Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and properties of metal trifluoride complexes with amide-functionalised tacn macrocycles and radiofluorination of [GaF3(L1)] by 18F/19F isotopic exchange†
Charley
O'Callaghan
a,
Victoria K.
Greenacre
 a,
Rhys P.
King
a,
Julian
Grigg
b,
Julie M.
Herniman
a,
Graeme
McRobbie
b and
Gillian
Reid
a,
Rhys P.
King
a,
Julian
Grigg
b,
Julie M.
Herniman
a,
Graeme
McRobbie
b and
Gillian
Reid
 *a
*a
aSchool of Chemistry, University of Southampton, Southampton SO17 1BJ, UK. E-mail: G.Reid@soton.ac.uk
bGE HealthCare, Pollards Wood, Nightingales Lane, Chalfont St Giles, Bucks, HP8 4SP, UK
First published on 22nd August 2024
Abstract
Three amide-functionalised tacn macrocyclic derivatives (tacn = 1,4,7-triazacyclononane) are reported, two tris-amide derivatives, L1 containing three –CH2C(O)NHPh pendant arms, L2 containing three –CH2CH2C(O)NHiPr pendant arms, and one mono-amide, L3, containing iPr groups on two of the tacn amine functions and a single –CH2C(O)NHPh function on the third. The reactions of these new ligands towards [MF3(dmso)(OH2)2] (M = Al, Ga) and towards FeF3·3H2O in alcoholic solution afford the complexes [MF3(L)] (L = L1–L3) in good yields as powdered solids. These complexes are characterised by IR and multinuclear NMR spectroscopy (diamagnetic species only) and mass spectrometry. [GaF3(L1)], [GaF3(L3)] and [FeF3(L3)] are also characterised by single crystal X-ray analysis. The corresponding reactions involving [InF3(dmso)(OH2)2] yield mixtures of products (along with F−), consistent with the M–F bond strengths decreasing as group 13 is descended. A few crystals of the target complex, [InF3(L2)], were also obtained from one such reaction. All of the complexes adopt fac-octahedral coordination via the amine N-donor atoms and retain the three fluoride ligands both in solution and in the solid state. Extensive intramolecular hydrogen-bonding involving the amide NH pendant groups and the MF3 moieties is evident in the crystal structures. In the isostructural [MF3(L3)] (M = Ga, Fe) complexes the head-to-tail C(O)NH⋯F H-bonded dimers observed in the solid state resemble those found frequently in organic compounds and that form the cornerstone of many supramolecular assemblies. This is consistent with the MF3 fragments being strong H-bond acceptors. Radiofluorination of [GaF3(L1)] by 18F/19F isotopic exchange in MeOH at 3 μmol mL−1 precursor concentration and using aqueous [18F]F− in target water (75%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25%) with gentle heating (80 °C, 10 min) gave ca. 20% radiochemical yield of [Ga18FF2(L1)]. In contrast, no 18F incorporation occurs with [GaF3(L3)] under any of the conditions explored.
25%) with gentle heating (80 °C, 10 min) gave ca. 20% radiochemical yield of [Ga18FF2(L1)]. In contrast, no 18F incorporation occurs with [GaF3(L3)] under any of the conditions explored.
Introduction
The last decade has seen considerable research into new metal–fluoride complexes, much of which has been motivated by their potential as carriers for the positron-emitting 18F radioisotope for positron emission tomography (PET) imaging in medicine.1–3 In selecting the target metal complexes, key requirements are that the metal–fluoride bond is sufficiently strong to allow easy and fast incorporation of the radiofluorine at a late stage in the procedure and for the radiolabelled complex to be stable to hydrolysis/substitution/decomposition under physiological conditions. Towards this objective, several systems incorporating aluminium(III), gallium(III), iron(III) and scandium(III) fluoride species bound to neutral4–7 or anionic8–12 tacn-based ligands (tacn = 1,4,7-triazacyclononane) have been reported.Notably, the [MF3(L)] (L = Me3-tacn, BnMe2-tacn, 1,4,7-tris(2-amino-3,5-di-tert-butylbenzyl)-1,4,7-triazacyclononane) complexes frequently form extended H-bonding networks with lattice water or MeOH molecules via F⋯HOH or F⋯HOCH3 interactions.13,14 These complexes can also function as metalloligands towards Lewis acids such as Gd3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 and alkali metal cations, as well as [NH4]+.16 We were therefore interested in expanding the coordination chemistry of these ‘MF3’ fragments (M = Al, Ga, In, Fe) with tacn-derivatives carrying pendant H-bond donor functions, since these may be relevant in facilitating the delivery of highly electronegative fluoride ions to the metal in the course of radiofluorination, and may also lead to unusual inorganic H-bonded assemblies in the final complexes.
15 and alkali metal cations, as well as [NH4]+.16 We were therefore interested in expanding the coordination chemistry of these ‘MF3’ fragments (M = Al, Ga, In, Fe) with tacn-derivatives carrying pendant H-bond donor functions, since these may be relevant in facilitating the delivery of highly electronegative fluoride ions to the metal in the course of radiofluorination, and may also lead to unusual inorganic H-bonded assemblies in the final complexes.
A plethora of tacn-derivatives with pendant arm functions is known. In considering possible H-bond donor pendant functions, amide groups were selected since these frequently feature in bioconjugates in medical imaging agents, for example, through coupling of a pendant carboxylic acid group to a peptide-based amine function and are therefore generally biocompatible.17
Several amide-functionalised tacn ligands have been reported in the literature and their coordination chemistry with (mostly) divalent metal salts described.18–23 Of these, the tris(amide)-tacn ligands typically function as hexadentate chelators via the three macrocyclic amine donor atoms and the three carboxamide oxygens atoms (N3O3 donor set), affording distorted octahedral dicationic metal species, that can be isolated with non-coordinating (e.g. nitrate, perchlorate or tetrafluoroborate) anions.
In the present study we describe the coordination of pyramidal ‘MF3’ fragments towards both mono- and tris(amide)-functionalised tacn ligands, L1–L3, specifically with a view to utilising the macrocycle as a neutral N3-donor ligand, intentionally leaving the pendant amide group(s) uninvolved in the metal coordination sphere and available as potential H-bond donor group(s). The synthesis and spectroscopic characterisation of L1–L3 (Scheme 1) derived from the nine-membered tacn (1,4,7-triazacyclononane) core are reported, L1 and L2 contain different linkers (–CH2C(O)NHPh and –CH2CH2C(O)NHiPr), while L3 contains a single –CH2C(O)NHPh pendant group.
We then explored their coordination towards various ‘MF3’ fragments (M = Al, Ga, In, Fe), using spectroscopic and crystallographic data to probe how the amide groups influence their molecular and extended structures, all of which are based upon fac-octahedral geometries with N3F3 donor sets at the metal, leaving the amide pendant arms uncoordinated. Single crystal X-ray structures for L1·HCl, L1·HNO3, along with four representative metal complexes, [GaF3(L1)]·1.5MeOH·0.5H2O, [InF3(L2)], [GaF3(L3)] and [FeF3(L3)], are reported, and the role of the H-bond donor amide groups in the solid state structures discussed. Experiments aimed at radiofluorination of the Ga(III) complexes containing L1 and L3via18F/19F isotopic exchange are also discussed.
