Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Lithium, sodium and potassium enolate aggregates and monomers: syntheses and structures†‡
Nathan
Davison
,
Jack M.
Hemingway
 *,
Paul G.
Waddell
and
Erli
Lu
*,
Paul G.
Waddell
and
Erli
Lu
 *
*
Chemistry-School of Natural and Environmental Sciences, Newcastle University, Newcastle upon Tyne, NE1 7RU, UK. E-mail: erli.lu@newcastle.ac.uk; jack.hemingway@newcastle.ac.uk; Web: https://www.erlilulab.org
First published on 12th February 2024
Abstract
In this Article, we report the syntheses and comparative structural studies of lithium, sodium, and potassium anthracen-9-yl enolates, as their aggregates (Li, Na: hexamer; K: tetramer) and ligand-stabilized monomers (for Li and Na). The monomers add new members to the rare collection of group-1 metal monomeric enolates. Moreover, the series covers different group-1 metal cations (Li+, Na+ and K+) and aggregate sizes, allowing comparative structural studies to elucidate how the metal identity and aggregate size influence the enolate structure.
Introduction
Enolates derive from α-deprotonation of organocarbonyls, such as ketones and aldehydes. They are widespread reaction intermediates in organic chemistry, and enable several textbook organic reactions, such as aldol addition.1 The most commonly used approach to generate enolates is the deprotonation of organocarbonyls using organo-alkali metal reagents, such as organolithium. The resultant alkali metal enolates are subsequently subjected to further transformations. It is quite understandable that the alkali metal enolate structures play an underpinning role in their following reactions. But the structural studies of isolated alkali metal enolates are not as well-established as one may expect: the state-of-the-art is covered in the following paragraph. Given the diverse scope of organocarbonyls (e.g., ketones, aldehydes, esters, amides and so on), their derivative enolates feature a high level of structural diversity. Beyond the hydrocarbon enolates derived from ketones and aldehydes, heteroatom-substituted enolates, such as the ones derived from esters,2 amides3 or α-hetero organocarbonyls (e.g., α-phosphino esters4), were also reported.A key factor to influence the coordination complex structure is metal identity. For the enolate complexes, among the most common alkali metal cations Li+, Na+ and K+, lithium enolates are the most studied. Since the 1980s, the groups of Williard,5 Mulvey,6 Reich,7 Collum,8 and Hevia,9 among others,10 reported a number of isolated and structurally characterized lithium enolate complexes, most of which are aggregates. In comparison with the relatively well-documented lithium enolates, sodium5e,11 and potassium5e,12 enolates are far less studied. A comparative structural study of Li, Na and K enolates within the same system, i.e., the same enolate substitutions and the ligand, would help to unravel the metal identity's structural directing role. In 1986, Williard and Carpenter reported such a remarkable comparative study on a series of Li, Na and K enolates of pinacolone (tert-butyl methyl ketone).13 In these cases, the Li and K pinacolone enolates were found to be hexamers (Li: no solvent coordination; K: one coordinated THF per K), while the Na enolate is a tetramer (no solvent coordination). The aggregates remain in solution as proven by NMR studies.13 Despite clearly revealing “subtle structural differences” (according to the authors) induced by the metal identity, the work from Williard and Carpenter did not include another crucial comparison dimension: aggregate size.
Aggregate size is one of the most important structural factors in alkali metal chemistry, playing underpinning roles in the structure–reactivity relationship.14 In the enolate chemistry specifically, as early as in 1981, Seebach and co-workers examined the mechanistic implications of the tetrameric cubic structure of Li enolates in addition reactions.15,16 In 2013, Cossío and co-workers conducted computational studies (DFT and molecular dynamics) on the relationship between alkali metal enolates aggregate sizes and their activity (reaction rate) in the aldol addition,17 where the monomers were found to be vastly superior to the dimers and tetramers. Out of the in silico chemical space, reported examples of crystallographically characterized alkali metal enolate monomers are rather limited. In 2009 and 2010, Reich and co-workers systematically studied the SCXRD and solution-state structures and the structural dynamics of several lithium enolates, with the presence of a series of ligands, including PMDTA, Me3TACN (also known as TMTAN), HMPA, and cryptand [2.1.1].7 The tridentate cyclic Me3TACN ligand was found to be able to stabilize a collection of lithium enolate monomers7 (Fig. 1A). Moving onto the heavier alkali metals, there is no sodium enolate monomer in literature, albeit a relevant sodium ethylacetoacetate monomer was reported as stabilized by 15-crown-5.18 A few monomers of potassium enolates and α-heteroatom substituted enolates were reported in 1993![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1994
4 and 1994![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12b,19 (Fig. 1B–F), including two separated ion pairs (Fig. 1C & E). Due to the scarcity of the alkali metal enolate monomers, an in-depth understanding of the structural differences between their aggregates and monomers remains an open question.
12b,19 (Fig. 1B–F), including two separated ion pairs (Fig. 1C & E). Due to the scarcity of the alkali metal enolate monomers, an in-depth understanding of the structural differences between their aggregates and monomers remains an open question.
