 Open Access Article
Open Access ArticleCapturing ammonium nitrate in a synthetic uranium oxide hydrate phase: revealing the role of ammonium ions and anion inclusions †
Yingjie
Zhang
 *a,
Timothy A.
Ablott
a,
Maria K.
Nicholas
a,
Inna
Karatchevtseva
a and
Jakub
Plášil
*a,
Timothy A.
Ablott
a,
Maria K.
Nicholas
a,
Inna
Karatchevtseva
a and
Jakub
Plášil
 b
b
aAustralian Nuclear Science and Technology Organisation, Locked Bag 2001, Kirrawee DC, NSW 2232, Australia. E-mail: yzx@ansto.gov.au
bDepartment of Structure Analysis, Institute of Physics of the CAS, Na Slovance 2, Praha 8, 182 00, Czech Republic
First published on 12th June 2024
Abstract
Although uranium oxide hydrate (UOH) minerals and synthetic phases have been extensively studied, the role of ammonium ions in the formation of UOH materials is not well understood. In this work, the stabilization of a synthetic UOH phase with ammonium ions and the inclusion of ammonium nitrate were investigated using a range of structural and spectroscopic techniques. Compound (NH4)2(NO3)[(UO2)3O2(OH)3] (U-N1) crystallises in the orthorhombic Pmn21 space group, having a layered structure with typical α-U3O8 type layers and interlayer (NH4)+ cations as well as (NO3)− anions. The presence of uranyl, (NH4)+ cations and (NO3)− anions were further confirmed with a combination of FTIR and Raman spectroscopies through characteristic vibrational modes. The roles of the (NH4)+ cations for charge compensation and facilitating the inclusion of (NO3)− anions via hydrogen bonding were revealed and discussed. The findings have implications for uranium geochemistry, reprocessing of spent nuclear fuel and possible spent nuclear fuel alteration pathways under geological disposal.
1. Introduction
As the secondary uranium minerals formed in the early stage of uraninite (UO2+x) weathering (oxidation and hydration), uranium oxide hydrate (UOH) minerals have attracted recent attention owing to them being direct natural analogues for the alterations of spent nuclear fuel (SNF) under geological disposal.1–3 It is well understood that both uraninite, and therefore UO2 (the key component of SNF), will gradually oxidise to U(VI) under oxidative conditions.4–6 The uranyl [(UO2)2+] cation, as a stable U(VI) form, can coordinate with O/OH ligands in the equatorial plane, forming tetragonal, pentagonal and hexagonal bipyramids as uranyl polyhedra that further connect to each other to form various uranyl oxyhydroxide layered structures with a variety of interlayer cations.7–10 Apart from the two dozen known UOH minerals identified in various uranium deposits,1,4 about the same but growing numbers of synthetic UOH compounds are being synthesised in the laboratory.1–4 Most synthetic UOH materials have layered structures with uranium oxide hydroxide layers and interlayer cations, differing mainly on O/OH ratio in the UOH layers and the interlayer cations. The UOH layer topologies have been widely reviewed.11 In terms of secondary cations for the synthetic UOH materials, alkali and alkaline earth metal ions dominate.12–18 However, UOHs with transition metals,19–22 lead23 and lanthanide ions24–26 have also been studied and reported recently.In addition to the dominant layered UOH structures, several other types of three-dimensional (3D) structures have also been revealed. Typical examples include complicated 3D architectures of UOH materials stabilized by Cs(I), Sr(II) and Ba(II) ions.16,17,27 Furthermore, open framework structures known as uranium oxide hydrate frameworks (UOHFs) can be formed with uranyl pentagonal bipyramid dimers acting as bridging ligands linking the β-U3O8 type uranium oxide hydroxide layers.28 These UOHFs have high structure flexibility and are capable of incorporating a range of additional cations from 1+ to 4+ valences including (NH4)+, Sr2+, Y3+, Er3+, Sm3+, Eu3+, Gd3+ and U4+ in the framework channels,29–32 highlighting the complex uranium hydrolysis chemistry in the presence of various secondary cations.
