Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Bidentate boron Lewis acids: synthesis by tin boron exchange reaction and host–guest complex formation†
J. Louis
Beckmann
 ,
Beate
Neumann
,
Hans-Georg
Stammler
,
Beate
Neumann
,
Hans-Georg
Stammler
 ,
Jan-Hendrik
Lamm
,
Jan-Hendrik
Lamm
 and
Norbert W.
Mitzel
and
Norbert W.
Mitzel
 *
*
Chair of Inorganic and Structural Chemistry, Center for Molecular Materials CM2, Faculty of Chemistry, University of Bielefeld, Universitätsstraße 25, 33615 Bielefeld, Germany. E-mail: mitzel@uni-bielefeld.de
First published on 17th April 2024
Abstract
Four bidentate boron Lewis acids based on the 1,8-diethynylanthracene backbone have been synthesized by a tin–boron exchange reaction with various chloroboranes, yielding the products in good to excellent yields. Complexation experiments of the host compounds with pyridine, pyrimidine and TMEDA demonstrated striking differences in terms of formation and solubility of the supramolecular adducts. The host–guest complexes were investigated by multinuclear NMR spectroscopy and structurally characterized by X-ray diffraction experiments, illustrating the adaptation of the host system upon adduct formation with different neutral guest molecules.
Introduction
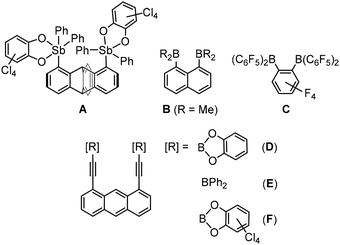
While poly-Lewis bases (e.g. crown ethers or cryptands) have been extensively studied for decades, their phenomenological counterparts, poly-Lewis acids (PLAs), have attracted increasing interest in recent years.1–3 Like their Lewis base counterparts, PLAs find applications in catalysis,4–8 anion sensing9–20 or small molecule recognition.21–23 PLAs combine multiple Lewis acid functions via a mostly organic backbone. Although a wide variety of elements are used in PLAs, such as silicon,24 tin,25,26 antimony11,27,28 or mercury,29 a high percentage of PLAs contain group 13 elements – particularly boron12,21,30–33 – due to their natural electron deficiency.Most PLAs are bidentate and therefore bind the corresponding guest molecule or ion in a pincer-like μ(1,2)-chelating mode. A rigid organic scaffold is required to ensure selective complexation of the guest molecule. To provide this rigidity, they are usually based on aromatic backbones such as phenylene,22 naphthalene13 or anthracene.33 The element which serves as Lewis acid component in such systems as well as the distance between the functions is crucial for their host–guest chemistry, as it determines the selectivity towards potential guest molecules. For example, the triptycene-based antimony(V) Lewis acid A (Scheme 1) by Gabbaï et al. can effectively bind fluoride ions in a chelating fashion.11 Among the bidentate boron Lewis acids, the naphthalene derivative B of Katz, commonly known as ‘hydride sponge’, is a prominent representative. This host system can chelate not only hydride ions, as the name suggests, but also fluoride and hydroxide ions.13 The perfluorinated system C of Collins and Piers, which has also been studied in detail in the context of isobutene polymerization, has been found to cooperatively complex chloride, fluoride, hydroxide, methanolate and azide ions.34–38
The organic backbone is sometimes substituted and extended with alkynyl spacers, which offers three advantages. First, the semi-rigid alkynes allow the Lewis acid functions to adapt to the spatial requirements of the guest molecule. Second, they prevent steric repulsion between the complexed guest molecule and the backbone, which can occur in some cases, such as with the 1,8-anthracenyl backbone. Finally, the alkynes can be easily substituted with various Lewis acid functions such as boryl33 and stibanyl39 groups. Some of these substitution reactions are not accessible for direct functionalization of aromatic systems. An established route for introducing such Lewis acid functions is either by lithiation, as used by Katz to synthesize the host system D,40 or, more elegantly, by a tin-element exchange reaction, as used to synthesize the anthracene-based diboranyl compounds D, E and F.33 A direct comparison of the two routes for the synthesis of D highlighted the advantages of the tin–boron exchange reaction.
The phenyl derivative E was found to form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-adduct with pyrimidine and TMEDA. Attempts to obtain a host with a significantly higher Lewis acidity in the form of the perchlorocatecholato derivative F were hampered by the insolubility of this compound.
