Open Access Article
Open Access ArticleSynthesis of N-heterocyclic carbene (NHC)-Au/Ag/Cu benzotriazolyl complexes and their catalytic activity in propargylamide cycloisomerization and carbonyl hydrosilylation reactions†
Entzy
Kaplanai
 a,
Nikolaos V.
Tzouras
a,
Nikolaos V.
Tzouras
 *ab,
Nikolaos
Tsoureas
a,
Nestor
Bracho Pozsoni
b,
Subhrajyoti
Bhandary
b,
Kristof
Van Hecke
*ab,
Nikolaos
Tsoureas
a,
Nestor
Bracho Pozsoni
b,
Subhrajyoti
Bhandary
b,
Kristof
Van Hecke
 a,
Steven P.
Nolan
a,
Steven P.
Nolan
 *b and
Georgios C.
Vougioukalakis
*b and
Georgios C.
Vougioukalakis
 *a
*a
aDepartment of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis, 15771, Athens, Greece. E-mail: nicktzouras@chem.uoa.gr; vougiouk@chem.uoa.gr
bDepartment of Chemistry and Centre of Sustainable Chemistry, Ghent University, Krijgslaan 281, S-3, 9000 Ghent, Belgium. E-mail: Steven.Nolan@ugent.be
First published on 11th June 2024
Abstract
Carbene–metal–amide (CMA) complexes of gold, silver, and copper have been studied extensively for their photochemical/photocatalytic properties and as potential (pre-)catalysts in organic synthesis. Herein, the design, synthesis, and characterization of five bench-stable Au-, Ag-, and Cu-NHC complexes bearing the benzotriazolyl anion as an amide donor, are reported. All complexes are synthesized in a facile and straightforward manner, using mild conditions. The catalytic activity of the Ag and Cu complexes was studied in propargylamide cycloisomerization and carbonyl hydrosilylation reactions. Both CMA-catalyzed transformations proceed under mild conditions and are highly efficient for a range of propargylamides and carbonyl compounds, respectively, affording the desired corresponding products in good to excellent yields.
Introduction
Carbene–metal–amides (CMAs) are a class of complexes consisting of a carbene type acceptor ligand and a coinage metal center (Au, Cu, Ag), coordinated by an amide donor.1,2 Over the last seven years, CMAs have attracted significant attention for their photonic applications, stemming from their uniquely simple structure, in combination with the easily modifiable donor and acceptor ligand properties. The option to employ a wide range of carbene and amido ligands has made them a valuable tool in various additional fields, such as catalysis, materials science, and medicinal chemistry.3–5For example, a plethora of Au-, Ag-, and Cu-CMA complexes now have shown promising results as emitters in the field of organic light emitting diodes (OLEDs).6,7 Recent studies have also revealed them as highly-efficient light-absorbing materials in solar cell applications, with improved performance and cost-effectiveness.2,8–12 In the field of medicine, a wide range of CMAs have shown anti-cancer, anti-inflammatory, antiviral, and/or antimicrobial properties, and are being explored for potential use in pharmaceuticals.13 Recently, the synthesis of a new series of carboline-derived CMAs with potent anticancer activity has also been reported.14
A general procedure for the synthesis of CMA complexes, particularly focusing on the metal–nitrogen bond formation, involves the use of a variety of bases, such as NaOtBu, KHMDS, KOH, or K2CO3, a well-defined [MCl(NHC)] complex and a (heterocyclic) amine in the presence of a solvent, such as acetone, THF, EtOH, etc. In some cases, the use of a phase transfer catalyst is essential for a positive reaction outcome.15,16 Another approach involves the direct use of the (heterocyclic) amine and a [M(OH)(NHC)] complex, without the need of exogenous bases.17,18 However, the existing procedures require in most cases the use of a strong base, toxic solvents, and inert conditions, limiting their applications, both in academia and industry.
