Highly porous boron nitride as a metal-free heterogeneous catalyst for cycloaddition of CO2 to epoxides†
Teppei
Miyamoto
a,
Atsushi
Takagaki
 *b,
Jun Tae
Song
*b,
Jun Tae
Song
 ac,
Motonori
Watanabe
ac,
Motonori
Watanabe
 ac and
Tatsumi
Ishihara
ac and
Tatsumi
Ishihara
 *ac
*ac
aDepartment of Applied Chemistry, Faculty of Engineering, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan. E-mail: ishihara@cstf.kyushu-u.ac.jp
bDivision of Materials Science and Chemical Engineering, Faculty of Engineering, Yokohama National University, 79-5 Tokiwadai, Hodogaya-ku, Yokohama 240-8501, Japan. E-mail: takagaki-atsushi-gw@ynu.ac.jp
cInternational Institute for Carbon-Neutral Energy Research (WPI-I2CNER), Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan
First published on 3rd October 2024
Abstract
Boron nitride has received much attention as an emerging heterogeneous catalyst. Porous boron nitride catalysts were synthesized using boric acid (B) and urea (U) at different molar ratios via a pyrolysis method and applied for cycloaddition of CO2 to epoxides as metal-free catalysts. The synthesized boron nitride samples had a turbostratic structure, and their porous properties, such as surface area, pore size distribution, and pore volume, largely depended on the molar ratio of B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U precursors. The sample synthesized at a molar ratio of B
U precursors. The sample synthesized at a molar ratio of B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 with the highest pore volumes among the samples prepared from boric acid and urea exhibited the highest activity for cycloaddition of CO2 to epoxides, epichlorohydrin and styrene oxide in the presence of tetrabutylammonium bromide (TBAB). There was a good correlation between the corresponding carbonate yield and the pore properties of the catalyst. The addition of melamine (M) during the synthesis greatly developed the porous structure, exceeding 1000 m2 g−1 surface area. The sample synthesized at a molar ratio of B
5 with the highest pore volumes among the samples prepared from boric acid and urea exhibited the highest activity for cycloaddition of CO2 to epoxides, epichlorohydrin and styrene oxide in the presence of tetrabutylammonium bromide (TBAB). There was a good correlation between the corresponding carbonate yield and the pore properties of the catalyst. The addition of melamine (M) during the synthesis greatly developed the porous structure, exceeding 1000 m2 g−1 surface area. The sample synthesized at a molar ratio of B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M
M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 having a large surface area of 1380 m2 g−1 with a high pore volume of 1.8 mL g−1 afforded a remarkable yield of 96% for the reaction of epichlorohydrin. The catalyst could be reused at least three times without a significant loss of activity. A cooperative reaction mechanism was proposed in which the hydroxyl groups of porous boron nitride catalysts as weak Brønsted acid sites polarize the oxygen atom of the epoxide, and the bromide ions of TBAB as Lewis base sites activate the carbon atom of the epoxide by the nucleophilic attack.
5 having a large surface area of 1380 m2 g−1 with a high pore volume of 1.8 mL g−1 afforded a remarkable yield of 96% for the reaction of epichlorohydrin. The catalyst could be reused at least three times without a significant loss of activity. A cooperative reaction mechanism was proposed in which the hydroxyl groups of porous boron nitride catalysts as weak Brønsted acid sites polarize the oxygen atom of the epoxide, and the bromide ions of TBAB as Lewis base sites activate the carbon atom of the epoxide by the nucleophilic attack.
Introduction
Since the discovery of the remarkable activity of boron nitride for oxidative dehydration of light alkanes,1–4 boron nitride has received much attention as a novel class of heterogeneous catalyst.5,6 Many efforts have been made to improve its catalytic activity and elucidate its reaction mechanisms, including studying its active sites.7–9 Apart from such a unique redox property of boron nitride, we disclosed that boron nitride functioned as a powerful solid acid–base bifunctional catalyst for a variety of base-catalyzed reactions, including the nitroaldol reaction, Knoevenagel condensation, and glucose–fructose isomerization.10,11 Two functional groups, amino and hydroxyl groups, which act as the Brønsted base and acid sites, respectively, were introduced through ball-milling treatment of hexagonal boron nitride with high crystallinity11,12 or spontaneously formed on the surface of porous boron nitride through synthesis via the pyrolysis method with boric acid and urea under NH3 flow at high temperatures.13–15 Despite its weak basicity and weak acidity, the boron nitride catalyst exhibited high activity for the above reactions, which is due to acid–base cooperativity. The presence of acid sites facilitates the activation of carbonyl compounds, resulting in the promotion of reactivity.Cycloaddition of CO2 with epoxides to cyclic carbonate is widely explored using heterogeneous catalysts and their homogeneous counterparts because unwanted CO2 is utilized as a carbon source. Two decades ago, solid base catalysts, including MgO and Mg–Al hydrotalcite, were reported to efficiently catalyze cycloaddition reactions.16,17 Lewis base sites of the catalysts, O2− for MgO, adsorb CO2, whereas Lewis acid sites, the metal cation on the surface, activate epoxide. The carbonate anion reacts with the carbon atom of the activated epoxide, resulting in the formation of cyclic carbonate. The reaction proceeds efficiently through the concerted action of the acid and base sites on the solid surface.
