Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Addressing the gaps in homeostatic mechanisms of copper and copper dithiocarbamate complexes in cancer therapy: a shift from classical platinum-drug mechanisms
Lydia W.
Njenga
a,
Simon N.
Mbugua
*b,
Ruth A.
Odhiambo
a and
Martin O.
Onani
c
aDepartment of Chemistry, University of Nairobi, P.O. Box 30197-00100, Nairobi, Kenya. E-mail: smbugua@kisiiuniversity.ac.ke
bDepartment of Chemistry, Kisii University, P.O. Box 408-40200, Kisii, Kenya
cDepartment of Chemical Sciences, University of the Western Cape, Private Bag X17, Belville, 7535, South Africa
First published on 11th July 2024
Abstract
Correction for ‘Addressing the gaps in homeostatic mechanisms of copper and copper dithiocarbamate complexes in cancer therapy: a shift from classical platinum-drug mechanisms’ by Lydia W. Njenga et al., Dalton Trans., 2023, 52, 5823–5847, https://doi.org/10.1039/D3DT00366C.
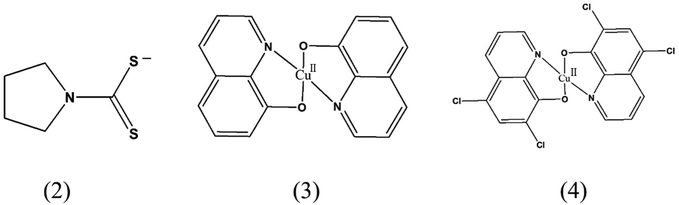
The authors regret that on page 5824 the structures of compounds 2, 3 and 4 were incorrect, showing protonated ligands. The corrected structures are shown below.
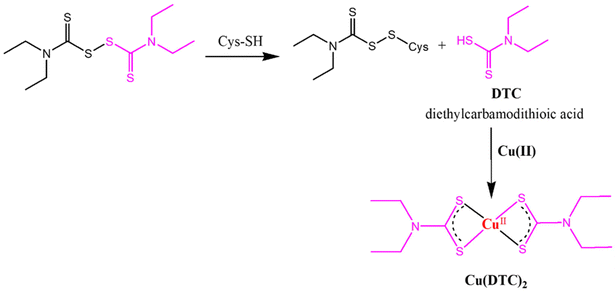
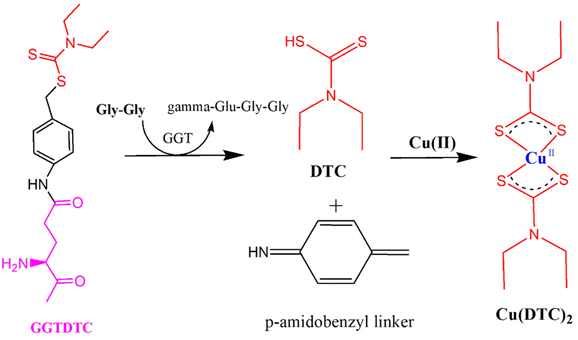
Fig. 8 also showed incorrect chemical structures; the correct figure is shown below.
On page 5825, the statement “Disulfiram (…) and clioquinol release copper in a reductive intracellular environment and have been shown to have anticancer effects in several clinical trials using mouse models.” was supported by incorrect references, and this statement should be supported by references 67, 68, and 16 of the original article.
On page 5831, the statement “The strong trans-influence of the sulphur atoms…” should be rephrased to refer to “the relatively strong trans-influence of the groups coordinated through sulphur atoms…”.
The authors regret that a large number of references used incorrect page numbers. These references are shown in their correct form here, with the following citation numbers:
| Reference here | Reference in original article | Reference here | Reference in original article |
|---|---|---|---|
| 1 | 11 | 21 | 136 |
| 2 | 21 | 22 | 143 |
| 3 | 27 | 23 | 144 |
| 4 | 39 | 24 | 165 |
| 5 | 52 | 25 | 172 |
| 6 | 66 | 26 | 177 |
| 7 | 67 | 27 | 181 |
| 8 | 68 | 28 | 191 |
| 9 | 71 | 29 | 196 |
| 10 | 73 | 30 | 202 |
| 11 | 74 | 31 | 203 |
| 12 | 76 | 32 | 228 |
| 13 | 81 | 33 | 259 |
| 14 | 83 | 34 | 260 |
| 15 | 85 | 35 | 262 |
| 16 | 97 | 36 | 275 |
| 17 | 107 | 37 | 276 |
| 18 | 114 | 38 | 282 |
| 19 | 115 | ||
| 20 | 133 |
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
References
- A. S. Oguntade, F. Al-Amodi, A. Alrumayh, M. Alobaida and M. Bwalya, Anti-angiogenesis in cancer therapeutics: the magic bullet, J. Egypt. Natl. Cancer Inst., 2021, 33(1), 15 CrossRef PubMed.
- F. Arnesano and G. Natile, Interference between copper transport systems and platinum drugs. In Seminars in Cancer Biology, Academic Press, 2021, vol. 76, pp. 173–188 Search PubMed.
- H. Zhu, S. Mao and W. Li, Association between Cu/Zn/Iron/Ca/Mg levels and Chinese children with cerebral palsy, Research Square, 2021, preprint (version 1), DOI:10.21203/rs.3.rs-703495/v1.
- H. Yang, X. Chen, K. Li, H. Cheaito, Q. Yang, G. Wu, J. Liu and Q. P. Dou, Repurposing old drugs as new inhibitors of the ubiquitin-proteasome pathway for cancer treatment, Semin. Cancer Biol., 2021, 68, 105–122 CrossRef CAS PubMed.
- C. Xiong, H. Ling, Q. Hao and X. Zhou, Cuproptosis: p53 regulated metabolic cell death?, Cell Death Differ., 2023, 30, 876–884 CrossRef CAS PubMed.
- P. S. da Costa, P. S. Ramos, C. Ferreira, J. L. Silva, T. El-Bacha and E. Fialho, Pro-Oxidant Effect of Resveratrol on Human Breast Cancer MCF-7 Cells is Associated with CK2 Inhibition, Nutr. Cancer, 2022, 74, 2142–2151 CrossRef PubMed.
- D. Denoyer, H. B. Pearson, S. A. S. Clatworthy, Z. M. Smith, P. S. Francis, R. M. Llanos, I. Volitakis, W. A. Phillips, P. M. Meggyesy and S. Masaldan, Copper as a target for prostate cancer therapeutics: copper-ionophore pharmacology and altering systemic copper distribution, Oncotarget, 2016, 7, 37064–37080 CrossRef PubMed.
- M. A. Cater, H. B. Pearson, K. Wolyniec, P. Klaver, M. Bilandzic, B. M. Paterson, A. I. Bush, P. O. Humbert, S. La Fontaine and P. S. Donnelly, Increasing intracellular bioavailable copper selectively targets prostate cancer cells, ACS Chem. Biol., 2013, 8(7), 1621–1631 CrossRef CAS PubMed.
- X. Chen, Q. Ping Dou, J. Liu and D. Tang, Targeting ubiquitin–proteasome system with copper complexes for cancer therapy, Front. Mol. Biosci., 2021, 8, 649151 CrossRef CAS PubMed.
- M. Y. Wani and M. A. Malik, Non-platinum Anticancer Agents, in Gold and Its Complexes in Anticancer Chemotherapy, Springer, Singapore, 2021, pp. 51–68, DOI:10.1007/978-981-33-6314-4_6.
- S. Yadav, Potential of Metal Complexes for the Treatment of Cancer: Current Update and Future Prospective, in Chemistry of Biologically Potent Natural Products and Synthetic Compounds, ed. S.-u. Islam and J. A. Banday, Wiley, 2021, ch. 7, pp. 183–204, DOI:10.1002/9781119640929.ch7.
- M. Badrooh, F. Shokrollahi and S. Javan, et al., Trigger of Apoptosis in Adenocarcinoma Gastric Cell Line (AGS) by a Complex of Thiosemicarbazone and Copper Nanoparticles, Mol. Biol. Rep., 2022, 49, 2217–2226 CrossRef CAS PubMed.
- C. Molinaro, A. Martoriati and K. Cailliau, Proteins from the DNA damage response: Regulation, dysfunction, and anticancer strategies, Cancers, 2021, 13, 3819 CrossRef CAS PubMed.
- Y. Huang, J. Fan, Y. Li, S. Fu, Y. Chen and J. Wu, Imaging of Tumor Hypoxia With Radionuclide-Labeled Tracers for PET, Front. Oncol., 2021, 11, 731503, DOI:10.3389/fonc.2021.731503.
- M. Pan, Q. Zheng, Y. Yu, H. Ai, Y. Xie, X. Zeng, C. Wang, L. Liu and M. Zhao, Seesaw conformations of Npl4 in the human p97 complex and the inhibitory mechanism of a disulfiram derivative, Nat. Commun., 2021, 12, 121 CrossRef CAS PubMed.
- S. Ritchie, D. A. Reed, B. A. Pereira and P. Timpson, The cancer cell secretome drives cooperative manipulation of the tumour microenvironment to accelerate tumourigenesis, Fac. Rev., 2021, 10, 4 CAS , PCMID: PMC7894270.
- J. Song, Z. Guan, C. Song, M. Li, Z. Gao and Y. Zhao, Apatinib suppresses the migration, invasion and angiogenesis of hepatocellular carcinoma cells by blocking VEGF and PI3K/AKT signaling pathways, Mol. Med. Rep., 2021, 23(6), 429 CrossRef CAS PubMed.
- D. Ma, J.-L. Zhang, Z.-H. Huang, G. Ai, G. Li and S.-N. Shu, Identification of novel compound ATP7B mutations in a child with rare Wilson disease: A case report, Research Square, 2023, preprint (version 1), DOI:10.21203/rs.3.rs-2434176/v1.
- S. S. Eben and J. A. Imlay, Excess copper catalyzes protein disulfide bond formation in the bacterial periplasm but not in the cytoplasm, Mol. Microbiol., 2023, 119(4), 423–438 CrossRef CAS PubMed.
- Y. Chen, L. Tang and W. Huang, et al., Identification of a prognostic cuproptosis-related signature in hepatocellular carcinoma, Biol. Direct, 2023, 18(1), 4 CrossRef CAS PubMed.
- Y. Yi and M. H. Lim, Current understanding of metal-dependent amyloid-β aggregation and toxicity, RSC Chem. Biol., 2023, 4, 121–131 RSC.
- I. Pal and S. G. Dey, The Role of Heme and Copper in Alzheimer's Disease and Type 2 Diabetes Mellitus, JACS Au, 2023, 3, 657–681 CrossRef CAS PubMed.
- L. Roldán-Martín, M. Sodupe and J.-D. Maréchal, Computational assessment of the impact of Cu(II) and Al(III) on β-amyloid42 fibrils: Binding sites, structural stability, and possible physiological implications, Front. Neurosci., 2023, 17, 1110311, DOI:10.3389/fnins.2023.1110311.
- T. S. Thind, Changing trends in discovery of new fungicides: a perspective, Indian Phytopathol., 2021, 74(4), 875–883 CrossRef.
- W.-L. Lee, J.-Y. Huang and L.-F. Shyur, Phytoagents for cancer management: regulation of nucleic acid oxidation, ROS, and related mechanisms, Oxid. Med. Cell. Longevity, 2013, 2013, 925804, DOI:10.1155/2013/925804.
- A. Kükürt, V. Gelen, Ö. F. Başer, H. A. Deveci and M. Karapehlivan, Thiols: Role in oxidative stress-related disorders, in Accenting Lipid Peroxidation, ed. P. Atukeren, IntechOpen, 2021, DOI:10.5772/intechopen.96682.
- G. Gurumoorthy, G. Mathu Bala and R. Hema, Synthesis Andcharacterization of Zinc Sulfide and Zinc-Iron Sulfide Nanoparticles from Zinc(II) Dithiocarbamate Complexes, Ann. Rom. Soc. Cell. Biol., 2021, 25(2), 2091–2095 Search PubMed.
- P. C. Rao, M. Prabu, Y. Son, J. Kim and M. Yoon, An Organic Guest Molecule Induced Ultrafast Breathing of Epitaxially Grown Metal-Organic Framework on Self-Assembled Monolayer, Chem. Commun., 2021, 57, 10158–10161 RSC.
- P. Pengo and L. Pasquato, Modified gold nanoparticles and surfaces, in The Supramolecular Chemistry of Organic-Inorganic Hybrid Materials, ed. K. Rurack and R. Martínez-Máñez, Wiley and Sons, New York, 2010, pp. 113–154, DOI:10.1002/9780470552704.ch4.
- O. M. El Yaagoubi, L. Oularbi, A. Bouyahya, H. Samaki, S. El Antri and S. Aboudkhil, The role of the ubiquitin-proteasome pathway in skin cancer development: 26S proteasome-activated NF-κB signal transduction, Cancer Biol. Ther., 2021, 22, 479–492 CrossRef CAS PubMed.
- S. Xiao-Xin, Y. Li, R. Sears and M. Dai, Targeting the MYC ubiquitination-proteasome degradation pathway for cancer therapy, Front. Oncol., 2021, 11, 679445 CrossRef PubMed.
- V. W. Karisma, W. Wu and M. Lei, et al., UVA-Triggered Drug Release and Photo-Protection of Skin, Front. Cell Dev. Biol., 2021, 9, 598717, DOI:10.3389/fcell.2021.598717.
- K. Dharmalingam, A. Birdi and S. Tomo, et al., Trace elements as immunoregulators in SARS-CoV-2 and other viral infections, Indian J. Clin. Biochem., 2021, 36(4), 416–426 CrossRef CAS PubMed.
- Y. Li, H. Liu, J. He, X. Shen, K. Zhao and Y. Wang, The Effects of Oral Administration of Molybdenum Fertilizers on Immune Function of Nanjiang Brown Goat Grazing on Natural Pastures Contaminated by Mixed Heavy Metal, Biol. Trace Elem. Res., 2022, 200, 2750–2757 CrossRef CAS PubMed.
- L. Chistoserdova, To methanotrophy and beyond! New insight into functional and ecological roles for copper chelators, ISME J., 2022, 16(1), 3–4 CrossRef CAS PubMed.
- J. F. Eichinger, L. J. Haeusel, D. Paukner, R. C. Aydin, J. D. Humphrey and C. J. Cyron, Mechanical homeostasis in tissue equivalents: a review, Biomech. Model. Mechanobiol., 2021, 20, 833–850 CrossRef PubMed.
- H. Liao, Y. Qi, Y. Ye, P. Yue, D. Zhang and Y. Li, Mechanotranduction Pathways in the Regulation of Mitochondrial Homeostasis in Cardiomyocytes, Front. Cell Dev. Biol., 2020, 8, 625089, DOI:10.3389/fcell.2020.625089.
- (a) K. A. Price, P. J. Crouch, I. Volitakis, B. M. Paterson, S.-C. Lim, P. S. Donnelly and A. R. White, Mechanisms controlling the cellular accumulation of copper bis (thiosemicarbazonato) complexes, Inorg. Chem., 2011, 50(19), 9594–9605 CrossRef CAS PubMed; (b) K. Gaur, A. M. Vázquez-Salgado and G. Duran-Camacho, et al., Iron and copper intracellular chelation as an anticancer drug strategy, Inorganics, 2018, 6(4), 126 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |