Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Retracted Article: Synthesis of a chiral dinuclear Cu(II)-benzothiazolamine complex: evidence of cuprophilic interaction in its structure and exploration of its electrochemical properties and catalytic performance†
O. Stephen
Ojo
 *,
Halilu
Sale
,
Mark D.
Symes
*,
Halilu
Sale
,
Mark D.
Symes
 and
Claire
Wilson
and
Claire
Wilson

WestCHEM, School of Chemistry, The Joseph Black Building, University of Glasgow, Glasgow, G12 8QQ, UK. E-mail: Oluwarotimi.ojo@glasgow.ac.uk
First published on 19th October 2023
Abstract
The synthesis of a chiral dinuclear [Cu(OAc)2(L1)]2 complex (A) and its analogues Cu(OAc)2(L1)2 (B), Cu(OAc)2(L1)PPh3 (C), CuBr(L1)PPh3 (D), and Cu(OAc)2(L2) (E) is described. The X-ray structure of A reveals a cuprophilic interaction (2.65 Å) and shows that L1 behaves as a monodentate ligand. The stereogenic centre in L1 aligns the NH group to form non-covalent interactions with the paddle-wheel acetate groups at variable distances (2.4–2.5 Å and 2.2–2.7 Å). Thermogravimetric analysis confirmed our hypothesis that two equivalents of L1 (B) or a combination of L1 and PPh3 (C) would disrupt the cuprophilic interaction. All complexes, except D, showed irreversible redox waves by cyclic voltammetry. Complexes C and E have lower oxidative peaks (at 10 V s−1) than complex A between +0.40 and +0.60 V. This highlights the influence of ligand(s) on the redox behaviour of Cu(II) complexes. The significance of this electrochemical behaviour was evident in the Chan–Lam (CL) coupling reaction, where 2.5 mol% of A successfully facilitated the formation of a C–N bond. This study showcased the structure, thermal stability, electrochemical properties and catalytic performance of a chiral dinuclear copper(II)-benzothiazolamine complex.
Introduction
Benzothiazole is a privileged heterocyclic scaffold that is less explored as a ligand in transition-metal catalysis,1a–c whereas similar motifs, such as benzimidazole and benzoxazole (Fig. 1A), have been studied extensively as ligands for the syntheses of metal complexes. For example, Pt(II) complexed with benzimidazole derivatives,2a benzoxazole-based ligands complexed with 3d-metal ions,2b and Cu(I) complexes constructed with different N-heterocyclic benzoxazole ligands2c have been reported. Benzothiazolamines (nitrogen at the 2-position) are medically important. They are present as core motifs in pharmaceutical drugs such as anti-HIV 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a and antibacterial 2
3a and antibacterial 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3b agents (Fig. 1B). Fluorinated benzothiazole derivatives have been investigated as PET imaging agents for breast cancer4a and as potential tracers for β-amyloid plaques in Alzheimer's disease.4b Crucially, benzothiazole derivatives complexed with transition metals have been shown to possess biological activities such as antimicrobial activity,5a antitumour activity,5b inhibition of enzymes,5c and in the treatment of Alzheimer's disease.5d A previous study1c has explored the synthesis and characterisation of palladium(II) complexed with achiral N-(benzothiazol-2-yl)benzamide 3 (Fig. 1D), which required the use of 1,2-bis(diphenylphosphino)ethane as a co-ligand. Herein, we report the synthesis and characterisation of a dinuclear paddle wheel copper(II) complex bearing (S)-L1 as a ligand (Fig. 1D). The research interest in paddle wheel dinuclear Cu(II) complexes has grown exponentially in recent years.6a,b This is due to their tunable redox chemistry, biological relevance and their striking structural features, which make them attractive as building blocks for supramolecular metal–organic frameworks (SMOFs).6c–g However, none of these examples possessed a chiral N-ancillary ligand. In addition to aurophility (AuI–AuI)7a and argentophilicity (AgI–AgI),7b cuprophilicity has been used to describe a closed-shell d10–d10 CuI–CuI interaction7c–e but not a d9–d9 CuII–CuII interaction. Cuprophilic interaction is considered to be present if the Cu–Cu separation is shorter than the sum of the van der Waals radii of two Cu atoms (2.80 Å).7f Ligands have been shown to support dinuclear copper complexes with Cu–Cu distances ranging from 2.5 to 4.0 Å.8a,b Interestingly, ligands9a–d complexed with copper(II) have rarely been used in CL reactions, and none of the reported examples are nitrogen-chelating monodentate chiral ligands.9e