Another acid–base bifunctional mechanism is also applicable to the fixation of CO2 with epoxides. In ionic liquid-based systems, such as betaine-based salts,18 choline chloride/urea,19 and [{(CH2)3COOH}2im]Br,20 it has been widely reported that Lewis base sites consisting of halide anions open the epoxy ring via nucleophilic attack, whereas Brønsted acid sites, such as carboxylic acid and hydroxyl groups of ionic liquids, cooperatively promote the ring-opening reaction.
Recently, the cycloaddition of CO2 to epoxides has been studied using new homogeneous and heterogeneous catalysts. Pintus et al. fabricated a hybrid material in which the amino acid L-arginine was immobilized on graphene oxide and used for the CO2 cycloaddition reactions.21 The reaction proceeded well due to the ring-opening of the epoxide by the Brønsted acid sites of the graphene oxide and the iodide anion of tetrabutylammonium iodide (TBAI) as an additive, and the activation of CO2 by L-arginine. Li et al. immobilized an alkylol amine on mesoporous silica and used it for the CO2 cycloaddition reactions.22 They claimed that the adjacent hydroxyl and amino groups acted as hydrogen bond donors (HBDs) to activate the epoxide. Ren et al. used quaternary ammonium salts of polyoxotungstate derivatives for the CO2 cycloaddition to styrene oxide as an alternative to quaternary ammonium halides.23 Gao et al. synthesized heteroatom-containing zeolites as solid Lewis catalysts.24 Using KI as an additive at 1.5 MPa CO2, the Ti-beta zeolite catalyst was highly active in the reaction. Yu et al. carried out the CO2 cycloaddition reactions under mild conditions using a binary organocatalyst consisting of an HBD and an ammonium salt, in particular, 3-(aminomethyl)phenol and TBAI or TBAB.25
Here, we synthesized porous boron nitride by pyrolysis and applied it to the CO2 fixation reaction. Porous boron nitride has both Brønsted acid and Brønsted base sites, of which the reaction was found to be promoted by Brønsted acid in the present study. This contrasts with our previous work, in which we observed the effect of the promotion of base-catalyzed reactions, while in the present study, the acidity of the boron nitride is of primary importance.
Experimental
Catalyst preparation
The samples were synthesized using boric acid (Kishida, 99.5%), melamine (Wako, 99.0%), and urea (Wako, 99.0%). These starting materials were physically mixed using a mortar. The resulting precursor powder was transferred to an alumina boat and heated at 1273 K for 3 h in an NH3 flow (100 cm3 (NTP) min−1). The molar ratios of boric acid (B), melamine (M), and urea (U) were varied and synthesized at B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M
M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x (x = 4, 5) or B
x (x = 4, 5) or B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y (y = 3–9).
y (y = 3–9).
Characterization
The crystal structure of the catalysts was determined by X-ray diffraction (XRD) analysis. The diffractometer (RINT-2500HLR+, Rigaku) was operated with Cu Kα radiation generated at 40 kV and 80 mA. Scans were obtained at a speed of 5° min−1 with a step width of 0.05° for 2θ values from 10 to 80°. The structure and functional groups of the catalysts were examined by Fourier transform infrared (FTIR) spectroscopy measurements (FT/IR-6600, JASCO). For the IR measurement, samples were pressed into pellets with KBr. The porous properties, including surface areas, pore volumes and pore size distributions, were evaluated by nitrogen sorption measurements (BELSORP-max, Microtrac-BEL). The samples were pretreated by evacuation at 473 K for 2 h before the measurement. The surface area and the pore size distribution of the sample were determined by applying the Brunauer–Emmett–Teller (BET) method and the Barrett, Joyner, and Halenda (BJH) method, respectively. CO2 adsorption was also conducted at 298 K to evaluate the micropores in the samples. The surface morphologies of the samples were observed by scanning electron microscopy (SEM, JSM-7900F, JEOL), and the elemental analysis of the samples was investigated using energy dispersive X-ray spectroscopy (EDS, X-MaxN80, OXFORD).Cycloaddition of CO2 to epoxide
The reaction was conducted with the catalyst (50 or 100 mg) in 1 mL toluene solution containing epoxide (epichlorohydrin or styrene oxide, 10 or 20 mmol) and tetrabutylammonium bromide (TBAB, 16 or 39 μmol) under 0.5 MPa CO2 in a PTFE lined stainless steel reactor vessel (50 mL, TAR-SR50, Taiatsu Techno Corp.). The reaction was performed at 403 K for 18 h under stirring. Aliquots of the solution were taken by a syringe and analyzed by gas chromatography using a flame ionization detector (GC-FID; GC-2025, column Rxi-1 HT, Shimadzu).Results and discussion
Fig. 1 shows the XRD patterns of boron nitride samples prepared by applying a pyrolysis method using boric acid and urea at various molar ratios. For all samples, especially B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and 1
4, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, two broad peaks at 25° and 42° were observed, which are attributed to the (002) and (100) planes of h-BN, respectively.26Fig. 1 shows the XRD pattern of commercial BN, a highly crystalline h-BN. The samples synthesized via pyrolysis have clear diffraction peaks, but they are less intense and broad than commercial h-BN. These broad patterns indicate the formation of a turbostratic boron nitride structure. Many studies have been carried out to synthesize turbostratic boron nitride by applying the pyrolysis method using boric acid and nitrogen-containing compounds, such as urea, as starting materials, and in all cases, similar XRD patterns were obtained.13–15,26–30 The peaks weakened and broadened with an increase in the molar ratio of urea, indicating lower crystallinity. The broadness of the peak corresponds to the crystallite size, which is related to the thickness of the layers for the (002) reflection. The decrease in crystallinity is in good agreement with the results of a previous study.13
