Activation of carbon disulfide by a hypersilyl germylene†‡
V. S.
Ajithkumar§
ab,
Nripen
Khilari§
 c,
Pratiksha B.
Ghanwat
ab,
Geethu
Venugopal
bd,
Debasis
Koley
c,
Pratiksha B.
Ghanwat
ab,
Geethu
Venugopal
bd,
Debasis
Koley
 *c and
Sakya S.
Sen
*c and
Sakya S.
Sen
 *ab
*ab
aInorganic Chemistry and Catalysis Division, CSIR-National Chemical Laboratory, Dr. Homi Bhabha Road, Pashan, Pune 411008, India. E-mail: ss.sen@ncl.res.in
bAcademy of Scientific and Innovative Research (AcSIR), New Ghaziabad 201002, India
cDepartment of Chemical Sciences, Indian Institute of Science Education and Research (IISER) Kolkata, Mohanpur, 741246, India. E-mail: koley@iiserkol.ac.in
dOrganic Chemistry Division, CSIR-National Chemical Laboratory, Dr. Homi Bhabha Road, Pashan, Pune 411008, India
First published on 30th May 2024
Abstract
In this work, the insertion of CS2 into the Ge–Si bond of PhC(NtBu)2Ge–Si(SiMe3)3 (1) has been investigated, resulting in the formation of PhC(NtBu)2Ge–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)–S–Si(SiMe3)3 (2). Interestingly, the addition of NHC to 2 allows the release of NHC·CS2 with concomitant regeneration of 1. Addition of another equivalent of 1 or an analogous hypersilyl silylene, [PhC(NtBu)2Si–Si(SiMe3)3], to 2 led to the formation of compounds with a Ge
S)–S–Si(SiMe3)3 (2). Interestingly, the addition of NHC to 2 allows the release of NHC·CS2 with concomitant regeneration of 1. Addition of another equivalent of 1 or an analogous hypersilyl silylene, [PhC(NtBu)2Si–Si(SiMe3)3], to 2 led to the formation of compounds with a Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S (3) or a Si
S (3) or a Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S (4) bond.
S (4) bond.
CS2, a heavier chalcogen analogue of CO2, is also a versatile building block for C,S-containing compounds in organic chemistry. It finds application in the industrial manufacturing of viscose fibers from cellulose and acts as a precursor for generating CCl4 and thiourea. CS2 has long been used as a sulfur source in organic chemistry. However, little is known about the reactivity of CS2 with low-valent group 14 compounds. Earlier work by Jutzi revealed the formation of a dithiasilethane from the reaction of decamethylsilicocene and CS2.1 The Tacke group later demonstrated the formation of an isolable silylene–CS2 adduct, an intermediate for a variety of sila-C,S-containing heterocycles.2 Driess and colleagues reported a unique CS2 activation by a bis-silylene leading to a 1,3-substituted cyclohexadiene derivative.3 Other studies by the groups of Okazaki, Weidenbruch and Kira reported reactions of stannylenes with CS2, resulting in tetrathioalkene derivatives and tetrathiabicyclo[3.3.0]octane derivatives.4–6 Aside low-valent group 14 species, the group of Moya-Cabrera disclosed the reactivity of CS2 with alanes and alumoxanes.7 Hoge and co-workers demonstrated the side-on addition of [Si(C2F5)3]− to CS2.8 Dostál et al. described the [2 + 2] cycloaddition of CS2 with bismuth sulphides,9 while Lichtenberg and co-workers reported the elegant insertion of CS2 into a Bi–N bond.10
In contrast, a vast number of germylene derivatives are not currently known to activate CS2. There are only two examples with germylenes, and the one reported by Okazaki shows that a germylene (Tbt(Tip)Ge: [Tbt = 2,4,6-tris(bis(trimethylsilyl)methyl)phenyl, Tip = 2,4,6-triisopropylphenyl]) reacts with CS2 to afford a germaketenedithioacetal derivative.11 The Jones group reported the reaction of a bis-germylene with CS2 and demonstrated the insertion of sulfur into the Ge(I)–Ge(I) bond.12 In contrast, the three-coordinate germylenes or bis-germylenes supported by N-donor ligands such as nacnac or amidinate have not been reported to react with CS2. With this in mind, our primary objective in the current work is to study the insertion chemistry of CS2 in a Ge–E bond because the insertion chemistry of CS2 remains virtually unexplored for germylenes.
