Open Access Article
Open Access ArticleSingle-molecule magnet behavior in heterometallic decanuclear [Ln2Fe8] (Ln = Y, Dy, Ho, Tb, Gd) coordination clusters†
Man-Ting
Chen
,
Hai-Xia
Zhao
 *,
La-Sheng
Long
*,
La-Sheng
Long
 * and
Lan-Sun
Zheng
* and
Lan-Sun
Zheng
Collaborative Innovation Center of Chemistry for Energy Materials, State Key Laboratory of Physical Chemistry of Solid Surfaces and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005, China. E-mail: hxzhao@xmu.edu.cn; lslong@xmu.edu.cn
First published on 9th January 2024
Abstract
Five decanuclear lanthanide–iron clusters, formulated as [Ln2Fe8(hmp)10(μ2-OH)4(μ3-OH)2(μ4-O)4(H2O)6]·6ClO4·xH2O (x ≈ 8, Ln = Y for 1; x ≈ 6, Ln = Dy for 2; x ≈ 6, Ln = Ho for 3; x ≈ 7, Ln = Tb for 4; x ≈ 7, Ln = Gd for 5, Hhmp = 2-(hydroxymethyl)pyridine), have been synthesized and structurally characterized. Single-crystal structural analysis reveals that the cluster consists of six face-sharing defective cubane units. Dynamic magnetic investigations indicated that cluster 2 exhibits single-molecule magnet behavior under a zero dc field eliciting an effective energy barrier of Ueff = 17.76 K and a pre-exponential factor of τ0 = 7.93 × 10−8 s. Investigation of the performance of a series of FeIII–DyIII SMMs indicates that the relatively low energy barrier in 2 is associated with the weak ferromagnetic coupling between FeIII and DyIII ions, while the strength of ferromagnetic interaction in these clusters is mainly related to the bond distances between DyIII and O atoms coordinated to FeIII ions. Clusters 3 and 4 exhibit similar dual relaxation pathways under their respective optimal external applied dc field, where the direct relaxation process occurs in the low-frequency area, which impedes the extraction of the Ueff, while the secondary relaxation process appears at a higher frequency, which is probably a connection with intermolecularly driven relaxation. Our findings offer a magneto-structural correlation model for further investigating the single-molecule magnet behavior in lanthanide–iron systems.
Introduction
A single-molecule magnet (SMM) is a nanomagnet of molecular size, characterized by potential energy barriers (Ueff) that facilitate the reversal of magnetic moments. It is distinguished by its slow magnetization relaxation, which stems from the high-spin ground state (S) and significant magnetic anisotropy (D) of an individual molecule.1 Since the discovery of the first Mn12-SMM, which exhibits slow paramagnetic relaxation within two bistable magnetic states by rendering the Ueff for the reversal of magnetization,2 SMMs have attracted considerable attention due to their potential applications in quantum computing,3 high-density data storage4 and molecular spintronics.5 To reveal the factors that affect the performance of SMMs, it was found that large magnetic anisotropy and large ground-state spin are of key importance in enhancing the performance of SMMs.6 The dysprosium (DyIII) ion, having a Kramers’ spin ground state doublet of 6H15/2, is expected to generate large magnetic anisotropy and is regarded as a promising candidate for obtaining SMMs with high Ueff values.7 The FeIII ion, on the other hand, has the largest single-site spin and is expected to attain large ground-state spin in 3d metals, in addition to contributing to the enhancement of magnetic exchange–coupling interactions.8 Therefore, the combination of DyIII ions and FeIII ions is expected to improve the performance of SMMs. However, although the first binuclear FeIII–DyIII SMM was reported in 2006,9 the highest Ueff value reported so far in any FeIII–DyIII cluster is only 65.1 K in the nonanuclear {FeIII6DyIII3} cluster.10 One of the reasons might be attributed to the stray magnetic fields generated by the FeIII spin center, which significantly increases the possibility of relaxation through the quantum tunneling mechanism. In addition, the weak or very weak magnetic coupling between FeIII and DyIII ions leads to low-energy relaxation pathways between the low-lying split sublevels, resulting in a low Ueff value.11 Here we report the syntheses, structures, and magnetic properties of five decanuclear 3d–4f clusters, formulated as [Ln2Fe8(hmp)10(μ2-OH)4(μ3-OH)2(μ4-O)4(H2O)6]·6(ClO4)·x(H2O) (x ≈ 8, Ln = Y for 1; x ≈ 6, Ln = Dy for 2; x ≈ 6, Ln = Ho for 3; x ≈ 7, Ln = Tb for 4; x ≈ 7, Ln = Gd for 5; Hhmp = 2-(hydroxymethyl)pyridine).Experimental
Materials and methods
