Exploitation of a 1D coordination polymer as a portable kit for an eye-catching fluorometric response towards sensing of trivalent cations†
Sourav
Datta
ab,
Sunanda
Dey
c,
Chittaranjan
Sinha
 c,
Basudeb
Dutta
c,
Basudeb
Dutta
 *ad,
Priyabrata
Banerjee
*ad,
Priyabrata
Banerjee
 *be and
Mohammad Hedayetullah
Mir
*be and
Mohammad Hedayetullah
Mir
 *a
*a
aDepartment of Chemistry, Aliah University, New Town, Kolkata 700 160, India. E-mail: dutta.basu11@gmail.com; chmmir@gmail.com
bElectric Mobility & Tribology Research Group, CSIR-Central Mechanical Engineering Research Institute, Mahatma Gandhi Avenue, Durgapur 713 209, India. E-mail: pr_banerjee@cmeri.res.in
cDepartment of Chemistry, Jadavpur University, Kolkata 700 032, India
dInstitute for Integrated Cell-Material Sciences, Kyoto University, Yoshida Ushinomiya-cho, Sakyo-ku, Kyoto 606-8501, Japan
eAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad 201 002, India
First published on 4th January 2024
Abstract
The development and utilization of coordination polymers (CPs) have drawn interest for potential applications in different fields. Detection of metal ions in efficient and selective manners is an important field of research. It paves the way to protect human health by balancing toxic metal ions and biologically active metal ions in the atmosphere. In this regard, a new one-dimensional (1D) 4-(1-naphthylvinyl)pyridine (4-nvp) based CP [Cd(NCS)2(4-nvp)2]n (1) was synthesized and characterized structurally by single-crystal X-ray diffraction. Interestingly, this 1D CP underwent supramolecular aggregation via π⋯π stacking interactions, which specifically generated an environment for a potent “turn on” response in the presence of trivalent cations (Fe3+, Al3+, and Cr3+) in the nanomolar range but remained silent in the presence of other metal ions. Density functional theory (DFT) computations and X-ray photoelectron spectroscopy (XPS) were performed to establish the sensing phenomena. Fascinatingly, utilizing the sensitivity of 1 in an aqueous medium, a hands-on portable cotton swab kit was developed for instant identification of these three important trivalent metal cations.
Introduction
Over the last few decades, coordination polymers (CPs) have attracted immense interest because of their pivotal role in structure–property relationship exhibited in their supramolecular assemblies.1–4 CPs are hybrid materials composed of metal ions and inorganic/organic ligands extended in an array. Diversity in the respect of structural dimensionality transforms these materials for different potential applications: sorption and separation of gases, drug delivery, catalysis, dye degradation, energy strategies, device fabrication, magnetism at variable temperatures, and ion replacing.5–14 Several CPs have been studied for sensor applications because of their ability to detect hazardous and physiologically vigorous metal ions.15–22 CPs are more attractive in the sensor field due to higher stability but also their recyclability and “tailor”-made easy synthetic procedure. During this stage of development, scientists expose the appropriate combination of metal ions and π-conjugated organic ligands that allow it to be functionalized as a fluorescence-based sensor.9 These types of fluorescence-based chemosensors are more efficient in terms of sharp and rapid specific changes. Ultimately, the signaling fluorescence changes involve several processes: Förster resonance energy transfer (FRET), inner filter effect (IFE), photo-induced electron transfer (PET), photo-induced charge transfer (PCT), and resonance energy transfer (RET).23 Furthermore, based on the direction of electron transport, PET is categorized mainly of two: reductive PET (R-PET) and oxidative PET (O-PET).As a result of increased urbanization and socioeconomic activity, traces of toxicity due to heavy metal ions have increased and pose a threat to human health. Mining industries, energy industries, and demanding agricultural activities increase toxic metal ions in the human diet indirectly through soil water or river water. In these circumstances, consuming food and water contaminated with such toxic metals is extremely dangerous, especially in developing countries. On the other hand, some trivalent metal ions are very important for humans because they have a wide variety of functions in the environment and biological systems. Among these trivalent metal ions, Fe3+, Al3+, and Cr3+ have prominent biological and environmental importance.24–29 They are also regarded as group-IIIA cations of analytical group.30 In this trivalent family, Fe3+ is the most abundant transition metal in cellular systems. It plays a vital part in tissue formation, cellular growth, as well as electron and oxygen transport through red blood cells in all tissues due to its acceptable redox potential.31 However, a deficiency or excess accumulation of Fe3+ can cause cell damage, Alzheimer's disease, Huntington's disease, Parkinson's disease, and anemia. Al3+ is the third most abundant element in the Earth's crust.32 According to a World Health Organization (WHO) report, the adult human intake of Al is 3–10 mg per day. Excessive consumption of Al3+ also leads to Alzheimer's disease, dementia, aluminum-induced bone disease, microcytic anemia, or amyotrophic lateral sclerosis.33–35 Another member of this family is Cr3+, which plays a vital part in the metabolism of carbohydrates, fats, and proteins; and its deficiency creates disturbances in lipid metabolism.32,36 Cr appears at trace levels in the human body, and the recommended intake range for adults is 25–35 μg per day. In the human body, the action of insulin is also regulated by Cr, which activates certain enzymes and influences nucleic acids and proteins. Insufficiency of this element can lead to lung problems, lower immunity, birth defects, sterility, and tumor creation. High levels of Cr(III) may even lead to cancer.
