 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Azidomethyl-bisoxadiazol-linked-1,2,3-triazole-(ABT)-based potential liquid propellant and energetic plasticizer†
Sohan
Lal
 a,
Richard J.
Staples
a,
Richard J.
Staples
 b and
Jean'ne M.
Shreeve
b and
Jean'ne M.
Shreeve
 *a
*a
aDepartment of Chemistry, University of Idaho, Moscow, Idaho 83844-2343, USA. E-mail: jshreeve@uidaho.edu; Fax: (+1) 208-885-5173
bDepartment of Chemistry, Michigan State University, East Lansing, Michigan 48824, USA
First published on 26th March 2024
Abstract
A scalable synthesis of azidomethyl bisoxadiazol linked-1,2,3-triazole-(ABT) based potential liquid propellant and energetic plasticizer is obtained from commercially available diaminomaleonitrile in excellent yield. Newly synthesized compounds were fully characterized by various spectroscopic techniques. These materials exhibit good densities (1.77 g cm−3) and high thermal stabilities (Td = 181 °C). Compound 5 has good detonation properties (5, P = 20.81 GPa, D = 7516 ms−1) and propulsive properties (Isp (neat) = 210 s). These are superior to TNT and GAP and comparable to BAMOD, making them potential green liquid rocket propellants and energetic plasticizers.
Polyazido heterocycles have been studied extensively due to their wide range of medical, pharmaceutical, and material science applications.1–5 The azide moiety, a well-known precursor in 3 + 2 cycloadditions and well known in the field of high energy density materials as one of the explosophoric groups (–N3, –NO2, –ONO2, –NHNO2, etc.), used to improve the nitrogen content, and heats of formation of these materials.6 These compounds produce green gaseous products (N2, N2O, HN3, NOX, etc.) during their combustion and deflagration.7 Polyazido compounds are utilized in various forms, such as energetic polymers, plasticizers, explosives, pyrotechnics, melt castable explosives, and hypergolic liquids.8 Recently a few heterocycles with the azidomethyl moiety with eminent properties have also been reported.9,10 1,2,3-Oxadiazole-derived energetic materials are popular because of their facile syntheses, readily available precursors, and high reaction yields, which help lower the total production cost of the newly designed materials. However, the stabilities and sensitivities of these molecules are a big concern. Therefore, the scientific community is continually working to develop high-performance and less sensitive energetic materials by structural modification or design of new skeletons. Isoxazole and 1,2,4-oxadiazole skeletons with appropriate explosophoric groups could be good choices to design new energetic materials because of the balance between energy and stability. Recently, several 1,2,3-triazole-based energetic compounds namely 4,5-di(1H-tetrazol-5-yl)-2H-1,2,3-triazole(I, H3BTT),11 4,5-bis(1-hydroxytetrazol-5-yl)-2H-1,2,3-triazoledihydrate (II, BHTT),11 dipotassium 4,5-bis(dinitromethyl)-2H-1,2,3-triazole (III, K2BDNMT),12 potassium-4-azido-5-(dinitromethyl)-2H-1,2,3-triazole (IV, KAzDNMT),13 4,5-bis(azidomethyl)-2-nitro-2H-1,2,3-triazole (V, BAzMNT)14 and (2-nitro-2H-1,2,3-triazole-4,5-diyl)bis (methylene) dinitrate (VI, BMDNNT)14 have been developed (Fig. 1).
 | ||
| Fig. 1 Recently developed 1,2,3-triazoles11–13 and this work. | ||
These compounds are highly mechanically sensitive materials (H3BTT: IS = 2J, BHTT: 1S = 1J, K2BDNMT: IS = 1J, KAzDNMT: IS = 2J, BAzMNT: IS = 1J, BMDNNT: IS = 1J), making them competitive candidates as green primary explosives. 5,5′-Bis(azidomethyl)-3,3′-bi(1,2,4-oxadiazole)9 exhibits good thermal stability (Td = 209 °C) and low impact sensitivity (IS = 40 J), which is a good secondary explosive candidate. Due to their high nitrogen content, H3BTT, BHTT and BAzMNT may well be erosion-reduced gun propellant ingredients or gas generators.
Now we report a unique combination of 1,2,4-oxadiazole and 1,2,3-triazole with azidomethyl functionalities to generate a potential liquid rocket propellants and energetic plasticizer with a better energetic performance and low sensitivity. The preparation of bisamidaoxime 3 is based on the literature procedure.11,15 Subsequently, compound 3 was treated with chloroacetyl chloride in refluxing toluene to give 4,5-bis(5-(chloromethyl)-1,2,4-oxadiazol-3-yl)-2H-1,2,3-triazole (4, BCMODAT) in excellent yield. Later compound 4, on treatment with NaN3, gave 4,5-bis(5-(azidomethyl)-1,2,4-oxadiazol-3-yl)-2H-1,2,3-triazole (5, AzM-BOLT) in excellent yield via a sluggish reaction (Scheme 1).
