Open Access Article
Open Access ArticleSubphthalocyanines as fluorescence sensors for metal cations†
Mary Angelia
Alfred
 a,
Kamil
Lang
a,
Kamil
Lang
 b,
Kaplan
Kirakci
b,
Kaplan
Kirakci
 b,
Pavel
Stuzhin
b,
Pavel
Stuzhin
 c,
Petr
Zimcik
c,
Petr
Zimcik
 a,
Jan
Labuta
a,
Jan
Labuta
 *d and
Veronika
Novakova
*d and
Veronika
Novakova
 *a
*a
aFaculty of Pharmacy in Hradec Kralove, Charles University, Ak. Heyrovskeho 1203, Hradec Kralove, 500 05 Czech Republic. E-mail: veronika.novakova@faf.cuni.cz
bInstitute of Inorganic Chemistry of the Czech Academy of Sciences, 250 68 Husinec-Řez, Czech Republic
cInstitute of Macroheterocycles, Ivanovo State University of Chemistry and Technology, Sheremetevskij Pr-t 7, 153000 Ivanovo, Russia
dResearch Center for Materials Nanoarchitectonics (MANA), National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan. E-mail: labuta.Jan@nims.go.jp
First published on 4th January 2024
Abstract
Subphthalocyanines (SubPcs) and their aza-analogues (SubTPyzPzs) are fluorophores with strong orange fluorescence emission; however, their sensing ability towards metal cations remains uncharted. To fill this gap, we have developed an efficient method for introducing aza-crown moieties at the axial position of SubPcs and SubTPyzPzs to investigate the structure–activity relationship for sensing alkali (Li+, Na+, K+) and alkaline earth metal (Ca2+, Mg2+, Ba2+) cations. SubPcs showed better photostability than SubTPyzPzs and even a commonly utilized dye, 6-carboxyfluorescein. Selectivity toward metal cations was driven by the size of the aza-crown, irrespective of the counter anion. The stoichiometry of binding was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in all cases, and the interaction between SubPcs and cations was characterized by the corresponding apparent binding constants (Ka). Notably, an unusually strong interaction of all sensoric SubPcs with Ba2+ compared to other studied cations was demonstrated. The role of the surrounding environment, i.e. the addition of water or methanol, in sensing cations is shown in detail as well. Selectivity towards K+ over Na+ was demonstrated in aqueous media with SubPcs bearing the 1-aza-6-crown-18 moiety in Tween 80 micelles. In this case, a 5-fold increase of the fluorescence quantum yield was observed upon binding K+ ions. The high brightness, photostability, and sensing activity in aqueous media make SubPc macrocycles promising fluorophores for metal cation sensing.
1 in all cases, and the interaction between SubPcs and cations was characterized by the corresponding apparent binding constants (Ka). Notably, an unusually strong interaction of all sensoric SubPcs with Ba2+ compared to other studied cations was demonstrated. The role of the surrounding environment, i.e. the addition of water or methanol, in sensing cations is shown in detail as well. Selectivity towards K+ over Na+ was demonstrated in aqueous media with SubPcs bearing the 1-aza-6-crown-18 moiety in Tween 80 micelles. In this case, a 5-fold increase of the fluorescence quantum yield was observed upon binding K+ ions. The high brightness, photostability, and sensing activity in aqueous media make SubPc macrocycles promising fluorophores for metal cation sensing.
Introduction
Metal cations are fundamental elements for sustaining plant, animal, and human life. Their absence or increase to toxic levels can cause growth disorders, severe malfunction, or even death.1,2 Various fluorescent probes are, therefore, being developed to enable their monitoring. Switching such probes on and off is typically driven by photo-induced electron transfer, charge transfer, fluorescence resonance energy transfer, excimer formation or metal-coordination-induced chemical reactions. The details of these switching mechanisms may be found in many reviews.3–5For the recognition of alkali and alkaline earth metal cations, crowns are probably the most suitable hosts, since their selectivity and sensitivity of recognition may be easily tuned by their size, exchange of oxygen for other heteroatoms or attachment of additional binding sites either in the form of a supporting ligand or as a cryptand.6–10 Wide diversity may be on the other hand found in signalling moieties, since many structurally different fluorophores can be found in the literature.11,12 Phthalocyanines and especially their aza-analogues have been shown to possess advantageous properties in sensing applications due to their spectral and photophysical properties.13,14 The lower homologues of phthalocyanines (i.e., subphthalocyanines, SubPcs) and of tetrapyrazinoporphyrazines (SubTPyzPzs), have been used as fluorophores for photochromic switches,15,16 and as photosensitizers for photodynamic therapy of cancer.17–21 The great application potential of these macrocycles has been summarized in recent reviews.22,23 To the best of our knowledge, no aza-crown containing SubPcs and only a limited number of crown containing SubPcs have been successfully prepared with a crown at the peripheral positions24 or a benzocrown21 at the axial position. Surprisingly, SubPcs have not yet been studied as fluorescence sensors for cation recognition. Thanks to promising literature notes about these fluorophores in the sensing of pH25 and fluorides,26 we decided to investigate the suitability of these macrocycles for cation recognition. We believe that they may extend the range of fluorophores for the visible area (around 590 nm) and that they may be superior to some of the fluorophores that suffer from notable photobleaching (e.g., cyanine dyes). Moreover, the great advantage of these compounds is the possibility of introducing a recognition moiety into the axial position, whereas peripheral sites may be used for tuning the spectral and physicochemical properties.
In this project, we, therefore, aimed to investigate for the first time SubPcs and SubTPyzPzs as signalling moieties for recognition of alkali and alkaline earth metal cations and to prove the sensing principle even in water employing Tween 80 micelles.
Results and discussion
Design and synthesis
The target compounds were designed to possess SubPc or SubTPyzPz as a signalling moiety, which ensures strong fluorescence emission at about 590 and 560 nm, respectively. Phenyl groups attached to the periphery of macrocycles enrich the π-system of the macrocycle, thus shifting the absorption maximum toward red. Both SubPc and SubTPyzPz cores were included to study the effect of aza-substitution. As axial ligands, they carry either 1-aza-4-crown-12, 1-aza-5-crown-15 or 1-aza-6-crown-18 capable of cation recognition. These recognition moieties were attached with a macrocycle through a phenylene linker to minimize the basicity of nitrogen responsible for switching and thus limit undesirable pH sensitivity (see also the discussion below). A control compound (always-ON) with phenol as the axial ligand was also introduced in the study.Ligands intended to be attached to the axial positions of macrocycles were prepared by a three-step procedure beginning from p-bromophenol (Scheme 1, i–iii). Its hydroxy group was protected with a tetrahydropyranyl ether followed by Buchwald–Hartwig coupling with an appropriate aza-crown ether. Finally, the protecting group was removed by acidic hydrolysis. Noteworthily, similar synthetic pathways with methyl as a protecting group and using BBr3 or HBr for deprotection were successful in our hands as well, but the yields differed significantly at each batch. By the above-mentioned procedure, we obtained the desired ligands 3a–c repeatedly in overall yields of about 80%.