5, two broad peaks at 25° and 42° were observed, which are attributed to the (002) and (100) planes of h-BN, respectively.26Fig. 1 shows the XRD pattern of commercial BN, a highly crystalline h-BN. The samples synthesized via pyrolysis have clear diffraction peaks, but they are less intense and broad than commercial h-BN. These broad patterns indicate the formation of a turbostratic boron nitride structure. Many studies have been carried out to synthesize turbostratic boron nitride by applying the pyrolysis method using boric acid and nitrogen-containing compounds, such as urea, as starting materials, and in all cases, similar XRD patterns were obtained.13–15,26–30 The peaks weakened and broadened with an increase in the molar ratio of urea, indicating lower crystallinity. The broadness of the peak corresponds to the crystallite size, which is related to the thickness of the layers for the (002) reflection. The decrease in crystallinity is in good agreement with the results of a previous study.13
 | ||
Fig. 1 XRD patterns for boron nitride samples prepared from boric acid and urea at different molar ratios. B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y (y = 3–9). y (y = 3–9). | ||
Table 1 shows the surface area and pore volume of the samples. Fig. 2 depicts the porous structure of boron nitride samples prepared from boric acid and urea. Sample B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 had a moderate surface area of 243 m2 g−1 with a pore volume of 0.37 mL g−1. With an increase in the molar ratio of urea, both the surface area and pore volume increased approximately threefold, which was mostly due to the development of micropores observed in the increase in uptake at a low relative pressure. The highest surface area of 779 m2 g−1 was obtained for sample B
3 had a moderate surface area of 243 m2 g−1 with a pore volume of 0.37 mL g−1. With an increase in the molar ratio of urea, both the surface area and pore volume increased approximately threefold, which was mostly due to the development of micropores observed in the increase in uptake at a low relative pressure. The highest surface area of 779 m2 g−1 was obtained for sample B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 among the samples. The surface area and pore volume were roughly correlated, and the largest pore volume of 1.40 mL g−1 was observed for sample B
8 among the samples. The surface area and pore volume were roughly correlated, and the largest pore volume of 1.40 mL g−1 was observed for sample B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. The large pore volume for samples B
5. The large pore volume for samples B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, and 1
5, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 is due to the emergence of mesopores, as shown in Fig. 2(c), which was in good agreement with our previous study.13
6 is due to the emergence of mesopores, as shown in Fig. 2(c), which was in good agreement with our previous study.13
| Sample | S BET/m2 g−1 | Pore volume/mL g−1 | |
|---|---|---|---|
| Commercial BN | 6 | 0.04 | |
| Synthesized BN | B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
243 | 0.37 |
B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
593 | 0.97 | |
B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
669 | 1.40 | |
B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
632 | 0.94 | |
B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
521 | 0.62 | |
B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
779 | 0.76 | |
B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
408 | 0.51 | |
The catalytic activity of boron nitride samples prepared from boric acid and urea for the cycloaddition of CO2 to epoxide was evaluated using styrene oxide and epichlorohydrin as reactants. Fig. 3 shows the results of the corresponding product yield. The yield for the reaction of styrene oxide increased with the increase in the boric acid: urea molar ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 1
3 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5; thereafter, the activity decreased with a further increase in the urea ratio. Sample B
5; thereafter, the activity decreased with a further increase in the urea ratio. Sample B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 afforded the highest yield among the samples tested. The pore volume of the samples is also shown in Fig. 3(a), which is correlated with the tendency of the yield. A similar dependence was observed for the reaction of epichlorohydrin in which the sample B
5 afforded the highest yield among the samples tested. The pore volume of the samples is also shown in Fig. 3(a), which is correlated with the tendency of the yield. A similar dependence was observed for the reaction of epichlorohydrin in which the sample B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 exhibited the highest yield (Fig. 3(b)). The dependence of the rate of formation of chloroethylene carbonate on CO2 pressure and epichlorohydrin concentration was also investigated (Fig. S1†). It was found that the reaction order for CO2 pressure was almost zero, whereas for epichlorohydrin concentration the order was 1.3. These results indicate that CO2 is well adsorbed on the solid catalyst surface.
5 exhibited the highest yield (Fig. 3(b)). The dependence of the rate of formation of chloroethylene carbonate on CO2 pressure and epichlorohydrin concentration was also investigated (Fig. S1†). It was found that the reaction order for CO2 pressure was almost zero, whereas for epichlorohydrin concentration the order was 1.3. These results indicate that CO2 is well adsorbed on the solid catalyst surface.
A good relationship between the catalytic activity and pore volume over boron nitride catalyst has been observed for the nitroaldol reaction, a base-catalyzed reaction,13 indicating that all boron nitride samples had similar active sites within the pores. The correlation between the catalytic activity and the surface area was also plotted (Fig. S2†). Although a rough correlation was observed, it was different for samples with higher urea ratios during synthesis, especially for sample B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 with the highest surface area. This may be mainly due to the amount of micropores. As shown by the pore size distribution estimated from the nitrogen sorption isotherm (Fig. 2), samples with a high urea ratio during the synthesis have well-developed micropores, resulting in high surface areas. The reaction does not occur in very small pores, thus the activity was low in these samples, even if the surface areas were high. In addition, the pores of the synthesized boron nitride do not have regular pore sizes as in zeolites and mesoporous materials but are mostly micropores, and their distribution decreases monotonically as the pore size increases. They also have negligible macropores. Therefore, the pore volume is considered to be roughly comparable to the integrated distribution of micropores. Moreover, for very small pores where the reaction cannot proceed, the integrated value is small. Consequently, an apparently good correlation between the pore volume and the activity can be interpreted.
8 with the highest surface area. This may be mainly due to the amount of micropores. As shown by the pore size distribution estimated from the nitrogen sorption isotherm (Fig. 2), samples with a high urea ratio during the synthesis have well-developed micropores, resulting in high surface areas. The reaction does not occur in very small pores, thus the activity was low in these samples, even if the surface areas were high. In addition, the pores of the synthesized boron nitride do not have regular pore sizes as in zeolites and mesoporous materials but are mostly micropores, and their distribution decreases monotonically as the pore size increases. They also have negligible macropores. Therefore, the pore volume is considered to be roughly comparable to the integrated distribution of micropores. Moreover, for very small pores where the reaction cannot proceed, the integrated value is small. Consequently, an apparently good correlation between the pore volume and the activity can be interpreted.
The correlation between the activity and pore volume indicates that the development of a pore structure is a suitable approach for further enhancing the activity. Fig. 4a shows the XRD patterns for boron nitride synthesized from boric acid and urea with and without melamine (M). The samples prepared with melamine, B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M
M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, showed much broader diffraction peaks than the samples without melamine, B
5, showed much broader diffraction peaks than the samples without melamine, B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, indicating that samples B
5, indicating that samples B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M
M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 had lower crystallinity with a turbostratic structure. Fig. 4b shows the FTIR spectra of the samples. The characteristic absorption bands for boron nitride were found at 1383 and 802 cm−1. The former is attributed to B–N stretching vibration27 and the latter to B–N–B out-of-plane bending vibrations.31,32 All samples had broad absorption bands around 3400 cm−1 and 3200 cm−1, which are attributed to O–H stretching vibrations and N–H stretching vibrations, respectively.33–35
5 had lower crystallinity with a turbostratic structure. Fig. 4b shows the FTIR spectra of the samples. The characteristic absorption bands for boron nitride were found at 1383 and 802 cm−1. The former is attributed to B–N stretching vibration27 and the latter to B–N–B out-of-plane bending vibrations.31,32 All samples had broad absorption bands around 3400 cm−1 and 3200 cm−1, which are attributed to O–H stretching vibrations and N–H stretching vibrations, respectively.33–35
 | ||
| Fig. 4 XRD patterns (a) and FTIR spectra (b) for boron nitride samples prepared using boric acid and urea with and without melamine. | ||
Fig. 5 shows N2 sorption isotherms and pore size distributions of the samples. The BET surface areas, SBET, the surface areas of the micropore obtained using the t-plot method, Smicro, and the pore volumes of the samples are listed in Table 2. It is apparent that the samples prepared using urea and melamine, B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M
M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, had a significantly high surface area, exceeding 1000 m2 g−1, twice that of the samples prepared using urea. The use of two nitrogen-containing precursors for the synthesis of boron nitride is helpful in enhancing porosity.15,26 Marchesini et al. demonstrated an increase in the surface area using urea and melamine for the synthesis of porous boron nitride using the pyrolysis method, which is due to the different thermal decomposition temperatures of the two materials.26 These nitrogen-containing materials act not only as reactants with boric acid but also as porogens during the synthesis. The increase in the BET surface area is mainly related to the emergence of micropores, Smicro. Fig. 6 shows the CO2 sorption isotherms of boron nitride samples measured at 298 K to evaluate their micropores. The increase in CO2 adsorption is in good agreement with the development of micropores. Among the samples prepared, sample B
5, had a significantly high surface area, exceeding 1000 m2 g−1, twice that of the samples prepared using urea. The use of two nitrogen-containing precursors for the synthesis of boron nitride is helpful in enhancing porosity.15,26 Marchesini et al. demonstrated an increase in the surface area using urea and melamine for the synthesis of porous boron nitride using the pyrolysis method, which is due to the different thermal decomposition temperatures of the two materials.26 These nitrogen-containing materials act not only as reactants with boric acid but also as porogens during the synthesis. The increase in the BET surface area is mainly related to the emergence of micropores, Smicro. Fig. 6 shows the CO2 sorption isotherms of boron nitride samples measured at 298 K to evaluate their micropores. The increase in CO2 adsorption is in good agreement with the development of micropores. Among the samples prepared, sample B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M
M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 exhibited the highest surface area (1378 m2 g−1) with the highest pore volume (1.8 mL g−1). Table 2 also lists the elemental compositions of the samples determined by EDS. Note that in the case of the equipment used in this study, it is possible to quantify light elements, including boron.14 All samples had similar compositions with a slight amount of carbon and oxygen.
