isoprene chemistry implementation
In addition to aromatics, the chemistry of isoprene is extended to incorporate the latest state-of-the-art knowledge, including subsequent reaction pathways of formed products such as methyl vinyl ketone (MVK) and methacrolein (MACR). These follow, e.g. the comprehensive study of Wennberg et al.30 but with a few adaptations. In the following, a brief description of these adaptations is presented, including changes to the isoprene NO3 and OH chemistry within the next two subsections. For isoprene + O3, only the treatment of the stable Criegee intermediate CH2OO changed as outlined in Sect. 2.2.1. For initial isoprene reactions with NO3, OH and ozone, the latest IUPAC task group reaction rate constant recommendations are used (Table S1-2†).Specific isoprene oxidation products are lumped to reduce the complexity of URMELL where feasible. The lumped products are labelled with an L as first letter of their lumped group name. Here, the reaction rate constant is a weighted constant based on the contribution to the lumping group and the individual rate constant of the single compound. The overall reaction product ratios are also calculated using the contribution of the single reaction pathways to the lumping group. Reactions of radicals with O2 are assumed to occur instantaneously, therefore these intermediate steps are skipped following the MCM3.3.1 implementation.
Within the NISOPBO2 scheme, the contribution from β-4,3-INO2 plays only a minor role. Therefore, only β-1,2-INO2 is considered for NISOPBO2 chemistry in URMELL including the formation of NISOPEOO1 isomer following Vereecken et al.87 The majority of oxidation steps of NISOPEOO1E produce MACRN + HCHO + HO2 and a product with a carbonyl, nitrate and epoxide group (ICNE) + HO2. In the temperature interval of 270 to 315 K, the yields for the MACRN and ICNE pathways range from 63–87% and 37–13%, respectively. For URMELL, fixed ratios for 298 K are implemented yielding 80% MACRN and 20% ICNE. For ICNE, the OH-pathway described by Wennberg et al.30 leading to OH + 2CO + 0.35NOA + 0.65MGLY + 0.65NO2 + 0.65HO2 is assumed to occur immediately. As this reaction directly regenerates OH, this has no effect on the OH budget. The NISOPEOO1Z pathways mainly leads to LIECHO + NO2 formation.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds of isoprene is possible at four positions: C1, C2, C3 and C4, whereby terminal OH-addition dominates. Thus, only the terminal products are considered (63% C1 and 37% C4) in URMELL. The remaining second double bond can take two alignments creating cis/trans alkyl radicals which undergo a reversible O2-addition creating a pool of various isoprene peroxy radicals (ISOPOO). The peroxy group of the ISOPOO-isomer is either in β- or δ-position. As the degradation pathways between the ISOPOO-isomers differ substantially, it is crucial to capture the ISOPOO-composition adequately. Teng et al.89 investigated this system and determined, that roughly 95% of ISOPOO is of β O2-addition under most atmospheric relevant conditions. This is much higher compared to the kinetic distribution and the reason for the shift in ISOPOO distribution between JAMv2b and URMELL. In JAMv2b, the reaction of isoprene and OH yields:
C double bonds of isoprene is possible at four positions: C1, C2, C3 and C4, whereby terminal OH-addition dominates. Thus, only the terminal products are considered (63% C1 and 37% C4) in URMELL. The remaining second double bond can take two alignments creating cis/trans alkyl radicals which undergo a reversible O2-addition creating a pool of various isoprene peroxy radicals (ISOPOO). The peroxy group of the ISOPOO-isomer is either in β- or δ-position. As the degradation pathways between the ISOPOO-isomers differ substantially, it is crucial to capture the ISOPOO-composition adequately. Teng et al.89 investigated this system and determined, that roughly 95% of ISOPOO is of β O2-addition under most atmospheric relevant conditions. This is much higher compared to the kinetic distribution and the reason for the shift in ISOPOO distribution between JAMv2b and URMELL. In JAMv2b, the reaction of isoprene and OH yields:| C5H8 + OH → 0.4LISOPACO2 + 0.35ISOPBO2 + 0.25ISOPDO2, | (R-23) |
| C5H8 + OH → 0.05LISOPACO2 + 0.6ISOPBO2 + 0.35ISOPDO2. | (R-24) |
The new branching is in agreement with Wennberg et al.30 and Müller et al.76 The contribution of δ O2-addition to the 1-OH system is about 5% and for the 4-OH system 6%.64 Within MAGRITTE76 and the reduced mechanisms of Wennberg et al.,30 the β- and δ-isomers are lumped within their OH system resulting in two ISOPOO reaction products. In URMELL, β-isomers are treated separately: ISOPBO2 for the 1-OH and ISOPDO2 for the 4-OH system. While the δ-isomers are grouped into LISOPACO2 with a ratio of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 for the 1-OH:4-OH system, respectively. For β-isomers, the 1,5 H-shift and for cis-δ-isomers the 1,6 H-shift is implemented following Wennberg et al.30 These H-shift reactions are a major HOx recycling pathways and can significantly impact OH, HO2, ozone and NO3 concentrations. This is further analyzed by a sensitivity study and discussed in the ESI S2.2.4.† Most parts of the OH-driven isoprene chemistry follow the full mechanism of Wennberg et al.30 Unfortunately, for some reaction products no subsequent reactions are listed, such as ISOP1OH4OH, ISOP1OH2OH and ISOP3OH4OH. Here, the reactions for ISOPAOH, ISOPBOH and ISOPDOH of the MCM3.3.1 are implemented in URMELL. To reduce the complexity of the system, also the recommendations for the reduced mechanism of Wennberg et al.30 are considered such as for aldehyde peroxyl radicals with (i) two hydroperoxyl or (ii) a hydroperoxyl and a hydroxyl group. Here, only H-shifts are considered as they are assumed to outcompete all bimolecular chemistry.30
40 for the 1-OH:4-OH system, respectively. For β-isomers, the 1,5 H-shift and for cis-δ-isomers the 1,6 H-shift is implemented following Wennberg et al.30 These H-shift reactions are a major HOx recycling pathways and can significantly impact OH, HO2, ozone and NO3 concentrations. This is further analyzed by a sensitivity study and discussed in the ESI S2.2.4.† Most parts of the OH-driven isoprene chemistry follow the full mechanism of Wennberg et al.30 Unfortunately, for some reaction products no subsequent reactions are listed, such as ISOP1OH4OH, ISOP1OH2OH and ISOP3OH4OH. Here, the reactions for ISOPAOH, ISOPBOH and ISOPDOH of the MCM3.3.1 are implemented in URMELL. To reduce the complexity of the system, also the recommendations for the reduced mechanism of Wennberg et al.30 are considered such as for aldehyde peroxyl radicals with (i) two hydroperoxyl or (ii) a hydroperoxyl and a hydroxyl group. Here, only H-shifts are considered as they are assumed to outcompete all bimolecular chemistry.30
2.6 URMELL compared to other mechanisms
In summary, the developed mechanism URMELL contains 313 species and 916 reactions. Compared to other reduced mechanisms this is much more sophisticated as for example RACM with around 100 species and 300 reactions, or JAMv2b with around 200 species and a bit more than 500 reactions. However, URMELL is still more condensed than the nearly explicit MCM3.3.1 with more than 5500 species and around 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 reactions, and applicable for 3D CTMs. While for aromatics most updates follow the MCM3.3.1, additional adjustments are performed based on recent findings which have not been included into a CTM chemical mechanism to this extent before or the MCM3.3.1. This already starts at the fundamental basics, the HOx, NOx and Ox systems itself, followed by changed chemical treatments of RC(O)O2 as well as ozonolysis reactions including stabilized Criegee intermediates and goes beyond isoprene. Although, newer mechanism focusing on isoprene exists, they were released before new findings on isoprene–NO3-chemistry became available.76,90 In the next sections, the impact of these changes on the tropospheric gas-phase composition are emphasized through both box model and CTM simulations.
000 reactions, and applicable for 3D CTMs. While for aromatics most updates follow the MCM3.3.1, additional adjustments are performed based on recent findings which have not been included into a CTM chemical mechanism to this extent before or the MCM3.3.1. This already starts at the fundamental basics, the HOx, NOx and Ox systems itself, followed by changed chemical treatments of RC(O)O2 as well as ozonolysis reactions including stabilized Criegee intermediates and goes beyond isoprene. Although, newer mechanism focusing on isoprene exists, they were released before new findings on isoprene–NO3-chemistry became available.76,90 In the next sections, the impact of these changes on the tropospheric gas-phase composition are emphasized through both box model and CTM simulations.
3 Design of model simulations
To evaluate the impact of all changes previously stated, the performance of URMELL is analyzed using box model simulations for several environmental scenarios and is compared with other chemical mechanisms of varying complexity (RACM, JAMv2b, MCM3.3.1). For this first task, the box model SPACCIM, the Spectral Cloud Chemistry Interaction Model is used (Sect. 3.1).91 Secondly, URMELL is applied in the chemical transport model COSMO-MUSCAT64,92 and examined through comparisons with multiple measurement sites for ozone, NO and NO2 (Sect. 3.2). For the evaluation, time series analyses are frequently used. To gain knowledge about the linear relationship between modeled and/or measured data, Pearson correlation values are calculated with Python. But, even though time series show a good agreement between their curve progression, they still may differ significantly in absolute values. Therefore, the coefficient of divergence (COD) is given in addition and calculated as follows:where n is the number of data points, xi is the concentration of one of the mechanisms to be compared with yi the reference data set concentration at time step i.93 The COD is a measure of variability and can reach values close to 1 for low similarity and values close to zero for high similarity or even equal zero if identical concentrations are present between the compared datasets. This is applied to box model simulations in Sect. 4 comparing the comprehensive MCM3.3.1 with the reduced mechanisms (RACM, JAMv2b, URMELL), as well as CTM simulations in Sect. 5.
3.1 Box model simulation with SPACCIM
For the mechanism comparison using the box model SPACCIM, the near explicit Master Chemical Mechanisms version 3.3.1 (MCM3.3.1) is used and functions as the benchmark mechanism throughout the box model analysis. Additionally, JAMv2.b, which was the starting point of URMELL, and an extension of the Regional Atmospheric Chemistry Mechanism RACM-MIM2-ext4,37,63 hereafter called RACM are considered. RACM has been extensively used for air quality studies within the chemical transport model COSMO-MUSCAT and is used as reference simulation for the 3D chemistry transport model simulations of Sect. 5.4,65,94,95 For all box model simulations, the MCM3.3.1 is considered as the reference point, as this is currently the most sophisticated mechanism available.96 Therefore, given correlation and COD values hereafter always refer to the MCM3.3.1, if not stated otherwise.The box model simulations are carried out for 45°N to define the period of solar radiation and zenith angle. Two different emission scenarios are considered: remote and urban which have been previously used with SPACCIM.99–103 The emission values are based on Guenther et al.,97 and EDGAR v2,98 respectively. But as RACM contains lumped species, the emissions are split into the individual species of the different chemical mechanisms (MCM, JAMv2b, URMELL) by using the fractions provided by Middleton et al.104
The urban case comprises higher anthropogenic emissions, e.g. for NOx and aromatics, while the remote case comprises lower NOx and higher BVOC emissions. The emissions are implemented as constant fluxes throughout the entire simulation period. Note, that this causes deviations from real emissions of anthropogenic as well as natural origins. However, this emission set-up is used for all mechanisms giving a systematic error and thus, for mechanism comparisons this approach is feasible.
To also account for deviating conditions, a summer (indicated by _s) and a winter (indicated by _w) case are simulated, which are initialized for the 19th of June and 19th of December with a constant boundary layer temperature of 280 K and 273 K, respectively. Additionally, different radiation conditions are investigated by either no attenuation of incoming radiation (indicated by _1), or an attenuation of incoming radiation by 50% (indicated by _05) resulting in halved photolysis rates. An overview about all scenarios is given in Table 1. The first 24 h are used as spin-up time. Therefore, the plots (Fig. 1, 2 and S2-1 till S2-6†) always start at 24 h and run until 96 h (showing 20.06. −22.06 or 20.12. −22.12.) and also the performed statistics (Tables 3, 4 and S2-1†) include these three model days, only.