Experimental
Infrared spectra were recorded as Nujol mulls between CsI plates using a PerkinElmer Spectrum 100 spectrometer over the range 4000–200 cm−1. 1H, 13C{1H}, 19F{1H}, 27Al and 71Ga NMR spectra were recorded from CD3OD solutions (unless otherwise stated) using a Bruker AV400 spectrometer and referenced to SiMe4via the residual solvent resonance (1H and 13C), external CFCl3 (19F), aqueous [Al(H2O)6]3+ (27Al) and aqueous [Ga(H2O)6]3+ (71Ga), respectively. Low resolution mass spectra were obtained in MeOH by positive ion electrospray MS using a Waters (Manchester, UK) Acquity TQD tandem quadrupole mass spectrometer. Samples were introduced to the mass spectrometer via an Acquity H-Class quaternary solvent manager (with TUV detector at 254 nm, sample and column manager). Ultra-high performance liquid chromatography was undertaken using Waters BEH C18 column (50 mm × 2.1 mm 1.7 μm). Gradient elution from 20% acetonitrile/80% water (0.2% formic acid) to 100% acetonitrile (0.2% formic acid) was performed over five min at a flow rate of 0.6 mL min−1. High resolution positive ion electrospray mass spectra were recorded using a MaXis (Bruker Daltonics, Bremen, Germany) time of flight (TOF) mass spectrometer. Samples were infused into the ion source using a syringe driver at a constant flow rate of 3 μL min−1. Duplicate microanalyses were undertaken at Medac Ltd, with the majority of measurements within ±0.4% of the theoretical value. However, in a few cases the values are slightly outside this range, reflecting the inherent variability of microanalytical measurements across different facilities.24 The purification of ligands L1 and L3 used a Biotage Selekt flash chromatography system (reverse-phase Sfär C18 column).All preparations were carried out under atmospheric conditions. Reagents FeF3·3H2O and InF3·3H2O (Sigma-Aldrich), GaF3·3H2O (Strem Chemicals) and AlF3·3H2O (Alfa Aesar) were used as received. 1,4,7-Triazacyclononane (tacn),25 and 1,4-diisopropyl-1,4,7-triazacyclononane (iPr2-tacn)26 were synthesised as described in the literature. The 2-chloro-N-phenylacetamide was synthesised as described in the literature and recrystallised from CH2Cl2/hexane before use.27N-Isopropylyacrylamide (Sigma-Aldrich) was used as supplied. The metal precursor complexes, [MF3(dmso)(OH2)2] (M = Al, Ga, In), were prepared using the methods reported.28
Ligand preparations
L1: Tacn (0.506 g, 3.92 mmol) was added to a rapidly stirring mixture of K2CO3 (6.50 g, 47.0 mmol) in acetone (80 mL) in a 250 mL round bottomed flask equipped with magnetic stirring. This was stirred for 10 min at room temperature. A solution of 2-chloro-N-phenylacetamide (2.00 g, 11.8 mmol, 3 mol. eq.) in acetone (40 mL) was then added dropwise and the reaction mixture stirred at room temperature overnight. The resulting yellow mixture was filtered and 1.5 M NaOH (ca. 150 mL) was added. This solution was extracted with CHCl3 (3 × 150 mL) and the organic extracts were combined. The solvent was removed using a rotary evaporator to leave a tan coloured oil. This was subsequently purified by flash chromatography. The crude ligand L1 was dissolved in a minimal volume of CHCl3 and four mass equivalents of silica was added to the solution, creating a slurry. The CHCl3 solvent was removed by rotary evaporation to leave a free-flowing orange powder. This was dry-loaded onto the Biotage (gradient = 40%–60% H2O/MeCN each containing 0.01% formic acid, over 4 column volumes where: CV = 164 mL and flow rate = 50 mL min−1). The fractions corresponding to L1 were combined and the solvent was removed in vacuo. The residue was washed with fresh MeCN, causing precipitation of a white solid, which was collected by filtration and dried. Yield: 1.96 g, 3.53 mmol (90%). 1H NMR (298 K, CD3OD): δ (ppm) = 8.54 (s, N![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 7.50–7.46 (m, [6H], Ar
), 7.50–7.46 (m, [6H], Ar![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 7.03–6.94 (m, [9H], Ar
), 7.03–6.94 (m, [9H], Ar![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 4.85 (s, H2O), 3.87 (br s, [6H], C
), 4.85 (s, H2O), 3.87 (br s, [6H], C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 3.14 (br s, [12H], tacn-C
2), 3.14 (br s, [12H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2). 13C{1H} NMR (298 K, CD3OD): δ (ppm) = 169.5 (
2). 13C{1H} NMR (298 K, CD3OD): δ (ppm) = 169.5 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 139.4 (Ar
O), 139.4 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 129.8 (Ar
), 129.8 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 125.4 (Ar
), 125.4 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 121.4 (Ar
), 121.4 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 59.5 (
), 59.5 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 51.1 (tacn-
H2), 51.1 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2). 13C 135-DEPT NMR (298 K, CD3OD): δ (ppm) = 129.6 (Ar
H2). 13C 135-DEPT NMR (298 K, CD3OD): δ (ppm) = 129.6 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 125.1 (Ar
), 125.1 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 121.1 (Ar
), 121.1 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 59.2 (
), 59.2 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 50.9 (tacn-
H2), 50.9 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2). HR ESI+ MS (CH3OH): found: m/z = 529.2921 [L1 + H]+ (calculated for [C30H37N6O3]+: m/z = 529.2927); 551.2738 [L1 + Na]+ (calculated for [C30H36N6NaO3]+: m/z = 551.2747). IR (Nujol, ν/cm−1): 3344 m, 3180 w (NH), 1682 s, 1596 s (C
H2). HR ESI+ MS (CH3OH): found: m/z = 529.2921 [L1 + H]+ (calculated for [C30H37N6O3]+: m/z = 529.2927); 551.2738 [L1 + Na]+ (calculated for [C30H36N6NaO3]+: m/z = 551.2747). IR (Nujol, ν/cm−1): 3344 m, 3180 w (NH), 1682 s, 1596 s (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
L1·HCl: L1 was converted to its HCl salt for X-ray structure analysis. One drop of 12 M HCl was added to a solution of L1 (0.01 g, 0.019 mmol) in deuterated methanol (2 mL). Crystals suitable for X-ray diffraction were grown via slow evaporation of solvent over a period of four weeks in the fridge. 1H NMR (298 K, CD3OD): δ (ppm) = 8.09 (s, N![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 7.56–7.53 (m, [6H], Ar
), 7.56–7.53 (m, [6H], Ar![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 7.25–7.21 (m, [6H], Ar
), 7.25–7.21 (m, [6H], Ar![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 7.10–7.06 (m, [3H], Ar
), 7.10–7.06 (m, [3H], Ar![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 5.03 (s, H2O), 4.23 (br s, [6H], C
), 5.03 (s, H2O), 4.23 (br s, [6H], C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 3.66 (br s, [12H], tacn-C
2), 3.66 (br s, [12H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2). 13C{1H} NMR (298 K, CD3OD): δ (ppm) = 167.8 (
2). 13C{1H} NMR (298 K, CD3OD): δ (ppm) = 167.8 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 139.0 (Ar
O), 139.0 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 130.0 (Ar
), 130.0 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 126.0 (Ar
), 126.0 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 121.6 (Ar
), 121.6 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 59.9 (
), 59.9 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 52.9 (tacn-
H2), 52.9 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2).
H2).
L2: Tacn (0.650 g, 5.03 mmol) and N-isopropylacrylamide (1.74 g, 15.3 mmol, 5% mol excess) were placed into a 250 mL round bottomed flask equipped with magnetic stirring and a reflux condenser and degassed MeOH (100 mL) was added. The mixture was heated at reflux for 18 h. The resulting pale-yellow solution was filtered through Celite to remove any particulates, and the volatiles were then removed via rotary evaporation, leaving a pale-yellow oil. This was dissolved in 1.0 M HCl (20.5 mL) and extracted with CHCl3 (3 × 50 mL) to remove the excess N-isopropylacrylamide. The organic phases were discarded. The pH of the remaining aqueous phase was adjusted to >12 using 2 M KOH (50 mL) and extracted with 3 × 50 mL CHCl3. The combined organic extracts were dried over MgSO4, filtered, and the volatiles removed via rotary evaporation. This left the product as a viscous yellow oil. Yield: 2.01 g, 4.46 mmol (89%). 1H NMR (298 K, CD3OD): δ (ppm) = 4.85 (s, H2O), 3.98–3.92 (septet, 3JH–H = 6.5 Hz, [3H], iPr-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 3.01–2.87 (br m, [6H], C
), 3.01–2.87 (br m, [6H], C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.83 (br s, [12H], tacn-C
2), 2.83 (br s, [12H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.38–2.34 (br t, [6H], C
2), 2.38–2.34 (br t, [6H], C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 1.14 (d, 3JH–H = 6.6 Hz, [18H], iPr-C
2), 1.14 (d, 3JH–H = 6.6 Hz, [18H], iPr-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 3). 1H NMR (298 K, CDCl3): δ (ppm) = 6.72 (br, [3H], N
3). 1H NMR (298 K, CDCl3): δ (ppm) = 6.72 (br, [3H], N![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 4.11–4.01 (septet, 3JH–H = 6.6 Hz, [3H], iPr-C
), 4.11–4.01 (septet, 3JH–H = 6.6 Hz, [3H], iPr-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 2.81–2.78 (t, 3JH–H = 6.5 Hz, [6H], C
), 2.81–2.78 (t, 3JH–H = 6.5 Hz, [6H], C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.76 (s, [12H], tacn-C
2), 2.76 (s, [12H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.29–2.26 (t, 3JH–H = 6.5 Hz, [6H], C
2), 2.29–2.26 (t, 3JH–H = 6.5 Hz, [6H], C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 1.14 (d, 3JH–H = 6.6 Hz, [18H], iPr-C
2), 1.14 (d, 3JH–H = 6.6 Hz, [18H], iPr-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 3). 13C{1H} NMR (298 K, CDCl3): δ (ppm) = 171.3 (
3). 13C{1H} NMR (298 K, CDCl3): δ (ppm) = 171.3 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 55.6 (tacn-
O), 55.6 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 54.8 (
H2), 54.8 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 41.0 (iPr-
H2), 41.0 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H), 34.3 (
H), 34.3 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 22.9 (iPr-
H2), 22.9 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H3). 13C DEPT-135 NMR (298 K, CDCl3): δ (ppm) = 55.4 (tacn-
H3). 13C DEPT-135 NMR (298 K, CDCl3): δ (ppm) = 55.4 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 54.6 (
H2), 54.6 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 40.8 (iPr-
H2), 40.8 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H), 34.1 (
H), 34.1 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 22.7 (iPr-
H2), 22.7 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H3). HR ESI+ MS (CH3OH): found: m/z = 491.3683 [L2 + Na]+ (calculated: m/z = 491.3680), 469.3863 [L2 + H]+ (calculated for [C24H49N6O3]+: m/z = 469.3861), 356.3019 [{iPrC(O)NH(CH2)2}2-tacn + H]+ (calculated for [C18H37N5O2]+: m/z = 356.30), 235.1967 [L2 + 2H]2+ (calculated for [C24H50N6O3]2+: m/z = 235.1967). IR (neat film, ν/cm−1): 3460 br, 3287 br (OH), 3078 br (NH), 1644 vs, 1554 s (C
H3). HR ESI+ MS (CH3OH): found: m/z = 491.3683 [L2 + Na]+ (calculated: m/z = 491.3680), 469.3863 [L2 + H]+ (calculated for [C24H49N6O3]+: m/z = 469.3861), 356.3019 [{iPrC(O)NH(CH2)2}2-tacn + H]+ (calculated for [C18H37N5O2]+: m/z = 356.30), 235.1967 [L2 + 2H]2+ (calculated for [C24H50N6O3]2+: m/z = 235.1967). IR (neat film, ν/cm−1): 3460 br, 3287 br (OH), 3078 br (NH), 1644 vs, 1554 s (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