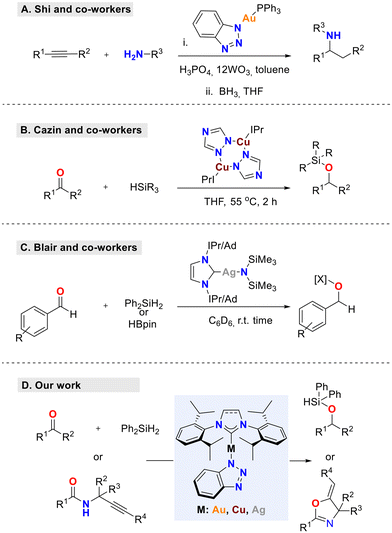
The catalytic activity of [AuCl(NHC)] and [CuCl(NHC)] complexes has been studied successfully in numerous transformations.19–23 Their high selectivity, allowing the use of very low catalytic loadings, especially in gold catalysis, has made them powerful and reliable tools. A crucial point in the case of CMA complexes, regardless of the ancillary ligand employed, is the formation of the M–N bond. Several studies have shown that the use of nitrogen-containing heterocyclic amines as donors provides stable CMAs with improved properties.3,5,24,25 In a key contribution, Shi and co-workers reported a new class of triazole-Au(I) complexes with improved reactivity and thermal stability that was synthesized and tested successfully in the intermolecular alkyne hydroamination (Scheme 1a).25 Along similar lines, in 2017, Cazin and co-workers developed a protocol for the synthesis of highly-stable neutral binuclear Cu(I)-NHC complexes, using 1,2,4-triazoles (trz) as bridging ligands (Scheme 1b).26 These [Cu(μ-trz)(NHC)]2 complexes were highly active in the hydrosilylation of ketones.
 | ||
| Scheme 1 Literature precedents using nitrogen containing compounds as amide donors, and our present approach. | ||
In contrast to the cases of gold and copper, silver-NHC catalysis remains relatively unexplored.27 On the other hand, the fact that the Ag-NHC bond is longer, and consequently weaker than that of the other coinage metals, makes silver suitable as a transmetallating agent in organometallic synthesis.28–30
This property has led to the widespread use of Ag-NHC complexes in organometallic chemistry.29,30 Furthermore, recent studies show significant promise for silver-NHC complexes as unique and highly selective catalysts in transformations including cycloisomerizations and hydrosilylations.5,28,30–32 In this regard, Blair and co-workers have reported the synthesis of rare monomeric phosphine silver(I) amido [Ag(Cy3P)(HMDS)] (HMDS = hexamethyldisilazane) complexes, both in the solid state and in solution, and explored their catalytic activities in hydroboration and the hydrosilylation of carbonyl compounds.31 These complexes are used as pre-catalysts, providing in situ access to silver(I) hydride species that are key in such transformations. More recently, the same group has synthesized two additional Ag(I) amido complexes, stabilized by ancillary N-heterocyclic carbene (NHC) ligands, and used them in hydroboration and hydrosilylation reactions (Scheme 1c).33 It was thus shown that upon changing the Lewis donor from PCy3 to a more electron donating NHC ligand, such as IPr [N,N′-bis(2,6-diisopropylphenyl)-imidazol-2-ylidene] or IAd [N,N′-bis-(1-adamantyl)imidazol-2-ylidene], improved catalytic efficiency and conversion in the hydroboration and hydrosilylation of carbonyl compounds is observed.
Inspired by these studies, we report the design, straightforward synthesis, and characterization of a new, air-, light- and moisture-stable and easily accessible CMA family of Au, Cu, and Ag complexes, bearing IPr and SIPr ligands, along with the benzotriazolyl anion as the amide component (Scheme 1d). The catalytic activity of these complexes was evaluated both in cycloisomerization and hydrosilylation reactions, thus providing a sustainable alternative to catalytic systems based on sensitive and not easily accessible catalysts.
Results and discussion
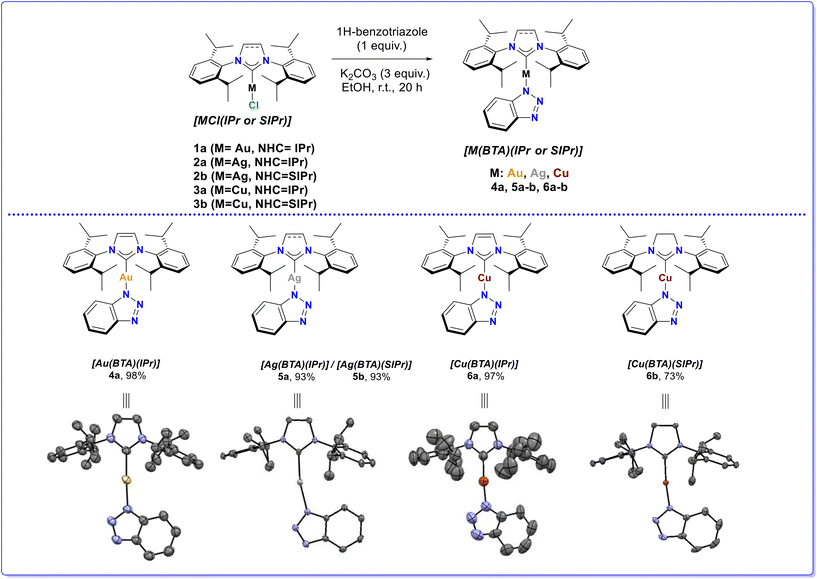
The Nolan group recently disclosed the sustainable synthesis of CMAs derived from Cu-, Ag-, or Au-NHC complexes bearing a carbazolyl moiety as the amide donor ([M(Cbz)(NHC)] where Cbz is carbazolyl).9 These complexes can be accessed via simple synthetic routes, using mild bases, such as K2CO3, and desirable greener solvents, such as ethanol and acetone. Using this work as a springboard, we aimed to address the synthesis of a new family of complexes, shown in Scheme 2, to study the possible incorporation and the impact of the benzotriazolyl anion. The synthesis of these complexes was achieved by utilizing the well-defined [MCl(IPr)] and [MCl(SIPr)] complexes 1a, 2a–b, 3a–b with 1H-benzotriazole (BTA) in the presence of K2CO3, with the aim of achieving the N–H metalation (Scheme 2). It is worth noting that the BTA-derived complex 6a can be accessed directly from the imidazolium salt and CuCl in a domino fashion, avoiding