The raw materials and reagents were all commercially purchased and used directly without further purification. Elemental analyses were carried out using a Flash Smart elemental analyzer. Powder X-ray diffraction (XRD) data were collected using a Rigaku Ultima IV powder X-ray diffractometer (Cu Kα, λ = 1.54184 Å). Thermogravimetric analysis (TGA) curves were obtained using an STA-449F5 thermal analyzer. The measurement of magnetic susceptibilities was conducted using a Quantum Design MPMS-XL5 superconducting quantum interference device (SQUID) and a physical property measurement system (PPMS-9T).Preparation of [Y2Fe8(C6H6NO)10(μ2-OH)4(μ3-OH)2(μ4-O)4(H2O)6]·6(ClO4)·8H2O (1)
2-Hydromethyl pyridine (1 mmol, 109.1 mg) and Fe(ClO4)3 (1 mmol, 372.2 mg) were mixed with a 1 mmol aqueous solution of Y(ClO4)3 (1.0 M) in methanol (8 mL) and 300 μL Et3N was added. The resulting solution was then heated and stirred at approximately 80 °C for 2 hours, and then the filtrate was volatilized in the air for about 1 day to obtain yellow block crystals. Yield: ca. 40% (based on Y3+). Anal. calcd (%) for C60H94N10Cl6Fe8Y2O58 (FW = 2720.7, based on 6 ClO4− and 8 guest water molecules): C, 26.49; N, 5.15; H, 3.48. Found (%): C, 26.72; N, 5.27; H, 3.39.Preparation of [Dy2Fe8(C6H6NO)10(μ2-OH)4(μ3-OH)2(μ4-O)4(H2O)6]·6(ClO4)·6H2O (2)
The synthesis scheme of 2 was similar to that of 1, only replacing Y(ClO4)3 with Dy(ClO4)3. Yield: ca. 40% (based on Dy3+). Anal. calcd (%) for C60H90N10Cl6Fe8Dy2O56 (FW = 2831.9, based on 6 ClO4− and 6 guest water molecules): C, 25.45; N, 4.95; H, 3.20. Found (%): C, 25.72; N, 5.08; H, 3.16.Preparation of [Ho2Fe8(C6H6NO)10(μ2-OH)4(μ3-OH)2(μ4-O)4(H2O)6]·6(ClO4)·6H2O (3)
The synthesis scheme of 3 was similar to that of 1, only replacing Y(ClO4)3 with Ho(ClO4)3. Yield: ca. 40% (based on Ho3+). Anal. calcd (%) for C60H90N10Cl6Fe8Ho2O56 (FW = 2836.7, based on 6 ClO4− and 6 guest water molecules): C, 25.40; N, 4.94; H, 3.20. Found (%): C, 25.77; N, 4.84; H, 3.19.Preparation of [Tb2Fe8(C6H6NO)10(μ2-OH)4(μ3-OH)2(μ4-O)4(H2O)6]·6(ClO4)·7H2O (4)
The synthesis scheme of 4 was similar to that of 1, only replacing Y(ClO4)3 with Tb(ClO4)3. Yield: ca. 40% (based on Tb3+). Anal. calcd (%) for C60H92N10Cl6Fe8Tb2O57 (FW = 2842.7, based on 6 ClO4− and 7 guest water molecules): C, 25.35; N, 4.93; H, 3.26. Found (%): C, 25.83; N, 4.81; H, 3.16.Preparation of [Gd2Fe8(C6H6NO)10(μ2-OH)4(μ3-OH)2(μ4-O)4(H2O)6]·6(ClO4)·7H2O (5)
The synthesis scheme of 5 was similar to that of 1, only replacing Y(ClO4)3 with Gd(ClO4)3. Yield: ca. 40% (based on Gd3+). Anal. calcd (%) for C60H92N10Cl6Fe8Gd2O57 (FW = 2839.4, based on 6 ClO4− and 7 guest water molecules): C, 25.38; N, 4.93; H, 3.27. Found (%): C, 25.42; N, 4.86; H, 3.15.X-ray crystallography
The X-ray diffraction data of clusters 1–4 were collected using a Rigaku Oxford Diffraction single-crystal X-ray diffractometer with Cu Kα radiation (λ = 1.54184 Å) at 100 K. Data for cluster 5 was collected using an Agilent SuperNova four-circle X-ray single-crystal diffractometer using Cu Kα radiation (λ = 1.54184 Å) at 100 K. The structures were solved and refined with full-matrix least-squares based on F2 using ShelXT and ShelXL programs on Olex2.12,13 Due to the high disorder of the guest H2O molecules, they were removed using SQUEEZE during structural refinement.14 Crystallographic data and structural refinements for 1–5 are summarized in Table S1.† CCDC 2195974 and 2195975 for 1 and 2, and 2314827–2314829 for 3–5, respectively.†Results and discussion
Crystal structures
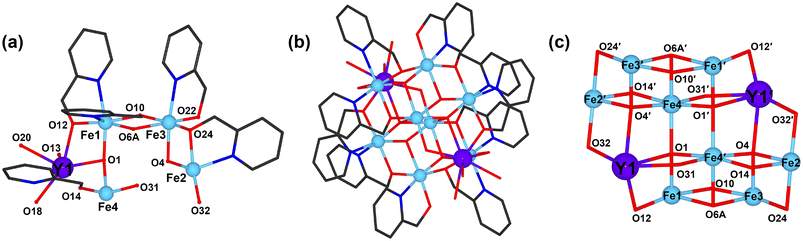
The experimental XRD patterns of clusters 1–5 were very similar to the simulated ones, confirming the phase purity of 1–5 (Fig. S1†). Single-crystal structural analysis reveals that clusters 1–5 are isostructural and crystallize in the monoclinic space group P21/n (Table S1†). Therefore, cluster 1 was used as a representative example to describe the configurational characteristics. From the chemical composition aspect, cluster 1 is composed of a cationic cluster of [Y2Fe8(hmp)10(μ2-OH)4(μ3-OH)2(μ4-O)4(H2O)6]6+, 6 ClO4− anions, and 8 H2O molecules. The number of guest water molecules was determined by elemental analysis, which is consistent with the thermogravimetric analysis result of 1. As shown in Fig. S2a,† the weight loss of 1 is approximately 5.19% at 140 °C, which corresponds to the calculated value of 5.29% for the removal of 8 guest water molecules. The XRD pattern confirmed that the final thermal decomposition product of 1 was a mixed phase, mainly including YFeO3 and Fe2O3 (Fig. S2b†).The