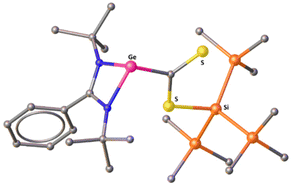
Recently, the groups of Baumgartner and Marschner reported a series of germanium(II) compounds attached to a hypersilyl moiety.13 While those silyl germylenes exhibit fascinating reactivity with alkynes and other unsaturated substrates, they have not been reported for CS2 activation. Inspired by their studies as well as our recent accomplishment in synthesizing sp2–sp3 disilenes using hypersilyl moieties,14 we have reported a hypersilyl germylene, PhC(NtBu)2Ge–Si(SiMe3)3 (1),15 which was chosen as the precursor because the Ge(II)–Si(IV) bond in this species exhibits sufficiently high reactivity.16 The reaction of 1 with CS2 led to immediate color change from yellow to dark purple and resulted in compound 2 in high yield, stemming from the selective insertion of CS2 into the Ge–Si bond (Scheme 1). The insertion of CS2 into the Ge–Si bond marks the first example of this type of insertion at a Ge(II) center. The 1H NMR spectrum of 2 reveals a resonance at 0.40 ppm for the Me3Si protons and another resonance at 1.10 ppm for the tBu protons. The corresponding 29Si NMR spectrum shows a peak at −9.7 ppm for the Me3Si groups and another resonance at −63.0 ppm for the Si atom bonded to sulfur. The IR spectrum of 2 displays three absorption bands at 1245, 1204, and 1180 cm−1 (Fig. S5 in the ESI†). Single-crystals of 2 were obtained from a supersaturated solution of 2 in toluene at room temperature. 2 crystallizes in the P1 space group of the triclinic crystal system, and the germanium center adopts a distorted trigonal pyramidal geometry (Fig. 1). Although there is no ambiguity in the constitution of 2, the quality of the X-ray structure is borderline, and hence the structural parameters should be regarded with caution. The Ge–C bond length is 2.02(2) Å, which is slightly longer than the typical Ge–C single bond.17
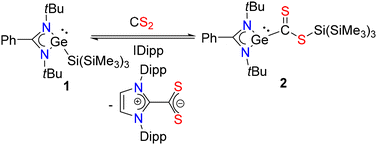 | ||
| Scheme 1 Activation of CS2 by PhC(NtBu)2Ge–Si(SiMe3)3 (1) and formation of 2. Subsequent release of CS2 as imidazolium-2-dithiocarboxylate upon addition of a NHC. | ||
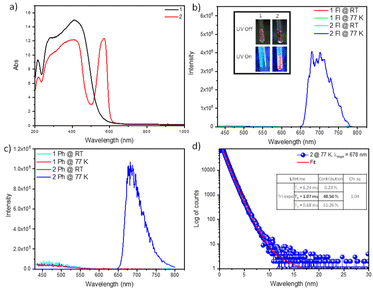
The solid state UV–vis absorption spectra of 1 show the absorption maximum at 412 nm, while for 2 there are two peaks at 408 nm and 570 nm, respectively (Fig. 2a). When 1 was excited at 412 nm at RT, a very weak fluorescence emission was detected around 460 nm. Similarly, 2 also shows fluorescence around 460 nm when excited at 408 nm and 570 nm at RT (Fig. 2b). However, at low temperature, a red color emission was observed under 365 nm UV light (inset, Fig. 2b). When the fluorescence emission profile was monitored at 77 K upon excitation at 570 nm, an intense emission band at 678 nm was observed. The phosphorescence emission was also recorded at 77 K after a 50 μs delay, which led to a phosphorescence emission at 678 nm (Fig. 2c). To further confirm this, delayed phosphorescence lifetime was also monitored at 77 K, revealing a lifetime of 1.07 ms for the 678 nm peak (Fig. 1d). This proves that the insertion of CS2 has an impact on the electronic properties, and these inorganic materials hold promise for further exploration in cryogenic thermometers and sensors. Based on the optimized geometries, we performed TDDFT calculations to explore the electronic excitation energies. In the case of 2, excitations at 399 nm (f = 0.0163) and 571 nm (f = 0.0014) represent electronic transitions of the type π→π* and n→π*, respectively (Fig. S20, Table S2†). The calculated vertical S–T gap is 0.11 eV for 1, while it is 0.35 eV for complex 2. Furthermore, perturbative spin–orbit coupling (pSOC) TDDFT calculation for 2 reveals the phosphorescence energy to be 1.71 eV with 1.88 ms radiation lifetime, in qualitative agreement with the experimental values.