Ammonium nitrate (AN), (NH4)NO3, has been widely utilised in the nuclear industry.33 Notably, production of UO2 fuel for nuclear reactors requires the precipitation of ammonium di-uranate (ADU), which is facilitated by the addition of ammonia and AN, followed by calcination to UO3 as the first uranium oxide. The quality and consistency of ADU precipitate are quite important in industrial operations.34 The processing parameters such as solution pH, addition of ammonia and AN affect the particle size, crystal structure and characteristics of ADU,35 and subsequently the properties of the UO3.36 As such, the effect of AN addition has been extensively investigated.37 The wide applications of AN in the current uranium-based nuclear fuel cycle (NFC) leads to its presence in various steps of the NFC. Therefore, its further interactions with UO3/UO2 warrant additional research efforts.
In nature, high ammonium concentrations in mineralizing fluids are likely derived from the breakdown of organic matters in sediments within the hydrothermal system,38 as well as from the bacterial activity (a process called DNRA – dissimilatory NO3− reduction to ammonium NH4+)39 connected with the oxidative dissolution of primary sulfidic minerals. Consequently, it is often found in uranium-containing minerals such as uranyl sulphates and phosphates.40 It is speculated that the key role of the ammonium ion would be the charge compensation, possibly replacing alkali ions. Even though the (NH4)+ ion has been found in nearly 15 uranyl minerals such as sulphates, phosphates etc.,40 its exact role in the formation of UOH minerals and synthetic phases has not been understood. Only one synthetic UOH compound with ammonium ions was reported in the literature.29 The compound (UOHF-NH4), forming as needle-like crystals, was synthesized hydrothermally at 220 °C using uranyl oxyacetate and ammonium carbonate in water. The crystal structure contains β-U3O8-type sheets parallel to (001) cross-linked through U2O12 pentagonal bipyramid dimers, resulting in an open framework structure composed only of uranium polyhedra. The (NH4)+ cations and H2O molecules are located in the framework channels.29 To our best knowledge, no layered structure of UOH minerals or synthetic phases with ammonium ions has been structurally documented.
What is also immediately apparent in reviewing the literature is that, despite the comprehensive studies on synthetic UOH materials with various cations across the periodic table, anion inclusions in synthetic UOH phases are much less studied.41 In this work, we report the synthesis and characterisation of a new UOH compound containing (NH4)+ ions facilitating the inclusion of (NO3)− anions. The layered structure with α-U3O8 type layers and interlayer (NH4)+ ions and (NO3)− anions was revealed by synchrotron single crystal X-ray diffraction. Subsequently, the presence of uranyl species, (NH4)+ cations and (NO3)− anions was further confirmed with a combination of FTIR and Raman spectroscopies. The roles of the (NH4)+ ions on the formation of layered UOH phases, e.g., charge compensation with interlayer hydrogen bonding and facilitating the inclusion of (NO3)− anions, were explored and discussed. In addition, its electronic structure was also investigated and reported.
2. Experimental
2.1. Material synthesis
Uranyl nitrate hexahydrate with uranium in natural isotopic abundance was used. Materials containing uranium are radioactive and should be handled with care in regulated facilities. Other chemicals in A.R. grade were purchased from Sigma-Aldrich (Merck).2.2. Characterisation
3. Results and discussion
3.1. Material synthesis and characterisation
A series of hydrothermal syntheses were performed to address the formation and stabilization of U-N1 with various solution pHs. Without pH adjustment, the synthesis (starting solution pH 3.32 and final pH 2.84) led to the formation of U-N1 in low yield (∼27 wt%). Gradually adjusting the starting solution pH to 4.0, 4.5 and 5.0 with a diluted NH3 solution increased the yield but with reduced crystal sizes. Further increasing the solution pH to above 5.0 led to the formation of a fine amorphous phase with a cloudy solution. As such, the optimized starting solution pH range for the synthesis of U-N1 would be between 4.0 and 5.0, with a trade-off between crystal quality and yield as pH increases across this range.The room temperature PXRD pattern of U-N1 matched the pattern simulated from the single crystal data, suggesting a pure phase material was obtained (Fig. S1, ESI†). The SEM-EDS examination of U-N1 confirmed the thin plate crystal morphology and the presence of U, N and O, with a U![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N atomic ratio 1
N atomic ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 1 and Table S1, ESI†). Note the quantification of O by EDS can be inaccurate and, as such, the O stoichiometry is likely to be underrepresented.
1 (Fig. 1 and Table S1, ESI†). Note the quantification of O by EDS can be inaccurate and, as such, the O stoichiometry is likely to be underrepresented.