1-adduct with pyrimidine and TMEDA. Attempts to obtain a host with a significantly higher Lewis acidity in the form of the perchlorocatecholato derivative F were hampered by the insolubility of this compound.
Since the synthesis and host–guest chemistry with anthracene derivatives is well established in our group,3,23,30,33,39 we chose the 1,8-diethinylanthracene backbone as a test system for our subsequent work, in which we tried to introduce other boranyl functions by a tin–boron exchange reaction, which offer additional properties such as an easier purification process, less steric repulsion of the substituents, better solubility or a higher Lewis acidity. We then investigated the host–guest chemistry of these new host systems.
Results and discussion
Tin–boron exchange reactions
The bidentate Lewis acids 3a–d were synthesized in good to excellent yields by a transmetallation reaction with the chloroboranes 2a–d (Scheme 2). A tin–boron exchange reaction with the chloroborane 2a41 gave product 3a in 89% yield. In the synthesis of 2a, the precursor compound I and the by-product II were structurally characterized (Scheme 2, for solid state structures see the ESI†). As reported, chloroborane 2a is unstable and was therefore used as a stabilized diethyl etherate; however, the product 3a was found to be stable for months as a solid under inert conditions at ambient temperature and for weeks as a solution in dry CDCl3. | ||
| Scheme 2 Synthesis of the bidentate boron host systems 3a–d, subsequent hydroboration of 3d and characterized intermediates I and II in the synthesis of 2a. | ||
For the dialkylborane derivatives 3b and 3c, comparable exchange reactions of stannylalkynes with Me2BBr and Et2BCl, respectively, are known from the literature.42,43 Since a reaction of stannane 1 with Me2BBr did not yield diborane 3b, Me2BCl (2b) was used instead. 2b was conveniently prepared in an analogous approach to the reported synthesis of Et2BCl (2c) by Breher et al.44 by reacting trimethylborane with boron trichloride in the presence of catalytic amounts of NaBH4.
Dimethylalkynylboranes have been reported to be highly unstable,43 and although we were able to isolate 3b, the reaction was found to be sensitive to reaction time. Longer reaction times (>30 min) lead to complete decomposition of the product, presumably due to ligand redistribution reactions of methyl and ethynyl groups. However, after removal of the formed chlorotrimethylstannane and excess Me2BCl (2b), the isolated compound 3b is stable for months as a solid under inert conditions at ambient temperature and for weeks as a solution in dry CDCl3. The transmetallation reaction with Et2BCl (2c) was found to be much more insensitive to unwanted ligand redistribution reactions. As the products 3b and 3c are non-volatile, purification was conveniently achieved by removal of the volatile reactants, giving both systems in quantitative yield.
A tin–boron exchange reaction with (C6F5)2BCl (2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45,46), as reported by Piers et al.47 could be applied to bisstannane 1 to give the highly electrophilic bidentate borane 3d, which could be further functionalized by hydroboration with two equivalents of Piers’ borane ((C6F5)2BH),46,48 leading to the tetraboranyl compound 4d. Further hydroboration of the CH
45,46), as reported by Piers et al.47 could be applied to bisstannane 1 to give the highly electrophilic bidentate borane 3d, which could be further functionalized by hydroboration with two equivalents of Piers’ borane ((C6F5)2BH),46,48 leading to the tetraboranyl compound 4d. Further hydroboration of the CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C[B(C6F5)2]2 groups does not occur even with an excess of (C6F5)2BH. This had already been observed by Piers for the hydroboration of diphenylacetylene, which also yielded the olefin without further hydroboration. This is due to either steric or electronic reasons, since both ethynyl functions in 1,8-diethynylanthracene undergo double hydroboration with Piers’ borane, ultimately forming the 1,8-diethylderivative bearing two CH2–CH[B(C6F5)2]2 groups.30 This again correlates with the observations regarding a feasible twofold hydroboration of phenylacetylene.46 As already mentioned by Piers, unsaturated CH
C[B(C6F5)2]2 groups does not occur even with an excess of (C6F5)2BH. This had already been observed by Piers for the hydroboration of diphenylacetylene, which also yielded the olefin without further hydroboration. This is due to either steric or electronic reasons, since both ethynyl functions in 1,8-diethynylanthracene undergo double hydroboration with Piers’ borane, ultimately forming the 1,8-diethylderivative bearing two CH2–CH[B(C6F5)2]2 groups.30 This again correlates with the observations regarding a feasible twofold hydroboration of phenylacetylene.46 As already mentioned by Piers, unsaturated CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C[B(C6F5)2]2 groups such as in 4d do not undergo β-elimination or retrohydroboration, which consistently was also not observed for compound 4d at ambient temperature.