Estimation of these aforementioned trivalent metal ions can be achieved using titrimetry, chromatography, flame atomic absorption spectrometry, inductively coupled plasma-atomic emission spectroscopy, potentiometry, X-ray fluorescence spectrometry, potentiometry, fluorimetry, and spectrophotometry. However, some of these instrumental methods have disadvantages, such as high cost of equipment, training for handling instruments, and being time-consuming. Fluorescence methods have high selectivity, enhanced sensitivity, high sampling frequency, low cost of equipment, operational simplicity, and direct visual perception. Few teams in this research field are developing different types of chemosensors and applying them in different ways.37–41 However, “sensing” of these metal ions using highly stable CPs has rarely been reported. “Turn on” responses are preferred to “turn off” responses because the human eye can see a small amount of light in the dark. In this regard, herein, we synthesized a Cd(II)-based one-dimensional coordination polymer (1D CP) [Cd(NCS)2(4-nvp)2]n (1) as a selective chemosensor for trivalent metal ions that show “turn on” responses in the presence of UV light. Sensing phenomena were validated by density functional theory (DFT) computations. The DFT calculation showed an interaction between compound 1 and trivalent cations, which affected the energy gap between the highest occupied molecular orbital and lowest unoccupied molecular orbital (HOMO–LUMO gap), thereby resulting “turn on” fluorescence phenomena. We also undertook X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), and energy dispersive X-ray (EDX) mapping studies to provide explanations for the adsorption of trivalent cations to 1. This sensory probe CP could detect the aforementioned metal ions with a low limit of detection (LOD) value as validated by DFT studies Furthermore, the synthesized 1 was made into a portable cotton swab kit for instant detection of trivalent metal ions.
General method for the SEM, XPS, and NMR studies
The SEM study of 1 was done after drying crystals for 2 h in an oven at 100 °C. Compound 1 was dipped in 5 mL of an aqueous solution (10−4 M) of metal ions (Fe3+, Al3+, Cr3+) for 2 h, washed, and dried for SEM study. For the XPS study, a similar type of sample preparations to that for SEM was carried out. For 1H NMR, a homogeneous mixture of 1 was prepared and titrated with Fe3+, Al3+, and Cr3+ in DMSO-d6 medium.Results and discussion
Structural description of [Cd(NCS)2(4-nvp)2] (1)
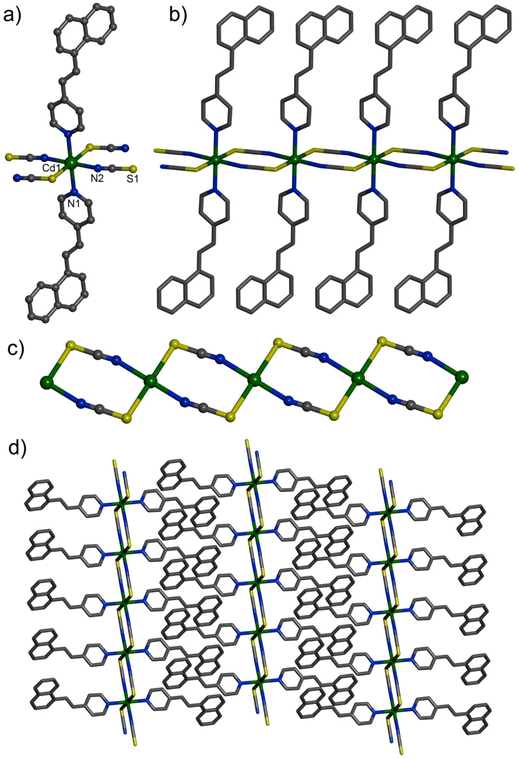
X-ray structural determination revealed that compound 1 crystallized in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with Z = 1. The asymmetric unit contained a Cd(II) ion, a 4-nvp ligand, and an thiocyanate anion.41 The coordination environment of the Cd(II) centre had a distorted octahedral geometry with a CdN4S2 core (Fig. 1a). Herein, the N(2) atom was slightly disordered and exhibited a 0.65 contribution, whereas the other part, N(3), exhibited a 0.35 contribution (Fig. S1, ESI†). Two N atoms and two S atoms from four bridging thiocyanate ligands formed the equatorial plane (Cd1–N2, 2.345(4); Cd1–S1, 2.746(1) Å), whereas trans-axial sites were occupied by two N atoms from two 4-nvp ligands. Cd(II) centres were bridged by end-to-end thiocyante ligands to generate a 1D polymeric chain (Fig. 1b). The closest Cd⋯Cd distance was 5.956 Å. Here, two Cd(II) centres and two thiocyanate ligands formed an eight-membered metal–ligand chelate ring [Cd2(μ-NCS-N,S)2]. These metal–ligand rings were interconnected to each other to generate a 1D tape-like structure (Fig. 1c). The 4-nvp ligands were projected from Cd(II) centres on both sides of the chain. These 4-nvp ligands formed adjacent chains which underwent π⋯π stacking interactions to produce two-dimensional (2D) supramolecular aggregates (Fig. 1d).
