 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
On the mechanism of sp2 C–H borylation using ortho-N-substituted pyridinium cations†
Nikita
Slesarchuk
 a,
Enlu
Ma
a,
Juan
Miranda-Pizarro
a,
Sami
Heikkinen
a,
Dieter
Schollmeyer
b,
Martin
Nieger
a,
Petra
Vasko
a,
Enlu
Ma
a,
Juan
Miranda-Pizarro
a,
Sami
Heikkinen
a,
Dieter
Schollmeyer
b,
Martin
Nieger
a,
Petra
Vasko
 a and
Timo
Repo
a and
Timo
Repo
 *a
*a
aDepartment of Chemistry, Laboratory of Inorganic Chemistry, University of Helsinki, P.O. Box 55, FIN-00014, Finland. E-mail: timo.repo@helsinki.fi
bJohannes Gutenberg-Universität Mainz, Department Chemie, Duesbergweg 10-14, D-55099 Mainz, Germany
First published on 6th May 2024
Abstract
ortho-N-Substituted pyridinium cations with the weakly coordinating anion [B(C6F5)4]− have been studied and crucial structural features in the sp2 C–H borylation catalysis of 3-methylthiophene have been identified. The electron-deficiency of the aromatic core of the cation is essential for activity together with accessible protons. The spectroscopic yield of the borylation of 3-methylthiophene with catecholborane (CatBH) was optimized up to 86% and the method was further applied to other substrates such as N-alkylbenzenes. A mechanistic DFT study revealed the rate-limiting step in the catalysis to be the liberation of molecular H2 (ΔG‡ = 27.5 kcal mol−1), whereas the overall reaction was found to be exergonic by 5.1 kcal mol−1.
Introduction
C–H borylation of an sp2 carbon is one of the most desired yet arduous chemical transformations.1,2 It is an effective way to access useful building blocks for further cross-coupling reactions. For example, taking advantage of boronic ester precursors, the Suzuki–Miyaura coupling ensures that many important pharmaceuticals become available.3 Currently, sp2 C–H borylation is accessible with platinum-group metal catalysts.1,2 Due to the low Earth-abundance of precious metals and meticulous removal of metal traces in the final product, the demand for metal-free borylation is growing.4 In fact, metal-free sp2 C–H borylations are not yet numerous but have already proven to be highly effective.5–14Metal-free, boron-based catalysts for sp2 C–H borylation take advantage of either the Frustrated Lewis Pair (FLP) approach or the high reactivity of borenium cations. Both strategies can facilitate the heterolytic splitting of H2,15–22 binding of CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23–26 and even breakage of some C–H bonds.5–9,12,13,27–29 Indeed, our group has recently reported that ortho-TMP-C6H4-BH2 (TMP = 2,2,6,6-tetramethylpiperid-1-yl) (Scheme 1a) is capable of stoichiometrically activating the sp2 C–H bond of thiophene at elevated temperatures, whereas the bulkier ansa-aminoborane with an electron withdrawing C6F5-substituent on the boron can react even at room temperature (Scheme 1b).5 Moreover, it has been shown that a 2-dimethylaminopyridinium cation with a weakly coordinating [B(C6F5)4]− anion can catalytically borylate 3-methylthiophene with catecholborane (Scheme 1c).5 Besides the 2-dimethylaminopyridinium salt, there are several other examples of catalytic metal-free sp2 C–H activation of different arenes and heterocycles using FLP or borenium catalysts.6–9,12–14 However, most examples have different shortcomings such as high air- and/or moisture-sensitivity, limitations to use only electron-rich heteroarene substrates or poor accessibility to the catalyst.