Can the inserted CS2 be liberated? Lichtenberg and co-workers have not only shown the insertion of CO2 into the Bi–N bond, but the elimination of CO2 at ambient temperature in pyridine or at 60 °C in THF. However, the methodology did not work for CS2.10 To our knowledge, there has been no report on the release of CS2 from a low-valent main-group species. Addition of 1 equiv. of IDipp to a toluene solution of 2 produced an immediate color change from purple to pale yellow, and analysis by 1H NMR spectroscopy confirmed the regeneration of 1 and the formation of the imidazolium-2-dithiocarboxylate, which was subsequently characterized by single crystal X-ray studies (see the ESI† for structural data).18 Regeneration of the starting germylene allows completion of a stoichiometric cycle (Scheme 1). Attempts to regenerate the germylene 1 from 2 using PCy3 or PPh3 led to a complicated reaction mixture as indicated by 31P NMR spectroscopy, and the generation of PCy3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S (31P: 61.1 ppm) and PPh3
S (31P: 61.1 ppm) and PPh3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S (31P: 42.7 ppm) along with other P-containing products (Fig. S11 and S12 in the ESI†). We also heated a benzene-d6 solution of compound 2 in a sealed NMR tube at 80 °C to see the elimination of CS2. The NMR spectrum indicates the formation of 1, although there are several additional peaks along with some amount of 2.
S (31P: 42.7 ppm) along with other P-containing products (Fig. S11 and S12 in the ESI†). We also heated a benzene-d6 solution of compound 2 in a sealed NMR tube at 80 °C to see the elimination of CS2. The NMR spectrum indicates the formation of 1, although there are several additional peaks along with some amount of 2.
Reaction of 2 with another equivalent of PhC(NtBu)2Ge–Si(SiMe3)3 (1) or the analogous silylene PhC(NtBu)2Si–Si(SiMe3)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 resulted in the formation of compounds with a Ge
19 resulted in the formation of compounds with a Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S (3) or a Si
S (3) or a Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S (4) double bond (Scheme 2). The observed formation of germanethione (3) from 2 contrasts previous syntheses of germanethiones,20–23 which are usually prepared from the reaction of the corresponding germylenes with elemental sulfur. It must be stated here that we were unable to identify the side product in this reaction as the NMR of the reaction mixture was quite complicated. In fact, we were unable to separate 3 completely from the reaction mixture, which resulted in some additional peaks in its 1H and 13C spectra.
S (4) double bond (Scheme 2). The observed formation of germanethione (3) from 2 contrasts previous syntheses of germanethiones,20–23 which are usually prepared from the reaction of the corresponding germylenes with elemental sulfur. It must be stated here that we were unable to identify the side product in this reaction as the NMR of the reaction mixture was quite complicated. In fact, we were unable to separate 3 completely from the reaction mixture, which resulted in some additional peaks in its 1H and 13C spectra.