3b agents (Fig. 1B). Fluorinated benzothiazole derivatives have been investigated as PET imaging agents for breast cancer4a and as potential tracers for β-amyloid plaques in Alzheimer's disease.4b Crucially, benzothiazole derivatives complexed with transition metals have been shown to possess biological activities such as antimicrobial activity,5a antitumour activity,5b inhibition of enzymes,5c and in the treatment of Alzheimer's disease.5d A previous study1c has explored the synthesis and characterisation of palladium(II) complexed with achiral N-(benzothiazol-2-yl)benzamide 3 (Fig. 1D), which required the use of 1,2-bis(diphenylphosphino)ethane as a co-ligand. Herein, we report the synthesis and characterisation of a dinuclear paddle wheel copper(II) complex bearing (S)-L1 as a ligand (Fig. 1D). The research interest in paddle wheel dinuclear Cu(II) complexes has grown exponentially in recent years.6a,b This is due to their tunable redox chemistry, biological relevance and their striking structural features, which make them attractive as building blocks for supramolecular metal–organic frameworks (SMOFs).6c–g However, none of these examples possessed a chiral N-ancillary ligand. In addition to aurophility (AuI–AuI)7a and argentophilicity (AgI–AgI),7b cuprophilicity has been used to describe a closed-shell d10–d10 CuI–CuI interaction7c–e but not a d9–d9 CuII–CuII interaction. Cuprophilic interaction is considered to be present if the Cu–Cu separation is shorter than the sum of the van der Waals radii of two Cu atoms (2.80 Å).7f Ligands have been shown to support dinuclear copper complexes with Cu–Cu distances ranging from 2.5 to 4.0 Å.8a,b Interestingly, ligands9a–d complexed with copper(II) have rarely been used in CL reactions, and none of the reported examples are nitrogen-chelating monodentate chiral ligands.9e
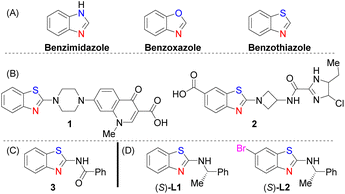 | ||
| Fig. 1 (A) Heterocyclic motifs for ligand design; (B) medically relevant benzothiazole derivatives 1 and 2; (C) achiral 3 and (D) chiral (S)-L1 and (S)-L2. | ||
The C–N bond formation via cross-coupling (transition metal catalysed) can be achieved using Buchwald–Hartwig (palladium),10a,b Ullmann-type (copper)10c or CL (copper)10d,e reaction conditions. Although these methods are complementary, the key difference is that aryl boronic acids are employed in CL reactions (Scheme 1) as one of the coupling partners and most reported examples required high loadings of copper (20–100%) to promote the reaction. Although a firm mechanistic understanding of CL reactions has been established, the roles of oxidant and CuI intermediate are poorly understood.11a,b
Results and discussion
Synthesis and structure
The synthesis of (S)-L1 was achieved using commercially available precursors (S)-1-phenylethylamine and 2-chlorobenzothiazole (Scheme 2).11c This method is column chromatography free, and it avoids the use of carbon disulfide (CS2) or thiols.11d,e The treatment of (S)-L1 with N-bromosuccinimide in dichloromethane (DCM) at room temperature (RT) for 12 h provided (S)-L2.11f | ||
| Scheme 2 Syntheses of (S)-L1, (S)-L2 and the Moiras complex (S)-A. For the synthesis of complexes B–E, see Scheme S1.† | ||
Moiras complex A was generated by mixing (S)-L1 with Cu(OAc)2, in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in tetrahydrofuran (THF) and methanol (MeOH) at 65 °C for 30 minutes. Its dinuclear and paddle wheel structure was confirmed by X-ray crystallography (Fig. 2). Also, (S)-L1 was shown to act as a monodentate ligand, chelated to copper via the endocyclic nitrogen (N1 or N3). The initial hypothesis expected N2 (or N4) also to coordinate with copper, but this postulated interaction did not occur. This is probably due to the bond angles of N1–C7–N2 or N3–C22–N4, which are 124.5° and 124.1°, respectively.
1, in tetrahydrofuran (THF) and methanol (MeOH) at 65 °C for 30 minutes. Its dinuclear and paddle wheel structure was confirmed by X-ray crystallography (Fig. 2). Also, (S)-L1 was shown to act as a monodentate ligand, chelated to copper via the endocyclic nitrogen (N1 or N3). The initial hypothesis expected N2 (or N4) also to coordinate with copper, but this postulated interaction did not occur. This is probably due to the bond angles of N1–C7–N2 or N3–C22–N4, which are 124.5° and 124.1°, respectively.
