Open Access Article
Open Access ArticleNi0.5Co0.5S nano-chains: a high-performing intercalating pseudocapacitive electrode in asymmetric supercapacitor (ASC) mode for the development of large-scale energy storage devices†
Vishal
Kushwaha
a,
K. D.
Mandal
a,
Asha
Gupta
 *a and
Preetam
Singh
*a and
Preetam
Singh
 *b
*b
aDepartment of Chemistry, Indian Institute of Technology (Banaras Hindu University) Varanasi, Uttar Pradesh 221005, India. E-mail: asha.chy@itbhu.ac.in; toashagupta@gmail.com; Tel: +91-6390363140
bDepartment of Ceramic Engineering, Indian Institute of Technology (Banaras Hindu University) Varanasi, Uttar Pradesh 221005, India. E-mail: preetamsingh.cer@itbhu.ac.in; preetamchem@gmail.com; Tel: +91-8118873748
First published on 12th February 2024
Abstract
Grid-scale energy storage solutions are necessary for using renewable energy sources efficiently. A supercapattery (supercapacitor + battery) has recently been introduced as a new variety of hybrid devices that engage both capacitive and faradaic charge storage processes. Nano-chain architectures of Ni0.5Co0.5S electrode materials consisting of interconnected nano-spheres are rationally constructed by tailoring the surface structure. Nano-chains of the bimetallic sulfide Ni0.5Co0.5S are presented to have a superior charge storage capacity. The Ni0.5Co0.5S nano-chain electrode presents a capacitance of 2001.6 F g−1 at 1 mV s−1, with a specific capacity of 267 mA h g−1 (1920 F g−1) at 1 A g−1 in 4 M KOH aqueous electrolyte through the galvanostatic charge–discharge (GCD) method. The reason behind the high charge storage capacity of the materials is the predominant redox-mediated diffusion-controlled pseudocapacitive mechanism coupled with surface capacitance (electrosorption), as the surface (outer) and intercalative (inner) charges stored by the Ni0.5Co0.5S electrodes are close to 46.0% and 54.0%, respectively. Additionally, a Ni0.5Co0.5S//AC two electrode full cell operating in asymmetric supercapacitor cell (ASCs) mode in 4 M KOH electrolyte exhibits an impressive energy density equivalent to 257 W h kg−1 and a power density of 0.73 kW kg−1 at a current rate of 1 A g−1.
Introduction
Clean, pollution-free renewable energy technologies including solar, wind, and tidal have been developed in response to the gradual depletion of fossil fuels and the increase in CO2 emissions but due to the time and weather dependent nature of these renewable energy technologies, a higher degree of current and voltage fluctuations is associated with these sources. Therefore, to get a steady electricity output from these sources, superior energy storage systems are needed to utilize such energy sources effectively. Thus, grid-scale energy storage devices are necessary to use renewable energy technology efficiently. Further large-scale energy storage systems at high current rates are also required for electric vehicles, smart grids, and different wearable and portable gadgets.1,2 Supercapacitors (SCs) have drawn a lot of attention due to their safer operation, rapid charge–discharge, and longer cycling life than traditional batteries and capacitors. The electrochemical characteristics of the electrode materials significantly impact the supercapacitor performance. Based on their energy storage mechanism, electrical double-layer capacitors (EDLCs) and pseudocapacitors (PCs) are the two main categories in their hybrid combinations to be used in SCs. Electrostatic charges accumulating at the electrode/electrolyte interface are the root of EDLC-type capacitance. Because the electrode materials used in pseudocapacitive energy storage with the accessibility of different oxidation states produce energy through swift and reversible redox processes, a substantially higher energy density than EDLCs is achieved.3–6 Supercapattery (supercapacitor + battery) which is another class of supercapacitors has recently been introduced as a new terminology to describe the variety of hybrid devices that engage both capacitive and faradaic charge storage processes either at the device level or the electrode material level. A supercapattery is also known as an asymmetric supercapacitor (ASC) or hybrid supercapacitor (HSC) or battery-supercapacitor hybrid device. In an HSC or ASC system, the power source of a capacitor-like electrode is combined with the energy source of a battery-like electrode in the same cell. The supercapattery bridges the gap between a supercapacitor and a battery by combining both capacitive and faradaic charge storage mechanisms into a single device that achieves optimal energy and power densities.7,8 While supercapacitors have a high power capability and batteries have a massive capacity for energy storage, a supercapattery comprises a wide variety of combinations that are currently under exploration to develop large-scale energy storage systems.9,10 To overcome low energy densities, low stability, and slow kinetics, strategic measures are required to be taken. In this context, significant research has been devoted to designing and fabricating several high-performance energy storage materials, such as Fe2O3,11,12 MoS2,13–15 Co3O4,16,17 NaVO3,18 V2O5,19 Nb2O5,20 MoO3,21–23 MnO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 and transition-metal sulfides25,26 in recent times. Due to their electronic structure and structural properties, TMSs have been extensively researched in optical, electrical and optoelectronic devices, including light-emitting diodes, solar cells, sensors and electrodes for lithium-ion batteries (LIBs).27–31 Due to their low electronegativity, high specific capacitance, and superior mechanical and thermal stability compared to their analogous oxide counterparts, sulfides of transition metals, such as WS2,32–34 FeS2,35,36 MoS2,13–15 CuS,37,38 CoS,39–43 and NiS,44–48 have been extensively employed as anode materials for supercapacitors. Because of its unique and distinctive properties, nickel sulfide is helpful in various applications and can serve as both the anode and cathode material for LIBs.49,50 The use of cobalt sulfides as the cathode in LIBs has been examined as a robust material with a low overpotential, extended cycle life, high resilience, and durability.51,52 Bimetallic sulfides are regarded as a promising class of electrode materials for high-performance energy storage devices due to their exceptional electrochemical activity and capacity compared to most mono-metal sulfides.53–58 Due to their distinctive physical, chemical, electrical, and optical properties, as well as their numerous potential applications in catalysis and electrochemical supercapacitors, Ni–Co–S compounds with different stoichiometric compositions, such as NiCo2S4,59–62 CoNi2S4,63–65 Ni1.5Co1.5S4,66,67 NixCo3−xS4,68,69 (Ni0.5Co0.5)9S8,70 and NixCo1−xS2,71–73 have drawn significant attention.
24 and transition-metal sulfides25,26 in recent times. Due to their electronic structure and structural properties, TMSs have been extensively researched in optical, electrical and optoelectronic devices, including light-emitting diodes, solar cells, sensors and electrodes for lithium-ion batteries (LIBs).27–31 Due to their low electronegativity, high specific capacitance, and superior mechanical and thermal stability compared to their analogous oxide counterparts, sulfides of transition metals, such as WS2,32–34 FeS2,35,36 MoS2,13–15 CuS,37,38 CoS,39–43 and NiS,44–48 have been extensively employed as anode materials for supercapacitors. Because of its unique and distinctive properties, nickel sulfide is helpful in various applications and can serve as both the anode and cathode material for LIBs.49,50 The use of cobalt sulfides as the cathode in LIBs has been examined as a robust material with a low overpotential, extended cycle life, high resilience, and durability.51,52 Bimetallic sulfides are regarded as a promising class of electrode materials for high-performance energy storage devices due to their exceptional electrochemical activity and capacity compared to most mono-metal sulfides.53–58 Due to their distinctive physical, chemical, electrical, and optical properties, as well as their numerous potential applications in catalysis and electrochemical supercapacitors, Ni–Co–S compounds with different stoichiometric compositions, such as NiCo2S4,59–62 CoNi2S4,63–65 Ni1.5Co1.5S4,66,67 NixCo3−xS4,68,69 (Ni0.5Co0.5)9S8,70 and NixCo1−xS2,71–73 have drawn significant attention.
Electrochemical activities can be maximized by altering the active material compositions, fine-tuning the electronic structures, and adjusting the active sites of materials. The ionic radius of Co2+ is close to that of Ni2+ as they are adjacent transition metal elements. Thus, doping Ni atoms onto the CoS molecule can regulate the electronic structure and stabilize Co species as the electrode material for supercapacitors. As the crystal structure and ionic radius are similar, a solid solution of NixCo1−xS is expected to be formed. Earlier, C. Huang et al. attempted to construct bundle-like NixCo1−xS/C nanostructures using 3D Ni–Co–BTC as the starting material to enhance the supercapacitive performance of the elctrode.73
This report presents the synthesis and characterization of a bimetallic sulfide Ni0.5Co0.5S nanostructure and the electrochemical performances of the developed Ni0.5Co0.5S nano-chain electrodes. Due to the combined advantages of Co/Ni–S electronic structures, the Ni0.5Co0.5S electrode demonstrated a significantly improved electrochemical performance. The electrode achieved a specific capacity of 267 mA h g−1 (1920 F g−1) at 1 A g−1 in 4 M KOH aqueous electrolyte through galvanostatic charge–discharge (GCD) experiments and a capacitance equivalent to 2001.6 F g−1 at 1 mV s−1 by cyclic voltammetry (CV). Furthermore, the assembled Ni0.5Co0.5S//AC asymmetric supercapacitors (ASCs), which employed the Ni0.5Co0.5S electrode as the positive electrode and activated carbon (AC) as the negative electrode, obtained an impressive energy density comparable to ∼257 W h kg−1 and a power density of ∼7.2 kW kg−1.