| Scenarios | Emission | Season | Photolysis rate | |||
|---|---|---|---|---|---|---|
| Urban | Remote | Summer | Winter | Full | Halved | |
| urb_s_1 | x | x | x | |||
| urb_s_05 | x | x | x | |||
| urb_w_1 | x | x | x | |||
| urb_w_05 | x | x | x | |||
| rem_s_1 | x | x | x | |||
| rem _s_05 | x | x | x | |||
| rem _w_1 | x | x | x | |||
| rem _w_05 | x | x | x | |||
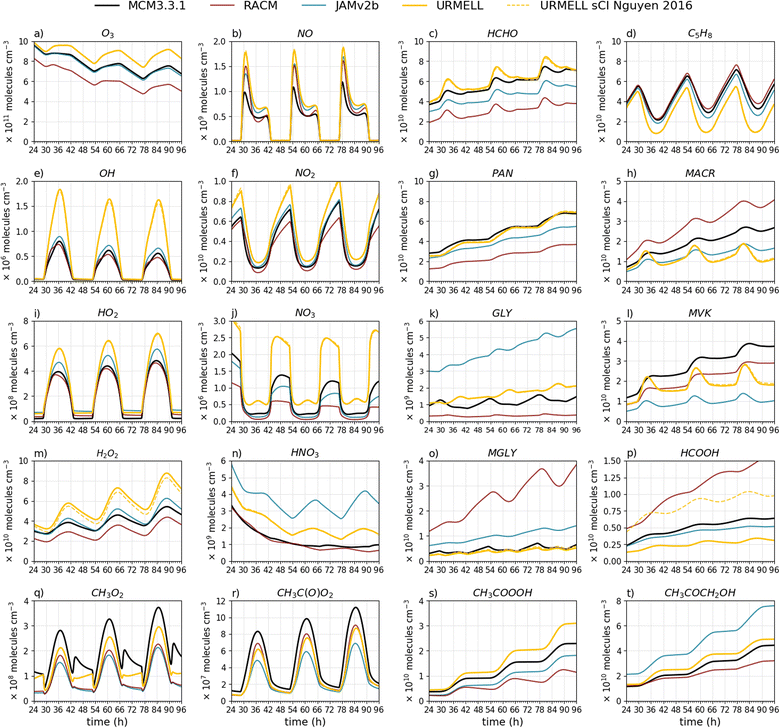 | ||
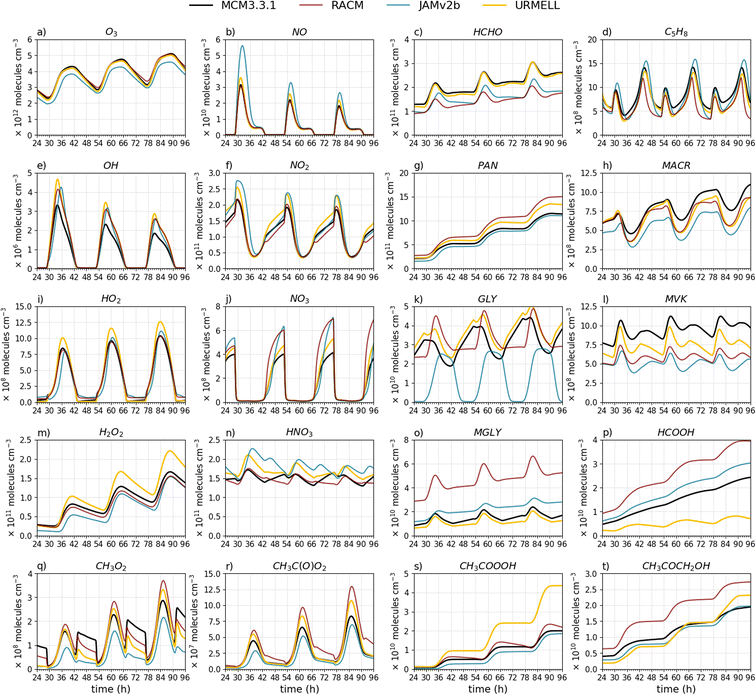
| Fig. 2 Time series of (a) O3, (b) NO, (c) HCHO, (d) C5H8, (e) OH, (f) NO2, (g) PAN, (h) MACR, (i) HO2, (j) NO3, (k) GLY, (l) MVK, (m) H2O2, (n) HNO3, (o) MGLY, (p) HCOOH, (q) CH3O2, (r) CH3(O)O2, (s) CH3COOOH and (t) CH3COCH2OH for the remote summer case with clear sky conditions modeled with MCM3.3.1 (black line), RACM (red line), JAMv2b (blue line) and URMELL (yellow line) as well as a sensitivity run including the branching ratios of Nguyen et al.79 for the Criegee intermediate CH2OO (yellow dashed line). | ||
Please note that the present study is a theoretical study to analyze the performance of the different mechanisms between each other under various environmental conditions. Thus, the results from the box model cannot directly be transferred to the reality and should be seen as extreme events to test the sensitivity of the mechanisms. But the studies can still provide insights on the concentration magnitude and help to identify important night- and day-time path ways and eventually on temporal profiles. For the mechanism comparison, 20 chemical compounds are considered. To investigate the effects of the updates to isoprene chemistry, isoprene and its reaction products MACR and MVK as well as the multi-generation product CH3COCH2OH are chosen. Additional common reaction products from various sources considered for the comparison are HCOOH, MGLY, GLY, HCHO, CH3COOOH, CH3O2 and CH3CO3. Other substances of interest are O3 and OH, HO2, H2O2, HNO3, NO, NO2, NO3 and PAN. All chemical compounds chosen are treated explicitly in the different mechanisms (no lumped compounds) except in RACM where MACR, PAN, CH3COOOH (PAA), CH3COCH2OH (HKET) and MGLY are lumped species.
3.2 CTM simulations with COSMO-MUSCAT
The comparison through box model comparisons has limitations with regard to advection, entrainment and deposition. In order to evaluate and verify the quality of URMELL for air quality modeling, it has been applied in the CTM COSMO-MUSCAT.64 Simulation results are compared with a simulation using the COSMO-MUSCAT default gas-phase mechanism RACM. The model system COSMO-MUSCAT and its good performance has been confirmed through multiple model intercomparison studies.105–108The period May 2014 is chosen for the model domain of Germany, as for this episode a detailed sensitivity study was carried out recently by Luttkus et al.4 The simulations are initialized for the 20th of April 2014. The time period until the 1st of May is used as spin-up while the entire May is used for the CTM simulation analysis. Additionally, a time period in spring enables a closer look at the model performance under varying meteorological conditions. At the 19th of May, a frontal system passes the model domain accompanied by rain and the formation of a high-pressure system afterwards. On the 20th of May calm winds, high solar radiation and warm temperatures boost BVOC emissions while at the same time their distribution is limited facilitating local degradation processes after a washout event. Therefore, the 20th of May is selected for further analysis. For both COSMO-MUSCAT simulations, the detailed land use data set with 138 land use categories is used together with the agricultural biomass density enhancement (for more detail about the model system and setup, the reader is referred to Luttkus et al.4 and references within). However, a few modifications have been made within the model setup compared to Luttkus et al.,4 which are outlined hereafter. Firstly, the outer European model domain providing the initial and boundary conditions for the inner German domain is enhanced and has a finer resolution of 14 km × 14 km now. Secondly, the deposition flux calculation changed from a bulk to a mosaic approach. Meaning, that instead of dominant or weighted average values for every grid cell, individual contributions of all appearing land use categories within a grid cell are considered, now. Both of these changes apply for RACM and URMELL simulations.
Additionally, URMELL considers more BVOCs as RACM and thus, for simulations with URMELL, the BVOC emission and deposition module are adapted as follows. The BVOC emission model distinguishes between 17 monoterpenes,109 while URMELL considers only four monoterpene clusters: α-pinene, β-pinene, myrcene and limonene. Therefore, all cyclic monoterpenes with a terminal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond (β-pinene, camphene, sabinene) are lumped into β-pinene (BPIN) and with an inner C
C double bond (β-pinene, camphene, sabinene) are lumped into β-pinene (BPIN) and with an inner C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond (α-pinene, Δ3-carene, α-thujene) into α-pinene (APIN). Cyclic monoterpenes with multiple C
C double bond (α-pinene, Δ3-carene, α-thujene) into α-pinene (APIN). Cyclic monoterpenes with multiple C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds (limonene, α- and γ-terpinene, α- and β-phellandrene) are grouped into limonene (LIMONENE) and acyclic monoterpenes (myrcene, trans- and cis-ocimene, linalool) into myrcene (MYRC). P-cymene is an aromatic compound and treated as xylene (XYL). 1,8-cineol is a bicyclic ether and a monoterpenoid from limonene, thus it is treated as the monoterpene reaction product TERPROD1, that represents all C10 secondary products of terpene oxidation. An overview of the monoterpene assignment is given in Table 2. Unlike RACM, the smaller BVOCs methanol, acetone, ethanol, acetaldehyde, formaldehyde, formic acid, and acetic acid are treated individually in URMELL. Therefore, the BVOC emission algorithm was adapted accordingly.
C double bonds (limonene, α- and γ-terpinene, α- and β-phellandrene) are grouped into limonene (LIMONENE) and acyclic monoterpenes (myrcene, trans- and cis-ocimene, linalool) into myrcene (MYRC). P-cymene is an aromatic compound and treated as xylene (XYL). 1,8-cineol is a bicyclic ether and a monoterpenoid from limonene, thus it is treated as the monoterpene reaction product TERPROD1, that represents all C10 secondary products of terpene oxidation. An overview of the monoterpene assignment is given in Table 2. Unlike RACM, the smaller BVOCs methanol, acetone, ethanol, acetaldehyde, formaldehyde, formic acid, and acetic acid are treated individually in URMELL. Therefore, the BVOC emission algorithm was adapted accordingly.
| URMELL species | APIN | BPIN | MYRC | LIMONENE | XYL | TERPROD1 |
|---|---|---|---|---|---|---|
| Emitted monoterpenes | α-Pinene, Δ3-carene, α-thujene | β-Pinene, camphen, sabinene | Myrcene, trans-ocimene, cis-ocimene, linalool | Limonene, α-terpinene, γ-terpinene, α-phellandrene, β-phellandrene | p-Cymene | 1,8-Cineol |
The dry deposition scheme implemented in COSMO-MUSCAT is based on Schlünzen et al.110–112 and essentially considers all RACM species. In each case, the dry deposition flux is the product of the species concentration and the deposition velocity, which is calculated based on a resistance model and is the reciprocal value of the aerodynamic, the sublayer and the surface resistance. Overall, the deposition depends on the atmospheric stability, land use type, season, solar irradiation, relative humidity and gaseous species (solubility, reactivity). The scheme used was adapted and extended to URMELL species based on functional group composition (see Table S1-1†). But unfortunately, information of the deposition velocity or resistances for multifunctional molecules are scarce. Therefore, measurements from Nguyen et al.113 for a temperate forest were used as a guideline. While for hydroperoxides with an additional hydroxyl or carbonyl group the surface resistance is small (as for methyl hydrogen peroxide (OP) and peroxyacetic acid (PAA), respectively), it gets higher for compounds with at least one carbonyl and hydroxyl and/or nitrooxy group (similar to formic acid (ORA)). All peroxy radicals are set to peroxides (RO2), all peroxyacetyl nitrates to PAN and substances with one or more carbonyl groups to acetaldehyde (MeCHO). Even though, more sophisticated deposition schemes are available,114–116 the implementation of a new deposition scheme into COSMO-MUSCAT is beyond the scope of this paper, but will be the subject of future model development.
In the following section, results from box model and CTM simulations are presented and discussed.
4 Box model results
To test the updates presented in the previous section multiple box model simulations are performed with SPACCIM for two emission scenarios (remote and urban) and various meteorological condition. Especially for the remote case URMELL predicts much higher peak oxidant concentrations e.g. O3, OH and NO3 radicals. To further quantify the sources leading to these increased oxidant concentrations, multiple sensitivity studies are performed for the rem_s_1 scenario and analyzed. The main results are presented hereafter and a more detailed analysis can be found in the ESI S2.3.†4.1 Sensitivity study for the remote case
In contrast to the updates on aromatic chemistry, most modifications in isoprene chemistry implemented in URMELL are not part of the current MCM3.3.1. Therefore, deviations within the remote scenario simulations will occur more likely (Fig. 2). For the OH radical, the modeled concentration peak is a factor of two to three higher. The modeled OH concentration levels of URMELL reach up to 1.75 × 106 molecules per cm3 for clear sky conditions (Fig. 2e) while the other mechanisms do not reach 106 molecules per cm3, which is in contrast to the urban case, where all mechanisms simulated noon values above 106 molecules per cm3 (Fig. 3e). But, measurements from isoprene-dominated regions would support higher OH values as modeled by URMELL.10,117–120For the rem_1_s sensitivity studies, the reactions of the updated reaction scheme (described in Sect. 2) are stepwise reset to JAMv2b reaction equations. At first, non-isoprene related updates are analyzed starting with the changed RC(O)O2 chemistry which comprises two simulations: (i) solely changed kNO2 rate coefficient and (ii) total RC(O)O2 related updates (Sect. 2.3). All reactions considered for these two studies are indicated by 1 and 2 in Table S1-2.† In a next step, the impact of changed photolysis rates and products are analyzed (Sect. 2.1, Table S1-3† indicated by 3). Finally, the change in GLY rate constant (Sect. 2.1) and ozonolysis implementations (Sect. 2.2) are investigated (Table S1-2† indicated by 4). Afterwards, the isoprene related effects including initial oxidation steps, H-shift reactions as well as MVK and MACR updates are examined (Sect. 2.5, indicated by 5 in Table S1-2†). Note, that due to the stepwise resetting to JAMv2b scenario 5 includes all changes indicated by step 1 through 5, for scenario 4 it is 1 through 4 and so on. For the original JAMv2b equations the reader is referred to the corresponding publication.39Table 3 summarizes the presented sensitivity studies of Fig. S2-1† and the considered adjustments.
| Sensitivity study | PAN | RC(O)O2 | Phot. | GLY | O3 | MVK & MACR | C5H8 | H-shift |
|---|---|---|---|---|---|---|---|---|
| 1: PAN | x | |||||||
| 2: RC(O)O2 | x | x | ||||||
| 3: Photolysis | x | x | x | |||||
| 4: GLY & O3 | x | x | x | x | x | |||
| 5: C5H8 | x | x | x | x | x | x | x | x |
In the following, the main results are summarized, but a more detailed analysis is given in the ESI S2.3.† Roughly half the difference of O3 (Fig. S2-1a†) and OH (Fig. S2-1e†) increase is caused by non-isoprene related changes mainly RC(O)O2 chemistry (Sect. S2.3.1†) and photolysis processes (Sect. S2.3.2†). The other half is due to isoprene chemistry primarily by the newly implemented H-shift reactions of ISOPOO and the ISOPOO pool distribution itself (Sect. 2.5.2 and S2.3.4†). The ISOPOO pool composition impacts O3 through LHC4ACCHO, MVK and MACR ozonolysis and indirectly through formation and degradation pathways of the isoprene nitrates LISOPACNO3, ISOPBNO3 and ISOPDNO3. NO3 (Fig. S2-1j†) reduces throughout all changes but most significantly for isoprene chemistry updates which is primarily caused by MPAN chemistry as well as lower NO2 (Fig. S2-1f†) and O3 (Fig. S2-1a†) concentrations mitigating the major NO3 production pathway (Sect. S2.3.4†). The increase in day-time NO3 is caused by the oxidation of MPAN (reaction product from MACR) by OH or O3. Additionally, the changed RC(O)O2kNO2 rate constant also notably impacts NO3 concentrations (Sect. S2.3.1†).
Photolysis processes impact the HOy budget (Fig. S2-1e, i and m†) as well as HCHO, CH3O2, CH3C(O)O2, GLY (Fig. S2-1k†) and MGLY (Fig. S2-1o†) concentrations (Sect. S2.3.2†). Compared to JAMv2b, the most significant effects on GLY is from the changed NO3 reaction rate constant (Sect. S2.3.3†). But in comparison to MCM3.3.1 changes to isoprene chemistry (internal OH addition channels) are the driving factors (Sect. S2.3.4†). A substantial increase in HCOOH (Fig. S2-1p†) is evoked by high JAMv2b yields from MVK ozonolysis (Sect. S2.3.4†). CH3C(O)O2 (Fig. S2-1r†) is governed by changes to its rate constants (Sect. S2.3.1†) and photolysis reactions (Sect. S2.3.2†). CH3C(O)O2 reaction product CH3COOOH (Fig. S2-1s†) is coupled to HO2 rate coefficient and CH3C(O)O2 concentration changes. Furthermore, CH3C(O)O2 chemistry governs day-time while ozonolysis processes dominate night-time CH3O2 production (Fig S2-1q†). In JAMv2b ozonolysis processes have no significant impact on CH3O2 concentrations. In URMELL a much higher CH3O2 yield of 0.407 for C5H8 ozonolysis is implemented and due to its fast reaction rate coefficient a continues rise until 5 a.m. is observed. But due to missing ozonolysis CH3O2 sources such as from BIGENE, CH3O2 is still lower than the MCM3.3.1 (this is addressed further in Sect. 4.3). On the other hand MCM3.3.1 may provide overestimated CH3O2 ozonolysis yields based on recommendations from Cox et al.73 Furthermore, BIGENE often have slower O3 reaction rate coefficients which makes them less efficient with decreasing O3 concentrations and therefore result in opposing night-time trends.