C[B(C6F5)2]2 groups such as in 4d do not undergo β-elimination or retrohydroboration, which consistently was also not observed for compound 4d at ambient temperature.
The solid state structures of 3a, 3d and 4d have been determined by single crystal X-ray diffraction (Fig. 1). The boron atoms in 3d and 4d are approximately trigonally planar with C–B–C angles ranging from 114.6(1)° to 125.1(3)° and angle sums of 360.0(3)°. In 3a, the deviation from the ideal 120° is most pronounced due to the bridging o-xylylene group: the five-membered ring causes the angles C(17)–B(1)–C(24) and C(27)–B(2)–C(34) to be significantly smaller than 120°, resulting in the other two C–B–C angles being larger than 120°. Despite this distortion, the boron atoms have trigonal planar geometry (d(B(1)⋯C(16)–C(17)–C(24)-plane) = 0.001(1) Å).
In 3d, the ethynyl spacer C(15)![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C(16)–B(1) is strongly distorted (angle C(15)–C(16)–B(1) = 166.8(2)°; torsion angle B(1)–C(1)–C(5)–C(8) = 156.6(1)°). This is most likely due to packing effects of the C6F5 groups. For example, one C6F5 group of the deformed ethynyl spacer shows an intramolecular parallel-displaced aryl stacking interaction with one C6F5 group of the other boranylalkynyl group (d(centroid–centroid) = 3.575(1) Å).
C(16)–B(1) is strongly distorted (angle C(15)–C(16)–B(1) = 166.8(2)°; torsion angle B(1)–C(1)–C(5)–C(8) = 156.6(1)°). This is most likely due to packing effects of the C6F5 groups. For example, one C6F5 group of the deformed ethynyl spacer shows an intramolecular parallel-displaced aryl stacking interaction with one C6F5 group of the other boranylalkynyl group (d(centroid–centroid) = 3.575(1) Å).
Additionally, the other C6F5 group of this deformed ethynyl spacer shows an intermolecular aryl stacking interaction with the anthracene backbone of another molecule (d(centroid–centroid) = 3.725(1) Å). Apart from these, there are several intermolecular aryl stacking interactions between the anthracene backbones. In 4d, the CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C[B(C6F5)2]2 groups are rotated away from each other due to steric repulsion. In addition, the vinyl groups are distorted and not in plane with the anthracenyl backbone. This is indicated by the torsion angle C(15)–C(1)–C(5)–C(17) of 22.2(3)°. Similar to 3d, there are also several intra- and intermolecular aryl stacking interactions in 4d.
C[B(C6F5)2]2 groups are rotated away from each other due to steric repulsion. In addition, the vinyl groups are distorted and not in plane with the anthracenyl backbone. This is indicated by the torsion angle C(15)–C(1)–C(5)–C(17) of 22.2(3)°. Similar to 3d, there are also several intra- and intermolecular aryl stacking interactions in 4d.
Host–guest chemistry of the Lewis-acids
The adduct formation of the host systems was then investigated in detail. The hydroborated species 4d was found to be unsuitable for the formation of distinguishable adducts, probably for steric reasons or due to strong aryl stacking interactions of the eight C6F5 groups. Furthermore, the host chemistry of 3b was found to be similar to that of 3c, but since the ethyl derivative 3c is easier accessible, this system was selected for further investigation. The adduct formation of 3a, 3c and 3d with pyridine (Py) and the bifunctional bases pyrimidine (Pym) and tetramethylethylenediamine (TMEDA) was analysed by means of NMR spectroscopy, single crystal X-ray diffraction and elemental analyses (Scheme 3). In general, the coordination sphere of the boron atoms of each adduct in the solid state is distorted tetrahedral by coordination of a nitrogen atom of the respective amine. Consistent with this, the adduct formation of each adduct in solution (except the insoluble ones) is indicated by a chemical shift in the 11B NMR spectra between −7 and +2 ppm, which is the typical shift range for tetracoordinated boron species. All three host systems formed the expected 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-adduct with pyridine. The signals in the 1H NMR spectra were shifted with respect to the corresponding free host system (Fig. 2).
2-adduct with pyridine. The signals in the 1H NMR spectra were shifted with respect to the corresponding free host system (Fig. 2).
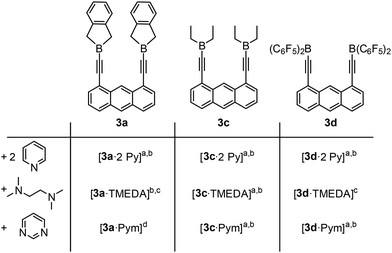 | ||
Scheme 3 Adduct formation of hosts 3a, 3c and 3d with pyridine, pyrimidine and TMEDA. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NMR spectra confirm the adduct formation; b NMR spectra confirm the adduct formation; b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a solid state structure was determined (see Fig. 4); c a solid state structure was determined (see Fig. 4); c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) the formed adduct precipitates quantitatively, therefore no NMR data could be collected; d the formed adduct precipitates quantitatively, therefore no NMR data could be collected; d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) the 1H NMR spectrum is strongly broadened, indicating oligomers or a dynamic exchange of different species in solution. the 1H NMR spectrum is strongly broadened, indicating oligomers or a dynamic exchange of different species in solution. | ||
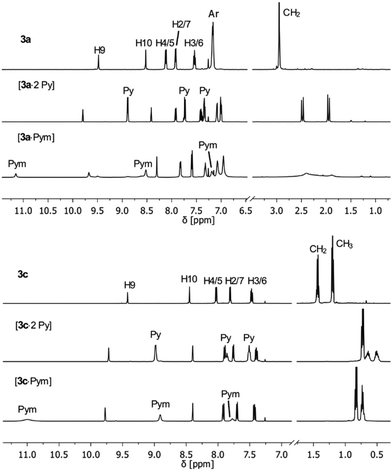 | ||
| Fig. 2 1H NMR spectra of diboranes 3a (top) and 3c (below) as free host systems and adducts with pyridine (Py) and pyrimidine (Pym) in CDCl3. | ||
In contrast to the free host, the methylene protons of the adducts [3a·2Py] and [3c·2Py] become diastereotopic, resulting in a prominent doublet in the 1H NMR spectra. In the 19F NMR spectrum of [3d·2Py], the signals of the C6F5 groups are shifted with respect to the free host compound (Fig. 3).
 | ||
| Fig. 3 19F NMR spectra of diborane 3d as free host system and adducts with pyridine (Py) and pyrimidine (Pym) in C6D6. | ||
Structural evidence for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-adduct formation of the pyridine adducts could be obtained by single crystal X-ray diffraction for the adducts of each host (Fig. 4). Among the pyridine adducts, [3d·2Py] shows the most pronounced distortion of the ethynyl spacers. This is expressed by the widest B⋯B distance among the adducts observed herein, by B(1)–C(1)–C(5) and B(2)–C(5)–C(1) angles well above 90° (indicating a strong lateral bending) and by a torsion angle B(1)–C(1)–C(5)–B(2) of 18.3(1)°, additionally indicating twisting of the alkyne units relative to the backbone (Table 1). This result can be explained by the higher steric demand of the C6F5 groups. Despite the higher Lewis acidity of the B(C6F5)2 functions, the B⋯N distances in [3d·2Py] are not significantly shorter than in [3a·2Py] or [3c·2Py].
2-adduct formation of the pyridine adducts could be obtained by single crystal X-ray diffraction for the adducts of each host (Fig. 4). Among the pyridine adducts, [3d·2Py] shows the most pronounced distortion of the ethynyl spacers. This is expressed by the widest B⋯B distance among the adducts observed herein, by B(1)–C(1)–C(5) and B(2)–C(5)–C(1) angles well above 90° (indicating a strong lateral bending) and by a torsion angle B(1)–C(1)–C(5)–B(2) of 18.3(1)°, additionally indicating twisting of the alkyne units relative to the backbone (Table 1). This result can be explained by the higher steric demand of the C6F5 groups. Despite the higher Lewis acidity of the B(C6F5)2 functions, the B⋯N distances in [3d·2Py] are not significantly shorter than in [3a·2Py] or [3c·2Py].