 | ||
| Fig. 2 X-ray structure of the Moiras complex (S)-A. Atomic displacement ellipsoids for non-H atoms drawn at the 50% probability level. | ||
The cuprophilic interaction between d9–d9 CuII–CuII in A is 2.65 Å, which is shorter than the sum of the van der Waals radii of two copper atoms (2.80 Å). The van der Waals radius for copper is 1.40 Å (Fig. 3 and S4†). Although complex A is C2-symmetrical, the N1–Cu2 and N3–Cu1 distances are relatively the same, being 2.17 and 2.18 Å, respectively. However, the distance of the intramolecular non-covalent interactions (O⋯H) between the paddle wheel acetate oxygen atoms and NH (next to the stereogenic centre) of L1 varied. The respective distances between O1 and O3 with H2 (in L1 on the right-hand side of the complex) are 2.4 Å and 2.5 Å, whereas the respective distances between O6 and O7 with H4 (in L1 on the left-hand side of the complex) are 2.2 Å and 2.7 Å (Fig. 3).
 | ||
| Fig. 3 X-ray structure of the Moiras complex (S)-A showing the van der Waals (vdw) radius of each atom (vdw radius for Cu = 1.40 Å) using PyMOL. | ||
With the knowledge of the behaviour of (S)-L1 as a ligand and the nature (paddle wheel, cuprophilic interaction and intramolecular hydrogen bonding) of the structure of complex A, we investigated the possibility of chelating two monodentate ligands to one copper atom, i.e., using two equivalents of L1 or a combination of one equivalent of L1 and triphenylphosphine (PPh3) each with one equivalent of Cu(OAc)2 (Scheme S1†). This endeavour generated complexes B and C. We also explored copper in the +1 oxidation state by mixing copper bromide dimethyl sulfoxide (CuBr·DMS) with one equivalent of L1 and one equivalent of PPh3 to generate complex D, which should provide a comparison with complex C (i.e., CuIvs. CuII). To understand the influence of halogen on the ligand and/or paddle wheel complexes, we generated complex E using one equivalent of (S)-L2 and one equivalent of Cu(OAc)2.
Thermal properties of complexes A–C and E
The influence of intra- or intermolecular non-covalent interactions on the boiling point of compounds has been well studied and established. The stronger the interaction the higher the boiling point. Herein, we conducted thermogravimetric analysis (TGA) on complex A to elucidate the influence of non-covalent interactions (cuprophilic and H-bond interactions) on its thermal stability. At 200 °C, A lost just 25% of its weight.In comparison, complex B has a lower thermal stability than A (Fig. 4). This confirmed our hypothesis that two equivalents of L1 would disrupt the non-covalent interactions observed in A. This result also highlights the influence of these interactions on the thermal properties of an organometallic complex. Overall complex C has higher thermal stability than A, possibly due to the presence of PPh3. However, A lost just 5% of its weight at 173 °C, whereas C lost 5% of its weight at a lower temperature (161 °C).
 | ||
| Fig. 4 TGA analysis. Comparison of the thermal properties of complexes A (green), B (red), and C (blue). For DSC analysis, see the ESI.† | ||
Electrochemical properties of complexes A–E
The electrochemical behaviour of complexes A–E was investigated using cyclic voltammetry (CV). The measurements were conducted within a potential range of +0.75 to −0.25 (V vs. SCE), employing different scan rates starting from 0.1 V s−1 to 10 V s−1. As expected, the anodic and cathodic currents generally increased with an increase in scan rate from 0.1 V s−1 to 10 V s−1 and there was a cathodic shift in the reduction peak positions and a slight anodic shift in the oxidation peak positions with an increase in scan rates. Complexes A, B, C and E displayed an irreversible process at approximately +0.5 V vs. SCE, which did not appear to become reversible as the scan rate increased.Previous electrochemical study has shown that the redox reaction of the CuII–CuIII couple was an irreversible process.12 Within the context of a copper-catalysed organic reaction catalytic cycle, this electrochemical behaviour displayed by complex A (B, C or E) would suggest a facile and irreversible oxidative addition. The oxidation of CuII to CuIII has been invoked and accepted as critical in the early step of the CL reaction mechanism,11a,b but the fate of CuIII afterwards (i.e., CuIII to CuI) is still debatable.9e Therefore, we explored the electrochemical behaviour of complex D (CuI) and compared it to that of complex C (CuII). Complex D possesses a fully reversible redox wave at +0.4 V vs. SCE and has a higher oxidative peak potential than complex C (Fig. 5). At 10 V s−1 scan rate, complex E has a lower oxidative peak potential than complex A from the range of +0.40 to +0.60 V (vs. SCE) (L1vs.L2), whereas the oxidative potentials of A and B are more similar. Interestingly, complex B has a slightly higher anodic potential than complex C at +0.45 V vs. SCE (L1vs. PPh3). These results demonstrate the influence of ligands on the electrochemical behaviour of CuII complexes.
 | ||
| Fig. 5 The overlay of cyclic voltammograms of complexes A–E at 10 V s−1. For the cyclic voltammogram of each complex at different scan rates, see Fig. S5–9.† | ||
Catalytic performance of complex A in the Chan–Lam reaction
The knowledge of the structure, thermal properties and electrochemical behaviour of complex A justified the exploration of its catalytic activity in the CL reaction. Critically, this study was carried out in a manner that should explain the roles of an oxidant, CuI (using complex D), and a base. Contentiously, a role for CuIV as a putative intermediate in the proposed CL reaction mechanism was considered.13a–c Notably, CuIII complexes were once deemed highly reactive, non-existent, and unstable. Now, there are numerous reports of isolable, crystallographically characterised, and even chemically inert complexes formally bearing CuIII centers.14a–e Initial studies tested the use of 2.5 mol% Cu(OAc)2 and conducted the reaction without complex A (Table 1, entries 1 and 2). The latter stage of the previously reported CL reaction mechanism11a suggested that oxygen (in air) acts as an oxidant that converts CuI into CuII.| Entry | R | Deviation from the reaction conditions | Product | Yieldc (%) |
|---|---|---|---|---|
| a Reaction conditions: 4 (1.57 mmol, 1.0 eq.), 5a–e (2.35 mmol, 1.5 eq.), pyridine (1.57 mmol, 1.0 eq.), PhMe (5 mL), the Moiras complex A (0.039 mmol, 0.025 eq.), with or without air, and room temperature (RT). b The reaction mixture was evacuated under vacuum and backfilled with N2 twice. c Isolated yields. | ||||
| 1 | 3-Me | Using Cu(OAc)2 | 6a | 10 |
| 2 | 3-Me | Without complex A | 6a | — |
| 3 | 3-Me | Using complex D | 6a | — |
| 4 | 3-Me | Without pyridine | 6a | — |
| 5b | 3-Me | Under N2 atmosphere | 6a | — |
| 6 | 3-Me | 1-Methylpyrrolidine as solvent | 6a | 12 |
| 7 | 3-Me | Pyridine as solvent | 6a | 8 |
| 8 | 3-Me | THF as solvent | 6a | 50 |
| 9 | 3-Me | DCM as solvent | 6a | 94 |
| 10 | 3-Me | None | 6b | 92 |
| 11 | 3-F | None | 6b | 90 |
| 12 | 4-F | None | 6c | 87 |
| 13 | 4-OMe | None | 6d | 85 |
| 14 | Napthyl | None | 6e | 90 |
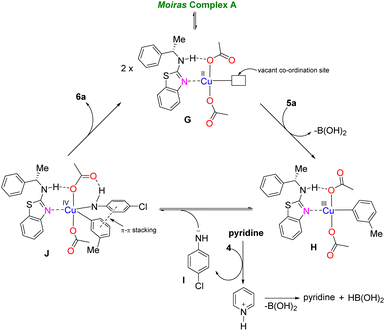
Therefore, entry 3 investigated the catalytic ability of CuI in the CL reaction in air, but it was ineffective. Entry 4 showcased the important role of a base (pyridine) towards the feasibility of the reaction – presumably, it helped generate anionic intermediate I (Scheme 3). Entry 5 obviates the relevance of, or the need for, an oxidant in this reaction. Entries 6 and 7 suggest that nitrogen-containing solvents can displace L1 from copper; hence, the yields of both reactions were like that of entry 1. Furthermore, entry 1 vs. entry 10 showcased the influence and importance of the ligand. A moderate yield was obtained if THF was used as solvent (entry 8), but DCM (entry 9) performed well like PhMe (entry 10; see Fig. S1–3† for the behaviour of complex A in different solvents). Notably, the reaction proceeded well with aromatic amine 4 and aryl boronic acids 5a–e (entries 10–14). However, we obtained homo-coupling products with aliphatic amines. Literature reports have shown that aryl boronic acids can be coupled with aliphatic amines as well as N-containing heterocycles.15 Presumably, the non-covalent interactions (π–π stacking) in the highly ordered ternary putative complex J (Scheme 3) are necessary for the C–N bond formation. Based on the electrochemical data (and using alkyl amines), G to H is irreversible, but H to J will likely be reversible due to the entropy penalty. In comparison, a prior convoluted mechanism11a proposed that the copper acetate dimer was deprotonated to the mononuclear square planar CuII catalyst, followed by Lewis-pairing, and then transmetallation and subsequent disproportionation to generate CuIII. This CuIII would then undergo reductive elimination to generate the product, as well as CuI species. The intermediate CuII species may then be regenerated under oxidative conditions from CuI. Overall, our work demonstrated the catalytic performance of a paddle wheel Cu(II) bearing a chiral benzothiazolamine ligand (complex A) in the Chan–Lam (CL) coupling reaction, which was driven by non-covalent interactions via a putative CuIV intermediate.