Experimental
Synthesis
All chemicals used in this experiment were purchased from Alfa Aesar and used without any further purification. To create a homogeneous solution, equimolar ratios of NiCl2·6H2O and CoCl2·6H2O were dissolved in 250 ml of deionized water and continuously stirred for 5–6 hours, followed by ultrasonication for 30 minutes. Then, a black fine precipitate was obtained when H2S gas was introduced into the solution using the Kipp generator technique. A schematic illustration of the synthesis of Ni0.5Co0.5S is shown in Fig. 1.The resultant fine precipitate was filtered and repeatedly washed with ethanol and deionized water. The product was then dried in a hot air oven (under an N2 atmosphere) at 120 °C for 24 hours and used for crystallographic and electrochemical characterization. The chemical reaction representing the formation of Ni0.5Co0.5S is given in eqn (1).
| CoCl2·6H2O + NiCl2·6H2O + 2H2S → 2Ni0.5Co0.5S + 12H2O + 4HCl | (1) |
Characterization
Using a Rigaku SmartLab 9 kW X-ray diffractometer (XRD) and Cu-Kα radiation (λ = 0.154 nm) in the range 2θ = 20°–70° with a step size of 0.02°, the material's phase purity and structural characteristics were investigated. Using FullProf Suite, the powder XRD patterns were Rietveld refined. The Xpert High Score (PANanylytical) programme identified the crucial phase. The surface morphology and structure of the sample were studied using SEM (EVO-Scanning Electron Microscope MA15/18, CARL ZEISS Microscopy Ltd). Microstructural analyses were carried out at a 200 kV acceleration voltage using a high-resolution transmission electron microscope (FEI Tecnai G2 20 TWIN, USA). Before placing a drop on a holey-carbon-coated copper grid (Pelco International, USA) for TEM imaging, samples were dissolved in 20 ml of anhydrous ethanol and sonicated for 30 minutes. Image J software was used to record and analyse HRTEM micrographs. Using an Agilent Cary 60 UV-visible spectrophotometer, the optical characteristics between 200–800 nm were investigated. Using a TGA thermal analyzer (STA 6000, PerkinElmer Pvt. Ltd), the analysis was conducted at a heating rate of 10 °C min−1 in the temperature range of 30 to 700 °C to determine the thermal stability of the as-prepared sample. The sample's Raman spectra were calculated using a 633 nm laser and a Renishaw Raman microscope. The Nicolet iS5 FT-IR spectrometer was used to capture the samples’ infrared spectra between 400 and 4000 cm−1. BET (BELCAT-II, MicrotracBEL Corp.) was used to measure the sample's pore size distribution and specific surface area. A standard three-electrode setup was used to conduct all electrochemical tests on the sample, including cyclic voltammetry (CV), galvanostatic charge–discharge (GCD) tests, and electrochemical impedance spectroscopy (EIS), and AfterMath software (WaveDriver-200 from Pine Research) was used to record the results.Preparation of the electrode
The appropriate amounts of the active material, activated carbon (AC), and polyvinylidene difluoride (PVDF) binder were combined in N-methyl-2-pyrrolidone (NMP) solvent in the ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to prepare working electrodes. Active material (Ni0.5Co0.5S) was preheated at 120 °C for 24 h in a hot air oven before making the electrode ink. To construct the working electrode, a homogeneous, viscous slurry was mixed using a mortar and pestle and then cast over a 1 cm2 area of Toray carbon paper. Additionally, the coated electrode was dried for 12 hours at 80 °C. In the fabrication of the electrodes, AC was employed to improve the conductivity of the electrodes. The electrode loading was estimated by weighing the electrode on an electronic balance (error limit: 0.01 mg). For that, the weight of the Toray paper was recorded, and then the Toray paper was coated with electrode ink (after the ink had dried across a 1 × 1 cm2 area on the Toray paper). One mg was found to represent the complete electrode load (0.7 mg active material, 0.2 mg AC, and 0.1 mg PVDF binder). Similarly, the required amounts of AC and PVDF binder were dissolved in NMP solvent in a 9
1 to prepare working electrodes. Active material (Ni0.5Co0.5S) was preheated at 120 °C for 24 h in a hot air oven before making the electrode ink. To construct the working electrode, a homogeneous, viscous slurry was mixed using a mortar and pestle and then cast over a 1 cm2 area of Toray carbon paper. Additionally, the coated electrode was dried for 12 hours at 80 °C. In the fabrication of the electrodes, AC was employed to improve the conductivity of the electrodes. The electrode loading was estimated by weighing the electrode on an electronic balance (error limit: 0.01 mg). For that, the weight of the Toray paper was recorded, and then the Toray paper was coated with electrode ink (after the ink had dried across a 1 × 1 cm2 area on the Toray paper). One mg was found to represent the complete electrode load (0.7 mg active material, 0.2 mg AC, and 0.1 mg PVDF binder). Similarly, the required amounts of AC and PVDF binder were dissolved in NMP solvent in a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio to produce AC electrodes.
1 ratio to produce AC electrodes.
Results and discussion
Structural characterization
The single-phase hexagonal Ni0.5Co0.5S sample was synthesized using a simple H2S-mediated two-phase (gas diffusion in liquid) precipitation route in an aqueous medium. The formation of Ni0.5Co0.5S in a single phase is facilitated by the controlled heterogeneous precipitation of the solid phase due to the gradual diffusion of H2S gas into the liquid phase. Fig. 2a shows the Rietveld refined XRD profile of the hexagonal Ni0.5Co0.5S. The crystal structure was refined using hexagonal CoS as the model structure. The sharp prominent diffraction peaks at 2θ values of 30.67, 35.34, 46.95, 54.52, 62.56, 63.87, 66.65, 73.12 and 74.75 represent the (100), (101), (102), (110), (103), (200), (201), (004), and (202) planes, respectively, of Ni0.5Co0.5S in the hexagonal cell (space group: P63/mmc) with lattice parameters a = b = 3.37 (1) Å, c = 5.18 (3) Å, α = β = 90° and γ = 120°, which match very well with the diffraction peaks of hexagonal CoS (ICDD File No: 01-075-0605). The VESTA images (Fig. 2b-I1, I2 and I3) of the Ni0.5Co0.5S nano-chains show the arrangement and sharing of cation polyhedra in a layered hexagonal structure. The Co2+/Ni2+ atom is bonded to six equivalents of S2− atoms to form a mixture of corner, edge and face sharing octahedra. Furthermore, the S2− atom is bonded in a 6 coordinate geometry to 6 equivalents of Co2+/Ni2+ atoms. The Debye–Scherrer equation was used to evaluate the crystallite size, and an average crystallite size of 21.07 (1) nm was found. Due to the substitution of smaller Co2+ ions (rCo2+ = 0.065 nm) with the larger Ni2+ ions (rNi2+ = 0.069 nm) in the hexagonal CoS structure, lattice parameters of Ni0.5Co0.5S are shifted to a higher value than those of hexagonal CoS.73 The XRD pattern of the Ni0.5Co0.5S sample confirms the presence of single-phase hexagonal (space group: P63/mmc) Ni0.5Co0.5S as no secondary diffraction phases are observed. | ||
| Fig. 2 (a) Rietveld refinement of the XRD profile of Ni0.5Co0.5S nano-chains, and (b) VESTA image of Ni0.5Co0.5S nano-chains. | ||
Thermogravimetric analysis (TGA) under an N2 atmosphere investigated the sample's thermal stability, as shown in ESI Fig. S1a.† The Ni0.5Co0.5S sample's black powder was subjected to heating from ambient temperature to 700 °C with a heating rate of 10 °C per minute. The weight loss process of the Ni0.5Co0.5S nano-chains may be separated into two distinct phases. The first phase of weight loss occurs within the temperature range of 65–135 °C, resulting in a weight loss of about 6.06%, equivalent to ∼0.45 H2O molecules. The observed reduction in weight may be ascribed to the presence of physically adsorbed moisture and the hydroxide group. From 135 °C to 533 °C, the sample is almost stable, with a slight weight loss, which implies that the Ni0.5Co0.5S nano-chains are stable up to a fairly high temperature. Additionally, at a temperature of 533 °C, a significant reduction in weight occurs due to the disintegration of the Ni0.5Co0.5S nano-chains, resulting in the release of sulfur. A weight loss of 17.81% accompanies this decomposition process.74,75 Hence to remove the water present in the sample, the sample was dried at 120 °C under an N2 atmosphere to make it moisture free to study the electrochemical performances of the electrode. Eqn (2) and (3) in the TGA study depict the observed weight losses.
| Ni0.5Co0.5S·xH2O → Ni0.5Co0.5S + xH2O; x ∼0.45 (T ∼135 °C) | (2) |
| Ni0.5Co0.5S → Ni0.5Co0.5Sx + (1 − x) S; x ∼ 0.45 (T ∼550 °C) | (3) |
The FT-IR spectrum of the as-prepared (without any pre-heating) Ni0.5Co0.5S nano-chains is shown in ESI Fig. S1b.† The spectral peaks at 3347 cm−1 and 1631 cm−1 may be attributed to the stretching and bending vibrations of the hydroxyl (OH) functional group of water molecules that have been adsorbed onto the surface. Furthermore, the bands at 407, 414, 421, and 433 cm−1 are related to the symmetrical stretching vibration, whereas those at 1016 and 1085 cm−1 are related to the asymmetrical stretching vibration assigned to Ni–S or Co–S bonds in the Ni0.5Co0.5S sample.4,76
For additional investigation on the structure and phase composition of the sample, Raman spectroscopy was performed on the pre-heated active material as shown in ESI Fig. S1c.† Peaks at 180, 455, 513, and 640 cm−1 can be indexed to the Raman-active vibrational peaks of the Ni0.5Co0.5S sample, which correspond to the typical phonon modes F2g, Eg, T2g and A1g, respectively.77,78 The Raman spectral lines of the Ni0.5Co0.5S sample exhibited a shift towards lower wave numbers, often referred to as a bathochromic shift or red shift. This shift indicates the phonon confinement effect, which may be attributed to the quantum confinement of optical phonons. This effect is further corroborated by a high-intensity stretching band seen at a wavenumber of 962 cm−1.4
The UV-visible absorption spectrum of the as-synthesized pre-heated Ni0.5Co0.5S was recorded at room temperature in the range of 200–800 nm (ESI Fig. S1d†). The absorption peak at a wavelength of 374 nm was seen, and the optical band gap (2.32 eV) was determined using the Tauc plot (shown in the inset of ESI Fig. S1d†).79 The surface area measurements of the Ni0.5Co0.5S sample using Brunauer–Emmett–Teller (BET) are displayed in ESI Fig. S1e.† The adsorption and desorption isotherms of the Ni0.5Co0.5S sample showed characteristics that correspond to type IV isotherms, indicating a combination of micro- and mesoporous structures. The calculated BET-specific surface area and the average diameters of pores were found to be 13.45 m2 g−1 and 14.27 nm, respectively. The Ni0.5Co0.5S sample exhibits mesopores characterized by a significantly enhanced surface area. This attribute facilitates an enhanced interaction with the electrolyte and improves electrochemical reaction kinetics. The increased concentration of charge carriers at the material's surface is responsible for this effect. Furthermore, it is shown that the measured mesopore diameter of the Ni0.5Co0.5S nano-chains is much larger than the dimensions of OH-ions in an aqueous KOH electrolyte.73,80
The elemental composition and chemical oxidation state of the Ni0.5Co0.5S nano-chains were studied using X-ray photoelectron spectroscopy (XPS). The spectrum shown in Fig. 3a exhibits the high-resolution Ni (2p) spectrum. It reveals the presence of two distinct peaks, namely 2p3/2 and 2p1/2, located at 856.74 and 874.34 eV, with their corresponding satellite peaks at 862.46 and 880.23 eV, respectively, assigned to the Ni2+ oxidation state. Likewise, the high-resolution Co (2p) spectrum (Fig. 3b) shows 2p3/2 and 2p1/2 at 782.05 and 797.96 eV, and the corresponding satellite peaks at 786.47 and 802.89 eV are assigned to the Co2+ oxidation state. The high-resolution S (2p) spectrum (Fig. 3c) shows two characteristic peaks at 168.98 and 170.12 eV, respectively. The peak observed at 168.98 eV corresponds to S (2p3/2) associated with the sulfur–metal bonding and the peak that appeared at 170.12 eV corresponds to S (2p1/2) and is attributed to the surface bonding of low-coordination divalent sulfur (S2−). Furthermore, the satellite peak observed at about 162.52 eV corresponds to the binding energies of metal–sulfur bonding (Ni–S and Co–S bonding). Thus, the XPS study confirms the formation of Co2+, Ni2+, and S2−oxidation states in the Ni0.5Co0.5S sample.64,67
 | ||
| Fig. 3 XPS plot of Ni0.5Co0.5S nano-chains, (a) Ni (2p) spectra, (b) Co (2p) spectra, and (c) S (2p) spectra. | ||
Fig. 4a shows the SEM image, which illustrates the particle dispersion and agglomerated arrangements of the Ni0.5Co0.5S sample containing interconnecting spheres of sub-micron sizes. The EDX (energy dispersive X-ray) image shown in Fig. 4b confirms the composition of the material. HR-TEM images shown in Fig. 4(c and d) confirm the formation of the nano-chain arrangement of the Ni0.5Co0.5S sample containing the interconnecting nanospheres. HR-TEM images of several other areas of the Ni0.5Co0.5S sample are provided in the ESI Fig. S2 (a, b and c)† further confirming the successful formation of nano-chains. In aqueous solution, reduction in coordination number of Ni2+/Co2+ cations located on the corner occurs during the sulphide formation reaction at surfaces. Hence, the edge sites of sulphide (Ni/CoS) nuclei act as the active sites to react with S2− anion relese through slow diffusion H2S in aqueous solution preferentially form chain-like sulfide nuclei resulting in formation of Ni0.5C0.5S nano-chains. The nano-chains’ average diameter was ∼60 nm as seen in the HRTEM images. The nano-chain structure can allow a fast electron transfer within the material required for a high rate/fast electrochemical capacitance/charge storage of the materials. The development of nano-chain architectures in Ni0.5Co0.5S electrode materials is significant because it has the potential to enhance the electrode's performance by reducing the diffusion length, facilitating a larger surface area for contact between the active materials and the electrolyte, and improving the kinetics of electrochemical reactions, leading to improved energy storage and conversion properties.81
 | ||
| Fig. 4 (a) SEM images, (b) EDX analysis image of the prepared Ni0.5Co0.5S nano-chains, (c and d) HRTEM images of Ni0.5Co0.5S nano-chains. | ||
Electrochemical studies
The electrochemical behavior was investigated using galvanostatic charge–discharge and cyclic voltammetry (CV) to evaluate the capability and suitability of the material for energy storage as supercapacitors. The charge-storage properties of the Ni0.5Co0.5S electrodes were evaluated using cyclic voltammetry (CV) in a three-electrode setup. The counter electrode consisted of a platinum electrode; the working electrode was a Ni0.5Co0.5S nano-chain coated carbon paper with an area of 1 cm2. In contrast, a saturated Hg/HgO electrode in 1 M KOH solution was used as the reference electrode in the KOH electrolyte. The electrochemical parameters of the working electrode can be analyzed by calculating the charge storage capacity of the materials using eqn (4) in conjunction with the CV curve. | (4) |
The calculation of the charge storage capacity in mA h g−1 can be determined using eqn (5).