23–26 and even breakage of some C–H bonds.5–9,12,13,27–29 Indeed, our group has recently reported that ortho-TMP-C6H4-BH2 (TMP = 2,2,6,6-tetramethylpiperid-1-yl) (Scheme 1a) is capable of stoichiometrically activating the sp2 C–H bond of thiophene at elevated temperatures, whereas the bulkier ansa-aminoborane with an electron withdrawing C6F5-substituent on the boron can react even at room temperature (Scheme 1b).5 Moreover, it has been shown that a 2-dimethylaminopyridinium cation with a weakly coordinating [B(C6F5)4]− anion can catalytically borylate 3-methylthiophene with catecholborane (Scheme 1c).5 Besides the 2-dimethylaminopyridinium salt, there are several other examples of catalytic metal-free sp2 C–H activation of different arenes and heterocycles using FLP or borenium catalysts.6–9,12–14 However, most examples have different shortcomings such as high air- and/or moisture-sensitivity, limitations to use only electron-rich heteroarene substrates or poor accessibility to the catalyst.
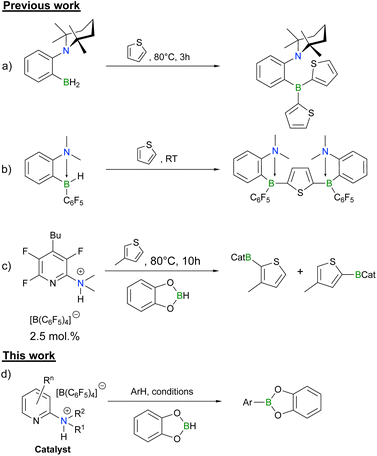 | ||
| Scheme 1 (a) sp2 C–H activation of thiophene by ortho-TMP-C6H4-BH2;5 (b) sp2 C–H activation of thiophene by ortho-Me2N-C6H4-BHC6F5;5 (c) catalytic borylation of 3-methylthiophene;5 (d) general reaction scheme of the borylation based on the pyridinium core used in this work. | ||
Herein, we report on the investigations of the structure–reactivity relationships and mechanistic understandings of the bench-stable 2-dimethylaminopyridinium derived catalyst, expand the substrate scope of the catalysis and discuss on the limitations of assembling CatB-derivatives from bench-stable pyridin-2-ylaminiums (Scheme 1d).
Results and discussion
Synthesis and optimization
In our previous research, we proposed that an o-N-substituted pyridinium cation, together with different hydroboranes, can liberate molecular hydrogen to form a compound that is reminiscent of an ansa-aminoborane-type FLP while also exhibiting borenium cation characteristics.5 Here the Lewis base component of the FLP directly affects the reactivity, which is clearly demonstrated in the previous reports.8,18 Hence, a systematic study of the effects of the o-N-substituent in the cation framework was first considered. Different o-N-substituted pyridinium compounds can be easily accessed from one precursor; our synthetic route5 was slightly modified from the previously reported to yield p-butyltetrafluoropyridine 1 (Scheme 2). Next, we could introduce different secondary amines in the ortho-position of 1 to yield compounds 2a–c. In the final step, a reported procedure5 was applied towards aminopyridines in order to synthesise the targeted salts incorporating the weakly coordinating anion [B(C6F5)4]− (3a–c). In a search for more simplified cations, other pyridine-based derivatives (3d–f) were also synthesized (see detailed syntheses in the ESI†). Compounds 1–3 were characterised in solution by multinuclear NMR spectroscopy to confirm the successful syntheses of the compounds (see the ESI†). In the case of 3a we were able to grow single crystals that were suitable for single-crystal X-ray diffraction studies. Crystals grown from a mixture of hexane/DCM (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) confirmed the molecular structure of 3a to be an ionic species where the acidic proton is located on the dimethylamino group rather than on the pyridyl nitrogen (Fig. 1).
1) confirmed the molecular structure of 3a to be an ionic species where the acidic proton is located on the dimethylamino group rather than on the pyridyl nitrogen (Fig. 1).