Conclusions
We have synthesised a novel paddle wheel Cu(II) complex A bearing a chiral benzothiazolamine monodentate ligand (L1). The single-crystal X-ray crystallography revealed a cuprophilic interaction between the two copper atoms at a distance (2.65 Å) lower than the sum of the van der Waals radii of two Cu atoms (2.80 Å). Within this complex four hydrogen bonds between NH (of L1) and oxygen atoms of the acetate group also exist, with varying distances. We investigated the influence of different ligands (L1vs. L2vs. PPh3) and the number of ligands (combination of L1 and PPh3) on the thermal properties and electrochemical behaviour of CuII complexes. The hydrogen-bond interaction within complex A and the π–π interaction between the aryl moieties of the two coupling partners on CuIV facilitated the formation of a C–N bond.Data availability
All experimental procedures, characterisation data, and NMR spectra are available in the ESI.†Author contributions
O. Stephen Ojo: conceptualisation, methodology, writing – original and final draft, data curation for all sections, and synthesis and characterisation of L1, L2, and complexes A–E. NMR and infra-red spectroscopy results and melting points comply with the ESI.† Halilu Sale: studied the electrochemical properties of complexes A–E and compiled their data. Mark D. Symes: reviewed, edited, and supervised the electrochemical study. Claire Wilson: X-ray crystallography analysis and data refinement.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the School of Chemistry, University of Glasgow is gratefully acknowledged. Thanks to Andrew Monaghan for TGA and DSC analysis of complexes A–C and E. Special thanks to Guilia Pellegrino for laboratory assistance and Professor Haralampos N. Moiras for helpful discussion. We have applied for a Creative Commons Attribution (CC BY) licence.References
- (a) L. Shadap, J. L. Tyagi, K. M. Poluri, S. Novikov, C.-W. T. Lo, Y. Mozharivskyj and M. R. Kollipara, Synthesis, and biological evaluation of some new class of benzothiazole–pyrazole ligands containing arene ruthenium, rhodium and iridium complexes, Transition Met. Chem., 2021, 46, 231–240 CrossRef CAS; (b) A. A. Irzoqi, F. A. Salman, Y. K. Alasadi and M. A. Alheety, Synthesis, and structural characterisation of Palladium(II) mixed ligand complexes of N–(benzothiazol-2-yl)benzamide and 1,2- bis(diphenylphosphino)ethane, Inorg. Chem., 2021, 60, 18854–18858 CrossRef CAS PubMed; (c) W. Xu, M. Arieno, H. Löw, K. Huang, X. Xie, T. Cruchter, Q. Ma, J. Xi, B. Huang, O. Wiest, L. Gong and E. Meggers, Metal-templated design: Enantioselective hydrogen-bond-driven catalysis requiring only parts-per-million catalyst loading, J. Am. Chem. Soc., 2016, 138, 8774–8780 CrossRef CAS.
- (a) H. Zhang, W. Wang, C. Liu, Z. Peng, C. Du and B. Zhang, Highly phosphorescent platinum(II) complexes supported by (2-(1H-benzimidazole)-phenyl)diphosphine oxide ancillary ligands, J. Mater. Chem. C, 2021, 9, 9627–9636 RSC; (b) O. Iasco, G. Novitchi, E. Jeanneau, J. B. Tommasino, N. Roques and D. Luneau, Versatile chemical transformations of benzoxazole based ligands on complexation with 3d-Metal Ions, Inorg. Chem., 2012, 51, 2588–2596 CrossRef CAS; (c) Y. Wu, X. Han, Y. Qu, K. Zhao, C. Wang, G. Huang and H. Wu, Two Cu(I) complexes constructed by different N-heterocyclic benzoxazole ligands: Syntheses, structures, and fluorescent properties, J. Mol. Struct., 2019, 1191, 95–100 CrossRef CAS.
- (a) S. Massari, D. Daele-mans, M. L. Barreca, A. Knezevich, S. Sabatini, V. Cecchetti, A. Marcello, C. Pannecouque and O. Tabarrini, J. Med. Chem., 2010, 53, 641 CrossRef CAS PubMed; (b) T. Soneda, H. Takeshita, Y. Kagoshima, Y. Yamamoto, T. Hosokawa, T. Konosu, N. Masuda, T. Uchida, I. Achiwa, J. Kuroyanagi, T. Fujisawa, A. Yokomizo and T. Noguchi, WO2009084614, 2009.
- (a) G.-Y. Li, D. D. Vaulina, J.-J. Li, O. S. Fedorova, H.-E. Wang, R.-S. Liu, R. N. Krasikova and C.-L. Chen, Synthesis, and biological evaluation of 2-(3,4-dimethoxyphenyl)- 6-(2-[18F]fluoroethoxy)benzothiazole ([18F]FEDBT) for PET imaging of breast cancer, Bioorg. Med. Chem. Lett., 2017, 27, 3460–3463 CrossRef CAS PubMed; (b) M.-Q. Zheng, D.-Z. Yin, J.-P. Qiao, L. Zhang and Y.-X. Wang, Syntheses, and evaluation of fluorinated benzothiazole anilines as potential tracers for β-amyloid plaques in Alzheimer's disease, J. Flour. Chem., 2008, 129, 210–216 CrossRef CAS.