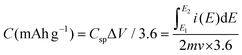 | (5) |
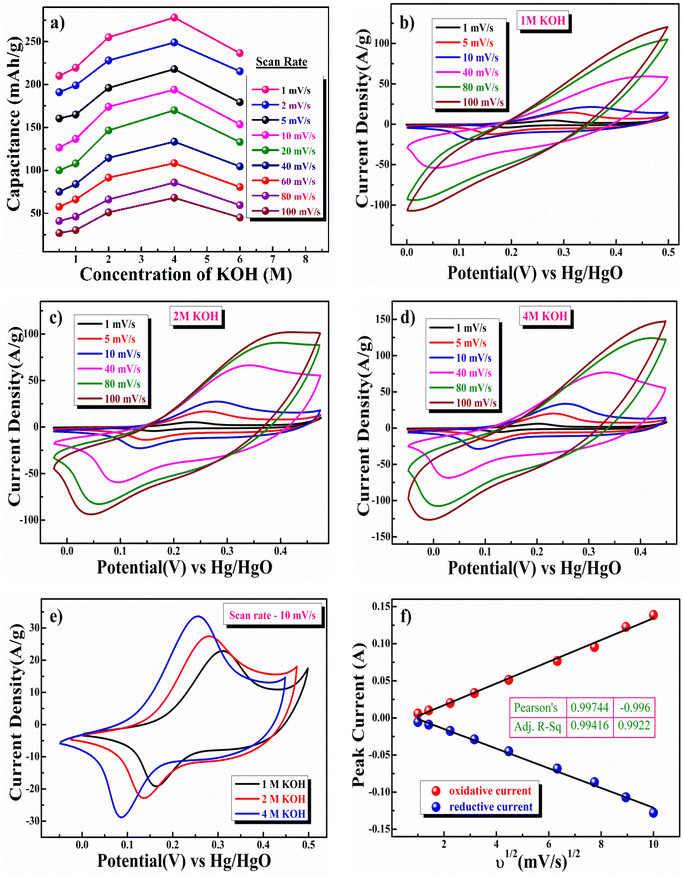
In this equation, Csp is the specific charge storage capacitance in F g−1, the integral  is the total voltammetric charge acquired by integrating both positive and negative sweeps in CV, (E2 − E1) is the width of the potential window (V), m is the mass (g) of the active material, and υ is the scan rate (V s−1). Although the capacitance values in the forward and backward directions do not precisely match, we have used the average value; as a result, the factor 1/2 is employed to create the holistic picture. The CV data were collected at different KOH concentrations, namely 0.5, 1, 2, 4, and 6 M. Fig. 5(a–d) illustrates the CV plot between the concentration of KOH and the mass-specific capacitance. The mass-specific capacitances were determined using eqn (4) and (5) and were found to be 1512, 1580, 1836, 2001.6, and 1703 F g−1 at a scan rate of 1 mV s−1 in KOH solutions with different concentrations ranging from 0.5, 1, 2, 4, to 6 M, respectively.
is the total voltammetric charge acquired by integrating both positive and negative sweeps in CV, (E2 − E1) is the width of the potential window (V), m is the mass (g) of the active material, and υ is the scan rate (V s−1). Although the capacitance values in the forward and backward directions do not precisely match, we have used the average value; as a result, the factor 1/2 is employed to create the holistic picture. The CV data were collected at different KOH concentrations, namely 0.5, 1, 2, 4, and 6 M. Fig. 5(a–d) illustrates the CV plot between the concentration of KOH and the mass-specific capacitance. The mass-specific capacitances were determined using eqn (4) and (5) and were found to be 1512, 1580, 1836, 2001.6, and 1703 F g−1 at a scan rate of 1 mV s−1 in KOH solutions with different concentrations ranging from 0.5, 1, 2, 4, to 6 M, respectively.
As seen in Fig. 5a, the specific capacitance exhibits an initial upward trend as the OH− concentration increases and reaches its peak capacitance in a 4 M KOH solution. Subsequently, the particular capacitance diminishes with further escalation of the OH− concentration. The Ni0.5Co0.5S sample has a higher specific capacitance in 4 M KOH due to the optimum number of charged ions trapped/stored on the porous surface. The electrolyte's ionic conductivity decreases when its concentration exceeds 4 M KOH. This decrease can be attributed to the slow migration of ions primarily due to the high probability of ion collision and the strong electrostatic interaction between anions and cations, leading to the sluggish formation of a double layer or surface absorptions of ions, resulting in a lower specific capacitance of the material in 6 M KOH electrolyte.82–85
One pair of clearly distinct redox peaks, located at +0.20/+0.12 V, is visible on the CV curve in Fig. 5d (4 M KOH). The pseudocapacitive behavior in conjunction with surface redox (electrosorption) is explained by the CV curve's nature.86 Thus non-rectangular and quasi-reversible curves were obtained in the potential range of −0.05 to +0.45 V, demonstrating that surface redox intercalation followed pseudocapacitive storage. Redox peaks are the result of the electrochemical charge transfer reaction involving the interconversion of Co2+ to Co3+ and Ni2+ to Ni3+ reversibly through electrosorption (redox) of OH− ions. Furthermore, the redox activity increases with the substitution of Ni atoms at the Co-site of the CoS base structure. The synergistic interaction between Co and Ni leads to a very high charge storage capacity of the Ni0.5Co0.5S electrode. The following chemical reaction equation is responsible for the redox-mediated charge storage on the electrode.73
| Ni0.52+Co0.52+S + OH− = Co0.53+Ni0.53+S(OH−) + e− | (6) |
Later studies were carried out in 4 M KOH electrolyte only as the best activity of the electrode was found in this electrolyte. The capacitance of a 4 M KOH solution was determined by measuring it at various scan rates ranging from 1, 5, 10, 20, 40, 80, and 100 mV s−1 and the corresponding capacitance values were found to be 2001.6, 1569.6, 1397, 1224, 960.5, 617, and 489.6 F g−1, respectively.
The specific capacitance decreases as the scan rates increase due to incomplete absorption coupled with surface redox at high scan rates. As the scan rate increases, the cathodic and anodic peaks shift linearly to a negative and positive potential, respectively. At the same time, the almost unchanged shapes of each curve demonstrated the high reversibility and rapid charge–discharge response of the Ni0.5Co0.5S electrode. The capacitance retention for a 4 M KOH solution decreases to 31.25% as the scan rate increases from 1 to 100 mV s−1. This decline may be attributed to the insufficient period available for the absorption and diffusion of ions into the active electrode surfaces at higher scan rates. Fig. 5e displays the comparative study of CV at different electrolyte concentrations, spanning from 1 M to 4 M. The redox potential is shifted to a lower value as the concentration of electrolyte (KOH) increases. This phenomenon is considered unfavourable in the context of high-voltage supercapacitor applications.73,82
The relationship between cathodic and anodic peak currents in Fig. 5f indicates a linear correlation with the square root of scan rate (υ1/2). This observation suggests that the CV curve exhibits a behaviour governed by semi-infinite diffusion. The computation of the diffusion coefficient may achieve a more comprehensive understanding of electrode diffusion. The diffusion coefficient of the electrode was obtained using the Randles–Sevick equation (eqn (7)).
| ip = 2.686 × 105 × n3/2AD1/2C0υ1/2 | (7) |
In this equation, ip is the peak current (A), n is the number of electrons transferred during the redox reaction (usually ∼1), A is the area of the electrode in cm2, D is the diffusion coefficient in cm2 s−1, C0 is the concentration of OH− ions in mol cm−3, and υ is the scan rate in V s−1. The hydroxide (OH−) ion diffusion coefficient (D) was found to be 3.14 × 10−8cm2 s−1 for oxidation and 2.50 × 10−8 cm2 s−1 for reduction, respectively.
With 1e−/OH− charge transfer associated with reversible intercalation/de-intercalation of OH− ions, the Ni0.5Co0.5S electrode has a theoretical capacity of 1062 F g−1, in the voltage window of −0.05 to 0.45 V. In our investigation, Ni0.5Co0.5S demonstrated a consistent capacitance of 278 F g−1 at the scan rate of 1 mV s−1. The Ni0.5Co0.5S electrode exhibited a capacitance of 960.5 F g−1 even at a scan rate of 40 mV s−1. Thus, the capacitance of Ni0.5Co0.5S can be described as a combination of double-layer formation coupled with diffusion-controlled surface redox reactions. The electrochemical charge storage reaction is depicted in eqn (8) representing the contribution of both (1) double-layer formation and (2) diffusion-controlled surface redox processes.
| Ni0.52+Co0.52+S + OH− + xKOH ↔ Kx+|Ni0.5−x2+Nix3+Co0.5−x2+Cox3+S|(OH)1+x− | (8) |
The value of x can vary with the scan rates. The contribution of both (1) double-layer formation and (2) diffusion-controlled surface redox processes are determined in later studies. As we know, the charge storage of the electrode is mainly composed of two parts: the surface-controlled double-layer formation and the diffusion-controlled redox process. The charge storage mechanisms/kinetics of the Ni0.5Co0.5S electrode was further evaluated qualitatively using the power law outlined in eqn (9).73,87
| i = avb | (9) |
| i(υ) = k1υ + k2υ1/2 | (10) |
To enhance understanding, eqn (10) was modified as follows:
 | (11) |
In eqn (11), k1υ and k2υ1/2 are the current contributions from the surface capacitive and diffusion-controlled intercalation processes, respectively. Therefore, by determining k1 and k2 from the slope and intercept of the y-axis from linear regression, we are able to measure their contribution to the current density at the fixed specific potential.91
The usual curve {(V)/υ1/2vs. υ1/2} in Fig. 6c represents the contribution of surface-mediated capacitance and diffusion-controlled intercalation processes at different scan rates. Specific contribution at a 10 mV s−1 scan rate is shown in Fig. 6d. Surface capacitance or electrosorption was found to contribute 46.0%, whereas diffusion-controlled intercalation contributed around 54.0%. The amount of charge stored in the outer and inner surfaces was also determined using the Trasatti plot. The total specific capacitance of the electrode, according to Trassati, is influenced by both the inner and outer surface capacitances. This can be expressed as:
| CTotal = Cin + Cout | (12) |
The charge storage mechanism depends on the scan rate used in the study of CV. The y-intercept in Fig. 6e represents the capacitance, which indicates the overall charge storage capacity of the electrode. This is determined by plotting the linear fit of C−1vs. υ1/2 at different scan rates. The outer surface capacitance or charge storage of the electrode is represented by the y-intercept in Fig. 6f, which illustrates the linear fit of C vs. υ−1/2. Cin's measured value was 1337.5 F g−1, accounting for 56% of the overall capacitance value. Similarly, Cout was estimated to be 1049 F g−1, representing 44% of the overall capacitance value. The total capacitance value (Ctotal) was determined to be 2386.5 F g−1. This confirms that the charge storage or capacitance of the Ni0.5Co0.5S electrodes is a combination of redox-mediated diffusion-controlled intercalating process and surface absorption processes.
To better understand the electrochemical characteristics of charge storage of the Ni0.5Co0.5S electrode, galvanostatic charge–discharge (GCD) studies in 4 M KOH aqueous electrolytes were performed. The electrode's specific capacitance can be determined by analysing the charge–discharge curve using eqn (13).71,91
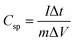 | (13) |
The calculation of the electrode's specific capacity in mA h g−1 requires the modification of eqn (13) as follows:
 | (14) |
Fig. 7a displays the galvanostatic specific capacity/capacitances corresponding to 1, 2, 3, 5, and 10 A g−1 current densities, measured as 267, 221.3, 191.2, 155.8, and 95.5 mA h g−1 (1920, 1593.4, 1376.6, 1121.7, 687.6 F g−1), respectively. The GCD, as mentioned above, showed that as the current density increases, the specific capacitance gradually decreases. The stability of the electrode is shown in Fig. 7b (capacitance value vs. cycle number at different current densities). The Ni0.5Co0.5S electrode's long-term cycling stability at 10 A g−1 for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles is shown in Fig. 7c resulting in 84.0% capacity retention. The electrode's specific capacitance did not vary significantly from its initial capacitance after 10
000 cycles is shown in Fig. 7c resulting in 84.0% capacity retention. The electrode's specific capacitance did not vary significantly from its initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. After continuous charge/discharge of 10
000 cycles. After continuous charge/discharge of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, the electrode's coulombic efficiency
000 cycles, the electrode's coulombic efficiency  was 92.2%, demonstrating the outstanding reversibility of the Ni0.5Co0.5S electrode. The comparison of galvanostatic specific capacitance in mA h g−1 at 1, 2, and 4 M KOH concentrations is shown in Fig. 7d. At a current density of 1 A g−1, the electrode demonstrates a higher specific capacitance in 4 M KOH electrolyte (with an extended discharge time) compared to 1 and 2 M KOH electrolytes. From the GCD experiment, the charge storage capacity (more realistic and applicable capacitance) of the electrode was measured using eqn (13) and (14), which are 214.8, 243.8, and 267 mA h g−1 (1547, 1756, and 1920 F g−1) for concentrations of 1, 2, and 4 M KOH electrolyte, respectively. The comparative specific capacitance observations confirmed an increase in specific capacitance value from 1 M to 4 M KOH concentration, which is similar to the findings of the CV results.
was 92.2%, demonstrating the outstanding reversibility of the Ni0.5Co0.5S electrode. The comparison of galvanostatic specific capacitance in mA h g−1 at 1, 2, and 4 M KOH concentrations is shown in Fig. 7d. At a current density of 1 A g−1, the electrode demonstrates a higher specific capacitance in 4 M KOH electrolyte (with an extended discharge time) compared to 1 and 2 M KOH electrolytes. From the GCD experiment, the charge storage capacity (more realistic and applicable capacitance) of the electrode was measured using eqn (13) and (14), which are 214.8, 243.8, and 267 mA h g−1 (1547, 1756, and 1920 F g−1) for concentrations of 1, 2, and 4 M KOH electrolyte, respectively. The comparative specific capacitance observations confirmed an increase in specific capacitance value from 1 M to 4 M KOH concentration, which is similar to the findings of the CV results.
The electrochemical impendence spectra (EIS) were studied (before and after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 repeated charge–discharge cycles) to evaluate the electrode kinetics and interface resistance at an open circuit potential in the frequency range of 100 kHz to 0.1 Hz. The impedance characteristic as a function of frequency is depicted in the Nyquist plot (Fig. 7e). This plot can be divided into two distinct sections. Firstly, there is a depressed arc observed in the high-frequency region, corresponding to the faradaic responses. Secondly, a straight line is observed in the low-frequency region, indicating a rapid OH− ion diffusion-controlled process. Furthermore, the impedance contribution was ascribed to the impedance distributions across internal series resistance (Rs), charge transfer resistance (Rct), and Warburg impedance (Rw). The internal series resistance (Rs) is represented by the point at which the curve intersects the real axis in the high-frequency region, which includes the resistance of the active material, electrolyte, and connected wires at the electrode–electrolyte interface. The Ni0.5Co0.5S electrode has Rs and Rct values of 1.01 and 0.72, respectively. The lower-frequency data provide insights into the Warburg diffusion resistance shown by the Ni0.5Co0.5S electrode.92 Based on the EIS spectra, the Ni0.5Co0.5S electrode shows good electrochemical stability after 10
000 repeated charge–discharge cycles) to evaluate the electrode kinetics and interface resistance at an open circuit potential in the frequency range of 100 kHz to 0.1 Hz. The impedance characteristic as a function of frequency is depicted in the Nyquist plot (Fig. 7e). This plot can be divided into two distinct sections. Firstly, there is a depressed arc observed in the high-frequency region, corresponding to the faradaic responses. Secondly, a straight line is observed in the low-frequency region, indicating a rapid OH− ion diffusion-controlled process. Furthermore, the impedance contribution was ascribed to the impedance distributions across internal series resistance (Rs), charge transfer resistance (Rct), and Warburg impedance (Rw). The internal series resistance (Rs) is represented by the point at which the curve intersects the real axis in the high-frequency region, which includes the resistance of the active material, electrolyte, and connected wires at the electrode–electrolyte interface. The Ni0.5Co0.5S electrode has Rs and Rct values of 1.01 and 0.72, respectively. The lower-frequency data provide insights into the Warburg diffusion resistance shown by the Ni0.5Co0.5S electrode.92 Based on the EIS spectra, the Ni0.5Co0.5S electrode shows good electrochemical stability after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 consecutive charge–discharge cycles compared to the initial. The phase angle of the electrode concerning the applied frequency is seen in Fig. 7f of the Bode plot. The Ni0.5Co0.5S electrode's phase angles were 52.2° at the beginning and 45.48° at the end of 10
000 consecutive charge–discharge cycles compared to the initial. The phase angle of the electrode concerning the applied frequency is seen in Fig. 7f of the Bode plot. The Ni0.5Co0.5S electrode's phase angles were 52.2° at the beginning and 45.48° at the end of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, respectively. The confirmation of the pseudocapacitive charge storage capacity of the Ni0.5Co0.5S electrode is shown by observing a reduced phase angle (about 50°), consistent with the findings depicted in the Nyquist plot.4,93 After 10
000 cycles, respectively. The confirmation of the pseudocapacitive charge storage capacity of the Ni0.5Co0.5S electrode is shown by observing a reduced phase angle (about 50°), consistent with the findings depicted in the Nyquist plot.4,93 After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles, the active electrode material of Ni0.5Co0.5S was extracted from the electrode and examined by powder XPS, Raman spectroscopy, and XRD to further investigate the long-term structural stability of the Ni0.5Co0.5S electrode during electrochemical testing. The inset in Fig. S1c† shows the Raman spectrum of the Ni0.5Co0.5S electrode after a continuous charge–discharge experiment. It shows peaks at 150.6, 394.5, 467, 513 and 635 cm−1 which can be indexed to the Raman-active vibrational peaks of the Ni0.5Co0.5S sample, which correspond to the typical phonon modes F2g, Eg, T2g and A1g respectively. The two A1g modes (150.6 and 635 cm−1) can be attributed to the stretching of the S atom in the S–M–S (M = Ni & Co) bond. The XPS spectra shown in ESI Fig. S3† reflect the electrochemical processes and the reversible conversion of the elemental states of Ni and Co during charge and discharge, contributing to higher capacitance. The binding energy is shifted to a lower value which can be attributed to the accumulation of charge at the electrode surface which is often associated with the adsorption of species or the formation of an electrical double layer. Fig. S4† shows the XRD spectra after continuous charge–discharge, indicating that the crystal structure of the electrode has led to a very small deterioration in the performance after continuous charge–discharge experiments as there is no change in the crystal structure of the electrode and the crystal structure of the parent Ni0.5Co0.5S remains intact even after 10
000 charge–discharge cycles, the active electrode material of Ni0.5Co0.5S was extracted from the electrode and examined by powder XPS, Raman spectroscopy, and XRD to further investigate the long-term structural stability of the Ni0.5Co0.5S electrode during electrochemical testing. The inset in Fig. S1c† shows the Raman spectrum of the Ni0.5Co0.5S electrode after a continuous charge–discharge experiment. It shows peaks at 150.6, 394.5, 467, 513 and 635 cm−1 which can be indexed to the Raman-active vibrational peaks of the Ni0.5Co0.5S sample, which correspond to the typical phonon modes F2g, Eg, T2g and A1g respectively. The two A1g modes (150.6 and 635 cm−1) can be attributed to the stretching of the S atom in the S–M–S (M = Ni & Co) bond. The XPS spectra shown in ESI Fig. S3† reflect the electrochemical processes and the reversible conversion of the elemental states of Ni and Co during charge and discharge, contributing to higher capacitance. The binding energy is shifted to a lower value which can be attributed to the accumulation of charge at the electrode surface which is often associated with the adsorption of species or the formation of an electrical double layer. Fig. S4† shows the XRD spectra after continuous charge–discharge, indicating that the crystal structure of the electrode has led to a very small deterioration in the performance after continuous charge–discharge experiments as there is no change in the crystal structure of the electrode and the crystal structure of the parent Ni0.5Co0.5S remains intact even after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles.94–96
000 charge–discharge cycles.94–96
The charge storage behaviour of Ni0.5Co0.5S electrodes was also investigated in a neutral 0.5 M Na2SO4 electrolyte. ESI Fig. S5a† shows well-separated redox peaks in the CV curve at various scan speeds (+0.64/+0.52 V at 1 mV s−1 scan rate). For the Ni0.5Co0.5S electrode in 0.5 M Na2SO4 electrolyte, the calculated specific capacitance (which depends on the scan rate) was found to be 414.4, 290, 207.5, 140, 95.6, and 82.8 F g−1 for the scan rates of 1, 5, 10, 40, 80, and 100 mV s−1 respectively. The quantitative GCD measurements findings are shown in ESI Fig. S5b,† and values were found to be 57, 43, 33.3, 19.3, and 9 mA h g−1 (410.4, 309.6, 239.8, 138.9, and 64.8 F g−1) at the current density of 1, 2, 3, 5, and 10 A g−1, respectively.