 | ||
| Scheme 2 General scheme of the syntheses of the catalysts 3a–c. R2NLi for 2b–c was prepared in situ. 2,3a: R = Me; 2,3b: R2 = –(CH2)5–; 2,3c: R2 = –(CH2)2O(CH2)2–. The [B(C6F5)4]− anion is omitted. All described compounds were characterized by NMR and HRMS (see the ESI†). | ||
To optimize the reaction conditions for the sp2 C–H borylation, 3-methylthiophene was chosen as the substrate and 3a as the catalyst (Table 1). The solvent screening (entries 1–5) revealed that aromatic solvents such as C6D6 and PhBr facilitate high conversions, although toluene appeared to be less effective than a neat reaction (entry 1 vs. 3). 1,2-Dichloroethane (DCE) resulted in moderate conversion, together with higher selectivity for 2-BCat-3-methylthiophene (see the ESI†). The reaction described in entry 7 was conducted by adding a second equivalent of catecholborane to entry 4 and heating for another 24 hours. In this case, the conversion improved significantly. To investigate the optimal time for the reaction, two samples prepared at the same time were heated either at 80 or 110 °C and monitored by 1H and 11B NMR spectroscopy at selected time points (see the ESI†). An increase in conversion was observed even beyond 24 hours, which indicates a high thermal stability of the catalyst. Finally, the catalyst loading was screened (entries 8–13) and an optimal conversion was observed at 5 mol% loading. In the entries 12 and 13, a significant increase in conversion was observed compared to entries 8 and 9, where the time was increased up to 120 hours, which again indirectly confirms the stability of the catalytic system. The last step of the optimization focussed on the screening of the amount of catecholborane, finding 1.5 equivalents to be ideal; thus entry 15 presents the optimised conditions used in the catalytic studies (vide infra).
| # | Temp. (°C) | Solvent | CatBH (eq.) | 3a (mol%) | Time (h) | Conv. (%) |
|---|---|---|---|---|---|---|
| a All reactions were carried out in J. Young NMR tubes. For detailed reaction conditions, see the ESI.† | ||||||
| 1 | 80 | PhMe | 1 | 4 | 24 | 54 |
| 2 | 80 | PhBr | 1 | 4 | 24 | 87.5 |
| 3 | 80 | Neat | 1 | 4 | 24 | 69 |
| 4 | 80 | C6D6 | 1 | 4 | 24 | 74 |
| 5 | 80 | DCE | 1 | 4 | 24 | 63 |
| 6 | 80 | Neat | 2 | 4 | 120 | 65 |
| 7 | 80 | C6D6 | 2 | 4 | 48 | 95 |
| 8 | 110 | C6D6 | 1 | 0.1 | 24 | 9 |
| 9 | 110 | C6D6 | 1 | 1 | 24 | 37 |
| 10 | 110 | C6D6 | 1 | 5 | 24 | 82 |
| 11 | 110 | C6D6 | 1 | 10 | 24 | 89 |
| 12 | 110 | C6D6 | 1 | 0.1 | 120 | 21 |
| 13 | 110 | C6D6 | 1 | 1 | 120 | 81 |
| 14 | 110 | DCE | 1 | 5 | 24 | 78 |
| 15 | 110 | DCE | 1.5 | 5 | 24 | 88 |
| 16 | 110 | DCE | 2 | 5 | 24 | 87 |
| 17 | 110 | DCE | 5 | 5 | 24 | 91 |
Subsequent screening of the catalysts 3a–c unfolded that replacement of the ortho-N-substituent from a dimethylamino- (3a) to piperidino- or morpholino-substituent (3b and 3c, respectively) decreased the yields of C–H borylation of 2-methylthiophene (Scheme 3). Interestingly, Fontaine et al. have reported that o-piperidino-C6H4-BH2 is a faster catalyst than o-NMe2-C6H4-BH2.8 Apparently, in the case of the aminopyridinium salts, o-N-substituent has only an auxiliary role in the C–H borylation. Non-fluorinated derivatives 3d–f showed only traces of borylated products under the same conditions and a very small conversion of 3-methylthiophene. It is likely that the electron-withdrawing effect of fluorine is essential in this type of catalysis.