Compound 4 is solid at room temperature while compound 5 is liquid at room temperature. Their thermal stabilities were determined using differential scanning calorimetry (DSC) at the heating rate of 5 °C min−1, (ESI, Fig. S13–S16†). Remarkably, compounds 5 exhibit high thermal stability (Td = 181 °C).
Compound 4·DMSO was crystallized by slow recrystallization in DMSO (Fig. 2). The crystal density of compound 4·DMSO is 1.635 g cm−3 at 100 K with single formula unit in the asymmetric unit (Z = 4, Z′ = 0.5), and orthorhombic Pnma space group as listed in Fig. S2–S5 (see ESI†).
The heats of formation ( (s)) of the newly synthesized compounds were calculated using the isodesmic method (Fig. S1†) with the Gaussian 03 suite of programs.16 Subsequently, their corresponding detonation and propulsive performance were estimated using (
(s)) of the newly synthesized compounds were calculated using the isodesmic method (Fig. S1†) with the Gaussian 03 suite of programs.16 Subsequently, their corresponding detonation and propulsive performance were estimated using ( (s)) and room temperature densities with the help of EXPLO5 V7.01 software.17 Compound 5 exhibits a very high positive enthalpy of formation (
(s)) and room temperature densities with the help of EXPLO5 V7.01 software.17 Compound 5 exhibits a very high positive enthalpy of formation ( (s)), 1010.21 kJ mol−1 which are superior to CL-20 (
(s)), 1010.21 kJ mol−1 which are superior to CL-20 (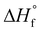 (s) = 608.70 kJ mol−1). Optimized structure, Mulliken and NBO charges of compound 5 are illustrated in Fig. 3 and 4 respectively.
(s) = 608.70 kJ mol−1). Optimized structure, Mulliken and NBO charges of compound 5 are illustrated in Fig. 3 and 4 respectively.
Additionally, a detailed study on the detonation and propulsive performance was carried out for the new materials shows that compound 5 has good potential as liquid rocket propellant and energetic plasticizer. Composite propellants (with AP/Al/HTPB) performed slightly lower than those of individual neat mono-propellant compounds. Whereas, the performance of the new compounds was notably better with aluminium (Al) as a fuel additive.
The weak interactions between the oxadiazole rings and the presence of spacer (–CH2) provided additional stability to compound 5. The ESP maxima and minima for compound 5 are 60.09 kcal mol−1 and −28.65 kcal mol−1, respectively (Fig. 5).
Furthermore, several non-covalent interactions and reduced density gradients were determined in compound 5 using B3LYP/6-311++G(d,p) level of theory (Fig. 6). This shows that compound 5 has good numbers of weak interactions (VDW and H-bonding) and corporately less steric effects, resulting in high thermal stability.
 | ||
| Fig. 6 Non-covalent interaction (NCI): (a) reduced density gradient (RDG) and (b) scatter diagram of compound 5. | ||
Additionally, the bond dissociation energy (BDE, gas-phase) of homolytic cleavage of the –CH2–N3 –N2 and –N3 bonds were predicted at B3LYP/6-311++G(d,p) level of theory. Compound 5 exhibits high BDEs 330.24 kJ mol−1 (–CH2–N3); 186.65 kJ mol−1 (–N2) and 202.3 kJ mol−1 (–N3), supports it high thermal stability (Fig. 7).
The detonation performance of compound 5 was calculated using its solid-phase heats of formation and experimental densities with EXPLO5 V 7.01 program17 and results are given in Table 1. Compound 5 (P = 20.81 GPa, D = 7516 ms−1, Q = 3925 kJ kg−1), which are comparable to those of compound BAMOD (P = 22.70 GPa, D = 7672 ms−1, Q = 4724 kJ kg−1) and superior to TNT (P = 18.56 GPa, D = 6839 ms−1, Q = 4395 kJ kg−1).