3.2. Crystal structure and discussion
The single crystal data and structure refinement details for U-N1 are summarised in Table 1, with selected bond lengths (Å) and angles (°) listed in Table 2. U-N1 crystallises in the orthorhombic Pmn21 space group. It has a layered crystal structure (Fig. 2 and Fig. S2, ESI†) based on α-U3O8 type uranium oxide hydroxide layers (Fig. 2b) and interlayer (NH4)+ cations and (NO3)− anions (Fig. 2a). There are two distinct uranyl centres in pentagonal bipyramidal coordination geometry, with U–O bond lengths from 1.802(10) to 1.840(17) Å and normal O–U–O angles from 178.4(7) to 179.0(6)°, and five U–O bonds in the equatorial plane range from 2.255(11) to 2.631(17) Å. The uranium polyhedra share edges to form the α-U3O8 type uranium oxide hydroxide layer (Fig. 2b). Both (NH4)+ cations and (NO3)− anions are located between the uranyl oxide hydroxide layers, with N–O bonds of the (NO3)− anion ranging from 1.259(19) to 1.26(4) Å. The distance between layers is ∼4.01 Å. The possible hydrogen bonds calculated using PLATON are listed in Table S2, ESI†. It is apparent that the (NH4)+ cation is involved in extensive hydrogen bonding with uranyl oxygens, herein called O–yl, (O1 and O2) and O (O3) inside the layer (Fig. 2c). In addition, the (NO3)− anion also has hydrogen bonding with (NH4)+ cation and, through this, also a uranyl oxygen (O7) (Fig. 2d). In the interlayer, (NH4)+ and (NO3)− in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio are packed into a layer via hydrogen bonding (H⋯O8/O9) (Table S2 and Fig. S2, ESI†).
1 ratio are packed into a layer via hydrogen bonding (H⋯O8/O9) (Table S2 and Fig. S2, ESI†).
| a R 1 = ∑‖Fo| − |Fc‖/|Fo|. b wR2 = {∑[w(Fo2 − Fc2)2]/∑[w(Fo2)2]}1/2. | |
|---|---|
| Compound | U-N1 |
| CCDC | 2353965 |
| Formula | H10N3O14U3 |
| Formula weight | 990.20 |
| Crystal system | Orthorhombic |
| Space group | Pmn21 |
| a (Å) | 12.387(3) |
| b (Å) | 7.4410(15) |
| c (Å) | 7.0020(14) |
| α (°) | 90 |
| β (°) | 90 |
| γ (°) | 90 |
| Volume (Å3) | 645.4(2) |
| Z/μ (mm−1) | 2/37.627 |
| Min./max. θ [°] | 2.738/24.989 |
| d calcd (g cm−3) | 5.085 |
| GOF | 1.006 |
| Final R1a [I > 2σ(I)] | 0.0346 |
| Final wR2b [I > 2σ(I)] | 0.0869 |
| Flack parameter | 0.006(18) |
| Bond | Length (Å) | Bond | Length (Å) | Bond | Length (Å) |
|---|---|---|---|---|---|
| a ½ − X, 1 − Y, −½ + Z. b ½ − X, 1 − Y, ½ + Z. c 1 − X, +Y, +Z. d 1 − X, +Y, 1 + Z. e +X, +Y, 1 + Z. | |||||
| U1–O1 | 1.802(10) | U1–O4b | 2.613(12) | U2–O4d | 2.397(14) |
| U1–O2 | 1.814(12) | O1–U1–O2 | 179.0(6) | U2–O4e | 2.397(14) |
| U1–O3 | 2.268(12) | U2–O6 | 1.815(17) | U2–O5 | 2.631(17) |
| U1–O3a | 2.270(11) | U2–O7 | 1.840(17) | O6–U2–O7 | 178.4(7) |
| U1–O5 | 2.462(5) | U2–O3 | 2.255(11) | O8–N1 | 1.259(19) |
| U1–O4 | 2.465(13) | U2–O3c | 2.255(11) | O9–N1 | 1.26(4) |
The bond valence sum (BVS) calculations (Table S3, ESI†), using literature values for U(VI) (RU–O = 2.051; B = 0.519),49 indicated that both U centres are present as U(VI) [U1 (5.76 vu) and U2 (5.81 vu)]. The unit cell contains 2N, 6U and 30O (Table 1), with the majority as O and two OH [O4 (1.28) and O5 (0.77)]. As such, the formula for U-N1 was determined to be (NH4)2(NO3)[(UO2)3O2(OH)3].
3.3. The role of ammonium ions
Unlike alkali metal ions (e.g., Na+ and K+), which are readily bonded to nearby O–yl and possibly OH and H2O extending into the interlayer, the (NH4)+ cation is only involved in H-bonding with adjacent O-donors. It is clear from Table S2, ESI† that the (NH4)+ cation is involved in possible H-bonding with O–yl (O1, O2, O6 and O7), O (O3) and the nitrate anion (O8 and O9). Such interactions between (NH4)+ cation and the nitrate anion at the interlayer facilitate the inclusion of the nitrate anions.3.4. Anion inclusion in UOH materials
The anion inclusion in UOH materials is much less studied. A recent work reported the inclusion of (IO3)− anions in a synthetic UOH material.41 In that study, Rb2K2[(UO2)6O4(OH)6]·(IO3)2 was synthesized hydrothermally at 220 °C using uranyl acetate, KIO3 and RbIO3 as precursors with initial solution pH 5. The compound crystallised in the trigonal space group P31m (a = 7.1406(5) Å and c = 7.4646(7) Å), has a layered structure with α-U3O8 layers and interlayer K(I) and Rb(I) ions, as well as (IO3)− anions. In this work, compound U-N1 crystallised in orthorhombic space group Pmn21 (a = 12.387(3) Å, b = 7.4410(15) Å and c = 7.0020(14) Å) has a similar layered structure with α-U3O8 layers but with incorporation of both (NH4)+ ions and (NO3)− anions.3.5. Electronic structures
The electronic structure of U-N1 was investigated using DRS. The DRS spectrum (Fig. 3) shows the broad and unresolved absorption bands in the UV region (300 nm–500 nm) with two strong maxima at ∼320 and 355 nm, and further two medium maxima at ∼425 and 480 nm, corresponding to the typical U–O charge transfer bands for the U(VI) containing materials.50,513.6. Vibrational modes
The linear (UO2)2+ group has three fundamental vibrational modes, namely the Raman active ν1 – symmetric stretching vibration, and two infrared active modes: ν2 – doubly degenerate bending vibration and ν3 – antisymmetric stretching vibration.52 The decrease of uranyl group symmetry could result in the splitting of the ν2(UO2)2+ and in activation of the ν1(UO2)2+ in the infrared spectrum and of ν3(UO2)2+ in the Raman spectrum.52The Raman spectrum of compound U-N1 (Fig. 4a) revealed two strong bands at 841 and 827 cm−1 attributed to the ν1(UO2)2+ symmetric stretching vibrations.53,54 These wavenumbers correspond to calculated U–O bond lengths of 1.770 Å to 1.784 Å for the uranyl centres,55 slightly shorter than the observed U–O bond lengths [1.802(10) Å–1.840(17) Å] determined from the crystal structure. Two medium-intensity Raman bands at 555 and 453 cm−1 are predominantly due to (U3O) bridge and (U–Oligand) stretching vibrations.52 Weak bands below 350 cm−1 could be due to various bending vibration modes, molecular deformation, and lattice modes.52 The presence of nitrate anions was confirmed with the characteristic Raman peak located at 1046 cm−1 assigned to the ν1 (NO3) symmetric stretching vibration (Fig. 4a).56 Furthermore, two shoulders located on the lower frequency side of the uranyl peak (∼817 and 804 cm−1) and a weak Raman peak at 704 cm−1 may be assigned to the ν2 ONO and ν4 (NO3) bending modes, respectively.
