 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effect of annealing conditions on the luminescence properties and thermometric performance of Sr3Al2O5Cl2:Eu2+ and SrAl2O4:Eu2+ phosphors
Simon N.
Ogugua
 *a,
Christopher
Abram
b,
Benoît
Fond
*a,
Christopher
Abram
b,
Benoît
Fond
 c,
Robin E.
Kroon
c,
Robin E.
Kroon
 a,
Frank
Beyrau
d and
Hendrik C.
Swart
a,
Frank
Beyrau
d and
Hendrik C.
Swart
 *a
*a
aDepartment of Physics, University of the Free State, Bloemfontein, ZA9300, South Africa. E-mail: Ogugua.sn@ufs.ac.za; Kroonre@ufs.ac.za; Swarthc@ufs.ac.za
bDepartment of Mechanical and Aerospace Engineering, Princeton University, NJ 08544, USA. E-mail: cabram@princeton.edu
cDepartment of Aeronautics, ONERA the French Aerospace Lab, 92190 Meudon, France. E-mail: benoit.fond@onera.fr
dLehrstuhl für Technische Thermodynamik, Otto-von-Guericke-Universität Magdeburg, 39106 Magdeburg, Germany. E-mail: frank.beyrau@ovgu.de
First published on 13th February 2024
Abstract
We report on the synthesis, photoluminescence optimization and thermometric properties of Sr3Al2O5Cl2:Eu2+ and SrAl2O4:Eu2+ phosphor powders. The photoluminescence of Sr2.9Al2O5Cl2:0.1Eu2+ phosphors exhibits a blue-shift with an increasing annealing temperature owing to a decrease in the crystal field strength of the host caused by evaporation of Cl from the material. The quenching of the blue band in favour of the red band observed in the luminescence spectra of Sr2.9Al2O5Cl2:0.1Eu2+ with an increased annealing temperature was explained using the mechanism of the Landau-Zener transitions. The quantum yield and the lifetime of the phosphors depend on the annealing temperature. Phosphor samples annealed at 850 °C, 1000 °C, 1200 °C and 1500 °C were found to be potential luminescence thermometers using the luminescence spectral method. For Sr3Al2O5Cl2:Eu2+ annealed at 1000 °C, the temperature-dependent dual-band intensity ratio demonstrated a high-temperature sensitivity of ∼1.47%/°C in the temperature range of 23 °C to 40 °C which is superior to other reported phosphors with a microsecond decay time, suggesting that the material has potential for sensitive thermometry applications at ambient temperatures.
1. Introduction
Temperature is an important parameter in our day-to-day lives. This spans from low-temperature environments of natural processes such as in biological systems1 and thermal convection in oceans and atmospheres, to high-temperature environments of industrial energy and manufacturing devices such as gas turbines and other reactive systems.2,3 Knowledge of the temperature is critical. Thermographic phosphors can provide robust solutions for remote temperature measurements. Phosphor thermometry utilizes temperature-sensitive powders which usually consist of host materials doped with small amounts of activator ions like rare-earth and/or transition metal ions.4 Thermographic phosphors exhibit changes in the luminescence emission intensity, the emission spectrum, or the lifetime with temperature, from which the temperature can be obtained after calibration. The temperature range, sensitivity and precision of measurements are determined by the intrinsic characteristics of the phosphor materials, and it is useful to discover and engineer new materials with enhanced temperature-dependent luminescence properties. To perform temperature measurements, either the temperature dependence of the luminescence decay time or the luminescence emission spectrum is exploited, referred to as the temporal and spectral methods, respectively.5,6 For two-dimensional measurements, the decay time method requires a camera with the ability to record at least two frames with a short interframe time,7 while the spectral method requires simultaneously recording two spectrally-filtered images but without specific requirements on the interframe time. Also with the latter method, the measurement time is flexible and can be longer in order to increase the temperature resolution. The spectral method has often been applied on single band phosphors such as ZnO, BAM:Eu, or (Sr,Mg)(PO4):Sn2+,8–10 the emission band of which broadens and shifts red or blue with temperature, but the resulting temperature sensitivity is typically below 1%/K near room temperature. Dual-band emission from levels in thermal equilibrium are also used, either from single dopant (YAG:Pr3+, MFG:Mn4+, YVO4:Dy3+ (ref. 11–13)) or from co-doping (YAG:Pr3+,Ce3+, Sr4Al14O25:Mn4+,Tb3+, YAG:Cr3+,Er3+,Nd3+ (ref. 14–16)). One particularly attractive aspect of the multi-band emission is the possibility to exploit the response with a colour camera, as shown in ref. 15. However, it is difficult to meet both requirements of short decay time of both emission bands for fluid thermometry and high-temperature sensitivity. In this paper, we exploit a dual-band thermometric response from a single dopant, Eu2+, with a decay time on the microsecond scale.Sr3Al2O5Cl2:Eu2+ is generally known for its red-orange or orange-yellow emission and persistent luminescence which lasts for a few seconds. To enhance the afterglow properties of this phosphor, it can be co-doped with rare-earth or post-transition metal ions such as Dy3+, Ce3+, Bi3+ and Tm3+.17–20 Temperature-dependent properties of Sr3Al2O5Cl2:Eu2+ have been investigated by Dutczak et al.17 The results show that the emission intensity of the red band of Sr3Al2O5Cl2:Eu2+ strongly decreases in the near-ambient temperature range (250 to 350 K). The annealing temperature after the synthesis of Sr3Al2O5Cl2:Eu2+ has a significant effect on the PL of the phosphor. When annealed at 850 °C, the phosphor showed two photoluminescence (PL) emission bands around 443 and 600 nm,21 but when annealed at higher temperatures (1100 °C to 1300 °C) it showed only the 600 nm emission.17,19,22 Although the luminescence properties and the temperature-dependent luminescence of Sr3Al2O5Cl2:Eu2+ has been reported in ref. 21 and 17, the thermometric properties of the material have not been reported, which is the focus of this research.
In this study, we explore the possibility of using Sr3Al2O5Cl2:Eu2+ and SrAl2O4:Eu2+ for dual-band and emission band shift thermometry, respectively. Sr3Al2O5Cl2:Eu2+ and SrAl2O4:Eu2+ phosphors were synthesised using the solid-state method specifically, with different concentrations of Eu2+ and annealing temperatures (850 °C to 1500 °C). We measured the effects of dopant concentration and annealing temperatures on the structure, morphologies, and temperature-dependence of the luminescence, and theoretically explored the potential of these phosphors for remote thermometry using the dual-band and emission band shift approach, with monochrome and colour sensors.