L3·HCl: In a 100 mL round bottomed flask equipped with magnetic stirring, iPr2-tacn (4.50 g, 21.1 mmol) was dissolved in degassed acetone (100 mL). Powdered K2CO3 (4.50 g, 32.5 mmol) was added. This was stirred at room temperature for 15 min and 2-chloro-N-phenylacetamide (3.57 g, 21.1 mmol) dissolved in degassed acetone (100 mL) was then added dropwise. The reaction mixture was stirred at room temperature overnight. After filtering through Celite, the filtrate was adjusted to pH 12 using aqueous 1.5 M NaOH (ca. 200 mL). This was extracted with 3 × 200 mL CHCl3, and the organic phases collected and combined. The solvent was removed via rotary evaporation to leave a tan-coloured oil. This crude product was purified in batches by flash chromatography using a Biotage Selekt flash chromatography system (dry-loaded; gradient = 30%–40% H2O/MeCN each containing 0.01% formic acid, over 4 column volumes where: CV = 164 mL and flow rate = 50 mL min−1). A portion of the crude product (3.00 g) was dissolved in a minimal amount of CHCl3. Then, four mass equivalents of chromatography-grade silica was added to the solution, creating a tan-coloured slurry. The CHCl3 solvent was removed via the rotary evaporator to leave a free-flowing orange powder. The pure fractions containing L3·HCl were collected, and the solvent was removed in vacuo leaving a viscous tan-coloured oil. After washing with MeCN and further drying, the product was isolated as the protonated ligand salt, L3·HCl. Yield = 2.50 g. 1H NMR (298 K, CD3OD): δ (ppm) = 8.32 (s, N![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 7.59–7.55 (m, [2H], Ar
), 7.59–7.55 (m, [2H], Ar![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 7.32–7.28 (m, [2H], Ar
), 7.32–7.28 (m, [2H], Ar![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 7.10–7.06, 4.90 (H2O), 3.59 (s, [2H], C
), 7.10–7.06, 4.90 (H2O), 3.59 (s, [2H], C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 3.35 (septet, [2H], iPr-C
2), 3.35 (septet, [2H], iPr-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 3.10–3.03 (br m, [4H], tacn-C
), 3.10–3.03 (br m, [4H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.96–2.87 (br m, [4H], tacn-C
2), 2.96–2.87 (br m, [4H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.84–2.74 (br m, [4H], tacn-C
2), 2.84–2.74 (br m, [4H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 1.25 (br d, [12H], iPr-C
2), 1.25 (br d, [12H], iPr-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 3). 13C{1H} NMR (298 K, CD3OD): δ (ppm) = 167.4 (
3). 13C{1H} NMR (298 K, CD3OD): δ (ppm) = 167.4 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 139.9 (Ar
O), 139.9 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 130.0 (Ar
), 130.0 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 125.3 (Ar
), 125.3 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 121.1 (Ar
), 121.1 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 58.5 (
), 58.5 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 55.3 (iPr-
H2), 55.3 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H), 50.3 (tacn-
H), 50.3 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 48.3 (tacn-
H2), 48.3 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 46.3 (tacn-
H2), 46.3 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 18.4 (iPr-
H2), 18.4 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H3), 18.1 (iPr-
H3), 18.1 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H3). 135-DEPT 13C NMR (298 K, CD3OD): δ (ppm) = 129.7 (Ar
H3). 135-DEPT 13C NMR (298 K, CD3OD): δ (ppm) = 129.7 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 125.0 (Ar
), 125.0 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 120.8 (Ar
), 120.8 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 58.2 (
), 58.2 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 55.0 (iPr-
H2), 55.0 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H), 50.0 (tacn-
H), 50.0 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 48.0 (tacn-
H2), 48.0 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 46.0 (tacn-
H2), 46.0 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 18.1 (iPr-
H2), 18.1 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H3), 17.8 (iPr-
H3), 17.8 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H3). HR ESI+ MS (CH3OH): found: m/z = 347.2812 [L3 + H]+ (calculated for [C20H35N4O]+: m/z = 347.2805).
H3). HR ESI+ MS (CH3OH): found: m/z = 347.2812 [L3 + H]+ (calculated for [C20H35N4O]+: m/z = 347.2805).
L3: L3·HCl (2.20 g, 5.74 mmol) was treated with a solution of NEt3 (20% by volume in deionised water) until pH > 12 (ca. 150 mL). A white precipitate formed initially, which then dissolved, and an orange oil was deposited. CHCl3 (100 mL) was added to dissolve the oil and the organic phase was separated and retained. Removal of the CHCl3 solvent in vacuo yielded a tan-coloured oil. Addition of pentane (25 mL) produced a dark-yellow solution, leaving a small amount of brown residue, which was discarded. The pentane was removed in vacuo, leaving the final product as a dark yellow oil (1.10 g, 55%). 1H NMR (298 K, CD3OD): δ (ppm) = 7.56–7.53 (m, [2H], Ar![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 7.35–7.29 (m, [2H], Ar
), 7.35–7.29 (m, [2H], Ar![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 7.13–7.08 (m, [1H], Ar
), 7.13–7.08 (m, [1H], Ar![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 4.85 (H2O), 2.91 (s, [2H], C
), 4.85 (H2O), 2.91 (s, [2H], C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 3.35 (septet, 3JH–H = 6.6 Hz, [2H], iPr-C
2), 3.35 (septet, 3JH–H = 6.6 Hz, [2H], iPr-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 2.86 (s, [4H], tacn-C
), 2.86 (s, [4H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.75–2.70 (br m, [8H], tacn-C
2), 2.75–2.70 (br m, [8H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 0.95 (d, 3JH–H = 6.6 Hz, [12H], iPr-C
2), 0.95 (d, 3JH–H = 6.6 Hz, [12H], iPr-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 3). 13C{1H} NMR (298 K, CD3OD): δ (ppm) = 173.6 (
3). 13C{1H} NMR (298 K, CD3OD): δ (ppm) = 173.6 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 139.4 (Ar
O), 139.4 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 129.8 (Ar
), 129.8 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 125.4 (Ar
), 125.4 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 121.5 (Ar
), 121.5 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 62.9 (
), 62.9 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 59.8 (tacn-
H2), 59.8 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 56.0 (iPr-
H2), 56.0 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H), 18.3 (iPr-
H), 18.3 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H3). 135-DEPT 13C NMR (298 K, CD3OD): δ (ppm) = 129.9 (Ar
H3). 135-DEPT 13C NMR (298 K, CD3OD): δ (ppm) = 129.9 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 125.5 (Ar
), 125.5 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 121.7 (Ar
), 121.7 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 63.0 (
), 63.0 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 59.9 (tacn-
H2), 59.9 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 56.1 (iPr-
H2), 56.1 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H), 49.7 (tacn-
H), 49.7 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 18.4 (iPr-
H2), 18.4 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H3). 1H NMR (298 K, CDCl3): δ (ppm) = 10.84 (s, [1H], N
H3). 1H NMR (298 K, CDCl3): δ (ppm) = 10.84 (s, [1H], N![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 7.59–7.56 (m, [2H], Ar
), 7.59–7.56 (m, [2H], Ar![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 7.34–7.28 (m, [2H], Ar
), 7.34–7.28 (m, [2H], Ar![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 7.11–7.05 (m, [1H], Ar
), 7.11–7.05 (m, [1H], Ar![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 3.37 (s, [2H], C
), 3.37 (s, [2H], C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.88 (septet, 3JH–H = 6.1 Hz [2H], iPr-C
2), 2.88 (septet, 3JH–H = 6.1 Hz [2H], iPr-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 2.82 (br s, [4H], tacn-C
), 2.82 (br s, [4H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.70 (br s, [8H], tacn-C
2), 2.70 (br s, [8H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 1.56 (H2O), 0.93 (d, 3JH–H = 6.6 Hz, [12H], iPr-C
2), 1.56 (H2O), 0.93 (d, 3JH–H = 6.6 Hz, [12H], iPr-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 3). 13C{1H} NMR (298 K, CDCl3): δ (ppm) = 171.3 (
3). 13C{1H} NMR (298 K, CDCl3): δ (ppm) = 171.3 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 139.6 (Ar
O), 139.6 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 128.7 (Ar
), 128.7 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 123.7 (Ar
), 123.7 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 119.9 (Ar
), 119.9 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 62.5 (
), 62.5 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 59.4 (tacn-
H2), 59.4 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 55.8 (iPr-
H2), 55.8 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H), 54.9 (tacn-
H), 54.9 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 49.1 (tacn-
H2), 49.1 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 18.0 (iPr-
H2), 18.0 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H3). HR ESI+ MS (CH3OH): found: m/z = 347.2812 [L3 + H]+ (calculated for [C20H35N4O]+m/z = 347.2805). IR (neat film, ν/cm−1): 3400 br, 3200 br (OH), 3053, 3033 w (NH), 2961, 2930, 2812 (C–H stretch), 1678 br s, 1600 (C
H3). HR ESI+ MS (CH3OH): found: m/z = 347.2812 [L3 + H]+ (calculated for [C20H35N4O]+m/z = 347.2805). IR (neat film, ν/cm−1): 3400 br, 3200 br (OH), 3053, 3033 w (NH), 2961, 2930, 2812 (C–H stretch), 1678 br s, 1600 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
Metal trifluoride complexes
![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 7.03–6.94 (m, [9H], Ar
), 7.03–6.94 (m, [9H], Ar![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 4.85 (H2O), 3.87 (s, [6H], C
), 4.85 (H2O), 3.87 (s, [6H], C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 3.21–3.09 (br m, [12H], tacn-C
2), 3.21–3.09 (br m, [12H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.66 (dmso). 19F{1H} NMR (CD3OD, 298 K): δ (ppm) = −174.1 (br s). 13C{1H} NMR (CD3OD, 298 K): δ (ppm) = 168.5 (
2), 2.66 (dmso). 19F{1H} NMR (CD3OD, 298 K): δ (ppm) = −174.1 (br s). 13C{1H} NMR (CD3OD, 298 K): δ (ppm) = 168.5 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 139.4 (Ar
O), 139.4 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 129.8 (Ar
), 129.8 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 125.4 (Ar
), 125.4 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 121.4 (Ar
), 121.4 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 59.5 (
), 59.5 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 51.1 (tacn-
H2), 51.1 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 40.6 (dmso). HR ESI+ MS (CH3OH): found: 529.2934 [L1 + H]+ (calculated: m/z = 529.2927), 551.2744 [L1 + Na]+ (calculated: m/z = 551.2747), 613.2666 [AlF3(L1) + H]+ (calculated: m/z = 613.2689). IR (Nujol, ν/cm−1): 3450 br, 3300 m (OH), 3146 w (NH), 1673 m (C
H2), 40.6 (dmso). HR ESI+ MS (CH3OH): found: 529.2934 [L1 + H]+ (calculated: m/z = 529.2927), 551.2744 [L1 + Na]+ (calculated: m/z = 551.2747), 613.2666 [AlF3(L1) + H]+ (calculated: m/z = 613.2689). IR (Nujol, ν/cm−1): 3450 br, 3300 m (OH), 3146 w (NH), 1673 m (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1621 w (HOH), 1600 m (C
O), 1621 w (HOH), 1600 m (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1032 br (S
O), 1032 br (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, dmso), 694 m, 673 sh (Al–F).
O, dmso), 694 m, 673 sh (Al–F).
![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 7.38–7.29 (br m, [6H], Ar
), 7.38–7.29 (br m, [6H], Ar![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 7.18 (br s, [N
), 7.18 (br s, [N![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ]), 7.10–7.08 (m, [3H], Ar
]), 7.10–7.08 (m, [3H], Ar![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 4.85 (s, H2O), 4.05 (s, [6H], C
), 4.85 (s, H2O), 4.05 (s, [6H], C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 3.45 (br s, [12H], tacn-C
2), 3.45 (br s, [12H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.66 (s, dmso). 13C{1H} NMR (CD3OD, 298 K): δ (ppm) = 168.3 (
2), 2.66 (s, dmso). 13C{1H} NMR (CD3OD, 298 K): δ (ppm) = 168.3 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 139.6 (Ar
O), 139.6 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 130.0 (Ar
), 130.0 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 125.5 (Ar
), 125.5 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 121.4 (Ar
), 121.4 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 58.5 (
), 58.5 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 50.2 (tacn-
H2), 50.2 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 40.6 (dmso). 19F{1H} NMR (CD3OD, 298 K): δ (ppm) = −180 (v br with partially resolved coupling to 69/71Ga). 71Ga NMR (CD3OD, 298 K): δ (ppm) = 46.6 (br quartet, 1J71Ga–19F ∼ 510 Hz). ESI+ MS (CH3OH): found: 635.4 (expected for [GaF2(L1)]+: m/z = 635.2). IR (Nujol, ν/cm−1): 3425 br (OH), 3195 w, 3133 w (NH), 1685 s (C
H2), 40.6 (dmso). 19F{1H} NMR (CD3OD, 298 K): δ (ppm) = −180 (v br with partially resolved coupling to 69/71Ga). 71Ga NMR (CD3OD, 298 K): δ (ppm) = 46.6 (br quartet, 1J71Ga–19F ∼ 510 Hz). ESI+ MS (CH3OH): found: 635.4 (expected for [GaF2(L1)]+: m/z = 635.2). IR (Nujol, ν/cm−1): 3425 br (OH), 3195 w, 3133 w (NH), 1685 s (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1621 sh (HOH), 1599 s (C
O), 1621 sh (HOH), 1599 s (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1015 m (S
O), 1015 m (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, dmso), 583, 543 w (Ga–F). Crystals suitable for X-ray diffraction were grown via vapour diffusion of diethyl ether into a methanol solution of the product.
O, dmso), 583, 543 w (Ga–F). Crystals suitable for X-ray diffraction were grown via vapour diffusion of diethyl ether into a methanol solution of the product.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 550, 537 w (Fe–F). ESI+ MS (MeOH): not observed.
O), 550, 537 w (Fe–F). ESI+ MS (MeOH): not observed.
![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 3.18–3.13 (m, [6H], C
), 3.18–3.13 (m, [6H], C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.85–3.03 (br m, [12H], tacn-C
2), 2.85–3.03 (br m, [12H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.66 (s, dmso), 2.51–2.48 (m, [6H], C
2), 2.66 (s, dmso), 2.51–2.48 (m, [6H], C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 1.14 (d, 3JH–H = 6.6 Hz, [18H], iPr-C
2), 1.14 (d, 3JH–H = 6.6 Hz, [18H], iPr-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 3). 1H NMR (D2O, 298 K): δ (ppm) = 3.93–3.83 (br septet, [3H], iPr-C
3). 1H NMR (D2O, 298 K): δ (ppm) = 3.93–3.83 (br septet, [3H], iPr-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 3.15–3.11 (br t, [6H], C
), 3.15–3.11 (br t, [6H], C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.89 (br s, [12H], tacn-C
2), 2.89 (br s, [12H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.51–2.48 (br t, [6H], C
2), 2.51–2.48 (br t, [6H], C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 1.11 (br d, [18H], iPr-C
2), 1.11 (br d, [18H], iPr-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 3). 13C{1H} NMR (CD3OD, 298 K): δ (ppm) = 172.7 (
3). 13C{1H} NMR (CD3OD, 298 K): δ (ppm) = 172.7 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 53.1 (
O), 53.1 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 51.2 (tacn-
H2), 51.2 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 42.7 (iPr-
H2), 42.7 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H), 33.4 (
H), 33.4 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 22.8 (iPr-
H2), 22.8 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H3). 13C{1H} NMR (D2O, 298 K): δ (ppm) = 172.4 (
H3). 13C{1H} NMR (D2O, 298 K): δ (ppm) = 172.4 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 50.9 (
O), 50.9 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 48.6 (tacn-
H2), 48.6 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 41.8 (iPr-
H2), 41.8 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H), 32.1 (
H), 32.1 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 21.4 (iPr-
H2), 21.4 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H3). 19F{1H} NMR (CD3OD, 298 K): δ (ppm) = −196 (br); (D2O, 298 K): δ (ppm) = −155 (br). 29Al NMR (CD3OD, 298 K): not observed. HR ESI+ MS (CH3OH): found: 553.3627 (expected for [AlF3(L2) + H]+: m/z = 553.3628), 491.3681 (expected for [L2 + Na]+: m/z = 491.3680), 469.3876 (expected for [L2 + H]+m/z = 469.3861). IR (Nujol, ν/cm−1): 3430 v br, 3290 br (OH), 3090 br, 3068 br (NH), 1644 s, 1551 s (C
H3). 19F{1H} NMR (CD3OD, 298 K): δ (ppm) = −196 (br); (D2O, 298 K): δ (ppm) = −155 (br). 29Al NMR (CD3OD, 298 K): not observed. HR ESI+ MS (CH3OH): found: 553.3627 (expected for [AlF3(L2) + H]+: m/z = 553.3628), 491.3681 (expected for [L2 + Na]+: m/z = 491.3680), 469.3876 (expected for [L2 + H]+m/z = 469.3861). IR (Nujol, ν/cm−1): 3430 v br, 3290 br (OH), 3090 br, 3068 br (NH), 1644 s, 1551 s (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1050 w (S
O), 1050 w (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, dmso), 667 br, 616 sh (Al–F).
O, dmso), 667 br, 616 sh (Al–F).
![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 3.42–3.38 (br m, [6H], C
), 3.42–3.38 (br m, [6H], C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 3.16–3.06 (br m, [6H], tacn-C
2), 3.16–3.06 (br m, [6H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 3.82–3.76 (br m, [6H], tacn-C
2), 3.82–3.76 (br m, [6H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.66 (dmso), 2.53–2.47 (br m, [6H], C
2), 2.66 (dmso), 2.53–2.47 (br m, [6H], C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 1.13 (d, 3JH–H = 6.4 Hz, [18H], iPr-C
2), 1.13 (d, 3JH–H = 6.4 Hz, [18H], iPr-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 3). 13C{1H} NMR (CD3OD, 298 K): δ (ppm) = 172.6 (
3). 13C{1H} NMR (CD3OD, 298 K): δ (ppm) = 172.6 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 54.6 (iPr-
O), 54.6 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H), 53.1 (
H), 53.1 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 51.2 (tacn-
H2), 51.2 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 42.7 (tacn-
H2), 42.7 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 40.6 (dmso), 33.4 (
H2), 40.6 (dmso), 33.4 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 22.8 (iPr-
H2), 22.8 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H3). 19F{1H} NMR (CD3OD, 298 K): δ (ppm) = −178.2 (br). 71Ga NMR (CD3OD, 298 K): δ (ppm) = 41.0 (br quartet, 1J71Ga–19F ∼ 520 Hz). ESI+ MS (CH3OH): found: 575.5 (expected for [GaF2(L2)]+: m/z = 575.3). IR (Nujol, ν/cm−1): 3438 br, 3267 br (OH), 3190 sh, 3060 br (NH), 1645 br s, 1548 s (C
H3). 19F{1H} NMR (CD3OD, 298 K): δ (ppm) = −178.2 (br). 71Ga NMR (CD3OD, 298 K): δ (ppm) = 41.0 (br quartet, 1J71Ga–19F ∼ 520 Hz). ESI+ MS (CH3OH): found: 575.5 (expected for [GaF2(L2)]+: m/z = 575.3). IR (Nujol, ν/cm−1): 3438 br, 3267 br (OH), 3190 sh, 3060 br (NH), 1645 br s, 1548 s (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1018 w (S
O), 1018 w (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, dmso), 528 m, 510 sh (Ga–F).