Substrate scope
The optimized reaction conditions were applied to a variety of substrates (Fig. 2), including those that are particularly averse to borylation. In the selected conditions, methylthiophenes can be borylated up to 76% spectroscopic yield (4a, 4b). The reaction also proceeds in the case of unsubstituted thiophene (4c) and benzothiophene (4d), but with low yields. Using N-phenylpyrrole as the substrate, we found that a complete reduction of the pyrrole ring and borylation occurred simultaneously (4f, Fig. 2b). Here we suggest that the N-phenylpyrrole is first reduced to N-phenylpyrrolidine after which borylation occurs. This pathway can be supported by the following facts; firstly, C–H borylation proceeds with phenyl-N-pyrrolidine; secondly, our system releases H2 (vide infra) and thirdly, pyrroles are acidophobic species. | ||
Fig. 2 Substrate scope for borylation under the optimized conditions with 3a. All reactions were carried out in J. Young NMR tubes. NMR yields were calculated based on HMB as an internal standard. For detailed experimental data see the ESI.† (a) sp2 C–H borylation of thiophene and N-alkylated derivatives; (b) reduction and sp2 C–H borylation of N-phenylpyrrole; (c) oligomerization of 3,4-ethylenedioxythiophene.30 * The selectivity of the 2-Bcat-product and 5-Bcat-product is 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13. 13. | ||
We also tested N-substituted benzenes as substrates (4e–4g) where a pyrrolidinobenzene derivative (4f) was the most affordable with 54% spectroscopic yield. The lower yields in the two other cases can be explained by the less activated ring (aniline 4e) and the presence of oxygen (morpholine 4g), which can interfere with the borane species and hamper the desired reactivity. We also tested the electron-rich 3,4-ethylenedioxythiophene (Fig. 2c) observing a colour change from yellow to orange and eventually to deep blue upon adding the catalyst to the substrate solution. Apparently, the catalyst is acidic enough to protonate this substrate directly and triggers its oligomerization instead of borylation.30 2-Bromo- and 3-bromothiophenes do not result in any products of borylation upon similar conditions, due to the electron-withdrawing effects of the bromide. Upon using pinacolborane (PinBH) as the boron source, ring-opening of the PinBH occurred in the same way as previously described,12 indirectly proving the high acidity of the catalyst 3a.
Borylation mechanism
To gain more insight into the mechanism of the sp2 C–H borylation, we continued our experiments with 3a as the catalyst and 3-methylthiophene as the substrate. When 3a is mixed with a stoichiometric amount of CatBH at RT, the 1H NMR spectrum of the mixture reveals a signal for molecular hydrogen and a new broad singlet can be found at 7.9 ppm along with aromatic signals originating from CatBH (see the ESI†). Here, additional 2D NMR experiments were performed to gain a better understanding of the reaction. The same mixture was cooled to −23 °C and the obtained data revealed cross-signals between the broad singlet at 7.9 ppm and the dimethylamino group in the COSY and NOESY spectra (in the negative area). Thus, the acidic proton is still located close to the dimethylamino group of the cation even upon mixing with CatBH. However, both species interact with each other, resulting in the liberation of H2 and decomposition of CatBH to CatB-oligomers which is evident in the 11B and 1H NMR spectra. Previously, this was attributed to Cat3B2.5 The formation of Cat3B2 together with BH3·L could occur following the Marder mechanism when CatBH is exposed to a Lewis base.31 However, this mechanism does not imply H2 formation and during our experimental work there was no evidence of formation of BH3. Apparently, the reaction of 3a with CatBH includes a second, competing pathway for the formation of H2 during the stoichiometric NMR studies. This observation follows the optimization study where 1.5 equiv. of CatBH were found to be optimal for the catalysis. After 24 h, the mixture of CatBH and 3a (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) includes the various CatB-oligomers which cannot react with 3-methylthiophene, even upon heating at 110 °C. Whereas these side reactions take place in RT, the borylation of 3-methylthiophene proceeds at higher temperatures.
1) includes the various CatB-oligomers which cannot react with 3-methylthiophene, even upon heating at 110 °C. Whereas these side reactions take place in RT, the borylation of 3-methylthiophene proceeds at higher temperatures.