Additionally, a comparative study compared the effects of the anion on the electrolyte between 0.5 M Na2SO4 and 4 M KOH. The Ni0.5Co0.5S electrode's comparative CV curve in 0.5 M Na2SO4 and 4 M KOH electrolytes is shown in ESI Fig. S5c.† There were observed shifts in the position of the redox peaks while transitioning from an OH electrolyte to a Na2SO4 electrolyte. Additionally, it was found that the redox peaks in 4 M KOH were more dominant than those in 0.5 M Na2SO4; this could be due to the size difference between the hydration radii of sulfate ions (3.79 Å) and hydroxyl ions (3 Å). The larger size of SO42− results in slow kinetics in diffusion-controlled processes resulting in lower charge storage in the neutral Na2SO4 electrolyte. Furthermore, the current response of the CV curve may be attributed to the greater molar conductance of OH− ions (198 cm2 Ω mol−1) in the KOH electrolyte, as compared to the conductivity of SO42− ions (79.8 cm2 Ω mol−1) in Na2SO4.80,84 GCD studies in 0.5 M Na2SO4 and 4 M KOH electrolytes were used to evaluate the electrode's capacitance quantitatively. In 4 M KOH compared to the 0.5 M Na2SO4 electrolyte, the electrode provides a greater specific capacitance value (longer discharge time), as illustrated in ESI Fig. S5d.† These investigations confirm the excellent electrode performance of the electrode in aqueous KOH electrolyte.
Two electrode full cell test in asymmetric supercapacitor cell (ASC) mode
To explore the real applicability of the Ni0.5Co0.5S electrode, the two-electrode full cell was fabricated in ASC mode in a 4 M KOH electrolyte where AC was employed as a counter electrode. The storage capacities of the positive and negative electrodes must be balanced using eqn (15) to estimate the maximum specific capacitance during the full-cell test. | (15) |
To balance the cell's capacity to store charges, eqn (16) was used to calculate the mass ratio (m+/m−) of the materials of an individual electrode that make up the positive and negative electrodes.
 | (16) |
With a fixed scan rate (10 mV s−1), separate CV curves for Ni0.5Co0.5S (positive electrode) and AC (negative electrode) are shown in Fig. 8a with a single operating potential window range. In the ESI (Fig. S6 and S7†), the electrochemical properties of the constructed AC electrode are shown using CV and GCD. The specific capacitance of the AC electrode was 96.4 mA h g−1 (347 F g−1) at 1 A g−1 from the GCD experiment and 91.3 mA h g−1 (328.5 F g−1) at a scan rate of 1 mV s−1 in CV. Because AC is employed to improve the conductivity of the active electrode, the contribution to capacitance caused by the AC electrode is subtracted when determining the Ni0.5Co0.5S electrode's electrochemical charge storage capacity. Thus, the acquired data solely displays the Ni0.5Co0.5S electrode's capacitive value. The calculated mass ratio (m+/m) for the asymmetric cell was 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.6, while the active material's weight was found to be 4.0 mg (activated carbon and PVDF weights excluded).
3.6, while the active material's weight was found to be 4.0 mg (activated carbon and PVDF weights excluded).
Two-electrode Ni0.5Co0.5S//AC, asymmetric supercapacitor cells (ASCs), are shown in Fig. 8b with their CV plots at scan rates ranging from 1 to 100 mV s−1 in the potential window 0 to 1.5 V. The GCD plot of the full cell at various current densities is shown in Fig. 8c. At constant current densities of 1, 2, 3, 5, and 10 A g−1, the specific capacitances of ASCs derived from GCD curves were 254, 202.8, 173.8, 149.7, and 106.5 mA h g−1 (609.6, 487, 417, 359.3, and 255.6 F g−1), respectively. The EIS (Nyquist and Bode) plots at an open circuit potential in the 100 kHz to 0.1 Hz frequency range are shown in Fig. 8d and e, confirming the superior charge transfer, higher specific capacitance, and excellent retention of the electronic structure of the Ni0.5Co0.5S electrode//AC full cell in ASC mode.
However, a slight reduction in capacity is due to the partially irreversible nature of the electrode, which was observed during the cycling test. The EIS result supports the evidence for cycling stability by displaying a slight variation in the cell's internal and charge transfer resistances before and after the cycling test.
The Ni0.5Co0.5S electrode//AC full cell, as shown in Fig. 8f, has exceptional long-term cycling stability, with 89.5% capacity retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Furthermore, after 10
000 cycles. Furthermore, after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, the Ni0.5Co0.5S electrode//AC full cell's coulombic efficiency has decreased by merely 5% from its initial value.
000 cycles, the Ni0.5Co0.5S electrode//AC full cell's coulombic efficiency has decreased by merely 5% from its initial value.
Eqns (17) and (18) were utilized to calculate the power and energy density of the ASC.
 | (17) |
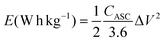 | (18) |
| Material | Energy density (W h kg−1) | Power density (kW kg−1) | Cycling stability (%) after “n” cycles | Capacitance (3 electrode system) | Electrolyte solution | Ref. |
|---|---|---|---|---|---|---|
| β-NiS | 163 | 0.5 | 90.0%, 2500 | 1578 F g−1 at 1 A g−1 | 2 M KOH | 4 |
| NiCo2S4 hollow spheres | 42.3 | 0.48 | 78.6%, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1036 F g−1 at 1 A g−1 | 6 M KOH | 60 |
| Ni1.5Co1.5S4@CNTs | 61.2 | 0.8 | 91.5% 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
210 mA h g−1 at 1 A g−1 | 2 M KOH | 66 |
| (Co0.5Ni0.5)9S8/N-C | — | — | 83%, 100 | 723.7 A h g−1 at 1 A g−1 | 1 M NaClO4 in (PC + FEC) | 70 |
| mNi0.32Co0.68S2 | 37 | 0.8 | 66.0%, 1000 | 1698 F g−1 at 2 A g−1 | 2 M KOH | 71 |
| NiXCo1−xS@C-3 | 45.31 | 0.75 | 91.0%, 8000 | 709 C g−1 at 1 A g−1 | 6 M KOH | 73 |
| Co0.5Ni0.5C2O4 | 283 | 0.82 | 90.7%, 2500 | 2396 F g−1 at 1 A g−1 | 2 M KOH | 91 |
| Ni-Co-S4 nanosheets | 60 | 1.8 | 90.1%, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1285 F g−1 at 100 A g−1 | 1 M KOH | 97 |
| Mn incorporated MoS2 | 48.8 | 5.0 | 77.0%, 5000 | 430 F g−1 at 5 mV s−1 | 0.5 M Na2SO4 | 98 |
| CoS-AC//AC | 44.2 | 0.4 | 77.5%, 2000 | 797.79 F g−1 at 10 A g−1 | K3Fe(CN)6 + KOH | 39 |
| FeCo-A-S | 46.1 | 8.0 | 79.3%, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2932.2 F g−1 at 1 A g−1 | 3 M KOH | 99 |
| 3D NiCo2S4 nanostructure | 38.64 | 1.33 | 72.7%, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2363.1 F g−1 at 2.5 A g−1 | 6 M KOH | 100 |
| Ni3S2/NF | 2.93 | 0.38 | 81.25%, 6000 | 255.3 mA h g−1 at 3 A g−1 | 1 M KOH | 101 |
| KNiPO4 | 200 | 0.82 | 92.3%, 2200 | 168.5 mA h g−1 at 1 A g−1 | 2 M KOH | 102 |
| SnS-SnS2@GO | — | — | 69.8%, 100 | 450.6 mA h g−1 at 0.1 A g−1 | 1 M NaClO4 in (EC + DMC + MEC) | 103 |
| Ni 0.5 Co 0.5 S nano-chains | 257 | 0.73 |
89.5%, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 000 000
|
267 mA h g −1 at 1 A g −1 | 4 M KOH | Present work |
Conclusions
In summary, single-phase hexagonal Ni0.5Co0.5S nano-chains consisting of interconnected nano-spheres were synthesized using a simple H2S-mediated heterogeneous two-phase (gas diffusion in liquid) precipitation route in an aqueous medium. Ni0.5Co0.5S nano-chains developed in a single phase due to the controlled precipitation of the solid phase caused by the slow diffusion of H2S gas into the liquid phase. The XRD pattern confirmed the single-phase hexagonal Ni0.5Co0.5S nano-crystalline material, and the average crystallite size, as determined by the Debye–Scherrer equation, was around 21 nm. The nano-chain structure may provide rapid electron transport inside the material for a high/fast rate of electrochemical charge storage/capacitance of the materials. Having a specific capacitance between −0.05 and 0.45 V of 2001.6 F g−1 at 1 mV s−1 in CV and 267 mA h g−1 (1920 F g−1) at 1 A g−1 in the GCD experiment, the Ni0.5Co0.5S electrode demonstrated an excellent pseudocapacitive charge storage performance. Furthermore, the surface (outer) and intercalative (inner) charges stored by the Ni0.5Co0.5S electrodes were 46.0%, and 54.0%, respectively. This suggests that a predominant redox-mediated diffusion-controlled pseudocapacitive mechanism in combination with surface capacitance (electrosorption) is the cause of the materials’ high charge storage capacity. An optimal energy density of 257.0 W h kg−1 and a power density of 0.73 kW kg−1 were attained by the Ni0.5Co0.5S//AC full cell in the 1.5 V voltage window with a 4 M KOH electrolyte at a current rate of 1 A g−1. The study suggests that the capacitance value and charge storage characteristics are better or on par with most previously reported transition-metal sulfide nanostructured pseudocapacitors and supercapacitors. These findings demonstrate the potential of Ni0.5Co0.5S nano-chains as a pseudocapacitive electrode for large-scale energy storage applications.Conflicts of interest
No conflicts of interest are disclosed by the authors.Acknowledgements
The authors express gratitude to the Department of Chemistry, IIT (BHU) for its support and resources. The authors would also like to thank the Central Instrument Facility Centre (CIFC), IIT-BHU, Varanasi, for providing characterization facilities and services. Dr Asha Gupta acknowledges financial assistance from the Science and Engineering Research Board (SERB) of India (Project no.: R&D/SERB/Chy/19-20/03; File. No: SRG/2019/000605).References
- Y. Jiang and J. Liu, Definitions of Pseudocapacitive Materials: A Brief Review, Energy Environ. Mater., 2019, 2, 30–37 CrossRef.
