Open Access Article
Open Access ArticleMolecular two-point recognition of fructosyl valine and fructosyl glycyl histidine in water by fluorescent Zn(II)-terpyridine complexes bearing boronic acids†
María K.
Salomón-Flores
 a,
Josue
Valdes-García
a,
Josue
Valdes-García
 a,
Alejandro O.
Viviano-Posadas
a,
Alejandro O.
Viviano-Posadas
 a,
Diego
Martínez-Otero
a,
Diego
Martínez-Otero
 ab,
Joaquín
Barroso-Flores
ab,
Joaquín
Barroso-Flores
 ab,
Iván J.
Bazany-Rodríguez
ab,
Iván J.
Bazany-Rodríguez
 a and
Alejandro
Dorazco-González
a and
Alejandro
Dorazco-González
 *a
*a
aInstitute of Chemistry, National Autonomous University of Mexico, Ciudad Universitaria, 04510, CDMX, Mexico. E-mail: adg@unam.mx
bCentro Conjunto de Investigación en Química Sustentable, UAEM-UNAM, Carretera Toluca-Atlacomulco Km 14.5, C. P. 50200, Toluca, Estado de México, Mexico
First published on 24th April 2024
Abstract
Selective recognition of fructosyl amino acids in water by arylboronic acid-based receptors is a central field of modern supramolecular chemistry that impacts biological and medicinal chemistry. Fructosyl valine (FV) and fructosyl glycyl histidine (FGH) occur as N-terminal moieties of human glycated hemoglobin; therefore, the molecular design of biomimetic receptors is an attractive, but very challenging goal. Herein, we report three novel cationic Zn-terpyridine complexes bearing a fluorescent N-quinolinium nucleus covalently linked to three different isomers of strongly acidified phenylboronic acids (ortho-, 2Zn; meta-, 3Zn and para-, 4Zn) for the optical recognition of FV, FGH and comparative analytes (D-fructose, Gly, Val and His) in pure water at physiological pH. The complexes were designed to act as fluorescent receptors using a cooperative action of boric acid and a metal chelate. Complex 3Zn was found to display the most acidic –B(OH)2 group (pKa = 6.98) and exceptionally tight affinity for FV (K = 1.43 × 105 M−1) with a strong quenching analytical response in the micromolar concentration range. The addition of fructose and the other amino acids only induced moderate optical changes. On the basis of several spectroscopic tools (1H, 11B NMR, UV-Vis, and fluorescence titrations), ESI mass spectrometry, X-ray crystal structure, and DFT calculations, the interaction mode between 3Zn and FV is proposed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 model through a cooperative two-point recognition involving a sp3 boronate–diol esterification with simultaneous coordination bonding of the carboxylate group of Val to the Zn atom. Fluorescence quenching is attributed to a static complexation photoinduced electron transfer mechanism as evidenced by lifetime experiments. The addition of FGH to 3Zn notably enhanced its emission intensity with micromolar affinity, but with a lower apparent binding constant than that observed for FV. FGH interacts with 3Zn through boronate–diol complexation and coordination of the imidazole ring of His. DFT-optimized structures of complexes 3Zn–FV and 3Zn–FGH show a picture of binding which shows that the Zn-complex has a suitable (B⋯Zn) distance to the two-point recognition with these analytes. Molecular recognition of fructosyl amino acids by transition-metal-based receptors has not been explored until now.
1 model through a cooperative two-point recognition involving a sp3 boronate–diol esterification with simultaneous coordination bonding of the carboxylate group of Val to the Zn atom. Fluorescence quenching is attributed to a static complexation photoinduced electron transfer mechanism as evidenced by lifetime experiments. The addition of FGH to 3Zn notably enhanced its emission intensity with micromolar affinity, but with a lower apparent binding constant than that observed for FV. FGH interacts with 3Zn through boronate–diol complexation and coordination of the imidazole ring of His. DFT-optimized structures of complexes 3Zn–FV and 3Zn–FGH show a picture of binding which shows that the Zn-complex has a suitable (B⋯Zn) distance to the two-point recognition with these analytes. Molecular recognition of fructosyl amino acids by transition-metal-based receptors has not been explored until now.
Introduction
The development of selective boronic acid-based receptors for glyco-amino acids related to glycated hemoglobin (HbA1c) such as fructosyl L-valine (FV) and fructosyl-valyl-histidine (FVH) remains a challenge in modern supramolecular chemistry and analytical sciences due to their potential application in sensing, separation, development of new concepts of glycopeptides recognition,1–5 as well as the diagnosis of sugar-related diseases and medicinal chemistry.6,7FV occurs as an N-terminal unit of human HbA1c inside β-subunits (fructosyl-Val-His-Leu-Thr…)8,9 and typically serves as a target test molecular moiety and chemical model to mimic the diagnosis of type 2 diabetes mellitus.1,10,11 To date, an overwhelming majority of FV and related fructosyl amino acid detection methods are based on chromatography separation-mass spectrometry,10,12 mutant proteins,13 electrochemical biosensors,2,9,14–18 and Pt/Au-amperometric nanomaterials5,9,19,20 that contain the fructosyl amino-acid oxidase. These enzymatic assay systems still suffer from some drawbacks such as multiple steps for their construction, long analysis times and the requirement for specialized labs. Additionally, FV sensing can be achieved by non-enzymatic systems using boronic acid-appended redox active species such as Au/C-electrodes functionalized with thiophene-boronic acid,21 ferrocenylboronic acid,22,23 and vinylphenylboronate-polymers24 where the FV recognition is driven by the formation of an sp3-boronated ester with a sugar moiety. It is well known that simple phenylboronic acid (PBA) has a higher affinity for catechol fragments, open-chain polyols and anionic species than for fructose in water (binding constant K = 160 M−1);25 therefore, these boronic acid-based materials are not particularly selective and interference from biogenic catecholamines, phosphorylated anions, dicarboxylates, and sorbitol/mannitol can be a problem.26–31 Furthermore, this affinity of PBA towards fructose is suitable to recognize/sense FV in the millimolar concentration range, but not lower, which is highly desired for its intended applications.
While the need for selective optical receptors for FV and FVH is evident, to the best of our knowledge, fluorescent molecular receptors for their quantification have not yet been developed.
Among different saccharide recognition techniques, fluorescence is particularly desired due to its known high sensitivity, quick and direct analytical response.32
Arylboronic acids can form reversible covalent bonds with 1,2-diols in basic aqueous media33 where the binding strength of boronic acid–1,2-diol ester depends on the orientation of the analyte's vicinal hydroxyl groups,34 the acidity of the boronic acid,35 and the influence of substituent groups to stabilize the sp3-boronate ester.28 In the last few decades, phenylboronic acid fluorophores have been demonstrated to be an outstanding tool for recognizing and sensing monosaccharides,36 catechol-based neurotransmitters,37 nucleotides,38 sialic acid,39 glucosamine,40 ginsenosides,41 glycated hemoglobin,42 and in general, 1,2-dihydroxy-substituted derivatives.33,43 However, the recognition of fructosyl amino acids (Amadori products)44 remains largely unexplored. In principle, it should be possible to have a selective optical chemosensor for FV by using a fluorescent receptor with multiple and high-affinity binding sites for FV or target derivatives.
On the other hand, reports in the context of coordination chemistry have shown that Zn(II)-terpyridine complexes are effective binding motifs for carboxylate anions and imidazole fragments45via direct coordination due to their versatile coordination number and strong Lewis acidity of the Zn atom.46,47 There is only one report in the literature showing that FV and some Amadori products, such as fructosyl-glycine (FG), can bind divalent transition metal ions (e.g. Cu(II), Ni(II)) with an affinity of about 103 M−1 in water.44
In this study, we have designed and prepared three novel cationic luminescent Zn-terpyridine complex-based receptors bearing a strongly acidified phenylboronic acid to bind through cooperative two sites to FV or fructosyl-Gly-His (FGH) which is a chemical model of the sequence fructosyl-Val-His from HbA1c with the same terminal residues.9 FV is the most common chemical model of HbA1c used in detection/recognition studies and clinical diagnostics.2 However, there is recent interest in cooperatively recognizing fructose and histidine residues to improve the specificity of sensors.5,48 In this work, for the first time, we explored developing a fluorescent receptor functional for FV and FGH through cooperative two-point recognition that includes a coordination bond and a covalent boronate–diol bond.
The baseline expectation for these Zn-receptors is that phenylboronic acid can form stable complexes with the fructosyl motif and the Zn(II) atom can interact with the carboxylate group from valine (FV) or the imidazole ring from histidine (FGH). The results obtained for a series of fluorescent Zn-terpyridine-4′-quinolinium complexes bearing three different isomers of boronic acids, including synthesis, crystal structures, acid–base properties, spectroscopic recognition and theoretical DFT calculations are summarized below.