5 exhibited the highest surface area (1378 m2 g−1) with the highest pore volume (1.8 mL g−1). Table 2 also lists the elemental compositions of the samples determined by EDS. Note that in the case of the equipment used in this study, it is possible to quantify light elements, including boron.14 All samples had similar compositions with a slight amount of carbon and oxygen.
 | ||
| Fig. 5 N2 sorption isotherms (a) and pore size distributions (b) of boron nitride synthesized from boric acid and urea with and without melamine. | ||
| Sample | S BET/m2 g−1 | S micro/m2 g−1 | Pore volume/mL g−1 | Atomic conc/% | |||
|---|---|---|---|---|---|---|---|
| B | C | N | O | ||||
B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
593 | 427 | 0.96 | 50 | 3 | 45 | 2 |
B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
669 | 536 | 1.4 | 49 | 4 | 45 | 2 |
B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1111 | 840 | 0.94 | 48 | 7 | 43 | 2 |
B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
1378 | 1060 | 1.8 | 49 | 4 | 45 | 2 |
 | ||
| Fig. 6 CO2 sorption isotherms for boron nitride synthesized from boric acid and urea with and without melamine measured at 298 K. | ||
The morphologies of the samples were investigated using an SEM apparatus. Fig. 7 shows the SEM images with the same magnification. The samples prepared using boric acid and urea only, B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, had sheet-like structures with mesopores, while the samples synthesized using boric acid, urea and melamine, B
5, had sheet-like structures with mesopores, while the samples synthesized using boric acid, urea and melamine, B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M
M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, had a more porous surface structure. The results of N2 and CO2 sorption isotherms are in good agreement with the change in the morphology.
5, had a more porous surface structure. The results of N2 and CO2 sorption isotherms are in good agreement with the change in the morphology.
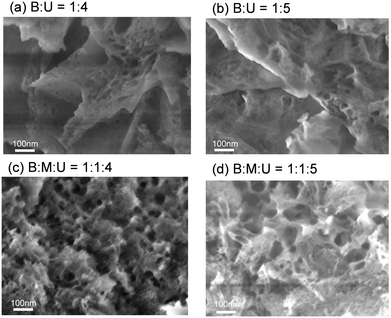 | ||
Fig. 7 SEM images of boron nitride synthesized from boric acid and urea with and without melamine: (a) B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, (b) B 4, (b) B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, (c) B 5, (c) B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and (d) B 4, and (d) B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. 5. | ||
The solid acid and base properties of the synthesized boron nitride samples were evaluated using color indicator reagents (Table 3). The use of color indicator reagents enables the determination of the Brønsted acid and base strength of the samples. Additionally, the acid or base amounts can be estimated by titration of adequate molecules (e.g. n-butylamine and benzoic acid) for colored samples. As previously reported by the authors, the porous boron nitride samples had weak basicity (H_ ≥ 7.2) and weak acidity (H0 ≤ +4.0).13 The solid acid amounts of samples B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 were 1.0 and 1.1 mmol g−1, respectively, while those of samples B
5 were 1.0 and 1.1 mmol g−1, respectively, while those of samples B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M
M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and B
4 and B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M
M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 were 1.4 and 1.8 mmol g−1, respectively. The base amounts were also increased for samples B
5 were 1.4 and 1.8 mmol g−1, respectively. The base amounts were also increased for samples B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M
M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. The increase in acid and base sites is due to the development of a porous structure. It is noteworthy that the molecular size of these color indicator reagents, including methyl red and bromocresol purple, is larger than that of epoxides and corresponding cyclic carbonate used in this study, indicating that the estimated acid and base sites are responsible for the reaction.
5. The increase in acid and base sites is due to the development of a porous structure. It is noteworthy that the molecular size of these color indicator reagents, including methyl red and bromocresol purple, is larger than that of epoxides and corresponding cyclic carbonate used in this study, indicating that the estimated acid and base sites are responsible for the reaction.
| Solid acid property | Solid base property | |||||
|---|---|---|---|---|---|---|
| Color indicator reagents | Acid amount/mmol g−1a | Color indicator reagents | Base amount/mmol g−1b | |||
| Phenylazonaphthyl amine (pKa = +4.0) | Methyl red (pKa = +4.8) | Bromothymol blue (pKa = +7.2) | Bromocresol purple (pKa = +6.3) | |||
| a H 0 ≤ +4.0. Determined by titration of n-butylamine for methyl red-adsorbed samples. b H _ ≥ 7.2. Determined by titration of benzoic acid for bromocresol purple-adsorbed samples. c +: Colored. −: Not colored. | ||||||
B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
−c | + | 1.0 | — | + | 0.08 |
B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
+ | + | 1.1 | + | + | 0.13 |
B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
— | + | 1.4 | — | + | 0.16 |
B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
— | + | 1.8 | — | + | 0.16 |
| MgO | — | — | 0 | + | + | 0.20 |
We have reported that the surface functional groups were hydroxyl and amino groups, with the amino groups actually being base sites that could interact when chloroform was adsorbed as an acidic probe molecule.14 Solid-state proton nuclear magnetic resonance (NMR) measurement showed that there were only two hydrogen species in boron nitride, and solid-state phosphorus NMR study using trimethylphosphine oxide as a probe molecule indicated that the boron nitride had weak acidity.10 In addition, the evaluation using color indicator reagents showed that the type of acid site was Brønsted acid. These results suggest that the solid acidity quantified corresponds to the hydroxyl group in boron nitride.
Graphitic carbon nitride g-C3N4, which exhibits semiconductor properties and is used in photocatalytic CO2 reduction reactions, is a compound similar to BN. Very recently, Li et al. demonstrated that acid modification using H3PO4 changed the bandgap of g-C3N4, resulting in an improvement of the photocatalytic activity.36 In contrast, the acidic hydroxyl groups in BN in the present study were naturally formed during synthesis, and BN had a very large bandgap, close to that of an insulator.
The reactivity results of the cycloaddition of CO2 to epichlorohydrin are listed in Table 4. A high yield for the corresponding cyclic carbonate was obtained for all boron nitride catalysts. Samples B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 afforded 76% and 90% yields, respectively (Table 4, entries 1 and 2). The improved activity was obtained for the samples prepared with melamine, B
5 afforded 76% and 90% yields, respectively (Table 4, entries 1 and 2). The improved activity was obtained for the samples prepared with melamine, B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M
M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, resulting in a higher carbonate yield (Table 4, entries 3 and 4). Sample B
5, resulting in a higher carbonate yield (Table 4, entries 3 and 4). Sample B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M
M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, which had the highest surface area and the highest acid amount, exhibited the highest carbonate yield (96%). MgO, as a comparison, showed lower activity under the same reaction conditions (Table 4, entry 5). Table 5 shows the results for the reaction with and without the addition of TBAB. As described, the coexistence of TBAB and boron nitride catalyst could afford the corresponding carbonate, resulting in a 23% product yield for a 1 h reaction (Table 5, entry 1). Without TBAB, the reaction hardly occurred (entry 2). Additionally, in the presence of TBAB without a boron nitride catalyst, no reaction proceeded (Table 5, entry 3). Thus, the addition of TBAB was necessary to produce the cyclic carbonate using a boron nitride catalyst.
5, which had the highest surface area and the highest acid amount, exhibited the highest carbonate yield (96%). MgO, as a comparison, showed lower activity under the same reaction conditions (Table 4, entry 5). Table 5 shows the results for the reaction with and without the addition of TBAB. As described, the coexistence of TBAB and boron nitride catalyst could afford the corresponding carbonate, resulting in a 23% product yield for a 1 h reaction (Table 5, entry 1). Without TBAB, the reaction hardly occurred (entry 2). Additionally, in the presence of TBAB without a boron nitride catalyst, no reaction proceeded (Table 5, entry 3). Thus, the addition of TBAB was necessary to produce the cyclic carbonate using a boron nitride catalyst.