Please note, that especially all the non- but also the isoprene related modifications effect not only the remote but also dominate the alterations in the urban cases for which no separate sensitivity study is presented.
4.2 Remote case
URMELL predicts much higher oxidant concentration (Fig. 2 and S2-5–S2-7†) linked to isoprene (e.g. OH recycling, ISOPOO pool) and non-isoprene related changes (e.g. RC(O)O2, photolysis) as described before. For all remote cases, correlation coefficients (see Table 4 and S2-1†) of O3, OH, NO, NO2, NO3 and HO2 are high for all mechanisms (R > 0.9). One exception is O3 for URMELL (R = 0.881), for which a slightly increasing trend is modeled in the end in comparison to the slightly decreasing trend of the MCM3.3.1 (Fig. 2a). In the case of reduced actinic radiation (Fig. S2-5†), the difference between peak O3, OH, NO, NO2, NO3 and HO2 concentrations decreases. This is also the case for the winter scenarios, but NO and NO2 estimates increase for all reduced mechanisms (Fig. S2-6 and S2-7†). In general, nearly all correlation coefficients of URMELL are above 0.8 (Table S2-1†) for the winter scenarios (Fig S2-6 and S2-7†). Here, the weaker photochemical activity and lower temperature reduce the diurnal variability, straightening most temporal profile, whereby the curve similarity increases. For MGLY, URMELL predicts very similar curves to the MCM3.3.1 for all scenarios (see Fig. 2o and S2-3o–S2-7o†).| Scenario | Compound | URMELL | JAMv2b | RACM | URMELL sens | ||||
|---|---|---|---|---|---|---|---|---|---|
| R | COD | R | COD | R | COD | R | COD | ||
| rem_s_1 | O3 | 0.881 | 0.071 | 0.998 | 0.010 | 0.986 | 0.115 | 0.985 | 0.077 |
| NO | 0.982 | 0.159 | 0.986 | 0.137 | 0.971 | 0.137 | 0.981 | 0.129 | |
| NO2 | 0.980 | 0.184 | 0.987 | 0.084 | 0.972 | 0.100 | 0.992 | 0.065 | |
| OH | 0.987 | 0.340 | 0.998 | 0.104 | 0.987 | 0.065 | 0.997 | 0.120 | |
| HO2 | 0.990 | 0.345 | 0.991 | 0.372 | 0.998 | 0.229 | 0.989 | 0.336 | |
| H2O2 | 0.992 | 0.185 | 0.995 | 0.041 | 0.997 | 0.146 | 0.989 | 0.045 | |
| NO3 | 0.954 | 0.379 | 0.986 | 0.202 | 0.967 | 0.448 | 0.973 | 0.161 | |
| HNO3 | 0.959 | 0.278 | 0.759 | 0.503 | 0.988 | 0.104 | 0.999 | 0.079 | |
| HCHO | 0.959 | 0.048 | 0.993 | 0.116 | 0.964 | 0.304 | 0.993 | 0.154 | |
| PAN | 0.998 | 0.037 | 0.999 | 0.105 | 0.999 | 0.332 | 0.998 | 0.211 | |
| GLY | 0.472 | 0.202 | 0.628 | 0.578 | 0.615 | 0.547 | 0.530 | 0.463 | |
| MGLY | 0.892 | 0.114 | 0.843 | 0.371 | 0.891 | 0.684 | 0.792 | 0.337 | |
| C5H8 | 0.892 | 0.333 | 0.989 | 0.072 | 0.990 | 0.036 | 0.989 | 0.082 | |
| MACR | 0.519 | 0.268 | 0.980 | 0.193 | 0.995 | 0.197 | 0.963 | 0.219 | |
| MVK | 0.711 | 0.223 | 0.757 | 0.492 | 0.999 | 0.149 | 0.780 | 0.471 | |
| HCOOH | 0.952 | 0.329 | 0.998 | 0.083 | 0.972 | 0.364 | 0.994 | 0.113 | |
| CH3O2 | 0.952 | 0.192 | 0.959 | 0.419 | 0.956 | 0.382 | 0.934 | 0.463 | |
| CH3C(O)O2 | 0.982 | 0.204 | 0.985 | 0.281 | 0.984 | 0.198 | 0.990 | 0.293 | |
| CH3COOOH | 0.999 | 0.116 | 0.999 | 0.178 | 0.988 | 0.299 | 0.999 | 0.131 | |
| CH3COCH2OH | 0.997 | 0.079 | 1.000 | 0.273 | 0.998 | 0.103 | 1.000 | 0.090 | |
All mechanisms show similar diurnal isoprene cycles (Fig. 2d). The increase in daytime OH lowers the isoprene minimum and causes a slightly earlier onset of afternoon isoprene rise for URMELL, which impacts the correlation and COD values (Table 4). This also holds for the rem_05_s case (Fig. S2-5d†). For the winter scenarios (Fig. S2-6d and S2-7d†), the OH concentration is much lower and only present for a shorter period of time which minimizes this shift and leads to higher R values (R > 0.9). For the isoprene reaction products MACR and MVK, stronger diurnal fluctuations are visible compared to all other mechanisms (Fig. 2h and l). This is caused by higher production from isoprene chemistry while at the same time degradation is intensified due to higher oxidant concentrations. But these variations attenuate for reduced actinic radiation and the winter scenarios (Fig. S2-5h/l–S2-7h/l†). MACR is linked to ISOPDO2 and MVK to ISOPBO2. For the rem_1_s scenario, MVK and MACR are directly impacted by the ISOPOO composition. In JAMv2b the reaction of C5H8 with OH yields 0.4/0.35/0.25 LISOPACO2/ISOPBO2/ISOPDO2 and in URMELL 0.05/0.6/0.35, respectively. Thus, higher MVK and similar MACR values are simulated. But, the increased oxidant concentration (Fig. 2a, e and j) intensifies the degradation. When reducing OH, MCM3.3.1 and URMELL adjust in the case of the rem_05_s scenario, while for the winter scenario URMELL simulates higher concentrations. Besides MACR and MVK, HCHO is e.g. linked to ISOPBO2 and ISOPDO2 chemistry. The simulated diurnal cycles for the rem_1_s scenario are similar for all mechanisms (Fig. 2c), but URMELL produces higher concentrations. For reduced photolysis and lower temperatures (Fig. S2-5d and S2-7d†) the impact of the ISOPOO pool composition manifest in even higher HCHO concentrations. CH3COCH2OH is on the other hand mainly produced from LISOPACO2. Therefore, due to the much lower LISOPACO2 production yield in URMELL lower CH3COCH2OH concentrations compared to JAMv2b are simulated.
Peroxy radical concentrations of CH3O2 and especially of CH3C(O)O2 are lower for all mechanisms compared to the MCM3.3.1 (Fig. 2q/r and S2-5q/r–S2-7q/r†). Important sources for both radicals are e.g. photolysis processes, ozonolysis reactions and for CH3O2 also CH3C(O)O2 reaction pathways. The CH3C(O)O2 offset between the MCM3.3.1 and the other mechanisms increases for the rem_05_s and the winter scenarios and is caused by changes to ozonolysis processes and the ISOPOO pool. This reduction in CH3C(O)O2 also lowers PAN (Fig. 2g and S2-5g–S2-7g†) and CH3COOOH (Fig. 2s and S2-5s–S2-7s†) but compared to JAMv2b PAN is slightly reduced due to slower kNO2 and CH3COOOH enhanced due to faster kHO2 reaction rate coefficient. With reduced photolysis and CH3C(O)O2 availability, day-time CH3O2 production shrinks. Night-time CH3O2 production is governed by ozonolysis processes. The higher CH3O2 yield from C5H8 + O3 oxidation and fast reaction rate coefficient generates a still partly increasing night-time trend for URMELL while the MCM3.3.1 predicts a fast increase (also see Sect. 4.1, 4.3) followed by a continuous decrease. This is producing opposing trends and results in low or even negative R values (Table S2-1†).
For GLY (Fig. 2k), the deviation from the diurnal cycle proposed by MCM3.3.1 is of specific interest and reveals low correlation values. URMELL simulates a minimum at 6:00, a maximum at 15:00, followed by a decrease until 19:00, after which somehow stable concentrations are reached (slightly increasing) until 5:00. The rather low GLY + NO3 reaction constant of URMELL hinders night-time GLY degradation (Fig. 2k). But compared to the MCM3.3.1, night-time URMELL GLY production from isoprene channels are much lower (Sect. S2.3.4†). The MCM3.3.1 simulates maximum GLY concentration around 6:00. URMELL shows a stronger day-time production mainly linked to newly implemented C5H8 chemistry (MVK, ISOPOO pool) with a strong photolysis component. Whereas the MCM3.3.1 simulates only minor day-time GLY production resulting in a saddle point instead of a maximum followed by a minimum at 19:00. Maximum concentrations of GLY coincide with minimum C5H8 concentrations confirming a link to C5H8 daytime chemistry, which is dominated by OH chemistry. In the cases of reduced actinic radiation (Fig. S2-5k†), GLY is negatively correlated. Now, the day-time GLY production of the MCM3.3.1 cannot outcompete the consumption, while URMELL still predicts a small increase. These opposing trends invoke a slightly negative R = −0.051 value. For the winter scenarios (Fig. S2-6k and S2-7k†), ozonolysis dominates for both scenarios, but with much higher production for the MCM3.3.1 (R = 0.9).
The increase in OH also boost day-time HNO3 formation via NO2 reaction (Fig. 2n) resulting in a clear diurnal cycle for the rem_1_s case. But with attenuating OH concentrations for the other scenarios this HNO3 day-time production diminishes. For HCOOH (Fig. 2p and S2-5p–S2-7p†), URMELL has a stronger day-time component and shows a clear diurnal cycle for the summer cases. Changes to HCOOH are mainly caused by the different treatment of the stabilized Criegee intermediate CH2OO (see Sect. 2.2.1) and changes to ozonolysis reactions especially for the reaction of MVK + O3. Measurements from remote high HOx and isoprene rich locations would support stronger day-time production strength of GLY, HCOOH, and HNO3 resulting in distinguishable diurnal cycles as simulated with URMELL.81,82,118,121 Changes to the abundance of these compounds alters the atmospheric oxidant budget mainly through their strong linkages to OH, HO2, RO2 and O3. GLY and HNO3 are additionally.
In conclusion, URMELL simulates concentration time series of various species in remote environments for different radiation and temperature regimes in the same manner as the benchmark MCM3.3.1 (R > 0.9 for most substances, but with partly higher COD values, Table 4). Overall, URMELL predicts a much higher remote tropospheric oxidative capacity. When resetting all mentioned updates (Table 3, case 5), O3, NO, NO2, NO3, OH, HO2 concentration time series are, of course in better accordance with JAMv2b, in most cases even closer to the MCM3.3.1 with excellent correlation (R > 0.98) and low COD values (Table 4). Moreover, for compounds (e.g. H2O2, NO3, HNO3) directly linked to the oxidants a close match to the MCM3.3.1 is reached.
The MCM3.3.1 contains more than 5500 species and OH is the dominant oxidizing agent for most non-radical species. Therefore, the MCM3.3.1 provides numerous additional OH sinks, not included in URMELL. Hence, further extension for example of the monoterpene chemistry might reduce the higher predicted concentration of OH and O3 in remote areas. Still, measurements in rural regions of the tropical forest support higher O3 and OH concentrations in isoprene-dominated regimes, even though this is currently a controversy and subject of recent scientific discussion.9,117,120 Therefore, CTMs that do not take these updates into account might not be able to predict concentrations comparable to ambient values in isoprene-dominated environments, such as the Amazonian rain forest.
4.3 Urban case
Fig. 3 illustrates the modeled concentrations of key gas-phase compounds for the urb_s_1 scenario (no attenuated actinic radiation) for all mechanisms. In addition, Table 5 shows all Pearson correlation coefficients (R) and COD values calculated between MCM3.3.1 and the other non-explicit mechanisms for the urb_s_1 scenario. The plots and tables for the other scenarios are provided in the ESI (Fig. S2-2 till S2-4 and Table S2-1).†| Scenario | Compound | URMELL | JAMv2b | RACM | |||
|---|---|---|---|---|---|---|---|
| R | COD | R | COD | R | COD | ||
| urb_s_1 | O3 | 0.999 | 0.012 | 0.987 | 0.064 | 0.994 | 0.014 |
| NO | 0.998 | 0.076 | 0.958 | 0.169 | 0.999 | 0.080 | |
| NO2 | 0.999 | 0.067 | 0.916 | 0.126 | 0.975 | 0.058 | |
| OH | 0.998 | 0.290 | 0.953 | 0.148 | 0.995 | 0.200 | |
| HO2 | 0.999 | 0.235 | 0.944 | 0.407 | 0.996 | 0.324 | |
| H2O2 | 1.000 | 0.105 | 0.989 | 0.235 | 0.998 | 0.063 | |
| NO3 | 0.996 | 0.055 | 0.959 | 0.175 | 0.968 | 0.179 | |
| HNO3 | 0.725 | 0.063 | 0.190 | 0.104 | 0.488 | 0.037 | |
| HCHO | 0.999 | 0.024 | 0.956 | 0.150 | 0.987 | 0.193 | |
| PAN | 1.000 | 0.065 | 0.999 | 0.085 | 1.000 | 0.122 | |
| GLY | 0.965 | 0.082 | −0.517 | 0.642 | 0.229 | 0.134 | |
| MGLY | 0.982 | 0.131 | 0.796 | 0.212 | 0.945 | 0.517 | |
| C5H8 | 0.964 | 0.104 | 0.924 | 0.107 | 0.871 | 0.179 | |
| MACR | 0.911 | 0.090 | 0.929 | 0.189 | 0.919 | 0.088 | |
| MVK | 0.890 | 0.121 | 0.411 | 0.309 | 0.899 | 0.232 | |
| HCOOH | 0.925 | 0.476 | 1.000 | 0.118 | 0.995 | 0.285 | |
| CH3O2 | 0.833 | 0.304 | 0.809 | 0.480 | 0.864 | 0.158 | |
| CH3C(O)O2 | 0.999 | 0.070 | 0.972 | 0.257 | 0.986 | 0.231 | |
| CH3COOOH | 0.999 | 0.317 | 0.994 | 0.325 | 0.994 | 0.101 | |
| CH3COCH2OH | 0.991 | 0.175 | 0.996 | 0.099 | 0.994 | 0.215 | |
The correlation values improved for nearly all species for the summer cases when comparing JAMv2b and URMELL with the MCM3.3.1 (Table 5 and S2-1†). In comparison with the MCMv3.3.1, all reduced mechanisms are able to reproduce the modeled cycle of the most frequently measured air quality components such as O3, NO and NO2 for clear sky conditions (Fig. 3) as well as OH, H2O2, NO3, HCHO, PAN, HCOOH, CH3C(O)O2, CH3COOOH and CH3COCH2OH with correlation values above 0.9. However, RACM and JAMv2b show deficits for HNO3 and GLY leading to lower or even negative correlation values. Even though, correlation values are high for most species, the magnitudes of certain species differ between the mechanisms. URMELL provides up to 50% higher peak HOy (H2O2, OH, HO2) concentrations than all other mechanisms. Based on performed sensitivity studies presented before (Sect. 4.1 and S2.2†), this difference is mainly linked to changes in RC(O)O2, isoprene chemistry and photolysis processes (same reactions as indicated for the sensitivity studies in Sect. 4.1 and listed in Tables S1-2 and S1-3† apply, therefore similar results occur). All reduced mechanisms tend to overestimate night time NO3 but URMELL shows the largest similarity to the MCM3.3.1 with R being greater than 0.99 for all summer scenarios (Tables 5 and S2-1†). Importantly, the diurnal cycle of GLY and HNO3 modeled by the MCM3.3.1 is reproduced by URMELL, only. For HCHO and MGLY, very similar concentrations are modeled with URMELL together with high R and low COD values. The highest deviation from the MCM3.3.1 is evident for HCOOH which is primarily caused by the changed Criegee intermediate treatment of the CH2OO biradical (see Sect. 2.2.1 for further details). As the only CH3COOOH source is the reaction of CH3C(O)O2 with HO2, the higher CH3COOOH with URMELL are caused by the faster production and the much slower degradation by OH. Higher night-time CH3O2 concentrations modeled with the MCM3.3.1 are due to ozonolysis reactions.