Results and discussion
Synthesis and crystal structure
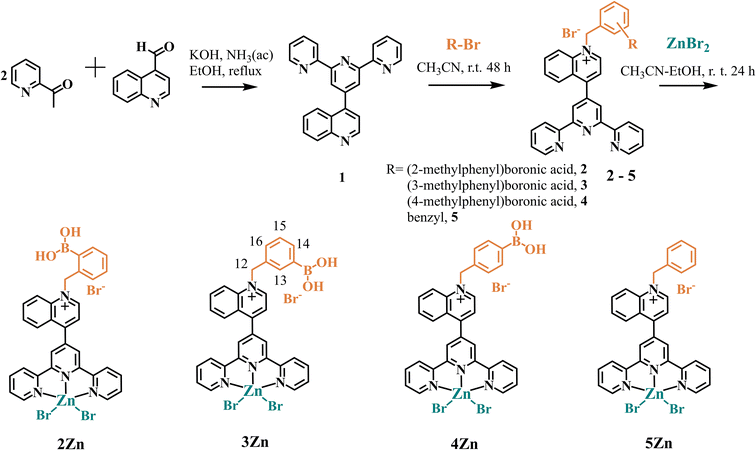
For these investigations, three isomers 2Zn, 3Zn and 4Zn involving two Lewis acids of different nature (B and Zn atoms) as binding sites for fructosyl-amino acids were successfully prepared by the three-step path described in Scheme 1. The synthesis was initiated with the preparation of a terpyridine bearing a quinoline moiety at position 4′ of the central pyridine ring by the Kröhnke reaction to give 1, and subsequent treatment with the corresponding isomer of (bromo-methyl)phenylboronic acid in dry CH3CN under a N2 atmosphere to afford the cationic ligands (L), 2–4. These ligands were characterized by 1H and 13C NMR spectroscopy, ESI-MS (+) and ATR-IR. The expected number of NMR signals and MS peak of these ligands were consistent with their structures (Fig. S1–S15†). For comparative purposes, ligand 5, which lacks boronic acid, was synthesized via the same synthetic route and characterized (Fig. S16–S19†), using benzyl bromide instead of a (bromo-methyl)phenylboronic acid. The Zn-complexes were obtained upon direct complexation with 1.1 equiv. of ZnBr2·2H2O in CH3CN–EtOH at r.t. In all cases, the bromide salts of Zn-complexes were obtained as pink pale crystalline powders and were pure according to 1H, 11B, and 13C NMR spectroscopy (Fig. S20–S37†), ESI-MS and elemental analysis (C, H, N). One charged state for the three isomers 2Zn, 3Zn and 4Zn, monoanionic species at m/z = 880.81, corresponding to {[ZnL]3+ + 4Br−}−, was clearly observed and isotopically resolved from their respective analysis by a negative scan of ESI (Fig. S23, S29 and S33†).The 11B NMR spectra of the three isomeric Zn-complexes in CD3OD–DMSO-d6 displayed only one signal at ∼27 ppm (Fig. S22, S27 and S32†). This value corresponds to the sp2-hybridized trigonal boron atom, however, this signal is upfield shifted compared to simple neutral PBA (δ = 30 ppm).49 As we have recently shown, this upfield shift of the signal in 11B NMR can be the result of the strong acidification of the phenylboronic groups by the delocalized positive charge on the quinolinium ring.50 Along this line, Lakowicz has previously reported strong acidification of phenylboronic acids when covalently linked to 6-methoxyquinolinium nuclei.51
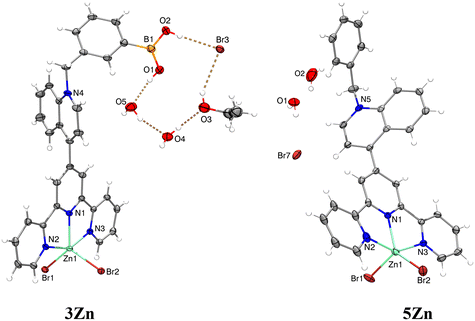
X-ray crystal structures were obtained for the hydrated bromide salts of 3Zn and 5Zn. Fig. 1 shows a perspective view of these molecules. Tables S1–S3† contain the crystallographic data and parameters of hydrogen bonds within the crystal packing of these complexes.
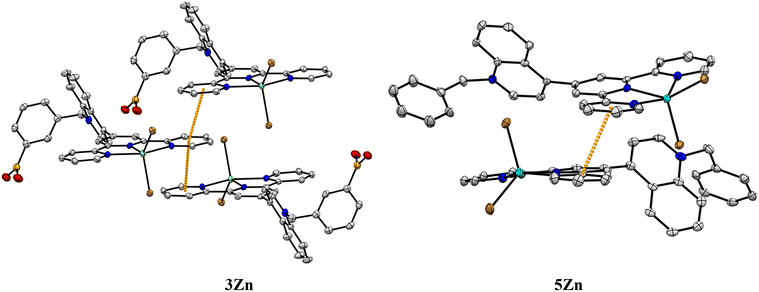
X-ray structural analysis of 3Zn confirms the presence of sp2-hybridized boronic acid and a trigonal planar geometry (∑∡(X–B–X) = 359.99°) which is consistent with its 11B NMR spectrum in solution. The angle between the –B(OH)2 plane and the phenyl ring is 46.55°. The –B(OH)2 group is stabilized by a Br− anion and a water molecule through hydrogen bonds (the parameters of hydrogen bonds are compiled in Table S2†). The structural analysis showed that the Zn(II) atoms in 3Zn and 5Zn have a distorted square-pyramidal geometry with a [ZnN3Br2] coordination sphere. For both crystals, the rings of central pyridine (terpy) and quinoline are significantly out of coplanarity. The angles are 63.25° and 58.42° for 3Zn and 5Zn, respectively. The distortion in the coplanarity between these rings seems to be primarily an electronic rather than steric effect.52
A review of the crystal packings of 3Zn and 5Zn reveals the presence of strong π⋯π stacking interactions between central and external pyridine rings (Fig. 2).
Optical and acid–base properties
The bromide salts of cationic complexes 2Zn, 3Zn, 4Zn and 5Zn are soluble in buffered water at pH = 7.4 (10 mM MOPS) and follow very well the Lambert–Beer law up to 100 μM by fluorescence spectroscopy; thus, these conditions were used for further spectroscopic studies. The absorption and fluorescence maxima of all Zn-complexes at physiological pH are compiled in Table 1. The blue emission at ∼450 nm in these quinolinium-based compounds is typically attributed to intramolecular charge transfers (ICTs) in the excited state.53| Complex |
λ
abs (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) ε) |
λ em | Fluorimetric titration, pKa |
|---|---|---|---|
| 2Zn | 278 (4.21); 285 (4.24); 330 (4.19) | 446 | 7.09 ± 0.02 |
| 3Zn | 279 (4.14); 286 (4.20); 329 (4.08) | 450 | 6.98 ± 0.03 |
| 4Zn | 278 (4.13); 285 (4.09); 329 (4.02) | 445 | 7.40 ± 0.04 |
The acid–base properties of Zn-complexes were explored by fluorimetric pH-titration experiments. Fig. 3A shows the family of fluorescence spectra of 3Zn at different pH values and its inset illustrates the fitting of the profile at a single wavelength vs. pH to the theoretical equation (see Fig. S46† for fluorescence spectra–pH of 2Zn and 4Zn).
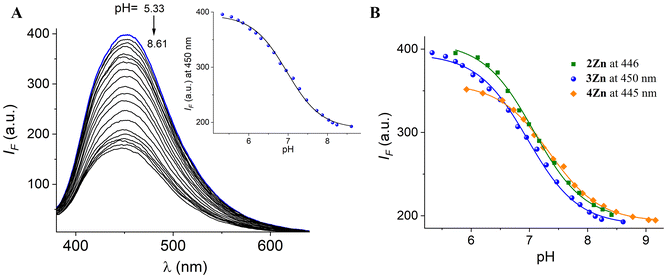 | ||
| Fig. 3 (A) Fluorescence spectra of 3Zn (20 μM) in buffered aqueous solution (λex = 330 nm) at different pH values. (B) The observed pH-titration profiles at the emission maximum of each Zn-complex. | ||
The pH-profiles of fluorescence intensity at 450 nm for the three isomers and their pKa values are shown in Fig. 3B and Table 2. The pKa values estimated for the –B(OH)2 group in 2Zn and 3Zn are close to 7.0. This finding is relevant to our objectives because it reveals that the boronic acid groups are in their sp3 anionic sugar bond form at pH = 7.40, enabling the fructosyl recognition under “physiological” conditions.
The calculated pKa values for 2Zn (pKa = 7.09) and 3Zn (pKa = 6.98) are lower than those reported for free phenylboronic acid (pKa = 8.8)25 and for typical boronic acid-based receptors for fructose that contain viologen or fluorene motifs.34,49,54 This fact is not unexpected because it is well known that the cationic nature of the quinolinium ring covalently appended to boronic acid reduces the pKa values up to two orders of magnitude.32,51,55
On the other hand, very low pKa values ranging from 6.70 to 7.90 have been reported previously for quinolinium51,55 and isoquinolinium50 nuclei-bearing isomers (ortho, meta) of phenylboronic acids. These values are consistent with those estimated for 2Zn and 3Zn.
Table 2 compiles the pKa results obtained from fluorescence spectroscopy of 2Zn, 3Zn and 4Zn and for comparison, lists pKa values found in the literature for related compounds containing a boronic acid-substituted benzyl pyridinium or benzyl quinolinium motif (see Fig. 4).
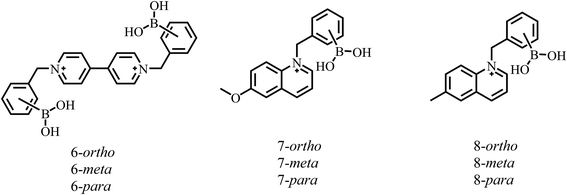 | ||
Fig. 4 Structure of compounds 6,497![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51 and 8 51 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 containing a boronic acid-substituted benzyl pyridinium or quinolinium fragment for which pKa values have been previously reported (Table 2). 55 containing a boronic acid-substituted benzyl pyridinium or quinolinium fragment for which pKa values have been previously reported (Table 2). | ||
An inspection of these data indicates that, in general, the pKa values of the meta and ortho isomers tend to be lower, between 0.2 and 1.1 units, as compared to the para isomer. This trend is also observed in our Zn-complexes.
Typically, this effect is ascribed to the fact that the quaternary nitrogen of the quinolinium moiety not only reduces the pKa of the boronic acids by an electron-withdrawing inductive effect, but also electrostatically stabilizes the sp3-boronate anion formed.49,51,55 Therefore, the pKa value of the para isomer is expected to be higher due to the greater distance between the –B(OH)2 group and the quinolinium ring compared to ortho or meta isomers, as observed in the value of 4Zn and with a different set of isomers in Table 2.
It is noteworthy that if the pKa were driven simply by interaction-induced stabilization and the distance between the quinolinium and the sp3-boronate anion formed as commonly described, one would expect the ortho isomer to always be the more acidic species; however, the data in Table 2 for 6 and 7 and the values of our complexes show that the meta isomer is slightly more acidic. Therefore, other side effects that stabilize the boronate anion cannot be ruled out as a steric effect which can be considerable, particularly for the ortho isomer.