O, dmso), 528 m, 510 sh (Ga–F).
![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 3.15 (br t, C
), 3.15 (br t, C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 3.12–3.02 (br m, [6H], tacn-C
2), 3.12–3.02 (br m, [6H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.99–2.88 (br m, [6H], tacn-C
2), 2.99–2.88 (br m, [6H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.74–2.69 (br m, [2H], C
2), 2.74–2.69 (br m, [2H], C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.66 (dmso), 2.65–2.61 (m, [2H], C
2), 2.66 (dmso), 2.65–2.61 (m, [2H], C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.52–2.45 (br m, [2H], C
2), 2.52–2.45 (br m, [2H], C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 1.14 (br d, [18H], iPr-C
2), 1.14 (br d, [18H], iPr-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 3). 19F{1H} NMR (CD3OD, 298 K): δ (ppm) = −197.8 (br) (a singlet is also observed at −132.6 ppm, suggesting significant liberation of F− from the indium(III) during the reaction, along with a minor species giving a broad resonance at −202 ppm). A few small crystals of [InF3(L2)] were grown via slow evaporation from the NMR solution of the product mixture in d4-methanol and were analysed by single crystal X-ray diffraction.
3). 19F{1H} NMR (CD3OD, 298 K): δ (ppm) = −197.8 (br) (a singlet is also observed at −132.6 ppm, suggesting significant liberation of F− from the indium(III) during the reaction, along with a minor species giving a broad resonance at −202 ppm). A few small crystals of [InF3(L2)] were grown via slow evaporation from the NMR solution of the product mixture in d4-methanol and were analysed by single crystal X-ray diffraction.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 512 (br, Fe–F). HR ESI+ MS (CH3OH): found: m/z = 562.3110 [FeF2(L2)]+ (calculated: m/z = 562.3105), 449.2263 [FeF2{(iPrC(O)NH(CH2)2)2-tacn} + H]+ (calculated: m/z = 449.2265), 262.1578 [Fe(L2)]2+ (calculated: m/z = 262.1563).
O), 512 (br, Fe–F). HR ESI+ MS (CH3OH): found: m/z = 562.3110 [FeF2(L2)]+ (calculated: m/z = 562.3105), 449.2263 [FeF2{(iPrC(O)NH(CH2)2)2-tacn} + H]+ (calculated: m/z = 449.2265), 262.1578 [Fe(L2)]2+ (calculated: m/z = 262.1563).
![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 7.33–7.28 (m, [2H], Ar
), 7.33–7.28 (m, [2H], Ar![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 7.10–7.06 (m, [1H], Ar
), 7.10–7.06 (m, [1H], Ar![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 4.86 (H2O), 3.59 (s, [2H], C
), 4.86 (H2O), 3.59 (s, [2H], C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 3.35 (br septet, overlapping with solvent peaks, iPr-C
2), 3.35 (br septet, overlapping with solvent peaks, iPr-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 3.15–3.02 (br m, [4H], tacn-C
), 3.15–3.02 (br m, [4H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.97–2.80 (br m, [8H], tacn-C
2), 2.97–2.80 (br m, [8H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.66 (dmso), 1.29 (d, 3JH–H = 6.5 Hz, [6H], iPr-C
2), 2.66 (dmso), 1.29 (d, 3JH–H = 6.5 Hz, [6H], iPr-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 3), 1.23 (d, 3JH–H = 6.6 Hz, [6H], iPr-C
3), 1.23 (d, 3JH–H = 6.6 Hz, [6H], iPr-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 3). 13C{1H} NMR (298 K, CD3OD): δ (ppm) = 164.6 (
3). 13C{1H} NMR (298 K, CD3OD): δ (ppm) = 164.6 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 140.0 (Ar
O), 140.0 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 130.0 (Ar
), 130.0 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 125.3 (Ar
), 125.3 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 121.1 (Ar
), 121.1 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 58.6 (
), 58.6 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 55.3 (iPr-
H2), 55.3 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H), 50.3 (tacn-
H), 50.3 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 48.3 (tacn-
H2), 48.3 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 46.3 (tacn-
H2), 46.3 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 18.5, 18.1 (iPr-
H2), 18.5, 18.1 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H3). 19F{1H} NMR (CD3OD, 298 K): δ (ppm) = −196 (br); (D2O, 298 K): δ (ppm) = −155.2 ([1F]), −156.1 ([2F]). 27Al NMR (CD3OD, 298 K): not observed. ESI MS+ (CH3OH) found: m/z = 431.2570 (expected for [AlF3(L3) + H]+: m/z = 431.2573), 347.2817 (expected for [L3 + H]+: m/z = 347.2805), 174.1441 (expected for [L3 + 2H]2+: m/z = 174.1439). IR (Nujol, ν/cm−1): 3430 (br, OH), 3266, 3177 (NH), 3155 (aromatic CH), 2727, 2676 (C–H stretch), 1693, 1615, 1600 (C
H3). 19F{1H} NMR (CD3OD, 298 K): δ (ppm) = −196 (br); (D2O, 298 K): δ (ppm) = −155.2 ([1F]), −156.1 ([2F]). 27Al NMR (CD3OD, 298 K): not observed. ESI MS+ (CH3OH) found: m/z = 431.2570 (expected for [AlF3(L3) + H]+: m/z = 431.2573), 347.2817 (expected for [L3 + H]+: m/z = 347.2805), 174.1441 (expected for [L3 + 2H]2+: m/z = 174.1439). IR (Nujol, ν/cm−1): 3430 (br, OH), 3266, 3177 (NH), 3155 (aromatic CH), 2727, 2676 (C–H stretch), 1693, 1615, 1600 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 642, 592 sh (Al–F).
O), 642, 592 sh (Al–F).
![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 7.32–7.27 (m, [2H], Ar
), 7.32–7.27 (m, [2H], Ar![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 7.11–7.05 (m, [1H], Ar
), 7.11–7.05 (m, [1H], Ar![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 4.86 (H2O), 3.60 (s, [2H], C
), 4.86 (H2O), 3.60 (s, [2H], C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 3.35 (septet, 3JH–H = 6.9 Hz, [2H], iPr-C
2), 3.35 (septet, 3JH–H = 6.9 Hz, [2H], iPr-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) ), 3.14–3.01 (m, [4H], tacn-C
), 3.14–3.01 (m, [4H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.99–2.79 (br m, [8H], tacn-C
2), 2.99–2.79 (br m, [8H], tacn-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 2), 2.66 (dmso), 1.29 (d, 3JH–H = 6.6 Hz, [6H], iPr-C
2), 2.66 (dmso), 1.29 (d, 3JH–H = 6.6 Hz, [6H], iPr-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 3), 1.23 (d, 3JH–H = 6.6 Hz, [6H], iPr-C
3), 1.23 (d, 3JH–H = 6.6 Hz, [6H], iPr-C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) 3). 13C{1H} NMR (298 K, CD3OD): δ (ppm) = 171.4 (
3). 13C{1H} NMR (298 K, CD3OD): δ (ppm) = 171.4 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 140.0 (Ar
O), 140.0 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 130.0 (Ar
), 130.0 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 125.5 (Ar
), 125.5 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 121.1 (Ar
), 121.1 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) ), 58.7 (
), 58.7 (![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 55.3 (iPr-
H2), 55.3 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H), 50.3 (tacn-
H), 50.3 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 48.3 (tacn-
H2), 48.3 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 46.3 (tacn-
H2), 46.3 (tacn-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H2), 40.6 (dmso), 18.5, 18.1 (iPr-
H2), 40.6 (dmso), 18.5, 18.1 (iPr-![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) H3). 19F{1H} NMR (CD3OD, 298 K): δ (ppm) = −171.5 (br s, [2F]) −172.3 (br s, [1F]). 71Ga NMR (298 K, MeOH): not observed. ESI+ MS (CH3OH): found: m/z = 473.3429 (expected for [GaF3(L3) + H]+: m/z = 473.2019); 453.1950 (expected for [GaF2(L3)]+: m/z = 453.1956), 347.2817 (expected for [L3 + H]+: m/z = 347.2805), 305.2331 (expected for [L3–iPr][H]+: m/z = 305.2336). IR (Nujol, ν/cm−1): 3420, 3307 (OH), 3193, 3132 (NH), 1689, 1622 (C
H3). 19F{1H} NMR (CD3OD, 298 K): δ (ppm) = −171.5 (br s, [2F]) −172.3 (br s, [1F]). 71Ga NMR (298 K, MeOH): not observed. ESI+ MS (CH3OH): found: m/z = 473.3429 (expected for [GaF3(L3) + H]+: m/z = 473.2019); 453.1950 (expected for [GaF2(L3)]+: m/z = 453.1956), 347.2817 (expected for [L3 + H]+: m/z = 347.2805), 305.2331 (expected for [L3–iPr][H]+: m/z = 305.2336). IR (Nujol, ν/cm−1): 3420, 3307 (OH), 3193, 3132 (NH), 1689, 1622 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1032 (S
O), 1032 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, dmso), 539, 520 (GaF). Crystals suitable for X-ray structure determination were grown via slow evaporation from a solution of the complex in acetonitrile.