- (a) J. Joseph and G. Boomadevi Janaki, Synthesis, structural characterisation, and biological studies of copper complexes with 2-aminobenzothiazole derivatives, J. Mol. Struct., 2014, 1063, 160–169 CrossRef CAS; (b) S. E. H. Etaiw, D. M. Abd El-Aziz, E. H. Abd El-Zaher and E. A. Ali, Synthesis, spectral, antimicrobial, and antitumor assessment of Schiff base derived from 2-aminobenzothiazole and its transition metal complexes, Spectrochim. Acta, Part A, 2011, 79, 1331–1337 CrossRef CAS; (c) X. Wang, K. Sarris, K. Kage, D. Zhang, S. P. Brown, T. Kolasa, C. Surowy, F. O. El Kouhen, S. W. Muchmore, J. D. Brioni and O. A. Stewart, Synthesis, and evaluation of benzothiazole-based analogues as novel, potent, and selective fatty acid amide hydrolase inhibitors, J. Med. Chem., 2009, 52, 170–180 CrossRef CAS; (d) L. Hroch, O. Benek, P. Guest, L. Aitken, O. Soukup, J. Janockova, K. Musil, V. Dohnal, R. Dolezal, K. Kuca, T. K. Smith, F. Gunn-Moore and K. Musilek, Design, synthesis and in vitro evaluation of benzothiazole-based ureas as potential ABAD/17βHSD10 modulators for Alzheimer's disease treatment, Bioorg. Med. Chem. Lett., 2016, 26, 3675–3678 CrossRef CAS PubMed.
- (a) P. Edelsbacher, G. Redhammer and U. Monkowius, Copper(II) complexes bearing cyclobutanecarboxylate and pyridine ligands: a new series of dinuclear paddle-wheel complexes, Monatsh. Chem., 2020, 151, 543–547 CrossRef CAS; (b) M. Hakimi, H. Rezaei, K. Moeini, Z. Mardani, V. Eigner and M. Dušek, Formation of a copper-copper bond in coordination of a cyclotriphosphazene ligand toward Cu(II): Structural, spectral, and docking studies, J. Mol. Struct., 2020, 1207, 127804 CrossRef CAS; (c) F. Sánchez-Férez, J. Soldevila-Sanmartín, J. A. Ayllón, T. Calvet, M. Font-Bardia and J. Pons, Synthesis, and characterisation of three new Cu(II) paddle-wheel compounds with 1,3-benzodioxole-5-carboxylic acid, Polyhedron, 2019, 164, 64–73 CrossRef; (d) M. Iqbal, A. Karim, S. Ali, M. N. Tahir and M. Sohail, Synthesis, characterisation, structural elucidation, electrochemistry, DNA binding, micellization behaviour and antioxidant activity of the Cu(II) carboxylate complexes, Polyhedron, 2020, 178, 114310 CrossRef CAS; (e) J. Soldevila-Sanmartín, M. Sanchez-Sala, T. Calvet, M. Font-Bardia, J. A. Ayllón and J. Pons, [Cu(μ-MeCO2)2(4-Bzpy)]2 (4-Bzpy = 4-benzylpyridine): Study of the intermolecular C-HÖ¼ Ö¼ Ö¼O hydrogen bonds at two temperatures, J. Mol. Struct., 2018, 1171, 808–814 CrossRef; (f) M. Guerrero, J. A. Ayllón, T. Calvet, M. Font-Bardia and J. Pons, Preparation, spectroscopic and structural study of copper(II) complexes derived from bulky pyridine ligands, Polyhedron, 2017, 134, 107–113 CrossRef CAS; (g) M. Sanchez-Sala, J. Pons, A. Alvarez-Larena, L. Bayés-García, M. Font-Bardia and J. A. Ayllón, Cu(II) 4-phenoxybenzoate dimers and monomer coordinated by pyridines: Synthesis and crystal structures, Polyhedron, 2018, 151, 545–553 CrossRef CAS.