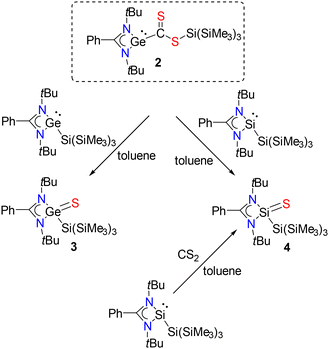 | ||
Scheme 2 Addition of another equivalent of germylene and silylene to 2 and formation of Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S (3) and Si S (3) and Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S (4) species. S (4) species. | ||
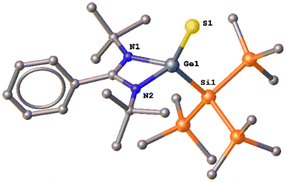
3 crystallized in the monoclinic crystal system with the Pc space group. The germanium center is bonded to the amidinate ligands, tris(trimethylsilyl), and sulfur atoms, resulting in a distorted tetrahedral geometry at the germanium center (Fig. 4). The Ge–S bond of 3 (2.100(3) Å) is slightly longer than the previously reported Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bonds (ranging from 2.048(2) to 2.066(1) Å).20–23 Compound 4 was previously reported and structurally authenticated by us through a direct reaction of the hypersilyl amidinate silylene, PhC(NtBu)2Si–Si(SiMe3)3 with elemental sulfur.19 Hence, we are not discussing here its structural and spectroscopic parameters. However, what is very interesting is that the reaction of the silylene PhC(NtBu)2Si–Si(SiMe3)319 with CS2 also led to the formation of 4, which is in stark contrast from the reaction of the analogous germylene (1).
S bonds (ranging from 2.048(2) to 2.066(1) Å).20–23 Compound 4 was previously reported and structurally authenticated by us through a direct reaction of the hypersilyl amidinate silylene, PhC(NtBu)2Si–Si(SiMe3)3 with elemental sulfur.19 Hence, we are not discussing here its structural and spectroscopic parameters. However, what is very interesting is that the reaction of the silylene PhC(NtBu)2Si–Si(SiMe3)319 with CS2 also led to the formation of 4, which is in stark contrast from the reaction of the analogous germylene (1).
Mechanistic aspects of the CS2 insertion in 1 were investigated using DFT calculations (Fig. 3). A detailed theoretical investigation (R-BP86-D3(SMD(toluene))/def2-TZVP//R-BP86/def2-SVP level) was conducted to explore the mechanistic insights into CS2 activation by 1. The initial nucleophilic coordination of 1 with CS2 affords the energetically stable intermediate INT1 (ΔGSL = −3.8 kcal mol−1), accompanying a facile activation barrier of 7.1 kcal mol−1. Similar interactions were computationally characterized for CO2 activation reactions catalyzed by base-stabilized Ge and Si complexes.24,25 Subsequent migration of the Si(SiMe3)3 unit from Ge → S generates the isomeric intermediate INT2, which is unstable by 3.3 kcal mol−1 compared to INT1. The step INT1 → INT2, with a rate-determining barrier of 14.5 kcal mol−1, involves a four-membered transition state TS2, where the concomitant cleavage and formation of the Ge–Si and Si–S bonds occur (Fig. 3). Subsequently, conformational changes constituting the Ge–C bond rotation will lead to a more stable Ge(II) complex 2 (Fig. 3 and S21†). In fact, an equilibrium mixture of 3 and INT3 will be generated from the combination of 2 with the starting germylene 1.