- M. Y. Worku, Recent Advances in Energy Storage Systems for Renewable Source Grid Integration: A Comprehensive Review, Sustainability, 2022, 14, 5985 CrossRef CAS.
- X. He and X. Zhang, A comprehensive review of supercapacitors: Properties, electrodes, electrolytes and thermal management systems based on phase change materials, J. Energy Storage, 2022, 56, 106023 CrossRef.
- V. Kushwaha, A. Gupta, R. B. Choudhary, K. D. Mandal, R. Mondal and P. Singh, Nanocrystalline β-NiS; A Redox-mediated Electrode in Aqueous Electrolyte for Pseudo-capacitor/Supercapacitor Applications, Phys. Chem. Chem. Phys., 2023, 25, 555 RSC.
- P. Simon and Y. Gogotsi, Materials for electrochemical capacitors, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- M. Winter and R. J. Brodd, What Are Batteries, Fuel Cells, and Supercapacitors?, Chem. Rev., 2004, 104, 4245–4269 CrossRef CAS PubMed.
- S. Balasubramaniam, A. Mohanty, S. K. Balasingam, S. J. Kim and A. Ramadoss, Comprehensive Insight into the Mechanism, Material Selection and Performance Evaluation of Supercapatteries, Nano-Micro Lett., 2020, 12, 1–46 CrossRef PubMed.
- L. Yu and G. Z. Chen, Supercapatteries as High-Performance Electrochemical Energy Storage Devices, Electrochem. Energy Rev., 2020, 3, 271–285 CrossRef.
- L. Yu and G. Z. Chen, Redox electrode materials for supercapatteries, J. Power Sources, 2016, 326, 604–612 CrossRef CAS.
- M. E. Sahin, F. Blaabjerg and A. Sangwongwanich, A Comprehensive Review on Supercapacitor Applications and Developments, Energies, 2022, 15, 674 CrossRef CAS.
- S. Cheng, Y. Zhang, Y. Liu, Z. Sun, P. Cui, J. Zhang, X. Hua, Q. Su, J. Fu and E. Xie, Energizing Fe2O3-based supercapacitors with tunable surface pseudocapacitance via, physical spatial-confining strategy, Chem. Eng. J., 2021, 406, 126875 CrossRef CAS.
- Y. Xiang, Z. Yang, S. Wang, M. S. A. Hossain, J. Yu, N. A. Kumar and Y. Yamauchi, Pseudocapacitive behavior of the Fe2O3 anode and its contribution to high reversible capacity in lithium ion batteries, Nanoscale, 2018, 10, 18010 RSC.
- M. Jia, T. Qi, Q. Yuan, P. Zhao and M. Jia, Polypyrrole Modified MoS2 Nanorod Composites as Durable Pseudocapacitive Anode Materials for Sodium-Ion Batteries, Nanomaterials, 2022, 12, 2006 CrossRef CAS PubMed.
- T. Yao, M. Yao and H. Wang, Porous graphene-like MoS2/carbon hierarchies for high-performance pseudocapacitive sodium storage, Sustainable Energy Fuels, 2022, 6, 822 RSC.
- P. Man, Q. Zhang, Z. Zhou, M. Chen, J. Yang, Z. Wang, Z. Wang, B. He, Q. Li, W. Gong, W. Lu, Y. Yao and L. Wei, Engineering MoS2 Nanosheets on Spindle-Like α-Fe2O3 as High-Performance Core-Shell Pseudocapacitive Anodes for Fiber-Shaped Aqueous Lithium-Ion Capacitors, Adv. Funct. Mater., 2020, 30, 2003967 CrossRef CAS.
- M. Mirzaeian, N. Akhanova, M. Gabdullin, Z. Kalkozova, A. Tulegenova, S. Nurbolat and K. Abdullin, Improvement of the Pseudocapacitive Performance of Cobalt Oxide-Based Electrodes for Electrochemical Capacitors, Energies, 2020, 13, 5228 CrossRef CAS.
- J. Li, G. Zanab and Q. Wu, An ultra-high-performance anode material for supercapacitors: self-assembled long Co3O4 hollow tube network with multiple heteroatom (C-, N- and S-) doping, J. Mater. Chem. A, 2016, 4, 9097 RSC.
- D. Yan, Y. Huang, Y. Yua and G. Cao, β-NaVO3 as a pseudocapacitive anode material for sodium-ion batteries, New J. Chem., 2023, 47, 1268 RSC.
- S. J. Patil, N. R. Chodankar, Y. K. Han and D. W. Lee, Carbon alternative pseudocapacitive V2O5 nanobricks and δ-MnO2 nanoflakes @ α-MnO2 nanowires hetero-phase for high-energy pseudocapacitor, J. Power Sources, 2020, 453, 227766 CrossRef CAS.
- S. Fu, Q. Yu, Z. Liu, P. Hu, Q. Chen, S. Feng, L. Mai and L. Zhou, Yolk-shell Nb2O5 microspheres as intercalation pseudocapacitive anode materials for high-energy Li-ion capacitors, J. Mater. Chem. A, 2019, 7, 11234 RSC.
- N. Zhaoa, H. Fana, M. Zhanga, J. Maa, Z. Dua, B. Yana, H. Lia and X. Jiang, Simple electrodeposition of MoO3 film on carbon cloth for high-performance aqueous symmetric supercapacitors, Chem. Eng. J., 2020, 390, 124477 CrossRef.
- L. Xu, W. Zhou, S. Chao, Y. Liang, X. Zhao, C. Liu and J. Xu, Advanced Oxygen-Vacancy Ce-Doped MoO3 Ultrathin Nanoflakes Anode Materials Used as Asymmetric Supercapacitors with Ultrahigh Energy Density, Adv. Energy Mater., 2022, 12, 2200101 CrossRef CAS.
- N. Naresh, P. Jena and N. Satyanarayana, Facile synthesis of MoO3/rGO nanocomposite as anode materials for high performance lithium-ion battery applications, J. Alloys Compd., 2019, 810, 151920 CrossRef CAS.
- N. Jabeen, Q. Xia, S. V. Savilov, S. M. Aldoshin, Y. Yu and H. Xia, Enhanced Pseudocapacitive Performance of α-MnO2 by Cation Preinsertion, ACS Appl. Mater. Interfaces, 2016, 8, 33732–33740 CrossRef CAS PubMed.
- P. Geng, S. Zheng, H. Tang, R. Zhu, L. Zhang, S. Cao, H. Xue and H. Pang, Transition Metal Sulfides Based on Graphene for Electrochemical Energy Storage, Adv. Energy Mater., 2018, 8, 1703259 CrossRef.
- W. Liu, H. Niu, J. Yang, K. Cheng, K. Ye, K. Zhu, G. Wang, D. Cao and J. Yan, Ternary Transition Metal Sulfides Embedded in Graphene Nanosheets as Both the Anode and Cathode for High-Performance Asymmetric Supercapacitors, Chem. Mater., 2018, 30, 1055–1068 CrossRef CAS.
- C. K. Kanade, H. Seok, V. K. Kanade, K. Aydin, H. U. Kim, S. B. Mitta, W. J. Yoo and T. Kim, Low-Temperature and Large-Scale Production of a Transition Metal Sulfide Vertical Heterostructure and Its Application for Photodetectors, ACS Appl. Mater. Interfaces, 2021, 13, 8710–8717 CrossRef CAS PubMed.
- P. Wang, D. Wu, K. Zhang and X. Wu, Two-Dimensional Quaternary Transition Metal Sulfide CrMoA2S6 (A = C, Si, or Ge): A Bipolar Antiferromagnetic Semiconductor with a High Néel Temperature, J. Phys. Chem. Lett., 2022, 13, 3850–3856 CrossRef CAS PubMed.
- Q. Zhu, Q. Xu, M. Du, X. Zeng, G. Zhong, B. Qiu and J. Zhang, Recent Progress of Metal Sulfide Photocatalysts for Solar Energy Conversion, Adv. Mater., 2022, 34, 2202929 CrossRef CAS PubMed.
- J. Chen, D. H. C. Chua and P. S. Lee, The Advances of Metal Sulfides and In Situ Characterization Methods beyond Li Ion Batteries: Sodium, Potassium, and Aluminum Ion Batteries, Small Methods, 2020, 4, 1900648 CrossRef CAS.
- J. Rehman, K. Eid, R. Ali, X. Fan, G. Murtaza, M. Faizan, A. Laref, W. Zheng and R. S. Varma, Engineering of Transition Metal Sulfide Nanostructures as Efficient Electrodes for High-Performance Supercapacitors, ACS Appl. Energy Mater., 2022, 5, 6481–6498 CrossRef CAS.
- O. Ola and K. Thummavichai, Layered tungsten-based composites and their pseudocapacitive and electrocatalytic performance, Mater. Chem. Front., 2022, 6, 737–747 RSC.
- A. D. Adhikari, S. Singh and I. Lahiri, WS2@PPy heterostructured high performance supercapacitor self-powered by PVDF piezoelectric separator, J. Alloys Compd., 2023, 939, 168713 CrossRef.
- V. Shrivastav, S. Sundriyal, V. Shrivastav, U. K. Tiwari and A. Deep, WS2/Carbon Composites and Nanoporous Carbon Structures Derived from Zeolitic Imidazole Framework for Asymmetrical Supercapacitors, Energy Fuels, 2021, 35, 15133–15142 CrossRef CAS.
- J. Chen, X. Zhou, C. Mei, J. Xu, S. Zhou and C. P. Wong, Pyrite FeS2 nanobelts as high-performance anode material for aqueous pseudocapacitor, Electrochim. Acta, 2016, 222, 172–176 CrossRef CAS.
- X. Xu, L. Li, H. Chen, X. Guo, Z. Zhang, J. Liu, C. Mao and G. Li, Constructing heterostructured FeS2/CuS nanospheres as high rate performance lithium ion battery anodes, Inorg. Chem. Front., 2020, 7, 1900 RSC.
- Y. Hu and L. Zhang, Boosting High-Rate Sodium Storage of CuS via a Hollow Spherical Nanostructure and Surface Pseudocapacitive Behavior, ACS Appl. Energy Mater., 2021, 4, 8901–8909 CrossRef CAS.
- R. Li, J. Tian, W. Wu, Q. Wang, C. Zhang, C. Zhou and L. Yang, CuS/polyaniline nanoarray electrodes for application in high-performance flexible supercapacitors, J. Energy Storage, 2022, 55, 105385 CrossRef.
- T. Xu, Z. Wang, G. Wang, L. Lu, S. Liu, S. Gao, H. Xu and Z. Yu, One-pot synthesis of a CoS-AC electrode in a redox electrolyte for high-performance supercapacitors, J. Appl. Electrochem., 2019, 49(11), 1069–1077 CrossRef CAS.
- P. Justin and G. R. Rao, CoS spheres for high-rate electrochemical capacitive energy storage application, Int. J. Hydrogen Energy, 2010, 35, 9709–9715 CrossRef CAS.
- K. Subramani, N. Sudhan, R. Divya and M. Sathish, All-solid-state asymmetric supercapacitors based on cobalt hexacyanoferrate-derived CoS and activated carbon, RSC Adv., 2017, 7, 6648–6659 RSC.
- N. Arsalani, L. S. Ghadimi, I. Ahadzadeh, A. G. Tabrizi and T. Nann, Green Synthesized Carbon Quantum Dots/Cobalt Sulfide Nanocomposite as Efficient Electrode Material for Supercapacitors, Energy Fuels, 2021, 35, 9635–9645 CrossRef CAS.
- B. Qu, Y. Chen, M. Zhang, L. Hu, D. Lei, B. Lu, Q. Li, Y. Wang, L. Chen and T. Wang, β-Cobalt sulfide nanoparticles decorated graphene composite electrodes for high capacity and power supercapacitors, Nanoscale, 2012, 4, 7810–7816 RSC.
- T. Zhu, Z. Wang, S. Ding, J. Chen and X. W. Lou, Hierarchical nickel sulfide hollow spheres for high performance supercapacitors, RSC Adv., 2011, 1, 397–400 RSC.
- J. Yang, X. Duan, W. Guo, D. Li, H. Zhang and W. Zheng, Electrochemical performances investigation of NiS/rGO composite as electrode material for supercapacitors, Nano Energy, 2014, 5, 74–81 CrossRef CAS.
- X. Lei, M. Li, M. Lu and X. Guan, Electrochemical Performances Investigation of New Carbon-Coated Nickel Sulfides as Electrode Material for Supercapacitors, Materials, 2019, 12, 3509 CrossRef CAS PubMed.
- P. Gaikar and S. P. Pawar, et al., Synthesis of nickel sulfide as a promising electrode material for pseudocapacitor application, RSC Adv., 2016, 6, 112589–112593 RSC.
- H. Wang, J. Wang and M. Liang, et al., Novel Dealloying-Fabricated NiS/NiO Nanoparticles with Superior Cycling Stability for Supercapacitors, ACS Omega, 2021, 6, 17999–18007 CrossRef CAS PubMed.
- Y. J. Lee, B. S. Reddy, H. A. Hong, K. W. Kim, S. J. Cho, H. J. Ahn, J. H. Ahn and K. K. Cho, Synthesis and electrochemical properties of nickel sulfide/carbon composite as anode material for lithium-ion and sodium-ion batteries, Int. J. Energy Res., 2022, 46, 16883–16895 CrossRef CAS.
- N. Feng, D. Hu, P. Wang, X. Sun, X. Li and D. He, Growth of nanostructured nickel sulfide films on Ni foam as high-performance cathodes for lithium ion batteries, Phys. Chem. Chem. Phys., 2013, 15, 9924 RSC.
- X. Zhang, Y. Zhao, C. Zhang, C. Wu, X. Li, M. Jin, W. Lan, X. Pan, J. Zhou and E. Xie, Cobalt sulfide embedded carbon nanofibers as a self-supporting template to improve lithium ion battery performances, Electrochim. Acta, 2021, 366, 137351 CrossRef CAS.
- B. Wang, Y. Cheng, H. Su, M. Cheng, Y. Li, H. Geng and Z. Dai, Boosting Transport Kinetics of Cobalt Sulfides Yolk-Shell Spheres by Anion Doping for Advanced Lithium and Sodium Storage, ChemSusChem, 2020, 13, 4078–4085 CrossRef CAS PubMed.
- Y. Liu, S. Niu and R. Hu, Preparation and supercapacitive performance of manganese-cobalt sulfide/silver nanowires/graphene ternary nanocomposites, J. Mater. Sci.: Mater. Electron., 2021, 32(11), 14337–14346 CrossRef CAS.
- M. S. Javed, H. Lei, J. Li, Z. Wang and W. Mai, Construction of highly dispersed mesoporous bimetallic-sulfide nanoparticles locked in N-doped graphitic carbon nanosheets for high energy density hybrid flexible pseudocapacitors, J. Mater. Chem. A, 2019, 7, 17435 RSC.
- M. R. Pallavolu, S. Vallem, R. R. Nallapureddy, S. Adem and S. W. Joo, Self Assembled Hierarchical Silkworm-Type Bimetallic Sulfide (NiMo3S4) Nanostructures Developed on S-g-C3N4 Sheets: Promising Electrode Material for Supercapacitors, ACS Appl. Energy Mater., 2023, 6, 812–821 CrossRef CAS.
- Q. Shao, X. Liu, J. Dong, L. Liang, Q. Zhang, P. Li, S. Yang, X. Zang and N. Cao, Vulcanization Conditions of Bimetallic Sulfides Under Different Sulfur Sources for Supercapacitors: A Review, J. Electron. Mater., 2023, 52, 1769–1784 CrossRef CAS.
- G. Fang, Z. Wu, J. Zhou, C. Zhu, X. Cao, T. Lin, Y. Chen, C. Wang, A. Pan and S. Liang, Observation of Pseudocapacitive Effect and Fast Ion Diffusion in Bimetallic Sulfides as an Advanced Sodium-Ion Battery Anode, Adv. Energy Mater., 2018, 8, 1703155 CrossRef.
- X. Chen, Q. Liu, T. Bai, W. Wang, F. He and M. Ye, Nickel and cobalt sulfide-based nanostructured materials for electrochemical energy storage devices, Chem. Eng. J., 2021, 409, 127237 CrossRef CAS.
- D. Li, Y. Gong and C. Pan, Facile synthesis of hybrid CNTs/NiCo2S4 composite for high performance supercapacitors, Sci. Rep., 2016, 6, 29788 CrossRef PubMed.
- L. Shen, L. Yu, H. B. Wu, X. Y. Yu, X. Zhang and X. W. Lou, Formation of nickel cobalt sulfide ball-in-ball hollow spheres with enhanced electrochemical pseudocapacitive properties, Nat. Commun., 2015, 6, 6694 CrossRef CAS PubMed.
- P. Wu, S. Cheng, M. Yao, L. Yang, Y. Zhu, P. Liu, O. Xing, J. Zhou, M. Wang, H. Luo and M. Liu, A Low-Cost, Self-Standing NiCo2S4@CNT/CNT Multilayer Electrode for Flexible Asymmetric Solid-State Supercapacitors, Adv. Funct. Mater., 2017, 27, 1702160 CrossRef.
- F. Lu, M. Zhou, W. Li, Q. Weng, C. Li, Y. Xue, X. Jiang, X. Zeng, Y. Bando and D. Golberg, Engineering sulfur vacancies and impurities in NiCo2S4 nanostructures toward optimal supercapacitive performance, Nano Energy, 2016, 26, 313–323 CrossRef CAS.
- Y. Han, S. Sun, W. Cui and J. Deng, Multidimensional structure of CoNi2S4 materials: structural regulation promoted electrochemical performance in a supercapacitor, RSC Adv., 2020, 10, 7541 RSC.
- W. Hu, R. Chen, W. Xie, L. Zou, N. Qin and D. Bao, CoNi2S4 Nanosheet Arrays Supported on Nickel Foams with Ultrahigh Capacitance for Aqueous Asymmetric Supercapacitor Applications, ACS Appl. Mater. Interfaces, 2014, 6, 19318–19326 CrossRef CAS PubMed.
- L. Chen, J. Wan, L. Fan, Y. Wei and J. Zou, Construction of CoNi2S4 hollow cube structures for excellent performance asymmetric supercapacitors, Appl. Surf. Sci., 2021, 570, 151174 CrossRef CAS.
- X. Zheng, X. He, J. Jiang, Z. Jia, Y. Li, Z. Wei and H. Yang, High-Performance Asymmetric Supercapacitors Based on the Ni1.5Co1.,5S4@CNTs Nanocomposites, NANO: Brief Rep. Rev., 2020, 15, 2050136 CrossRef CAS.
- F. Jin, X. He, J. Jiang, W. Zhu, J. Dai and H. Yang, Synthesis of Hierarchical Porous Ni1.5Co1.5S4/g-C3N4 Composite for Supercapacitor with Excellent Cycle Stability, Nanomaterials, 2020, 10, 1631 CrossRef CAS PubMed.
- L. Yu, L. Zhang, H. B. Wu and X. W. Lou, Formation of NixCo3−xS4 Hollow Nanoprisms with Enhanced Pseudocapacitive Properties, Angew. Chem., Int. Ed., 2014, 53, 3711–3714 CrossRef CAS PubMed.
- F. Yang, Z. Fang, K. Xu, J. Yang and J. Hu, NixCo3−xS4@NiCo2O4 hybrid composites as supercapacitors electrode material, Mater. Lett., 2017, 191, 101–104 CrossRef CAS.
- D. Cao, W. Kang, S. Wang, Y. Wang, K. Sun, L. Yang, X. Zhou, D. Sun and Y. Cao, In situ N-doped carbon modified (Co0.5Ni0.5)9S8 solid-solution hollow spheres as high-capacity anodes for sodium-ion batteries, J. Mater. Chem. A, 2019, 7, 8268 RSC.
- L. Jin, B. Liu, Y. Wu, S. Thanneeru and J. He, Synthesis of Mesoporous CoS2 and NixCo1−xS2 with Superior Supercapacitive Performance Using a Facile Solid-Phase Sulfurization, ACS Appl. Mater. Interfaces, 2017, 9, 36837–36848 CrossRef CAS PubMed.
- G. Li and C. Xu, Hydrothermal synthesis of 3D NixCo1−xS2 particles/graphene composite hydrogels for high performance supercapacitors, Carbon, 2015, 90, 44–52 CrossRef CAS.
- C. Huang, A. Gao, Z. Zhu, F. Yi, M. Wang, J. Hao, H. Cheng, J. Ling and D. Shu, Metal organic frameworks derive d Ni-dope d hierarchical NixCo1−xS@C bundled-like nanostructures for enhanced supercapacitors, Electrochim. Acta, 2022, 406, 139872 CrossRef CAS.
- J. Yang, M. Ma, C. Sun, Y. Zhang, W. Huang and X. Dong, Hybrid NiCo2S4@MnO2 heterostructures for high-performance supercapacitor electrodes, Mater. Chem. A, 2015, 3, 1258 RSC.
- D. Wang, Y. X. Chang, Y. R. Li, S. L. Zhang and S. L. Xu, Well-dispersed NiCoS2 nanoparticles/rGO composite with a large specific surface area as an oxygen evolution reaction electrocatalysts, Rare Met., 2021, 40(11), 3156–3165 CrossRef CAS.
- T. Zhu, G. Zhang, T. Hu, Z. He, Y. Lu, G. Wang, H. Guo, J. Luo, C. Lin and Y. Chen, Synthesis of NiCo2S4-based nanostructured electrodes supported on nickel foams with superior electrochemical performance, J. Mater. Sci., 2016, 51, 1903–1913 CrossRef CAS.
- M. Dong, Z. Chai1, J. Li and Z. Wang, One-step potentiostatic electrodeposition of cross-linked bimetallic sulfide nanosheet thin film for supercapacitors, Ionics, 2020, 26, 4095–4102 CrossRef CAS.
- N. Jabeen, Q. Xia, M. Yang and H. Xia, Unique Core-Shell Nanorod Arrays with Polyaniline Deposited into Mesoporous NiCo2O4 Support for High-Performance Supercapacitor Electrodes, ACS Appl. Mater. Interfaces, 2016, 8, 6093–6100 CrossRef CAS PubMed.
- S. Ahmed, M. Ahmad, M. H. Yousaf, S. Haider, Z. Imran, S. S. Batool, I. Ahmad, M. I. Shahzad and M. Azeem, Solvent-free synthesis of NiCo2S4 having the metallic nature, Front. Chem., 2022, 10, 1027024 CrossRef CAS PubMed.
- N. K. Mishra, A. K. Singh, R. Mondal and P. Singh, NiC2O4·2H2O Nanoflakes: A Novel Redox-mediated Intercalative Pseudocapacitive Electrode for Supercapacitor Applications in Aqueous KOH and Neutral Na2SO4 electrolytes, ChemistrySelect, 2022, 7(21), e202201134 CrossRef CAS.
- B. Zhang, X. Zhang, Y. Wei, L. Xia, C. Pi, H. Song, Y. Zheng, B. Gao, J. Fu and P. K. Chu, General synthesis of NiCo alloy nanochain arrays with thin oxide coating: a highly efficient bifunctional electrocatalyst for overall water splitting, J. Alloys Compd., 2019, 797, 1216–1223 CrossRef CAS.
- A. Gupta and V. Kushwaha, et al., SrFeO3−δ: a novel Fe4+-Fe2+ redox mediated pseudocapacitive electrode in aqueous electrolyte, Phys. Chem. Chem. Phys., 2022, 24, 11066–11078 RSC.
- B. Pattanayak, P. A. Le, D. Panda, F. M. Simanjuntak, K. H. Wei, T. Winie and T. Y. Tseng, Ion accumulation-induced capacitance elevation in a microporous graphene-based supercapacitor, RSC Adv., 2022, 12, 27082 RSC.
- B. Pal, S. Yang, S. Ramesh, V. Thangadurai and R. Jose, Electrolyte selection for supercapacitive devices: a critical review, Nanoscale Adv., 2019, 1(10), 3807–3835 RSC.
- L. Zhang, Z. Zeng, D. W. Wang, Y. Zuo, J. Chen and X. Yan, Magnetic field-induced capacitance change in aqueous carbon-based supercapacitors, Cell Rep. Phys. Sci., 2021, 2, 100455 CrossRef CAS.
- B. Evanko, S. W. Boettcher, S. J. Yoo and G. D. Stucky, Redox-Enhanced Electrochemical Capacitors: Status, Opportunity, and Best Practices for Performance Evaluation, ACS Energy Lett., 2017, 2, 2581–2590 CrossRef CAS.
- S. Fleischmann, J. B. Mitchell, R. Wang, C. Zhan, D. Jiang, V. Presser and V. Augustyn, Pseudocapacitance: From Fundamental Understanding to High Power Energy Storage Materials, Chem. Rev., 2020, 120, 6738–6782 CrossRef CAS PubMed.
- S. Ardizzone, G. Fregonara and S. Trasatti, “Inner” And “Outer” Active Surface of RuO2 Electrodes, Electrochim. Acta, 1990, 35, 263–267 CrossRef CAS.
- J. Wang, J. Polleux, J. Lim and B. Dunn, Pseudocapacitive Contributions to Electrochemical Energy Storage in TiO2 (Anatase) Nanoparticles, J. Phys. Chem. C, 2007, 111, 14925–14931 CrossRef CAS.
- R. Kumar and M. Bag, Quantifying Capacitive and Diffusion-Controlled Charge Storage from 3D Bulk to 2D Layered Halide Perovskite-Based Porous Electrodes for Efficient Supercapacitor Applications, J. Phys. Chem. C, 2021, 125, 16946–16954 CrossRef CAS.
- N. K. Mishra, R. Mondal, T. Maiyalagan and P. Singh, Synthesis, Characterizations, and Electrochemical Performances of Highly Porous, Anhydrous Co0.5Ni0.5C2O4 for Pseudocapacitive Energy Storage Applications, ACS Omega, 2022, 7, 1975–1987 CrossRef CAS PubMed.
- A. N. Singh, K. G. Nigam, R. Mondal, V. Kushwaha, A. Gupta, C. Rath and P. Singh, Effect of strontium doping on the electrochemical pseudocapacitance of Y1−xSrxMnO3−δ perovskites, Phys. Chem. Chem. Phys., 2023, 25, 326 RSC.
- D. Mohanadas and M. A. A. M. Abdah, Facile synthesis of PEDOT-rGO/HKUST-1 for high performance symmetrical supercapacitor device, Sci. Rep., 2021, 11, 11747 CrossRef CAS PubMed.
- P. Wu, S. Cheng, M. Yao, L. Yang, Y. Zhu, P. Liu, O. Xing, J. Zhou, M. Wang, H. Luo and M. Liu, A Low-Cost, Self-Standing NiCo2O4@CNT/CNT Multilayer, Adv. Funct. Mater., 2017, 27, 1702160 CrossRef.
- C. Xia, P. Li, A. N. Gandi, U. Schwingenschlögl and H. N. Alshareef, Is NiCo2S4 Really a Semiconductor, Chem. Mater., 2015, 27, 6482–6485 CrossRef CAS.
- Y. Gao, L. Mi, W. Wei, S. Cui, Z. Zheng, H. Hou and W. Chen, Double Metal Ions Synergistic Effect in Hierarchical Multiple Sulfide Microflowers for Enhanced Supercapacitor Performance, ACS Appl. Mater. Interfaces, 2015, 7, 4311–4319 CrossRef CAS PubMed.
- W. Chen, C. Xia and H. N. Alshareef, One-Step Electrodeposited Nickel Cobalt Sulfide Nanosheet Arrays for High-Performance Asymmetric Supercapacitors, ACS Nano, 2014, 8(9), 9531–9541 CrossRef CAS PubMed.
- S. S. Singha, S. Rudra, S. Mondal, M. Pradhan, A. K. Nayak, B. Satpati, P. Pal, K. Das and A. Sinha, Mn incorporated MoS2 nanoflowers: A high-performance electrode material for symmetric supercapacitor, Electrochim. Acta, 2020, 338, 135815 CrossRef CAS.
- S. X. Yan, S. H. Luo, J. Feng, L. Yang, P.-W. Li, Q. Wang, Y. H. Zhang, X. Liu and L. J. Chang, Asymmetric, Flexible Supercapacitor Based on Fe−Co Alloy@Sulfide with High Energy and Power Density, ACS Appl. Mater. Interfaces, 2021, 13, 49952–49963 CrossRef CAS PubMed.
- F. Lu, M. Zhou, W. Li, Q. Weng, C. Li, Y. Xue, X. Jiang, X. Zeng, Y. Bando and D. Golberg, Engineering sulfur vacancies and impurities in NiCo2S4 nanostructures toward optimal supercapacitive performance, Nano Energy, 2016, 26, 313–323 CrossRef CAS.
- Q. Xia, L. Si, K. Liu, A. Zhou, C. Su, N. M. Shinde, G. Fan and J. Dou, In Situ Preparation of Three-Dimensional Porous Nickel Sulfide as a Battery-Type Supercapacitor, Molecules, 2023, 28, 4307 CrossRef CAS PubMed.
- M. Singh, S. Kumar, R. Mondal, P. Singh, R. Prakash and N. Sharma, Combustion-Synthesized KNiPO4: A Non-toxic, Robust, Intercalating Battery-Type Pseudocapacitive Electrode for Hybrid Supercapacitors as a Large-Scale Energy Storage Solution, Energy Fuels, 2023, 37, 4094–4105 CrossRef CAS.
- Q. Li, F. Yu, Y. Cui, J. Wang, Y. Zhao and J. Peng, Multilayer SnS-SnS2@GO heterostructures nanosheet as anode material for Sodium ion battery with high capacity and stability, J. Alloys Compd., 2023, 937, 168392 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3dt04184k |
| This journal is © The Royal Society of Chemistry 2024 |