asymmetric unit in the cationic cluster contains 5 hmp− ligands, 1 Y3+ ion, 4 Fe3+ ions, and 3 coordinated H2O molecules (O13, O18 and O20) (Fig. 1a). The eight hmp− ligands allow the alkoxide O atoms to bridge Fe2–Fe3(O24), Fe2–Fe4′(O14), Fe3–Fe1(O10), and Fe1–Y1(O12) atoms in μ2-η1:η2 coordination modes (Fig. S3a and S3b†), the remaining two hmp− ligands only coordinate with Fe3 atoms in a μ1-η1:η1 pattern (Fig. S3c†). The connection of two asymmetric units through O1 and O4 atoms from one asymmetric unit coordinated with the Fe4′ and Y1′ atoms from another asymmetric unit, respectively, generates a cationic cluster of [Y2Fe8(hmp)10(μ2-OH)4(μ3-OH)2(μ4-O)4(H2O)6]6+ (Fig. 1b). Therefore, the cationic cluster core in 1 (Fig. 1c) can be viewed as six face-sharing defective cubane units of two Fe3(μ2-OR)2(μ3-OH)(μ4-O) units, two Fe3(μ2-OR)(μ3-OH)(μ4-O)2 units, and two YFe2(μ2-OR)(μ2-OH)(μ3-OH)(μ4-O) units linked together by two μ2-OH− groups and four μ4-O2− groups (where the O atoms are from hmp− ligands in the OR group). Such a face-sharing defective cubane unit is very similar to that observed in Mn clusters.15
The Y13+ ion in 1 is octa-coordinated with a triangular dodecahedron configuration from one O atom from the hmp− ligand, two μ2-OH− groups, two μ4-O2− groups, and three coordination water molecules (Fig. S4a†). All Fe atoms are hexa-coordinated in a distorted octahedral coordination sphere: Fe1 and Fe2 are surrounded by two N and two O atoms from two hmp− ligands and one μ4-O2− group, respectively. The coordination environment of Fe1 is slightly different from that of Fe2, that is, one μ3-OH− group in Fe1 is replaced by one μ2-OH− group in Fe2 (Fig. S4b and S4c†). Fe3 is coordinated with one N and three O atoms, respectively, from three hmp− ligands, one μ3-OH− group, and one μ4-O2− group (Fig. S4d†), while the Fe4 atom is coordinated with one O atom from the hmp− ligand, one μ2-OH− group, one μ3-OH− group, and three μ4-O2− groups (Fig. S4e†). According to bond valence sum (BVS) calculations and the charge balance principle, all iron ions are trivalent (Table S2†). The Y⋯Fe distances are between 3.387(15) Å (Y1⋯Fe2′) and 3.498(6) Å (Y1⋯Fe4). The bond lengths of Fe–O and Fe–N are in the range of 1.887(6)–2.231(9) Å and 1.934(9)–2.184(7) Å, respectively. The Fe–O bond lengths in 1 are comparable to the YFe6 cluster of Fe–O bond lengths of 1.936(2)–2.243(2) Å16 and the Fe–N bond distances correspond to the Y2Fe8 cluster of 2.091(5)–2.216(6) Å.17 The Y–O bond length falls in the range of 2.279(5)–2.493(4) Å and the O–Y–O bond angles vary from 65.99(16)–152.63(18)°, respectively, in agreement with the bond lengths of 2.245 (9)–2.468 (9) Å and bond angles of 66.1 (3)–159.2 (4)° reported for the Y4Fe4 cluster.18
The structure of cluster 2 is very similar to that of cluster 1. The bond distances of Dy⋯Fe are in the range of 3.396(14)–3.412(11) Å, while the Fe–O and Fe–N bond lengths are in the range of 1.890(6)–2.180(2) Å and 1.938(9)–2.188(7) Å, respectively. The bond distance values of Fe–O and Fe–N in 2 are comparable to those of 1.880(7)–2.143(7) Å and 1.971(9)–2.240(10) Å, respectively, in the Dy3Fe7 cluster reported previously.19 The distances of Dy–O are in the range of 2.297 (5)–2.504 (4) Å and the O–Dy–O bond angles vary from 66.32(16)–152.65(18)°, which agree well with the Dy–O bond distances of 2.268(7)–2.523(7) Å and O–Dy–O bond angles of 55.4(3)°–157.8(3)° reported for the Dy8Fe4 cluster.20
Magnetic properties
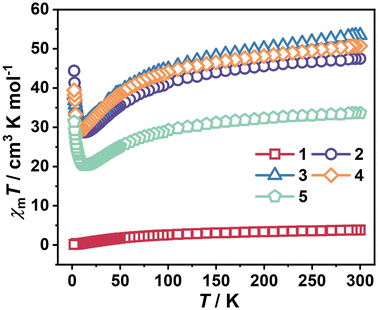
Temperature-dependent magnetic susceptibility of clusters 1–5 was measured between 2 and 300 K by applying a direct-current (dc) field of 1000 Oe. As shown in Fig. 2, upon cooling, the χmT value of 1 continuously decreases and reaches 0.11 cm3 K mol−1 at 2 K. The χmT value of 3.78 cm3 K mol−1 at room temperature is much smaller than the expected value of 35 cm3 K mol−1 based on 8 noninteracting FeIII ions (S = 5/2; g = 2). The significant discrepancy between experimental and theoretical χmT values at room temperature is attributed to the antiferromagnetic interaction between FeIII ions.21 The magnetic susceptibility data above 100 K were fitted using the Curie–Weiss law, generating the Curie constant C = 4.93 cm3 mol−1 and the Weiss constant θ = −92.07 K (Fig. S5a†). The negative θ value indicates that antiferromagnetic interactions exist in 1.22 The χmT value for 2 slowly decreases from 47.48 cm3 K mol−1 at 300 K to a minimum value of 28.74 cm3 K mol−1 at 14 K and then steeply increases