- (a) H. Schmidbaur and A. Schier, Aurophilic interactions as a subject of current research: an up-date, Chem. Soc. Rev., 2012, 41, 370–412 RSC; (b) H. Schmidbaur, Is Gold chemistry a topical field of study?, Angew. Chem., Int. Ed. Engl., 1976, 15, 728–740 CrossRef; (c) N. V. S. Harisomayajula, S. Makovetskyi and Y.-C. Tsai, Cuprophilic interactions in and between molecular entities, Chem. – Eur. J., 2019, 25, 8936–8954 CrossRef CAS PubMed; (d) J. M. Goodwin, P.-C. Chiang, M. Brynda, K. Penkina, M. M. Olmstead and T. E. Patten, Asymmetric dinuclear copper(I) complexes of bis-(2-(2-pyridyl)ethyl)-2-(N-toluenesulfonylamino)ethylamine with short copper-copper distances, Dalton Trans., 2007, 3086–3092 RSC; (e) M. Kato, T. Tanase and M. Mikuriya, Dinuclear copper(II) complexes with {Cu2(μ-hydroxo)bis(μ-carboxylato)}+ cores and their reactions with sugar phosphate esters: A substrate binding model of fructose-1,6-bisphosphatase, Inorg. Chem., 2006, 45(7), 2925–2941 CrossRef CAS PubMed; (f) G. van Koten, A view of organocopper compounds and cuprates, J. Organomet. Chem., 1990, 400, 283–301 CrossRef CAS.
- (a) T. C. Davenport and T. D. Tilley, Dinucleating naphthyridine-based ligand for assembly of bridged dicopper(I) centres: Three-centre two-electron bonding involving an acetonitrile donor, Angew. Chem., 2011, 123, 12413–12416 CrossRef; (b) C. He, J. L. DuBois, B. Hedman, K. O. Hodgson and S. J. Lippard, A short copper-copper distance in a (μ-1,2-peroxo)dicopper(II) Complex having a 1,8-Naphthyridine Unit as an additional bridge, Angew. Chem., Int. Ed., 2001, 40, 1484–1487 CrossRef CAS.
- (a) J. P. Collman, M. Zhong, C. Zhang and S. Costanzo, Catalytic activities of Cu(II) complexes with nitrogen-chelating bidentate ligands in the coupling of imidazoles with arylboronic acids, J. Org. Chem., 2001, 66, 7892–7897 CrossRef CAS; (b) B. Liu, B. Liu, Y. Zhou and W. Chen, Copper(II) hydroxide complexes of N-heterocyclic carbenes and catalytic oxidative amination of arylboronic acids, Organometallics, 2010, 29, 1457–1146 CrossRef CAS; (c) A. Gogoi, G. Sarmah, A. Dewan and U. Bora, Unique copper-salen complex: An efficient catalyst for N-arylation of anilines and imidazole at room temperature, Tetrahedron Lett., 2014, 55, 31–35 CrossRef CAS; (d) V. H. Duparc, G. L. Bano and F. Schaper, ACS Catal., 2018, 8, 7308–7325 CrossRef; (e) N. Akatyev, M. Il'in, M. Il'in Jr., S. Peregudova, A. Peregudov, A. Buyanovskaya, K. Kudryavtsev, A. Dubovik, V. Grinberg, V. Orlov, A. Pavlov, V. Novikov, I. Volkov and Y. Belokon, Chan-Evans-Lam C-N Coupling promoted by a dinuclear positively charged Cu(II) Complex. Catalytic performance and some evidence for the mechanism of CEL Reaction obviating Cu(III)/Cu(I) Catalytic Cycle, ChemCatChem, 2020, 12, 3010–3021 Search PubMed.
- (a) F. Paul, J. Patt and J. F. Hartwig, Palladium-catalysed formation of carbon-nitrogen bonds. Reaction intermediates and catalyst improvements in the hetero cross-coupling of aryl halides and tin amides, J. Am. Chem. Soc., 1994, 116, 5969–5970 CrossRef CAS; (b) A. S. Guram and S. L. Buchwald, Palladium-catalysed aromatic aminations with in-situ generated amino stannanes, J. Am. Chem. Soc., 1994, 116, 7901–7902 CrossRef CAS; (c) J. Hassan, M. Sévignon, C. Gozzi, E. Schulz and M. Lemaire, Aryl-aryl bond formation one century after the discovery of the Ullmann Reaction, Chem. Rev., 2002, 102, 1359–1469 CrossRef CAS PubMed; (d) P. Y. S. Lam, C. G. Clark, S. Saubern, J. Adams, M. P. Winters, D. M. T. Chan and A. Combs, New aryl/heteroaryl C-N bond cross-coupling reactions via arylboronic acid/cupric acetate arylation, Tetrahedron Lett., 1998, 39, 2941–2944 CrossRef CAS; (e) D. A. Evans, J. L. Katz and T. R. West, Synthesis of diaryl ethers through the copper-promoted arylation of phenols with arylboronic Acids. An expedient synthesis of thyroxine, Tetrahedron Lett., 1998, 39, 2937–2940 CrossRef CAS.