with Z = 1. The asymmetric unit contained a Cd(II) ion, a 4-nvp ligand, and an thiocyanate anion.41 The coordination environment of the Cd(II) centre had a distorted octahedral geometry with a CdN4S2 core (Fig. 1a). Herein, the N(2) atom was slightly disordered and exhibited a 0.65 contribution, whereas the other part, N(3), exhibited a 0.35 contribution (Fig. S1, ESI†). Two N atoms and two S atoms from four bridging thiocyanate ligands formed the equatorial plane (Cd1–N2, 2.345(4); Cd1–S1, 2.746(1) Å), whereas trans-axial sites were occupied by two N atoms from two 4-nvp ligands. Cd(II) centres were bridged by end-to-end thiocyante ligands to generate a 1D polymeric chain (Fig. 1b). The closest Cd⋯Cd distance was 5.956 Å. Here, two Cd(II) centres and two thiocyanate ligands formed an eight-membered metal–ligand chelate ring [Cd2(μ-NCS-N,S)2]. These metal–ligand rings were interconnected to each other to generate a 1D tape-like structure (Fig. 1c). The 4-nvp ligands were projected from Cd(II) centres on both sides of the chain. These 4-nvp ligands formed adjacent chains which underwent π⋯π stacking interactions to produce two-dimensional (2D) supramolecular aggregates (Fig. 1d).
Photoluminescence property and fluorescence sensing
Selectivity is an essential criterion of chemosensors. Herein, our synthesized compound 1 selectively identified Fe3+, Al3+, and Cr3+ over 14 other biologically important metal ions (K+, Hg2+, Mn2+, Co2+, Cu2+, Zn2+, Pb2+, Ca2+, Pd2+, Na+, Cd2+, Ni2+, Ba2+, and Mg2+). Therefore, compound 1 was regarded as a sensor for trivalent ions. Fluorescence spectra revealed that compound 1 (1 mg ml−1, in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH3CN/H2O, v/v) did not show emission, but gave strong green emission for selective M3+ (Fe3+, Al3+, and Cr3+) ions (Fig. 2). However, emission was not observed with the other 14 metal ions. Gradual addition of a M3+ ion to a non-emissive solution of 1 led to excessive enhancement at 517 nm upon excitation at 400 nm. Herein, 0–240 μL of an aqueous solution (10−4 M) of each metal ion showed emission (except Cr3+; 130 μL) until saturation was reached.
1 CH3CN/H2O, v/v) did not show emission, but gave strong green emission for selective M3+ (Fe3+, Al3+, and Cr3+) ions (Fig. 2). However, emission was not observed with the other 14 metal ions. Gradual addition of a M3+ ion to a non-emissive solution of 1 led to excessive enhancement at 517 nm upon excitation at 400 nm. Herein, 0–240 μL of an aqueous solution (10−4 M) of each metal ion showed emission (except Cr3+; 130 μL) until saturation was reached.