Computational investigations using density functional theory (DFT) at the PBE1PBE-GD3BJ/Def2-TZVP level of theory were undertaken to clarify the mechanism of the C–H borylation further. In the calculations, we omitted the effect of the weakly coordinating anion and focused only on the cationic part of 3a (see the ESI† for full computational details). The cation in 3a and CatBH interact only weakly, but when in correct orientation, molecular hydrogen can be liberated. The activation barrier for hydrogen release (TS1 in Fig. 3) was calculated to be 27.5 kcal mol−1, which is in agreement with the experimental observations of obtaining borylation only at high reaction temperatures (80–110 °C). The high barrier also confirms that the liberation of H2 observed in the stoichiometric reactions performed at RT occurs via a different pathway producing the CatB-oligomers.
The formation of intermediate Int1 is calculated to be exergonic by 3.1 kcal mol−1. The structure of Int1 is analogous to the one calculated by Ingleson and co-workers, who reported the effective haloboration of internal alkynes by cationic boronium and borenium compounds.32 Identifying the relative energy of Int1 compared to the starting materials, we tried to isolate it experimentally via a hydride cleavage of CatBH in the presence of 2a and BCF. Upon mixing 2a, BCF and CatBH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), we observed an equilibrium between CatBH, 2a and the adduct [CatBH-2a] (see the ESI†). However, this mixture did not afford the Int1 cation and [HB(C6F5)3]− anion, which are usually formed in combination with other Lewis bases, CatBH and BCF.33 Furthermore, it is known that at elevated temperatures BCF and CatBH react forming CatBC6F5 which is detrimental to the catalysis.12
1), we observed an equilibrium between CatBH, 2a and the adduct [CatBH-2a] (see the ESI†). However, this mixture did not afford the Int1 cation and [HB(C6F5)3]− anion, which are usually formed in combination with other Lewis bases, CatBH and BCF.33 Furthermore, it is known that at elevated temperatures BCF and CatBH react forming CatBC6F5 which is detrimental to the catalysis.12
The high calculated barrier for the H2 liberation prompted us to test whether the required activation energy could be lowered by mixing 2a, 3a and CatBH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in order to form Int1 through liberation of H2 from 3a and [CatBH-2a]. However, the NMR spectra of the mixture revealed no new signals that could be attributed to Int1. Apparently, intramolecular liberation of H2viaTS1 is more accessible in this case compared to an intermolecular H2 liberation from [CatBH-2a] and 3a.
1) in order to form Int1 through liberation of H2 from 3a and [CatBH-2a]. However, the NMR spectra of the mixture revealed no new signals that could be attributed to Int1. Apparently, intramolecular liberation of H2viaTS1 is more accessible in this case compared to an intermolecular H2 liberation from [CatBH-2a] and 3a.
The next step in the calculated mechanism involves the interaction of Int1 with the 3-methylthiophene and formation of a new B–C bond via a transition state TS2 at 12.0 kcal mol−1. A corresponding local minimum structure Int2 is stable, albeit with a very similar relative energy to TS2 (ΔGInt2–TS2 = −0.7 kcal mol−1). A subsequent N–B bond breakage (TS3) occurs easily as the barrier is only 13.5 kcal mol−1. The local minimum (Int2) and transition state (TS2 and TS3) energies are significantly lower than the barrier for TS1; hence, we can determine the initial hydrogen release as the rate determining step in the catalysis. The final proton transfer from the 3-methylthiophene to form the cationic 3a is thought to be barrierless; at least we were not able to find the corresponding transition state for this final process. Overall, the formation of product 4a is calculated to be an exergonic process (ΔG = −5.1 kcal mol−1) corroborating well with the experimental findings.