| Entry | Catalyst | S BET/m2 g−1 | Acid amount/mmol g−1 | Acid density/μmol m−2 | Yield/% | TONb |
|---|---|---|---|---|---|---|
| a Reaction conditions: epichlorohydrin (10 mmol), TBAB (39 μmol), CO2 (0.5 MPa), catalyst (50 mg), toluene (1 mL), 403 K, and 18 h. b Turnover number. Calculated based on the amount of acid. | ||||||
| 1 | B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
593 | 1.0 | 1.69 | 76 | 152 |
| 2 | B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
669 | 1.1 | 1.64 | 90 | 164 |
| 3 | B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1111 | 1.4 | 1.26 | 87 | 124 |
| 4 | B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
1378 | 1.8 | 1.31 | 96 | 107 |
| 5 | MgO | 20 | — | — | 80 | — |
| Entry | Catalyst | Yield/% |
|---|---|---|
| a Reaction conditions: epichlorohydrin (10 mmol), toluene (1 mL), CO2 (0.5 MPa), catalyst (50 mg) and/or TBAB (39 μmol), 403 K, and 1 h. | ||
| 1 | B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 + TBAB 4 + TBAB |
23 |
| 2 | B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1 U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1.1 |
| 3 | TBAB | 0 |
According to the results, a reaction mechanism using a boron nitride catalyst in the presence of TBAB is proposed in Scheme 1. The Brønsted acidic hydroxyl groups of boron nitride catalyst polarize the oxygen atom of the epoxide, while the Lewis basic Br ions of TBAB activate the carbon atom by nucleophilic attack, resulting in the ring opening of the epoxide. Then, the negatively charged oxygen atom of the ring-opened epoxide activates CO2, followed by the formation of the corresponding cyclic carbonate. Our previous study explored the base-catalyzed reactions, including nitroaldol reaction and Knoevenagel condensation using ball-milled boron nitride catalysts as well as synthesized porous boron nitride via pyrolysis method.10,13 In these reactions, the boron nitride catalysts exhibited acid–base bifunctionality that promoted the reactivity. Brønsted base sites, namely amino groups on the surface functioned as the main active sites for those reactions, and Brønsted acid sites, hydroxyl groups, facilitated the activation of the carbonyl groups of the substrate.10,11,13 In contrast, the present study utilizes the acidic hydroxyl groups as the main active sites for the cycloaddition of CO2 to epoxide. Based on the acid amounts of the boron nitride catalyst, the acid density and turnover numbers (TON) were calculated (Table 4). The acid densities of the B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U samples were 1.69 and 1.64 μmol m−2 for B
U samples were 1.69 and 1.64 μmol m−2 for B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, respectively, indicating that the functional groups were uniformly distributed independently of the B
5, respectively, indicating that the functional groups were uniformly distributed independently of the B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U molar ratio. The same results were found for the B
U molar ratio. The same results were found for the B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M
M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U samples although these values were lower than those of the B
U samples although these values were lower than those of the B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U samples. The obtained TON values were 152 and 164 for the samples of B
U samples. The obtained TON values were 152 and 164 for the samples of B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, respectively. There is a correlation between the acid density and TON. Thus, in terms of turnover, sample B
5, respectively. There is a correlation between the acid density and TON. Thus, in terms of turnover, sample B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 was the most catalytically active among the samples tested although the highest yield was achieved for the sample B
5 was the most catalytically active among the samples tested although the highest yield was achieved for the sample B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M
M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.
5.
 | ||
| Scheme 1 Proposed reaction mechanism for cycloaddition of CO2 to epoxide using the boron nitride catalyst in the presence of TBAB. | ||
The reusability of the boron nitride catalyst was investigated. Fig. S3† shows the results of the product yields after the initial reaction and after the first and second recycling in the cycloaddition of CO2 to epichlorohydrin. The catalyst was recovered by centrifugation and washed with toluene, followed by drying. The catalyst could be recyclable at least three times without a significant loss of activity. XRD measurement confirmed that there is no apparent structural change in the catalyst after the reaction.
Conclusions
Porous boron nitride catalysts were synthesized using boric acid, urea, and melamine using the pyrolysis method. The activity of the boron nitride catalysts for the cycloaddition of CO2 to epoxide was well correlated with their porous properties. Adding melamine as precursors significantly increased the surface areas of the boron nitride materials, twice that of the samples using urea. The sample of B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M
M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U = 1
U = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 showed a remarkable surface area of 1378 m2 g−1, resulting in the highest product yield of 96% for the reaction of epichlorohydrin in the presence of TBAB. The Brønsted acid sites and hydroxyl groups on the surface of the catalyst were the main active sites to promote the activation of the epoxide.
5 showed a remarkable surface area of 1378 m2 g−1, resulting in the highest product yield of 96% for the reaction of epichlorohydrin in the presence of TBAB. The Brønsted acid sites and hydroxyl groups on the surface of the catalyst were the main active sites to promote the activation of the epoxide.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.Author contributions
AT: conceptualization; AT, TM and MW: investigation and methodology; JTS and MW: formal analysis; AT and TI: supervision; AT: funding acquisition; TM and AT: writing – original draft; AT, JTS, MW and TI: writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Grant-in-Aids for Challenging Research (Exploratory) (No. 21K18853) of JSPS, Japan and Yashima Environment Technology Foundation.Notes and references
- J. T. Grant, C. A. Carrero, F. Goeltl, J. Venegas, P. Muller, S. P. Burt, S. E. Specht, W. P. McDermott, A. Chieregato and I. Hermans, Science, 2016, 354, 1570 CrossRef CAS PubMed.
- L. Shi, D. Wang, W. Song, D. Shao, W.-P. Zhang and A.-H. Lu, ChemCatChem, 2017, 9, 1788 CrossRef CAS.