Precursors 4 and 5 were prepared according to the literature27–29 but crystallization from benzene and hexane instead of sublimation as a final purification step of 4 was used to get this compound in an improved yield of 78%.
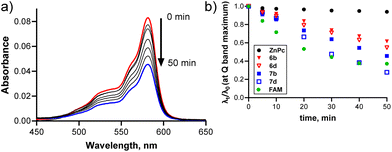
Cyclotrimerization of 4,5-diphenylphthalonitrile (4) followed by direct substitution with the respective ligands as a one-pot reaction25 led to the target macrocycles 6a–d in yields of about 23% (Scheme 2). Toluene and p-xylene were chosen as solvents to avoid chlorination by Cl2 evolved from BCl3. The similar conditions applied for 5,6-diphenylpyrazine-2,3-dicarbonitrile (5) and ligand 3b produced the desired SubTPyzPz 7b, which was, however, found to be less stable than the SubPc analogue 6b. The decomposition products made the purification difficult, so we obtained 7b in a yield of only 11%. Based on this experience, we decided to study the (photo)stability of the target derivatives by UV–vis spectroscopy by observing the decrease in the Q band in THF both in the dark or upon light exposure (100 W Xe-ozone free lamp, Newport) (Fig. 1). Sensors 6b and 7b, and controls 6d and 7d were chosen as model compounds and compared with unsubstituted zinc(II) phthalocyanine (ZnPc) and 6-carboxyfluorescein (FAM) under the same experimental conditions. All the studied derivatives were stable in the dark but decomposed in various degrees under light irradiation. As expected, all the studied derivatives were less stable than ZnPc, which is known for great stability,30,31 but had better photostability than FAM, which is a common commercially available fluorophore widely used in practice. Obviously, the isosteric replacement of benzenes in SubPcs with pyrazines decreased the stability of the macrocycle. Decomposition produced no distinct spots on the TLC, so the decomposition products could not be determined. From the fact that only a decrease of all absorption bands was observed without the formation of new bands (Fig. S11†), we can only assume that the macrocycle decomposes into low-molecular-weight substances. Many factors influence the photostability of SubPcs and their analogues;32 thus, a clear explanation is difficult. It is, however, worth noting that long irradiation times were used to clearly observe the differences between compounds. Such light doses are unnecessary in practical applications and were used to test that the target sensor can be considered stable under biologically relevant conditions. Nevertheless, these experiments clearly demonstrated that 6b is superior to its aza-analogue 7b in photostability.
 | ||
| Scheme 2 Synthesis of target macrocycles – (i) BCl3, p-xylene, argon, reflux, 2 h; followed by 3a–c or phenol, anhydrous toluene, argon, reflux, 12 h, 20–25%. | ||
UV–vis spectral properties
The characteristic Q band was observed for SubPcs and SubTPyzPzs at about 582 nm and 550 nm, respectively (Table 1). Peripheral phenyls contributed to the π-system of the parent macrocycle, resulting in an ∼20 nm red shift in comparison with nonsubstituted macrocycles.16,25,33 The identical positions of Q bands of 6a–d indicated no involvement of the axial ligands, which is in accordance with the literature.25 Since 6b has a more straightforward purification procedure, better (photo)stability and a red-shifted absorption band (in comparison with 7b), which is advantageous for any biological experiments, we decided to exclude aza-analogue 7b from further fluorescence studies and focus on SubPc derivatives only, which seem to have better potential in this application.| Compound | Axial ligand | λ A (nm) | λ F (nm) | Φ F (OFF) | Φ F (ON) | FEF |
K
a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (M−1)
(M−1) |
|---|---|---|---|---|---|---|---|
a Absorption maximum (λA), fluorescence emission maximum (λF), fluorescence quantum yield (ΦF) – either without any analyte (ΦF (OFF)) or at the plateau phase of titration in excess of appropriate salt (in the form of triflates), fluorescence enhancement factor (FEF, i.e. the ratio between ΦF (ON) and ΦF (OFF)), and apparent association constant (Ka) determined using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest binding model. ΦF values were determined by a comparison method using rhodamine 6G as the reference (ΦF = 0.94, EtOH34). For the details of titration experiments, see the Experimental part.
b Values determined from fluorescence emission data. All values have a relative error <15%.
c No changes in ΦF of statistical significance were observed upon salt addition.
d Value determined from NMR is Ka = 34 1 host–guest binding model. ΦF values were determined by a comparison method using rhodamine 6G as the reference (ΦF = 0.94, EtOH34). For the details of titration experiments, see the Experimental part.
b Values determined from fluorescence emission data. All values have a relative error <15%.
c No changes in ΦF of statistical significance were observed upon salt addition.
d Value determined from NMR is Ka = 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 M−1.
e Value determined from NMR is Ka > 230 800 M−1.
e Value determined from NMR is Ka > 230![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 (axial phenyl linker resonances at 5.33 and 6.18 ppm were used for analyses). 000 M−1 (axial phenyl linker resonances at 5.33 and 6.18 ppm were used for analyses).
|
|||||||
| 6a | 1-Aza-4-crown-12 | 583 | 593 | 0.0023 | 0.097 Li+ | 42 | 9.9 |
| 0.0023 Na+ | —c | —c | |||||
| 0.0023 K+ | —c | —c | |||||
| 0.10 Ca2+ | 43 | 500 | |||||
| 0.013 Mg2+ | 5.7 | 9080 | |||||
| 0.14 Ba2+ | 61 | 192![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|||||
| 6b | 1-Aza-5-crown-15 | 582 | 593 | 0.0014 | 0.0117 Li+ | 8.4 | 5.5 |
| 0.019 Na+ | 14 | 615 | |||||
| 0.0053 K+ | 3.8 | 76 | |||||
| 0.0006 Ca2+ | 11 | 58 | |||||
| 0.0041 Mg2+ | 2.9 | 520 | |||||
| 0.15 Ba2+ | 107 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
|||||
| 6c | 1-Aza-6-crown-18 | 583 | 593 | 0.0022 | 0.089 Li+ | 40 | 5.7 |
| 0.012 Na+ | 5.5 | 1940 | |||||
| 0.097 K+ | 44 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 d 100 d |
|||||
| 0.10 Ca2+ | 45 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
|||||
| 0.094 Mg2+ | 43 | 6 200 | |||||
| 0.090 Ba2+ | 41 | 384![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 e 000 e |
|||||
| 6d | Phenol | 582 | 592 | 0.12 | 0.11 Li+ | —c | —c |
| 0.12 Na+ | — c | —c | |||||
| 0.12 K+ | —c | —c | |||||
| 0.12 Ca2+ | —c | —c | |||||
| 0.12 Mg2+ | —c | —c | |||||
| 0.13 Ba2+ | —c | —c | |||||
| 7b | 1-Aza-5-crown-15 | 550 | 563 | ||||
| 7d | Phenol | 550 | 562 | ||||
Metal cation sensing in tetrahydrofuran
Switching fluorescence between ON and OFF states is typically based on blocking and facilitating a quenching process, respectively. Highly efficient quenching in the OFF state is necessary for good signal-to-noise ratios during analyte detection. In our case, quenching in the OFF state was enabled by photo-induced electron transfer (PET) occurring between a nitrogen in the aza-crown (serving as a donor of electrons) and the SubPc macrocycle (serving as an acceptor). By coordinating the cation to the recognition site of the aza-crown, the lone electron pair of the nitrogen of the aza-crown cannot provide electrons for the PET process, which results in the restoration of strong fluorescence emission. The selectivity of recognition of a given cation should be driven by the size of the aza-crown cavity while the increase in fluorescence (fluorescence enhancement factor, FEF) should be driven by the strength of the coordination of the lone pair to the cation. To prove this mechanism, extensive fluorescence studies were performed.Upon excitation, SubPcs 6a–c in THF emitted only weak fluorescence at 593 nm with a Stokes shift of 10 nm.19,25 Fluorescence quantum yields (ΦF) were below 0.003 due to highly efficient quenching by PET from the axial nitrogen to the macrocyclic core. The always-ON control 6d possessed, on the other hand, strong fluorescence emission with the maximum at 592 nm and ΦF = 0.12 (THF), which correspond to the values published for SubPcs and their analogues (for example ΦF in acetone for unsubstituted SubPc with the phenoxy group at the axial position, 6d and 7d were reported to be 0.18, 0.28 and 0.13, respectively25).