 | ||
Fig. 2 Change in the emission spectrum of 1 (100 μL of 1 mg ml−1 stock solution) upon addition of different metal ions (240 μL for each salt except Cr3+; 130 μL) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH3CN/H2O (v/v), λex, 400 nm. 1 CH3CN/H2O (v/v), λex, 400 nm. | ||
Therefore, in the fluorescence titration of 1, we found smooth spectra that displayed continuous enhancement of fluorescence intensity upon addition of selective M3+ (Fe3+, Al3+, and Cr3+) ions (Fig. 3). To check the sensitivity of M3+ ions, LOD values were calculated using 3σ/K methods. The σ value (1.98) was obtained by undertaking six blank titrations of compound 1 using a high concentration of solution (3 ml of a 1 mg ml−1 solution) (Fig. S2, ESI†). The LOD values of Fe3+, Al3+, and Cr3+ were determined by following 3σ methods, and found to be 73.7 nM, 74.1 nM, and 43.1 nM, respectively (Fig. S3 and Table S4, ESI†). These values indicated that compound 1 was highly sensitive towards M3+ ions. Compound 1 was non-emissive to metal ions such as K+, Hg2+, Mn2+, Co2+, Cu2+, Zn2+, Pb2+, Ca2+, Pd2+, Na+, Cd2+, Ni2+, Ba2+, and Mg2+, but was selective for the trivalent ions Fe3+, Al3+, and Cr3+. These observations suggested practical application of 1 to detect selective metal ions only in the presence of other co-existing metal ions. Interference of emission enhancement was examined with other metal ions (Fig. 2), but significant interference in the detection of selective metal ions was not observed. A cross-interference study confirmed the selectivity of compound 1 towards the Fe3+, Al3+, and Cr3+ only in presence of other ions (Fig. S4–S6, ESI†).
The measured LOD values came into competition with recently established fluorometric detection of these metal ions. Thus, the modified Stern–Volmer (SV) equation was introduced to obtain the fluorescence enhancement constant. The SV equation is (I0/I) = A ek[Q] + B [where I0 and I were the emission intensities of 1 in the absence and presence of the metal ions (Fe3+, Al3+, and Cr3+) respectively, and k, A, and B are constants]. The SV plot was nonlinear (i.e., I0/I = 1 + B1[M3+] + B2[M3+]2) (Fig. S7a–c, ESI†). The calculated Ksv values were 48.97 × 106 M−1, 28.68 × 106 M−1, and 36.69 × 106 M−1 for Fe3+, Al3+, and Cr3+ respectively, which highlighted the high efficiency of fluorescence enhancement of 1 towards these metal ions (Fig. S7a–c, ESI†).
Sensing mechanism
The crystal structure of 1 displayed a “dangling” naphthalene moiety in the crystal lattice. This could be used to construct a host–guest interaction with the highly π electron-rich naphthalene moiety to trivalent metal ions. Herein, the intense enhancement of fluorescence intensity of 1 in the presence of M3+ (Fe3+, Al3+, and Cr3+) ions could be ascribed to intramolecular charge transfer (ICT).42 The latter occurs in rigid structures, and the strong interaction between the electron cloud of the dangling naphthalene moiety of 1 and trivalent metal ions provided rigidity to the structure. The trivalent metal ion fitted in the hole which was created by two parallel naphthalene moieties (Fig. S8, ESI†),43–45 making it rigid and diminishing intramolecular rotational and vibrational energy losses. Fluorescence titration with all cations (single positive, double positive, and triple positive) revealed that there was dramatic enhancement of 1 in the presence of trivalent cations only in the presence of an appropriate size and electron affinity to fit the hole. This interaction was also elucidated by 1H NMR spectroscopy conducted in DMSO-d6 medium with varying amounts of metal ions (Fe3+, Al3+, and Cr3+). The outcomes of these results affirmed the interaction of metal ions with naphthalene moieties via shifting the aromatic protons towards a downfield region. Electron-deficient metal ions withdrew the electron cloud of the electron-rich naphthalene moiety that shifted protons in de-shielded regions to keep other peaks intact (Fig. S9–S12, ESI†). Peak