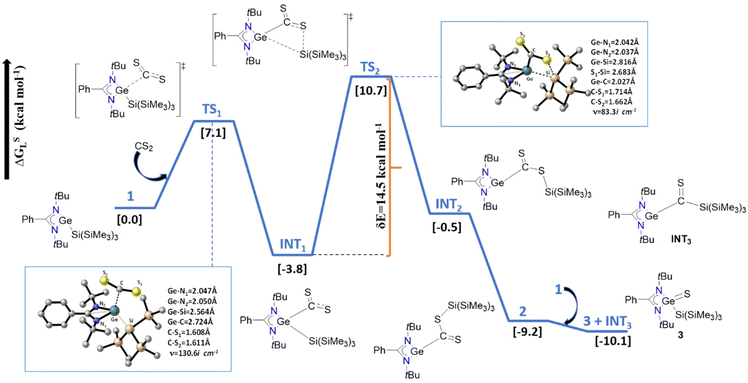 | ||
| Fig. 3 Energy profile for the CS2 activation reaction. All the energies (ΔGSL) are in kcal mol−1. Optimized geometries of transition states with the key geometric parameters. | ||
In summary, our current study unveils the insertion of CS2 into a Ge–Si bond of a Ge(II) compound, transforming the silyl group into a dithiocarboxylate ligand and resulting in 2. Subsequently, the resulting CS2 moiety can be released as NHC·CS2 from the germanium center upon the addition of an NHC. This CS2 insertion is in contrast to prior studies involving a low-valent heavier group 14 element, where carbon–sulfur bond reduction occurred, but no liberation of CS2 from the low-valent group 14 center is reported. Treatment of 2 with an additional equivalent of 1 or a related silylene yields compounds featuring Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S (3) or Si
S (3) or Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S (4) bonds – a novel way for the preparation of heavy ketones.
S (4) bonds – a novel way for the preparation of heavy ketones.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Scheme for Translational and Advanced Research in Sciences (STARS), the Ministry of Education, and the Government of India (grant MoE-STARS/STARS-1/255) (S. S. S. and D. K.). V. S. A. and G. V. thank DST, INSPIRE (IF190035 and IF190408, respectively) India for their research fellowships. P. B. G. and N. K. are grateful to UGC-India for their research fellowships. D. K. and N. K. thank IISER Kolkata for computational facility.References
- P. Jutzi, D. Eikenberg, A. Möhrke, B. Neumann and H.-G. Stammler, Organometallics, 1996, 15, 753 CrossRef CAS.
- F. M. Mgck, J. A. Baus, M. Nutz, C. Burschka, J. Poater, F. M. Bickelhaupt and R. Tacke, Chem. – Eur. J., 2015, 21, 16665 CrossRef.
- M.-P. Luecke, L. Giarrana, A. Kostenko, T. Gensch, S. Yao and M. Driess, Angew. Chem., Int. Ed., 2022, 61, e202110398 CrossRef CAS.
- M. Saito, N. Tokitoh and R. Okazaki, Organometallics, 1995, 14, 3620–3622 CrossRef CAS.
- M. Weidenbruch, U. Grobecker, W. Saak, E.-M. Peters and K. Peters, Organometallics, 1998, 17, 5206–5208 CrossRef CAS.
- C. Yan, Z. Xu, X.-Q. Xiao, Z. Li, Q. Lu, G. Lai and M. Kira, Organometallics, 2016, 35, 1323–1328 CrossRef CAS.
- S. González-Gallardo, V. Jancik, D. G. Díaz-Gómez, F. Cortés-Guzmán, U. Hernández-Balderas and M. Moya-Cabrera, Dalton Trans., 2019, 48, 5595–5603 RSC.
- N. Tiessen, M. Keßler, B. Neumann, H.-G. Stammler and B. Hoge, Angew. Chem., Int. Ed., 2021, 60, 12124–12131 CrossRef CAS PubMed.
- L. Dostál, R. Jambor, A. Růžička, R. Jirásko, E. Černošková, L. Beneš and F. de Proft, Organometallics, 2010, 29, 4486–4490 CrossRef.
- K. Oberdorf, A. Hanft, X. Xie, F. M. Bickelhaupt, J. Poater and C. Lichtenberg, Chem. Sci., 2023, 14, 5214–5219 RSC.
- N. Tokitoh, H. Suzuki and R. Okazaki, J. Chem. Soc., Chem. Commun., 1995, 1425–1426 RSC.
- J. Li, M. Hermann, G. Frenking and C. Jones, Angew. Chem., Int. Ed., 2012, 51, 8611–8614 CrossRef CAS PubMed.
- (a) M. Walewska, J. Baumgartner and C. Marschner, Chem. Commun., 2015, 51, 276–278 RSC; (b) M. Walewska, J. Baumgartner, C. Marschner, L. Albers and T. Müller, Chem. – Eur. J., 2016, 22, 18512–18521 CrossRef CAS PubMed; (c) T. Sugahara, A. E. Ferao, A. Rey, J.-D. Guo, S. Aoyama, K. Igawa, K. Tomooka, T. Sasamori, D. Hashizume, S. Nagase and N. Tokitoh, Dalton Trans., 2020, 51, 8881–8885 Search PubMed; (d) M. Walewska, J. Baumgartner and C. Marschner, Molecules, 2020, 25, 686 CrossRef CAS PubMed.
- (a) M. K. Bisai, T. Das, K. Vanka, R. G. Gonnade and S. S. Sen, Angew. Chem., Int. Ed., 2021, 60, 20706–20710 CrossRef CAS PubMed; (b) M. K. Bisai, V. S. Ajithkumar, K. V. Raj, K. Vanka, R. G. Gonnade and S. S. Sen, Chem. Commun., 2023, 59, 1669–1672 RSC.
- M. K. Bisai, V. S. Ajithkumar, R. G. Gonnade and S. S. Sen, Organometallics, 2021, 40, 2651–2657 CrossRef CAS.
- V. S. Ajithkumar, P. B. Ghanwat, K. V. Raj, K. Vanka, R. G. Gonnade and S. S. Sen, Organometallics, 2023, 42, 2983–2990 CrossRef CAS.
- S. Pahar, V. Sharma, S. Tothadi and S. S. Sen, Dalton Trans., 2021, 50, 16678–16684 RSC.
- L. Delaude, A. Demonceau and J. Wouters, Eur. J. Inorg. Chem., 2009, 1882–1891 CrossRef CAS.
- M. K. Bisai, V. S. V. S. N. Swamy, T. Das, K. Vanka, R. G. Gonnade and S. S. Sen, Inorg. Chem., 2019, 58, 10536–10542 CrossRef CAS PubMed.
- Y. Ding, Q. Ma, I. Usón, H. W. Roesky, M. Noltemeyer and H.-G. Schmidt, J. Am. Chem. Soc., 2002, 124, 8542–8543 CrossRef CAS PubMed.
- B. Prashanth and S. Singh, Dalton Trans., 2016, 45, 6079–6087 RSC.
- S. Sinhababu, R. K. Siwatch, G. Mukherjee, G. Rajaraman and S. Nagendran, Inorg. Chem., 2012, 51, 9240–9248 CrossRef CAS PubMed.
- Y. Xiong, S. Yao, S. Inoue, A. Berkefeld and M. Driess, Chem. Commun., 2012, 48, 12198–12200 RSC.
- D. Sarkar, C. Weetman, S. Dutta, E. Schubert, C. Jandl, D. Koley and S. Inoue, J. Am. Chem. Soc., 2020, 142, 15403–15411 CrossRef CAS PubMed.
- S. Dutta, K. Singh and D. Koley, Chem. – Asian J., 2021, 16, 3492–3508 CrossRef CAS PubMed.
Footnotes |
| † The paper is dedicated to Prof. Amitava Das on the occasion of his 65th birthday. |
| ‡ Electronic supplementary information (ESI) available: Experimental details, spectroscopic data, theoretical calculations, and representative NMR spectra. CCDC 2339965 (2) and 2339967 (3). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt01573h |
| § These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |