Open Access Article
Open Access ArticleSolvent vapour-responsive structural transformations in molecular crystals composed of a luminescent mononuclear aluminium(III) complex†
Fumiya
Kobayashi
 *,
Azuki
Yoshida
,
Misato
Gemba
,
Yuta
Takatsu
and
Makoto
Tadokoro
*,
Azuki
Yoshida
,
Misato
Gemba
,
Yuta
Takatsu
and
Makoto
Tadokoro
 *
*
Department of Chemistry, Faculty of Science, Tokyo University of Science, 1-3, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan
First published on 5th June 2024
Abstract
Investigations into the construction of functional molecular crystals and their external stimuli-induced structural transformations represent compelling research topics, particularly for the advancement of sensors and memory devices. However, reports on the development of molecular crystals constructed from discrete mononuclear complex units and exhibiting structural transformations via the adsorption/desorption of guest molecules are scarce. In this study, we synthesised three molecular crystals composed of [Al(sap)(acac)(H2O)]·(solvent) (H2sap = 2-salicylideneaminophenol, acac = acetylacetonate, solvent = Me2CO (Al·Me2CO), MeCN (Al·MeCN), or DMSO (Al·DMSO)), and demonstrated solvent vapour-responsive reversible crystal-to-crystal structural transformations in Al·Me2CO and Al·MeCN. For Al·DMSO, exposure to DMSO vapour led to the formation of DMSO-coordinated compound [Al(sap)(acac)(DMSO)], indicating an irreversible structural transformation. This solvent vapour-responsive system incorporates a luminescent mononuclear aluminium(III) complex (λmax = 539–552 nm, Φem = 0.07–0.27) as the molecular building unit for the porous-like framework. Therefore, we synthesised a new functional molecular material and a potential molecular building unit that facilitates guest fixation through hydrogen-bonding.
Introduction
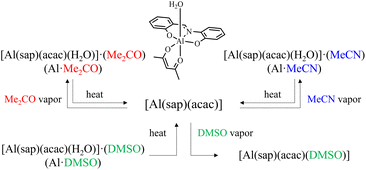
Molecular materials that respond to external stimuli have attracted significant attention owing to their potential applications in sensors and memory devices. These external stimuli, such as heat, light, and pressure, induce functional changes in the materials, including alterations in magnetic,1–9 luminescent,10–14 and dielectric properties.15–18 In recent years, several molecules responsive to external stimuli and their functional control have been reported within a group of compounds referred to as “soft crystals”.19 Among them, molecules responsive to solvent vapours are particularly intriguing as they allow reversible functional control in the solid state, without the need for recrystallization from solution.20–35 In conventional molecular crystals, unlike rigid frameworks such as metal–organic frameworks (MOFs) and porous coordination polymers (PCPs),36–44 the removal or desorption of solvent molecules from the pores can induce structural changes, such as shrinkage or collapse, which may then lead to closing of the pores. This typically prevents subsequent reversible re-adsorption. However, reversible guest adsorption and desorption, along with functional control, have been reported in several systems comprising discrete molecules.44–56 For example, Ito et al. reported solvent vapour-induced reversible structural rearrangements and luminescent colour changes in crystals composed of a mononuclear gold(I) isocyanide complex.57 However, intentionally incorporating guest molecules, such as solvent molecules, and designing molecules that exhibit reversible adsorption and desorption characteristics can be highly challenging.In our previous study, we reported a solvent vapor-responsive molecular system consisting of a mononuclear complex of type [M(sap)(acac)(solvent)] (where M = FeIII or AlIII, H2sap = 2-salicylideneaminophenol, acac = acetylacetonate, solvent = MeOH, DMSO, or pyridine), with coordination sites for solvent molecules.58,59 In this system, molecular arrangement and associated functionality can be controlled through solvent vapor exposure-induced substitution reactions of coordinating solvents. In addition, intentional incorporation of solvent vapor responsiveness into this molecular system is possible, potentially enabling the development of highly functional molecular materials by combining it with functionality arising from metal ions and aggregated structures. As a part of this research, we report the synthesis of new solvent vapour-responsive molecular crystals of [Al(sap)(acac)(H2O)]·(solvent) (solvent = Me2CO, MeCN, or DMSO), their crystal-to-crystal structural transformations, and their photo-luminescence properties. The hydrogen-bonded dimers of [Al(sap)(acac)(H2O)] units formed a one-dimensional (1D) channel structure, securing solvent molecules within the pores through hydrogen-bonding. The channel structures and lattice solvents exhibited reversible recovery upon exposure to solvent vapours (Scheme 1).
Results and discussion
Syntheses and single-crystal structural analyses
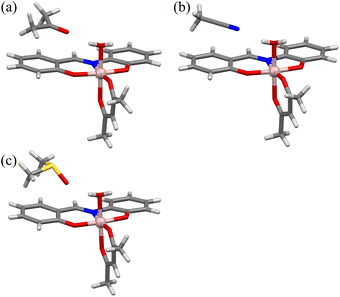
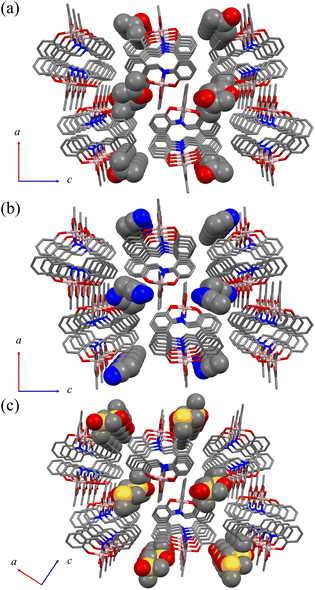
[Al(sap)(acac)(MeOH)] was synthesised according to our previously reported method.58,59 Single crystals of [Al(sap)(acac)(H2O)]·(Me2CO) (Al·Me2CO), [Al(sap)(acac)(H2O)]·(MeCN) (Al·MeCN), and [Al(sap)(acac)(H2O)]·(DMSO) (Al·DMSO) were obtained by recrystallising [Al(sap)(acac)(MeOH)] from Me2CO, MeCN, and DMSO, respectively (see Experimental section for details). All compounds were then characterised using elemental analysis, single-crystal X-ray diffraction (XRD), powder X-ray diffraction (PXRD), and Fourier-transform infrared (FT-IR) spectroscopy (Fig. S1 and S2, ESI†).The single-crystal X-ray structures of Al·Me2CO, Al·MeCN, and Al·DMSO were obtained at 173 K, and the crystallographic data are displayed in Table S1 (ESI†). Al·Me2CO crystallised in the orthorhombic Pbca space group with an asymmetric unit containing one lattice Me2CO molecule (Fig. 1a and Fig. S3 and S4, ESI†). The structure of Al·Me2CO was found to be similar to the previously reported structure of the aluminium(III) complex [Al(sap)(acac)(MeOH)].59 The aluminium(III) centre possesses a NO5-donor coordination sphere with a distorted octahedral geometry. The metal centre has a coordinated NO3-donor set equatorially arising from the sap and acac ligands, while the axial positions are occupied by O and O′ donors from acac and H2O, respectively. Each [Al(sap)(acac)(H2O)] molecule exhibits stereoisomers and forms dimers with neighbouring isomers by complementary hydrogen-bonding between coordinated water molecules and phenol oxygen atoms from the sap ligands (O(2)–O(5) = 2.645(2) Å) (Fig. 2 and Fig. S5a, ESI†). Each dimer interacts with neighbouring dimers by CH–π, CH–O and CH–N interactions, leading to a supramolecular framework and a 1D channel structure along the b axis (Fig. 3a and Fig. S6a, Table S2, ESI†). The lattice Me2CO molecules present in the channel pores are stabilised by hydrogen-bonding with the coordinated water molecules (O(5)–O(6) = 2.845(2) Å) (Fig. S7a, ESI†). Compared to the previously reported crystal structure of the non-porous [Al(sap)(acac)(MeOH)] complex,59 the bidirectional hydrogen-bonding of the coordinated water molecules in Al·Me2CO contributes to the fixation of guest molecules and the formation of a porous framework. Al·MeCN has an identical structure to that of Al·Me2CO and also forms dimers through hydrogen-bonding (O(2)–O(5) = 2.654(3) Å) (Fig. 1b and Fig. S8, ESI†). Therefore, Al·MeCN crystallises in the orthorhombic space group Pbca with an asymmetric unit containing one lattice MeCN molecule and forms a 1D channel structure along the b axis (Fig. 3b and Fig. S6b, S9, Table S3, ESI†). The lattice MeCN molecules present in the channel pores are stabilised by hydrogen-bonding with the coordinated water molecules (N(2)–O(5) = 2.886(2) Å) (Fig. S5b and S7b, ESI†). Al·DMSO has a similar molecular structure to that of Al·Me2CO and Al·MeCN; however, it crystallises in the monoclinic space group P21/n with an asymmetric unit containing one lattice DMSO molecule (Fig. 1c and Fig. S10, S11, ESI†). The intermolecular interactions observed in Al·DMSO are also similar to those observed in Al·Me2CO and Al·MeCN, resulting in the formation of a 1D channel structure along the b axis (Fig. 3c and Fig. S5c, S6c, Table S4, ESI†). The decrease in the symmetry of the crystal system in Al·DMSO can be attributed to the intermolecular interactions involving the low-symmetry DMSO molecule (Fig. S7c, ESI†). The results of Hirshfeld dnorm surface analysis for Al·Me2CO, Al·MeCN and Al·DMSO are shown in Fig. S12,† displaying the observed intermolecular interactions visibly. Thermogravimetric analysis (TGA) was used to determine the number of lattice solvents in Al·Me2CO, Al·MeCN, and Al·DMSO and their thermal stabilities. The TGA results for Al·Me2CO and Al·MeCN showed 17.7% and 15.1% weight losses corresponding to one water molecule and one solvent molecule (Me2CO and MeCN) at temperatures above 110 °C, respectively, thus corroborating the results of single-crystal X-ray diffraction (Fig. S13a and b, ESI†). Moreover, the de-solvated compound [Al(sap)(acac)] exhibited high thermal stability at temperatures below 250 °C. In contrast, a two-step weight loss of 22.2% was observed for Al·DMSO at 145 and 230 °C, corresponding to one water molecule and one DMSO molecule. Then, further weight loss was observed, which was attributed to the decomposition of Al·DMSO (Fig. S13c, ESI†). Thermogravimetric mass spectrometry (TG-MS) results for Al·DMSO indicate that both the observed first and second steps can be attributed to the de-solvation of a water molecule and a DMSO molecule, respectively.
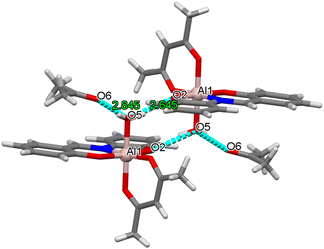 | ||
| Fig. 2 Dimeric structure of Al·Me2CO constructed by hydrogen-bonding. Blue-dashed lines represent the hydrogen-bonding. Colour code: C, grey; N, blue; O, red; Al, pink. | ||
Crystal-to-crystal structural transformations
Owing to the porous-like nature and high thermal stabilities of Al·Me2CO and Al·MeCN, we investigated their solvent vapour-responsive structural transformations. When the Me2CO and MeCN-containing complexes Al·Me2CO and Al·MeCN were annealed at 100 °C for 1 h, their initial PXRD patterns changed (Fig. 4). These patterns were found to be identical and corresponded to the de-solvated five-coordinated compound [Al(sap)(acac)]. When the de-solvated compounds were exposed to Me2CO or MeCN vapour for 1 h, the PXRD patterns changed again (Fig. 4 and S14, ESI†). These PXRD patterns corresponded to the original patterns for Al·Me2CO and Al·MeCN, respectively. This result is in accordance with the coordination of water molecules upon exposure to solvent vapour, resulting in structural rearrangements to the 1D channel structure. The TGA results for Al·reMe2CO and Al·reMeCN showed 17.3% and 14.1% weight losses, corresponding to adsorbed water molecules and solvent molecules (Me2CO, MeCN), respectively (Fig. S15, ESI†). Notably, the observed PXRD transformations and de-sorption/adsorption process were found to be reversible, leading to crystal-to-crystal structural transformations.60–63 Unlike the results of Al·Me2CO and Al·MeCN mentioned above, Al·DMSO exhibited different structural transformations. When the DMSO-containing complex Al·DMSO was annealed at 200 °C for 1 h, the initial PXRD pattern changed to those of de-solvated compound [Al(sap)(acac)]. After exposing the de-solvated compound to DMSO vapour for 1 day, the PXRD pattern changed again. However, the obtained pattern corresponded to that of the DMSO-coordinated compound [Al(sap)(acac)(DMSO)]59 rather than to Al·DMSO (Fig. 4c). This result indicates that the structural transformation to [Al(sap)(acac)(DMSO)] takes precedence over that to Al·DMSO under DMSO vapour. This trend is in accordance with the spontaneous DMSO vapour-induced coordinated solvent molecule exchange from [Al(sap)(acac)(MeOH)] and [Al(sap)(acac)(EtOH)] to [Al(sap)(acac)(DMSO)], as previously reported by us.59 The TGA result for Al·reDMSO supported the above result and showed one-step weight loss of 19.3%, corresponding to the coordinated DMSO molecule (Fig. S15c, ESI†). Thus, Al·DMSO exhibited irreversible structural transformations.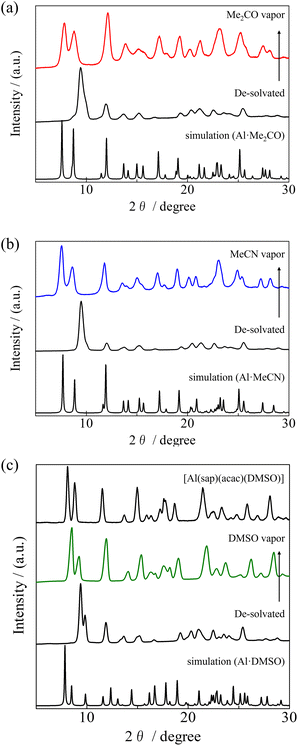 | ||
| Fig. 4 PXRD patterns for the de-solvated compounds [Al(sap)(acac)] and those obtained after exposure to (a) Me2CO, (b) MeCN, and (c) DMSO vapours. | ||
Luminescence properties
We also investigated the luminescence properties of the aluminium(III) complexes.64–67 The solid-state excitation and emission spectra of Al·Me2CO, Al·MeCN, and Al·DMSO obtained at 298 K are shown in Fig. S16† and Fig. 5 (Table S5, ESI†), respectively. At 298 K, the crystalline solid samples of Al·Me2CO, Al·MeCN, and Al·DMSO exhibited strong yellow emission. The emission spectra of Al·Me2CO, Al·MeCN, and Al·DMSO were similar and showed unstructured broad emission bands. However, their maxima (λmax) were slightly different; 539 nm for Al·Me2CO, 540 nm for Al·MeCN, and 552 nm for Al·DMSO. Emission quantum yield (Φem) measurements in the solid state revealed strong emissions from Al·Me2CO (0.27), Al·MeCN (0.22), and Al·DMSO (0.07) at 298 K. The observed differences in the photophysical properties of Al·Me2CO, Al·MeCN, and Al·DMSO can be attributed to the differences in their crystal structure and their resulting intermolecular interactions. The emission spectra of Al·Me2CO in Me2CO, Al·MeCN in MeCN and Al·DMSO in DMSO at 298 K (1.0 × 10−5 M) are shown in Fig. S18 (Fig. S17 and Table S6, ESI†). The emission maxima for Al·Me2CO, Al·MeCN, and Al·DMSO were 523, 526, and 527 nm, respectively. The emission quantum yields for Al·Me2CO, Al·MeCN, and Al·DMSO in solution at 298 K were 0.47, 0.42, and 0.76, respectively, revealing stronger emissions than those in the solid state. The emission intensity of Al·DMSO was significantly higher than those of Al·Me2CO and Al·MeCN. This can be attributed to the coordination of the DMSO molecule in the aluminium(III) complex instead of the water molecule. This coordination decreases the nonradiative rate associated with the OH vibrations in water molecules. The previously reported DMSO-coordinated aluminium(III) complex, [Al(sap)(acac)(DMSO)], exhibits strong green emission even in the solid state (Φem = 0.42, at 298 K).59 This result suggests that replacing the coordinated water molecules with other coordinating solvents decreases the nonradiative rates.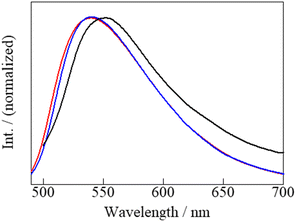 | ||
| Fig. 5 Emission spectra of Al·Me2CO (red), Al·MeCN (blue), and Al·DMSO (black) in the solid state at 298 K. | ||
DFT and TDDFT calculations
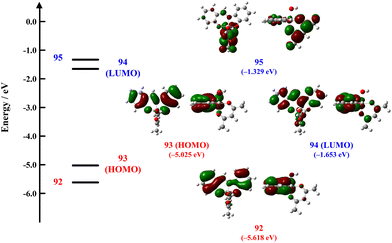
Density functional theory (DFT) and time-dependant DFT (TDDFT) calculations for the [Al(sap)(acac)(H2O)] (Al) unit were performed to investigate the relationship between its structures and emission origins. The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energies for Al are shown in Fig. 6 (Tables S7 and S8, ESI†). The singlet excited states of Al were also calculated using TDDFT. The lowest energy of the spin-allowed electronic vertical transition for Al [ΔE(S0–S1)] was 2.93 eV (424 nm). The intraligand transition occurs from the π HOMO delocalised in the sap ligand to the π* LUMO delocalised in the sap ligand. In addition, the ligand-to-ligand charge transfer (LLCT) occurs from the HOMO delocalised in the sap ligand to the LUMO+1 delocalised in the acac ligand. These results indicate that the S1 states of Al include singlet ligand-centred (1LC) and 1LLCT excited states. The above results are similar to those reported for [Al(sap)(acac)(MeOH)].59 We also performed DFT and TDDFT calculations for the hydrogen-bonded dimer of [Al(sap)(acac)(H2O)]2 ([Al]2). The HOMO and LUMO orbitals for [Al]2 are very similar with those of Al (Fig. S19 and Tables S9, S10, ESI†). Comparing the molecular orbital energies of Al and [Al]2, dimerisation via hydrogen bonding leads to the stabilisation of the HOMO energy. As a result, the lowest energy of the spin-allowed electronic vertical transition for [Al]2 [ΔE(S0–S1)] was 3.05 eV (406 nm). Thus, the differences in intermolecular interactions observed in Al·Me2CO, Al·MeCN, and Al·DMSO are reflected in the photophysical properties. In particular, because the molecular orbital of the water molecule is included in the HOMO in Al, the short hydrogen bond between the coordinated water molecule and DMSO (O(5)–O(6) = 2.722(8) Å) may affect the red-shifted emission bands of Al·DMSO.Conclusions
In conclusion, we demonstrated the solvent vapour-induced structural transformations of molecular crystals composed of [Al(sap)(acac)(H2O)]·(solvent) (solvent = Me2CO (Al·Me2CO), MeCN (Al·MeCN), DMSO (Al·DMSO)). Interestingly, this solvent vapour-responsive system was formed using a versatile mononuclear aluminium(III) complex that exhibits strong yellow emission in the solid state (λmax = 539–552 nm, Φem = 0.07–0.27, at 298 K). This study presents the first example of a solvent vapour-responsive luminescent porous-like crystal constructed from a mononuclear aluminium(III) complex. Our results also suggest the versatility of the activated five-coordinated compound [Al(sap)(acac)], which possesses a metal open site, as a molecular building block unit for the development of an advanced switching system associated with structural rearrangements. For example, these structural rearrangements can be induced by not only solvent vapour but also by the sublimation of the coordination compound. Further development of a novel solvent vapour-responsive system using the activated Al(III) unit [Al(sap)(acac)] and the hydrogen-bonded unit [Al(sap)(acac)(H2O)] is currently underway in our laboratory.Experimental
General
All reagents and solvents were obtained from Tokyo Kasei Co. and Wako Pure Chemical Industries and were of reagent grade; they were used without further purification. All reactions were carried out under ambient atmosphere.Syntheses
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) 2-salicylideneaminophenol) ligand and [Al(sap)(acac)(MeOH)].
H2sap and [Al(sap)(acac)(MeOH)] were synthesised according to the method we described previously.58,59
2-salicylideneaminophenol) ligand and [Al(sap)(acac)(MeOH)].
H2sap and [Al(sap)(acac)(MeOH)] were synthesised according to the method we described previously.58,59
Solvent-vapour exposure experiments
A small amount of the crystalline sample chosen from de-solvated crystals [Al(sap)(acac)] was added to a small Petri dish. The Petri dish was placed in a larger closed Petri dish together with a Kimwipe and required solvent (Me2CO or MeCN or DMSO) as the vapor source. The closed system was left to stand for 1 h or 1 day at room temperature (25 °C) (Fig. S14†).Physical measurements
Author contributions
F. K. and M. T. planned and directed the project; A. Y. and M. G. synthesised the compounds and carried out all the measurements. Y. T. assisted during XRD and luminescence measurements and analyses. F. K. drafted the manuscript and all authors contributed to revising the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the JSPS KAKENHI Grant-in-Aid for Early-Career Scientists JP23K13767, the Tokuyama Science Foundation and the Izumi Science and Technology Foundation.References
- O. Sato, J. Tao and Y. Z. Zhang, Angew. Chem., Int. Ed., 2007, 46, 2152–2187 CrossRef CAS PubMed.
- S. Hayami, K. Hiki, T. Kawahara, Y. Maeda, D. Urakami, K. Inoue, M. Ohama, S. Kawata and O. Sato, Chem. – Eur. J., 2009, 15, 3497–3508 CrossRef CAS PubMed.
- X. Feng, C. Mathoniere, R. Jeon Ie, M. Rouzieres, A. Ozarowski, M. L. Aubrey, M. I. Gonzalez, R. Clerac and J. R. Long, J. Am. Chem. Soc., 2013, 135, 15880–15884 CrossRef CAS PubMed.
- E. Milin, V. Patinec, S. Triki, E. E. Bendeif, S. Pillet, M. Marchivie, G. Chastanet and K. Boukheddaden, Inorg. Chem., 2016, 55, 11652–11661 CrossRef CAS PubMed.
- H. Zheng, Y. S. Meng, G. L. Zhou, C. Y. Duan, O. Sato, S. Hayami, Y. Luo and T. Liu, Angew. Chem., Int. Ed., 2018, 57, 8468–8472 CrossRef CAS PubMed.
- H. Y. Sun, Y. S. Meng and T. Liu, Chem. Commun., 2019, 55, 8359–8373 RSC.
