Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A nested spin structure and single molecule magnet behaviour in an Fe8Dy12 heterometallic cyclic coordination cluster†
Yan
Peng
 *ab,
Jonas
Braun
*ab,
Jonas
Braun
 acd,
Michael
Schulze
acd,
Michael
Schulze
 e,
Hagen
Kaemmerer
e,
Hagen
Kaemmerer
 ad,
Yannik F.
Schneider
ad,
Yannik F.
Schneider
 ac,
Christopher E.
Anson
ac,
Christopher E.
Anson
 a,
Wolfgang
Wernsdorfer
a,
Wolfgang
Wernsdorfer
 de and
Annie K.
Powell
de and
Annie K.
Powell
 *abd
*abd
aInstitute of Inorganic Chemistry, Karlsruhe Institute of Technology (KIT), Kaiserstr. 12, 76131 Karlsruhe, Germany. E-mail: annie.powell@kit.edu
bSchool of Chemistry and Chemical Engineering, Jiangxi University of Science and Technology, Ganzhou 341000, P.R. China
cInstitute of Nanotechnology (INT), Karlsruhe Institute of Technology (KIT), Kaiserstr. 12, 76131 Karlsruhe, Germany
dInstitute for Quantum Materials and Technologies (IQMT), Karlsruhe Institute of Technology (KIT), Kaiserstr. 12, 76131 Karlsruhe, Germany
ePhysikalisches Institut, Karlsruhe Institute of Technology (KIT), Kaiserstr. 12, 76131 Karlsruhe, Germany
First published on 20th December 2023
Abstract
The 20-nuclearity compound [Fe8Dy12(tea)8(teaH)12(NO3)12]·8MeCN (where teaH3 = triethanolamine) was synthesised and characterised through single crystal X-ray diffraction and magnetic measurements. The shape of the magnetic hysteresis in the microSQUID measurements was rationalised using the MAGELLAN program.
Investigations on coordination clusters (CCs) based on either 3d or 4f ions have attracted great attention over the past decades because of their beautiful molecular architectures and interesting magnetic properties, such as single molecule magnet (SMM) behaviour,1–3 the magnetocaloric effect4–8 and spintronics.9 Particular attention has been paid to cyclic coordination clusters (CCCs), whose closed but infinite electronic structure could have applications in molecular electronics/spintronics and have, for example, been identified as qubits.10 Pioneering research has shown many examples of homometallic wheel-shaped clusters such as {Mo154},11 {Mn84},12 {Ni24},13 {Dy21}14 and {Gd140}.15 However, heterometallic cyclic clusters containing cooperatively coupled 3d and 4f ions have rarely been reported. Most of the examples, including {M4Ln4} (M = FeIII, CrIII, MnIII),16–19 {Fe4Ln2},20,21 {Fe5Yb3},21 {Fe3Yb2},21 {Fe16Ln4},22 {Fe10Ln10}21,23,24 and {Fe18Dy6}25,26 complexes, were reported by Powell and co-workers. Examples of other 3d–4f wheel motifs include {Mn8Ln8},27 {Cr8Ln8},28 {Cu36Ln24}29 and {Co16Ln24}30 complexes reported by other groups. It is noted that the common features in the synthesis of these 3d–4f cyclic structures are the use of amine polyalcohol ligands such as triethanolamine and N-nbutyldiethanolamine, and small bridging co-ligands, like acetate, benzoate and α-amino acids.
Cyclic systems containing paramagnetic ions, in particular DyIII, have the possibility to show toroidal moments which lead to non-magnetic states. Such a toroidal moment can have a spin structure which is either a cyclic arrangement of the spin in the metal ion plane or a solenoidal arrangement, both of which lead to non-magnetic states. For coordination systems the first molecule to be identified showing such an arrangement was a Dy3 triangle.31–33 Since then many more molecular systems have been identified, as recently summarised in Murray's book Single Molecule Toroics.34
The compound we report here has the same overall nuclearity as the Fe10Ln10 clusters we previously described. These exhibited quantum properties such as proximity to the quantum critical point, exciton formation and intermolecular charge hopping.21,23 By slightly modifying the synthetic procedure we obtained an Fe8Dy12 coordination cluster and report here the investigation of its magnetic properties.
The reaction of Fe(NO3)3·9H2O and Dy(NO3)3·6H2O with teaH3 in MeOH and MeCN in the presence of Et3N at ambient temperature yielded [Fe8Dy12(Htea)8(tea)12(NO3)12]·8MeCN (1). Single-crystal X-ray diffraction studies reveal that compound (1) crystallises in the tetragonal space group I41/acd (Fig. 1). The molecule is on a twofold axis in the crystal and thus the asymmetric unit is half the molecule (Fig. S1†).
Compound 1 is related to the previously reported [Fe10Ln10(Me-tea)10(Me-teaH)10(NO3)10].21,23,24 The repeating units of both nanoscale compounds are extremely similar as both show Dy–Fe–Dy–Fe–Dy chains (Fig. 2), but rather than the FeIII ion that follows, leading to alternating Fe–Dy in the {Fe10Dy10} ring, these units are linked by an additional DyIII ion in {Fe8Dy12} (1). This also influences the topology of the cluster. While {Fe10Dy10} can be described as a cyclic standing wave, the presence of additional DyIII ions leads to the cluster folding into a saddle shape (Fig. 2). Within 1, the FeIII ion is chelated by the nitrogen and three oxygen atoms of a fully deprotonated tea3− ligand and the DyIII ions by a doubly deprotonated teaH2− ligand with one alcohol arm remaining protonated. The two deprotonated alcohol arms of each ligand form μ-alkoxo bridges to adjacent metal centres in the ring.
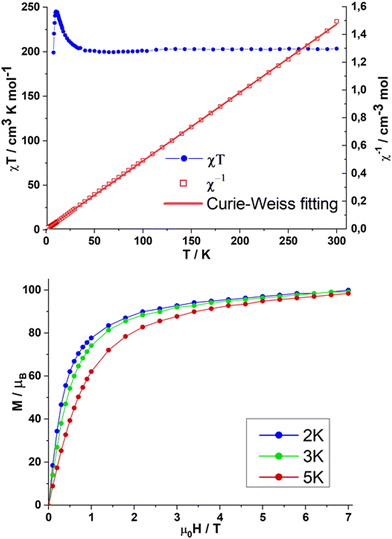
The static magnetic susceptibilities of compound 1 were collected over the temperature range 1.8–300 K under an applied magnetic field of 0.1 T. Magnetisation data were collected at fields of 0–7 T at temperatures of 2, 3 and 5 K. The χT value of 1 is 204.7 cm3 K mol−1 at 300 K, close to the expected value of 205.1 cm3 K mol−1 for eight uncoupled FeIII and twelve DyIII ions (Fig. 2). Upon cooling, the χT value stays nearly constant before increasing sharply below 30 K reaching a maximum value of 246.9 cm3 K mol−1 at 4.3 K. This is followed by an abrupt decrease at lower temperature to 200.6 cm3 K mol−1 at 1.8 K. The increase of the χT value suggests ferromagnetic coupling between FeIII and DyIII ions, while the subsequent decrease is likely due to the presence of magnetic anisotropy and/or intermolecular weak antiferromagnetic interactions. The presence of dominant ferromagnetic intramolecular interactions is supported by the temperature dependence of the reciprocal susceptibility (1/χ) over the whole temperature range following the Curie–Weiss law (χ = C/(T − θ)) with a Weiss constant θ = +0.54 K and Curie Constant C = 203 cm3 K mol−1 (Fig. 3, top). The field-dependent magnetisation measurements for 1 (Fig. 3, bottom) increase sharply at low fields and reach 95.80μB at 7 T without reaching saturation, suggesting the presence of significant magnetic anisotropy.
To probe the magnetic anisotropy, ac susceptibility measurements were carried out under zero dc field for compound 1. Out-of-phase signals were detected below 5 K (Fig. S3†) indicative of the slow relaxation of the magnetisation behaviour characteristic of potential SMMs. Under a small external dc field, the magnetic slow relaxation was still too fast for maxima to be observed (Fig. S4†) within the parameters of our SQUID.
MicroSQUID measurements were performed at different temperatures and scan rates (Fig. 4 and S5†). In order to investigate the energy barrier and relaxation time, dc magnetisation decay measurements were performed under zero applied field in the sub-kelvin temperature range (Fig. S5†). The relaxation times were fitted using the following equation:
τ−1 = AT + B + CTn + τ0−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ueff/kBT) exp(−Ueff/kBT) |
In order to understand the hysteresis behaviour seen in the microSQUID measurement we performed the electrostatic field-based MAGELLAN35 analysis to locate the Ising easy-axes of the DyIII ions. Although this program was designed for purely DyIII containing molecules, nevertheless we have found that coordination clusters containing FeIII and DyIII can be safely analysed. Furthermore, a cluster of this size cannot be calculated using ab initio methods; therefore MAGELLAN provides the only opportunity to help rationalise the magnetic behaviour. Whereas in the Fe10Dy10 cluster the axes are arranged in a regular arrangement (see Fig. S6†), the saddle-shape of 1 leads to an apparent jumble of directions (see Fig. 5). However, a closer analysis reveals that in fact the twelve DyIII ions can be divided into three sets of four ions (see Fig. 5). The innermost set of four corresponds to a slightly distorted tetrahedron with its MAGELLAN axes oriented in a spin-ice-like arrangement (pink in Fig. 5).36,37 The middle set of axes form what can be described as a toroidal arrangement around a slightly distorted square as seen for Dy4.38 The “square” is neither completely planar nor completely square, but with angles of 86.3° and 93.7° as well as distances of 12.8 Å and 13.3 Å, and the deviation from planarity for each of the DyIII ions of 0.32 Å is not far off (burgundy in Fig. 5). The outer set is arranged in a boat-like shape. Two of the axes are parallel and essentially perpendicular to the plane of the “square” of the middle layer. The other two are at an angle of 30.1° to the perpendicular ones (cyan in Fig. 5).
These arrangements may lead to the following characteristics of the microSQUID hysteresis curve. The easiest part to assess is the toroidal nature of the middle set which leads to a narrowing and a change in gradient near 0 T. The alignment of the outer set is likely responsible for the sharp increase in magnetisation at fields higher than 0.1 T. The inner spin-ice-like set remains a “dark horse” in the sense that its contribution to the magnetic hysteresis behaviour is impossible to judge but it might possibly be non-magnetic.
In conclusion, disrupting the alternating pattern of the ions in the Fe10Ln10 ring system through slightly altered reaction conditions leads to a folding of the ring into a saddle shape. This in turn alters the orientations of the MAGELLAN axes. These axes help rationalise the magnetic hysteresis observed in the microSQUID measurements.
Author contributions
Y.P. and Y.F.S.: synthesis and characterisation; J.B., H.K., C.E.A. and A.K.P.: paper conceptualisation and writing; C.E.A.: crystallography; M.S. and W.W.: microSQUID measurement and interpretation; and A.K.P.: supervision and funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge funding through DFG CRC 1573 “4f For Future” and the POF MSE Helmholtz Foundation, and J. B. and Y. F. S. acknowledge support from the Landesgraduiertenförderung Baden-Württemberg.References
- R. Sessoli, D. Gatteschi, A. Caneschi and M. A. Novak, Nature, 1993, 365, 141–143 CrossRef CAS.
- R. Sessoli, H.-L. Tsai, A. R. Schake, S. Wang, J. B. Vincent, K. Folting, D. Gatteschi, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1993, 115, 1804–1816 CrossRef CAS.