2. Experimental
2.1. Synthesis
Divalent europium doped strontium aluminate chloride (Sr3−xAl2O5Cl2:x%Eu2+, x = 0.05, 0.08, 0.1 and 0.15) phosphor particles were prepared by the conventional high-temperature solid-state reaction method. The stoichiometric amount of the starting materials 8.283 mmol of SrCO3 (analytical reagent (A. R.)), 4.141 mmol of SrCl2·H2O (A. R.), Al2O3 (A. R.) and Eu2O3 (99.99%) and acetone were used to make a slurry mixture inside a Pyrex borosilicate glass mortar. The mixture was ground using a pestle for about 30 min and annealed using a ceramic crucible in a reducing gas (95% Ar/5% H2) at 1000 °C for 4 h, allowed to cool to room temperature and the final product was ground for 30 min. A similar process was repeated for other samples, with the concentration of Eu2O3 fixed at x = 0.1 for different annealing temperatures, namely 850, 1200 and 1500 °C.2.2. Characterization
The structure of the samples was analyzed using a Bruker D8 Advance X-ray diffractometer. The morphology studies and the elemental analyses were carried out using a Jeol JSM-7800F field emission scanning electron microscope (FE-SEM) fitted with an Oxford X-MaxN 80 energy-dispersive X-ray spectrometer (EDS). The PL spectra were measured using an FLS980 fluorescence spectrometer (Edinburgh Instruments) equipped with a 450 W xenon lamp as a steady-state excitation source and a Pulsed Light Emitting Diode at 360 nm incorporated in an FLS980 fluorescence spectrometer for lifetime measurements. The quantum yield (QY) measurements were performed using an integrating sphere incorporated in the FLS980 spectrometer. The temperature-dependent PL was measured with a system consisting of a 325 nm He–Cd gas laser excitation source, a spectrometer, a photomultiplier tube detector, and a lock-in amplifier.3. Results and discussion
3.1. Structure
X-ray diffraction (XRD) patterns of Sr3−xAl2O5Cl2:xEu2+ (x = 0.05, 0.08, 0.1 and 0.15) annealed at 1000 °C are shown in Fig. 1(a). The samples crystallized as single phase Sr3Al2O5Cl2 consistent with JCPDS file no: 80-0564. The variation in Eu2+ concentration did not have a significant effect on the patterns. This is evidence that Eu2+ ions are incorporated well in the Sr2+ sites, as expected considering their relatively close ionic radii of 1.30 and 1.31 Å, respectively, in their 9-coordinated sites.23 Sr3Al2O5Cl2 belongs to the P212121 space group having an orthorhombic crystal structure with three different strontium sites24,25 as shown in the unit cell in Fig. 1(b). The individual Sr2+ site is coordinated by five O2− and four Cl− ligands (Fig. 1(c)) with differences in the average Sr–O and Sr–Cl bond lengths. In addition, Sr1 and Sr3 form a non-enclosed one-dimensional ring along the [100] plane. Sr2 and Cl are distributed in the large tunnel formed by the Sr1–Sr3 screwed tetrahedron framework.26 The three Sr sites are linked through AlO4 tetrahedral matrices.27Images of the morphologies of Sr3−xAl2O5Cl2:xEu2+ (x = 0.05 and 0.15) phosphors obtained from FE-SEM are shown in Fig. 1(d and e), respectively, showing rod-like structures. Variation in Eu2+ content in the samples did not have a significant effect on the morphologies of the phosphors.
Further work focused on a doping concentration of 0.1 mol% which emission intensity was found later in this article (see Fig. 5(b)), to be the highest. Samples with this doping concentration were prepared using different annealing temperatures (850, 1000, 1200 and 1500 °C, represented as SAO:0.1Eu850, SAO:0.1Eu1000, SAO:0.1Eu1200 and SAO:0.1Eu1500, respectively, subsequently) and the XRD patterns are shown in Fig. 2(a). Apart from the crystal planes from Sr3Al2O5Cl2 (JCPDS file no: 80-0564), Fig. 2(b) (enlarged pattern from 15 to 40 2θ) shows that SAO:0.1Eu850 contained some planes from SrO (JCPDS file no: 75-0263) and SrCl2 (JCPDS file no: 77-2165). The presence of SrO and SrCl2 phase is owing to the low annealing temperature. The SAO:0.1Eu1000 and SAO:0.1Eu1200 crystalized as a single phase Sr3Al2O5Cl2 and are consistent with the JCPDS file no: 80-0564. For the higher temperature annealing of 1500 °C, the pattern of the SAO:0.1Eu1500 matched with the JCPDS file no: 74-0794 for SrAl2O4, indicating the elimination of Cl from the structure. The Rietveld refined results of the SAO:0.1Eu1000 (cell parameters: a = 9.4176 (9) Å, b = 9.3989(6) Å, c = 9.3996(6) Å, and V = 832.05(1) Å3) and SAO:0.1Eu1200 (cell parameters: a = 9.4064(5), b = 9.3979(4), c = 9.4233(8) Å, and V = 833.03(8) Å3), Fig. 2(c and d), revealed that the samples crystallized as an orthorhombic structure with a space group of P212121. Compared to the standard cell parameters, a = b = c = 9.422 Å, and V = 836.43 Å3, reported in JCPDS file no: 80-0564, it is seen that the Rietveld parameters showed only a slight reduction. The Rietveld refined result of the SAO:0.1Eu1500, Fig. 2(e) exhibited a monoclinic structure with the P21 space group belonging to SrAl2O4. The Rietveld cell parameters: a = 8.4258(7) Å, b = 8.8039(6) Å, c = 5.1497(9) Å, and V = 381.35 Å3, are in close agreement with the standard cell parameters a = 8.447 Å, b = 8.816 Å, c = 5.163 Å, and V = 383.80 Å3, reported in JCPDS file no: 74-0794. The crystal structure of SrAl2O4 contains rings formed by six corners sharing AlO4 tetrahedra, as displayed in Fig. 2(f). Two non-equivalent strontium sites, Sr1 and Sr2, are situated within the channels formed by the AlO4 tetrahedra. Both strontium sites are coordinated to seven oxygen ions with average distances of 2.69 Å and 2.67 Å for Sr1 and Sr2, respectively.28
3.2. Morphology
FE-SEM micrographs of the SAO:0.1Eu850, SAO:0.1Eu1000, SAO:0.1Eu1200 and SAO:0.1Eu1500 phosphors annealed at different temperatures are shown in Fig. 3. The image of the SAO:0.1Eu850, Fig. 3(a), exhibited irregular morphology comprising of rod-like, plate-like, and agglomerated structures of different sizes. This is confirmation that the sample consists of mixed phases, as revealed by the XRD result in Fig. 2. In Fig. 3(b and c), the morphology of the SAO:0.1Eu1000 and SAO:0.1Eu1200 exhibited regular rod-like shapes. It is evident from Fig. 3(b and c) that the diameter of the rods reduced, and the shapes became more defined when the sample was annealed at 1200 °C. For the SAO:0.1Eu1500, the particles exhibited different morphology entirely, as shown in Fig. 3(d). The image showed agglomerates of plate-like structures of various sizes.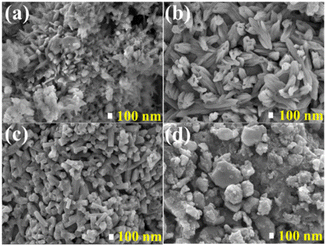 | ||
| Fig. 3 The FE-SEM images showing the morphologies of the (a) SAO:0.1Eu850, (b) SAO:0.1Eu1000, (c) SAO:0.1Eu1200 and (d) SAO:0.1Eu1500. | ||
3.3. Element analysis
Energy dispersive X-ray spectroscopy (EDS) was used for the identification of elements in the samples. The EDS spectra of the SAO:0.1Eu850, SAO:0.1Eu1000 and SAO:0.1Eu1200, shown in Fig. 4 exhibits peaks from Sr, Al, O, C, Cl and Eu, while the spectra of the SAO:0.1Eu1500 contain all these elements except Cl. The absence of Cl suggests that it evaporated completely from the sample at 1500 °C annealing temperature which resulted in the change in the structure and morphologies of the samples as observed in the XRD (Fig. 2(a)) and FE-SEM (Fig. 3(d)) results and is consistent with the XRD identification of SrAl2O4. The atomic ratios are in good agreement with the compositions identified for annealing at 1000 °C, 1200 °C and 1500 °C (Table 1). Also, note that the elemental composition (Table 1) derived from the EDS spectra (Fig. 4), changed with the increase in the annealing temperature in favour of Al. The decrease in the Sr intensity could be due to a gradual evaporation of the element from the surface of the material as the annealing temperature was increased. This is logical, considering that the boiling point of Sr is 1382 °C and that of Al is 2478 °C. The change in the Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sr ratio at a particular annealing temperature adjusts in a manner that maintains charge neutrality at each phase of the material. Jafer et al.29 and Jaffar et al.30 have reported similar phenomena on Y2O3:Bi3+ and La2O3:Bi3+, respectively. In these cases, Bi3+ evaporated from the materials at elevated annealing temperatures.
Sr ratio at a particular annealing temperature adjusts in a manner that maintains charge neutrality at each phase of the material. Jafer et al.29 and Jaffar et al.30 have reported similar phenomena on Y2O3:Bi3+ and La2O3:Bi3+, respectively. In these cases, Bi3+ evaporated from the materials at elevated annealing temperatures.
| Annealing temperature (°C) | Elemental composition (a.u) | Ratios of element to Al | ||||||
|---|---|---|---|---|---|---|---|---|
| Sr | Al | O | Cl | Eu | Sr/Al | Cl/Al | Eu/Al | |
| 850 | 0.47 | 0.08 | 1.39 | 0.23 | 0.01 | 5.87 | 2.88 | 0.13 |
| 1000 | 0.33 | 0.18 | 1.42 | 0.17 | 0.01 | 1.83 | 0.94 | 0.06 |
| 1200 | 0.33 | 0.18 | 1.45 | 0.15 | 0.01 | 1.83 | 0.83 | 0.06 |
| 1500 | 0.36 | 0.69 | 1.87 | — | 0.01 | 0.52 | 0.01 | |
3.4. Effect of dopant concentration on photoluminescence
The room temperature PL excitation spectra of the Sr3−xAl2O5Cl2:xEu2+ (x = 0.05, 0.08, 0.1 and 0.15) annealed at 1000 °C when monitoring the 600 nm emission band of Eu2+ are shown in Fig. 5(a). The spectra consist of two broad bands stretching from the deep ultraviolet to the near visible range of the electromagnetic spectrum with maxima around 250 and 325 nm. These bands are assigned to the electric-dipole transition from the 8S7/2 ground state of the 4f7 configuration to the 4f65d1 excited states of Eu2+.17,21,25 It appears from the excitation spectra that the 250 nm band has higher intensity at a low concentration of Eu2+ (x = 0.05 and 0.08), and the 325 nm band showed dominance at a higher Eu2+ concentration (x = 0.15). Balance in intensity between these two bands and what appeared to be the best intensity was attained in the sample doped with x = 0.1 of Eu2+.Fig. 5(b) displays the emission spectra of the phosphors doped with different concentrations of Eu2+ excited at 325 nm. The broad bands cover the visible spectrum with maxima at 443 and 600 nm and are assigned to the 4f 65d1 → 4f7 electric dipole transitions of Eu2+ ions.21 The origin of the blue and red bands in Sr3Al2O5Cl2:Eu2+ is still a topic of discussion. Wang et al.21 published the luminescence of Sr3Al2O5Cl2:Eu2+,Dy3+ with emission at 440 nm and 590 nm and assigned the two bands to Eu2+ sitting at different sites in the host lattice. In addition, Dutczak et al.17 observed a broad red band with a maximum around 615 nm from Sr3Al2O5Cl2:Eu2+,Dy3+ and they believe that the broadband originated from overlapping emission bands from Eu2+ ions sitting at three different Sr sites (Sr1, Sr2 and Sr3) in the host lattice. The fitted emission spectrum of the sample doped with x = 0.1 of Eu2+ (Fig. 5(c)) shows that the broadband with a maximum at 600 nm can be separated into two bands located at 2.10 eV (590 nm) and 2.07 eV (600 nm) and the blue band has a single peak at 2.80 eV (443 nm), which supplements the idea that the emission band is generated from Eu2+ occupying different sites in the host lattice. Similar results have been published by Wang et al.21 To further support this view, the excitation spectra of the sample with x = 0.1 recorded at 443 nm and 600 nm emissions were compared in Fig. 5(d). The excitation spectrum recorded at 600 nm emission showed broader band with maximums at 250 and 325 nm, while the one recorded at 443 nm showed narrower band with maximums at 296 and 325 nm. The excitation spectrum under 443 nm emission is assigned to 4f7 → 4f65d1 excitation bands for Eu2+ occupying Sr sites with higher coordination numbers (stronger crystal field), whereas the broad spectrum under 600 nm emission contains contributions from 4f7 → 4f65d1 excitation bands of Eu2+ occupying all Sr sites, due to partial energy transfer from Eu2+ occupying the high energy Sr sites to the red emitting Eu2+ ions.28Fig. 5(e) shows the plot of the emission intensity versus Eu2+ concentration for the 443 and 600 nm bands, with the sample doped with x = 0.1 Eu2+ having the highest intensity in both bands. It is also evident from Fig. 5(e) that the luminescence intensity ratio (LIR) of the 443 nm bands with respect to the 600 nm bands increased with Eu2+ concentration and attained a maximum when x = 0.1 and quenched completely when x = 0.15. The latter phenomenon could be due to energy transfer among Eu2+ ions occupying different Sr sites in the host lattice.
3.5. Effect of annealing on photoluminescence
The normalized room temperature PL excitation spectra of the SAO:0.1Eu850, SAO:0.1Eu1000, SAO:0.1Eu1200 and SAO:0.1Eu1500 when monitoring the 600 nm emission bands are shown in Fig. 6(a). The spectra extended from the ultraviolet region to the visible wavelength with maxima at 250 and 325 nm, showing similar features as the spectra shown in Fig. 5(a). However, the spectrum of the SAO:0.1Eu1500 broadened towards a higher wavelength compared to the rest.The normalized room temperature PL emission spectra of SAO:0.1Eu850, SAO:0.1Eu1000, SAO:0.1Eu1200, and SAO:0.1Eu1500 (measured at 325 nm excitation) exhibited broad bands which displayed different features in the visible wavelength region as shown in Fig. 6(b). The SAO:0.1Eu850 shared similar features with the SAO:0.1Eu1000, showing a blue and red band with maxima at 443 nm and 600 nm, respectively.
The SAO:0.1Eu1200 showed a similar PL emission band at 600 nm, but the 443 nm band was quenched completely. The SAO:0.1Eu1500 exhibited greenish emission with a maximum at 520 nm, which is characteristic of SrAl2O4:Eu2+.28 Note that the emission spectra reduced from dual to single-band for annealing temperatures over 1000 °C. The plot of the integrated emission intensity of the phosphors versus the annealing temperatures (inset of Fig. 6(b)) shows almost a logarithmic regression. This makes sense since the sample annealed at 850 °C is only partially the target phase. The fitted PL spectra of the SAO:0.1Eu1200 and SAO:0.1Eu1500, Fig. 6(c and d) shows two bands each at 1.88 eV (660 nm) and 2.09 eV (593 nm), and 2.27 eV (546 nm) and 2.40 eV (517 nm), respectively. The two bands observed from the fitted PL spectrum of the SAO:0.1Eu1500 is assigned to the two Sr sites (Sr1 and Sr2) in SrAl2O4 as shown in the crystal structure in Fig. 2(e).
The mechanism behind the quenching of the blue band in the SAO:0.1Eu1200 has not been fully understood. Nevertheless, using other features observed in the emission spectra and the results from different techniques, the underlying mechanism of the quenching can be explained. In Fig. 6(b), there is a slight blue broadening of the 600 nm emission band as the annealing temperature was increased. A similar phenomenon has been reported for Eu2+ doped in host lattices such as BaMgAl10O17 and SrMgAl10O17,31 BaCN2,32 Ca2SiO4 and NaBaPO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 when the luminescence of the phosphors was measured at different temperatures, and it was assigned to reduced lattice vibration,31,34 which leads to a decrease in crystal field strength of the lattice.35 Dutczak et al.17 reported a similar phenomenon in Sr3Al2O5Cl2:Eu2+ and attributed it to the blue-shift in the emission spectrum due to faster quenching of the red emission. However, they excluded the possibility of inter-site energy transfer between Eu2+ ions. The quenching of the blue band has been widely studied in SrAl2O4:Eu2+, and there is a general agreement that it is due to the thermal activated non-radiative energy transfer between Eu2+ ions occupying different Sr sites in the host lattice.36,37 However, instead of the blue-shift as observed in Sr3Al2O5Cl2:Eu2+, a red-shift was observed in SrAl2O4:Eu2+, which suggests that the splitting of the 5d state of the Eu2+ ions is due to the increase in the crystal field strength of the lattice which results to the reduction in the energy between the 4f65d1 and 4f7 states.38 The sample annealed at a very high temperature (1500 °C) changed from Sr3Al2O5Cl2:Eu2+ to SrAl2O4:Eu2+, as confirmed by the XRD, EDS, and PL results. This is due to the evaporation of Cl in the material during synthesis as the annealing temperature increases. As seen from the EDS spectra (see Fig. 4), the Cl peak totally disappeared from the spectrum of the SAO:0.1Eu1500. The gradual evaporation of Cl from the material probably will result in a decrease in the crystal field strength of the host which leads to the blue-shift observed in the PL as the annealing temperature was increased in Fig. 6(b). Indeed, the ratio of Cl to Al and Sr is lower for the sample annealed at 1200 °C than the one annealed at 1000 °C. Furthermore, considering the SAO:0.1Eu850, SAO:0.1Eu1000 and SAO:0.1Eu1200, the higher the annealing temperature, the more crystalline they become. This strongly influenced the PL characteristics since lanthanide ions in a host with higher crystallinity experience a stronger crystal field.39,40 Therefore, at a higher temperature, the system will try to attain equilibrium between the decreasing crystal field strength due to the Cl vacancies and the increasing crystal field strength due to the improved crystallinity of the material. At the point of equilibrium in the system, there could be energy transfer from the Eu2+ occupying the Sr1 site in the host to the Eu2+ occupying the Sr2 site through Landau-Zener transitions36,41 as illustrated in Fig. 7. It is well known that the energy level difference between the 4f65d1 and 4f7 states for divalent and trivalent rare earth ions decreases with increase in the crystal field strength of a host.42,43 The reduction in energy difference between the ground state and the first excited state will cause efficient energy transfer between the Eu2+ ions occupying different sites in the host lattice, hence quenching the higher energy luminescence centers.44,45
33 when the luminescence of the phosphors was measured at different temperatures, and it was assigned to reduced lattice vibration,31,34 which leads to a decrease in crystal field strength of the lattice.35 Dutczak et al.17 reported a similar phenomenon in Sr3Al2O5Cl2:Eu2+ and attributed it to the blue-shift in the emission spectrum due to faster quenching of the red emission. However, they excluded the possibility of inter-site energy transfer between Eu2+ ions. The quenching of the blue band has been widely studied in SrAl2O4:Eu2+, and there is a general agreement that it is due to the thermal activated non-radiative energy transfer between Eu2+ ions occupying different Sr sites in the host lattice.36,37 However, instead of the blue-shift as observed in Sr3Al2O5Cl2:Eu2+, a red-shift was observed in SrAl2O4:Eu2+, which suggests that the splitting of the 5d state of the Eu2+ ions is due to the increase in the crystal field strength of the lattice which results to the reduction in the energy between the 4f65d1 and 4f7 states.38 The sample annealed at a very high temperature (1500 °C) changed from Sr3Al2O5Cl2:Eu2+ to SrAl2O4:Eu2+, as confirmed by the XRD, EDS, and PL results. This is due to the evaporation of Cl in the material during synthesis as the annealing temperature increases. As seen from the EDS spectra (see Fig. 4), the Cl peak totally disappeared from the spectrum of the SAO:0.1Eu1500. The gradual evaporation of Cl from the material probably will result in a decrease in the crystal field strength of the host which leads to the blue-shift observed in the PL as the annealing temperature was increased in Fig. 6(b). Indeed, the ratio of Cl to Al and Sr is lower for the sample annealed at 1200 °C than the one annealed at 1000 °C. Furthermore, considering the SAO:0.1Eu850, SAO:0.1Eu1000 and SAO:0.1Eu1200, the higher the annealing temperature, the more crystalline they become. This strongly influenced the PL characteristics since lanthanide ions in a host with higher crystallinity experience a stronger crystal field.39,40 Therefore, at a higher temperature, the system will try to attain equilibrium between the decreasing crystal field strength due to the Cl vacancies and the increasing crystal field strength due to the improved crystallinity of the material. At the point of equilibrium in the system, there could be energy transfer from the Eu2+ occupying the Sr1 site in the host to the Eu2+ occupying the Sr2 site through Landau-Zener transitions36,41 as illustrated in Fig. 7. It is well known that the energy level difference between the 4f65d1 and 4f7 states for divalent and trivalent rare earth ions decreases with increase in the crystal field strength of a host.42,43 The reduction in energy difference between the ground state and the first excited state will cause efficient energy transfer between the Eu2+ ions occupying different sites in the host lattice, hence quenching the higher energy luminescence centers.44,45
Fig. 7 illustrates the energy configuration diagram of the system as a function of configuration coordinate, q, according to the fitted PL spectrum in Fig. 5(c). The ground state (4f7 level) is made up of a parabola and the excited states (4f65d1 level) are made up of three parabolas representing the energy levels of the Eu2+ ions occupying the Sr1 (443 nm), Sr2 (590 nm) and Sr3 (600 nm) sites (represented as Sr1, Sr2 and Sr3 levels henceforth) in the matrix. The Sr1 and Sr2 levels demonstrate the avoided crossing phenomenon (the inset of Fig. 7 illustrates the probability of diabatic transition between the two energy levels). In this model, once excited, electrons will populate the three sites. For the sample annealed at 850 °C and 1000 °C, the three sites can contribute to the emission. At higher annealing temperature, when the system has attained equilibrium in crystal field strength, the energy difference between the Sr1 and Sr2 levels would be minimal, and due to the electron-vibrational coupling and interaction between the Eu2+ ions occupying these sites, energy could be transferred non-radiatively from Sr1 to Sr2. The closer the energy gap between Sr1 and Sr2 levels, the more energy will be transferred from Sr1 to Sr2 site until the emission from the Sr1 site is quenched completely. This energy transfer from the Sr1 to Sr2 site explains why the intensity and the area covered by the emission from the Sr2 site increased in Fig. 6(c) when compared to that of Fig. 5(c). Additionally, the red-shift in the peak positions in Fig. 6(c) compared to the peak positions in the fitted red band in Fig. 5(c) can be explained by increased crystal field strength as the phosphor crystallinity increases.
3.6. Lifetime
The fitted decay curves of SAO:0.1Eu850, SAO:0.1Eu1000, and SAO:0.1Eu1200, (monitoring the emission at 600 nm) and SAO:0.1Eu1500 (monitoring the emission at 515 nm) are shown in Fig. 8(a). Also, the fitted decay curves of the 443 nm band of SAO:0.1Eu850 and SAO:0.1Eu1000 are shown in Fig. 8(b).In both cases, the lifetimes were excited using an LED 360 nm incorporated in the FLS980 fluorescence spectrometer. For a material with an Nth order exponential decay and luminescent intensity I(t) at time ‘t’, the decay curve can be represented by eqn (1) as
 | (1) |
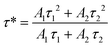 | (2) |
The fitted first and second components of the lifetimes and the calculated average lifetimes of the SAO:0.1Eu850, SAO:0.1Eu1000, SAO:0.1Eu1200, and SAO:0.1Eu1500 are shown in Table 2. The average lifetime showed annealing temperature-dependent behaviour, with the SAO:0.1Eu1500 having a lifetime significantly shorter (0.6 μs) than the SAO:0.1Eu 1000 (>2.5 μs). For the 443 nm band, the lifetime of SAO:0.1Eu850 has a value twice that of SAO:0.1Eu1000. The reduction in the lifetime could be ascribed to a change in the multiphonon relaxation rate due to a change in the elemental composition of the host materials at different annealing temperatures.
| Annealing temperatures (°C) | λ Emi. (nm) | τ 1 (μs) | τ 2 (μs) | τ* (μs) | λ Emi. (nm) | τ 1 (μs) | τ 2 (μs) | τ* (μs) |
|---|---|---|---|---|---|---|---|---|
| 850 | 600 | 2.1 | — | 2.1 | 443 | 0.2 | 0.6 | 0.6 |
| 1000 | 600 | 1.2 | 2.8 | 2.5 | 443 | 0.04 | 0.4 | 0.3 |
| 1200 | 600 | 1.0 | 2.4 | 2.0 | — | — | — | — |
| 1500 | 515 | 0.3 | 1.0 | 0.6 | — | — | — | — |
The changes observed in the luminescence spectra of the SAO:0.1Eu850, SAO:0.1Eu1000, SAO:0.1Eu1200, and SAO:0.1Eu1500 (see Fig. 6(b)) are evident in the tunable colours shown in the CIE coordinates in Fig. 8(c). The (x, y) components of the CIE coordinates of the SAO:0.1Eu850, SAO:0.1Eu1000, SAO:0.1Eu1200, and SAO:0.1Eu1500 are shown in Table 3. The emitted colours of the samples are tuned from red, through yellow to green as the annealing temperature was increased.
| Annealing temperatures (°C) | CIE coordinates (x, y) | QY (%) |
|---|---|---|
| 850 | (0.477, 0.403) | 28 |
| 1000 | (0.484, 0.412) | 35 |
| 1200 | (0.518, 0.457) | 27 |
| 1500 | (0.289, 0.564) | 27 |
The quantum yield (QY) of the samples was measured using an integrating sphere incorporated in the FLS980 spectrometer and the results are tabulated in Table 3. A detailed setup for the QY was discussed in our previous work.47 The QY increased with annealing temperature from 28% to 35%, for the SAO:0.1Eu850 and SAO:0.1Eu1000, respectively, and decreased to 27% for the SAO:0.1Eu1200 and SAO:0.1Eu1500.
3.7. Temperature-dependent luminescence
The temperature-dependent PL spectra of the SAO:0.1Eu850, SAO:0.1Eu1000, SAO:0.1Eu1200, and SAO:0.1Eu1500 were recorded using a 325 nm He–Cd laser system and the results are shown in Fig. 9. The PL intensity displayed a quenching of the red emission band in the measurement range of 26 °C to 100 °C for SAO:0.1Eu850, SAO:0.1Eu1000 and SAO:0.1Eu1200, and the green band for SAO:0.1Eu1500. This quenching is more pronounced for the low annealing temperature (<1200 °C) samples than for the 1200 °C and 1500 °C samples. The blue emission band, which is only found in the low annealing temperature samples, is unaffected by quenching, which offers a suitable reference for ratiometric measurements. With regards to the shape of the red emission band, the SAO:0.1Eu1200 shows a pronounced shift and broadening of the band which is much less pronounced in the other samples. | ||
| Fig. 9 Temperature-dependent photoluminescence spectra of the (a) SAO:0.1Eu850, (b) SAO:0.1Eu1000, (c) SAO:0.1Eu1200, and (d) SAO:0.1Eu1500 excited using a 325 nm Cd–He laser. | ||
In all cases, the broad red emission bands are assigned to the 4f65d1 → 4f7 electric dipole transitions of Eu2+ ions.21 The shape of the emission bands of the SAO:0.1Eu850 suggests the presence of some Eu3+ emission lines around 648 nm and 730 nm, which are assigned to the 5D0 → 7F3 and 5D0 → 7F5 transitions, respectively.48,49 The mechanism behind the blue-shift observed mainly in the SAO:0.1Eu1200 from red to green (600–586 nm) could be due to the decrease in the crystal field of the host around the Eu2+ ions, as explained earlier.
The values of the full width at half maximum (FWHM) of the red emission band (green band for SAO:0.1Eu1500) increased with increasing temperature for all samples but are much more pronounced for the SAO:0.1Eu1200, as shown in Fig. 10(a). This phenomenon has been reported in different phosphor materials8,9,31,32,34,50–52 and can be attributed to the reduced lattice vibration on decreasing the temperature31,34,50 due to the temperature dependence of the electron–phonon interaction.51
The phenomena underlying a usable luminescence thermometry response of the phosphor materials include the temperature quenching of luminescence when the temperature of a material is increased,53 temperature-dependent blue or red shift of emission position and9 back energy transfer between two states.54 The temperature quenching effect can be explained using the Mott–Seitz theory.53 Here, the temperature-dependent luminescence spectrum of the SAO:0.1Eu1000 (Fig. 9(b)) is used as an example, and the underlying mechanism is illustrated using a configuration coordinate model consisting of four parabolas representing the 4f7 ground state of Eu2+, and the Eu2+ ions occupying the Sr1, Sr2 and the Sr3 sites in the 4f65d1 excited state, Fig. 10(c). According to this model, an electron in the Sr1 site, assisted by thermal energy, non-radiatively deexcites to the 4f7 ground state through the intersection I of the Sr1 and 4f7 parabolas53,55 with the temperature-dependent rate described by eqn (3):15
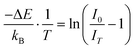 | (3) |
The temperature-sensitive nature of the emission spectra of the SAO:0.1Eu850, SAO:0.1Eu1000, SAO:0.1Eu1200 and SAO:0.1Eu1500 phosphor powders suggests that the materials have potential for thermometric applications. Thermometric parameters for the LIR technique were derived. For the SAO:0.1Eu850 and SAO:0.1Eu1000, the two-band approach was applied to the temperature-dependent luminescence data (Fig. 9(a and b)) by taking the LIR of the red band (500–800 nm) to the blue band (400–500 nm) defined as band 2 and band 1, respectively. In this case, the LIR is defined by eqn (4) (ref. 6, 57 and 58) as
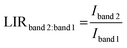 | (4) |
 | (5) |
| Phosphors | Band 1 (nm) | Band 2 (nm) | I band 1 | I band 2 |
|---|---|---|---|---|
| SAO:0.1Eu850 | 400–500 | 500–800 | I 400–500 | I 500–800 |
| SAO:0.1Eu1000 | 400–500 | 500–800 | I 400–500 | I 500–800 |
| λ1 (nm) | λ2 (nm) | I λ1 | I λ2 | |
|---|---|---|---|---|
| SAO:0.1Eu1200 | 470–560 | 560–800 | I 470–500 | I 560–800 |
| SAO:0.1Eu1500 | 420–485 | 485–800 | I 420–500 | I 485–800 |
The LIR obtained from the temperature-dependent luminescence emission spectra of the SAO:0.1Eu850, SAO:0.1Eu1000, SAO:0.1Eu1200 and SAO:0.1Eu1500 (Fig. 9(a–c)) decreased monotonically with temperature as shown in Fig. 11(a). The LIR of the temperature-dependent luminescence was fitted using a cubic polynomial function given by eqn (6) and plotted in Fig. 11(a).
| A + BT + CT2 + DT3 | (6) |
 | ||
| Fig. 11 (a) LIR, (b) Sa and (c) Sr as a function of temperature for the SAO:0.1Eu850, SAO:0.1Eu1000, SAO:0.1Eu1200 and SAO:0.1Eu1500. | ||
The absolute and relative sensitivities Sa and Sr are defined by eqn (7) and (8) as
 | (7) |
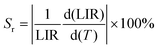 | (8) |
The colour response of the four phosphors is shown in Fig. 12(a–d). SAO:0.1Eu1000 has the most pronounced colour response, followed by SAO:0.1Eu850. This colour response is well suited for temperature imaging with a colour camera, e.g., ref. 15. As a thermometric parameter we determined the relative sensitivities of the colour coordinates x, and y, as in ref. 15. It was found to be as high as 0.88%/°C and 1.0%/°C at 90 °C from SAO:0.1Eu1000, which is higher than Sr4Al14O25:Mn4+,Tb3+ used with a colour camera in ref. 15. The colour response of SAO:0.1Eu1200 and SAO:0.1Eu1500 are comparatively far less pronounced. In the absence of a reference band, their thermometric performance as a luminescence thermometer is expected to be worse than the two other samples.