intensity narrowed continuously upon addition of excess metal ions.SEM, EDX mapping, and XPS also revealed the sorption of M3+ ions on the surface of compound 1. The strong interaction between the naphthalene moiety and trivalent metal ion helped the metal ion to fit into the crystal lattice of 1, which disturbed the crystal lattice and the broken surface was observed by SEM (Fig. S13–S16, ESI†). EDX mapping also revealed the presence of trivalent metal ions in the corresponding samples. Mapping and pictorial data surveys of elements confirm trivalent metal ions to be present in compound 1. Deconvolution of XPS data showed metal ions on the CP surface. A plot of 1 + Fe(III) spectrum showed peaks at 726.11 eV and 711.85 eV for 2p1/2 and 2p3/2, respectively, which corroborated the presence of iron in CP 1. Two other spectra of 1 + Al(III) showed a peak at 75.2 eV for Al 2p, and 1 + Cr(III) gave peaks at 586.76 eV and 577.13 eV for 2p1/2 and 2p3/2, respectively, which clarified the presence of corresponding metal ions (Fig. 4).46–51
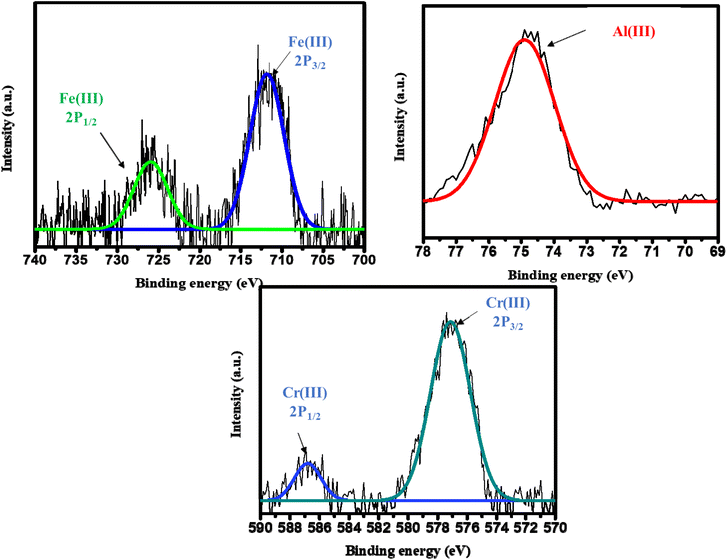 | ||
| Fig. 4 High-resolution XPS spectra of Fe(III) [2p1/2 and 2p3/2], Al(III) [2p], and Cr(III) [2p1/2 and 2p3/2]. | ||
In addition, the C 1s spectrum in all cases suggested an electron cloud flowing from the electron-rich naphthalene moiety to electron-deficient M3+ ions. In Fig. S17 (ESI†), the “C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C” binding energy at 285.9 eV changed slightly and also increased with respect to 1 alone, thereby confirming a small interaction between M3+ ions and naphthalene moities.52–54
C” binding energy at 285.9 eV changed slightly and also increased with respect to 1 alone, thereby confirming a small interaction between M3+ ions and naphthalene moities.52–54
Theoretical substantiation
A DFT study was performed in Turbomole (v7.0) using the B3LYP functional to enlarge an obvious observation for this type of fluorescence enhancement.55–57 The asymmetric unit of the CIF file of 1 was used to perform the DFT study. The optimized geometry of compound 1 showed that the total electron cloud of the whole single unit resided in the naphthalene moiety that promoted the interaction between the electron-rich naphthalene moiety and highly electron-deficient M3+ metal ions (Fig. S18, ESI†). Geometrical optimization of this coordination unit in the presence of M3+ (Fe3+, Al3+, and Cr3+) ions in Turbomole v7 revealed three explanations. First, a lower HOMO–LUMO gap in the presence of M3+ metal ions influenced fluorescence enhancement (Fig. 5).58,59 Second, geometrical optimization of 1 in the presence of different ions resulted in a <3 Å distance between the pi-electron cloud of the naphthalene moiety and metal ions (Fig. S19, ESI†), implying electrostatic forces. Third, the total optimized energy in the DFT study in 1 in the presence of M3+ ions was much less than that for the single coordination unit, resulting in more rigidity that diminished intramolecular rotational and vibrational energy losses. The extremely low HOMO–LUMO gap was the key factor for the highly sensitivity towards Cr3+ with respect to other trivalent metal ions. The very low LOD (43.1) of Cr3+ compared with that of Fe3+ and Al3+ was explainable by the result of DFT calculations.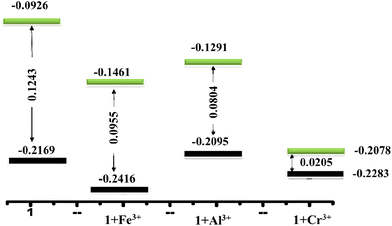 | ||
| Fig. 5 Energy-profile diagram of compound 1 with metal ions (Fe3+, Al3+, and Cr3+). HOMO (black) and LUMO (green) energy levels are calculated in the Hartree unit from DFT computations. | ||
Hands-on detection of an environmental specimen