the sequential addition of substrates, in high yield (for further details see the ESI†). After screening several solvents, the optimal choice proved to be ethanol, as moving away from ethanol led to side-product formation in the case of Ag. All reactions were performed at room temperature, with no need of inert conditions or special handling, and the synthetic methodology was applied successfully not only to the Au-, but also to Ag- and Cu-CMAs. We therefore isolated complexes 4a, 5a–b, and 6a–b ([M(BTA)(IPr or SIPr)]) in pure form, and in excellent yields. It should also be noted that the synthesis of complex 4a has been previously mentioned in the literature, albeit following a different approach.34 The molecular structures of complexes 4a, 5a, and 6a–b were unambiguously established by X-ray diffraction analysis of single crystals, which revealed coordination of the metal (Au, Ag, Cu) to the terminal nitrogen (N1) of the BTA anion. The 1H and 13C{1H} NMR spectra of 4a are in full agreement with this binding motif, with distinct, clear sharp signals of the nuclei in the aromatic ring. The 1H and 13C{1H} NMR spectra of 5a and 6a–b show broad signals of the BTA anion. According to the literature, these broad signals suggest a rapid (on the NMR time-scale) equilibrium exchange between N1 and N2 of the BTA anion.25 To examine if this equilibrium is also observed in our systems, we performed variable temperature NMR experiments, which should thus result in the appearance of two signals at lower temperatures. However, two distinct signals were not observed, even at very low temperatures (−50 °C), suggesting that the only equilibrium taking place under these conditions is between N1 and N3 of the benzotriazole, which are equivalent, thus not affording distinct signals in the NMR spectra. These experiments are shown and further discussed in the ESI.†With the new family of 1H-benzotriazole-derived CMAs in hand, we explored their application in two useful organic transformations: the intramolecular cyclization of propargyl amide, leading selectively to the corresponding 5-exo-dig products, and the hydrosilylation of a range of carbonyl compounds (both aldehydes and ketones). Considering the numerous studies based on the catalytic activity of Au-based35 CMAs,36,37 we focused mostly on the study of the catalytic activity of Ag- and Cu-based complexes 5a–b and 6a–b.38–40 As the initial benchmark reaction, we studied the cyclization of the electron rich N-(2-methylbut-3-yn-2-yl)benzamide 7a to alkylidene oxazoline 8a (5-exo-dig product), monitoring the reaction via1H NMR spectroscopy, using an internal standard (1,3,5 trimethoxybenzene) as reference. In our first attempt, the reaction of 7a in the presence of 2 mol% of [Ag(BTA)(IPr)] (5a) and DCE as the solvent, at room temperature for 16 hours, afforded 8a in 94% yield (Table 1, entry 1). Notably, all the experiments were set up and performed outside of a glovebox, as the CMA catalysts are air and moisture stable, in contrast to other literature works using catalysts that require special handling.26,31,33 Solvent screening revealed that DCE was the most suitable choice for this reaction (entries 2–4). Moreover, time control experiments showed that only 1 hour, instead of 16, is long enough to achieve the formation of the cyclized product 8a in excellent yields (entry 5). Finally, reducing the catalytic loading from 2 to 1 mol% afforded the desired product in the same yield (entry 6). Complex [Ag(BTA)SIPr] (5b) was also tested under the same reaction conditions successfully (entry 7). On the other hand, the same cycloisomerization using the copper-based complexes was more challenging. More specifically, the use of 2 mol% [Cu(BTA)(IPr)] (6a) in DCE for 16 hours furnished 8a in 65% yield (entry 8). When the reaction was heated to 50 °C, the yield increased to 76% (entry 9). The yield of the desired product was significantly lower with the use of [Cu(BTA)(SIPr)] (6b) (entry 10). To test whether the 1H-benzotriazolyl moiety was necessary in our complexes for them to show their high catalytic efficiency, the catalytic ability of [Ag(IPr)Cl] (2a) and [Cu(IPr)Cl] (3a) was also probed, under identical reaction conditions. Interestingly, 2a afforded the targeted alkylidene oxazoline in the same yield as 5a (entry 11), while no reaction took place when 3a was used (entry 12).
| Entrya | [Cat] (loading, mol%) | Solvent | Time (h) | Yield of 8b (%) |
|---|---|---|---|---|
| a Reaction conditions: 0.5 mmol of 7a, 0.005 mmol (1 mol%) or 0.01 mmol (2 mol%) catalyst, 0.250 mL of solvent, 25 °C, time, under air. b Reaction yields were determined by 1H-NMR, using 1,3,5-trimethoxybenzene as internal standard. c The reaction was heated to 50 °C. d N.R.: no reaction. | ||||
| 1 | 5a (2) | DCE | 16 | 94 |
| 2 | 5a (2) | 1,4 dioxane | 16 | 92 |
| 3 | 5a (2) | DCM | 16 | 90 |
| 4 | 5a (2) | HFIP | 1 | 93 |
| 5 | 5a (2) | DCE | 1 | 97 |
| 6 | 5a (1) | DCE | 1 | 97 |
| 7 | 5b (2) | DCE | 1 | 98 |
| 8 | 6a (2) | DCE | 16 | 65 |
| 9c | 6a (2) | DCE | 16 | 76 |
| 10 | 6b (2) | DCE | 16 | 35 |
| 11 | 2a (2) | DCE | 1 | 96 |
| 12 | 3a (2) | DCE | 16 | N.R.d |
To examine if these results are relevant only for electron rich substrates, like 7a, and to expand the substrate scope study, propargyl amides bearing either electron-donating (EDG) or electron-withdrawing (EWG) groups on the benzene ring, or having no substitution at all, were also investigated (Scheme 3). In all cases, only the 5-exo-dig alkylidene oxazoline products (7b-f) were obtained. Initially, N-propargyl benzamides 7b–7f were found to be unreactive in DCE. However, replacing DCE with hexafluoro-2-propanol (HFIP), in combination with a longer reaction time (16 hours), by employing catalyst 5a, afforded the desired product 8b in 67% yield. On the other hand, catalyst 5b is more efficient in the intramolecular cyclization of 7b, leading to 8b in an excellent, 96% yield. Copper based complexes 6a and 6b, as well as chloride-based complexes 2a and 3a proved ineffective (see Table S2 in the ESI† for more details on optimization).
To examine if these results are relevant only for electron rich substrates, like 7a, and to expand the substrate scope study, propargyl amides bearing either electron-donating (EDG) or electron-withdrawing (EWG) groups on the benzene ring, or having no substitution at all, were also investigated (Scheme 3). In all cases, only the 5-exo-dig alkylidene oxazoline products (7b–f) were obtained. Initially, N-propargyl benzamides 7b–7f were found to be unreactive in DCE. However, replacing DCE with hexafluoro-2-propanol (HFIP), in combination with a longer reaction time (16 hours), by employing catalyst 5a, afforded the desired product 8b in 67% yield. On the other hand, catalyst 5b is more efficient in the intramolecular cyclization of 7b, leading to 8b in an excellent, 96% yield. Copper based complexes 6a and 6b, as well as chloride-based complexes 2a and 3a proved ineffective (see Table S2 in the ESI† for more details on optimization).
HFIP has been shown to exert unique properties as solvent, due to the strong electron-withdrawing character of its fluoroalkyl groups.35 Its mildly acidic character in combination with its strong hydrogen-bond donating ability make it ideal for several transformations.41 For example, we have recently explored the dual role of HFIP as a solvent and an activator, achieving the activation of Au–Cl bonds through hydrogen bonding alone, in gold-catalysed cycloisomerizations.35 The role of HFIP could be analogous in the present systems, as it can aid towards the removal of the benzotriazolyl anion via protodemetallation and/or hydrogen bonding, thus generating the reactive species.
The presence of a fluoride substituent on the aromatic ring of the precursor to alkylidene oxazoline 8c led to very good to excellent results upon using complexes 5a or 5b, whereas very low yields of the corresponding product were observed when using complexes 6a or 6b (Scheme 3). When using a substrate bearing two chlorides at the meta positions of the aromatic ring, oxazoline 8d was obtained in 99% yield, in only 1 hour, in the presence of 5a; catalyst 5b proved successful as well. Both [Cu(BTA)(IPr)] (6a) and [Cu(BTA)(SIPr)] (6b) performed exceedingly well in this transformation, resulting in the formation of 8d in 99% yield in 16 hours. We also utilized an alkyl-substituted propargyl amide, leading to oxazoline 8e in a very good yield in 16 hours, both with 5a and 5b. In this case, no product formation was observed when employing complexes 6a or 6b. In addition, the use of a bulkier, non-terminal alkyne, towards oxazoline 8f, is not amenable to our protocol, as no product formation was observed, even at 50 °C for 16 hours. Finally, substates 7b–f remained unreactive when chloride-based complexes 2a and 3a were used as catalysts, under the optimal reaction conditions (see Table S2 in the ESI† for more details on optimization).
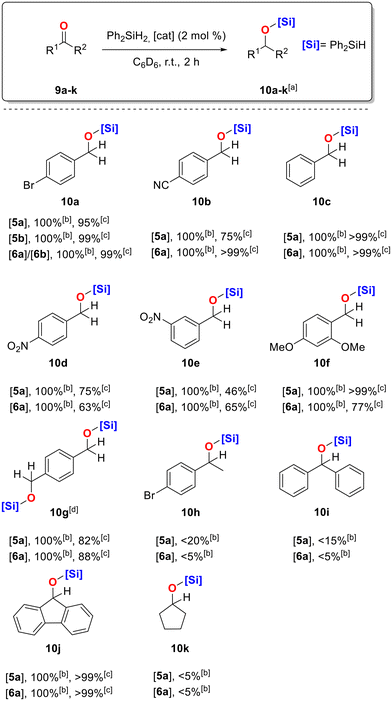
We then extended our study to the hydrosilylation of carbonyl compounds. The optimal reaction conditions were probed employing 4-bromobenzaldehyde as a benchmark substrate (Table 2). Reactions were performed using a hydrosilane reagent, 2 mol% of catalyst, at room temperature, in a J. Young NMR tube, and reaction progress was monitored by 1H NMR spectroscopy. Initial studies were performed with 3 equivalents of diphenylsilane (Ph2SiH2) and catalyst 5a, for 2 hours, either in THF-d8 or in benzene-d6, with the later providing better results (Table 2, entries 1 and 2). Diphenylsilane is a suitable hydride source in this type of transformations, due to its low cost and its ability to be used safely, both in small and large scale reactions.42,43 Attempts to further reduce the catalyst loading were not successful. An increase in the number of Ph2SiH2 equivalents was not beneficial either, while 2 hours were enough to reach full conversion of 9a to 10a. Furthermore, as is shown in entries 6 and 7 (Table 2), employing alternative silane sources, such as MePh2SiH or Si(OEt)3H, did not lead to the desired product. Finally, to confirm catalyst activity, we performed a blank experiment revealing that in the absence of 5a no reaction takes place (Table 2, entry 8). Catalysts 5b, 6a, and 6b were also tested in the same reaction under the optimal reaction conditions, all affording 10a in yields >99% (Table 2, entries 9–11). It is worth mentioning that, compared to Ag-based CMAs, the applications of Cu-based CMA complexes in the hydrosilylation of carbonyl compounds are rare in the literature, and, therefore, these results showcase the potential of more sustainable systems for the synthesis industrially interesting silyl ethers.26,31,33 On the other hand, complex 2a was inactive under these reaction conditions, underlining the necessity of the 1H-benzotriazolyl anion in the complex scaffold (Table 2, entry 12). A wide range of aldehydes and ketones were then tested, using mainly catalysts 5a and 6a (Scheme 4). The present protocol works very efficiently for several substituted aromatic aldehydes, such as 9a. The corresponding silyl ethers were obtained in nearly quantitative yields or in higher yields than those reported in literature precedents, using only 2 mol% of 5a,b or 6a,b, in 2 hours at room temperature, while similar protocols use at least 5 mol% catalyst loading, heating to accelerate the reaction, and/or require longer reaction times.26,31,33 Thus, our system competes with the state-of-the-art catalytic systems, while offering the advantage of lower-than-average catalyst loading, easily accessible and indefinitely stable (pre)catalysts, and mild reaction conditions. Silylether 10a was further hydrolyzed to the corresponding primary alcohol 11a in 97% yield (Scheme 5) and the overall yield of the two step procedure, beginning from 9a, is 92%. Moreover, benzaldehyde 9c, 2,4-dimethoxybenzaldehyde 9f, and terephaldehyde 9g underwent successful hydrosilylation with catalyst 5a, resulting in the corresponding silylated ethers 10c, 10f, and 10g, respectively, in very good to excellent yields (82–99%, Scheme 5). In the presence of 5a, benzaldehydes bearing electron-withdrawing substituents, such as –cyano or –nitro, regardless of the substituents’ position, afforded the desired products 10b, 10d, and 10e in lower, but very good yields, ranging from 75% to 86%. The same range of substituted benzaldehydes underwent successful hydrosilylation with catalyst 6a having better results than 5a on yields except of substrates 9d and 9f. Ketones are generally more challenging, due to electronic factors and the increased steric hindrance around the carbonyl group; therefore, para-bromoacetophenone (9h), benzophenone (9i), and cyclopentanone (9k) provided low conversions under our standard conditions, even with longer reaction times (10h, 10i, 10k, 5–20%), when 5a or 6a were applied. On the other hand, 9-fluorenone resulted in the desired silylated ether 10j in excellent yield (>99%) with both catalysts.