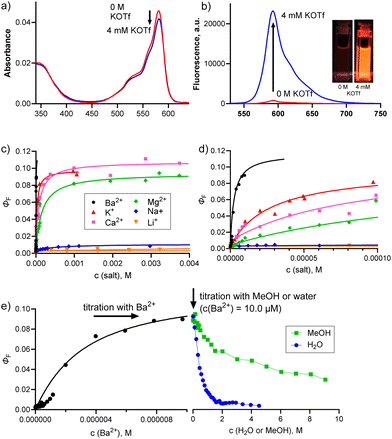
Before studying the cation binding to azacrown SubPcs, we focused first on the potential effect of the counter anion. In our previous project with zinc(II) azaphthalocyanines,9 we described that the sensitivity toward cations is strongly dependent on the counter anion used and increased as follows: NO3− < Br− < CF3SO3− < ClO4− ≪ SCN−. Extraordinary high sensitivity toward SCN− resulted from coordination of these anions to the central zinc(II) atom. To select the most suitable counter anion for the current series of SubPcs, we first titrated 6c as a model compound with different potassium salts (Fig. S12†). Since only negligible differences were observed between thiocyanates and other analytes, we further confirmed the role of zinc(II) in the coordination of the counter anion described in our previous project (because such an interaction is impossible with the central boron atom of 6c). Overall, the effect of the counter anion was rather limited; therefore, triflate (CF3SO3−) was selected as a counter anion for the following experiments due to high ΦF in the ON state.
The ability of 6a–c to serve as fluorescence sensors by the above-described mechanism was studied in detail by titrations with Li+, Na+, K+, Mg2+, Ca2+ and Ba2+ triflates. In the case of sensitive analytes, the stepwise addition of the salt (in a MeOH stock solution) to the THF solution of the studied SubPcs led to a steep increase in fluorescence emission (Table 1, FEF values; Fig. 2c, d and Fig. S16†), reaching a value of ΦF ∼0.1. This ΦF is close to the value of the always-ON control 6d, i.e. the maximum possible value, which suggests strong binding of particular cations with efficient blocking of PET. Expectedly, no changes were observed in the absorption spectra (Fig. 2a) since electrons of the aza-crown are not involved in the π-conjugated system of the macrocycle.
The stoichiometry of binding of sensitive analytes was studied by both NMR titration studies (Fig. 3, S13 and S14†) and Job's method of continuous variations with fluorescence monitoring (Fig. S15†). In the 1H NMR spectra of 6c, protons on the axial phenylene linker at 5.33 and 6.18 ppm shifted downfield upon the addition of K+ up to 5.46 and 6.62 at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 6c
1 ratio of 6c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOTf with Δδ = 0.13 and 0.44 ppm, respectively (Fig. 3). Further addition of K+ did not alter the position of the resonances. The shift can be explained by the fact that coordination of the cation leads to a lower ability of nitrogen to donate electrons to phenylene, which leads to a magnetic deshielding effect. Similarly, the signal at approximately 3.27 ppm corresponding to the protons of –CH2– next to the nitrogen in the aza-crown shifted upfield to 2.97 ppm (Δδ = −0.30 ppm) at a 1
KOTf with Δδ = 0.13 and 0.44 ppm, respectively (Fig. 3). Further addition of K+ did not alter the position of the resonances. The shift can be explained by the fact that coordination of the cation leads to a lower ability of nitrogen to donate electrons to phenylene, which leads to a magnetic deshielding effect. Similarly, the signal at approximately 3.27 ppm corresponding to the protons of –CH2– next to the nitrogen in the aza-crown shifted upfield to 2.97 ppm (Δδ = −0.30 ppm) at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 6c
1 ratio of 6c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOTf and did not move further at higher salt concentrations. This opposite shift of 6c resonances was probably caused by the reduction of aza-crown conformational flexibility in conjunction with changes in the electron density on the nitrogen atom upon cation encapsulation. The interaction of 6c with Ba2+ (Fig. S13†) showed a similar pattern with chemical shift differences of Δδ = 0.16 and 0.61 ppm for phenylene and Δδ = −0.19 ppm for aliphatic signals of aza-crown protons. Similar cation-induced NMR shifts were also observed in the literature for aza-crown containing benzyl sidearms.35 No changes in the 1H NMR of the always-ON control 6d were noticed (Fig. S14†). NMR studies with 6c and its negative control 6d, thus, unequivocally proved 1
KOTf and did not move further at higher salt concentrations. This opposite shift of 6c resonances was probably caused by the reduction of aza-crown conformational flexibility in conjunction with changes in the electron density on the nitrogen atom upon cation encapsulation. The interaction of 6c with Ba2+ (Fig. S13†) showed a similar pattern with chemical shift differences of Δδ = 0.16 and 0.61 ppm for phenylene and Δδ = −0.19 ppm for aliphatic signals of aza-crown protons. Similar cation-induced NMR shifts were also observed in the literature for aza-crown containing benzyl sidearms.35 No changes in the 1H NMR of the always-ON control 6d were noticed (Fig. S14†). NMR studies with 6c and its negative control 6d, thus, unequivocally proved 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry in the case of 6c/K+ and 6c/Ba2+. This is in agreement with the results from Job's plot (i.e., method of continuous variations, Fig. S15†), where the 1
1 binding stoichiometry in the case of 6c/K+ and 6c/Ba2+. This is in agreement with the results from Job's plot (i.e., method of continuous variations, Fig. S15†), where the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry was further confirmed independently for 6b/Na+, 6b/Ba2+, 6c/K+ and 6c/Ba2+ using the fluorescence emission intensity.