 | ||
| Fig. 12 The CIE coordinates showing the colour response of the (a) SAO:0.1Eu850, (b) SAO:0.1Eu1000, (c) SAO:0.1Eu1200 and (d) SAO:0.1Eu1500. | ||
Furthermore, all the samples exhibited excellent thermometric reproducibility during multiple heating–cooling cycles between 26 to 100 C, as shown in Fig. 13(a–d). This is evidence that the thermometric properties of these materials are sustainable in this temperature range.
 | ||
| Fig. 13 Repeatability heating–cooling cycles between 26 and 100 °C For (a) SAO:0.1Eu850, (b) SAO:0.1Eu1000, (c) SAO:0.1Eu1200 and (d) SAO:0.1Eu1500. | ||
The thermometric performances of the phosphors at different temperature ranges and 30 °C and their room temperature lifetimes are compared to earlier reported phosphors in Table 5. The maximum value of the relative sensitivity (Sr,max) of SAO:0.1Eu1000 is among the best-reported values. Also, only a few phosphors have better Sr value at 30 °C than this phosphor, but they involved either a very long band >100 μs (e.g., the emission for Eu3+ (ref. 76 and 81) or Dy3+ (ref. 53)) or a very short band <10 ns (emission from Ce3+ (ref. 74) or a charge transfer band75). Here, the decay time of both bands is in the microsecond range, which is ideally suited for imaging fast moving objects, e.g., gas and liquid flows using phosphor particles as tracers, and also for time-gated imaging in applications where fluorescence must be avoided.83 In summary, Sr3Al2O5Cl2:Eu2+ is an excellent candidate phosphor for sensitive dual-band thermometry at ambient temperatures where lifetimes in the microsecond range are required.
| Phosphor | S r, max%/°C | T(Sr, max) °C | S r@30 °C | Tau (Sr, max) | λ Exc. (nm) | Ref. |
|---|---|---|---|---|---|---|
| Row with a pair of lifetimes represents the lifetimes of the two dopant ions, respectively, or in the case of a single dopant, the lifetime at two emission wavelengths. | ||||||
| ZnO | 2.00 | 227 | 0.75 | 700 ps | 355 | 8 |
| BAM:Eu2+ | 0.25 | 22 | 0.23 | 1.17 μs | 355 | 8 and 63 |
| Sr4Al14O25:Mn4+,Tb3+ | 2.8 | 150 | 0.42 | 2.1, 2.1 (ms) | 266 | 15 |
| YVO4:Dy3+ | 1.8 | 25 | 1.6 | 140 (μs) | 310 | 13 |
| NaYF4:Er3+,Yb3+ | 1.20 | 27 | — | — | 980 | 64 |
| Ca6BaP4O17:Eu2+ | 1.53 | 227 | 0.25 | 2.1 (μs) | 300 | 65 |
| Na3Sc2P3O12:Eu2+,Mn2+ | 1.56 | 200 | 0.42 | 364.27 (ns), 16.83 (ms) | 340 | 66 |
| LaAlO3:Eu2+,Eu3+ | 1.19 | 130 | 0.39 | 482.68 (ns), 1.64 (ms) | 360 | 67 |
| Li4SrCaSi2O4N8/3:Eu2+ | 1.76 | 200 | 0.15 | 178.3, 269.8 (ns) | 363 | 68 |
| Sr4Al14O25:Eu2+ | 0.62 | 100 | 0.05 | 34.41, 657.27 (ns) | 290 | 69 |
| Sr4La(PO4)3OEu2+,Sm3+ | 0.48 | 300 | 0.15 | 326.00 (ns), – | 412 | 70 |
| Ca8Mg3Al2Si7O28:Ce3+,Eu2+ | 1.35 | 227 | 0.52 | 30.10, 890.00 (ns) | 320 | 71 |
| CsPbBr3/EuPO4 | 1.80 | 177 | 0.20 | — | 393 | 72 |
| Ba5SiO4Cl6:Eu2+ | 0.87 | 225 | 0.07 | 10.50, 9.60 (μs) | 397 | 73 |
| La5Si2BO13:Ce3+,Eu2+ | 3.22 | 20 | 3.00 | 13.85 (ns), — | 345 | 74 |
| CsCu2I3:Eu2+ | 2.60 | −13 | 1.95 | 1.66.00 (μs), 87.04 (ns) | 310 | 75 |
| La0.17Gd0.8AlO3:Eu2+,Eu3+ | 3.23 | 30 | 3.23 | 352.80 (ns), 1.63 (ms) | 315 | 76 |
| MgAl2O4:Eu2+,Eu3+ | 0.83 | 200 | 0.26 | 2.12 (μs), 1.26 (ms) | 358 | 77 |
| Ca9Zn1.5(PO4)7:Eu2+,Eu3+ | 1.40 | 180 | 0.14 | 258.72 (ns), 0.68 (ms) | 362 | 78 |
| SrAl2Si2O8:Eu2+,Eu3+ | 0.22 | 300 | 0.01 | 5.80 (μs), 1.87 (ms) | 324 | 79 |
| Sr2.94Eu0.01La1.05P2.95Si0.05O12 | 1.30 | 30 | 1.30 | 220.43, 429.10 (ns) | 375 | 80 |
| Ca2Al2SiO7:Eu2+,Eu3+ | 2.46 | 170 | 1.35 | 210.30 (ns), 2.25 (ms) | 394 | 81 |
| LiSr4(BO3)3:Ce3+,Eu2+ | 4.02 | 20 | 3.80 | 12.6 (ns), 286.10 (ns) | 356 | 82 |
| SAO:0.1Eu800 | 1.75 | 75 | 1.16 | 0.6, 2.1 (μs) | 325 | This work |
| SAO:0.1Eu1000 | 4.00 | 100 | 1.49 | 0.3, 2.5 (μs) | 325 | This work |
| SAO:0.1Eu1200 | 1.32 | 100 | 0.21 | 2.0 (μs) | 325 | This work |
| SAO:0.1Eu1500 | 0.65 | 100 | 0.67 | 0.6 (μs) | 325 | This work |
4. Conclusion
Sr3Al2O5Cl2:Eu2+ and SrAl2O4:Eu2+ thermographic phosphor particles were prepared and demonstrated to be highly sensitive to temperature in the ambient region. The optimal emission was obtained from the sample doped with 0.1 mol% of Eu2+. The structures, morphologies, chemical compositions, luminescence spectra, lifetimes, quantum yields, and temperature-sensitivity properties were dependent on the annealing temperature. The material contained impurity phases from SrO and SrCl2 when annealed at low temperature (850 °C) and a single phase, (Sr3Al2O5Cl2) at higher annealing temperatures, 1000 and 1200 °C. At the highest annealing temperature (1500 °C), a change of phase to SrAl2O4 was confirmed from the XRD patterns and the absence of Cl from the EDS spectrum. The luminescence of Sr3Al2O5Cl2:Eu2+ was characterized by broad blue and red band emissions and it exhibited complete quenching of the blue band with an increase in the Eu2+-doping concentration and the annealing temperature. In terms of temperature response, the red band showed significant thermal quenching, which was more pronounced in the samples annealed at lower temperatures. Both samples annealed at 850 °C and 1000 °C offer good performance as luminescence thermometers, as the ratio of the temperature-sensitive red band to the temperature-insensitive blue band has a temperature sensitivity well above 1%/°C in the 30 to 100 °C range. This results in the most pronounced colour response among reported phosphors with decay times in the microsecond range, which makes Sr3Al2O5Cl2:Eu2+ very well suited for temperature imaging of fast-moving objects, e.g., fluid temperature imaging with a colour camera, e.g., ref. 15.Author contributions
Hendrik C. Swart: Funding acquisition, supervision, resources and writing – review & editing. Robin E. Kroon: Supervision, resources and writing – review & editing. Frank Beyrau: Resources and writing – review & editing. Christopher Abram and Benoît Fond: Funding acquisition, supervision, conceptualization, investigation, methodology, validation, visualization and writing – review & editing. Simon N. Ogugua: Writing – original draft, conceptualization, investigation, methodology and validation, visualization.Conflicts of interest
There are no conflicts to declare.Acknowledgements
South African Research Chairs Initiative of the Department of Science and Technology and the National Research Foundation of South Africa (84415). The World Academy of Science/Deutsche Forschungsgemeinschaft (AB668/1-1). European Commission Marie Curie (708068).References
- K. Okabe, N. Inada, C. Gota, Y. Harada, T. Funatsu and S. Uchiyama, Nat. Commun., 2012, 3, 705 CrossRef PubMed
.