to 44.36 cm3 K mol−1 at 2 K on lowering the temperature. The decrease of the χmT value in the range of 14–300 K could be attributed to the depopulation of the Stark sublevels of the DyIII ions23 and the existence of weak antiferromagnetic interactions between spin carriers, and the increase of the χmT value below 14 K observed in 2 shows the presence of ferromagnetic interactions. It is worth noting that the ferro- or antiferromagnetic interaction may be induced by weak DyIII–FeIII coupling.24 At 300 K, the χmT product is 47.48 cm3 K mol−1, which is lower than the expected value of 63.33 cm3 K mol−1 for 2 uncoupled DyIII (J = 15/2; g = 4/3) and 8 FeIII (S = 5/2; g = 2) ions, indicating antiferromagnetic interaction between spin carriers in 2.25 The plot of 1/χmT vs. T obeys the Curie–Weiss law above 100 K, leading to the values of C = 51.28 cm3 mol−1 and θ = −24.77 K for 2 (Fig. S5b†). It has been mentioned that the θ value in 1 is more negative than that in 2, suggesting that the antiferromagnetic coupling in 1 is stronger than that in 2. This fact indicates the presence of ferromagnetic interactions between DyIII–FeIII ions.The magnetic behavior of clusters 3–5 reflected by their χmT values is rather similar to that in cluster 2. The magnetic data are summarized in Table 1. Cluster 5 contains two isotropic GdIII ions without orbital contributions to the ground state. The subtraction of the χmT value of cluster 1, containing the diamagnetic YIII ions and thus revealing the FeIII interactions, could provide insights into magnetic interactions. The shape of the subtracted ΔχmT curves is shown in Fig. S6,† where the ΔχmT value of χmT (5)–χmT (1) gradually decreases to reach the minimum value of 19.81 cm3 K mol−1 from 300 to 14 K due to the antiferromagnetic interactions between GdIII–FeIII ions and/or GdIII–GdIII ions, and then increases significantly in the range of 14–2 K indicating a ferromagnetic arrangement in cluster 5, which may be attributed to the interactions of GdIII–FeIII ions and/or GdIII–GdIII ions.17 For clusters 2–4, the thermal behavior of ΔχmT will become more complicated due to the depopulation of Stark sublevels of the anisotropic DyIII, HoIII and TbIII ions, which can also contribute to the decrease of ΔχmT.23
| 1 (Y) | 2 (Dy) | 3 (Ho) | 4 (Tb) | 5 (Gd) | |
|---|---|---|---|---|---|
| Ground-state term of LnIII ion | 1S0 | 6H15/2 | 5I8 | 7F6 | 8S7/2 |
| g for LnIII ion | 0 | 4/3 | 5/4 | 3/2 | 2 |
| X m T (cm3 K mol−1) expected value at 300 K | 35 | 63.33 | 63.13 | 58.63 | 50.75 |
| X m T (cm3 K mol−1) experimental value at 300 K | 3.78 | 47.48 | 53.44 | 50.71 | 33.62 |
| X m T (cm3 K mol−1) experimental value at 2 K | 0.11 | 44.36 | 38.14 | 39.45 | 31.40 |
| Magnetization (NμB) observed at 7 T and 2 K | 2.62 | 46.03 | 18.23 | 16.20 | 15.30 |
| Curie constant (cm3 K mol−1) above 100 K | 4.93 | 51.28 | 59.14 | 55.22 | 36.34 |
| Weiss constant, θ (K), above 100 K | −92.07 | −24.77 | −34.41 | −27.65 | −24.88 |
The field dependence of the magnetization for 1–5 was measured at 2 K in the field range of 0–7 T (Fig. S7†). For 1, the observed value of 2.62NμB at 7 T was much lower than the calculated saturation value of 40NμB based on the 8 uncorrelated FeIII ions, which might be ascribed to the low-lying excited states resulting from weak intra-cluster magnetic coupling in magnetic centers.26 The magnetization for 2–5 occurred at 7 T with no real sense of saturation, and for all of them saturation values are lower than the theoretical values if all the spins are ferromagnetically aligned. The lack of saturation indicates the existence of low-lying excited states and/or intrinsic magnetic anisotropy.8b,27
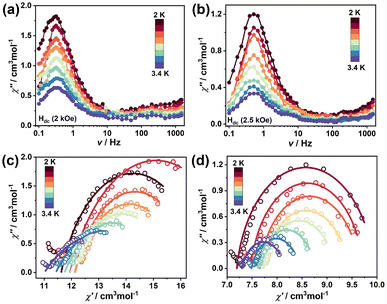
To gain insights into the magnetization dynamics in 2, ac susceptibilities were measured under zero external field. The temperature dependence plots of the in-phase (χ′) and out-of-phase (χ′′) ac susceptibility signals under the zero dc field vary with frequencies (Fig. S8a and S8b†) and the frequency dependence plots of χ′ and χ′′ are shown in Fig. 3a and b. A series of continuous peaks was observed within a temperature range of 2–3.4 K, indicating the presence of slow relaxation of the magnetization and the SMM response. Cole–Cole plots at fixed temperatures in the range of 2–3.4 K exhibit typical semicircular profiles, which are well fitted by the generalized Debye model28 (Fig. 3c) and give the α parameters of 0.15–0.29 (Table S3†), suggesting the narrow distribution in the relaxation times of the system. The plot of the natural logarithm of relaxation time ln(τ) versus 1/T for 2 remains linear (Fig. 3d), indicating that an Orbach process is dominant for slow magnetic relaxation. Fitting of the plot of ln(τ) versus 1/T with the Arrhenius law afforded a characteristic Ueff value of 17.76 K with a pre-exponential factor τ0 value of 7.93 × 10−8 s for 2. Cluster 1 reveals antiferromagnetic coupling containing two diamagnetic YIII ions and does not exhibit SMM behavior, whereas cluster 2 displays χ′′ ac susceptibility signals, which is typical of SMMs due to the introduction of anisotropic DyIII ions, the relatively low Ueff value likely being due to the weak magnetic coupling between FeIII−DyIII ions.29
Table 2 lists the SMM performances of FeIII–DyIII SMMs reported previously. The Ueff of 2 is lower than that of {FeIII6DyIII3},10 {FeIII4DyIII2},30,33 {FeIII2DyIII4},31 {FeIII7DyIII3},32 and {FeIII4DyIII4},34 but higher than that of some clusters, such as {FeIII7DyIII4},35 {FeIII18DyIII6},36 {FeIII6DyIII3},37 {FeIIIDyIII},23 {FeIII2DyIII3},38 {FeIII8DyIII2},17 and {FeIII4DyIII2}.39 Based on Table 2, it is difficult to judge the SMM performance of these clusters according to the macroscopic magnetic properties of these clusters. To further reveal the factors that affect the performance of SMMs in this system, the performance of SMMs in {FeIII4DyIII2},30,33 {FeIII4DyIII2}39 and cluster 2 was compared, not only because they all contain two discrete DyIII ions that are symmetrically related, but also because the iron clusters themselves within them do not show SMM behavior. Based on the performance of these SMMs, it is clear that the performance of SMMs in these clusters is in the order of {FeIII4DyIII2}30 > {FeIII4DyIII2}33 > 2 > {FeIII4DyIII2}.39 This result indicates that the strength of ferromagnetic interaction is conducive to obtaining excellent SMMs, because the strength of the ferromagnetic interaction in these clusters in the temperature range of 2–10 K is in the order of {FeIII4DyIII2}30 > {FeIII4DyIII2}33 > 2 > {FeIII4DyIII2}.39 To reveal the key factor that influences the ferromagnetic interaction in these clusters, their bond distances between DyIII and O atoms coordinated to FeIII ions and bond angles of Dy–O–Fe are investigated. The bond distances between DyIII and O atoms coordinated to FeIII ions and bond angles of Dy–O–Fe are respectively 2.288(4)–2.307(5) Å and 105.42(18)–105.92(19)° for {FeIII4DyIII2},30 2.248(3)−2.353(3) Å and 100.79(13)–128.89(16)° for {FeIII4DyIII2},33 2.297(5)–2.504(4) Å and 100.43(18)−110.3(2)° for 2, and 2.405(6)−2.508(5) Å and 100.49(18)−127.7(2)° for {FeIII4DyIII2}.39 These results indicate that the strength of the ferromagnetic interaction in these clusters is mainly related to the bond distances between DyIII and O atoms coordinated to FeIII ions.
| FeIII–DyIII SMM | U eff/K [Hdc ≠ 0, Oe] | τ 0/s | Ref. |
|---|---|---|---|
| Fe6IIIDy3III | 65.1 | 1.64 × 10−12 | 10 |
| Fe4IIIDy2III | 36.9 | 6.8 × 10−10 | 30 |
| Fe2IIIDy4III | 34 | 2.0 × 10−11 | 31 |
| Fe7IIIDy3III | 30.9 | 1.3 × 10−7 | 32 |
| Fe4IIIDy2III | 30.85 (1200) | 3.70 × 10−8 | 33 |
| Fe4IIIDy4III | 30.5 | 2.0 × 10−9 | 34 |
| Fe7IIIDy4III | 16.9 | 4.6 × 10−7 | 35 |
| Fe18IIIDy6III | 14.7 | 2.98 × 10−7 | 36 |
| Fe6IIIDy3III | 12.4 (2000) | 8.0 × 10−5 | 37 |
| FeIIIDyIII | 9.72 (1000) | 3.69 × 10−6 | 23 |
| Fe2IIIDy3III | 6.78 (1200) | 2.01 × 10−5 | 38 |
| Fe8IIIDy2III | 4.1 (600) | 4.90 × 10−5 | 17 |
| Fe4IIIDy2III | 0.44 | 1.60 × 10−6 | 39 |
| 2 | 17.76 | 7.93 × 10−8 | This work |
The dynamic magnetic behavior of clusters 3 and 4 was further investigated by conducting ac susceptibility measurements within the temperature range of 2–10 K. In the absence of an external magnetic field, frequency-dependent χ′′ signals were observed below 4 K (Fig. S9†), indicating the onset of the slow magnetization relaxation. However, no expected maximum peaks in χ′′ signals are indicative of a quantum tunneling relaxation of the magnetization (QTM) driven by inter-cluster dipolar interactions.40 The frequency dependence of χ′′ ac susceptibility for clusters 3 and 4 have been recorded in an external applied dc field from 1 to 4 kOe at 2 K (Fig. S10†), where the optimal field is observed at 2 kOe for 3 and 2.5 kOe for 4 (Fig. S11†). The temperature dependence of χ′ and χ′′ ac susceptibilities of 3 and 4 were measured under optimal 2 kOe and 2.5 kOe dc fields, respectively. Both the χ′ and χ′′ signals are frequency dependent, indicating the slow magnetic relaxation (Fig. S12†). As shown in Fig. 4a and b, the intensity of the χ′′ signals increased with decreasing temperature, but frequency-independent peaks almost appeared in the low-frequency range of 0.1–10 Hz for 3 and 4, which were probably induced by low-lying excited exchange states promoting the QTM,41 causing a direct relaxation process rather than thermal activation relaxation.42 Moreover, a small tail of peaks was observed in the high-frequency region of 100–1500 Hz revealing that secondary relaxation occurs.43 The Cole–Cole plots were fitted using the generalized Debye model28 and show pronounced asymmetric semicircle shapes (Fig. 4c and d). The distribution coefficient α values range from 0.26 to 0.60 for 3, indicating a broader distribution of relaxation times, while the smaller α values of 0.08–0.22 for 4 show a narrow relaxation time distribution. A minor second tail of peaks could also be observed in the Cole–Cole plots,44 thus supporting the presence of secondary relaxation pathways in the high-frequency region.42 This phenomenon is commonly found in lanthanide-based molecular magnets, which presumably originates from intermolecular interactions.45
Conclusions
In conclusion, we have synthesized and characterized five decanuclear 3d–4f clusters [Ln2Fe8] {Ln = Y (1)/Dy (2)/Ho (3)/Tb (4)/Gd (5)} featuring six face-sharing defective cubane cores. Cluster 2 exhibits SMM behavior under a zero dc field, giving an energy barrier of 17.76 K. Investigation of the performance of a series of FeIII–DyIII SMMs indicates that the energy barrier in these clusters is associated with the strength of the ferromagnetic interaction between FeIII and DyIII ions, while the strength of the ferromagnetic interaction in these clusters is mainly related to the bond distances between Dy3+ and O atoms coordinated to Fe3+ ions. Clusters 3 and 4 exhibit two similar relaxation processes under optimal 2 kOe and 2.5 kOe dc fields, respectively. Frequency-independent behavior was almost observed in the low-frequency region, implying that direct relaxation dominates, while the weak tail signal at higher frequencies may be ascribed to the presence of secondary relaxation pathways, which may be related to the intermolecularly driven relaxation process. The current work unravels the magneto-structural correlations in heterometallic lanthanide–iron coordination clusters and provides the opportunity to identify SMM behavior under a magnetic field.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grants 21971214 and 92161203).References
-
(a) S. M. J. Aubin, N. R. Dilley, L. Pardi, J. Krzystek, M. W. Wemple, L.-C. Brunel, M. B. Maple, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1998, 120, 4991–5004 Search PubMed
; (b) F. R. Renani and G. Kirczenow, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 121403 CrossRef
.
- R. Sessoli, H. L. Tsai, A. R. Schake, S. Wang, J. B. Vincent, K. Folting, D. Gatteschi, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1993, 115, 1804–1816 CrossRef CAS
.
-
(a) M. N. Leuenberger and D. Loss, Nature, 2001, 410, 789–793 CrossRef CAS PubMed
; (b) N. Roch, S. Florens, V. Bouchiat, W. Wernsdorfer and F. Balestro, Nature, 2008, 453, 633–637 CrossRef CAS PubMed
.
-
(a) S. Thiele, F. Balestro, R. Ballou, S. Klyatskaya, M. Ruben and W. Wernsdorfer, Science, 2014, 344, 1135–1138 CrossRef CAS PubMed
; (b) R. Sessoli, Nature, 2017, 543, 189–190 CrossRef CAS PubMed
.
-
(a) L. Bogani and W. Wernsdorfer, Nat. Mater., 2008, 7, 179–186 CrossRef CAS PubMed
; (b) M. Mannini, F. Pineider, P. Sainctavit, C. Danieli, E. Otero, C. Sciancalepore, A. M. Talarico, M.-A. Arrio, A. Cornia, D. Gatteschi and R. Sessoli, Nat. Mater., 2009, 8, 194–197 CrossRef CAS PubMed
.
-
(a) D. Gatteschi and R. Sessoli, Angew. Chem., Int. Ed., 2003, 42, 268–297 CrossRef CAS PubMed
; (b) T. Gupta, M. F. Beg and G. Rajaraman, Inorg. Chem., 2016, 55, 11201–11215 CrossRef CAS PubMed
.
-
(a) S.-D. Jiang, B.-W. Wang, G. Su, Z.-M. Wang and S. Gao, Angew. Chem., 2010, 122, 7610–7613 Search PubMed
; (b) J.-L. Liu, Y.-C. Chen, Y.-Z. Zheng, W.-Q. Lin, L. Ungur, W. Wernsdorfer, L. F. Chibotaru and M.-L. Tong, Chem. Sci., 2013, 4, 3310 RSC
; (c) D. N. Woodruff, R. E. P. Winpenny and R. A. Layfield, Chem. Rev., 2013, 113, 5110–5148 CrossRef CAS PubMed
; (d) Y. Huo, Y.-C. Chen, J.-L. Liu, J.-H. Jia, W.-B. Chen, S.-G. Wu and M.-L. Tong, Dalton Trans., 2017, 46, 16796–16801 RSC
; (e) J.-H. Jia, Q.-W. Li, Y.-C. Chen, J.-L. Liu and M.-L. Tong, Coord. Chem. Rev., 2019, 378, 365–381 CrossRef CAS
.
-
(a) M. K. Singh and G. Rajaraman, Inorg. Chem., 2019, 58, 3175–3188 CrossRef CAS PubMed
; (b) H.-L. Zhang, Y.-Q. Zhai, L. Qin, L. Ungur, H. Nojiri and Y.-Z. Zheng, Matter, 2020, 2, 1481–1493 CrossRef
.
- M. Ferbinteanu, T. Kajiwara, K.-Y. Choi, H. Nojiri, A. Nakamoto, N. Kojima, F. Cimpoesu, Y. Fujimura, S. Takaishi and M. Yamashita, J. Am. Chem. Soc., 2006, 128, 9008–9009 CrossRef CAS PubMed
.
- S. Schmidt, D. Prodius, V. Mereacre, G. E. Kostakis and A. K. Powell, Chem. Commun., 2013, 49, 1696–1698 Search PubMed
.
-
(a) L. R. Piquer and E. C. Sañudo, Dalton Trans., 2015, 44, 8771–8780 RSC
; (b) H.-S. Wang, Q.-Q. Long, Z.-B. Hu, L. Yue, F.-J. Yang, C.-L. Yin, Z.-Q. Pan, Y.-Q. Zhang and Y. Song, Dalton Trans., 2019, 48, 13472–13482 RSC
; (c) A. Dey, J. Acharya and V. Chandrasekhar, Chem. – Asian J., 2019, 14, 4433–4453 CrossRef CAS PubMed
; (d) S. G. Baca, J. van Leusen, M. Speldrich and P. Kögerler, Inorg. Chem. Front., 2016, 3, 1071–1075 RSC
.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. a. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS
.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3–8 CrossRef PubMed
.
-
(a) A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7–13 CrossRef CAS
; (b) A. L. Spek, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 9–18 Search PubMed
.
-
(a) N. C. Harden, M. A. Bolcar, W. Wernsdorfer, K. A. Abboud, W. E. Streib and G. Christou, Inorg. Chem., 2003, 42, 7067–7076 Search PubMed
; (b) J. Liu, C. Ma, H. Chen, M. Hu, H. Wen, H. Cui, X. Song and C. Chen, Dalton Trans., 2013, 42, 2423–2430 RSC
; (c) L. Sun, H. Chen, C. Ma and C. Chen, Inorg. Chem. Commun., 2017, 77, 77–79 CrossRef CAS
.
- M. N. Akhtar, M. A. AlDamen, M. Fitta, M. Shahid and A. M. Kirillov, Cryst. Growth Des., 2022, 22, 608–614 CrossRef CAS
.
- X. Zhao, Y. Wang, D. Bao, F. Zhang, W. Wu, F. Xu, Q. Zhang and Y. Li, Appl. Organomet. Chem., 2019, 33, e5222 CrossRef CAS
.
- A. Mondal, M. Raizada, P. K. Sahu and S. Konar, Inorg. Chem. Front., 2021, 8, 4625–4633 RSC
.
- G. Abbas, Y. Lan, V. Mereacre, W. Wernsdorfer, R. Clérac, G. Buth, M. T. Sougrati, F. Grandjean, G. J. Long, C. E. Anson and A. K. Powell, Inorg. Chem., 2009, 48, 9345–9355 CrossRef CAS PubMed
.
- X. He, W. Cheng, Q. Lin, Y. Dong and Y. Xu, Cryst. Growth Des., 2017, 17, 347–354 CrossRef CAS
.
- M. N. Akhtar, M. A. AlDamen, C. D. McMillen, A. Escuer and J. Mayans, Inorg. Chem., 2021, 60, 9302–9308 Search PubMed
.
- A. Baniodeh, Y. Lan, G. Novitchi, V. Mereacre, A. Sukhanov, M. Ferbinteanu, V. Voronkova, C. E. Anson and A. K. Powell, Dalton Trans., 2013, 42, 8926–8938 RSC
.
- A. Topor, D. Liu, C. Maxim, G. Novitchi, C. Train, Z. A. AlOthman, A. A. S. Al-Kahtani, L. Ungur, L. T. A. Ho, L. F. Chibotaru and M. Andruh, J. Mater. Chem. C, 2021, 9, 10912–10926 RSC
.
- D. Prodius, V. Mereacre, P. Singh, Y. Lan, S. Mameri, D. D. Johnson, W. Wernsdorfer, C. E. Anson and A. K. Powell, J. Mater. Chem. C, 2018, 6, 2862–2872 Search PubMed
.
- S. Chen, V. Mereacre, D. Prodius, G. E. Kostakis and A. K. Powell, Inorg. Chem., 2015, 54, 3218–3227 CrossRef CAS PubMed
.
- A. M. Ako, V. Mereacre, R. Clérac, I. J. Hewitt, Y. Lan, C. E. Anson and A. K. Powell, Dalton Trans., 2007, 45, 5245–5247 Search PubMed
.