- D. Gatteschi and R. Sessoli, Angew. Chem., Int. Ed., 2003, 42, 268–297 CrossRef CAS PubMed.
- M. Evangelisti, F. Luis, L. J. de Jongh and M. Affronte, J. Mater. Chem., 2006, 16, 2534–2549 RSC.
- J. W. Sharples, Y. Z. Zheng, F. Tuna, E. J. McInnes and D. Collison, Chem. Commun., 2011, 47, 7650–7652 RSC.
- J. W. Sharples, D. Collison, E. J. L. McInnes, J. Schnack, E. Palacios and M. Evangelisti, Nat. Commun., 2014, 5, 5321–5326 CrossRef CAS.
- Y. Z. Zheng, G. J. Zhou, Z. Zheng and R. E. Winpenny, Chem. Soc. Rev., 2014, 43, 1462–1475 RSC.
- T. G. Tziotzi, D. Gracia, S. J. Dalgarno, J. Schnack, M. Evangelisti, E. K. Brechin and C. J. Milios, J. Am. Chem. Soc., 2023, 145, 7743–7747 CrossRef CAS.
- L. Bogani and W. Wernsdorfer, Nat. Mater., 2008, 7, 179–186 CrossRef CAS PubMed.
- G. A. Timco, S. Carretta, F. Troiani, F. Tuna, R. J. Pritchard, C. A. Muryn, E. J. McInnes, A. Ghirri, A. Candini, P. Santini, G. Amoretti, M. Affronte and R. E. Winpenny, Nat. Nanotechnol., 2009, 4, 173–178 CrossRef CAS PubMed.
- T. Liu, E. Diemann, H. Li, A. W. M. Dress and A. Müller, Nature, 2003, 426, 59–62 CrossRef CAS PubMed.
- A. J. Tasiopoulos, A. Vinslava, W. Wernsdorfer, K. A. Abboud and G. Christou, Angew. Chem., Int. Ed., 2004, 43, 2117–2121 CrossRef CAS.
- A. L. Dearden, S. Parsons and R. E. P. Winpenny, Angew. Chem., Int. Ed., 2001, 40, 151–154 CrossRef CAS.
- S. Biswas, S. Das, J. Acharya, V. Kumar, J. van Leusen, P. Kogerler, J. M. Herrera, E. Colacio and V. Chandrasekhar, Chem. – Eur. J., 2017, 23, 5154–5170 CrossRef CAS PubMed.
- X.-Y. Zheng, Y.-H. Jiang, G.-L. Zhuang, D.-P. Liu, H.-G. Liao, X.-J. Kong, L.-S. Long and L.-S. Zheng, J. Am. Chem. Soc., 2017, 139, 18178–18181 CrossRef CAS PubMed.
- M. Li, A. M. Ako, Y. Lan, W. Wernsdorfer, G. Buth, C. E. Anson, A. K. Powell, Z. Wang and S. Gao, Dalton Trans., 2010, 39, 3375–3377 RSC.
- M. Li, Y. Lan, A. M. Ako, W. Wernsdorfer, C. E. Anson, G. Buth, A. K. Powell, Z. Wang and S. Gao, Inorg. Chem., 2010, 49, 11587–11594 CrossRef CAS PubMed.
- J. Rinck, G. Novitchi, W. Van den Heuvel, L. Ungur, Y. Lan, W. Wernsdorfer, C. E. Anson, L. F. Chibotaru and A. K. Powell, Angew. Chem., Int. Ed., 2010, 49, 7583–7587 CrossRef CAS PubMed.
- D. Schray, G. Abbas, Y. Lan, V. Mereacre, A. Sundt, J. Dreiser, O. Waldmann, G. E. Kostakis, C. E. Anson and A. K. Powell, Angew. Chem., Int. Ed., 2010, 49, 5185–5188 CrossRef CAS PubMed.
- S. Schmidt, D. Prodius, G. Novitchi, V. Mereacre, G. E. Kostakis and A. K. Powell, Chem. Commun., 2012, 48, 9825–9827 RSC.