Fructosyl amino acid binding studies
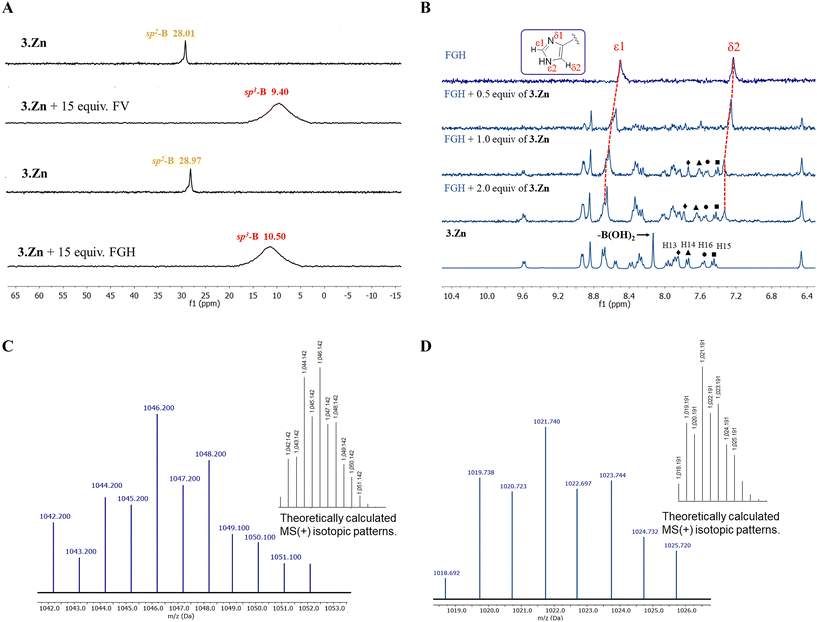
Taking advantage of the fact that the 3Zn complex possesses the most acidic –B(OH)2 group (pKa = 6.98), this compound was studied as a model receptor for FV and FGH by different spectroscopic experiments. The first evidence for the high affinity of 3Zn for these two fructosyl amino acids was obtained by 11B and 1H NMR measurements. Fig. 5A illustrates the experiments of 3Zn with FV and FGH monitored by 11B NMR spectroscopy. Upon the addition of 15 equiv. of FV to a solution of 3Zn (2.0 mM) the initial signal for the sp2-B atom at 28.01 ppm disappeared and a new broadened signal at 9.40 ppm arose for the sp3-B atom. A similar 11B NMR response was observed with FGH. This chemical shift (Δδ = 18.61 ppm for FV and 18.47 ppm for FGH) of the 11B NMR signals is characteristic of the formation of an sp3-boronate complex with sugars.49,56 Thus, the shift in 11B NMR signals from sp2-B to sp3-B confirms the binding of the fructosyl moieties for both analytes to the 3Zn complex through the formation of a boronate ester.Particularly, for the case of FGH, we investigated the potential coordination of the imidazole ring of His with the Zn(II) center by a 1H NMR titration experiment in D2O–DMSO-d6. The addition of 3Zn (0–2.0 equiv.) to the solution of FGH (3.0 mM) induces a notable downfield shift (Δδ = 0.31 ppm) of the ε1-proton corresponding to the imidazole ring as is shown in Fig. 5B. The downfield shift can be assigned to the effective coordination of the imidazole ring to the Zn(II) center. This metal binding to the heterocycle is not unexpected because the Nε2-atom of imidazole is undoubtedly the strongest bonding donor atom due to its high basicity and a favorable electrostatic potential as is evidenced by the great occurrence of this coordination bond (Zn–Nε2) in Zn-histidine complexes and Zn-proteins by NMR spectroscopic studies.57
Furthermore, a comparison between the 1H NMR spectra of free 3Zn and FGH + 3Zn shows that only the aromatic H13–16 protons of the phenylboronic acid ring (see Scheme 1 for numbering of the structure) are affected. Reversible esterification of boronic acid with the fructosyl fragment induces an upfield shift of ∼0.22 ppm for the aromatic H13–16 protons with a simultaneous disappearance of the signal of –B(OH)2 at 8.12 ppm; practically, the rest of the protons of 3Zn are not affected.
Mass spectrometry has been used in the past to study boronate–diol complexes.32 The mass spectra of 3Zn in the presence of 3.0 equiv. of FV or FGH obtained by a positive scan of ESI in aqueous media showed practically one charged species at m/z = 1046.2 and 1021.7, respectively (Fig. 5C and D).
These peaks were isotopically resolved and match very well the theoretical distribution for the monocationic complexes {[3Zn] + 2(Br)− + FV + 2(CH3OH) + H2O}+ (calc. C44H51BBr2N5O10Zn, m/z 1046.14) and {[3Zn] + Br− + FGH + 1(EtOH)}+ (calc. C47H47BBrN8O9Zn, m/z = 1021.20) corresponding to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 supramolecular complexes 3Zn–FV and 3Zn–FGH, respectively. [3Zn] species corresponds to the tricationic Zn-complex without bromide ions. In both cases, boronate/carboxylate groups balance the charges.
1 supramolecular complexes 3Zn–FV and 3Zn–FGH, respectively. [3Zn] species corresponds to the tricationic Zn-complex without bromide ions. In both cases, boronate/carboxylate groups balance the charges.
Fluorescent chemosensing studies of fructosyl amino acids
Addition of FV, FGH, Fru, His, Gly and Val in a micromolar concentration range to aqueous solutions of 2Zn, 3Zn and 4Zn at pH = 7.4 induced small changes, but changes in the UV-Vis spectra of Zn-complexes with FV and FGH were clearly observed. As an example, Fig. S47† shows the titration experiment for 3Zn with increasing concentrations of FV (0–180 μM), from which the apparent binding constant, K = (1.36 ± 0.12) × 105 M−1, can be well calculated by fitting to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 model (see inset, Fig. S47†). Three isosbestic points at 290, 310 and 344 nm were observed, which indicates that only two species are in equilibrium (free complex 3Zn and its complexed form with FV).
1 model (see inset, Fig. S47†). Three isosbestic points at 290, 310 and 344 nm were observed, which indicates that only two species are in equilibrium (free complex 3Zn and its complexed form with FV).
To obtain a more sensitive optical response, we tested the affinity/sensing of 3Zn towards FV, FGH and comparative analytes (Fru, Val, Gly and His) by steady-state fluorescence spectroscopy in pure water.
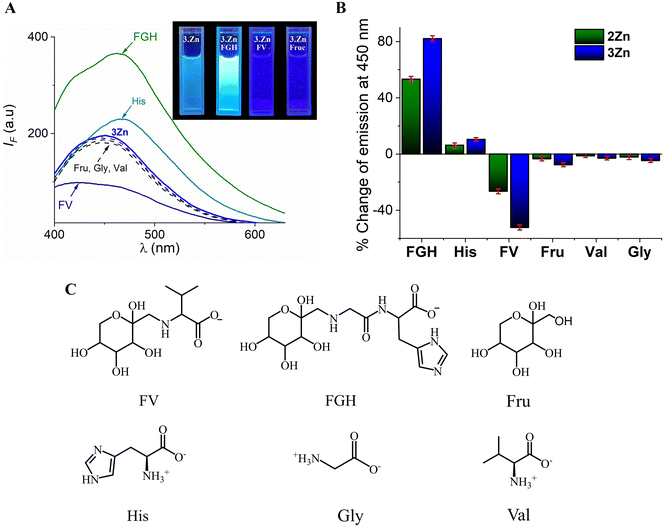
Initially, the relative fluorescence selectivity was analyzed. FV, FGH, Fru, Val, Gly, and His ([analyte]final = 100 μM) were added to an aqueous solution (10 mM MOPS, pH = 7.4) of 3Zn (20 μM) and the emission spectra (λex = 330) was recorded (Fig. 6A). In addition, Fig. 6B shows the emission intensity change at 450 nm of 2Zn and 3Zn upon the addition of these analytes. Overall, Fru, Val and Gly gave a low quenching response IF < 7% of its starting emission I0. The addition of FV notably decreases the emission intensity by ca. ∼29% and 52% for 2Zn and 3Zn, respectively. This quenching effect is not unexpected because the boronic acid fluorophores or Zn-complexes typically show a complexation-induced quenching analytical response as a result of a photoelectron transfer (PET) mechanism when forming a boronate–diol ester36,58 or by the coordination of carboxylates to the Zn atom.59
In contrast, the addition of His induced a slight enhancement in intensity (∼10%) for both Zn-complexes. Optical recognition of His by Zn-terpy complexes with the turn-on signal has been previously reported60 and the fluorescence enhancement was attributed to (1) the increase in the rigidity of the system by effective coordination of the imidazole ring of His and (2) the disappearance of any intramolecular charge transfer processes in the receptor-anion complex.60 Furthermore, the coordination of His decreases the degree of hydration of the complex which should favor emission.47
The addition of FGH to the buffered solution of 3Zn generated a strong enhancement fluorescence of approximately 90% with a bathochromic effect (Δλ = 12 nm) in the emission maxima (Fig. 6B). A similar effect was observed for 2Zn but with a smaller intensity improvement (53%) than that observed for 3Zn.
The strong fluorescence change induced by FV and FGH suggests that fructosyl amino acids have greater affinity than their chemical constituents (Fru or amino acids) separately which can be explained by a two-point cooperative recognition which does not seem feasible for Fru or amino acids. To verify this selectivity, we determined the binding constants for all analytes through fluorimetric titration experiments. Fig. 7 shows the fluorimetric titrations of the most acidic complex, 3Zn with FV and FGH.
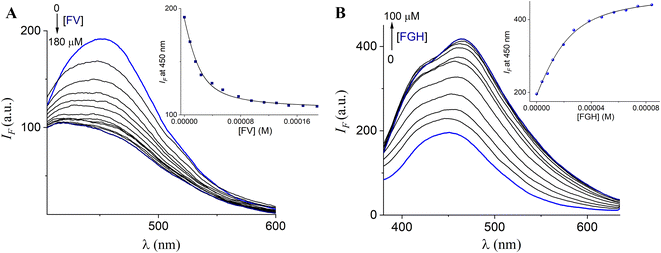 | ||
| Fig. 7 Changes in the emission spectra (λex = 330 nm) of buffered (10 mM, MOPS, pH = 7.4) aqueous solutions of 3Zn (20 μM) upon the addition of increasing amounts of (A) FV and (B) FGH. The inset shows the curve at 450 nm (average of triplicate experiments). The solid line was obtained by fitting to eqn (1). | ||
In general, the profile curves (shown in the insets) can be well fitted to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
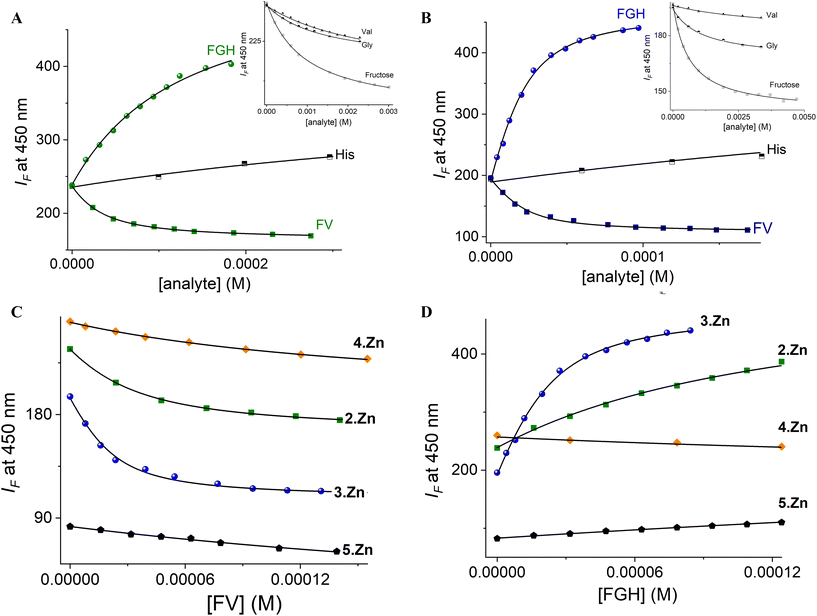
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model with non-linear least-squares treatment using eqn (1) to obtain apparent binding constants K3Zn–FV = (1.43 ± 0.10) × 105 and K3Zn–FGH = (1.02 ± 0.07) × 105, where IF is the observed intensity, I0 is the intensity of free 3Zn (R), ΔI∞ is the change induced by the analyte at saturation, [A]T is the total concentration of the guest and K is the apparent binding constant.
1 binding model with non-linear least-squares treatment using eqn (1) to obtain apparent binding constants K3Zn–FV = (1.43 ± 0.10) × 105 and K3Zn–FGH = (1.02 ± 0.07) × 105, where IF is the observed intensity, I0 is the intensity of free 3Zn (R), ΔI∞ is the change induced by the analyte at saturation, [A]T is the total concentration of the guest and K is the apparent binding constant.
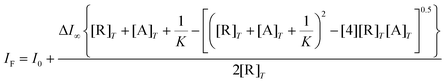 | (1) |
The apparent complexation constant determined from fluorescence matches well with the value estimated by the UV-Vis titration (vide supra), indicating that the interaction occurs mainly in the ground state.
The fitted fluorimetric curves to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 model for all analytes with 2Zn and 3Zn are shown in Fig. 8A and B (see Fig. S48† for 4Zn). The estimated apparent binding constants (error < 10%) are compiled in Table 3.
1 model for all analytes with 2Zn and 3Zn are shown in Fig. 8A and B (see Fig. S48† for 4Zn). The estimated apparent binding constants (error < 10%) are compiled in Table 3.
| Analyte |
2Zn, K(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
3Zn, K(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
4Zn, K(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
5Zn, K(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
|---|---|---|---|---|
| a Not calculated. | ||||
| FV | (4.16 ± 0.10) × 104 | (1.43 ± 0.10) × 105 | (2.89 ± 0.09) × 103 | (2.39 ± 0.13) × 103 |
| FGH | (9.07 ± 0.07) × 103 | (1.02 ± 0.07) × 105 | (2.58 ± 0.11) × 103 | (1.23 ± 0.11) × 103 |
| His | (1.28 ± 0.09) × 103 | (1.41 ± 0.08) × 103 | (8.02 ± 0.12) × 102 | —a |
| Gly | (7.19 ± 0.07) × 102 | (9.24 ± 0.09) × 102 | (6.28 ± 0.06) × 102 | —a |
| Val | (3.55 ± 0.12) × 102 | (2.66 ± 0.12) × 102 | (3.27 ± 0.07) × 102 | —a |
| Fru | (1.10 ± 0.03) × 103 | (1.68 ± 0.07) × 103 | (1.25 ± 0.12) × 103 | —a |
 | ||
| Fig. 8 Fluorimetric titration (λex = 330 nm) of aqueous solutions of (A) 2Zn and (B) 3Zn (20 μM) upon the addition of increasing amounts of fructosyl amino acids, His, Gly, Val and fructose at pH 7.4. The solid lines were obtained by fitting to eqn (1). Fluorimetric titration profiles of all Zn-complexes with (C) FV and (D) FGH. | ||
A detailed inspection of the results in Table 3 shows that, indeed, tendencies in affinity constants are similar for the most acidic and effective receptors (3Zn and 2Zn). For example, 3Zn binds FV two or three orders of magnitude tighter than it binds Fru or Val (<103 M−1) and 3Zn binds FGH two orders of magnitude larger than Fru or His (∼103 M−1).
One can deduce that the combination of the metal coordination with boronate–diol in the meta isomer of phenylboronic acid leads to substantial increases in the complexation between 3Zn and FV or FGH.
Although Val or His have potential metal binding sites (carboxylate/imidazole) and Fru can interact with boronic acid, these seem to only involve the recognition of one point with the Zn-complexes.
Reversible two-point recognition with derivatives of Zn complexes bearing a phenylboronic acid has been used in binding flexible biological polyol-appended carboxylic acids such as sialic acid59 and uronic acid.61
To support the two-point recognition mechanism we estimated the affinity constant of FV and FGH towards the reference compound 5Zn which lacks boronic acid under the same conditions (Fig. 8C and D). In both cases, the affinity and effect on the intensity are like that observed for pure amino acids (Val or His) and drops three orders of magnitude when compared to the affinity between the fructosyl amino acids and 3Zn.
On the other hand, the affinity of 3Zn and 2Zn towards FV in the range of K = (1.43–0.41) × 105 M−1 is considerably greater than the stability constant of phenylboronic acid and Fru (K = 160 M−1, taken from the literature)25 which is consistent with (1) the increase of stability of the boronate–diol interaction promoted by the strong Lewis acidity of the phenylboronic groups in these Zn-complexes and (2) the participation of interaction carboxylate-metal as a cooperative binding site.
From the data in Table 3, the order of preference of FV/FGH towards the receptors is 3Zn > 2Zn > 4Zn which correlates with the acidity of their –B(OH)2 groups. This correlation strongly indicates that the diol binding boronic acid site drives the interaction between Zn-complexes and studied fructosyl amino acids as evidenced by 11B NMR measurements. Among Zn-complexes, the most efficient receptor 3Zn binds FV about 1.5 times more tightly than does FGH, probably due to the electrostatic contribution of the carboxylate anion from Val.
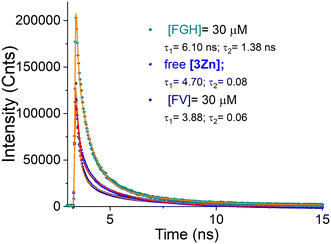
In order to explore the nature of the fluorescence response induced by fructosyl amino acids, the lifetimes of 3Zn in the absence and presence of FV and FGH were recorded. An aqueous solution of 3Zn upon excitation with a 354 laser displayed a bi-exponential decay with lifetimes τ1 = 4.70 ns and τ = 0.08 ns (Fig. 9).
The 3Zn complex with 3.0 equiv. of FV exhibited a biexponential decay with nearly similar values, τ1 = 3.98 ns and τ = 0.06 ns, suggesting a mainly static quenching by complexation (3Zn–FV) as evidenced by NMR, ESI-MS and UV-Vis experiments.
It is well known that a static quenching mechanism in a system fluorophore–quencher does not change the lifetime values.62 In contrast, the addition of FGH modestly increases the lifetimes up to τ1 = 6.10 ns and τ2 = 1.38 ns. These longer values can be ascribed to the disappearance of any non-emitting charge transfer caused by the coordination of the imidazole ring as well as the formation of a large rigid extended π-conjugated system involving ligand–ligand charge transfers in the final coordination complex.63
Considering all foregoing spectroscopic evidence, the crystal structure and ESI-MS results, the molecular recognition modes of 3Zn with fructosyl amino acids are illustrated in Fig. 10. Therein, 3Zn can complex with FV or FGH through two-point recognition, one diol-binding boronic acid site and the other at the carboxylate anion or imidazole ring binding Zn atom. According to the best of our knowledge, this is the first example of an artificial fluorescent receptor for fructosyl amino acids and the sole for FV.
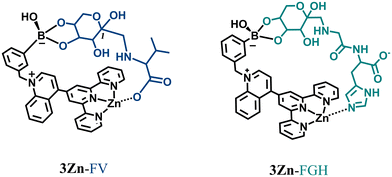 | ||
| Fig. 10 The complexation mode proposed for binding of the two-points of FV and FGH to the 3Zn complex. | ||
The reversibility of the boronic acid–diol interaction is a key aspect of molecular recognition because it serves as a basis for understanding and improving the sensing response of the target analyte,28,33 therefore, we investigated the reversibility of the interaction of 3Zn with FV through a qualitative experiment using the Alizarin Red S (ARS) dye.
It is well known that ARS binds reversibly to arylboronic acids and is commonly used for the formation of dynamic chromogenic assemblies with application in the detection of saccharides.25,64
In a typical experiment, an aqueous solution of ARS displays a dramatic change in color in response to the binding of a boronic acid from deep red to yellow. The displacement of the maxima occurs from ∼520 cm (free ARS, deep red) to ∼460 cm (ARS + boronic acid, yellow) as described in detail by Wang.25
We initially monitored the color change of free ARS (100 mM) upon the addition of our complex containing the boronic acid, 3Zn (1.1 equiv.) in a buffered aqueous solution at pH = 7.4. As shown in Fig. S49,† there is a hypsochromic shift in the maxima, from 521 nm to 458 nm, similar to that reported for the ARS–boronic acid systems, induced by the formation of the sp3 boronic ester. Subsequently, the addition of an excess of FV (10 equiv.) practically restored the spectrum of free ARS both in its form and at its maximum, suggesting that the ARS dye was displaced by the FV analyte which shows the reversibility of the ARS–3Zn complex.
Furthermore, the reversibility of the supramolecular 3Zn–FV complex qualitatively was verified as described below.
The complex prepared in situ3Zn–FV (110 μM, each component) was added to an aqueous buffered solution of ARS (100 μM) and as seen in Fig. S50,† the initial absorption maximum at 520 nm was displaced to 461 nm, corresponding to the formation of the ARS–3Zn complex, which is only possible if the dye displaces the FV analyte. These experiments provide strong evidence of reversibility in the complexation of the molecular receptor 3Zn with FV in water.
DFT calculation
Finally, to gain further insight into the cooperative binding mode of 3Zn with FV and FGH, density functional theory calculations were carried out for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 supramolecular complexes. All calculations were carried out with the Gaussian16 suite of programs65 at the ωB97-XD/LANL2DZ level of theory with the PCM implicit solvent model (water). Thus, complexes 3Zn–FV and 3Zn–FGH were optimized in order to assess their coordination preferences (Fig. 11). Frequency calculations reveal both compounds to be minimal on their respective potential energy surfaces.
1 supramolecular complexes. All calculations were carried out with the Gaussian16 suite of programs65 at the ωB97-XD/LANL2DZ level of theory with the PCM implicit solvent model (water). Thus, complexes 3Zn–FV and 3Zn–FGH were optimized in order to assess their coordination preferences (Fig. 11). Frequency calculations reveal both compounds to be minimal on their respective potential energy surfaces.
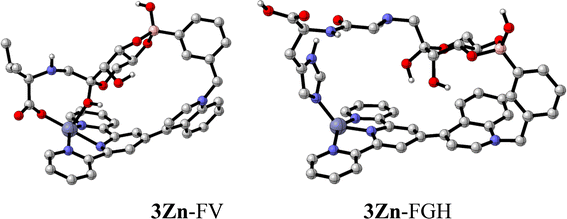 | ||
| Fig. 11 Optimized structures of 3Zn–FV and 3Zn–FGH at the ωB97-XD/LANL2DZ level of theory (hydrogen atoms not shown for clarity). | ||
FV is long enough to coordinate with two –OH groups of the fructose moiety with the tetrahedral sp3-boronate anion and cooperatively coordinate with the carboxylate group from Val with the Zn atom.
Additionally, a close (Fru)C1–OH⋯Zn interaction is observed which generates a pentacoordinate Zn complex. Therefore, a three-point recognition between 3Zn and FV is sketched, which may explain their high affinity.
On the other hand, a bent conformation of FGH interacts with 3Zn through the boronate ester with two OH groups from the Fru moiety and a coordination bond between the imidazole ring and the metallic atom to give a tetracoordinated Zn complex. Thermochemistry calculations derived from the vibrational analysis reveals that 3Zn–FV is more stable than compound 3Zn–FGH by ∼11.24 kcal mol−1, which makes the former the best candidate for a receptor from a theoretical standpoint. The B⋯Zn distances for the optimized structures are 9.79 Å and 10.59 Å for 3Zn–FV and 3Zn–FGH, respectively. These values are close to the B⋯Zn distance in the crystal structure (12.44 Å). The narrowing of the B⋯Zn distance in the receptor induced by the coordination of the fructosyl amino acids is plausible due to the flexible methylene group of the receptor that can rotate and bring these atoms closer together.
Conclusions
We have described the first example of a set of fluorescent Zn-terpyridine complexes appended to strongly acidified phenylboronic acid groups for optical recognition of biochemically relevant fructosyl amino acids that occur in HbA1c such as FV and FGH.These cationic Zn-complexes possess a high-water solubility, photostability and strong ability for fluorescence sensing of FV and FGH in a micromolar concentration range at physiological pH.
The complex bearing meta-substituted boronic acid 3Zn stood out from the other two (ortho-2Zn and para-4Zn) for having the most acidic –B(OH)2 group with pKa = 6.98 and exceptionally strong affinity for FV (K = 1.43 × 105 M−1) and FGH (K = 1.05 × 105 M−1) with selectivity over their separate molecular constituents such as fructose, Val, Gly and His.
1H, 11B NMR, UV-Vis and fluorescence titration experiments, MS measurements, X-ray crystal structure and DFT calculations showed that FV and FGH are bound to 3Zn in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mode through two-point recognition that involves (1) a boronate–diol complexation and (2) the coordination bond of the carboxylate anion (FV) or the imidazole ring (FGH) to the Zn atom.
1 mode through two-point recognition that involves (1) a boronate–diol complexation and (2) the coordination bond of the carboxylate anion (FV) or the imidazole ring (FGH) to the Zn atom.
Addition of FV to an aqueous solution of 3Zn displays an efficient quenching response which can be explained by a static PET mechanism possibly in both the excited and the ground state as evidenced by lifetime measurements and spectroscopic titrations. In contrast, addition of FGH induced a notable turn-on signal which can be rationalized by a rigidification of the complex and a decrease in the hydration grade of the final 3Zn–FGH complex.
Overall, these results further highlight the usefulness of a new set of water-soluble and fluorescent receptors based on two different Lewis acids as analytical tools for the selective and direct sensing of fructosyl amino acids with biochemical and medical relevance.
Experimental section
General conditions, chemicals and equipment are described in the ESI.† Isomers 2–4 and compound 5 were prepared according to a modified methodology reported previously.50Chemical synthesis
The solvent was reduced under vacuum up to ∼20% of the initial volume and cold distilled water (60.0 mL) was added to give a precipitate. This precipitate was filtered off, washed with water twice (5.0 mL) and recrystallized in EtOH to obtain 1 as pale pink crystalline powder. Yield: 344.0 mg (75.0%).
1H NMR (300 MHz, 25 °C, DMSO-d6) δ (ppm) 9.05 (d, J = 6.37 Hz, 1H), 8.74–8.70 (m, 4H), 8.56 (s, 2H), 8.18 (d, J = 8.32 Hz, 1H), 8.07 (m, 2H), 7.93 (dd, J = 8.56, 1.36 Hz, 1H), 7.86 (m, 1H), 7.69–7.63 (m, 2H), 7.53 (m, 2H).
13C NMR (75 MHz, 25 °C, DMSO-d6) δ (ppm) 155.54, 154.67, 150.45, 149.52, 148.16, 147.39, 145.07, 137.68, 129.96, 129.84, 127.72, 125.11, 124.85, 121.42, 121.16, 121.04 (one signal was not detected).
ESI(+)-MS (m/z) [1]+: calculated for [C24H17N4]+: 361.15, found: 361.30. Anal. calcd for C24H16N4 (360.42); C, 79.98; H, 4.47; N, 15.15. Found: C, 79.97; H, 4.56; N, 15.04.
1H NMR (300 MHz, 25°, DMSO-d6) δ (ppm) 9.62 (d, J = 6.14 Hz, 1H), 8.79 (d, J = 7.73 Hz, 2H), 8.75–8.70 (m, 4H), 8.62–8.50 (m, 2H), 8.44 (d, J = 6.04 Hz, 1H), 8.29–8.18 (m, 2H), 8.11 (m, 2H), 8.01 (t, J = 7.68 Hz, 1H), 7.87 (dd, J = 7.16, 1.65 Hz, 1H), 7.58 (m, 2H), 7.43–7.29 (m, 3H), 6.85 (d, J = 7.72 Hz, 1H), 6.63 (s, 2H).
13C NMR (126 MHz, 25 °C, DMSO) δ (ppm) 156.04, 155.61, 154.26, 149.74, 149.52, 145.19, 138.40, 137.89, 135.56, 135.43, 134.05, 130.63, 130.40, 128.18, 127.89, 127.72, 126.50, 125.12, 122.74, 121.26, 120.95, 119.90, 60.85 (one signal was not detected).
ESI(+)-MS (m/z): calculated for [C31H24BN4O2]+: 495.20, found: 495.20. ATR-IR ν (cm−1): 3329br, 3055m, 1579m, 1366s, 764s.
1H NMR (300 MHz, 25 °C, DMSO-d6) δ (ppm) 9.86 (d, J = 5.89 Hz, 1H), 8.78 (d, J = 7.38 Hz, 2H), 8.76–8.68 (m, 4H), 8.57 (d, J = 9.61 Hz, 1H), 8.51 (d, J = 6.00 Hz, 1H), 8.30–8.21 (m, 2H), 8.17 (s, 2H), 8.10 (m, 2H), 8.00 (t, J = 7.73 Hz, 1H), 7.82–7.75 (m, 2H), 7.57 (m, 2H), 7.48–7.36 (m, 2H), 6.48 (s, 2H).
13C NMR (101 MHz, 25 °C, DMSO-d6) δ (ppm) 156.31, 155.69, 154.33, 150.01, 149.58, 148.07, 145.17, 138.11, 137.88, 135.63, 134.49, 133.07, 132.61, 130.72, 128.93, 128.30, 128.07, 125.14, 122.93, 121.28, 120.97, 120.04, 60.46, (one signal was not detected).
ESI(+)-MS (m/z): calculated for [C31H24BN4O2]+: 495.20, found: 495.2. ATR-IR ν (cm−1): 3309br, 3058m, 2960w, 1579m, 1368m, 1329m, 707m.
1H NMR (301 MHz, 25 °C, DMSO-d6) δ (ppm) 9.86 (d, J = 6.15 Hz, 1H), 8.78 (d, J = 7.92 Hz, 2H), 8.75–8.70 (m, 4H), 8.56–8.47 (m, 2H), 8.28–8.19 (m, 2H), 8.16 (s, 2H), 8.11 (m, 2H), 7.99 (t, J = 7.79 Hz 1H), 7.81 (d, J = 8.17 Hz, 2H), 7.57 (m, 2H), 7.37 (d, J = 8.04 Hz, 2H), 6.48 (s, 2H). 13C NMR (126 MHz, 25 °C, DMSO-d6) δ (ppm) 156.40, 155.62, 154.32, 150.04, 149.53, 145.20, 138.04, 137.83, 135.78, 135.53, 134.76, 130.63, 128.25, 128.11, 126.04, 125.09, 122.96, 121.24, 120.97, 120.28, 119.98, 60.28.
ESI(+)-MS (m/z): calculated for [C31H24BN4O2]+: 495.2, found: 495.2. ATR-IR ν (cm−1): 3313br, 3058m, 1567s, 1367m, 1334m, 1015m, 765m.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) system, obtaining white needle-shaped crystals. Yield: 94.4 mg (60%).
1, v/v) system, obtaining white needle-shaped crystals. Yield: 94.4 mg (60%).
1H NMR (300 MHz, 25 °C, DMSO-d6) δ (ppm) 9.90 (d, J = 6.07 Hz, 1H), 8.79 (d, J = 7.94 Hz, 2H), 8.74–8.72 (m, 2H), 8.72 (s, 2H), 8.59 (d, J = 9.04 Hz, 1H), 8.51 (d, J = 5.98 Hz, 1H), 8.27–8.23 (m, 2H), 8.11 (m, 2H), 8.00 (m, 1H), 7.58 (m, 2H), 7.46–7.37 (m, 5H), 6.51 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ (ppm) 156.37, 155.61, 154.30, 150.00, 149.52, 145.16, 138.01, 137.81, 135.55, 134.07, 130.64, 129.10, 128.73, 128.25, 128.10, 127.13, 125.08, 122.93, 121.22, 120.93, 119.96, 60.13.
MALDI(+)-TOF (m/z): calculated for [C31H23N4]+: 451.19, found: 452.351. ATR-IR ν (cm−1): 3381br, 3056m, 2954m, 1732s, 1580s, 1370m, 1236s, 745m.
General synthesis path for the Zn-complexes
The corresponding ligand 2–4 and 1.10 equiv. of the ZnBr2·2H2O salt were placed in a balloon flask with 5.0 mL of the mixture EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (1
CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The reaction mixture was stirred for 24 h at r.t. to give a white solid, (in all cases). The solvents were removed under reduced pressure, the precipitate was washed twice with cold EtOH (10 mL) and dried under vacuum for ∼2 h to give the bromide salt of the corresponding Zn(II) complex.
