 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dial-a-base mechanochemical synthesis of N-heterocyclic carbene copper complexes†
Dawid J.
Babula
ab,
Rex S. C.
Charman
a,
Josie A.
Hobson
a,
Mary F.
Mahon
a and
David J.
Liptrot
 *a
*a
aDepartment of Chemistry, University of Bath, Bath, BA2 7AY, UK. E-mail: d.j.liptrot@bath.ac.uk
bInstitute for Sustainability, University of Bath, Bath, BA2 7AY, UK
First published on 12th February 2024
Abstract
Liquid assisted ball milling of [NHC]HBr (NHC = N-heterocyclic carbene) salts with copper(I) chloride, and a range of alkali metal complexes was shown to efficiently produce (NHC)CuX (NHC = normal or RE-NHC, X = halide, alkoxide, amide, alkyl, aryl; RE-NHC = ring-expanded NHC).
Mechanochemistry continues to revolutionise fields from organic synthesis1 to polymer chemistry.2 Its impact is also increasingly evident in the area of organometallic synthesis, with ball milling providing access to both unprecedented new motifs and widespread utility reagents.3 Ball milling has numerous attractive properties from a green chemistry perspective; it results in significantly reduced solvent demand and can reduce energy costs in terms of heating and lower reaction times.4 Another key approach to maximising sustainability is catalysis. N-heterocyclic carbene (NHC) copper alkoxides are widely implicated as precatalysts in a huge swathe of reactions.5 Moreover, the ring-expanded NHC (RE-NHC) 6-Dipp has been found to be a privileged motif in isolating and structurally characterising a wide range of interesting copper complexes. Routes to these generally start from (6-Dipp)CuOtBu and have provided access to an isolable NHC-copper hydride dimer,6 a range of highly stable NHC-copper boryls,7–9 as well as a copper formimidoyl,10 copper phosphide,11 copper stannyl,12 and copper germyl,13 amongst others.
Synthetic access to NHC-copper complexes has been the subject of intensive study. Initial work employed strong bases such as NaH or NaOtBu to generate the corresponding free carbene from a carbene salt, followed by addition of CuCl. Subsequent work showed that Cu2O could do double duty as both the copper source and a “built-in base”.14 Most recently, generation of a metallate, [NHC-H][CuClX], via reaction of the NHC precursor salt with CuCl, and subsequent deprotonation by a weak base has been established as a widely applicable solution-based route to (NHC)CuCl systems.15,16 This strategy is attractive as it is tolerant to air, and exploits inexpensive bases such as potassium carbonate. Alongside a single report of a microwave assisted weak base method,17 access to RE-NHC systems relies on the strong base approach. For example, synthesis of (6-Dipp)CuCl generally occurs via the deprotonation of [6-Dipp]HCl with NaHMDS followed by addition of CuCl. Access to (6-Dipp)CuOtBu can then be achieved via the addition of the corresponding alkali metal alkoxide6 or, alternatively, CuCl can be replaced by CuMes in the first step to provide (6-Dipp)CuMes which is then a competent base towards tert-butanol.18
Mechanochemical approaches have contributed to the synthesis of NHC-copper(I) systems. An initial report by Lamaty validated formation of (NHC)CuCl systems from metallic copper and the corresponding [NHC]HCl salt by ball milling under air.19 They also reported mechanochemical transmetallation of NHC-silver complexes to copper.20 Cazin and co-worker reported that ball milling an appropriate carbene precursor salt plus copper(I) chloride with subsequent addition of potassium carbonate provided access to (NHC)CuCl systems.21 This extension of their well-established “weak base” method22 tolerated a range of 5-membered saturated and unsaturated NHCs encompassing imidazolin-2-ylidene, imidazolidin-2-ylidene, and triazolylidene systems. They showed the reaction could be scaled to generate 5 g of (IPr)CuCl in good yields, and subsequently to a range of metals.23 Expanding on this work, Teichert and co-workers showed that K3PO4 provided similar access to (NHC)CuCl systems.24 They further noted that strong bases such as NaOtBu, NaHMDS and NaH provided no desired product in the first two cases and an intractable mixture containing the targetted (NHC)CuCl in the third instance under mechanochemical conditions.