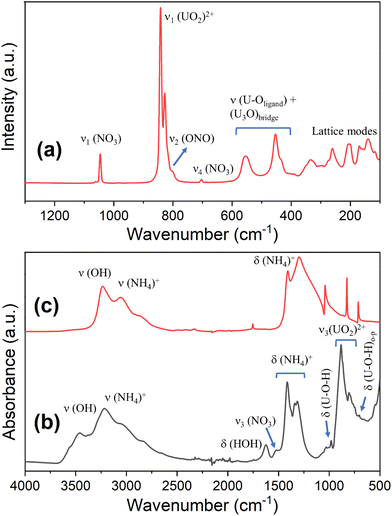 | ||
| Fig. 4 Vibrational spectra of compound U-N1: Raman (a), FTIR (b) together with FTIR of (NH4)NO3 (c) as a reference. | ||
The FTIR spectrum of compound U-N1 (Fig. 4b), in comparison with that of (NH4)NO3 (Fig. 4c), showed characteristic bands for both the expected UOH phase and (NH4)NO3, confirming that the assignment of (NH4)NO3 as the interlayer species is correct. The characteristic infrared bands for a typical UOH phase are: ν(OH) – stretching vibrations of hydroxyl ions (including water molecules) in the region 3600–3460 cm−1; the corresponding bending vibrations of δ(U–O–H) around 1030–980 cm−1, and bands in the range 760–700 cm−1, presumed to mostly be due to out-of-plane bending vibrations of U–O–H. Two strong infrared peaks at 883 and 805 cm−1 were attributed to the ν3(UO2)2+ antisymmetric stretching vibrations. There are several characteristic infrared bands for (NH4)NO3: the strong, broad bands at ∼3200 cm−1 are typical of the absorption of the (NH4)+ ions, with the corresponding bending modes evident in the region 1415–1315 cm−1. Additionally, the bending vibration mode of HOH is centred around 1624 cm−1 and a weak IR peak at 1524 cm−1 is due to ν3(NO3) antisymmetric stretching vibrations.
The combination of micro-Raman and FTIR makes it evident that present in the compound U-N1 are the expected uranyl and hydroxyl vibrations typical for a UOH phase, but also containing additional spectral features that can be assigned to the presence of both (NH4)+ and (NO3)− consistent with the structure assigned using SC-XRD.
3.7. Thermal stability
The thermal stability of compound U-N1 was also investigated. From the thermogravimetric (TG) and 1st derivatives curves (Fig. S3, ESI†), U-N1 lost about 9.4 wt% absorbed H2O (equivalent to 5–6 lattice H2O molecules per formula) up to 200 °C, followed by the gradual decomposition of (NH4)+ and (NH4)NO3 from 200 °C to 450 °C, then losing NO2 and (OH)− from 500 °C to 700 °C, losing O to form UO2 as the final residue at ∼800 °C (calc. 75.0%; obs. 74.7%).The higher temperature at which much of the water was lost is strongly indicative of their inclusion in the crystal lattice of U-N1. Due to their disorder, such waters would not be visible in SC-XRD and, as such, variable temperature PXRD experiment was performed in order to elucidate the impact of these water molecules on stabilising the structure. PXRD data were collected after successively heating U-N1 to 50 °C, 100 °C, 150 °C and 200 °C. The patterns (Fig. S4, ESI†) reveal that the structure remains unchanged at 100 °C, after which a complete decomposition occurs, as evidenced by only broad undefined peaks remaining visible in the PXRD patterns at 150 °C and 200 °C. This strongly suggests that the lattice water plays a crucial role in stabilising the structure, likely involving H-bonding interactions in addition to those proposed (Table S2, ESI†) between the U3O8-type layers involving NH4+ and NO3− species in the interlayer.
3.8. Implications and perspectives
Apart from SNF, other uranium-rich radioactive wastes are also generated from the nuclear fuel cycle such as depleted uranium from the U-235 enrichment for fuel fabrication, and nuclear medicine sector such as the production of Mo-99 via nuclear fission of U-235 targets in reactors.58 They require proper treatment and conditioning to form solid waste forms for geological disposal.58 Like the circumstance for SNF under geological disposal, all other uranium-rich waste forms might also be exposed to fluids rich in ammonium. As such, further research is necessary to understand better the effect of ammonium ions on the alteration of various uranium-rich waste forms.
4. Conclusions
Although ammonium exists in high concentrations in nature, its role on the formation and stabilization of UOH minerals and synthetic phases is not well defined. This work accounts for the first in the limited synthetic studies trying to reveal the role of ammonium ions in UOH systems.29 Consequently, a UOH phase containing α-U3O8 layers and interlayer (NH4)+ ions, introduced via the inclusion of (NH4)NO3, was successfully synthesized under mild hydrothermal conditions and characterised using both structural and spectroscopic techniques. While a combination of micro-Raman and FTIR provided evidence for the presence of uranyl and (NH4)+ cations along with OH− and (NO3)− anions, synchrotron single crystal XRD revealed the role of (NH4)+ ions as a single charge cation involved in extensive hydrogen bonding with both the α-U3O8 type layer and also neighbouring nitrate anions, facilitating their inclusion into the interlayer.The findings have implications for uranium geochemistry, possible SNF alteration pathway under geological disposal, and the treatment of wastes arising from nuclear fuel fabrications. Further work is necessary to establish the role of (NH4)+ ions in the stabilisation of UOH materials at lower temperatures and their utilisation as a means of possible inclusion for anions of interest across a wide range of experimental parameters.