- A. Baniodeh, C. E. Anson and A. K. Powell, Chem. Sci., 2013, 4, 4354–4361 RSC.
- A. Baniodeh, I. J. Hewitt, V. Mereacre, Y. Lan, G. Novitchi, C. E. Anson and A. K. Powell, Dalton Trans., 2011, 40, 4080–4086 RSC.
- A. Baniodeh, Y. Liang, C. E. Anson, N. Magnani, A. K. Powell, A.-N. Unterreiner, S. Seyfferle, M. Slota, M. Dressel, L. Bogani and K. Goß, Adv. Funct. Mater., 2014, 24, 6280–6290 CrossRef CAS.
- J. R. Machado, A. Baniodeh, A. K. Powell, B. Luy, S. Kramer and G. Guthausen, ChemPhysChem, 2014, 15, 3608–3613 CrossRef CAS PubMed.
- O. Botezat, J. van Leusen, V. C. Kravtsov, P. Kögerler and S. G. Baca, Inorg. Chem., 2017, 56, 1814–1822 CrossRef CAS PubMed.
- H. Kaemmerer, A. Baniodeh, Y. Peng, E. Moreno-Pineda, M. Schulze, C. E. Anson, W. Wernsdorfer, J. Schnack and A. K. Powell, J. Am. Chem. Soc., 2020, 142, 14838–14842 CrossRef CAS PubMed.
- K. R. Vignesh, S. K. Langley, B. Moubaraki, K. S. Murray and G. Rajaraman, Chem. – Eur. J., 2015, 21, 16364–16369 CrossRef CAS PubMed.
- L. Qin, J. Singleton, W. P. Chen, H. Nojiri, L. Engelhardt, R. E. P. Winpenny and Y. Z. Zheng, Angew. Chem., Int. Ed., 2017, 56, 16571–16574 CrossRef CAS.
- J. D. Leng, J. L. Liu and M. L. Tong, Chem. Commun., 2012, 48, 5286–5288 RSC.
- Z.-M. Zhang, L.-Y. Pan, W.-Q. Lin, J.-D. Leng, F.-S. Guo, Y.-C. Chen, J.-L. Liu and M.-L. Tong, Chem. Commun., 2013, 49, 8081–8083 RSC.
- J. Tang, I. Hewitt, N. T. Madhu, G. Chastanet, W. Wernsdorfer, C. E. Anson, C. Benelli, R. Sessoli and A. K. Powell, Angew. Chem., 2006, 118, 1761 CrossRef.
- L. F. Chibotaru, L. Ungur and A. Soncini, Angew. Chem., 2008, 120, 4194–4197 CrossRef.
- J. Luzon, K. Bernot, I. J. Hewitt, C. E. Anson, A. K. Powell and R. Sessoli, Phys. Rev. Lett., 2008, 100, 247205 CrossRef PubMed.
- K. Murray, Single Molecule Toroics, Springer Nature Switzerland AG, Cham, 2022 Search PubMed.
- N. F. Chilton, D. Collison, E. J. McInnes, R. E. Winpenny and A. Soncini, Nat. Commun., 2013, 4, 2551 CrossRef PubMed.
- C. Kachi-Terajima, T. Eiba, R. Ishii, H. Miyasaka, Y. Kodama and T. Saito, Angew. Chem., Int. Ed., 2020, 59, 22048–22053 CrossRef CAS PubMed.
- C. Castelnovo, R. Moessner and S. L. Sondhi, Annu. Rev. Condens. Matter Phys., 2012, 3, 35–55 CrossRef CAS.
- G. Abbas, Y. Lan, G. E. Kostakis, W. Wernsdorfer, C. E. Anson and A. K. Powell, Inorg. Chem., 2010, 49, 8067–8072 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and further magnetic data together with crystallographic data in CIF format. CCDC 2306455. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3dt04141g |
| This journal is © The Royal Society of Chemistry 2024 |