- (a) J. C. Vantourout, H. N. Miras, A. Isidro-Llobet, S. Sproules and A. J. B. Watson, Spectroscopic studies of the Chan–Lam amination: A mechanism-inspired solution to boronic ester reactivity, J. Am. Chem. Soc., 2017, 139, 4769–4779 CrossRef CAS PubMed; (b) A. E. King, T. C. Brunold and S. S. Stahl, Mechanistic study of copper-catalysed aerobic oxidative coupling of arylboronic esters and methanol: Insights into an organometallic oxidase reaction, J. Am. Chem. Soc., 2009, 131, 5044–5045 CrossRef CAS; (c) D. Ma, X. Lu, L. Shi, H. Zhang, Y. Jiang and X. Liu, Domino condensation/S-arylation/heterocyclisation reactions: Copper-catalysed three-component synthesis of 2-N-substituted benzothiazoles, Angew. Chem., 2011, 123, 1150–1153 CrossRef; (d) S. K. Rout, S. Guin, J. Nath and B. K. Patel, An “on-water” exploration of CuO nanoparticle catalysed synthesis of 2-aminobenzothiazoles, Green Chem., 2012, 14, 2491 RSC; (e) G. W. Stewart, C. A. Baxter, E. Cleator and F. J. Sheen, A mild and efficient one-pot synthesis of 2-aminated benzoxazoles and benzothiazoles, J. Org. Chem., 2009, 74, 3229–3231 CrossRef CAS; (f) R. A. Judge, A. Vasudevan, V. E. Scott, G. H. Simler, S. D. Pratt, M. T. Namovic, C. B. Putman, A. Aguirre, V. S. Stoll, M. Mamo, S. I. Swann, S. C. Cassar, C. R. Faltynek, K. L. Kage, J. M. Boyce-Rustay and A. D. Hobson, Design of aminobenzothiazole inhibitors of rho kinases 1 and 2 by using protein kinase A as a structure surrogate, ChemBioChem, 2018, 19, 613–621 CrossRef CAS PubMed.
- Z. Wu, Z. Zhang and L. Liu, Electrochemical studies of a Cu(II)-Cu(III) couple: Cyclic voltammetry and chronoamperometry in a strong alkaline medium and in the presence of periodate anions, Electrochim. Acta, 1997, 42, 2719–2723 CrossRef CAS.
- (a) G. Demazeau, S. Darracq and J. H. Choy, High Oxygen pressure and the stabilisation of a new mixed valence Cu(III)/Cu(IV), High Pressure Res., 2006, 12, 323–328 CrossRef; (b) S. Darracq, S. G. Kang, J. H. Choy and G. Demazeau, Stabilisation of the mixed valence Cu(III)/Cu(IV) in the perovskite lattice of La1-xSrxCuO3 under high oxygen pressure, J. Solid State Chem., 1995, 114, 88–94 CrossRef CAS; (c) O. V. Mikhailov and D. V. Chachkov, About possibility of stabilization of unusual copper(IV) oxidation state in complexes with porphyrazine and two fluorine ligands: Quantum-chemical design, Inorg. Chem. Commun., 2019, 106, 224–227 CrossRef CAS.
- (a) M. A. Willert-Porada, D. J. Burton and N. C. Baenziger, Synthesis and X-ray structure of bis(trifluoromethyl)(N,N-diethyldithiocarbamato)-copper; a remarkably stable Perfluoroalkylcopper(III) complex, J. Chem. Soc., Chem. Commun., 1989, 1633–1634 RSC; (b) J. Hanss and H.-J. Krüger, The first stable copper(III) complex containing aliphatic thiolates as ligands: Structural and spectroscopic evidence for CuII and CuIII ions in complexes with square-planar CuN2S2 coordination environments, Angew. Chem., Int. Ed. Engl., 1996, 35, 2827–2830 CrossRef CAS; (c) A. M. Romine, N. Nebra, A. I. Konovalov, E. Martin, J. Benet-Buchholz and V. V. Grushin, Angew. Chem., Int. Ed., 2015, 54, 2745–2749 CrossRef CAS; (d) S.-L. Zhang and W.-F. Bie, Isolation and characterization of copper(III) trifluoromethyl complexes and reactivity studies of aerobic trifluoromethylation of arylboronic acids, RSC Adv., 2016, 6, 70902–70906 RSC; (e) D. Naumann, T. Roy, K.-F. Tebbe and W. Crump, Synthesis, and structure of surprisingly stable Tetrakis(trifIuoromethyl)cuprate(III) salts, Angew. Chem., Int. Ed. Engl., 1993, 32, 1482–1483 CrossRef.
- (a) V. H. Duparc and F. Schaper, Sulfonato-diketimine Copper(II) complexes: Synthesis and application as catalysts in Chan–Evans–Lam couplings, Organometallics, 2017, 36, 3053–3060 CrossRef; (b) X. Jia and P. Peng, N,O-Bidentate ligand-tunable copper(II) complexes as a catalyst for Chan–Lam coupling reactions of arylboronic acids with 1H-imidazole derivatives, Org. Biomol. Chem., 2018, 16, 8984–8988 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2292157. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3dt02994h |
| This journal is © The Royal Society of Chemistry 2024 |