This becomes even more evident for the winter scenarios (Fig S2-3q and S2-4q†), when photolysis processes are insignificant loss processes and ozonolysis reactions gain in importance. Further investigation of the modeled CH3O2 concentration by the MCM3.3.1 revealed additional sources (e.g. CH3CHOOA, CH3CHOOB, CH3CHOOC) from alkene ozonolysis. But based on Cox et al.73 the degradation channels of Criegee intermediates have much lower CH3O2 production rates. As for the ozonolysis of C3H6 which does not include a direct CH3O2 pathway (see Sect. 2.2) but in the MCM3.3.1 would initiate the production of CH3CHOOA. Furthermore, in JAMv2b most alkenes are lumped into BIGENE, for which no reaction with O3 is implemented and therefore, no CH3CHOOB and CH3CHOOC formation is considered. Here, more knowledge is needed to adjust the representation of alkenes in URMELL, including the lumping and adequate reaction equations. However, this is out of scope of this study, it will be the focus of further mechanism development. Ozonolysis reactions are also a major night-time HO2 source. While during summer, HO2 would peak around noon, in winter the night-time HO2 ozonolysis peak exceeds the day-time peak for the MCM3.3.1 (Fig. S2-3i and S2-4i†). For URMELL, the night-time peak is much lower compared to the MCM3.3.1 and for the urb_w_05 scenario even lower than the day-time peak.
The high NOx concentrations of the urban scenarios result in HOx limit regimes, which makes the winter scenarios highly sensitive to any kind of HOx changes. As the HOx and NOx cycling is linked to O3, it is impacted, too. Furthermore, the reduction in CH3O2 and HO2 boost O3 (Fig. S2-3a and S2-4a†) and NO3 (Fig. S2-3j and S2-4j†) night-time oxidation which decrease their concentrations and in turn enhances NOx (Fig. S2-3b/f, S2-4b/f†). The stronger O3 depletion especially for the urb_w_05 scenario also causes a shift towards CH3C(O)O2 degradation channels resulting into reduced CH3C(O)O2 concentrations (Fig. S2-3r and S2-4r†). Nevertheless, compared to JAMv2b URMELL still provides better R and COD values for both winter scenarios (Table S2-1†).
The intensified NO3 oxidation for the urb_05_w scenario also highlights the importance of the NO3 reaction with monohydroxy phenols. This reaction produces ring-opening products with an ON(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)
O)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group (NPHENOLO2, NCRESO2) for which various approximations are available and a sensitivity study testing the different approaches is presented in the ESI (S2.2†).
O group (NPHENOLO2, NCRESO2) for which various approximations are available and a sensitivity study testing the different approaches is presented in the ESI (S2.2†).
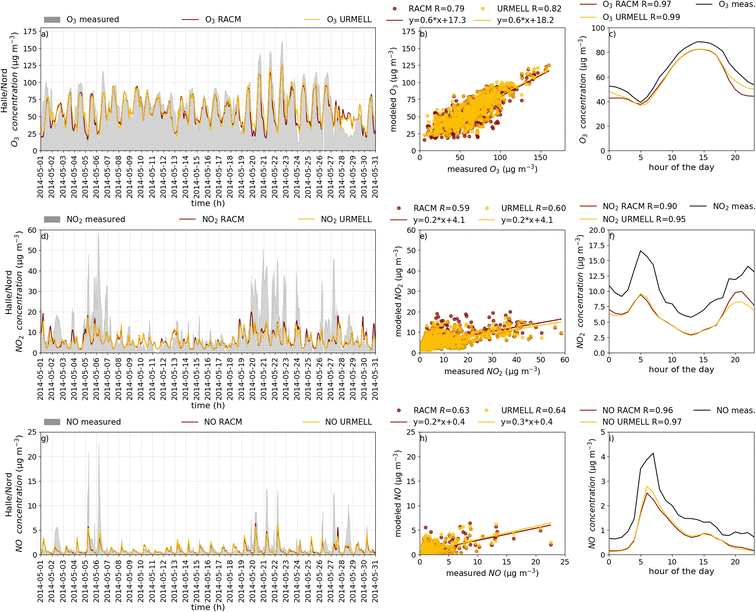
5 CTM results
For the CTM simulation, similar results as with the well-established mechanism RACM are desired, when comparing with NO, NO2 and O3 measurements. To analyze the model data of these three compounds, 62 measurement sites are selected throughout Germany comprising a variety of different VOC and NOx regimes. Due to data gaps or episodes of constant values when the detection limit of the measurement device is reached, only 57 stations are considered for the comparisons. These include 24 remote background (BR), 14 urban background (BU), 8 traffic (T) and 11 industrial/traffic and industrial (IR/IU/TI) sites. While for nearly all stations O3 data is available, NO2 and NO data is limited to 40 and 14 stations, respectively. An overview of the sites is provided in Table S3-1† and a map containing all measurement sites is given in Fig. S3-1b.†Note, that the accuracy of the different simulated time series depend on multiple factors, such as quality of anthropogenic and biogenic emission fields, orographic effects, deposition processes, meteorological parameters which influence the dispersion and boundary layer height, and not primarily on the applied chemical mechanism. Another very important aspect is the model resolution, as a grid cell typically comprises multiple land use categories and/or structures. Especially in cities, buildings or other obstacles influence the transport of airborne substances such as air pollutants resulting in accumulation and removal areas. Also transition areas between, for example, cities and natural vegetation holds some uncertainties with regard to exactly predicting concentrations at a specific point. Additionally, local deviation from the standard emission profile used to calculate the emissions are hard to capture. Furthermore, the highly temporal fluctuation of measurements is often more pronounced compared to model predictions. Therefore, multiple aspects have to be considered when analyzing time series of modeled and measured data. To reduce measurement noise and to compensate outliers (extreme events, special events etc.) also the average diurnal concentration cycles for May 2014 are calculated. Please also note, that the obtained mechanistic differences presented in Sect. 4.1 cannot be directly transferred to the CTM simulations as emissions and meteorological conditions change with space and time. Therefore, the high increases in OH and ozone as seen from the remote case studies above are not presumed.
Table S3-2† summarizes the minimum, mean, maximum and standard deviation for all measurements and model simulation time series of O3, NO2 and NO, as well as the associated correlation and COD values between the measurement and model simulations for the selected stations. Both RACM and URMELL predict quite similar concentration time series with correlation and COD values in the range of 0.1 of each other for most stations. In the case of O3, correlation values are above 0.5 for all stations. When looking at the entire time series, RACM provides more often slightly higher correlation (25 out of 56 for O3, 20 out of 40 for NO2, 11 out of 14 for NO) and almost always slightly lower COD values. Note that for RRACM–RURMELL<0.01 both time series are considered too similar to count for either mechanism (RRACM–RURMELL<0.01 16, 9 and 2 times, respectively) and are indicated by yellow coloring in Fig S3-1b.† However, average diurnal concentration cycles provide more often (21, 18, 0 and RRACM–RURMELL <0.01 25, 9 and 6 times, respectively) closer agreement to the measurements with correlation values for O3 of 0.8 or higher (Table S3-3†) for URMELL. In Fig. S3-1b,† the color indicates if RACM (red) or URMELL (green) provides higher correlation values with O3 measurements. This will be further elaborated within in the next subsection.
5.1 Remote sites
For the remote sites, only O3 is further evaluated, because of the lack of NOx data. In the north east of the domain, a cluster where RACM gives better O3 correlation values for remote sites (Fig. S3-1b†) is evident. Further investigations indicate a link between the O3 cycles and the land use type, e.g. different tree species. Therefore, measurement sites within or close to forests of the four most common German tree species are investigated further: Spreewald (pine; Fig. S3.2-1 and S3.2-5†), Kellerwald (beech; Fig. S3.2-2 and S3.2-6†), Simmerath (oak; Fig. S3.2-3 and S3.2-7†) and Schmuecke (spruce; Fig. S3-4 and S3-8†). A brief description of the BVOC emissions is given in the ESI S.3.2.1† (a more detailed description of the implemented BVOC emission algorithm can be found in Luttkus et al.4). Especially in the east, continuous pine induced monoterpene and the lack of pine isoprene emissions invoke e.g. slightly lower correlation values for URMELL. This also holds for urban stations within monoterpene dominated areas. Whereas in isoprene, OVOC and synthesis monoterpene emission dominated regions (oak, beech, spruce, mixed forest) similar or higher correlation values occur (Fig. S3-1b†).The time series (Fig. 4, 5 and S3.2-1–S3.2-4†) indicate an intense O3 episode from the 19th until the 23rd of May 2014. During this episode calm winds, high solar radiation and warm temperatures boost BVOC emissions and facilitate local degradation processes. Both RACM and URMELL underestimate this O3 episode for all stations. Possible reasons for the underestimated ozone concentration are: (i) under predicted BVOC emissions, (ii) location at higher altitude (which is the case for all spruce forest within the lower mountain range) which impacts several terrain related factors, e.g., stratospheric ozone intrusion, meteorological parameters (wind speed, wind direction, atmospheric stability, planetary boundary layer height). Especially during this episode, large deviation between RACM and URMELL are modeled for spruce, beech and mixed forests. Here URMELL predicts higher night-time O3 concentrations which are in better agreement with the measurements (Fig. 4, S3.2-2 and S3.2-4†) and result in higher correlation values (all green dots in Fig. S3-1b†). The O3 increase for spruce is so high (Fig. S3.2-4†), that a complete offset between measured and modeled O3 concentration is observed, which needs to be further investigated.
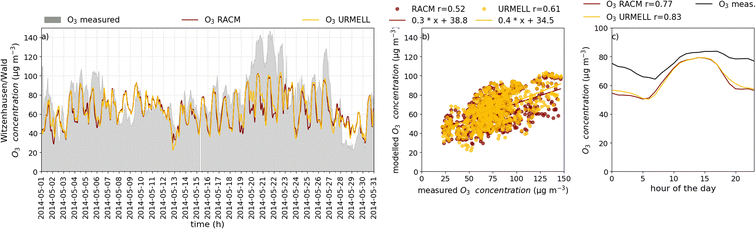 | ||
| Fig. 4 Time series of O3 in (a)–(c) for Witzenhausen (mixed forest); measurements in grey for (a), black line for (c) as well as modeled concentrations using RACM (red line) and URMELL (yellow line). | ||
5.2 Urban sites
For the urban stations (squares and triangles), 13 (7) out of 32 stations show better correlations for the mean diurnal O3 cycle with URMELL and 16 (11) with similar values (entire time series). All three stations with worse correlations are within the north east of Germany again. In general, URMELL produces a slower O3 decay after approximately 18 UTC until midnight resulting in higher O3 concentrations during the first half of the night (exemplary see Fig. 5). However, minimum O3 concentration between 4 and 6 UTC is often lower in the model due to faster and/or prolonged decay due to enhanced NO concentrations. Maximum concentrations are similar. The diurnal NO2 cycle shows two peaks one around 5 UTC and a second one around 20 UTC. For the first peak, similar or slightly increased concentration are reached for URMELL, while for the second NO2 peak URMELL generally produces lower concentrations. These changes can be mainly attributed to RC(O)O2 changes. For most sites, this change in NO2 peak concentrations better reproduce the measured data. But in cases where measurements have a higher second peak, RACM has higher correlation values (see Table S3-2†). For NO, there is no clear differentiation between RACM and URMELL possible. All stations show a clear peak around 6 UTC, but depending on the sites, sometimes RACM and sometimes URMELL shows higher/lower concentrations and may produce a second smaller NO peak in the afternoon. Additionally, due to the limited amount of data, no definite trend can be derived.5.3 Spatial (O3) concentration distribution on May 20th
To further elaborate the deviation during the O3 episode, O3 map plots for 3 UTC, 13 UTC and 19 UTC of the 20th of May are presented. This allows insights into the spatial distribution of the identified simulated O3 deviation. Due to the interwoven links between HOx, NOx, O3, isoprene and monoterpenes, an assessment of purely individual impacts is not feasible.At 3 UTC, highest O3 concentrations are reached for beech- and spruce-dominated areas independent of the chemical mechanism. However, URMELL simulates higher O3 concentrations (Fig. 6) for most of the domain compared to RACM. In lower NOx environments (Fig. S4-1†), highest OH and monoterpene concentrations are reached with URMELL for pine (Fig. S4-2 and S4-3†).
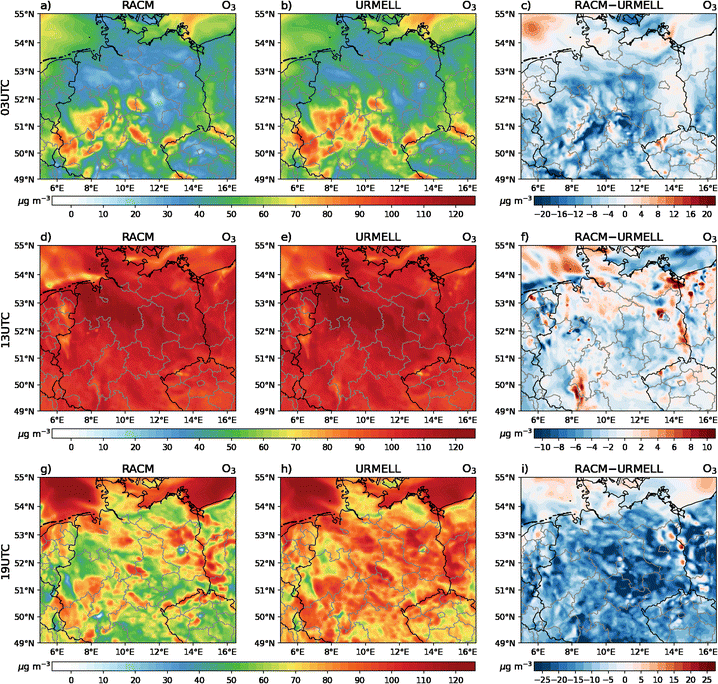 | ||
| Fig. 6 Ozone concentration for the 20th of May 2014 at 3 UTC (a–c)), 13 UTC (d–f) and 19 UTC (g–i) for RACM (a, d and g), URMELL (b, e and h) and the difference between RACM and URMEL (c, f and i). | ||
At 13 UTC, URMELL mainly predicts lower OH concentration, but in pine dominated areas a certain fraction of higher OH concentration occurs. Monoterpene concentrations are highest for beech followed by pine with URMELL. Here, pine emit high quantities of APIN while beech emit BPIN with individual degradation pathways, whereas in RACM both are treated as API. Furthermore, the strong OVOC emissions of beech support the changes to the OVOC emission split including the treatment of various individual species and their chemistry. The new OVOC as well as monoterpene treatment is essentially involved in OH and O3 concentration degradation processes. Isoprene emission is rather low for most parts, but gain in importance for oaks (Fig. S3.2-3 and S3.2-7†). Unfortunately, oaks often coincide with high NOx and monoterpene emissions, which limits the localization of isoprene dominated regimes where changes described in Sect. 4.1 could be identified. One area of isoprene-dominated chemistry could be clearly identified (red circled area in Fig. 3a). Here, the differential plot of isoprene (Fig. S4-4f†) indicates lower concentrations with URMELL while at the same time OH (Fig. S4-2f†) and O3 (Fig. 6f) values are higher. The enhanced oxidant concentrations also boost monoterpene degradation resulting in lower concentration values with URMELL (Fig. S4-3f†). For higher NOx concentrations, higher NO and NO2 (Fig. S4-1f†) values result in lower O3 concentration and vice versa, independent of the higher isoprene concentrations simulated with URMELL (see blue areas in the Netherlands and north of Berlin of Fig. S4-4f†).
At 19 UTC, pine forests become discernible in the O3 plot (Fig. 6), but again with higher values for URMELL due to a slower O3 decay. Monoterpene concentrations remain higher for beech, while much lower concentrations are reached for pine in combination with lower OH values than for beech-dominated areas. For further investigation of the monoterpene-related impacts, more knowledge about the individual monoterpenes is required.
6 Summary and outlook
In this study, we presented the new chemical mechanism URMELL, which was developed for better representation of the atmospheric chemistry of anthropogenic as well as biogenic VOCs in atmospheric models aiming at advanced air quality assessments and direct and explicit SOA modelling. The focus of the URMELL development was on isoprene and aromatics, as they are strongly linked to the budget of key pollutants and oxidants, such as O3, NOx and HOx.Originating from JAMv2b e.g., photolysis rates, certain reaction rate constants, the ozonolysis of ethene and propene, the treatment of RC(O)O2, aromatics, sesquiterpenes and isoprene were updated and extended. The impact of these changes was analyzed for specific gas-phase compounds under various meteorological conditions in urban and remote environments using the box model SPACCIM. In the analyses, compound concentrations predicted by the reduced gas-phase mechanisms JAMv2b, RACM or the near explicit mechanism MCM3.3.1 were compared with URMELL. For all urban scenarios, URMELL produces high correlation values above (or close to) 0.8 for most compounds compared to the MCM3.3.1. For the remote scenarios, URMELL simulates much higher oxidant concentrations than all other mechanisms. Based on performed sensitivity studies, approximately half of the increase can be addressed to non-isoprene (mainly photolysis and RC(O)O2) and the other half to isoprene-related mechanism changes. These adjustments in URMELL may help to overcome the recent shortage in the modeled HOx budget compared to measurements. Furthermore, the model performance is also improved for not so commonly studied compounds, such as glyoxal or methylglyoxal, due to better depiction of their diurnal cycles. From these box model analyses, it was possible to identify several aspects that require further investigation and more knowledge. The most relevant non-isoprene related aspects are: (i) formic acid gas-phase formation pathways, (ii) stable Criegee intermediate chemistry, (iii) verification of the degradation of aromatic ring-opening radicals with an ON(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)
O)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, (iv) alkene ozonolysis and (v) monoterpene treatment.
O group, (iv) alkene ozonolysis and (v) monoterpene treatment.
Even though a lot of research has been done on isoprene during the last decade, there are still uncertainties. For example, no explicit recommendation exists for ISOPOO pool composition. So far there is a multitude of varying yields for the complex C5H8 + OH chemistry applied in reduced chemical mechanisms. But, these ratios are crucial as they control the production of the most important reaction products MACR, MVK and HPALDs. Furthermore, the ISOPOO pool also determines the HOx recycling ability through H-shift reactions and, as the implementation of these H-shift reactions significantly increases the HOx concentration, but the rate constants need to be further determined.
URMELL has further been successfully applied in the chemical transport model COSMO-MUSCAT with good results in both urban and rural environments. Time series comparisons for O3 and NO2 confirm a good agreement between RACM and URMELL with correlation and COD values in close approximation (0.1 of each other). While RACM predicts slightly better correlation values for pine, URMELL better predicts O3 concentration during intense O3 episodes for OVOC, light-dependent monoterpene and isoprene emission dominated regions. A slower O3 decay and a lower NO2 peak are identified post meridiem for remote low NOx environments. This improves the model performance for most remote non-pine stations compared to RACM. A complete offset between measured and modeled O3 concentration is observed in spruce-dominated areas, which certainly requires further investigation. No improvement has been achieved for low isoprene emitting environments, such as pine forests. This also transfers to urban environments in isoprene-limited regions. The updates to anthropogenic chemistry improved the correlation values of the mean daily concentration cycles for urban and traffic sites not just for O3 but also for NO2. Therefore, URMELL is capable of predicting gas-phase concentrations of the air pollutants in urban and remote environments for different temperature and radiation regimes. As URMELL is designed to also enable direct and explicit SOA formation, it can be considered a suitable tool for simulating regional air quality in a changing atmosphere, because of the more sophisticated description of NMVOC oxidation compared to RACM.
Especially during stable, warm and sunny conditions, URMELL simulates higher O3 night-time concentrations than RACM, which reduces the offset between measured and modeled values. However, O3 maximum concentrations are still not reached mainly in monoterpene-dominated regimes, which could be related to the incomplete monoterpene oxidation schemes. The analyses allow to suggest a tree species dependent O3 cycle, but to verify this hypothesis additional research is needed. This includes the continuing development of more detailed chemical mechanisms capable of treating a variety of BVOCs including diverse monoterpenes, which are dominating the BVOC composition in coniferous forests.
Overall, it has to be noted that URMELL provides similar NOx and O3 concentration time series for all urban box model simulations compared to the MCM3.3.1 and RACM. However, the better agreement of frequently produced organic reaction products such as HCHO, GLY and MGLY of URMELL with the MCM3.3.1 indicates improved chemical degradation schemes. The improved isoprene HOx recycling in remote environments raise OH concentrations to the range of measured values (∼106 molecules per cm3). Furthermore, in CTM simulations URMELL produces similar O3 and NOx concentrations to RACM with slightly higher correlation values for urban and non-monoterpene-dominated areas. In addition, the integration of highly oxidized reaction products produces a much higher variety of species in URMELL and suggest a direct and explicit SOA formation potential. This will be addressed further in a follow-up study. Next to the anthropogenic and isoprene adjustments we want to emphasis the changes to RC(O)O2, especially the reaction rate constant kNO2.
A better understanding of the impact of specific characteristics of individual tree species on atmospheric chemistry is key for a livable future as many trees have been and are being planted in both urban and rural areas as part of re- and afforestation programs for climate adaptation and mitigation measures. As a warming climate is likely to increase BVOC emissions, their importance on atmospheric chemistry advances. Therefore, there is an urgent need to expand the knowledge on BVOC chemical degradation such as: (i) the identification of significant deviations between the reaction pathways of individual BVOCs within OVOCs and monoterpene clusters, (ii) the differentiation of the BVOC diversity in chemistry mechanisms and (iii) the formation of stable accretion products (ROOR) from the reaction between two peroxy radicals for varying BVOCs, as they are important SOA sources.
Despite mechanism development, there is also an essential demand for comprehensive and time-resolved measurements, particularly of VOCs and OVOCs, over long-time periods and vast spatial coverage to further evaluate model results. This is especially true for yet not so frequently measured substances such as formaldehyde, glyoxal, methylglyoxal and formic acid. Measurements of their main BVOC precursors, isoprene and the most common monoterpenes, would additionally help to constrain BVOC emission parameterizations in models and possibly help to identify stress induced BVOC emissions, such as under heat and drought conditions which alters the BVOC emission strength and composition. As heat and drought are predicted to increase in frequency and intensity with progressing climate warming in many regions such as Central Europe, possible feedback mechanisms can only be evaluated with a comprehensive chemical mechanism still applicable in chemical transport and climate models.
Author contributions
Conceptualization: MLL, RW, AT, EHH. Data curation: MLL. Formal Analysis: MLL. Funding acquisition: MLL. Investigation: MLL. Methodology: MLL, RW, AT, EHH. Project administration: MLL, RW. Software: MLL, RW. Supervision: MLL, RW. Validation: MLL. Visualization: MLL. Writing – original draft: MLL. Writing – review & editing: MLL, EHH, AT, RW, HH, IT.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the PhD scholarship of the German Federal Environmental Foundation (Deutsche Bundesstiftung Umwelt, DBU) granted to MLL (AZ 20016/452). We thank the German Environment Agency (Umweltbundesamt - UBA) for the access to the measurement data.References
- G. Curci, M. Beekmann, R. Vautard, G. Smiatek, R. Steinbrecher, J. Theloke and R. Friedrich, Modelling study of the impact of isoprene and terpene biogenic emissions on European ozone levels, Atmos. Environ., 2009, 43, 1444–1455 CrossRef CAS.
- O. J. Squire, A. T. Archibald, P. T. Griffiths, M. E. Jenkin, D. Smith and J. A. Pyle, Influence of isoprene chemical mechanism on modelled changes in tropospheric ozone due to climate and land use over the 21st century, Atmos. Chem. Phys., 2015, 15, 5123–5143 CrossRef CAS.
- K. N. Sartelet, F. Couvidat, C. Seigneur and Y. Roustan, Impact of biogenic emissions on air quality over Europe and North America, Atmos. Environ., 2012, 53, 131–141 CrossRef CAS.
- M. L. Luttkus, E. H. Hoffmann, L. Poulain, A. Tilgner and R. Wolke, The Effect of Land Use Classification on the Gas-Phase and Particle Composition of the Troposphere: Tree Species Versus Forest Type Information, J. Geophys. Res.: Atmos., 2022, 127, e2021JD035305 CrossRef CAS.
- M. Kanakidou, J. H. Seinfeld, S. N. Pandis, I. Barnes, F. J. Dentener, M. C. Facchini, R. V. Dingenen, B. Ervens, A. Nenes, C. J. Nielsen, E. Swietlicki, J. P. Putaud, Y. Balkanski, S. Fuzzi, J. Horth, G. K. Moortgat, R. Winterhalter, C. E. L. Myhre, K. Tsigaridis, E. Vignati, E. G. Stephanou and J. Wilson, Organic aerosol and global climate modelling: a review, Atmos. Chem. Phys., 2005, 5, 1053–1123 CrossRef CAS.
- E. von Schneidemesser, P. S. Monks, J. D. Allan, L. Bruhwiler, P. Forster, D. Fowler, A. Lauer, W. T. Morgan, P. Paasonen, M. Righi, K. Sindelarova and M. A. Sutton, Chemistry and the Linkages between Air Quality and Climate Change, Chem. Rev., 2015, 115, 3856–3897 CrossRef CAS.
- R. Atkinson, Atmospheric chemistry of VOCs and NOV, Atmos. Environ., 2000, 34, 2063–2101 CrossRef CAS.
- R. Atkinson and J. Arey, Gas-phase tropospheric chemistry of biogenic volatile organic compounds: a review, Atmos. Environ., 2003, 37, 197–219 CrossRef.
- J. Lelieveld, T. M. Butler, J. N. Crowley, T. J. Dillon, H. Fischer, L. Ganzeveld, H. Harder, M. G. Lawrence, M. Martinez, D. Taraborrelli and J. Williams, Atmospheric oxidation capacity sustained by a tropical forest, Nature, 2008, 452, 737–740 CrossRef CAS PubMed.
- L. K. Whalley, P. M. Edwards, K. L. Furneaux, A. Goddard, T. Ingham, M. J. Evans, D. Stone, J. R. Hopkins, C. E. Jones, A. Karunaharan, J. D. Lee, A. C. Lewis, P. S. Monks, S. J. Moller and D. E. Heard, Quantifying the magnitude of a missing hydroxyl radical source in a tropical rainforest, Atmos. Chem. Phys., 2011, 11, 7223–7233 CrossRef CAS.
- J. F. Pankow, An absorption model of gas/particle partitioning of organic compounds in the atmosphere, Atmos. Environ., 1994, 28, 185–188 CrossRef CAS.
- K. Lehtipalo, C. Yan, L. Dada, F. Bianchi, M. Xiao, R. Wagner, D. Stolzenburg, L. R. Ahonen, A. Amorim, A. Baccarini, P. S. Bauer, B. Baumgartner, A. Bergen, A.-K. Bernhammer, M. Breitenlechner, S. Brilke, A. Buchholz, S. B. Mazon, D. Chen, X. Chen, A. Dias, J. Dommen, D. C. Draper, J. Duplissy, M. Ehn, H. Finkenzeller, L. Fischer, C. Frege, C. Fuchs, O. Garmash, H. Gordon, J. Hakala, X. He, L. Heikkinen, M. Heinritzi, J. C. Helm, V. Hofbauer, C. R. Hoyle, T. Jokinen, J. Kangasluoma, V.-M. Kerminen, C. Kim, J. Kirkby, J. Kontkanen, A. Kürten, M. J. Lawler, H. Mai, S. Mathot, R. L. Mauldin, U. Molteni, L. Nichman, W. Nie, T. Nieminen, A. Ojdanic, A. Onnela, M. Passananti, T. Petäjä, F. Piel, V. Pospisilova, L. L. J. Quéléver, M. P. Rissanen, C. Rose, N. Sarnela, S. Schallhart, S. Schuchmann, K. Sengupta, M. Simon, M. Sipilä, C. Tauber, A. Tomé, J. Tröstl, O. Väisänen, A. L. Vogel, R. Volkamer, A. C. Wagner, M. Wang, L. Weitz, D. Wimmer, P. Ye, A. Ylisirniö, Q. Zha, K. S. Carslaw, J. Curtius, N. M. Donahue, R. C. Flagan, A. Hansel, I. Riipinen, A. Virtanen, P. M. Winkler, U. Baltensperger, M. Kulmala and D. R. Worsnop, Multicomponent new particle formation from sulfuric acid, ammonia, and biogenic vapors, Sci. Adv., 2018, 4, eaau5363 CrossRef CAS PubMed.