Since the 2020s, our group has focused on unravelling the structural and reactivity directing roles of the two key factors in alkali metal chemistry: (1) aggregate size;20,21 (2) metal identity.22 Herein, we bring the effort into Li, Na and K enolate chemistry by reporting the synthesis and characterisation of a series of Li, Na, K anthracen-9-yl enolates as their aggregates and ligand-stabilised monomers. We found that without external ligand, the Li and Na anthracen-9-yl enolates exist as hexamers but with different coordination geometries, while the K enolate exists as a tetramer. Employing the Me6Tren ligand, we isolated and characterized two rare examples of Li and Na enolate monomers. Based on the complexes, the enolates structural trends are studied on two dimensions of comparison: (1) metal identity; (2) aggregate size. Our findings are elaborated in the following sections.
Results and discussion
Synthesis of the Li, Na and K enolates
As a part of our systematic studies of the Brønsted basicity and nucleophilicity of organo-alkali metal reagents,20–22 9-acetylanthracene (1) was treated with lithium, sodium and potassium alkyl complexes [MCH2SiMe3]n (M = Li, 2-Li;23 M = Na, 2-Na;24 M = K, 2-K25) in d6-benzene (for the NMR-scale reactions) or benzene (for the scale-up reactions) (Scheme 1). 9-Acetylanthracene was chosen as the substrate for its superior steric protection over the products, as well as the crystallinity induced by the anthracen-9-yl functional group. All the reactions occurred as deprotonation at the methyl position exclusively – we did not observe the possible nucleophilic addition side reaction towards the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. The corresponding Li, Na and K enolates, [M{OC(
O bond. The corresponding Li, Na and K enolates, [M{OC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(C14H9)}]n (3-Li/Na/K, n = 6 for Li and Na, n = 4 for K), were isolated in 73–97% crystalline yields. Among them, 3-Li and 3-Na crystallize into hexamers, while 3-K crystallizes as a tetramer. The 1H and 7Li (for 3-Li) NMR spectra of 3-Li/Na/K are reported in the ESI,‡ from the in situ1H NMR monitoring of the corresponding NMR-scale reaction. Upon formation, 3-Li/Na/K crystallise from the C6D6/C6H6 solutions rapidly, and can not be re-dissolved. For this reason, their 13C and 1H DOSY NMR spectra are absent.
CH2)(C14H9)}]n (3-Li/Na/K, n = 6 for Li and Na, n = 4 for K), were isolated in 73–97% crystalline yields. Among them, 3-Li and 3-Na crystallize into hexamers, while 3-K crystallizes as a tetramer. The 1H and 7Li (for 3-Li) NMR spectra of 3-Li/Na/K are reported in the ESI,‡ from the in situ1H NMR monitoring of the corresponding NMR-scale reaction. Upon formation, 3-Li/Na/K crystallise from the C6D6/C6H6 solutions rapidly, and can not be re-dissolved. For this reason, their 13C and 1H DOSY NMR spectra are absent.
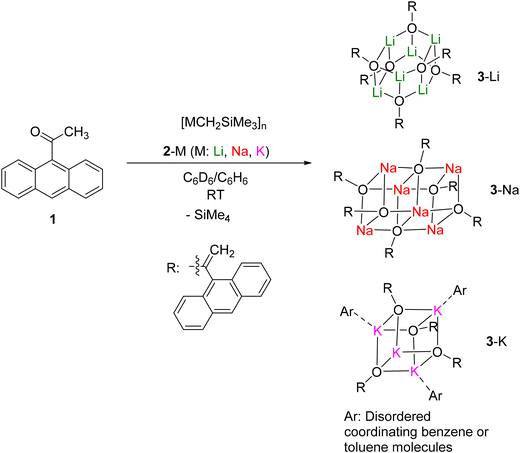 | ||
| Scheme 1 Syntheses of Li, Na and K enolate clusters, 3-Li (hexamer), 3-Na (hexamer) and 3-K (tetramer). | ||
Following the successful syntheses of the enolate clusters 3-Li/Na/K, reactions between 1 and the monomeric organo-alkali metal complexes, [M(CH2SiMe3)(Me6Tren)] (M = Li, 4-Li;26 M = Na, 4-Na22,27) and [K(CH2Ph)(Me6Tren)] (5-K28), were tested (Scheme 2). The 4-Li/Na reactions yielded corresponding Me6Tren-coordinated Li/Na enolate monomers, 6-Li and 6-Na, respectively. In comparison, reaction between a benzyl potassium monomer 5-K28 and 1 produced the potassium enolate tetramer 3-K, while the Me6Tren ligand dissociated and was observed in the 1H NMR spectroscopy as coordination-free. We attribute the Me6Tren ligand dissociation in the potassium reaction to two factors: (1) the relatively low affinity between the Me6Tren ligand and K+ (comparing with Li+/Na+): the low affinity between Me6Tren and K+ allowed us to realize the first alkali metal cations selective reduction between K+ and Li+;29 (2) The K enolate tetramer 3-K structure features much more significant cation–arene interactions (vide infra, Fig. 4) comparing with the structures of 3-Li (Fig. 2) and 3-Na (Fig. 3). The alkali metal cation–arene interaction is known to be more pronounced for heavier alkali metal cations.21b,28,30
Single-crystal X-ray diffraction (SCXRD) structures of the Li, Na and K enolates
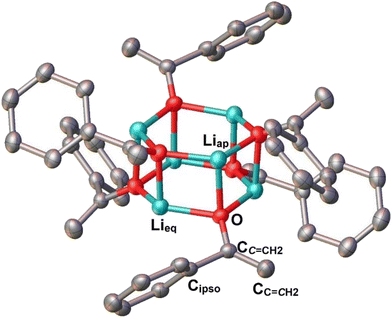
The hexameric (3-Li, 3-Na), tetrameric (3-K) and monomeric (6-Li, 6-Na) structures were confirmed by the SCXRD studies: their solid-state molecular structures are presented in Fig. 2–6. In the following section, we will focus on the structural influences induced by two factors: (i) the metal identity; (ii) the aggregate size. In the aggregate clusters 3-Li/Na/K, we did not observe pronounced π–π stacking31 between the anthracenyl substitutes, or with the coordinated arene solvents. Hence, the π–π stacking is not a major structural contributor and therefore, we are confident that the trends summarized in the following section are not only limited to the anthracen-9-yl enolate, but also extrapolatable to other enolates.The influence of the metal's identity is obvious for enolate clusters 3-Li/Na/K, especially in their aggregate sizes and geometries. For the smallest Li+, the enolate hexamer 3-Li features a Li6O6 hexagonal prismatic core (Fig. 2), which is similar to a Li pinacolone enolate hexamer reported by Williard and co-workers.5h In 3-Li, each Li center coordinates with three O sites and features a coordination number of three. Upon moving from Li+ to Na+, one may expect the metal's coordination number to increase with the increase of metal's ionic radius. Indeed, 3-Na features a three-deck square prismatic structure (Fig. 3), with the inner deck Na+ centers featuring a coordination number of four, while the outer decks Na+ centers’ coordination number is three. While 3-Li and 3-Na can also be considered as two stacked layers of M3O3 motif, it is interesting to compare the M3O3 layer structures (Scheme 3). The larger Na+ cation leads to longer Na–O bond, which “tethers” the M and O in the middle of the M3O3 unit (Scheme 3). From another perspective, 3-Li is a “ring-laddering” loop, while 3-Na features a “ring-stacking” structure (Scheme 3). It worth mentioning that the ring-laddering and ring-stacking are ubiquitous structural features in group-1 metal coordination chemistry and have been well documented.32 Reviewing the literature33 suggests that the determining factor is metal identity, or more specifically, the metal ionic radius, as we observed herein; though influences from ligand atom (e.g., C vs. N vs. O) and ligand steric profile should also be considered.
 | ||
| Scheme 3 The M3O3 unit in 3-Li and 3-Na, and the ring-laddering (3-Li) and ring-stacking (3-Na) representation of the complexes. | ||
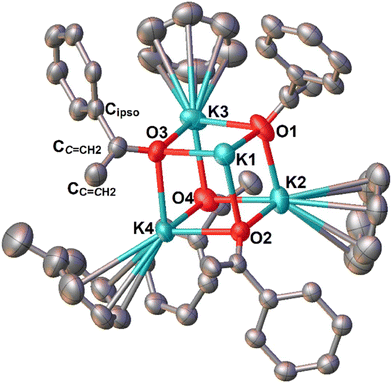
A further increase in the ionic radius going from Na+ to K+ leads into a distorted cubic K4O4 core in 3-K (Fig. 4), which features significant cation–π interactions with the arene solvent molecules. It is interesting to note that both benzene and toluene solvent molecules coordinate to the K centers (albeit disordered), and only three out of the four K centers are coordinated by the arene solvent molecules. This solvent molecule coordination discrimination, i.e., not all the K centres are coordinated, results in an asymmetric K4O4 core, and could likely be a result of steric crowding. The cation–π interaction is much more pronounced in 3-K than in 3-Li/Na (not at all for 3-Li, very little Na-anthracenyl interactions in 3-Na), which can be attributed to the stronger π-affinity of K+.21b,28 Compared with the pronounced metal identity structural influence in the aggregate size and geometry, the anionic enolate ligand structure, [OC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(C14H9)]−, in 3-Li/Na/K, is largely unaffected by changes of the metal identity and the resultant changes of aggregate size. But we did observe non-negligible differences of the anionic enolate ligand structure between the clusters 3-Li/Na and the monomers 6-Li/Na, which will be elaborated in the following paragraphs.
CH2)(C14H9)]−, in 3-Li/Na/K, is largely unaffected by changes of the metal identity and the resultant changes of aggregate size. But we did observe non-negligible differences of the anionic enolate ligand structure between the clusters 3-Li/Na and the monomers 6-Li/Na, which will be elaborated in the following paragraphs.
The enolate monomers 6-Li/Na share a similar five-coordinate core structure (Fig. 5), where the Me6Tren ligand's four nitrogen atoms and the oxygen atom of the enolate ligand furnish the inner coordination sphere. The Na–N/O bonds in 6-Na are longer than the corresponding Li–N/O bonds in 6-Li, reflecting sodium's larger ionic radius. The geometries of enolate ligand in 6-Li/Na are very similar, which is also reflected by the density functional theory (DFT) charge distribution studies. Examining the natural bonding orbital (NBO) atomic charges for the modelled monomers 6′-Li/Na indicates little differences between the Li and Na complexes (Fig. 6). For both 6′-Li and 6′-Na, a positive charge of approximately 0.9 is located on the metal cation, and a countering negative charge is located on the oxygen atom.
However, despite being largely immune to the change of metal identity, the enolate ligand structure can be influenced by the aggregate size. By comparing the enolate units in the hexamers (3-Li/Na) and monomers (6-Li/Na), we noted that the C–O bonds are significantly shorter in the monomers (monomers 1.27–1.28 Å vs. hexamers 1.32–1.35 Å) (Scheme 4). While the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–Ar bonds appear as slightly longer in the monomers, these elongation (0.01–0.02 Å) are too small to be considered as pronounced. The shortening of C–O bond in the monomers could be attributed to a weaker stabilization of the negative charge on the oxygen cf. the hexamers: In the hexamers, each anionic oxygen is coordinated by three (3-Li) or four (3-Na) metal cations, but the number is one in the monomers.
C and C–Ar bonds appear as slightly longer in the monomers, these elongation (0.01–0.02 Å) are too small to be considered as pronounced. The shortening of C–O bond in the monomers could be attributed to a weaker stabilization of the negative charge on the oxygen cf. the hexamers: In the hexamers, each anionic oxygen is coordinated by three (3-Li) or four (3-Na) metal cations, but the number is one in the monomers.
To further understand whether there is a thermodynamic preference between the liner and the bent M–O–C angles, or if it is largely kinetic-driven (i.e., depends on the steric congestion), we conducted potential energy surface scans. The methodology has been well-established to probe the energetic preference for cis or trans conformations in coordination chemistry.34 The calculations are based on the hypothetical model of the monomeric M–O–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)Ar (Ar: anthracenyl), which removes all the ligand and secondary bridging units, minimizing the steric influence. The M–O–C angle was initially set to 90° leaving the remainder of the M–O–C(
CH2)Ar (Ar: anthracenyl), which removes all the ligand and secondary bridging units, minimizing the steric influence. The M–O–C angle was initially set to 90° leaving the remainder of the M–O–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)Ar moiety unchanged from the optimized structure and was then sequentially increased by 5° until equal to 270°. All geometric parameters were relaxed to loose convergence criteria at each angle step except the M–O–C angle itself which was fixed. It was found that the minimum energy of M–O–C(
CH2)Ar moiety unchanged from the optimized structure and was then sequentially increased by 5° until equal to 270°. All geometric parameters were relaxed to loose convergence criteria at each angle step except the M–O–C angle itself which was fixed. It was found that the minimum energy of M–O–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)Ar was at 205° regardless of the metal identity, with the near linear structures being favored over the more bent structures. The resulting plots are shown in Fig. 7. We also observed that the 90° angle (metal cation pointing towards the C
CH2)Ar was at 205° regardless of the metal identity, with the near linear structures being favored over the more bent structures. The resulting plots are shown in Fig. 7. We also observed that the 90° angle (metal cation pointing towards the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 unit) is preferred over the 270° angle (metal cation pointing towards the aryl), which could be a result of steric repulsion between the bulky anthracenyl and the metal cation.
CH2 unit) is preferred over the 270° angle (metal cation pointing towards the aryl), which could be a result of steric repulsion between the bulky anthracenyl and the metal cation.
 | ||
Fig. 7 Potential energy surface scans of the M–O–C angle M–O–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)Ar (M = Li – left and M = Na – right). CH2)Ar (M = Li – left and M = Na – right). | ||
Conclusion
Herein, we report the syntheses and structures of a series of Li, Na and K enolates. Without any external ligand, the enolates aggregate into hexamers (Li and Na) and tetramer (K), and their geometries are heavily influenced by the metal identity, as well as the particularly pronounced metal cation–arene interactions in the potassium case. With the presence of the Me6Tren ligand, the Li and Na enolates exist as monomers with similar structures. The coordination of the Me6Tren ligand to Li+ or Na+ is the key to form the monomeric structures. In comparison, the Me6Tren ligand failed to coordinate to K+, hence the corresponding K enolate monomer is not available.This work adds five new members to the alkali metal enolate family. In particular, the two monomers 6-Li and 6-Na are the newest examples of the scarce collection of alkali metal enolate monomers. The aggregates, 3-Li/Na/K, exhibit a clear structural trend dictated by the metal identity, specifically, the metal ionic radius. Comparisons between the monomers 6-Li/Na and their corresponding hexamers 3-Li/Na unveils an interesting influence of the aggregate size towards the carbon–oxygen bond length in the enolate ligand. It would be interesting to see how the structural observations in this work would be translated into the enolate reactivity – more investigations are underway and will be reported in due course.