Cognisant of the fact that, thus far, no group have described a mechanochemical synthesis of RE-NHC copper complexes, and that a direct route to systems where the copper is bound to a range of anionic ligands other than chloride would be useful, we sought to develop a “Dial-a-Base” method as a means of general access to (NHC)CuX (NHC = normal or RE-NHC, X = halide, alkoxide, amide, alkyl, aryl). Using the synthesis of (6-Dipp)CuOtBu as a model system, we initially investigated the addition of 3 equivalents of KOtBu to a pre-ground mixture of [6-Dipp]HCl and CuCl followed by ball-milling. This gave conversion to the desired product, albeit contaminated with significant amounts of free 6-Dipp. A switch to liquid assisted grinding (toluene, η ≈ 0.5) provided complete conversion by NMR and a 77% yield of (6-Dipp)CuOtBu. As workup was performed by extraction into toluene, filtration, and removal of all volatiles we settled on a slurrying regime (η ≈ 5) as our optimised conditions as doing so provided no deleterious effects on yield, but greatly facilitated work-up providing an 83% isolated yield. We extended these conditions to a range of 5-, 6- and 7-membered NHCs which provided moderate to good yields in all cases on a synthetically useful 2 mmol scale (Scheme 1, 1–3).
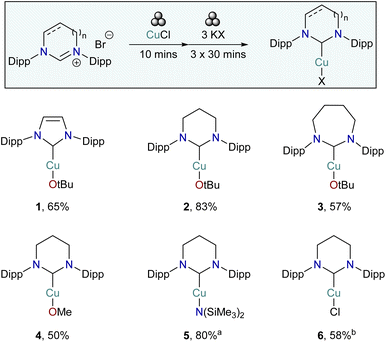 | ||
| Scheme 1 Mechanochemical synthesis of a range of 5-, 6-, and 7-membered NHC-copper alkoxides, an amide and a halide. (a) 2 KHMDS, t = 16 h. (b) KX = K2CO3, t = 16 h. | ||
Conscious of the dual role played by the KOtBu in this reaction, we then set out to extend the range of bases exploited in the reaction, once again focussing on the 6-Dipp ligand. Exploitation of NaOMe provided access to an additional alkoxide, (6-Dipp)CuOMe, 4, in marginally reduced yields. The use of KOH failed to provide the 6-Dipp analogue of Nolan's cuprous synthon, (IPr)CuOH,25 which we attribute to the effect of water on RE-NHCs. The reaction tolerated KHMDS to generate the corresponding amide, 5, in good yield, albeit with a minor tweak to the conditions applied. Use of potassium carbonate provided a mixture of products when reacted with [6-Dipp]HBr and CuCl, but switching the carbene precursor to [6-Dipp]HCl gave good conversion overnight (58%) to (6-Dipp)CuCl, 6. Finally, attempts to exploit KH in this reaction provided crude NMR data consistent with the formation of some (6-Dipp)CuH however no appreciable quantity could be accessed upon workup.
Extension of this reactivity to MeLi, BuLi, tBuLi and PhLi gave access to the corresponding alkyl and aryl systems (Scheme 2, 7–10), all four of which were structurally characterised (Fig. 1). For 7–9 organolithium reagents were added as commercially available solutions, affecting the η parameter (η ≈ 10). MesLi, on the other hand, provided poor conversion and reaction mixtures containing a large range of products.
 | ||
| Fig. 1 Plots depicting the molecular structures of 7–10. Ellipsoids are depicted at 30% probability. Hydrogen atoms and minor disordered components (9 and 10) and have been omitted and Dipp substituents are depicted as wireframes, for visual ease. 7: symmetry operations: 1x, 3/2 − y, z. 10: symmetry operations: 1 1/3 − y + x, 2/3 − y, 7/6 − z.† | ||
The synthesis of compounds 7–10 gives an opportunity to interrogate a homologous set of NHC-copper carbohydryls. Whilst NHC-copper n-alkyls are well known, and aryls are common, 9 constitutes the first structurally characterised example of a tert-butyl complex of an NHC-stabilised copper(I) fragment, and in fact the third copper(I) tert-butyl compound to ever be structurally characterised and reported. The geometry at the metal is close to linear. The carbene carbon–copper bond is marginally shorter than that of the alkyl carbon–copper bond (9: CNHC–Cu, 1.9148(18) Å; Canion–Cu, 1.949(2) Å; C–Cu–C, 177.18(8)°). The 5-membered analogue of 7, (IPr)CuMe, described by Sadighi and co-workers26 exhibits ideal linearity and marginally shortened copper–carbon bond distances (IPrCuMe: Canion–Cu, 1.913(6) Å; CNHC–Cu, 1.887(5) Å; C–Cu–C, 180.000(1)°). 7: CNHC–Cu, 1.9229(16) Å; Canion–Cu, 1.926(2) Å; C–Cu–C, 175.45(9)°, an effect commonly observed when comparing 5- and 6-membered carbene copper complexes. The closest literature analogue of 8, (IPr*Me)Cu-hexyl was reported by Tran, Bullock and co-workers27 and it contains two distinct molecules in the asymmetric unit, with near-linear C–Cu–C angles (C–Cu–C, 177.7(2), 177.1(2)°) and copper–carbon bond distances which are, once again, shorter for the carbene carbon to copper bond (CNHC–Cu, 1.890(4), 1.900(4) Å) than the hexyl carbon to copper bond (Cu–C, 1.931(5), 1.927(5) Å). This contrasts with 8 (CNHC–Cu, 1.9264(17) Å; Cu–Canion, 1.939(2) Å; C–Cu–C 171.80(9)°), wherein the ring-expansion of the carbene elongates the carbon–copper bonds once more, but less so when comparing the alkyl–carbon copper bonds than the carbene–carbon copper bonds, possibly due to steric demand of the flanking groups in IPr*Me.