- I. Riipinen, J. R. Pierce, T. Yli-Juuti, T. Nieminen, S. Häkkinen, M. Ehn, H. Junninen, K. Lehtipalo, T. Petäjä, J. Slowik, R. Chang, N. C. Shantz, J. Abbatt, W. R. Leaitch, V.-M. Kerminen, D. R. Worsnop, S. N. Pandis, N. M. Donahue and M. Kulmala, Organic condensation: a vital link connecting aerosol formation to cloud condensation nuclei (CCN) concentrations, Atmos. Chem. Phys., 2011, 11, 3865–3878 CrossRef CAS.
- J. Kirkby, J. Duplissy, K. Sengupta, C. Frege, H. Gordon, C. Williamson, M. Heinritzi, M. Simon, C. Yan, J. Almeida, J. Tröstl, T. Nieminen, I. K. Ortega, R. Wagner, A. Adamov, A. Amorim, A.-K. Bernhammer, F. Bianchi, M. Breitenlechner, S. Brilke, X. Chen, J. Craven, A. Dias, S. Ehrhart, R. C. Flagan, A. Franchin, C. Fuchs, R. Guida, J. Hakala, C. R. Hoyle, T. Jokinen, H. Junninen, J. Kangasluoma, J. Kim, M. Krapf, A. Kürten, A. Laaksonen, K. Lehtipalo, V. Makhmutov, S. Mathot, U. Molteni, A. Onnela, O. Peräkylä, F. Piel, T. Petäjä, A. P. Praplan, K. Pringle, A. Rap, N. A. D. Richards, I. Riipinen, M. P. Rissanen, L. Rondo, N. Sarnela, S. Schobesberger, C. E. Scott, J. H. Seinfeld, M. Sipilä, G. Steiner, Y. Stozhkov, F. Stratmann, A. Tomé, A. Virtanen, A. L. Vogel, A. C. Wagner, P. E. Wagner, E. Weingartner, D. Wimmer, P. M. Winkler, P. Ye, X. Zhang, A. Hansel, J. Dommen, N. M. Donahue, D. R. Worsnop, U. Baltensperger, M. Kulmala, K. S. Carslaw and J. Curtius, Ion-induced nucleation of pure biogenic particles, Nature, 2016, 533, 521–526 CrossRef CAS.
- F. Riccobono, S. Schobesberger, C. Scott, J. Dommen, I. Ortega, L. Rondo, J. Almeida, A. Amorim, F. Bianchi, M. Breitenlechner, A. David, A. Downard, E. Dunne, J. Duplissy, S. Ehrhart, R. Flagan, A. Franchin, A. Hansel, H. Junninen, M. Kajos, H. Keskinen, A. Kupc, A. Kürten, A. Kvashin, A. Laaksonen, K. Lehtipalo, V. Makhmutov, S. Mathot, T. Nieminen, A. Onnela, T. Petäjä, A. Praplan, F. Santos, S. Schallhart, J. Seinfeld, M. Sipilä, D. Spracklen, Y. Stozhkov, F. Stratmann, A. Tomé, G. Tsagkogeorgas, P. Vaattovaara, Y. Viisanen, A. Vrtala, P. Wagner, E. Weingartner, H. Wex, D. Wimmer, K. Carslaw, J. Curtius, N. Donahue, J. Kirkby, M. Kulmala, D. Worsnop and U. Baltensperger, Oxidation products of biogenic emissions contribute to nucleation of atmospheric particles, Science, 2014, 344, 717–721 CrossRef CAS PubMed.
- F. Bianchi, J. Tröstl, H. Junninen, C. Frege, S. Henne, C. R. Hoyle, U. Molteni, E. Herrmann, A. Adamov, N. Bukowiecki, X. Chen, J. Duplissy, M. Gysel, M. Hutterli, J. Kangasluoma, J. Kontkanen, A. Kürten, H. E. Manninen, S. Münch, O. Peräkylä, T. Petäjä, L. Rondo, C. Williamson, E. Weingartner, J. Curtius, D. R. Worsnop, M. Kulmala, J. Dommen and U. Baltensperger, New particle formation in the free troposphere: A question of chemistry and timing, Science, 2016, 352, 1109–1112 CrossRef CAS PubMed.
- J. Tröstl, W. K. Chuang, H. Gordon, M. Heinritzi, C. Yan, U. Molteni, L. Ahlm, C. Frege, F. Bianchi, R. Wagner, M. Simon, K. Lehtipalo, C. Williamson, J. S. Craven, J. Duplissy, A. Adamov, J. Almeida, A.-K. Bernhammer, M. Breitenlechner, S. Brilke, A. Dias, S. Ehrhart, R. C. Flagan, A. Franchin, C. Fuchs, R. Guida, M. Gysel, A. Hansel, C. R. Hoyle, T. Jokinen, H. Junninen, J. Kangasluoma, H. Keskinen, J. Kim, M. Krapf, A. Kürten, A. Laaksonen, M. Lawler, M. Leiminger, S. Mathot, O. Möhler, T. Nieminen, A. Onnela, T. Petäjä, F. M. Piel, P. Miettinen, M. P. Rissanen, L. Rondo, N. Sarnela, S. Schobesberger, K. Sengupta, M. Sipilä, J. N. Smith, G. Steiner, A. Tomè, A. Virtanen, A. C. Wagner, E. Weingartner, D. Wimmer, P. M. Winkler, P. Ye, K. S. Carslaw, J. Curtius, J. Dommen, J. Kirkby, M. Kulmala, I. Riipinen, D. R. Worsnop, N. M. Donahue and U. Baltensperger, The role of low-volatility organic compounds in initial particle growth in the atmosphere, Nature, 2016, 533, 527–531 CrossRef PubMed.
- A. M. Dunker, B. Koo and G. Yarwood, Ozone sensitivity to isoprene chemistry and emissions and anthropogenic emissions in central California, Atmos. Environ., 2016, 145, 326–337 CrossRef CAS.
- M. Trainer, E. J. Williams, D. D. Parrish, M. P. Buhr, E. J. Allwine, H. H. Westberg, F. C. Fehsenfeld and S. C. Liu, Models and observations of the impact of natural hydrocarbons on rural ozone, Nature, 1987, 329, 705–707 CrossRef CAS.
- W. L. Chameides, R. W. Lindsay, J. Richardson and C. S. Kiang, The Role of Biogenic Hydrocarbons in Urban Photochemical Smog: Atlanta as a Case Study, Science, 1988, 241, 1473–1475 CrossRef CAS PubMed.
- N. R. Council, Rethinking the Ozone Problem in Urban and Regional Air Pollution, The National Academies Press, Washington, DC, 1991 Search PubMed.
- N. L. Ng, S. S. Brown, A. T. Archibald, E. Atlas, R. C. Cohen, J. N. Crowley, D. A. Day, N. M. Donahue, J. L. Fry, H. Fuchs, R. J. Griffin, M. I. Guzman, H. Herrmann, A. Hodzic, Y. Iinuma, J. L. Jimenez, A. Kiendler-Scharr, B. H. Lee, D. J. Luecken, J. Mao, R. McLaren, A. Mutzel, H. D. Osthoff, B. Ouyang, B. Picquet-Varrault, U. Platt, H. O. T. Pye, Y. Rudich, R. H. Schwantes, M. Shiraiwa, J. Stutz, J. A. Thornton, A. Tilgner, B. J. Williams and R. A. Zaveri, Nitrate radicals and biogenic volatile organic compounds: oxidation, mechanisms, and organic aerosol, Atmos. Chem. Phys., 2017, 17, 2103–2162 CrossRef CAS.
- A. Zare, P. S. Romer, T. Nguyen, F. N. Keutsch, K. Skog and R. C. Cohen, A comprehensive organic nitrate chemistry: insights into the lifetime of atmospheric organic nitrates, Atmos. Chem. Phys., 2018, 18, 15419–15436 CrossRef CAS.
- T. Berndt, S. Richters, T. Jokinen, N. Hyttinen, T. Kurtén, R. V. Otkjær, H. G. Kjaergaard, F. Stratmann, H. Herrmann, M. Sipilä, M. Kulmala and M. Ehn, Hydroxyl radical-induced formation of highly oxidized organic compounds, Nat. Commun., 2016, 7, 13677 CrossRef CAS PubMed.
- T. Jokinen, T. Berndt, R. Makkonen, V.-M. Kerminen, H. Junninen, P. Paasonen, F. Stratmann, H. Herrmann, A. B. Guenther, D. R. Worsnop, M. Kulmala, M. Ehn and M. Sipilä, Production of extremely low volatile organic compounds from biogenic emissions: Measured yields and atmospheric implications, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 7123–7128 CrossRef CAS PubMed.
- A. B. Guenther, X. Jiang, C. L. Heald, T. Sakulyanontvittaya, T. Duhl, L. K. Emmons and X. Wang, The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1): an extended and updated framework for modeling biogenic emissions, Geosci. Model Dev., 2012, 5, 1471–1492 CrossRef.
- J. Peeters, T. L. Nguyen and L. Vereecken, HOx radical regeneration in the oxidation of isoprene, Phys. Chem. Chem. Phys., 2009, 11, 5935–5939 RSC.
- X. Ren, J. R. Olson, J. H. Crawford, W. H. Brune, J. Mao, R. B. Long, Z. Chen, G. Chen, M. A. Avery, G. W. Sachse, J. D. Barrick, G. S. Diskin, L. G. Huey, A. Fried, R. C. Cohen, B. Heikes, P. O. Wennberg, H. B. Singh, D. R. Blake and R. E. Shetter, HOx chemistry during INTEX-A 2004: Observation, model calculation, and comparison with previous studies, J. Geophys. Res.: Atmos., 2008, 113, D05310 Search PubMed.
- D. Taraborrelli, M. G. Lawrence, T. M. Butler, R. Sander and J. Lelieveld, Mainz Isoprene Mechanism 2 (MIM2): an isoprene oxidation mechanism for regional and global atmospheric modelling, Atmos. Chem. Phys., 2009, 9, 2751–2777 CrossRef CAS.
- P. O. Wennberg, K. H. Bates, J. D. Crounse, L. G. Dodson, R. C. McVay, L. A. Mertens, T. B. Nguyen, E. Praske, R. H. Schwantes, M. D. Smarte, J. M. St Clair, A. P. Teng, X. Zhang and J. H. Seinfeld, Gas-Phase Reactions of Isoprene and Its Major Oxidation Products, Chem. Rev., 2018, 118, 3337–3390 CrossRef CAS.
- M. A. H. Khan, M. E. Jenkin, A. Foulds, R. G. Derwent, C. J. Percival and D. E. Shallcross, A modeling study of secondary organic aerosol formation from sesquiterpenes using the STOCHEM global chemistry and transport model: SOA FORMATION FROM SESQUITERPENES, J. Geophys. Res.: Atmos., 2017, 122, 4426–4439 CrossRef CAS.
- R. Baraldi, C. Chieco, L. Neri, O. Facini, F. Rapparini, L. Morrone, A. Rotondi and G. Carriero, An integrated study on air mitigation potential of urban vegetation: From a multi-trait approach to modeling, Urban For. Urban Green., 2019, 41, 127–138 CrossRef.
- W. P. L. Carter, Updated Maximum Incremental Reactivity Scale and Hydrocarbon Bin Reactivities for Regulatory Applications (California Air Resources Board Contract 07-339), College of Engineering Center for Environmental Research and Technology University of California, Riverside, CA, 2010 Search PubMed.
- S. Gu, A. Guenther and C. Faiola, Effects of Anthropogenic and Biogenic Volatile Organic Compounds on Los Angeles Air Quality, Environ. Sci. Technol., 2021, 55, 12191–12201 CrossRef CAS PubMed.
- M. L. Luttkus, R. Wolke, B. Heinold, A. Tilgner, L. Poulain and H. Herrmann, in Air Pollution Modeling and its Application XXVII, ed. C. Mensink and V. Matthias, Springer Berlin Heidelberg, Berlin, Heidelberg, 2021, pp. 11–17 Search PubMed.
- H. Li, M. R. Canagaratna, M. Riva, P. Rantala, Y. Zhang, S. Thomas, L. Heikkinen, P.-M. Flaud, E. Villenave, E. Perraudin, D. Worsnop, M. Kulmala, M. Ehn and F. Bianchi, Atmospheric organic vapors in two European pine forests measured by a Vocus PTR-TOF: insights into monoterpene and sesquiterpene oxidation processes, Atmos. Chem. Phys., 2021, 21, 4123–4147 CrossRef CAS.
- W. R. Stockwell, F. Kirchner, M. Kuhn and S. Seefeld, A new mechanism for regional atmospheric chemistry modeling, J. Geophys. Res., 1997, 102, 25847–25879 CrossRef CAS.
- W. S. Goliff, W. R. Stockwell and C. V. Lawson, The regional atmospheric chemistry mechanism, version 2, Atmos. Environ., 2013, 68, 174–185 CrossRef CAS.
- M. G. Schultz, S. Stadtler, S. Schröder, D. Taraborrelli, B. Franco, J. Krefting, A. Henrot, S. Ferrachat, U. Lohmann, D. Neubauer, C. Siegenthaler-Le Drian, S. Wahl, H. Kokkola, T. Kühn, S. Rast, H. Schmidt, P. Stier, D. Kinnison, G. S. Tyndall, J. J. Orlando and C. Wespes, The chemistry–climate model ECHAM6.3-HAM2.3-MOZ1.0, Geosci. Model Dev., 2018, 11, 1695–1723 CrossRef CAS.
- L. K. Emmons, S. Walters, P. G. Hess, J.-F. Lamarque, G. G. Pfister, D. Fillmore, C. Granier, A. Guenther, D. Kinnison, T. Laepple, J. Orlando, X. Tie, G. Tyndall, C. Wiedinmyer, S. L. Baughcum and S. Kloster, Description and evaluation of the Model for Ozone and Related chemical Tracers, version 4 (MOZART-4), Geosci. Model Dev., 2010, 3, 43–67 CrossRef.
- G. Yarwood, Y. Shi and R. Beardsley, Develop CB7 Chemical Mechanism for CAMx Ozone Modeling, Final Rep., 2021, 582-21-21802-020 Search PubMed.