- Y.-Z. Zheng, Y. Lan, C. E. Anson and A. K. Powell, Inorg. Chem., 2008, 47, 10813–10815 CrossRef CAS PubMed
.
- K. S. Cole and R. H. Cole, J. Chem. Phys., 1941, 9, 341–351 CrossRef CAS
.
- K.-Q. Hu, X. Jiang, S.-Q. Wu, C.-M. Liu, A.-L. Cui and H.-Z. Kou, Inorg. Chem., 2015, 54, 1206–1208 CrossRef CAS PubMed
.
- S. F. M. Schmidt, C. Koo, V. Mereacre, J. Park, D. W. Heermann, V. Kataev, C. E. Anson, D. Prodius, G. Novitchi, R. Klingeler and A. K. Powell, Inorg. Chem., 2017, 56, 4796–4806 CrossRef CAS PubMed
.
- C. D. Polyzou, A. Baniodeh, N. Magnani, V. Mereacre, N. Zill, C. E. Anson, S. P. Perlepes and A. K. Powell, RSC Adv., 2015, 5, 10763–10767 RSC
.
- G. Abbas, Y. Lan, V. Mereacre, W. Wernsdorfer, R. Clérac, G. Buth, M. T. Sougrati, F. Grandjean, G. J. Long, C. E. Anson and A. K. Powell, Inorg. Chem., 2009, 48, 9345–9355 CrossRef CAS PubMed
.
- M. N. Akhtar, V. Mereacre, G. Novitchi, J.-P. Tuchagues, C. E. Anson and A. K. Powell, Chem. – Eur. J., 2009, 15, 7278–7282 Search PubMed
.
- D. Schray, G. Abbas, Y. Lan, V. Mereacre, A. Sundt, J. Dreiser, O. Waldmann, G. E. Kostakis, C. E. Anson and A. K. Powell, Angew. Chem., Int. Ed., 2010, 49, 5185–5188 Search PubMed
.
- D. Prodius, V. Mereacre, P. Singh, Y. Lan, S. Mameri, D. D. Johnson, W. Wernsdorfer, C. E. Anson and A. K. Powell, J. Mater. Chem. C, 2018, 6, 2862–2872 Search PubMed
.
- H. Kaemmerer, A. Baniodeh, Y. Peng, E. Moreno-Pineda, M. Schulze, C. E. Anson, W. Wernsdorfer, J. Schnack and A. K. Powell, J. Am. Chem. Soc., 2020, 142, 14838–14842 CrossRef CAS PubMed
.
- I. A. Kühne, V. Mereacre, C. E. Anson and A. K. Powell, Chem. Commun., 2016, 52, 1021–1024 Search PubMed
.
- H. Li, W. Shi, Z. Niu, J.-M. Zhou, G. Xiong, L.-L. Li and P. Cheng, Dalton Trans., 2015, 44, 468–471 RSC
.
- F.-W. Zheng, H.-T. Chen, D.-J. Li, F.-J. Han and L. Yang, J. Cluster Sci., 2021, 32, 461–467 CrossRef CAS
.
-
(a) D. Grebenyuk, M. Zobel, M. Polentarutti, L. Ungur, M. Kendin, K. Zakharov, P. Degtyarenko, A. Vasiliev and D. Tsymbarenko, Inorg. Chem., 2021, 60, 8049–8061 CrossRef CAS PubMed
; (b) G. Brunet, F. Habib, I. Korobkov and M. Murugesu, Inorg. Chem., 2015, 54, 6195–6202 CrossRef CAS PubMed
.
-
(a) K. L. M. Harriman, J. J. Le Roy, L. Ungur, R. J. Holmberg, I. Korobkov and M. Murugesu, Chem. Sci., 2017, 8, 231–240 RSC
; (b) J. D. Rinehart, K. R. Meihaus and J. R. Long, J. Am. Chem. Soc., 2010, 132, 7572–7573 CrossRef CAS PubMed
.
- M. Li, H. Wu, Q. Wei, H. Ke, B. Yin, S. Zhang, X. Lv, G. Xie and S. Chen, Dalton Trans., 2018, 47, 9482–9491 RSC
.
- Y.-N. Guo, G.-F. Xu, P. Gamez, L. Zhao, S.-Y. Lin, R. Deng, J. Tang and H.-J. Zhang, J. Am. Chem. Soc., 2010, 132, 8538–8539 CrossRef CAS PubMed
.
-
(a) X.-L. Li, H. Li, D.-M. Chen, C. Wang, J. Wu, J. Tang, W. Shi and P. Cheng, Dalton Trans., 2015, 44, 20316–20320 RSC
; (b) H.-J. Lun, X.-J. Kong, L.-S. Long and L.-S. Zheng, Dalton Trans., 2020, 49, 2421–2425 RSC
.
-
(a) G. Cosquer, F. Pointillart, S. Golhen, O. Cador and L. Ouahab, Chem. – Eur. J., 2013, 19, 7895–7903 CrossRef CAS PubMed
; (b) M. Dolai, M. Ali, J. Titiš and R. Boča, Dalton Trans., 2015, 44, 13242–13249 RSC
; (c) K. R. Meihaus, J. D. Rinehart and J. R. Long, Inorg. Chem., 2011, 50, 8484–8489 CrossRef CAS PubMed
; (d) F. Habib and M. Murugesu, Chem. Soc. Rev., 2013, 42, 3278–3288 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, tables and additional figures. CCDC 2195974, 2195975 and 2314827–2314829. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3dt03832g |
| This journal is © The Royal Society of Chemistry 2024 |