The remarkable capacity of compound 1 with regard to sensitive and selective detection of trivalent metal ions in an aqueous medium prompted study of its practical usefulness. Fe3+, Al3+, and Cr3+ were spiked in tap water. The water samples were similar to analytic samples. In this regard, we developed a cotton swab-based sensing probe. A well-dispersed solution of compound 1 in CH3CN + H2O mixed-solvent was prepared for this study. Then, commercially available air-dried cotton swabs were dipped in the solution and dried to make the kit. Then, the treated cotton swab functioned as a detection probe for trivalent metal ions, and did not exhibit any color in visible light or UV light. However, when it was immersed in an aqueous solution of trivalent metal ions (Fe3+, Al3+, or Cr3+) in the presence of UV light, it showed an outstanding eye-catching green fluorescence (Fig. 6). Therefore, our material could be used to identify trivalent metal ions in universal media, which facilitated the practical utility of compound 1. | ||
| Fig. 6 Cotton ball-derived treated cotton swab dipped into solutions of 1 + Fe3+, 1 + Al3+, and 1 + Cr3+ under visible light and UV light. | ||
Comparative literature survey
An extensive literature review was performed to investigate the advantages of our chemosensor. Reports suggested that the synthesized material was a effective and robust CP that could be employed to identify all trivalent metal ions in the ppb range (Table S5, ESI†). The “turn on” phenomenon was the most importance feature of the synthesized material. Identification but also verification of the mechanism (via morphological explanation, XPS, and theoretical DFT calculations) made our study unique. Furthermore, implementation of a cotton swab as a hands-on experimental kit was a fascinating development in the field of sensing. Thus, our synthesized material could be used as a sensor (especially for group-IIIA metal ions).Conclusions
We demonstrated a supramolecular assembly of a 4-nvp appended Cd(II)-based 1D CP as a single fluorescent molecular probe that could detect trivalent ions (Fe3+, Al3+, and Cr3+) in the nanomolar range in aqueous medium. Thus, the synthesized compound was important with regard to biological applications and biocompatibility. Fe3+, Al3+, and Cr3+ could be identified by a group-separation technique. Our synthesized compound could be used for the detection of group-IIIA cations based on a fluorescence-enhancement method. Moreover, compound 1 was utilized to fabricate a low-cost cotton swab kit for the treatment of real samples in the detection of trivalent metal cations.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by SERB India (CRG/2022/001842, 19/12/2022). S. D. acknowledges the Department of Science and Technology, Government of India for his fellowship (IF200416). S. D. is also thankful to Mrs Somrita Nag (research scholar, CSIR-CMERI) for her scientific inputs to draw the TOC. P. B. acknowledges a DST SERB-CRG sponsored project (GAP-240712; CRG/2022/001679).References
- V. Chandrasekhar, B. M. Pandian, R. Boomishankar, A. Steiner, J. J. Vittal, A. Houri and R. Clerac, Inorg. Chem., 2008, 47, 4918–4929 CrossRef CAS PubMed.
- M. H. Mir, L. Wang, M. W. Wong and J. J. Vittal, Chem. Commun., 2009, 4539–4541 RSC.
- W. L. Leong and J. J. Vittal, J. Inclusion Phenom. Macrocyclic Chem., 2011, 71, 557–566 CrossRef CAS.
- A. Hazra, A. S. Bej, A. Mondal, N. C. Murmu and P. Banerjee, ACS Omega, 2020, 5, 15949–15961 CrossRef CAS PubMed.
- K. L. Haas and K. J. Franz, Chem. Rev., 2009, 109, 4921–4960 CrossRef CAS PubMed.
- A. Müller, A. M. Dommröse, W. Jaegermann, E. Krickemeyer and S. Sarkar, Angew. Chem., Int. Ed. Engl., 1981, 20, 1061–1063 CrossRef.
- M. Villa, M. Roy, G. Bergamini, M. Gingras and P. Ceroni, Dalton Trans., 2019, 48, 3815–3818 RSC.
- B. Dutta, S. Maity, S. Ghosh, C. Sinha and M. H. Mir, New J. Chem., 2019, 43, 5167–5172 RSC.
- B. Dutta, A. Hazra, A. Dey, C. Sinha, P. P. Ray, P. Banerjee and M. H. Mir, Cryst. Growth Des., 2020, 20, 765–776 CrossRef CAS.
- N. Stock and S. Biswas, Chem. Rev., 2012, 112, 933–969 CrossRef CAS PubMed.
- M. P. Suh, H. J. Park, T. K. Prasad and D. W. Lim, Chem. Rev., 2012, 112, 782–835 CrossRef CAS PubMed.
- P. Ghosh, A. Roychowdhury, M. Corbella, A. Bhaumik, P. Mitra, S. M. Mobin, A. Mukherjee, S. Basu and P. Banerjee, Dalton Trans., 2014, 43, 13500–13508 RSC.
- X. Z. Song, S. Y. Song, S. N. Zhao, Z. M. Hao, M. Zhu, X. Meng, L. L. Wu and H. J. Zhang, Adv. Funct. Mater., 2014, 24, 4034–4041 CrossRef CAS.
- J. D. Xiao, L. G. Qiu, F. Ke, Y. P. Yuan, G. S. Xu, Y. M. Wang and X. J. Jiang, Mater. Chem. A, 2013, 1, 8745–8752 RSC.
- J. N. Wilson and U. Bunz, J. Am. Chem. Soc., 2005, 127, 4124–4125 CrossRef CAS PubMed.
- A. P. R. de Souza, C. W. Foster, A. V. Kolliopoulos, M. Bertotti and C. E. Banks, Analyst, 2015, 140, 4130–4136 RSC.
- B. L. Hou, D. Tian, J. Liu, L. Z. Dong, S. L. Li, D. S. Li and Y. Q. Lan, Inorg. Chem., 2016, 55, 10580–10586 CrossRef CAS PubMed.
- Y. Z. Chen and H. L. Jiang, Chem. Mater., 2016, 28, 6698–6704 CrossRef CAS.
- N. K. Das, S. Ghosh, A. Priya, S. Datta and S. Mukherjee, J. Phys. Chem. C, 2015, 119, 24657–24664 CrossRef CAS.
- L. I. Szekeres, S. Bálint, G. Galbács, I. Kálomista, T. Kiss, F. H. Larsen, L. Hemmingsen and A. Jancsó, Dalton Trans., 2019, 48, 8327–8339 RSC.
- S. Zhang, B. Wang, S. Li, X. Li, G. Liu, Z. Zhang and X. Wang, J. Mol. Struct., 2024, 1297, 136929 CrossRef CAS.
- Z. G. Liang, G. M. Li, X. Y. Ren, J. H. Li, J. Pan and S. D. Han, Inorg. Chem., 2023, 62, 8663–8669 CrossRef CAS PubMed.
- R. Das, R. Pal, S. Bej, M. Mondal, K. Kundu and P. Banerjee, Mater. Adv., 2022, 3, 4421–4459 RSC.
- D. Das, R. Alam, A. Katarkar and M. Ali, Photochem. Photobiol. Sci., 2019, 18, 242–252 CrossRef CAS PubMed.
- T. Anand, S. K. A. Kumar and S. K. Sahoo, Spectrochim. Acta, Part A, 2018, 204, 105–112 CrossRef CAS PubMed.
- S. Samanta, S. Goswami, A. Ramesh and G. Das, Sens. Actuators, B, 2014, 194, 120–126 CrossRef CAS.
- X. Chen, X. Y. Shen, E. Guan, Y. Liu, A. Qin, J. Z. Sun and B. Z. Tang, Chem. Commun., 2013, 49, 1503–1505 RSC.
- N. R. Chereddy, P. Nagaraju, M. V. NiladriRaju, V. R. Krishnaswamy, P. S. Korrapati, P. R. Bangal and V. J. Rao, Biosens. Bioelectron., 2015, 68, 749–756 CrossRef CAS PubMed.
- A. Barba-Bon, A. M. Costero, S. Gil, M. Parra, J. Soto, R. M. Máñez and F. Sancenón, Chem. Commun., 2012, 48, 3000–3002 RSC.
- G. Svehla, Vogel's qualitative inorganic analysis, Longman, England, 7th edn, 1997 Search PubMed.
- B. L. Su, N. Moniotte, N. Nivarlet, L. H. Chen, Z. Y. Fu, J. Desmet and J. Li, J. Colloid Interface Sci., 2011, 358, 136–145 CrossRef CAS PubMed.
- X. Chen, X. Y. Shen, E. Guan, Y. Liu, A. Qin, J. Z. Sun and B. Z. Tang, Chem. Commun., 2013, 49, 1503–1505 RSC.
- T. P. Flaten, Brain Res. Bull., 2001, 55, 187–196 CrossRef CAS PubMed.
- B. Wang, W. Xing, Y. Zhao and X. Deng, Environ. Toxicol. Pharmacol., 2010, 29, 308–313 CrossRef CAS PubMed.
- C. C. Willhite, N. A. Karyakina, R. A. Yokel, N. Yenugadhati, T. M. Wisniewski, I. M. F. Arnold, F. Momoli and D. Krewski, Crit. Rev. Toxicol., 2014, 44, 1–80 CrossRef CAS PubMed.