O, dmso), 539, 520 (GaF). Crystals suitable for X-ray structure determination were grown via slow evaporation from a solution of the complex in acetonitrile.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1617 sh (HOH), 1598 (C
O), 1617 sh (HOH), 1598 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 539, 521 (FeF). HR ESI+ MS (CH3OH): found: m/z = 440.2043 [FeF2(L3)]+ (calculated: m/z = 440.2050), 347.2809 (expected for [L3 + H]+: m/z = 347.2805). Crystals suitable for X-ray diffraction were grown via slow evaporation from a solution of the product in methanol/diethyl ether.
O), 539, 521 (FeF). HR ESI+ MS (CH3OH): found: m/z = 440.2043 [FeF2(L3)]+ (calculated: m/z = 440.2050), 347.2809 (expected for [L3 + H]+: m/z = 347.2805). Crystals suitable for X-ray diffraction were grown via slow evaporation from a solution of the product in methanol/diethyl ether.
Results and discussion
The target amide-functionalised tacn ligands, L1–L3 were selected to explore the effects of (i) varying the linker between the amine and amide groups (i.e.L1vs. L2) and (ii) the number of amide groups present (i.e.L1vs. L3). The phenyl groups present in L1 and L3 also provide a convenient chromophore to track the complexes in subsequent radiochemistry experiments. L1–L3 were prepared as shown in Scheme 2, via reaction of tacn with K2CO3 and three mol. equiv. of 2-chloro-N-phenylacetamide in acetone at room temperature (18 h) (L1), N-isopropylacrylamide in refluxing MeOH (L2), or by reaction of preformed iPr2-tacn with one mol. equiv. of 2-chloro-N-phenylacetamide and K2CO3 in acetone. Following work-up and flash chromatography (L1 and L3), the pure ligands were isolated as a white powder (L1), yellow oil (L2) or orange oil (L3) and characterised by 1H and 13C{1H} NMR and IR spectroscopy, UV-HPLC analysis (L1 and L3) and via high resolution ESI+ MS. The IR spectra show two strong C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations for each of L1–L3, along with the expected ν(NH) bands.
O stretching vibrations for each of L1–L3, along with the expected ν(NH) bands.
To further confirm the identity of L1, a small sample was converted to its protonated form by addition of HClaq to a solution of L1 in MeOH and crystallised from via slow evaporation over a few weeks. The structure of L1·HCl was then determined by X-ray crystallography, which confirmed the presence of the tris-amide tacn moiety (L1) and showed (Fig. 1) mono-protonation of the tacn ring. Intramolecular N6–H6⋯O1(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) H-bonding between the amide groups is evident between two of the pendant arms of L1·HCl, while intermolecular H-bonding involving the chloride anion and involving the third amide arm in two adjacent L1 moieties, C(O)N4–H4⋯Cl1⋯H5–N5C(O) gives rise to weakly associated dimers in the solid state (N4H4⋯Cl = 2.130, N5H5⋯Cl = 2.166 Å).
C) H-bonding between the amide groups is evident between two of the pendant arms of L1·HCl, while intermolecular H-bonding involving the chloride anion and involving the third amide arm in two adjacent L1 moieties, C(O)N4–H4⋯Cl1⋯H5–N5C(O) gives rise to weakly associated dimers in the solid state (N4H4⋯Cl = 2.130, N5H5⋯Cl = 2.166 Å).
A few crystals of L1·HNO3 were also isolated as a minor by-product during this study, from an attempt to the react L1 with Ga(NO3)3·9H2O in MeOH; the structure of this salt is shown in ESI Fig. S16.†
Reactions of L1–L3 with [MF3(dmso)(OH2)2] (M = Al, Ga, In) and FeF3·3H2O
While the poorly soluble (and usually polymeric)28 MF3·3H2O (M = Al, Ga, In) precursors can provide a source of MF3 for coordination to certain ligands under high temperature and pressure (solvothermal) conditions,4,30 we have shown previously that the molecular [MF3(dmso)(OH2)2] are often more suitable precursors due their higher solubilities under milder reaction conditions, and therefore better compatibility with a wider range of ligand types and functionalities.29,30 Since the pendant amide functions may be susceptible to hydrolysis, the [MF3(dmso)(OH2)2] complexes were chosen as the metal trifluoride precursors for the present study to facilitate the coordination chemistry under milder reaction conditions, as illustrated in Scheme 3. Treatment of [MF3(dmso)(OH2)2] (M = Al, Ga) with one mol. eq. of L (L = L1–L3) at room temperature or with gentle heating (60 °C, M = Ga) affords the distorted octahedral complexes fac-[MF3(L)] as white powdered solids and their spectroscopic and structural data are discussed below. For M = Al, short reaction times (2–6 h at room temperature) gave higher yields of the target complexes, while refluxing in MeOH overnight led to precipitation of some white solid that needed to be separated before the target complexes were isolated from the filtrate. The isolated yields for the Al(III) and Ga(III) complexes were typically in the range 65–80%. However, for In(III), despite several attempts and varying the reaction conditions, the reaction of [InF3(dmso)(OH2)2] with L1 repeatedly gave a mixture of products and a pure sample of [InF3(L1)] could not be isolated. Also, in the case of the [InF3(dmso)(OH2)2]/L2 reaction, elemental analysis on samples from different batches did not match the expected values, and while 1H and 19F{1H} NMR spectroscopic analysis indicated the presence of [InF3(L2)] (which was also confirmed by a single crystal X-ray structure analysis – discussed below), a second, unidentified product, along with a significant amount of free F− were also present. The production of a mixture of species may be a consequence of the weaker M–F bonds present in the In(III) species (compared to Al(III) and Ga(III)), resulting in competition for coordination to In(III) of one or more amide pendant groups and loss of F−. However, given these results, the indium chemistry was not pursued further.In the case of the Fe(III) complexes, the precursor, FeF3·3H2O, was reacted directly with L1–L3, in EtOH solution at reflux. The three [FeF3(L)] complexes were isolated in good yields as pale-yellow solids. While the expected ν(Fe–F) bands are present in the IR spectra and the expected peaks are evident in the ESI+ MS for [FeF3(L2)], the paramagnetic nature of these complexes precludes any useful NMR analysis.
The powdered [MF3(L)] products show either two (a1 + e) or one broad M–F stretching vibration in the far IR regions as expected, and the observed frequencies compare well with the literature data for [MF3(Me3-tacn)] (M = Al, Ge, Fe).4,13 The IR spectra also confirm the presence of H2O and in some cases dmso in the isolated products. This was also consistent with microanalytical data, while ESI-MS typically showed peaks with the expected isotopic pattern associated with [MF2(L)]+ or in some cases [MF3(L) + H]+, as expected, although often with low intensities; peaks for [L + H]+ are also observed in a number of cases.
Solution multinuclear NMR studies were hindered somewhat by the limited solubilities of the new complexes, especially in non-protic solvents. Hence spectra were mostly obtained from H2O or MeOH solutions. For the GaF3 complexes involving L1 and L2 the fac-octahedral geometry is unambiguously assigned from the 71Ga and 19F{1H} NMR spectra. Thus, the 71Ga spectra each show a broadened 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 quartet in the range +40 to +50 ppm, arising from coupling of the quadrupolar 71Ga nucleus to the three facial fluorides, 1J71Ga–19F ∼ 510–520 Hz (Fig. 2), and a very broad resonance for each complex in the 19F{1H} NMR spectra at ∼−180 ppm, caused by two overlapping, partially resolved 1
1 quartet in the range +40 to +50 ppm, arising from coupling of the quadrupolar 71Ga nucleus to the three facial fluorides, 1J71Ga–19F ∼ 510–520 Hz (Fig. 2), and a very broad resonance for each complex in the 19F{1H} NMR spectra at ∼−180 ppm, caused by two overlapping, partially resolved 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 quartets due to coupling of the 19F to both the 69Ga and 71Ga nuclei (each of which has I = 3/2), respectively, in the approximately C3v symmetry molecules. These chemical shifts and couplings are comparable with those reported for [GaF3(Me3-tacn)] and [GaF3(BnMe2-tacn)].4
1 quartets due to coupling of the 19F to both the 69Ga and 71Ga nuclei (each of which has I = 3/2), respectively, in the approximately C3v symmetry molecules. These chemical shifts and couplings are comparable with those reported for [GaF3(Me3-tacn)] and [GaF3(BnMe2-tacn)].4
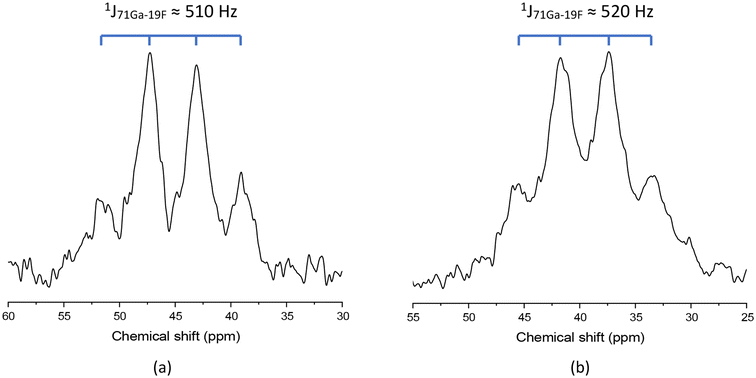 | ||
Fig. 2
71Ga NMR spectra of (a) [GaF3(L1)] and (b) [GaF3(L2)], each showing the expected broad 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 quartet 1J71Ga19F coupling (CD3OD). 1 quartet 1J71Ga19F coupling (CD3OD). | ||
Two 19F NMR resonances are expected for the lower symmetry [MF3(L3)] complexes, these are observed at −155.2 ([1F]), −156.1 ([2F]) for M = Al (D2O), and at −171.5 ([2F]) and −172.3 ppm ([1F]) for M = Ga (MeOH), although the F–F couplings are lost in the line widths. No 71Ga NMR resonance was observed for [GaF3(L3)], probably because of the lower symmetry arising from the asymmetrically substituted tacn N-donor atoms. The 1H and 13C{1H} NMR spectra for [MF3(L3)] (M = Al, Ga) also show that the two CH3 groups in the iPr pendant groups become diastereotopic in the complexes, as expected, providing further supporting evidence for the successful complexation of the MF3 fragments to L3.31
The 19F NMR shifts for the complexes show a significant solvent dependence in MeOH and H2O. This is attributed to the highly polar nature of the pyramidal MF3 units present and their strong tendency to hydrogen bond to adjacent H-bond donors, including both the pendant amide groups and protic solvent molecules (as is also observed in the crystal structures – below).