 | ||
| Fig. 4 Molecular structures of the adducts [3a·2Py], [3a·TMEDA], [3d·Pym], [3c·2Py], [3c·TMEDA], [3c·Pym] and [3d·2Py] in the solid state. B⋯N interactions are shown as red dotted lines. Hydrogen atoms, solvent molecules (in case of [3d·2Py] and [3c·Pym]) and one disordered C6F5 group (in case of [3d·2Py]) are omitted for clarity. For [3a·2Py], one of two independent molecules in the asymmetric unit is shown. Ellipsoids are set at 50% probability. Relevant data for these structures are listed in Table 1. | ||
| Adduct | d(B(1)⋯B(2)) | d(B(1)–N(1)) | d(B(2)–N(2)) | ∡(B(1)–C(1)–C(5)) | ∡(B(2)–C(5)–C(1)) | τ(B(1)–C(1)–C(5)–B(2)) |
|---|---|---|---|---|---|---|
| [3a·2Py] | 5.706(7) | 1.638(6) | 1.638(5) | 98.8(1) | 90.0(1) | 13.7(1) |
| [3c·2Py] | 5.808(1) | 1.645(1) | 1.636(1) | 95.4(1) | 92.8(1) | 21.6(1) |
| [3d·2Py] | 6.403(6) | 1.596(6) | 1.629(5) | 101.9(1) | 95.8(1) | 18.3(1) |
| [3a·TMEDA] | 5.453(2) | 1.659(2) | 1.678(2) | 92.0(1) | 92.6(1) | 17.1(1) |
| [3c·TMEDA] | 5.494(1) | 1.717(1) | 1.719(1) | 93.7(1) | 92.1(1) | 13.9(1) |
| [3c·Pym] | 5.231(2) | 1.680(2) | 1.673(2) | 90.7(1) | 92.5(1) | 8.5(1) |
| [3d·Pym] | 5.085(3) | 1.669(2) | 1.671(3) | 91.3(1) | 90.7(1) | 4.7(1) |
The formation of supramolecular complexes was then tested with the flexible bifunctional base TMEDA, which can adapt to the (mostly) fixed B⋯B distance in the host system. All three host systems quantitatively form an adduct with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 composition in the form of a poorly soluble or insoluble precipitate. Even when a large excess of TMEDA was applied, the 1
1 composition in the form of a poorly soluble or insoluble precipitate. Even when a large excess of TMEDA was applied, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adducts remained precipitated. Elemental analyses confirmed the 1
1 adducts remained precipitated. Elemental analyses confirmed the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 composition of the three TMEDA adducts.
1 composition of the three TMEDA adducts.
While [3a·TMEDA] and [3d·TMEDA] are completely insoluble in CDCl3, the adduct [3c·TMEDA] remains partially dissolved. This corresponds to the observation, that the adducts of the ethyl derivative 3c are the most soluble ones studied here. In case of [3c·TMEDA], the 1H and 11B NMR spectroscopic data additionally support the existence of a stable adduct in solution. For [3a·TMEDA] and [3c·TMEDA], the precipitation process provided single crystals suitable for X-ray diffraction. The molecular structures in the solid state confirm the presence of chelate-like 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-adducts, in which the two amine functions of one TMEDA molecule are coordinated by the two boron atoms of a host molecule.
1-adducts, in which the two amine functions of one TMEDA molecule are coordinated by the two boron atoms of a host molecule.
The pincer-like complexation of TMEDA results in less bent alkyne spacers and consequently a smaller B⋯B distance compared to the pyridine adducts (Table 1). Although the results for [3c·TMEDA] confirm a chelate-like 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-adduct and strongly suggest such an adduct for [3a·TMEDA], it cannot be conclusively ruled out for [3d·TMEDA] whether it is a chelate-like 1
1-adduct and strongly suggest such an adduct for [3a·TMEDA], it cannot be conclusively ruled out for [3d·TMEDA] whether it is a chelate-like 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-adduct or a polymer with a 1
1-adduct or a polymer with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 composition.
1 composition.