1 stoichiometry was further confirmed independently for 6b/Na+, 6b/Ba2+, 6c/K+ and 6c/Ba2+ using the fluorescence emission intensity.
The binding strength between SubPcs 6a–c and individual cations was then analyzed using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest binding model,36,37 with the apparent binding constant Ka. The constant is apparent due to the presence of MeOH in the cation stock solution, which also interacts with SubPc's aza-crown38 unit (more details are given below). The binding isotherms are plotted in Fig. 2c, d and Fig. S16† and the actual values are given in Table 1 and Table S1.†
1 host–guest binding model,36,37 with the apparent binding constant Ka. The constant is apparent due to the presence of MeOH in the cation stock solution, which also interacts with SubPc's aza-crown38 unit (more details are given below). The binding isotherms are plotted in Fig. 2c, d and Fig. S16† and the actual values are given in Table 1 and Table S1.†
Size-driven recognition of cations took place only for alkali metal cations, whereas limited size preference was observed in the group of alkaline earth metal cations. For example, 6c bearing a 1-aza-6-crown-18 moiety is switched ON by K+ and not by smaller cations, such as Na+ and Li+ (where the binding constants are small). Although the FEF increases in the presence of both K+ and Li+, in the case of Li+ this occurs only at high concentrations since Ka = 5.7 M−1.
SubPc 6b with a 1-aza-5-crown-15 moiety prefers, on the other hand, Na+ over K+, which is documented by higher FEF and Ka values for 6b/Na+ interaction than those for 6b/K+. The smallest analogue 6a with a 1-aza-4-crown-12 moiety recognized Li+ over the others; however, Ka was 9.9 M−1 only, indicating that this sensor is selective for Li+ but can be switched ON only at high Li+ concentrations.
Alkaline earth metal cations were coordinated to 6a–c more tightly than alkali metal cations, which was obvious from both high FEF values reaching the value of the control compound 6d and the high Ka values (approximately in thousands of M−1) (see Table 1 and Table S1†). A similar trend has been published before using different methods (i.e., absorption39 and fluorescence8 spectroscopy, polarography40 or laser infrared multiple photon dissociation spectroscopy41). This can be explained by the fact that alkaline earth metal cations are roughly similar in size to alkali metals, but being divalent cations, they possess a larger positive charge. As a result, their interaction with the aza-crown moiety is stronger, resulting in higher FEF and Ka values. Surprisingly, the binding of Ba2+ was, in our case, abnormally strong since the Ka values were determined to be 192![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 13
000, 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 and 384
900 and 384![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 for 6a, 6b and 6c, respectively. This gives us a great starting point for follow-up studies on Ba2+ sensors.
000 M−1 for 6a, 6b and 6c, respectively. This gives us a great starting point for follow-up studies on Ba2+ sensors.
Importantly, there was a decrease in the fluorescence emission upon the addition of water or MeOH into the solution of the already switched-ON SubPc 6c (1 μM) by Ba2+ (10.0 μM), as seen in Fig. 2e. This effect was moderate for MeOH and more substantial for water. This indicates that more polar molecules bind more strongly to the aza-crown unit38 and knock out the Ba2+ cation, which enables fluorescence quenching via PET. This is further depicted by the weakened fluorescence response (and lower sensitivity) of a 6c solution containing a defined amount of water when titrated with Ba2+ cations (Fig. S17a†). Similar behaviour can also be observed for K+ (Fig. S17b†). These results suggest that the ambient environment plays a very important role in cation sensing, and it is essential to investigate the sensing capability of the sensors in aqueous media to assess their impact as fluorescence sensors.
Metal cation sensing in water
SubPcs are too hydrophobic sensor molecules to be used in water without losing their monomeric character and fluorescence properties. To overcome these limitations, compound 6c, chosen as a model compound of the series, and 6d, a control compound always in the ON state, were integrated into ∼10 nm-sized Tween 80 micelles. Tween 80 is a common nonionic surfactant and emulsifier, and the characteristics of prepared micelles in water are presented in Table S2 and Fig. S18 and S19.† The absorption spectra of 6c and 6d in micelles corresponded well with their absorption spectra in THF (Fig. 4a), which proves the monomeric character of both compounds under aqueous conditions. Surprisingly, the fluorescence intensity of 6c/Tween 80 was not efficiently quenched (ΦF = 0.04), indicating a specific interaction of the 1-aza-6-crown-18 moiety with the solvent. As a result of the high initial ΦF value, the addition of KCl led only to a small increase in the fluorescence intensity (data not shown). Explanation consists of the fact that aza-crown nitrogen has the character of weakly basic aromatic amine and can be partially protonated in used deionized water of approximately pH 6. We assumed that nitrogen protonation leads to the reduction of the efficacy of PET even in the OFF state and, therefore, to a high initial ΦF value. To prove that, we investigated the fluorescence intensities of the 6c/Tween 80 and 6d/Tween80 at different pH values, and indeed, the a considerable decrease of fluorescence intensities, i.e., high efficacy of PET, of 6c/Tween80 was restored at pH values above 7 (Fig. 4b). Since the physiological pH is 7.4, the effect of protonation should not affect the sensor's properties in the biological environment.Based on the above results, the sensing ability of 6c/Tween 80 was investigated in TRIS buffer at approximately pH 8 to eliminate protonation of aza-crown nitrogens. In agreement with the fluorescence behaviour of 6c in THF, the fluorescence emission of 6c/Tween 80 in Tris buffer was weak (the OFF state, ΦF = 0.014), and responded to the addition of KCl (up to ΦF = 0.072) (Fig. 4c and d), but not to the addition of NaCl (ΦF = 0.017) (Fig. S20†). In contrast, the always-ON control 6d/Tween 80 exhibited constant fluorescence emission (ΦF = 0.10) irrespective of the pH value (Fig. 4b), and slight fluorescence quenching with increased concentrations of NaCl (ΦF = 0.089) or KCl (ΦF = 0.095) (Fig. 4d, Fig. S20†). The results of 6c/Tween 80, showing approximately a 5-time increase of ΦF in the presence of KCl, proved that prepared derivatives can be transferred to aqueous media using micelles as delivery systems without losing their sensing abilities.
Conclusions
A straightforward synthetic method for introducing aza-crown moieties at the axial position of SubPcs or SubAzaPcs was developed. Since the synthesized SubAzaPcs had blue-shifted absorption and emission spectra, were less stable during purification processes, and were less photostable when compared with the corresponding SubPc derivatives, the sensing ability towards alkali and alkaline earth metal cations was studied in detail only in a series of SubPc aza-crown derivatives 6a–6c. SubPc sensors were switched ON in the presence of sensitive analytes, that was monitored as an increase in ΦF. Size preference was observed only in the case of alkali metal cations, while alkaline earth metal cations were bonded regardless of their size. High molar absorption coefficients of the studied SubPc derivatives (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε ∼4.7 dm3 mol−1 cm−1), high fluorescence enhancement factors of selective anions (FEF up to 107), and high fluorescence quantum yields in the ON state (ΦF up to 0.15) rank these derivatives among promising fluorescence sensors. Interestingly, the binding of Ba2+ was extraordinarily strong, making some of these derivatives promising targets for follow-up studies aimed to monitor Ba2+ in the environment.