1, v/v). The reaction mixture was stirred for 24 h at r.t. to give a white solid, (in all cases). The solvents were removed under reduced pressure, the precipitate was washed twice with cold EtOH (10 mL) and dried under vacuum for ∼2 h to give the bromide salt of the corresponding Zn(II) complex.
1H NMR (300 MHz, 25 °C, DMSO-d6) δ (ppm) 9.76 (d, J = 5.07 Hz, 1H), 9.19 (s, 2H), 8.94 (s, 2H), 8.78 (d, J = 8.00 Hz, 2H), 8.66–8.62 (m, 2H), 8.55 (d, J = 5.75 Hz, 2H), 8.47 (d, J = 8.50 Hz, 1H), 8.37 (t, J = 7.58 Hz, 2H), 8.31 (t, J = 7.98 Hz, 1H), 8.04 (t, J = 7.67 Hz, 1H), 7.98–7.91 (m, 2H), 7.92 (d, J = 7.29 Hz, 1H), 7.45–7.35 (m, 2H), 6.81 (d, J = 7.66 Hz, 1H), 6.70 (s, 2H).
13C NMR (75 MHz, 25 °C, DMSO-d6) δ (ppm) 149.73, 149.53, 148.97, 148.80 (shoulder), 146.33, 140.90, 138.22, 137.60, 135.99, 135.63, 135.40, 130.93, 130.42, 130.22, 128.91, 127.94, 127.79, 127.53, 123.82, 123.00, 122.81, 119.80, 60.96. 11B NMR (96 MHz, 25 °C, CD3OD–DMSO-d6, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) δ (ppm) 28.62.
1, v/v) δ (ppm) 28.62.
ESI(−)-MS (m/z): calculated [C31H24BBr4N4O2Zn]−: 880.81, found: 880.5. ESI(+)-MS (m/z): calculated [C31H26BBr2N4O3Zn]+: 738.98, found: 739.0. ATR-IR ν (cm−1): 3324br, 3056m, 1597s, 1367s, 763s, 637m. Far IR ν (cm−1): 408m, 315w, 202br, 171s. Anal. calcd for C31H24BBr3N4O2Zn (800.46); C, 46.52; H, 3.02; N, 7.00. Found: C, 46.97; H, 3.36; N, 6.84.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution after 4 days at low temperature (∼4 °C). Yield: 64.0 mg, (92%).
1, v/v) solution after 4 days at low temperature (∼4 °C). Yield: 64.0 mg, (92%).
1H NMR (300 MHz, 25 °C, DMSO-d6) δ (ppm) 10.11 (d, J = 5.91 Hz, 1H), 9.18 (s, 2H), 8.93 (d, J = 4.34 Hz, 2H), 8.79–8.70 (m, 4H), 8.62 (d, J = 5.75 Hz, 1H), 8.47 (d, J = 8.59 Hz, 1H), 8.40–8.30 (m, 3H), 8.20 (s, 2H), 8.03 (t, J = 7.79 Hz, 1H), 7.99–7.89 (m, 2H), 7.83 (d, J = 7.29 Hz, 1H), 7.54 (d, J = 7.78 Hz, 1H), 7.43 (t, J = 7.50 Hz, 1H), 6.53 (s, 2H).
13C NMR (126 MHz, DMSO-d6) δ (ppm) 154.43, 150.09, 149.52, 149.02, 148.91, 146.37, 140.98, 137.88, 136.09, 135.37, 134.71, 133.11, 132.95, 131.05, 129.28, 129.07, 128.35, 127.86, 127.77, 123.90, 123.18, 122.84, 120.01, 60.71. 11B NMR (96 MHz, 25 °C, CD3OD–DMSO-d6, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) δ (ppm) 28.50.
1, v/v) δ (ppm) 28.50.
ESI(−)-MS (m/z): calculated [C31H24BBr4N4O2Zn]−: 880.81, found: 880.2. ESI(+)-MS (m/z): calculated [C31H24BBr2N4O2Zn]+: 720.97, found: 720.4. ATR-IR ν (cm−1): 3397br, 3064m, 2972w, 1599s, 1368s, 1333s, 1014m, 764s, 706s, 637m. Far IR ν (cm−1): 410w, 209br, 171m. Anal. calcd for the X-ray single crystal sample C33H34BBr3N4O5Zn (882.54); C, 44.91; H, 3.88; N, 6.35. Found: C, 44.96; H, 3.93; N, 6.32.
1H NMR (300 MHz, 25° C, DMSO-d6) δ (ppm) 10.09 (d, J = 4.87 Hz, 1H), 9.18 (s, 2H), 8.95 (s, 2H), 8.80–8.67 (m, 3H), 8.61 (d, J = 5.33 Hz, 1H), 8.48–8.28 (m, 4H), 8.15 (s, 2H), 8.06–7.93 (m, 3H), 7.83 (d, J = 7.96 Hz, 2H), 7.44 (d, J = 7.33 Hz, 2H), 6.54 (s, 2H). 13C NMR (75 MHz, 25 °C, DMSO-d6) δ (ppm) 154.51, 150.16, 149.49, 148.99, 148.85, 146.35, 140.94, 137.79, 136.04, 135.56, 134.80, 131.00, 129.02, 127.80, 126.36, 123.83, 123.14, 122.77, 121.41, 119.96, 115.86, 60.53. 11B NMR (96 MHz, 25 °C, CD3OD–DMSO-d6, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) δ 28.73.
1, v/v) δ 28.73.
ESI(−)-MS (m/z): calculated [C31H24BBr4N4O2Zn]−: 880.81, found: 880.5. ATR-IR ν (cm−1): 3346br, 3057m, 3032m, 1598s, 1366s, 1334s, 1013s, 792s, 636s. Far IR ν (cm−1): 410m, 209br, 197br, 177m. Anal. calcd for C31H24BBr3N4O2Zn (800.46); C, 46.52; H, 3.02; N, 7.00. Found: C, 46.82; H, 3.27; N, 6.95.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution after 7 days at r.t. Yield: 55.5 mg, (68%).
1, v/v) solution after 7 days at r.t. Yield: 55.5 mg, (68%).
1H NMR (301 MHz, 25 °C, DMSO-d6) δ (ppm) 9.90 (d, J = 6.07 Hz, 1H), 8.79 (d, J = 7.94 Hz, 2H), 8.74–8.72 (m, 2H), 8.72 (s, 2H), 8.59 (d, J = 9.04 Hz, 1H), 8.51 (d, J = 5.98 Hz, 1H), 8.27–8.23 (m, 2H), 8.11 (td, JHH = 7.75, 1.80 Hz, 2H), 8.00 (m, 1H), 7.58 (m, 2H), 7.46–7.37 (m, 5H), 6.51 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ (ppm) 156.37, 155.61, 154.30, 150.00, 149.52, 145.16, 138.01, 137.81, 135.55, 134.07, 130.64, 129.10, 128.73, 128.25, 128.10, 127.13, 125.08, 122.93, 121.22, 120.93, 119.96, 60.13.
ESI(−)-MS (m/z): calculated for negative scan [C31H23Br4N4Zn]−: 836.79, found: 836.5. ESI(+)-MS (m/z): calculated [C31H23Br2N4Zn]+: 676.9, found: 676.8. ATR-IR ν (cm−1): 3381br, 3056m, 2954m, 1732s, 1580s, 1370m, 1236s, 745m. Anal. calcd for C31H23Br3N4Zn (756.65); C, 49.21; H, 3.06; N, 7.40. Found: C, 49.53; H, 3.39; N, 7.24.
1H NMR (300 MHz, 25 °C, DMSO-d6) δ 4.09–4.00 (m, 2H), 3.96–3.87 (m, 1H), 3.81 (s, 1H), 3.77 (s, 1H), 3.67 (d, JHH = 4.21 Hz, 1H), 3.34 (s, 2H), 2.31 (m, 1H), 1.07 (dd, JHH = 20.97, 6.91 Hz, 6H). 13C NMR (76 MHz, D2O) δ (ppm) 172.20, 95.25, 70.12, 69.32, 69.04, 68.87, 63.86, 53.34, 29.02, 18.45, 16.90.
ESI-MS (m/z): calculated for [C11H22NO7]+: 280.14, found: 280.2. ATR-IR ν (cm−1): 3449m, 3417m, 3212w, 2966s, 2903w, 1569s, 1328s, 1084s, 762m, 562s. M.p. 155 °C (from the literature 154 °C).
1H NMR (300 MHz, 25 °C, D2O) δ 8.59 (s, 1H), 7.29 (s, 1H), 4.56 (m, 1H), 4.12–3.54 (m, 7H), 3.52–2.94 (m, 4H). 13C NMR (101 MHz, D2O) δ (ppm) 176.41, 133.74, 133.41, 130.25, 116.91, 69.43, 68.97, 63.62, 57.40, 54.23, 49.73, 27.62, 27.36, 16.74. HRMS-ESI+ (m/z): calculated for [C14H23N4O8]+: 375.15, found: 375.150916. ATR-IR ν (cm−1): 3258br, 3144br, 2879m, 1588s, 1391s, 1078m, 1028m, 623br, 532br.
Fluorescence experiments and lifetime measurements
Titration experiments were carried out by adding aliquots of stock solutions of the analyte to a neutral aqueous solution (10 mM of MOPS, pH 7.4) of the corresponding Zn-complex (20 μM). After the addition of analytes, the solution was equilibrated for 1 min at r.t. before recording the emission spectrum (λex = 330 nm) using a quartz cuvette. Lifetime measurements were made according to ref. 62 and the details are described in the ESI.†11B, 1H NMR titration experiments
NMR titration experiments were performed using a 300 MHz spectrometer. 11B NMR spectra were recorded at 96 MHz. Aliquots of concentrated stock solutions of FGH were added directly to the corresponding Zn-complex (10 mM) solution in CD3OD–DMSO-d6 (v/v, 4/1) at 25 °C using quartz NMR tubes. In the case of 1H NMR experiments, the spectra were recorded after the addition of aliquots of a concentrated solution of 3Zn to FHG solution in CD3OD, directly in an NMR tube.Crystallographic experiments
The relevant details of the crystals 3Zn and 5Zn, data collection and structure refinement can be found in Table S1.† These single-crystals were collected on a Bruker APEX II CCD diffractometer at 100 K, using Cu-Kα radiation (k = 1.54178 Å) from an Incoatec ImuS source and Helios optic monochromator.66 Suitable single-crystals were coated with hydrocarbon oil, picked up with a nylon loop, and mounted in the cold nitrogen stream of the diffractometer. The structures were solved by direct methods67 and refined by full-matrix least-squares on F2 using the ShelXle GUI.68,69The hydrogen atoms of the C–H and O–H bonds were placed in idealized positions, as it was not possible to find the hydrogen atoms from the O–H moiety in the map of residual density, and their position was refined with Uiso = aUeq, where a is 1.5 for –CH3 and –OH moieties and 1.2 for others.