At least the formation of supramolecular adducts was tested with pyrimidine, which is a more rigid guest molecule and has a smaller N⋯N distance than TMEDA. When added to a solution of 3a, a strong broadening of the signals in the NMR spectra is observed (Fig. 2). The appearance of only one 11B NMR signal at 0.7 ppm of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 3a and pyrimidine confirms the saturation of each boron atom with an amine function. The broadening of the 1H NMR resonances indicates the formation of oligomers with a 1
1 mixture of 3a and pyrimidine confirms the saturation of each boron atom with an amine function. The broadening of the 1H NMR resonances indicates the formation of oligomers with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 composition. Despite all attempts, single crystals of such an adduct could not be obtained.
1 composition. Despite all attempts, single crystals of such an adduct could not be obtained.
Both 3c and 3d bind pyrimidine in a pincer-like fashion, as confirmed by the solid-state structures of the adducts [3c·Pym] and [3d·Pym]. Since the N⋯N distance in pyrimidine is smaller than in TMEDA, the B⋯B distances in the pyrimidine adducts are the shortest observed in this study. The alkyne spacers of the pyrimidine adducts are also the least distorted, as indicated by angles B(1)–C(1)–C(5) and B(2)–C(5)–C(1) close to 90° and a small torsion angle B(1)–C(1)–C(5)–B(2) (Table 1).
A doublet of the diastereotopic methylene protons can be observed in the 1H NMR spectrum of [3c·Pym] (Fig. 2), similar but shifted with respect to [3c·2Py]. The 1H and 19F NMR spectroscopic data of [3d·Pym] are also consistent with the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-adduct. Comparable to the 19F NMR spectrum of the pyridine adduct of 3d, the signals of the C6F5 groups in [3d·Pym] are shifted with respect to the free host 3d (Fig. 3). When more than 1.0 equivalents of pyrimidine are added, signals of [3d·2Pym] can be observed in addition to [3d·Pym] as a distinct second species in solution. In the solid state, the B⋯B distance is even smaller than for [3c·Pym], despite the higher steric demand of C6F5 groups compared to ethyl groups.
1-adduct. Comparable to the 19F NMR spectrum of the pyridine adduct of 3d, the signals of the C6F5 groups in [3d·Pym] are shifted with respect to the free host 3d (Fig. 3). When more than 1.0 equivalents of pyrimidine are added, signals of [3d·2Pym] can be observed in addition to [3d·Pym] as a distinct second species in solution. In the solid state, the B⋯B distance is even smaller than for [3c·Pym], despite the higher steric demand of C6F5 groups compared to ethyl groups.
Conclusions
The tin–boron exchange reaction with the chloroboranes 2a–d allowed the efficient synthesis of four bidentate Lewis acids based on the 1,8-diethynylanthracene backbone. In addition, host 3d was hydroborated to give tetraborane 4d, with which no further hydroboration reaction was possible. Host–guest experiments with pyrimidine and TMEDA in solution and in the solid state demonstrated the formation of supramolecular chelate-like host–guest complexes. In the solid state structures, different steric requirements of the substituents were expressed by different degrees of bending of the alkyne spacers. This backbone distortion allows the host system to adapt to the guest molecules to a certain extent. The different substituents of the boranyl functions resulted in significantly different solubilities of the adducts. While the partially fluorinated system 3d and its adducts were moderately soluble, the adducts of the ethyl derivative 3c were the most soluble. The TMEDA adducts were completely or nearly insoluble in chloroform or benzene and immediately precipitated as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adducts. For 3c and 3d, pincer-like host–guest complexes with the smaller pyrimidine were observed in solution and the solid state. In contrast to 3c and 3d, system 3a, despite its similarity to 3c, did not form a distinct adduct with pyrimidine, but rather showed a dynamic exchange of different species in solution. The highly Lewis-acidic system 3d forms a 1
1 adducts. For 3c and 3d, pincer-like host–guest complexes with the smaller pyrimidine were observed in solution and the solid state. In contrast to 3c and 3d, system 3a, despite its similarity to 3c, did not form a distinct adduct with pyrimidine, but rather showed a dynamic exchange of different species in solution. The highly Lewis-acidic system 3d forms a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct with pyrimidine, but also shows two distinguishable species ([3d·Pym] and [3d·2Pym]) upon addition of more than one equivalent pyrimidine.
1 adduct with pyrimidine, but also shows two distinguishable species ([3d·Pym] and [3d·2Pym]) upon addition of more than one equivalent pyrimidine.