- W. P. L. Carter, Development of the SAPRC-07 chemical mechanism, Atmos. Environ., 2010, 44, 5324–5335 CrossRef CAS.
- W. P. L. Carter, Documentation of the SAPRC-18 Mechanism (Report to California Air Resources Board Contract No 11-761), College of Engineering Center for Environmental Research and Technology University of California, Riverside, CA, 2020 Search PubMed.
- A. Mutzel, Y. Zhang, O. Böge, M. Rodigast, A. Kolodziejczyk, X. Wang and H. Herrmann, Importance of secondary organic aerosol formation of α-pinene, limonene, and m-cresol comparing day- and nighttime radical chemistry, Atmos. Chem. Phys., 2021, 21, 8479–8498 CrossRef CAS.
- J. H. Seinfeld, G. B. Erdakos, W. E. Asher and J. F. Pankow, Modeling the Formation of Secondary Organic Aerosol (SOA). 2. The Predicted Effects of Relative Humidity on Aerosol Formation in the α-Pinene-, β-Pinene-, Sabinene-, Δ 3 -Carene-, and Cyclohexene-Ozone Systems, Environ. Sci. Technol., 2001, 35, 1806–1817 CrossRef CAS PubMed.
- J. F. Pankow, J. H. Seinfeld, W. E. Asher and G. B. Erdakos, Modeling the Formation of Secondary Organic Aerosol. 1. Application of Theoretical Principles to Measurements Obtained in the α-Pinene/, β-Pinene/, Sabinene/, Δ 3 -Carene/, and Cyclohexene/Ozone Systems, Environ. Sci. Technol., 2001, 35, 1164–1172 CrossRef CAS.
- D. Thomsen, J. Elm, B. Rosati, J. T. Skønager, M. Bilde and M. Glasius, Large Discrepancy in the Formation of Secondary Organic Aerosols from Structurally Similar Monoterpenes, ACS Earth Space Chem., 2021, 5, 632–644 CrossRef CAS.
- C. J. Colville and R. J. Griffin, The roles of individual oxidants in secondary organic aerosol formation from Δ3-carene: 2. soa formation and oxidant contribution, Atmos. Environ., 2004, 38, 4013–4023 CrossRef CAS.
- J. D. Blande, J. K. Holopainen and Ü. Niinemets, Plant volatiles in polluted atmospheres: stress responses and signal degradation: Plant volatiles in a polluted atmosphere, Plant, Cell Environ., 2014, 37, 1892–1904 CrossRef CAS PubMed.
- J. K. Holopainen and J. Gershenzon, Multiple stress factors and the emission of plant VOCs, Trends Plant Sci., 2010, 15, 176–184 CrossRef CAS PubMed.
- Ü. Niinemets, Mild versus severe stress and BVOCs: thresholds, priming and consequences, Trends Plant Sci., 2009, 15, 145–153 CrossRef PubMed.
- Ü. Niinemets, A. Arneth, U. Kuhn, R. K. Monson, J. Peñuelas and M. Staudt, The emission factor of volatile isoprenoids: stress, acclimation, and developmental responses, Biogeosciences, 2010, 7, 2203–2223 CrossRef.
- R. Grote, M. Sharma, A. Ghirardo and J.-P. Schnitzler, A New Modeling Approach for Estimating Abiotic and Biotic Stress-Induced de novo Emissions of Biogenic Volatile Organic Compounds From Plants, Front. For. Glob. Change, 2019, 2, 26 CrossRef.
- C. Bloss, V. Wagner, M. E. Jenkin, R. Volkamer, W. J. Bloss, J. D. Lee, D. E. Heard, K. Wirtz, M. Martin-Reviejo, G. Rea, J. C. Wenger and M. J. Pilling, Development of a detailed chemical mechanism (MCMv3.1) for the atmospheric oxidation of aromatic hydrocarbons, Atmos. Chem. Phys., 2005, 5, 641–664 CrossRef CAS.
- M. E. Jenkin, S. M. Saunders and M. J. Pilling, The tropospheric degradation of volatile organic compounds: a protocol for mechanism development, Atmos. Environ., 1997, 31, 81–104 CrossRef CAS.
- M. E. Jenkin, S. M. Saunders, V. Wagner and M. J. Pilling, Protocol for the development of the Master Chemical Mechanism, MCM v3 (Part B): tropospheric degradation of aromatic volatile organic compounds, Atmos. Chem. Phys., 2003, 3, 181–193 CrossRef CAS.
- M. E. Jenkin, K. P. Wyche, C. J. Evans, T. Carr, P. S. Monks, M. R. Alfarra, M. H. Barley, G. B. McFiggans, J. C. Young and A. R. Rickard, Development and chamber evaluation of the MCM v3.2 degradation scheme for β-caryophyllene, Atmos. Chem. Phys., 2012, 12, 5275–5308 CrossRef CAS.
- M. E. Jenkin, J. C. Young and A. R. Rickard, The MCM v3.3.1 degradation scheme for isoprene, Atmos. Chem. Phys., 2015, 15, 11433–11459 CrossRef CAS.
- S. M. Saunders, M. E. Jenkin, R. G. Derwent and M. J. Pilling, Protocol for the development of the Master Chemical Mechanism, MCM v3 (Part A): tropospheric degradation of non-aromatic volatile organic compounds, Atmos. Chem. Phys., 2003, 3, 161–180 CrossRef CAS.
- D. E. Kinnison, G. P. Brasseur, S. Walters, R. R. Garcia, D. R. Marsh, F. Sassi, V. L. Harvey, C. E. Randall, L. Emmons, J. F. Lamarque, P. Hess, J. J. Orlando, X. X. Tie, W. Randel, L. L. Pan, A. Gettelman, C. Granier, T. Diehl, U. Niemeier and A. J. Simmons, Sensitivity of chemical tracers to meteorological parameters in the MOZART-3 chemical transport model, J. Geophys. Res.: Atmos., 2007, 112, D20302 CrossRef.
- F. Paulot, J. D. Crounse, H. G. Kjaergaard, A. Kürten, J. M. S. Clair, J. H. Seinfeld and P. O. Wennberg, Unexpected Epoxide Formation in the Gas-Phase Photooxidation of Isoprene, Science, 2009, 325, 730–733 CrossRef CAS PubMed.
- G. M. Wolfe, J. D. Crounse, J. D. Parrish, J. M. St. Clair, M. R. Beaver, F. Paulot, T. P. Yoon, P. O. Wennberg and F. N. Keutsch, Photolysis, OH reactivity and ozone reactivity of a proxy for isoprene-derived hydroperoxyenals (HPALDs), Phys. Chem. Chem. Phys., 2012, 14, 7276–7286 RSC.
- M. Karl, H.-P. Dorn, F. Holland, R. Koppmann, D. Poppe, L. Rupp, A. Schaub and A. Wahner, Product study of the reaction of OH radicals with isoprene in the atmosphere simulation chamber SAPHIR, J. Atmos. Chem., 2006, 55, 167–187 CrossRef CAS.
- R. Wolke, W. Schröder, R. Schrödner and E. Renner, Influence of grid resolution and meteorological forcing on simulated European air quality: A sensitivity study with the modeling system COSMO–MUSCAT, Atmos. Environ., 2012, 53, 110–130 CrossRef CAS.
- R. Stern, P. Builtjes, M. Schaap, R. Timmermans, R. Vautard, A. Hodzic, M. Memmesheimer, H. Feldmann, E. Renner, R. Wolke and A. Kerschbaumer, A model inter-comparison study focussing on episodes with elevated PM10 concentrations, Atmos. Environ., 2008, 42, 4567–4588 CrossRef CAS.
- E. Renner and R. Wolke, Modelling the formation and atmospheric transport of secondary inorganic aerosols with special attention to regions with high ammonia emissions, Atmos. Environ., 2010, 44, 1904–1912 CrossRef CAS.
- R. Atkinson, D. L. Baulch, R. A. Cox, J. N. Crowley, R. F. Hampson, R. G. Hynes, M. E. Jenkin, M. J. Rossi and J. Troe, Evaluated kinetic and photochemical data for atmospheric chemistry: Volume I - gas phase reactions of Ox, HOx, NOx and SOx species, Atmos. Chem. Phys., 2004, 4, 1461–1738 CrossRef CAS.
- R. Atkinson, D. L. Baulch, R. A. Cox, J. N. Crowley, R. F. Hampson, R. G. Hynes, M. E. Jenkin, M. J. Rossi, J. Troe and I. Subcommittee, Evaluated kinetic and photochemical data for atmospheric chemistry: Volume II – gas phase reactions of organic species, Atmos. Chem. Phys., 2006, 6, 3625–4055 CrossRef CAS.
- R. Atkinson, D. L. Baulch, R. A. Cox, J. N. Crowley, R. F. Hampson, R. G. Hynes, M. E. Jenkin, M. J. Rossi and J. Troe, Evaluated kinetic and photochemical data for atmospheric chemistry: Volume III – gas phase reactions of inorganic halogens, Atmos. Chem. Phys., 2007, 7, 981–1191 CrossRef CAS.
- R. Atkinson, D. L. Baulch, R. A. Cox, J. N. Crowley, R. F. Hampson, R. G. Hynes, M. E. Jenkin, M. J. Rossi, J. Troe and T. J. Wallington, Evaluated kinetic and photochemical data for atmospheric chemistry: Volume IV – gas phase reactions of organic halogen species, Atmos. Chem. Phys., 2008, 8, 4141–4496 CrossRef CAS.
- J. N. Crowley, M. Ammann, R. A. Cox, R. G. Hynes, M. E. Jenkin, A. Mellouki, M. J. Rossi, J. Troe and T. J. Wallington, Evaluated kinetic and photochemical data for atmospheric chemistry: Volume V – heterogeneous reactions on solid substrates, Atmos. Chem. Phys., 2010, 10, 9059–9223 CrossRef CAS.
- M. Ammann, R. A. Cox, J. N. Crowley, M. E. Jenkin, A. Mellouki, M. J. Rossi, J. Troe and T. J. Wallington, Evaluated kinetic and photochemical data for atmospheric chemistry: Volume VI – heterogeneous reactions with liquid substrates, Atmos. Chem. Phys., 2013, 13, 8045–8228 CrossRef.
- R. A. Cox, M. Ammann, J. N. Crowley, H. Herrmann, M. E. Jenkin, V. F. McNeill, A. Mellouki, J. Troe and T. J. Wallington, Evaluated kinetic and photochemical data for atmospheric chemistry: Volume VII – Criegee intermediates, Atmos. Chem. Phys., 2020, 20, 13497–13519 CrossRef CAS.
- A. Mellouki, M. Ammann, R. A. Cox, J. N. Crowley, H. Herrmann, M. E. Jenkin, V. F. McNeill, J. Troe and T. J. Wallington, Evaluated kinetic and photochemical data for atmospheric chemistry: volume VIII – gas-phase reactions of organic species with four, or more, carbon atoms ( ≥ C4), Atmos. Chem. Phys., 2021, 21, 4797–4808 CrossRef CAS.
- M. E. Jenkin, R. Valorso, B. Aumont, A. R. Rickard and T. J. Wallington, Estimation of rate coefficients and branching ratios for gas-phase reactions of OH with aliphatic organic compounds for use in automated mechanism construction, Atmos. Chem. Phys., 2018, 18, 9297–9328 CrossRef CAS.
- J.-F. Müller, T. Stavrakou and J. Peeters, Chemistry and deposition in the Model of Atmospheric composition at Global and Regional scales using Inversion Techniques for Trace gas Emissions (MAGRITTE v1.1) – Part 1: Chemical mechanism, Geosci. Model Dev., 2019, 12, 2307–2356 CrossRef.
- H. Hou, B. Wang and Y. Gu, Mechanism of the OH+CH2CO reaction, Phys. Chem. Chem. Phys., 2000, 2, 2329–2334 RSC.
- L. Sheps, B. Rotavera, A. J. Eskola, D. L. Osborn, C. A. Taatjes, K. Au, D. E. Shallcross, M. A. H. Khan and C. J. Percival, The reaction of Criegee intermediate CH 2 OO with water dimer: primary products and atmospheric impact, Phys. Chem. Chem. Phys., 2017, 19, 21970–21979 RSC.
- T. B. Nguyen, G. S. Tyndall, J. D. Crounse, A. P. Teng, K. H. Bates, R. H. Schwantes, M. M. Coggon, L. Zhang, P. Feiner, D. O. Milller, K. M. Skog, J. C. Rivera-Rios, M. Dorris, K. F. Olson, A. Koss, R. J. Wild, S. S. Brown, A. H. Goldstein, J. A. de Gouw, W. H. Brune, F. N. Keutsch, J. H. Seinfeld and P. O. Wennberg, Atmospheric fates of Criegee intermediates in the ozonolysis of isoprene, Phys. Chem. Chem. Phys., 2016, 18, 10241–10254 RSC.
- B. Franco, T. Blumenstock, C. Cho, L. Clarisse, C. Clerbaux, P.-F. Coheur, M. De Mazière, I. De Smedt, H.-P. Dorn, T. Emmerichs, H. Fuchs, G. Gkatzelis, D. W. T. Griffith, S. Gromov, J. W. Hannigan, F. Hase, T. Hohaus, N. Jones, A. Kerkweg, A. Kiendler-Scharr, E. Lutsch, E. Mahieu, A. Novelli, I. Ortega, C. Paton-Walsh, M. Pommier, A. Pozzer, D. Reimer, S. Rosanka, R. Sander, M. Schneider, K. Strong, R. Tillmann, M. Van Roozendael, L. Vereecken, C. Vigouroux, A. Wahner and D. Taraborrelli, Ubiquitous atmospheric production of organic acids mediated by cloud droplets, Nature, 2021, 593, 233–237 CrossRef CAS PubMed.
- D. B. Millet, M. Baasandorj, D. K. Farmer, J. A. Thornton, K. Baumann, P. Brophy, S. Chaliyakunnel, J. A. de Gouw, M. Graus, L. Hu, A. Koss, B. H. Lee, F. D. Lopez-Hilfiker, J. A. Neuman, F. Paulot, J. Peischl, I. B. Pollack, T. B. Ryerson, C. Warneke, B. J. Williams and J. Xu, A large and ubiquitous source of atmospheric formic acid, Atmos. Chem. Phys., 2015, 15, 6283–6304 CrossRef CAS.
- B. Yuan, P. R. Veres, C. Warneke, J. M. Roberts, J. B. Gilman, A. Koss, P. M. Edwards, M. Graus, W. C. Kuster, S.-M. Li, R. J. Wild, S. S. Brown, W. P. Dubé, B. M. Lerner, E. J. Williams, J. E. Johnson, P. K. Quinn, T. S. Bates, B. Lefer, P. L. Hayes, J. L. Jimenez, R. J. Weber, R. Zamora, B. Ervens, D. B. Millet, B. Rappenglück and J. A. de Gouw, Investigation of secondary formation of formic acid: urban environment vs. oil and gas producing region, Atmos. Chem. Phys., 2015, 15, 1975–1993 CrossRef CAS.