 | ||
Scheme 5 Hydrolysis of silylether 10a with TBAF, 1 M in THF: a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Reaction yields were determined by 1H-NMR using 1,3,5-trimethoxybenzene as internal standard. Reaction yields were determined by 1H-NMR using 1,3,5-trimethoxybenzene as internal standard. | ||
| Entrya | [Cat] (loading, mol%) | Silane (equiv.) | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 0.5 mmol of 9a, 0.01 mmol (2 mol%) or 0.015 mmol (3 mol%) catalyst, silane, 0.250 mL of solvent, 25 °C, time, under Ar. b The reaction yields were determined by 1H-NMR using 1,3,5-trimethoxybenzene as internal standard. c N.R.: no reaction. | |||||
| 1 | 5a (2) | Ph2SiH2 (3) | THF-d8 | 2 | 84 |
| 2 | 5a (2) | Ph2SiH2 (3) | C6H6 | 2 | 95 |
| 3 | 5a (2) | Ph2SiH2 (2) | C6H6 | 2 | 89 |
| 4 | 5a (3) | Ph2SiH2 (3) | C6H6 | 2 | 84 |
| 5 | 5a (2) | Ph2SiH2 (3) | C6H6 | 3 | 95 |
| 6 | 5a (2) | MePh2SiH (3) | C6H6 | 2 | N.R.c |
| 7 | 5a (2) | (OEt)3SiH (3) | C6H6 | 2 | N.R. |
| 8 | — | Ph2SiH2 (3) | C6H6 | 2 | N.R. |
| 9 | 5b (2) | Ph2SiH2 (3) | C6H6 | 2 | >99 |
| 10 | 6a (2) | Ph2SiH2 (3) | C6H6 | 2 | >99 |
| 11 | 6b (2) | Ph2SiH2 (3) | C6H6 | 2 | >99 |
| 12 | 2a (2) | Ph2SiH2 (3) | C6H6 | 2 | N.R. |
Conclusions
We have designed, synthesized, and thoroughly characterized a new, air-, light-, and moisture-stable family of Au-, Ag-, and Cu-NHC complexes, bearing the benzotriazolyl anion as an amide donor. All complexes can be accessed through a facile, sustainable, and straightforward manner, using commercially available starting materials under mild reaction conditions, with no need for an inert atmosphere. These CMA complexes are obtained in almost quantitative yields in a highly pure form. The potential of these complexes as pre-catalysts was explored in the intramolecular cyclization of propargylamides, as well as in the hydrosilylation of carbonyl compounds. Both reactions are catalyzed using low catalyst loadings (1–2 mol%), under mild reaction conditions. The desired products from both synthetic methodologies were generally obtained in very good to excellent yields, ranging from 46–99%. The catalytic activity of our new CMA complexes is comparable to that of the state-of-the-art catalysts in the case of aldehyde hydrosilylation and Cu- or Ag-catalyzed propargylamide cycloisomerization, while the hydrosilylation of ketones requires the use of pre-catalysts bearing more nucleophilic and strongly basic triazolyl anions.14,26,31,33 However, the advantage of the design of the CMA complexes examined herein, lies in their stability and ease of access, which is substantially improved in comparison to the current state-of-the-art.Data availability
The data that support the findings of this study are available in the ESI† of this article.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The research project was financially supported by the Hellenic Foundation for Research and Innovation (H.F.R.I.) under the “1st Call for H.F.R.I. Research Projects to support Faculty Members & Researchers and the procurement of high-cost research equipment grant” (Project Number: 16). The Research Foundation – Flanders (FWO) is acknowledged for a Fundamental Research PhD fellowship to NVT (11I6921N), research grant G0A6823N to SPN and 127522N for SB, and project AUGE/11/029 to KVH. We are grateful to the BOF (starting and senior grants to SPN) as well as the iBOF C3 project for financial support. NVT is the recipient of the 2023 Incentive Award from C.G.B.-C.B.B., the Management Committee of the Bulletin of the Belgian Chemical Societies: Koninklijke Vlaamse ChemischeVereniging (KVCV) and Société Royale de Chimie (SRC). Umicore AG is gratefully thanked for generous gifts of materials.References
- E. J. Taffet, Y. Olivier, F. Lam, D. Beljonne and G. D. Scholes, J. Phys. Chem. Lett., 2018, 9, 1620–1626 CrossRef CAS PubMed.