ε ∼4.7 dm3 mol−1 cm−1), high fluorescence enhancement factors of selective anions (FEF up to 107), and high fluorescence quantum yields in the ON state (ΦF up to 0.15) rank these derivatives among promising fluorescence sensors. Interestingly, the binding of Ba2+ was extraordinarily strong, making some of these derivatives promising targets for follow-up studies aimed to monitor Ba2+ in the environment.
Importantly, we have shown that assessment of the selectivity of a sensor towards an analyte requires collecting characteristics such as the FEF, ΦF in the ON state, and the corresponding Ka values and evaluating them together. It can be documented by the behavior of 6c. This derivative showed a strong fluorescence emission increase upon meeting Li+, K+, Ca2+, Mg2+ and Ba2+; however, only K+ and Ba2+ ions exhibited reasonable Ka values. Since the levels of Ba2+ in plasma are negligible,426c can be considered as a promising fluorescence sensor candidate for the selective recognition of K+ levels in human fluids.
This work also highlighted the need to study the sensing ability of sensors in aqueous environments, since the presence of water (and other polar molecules such as MeOH) significantly altered the binding of cations to aza-crowns. Finally, the present study opened new directions for broader applications of SubPcs in fluorescence sensing, since these derivatives provide photostable fluorophores with advantageous spectral properties, and the sensing ability can be transposed into aqueous media. Their great advantage is that recognition moieties can be attached at the axial position, whereas their peripheral sites on signalling moieties may be used to tune the spectral and physico-chemical properties.
Experimental
General
All chemical reagents were purchased from certified suppliers (Sigma-Aldrich, TCI, Acros Organic, Fluorochem) and used as received. All the organic solvents used in the synthesis were of analytical grade. Thin-layer chromatography was performed on Merck aluminum sheets coated with silica gel 60 F254. Merck Kieselgel 60 (0.040–0.063 mm) was used for column chromatography. The infrared spectra were measured on a Nicolet 6700 spectrometer in the ATR mode. The 1H and 13C NMR spectra were recorded on a VNMR S500 NMR spectrometer or a Jeol JNM-ECZ600R. The chemical shifts are reported as δ values in ppm and are indirectly referenced to Si(CH3)4via the signal from the solvent. J values are given in Hz. The UV–vis spectra were recorded using a Shimadzu UV-2600 spectrophotometer (Shimadzu, Kyoto, Japan). Fluorescence spectra were recorded using an FS5 spectrofluorometer (Edinburgh Instruments, Edinburgh, UK). A UHPLC Acquity UPLC I-class system (Waters, Millford, USA) coupled to a high-resolution mass spectrometer (HRMS) Synapt G2Si (Waters, Manchester, UK) based on Q-TOF were used for HRMS spectra measurement. Chromatography was carried out using an Acquity UPLC BEH C18 column (2.1 × 50 mm, 1.7 μm) or an Acquity UPLC BEH C4 (2.1 × 50 mm, 1.7 μm, 300 Å) columns for molecules up to or above 1200 m/z, respectively, using gradient elution with acetonitrile and 0.1% formic acid at a flow rate of 0.4 ml min−1. Electrospray ionization was operated in positive mode. The ESI spectra were recorded using leucine-enkephalin as a lock mass reference and sodium formate (in the range 50–1200 m/z) or sodium iodide (in the range 50–2000 m/z) for mass calibration.The compounds 1,434,27,285,296d25 and 7d25 were synthesized according to the previously reported procedures.
Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45; yield 15 mg (74%) as a light pink oil. 1H NMR (500 MHz, DMSO-d6) δ 8.48 (s, 1H), 6.61–6.57 (m, 2H), 6.57–6.54 (m, 2H), 3.67 (t, J = 5.0 Hz, 4H), 3.56–3.50 (m, 8H), 3.36 (t, J = 5.0 Hz, 4H). 13C NMR (126 MHz, DMSO-d6) δ 148.26, 141.92, 115.69, 113.77, 70.83, 69.30, 69.04, 52.07. IR (ATR): νmax (cm−1) 2924, 2856, 1514, 1360, 1226, 1125, 1089, 1067, 817, 677, 646. HRMS (ESI) calculated for C14H21NO4 + H+ 268.1544, found 268.1547 [M + H]+, 290.1363 [M + Na]+.
45; yield 15 mg (74%) as a light pink oil. 1H NMR (500 MHz, DMSO-d6) δ 8.48 (s, 1H), 6.61–6.57 (m, 2H), 6.57–6.54 (m, 2H), 3.67 (t, J = 5.0 Hz, 4H), 3.56–3.50 (m, 8H), 3.36 (t, J = 5.0 Hz, 4H). 13C NMR (126 MHz, DMSO-d6) δ 148.26, 141.92, 115.69, 113.77, 70.83, 69.30, 69.04, 52.07. IR (ATR): νmax (cm−1) 2924, 2856, 1514, 1360, 1226, 1125, 1089, 1067, 817, 677, 646. HRMS (ESI) calculated for C14H21NO4 + H+ 268.1544, found 268.1547 [M + H]+, 290.1363 [M + Na]+.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; yield 21 mg (87%) as a light pink oil. 1H NMR (500 MHz, DMSO-d6) δ 8.45 (s, 1H), 6.64–6.57 (m, 2H), 6.51–6.44 (m, 2H), 3.58 (t, J = 6.1 Hz, 4H), 3.55–3.47 (m, 12H), 3.37 (t, J = 6.1 Hz, 4H). 13C NMR (126 MHz, DMSO-d6) δ 148.01, 140.99, 115.87, 112.78, 70.35, 69.44, 69.19, 68.36, 52.21. IR (ATR): νmax (cm−1) 2865, 1734, 1514, 1355, 1222, 1121, 938, 814, 734. HRMS (ESI) calculated for C16H25NO5 + H+ 312.1806, found 312.1810 [M + H]+, 334.1629 [M + Na]+.