The disorder moieties in complexes 3Zn and 5Zn were modeled using RIGU, SIMU, SAME and EADP instructions described in SHELXL.67 For complex 3Zn, the molecule of ethanol exhibits positional disorder with an 85/15 occupancy ratio between the two positions, while the bromide anion is modeled in 2 positions with 95/5 occupancy ratio. For complex 5Zn, the occupancy where three or more positions are modeled was obtained using free variables and the sump instruction and subsequently set to restrict to unity. The asymmetric unit consists of three independent molecules, in the first molecule the positional disorder of a benzene ring was modeled, in the second and third molecule the positional disorder of pyridine rings was modeled; in all cases two positions were modeled using the same free variable, with a 63/37 occupancy ratio between the two positions, the anion [ZnBr4]2− was modeled in three positions, with a 57/37/6 occupancy ratio respectively. The cavities where the solvents are housed are larger than the volume of the solvent molecule, so in the three cavities the solvent presents occupational disorder with an occupation relationship as follows: 78/22, 35/32/17/15, and 52/25/23 respectively.
The molecular graphics were prepared using Ortep3, POV-RAY and GIMP.70 Crystallographic data for the two crystal structures have been deposited with the Cambridge Crystallographic Data Centre, CCDC no. 2279289 and 2279290.† X-ray crystallographic data in the CIF format are available in the ESI.†
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank M. Sc. Eréndira García Rios, M. Sc. Lucero Mayra Ríos Ruiz, M. Sc. Lucía del Carmen Márquez Alonso, Dra. Beatriz Quiroz-García, Dra. Adriana Romo Pérez, Dra. Isabel Chávez Uribe and M. Sc. Elizabeth Huerta Salazar for technical assistance. We thank PAPIIT-UNAM 220023 and CONACYT PRONACES-160671 for financial support. M. K. S.-F. and A. O. V.-P. are grateful to CONAHCyT for scholarships 848759 and 868013. J. B. F. is grateful to DGTIC-UNAM for the use of the supercomputer Miztli.References
- M. Li, W. Zhu, F. Marken and T. D. James, Electrochemical sensing using boronic acids, Chem. Commun., 2015, 51, 14562–14573 RSC.
- S. Yazdanpanah, M. Rabiee, M. Tahriri, M. Abdolrahim and L. Tayebi, Glycated hemoglobin-detection methods based on electrochemical biosensors, TrAC, Trends Anal. Chem., 2015, 72, 53–67 CrossRef CAS.
- J. Y. Wang, T. C. Chou, L. C. Chen and K. C. Ho, Using poly(3-aminophenylboronic acid) thin film with binding-induced ion flux blocking for amperometric detection of hemoglobin A1c, Biosens. Bioelectron., 2014, 63, 317–324 CrossRef PubMed.
- J. I. Anzai, Recent progress in electrochemical biosensors based on phenylboronic acid and derivatives, Mater. Sci. Eng., C, 2016, 67, 737–746 CrossRef CAS PubMed.
- H. Shahbazmohammadi, S. Sardari and E. Omidinia, An amperometric biosensor for specific detection of glycated hemoglobin based on recombinant engineered fructosyl peptide oxidase, Int. J. Biol. Macromol., 2020, 142, 855–865 CrossRef CAS PubMed.
- J. S. Hansen, M. Ficker, J. F. Petersen, J. B. Christensen and T. Hoeg-Jensen, Ortho-Substituted fluorescent aryl monoboronic acid displays physiological binding of D-glucose, Tetrahedron Lett., 2013, 54, 1849–1852 CrossRef CAS.
- A. Stubelius, S. Lee and A. Almutairi, The Chemistry of Boronic Acids in Nanomaterials for Drug Delivery, Acc. Chem. Res., 2019, 52, 3108–3119 CrossRef CAS PubMed.
- P. Keil, H. B. Mortensen and C. Christophersen, Fructosilvaline. A simple model of the N-terminal residue of human haemoglobin A1c, Acta Chem. Scand., Ser. B, 1985, 39, 191–193 CrossRef CAS PubMed.
- S. Chawla and C. S. Pundir, An electrochemical biosensor for fructosyl valine for glycosylated hemoglobin detection based on core-shell magnetic bionanoparticles modified gold electrode, Biosens. Bioelectron., 2011, 26, 3438–3443 CrossRef CAS PubMed.
- C. Gerke, M. Buchholz, H. Müller, R. Meusinger, M. Grimmler and E. Metzmann, Direct glucosone-based synthesis and HILIC-ESI-MS/MS characterization of N-terminal fructosylated valine and valylhistidine for validation of enzymatic HbA1c assays in the diagnosis of diabetes mellitus, Anal. Bioanal. Chem., 2019, 411, 7967–7979 CrossRef CAS PubMed.
- S. Liu, J. Leng and T. C. Aquino, Development of Disposable Single-Use Biosensor for Fructosyl Valine and Glycated Hemoglobin A1c, J. Sens. Technol., 2019, 09, 45–53 CrossRef CAS.
- A. D. Troise, A. Fiore, G. Roviello, S. M. Monti and V. Fogliano, Simultaneous quantification of amino acids and Amadori products in foods through ion-pairing liquid chromatography-high-resolution mass spectrometry, Amino Acids, 2015, 47, 111–124 CrossRef CAS PubMed.
- A. Sakaguchi, S. Ferri, W. Tsugawa and K. Sode, Novel fluorescent sensing system for α-fructosyl amino acids based on engineered fructosyl amino acid binding protein, Biosens. Bioelectron., 2007, 22, 1933–1938 CrossRef CAS PubMed.
- M. Hatada, S. Saito, S. Yonehara, W. Tsugawa, R. Asano, K. Ikebukuro and K. Sode, Development of glycated peptide enzyme sensor based flow injection analysis system for haemoglobin A1c monitoring using quasi-direct electron transfer type engineered fructosyl peptide oxidase, Biosens. Bioelectron., 2021, 177, 112984 CrossRef CAS PubMed.
- C. S. Pundir and S. Chawla, Determination of glycated hemoglobin with special emphasis on biosensing methods, Anal. Biochem., 2014, 444, 47–56 CrossRef CAS PubMed.
- Z. Zhan, Y. Li, Y. Zhao, H. Zhang, Z. Wang, B. Fu and W. J. Li, A Review of Electrochemical Sensors for the Detection of Glycated Hemoglobin, Biosensors, 2022, 12, 1–23 Search PubMed.
- B. Wang and J. I. Anzai, Recent progress in electrochemical HbA1c sensors: A review, Materials, 2015, 8, 1187–1203 CrossRef PubMed.
- U. Jain, S. Gupta and N. Chauhan, Construction of an amperometric glycated hemoglobin biosensor based on Au–Pt bimetallic nanoparticles and poly (indole-5-carboxylic acid) modified Au electrode, Int. J. Biol. Macromol., 2017, 105, 549–555 CrossRef CAS PubMed.
- U. Jain, S. Gupta and N. Chauhan, Detection of glycated hemoglobin with voltammetric sensing amplified by 3D-structured nanocomposites, Int. J. Biol. Macromol., 2017, 101, 896–903 CrossRef CAS PubMed.
- S. Chawla and C. S. Pundir, An amperometric hemoglobin A1c biosensor based on immobilization of fructosyl amino acid oxidase onto zinc oxide nanoparticles-polypyrrole film, Anal. Biochem., 2012, 430, 156–162 CrossRef CAS PubMed.
- J. Y. Park, B. Y. Chang, H. Nam and S. M. Park, Selective electrochemical sensing of glycated hemoglobin (HbA1c) on thiophene-3-boronic acid self-assembled monolayer covered gold electrodes, Anal. Chem., 2008, 80, 8035–8044 CrossRef CAS PubMed.
- H. C. Chien and T. C. Chou, A Nonenzymatic Amperometric Method for Fructosyl-Valine Sensing Using Ferroceneboronic Acid, Electroanalysis, 2011, 23, 402–408 CrossRef CAS.
- S. Liu, U. Wollenberger, M. Katterle and F. W. Scheller, Ferroceneboronic acid-based amperometric biosensor for glycated hemoglobin, Sens. Actuators, B, 2006, 113, 623–629 CrossRef CAS.
- R. Rajkumar, A. Warsinke, H. Möhwald, F. W. Scheller and M. Katterle, Development of fructosyl valine binding polymers by covalent imprinting, Biosens. Bioelectron., 2007, 22, 3318–3325 CrossRef CAS PubMed.
- G. Springsteen and B. Wang, A detailed examination of boronic acid-diol complexation, Tetrahedron, 2002, 58, 5291–5300 CrossRef CAS.
- J. Zamora-Moreno, M. K. Salomón-Flores, J. Valdes-García, C. Pinzón-Vanegas, D. Martínez-Otero, J. Barroso-Flores, R. Villamil-Ramos, M. A. Romero-Solano and A. Dorazco-González, Water-soluble fluorescent chemosensor for sorbitol based on a dicationic diboronic receptor. Crystal structure and spectroscopic studies, RSC Adv., 2023, 13, 32185–32198 RSC.
- L. R. Ortega-Valdovinos, J. Valdes-García, I. J. Bazany-Rodríguez, J. C. Lugo-González, A. Dorazco-González and A. K. Yatsimirsky, Anion recognition by anthracene appended ortho-aminomethylphenylboronic acid: a new PET-based sensing mechanism, New J. Chem., 2021, 45, 15618–15628 RSC.
- J. A. Peters, Interactions between boric acid derivatives and saccharides in aqueous media: Structures and stabilities of resulting esters, Coord. Chem. Rev., 2014, 268, 1–22 CrossRef CAS.
- Z. Guo, I. Shin and J. Yoon, Recognition and sensing of various species using boronic acid derivatives, Chem. Commun., 2012, 48, 5956–5967 RSC.
- T. Pradhan, H. S. Jung, J. H. Jang, T. W. Kim, C. Kang and J. S. Kim, Chemical sensing of neurotransmitters, Chem. Soc. Rev., 2014, 43, 4684–4713 RSC.
- C. W. Gray and T. A. Houston, Boronic acid receptors for α-hydroxycarboxylates: High affinity of Shinkai's glucose receptor for tartrate, J. Org. Chem., 2002, 67, 5426–5428 CrossRef CAS PubMed.