- N. V. Tzouras, E. A. Martynova, X. Ma, T. Scattolin, B. Hupp, H. Busen, M. Saab, Z. Zhang, L. Falivene, G. Pisanò, K. Van Hecke, L. Cavallo, C. S. J. Cazin, A. Steffen and S. P. Nolan, Chem. – Eur. J., 2021, 27, 11904–11911 CrossRef CAS PubMed.
- A. S. Romanov, S. T. E. Jones, Q. Gu, P. J. Conaghan, B. H. Drummond, J. Feng, F. Chotard, L. Buizza, M. Foley, M. Linnolahti, D. Credgington and M. Bochmann, Chem. Sci., 2020, 11, 435–446 RSC.
- J. Zhang, T. Li, X. Li, A. Lv, X. Li, Z. Wang, R. Wang, Y. Ma, R. Fang, R. Szostak and M. Szostak, Commun. Chem., 2022, 5, 1–11 CrossRef CAS PubMed.
- S. Yang, T. Zhou, X. Yu and M. Szostak, Molecules, 2023, 28, 34–37 Search PubMed.
- J. Hossain, R. Akhtar and S. Khan, Polyhedron, 2021, 201, 115151 CrossRef CAS.
- H. Duan, S. Sengupta, J. L. Petersen, N. G. Akhmedov and X. Shi, J. Am. Chem. Soc., 2009, 131, 12100–12102 CrossRef CAS PubMed.
- S. G. Guillet, A. A. Logvinov, V. A. Voloshkin, E. A. Martynova and S. P. Nolan, Org. Lett., 2023, 25, 1403–1408 CrossRef CAS PubMed.
- I. I. Hashim, N. V. Tzouras, W. Janssens, T. Scattolin, L. Bourda, S. Bhandary, K. Van Hecke, S. P. Nolan and C. S. J. Cazin, Chem. – Eur. J., 2022, 28, e202201224 CrossRef PubMed.
- J. Feng, E. J. Taffet, A. P. M. Reponen, A. S. Romanov, Y. Olivier, V. Lemaur, L. Yang, M. Linnolahti, M. Bochmann, D. Beljonne and D. Credgington, Chem. Mater., 2020, 32, 4743–4753 CrossRef CAS.
- C. N. Muniz, C. A. Archer, J. S. Applebaum, A. Alagaratnam, J. Schaab, P. I. Djurovich and M. E. Thompson, J. Am. Chem. Soc., 2023, 145, 13846–13857 CrossRef CAS PubMed.
- X. F. Song, Z. W. Li, W. K. Chen, Y. J. Gao and G. Cui, Inorg. Chem., 2021, 60, 9368–9377 CrossRef PubMed.
- K. M. Hindi, M. J. Panzner, C. A. Tessier, C. L. Cannon and W. J. Youngs, Chem. Rev., 2009, 109, 3859–3884 CrossRef CAS PubMed.
- N. V. Tzouras, T. Scattolin, A. Gobbo, S. Bhandary, F. Rizzolio, E. Cavarzerani, V. Canzonieri, K. Van Hecke, G. C. Vougioukalakis, C. S. J. Cazin and S. P. Nolan, ChemMedChem, 2022, 17, 1–7 CrossRef PubMed.
- A. S. Romanov, C. R. Becker, C. E. James, D. Di, D. Credgington, M. Linnolahti and M. Bochmann, Chem. – Eur. J., 2017, 23, 4625–4637 CrossRef CAS PubMed.
- R. Hamze, M. Idris, M. R. Daniel Sylvinson, M. Chul Jung, R. Haiges, P. I. Djurovich and M. E. Thompson, Front. Chem., 2020, 8, 401 CrossRef CAS PubMed.
- P. J. Conaghan, C. S. B. Matthews, F. Chotard, S. T. E. Jones, N. C. Greenham, M. Bochmann, D. Credgington and A. S. Romanov, Nat. Commun., 2020, 11, 1–8 CrossRef PubMed.
- A. Gómez-Suárez, D. J. Nelson, D. G. Thompson, D. B. Cordes, D. Graham, A. M. Z. Slawin and S. P. Nolan, Beilstein J. Org. Chem., 2013, 9, 2216–2223 CrossRef PubMed.