Author contributions
YZ: concept, resource, project management, supervision, data curation, data analysis, paper drafting; TAA/MKN/IK: data curation, data analysis, paper drafting; JP: concept, data analysis, paper drafting.Data availability
Data can be made available through a request to the corresponding author.Conflicts of interest
The authors are not aware of any conflict of interest.Acknowledgements
MKN is grateful to the University of Sydney for the Australian Government Research Training Program (RTP) scholarship, to the Australian Institute of Nuclear Science and Engineering (AINSE) Ltd for the Residential Student Scholarship (RSS). The synthesis and characterization of materials were carried out in the facilities under Nuclear Science and Technology (NST) at ANSTO. The crystallographic data for compound U-N1 were collected on the MX1 beamline at the Australian Synchrotron, a part of ANSTO. JKP acknowledges the support by the project CzechNanoLab by MEYS CR (LM2018110).References
- P. C. Burns, Hydrated uranium oxides, in Comprehensive Nuclear Materials, ed. R. Konings and R. Stoller, Elsevier, 2nd edn, 2020 Search PubMed.
- Y. Zhang, K. T. Lu and R. Zheng, Dalton Trans., 2022, 51, 2158–2169 RSC.
- (a) R. J. Baker, Coord. Chem. Rev., 2014, 266, 123–136 CrossRef; (b) J. Plášil, J. Geosci., 2014, 59, 99–114 CrossRef.
- J. Plášil, Eur. J. Mineral., 2017, 29, 1–15 CrossRef.
- R. J. Finch and R. C. Ewing, J. Nucl. Mater., 1992, 190, 133–156 CrossRef CAS.
- J. Janeczek and R. Ewing, J. Nucl. Mater., 1992, 190, 157–173 CrossRef CAS.
- T. A. Olds, J. Plášil, A. R. Kampf, T. Spano, P. Haynes, S. M. Carlson, P. C. Burns, A. Simonetti and O. P. Mills, Am. Mineral., 2018, 103, 143–150 CrossRef.
- K.-A. Hughes, P. C. Burns and U. Kolitsch, Can. Mineral., 2003, 41, 677–685 CrossRef CAS.
- K. T. Lu, Y. Zhang, T. Wei, J. Čejka and R. Zheng, Dalton Trans., 2020, 49, 5832–5841 RSC.
- P. C. Burns and J. Hanchar, Can. Mineral., 1999, 37, 1483–1491 CAS.
- (a) M. L. Miller, R. J. Finch, P. C. Burns and R. C. Ewing, J. Mater. Res., 1996, 11(12), 3048–5056 CrossRef CAS; (b) S. V. Krivovichev and J. Plášil, CHAPTER 3: Mineralogy and crystallography of uranium, Mineralogical Association of Canada Short Course 43, Winnipeg MB, 2013, 15–119 Search PubMed.
- P. C. Burns and F. C. Hill, Can. Mineral., 2000, 38, 163–173 CrossRef CAS.
- F. C. Hill and P. C. Burns, Can. Mineral., 1999, 37, 1283–1288 CAS.
- R. E. Glatz, Y. Li, K.-A. Hughes, C. L. Cahill and P. C. Burns, Can. Mineral., 2002, 40, 217–224 CrossRef CAS.
- P. C. Burns and F. C. Hill, Can. Mineral., 2000, 38, 175–181 CrossRef CAS.
- K. T. Lu, Y. Zhang, T. Wei, T. A. Ablott, T. H. Nguyen and R. Zheng, New J. Chem., 2022, 46, 1371–1380 RSC.
- K. T. Lu, Y. Zhang, T. Wei, T. A. Ablott, J. Plasil, I. Karatchevtseva and R. Zheng, New J. Chem., 2023, 47, 13286–13296 RSC.
- Y. Zhang, K. T. Lu, T. Wei, I. Karatchevtseva and R. Zheng, Dalton Trans., 2023, 52, 17942–17953 RSC.