| Compound | 5 | GAPa | BAMODb | TNTc |
|---|---|---|---|---|
| a Ref. 17. b Ref. 9. c Ref. 17. d Molecular formula. e Molecular weight. f CO based oxygen balance. g CO2 based oxygen balance. h N + O contents in %. i Nitrogen content in %. j Calculated enthalpy of formation. k Measured densities, gas pycnometer at room temperature. l Melting point and decomposition temperature (onset) under nitrogen gas (DSC, 5 °C min−1). m Calculated detonation velocity. n Calculated detonation pressure. o Heat of detonation. p Mole of gaseous products. q Measured impact sensitivity (IS). r Measured friction sensitivity (FS). s I sp = Specific impulse of neat compound (monopropellant). t ρI sp = Density specific impulse of neat compound (monopropellant). u Characteristic velocity. v I sp = Specific impulse at 88% compound and 12% Al. w I sp = Specific impulse at 78% compound, 12% Al (fuel additive) and 10% binder (HTPB). x Specific impulse calculated at an isobaric pressure of 70 bar and initial temperature of 3300 K using EXPLO5 V 7.01. y This work. | ||||
| Formulad | C8H5N13O2 | C3H5N3O | C8H4N12O3 | C7H5N3O6 |
| FW [g mol−1]e | 315.21 | — | 316.21 | 227.13 |
| OBCO (%)f | −43.15 | −72.66 | −35.42 | −24.66 |
| OBCO2 (%)g | −83.75 | −121.10 | −75.90 | −73.97 |
| N + O [%]h | 67.92 | 16.15 | 68.34 | 60.76 |
| N [%]i | 57.77 | 42.41 | 53.16 | 18.50 |
| ΔHf [kJ mol−1]j | 1010.21 | 141.94 | 1072.90 | 70.30 |
| ρ [g cm−3]k | 1.77 | 1.29 | 1.75 | 1.65 |
| T d [°C]l | 181 | −45 | 193 | 300 |
| D [ms−1]m | 7516 | 6303 | 7672 | 6839 |
| P [GPa]n | 20.81 | 15.41 | 22.70 | 18.56 |
| −Q [kJ kg−1]o | 3925 | 3635 | 4724 | 4395 |
| M[g mol−1]p | 27.78 | 32.07 | 27.65 | 26.47 |
| IS [J]q | 4 | 9 | — | 15 |
| FS [N]r,x | 120 | 444 | 80 | 353 |
| I sp [s]s,x | 209.57 | 198.46 | 229.17y | 206.49y |
| ρI sp [s]t,x | 370.95 | 256.60 | 401.05y | 341.54y |
| C* [ms−1]u,x | 1283.4 | 1207.8 | 1399.5y | 1283.5y |
| I sp [s]v,x | 217.84 | 224.79 | 231.56y | 232.00y |
| I sp [s]w,x | 207.56 | 218.97 | 220.78y | 225.11y |
In summary, a facile synthesis of compound 5 from commercially available diaminomaleonitrile (DAMN) was developed. The newly synthesized compounds were fully characterized by FTIR, NMR, and elemental analyses. A detailed study of energetic performance and thermal behaviour of compound 5 was also carried out. Compound 5 possess high thermal stabilities (Td, 181 °C) and low sensitivities toward friction (120 N) and impact (4 J). Compound 5 (P = 20.81 GPa, D = 7516 ms−1, Isp = 210 s), has potential as a liquid propellant and energetic plasticizer in rocket propulsion.
Experimental section
Compounds 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 and 3
15 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 were prepared according to literature procedure (see ESI†).
11 were prepared according to literature procedure (see ESI†).