- V. Garcia-Lopez, M. Palacios-Corella, S. Cardona-Serra, M. Clemente-Leon and E. Coronado, Chem. Commun., 2019, 55, 12227–12230 RSC.
- T. Nakanishi, Y. Hori, H. Sato, S. Q. Wu, A. Okazawa, N. Kojima, T. Yamamoto, Y. Einaga, S. Hayami, Y. Horie, H. Okajima, A. Sakamoto, Y. Shiota, K. Yoshizawa and O. Sato, J. Am. Chem. Soc., 2019, 141, 14384–14393 CrossRef CAS PubMed.
- M. Cammarata, S. Zerdane, L. Balducci, G. Azzolina, S. Mazerat, C. Exertier, M. Trabuco, M. Levantino, R. Alonso-Mori, J. M. Glownia, S. Song, L. Catala, T. Mallah, S. F. Matar and E. Collet, Nat. Chem., 2021, 13, 10–14 CrossRef CAS PubMed.
- O. S. Wenger, Chem. Rev., 2013, 113, 3686–3733 CrossRef CAS PubMed.
- C. Jobbágy and A. Deák, Eur. J. Inorg. Chem., 2014, 2014, 4434–4449 CrossRef.
- A. Kobayashi and M. Kato, Chem. Lett., 2017, 46, 154–162 CrossRef CAS.
- M. Yoshida and M. Kato, Coord. Chem. Rev., 2018, 355, 101–115 CrossRef CAS.
- T. Seki, N. Hoshino, Y. Suzuki and S. Hayashi, CrystEngComm, 2021, 23, 5686–5696 RSC.
- X. Y. Dong, B. Li, B. B. Ma, S. J. Li, M. M. Dong, Y. Y. Zhu, S. Q. Zang, Y. Song, H. W. Hou and T. C. Mak, J. Am. Chem. Soc., 2013, 135, 10214–10217 CrossRef CAS PubMed.
- K. Pasińska, A. Ciupa, A. Pikul, A. Gągor, A. Pietraszko and A. Ciżman, J. Mater. Chem. C, 2020, 8, 6254–6263 RSC.
- Y. Y. Tang, J. C. Liu, Y. L. Zeng, H. Peng, X. Q. Huang, M. J. Yang and R. G. Xiong, J. Am. Chem. Soc., 2021, 143, 13816–13823 CrossRef CAS PubMed.
- J. Yanagisawa, T. Aoyama, K. Fujii, M. Yashima, Y. Inaguma, A. Kuwabara, K. Shitara, B. Le Ouay, S. Hayami, M. Ohba and R. Ohtani, J. Am. Chem. Soc., 2024, 146, 1476–1483 CrossRef CAS PubMed.
- M. Kato, H. Ito, M. Hasegawa and K. Ishii, Chem. – Eur. J., 2019, 25, 5105–5112 CrossRef CAS PubMed.
- S. Ohkoshi, K. Arai, Y. Sato and K. Hashimoto, Nat. Mater., 2004, 3, 857–861 CrossRef CAS PubMed.
- T. Liu, Y. J. Zhang, S. Kanegawa and O. Sato, Angew. Chem., Int. Ed., 2010, 49, 8645–8648 CrossRef CAS PubMed.
- H. Iguchi, S. Takaishi, H. Miyasaka, M. Yamashita, H. Matsuzaki, H. Okamoto, H. Tanaka and S. Kuroda, Angew. Chem., Int. Ed., 2010, 49, 552–555 CrossRef CAS PubMed.
- J. Liu, X. P. Zhang, T. Wu, B. B. Ma, T. W. Wang, C. H. Li, Y. Z. Li and X. Z. You, Inorg. Chem., 2012, 51, 8649–8651 CrossRef CAS PubMed.
- X. Zhang, B. Li, Z.-H. Chen and Z.-N. Chen, J. Mater. Chem., 2012, 22, 11427–11441 RSC.
- A. D. Naik, K. Robeyns, C. F. Meunier, A. F. Leonard, A. Rotaru, B. Tinant, Y. Filinchuk, B. L. Su and Y. Garcia, Inorg. Chem., 2014, 53, 1263–1265 CrossRef CAS PubMed.
- D. Shao, L. Shi, L. Yin, B. L. Wang, Z. X. Wang, Y. Q. Zhang and X. Y. Wang, Chem. Sci., 2018, 9, 7986–7991 RSC.
- D. Shao, L. Shi, F. X. Shen, X. Q. Wei, O. Sato and X. Y. Wang, Inorg. Chem., 2019, 58, 11589–11598 CrossRef CAS PubMed.