- M. Rivenet, N. Vigier, P. Roussel and F. Abraham, J. Solid State Chem., 2009, 182, 905–912 CrossRef CAS.
- N. G. Chernorukov, O. V. Nipruk, K. A. Klinshova, O. N. Tumaeva and D. V. Sokolov, New J. Chem., 2021, 45, 9922–9935 RSC.
- T. A. Ablott, K. T. Lu, T. Wei and Y. Zhang, Dalton Trans., 2023, 52, 6629–6640 RSC.
- Y. Zhang, J. Čejka, G. R. Lumpkin, T. T. Tran, I. Aharonovich, I. Karatchevtseva, J. R. Price, N. Scales and K. Lu, New J. Chem., 2016, 40, 5357–5357 RSC.
- Y. Li and P. C. Burns, Can. Mineral., 2000, 38, 1433–1441 CrossRef CAS.
- Y. Zhang, R. Aughterson, I. Karatchevtseva, L. Kong, T. T. Tran, J. Čejka, I. Aharonovich and G. R. Lumpkin, New J. Chem., 2018, 42, 12386–12393 RSC.
- Y. Zhang, R. D. Aughterson, Z. Zhang, T. Wei, K. Lu, J. Čejka and I. Karatchevtseva, Inorg. Chem., 2019, 58, 10812–10821 CrossRef CAS PubMed.
- Y. Zhang, K. T. Lu, T. A. Ablott, T. Wei and R. Zheng, Chem. – Asian J., 2024, 19, e202400101 CrossRef CAS PubMed.
- K.-A. Kubatko and P. C. Burns, Inorg. Chem., 2006, 45, 10277–10281 CrossRef CAS PubMed.
- Y. Zhang, T. Wei, T. T. Tran, K. T. Lu, Z. Zhang, J. R. Price, I. Aharonovich and R. Zheng, Inorg. Chem., 2020, 59, 12166–12175 CrossRef CAS PubMed.
- Y. Li, C. L. Cahill and P. C. Burns, Chem. Mater., 2001, 13, 4026–4031 CrossRef CAS.
- T. A. Ablott, K. T. Lu, R. D. Aughterson and Y. Zhang, Dalton Trans., 2022, 51, 15965–15973 RSC.
- K. T. Lu, Y. Zhang, T. Wei, Z. Wang, D. T. Oldfield and R. Zheng, Inorg. Chem., 2021, 60, 13233–13241 CrossRef CAS PubMed.
- K. T. Lu, Y. Zhang, R. D. Aughterson and R. Zheng, Dalton Trans., 2020, 49, 15854–15863 RSC.
- S. Paik, S. Biswas, S. Bhattacharya and S. B. Roy, J. Radioanal. Nucl. Chem., 2013, 295, 2141–2146 CrossRef CAS.
- (a) B. Tomazic and M. Branica, J. Inorg. Nucl. Chem., 1972, 34, 1319–1332 CrossRef CAS; (b) J. Sutton, J. Inorg. Nucl. Chem., 1955, 1, 68–74 CrossRef CAS; (c) J. Maly and V. Vesely, J. Inorg. Nucl. Chem., 1958, 7, 119–128 CrossRef CAS.
- J. B. Ainscough and B. W. Oldfield, J. Appl. Chem., 2007, 12(9), 418–424 CrossRef.
- S. Manna, S. B. Roy and J. B. Joshi, J. Nucl. Mater., 2012, 424, 94–100 CrossRef CAS.
- S. Paik, S. Biswas, S. Bhattacharya and S. B. Roy, J. Nucl. Mater., 2013, 440, 34–38 CrossRef CAS.
- J. Jo, T. Yamanaka, T. Kashimura, Y. Okunishi, Y. Kuwahara, I. Kadota, Y. Miyoshi, J.-I. Ishibashi and H. Chiba, Geochem. J., 2018, 52, 317–333 CrossRef CAS.
- J. Friedl, D. De Rosa, D. W. Rowlings, P. R. Grace, Ch. Müller and C. Scheer, Soil Biol. Biochem., 2018, 125, 340–349 CrossRef CAS.
- Mineral data: https://www.mindat.org/chemsearch.php?inc=N%2CU%2C&exc=&ima=0&sub=Search±for±Minerals (accessed on the 8th of May 2024).
- G. L. Murphy, P. Kegler, M. Klinkenberg, A. Wilden, M. Henkes, D. Schneider and E. V. Alekseev, Dalton Trans., 2021, 50, 17257–17264 RSC.