Author contributions
J. L. Beckmann: synthesis, data analysis, writing the manuscript. H.-G. Stammler, B. Neumann and J.-H. Lamm: determination of SC-XRD structures, XRD data analysis. N. W. Mitzel: writing, reviewing, and editing the manuscript, supervision, project administration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Marco Wißbrock and Dr Andreas Mix for recording NMR spectra and Barbara Teichner for performing elemental analyses. This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – grant MI477-25/3, project no. 2458859450.References
- V. M. Gonzalez, G. Park, M. Yang and F. P. Gabbaï, Dalton Trans., 2021, 50, 17897 RSC.
- D. You, B. Zhou, M. Hirai and F. P. Gabbaï, Org. Biomol. Chem., 2021, 19, 4949 RSC.
- N. Aders, J.-H. Lamm, J. L. Beckmann, B. Neumann, H.-G. Stammler and N. W. Mitzel, Dalton Trans., 2022, 51, 12943 RSC.
- M. Yang, M. Hirai and F. P. Gabbaï, Dalton Trans., 2019, 48, 6685 RSC.
- S. N. Kessler, M. Neuburger and H. A. Wegner, Eur. J. Org. Chem., 2011, 3238 CrossRef CAS.
- S. N. Kessler, M. Neuburger and H. A. Wegner, J. Am. Chem. Soc., 2012, 134, 17885 CrossRef CAS PubMed.
- S. P. Lewis, L. D. Henderson, B. D. Chandler, M. Parvez, W. E. Piers and S. Collins, J. Am. Chem. Soc., 2005, 127, 46 CrossRef CAS PubMed.
- L. Schweighauser, I. Bodoky, S. N. Kessler, D. Häussinger, C. Donsbach and H. A. Wegner, Org. Lett., 2016, 18, 1330 CrossRef CAS PubMed.
- H. Zhao and F. P. Gabbaï, Nat. Chem., 2010, 2, 984 CrossRef CAS PubMed.
- M. Hirai and F. P. Gabbaï, Angew. Chem., Int. Ed., 2015, 54, 1205 CrossRef CAS PubMed.
- C.-H. Chen and F. P. Gabbaï, Angew. Chem., Int. Ed., 2017, 56, 1799 CrossRef CAS PubMed.
- T. W. Hudnall, C.-W. Chiu and F. P. Gabbaï, Acc. Chem. Res., 2009, 42, 388 CrossRef CAS PubMed.
- H. E. Katz, J. Org. Chem., 1985, 50, 5027 CrossRef CAS.
- N. Busschaert, C. Caltagirone, W. van Rossom and P. A. Gale, Chem. Rev., 2015, 115, 8038 CrossRef CAS PubMed.
- N. H. Evans and P. D. Beer, Angew. Chem., Int. Ed., 2014, 53, 11716 CrossRef CAS PubMed.
- P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486 CrossRef CAS PubMed.
- J. Y. Lim and P. D. Beer, Chem, 2018, 4, 731 CAS.
- M. S. Taylor, Coord. Chem. Rev., 2020, 413, 213270 CrossRef CAS.
- A. Brown and P. D. Beer, Chem. Commun., 2016, 52, 8645 RSC.
- M. Hirai and F. P. Gabbaï, Chem. Sci., 2014, 5, 1886 RSC.
- C.-H. Chen and F. P. Gabbaï, Chem. Sci., 2018, 9, 6210 RSC.
- J. Rudlof, T. Glodde, A. Mix, B. Neumann, H.-G. Stammler and N. W. Mitzel, Eur. J. Inorg. Chem., 2022, e202100842 CrossRef CAS.
- N. Aders, P. C. Trapp, J.-H. Lamm, J. L. Beckmann, B. Neumann, H.-G. Stammler and N. W. Mitzel, Organometallics, 2022, 41, 3600 CrossRef CAS.
- J. Horstmann, M. Niemann, K. Berthold, A. Mix, B. Neumann, H.-G. Stammler and N. W. Mitzel, Dalton Trans., 2017, 46, 1898 RSC.
- A. S. Wendji, C. Dietz, S. Kühn, M. Lutter, D. Schollmeyer, W. Hiller and K. Jurkschat, Chem. – Eur. J., 2016, 22, 404 CrossRef CAS PubMed.