Further confirmation of the molecular structures and the nature and extent of hydrogen-bonding present in [GaF3(L1)]·1.5MeOH·0.5H2O, [InF3(L2)], [GaF3(L3)] and [FeF3(L3)] were obtained by single crystal X-ray analyses.
The structure of [GaF3(L1)]·1.5MeOH·0.5H2O (Fig. 3) shows the Ga(III) atom in a distorted octahedral coordination environment, with the tridentate tacn ring occupying one face of the Ga (Ga–N1 = 2.157(2), Ga–N2 = 2.162(2), Ga–N3 = 2.165(3) Å), and the three facial fluorides lying trans to the amine N-donor atoms, Ga–F1 = 1.8287(18), Ga–F2 = 1.8487(17), Ga–F3 = 1.8493(16) Å, in accord with the corresponding bond distances reported for [GaF3(Me3-tacn)]·4H2O.4 The structure is disordered, with two distinct forms modelled (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 occupancy) displaying different orientations for one of the pendant amide arms (see Experimental). Hydrogen bonding is evident in one of the components between an amide N–H group or lattice water molecule and the F ligands in an adjacent molecule.
50 occupancy) displaying different orientations for one of the pendant amide arms (see Experimental). Hydrogen bonding is evident in one of the components between an amide N–H group or lattice water molecule and the F ligands in an adjacent molecule.
The reaction of [InF3(dmso)(H2O)2] with L1–L3 under similar conditions to the Al(III) and Ga(III) complex syntheses produced a mixture of products. In one case we were able to obtain a few crystals of [InF3(L2)] from the product mixture and confirmed its structure by single crystal X-ray analysis. [InF3(L2)] crystallises in the trigonal space group R3c, with three-fold crystallographic symmetry. The structure (Fig. 4(a)) shows the distorted octahedral coordination at the metal ion via three facial fluorides and the three N-donor atoms from the tacn ring, d(In–F) = 2.071(2), d(In–N) = 2.299(3) Å. The F–In–F angles are 97.99(8)°, while the N–In–N angles involving the macrocycle are much more acute (78.04(10)°). These values are in good accord with the corresponding metrics reported for [InF3(Me3-tacn)]·4H2O and [InF3(BnMe2-tacn)]·1.2H2O.4 Similarly to the case of [GaF3(L1)] discussed above, the pendant amide groups (in this case, –CH2CH2C(O)NHiPr, i.e. with the amide groups extended further from the macrocyclic amine functions by the extra CH2 unit present in each pendant arm in L2) are not involved in coordination to indium(III), however, they each form one intermolecular N–H⋯F H-bond, d(N⋯F) = 1.888 Å, to an adjacent molecule, as illustrated in Fig. 4(b), to generate 2D sheets.
Crystals of both [GaF3(L3)] (Fig. 5(a)) and [FeF3(L3)] (Fig. 5(b)) were obtained as described in the Experimental section and are isostructural. Each complex shows fac-tridentate coordination of L3 to the metal ion via its tacn N(amine) donor atoms, with the three F− ligands occupying the other face and giving a distorted octahedral species. Both complexes form ‘head-to-tail’ H bonded dimers via hydrogen bonding from the amide NH group is one molecule with one F atom in the second molecule (M = Fe: F⋯HN distance = 1.787 Å; M = Ga: F⋯HN distance = 1.769 Å).
Looking at the extended structures shows that the dimer units are arranged in a hexagonal ‘windmill’-like array when viewed down the c-axis (Fig. 6). This leaves solvent accessible voids in the lattice containing disordered H2O, which was modelled using a solvent mask, and consistent with ca. 1.20 and 2.40 H2O molecules per unit cell for the Ga and Fe species, respectively. These complexes are also extremely hygroscopic, with the powders and crystals rapidly becoming sticky upon exposure to moist air over a few minutes.
 | ||
| Fig. 6 View of the structure of [GaF3(L3)] viewed down the c-axis showing the hexagonal arrangement adopted by the weakly associated dimers (the same arrangement is present in [FeF3(L3)]). | ||
Radiofluorination of [GaF3(L1)] and [GaF3(L3)]
We have previously reported the radiofluorination of several gallium(III) macrocyclic complexes via both Cl/18F4 and 19F/18F6 exchange reactions in partially aqueous MeCN or EtOH solvent, including for the production of [Ga18F19F2(BnMe2-tacn)], which resulted in good radiochemical yields, and high radiochemical stability when formulated in EtOH with (aqueous) phosphate buffered saline at pH 7.4. We were therefore interested to explore the radiofluorination of both the [GaF3(L1)] and [GaF3(L3)] complexes by 18F/19F isotopic exchange to determine whether the presence of the strong H-bond donor pendant amide groups would affect the radiochemistry. Both of the precursor complexes also contain (at least) one Ph group, providing a chromophore for correlation of the UV trace of the precursor with the radioproduct(s).Radiofluorination experiments using [GaF3(L1)] were performed by 18F/19F isotopic exchange in MeOH solution due to the poor solubility of the complex in other solvents such as MeCN and EtOH. While several different conditions were explored (Table 1), the highest RCY of ca. 20% was achieved reproducibly using 2 mg of the complex in MeOH, followed by the addition of [18F]F− in target water (to give a 3.0 μmol mL−1 solution in 75%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25% MeOH
25% MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O) and heating this to 80 °C for 10 min (Table 2).
H2O) and heating this to 80 °C for 10 min (Table 2).
| Precursor concentration (μmol mL−1) | Temperature (°C) | Time (min) | RCY (%) |
|---|---|---|---|
| 1.5 | 80 | 10 | 7 |
| 1.5 | 80 | 10 | 6 |
| 3 | 80 | 10 | 20 |
| 3 | 80 | 10 | 19 |
| 3 | 80 | 10 | 18 |
| 3 | 80 | 10 | 15 |
| 3 | 80 | 20 | 15 |
| 3 | 80 | 30 | 17 |
| 3 | 60 | 10 | 9 |
| 3 | 60 | 20 | 13 |
| 3 | 60 | 30 | 16 |
| 3 | RT | 15 | 7 |
| 3 | RT | 30 | 10 |
| 3 | RT | 45 | 11 |
| 3 | RT | 60 | 14 |
[Ga18FF2(L1)] in 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 H2O 10 H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH EtOH |
[Ga18FF2(L1)] in 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 PBS 10 PBS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH EtOH |
||
|---|---|---|---|
| Time/min | RCP (%) | Time/min | RCP (%) |
| 0 | 68 | 0 | 64 |
| 30 | 67 | 30 | 61 |
| 80 | 59 | 80 | 57 |
| 120 | 61 | 120 | 59 |
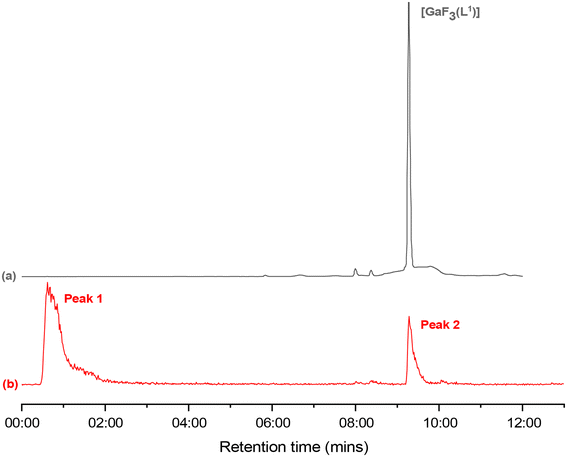
The radioproduct, [Ga18FF2(L1)], was identified by comparison of the Rt for the radiotrace and UV-HPLC trace of the radioproduct, and matching the latter with the UV-HPLC trace of the reference complex, [GaF3(L1)] (Fig. 7). While using a lower precursor concentration (1.5 μmol mL−1) also showed some radiofluorine incorporation, the RCY was lower (typically ca. 7%).
Purification was attempted using a solid phase extraction (SPE) cartridge method (see ESI†) before formulating the radioproduct in either 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 H2O/EtOH or 90
10 H2O/EtOH or 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 PBS/EtOH to investigate the radiochemical stability over a period of 2 h (Table 2). The RCP decreases by ca. 7% in EtOH/H2O, and by ca. 5% in EtOH/PBS.