2; yield 21 mg (87%) as a light pink oil. 1H NMR (500 MHz, DMSO-d6) δ 8.45 (s, 1H), 6.64–6.57 (m, 2H), 6.51–6.44 (m, 2H), 3.58 (t, J = 6.1 Hz, 4H), 3.55–3.47 (m, 12H), 3.37 (t, J = 6.1 Hz, 4H). 13C NMR (126 MHz, DMSO-d6) δ 148.01, 140.99, 115.87, 112.78, 70.35, 69.44, 69.19, 68.36, 52.21. IR (ATR): νmax (cm−1) 2865, 1734, 1514, 1355, 1222, 1121, 938, 814, 734. HRMS (ESI) calculated for C16H25NO5 + H+ 312.1806, found 312.1810 [M + H]+, 334.1629 [M + Na]+.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15; yield 24 mg (89%) as a light pink oil. 1H NMR (600 MHz, DMSO-d6) δ 8.50 (s, 1H), 6.64–6.58 (m, 2H), 6.57–6.51 (m, 2H), 3.55–3.52 (m, 20H), 3.38 (t, J = 6.1 Hz, 4H). 13C NMR (151 MHz, DMSO-d6) δ 148.36, 141.27, 115.82, 113.67, 70.07, 70.06, 69.98, 69.92, 68.39, 51.40. IR (ATR): νmax (cm−1) 2866, 1514, 1351, 1249, 1112, 945, 816. HRMS (ESI) calculated for C18H29NO6 + H+ 356.2068, found 356.2065 [M + H]+, 378.1887 [M + Na]+.
15; yield 24 mg (89%) as a light pink oil. 1H NMR (600 MHz, DMSO-d6) δ 8.50 (s, 1H), 6.64–6.58 (m, 2H), 6.57–6.51 (m, 2H), 3.55–3.52 (m, 20H), 3.38 (t, J = 6.1 Hz, 4H). 13C NMR (151 MHz, DMSO-d6) δ 148.36, 141.27, 115.82, 113.67, 70.07, 70.06, 69.98, 69.92, 68.39, 51.40. IR (ATR): νmax (cm−1) 2866, 1514, 1351, 1249, 1112, 945, 816. HRMS (ESI) calculated for C18H29NO6 + H+ 356.2068, found 356.2065 [M + H]+, 378.1887 [M + Na]+.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, yield 18 mg (23%) of violet solid. 1H NMR (600 MHz, CDCl3) δ 8.89 (s, 6H), 7.32–7.29 (m, 12H), 7.27–7.25 (m, 18H), 6.21–6.18 (m, 2H), 5.42–5.39 (m, 2H), 3.68–3.66 (m, 4H), 3.60 (dd, J = 4.3, 2.3 Hz, 4H), 3.55 (td, J = 3.9, 2.4 Hz, 4H), 3.32 (t, J = 4.9 Hz, 4H). 13C NMR (151 MHz, CDCl3) δ 151.64, 143.04, 141.25, 130.23, 130.11, 128.15, 127.14, 124.21, 119.79, 113.50, 71.60, 70.03, 69.94, 52.99. IR (ATR): νmax (cm−1) 3648, 3545, 3524, 3502, 2926, 2854, 1731, 1511, 1455, 1439, 1163, 1068, 762, 724, 656. HRMS (ESI) calculated for C78H64BN7O6 + H+, 1118.4560 found 1118.4568 [M + H]+ 1118.4388 [M + Na]+. λmax (THF, log
4, yield 18 mg (23%) of violet solid. 1H NMR (600 MHz, CDCl3) δ 8.89 (s, 6H), 7.32–7.29 (m, 12H), 7.27–7.25 (m, 18H), 6.21–6.18 (m, 2H), 5.42–5.39 (m, 2H), 3.68–3.66 (m, 4H), 3.60 (dd, J = 4.3, 2.3 Hz, 4H), 3.55 (td, J = 3.9, 2.4 Hz, 4H), 3.32 (t, J = 4.9 Hz, 4H). 13C NMR (151 MHz, CDCl3) δ 151.64, 143.04, 141.25, 130.23, 130.11, 128.15, 127.14, 124.21, 119.79, 113.50, 71.60, 70.03, 69.94, 52.99. IR (ATR): νmax (cm−1) 3648, 3545, 3524, 3502, 2926, 2854, 1731, 1511, 1455, 1439, 1163, 1068, 762, 724, 656. HRMS (ESI) calculated for C78H64BN7O6 + H+, 1118.4560 found 1118.4568 [M + H]+ 1118.4388 [M + Na]+. λmax (THF, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 581 (4.762), 525 (sh), and 323 nm (4.441 dm3 mol−1 cm−1).
ε) 581 (4.762), 525 (sh), and 323 nm (4.441 dm3 mol−1 cm−1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, yield 17 mg (22%) of violet solid. 1H NMR (600 MHz, THF-d8) δ 8.85 (s, 6H), 7.30–7.28 (m, 12H), 7.23–7.19 (m, 18H), 6.11–6.09 (m, 2H), 5.37–5.34 (2H, m), 3.51–3.49 (m, 8H), 3.47–3.44 (m, 8H), 3.27 (t, J = 6.1 Hz, 4H). 13C NMR (151 MHz, THF-d8) δ 152.926, 143.94, 142.474, 131.281, 131.142, 128.987, 127.948, 124.846, 120.676, 112.924, 72.377, 71.266, 71.118, 70.007, 53.599. IR (ATR): νmax (cm−1) 2927, 2854, 1732, 1454, 1439, 1242, 1163, 1123, 1069, 763, 701, 670. HRMS (ESI) calculated for C78H64BN7O6 + H+, 1162.4822 found 1162.4833 [M + H]+ 1184.4651 [M + Na]+. λmax (THF, log
1, yield 17 mg (22%) of violet solid. 1H NMR (600 MHz, THF-d8) δ 8.85 (s, 6H), 7.30–7.28 (m, 12H), 7.23–7.19 (m, 18H), 6.11–6.09 (m, 2H), 5.37–5.34 (2H, m), 3.51–3.49 (m, 8H), 3.47–3.44 (m, 8H), 3.27 (t, J = 6.1 Hz, 4H). 13C NMR (151 MHz, THF-d8) δ 152.926, 143.94, 142.474, 131.281, 131.142, 128.987, 127.948, 124.846, 120.676, 112.924, 72.377, 71.266, 71.118, 70.007, 53.599. IR (ATR): νmax (cm−1) 2927, 2854, 1732, 1454, 1439, 1242, 1163, 1123, 1069, 763, 701, 670. HRMS (ESI) calculated for C78H64BN7O6 + H+, 1162.4822 found 1162.4833 [M + H]+ 1184.4651 [M + Na]+. λmax (THF, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 581 (4.732), 525 (sh), and 328 nm (4.334 dm3 mol−1 cm−1).