- D. Aragão, J. Aishima, H. Cherukuvada, R. Clarken, M. Clift, N. P. Cowieson, D. J. Ericsson, C. L. Gee, S. Macedo, N. Mudie, S. Panjikar, J. R. Price, A. Riboldi-Tunnicliffe, R. Rostan, R. Williamson and T. T. Caradoc-Davies, J. Synchrotron Radiat., 2018, 25, 885–891 CrossRef PubMed.
- W. Kabsch, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2010, 66, 133–144 CrossRef CAS PubMed.
- G. M. Sheldrick, SADABS, Empirical Absorption and Correction Software, Göttingen, University of Göttingen, 1996 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3–8 CrossRef PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- A. L. Spek, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2009, D65, 148–155 CrossRef PubMed.
- (a) I. D. Brown, Chem. Rev., 2009, 109(12), 6858–6919 CrossRef CAS PubMed; (b) P. C. Burns, P. C. Burns, R. C. Ewing and F. C. Hawthorne, Can. Mineral., 1997, 35, 1551–1570 CAS.
- (a) N. D. Shepherd, Y. Zhang, I. Karatchevtseva, J. R. Price, L. Kong, N. Scales and G. R. Lumpkin, Polyhedron, 2016, 113, 88–95 CrossRef CAS; (b) Y. Zhang, D. J. Fanna, N. D. Shepherd, I. Karatchevtseva, K. Lu, L. Kong and J. R. Price, RSC Adv., 2016, 6, 75045–75053 RSC.
- (a) E. R. Vance, Y. Zhang and Z. Zhang, J. Nucl. Mater., 2010, 400(1), 8–14 CrossRef CAS; (b) Y. Zhang, T. Wei, Z. Zhang, L. Kong, P. Dayal and D. J. Gregg, J. Am. Ceram. Soc., 2019, 102(12), 7699–7709 CrossRef CAS.
- R. L. Frost, J. Čejka and M. L. Weier, J. Raman Spectrosc., 2007, 38(4), 460–466 CrossRef CAS.
- (a) Y. Zhang, L. Kong, I. Karatchevtseva, R. D. Aughterson, D. J. Gregg and G. Triani, J. Am. Ceram. Soc., 2017, 100(9), 4341–4351 CrossRef CAS; (b) Y. Zhang, L. Kong, R. D. Aughterson, I. Karatchevtseva and R. Zheng, J. Am. Ceram. Soc., 2017, 100(11), 5335–5346 CrossRef CAS.
- (a) Y. Zhang, J. Čejka, I. Karatchevseva, M. Qin, L. Kong, K. Short, S. C. Middleburg and G. R. Lumpkin, J. Nucl. Mater., 2014, 446, 68–72 CrossRef CAS; (b) Y. Zhang, I. Karatchevtseva, M. Qin, S. C. Middleburgh and G. R. Lumpkin, J. Nucl. Mater., 2013, 437, 149–153 CrossRef CAS.
- J. R. Bartlett and R. P. Cooney, J. Mol. Struct., 1989, 193, 295–300 CrossRef CAS.
- G. Socrates, Infrared and Raman Characteristic Group Frequencies: Tables and Charts, John Wiley & Sons Hoboken, NY, 3rd edn, 2001 Search PubMed.
- (a) J. Brugger, N. Meisser and P. C. Burns, Am. Mineral., 2003, 88, 676–685 CrossRef CAS; (b) J. Plášil, J. Sejkora, R. Škoda and P. Škácha, J. Geosci., 2014, 59, 223–253 CrossRef; (c) J. Plášil, G. Steciuk, J. Majzlan, R. Škoda, J. Filip, M. Petr, J. Kolařík, M. Klementová, O. Bähre, G. Klöβ and L. Lapčák, ACS Earth Space Chem., 2022, 6(5), 1250–1258 CrossRef.
- (a) Y. Zhang, L. Kong, M. Ionescu and D. J. Gregg, J. Eur. Ceram. Soc., 2022, 42(5), 1852–1876 CrossRef CAS; (b) Y. Zhang and A. H. Mir, J. Nucl. Mater., 2023, 583, 154512 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Supporting figures and tables. CCDC 2353965 (U-N1). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt01372g |
| This journal is © The Royal Society of Chemistry 2024 |