(IPr)CuPh was reported by Kleeberg and co-workers28 and shows shorter copper–carbon bonds and a narrow C–Cu–C angle than 10 (CNHC–Cu, 1.906(3) Å, 1.892(3) Å; Canion–Cu: 1.898(3) Å, 1.907(3) Å; C–Cu–C: 174.1(1)°, 173.9(1)°; 10: CNHC–Cu 1.919(3) Å Canion–Cu 1.926(3)° C–Cu–C 180.0°). Comparison of 7–10 shows no clear trend in C–Cu–C angles, or CNHC–Cu distances, but a steady elongation of the alkyl copper–carbon distance from 7–9 is observed, corresponding to increased steric bulk at the anionic carbon. In contrast, the phenyl carbon in 10 is similarly distant from the copper as the methyl carbon of 7. This may be a consequence of the flat phenyl ring in 10 being more capable of orienting itself to evade steric clash with the flanking Dipp groups compared to the primary and tertiary alkyl groups in 8 and 9.
Whilst these routes that rely on inexpensive commercial bases are attractive, the use of 3 equivalents of base/proligand would be prohibitively expensive in the case of more complex anionic ligands, and would also have negative sustainability impacts. We thus investigated the mechanochemical reaction of (6-Dipp)CuCl, 6, with protic substrates in the presence of K2CO3 to provide an inexpensive route to complexes of the form (6-Dipp)CuX (X = acetylide, amide, alkoxide). This approach tolerated an acetylene and hexafluoro-iso-propanol,29 providing the respective acetylide (11) and alkoxide (12) in good yields (Scheme 3).
The formation of 11 is somewhat surprising given the respective pKa values of phenyl acetylene, and the conjugate acid of K2CO3. We propose that the formation of potassium phenylacetylide under these conditions is unlikely although not impossible, and a reaction analogous to the formation of 6 from an NHC-salt is operant.
In conclusion, we have developed a simple “Dial-a-Base” route to NHC-copper complexes, providing access to a wide-range of systems of the form (NHC)CuX under mechanochemical conditions. The reaction tolerates both normal and ring-expanded NHCs, and in our hands provides convenient access to examples of copper alkyl, aryl, acetylide, amide, alkoxide, and chloride complexes, many of which are well-established (pre)catalysts, or have otherwise useful properties.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
DJL thanks the Royal Society for the support of a University Research Fellowship.References
- J. Andersen and J. Mack, Green Chem., 2018, 20, 1435–1443 RSC.
- A. Krusenbaum, S. Grätz, G. T. Tigineh, L. Borchardt and J. G. Kim, Chem. Soc. Rev., 2022, 51, 2873–2905 RSC.
- D. V. Aleksanyan and V. A. Kozlov, Mendeleev Commun., 2023, 33, 287–301 CrossRef CAS.
- N. Fantozzi, J.-N. Volle, A. Porcheddu, D. Virieux, F. García and E. Colacino, Chem. Soc. Rev., 2023, 52, 6680–6714 RSC.
- F. Lazreg, F. Nahra and C. S. J. Cazin, Coord. Chem. Rev., 2015, 293–294, 48–79 CrossRef CAS.
- A. J. Jordan, C. M. Wyss, J. Bacsa and J. P. Sadighi, Organometallics, 2016, 35, 613–616 CrossRef CAS.
- T. M. Horsley Downie, R. S. C. Charman, J. W. Hall, M. F. Mahon, J. P. Lowe and D. J. Liptrot, Dalton Trans., 2021, 50, 16336–16342 RSC.
- D. J. Babula, R. S. C. Charman, T. H. Jerome, T. M. Horsley Downie, M. F. Mahon and D. J. Liptrot, Eur. J. Inorg. Chem., 2023, 26, e202300043 CrossRef CAS.
- R. S. C. Charman, J. A. Hobson, R. A. Jackson, M. F. Mahon, S. E. Neale and D. J. Liptrot, Chem. – Eur. J., 2024, 30, e202302704 CrossRef CAS PubMed.
- R. S. C. Charman, T. M. Horsley Downie, T. H. Jerome, M. F. Mahon and D. J. Liptrot, Inorganics, 2022, 10, 135 CrossRef CAS.
- T. M. Horsley Downie, J. W. Hall, T. P. Collier Finn, D. J. Liptrot, J. P. Lowe, M. F. Mahon, C. L. McMullin and M. K. Whittlesey, Chem. Commun., 2020, 56, 13359–13362 RSC.
- R. S. C. Charman, M. F. Mahon, J. P. Lowe and D. J. Liptrot, Dalton Trans., 2022, 51, 831–835 RSC.
- R. S. C. Charman, N. J. Evans, L. E. English, S. E. Neale, P. Vasko, M. F. Mahon and D. J. Liptrot, Chem. Sci., 2024, 15, 584–593 RSC.
- C. A. Citadelle, E. L. Nouy, F. Bisaro, A. M. Z. Slawin and C. S. J. Cazin, Dalton Trans., 2010, 39, 4489–4491 RSC.
- C. Gibard, H. Ibrahim, A. Gautier and F. Cisnetti, Organometallics, 2013, 32, 4279–4283 CrossRef CAS.
- O. Santoro, A. Collado, A. M. Z. Slawin, S. P. Nolan and C. S. J. Cazin, Chem. Commun., 2013, 49, 10483–10485 RSC.
- J. W. Hall, D. Bouchet, M. F. Mahon, M. K. Whittlesey and C. S. J. Cazin, Organometallics, 2021, 40, 1252–1261 CrossRef CAS.
- J. W. Hall, D. M. L. Unson, P. Brunel, L. R. Collins, M. K. Cybulski, M. F. Mahon and M. K. Whittlesey, Organometallics, 2018, 37, 3102–3110 CrossRef CAS.
- A. Beillard, T.-X. Métro, X. Bantreil, J. Martinez and F. Lamaty, Chem. Sci., 2017, 8, 1086–1089 RSC.
- A. Wróblewska, G. Lauriol, G. Mlostoń, X. Bantreil and F. Lamaty, J. Organomet. Chem., 2021, 949, 121914 CrossRef.
- G. Pisanò and C. S. J. Cazin, Green Chem., 2020, 22, 5253–5256 RSC.
- E. A. Martynova, N. V. Tzouras, G. Pisanò, C. S. J. Cazin and S. P. Nolan, Chem. Commun., 2021, 57, 3836–3856 RSC.
- G. Pisanò and C. S. J. Cazin, ACS Sustainable Chem. Eng., 2021, 9, 9625–9631 CrossRef.
- I. Remy-Speckmann, B. M. Zimmermann, M. Gorai, M. Lerch and J. F. Teichert, Beilstein J. Org. Chem., 2023, 19, 440–447 CrossRef CAS PubMed.
- G. C. Fortman, A. M. Z. Slawin and S. P. Nolan, Organometallics, 2010, 29, 3966–3972 CrossRef CAS.
- N. P. Mankad, T. G. Gray, D. S. Laitar and J. P. Sadighi, Organometallics, 2004, 23, 1191–1193 CrossRef CAS.
- A. L. Speelman, B. L. Tran, J. D. Erickson, M. Vasiliu, D. A. Dixon and R. M. Bullock, Chem. Sci., 2021, 12, 11495–11505 RSC.
- J. Plotzitzka and C. Kleeberg, Inorg. Chem., 2016, 55, 4813–4823 CrossRef CAS PubMed.
- S. Ostrowska, P. Arnaut, D. J. Liptrot, C. S. J. Cazin and S. P. Nolan, Chem. Commun., 2023, 59, 9126–9129 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Full synthetic and characterisation details. CCDC 2314951 (7), 2314952 (8), 2314953 (9) and 2314954 (10). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt00334a |
| This journal is © The Royal Society of Chemistry 2024 |