4,5-Bis(5-(chloromethyl)-1,2,4-oxadiazol-3-yl)-2H-1,2,3-triazole (4)
Compound 3 (2.590 g, 14 mmol, 1.0 equiv.) was dissolved in toluene (10 mL), and the resulting mixture was cooled to 0 °C. Then chloroacetyl chloride (5.53 g, 3.9 mL, 49 mmol, 3.5 equiv.) was added dropwise. The resulting reaction mixture was stirred at room temperature for 12 h and was subsequently refluxed for 6 h. After cooling to room temperature, the solvent was evaporated under reduced pressure, and the crude product was washed with water (5 × 20 mL), precipitate was collected by filtration, which was further recrystallized from ethanol. Crystalline, brown solid; yield: 4.0 g, 95%, DSC (5 °C min−1): Tm (onset) 71 °C, Td (onset) 316 °C; IR (KBr pellet) υ 3615 (s), 3354 (s), 3186 (s), 2948 (s), 2806 (s), 2745 (s), 2626 (m), 1625 (s), 1581 (s), 1427 (s), 1397 (s), 1370 (m), 1319 (w), 1270 (m), 1237 (m), 1205 (m), 1096 (s), 1022 (w), 996 (s), 952 (s), 907 (s), 892 (s), 860 (m), 790 (s), 732 (m), 641 (w), 489 (w) cm−1; 1H NMR (500.19 MHz, DMSO-d6): δ 5.20 (s, 4H), 16.61 (s, 1H); 13C NMR (125.8 MHz, DMSO-d6): δ 33.7, 133.2, 161.4, 176.1; elemental analysis: calcd (%) for C8H5Cl2N7O2 (302.08): C, 31.8; H, 1.67; N, 32.46. Found C, 31.90; H, 1.80; N, 32.37.4,5-Bis(5-(azidomethyl)-1,2,4-oxadiazol-3-yl)-2H-1,2,3-triazole (5)
Compound 4 (2.416 g, 8.0 mmol, 1.0 equiv.) was dissolved in acetonitrile (20 mL), and sodium azide (2.080 g, 32.00 mmol, 4.0 equiv.) was added. The resulting mixture was stirred at room temperature for 120 h. Then, the solid was filtered and washed with (5 × 20 mL) of acetonitrile. The filtrate was dried over anhydrous Na2SO4 and the solvent was evaporated under reduced pressure at room temperature. The resulting crude material was further purified by flash column chromatography or trituration. Yellow viscous liquid; Yield: 2.21 g, 88%, DSC (5 °C min−1): Td (onset) 181 °C; IR (KBr pellet) υ 3211 (s), 2965 (s), 2920 (s), 2847 (s), 2107 (s), 1581 (s), 1429 (s), 1326 (s), 1247 (s), 1247 (s), 1200 (s), 1097 (s), 1019 (m), 990 (m), 953 (s), 901 (s), 767 (s), 742 (s), 671 (m), 555 (m) cm−1; 1H NMR (500.19 MHz, DMSO-d6): δ 4.98 (s, 4H); 13C NMR (125.77 MHz, DMSO-d6): δ 44.5, 132.8, 161.7, 175.6; elemental analysis: calcd (%) for C8H5N13O2·0.6H2O (326.03): C, 29.47; H, 1.92; N, 55.85. Found C, 29.79; H, 1.94; N, 55.51.Author contributions
S. L. investigation, methodology, conceptualization and manuscript writing. R. J. S. X-ray data collection and structure solving. S. L. and J. M. S. conceptualization, manuscript writing-review and editing, supervision.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The Rigaku Synergy S Diffractometer was purchased with support from the National Science Foundation MRI program (1919565). We are grateful for the support of the Fluorine-19 fund.References
- H. Gao and J. M. Shreeve, Azole-based energetic salts, Chem. Rev., 2011, 111, 7377–7436 CrossRef CAS PubMed.
- (a) S. Lal, L. Mallick, S. Rajkumar, O. P. Oommen, S. Reshmi, N. Kumbhakarna, A. Chowdhury and I. N. N. Namboothiri, Synthesis and energetic properties of high-nitrogen substituted bishomocubanes, J. Mater. Chem. A, 2015, 3, 22118–22128 RSC; (b) S. Lal, R. J. Staples and J. M. Shreeve, Design and synthesis of phenylene-bridged isoxazole and tetrazole-1-ol based energetic materials of low sensitivity, Dalton Trans., 2023, 52, 3449–3457 RSC; (c) S. Lal, R. J. Staples and J. M. Shreeve, Design and synthesis of high-performance planar explosives and solid propellants with tetrazole moieties, Org. Lett., 2023, 25, 5100–5104 CrossRef CAS PubMed.
- (a) Y. Tang and J. M. Shreeve, Nitroxy/azido-functionalized triazoles as potential energetic plasticizers, Chem. – Eur. J., 2015, 21, 7285–7291 CrossRef CAS PubMed; (b) J. P. Agrawal, High Energy Materials: Propellants, Explosives and Pyrotechnics, Wiley-VCH, Weinheim, 1st edn, 2010 CrossRef.
- (a) M.-H. V. Huynh, M. A. Hiskey, E. L. Hartline, D. P. Montoya and R. Gilardi, Polyazido high-nitrogen compounds: Hydrazo and azo-1,3,5-triazine, Angew. Chem., Int. Ed., 2004, 43, 4924–4928 CrossRef CAS PubMed; (b) K. A. Lyssenko, Y. V. Nelubina, D. V. Safronov, O. I. Haustova, R. G. Kostyanovsky, D. A. Lenev and M. Y. Antipin, Intermolecular N3⋯N3 interactions in the crystal of pentaerythrityl tetraazide, Mendeleev Commun., 2005, 15, 232–234 CrossRef.