Conclusions
In conclusion, vital parts of the catalyst structure based on the ortho-N-substituted pyridine core were established. For such type of a borylation catalyst, there is a high requirement for electron-deficiency that can be accomplished by adding fluorine substituents in the pyridine core. According to DFT, H2 liberation is the rate-determining step in the proposed catalytic cycle. Thus, the acidic ortho-N-substituent plays a role of a “proton shell”, in which the proton has to be in an accessible orientation together with hydroborane for hydrogen liberation. Methylthiophenes and N-alkylbenzenes are available for sp2 C–H borylation through the described catalytic cycle using bench-stable catalyst 3a. The reaction proceeds only with acid-resistant hydroboranes, such as CatBH. The main limitation of the studied borylation is oligomerization of CatBH as a parallel pathway. Due to CatBH and its products, the reaction has to be carried out under an air-free atmosphere, although the catalyst is bench-stable.Author contributions
N. S. – conceptualization, data curation, investigation, methodology, visualization, and writing – original draft. E. M. – formal analysis and validation. J. M. P. – formal analysis, validation, methodology, and writing – review & editing. S. H. – NMR analysis and data curation. D. S. and M. N. – X-ray structure analysis, data curation, formal analysis, and visualisation. P. V. – methodology, data curation, formal analysis, and writing – review & editing. T. R. – supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been supported by CHEMS – The Doctoral Programme in Chemistry and Molecular Sciences at University of Helsinki. PV wishes to thank the Jenny and Antti Wihuri Foundation and the Research Council of Finland (grant no. 338271 and 346565) for funding. The authors wish to acknowledge CSC – IT Center for Science, Finland for computational resources. A. Eronen is thanked for help with HRMS measurements and J. Mannisto for discussions and corrections during the preparation of the manuscript.References
- T. Dalton, T. Faber and F. Glorius, ACS Cent. Sci., 2021, 7, 245–261 CrossRef CAS.
- I. A. I. Mkhalid, J. H. Barnard, T. B. Marder, J. M. Murphy and J. F. Hartwig, Chem. Rev., 2010, 110, 890–931 CrossRef CAS.
- N. Schneider, D. M. Lowe, R. A. Sayle, M. A. Tarselli and G. A. Landrum, J. Med. Chem., 2016, 59, 4385–4402 CrossRef CAS PubMed.
- C.-L. Sun and Z.-J. Shi, Chem. Rev., 2014, 114, 9219–9280 CrossRef CAS.
- K. Chernichenko, M. Lindqvist, B. Kótai, M. Nieger, K. Sorochkina, I. Pápai and T. Repo, J. Am. Chem. Soc., 2016, 138, 4860–4868 CrossRef CAS PubMed.
- Q. Yin, H. F. T. Klare and M. Oestreich, Angew. Chem., Int. Ed., 2017, 56, 3712 CrossRef CAS PubMed.
- M.-A. Légaré, M.-A. Courtemanche, É. Rochette and F.-G. Fontaine, Science, 2015, 349, 513–516 CrossRef PubMed.
- J. Légaré Lavergne, A. Jayaraman, L. C. Misal Castro, É. Rochette and F.-G. Fontaine, J. Am. Chem. Soc., 2017, 139, 14714–14723 CrossRef PubMed.
- É. Rochette, V. Desrosiers, Y. Soltani and F.-G. Fontaine, J. Am. Chem. Soc., 2019, 141, 12305–12311 CrossRef PubMed.
- J. Lv, X. Chen, X.-S. Xue, B. Zhao, Y. Liang, M. Wang, L. Jin, Y. Yuan, Y. Han, Y. Zhao, Y. Lu, J. Zhao, W.-Y. Sun, K. N. Houk and Z. Shi, Nature, 2019, 575, 336–340 CrossRef CAS PubMed.
- V. Iashin, D. Berta, K. Chernichenko, M. Nieger, K. Moslova, I. Papai and T. Repo, Angew. Chem., Int. Ed., 2020, 26, 13873–13879 CAS.
- A. Del Grosso, R. G. Pritchard, C. A. Muryn and M. J. Ingleson, Organometallics, 2010, 29, 241–249 CrossRef CAS.
- X. Tan, X. Wang, Z. H. Li and H. Wang, J. Am. Chem. Soc., 2022, 144, 23286–23291 CrossRef CAS PubMed.
- V. Desrosiers, A. Gaudy, K.-A. Giroux and F.-G. Fontaine, Z. Anorg. Allg. Chem., 2023, 649, e202300006 CrossRef CAS.
- D. W. Stephan, Org. Biomol. Chem., 2008, 6, 1535 RSC.
- V. Sumerin, F. Schulz, M. Atsumi, C. Wang, M. Nieger, M. Leskelä, T. Repo, P. Pyykkö and B. Rieger, J. Am. Chem. Soc., 2008, 130, 14117–14119 CrossRef CAS PubMed.
- D. Stephan and G. Erker, Angew. Chem., Int. Ed., 2010, 49, 46–76 CrossRef CAS PubMed.
- V. Sumerin, K. Chernichenko, M. Nieger, M. Leskelä, B. Rieger and T. Repo, Adv. Synth. Catal., 2011, 353, 2093–2110 CrossRef CAS.
- K. Chernichenko, B. Kótai, I. Pápai, V. Zhivonitko, M. Nieger, M. Leskelä and T. Repo, Angew. Chem., Int. Ed., 2015, 54, 1749–1753 CrossRef CAS PubMed.
- V. V. Zhivonitko, K. Sorochkina, K. Chernichenko, B. Kótai, T. Földes, I. Pápai, V.-V. Telkki, T. Repo and I. Koptyug, Phys. Chem. Chem. Phys., 2016, 18, 27784–27795 RSC.
- K. Sorochkina, V. V. Zhivonitko, K. Chernichenko, V.-V. Telkki, T. Repo and I. V. Koptyug, J. Phys. Chem. Lett., 2018, 9, 903–907 CrossRef CAS PubMed.
- J. Zheng, Z. H. Li and H. Wang, Chem. Sci., 2018, 9, 1433–1438 RSC.
- C. Mömming, E. Otten, G. Kehr, R. Fröhlich, S. Grimme, D. Stephan and G. Erker, Angew. Chem., Int. Ed., 2009, 48, 6643–6646 CrossRef PubMed.
- I. Peuser, R. C. Neu, X. Zhao, M. Ulrich, B. Schirmer, J. A. Tannert, G. Kehr, R. Fröhlich, S. Grimme, G. Erker and D. W. Stephan, Chem. – Eur. J., 2011, 17, 9640–9650 CrossRef CAS PubMed.
- D. Voicu, M. Abolhasani, R. Choueiri, G. Lestari, C. Seiler, G. Menard, J. Greener, A. Guenther, D. W. Stephan and E. Kumacheva, J. Am. Chem. Soc., 2014, 136, 3875–3880 CrossRef CAS PubMed.
- M.-A. Légaré, M.-A. Courtemanche and F.-G. Fontaine, Chem. Commun., 2014, 50, 11362–11365 RSC.
- V. Bagutski, A. Del Grosso, J. A. Carrillo, I. A. Cade, M. D. Helm, J. R. Lawson, P. J. Singleton, S. A. Solomon, T. Marcelli and M. J. Ingleson, J. Am. Chem. Soc., 2013, 135, 474–487 CrossRef CAS PubMed.
- É. Rochette, M. Courtemanche and F. Fontaine, Chem. – Eur. J., 2017, 23, 3567–3571 CrossRef PubMed.
- A. Prokofjevs and E. Vedejs, J. Am. Chem. Soc., 2011, 133, 20056–20059 CrossRef CAS PubMed.
- C.-C. Han, et al., United States Patent Application Publication, US2014/0293514A1, 2014 Search PubMed.
- S. A. Westcott, H. P. Blom, T. B. Marder, R. T. Baker and J. C. Calabrese, Inorg. Chem., 1993, 32, 2175–2182 CrossRef CAS.
- J. R. Lawson, E. R. Clark, I. A. Cade, S. A. Solomon and M. J. Ingleson, Angew. Chem., Int. Ed., 2013, 52, 7518–7522 CrossRef CAS PubMed.
- E. R. Clark, A. Del Grosso and M. J. Ingleson, Chem. – Eur. J., 2013, 19, 2462–2466 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR spectra, results of supplementary experiments, and computational details. CCDC 2288120 (3a). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt00853g |
| This journal is © The Royal Society of Chemistry 2024 |