ε) 581 (4.732), 525 (sh), and 328 nm (4.334 dm3 mol−1 cm−1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, yield 21 mg (26%) of violet solid. 1H NMR (600 MHz, THF-d8) δ 8.84 (s, 6H), 7.33–7.30 (m, 12H), 7.26–7.22 (m, 18H), 6.17–6.14 (m, 2H), 5.37–5.33 (m, 2H), 3.53–3.50 (m, 12H), 3.48–3.45 (m, 8H), 3.31 (t, J = 6.1 Hz, 4H). 13C NMR (151 MHz, THF-d8) δ 152.93, 143.96, 142.48, 131.28, 131.16, 129.02, 127.98, 124.83, 120.65, 113.62, 71.96, 71.91, 71.89, 71.70, 70.15, 52.82. IR (ATR): νmax (cm−1) 3409, 2925, 2853, 1732, 1576, 1454, 1438, 1163, 1068, 1015, 762, 701, 612. HRMS (ESI) calculated for C78H64BN7O6 + H+, 1206.5084 found 1206.5094 [M + H]+ 1228.4918 [M + Na]+. λmax (THF, log
2, yield 21 mg (26%) of violet solid. 1H NMR (600 MHz, THF-d8) δ 8.84 (s, 6H), 7.33–7.30 (m, 12H), 7.26–7.22 (m, 18H), 6.17–6.14 (m, 2H), 5.37–5.33 (m, 2H), 3.53–3.50 (m, 12H), 3.48–3.45 (m, 8H), 3.31 (t, J = 6.1 Hz, 4H). 13C NMR (151 MHz, THF-d8) δ 152.93, 143.96, 142.48, 131.28, 131.16, 129.02, 127.98, 124.83, 120.65, 113.62, 71.96, 71.91, 71.89, 71.70, 70.15, 52.82. IR (ATR): νmax (cm−1) 3409, 2925, 2853, 1732, 1576, 1454, 1438, 1163, 1068, 1015, 762, 701, 612. HRMS (ESI) calculated for C78H64BN7O6 + H+, 1206.5084 found 1206.5094 [M + H]+ 1228.4918 [M + Na]+. λmax (THF, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 582 (4.665), 525 (sh), and 328 nm (4.332 dm3 mol−1 cm−1).
ε) 582 (4.665), 525 (sh), and 328 nm (4.332 dm3 mol−1 cm−1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, yield 9 mg (11%) of violet solid. 1H NMR (600 MHz, THF-d8) δ 7.79–7.75 (m, 12H), 7.43–7.37 (m, 18H), 6.14–6.10 (m, 2H), 5.38–5.33 (2H, m), 3.51–3.47 (m, 12H), 3.46–3.44 (m, 4H), 3.28 (t, J = 6.2 Hz, 4H). 13C NMR (151 MHz, THF-d8) δ 155.78, 149.92, 141.86, 140.22, 131.54, 130.21, 129.22, 122.57, 120.55, 113.06, 72.39, 71.29, 71.13, 69.96, 53.59. HRMS (ESI) calculated for C78H64BN7O6 + H+, 1168.4537 found 1168.4543 [M + H]+ 1190.4364 [M + Na]+. λmax (THF, log
1, yield 9 mg (11%) of violet solid. 1H NMR (600 MHz, THF-d8) δ 7.79–7.75 (m, 12H), 7.43–7.37 (m, 18H), 6.14–6.10 (m, 2H), 5.38–5.33 (2H, m), 3.51–3.47 (m, 12H), 3.46–3.44 (m, 4H), 3.28 (t, J = 6.2 Hz, 4H). 13C NMR (151 MHz, THF-d8) δ 155.78, 149.92, 141.86, 140.22, 131.54, 130.21, 129.22, 122.57, 120.55, 113.06, 72.39, 71.29, 71.13, 69.96, 53.59. HRMS (ESI) calculated for C78H64BN7O6 + H+, 1168.4537 found 1168.4543 [M + H]+ 1190.4364 [M + Na]+. λmax (THF, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 549 (4.557), 500 (sh) and 377 nm (4.420 dm3 mol−1 cm−1).
ε) 549 (4.557), 500 (sh) and 377 nm (4.420 dm3 mol−1 cm−1).
Job's method of continuous variation
Stock solutions of a SubPc (10 μM) and salt (10 μM) in a mixture of THF/MeOH 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 were prepared. A series of fluorescence measurements with different SubPc/salt ratios (18 measurements ranging between 1
2 were prepared. A series of fluorescence measurements with different SubPc/salt ratios (18 measurements ranging between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 4
4 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios) with a constant total volume of 2.5 mL and total concentration of components of 10 μM ([SubPc] + [salt] = 10 μM) was performed. The final stoichiometry was determined from the Job's plot constructed from the dependence of Fx − F0 on [analyte]/([analyte] + [SubPc]), where Fx refers to the fluorescence emission intensity of the SubPc/salt mixture and F0 to the mixture where THF/MeOH 8
1 ratios) with a constant total volume of 2.5 mL and total concentration of components of 10 μM ([SubPc] + [salt] = 10 μM) was performed. The final stoichiometry was determined from the Job's plot constructed from the dependence of Fx − F0 on [analyte]/([analyte] + [SubPc]), where Fx refers to the fluorescence emission intensity of the SubPc/salt mixture and F0 to the mixture where THF/MeOH 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (without dissolved salt) was used instead of a salt stock solution.
2 (without dissolved salt) was used instead of a salt stock solution.
Author contributions
MAA – conducted the research and investigation process, specifically performed the experiments; JL – performed the calculations (association constants, etc.) and wrote the manuscript, KL and KK – prepared and characterized the micelles, and edited the manuscript; PS and PZ – conduced a critical review of the research hypothesis and wrote the draft of the manuscript; VN – formulated the research goals, provided mentorship, and wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The financial support from the Czech Science Foundation (21-14919J), Russian Foundation for Basic Research (20-53-26004) and Charles University (SVV 260 666, PRIMUS/20/SCI/013) is gratefully acknowledged.References
- A. Sigel, H. Sigel and R. K. O. Sigel, in The Alkali Metal Ions: Their Role for Life, Springer, Cham, 2016, vol. 16, p. 628, DOI:10.1007/978-3-319-21756-7.
- P. Pathak, R. R. Srivastava, G. Keceli and S. Mishra, in Strontium Contamination in the Environment, ed. P. Pathak and D. K. Gupta, Springer International Publishing, Cham, 2020, pp. 227–243, DOI:10.1007/978-3-030-15314-4_12.
- M. Rajasekar, V. Ranjitha and K. Rajasekar, Results Chem., 2023, 5, 100850 CrossRef CAS.
- A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515–1566 CrossRef CAS PubMed.
- H. Niu, J. Liu, H. M. O'Connor, T. Gunnlaugsson, T. D. James and H. Zhang, Chem. Soc. Rev., 2023, 52, 2322–2357 RSC.
- J. Li, D. Yim, W.-D. Jang and J. Yoon, Chem. Soc. Rev., 2017, 46, 2437–2458 RSC.
- X. X. Zhang, R. M. Izatt, J. S. Bradshaw and K. E. Krakowiak, Coord. Chem. Rev., 1998, 174, 179–189 CrossRef CAS.
- L. Lochman, J. Svec, J. Roh and V. Novakova, Dyes Pigm., 2015, 121, 178–187 CrossRef CAS.
- L. Lochman, M. Machacek, M. Miletin, S. Uhlirova, K. Lang, K. Kirakci, P. Zimcik and V. Novakova, ACS Sens., 2019, 4, 1552–1559 CrossRef CAS PubMed.