- L. Chen, Y. Huang, Y. Xue, Z. Jia and W. Wang, OH-initiated atmospheric degradation of hydroxyalkyl hydroperoxides: mechanism, kinetics, and structure–activity relationship, Atmos. Chem. Phys., 2022, 22, 3693–3711 CrossRef CAS.
- M. E. Jenkin, M. D. Hurley and T. J. Wallington, Investigation of the radical product channel of the CH3C(O)O2 + HO2 reaction in the gas phase, Phys. Chem. Chem. Phys., 2007, 9, 3149–3162 RSC.
- M. E. Jenkin, R. Valorso, B. Aumont and A. R. Rickard, Estimation of rate coefficients and branching ratios for reactions of organic peroxy radicals for use in automated mechanism construction, Atmos. Chem. Phys., 2019, 19, 7691–7717 CrossRef CAS.
- K. H. Bates, D. J. Jacob, K. Li, P. D. Ivatt, M. J. Evans, Y. Yan and J. Lin, Development and evaluation of a new compact mechanism for aromatic oxidation in atmospheric models, Atmos. Chem. Phys., 2021, 21, 18351–18374 CrossRef CAS.
- L. Vereecken, P. T. M. Carlsson, A. Novelli, F. Bernard, S. S. Brown, C. Cho, J. N. Crowley, H. Fuchs, W. Mellouki, D. Reimer, J. Shenolikar, R. Tillmann, L. Zhou, A. Kiendler-Scharr and A. Wahner, Theoretical and experimental study of peroxy and alkoxy radicals in the NO 3 -initiated oxidation of isoprene, Phys. Chem. Chem. Phys., 2021, 23, 5496–5515 RSC.
- J. H. Seinfeld and S. N. Pandis, Atmospheric Chemistry and Physics: from Air Pollution to Climate Change, J. Wiley, Hoboken, N.J, 2nd edn, 2006 Search PubMed.
- A. P. Teng, J. D. Crounse and P. O. Wennberg, Isoprene Peroxy Radical Dynamics, J. Am. Chem. Soc., 2017, 139, 5367–5377 CrossRef CAS PubMed.
- R. H. Schwantes, L. K. Emmons, J. J. Orlando, M. C. Barth, G. S. Tyndall, S. R. Hall, K. Ullmann, J. M. St. Clair, D. R. Blake, A. Wisthaler and T. P. V. Bui, Comprehensive isoprene and terpene gas-phase chemistry improves simulated surface ozone in the southeastern US, Atmos. Chem. Phys., 2020, 20, 3739–3776 CrossRef CAS.
- R. Wolke, A. M. Sehili, M. Simmel, O. Knoth, A. Tilgner and H. Herrmann, SPACCIM: A parcel model with detailed microphysics and complex multiphase chemistry, Atmos. Environ., 2005, 39, 4375–4388 CrossRef CAS.
- R. Wolke, O. Knoth, O. Hellmuth, W. Schröder and E. Renner, in Parallel Computing, ed. G. R. Joubert, W. E. Nagel, F. J. Peters and W. V. Walter, North-Holland, 2004, vol. 13, pp. 363–369 Search PubMed.
- M. L. Bell, K. Ebisu and R. D. Peng, Community-level spatial heterogeneity of chemical constituent levels of fine particulates and implications for epidemiological research, J. Exposure Sci. Environ. Epidemiol., 2011, 21, 372–384 CrossRef CAS PubMed.
- R. Schrödner, A. Tilgner, R. Wolke and H. Herrmann, Modeling the multiphase processing of an urban and a rural air mass with COSMO–MUSCAT, Urban Climate, 2014, 10, 720–731 CrossRef.
- Y. Chen, R. Wolke, L. Ran, W. Birmili, G. Spindler, W. Schröder, H. Su, Y. Cheng, I. Tegen and A. Wiedensohler, A parameterization of the heterogeneous hydrolysis of N2O5 \hack\break for mass-based aerosol models: improvement of \hack\break particulate nitrate prediction, Atmos. Chem. Phys., 2018, 18, 673–689 CrossRef CAS.
- M. E. Jenkin, M. A. H. Khan, D. E. Shallcross, R. Bergström, D. Simpson, K. L. C. Murphy and A. R. Rickard, The CRI v2.2 reduced degradation scheme for isoprene, Atmos. Environ., 2019, 212, 172–182 CrossRef CAS.
- A. Guenther, C. N. Hewitt, D. Erickson, R. Fall, C. Geron, T. Graedel, P. Harley, L. Klinger, M. Lerdau, W. A. Mckay, T. Pierce, B. Scholes, R. Steinbrecher, R. Tallamraju, J. Taylor and P. Zimmerman, A global model of natural volatile organic compound emissions, J. Geophys. Res.: Atmos., 1995, 100, 8873–8892 CrossRef CAS.
- J. G. J. Olivier, A. F. Bouwman, C. W. M. Van derMaas, J. J. M. Berdowski, C. Veldt, J. P. J. Bloos, A. J. H. Visschedijk, P. Y. J. Zandveld and J. L. Haverlag, Description of EDGAR Version 2.0. A Set of Global Emission Inventories of Greenhouse Gases and Ozone-Depleting Substances for All Anthropogenic and Most Natural Sources on a Per Country Basis and on 1degree X1degree Grid (RIVM Rapport 771060002, RIVM, Bilthoven, Netherlands, 1996 Search PubMed.
- B. Ervens, CAPRAM 2.4 (MODAC mechanism): An extended and condensed tropospheric aqueous phase mechanism and its application, J. Geophys. Res., 2003, 108, 4426 CrossRef.
- H. Herrmann, D. Hoffmann, T. Schaefer, P. Bräuer and A. Tilgner, Tropospheric Aqueous-Phase Free-Radical Chemistry: Radical Sources, Spectra, Reaction Kinetics and Prediction Tools, ChemPhysChem, 2010, 11, 3796–3822 CrossRef CAS PubMed.
- E. H. Hoffmann, A. Tilgner, R. Wolke, O. Böge, A. Walter and H. Herrmann, Oxidation of substituted aromatic hydrocarbons in the tropospheric aqueous phase: kinetic mechanism development and modelling, Phys. Chem. Chem. Phys., 2018, 20, 10960–10977 RSC.
- A. Tilgner, P. Bräuer, R. Wolke and H. Herrmann, Modelling multiphase chemistry in deliquescent aerosols and clouds using CAPRAM3.0i, J. Atmos. Chem., 2013, 70, 221–256 CrossRef CAS.
- A. Tilgner and H. Herrmann, Radical-driven carbonyl-to-acid conversion and acid degradation in tropospheric aqueous systems studied by CAPRAM, Atmos. Environ., 2010, 44, 5415–5422 CrossRef CAS.
- P. Middleton, W. R. Stockwell and W. P. L. Carter, Aggregation and analysis of volatile organic compound emissions for regional modeling, Atmos. Environ., Part A, 1990, 24, 1107–1133 CrossRef.
- U. Im, R. Bianconi, E. Solazzo, I. Kioutsioukis, A. Badia, A. Balzarini, R. Baró, R. Bellasio, D. Brunner, C. Chemel, G. Curci, J. Flemming, R. Forkel, L. Giordano, P. Jiménez-Guerrero, M. Hirtl, A. Hodzic, L. Honzak, O. Jorba, C. Knote, J. J. P. Kuenen, P. A. Makar, A. Manders-Groot, L. Neal, J. L. Pérez, G. Pirovano, G. Pouliot, R. S. Jose, N. Savage, W. Schroder, R. S. Sokhi, D. Syrakov, A. Torian, P. Tuccella, J. Werhahn, R. Wolke, K. Yahya, R. Zabkar, Y. Zhang, J. Zhang, C. Hogrefe and S. Galmarini, Evaluation of operational on-line-coupled regional air quality models over Europe and North America in the context of AQMEII phase 2. Part I: Ozone, Atmos. Environ., 2015, 115, 404–420 CrossRef CAS.
- U. Im, R. Bianconi, E. Solazzo, I. Kioutsioukis, A. Badia, A. Balzarini, R. Baró, R. Bellasio, D. Brunner, C. Chemel, G. Curci, H. D. van der Gon, J. Flemming, R. Forkel, L. Giordano, P. Jiménez-Guerrero, M. Hirtl, A. Hodzic, L. Honzak, O. Jorba, C. Knote, P. A. Makar, A. Manders-Groot, L. Neal, J. L. Pérez, G. Pirovano, G. Pouliot, R. S. Jose, N. Savage, W. Schroder, R. S. Sokhi, D. Syrakov, A. Torian, P. Tuccella, K. Wang, J. Werhahn, R. Wolke, R. Zabkar, Y. Zhang, J. Zhang, C. Hogrefe and S. Galmarini, Evaluation of operational online-coupled regional air quality models over Europe and North America in the context of AQMEII phase 2. Part II: Particulate matter, Atmos. Environ., 2015, 115, 421–441 CrossRef CAS.
- E. Solazzo, R. Bianconi, G. Pirovano, V. Matthias, R. Vautard, M. D. Moran, K. W. Appel, B. Bessagnet, J. Brandt, J. H. Christensen, C. Chemel, I. Coll, J. Ferreira, R. Forkel, X. V. Francis, G. Grell, P. Grossi, A. B. Hansen, A. I. Miranda, U. Nopmongcol, M. Prank, K. N. Sartelet, M. Schaap, J. D. Silver, R. S. Sokhi, J. Vira, J. Werhahn, R. Wolke, G. Yarwood, J. Zhang, S. T. Rao and S. Galmarini, Operational model evaluation for particulate matter in Europe and North America in the context of AQMEII, Atmos. Environ., 2012, 53, 75–92 CrossRef CAS.
- E. Solazzo, R. Bianconi, R. Vautard, K. W. Appel, M. D. Moran, C. Hogrefe, B. Bessagnet, J. Brandt, J. H. Christensen, C. Chemel, I. Coll, H. D. van der Gon, J. Ferreira, R. Forkel, X. V. Francis, G. Grell, P. Grossi, A. B. Hansen, A. Jeričević, L. Kraljević, A. I. Miranda, U. Nopmongcol, G. Pirovano, M. Prank, A. Riccio, K. N. Sartelet, M. Schaap, J. D. Silver, R. S. Sokhi, J. Vira, J. Werhahn, R. Wolke, G. Yarwood, J. Zhang, S. T. Rao and S. Galmarini, Model evaluation and ensemble modelling of surface-level ozone in Europe and North America in the context of AQMEII, Atmos. Environ., 2012, 53, 60–74 CrossRef CAS.
- R. Steinbrecher, G. Smiatek, R. Köble, G. Seufert, J. Theloke, K. Hauff, P. Ciccioli, R. Vautard and G. Curci, Intra- and inter-annual variability of VOC emissions from natural and semi-natural vegetation in Europe and neighbouring countries, Atmos. Environ., 2009, 43, 1380–1391 CrossRef CAS.
- K. H. Schlünzen and S. Pahl, Modification of dry deposition in a developing sea-breeze circulation—A numerical case study, Atmos. Environ., Part A, 1992, 26, 51–61 CrossRef.
- K. H. Schlünzen, U. Bungert, D. D. Flagg, B. H. Fock, A. Gierisch, D. Grawe, P. Kirschner, C. Lüpkes, V. Reinhardt, H. Ries, R. Schoetter, C. Spensberger and M. Uphoff, Technical Documentation of the Multiscale Model System M-SYS Search PubMed.
- K. H. Schlunzen and J. J. Katzfey, Relevance of sub-grid-scale land-use effects for mesoscale models, Tellus A, 2003, 55, 232–246 CrossRef.
- T. B. Nguyen, J. D. Crounse, A. P. Teng, J. M. St. Clair, F. Paulot, G. M. Wolfe and P. O. Wennberg, Rapid deposition of oxidized biogenic compounds to a temperate forest, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, E392–E401 CAS.
- T. Emmerichs, A. Kerkweg, H. Ouwersloot, S. Fares, I. Mammarella and D. Taraborrelli, A revised dry deposition scheme for land–atmosphere exchange of trace gases in ECHAM/MESSy v2.54, Geosci. Model Dev., 2021, 14, 495–519 CrossRef CAS.
- Z. Wu, L. Zhang, J. T. Walker, P. A. Makar, J. A. Perlinger and X. Wang, Extension of a gaseous dry deposition algorithm to oxidized volatile organic compounds and hydrogen cyanide for application in chemistry transport models, Geosci. Model Dev., 2021, 14, 5093–5105 CrossRef CAS PubMed.
- L. Zhang, J. R. Brook and R. Vet, A revised parameterization for gaseous dry deposition in air-quality models, Atmos. Chem. Phys., 2003, 3, 2067–2082 CrossRef CAS.
- D. Jeong, R. Seco, L. Emmons, R. Schwantes, Y. Liu, K. A. McKinney, S. T. Martin, F. N. Keutsch, D. Gu, A. B. Guenther, O. Vega, J. Tota, R. A. F. Souza, S. R. Springston, T. B. Watson and S. Kim, Reconciling Observed and Predicted Tropical Rainforest OH Concentrations, J. Geophys. Res.: Atmos., 2022, 127, e2020JD032901 CrossRef CAS.
- S. M. MacDonald, H. Oetjen, A. S. Mahajan, L. K. Whalley, P. M. Edwards, D. E. Heard, C. E. Jones and J. M. C. Plane, DOAS measurements of formaldehyde and glyoxal above a south-east Asian tropical rainforest, Atmos. Chem. Phys., 2012, 12, 5949–5962 CrossRef CAS.
- F. L. Eisele, D. J. Tanner, C. A. Cantrell and J. G. Calvert, Measurements and steady state calculations of OH concentrations at Mauna Loa Observatory, J. Geophys. Res.: Atmos., 1996, 101, 14665–14679 CrossRef CAS.
- F. Holland, A. Hofzumahaus, J. Schäfer, A. Kraus and H.-W. Pätz, Measurements of OH and HO2 radical concentrations and photolysis frequencies during BERLIOZ, J. Geophys. Res.: Atmos., 2003, 108, PHO 2-1–PHO 2-23 CrossRef.
- V. Michoud, S. Sauvage, T. Léonardis, I. Fronval, A. Kukui, N. Locoge and S. Dusanter, Field measurements of methylglyoxal using proton transfer reaction time-of-flight mass spectrometry and comparison to the DNPH–HPLC–UV method, Atmos. Meas. Tech., 2018, 11, 5729–5740 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: URMELL species list, complex rate coefficients, chemical and photolysis reactions, additional information about box and CTM model simulation with SPACCIM and COSMO-MUSCAT including figures and statistics. See DOI: https://doi.org/10.1039/d3ea00094j |
| This journal is © The Royal Society of Chemistry 2024 |