- J. Valdes-García, J. Zamora-Moreno, M. K. Salomón-Flores, D. Martínez-Otero, J. Barroso-Flores, A. K. Yatsimirsky, I. J. Bazany-Rodríguez and A. Dorazco-González, Fluorescence Sensing of Monosaccharides by Bis-boronic Acids Derived from Quinolinium Dicarboxamides: Structural and Spectroscopic Studies, J. Org. Chem., 2023, 88, 2174–2189 CrossRef PubMed.
- Y. Kubo, F. Marken, K. Sakurai, J. Zhao and T. D. James, Exploiting the Reversible Covalent Bonding of Boronic Acids: Recognition, Sensing, and Assembly, Acc. Chem. Res., 2013, 46, 312–326 CrossRef PubMed.
- W. L. A. Brooks, C. C. Deng and B. S. Sumerlin, Structure-Reactivity Relationships in Boronic Acid-Diol Complexation, ACS Omega, 2018, 3, 17863–17870 CrossRef CAS PubMed.
- J. Yan, G. Springsteen, S. Deeter and B. Wang, The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols-it is not as simple as it appears, Tetrahedron, 2004, 60, 11205–11209 CrossRef CAS.
- X. Zhang, G. Liu, Z. Ning and G. Xing, Boronic acid-based chemical sensors for saccharides, Carbohydr. Res., 2017, 452, 129–148 CrossRef CAS PubMed.
- A. Chaicham, S. Sahasithiwat, T. Tuntulani and B. Tomapatanaget, Highly effective discrimination of catecholamine derivatives via FRET-on/off processes induced by the intermolecular assembly with two fluorescence sensors, Chem. Commun., 2013, 49, 9287–9289 RSC.
- A. M. Agafontsev, A. Ravi, T. A. Shumilova, A. S. Oshchepkov and E. A. Kataev, Molecular Receptors for Recognition and Sensing of Nucleotides, Chem. – Eur. J., 2019, 25, 2684–2694 CrossRef CAS PubMed.
- H. Otsuka, E. Uchimura, H. Koshino, T. Okano and K. Kataoka, Anomalous Binding Profile of Phenylboronic Acid with N-Acetylneuraminic Acid (Neu5Ac) in Aqueous Solution with Varying pH, J. Am. Chem. Soc., 2010, 11, 3493–3502 Search PubMed.
- C. R. Cooper and T. D. James, Selective D-glucosamine hydrochloride fluorescence signalling based on ammonium cation and diol recognition, Chem. Commun., 1997, 15, 1419–1420 RSC.
- A. E. Hargrove, R. N. Reyes, I. Riddington, E. V. Anslyn and J. L. Sessler, Boronic acid porphyrin receptor for ginsenoside sensing, Org. Lett., 2010, 12, 4804–4807 CrossRef CAS PubMed.
- K. S. Ahn, J. H. Lee, J. M. Park, H. N. Choi and W. Y. Lee, Luminol chemiluminescence biosensor for glycated hemoglobin (HbA1c) in human blood samples, Biosens. Bioelectron., 2016, 75, 82–87 CrossRef CAS PubMed.
- X. Huang, Y. Han, J. Li, M. Tang and G. Qing, Sensitive and specific detection of saccharide species based on fluorescence: update from 2016, Anal. Bioanal. Chem., 2023, 415, 4061–4077 CrossRef CAS PubMed.
- B. Gyurcsik, T. Gajdab, L. Nagyb, K. Burger, A. Rockenbauer and L. Korecz, Proton, copper(II) and nickel(II) complexes of some amadori rearrangement products of D-glucose and amino acids, Inorg. Chim. Acta, 1993, 214, 57–66 CrossRef CAS.
- Y. Zhou and J. Yoon, Recent progress in fluorescent and colorimetric chemosensors for detection of amino acids, Chem. Soc. Rev., 2012, 41, 52–67 RSC.
- H. Aït-Haddou, S. L. Wiskur, V. M. Lynch and E. V. Anslyn, Achieving large color changes in response to the presence of amino acids: A molecular sensing ensemble with selectivity for aspartate, J. Am. Chem. Soc., 2001, 123, 11296–11297 CrossRef PubMed.
- I. J. Bazany-Rodríguez, M. K. Salomón-Flores, J. M. Bautista-Renedo, N. González-Rivas and A. Dorazco-González, Chemosensing of Guanosine Triphosphate Based on a Fluorescent Dinuclear Zn(II)-Dipicolylamine Complex in Water, Inorg. Chem., 2020, 59, 7739–7751 CrossRef PubMed.
- B. Watanabe, A. Ichiyanagi, K. Hirokawa, K. Gomi, T. Nakatsu and H. Kato, Synthesis and inhibitory activity of substrate-analog fructosyl peptide oxidase inhibitors, Bioorg. Med. Chem. Lett., 2015, 25, 3910–3913 CrossRef CAS PubMed.
- S. Gamsey, N. A. Baxter, Z. Sharrett, D. B. Cordes, M. M. Olmstead, R. A. Wessling and B. Singaram, The effect of boronic acid-positioning in an optical glucose-sensing ensemble, Tetrahedron, 2006, 62, 6321–6331 CrossRef CAS.
- I. J. Bazany-Rodríguez, M. K. Salomón-Flores, A. O. Viviano-Posadas, M. A. García-Eleno, J. Barroso-Flores, D. Martínez-Otero and A. Dorazco-González, Chemosensing of neurotransmitters with selectivity and naked eye detection of L-DOPA based on fluorescent Zn(II)-terpyridine bearing boronic acid complexes, Dalton Trans., 2021, 50, 4255–4269 RSC.
- R. Badugu, J. R. Lakowicz and C. D. Geddes, Boronic acid fluorescent sensors for monosaccharide signaling based on the 6-methoxyquinolinium heterocyclic nucleus: Progress toward noninvasive and continuous glucose monitoring, Bioorg. Med. Chem., 2005, 13, 113–119 CrossRef CAS PubMed.
- I. J. Bazany-Rodríguez, D. Martínez-Otero, J. Barroso-Flores, A. K. Yatsimirsky and A. Dorazco-González, Sensitive water-soluble fluorescent chemosensor for chloride based on a bisquinolinium pyridine-dicarboxamide compound, Sens. Actuators, B, 2015, 221, 1348–1355 CrossRef.
- W. F. Jager, T. S. Hammink, O. van den Berg and F. C. Grozema, Highly sensitive water-soluble fluorescent pH sensors based on the 7-amino-1-methylquinolinium chromophore., J. Org. Chem., 2010, 75, 2169–2178 CrossRef CAS PubMed.
- R. Hosseinzadeh, M. Mohadjerani, M. Pooryousef, A. Eslami and S. Emami, A new boronic acid fluorescent sensor based on fluorene for monosaccharides at physiological pH, Spectrochim. Acta, Part A, 2015, 144, 53–60 CrossRef CAS PubMed.
- R. Badugu, J. R. Lakowicz and C. D. Geddes, Fluorescence sensors for monosaccharides based on the 6-methylquinolinium nucleus and boronic acid moiety: Potential application to ophthalmic diagnostics, Talanta, 2005, 65, 762–768 CrossRef CAS PubMed.
- X. Chen, S. L. Chew, F. M. Kerton and N. Yan, Direct conversion of chitin into a N-containing furan derivative, Green Chem., 2014, 16, 2204–2212 RSC.
- P. Barraud, M. Schubert and F. H. T. Allain, A strong 13C chemical shift signature provides the coordination mode of histidines in zinc-binding proteins, J. Biomol. NMR, 2012, 53, 93–101 CrossRef CAS PubMed.
- G. Fang, H. Wang, Z. Bian, J. Sun, A. Liu, H. Fang, B. Liu, Q. Yao and Z. Wu, Recent development of boronic acid-based fluorescent sensors, RSC Adv., 2018, 8, 29400–29427 RSC.
- M. Yamamoto, M. Takeuchi and S. Shinkai, Molecular design of a PET-based chemosensor for uronic acids and sialic acids utilizing a cooperative action of boronic acid and metal chelate, Tetrahedron, 1998, 54, 3125–3140 CrossRef CAS.
- J. Du, Z. Huang, X.-Q. Yu and L. Pu, Highly selective fluorescent recognition of histidine by a crown ether–terpyridine–Zn(II) sensor, Chem. Commun., 2013, 49, 5399–5401 RSC.
- M. Takeuchi, M. Yamamoto and S. Shinkai, Fluorescent sensing of uronic acids based on a cooperative action of boronic acid and metal chelate, Chem. Commun., 1997, 18, 1731–1732 RSC.
- M. K. Salomón-Flores, C. L. Hernández-Juárez, I. J. Bazany-Rodríguez, J. Barroso-Flores, D. Martínez-Otero, R. López-Arteaga, J. Valdés-Martínez and A. Dorazco-González, Efficient fluorescent chemosensing of iodide based on a cationic meso-tetraarylporphyrin in pure water, Sens. Actuators, B, 2019, 281, 462–470 CrossRef.
- Q. Zhang, X. Tian, Z. Hu, C. Brommesson, J. Wu, H. Zhou, S. Li, J. Yang, Z. Sun, Y. Tian and K. Uvdal, A series of Zn(II) terpyridine complexes with enhanced two-photon-excited fluorescence for in vitro and in vivo bioimaging, J. Mater. Chem. B, 2015, 3, 7213–7221 RSC.
- G. Springsteen and B. Wang, Alizarin Red S. as a general optical reporter for studying the binding of boronic acids with carbohydrates, Chem. Commun., 2001, 1608–1609 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gauss View 5.0, Gaussian, Inc., Wallingford, USA, 2016 Search PubMed.
- APEX 2 Softw. Suite, Bruker AXS Inc., Madison, Wisconsin, USA, 2004 Search PubMed.
- G. M. Sheldrick, SHELXT – Integrated space-group and crystal-structure determination, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3–8 CrossRef PubMed.
- C. B. Hübschle, G. M. Sheldrick and B. Dittrich, ShelXle: A Qt graphical user interface for SHELXL, J. Appl. Crystallogr., 2011, 44, 1281–1284 CrossRef PubMed.
- G. M. Sheldrick, SHELXL-97, Progr. Cryst. Struct. Refinement, Univ. Göttingen Search PubMed.
- L. J. Farrugia, Ortep-3 for Windows, Ortep-3 Wind, https://www.chem.gla.ac.uk/~louis/software/ortep/ Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General information, X-ray crystallographic data, 1H, 13C, 11B NMR spectra for all compounds and UV-Vis and fluorescence titration experiments. CCDC 2279289 and 2279290. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt00260a |
| This journal is © The Royal Society of Chemistry 2024 |