- L. Lochman, J. Svec, J. Roh, K. Kirakci, K. Lang, P. Zimcik and V. Novakova, Chem. – Eur. J., 2016, 22, 2417–2426 CrossRef CAS PubMed.
- L. D. Lavis and R. T. Raines, ACS Chem. Biol., 2008, 3, 142–155 CrossRef CAS PubMed.
- R. Jenkins, M. K. Burdette and S. H. Foulger, RSC Adv., 2016, 6, 65459–65474 RSC.
- V. Novakova, M. P. Donzello, C. Ercolani, P. Zimcik and P. A. Stuzhin, Coord. Chem. Rev., 2018, 361, 1–73 CrossRef CAS.
- V. Novakova, J. Porphyrins Phthalocyanines, 2022, 26, 765–782 CrossRef CAS.
- M. H. Shi, J. Z. Chen and Z. Shen, J. Porphyrins Phthalocyanines, 2016, 20, 1082–1089 CrossRef CAS.
- H. Gotfredsen, M. D. Kilde, M. Santella, A. Kadziola and M. B. Nielsen, Mol. Syst. Des. Eng., 2019, 4, 199–205 RSC.
- E. van de Winckel, M. Mascaraque, A. Zamarron, A. J. de la Fuente, T. Torres and A. de la Escosura, Adv. Funct. Mater., 2018, 28, 10 CrossRef.
- H. Xu, X. J. Jiang, E. Y. M. Chan, W. P. Fong and D. K. P. Ng, Org. Biomol. Chem., 2007, 5, 3987–3992 RSC.
- H. Xu and D. K. P. Ng, Chem. – Asian J., 2009, 4, 104–110 CrossRef CAS PubMed.
- F. Yurt, T. Arslan, Z. Biyiklioglu, A. Tuncel, D. Ozel and K. Ocakoglu, J. Drug Delivery Sci. Technol., 2020, 56, 101567 CrossRef CAS.
- J. Demuth, L. Gallego, M. Kozlikova, M. Machacek, R. Kucera, T. Torres, M. V. Martinez-Diaz and V. Novakova, J. Med. Chem., 2021, 64, 17436–17447 CrossRef CAS PubMed.
- J. Labella and T. Torres, Trends Chem., 2023, 5, 353–366 CrossRef CAS.
- G. Lavarda, J. Labella, M. V. Martínez-Díaz, M. S. Rodríguez-Morgade, A. Osuka and T. Torres, Chem. Soc. Rev., 2022, 51, 9482–9619 RSC.
- N. Kobayashi, T. Ishizaki, K. Ishii and H. Konami, J. Am. Chem. Soc., 1999, 121, 9096–9110 CrossRef CAS.
- I. A. Skvortsov, P. Zimcik, P. A. Stuzhin and V. Novakova, Dalton Trans., 2020, 49, 11090–11098 RSC.
- S. Xu, K. C. Chen and H. Tian, J. Mater. Chem., 2005, 15, 2676–2680 RSC.
- T. V. Dubinina, S. A. Trashin, N. E. Borisova, I. A. Boginskaya, L. G. Tomilova and N. S. Zefirov, Dyes Pigm., 2012, 93, 1471–1480 CrossRef CAS.
- T. V. Dubinina, M. S. Belousov, S. S. Maklakov, V. I. Chernichkin, M. V. Sedova, V. A. Tafeenko, N. E. Borisova and L. G. Tomilova, Dyes Pigm., 2019, 170, 107655 CrossRef CAS.
- H. W. Rothkopf, D. Wohrle, R. Muller and G. Kossmehl, Chem. Ber., 1975, 108, 875–886 CrossRef CAS.
- V. Novakova, P. Reimerova, J. Svec, D. Suchan, M. Miletin, H. M. Rhoda, V. N. Nemykin and P. Zimcik, Dalton Trans., 2015, 44, 13220–13233 RSC.
- H. Xu, W. K. Chan and D. K. P. Ng, Synthesis, 2009, 1791–1796 CAS.
- V. V. Travkin, D. A. Semikov, P. A. Stuzhin, I. A. Skvortsov and G. L. Pakhomov, Appl. Sci., 2023, 13, 1211 CrossRef CAS.
- I. A. Skvortsov, U. P. Kovkova, Y. A. Zhabanov, I. A. Khodov, N. V. Somov, G. L. Pakhomov and P. A. Stuzhin, Dyes Pigm., 2021, 185, 108944 CrossRef.
- R. F. Kubin and A. N. Fletcher, J. Lumin., 1982, 27, 455–462 CrossRef.
- K. W. Chi, K. T. Shim, H. H. U. Lee and Y. J. Park, Bull. Korean Chem. Soc., 2005, 26, 393–398 CrossRef CAS.
- K. Hirose, J. Inclusion Phenom. Macrocyclic Chem., 2001, 39, 193–209 CrossRef CAS.
- T. H. Ngo, J. Labuta, G. N. Lim, W. A. Webre, F. D'Souza, P. A. Karr, J. E. M. Lewis, J. P. Hill, K. Ariga and S. M. Goldup, Chem. Sci., 2017, 8, 6679–6685 RSC.
- H.-J. Buschmann, R.-C. Mutihac and E. Schollmeyer, J. Solution Chem., 2010, 39, 291–299 CrossRef CAS.
- S. H. Mashraqui, S. Sundaram, T. Khan, S. Ghadigaonkar and K. Poonia, J. Inclusion Phenom. Macrocyclic Chem., 2008, 62, 81–90 CrossRef CAS.
- M. Hojo, I. Hisatsune, H. Tsurui and S.-i. Minami, Anal. Sci., 2000, 16, 1277–1284 CrossRef CAS.
- F. Gámez, P. Hurtado, B. Martínez-Haya, G. Berden and J. Oomens, Int. J. Mass Spectrom., 2011, 308, 217–224 CrossRef.
- M. Łukasik-Głębocka, K. Sommerfeld, A. Hanć, A. Grzegorowski, D. Barałkiewicz, M. Gaca and B. Zielińska-Psuja, J. Anal. Toxicol., 2014, 38, 380–382 CrossRef PubMed.
- S. Xu, L. Mao, P. Ding, X. Zhuang, Y. Zhou, L. Yu, Y. Liu, T. Nie, T. Xu, Y. Xu, J. Liu, J. Smaill, X. Ren, D. Wu and K. Ding, Bioorg. Med. Chem., 2015, 23, 3751–3760 CrossRef CAS PubMed.
- X.-X. Zhang and S. L. Buchwald, J. Org. Chem., 2000, 65, 8027–8031 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: NMR spectra, effect of the counter anion, stoichiometry studies, summary of obtained apparent binding constants, binding isotherms from fluorescence measurements, effect of water, characterization of micelles, and sensing in water. See DOI: https://doi.org/10.1039/d3dt03839d |
| This journal is © The Royal Society of Chemistry 2024 |