Author contributions
E. L. and N. D. conceived and conceptualised the studies. N. D. conducted the syntheses and experimental characterisations under E. L.'s supervision. J. M. H. designed and conducted the computational studies under the guidance of E. L. P. G. W. collected and refined and the single-crystal X-ray diffraction data. E. L. wrote the manuscript with input from all the authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Chemistry Technical Support Team (Drs Laura McCorkindale and Amy Roberts, Mr Niall Straughan) at Newcastle University for supporting our research. E. L., N. D. and J. M. H. thank the Newcastle University Academic Track (NUAcT) Fellowship (E. L. and N. D.) and the Leverhulme Trust (RPG-2022-231) for financial support.References
- For example, a lithium “aldolate”, i.e., the aldol addition intermediate, was isolated and characterized in 1985: P. G. Williard and J. M. Salvino, X-Ray Crystal Structure of a Lithium Aldolate – A Tetrameric Aggregate, Tetrahedron Lett., 1985, 26, 3931–3934 CrossRef CAS.
- D. Seebach, R. Amstutz, T. Laube, B. Schweizer and J. D. Dunitz, Structures of Three Lithium Ester Enolates by X-ray Diffraction: Derivation of Reaction Path for Cleavage into Ketene and Alcoholate, J. Am. Chem. Soc., 1985, 107, 5403–5409 CrossRef CAS.
- W. Bauer, T. Laube and D. Seebach, Crystal and molecular structure of a THF-solvated lithium amide enolate dimer, Chem. Ber., 1985, 764–773 CrossRef CAS.
- P. Veya, C. Floriani, A. Chiesi-Villa, C. Guastini, A. Dedieu, F. Ingold and P. Braunstein, Experimental and Theoretical Studies on the Reactivity of α-Phosphino Enolates. Crystal Structures of [K(18-crown-6)][Ph2PCHC(O)Ph] and [K(Kryptofix-2,2,2)][Ph2PCHC(O)Ph], Organometallics, 1993, 12, 4359–4367 CrossRef CAS.
- (a) P. G. Williard and G. B. Carpenter, X-ray Crystal Structure of an Unsolvated Lithium Enolate Anion, J. Am. Chem. Soc., 1985, 107, 3345–3346 CrossRef CAS; (b) P. G. Williard and M. J. Hintze, The First Structural Characterization of a Dimeric Lithium Ketone Enolate-Lithium Diisopropylamide Complex, J. Am. Chem. Soc., 1987, 109, 5539–5541 CrossRef CAS; (c) P. G. Williard and G. J. MacEwan, Crystal Structure of a Unique Aggregate Containing Lithium and Potassium Cations, Ketone Enolate, and tert-Butoxide, J. Am. Chem. Soc., 1989, 111, 7671–7672 CrossRef CAS; (d) P. G. Williard and M. J. Hintze, Mixed Aggregates: Crystal Structures of a Lithium Ketone Enolate/Lithium Amide and of a Sodium Ester Enolate/Sodium Amide, J. Am. Chem. Soc., 1990, 112, 8602–8604 CrossRef CAS; (e) K. W. Henderson, P. G. Williard and P. R. Bernstein, Synthesis and Characterization of the First Mixed Alkali Metal Enolate Containing Amine Ligands: A Novel “Open-Stack” Structure and Its Implications for Aldol Addition, Angew. Chem., Int. Ed. Engl., 1995, 34, 1117–1119 CrossRef CAS; (f) K. W. Henderson, A. E. Dorigo, P. G. Williard and P. R. Bernstein, A Triple Anion Complex Containing Enolate, Amide, and Halide – A New Structural Type in Lithium Chemistry, Angew. Chem., Int. Ed. Engl., 1996, 35, 1322–1324 CrossRef CAS; (g) J. Guang, Q. P. Liu, R. Hopson and P. G. Williard, Lithium Pinacolone Enolate Solvated by Hexamethylphosphoramide, J. Am. Chem. Soc., 2015, 137, 7347–7356 CrossRef CAS PubMed; (h) J. Guang, Q. Liu, R. Hopson, G. Kagan, W. Li, T. B. Monroe and P. G. Williard, Conformational Polymorphism of Lithium Pinacolone Enolate, J. Am. Chem. Soc., 2016, 138, 15177–15188 CrossRef CAS PubMed.
- W. Clegg, L. Horsburgh, R. E. Mulvey and M. J. Ross, Crystal Structure of Dilithioacetylacetone: A Twenty-Four Vertex Li-O Cage Molecule with Limited Li-C(terminal) Bonding and Selective THF Solvation, Angew. Chem., Int. Ed. Engl., 1995, 34, 1233–1234 CrossRef CAS.