- N. Li, J. P. Xue, J. L. Liu, Y. Y. Wang, Z. S. Yao and J. Tao, Dalton Trans., 2020, 49, 998–1001 RSC.
- F. Kobayashi, Y. Komatsumaru, R. Akiyoshi, M. Nakamura, Y. Zhang, L. F. Lindoy and S. Hayami, Inorg. Chem., 2020, 59, 16843–16852 CrossRef CAS PubMed.
- H. Zenno, F. Kobayashi, M. Nakamura, Y. Sekine, L. F. Lindoy and S. Hayami, Dalton Trans., 2021, 50, 7843–7853 RSC.
- F. Kobayashi, K. Iwaya, H. Zenno, M. Nakamura, F. Li and S. Hayami, Bull. Chem. Soc. Jpn., 2021, 94, 158–163 CrossRef CAS.
- M. Reczynski, D. Pinkowicz, K. Nakabayashi, C. Nather, J. Stanek, M. Koziel, J. Kalinowska-Tluscik, B. Sieklucka, S. I. Ohkoshi and B. Nowicka, Angew. Chem., Int. Ed., 2021, 60, 2330–2338 CrossRef CAS PubMed.
- D. Shao, W.-J. Tang, Z. Ruan, X. Yang, L. Shi, X.-Q. Wei, Z. Tian, K. Kumari and S. K. Singh, Inorg. Chem. Front., 2022, 9, 6147–6157 RSC.
- J. Yanagisawa, K. Tanaka, H. Kano, K. Miyata, B. Le Ouay, R. Ohtani and M. Ohba, Inorg. Chem., 2022, 61, 15638–15644 CrossRef CAS PubMed.
- A. M. T. Muthig, O. Mrozek, T. Ferschke, M. Rodel, B. Ewald, J. Kuhnt, C. Lenczyk, J. Pflaum and A. Steffen, J. Am. Chem. Soc., 2023, 145, 4438–4449 CrossRef CAS PubMed.
- J. R. Li, J. Yu, W. Lu, L. B. Sun, J. Sculley, P. B. Balbuena and H. C. Zhou, Nat. Commun., 2013, 4, 1538 CrossRef PubMed.
- J. P. Zhang, P. Q. Liao, H. L. Zhou, R. B. Lin and X. M. Chen, Chem. Soc. Rev., 2014, 43, 5789–5814 RSC.
- R. Ohtani and S. Hayami, Chem. – Eur. J., 2017, 23, 2236–2248 CrossRef CAS PubMed.
- W. Liu, Y. Y. Peng, S. G. Wu, Y. C. Chen, M. N. Hoque, Z. P. Ni, X. M. Chen and M. L. Tong, Angew. Chem., Int. Ed., 2017, 56, 14982–14986 CrossRef CAS PubMed.
- H. Y. Wang, J. Y. Ge, C. Hua, C. Q. Jiao, Y. Wu, C. F. Leong, D. M. D'Alessandro, T. Liu and J. L. Zuo, Angew. Chem., Int. Ed., 2017, 56, 5465–5470 CrossRef CAS PubMed.
- M.-Y. Chao, J. Chen, Z.-M. Hao, X.-Y. Tang, L. Ding, W.-H. Zhang, D. J. Young and J.-P. Lang, Cryst. Growth Des., 2018, 19, 724–729 CrossRef.
- J. W. Shin, A. R. Jeong, S. Jeoung, H. R. Moon, Y. Komatsumaru, S. Hayami, D. Moon and K. S. Min, Chem. Commun., 2018, 54, 4262–4265 RSC.
- J. Zhang, W. Kosaka, K. Sugimoto and H. Miyasaka, J. Am. Chem. Soc., 2018, 140, 5644–5652 CrossRef CAS PubMed.
- J. Zhang, W. Kosaka, Y. Kitagawa and H. Miyasaka, Angew. Chem., Int. Ed., 2019, 58, 7351–7356 CrossRef CAS PubMed.
- A. Das, F. J. Klinke, S. Demeshko, S. Meyer, S. Dechert and F. Meyer, Inorg. Chem., 2012, 51, 8141–8149 CrossRef CAS PubMed.
- J. S. Costa, S. Rodriguez-Jimenez, G. A. Craig, B. Barth, C. M. Beavers, S. J. Teat and G. Aromi, J. Am. Chem. Soc., 2014, 136, 3869–3874 CrossRef CAS PubMed.
- R. G. Miller and S. Brooker, Chem. Sci., 2016, 7, 2501–2505 RSC.
- S. Rodriguez-Jimenez, H. L. Feltham and S. Brooker, Angew. Chem., Int. Ed., 2016, 55, 15067–15071 CrossRef CAS PubMed.
- W. B. Chen, J. D. Leng, Z. Z. Wang, Y. C. Chen, Y. Miao, M. L. Tong and W. Dong, Chem. Commun., 2017, 53, 7820–7823 RSC.
- L. Z. Cai, X. M. Jiang, Z. J. Zhang, P. Y. Guo, A. P. Jin, M. S. Wang and G. C. Guo, Inorg. Chem., 2017, 56, 1036–1040 CrossRef CAS PubMed.
- P. Kar, M. Yoshida, Y. Shigeta, A. Usui, A. Kobayashi, T. Minamidate, N. Matsunaga and M. Kato, Angew. Chem., Int. Ed., 2017, 56, 2345–2349 CrossRef CAS PubMed.
- V. Jornet-Molla, Y. Duan, C. Gimenez-Saiz, Y. Y. Tang, P. F. Li, F. M. Romero and R. G. Xiong, Angew. Chem., Int. Ed., 2017, 56, 14052–14056 CrossRef CAS PubMed.
- M. du Plessis, V. I. Nikolayenko and L. J. Barbour, Inorg. Chem., 2018, 57, 12331–12337 CrossRef CAS PubMed.
- W. B. Chen, Y. C. Chen, G. Z. Huang, J. L. Liu, J. H. Jia and M. L. Tong, Chem. Commun., 2018, 54, 10886–10889 RSC.
- C. D. Ene, C. Maxim, M. Rouzieres, R. Clerac, N. Avarvari and M. Andruh, Chem. – Eur. J., 2018, 24, 8569–8576 CrossRef CAS PubMed.
- M. Nakaya, W. Kosaka, H. Miyasaka, Y. Komatsumaru, S. Kawaguchi, K. Sugimoto, Y. Zhang, M. Nakamura, L. F. Lindoy and S. Hayami, Angew. Chem., Int. Ed., 2020, 59, 10658–10665 CrossRef CAS PubMed.
- M. Jin, T. Sumitani, H. Sato, T. Seki and H. Ito, J. Am. Chem. Soc., 2018, 140, 2875–2879 CrossRef CAS PubMed.
- F. Kobayashi, R. Akiyoshi, D. Kosumi, M. Nakamura, L. F. Lindoy and S. Hayami, Chem. Commun., 2020, 56, 10509–10512 RSC.
- F. Kobayashi, M. Gemba, S. Hoshino, K. Tsukiyama, M. Shiotsuka, T. Nakajima and M. Tadokoro, Chem. – Eur. J., 2023, 29, e2022039377 Search PubMed.
- Z. Duan, Y. Zhang, B. Zhang and D. Zhu, J. Am. Chem. Soc., 2009, 131, 6934–6935 CrossRef CAS PubMed.
- J. Shi, Y. Zhang, B. Zhang and D. Zhu, Dalton Trans., 2016, 45, 89–92 RSC.
- D.-D. Zhou, Y.-T. Xu, R.-B. Lin, Z.-W. Mo, W.-X. Zhang and J.-P. Zhang, Chem. Commun., 2016, 52, 4991–4994 RSC.
- X.-K. Yang and J.-D. Chen, CrystEngComm, 2019, 21, 7437–7446 RSC.
- J. Qiao, L. D. Wang, J. F. Xie, G. T. Lei, G. S. Wu and Y. Qiu, Chem. Commun., 2005, 4560–4562 RSC.
- K. Y. Hwang, H. Kim, Y. S. Lee, M. H. Lee and Y. Do, Chem. – Eur. J., 2009, 15, 6478–6487 CrossRef CAS PubMed.
- S. W. Kwak, H. Jin, H. Shin, J. H. Lee, H. Hwang, J. Lee, M. Kim, Y. Chung, Y. Kim, K. M. Lee and M. H. Park, Chem. Commun., 2018, 54, 4712–4715 RSC.
- T. Ono, K. Ishihama, A. Taema, T. Harada, K. Furusho, M. Hasegawa, Y. Nojima, M. Abe and Y. Hisaeda, Angew. Chem., Int. Ed., 2021, 60, 2614–2618 CrossRef CAS PubMed.
- P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, J. Phys. Chem., 1994, 98, 11623 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys, 1988, 37, 785 CrossRef CAS PubMed.
- J. S. Binkley, J. A. Pople and W. J. Hehre, J. Am. Chem. Soc., 1980, 102, 939 CrossRef CAS.
- E. Runge and E. K. U. Gross, Phys. Rev. Lett., 1984, 52, 997 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: PXRD patterns, IR spectra, crystallographic data, crystal structures, luminescence properties, TGA, DFT and TD-DFT calculations. CCDC 2337339–2337341. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt00747f |
| This journal is © The Royal Society of Chemistry 2024 |