- T. Y. Gu, M. Dai, D. J. Young, Z. G. Ren and J. P. Lang, Inorg. Chem., 2017, 56, 4668–4678 CrossRef PubMed.
- K. P. Wang, Y. Lei, S. J. Zhang, W. J. Zheng, J. P. Chen, S. Chen, Q. Zhang, Y. B. Zhang and Z. Q. Hu, Sens. Actuators, B, 2017, 252, 1140–1145 CrossRef CAS.
- K. P. Wang, J. P. Chen, S. J. Zhang, Y. Lei, H. Zhong, S. Chen, X. H. Zhou and Z. Q. Hu, Anal. Bioanal. Chem., 2017, 409, 5547–5554 CrossRef CAS PubMed.
- S. Goswami, K. Aich, S. Das, A. K. Das, D. Sarkar, S. Panja, T. K. Mondal and S. Mukhopadhyay, Chem. Commun., 2013, 49, 10739–10741 RSC.
- R. Chandra, A. K. Manna, K. Rout, J. Mondal and G. K. Patra, RSC Adv., 2018, 8, 35946–35958 RSC.
- P. Ghorai, A. Dey, A. Hazra, B. Dutta, P. Brandão, P. P. Ray, P. Banerjee and A. Saha, Cryst. Growth Des., 2019, 19, 6431–6447 CrossRef CAS.
- B. Dutta, S. Dey, K. Pal, S. Bera, S. Naaz, K. Jana, C. Sinha and M. H. Mir, New J. Chem., 2020, 44, 13163–13171 RSC.
- S. Paul, A. Manna and S. Goswami, Dalton Trans., 2015, 44, 11805–11810 RSC.
- R. Chandra, A. K. Manna, K. Rout, J. Mondal and G. K. Patra, RSC Adv., 2018, 8, 35946–35958 RSC.
- B. Dutta, R. Jana, A. K. Bhanja, P. P. Ray, C. Sinha and M. H. Mir, Inorg. Chem., 2019, 58, 2686–2694 CrossRef CAS PubMed.
- S. Choi, C. Kim, J. M. Suh and H. W. Jang, Carbon Energy, 2019, 1, 85–108 CrossRef CAS.
- A. Mukherjee, S. Chakrabarty, N. Kumari, W. N. Su and S. Basu, ACS Omega, 2018, 3, 5946–5957 CrossRef CAS PubMed.
- J. Wei, Y. Hu, Y. Liang, B. Kong, Z. Zheng, J. Zhang, Y. Zhao and H. Wang, J. Mater. Chem. A, 2017, 5, 10182–10189 RSC.
- C. Zhang, C. Zhang, Y. Xie, J. W. Su, X. He, J. D. Demaree, M. H. Griep, J. L. Atwood and J. Lin, Chem. – Eur. J., 2019, 25, 4036–4039 CrossRef CAS PubMed.
- N. Kumar and K. Biswas, J. Mater. Res. Technol., 2019, 8, 63–74 CrossRef CAS.
- Y. Chen, D. An, S. Sun, J. Gao and L. Qian, Materials, 2018, 11, 269 CrossRef PubMed.
- K. Ranganathan, A. Morais, I. Nongwe, C. Longo, A. F. Nogueira and N. J. Coville, J. Mol. Catal. A: Chem., 2016, 422, 165–174 CrossRef CAS.
- N. Dwivedi, R. J. Yeo, N. Satyanarayana, S. Kundu, S. Tripathy and C. S. Bhatia, Sci. Rep., 2015, 5, 7772 CrossRef CAS PubMed.
- F. Zhao, A. Vrajitoarea, Q. Jiang, X. Han, A. Chaudhary, J. O. Welch and R. B. Jackman, Sci. Rep., 2015, 5, 13771 CrossRef PubMed.
- P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, J. Phys. Chem., 1994, 98, 11623–11627 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1992, 96, 2155–2160 CrossRef CAS.
- S. H. Vosko, L. Wilk and M. Nusair, Can. J. Phys., 1980, 58, 1200–1211 CrossRef CAS.
- S. Khan, A. Hazra, B. Dutta, Akhtaruzzaman, M. J. Raihan, P. Banerjee and M. H. Mir, Cryst. Growth Des., 2021, 21, 3344–3354 CrossRef CAS.
- B. Dutta, A. Hazra, S. Datta, C. Sinha, P. Banerjee and M. H. Mir, ACS Appl. Polym. Mater., 2022, 4, 2841–2850 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, Tables S1–S5, Fig. S1–S20 and X-ray crystallographic data in CIF format for compound 1. CCDC 2307019. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3dt03939k |
| This journal is © The Royal Society of Chemistry 2024 |