- (a) D. Chen, H. Yang, Z. Yi, H. Xiong, L. Zhang, S. Zhu and G. Cheng, C8N26H4: An environmentally friendly primary explosive with high heat of formation, Angew. Chem., Int. Ed., 2018, 57, 2081–2084 CrossRef CAS PubMed; (b) M. H. H. Wurzenberger, M. S. Gruhne, M. Lommel, N. Szimhardt, T. M. Klapötke and J. Stierstorfer, Comparison of 1-Ethyl 5H-tetrazole and 1-azidoethyl-5H-tetrazole as ligands in energetic transition metal complexes, Chem. - Asian J., 2019, 14, 2018–2028 CrossRef CAS PubMed.
- (a) J. Zhou, J. Zhang, B. Wang, L. Qiu, R. Xu and A. B. Sheremetev, Recent synthetic efforts towards high energy density materials: How to design high performance energetic structures?, FirePhysChem, 2022, 2, 83–139 CrossRef; (b) S. Lal, A. Bhattacharjee, A. Chowdhury, N. Kumbhakarna and I. N. N. Namboothiri, Approaches to 1,4-disubstituted cubane derivatives as energetic materials: Design, theoretical studies and synthesis, Chem. – Asian J., 2022, 17, e202200489 CrossRef CAS PubMed; (c) L. Mallick, S. Lal, S. Reshmi, I. N. N. Namboothiri, A. Chowdhury and N. Kumbhakarna, Theoretical studies on the propulsive and explosive performance of strained polycyclic cage compounds, New J. Chem., 2017, 41, 920–930 RSC.
- H. Gao, Q. Zhang and J. M. Shreeve, Fused heterocycle-based energetic materials (2012–2019), J. Mater. Chem. A, 2020, 8, 4193–4216 RSC.
- F. Chen, Y. Wang and Q. Zhang, Recent advances in the synthesis and properties of energetic plasticizers, New J. Chem., 2022, 46, 20540–20553 RSC.
- L. Bauer, M. Benz, T. M. Klapötke, T. Lenz and J. Stierstorfer, Polyazido-methyl derivatives of prominent oxadiazole and isoxazole scaffolds: Synthesis, explosive properties, and evaluation, J. Org. Chem., 2021, 86, 6371–6380 CrossRef CAS PubMed.
- L. Z. -Xiong, O. Y. -Xiang and C. B. -Ren, Synthesis of two furazano azides, J. Beijing Inst. Technol., 2001, 10, 322–325 Search PubMed.
- A. A. Dippold, D. Izsak, T. M. Klapotke and C. Pfluger, Combining the advantages of tetrazoles and 1,2,3-triazoles: 4,5-bis(tetrazol-5-yl)-1,2,3-triazole, 4,5-bis(1-hydroxytetrazol-5-yl)- 1,2,3-triazole, and their energetic derivatives, Chem. – Eur. J., 2016, 22, 1768–1778 CrossRef CAS PubMed.
- H. Gu, Q. Ma, S. Huang, Z. Zhang, Q. Zhang, G. Cheng, H. Yang and G. Fan, Gem-dinitromethyl-substituted energetic metal organic framework based on 1,2,3-Triazole from in situ controllable synthesis, Chem. – Asian J., 2018, 13, 2786–2790 CrossRef CAS PubMed.
- Y. Cao, H. Huang, L. Wang, X. Lin and J. Yang, Synthesis and Characterization of Azido-Functionalized 1,2,3- Triazole and Fused 1,2,3-Triazole, J. Org. Chem., 2023, 88, 4301–4308 CrossRef CAS PubMed.
- M. Benz, S. Dörrich, T. M. Klapötke, T. Lenz, M. Mouzayek and J. Stierstorfer, 4,5-Bisnitratomethyl and similar representatives of the rare class of 2 nitro-1,2,3-triazoles, Org. Lett., 2023, 25, 5974–5977 CrossRef CAS PubMed.
- M.-J. Crawford, K. Karaghiosoff, T. M. Klapotke and F. A. Martin, Synthesis and characterization of 4,5-dicyano-2H-1,2,3-triazole and its sodium, ammonium, and guanidinium salts, Inorg. Chem., 2009, 48, 1731–1743 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. L. Caricato, X. H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, N. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Revision D.01, Gaussian, Inc., Wallingford CT, 2003.
- EXPLO5, OZMResearchs.r.o.: Hro̊chuvTÿnec, CzechRepublic. https://www.ozm.cz/explosives-performancetests/thermochemical-computer-code-explo5/(accessed on 2022-10-20).
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2330491. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt00638k |
| This journal is © The Royal Society of Chemistry 2024 |