-
J. P. Feist and A. L. Heyes, Development of the phosphor thermometry technique for the application in gas turbines,10th international symposium on applications of laser techniques to fluid mechanics, Lisbon, Portugal, 2000 Search PubMed
.
- S. W. Allison and G. T. Gillies, Rev. Sci. Instrum., 1997, 62, 2615–2650 CrossRef
.
- T. Justel, H. Nikol and C. Ronda, Angew. Chem., Int. Ed., 1998, 37, 3084–3103 CrossRef CAS PubMed
.
- S. Zhang, Y. Nakai, T. Tsuboi, Y. Huang and H. J. Seo, Chem. Mater., 2011, 23, 1216–1224 CrossRef CAS
.
- R. Zhou, C. Liu, L. Lin, Y. Huang and H. Liang, Chem. Eng. Sci., 2019, 369, 376–385 CrossRef CAS
.
- C. Abram, I. W. Panjikkaran, S. N. Ogugua and B. Fond, Opt. Lett., 2020, 45, 3893–3896 CrossRef PubMed
.
- C. Abram, B. Fond and F. Beyrau, Opt. Express, 2015, 23, 19453–19468 CrossRef CAS PubMed
.
- B. Fond, C. Abram and F. Beyrau, Appl. Phys. B, 2015, 121, 495–509 CrossRef CAS
.
- B. Fond, C. Abram and F. Beyrau, Opt. Mater., 2019, 89, 615–622 CrossRef CAS
.
- J. Jordan and D. A. Rothamer, Appl. Phys. B: Lasers Opt., 2013, 110, 285–291 CrossRef CAS
.
- J. Brübach, J. P. Feist and A. Dreizler, Meas. Sci. Technol., 2008, 19, 025602 CrossRef
.
- I. E. Kolesnikov, A. A. Kalinichev, M. A. Kurochkin, E. V. Golyeva, A. S. Terentyeva, E. Y. Kolesnikov and E. Lähderanta, Sci. Rep., 2019, 9, 2043 CrossRef CAS PubMed
.
- D. Witkowski and D. A. Rothamer, Proc. Combust. Inst., 2019, 37, 1393–1400 CrossRef CAS
.
- W. Piotrowski, K. Trejgis, K. Maciejewska, K. Ledwa, B. Fond and L. Marciniak, ACS Appl. Mater. Interfaces, 2020, 12, 44039–44048 CrossRef CAS PubMed
.
- W. Piotrowski, L. Dalipi, R. Szukiewicz, B. Fond, M. Dramićanin and L. Marciniak, J. Mater. Chem. C, 2021, 9, 12671–12680 RSC
.
- D. Dutczak, C. Ronda, A. Meijerink and T. Jüstel, J. Lumin., 2013, 141, 150–154 CrossRef CAS
.
- R. Chen, Y. Hun, L. Chen, X. Wang, Y. Jin and H. Wu, Ceram. Int., 2014, 40, 8229–8236 CrossRef CAS
.
- W. Xie, C. Zou, S. Li, J. Sun, F. Kang and G. Sun, Phys. Chem. Chem. Phys., 2018, 20, 13983–13993 RSC
.
- W. Zeng, Y. Wang, Y. Li and X. Xu, Mater. Res. Soc. Symp. Proc., 2014, 1592, 6466 CrossRef
.
- Z. Wang, Q. Zhang, M. Rong, H. Tan, Q. Wang, Q. Zhou and G. Chen, Chem. Phys. Lett., 2016, 658, 324–327 CrossRef CAS
.
- Z. Wang, X. Li, L. Xiong, M. Zhou, F. Hu, Y. Zhu and Y. Zhang, ECS J. Solid State Sci. Technol., 2012, 1, R143–R146 CrossRef CAS
.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef
.
-
M. Sato, S. W. Kim, Y. Shimomura, T. Hasegawa, K. Toda and G. Adachi, Rare earth-doped phosphors for white light-emitting diodes, in Handbook on the physics and chemistry of rare earths, ed. J.-C. Bünzli and V. K. Pecharsky, Elsevier B.V., 2016, vol. 49, pp. 68–70 Search PubMed
.
- L. Ning, C. Zhou, W. Chen, Y. Huang, C. Duan, P. Dorenbos, Y. Tao and H. Liang, J. Phys. Chem. C, 2015, 119, 6785–6792 CrossRef CAS
.
- Y. Lu, X. Li, Y. Dong, Z. Ma, T. Hou, D. Jiao, Q. Guo, L. Guan and F. Teng, Optik, 2016, 127, 4625–4629 CrossRef CAS
.
- A. Vegas, R. L. Martin and D. J. M. Bevan, Acta Crystallogr., Sect. B: Struct. Sci., 2009, 65, 11–21 CrossRef CAS PubMed
.
- D. Dutczak, T. Jüstel, C. Ronda and A. Meijerink, Phys. Chem. Chem. Phys., 2015, 17, 15236–15249 RSC
.
- R. M. Jafer, H. C. Swart, A. Yousif, V. Kumar and E. Coetsee, J. Lumin., 2016, 180, 198–203 CrossRef CAS
.
- B. M. Jaffar, H. C. Swart, H. A. A. S. Ahmed, A. Yousif and R. E. Kroon, J. Lumin., 2019, 209, 217–224 CrossRef CAS
.
- H. Tanno, S. Zhang, T. Shinoda and H. Kajiyama, J. Lumin., 2010, 130, 82–86 CrossRef CAS
.
- Y. Masubuchi, S. Nishitani, A. Hosono, Y. Kitagawa, J. Ueda, S. Tanabe, H. Yamane, M. Higuchi and S. Kikkawa, J. Mater. Chem. C, 2018, 6, 6370–6377 RSC
.
- J. S. Kim, Y. H. Park, S. M. Kim, J. C. Choi and H. L. Park, Solid State Commun., 2005, 133, 445–448 CrossRef CAS
.
-
G. Arnaoutakis, Temperature dependence of phosphors via photo-and thermo- luminescence, Edinburgh Instruments AN_P38, 2017, DOI:10.13140/RG.2.2.10709.52967
.
- J. S. Kim, Y. H. Park, J. C. Choi and H. L. Park, J. Electrochem. Soc., 2005, 152, H135–H137 CrossRef CAS
.
- M. Nazarov, M. G. Brik, D. Spassky and B. Tsukerblat, J. Lumin., 2017, 182, 79–86 CrossRef CAS
.
- J. Botterman, J. J. Joos and P. F. Smet, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 90, 085147 CrossRef CAS
.
- S. N. Ogugua, H. C. Swart and O. M. Ntwaeaborwa, Sens. Actuators, B, 2017, 250, 285–299 CrossRef CAS
.
- M. Takesue, H. Hayashi and R. L. Smith Jr., J. Prog. Cryst. Growth Charact. Mater., 2009, 55, 98–124 CrossRef CAS
.