- (a) K. J. Kolonko, M. M. Biddle, I. A. Guzei and H. J. Reich, Solution Structures of Lithium Enolates of Cyclopentanone, Cyclohexanones, Acetophenones, and Benzyl Ketones. Triple Ions and Higher Lithiate Complexes, J. Am. Chem. Soc., 2009, 131, 11525–11534 CrossRef CAS PubMed; (b) K. J. Kolonko, I. A. Guzei and H. J. Reich, Structure and Dynamics of α-Aryl Amide and Ketone Enolates: THF, PMDTA, TMTAN, HMPA, and Crypt-Solvated Lithium Enolates, and Comparison with Phosphazenium Analogues, J. Org. Chem., 2010, 75, 6163–6172 CrossRef CAS PubMed.
- N. M. Lui, S. N. MacMillan and D. B. Collum, Lithiated Oppolzer Enolates: Solution Structures, Mechanism of Alkylation, and Origin of Stereoselectivity, J. Am. Chem. Soc., 2022, 144, 23379–23395 CrossRef CAS PubMed.
- D. R. Armstrong, A. M. Drummond, L. Balloch, D. V. Graham, E. Hevia and A. R. Kennedy, Metalation of 2,4,6-Trimethylacetophenone Using Organozinc Reagents: The Role of the Base in Determining Composition and Structure of the Developing Enolate, Organometallics, 2008, 27, 5860–5866 CrossRef CAS.
- (a) J. T. B. H. Jastrzebski, G. van Koten, M. J. N. Christophersen and C. H. Stam, The Unexpected Formation of a Lithium-Enolate from Bis{2-[(Dimethylamino)Methyl]Phenyl} Copper Lithium. X-ray Structure of Li4[OC(=CH2)C6H4CH2NMe2-2]4, J. Organomet. Chem., 1985, 292, 319–324 CrossRef CAS; (b) P. J. Posposil, S. R. Wilson and E. N. Jacobsen, A Substoichiometric Pyridine-Lithium Enolate Complex: Solution and X-ray Data and Implications for Catalysis in Aldol Reaction, J. Am. Chem. Soc., 1992, 114, 7585–7587 CrossRef; (c) M.-L. Hsueh, B.-T. Ko, T. Athar, C.-C. Lin, T.-M. Wu and S.-F. Hsu, Synthesis, Structure, and Catalysis Studies of Mixed Lithium-Magnesium and Sodium-Magnesium Complexes: Highly Isospecific Initiators for Polymerization of Methyl Methacrylate, Organometallics, 2006, 25, 4144–4149 CrossRef CAS; (d) R. Knorr and M. Knittl, Unusual (3 THF)-microsolvated dimers and donor-free aggregates of two lithium enolates, Tetrahedron, 2021, 102, 132500 CrossRef CAS; (e) S. Zhou, X. Xu, X. Zhu, Y. Zheng, S. Chen and M. Xue, A facile approach to C-functionalized β-ketoimine compounds via terminal alkylation of a tetralithiated intermediate, Org. Biomol. Chem., 2022, 20, 4289–4292 RSC.
- (a) E. Hevia, K. W. Henderson, A. R. Kennedy and R. E. Mulvey, Synthesis and Characterization of New Mixed-Metal Sodium-Magnesium Enolates Derived from 2,4,6-Trimethylacetophenone, Organometallics, 2006, 25, 1778–1785 CrossRef CAS; (b) S. E. Baillie, E. Hevia, A. R. Kennedy and R. E. Mulvey, Synthesis of Mixed Alkali-Metal-Zinc Enolate Complexes Derived from 2,4,6-Trimethylacetophenone: New Inverse Crown Structures, Organometallics, 2007, 26, 204–209 CrossRef CAS; (c) S. K. Ray, A. Homberg, M. Vishe, C. Besnard and J. Lacour, Efficient Synthesis of Ditopic Polyamide Receptors for Cooperative Ion Pair Recognition in Solution and Solid States, Chem. – Eur. J., 2018, 24, 2944–2951 CrossRef CAS PubMed; (d) A. Homberg, R. Hrdina, M. Vishe, L. Guénée and J. Lacour, Stereoselective deconjugation of macrocyclic α,β-unsaturated esters by sequential amidation and olefin transposition: application to enantioselective phase-transfer catalysis, Org. Biomol. Chem., 2019, 17, 6905–6910 RSC; (e) R. A. Woltornist and D. B. Collum, Ketone Enolization with Sodium Hexamethyldisilazide: Solvent- and Substrate-Dependent E−Z Selectivity and Affiliated Mechanisms, J. Am. Chem. Soc., 2021, 143, 17452–17464 CrossRef CAS PubMed.