10 PBS/EtOH to investigate the radiochemical stability over a period of 2 h (Table 2). The RCP decreases by ca. 7% in EtOH/H2O, and by ca. 5% in EtOH/PBS.
Several attempts to radiolabel solutions of [GaF3(L3)] in MeOH, MeCN or EtOH using target water containing [18F]F−, either at room temperature for 30–60 min, or with heating (80 °C for 10–60 min), gave no evidence for radiofluorine uptake in any of these experiments.
Conclusions
This work has explored how tacn-based ligands incorporating one or more amide pendant functions coordinate to trivalent Group 13 metal trifluoride reagents, leading to three series of distorted octahedral complexes, fac-[MF3(L)] for M = Al, Ga and Fe; L = L1–L3, in which the macrocycle coordinates via the three tacn amine donor groups only, leaving the amide functions (potential H-bond donors) uncoordinated, as confirmed by a combination of spectroscopic analyses and X-ray crystallographic studies on representative examples. In the solid state, significant intermolecular H-bonding involving the amide groups and one or more coordinated fluoride ligand is evident in all of the complexes producing an extended 3D polymer array for [InF3(L2)]. The L3 complexes, [GaF3(L3)] and [FeF3(L3)] are isostructural and formed of ‘head-to-tail’ dimers that can be considered as inorganic analogues of the H-bonded dimers formed by amine thioureas with carboxylates that are observed frequently as ‘building blocks’ in supramolecular chemistry.Radiofluorination experiments using [GaF3(L1)] in aqueous MeOH and [18F]F− in target water with brief heating (80 °C/10 min) showed modest uptake, giving a radiochemical yield (RCY) of ∼20%, which was lower than observed previously for [GaF3(BnMe2-tacn)].6 Partial purification using a SPE protocol resulted in a RCP of 68% in H2O/EtOH, and 64% in PBS/EtOH, however, both formulations showed some loss of 18F− over 2 h. Therefore, further efforts to remove unreacted [18F]F− were not pursued. The monoamide complex, [GaF3(L3)], showed no clear evidence for [18F]F− uptake under similar conditions.
Author contributions
Project conceptualisation and funding (GR, GMcR), ligand and complex synthesis (COC) and characterisation (COC, VKG, RPK, JMH), radiochemistry (COC, GMcR), data analysis, manuscript preparation and reviewing (all authors).Data availability
The spectroscopic data for all of the new ligands and complexes are presented in the ESI† for this manuscript, along with X-ray crystallographic details and a table of crystallographic parameters, the radiolabelling methods and data. The cif files and checkcifs are available via the CCDC with reference numbers 2355936–2355941.† All of these data will also be publicly available via the University of Southampton's permanent repository with a unique doi number.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the EPSRC for support through the Mithras Programme Grant (EP/SO32789/1) and through DTP grant number EP/T517859/1 (COG).References
- K. Chansaenpak, B. Vabre and F. P. Gabbaï, Chem. Soc. Rev., 2016, 45, 954 RSC.
- W. Levason, F. M. Monzittu and G. Reid, Coord. Chem. Rev., 2019, 391, 90 CrossRef.
- S. Schmitt and E. Moreau, Coord. Chem. Rev., 2023, 480, 215028 CrossRef.
- R. Bhalla, C. Darby, W. Levason, S. K. Luthra, G. McRobbie, G. Reid, G. Sanderson and W. Zhang, Chem. Sci., 2014, 5, 381 RSC.
- W. Levason, S. K. Luthra, G. McRobbie, F. M. Monzittu and G. Reid, Dalton Trans., 2017, 46, 14519 RSC.
- F. M. Monzittu, I. Khan, W. Levason, S. K. Luthra, G. McRobbie and G. Reid, Angew. Chem., Int. Ed., 2018, 57, 6658 CrossRef.
- M. S. Woodward, D. E. Runacres, J. Grigg, I. Khan, W. Levason, G. McRobbie and G. Reid, Pure Appl. Chem., 2024, 96, 57 CrossRef.
- (a) P. Laverman, W. McBride, R. Sharkey, A. Eek, L. Joosten, W. Oyen, D. Goldenberg and O. Boerman, J. Nucl. Med., 2010, 51, 454 CrossRef PubMed; (b) W. McBride, C. D'Souza, R. Sharkey, H. Karacay, E. Rossi, C. Chang and D. Goldenberg, Bioconjugate Chem., 2010, 21, 1331 CrossRef PubMed; (c) W. McBride, R. Sharkey, H. Karacay, C. D'Souza, E. Rossi, P. Laverman, C. Chang, O. Boerman and D. Goldenberg, J. Nucl. Med., 2009, 50, 991 CrossRef CAS PubMed; (d) W. McBride, C. D'Souza, H. Karacay, R. Sharkey and D. Goldenberg, Bioconjugate Chem., 2012, 23, 538 CrossRef CAS PubMed.
- S. Schmitt and E. Moreau, Coord. Chem. Rev., 2023, 480, 215028 CrossRef CAS.
- R. Bhalla, W. Levason, S. K. Luthra, G. McRobbie, G. Sanderson and G. Reid, Chem. – Eur. J., 2015, 21, 4688 CrossRef CAS PubMed.
- J. N. Whetter, B. A. Vaughn, A. J. Koller and E. Boros, Angew. Chem., Int. Ed., 2022, 61, e202114203 CrossRef CAS.
- D. E. Runacres, V. K. Greenacre, J. M. Dyke, J. Grigg, G. Herbert, W. Levason, G. McRobbie and G. Reid, Inorg. Chem., 2023, 62, 20844 CrossRef CAS PubMed.
- P. J. Blower, W. Levason, S. Luthra, G. McRobbie, F. M. Monzittu, T. O. Mules, G. Reid and M. N. Subhan, Dalton Trans., 2019, 48, 6767 RSC.
- F. N. Penkert, T. Weyhermuller and K. Wieghardt, Chem. Commun., 1998, 557 CAS.
- K. S. Pedersen, G. Lorusso, J. J. Morales, T. Weyhermüller, S. Piligkos, S. K. Singh, D. Larsen, M. Schau-Magnussen, G. Rajaraman, M. Evangelisti and J. Bendix, Angew. Chem., Int. Ed., 2014, 53, 2394 CrossRef PubMed.
- R. Bhalla, W. Levason, S. K. Luthra, G. McRobbie, G. Reid, G. Sanderson and W. Zhang, Chem. Commun., 2014, 50, 12673 RSC.
- (a) D. Shetty, S. Y. Choi, J. M. Jeong, L. Hoigebazar, Y.-S. Lee, D. S. Lee, J.-K. Chung, M. C. Lee and Y. K. Chung, Eur. J. Inorg. Chem., 2010, 5432–5438 CrossRef; (b) R. D. Hancock, H. Maumela and A. S. de Sousa, Coord. Chem. Rev., 1996, 148, 315 CrossRef.
- T. Weyhermuller, K. Wieghardt and P. Chaudhuri, J. Chem. Soc., Dalton Trans., 1998, 3805 RSC.
- J. Huskens and A. Dean Sherry, J. Chem. Soc., Dalton Trans., 1998, 177 RSC.
- A. J. Blake, I. A. Fallis, A. Heppeler, S. Parsons, S. A. Ross and M. Schroder, J. Chem. Soc., Dalton Trans., 1996, 31 RSC.
- Z. Zhang, Y. He, Q. Zhao, W. Xu, Y.-Z. Li and Z.-L. Wang, Inorg. Chem. Commun., 2006, 9, 269 CrossRef CAS.
- S. Amin, C. Marks, L. M. Toomey, M. R. Churchill and J. R. Morrow, Inorg. Chim. Acta, 1996, 246, 99 CrossRef CAS.
- P. B. Tsitovich and J. R. Morrow, Inorg. Chim. Acta, 2012, 393, 3 CrossRef CAS.
- R. E. H. Kuveke, L. Barwise, Y. van Ingen, K. Vashisth, N. Roberts, S. S. Chitnis, J. L. Dutton, C. D. Martin and R. L. Melen, ACS Cent. Sci., 2022, 8, 855 CrossRef CAS PubMed.
- J. E. Richman and T. J. Atkins, J. Am. Chem. Soc., 1974, 96, 2268 CrossRef CAS.
- A. E. Martin, T. M. Ford and J. E. Bulkowski, J. Org. Chem., 1982, 47, 412 CrossRef CAS.
- L. Zhaowen, Z. Li, X. Chunfen, Y. Yong, Z. Fanbo and H. Kaixun, Med. Chem. Res., 2007, 16, 380 CrossRef.
- R. Bhalla, J. Burt, A. L. Hector, W. Levason, S. K. Luthra, G. McRobbie, F. M. Monzittu and G. Reid, Polyhedron, 2016, 106, 65 CrossRef.
- R. Bhalla, W. Levason, S. K. Luthra, G. McRobbie, F. M. Monzittu, J. Palmer, G. Reid, G. Sanderson and W. Zhang, Dalton Trans., 2015, 44, 9569 RSC.
- F. M. Monzittu, I. Khan, W. Levason, S. K. Luthra, G. McRobbie and G. Reid, Angew. Chem., Int. Ed., 2018, 57, 6658 CrossRef PubMed.
- G. R. Giesbrecht, A. Gebauer, A. Shafir and J. Arnold, Dalton Trans., 2000, 4018 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: NMR, IR and mass spectra associated with ligands L1–L3 and the metal trifluoride complexes described in this work (Fig. S1–S17), together with the crystal structure of L1·HNO3. CCDC 2355936–2355941. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt02074j |
| This journal is © The Royal Society of Chemistry 2024 |