- A. Schwartzen, L. Siebe, J. Schwabedissen, B. Neumann, H.-G. Stammler and N. W. Mitzel, Eur. J. Inorg. Chem., 2018, 2018, 2533 CrossRef CAS.
- J. L. Beckmann, J. Krieft, Y. V. Vishnevskiy, B. Neumann, H.-G. Stammler and N. W. Mitzel, Chem. Sci., 2023, 14, 13551 RSC.
- J. Qiu, D. K. Unruh and A. F. Cozzolino, J. Phys. Chem. A, 2016, 120, 9257 CrossRef CAS PubMed.
- M. Fleischmann, J. S. Jones, F. P. Gabbaï and M. Scheer, Chem. Sci., 2015, 6, 132 RSC.
- J.-H. Lamm, J. Horstmann, J. H. Nissen, J.-H. Weddeling, B. Neumann, H.-G. Stammler and N. W. Mitzel, Eur. J. Inorg. Chem., 2014, 2014, 4294 CrossRef CAS.
- M. Melaïmi, S. Solé, C.-W. Chiu, H. Wang and F. P. Gabbaï, Inorg. Chem., 2006, 45, 8136 CrossRef PubMed.
- S. Solé and F. P. Gabbaï, Chem. Commun., 2004, 1284 RSC.
- P. Niermeier, S. Blomeyer, Y. K. J. Bejaoui, J. L. Beckmann, B. Neumann, H.-G. Stammler and N. W. Mitzel, Angew. Chem., Int. Ed., 2019, 58, 1965 CrossRef CAS PubMed.
- V. C. Williams, W. E. Piers, W. Clegg, M. R. J. Elsegood, S. Collins and T. B. Marder, J. Am. Chem. Soc., 1999, 121, 3244 CrossRef CAS.
- V. C. Williams, G. J. Irvine, W. E. Piers, Z. Li, S. Collins, W. Clegg, M. R. J. Elsegood and T. B. Marder, Organometallics, 2000, 19, 1619 CrossRef CAS.
- L. D. Henderson, W. E. Piers, G. J. Irvine and R. McDonald, Organometallics, 2002, 21, 340 CrossRef CAS.
- S. P. Lewis, N. J. Taylor, W. E. Piers and S. Collins, J. Am. Chem. Soc., 2003, 125, 14686 CrossRef CAS PubMed.
- J. Chai, S. P. Lewis, S. Collins, T. J. J. Sciarone, L. D. Henderson, P. A. Chase, G. J. Irvine, W. E. Piers, M. R. J. Elsegood and W. Clegg, Organometallics, 2007, 26, 5667 CrossRef CAS.
- J. L. Beckmann, J. Krieft, Y. V. Vishnevskiy, B. Neumann, H.-G. Stammler and N. W. Mitzel, Angew. Chem., Int. Ed., 2023, 62, e202310439 CrossRef CAS PubMed.
- H. E. Katz, J. Org. Chem., 1989, 54, 2179 CrossRef CAS.
- G. E. Herberich, U. Eigendorf and U. Englert, Chem. Ber., 1993, 126, 1397 CrossRef CAS.
- B. Wrackmeyer, H.-J. Schanz and W. Milius, Angew. Chem., Int. Ed. Engl., 1997, 36, 1117 CrossRef CAS.
- B. Wrackmeyer and H. Nöth, Chem. Ber., 1977, 110, 1086 CrossRef CAS.
- M. Radius and F. Breher, Chem. – Eur. J., 2018, 24, 15744 CrossRef CAS PubMed.
- R. D. Chambers and T. Chivers, J. Chem. Soc., 1965, 3933 RSC.
- D. J. Parks, W. E. Piers and G. P. A. Yap, Organometallics, 1998, 17, 5492 CrossRef CAS.
- K. Köhler, W. E. Piers, A. P. Jarvis, S. Xin, Y. Feng, A. M. Bravakis, S. Collins, W. Clegg, G. P. A. Yap and T. B. Marder, Organometallics, 1998, 17, 3557 CrossRef.
- D. J. Parks, R. E. von Spence and W. E. Piers, Angew. Chem., Int. Ed. Engl., 1995, 34, 809 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic protocols, spectra, crystallographic data. CCDC 2337388–2337399. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt00782d |
| This journal is © The Royal Society of Chemistry 2024 |