- J. El Ghoul and L. El Mir, J. Mater. Sci.: Mater. Electron., 2015, 26, 3550–3557 CrossRef CAS
.
- J. R. Rubbmark, M. M. Kash, M. G. littman and D. Kleppner, Phys. Rev. A, 1981, 23, 3107–3117 CrossRef CAS
.
- T. Justel, W. Mayr and P. J. Schmidt, Tuning the 4f15d-4f2 UV emission of Pr3+, in: 200th meeting of the electrochemical society, San Francisco, CA, USA, 2001.
- L. G. Van Uitert, J. Lumin., 1984, 29, 1–9 CrossRef CAS
.
- S. H. M. Poort, W. P. Blocpoel and G. Blasse, Chem. Mater., 1995, 7, 1547–1551 CrossRef CAS
.
- T. Aitasalo, J. Hölsä, H. Jungner, J.-C. Krupa, M. Lastusaari, J. Legendziewicz and J. Niittykoski, Radiat. Meas., 2004, 38, 727–730 CrossRef CAS
.
- J. Włodarczyk and B. Kierdaszuk, Biophys. J., 2003, 85, 589–598 CrossRef PubMed
.
- S. N. Ogugua, S. K. K. Shaat, H. C. Swart, R. E. Kroon and O. M. Ntwaeaborwa, J. Alloys Compd., 2019, 775, 950–968 CrossRef CAS
.
- S. K. Gupta, C. Reghukumar and R. M. Kadam, RSC Adv., 2016, 6, 53614–53624 RSC
.
- C. Cascales, J. Fernández and R. Balda, Opt. Express, 2005, 13, 2141–2152 CrossRef CAS PubMed
.
- S. Tombe, G. Adam, H. Heilbrunner, D. H. Apaydin, C. Ulbricht, N. S. Sariciftci, C. J. Arendse, E. Iwuoha and M. C. Scharber, J. Mater. Chem. C, 2017, 5, 1714–1723 RSC
.
- J. S. Kim, Y. H. Park, J. C. Choi and H. L. Park, ESL, 2005, 8, H65–H67 CAS
.
- J. Ni, Q. Liu, Z. Zhou and G. Liu, RSC Adv., 2017, 7, 42211–42217 RSC
.
-
M. Dramićanin, Luminescence thermometry: methods, materials, and applications, Woodhead Publ. ser. Electron, 2018, pp. 41–42 Search PubMed
.
- B. Cao, Y. Bao, Y. Liu, J. Shang, Z. Zhang, Y. He, Z. Feng and B. Dong, Chem. Eng. J., 2020, 385, 123906 CrossRef CAS
.
- C. Matuszewska, K. Elzbieciak-Piecka and L. Marciniak, J. Phys. Chem. C, 2019, 123, 18646–18653 CrossRef CAS
.
- R. C. E. Sia, R. A. Arellano-Reyes, T. E. Keyes and J. Guthmuller, Phys. Chem. Chem. Phys., 2021, 23, 26324–26335 RSC
.
- E. J. McLaurin, L. R. Bradshaw and D. R. Gamelin, Chem. Mater., 2013, 25, 1283–1292 CrossRef CAS
.
- W. Xu, Z. Zhang and W. Cao, Opt. Lett., 2012, 37, 4865–4867 CrossRef CAS PubMed
.
- D. K. Amarasinghe and F. A. Rabuffetti, Chem. Mater., 2019, 31, 10197–10204 CrossRef CAS
.
- R. Liang, R. Tian, W. Shi, Z. Liu, D. Yan, M. Wei, D. G. Evans and X. Duan, Chem. Commun., 2013, 49, 969–971 RSC
.
- T. P. van Swieten, A. Meijerink and F. T. Rabouw, ACS Photonics, 2022, 9, 1366–1374 CrossRef CAS PubMed
.
- M. Suta and A. Meijerink, Adv. Theory Simul., 2020, 3, 2000176 CrossRef CAS
.
- M. Lawrence, H. Zhao and L. Ganippa, Opt. Express, 2013, 21, 12260–12281 CrossRef CAS PubMed
.
- S. Zhou, K. Deng, X. Wei, G. Jiang, C. Duan, Y. Chen and M. Yin, Opt. Commun., 2013, 291, 138–142 CrossRef CAS
.
- R. F. Zhou, C. M. Liu, L. T. Lin, Y. Huang and H. B. Liang, Chem. Eng. J., 2019, 369, 376 CrossRef CAS
.
- X. G. Zhang, Z. P. Zhu, Z. Y. Guo, Z. S. Sun and Y. B. Chen, Chem. Eng. J., 2019, 356, 413–422 CrossRef CAS
.
- B. Chen, C. Li, D. Deng, F. Ruan, M. Wu, L. Wang, Y. Zhu and S. Xu, J. Alloys Compd., 2019, 792, 702–712 CrossRef CAS
.
- H. Yu, L. Zhou, R. Ye, D. Deng and S. Xu, Dalton Trans., 2022, 51, 7333–7342 RSC
.
- M. Wu, D. Deng, F. Ruan, B. Chen, L. Lei, R. Lei and S. Xu, Opt. Mater., 2019, 88, 704–710 CrossRef CAS
.
- F. Liu, D. Deng, M. Wu, B. Chen, L. Zhou and S. Xu, J. Rare Earths, 2021, 39, 261–268 CrossRef CAS
.
- R. Zhou, L. Lin, H. Zhao, T. Deng and J. Li, Mater. Chem. Front., 2021, 5, 6071–6081 RSC
.
- C. Wang, H. Lin, X. Xiang, Y. Cheng, Q. Huang, Y. Gao, X. Cui and Y. Wang, J. Mater. Chem. C, 2018, 6, 9964–9971 RSC
.
- P. Du, L. Luo, W. Li, F. Yan and G. Xing, J. Alloys Compd., 2020, 826, 154233 CrossRef CAS
.
- F. Liu, Y. Tian, D. Deng, M. Wu, B. Chen, L. Zhou and S. Xu, J. Rare Earths, 2021, 39, 1311–1319 CrossRef CAS
.
- X. Li, G. Gao, K. Wang, Z. Chen, Z. Gao, Q. Qin, L. Chen and B. Zou, A ratiometric optical thermometer with dual-color emission based on Eu2+-doped CsCu2I3 microcrystals, J. Rare Earths, 2023 DOI:10.1016/j.jre.2023.07.016
.
- C. Li, B. Chen, D. Deng, H. Yu, H. Li, C. Shen, L. Wang and S. Xu, J. Lumin., 2020, 221, 117036 CrossRef CAS
.
- E. I. Kolesnikov, E. V. Afanaseva, M. A. Kurochkin, E. Y. Kolesnikov and E. Lähderanta, Phys. B, 2022, 624, 413456 CrossRef
.
- H. Yu, F. Ruan, L. Chen, D. Deng and D. Deng, Opt. Mater., 2020, 100, 109678 CrossRef CAS
.
- J. Xue, M. Song, H. M. Noh, S. H. Park, B. C. Choi, J. H. Kim, J. H. Jeong and P. Du, J. Alloys Compd., 2020, 843, 155858 CrossRef CAS
.
- T. Yu, B. Liu, Z. Ma, Y. Jiang, Q. Zeng, D. Wen and Y. Guo, Mater. Chem. Front., 2021, 5, 6256–6264 RSC
.
- T. Hu, Y. Gao, M. Molokeev, Z. Xia and Q. Zhang, Sci. China Mater., 2019, 62, 1807–1814 CrossRef CAS
.
- C. Li, B. Chen, D. Deng, M. Wu, H. Yu, H. Li, C. Shen, L. Wang and S. Xu, J. Alloys Compd., 2020, 838, 155675 CrossRef CAS
.
- A. Mendieta, B. Fond, P. Dragomirov and F. Beyrau, Meas. Sci. Technol., 2019, 30, 074002 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2024 |