- M. Teci, D. Hueber, P. Pale, L. Toupet, A. Blanc, E. Brenner and D. Matt, Chem. – Eur. J., 2017, 23, 7809–7818 CrossRef CAS PubMed.
- B. Ranieri, I. Escofet and A. M. Echavarren, Org. Biomol. Chem., 2015, 13, 7103–7118 RSC.
- A. Mariconda, M. Sirignano, R. Troiano, S. Russo and P. Longo, Catalysts, 2022, 12, 836 CrossRef CAS.
- S. Ando, H. Matsunaga and T. Ishizuka, J. Org. Chem., 2015, 80, 9671–9681 CrossRef CAS PubMed.
- L. Kuehn, A. F. Eichhorn, T. B. Marder and U. Radius, J. Organomet. Chem., 2019, 881, 25–33 CrossRef CAS.
- M. Trose, F. Nahra, D. B. Cordes, A. M. Z. Slawin and C. S. J. Cazin, Chem. Commun., 2019, 55, 12068–12071 RSC.
- H. Duan, S. Sengupta, J. L. Petersen, N. G. Akhmedov and X. Shi, J. Am. Chem. Soc., 2009, 131, 12100–12102 CrossRef CAS PubMed.
- M. Trose, F. Lazreg, T. Chang, F. Nahra, D. B. Cordes, A. M. Z. Slawin and C. S. J. Cazin, ACS Catal., 2017, 7, 238–242 CrossRef CAS.
- N. A. Johnson, M. R. Southerland and W. J. Youngs, Molecules, 2017, 22, 1–20 Search PubMed.
- Z. Wang, N. V. Tzouras, S. P. Nolan and X. Bi, Trends Chem., 2021, 281, 674–685 CrossRef.
- J. C. Garrison and W. J. Youngs, Chem. Rev., 2005, 105, 3978–4008 CrossRef CAS PubMed.
- S. Budagumpi, R. S. Keri, G. Achar and K. N. Brinda, Adv. Synth. Catal., 2020, 362, 970–997 CrossRef CAS.
- S. A. Orr, J. A. Kelly, A. J. Boutland and V. L. Blair, Chem. – Eur. J., 2020, 26, 4947–4951 CrossRef CAS PubMed.
- V. H. L. Wong, S. V. C. Vummaleti, L. Cavallo, A. J. P. White, S. P. Nolan and K. K. M. Hii, Chem. – Eur. J., 2016, 22, 13320–13327 CrossRef CAS PubMed.
- C. P. Giarrusso, D. V. Zeil and V. L. Blair, Dalton Trans., 2023, 52, 7828–7835 RSC.
- Q. Wang, S. E. Motika, N. G. Akhmedov, J. L. Petersen and X. Shi, Angew. Chem., Int. Ed., 2014, 53, 5418–5422 CrossRef CAS PubMed.
- N. V. Tzouras, L. P. Zorba, E. Kaplanai, N. Tsoureas, D. J. Nelson, S. P. Nolan and G. C. Vougioukalakis, ACS Catal., 2023, 13, 8845–8860 CrossRef CAS.
- A. S. K. Hashmi, M. C. Blanco Jaimes, A. M. Schuster and F. Rominger, J. Org. Chem., 2012, 77, 6394–6408 CrossRef CAS PubMed.
- Y. Liu, Y. Shi, L. Wei, K. Zhao, J. Zhao, P. Zhang, X. Xu and P. Li, Chem. – Asian J., 2021, 16, 2417–2420 CrossRef CAS PubMed.
- K. Yamamoto, Y. Tsuda, M. Kuriyama, Y. Demizu and O. Onomura, Chem. – Asian J., 2020, 15, 840–844 CrossRef CAS PubMed.
- A. Alhalib and W. J. Moran, Org. Biomol. Chem., 2014, 12, 795–800 RSC.
- V. H. L. Wong, A. J. P. White, T. S. Hor and K. K. Hii, Adv. Synth. Catal., 2015, 357, 3943–3948 CrossRef CAS.
- V. Pozhydaiev, M. Power, V. Gandon, J. Moran and D. Lebœuf, Chem. Commun., 2020, 56, 11548–11564 RSC.
- K. Kuciński and G. Hreczycho, Green Chem., 2020, 22, 5210–5224 RSC.
- F. Uhlig and D. Dortmund, Inorg. Chem., 2000, 2, 208–222 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 4a, 5a, 6a, and 6b are 2333564, 2333769, 2333767, and 2333768. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt01414f |
| This journal is © The Royal Society of Chemistry 2024 |