- (a) E. Hevia, K. W. Henderson, A. R. Kennedy and R. E. Mulvey, Synthesis and Characterization of New Mixed-Metal Sodium-Magnesium Enolates Derived from 2,4,6-Trimethylacetophenone, Organometallics, 2006, 25, 1778–1785 CrossRef CAS; (b) S. E. Baillie, E. Hevia, A. R. Kennedy and R. E. Mulvey, Synthesis of Mixed Alkali-Metal-Zinc Enolate Complexes Derived from 2,4,6-Trimethylacetophenone: New Inverse Crown Structures, Organometallics, 2007, 26, 204–209 CrossRef CAS; (c) S. K. Ray, A. Homberg, M. Vishe, C. Besnard and J. Lacour, Efficient Synthesis of Ditopic Polyamide Receptors for Cooperative Ion Pair Recognition in Solution and Solid States, Chem. – Eur. J., 2018, 24, 2944–2951 CrossRef CAS PubMed; (d) A. Homberg, R. Hrdina, M. Vishe, L. Guénée and J. Lacour, Stereoselective deconjugation of macrocyclic α,β-unsaturated esters by sequential amidation and olefin transposition: application to enantioselective phase-transfer catalysis, Org. Biomol. Chem., 2019, 17, 6905–6910 RSC; (e) R. A. Woltornist and D. B. Collum, Ketone Enolization with Sodium Hexamethyldisilazide: Solvent- and Substrate-Dependent E−Z Selectivity and Affiliated Mechanisms, J. Am. Chem. Soc., 2021, 143, 17452–17464 CrossRef CAS PubMed.
- P. J. Williard and G. B. Carpenter, X-ray Crystal Structures of Lithium, Sodium, and Potassium Enolates of Pinacolone, J. Am. Chem. Soc., 1986, 108, 462–468 CrossRef CAS PubMed.
- H. J. Reich, Role of Organolithium Aggregates and Mixed Aggregates in Organolithium Mechanisms, Chem. Rev., 2013, 113, 7130–7178 CrossRef CAS PubMed.
- D. Seebach, R. Amstutz and J. D. Dunitz, Tetrameric Cubic Structures of Two Solvated Lithium Enolates, Helv. Chim. Acta, 1981, 64, 2617–2621 CrossRef.
- R. Amstutz, W. B. Schweizer and D. Seebach, Mechanistic Implications of the Tetrameric Cubic Structure of Lithium Enolates, Helv. Chim. Acta, 1981, 64, 2622–2626 CrossRef.
- O. Larrañaga, A. de Cózer, F. M. Bickelhaupt, R. Zangi and F. P. Cossío, Aggregation and Cooperative Effects in the Aldol Reactions of Lithium Enolates, Chem. – Eur. J., 2013, 19, 13761–13773 CrossRef PubMed.
- C. Cambillau and G. Bram, Complexes formés par addition d’éthers-couronnes aux énolates de Na+ et K+ de l'acétylacétate d’éthyle: structures cristallines et système d’équilibres en solution dans le THF et le DMSO, Can. J. Chem., 1982, 60, 2554–2565 CrossRef CAS.
- P. Veya and C. Floriani, Reaction of Metal Carbonyls with Naked Enolates to Make a Metallocarbene Enolate and ALkylcarbonylmetalates, Organometallics, 1994, 13, 214–223 CrossRef CAS.
- For a review, see: N. Davison and E. Lu, The quest for organo-alkali metal monomers: unscrambling the structure-reactivity relationship, Dalton Trans., 2023, 52, 8172–8192 RSC.
- For representative reports, see: (a) N. Davison, E. Falbo, P. G. Waddell, T. J. Penfold and E. Lu, A monomeric methyllithium complex: synthesis and structure, Chem. Commun., 2021, 57, 6205–6208 RSC; (b) J. Barker, N. Davison, P. G. Waddell and E. Lu, Monomeric lithium and sodium silylbenzyl complexes: synthesis, structures, and C=O bond olefination, Chem. Commun., 2023, 59, 8083–8086 RSC.
- N. Davison, C. L. McMullin, L. Zhang, S.-X. Hu, P. G. Waddell, C. Wills, C. Dixon and E. Lu, Li vs Na: Distinct Reaction Patterns of Organo-Lithium and -Sodium Monomeric Complexes, and Ligand-Catalyzed Ketone/Aldehyde Methylenation, J. Am. Chem. Soc., 2023, 145, 6562–6576 CrossRef CAS PubMed.
- P. J. Davidson, D. H. Harris and M. F. Lappert, Subvalent Group 4B metal alkyls and amides. Part I. The synthesis and physical properties of kinetically stable bis[bis(trimethysilyl)methyl]-germanium(II), -tin(II), and -lead(II), J. Chem. Soc., Dalton Trans., 1976, 2268–2274 RSC.
- (a) S. E. Baillie, W. Clegg, P. García-Álvarez, E. Hevia, A. R. Kennedy, J. Klett and L. Russo, Synthesis and characterization of an infinite sheet of metal–alkyl bonds: unfolding the elusive structure of an unsolvated alkali-metal trisalkylmagnesiate, Chem. Commun., 2011, 47, 388–390 RSC; (b) J. R. Lynch, A. R. Kennedy, J. Barker, J. Reid and R. E. Mulvey, Crystallographic Characterisation of Organolithium and Organomagnesium Intermediates in Reactions of Aldehydes and Ketones, Helv. Chim. Acta, 2022, 105, e202200082 CrossRef CAS.
- W. Clegg, B. Conway, A. R. Kennedy, J. Klett, R. E. Mulvey and L. Russo, Synthesis and Structures of [(Trimethylsilyl)methyl]sodium and -potassium with Bi- and Tridentate N-Donor Ligands, Eur. J. Inorg. Chem., 2011, 721–726 CrossRef CAS.
- N. Davison, P. G. Waddell, C. Dixon, C. Wills, T. J. Penfold and E. Lu, A monomeric (trimethylsilyl)methyl lithium complex: synthesis, structure, decomposition and preliminary reactivity studies, Dalton Trans., 2022, 51, 10707–10713 RSC.
- D. E. Anderson, A. Tortajada and E. Hevia, Highly Reactive Hydrocarbon Soluble Alkylsodium Reagents for Benzylic Aroylation of Toluenes using Weinreb Amides, Angew. Chem., Int. Ed., 2023, 62, e202218498 CrossRef CAS PubMed.
- M. G. Davidson, D. Garvia-Vivo, A. R. Kennedy, R. E. Mulvey and S. D. Robertson, Exploiting σ/π Coordination Isomerism to Prepare Homologous Organoalkali Metal (Li, Na, K) Monomers with Identical Ligand Sets, Chem. – Eur. J., 2011, 17, 3364–3369 CrossRef CAS PubMed.
- N. Davison, P. G. Waddell and E. Lu, Reduction of K+ or Li+ in a Heterobimetallic Electride K+[LiN(SiMe3)2]e−, J. Am. Chem. Soc., 2023, 145, 17007–17012 CrossRef CAS PubMed.
- D. E. Anderson, A. Tortajada and E. Hevia, New Frontiers in Organosodium Chemistry as Sustainable Alternatives to Organolithium Reagents, Angew. Chem., Int. Ed., 2023, e202313556 Search PubMed.
- For a recent review on π–π and other stacking effects in coordination chemistry, especially their structure-directing roles, see: D. P. Malenov and S. D. Zarić, Stacking interactions of aromatic ligands in transition metal complexes, Coord. Chem. Rev., 2020, 419, 213338 CrossRef CAS.
- For the ring-laddering and ring-stacking concept, see: (a) D. Barr, W. Clegg, R. E. Mulvey, R. Snaith and K. Wade, Bonding implications of interatomic distances and ligand orientations in the iminolithium hexamers [LiN=C(Ph)tBu]6 and [LiN=C(Ph)NMe2]6: a stacked-ring approach to these and related oligomeric organolithium systems, J. Chem. Soc., Chem. Commun., 1986, 295–297 RSC; (b) R. E. Mulvey, Ring-Stacking and Ring-Laddering in Organonitrogenlithium Compounds: The Development of Concepts with Wide Applicability throughout Lithium Structural Chemistry, Chem. Soc. Rev., 1991, 20, 167–209 RSC; (c) R. E. Mulvey, Synthetic and structural developments in hetero-s-block-metal chemistry: new ring-laddering, ring-stacking and other architectures, Chem. Soc. Rev., 1998, 27, 339–346 RSC; (d) A. D. Bond, Ring-Laddering and Ring-Stacking: Unifying Concepts in the Structural Chemistry of Organic Ammonium Halides, Cryst. Growth Des., 2005, 5, 755–771 CrossRef CAS.
- (a) W. N. Setzer and P. v. R. Schleyer, X-Ray Structural Analyses of Organolithium Compounds, Adv. Organomet. Chem., 1985, 24, 353–451 CrossRef CAS; (b) C. Schade and P. v. R. Schleyer, Sodium, Potassium, Rubidium, and Cesium: X-Ray Structurai Analysis of Their Organic Compounds, Adv. Organomet. Chem., 1987, 27, 169–278 CrossRef CAS; (c) K. Gregory, P. v. R. Schleyer and R. Snaith, Structures of Organonitrogen—Lithium Compounds: Recent Patterns and Perspectives in Organolithium Chemistry, Adv. Inorg. Chem., 1991, 37, 47–142 CrossRef CAS.
- (a) E. Lu, O. J. Cooper, F. Tuna, A. J. Wooles, N. Kaltsoyannis and S. T. Liddle, Uranium-Carbene-Imido Metalla-Allenes: Ancillary-Ligand-Controlled cis-/trans-Isomerisation and Assessment of trans Influence in the R2C=U(IV)=NR’ Unit (R=Ph2PNSiMe3; R’=CPh3), Chem. – Eur. J., 2016, 22, 11559–11563 CrossRef CAS PubMed; (b) E. Lu, S. Sajjad, V. E. J. Berryman, A. J. Wooles, N. Kaltsoyannis and S. T. Liddle, Emergence of the structure-directing role of f-orbital overlap-driven covalency, Nat. Commun., 2019, 10, 634 CrossRef CAS PubMed.
Footnotes |
| † This article is dedicated to Professor Chang-Tao Qian, Shanghai Institute of Organic Chemistry, on the occasion of his 90th birthday. |
| ‡ Electronic supplementary information (ESI) available. CCDC CCDC 2307285 (3-Li), 2307287 (3-Na), 2307288 (3-K), 2307289 (6-Li) and 2307290 (6-Na). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt00211c |
| This journal is © The Royal Society of Chemistry 2024 |