- R. Huang, B. Zhang, J. Wang, K.-H. Wu, W. Shi, Y. Zhang, Y. Liu, A. Zheng, R. Schlögl and D. S. Su, ChemCatChem, 2017, 9, 3293 CrossRef CAS.
- Y. Honda, A. Takagaki, R. Kikuchi and S. T. Oyama, Chem. Lett., 2018, 47, 1090 CrossRef CAS.
- J. M. Venegas, W. P. McDermott and I. Hermans, Acc. Chem. Res., 2018, 51, 2556 CrossRef CAS.
- L. Shi, Y. Wang, B. Yan, W. Song, D. Shao and A.-H. Lu, Chem. Commun., 2018, 54, 10936 RSC.
- A. M. Love, B. Thomas, S. E. Specht, M. P. Hanrahan, J. M. Venegas, S. P. Burt, J. T. Grant, M. C. Cendejas, W. P. McDermott, A. J. Rossini and I. Hermans, J. Am. Chem. Soc., 2019, 141, 182 CrossRef CAS.
- R. W. Dorn, L. O. Mark, I. Huang, M. C. Cendejas, Y. Xu, P. L. Gor'kov, W. Mao, F. Ibrahim, Z. Gan, I. Hermans and A. J. Rossini, J. Am. Chem. Soc., 2022, 144, 18766 CrossRef CAS PubMed.
- P. Han, R. Yan, Y. Wei, L. Li, J. Luo, Y. Pan, B. Wang, J. Lin, S. Wan, H. Xiong, Y. Wang and S. Wang, J. Am. Chem. Soc., 2023, 145, 10564 CrossRef CAS.
- S. Torii, K. Jimura, S. Hayashi, R. Kikuchi and A. Takagaki, J. Catal., 2017, 355, 176 CrossRef CAS.
- S. Namba, A. Takagaki, K. Jimura, S. Hayashi, R. Kikuchi and S. T. Oyama, Catal. Sci. Technol., 2019, 9, 302 RSC.
- A. Takagaki, Catal. Today, 2020, 352, 279 CrossRef CAS.
- S. Nakamura, A. Takagaki, M. Watanabe, K. Yamada, M. Yoshida and T. Ishihara, ChemCatChem, 2020, 12, 6033 CrossRef CAS.
- A. Takagaki, S. Nakamura, M. Watanabe, Y. Kim, J. T. Song, K. Jimura, K. Yamada, M. Yoshida, S. Hayashi and T. Ishihara, Appl. Catal., A, 2020, 608, 117843 CrossRef CAS.
- A. Takagaki, S. Nakamura, S. Ashimura, M. Yoshida, J. T. Song, M. Watanabe, S. Hayashi and T. Ishihara, Appl. Catal., A, 2022, 638, 118635 CrossRef CAS.
- M. Tu and R. J. Davis, J. Catal., 2001, 199, 85 CrossRef CAS.
- K. Yamaguchi, K. Ebitani, T. Yoshida, H. Yoshida and K. Kaneda, J. Am. Chem. Soc., 1999, 121, 4526 CrossRef CAS.
- Y. Zhou, S. Hu, X. Ma, S. Liang, T. Jiang and B. Han, J. Mol. Catal. A: Chem., 2008, 284, 52 CrossRef CAS.
- A. Zhu, T. Jiang, B. Han, J. Zhang, Y. Xie and X. Ma, Green Chem., 2007, 9, 169 RSC.
- J. Sun, L. Han, W. Cheng, J. Wang, X. Zhang and S. Zhang, ChemSusChem, 2011, 4, 502 CrossRef CAS PubMed.
- A. Pintus, S. Mantovani, A. Kovtun, G. Bertuzzi, M. Melucci and M. Bandini, Chem. – Eur. J., 2022, e202202440 Search PubMed.
- C. Li, W. Xiong, T. Zhao, F. Liu, H. Cai and P. Chen, Appl. Catal., B, 2023, 324, 122217 CrossRef CAS.
- J. Ren, A. Proust, F. Launay and R. Villanneau, Chem. Commun., 2024, 60, 4549 RSC.
- B. Gao, W. Li, Y. Chai, G. Wu and L. Li, ChemCatChem, 2024, e202401385 CrossRef.
- W.-W. Yu, X.-G. Meng, Z.-Y. Gan, W. Li, Y.-L. Zhang and J. Zhou, Catal. Sci. Technol. 10.1039/D4CY00639A.
- S. Marchesini, C. M. McGilvery, J. Bailey and C. Petit, ACS Nano, 2017, 11, 10003–10011 CrossRef CAS.
- X. Li, X. Wang, J. Zhang, N. Hanagata, X. Wang, Q. Weng, A. Ito, Y. Bando and D. Golberg, Nat. Commun., 2017, 8, 13936 CrossRef CAS PubMed.
- A. Nag, N. Raidongia, K. P. S. S. Hembram, R. Datta, U. V. Waghmare and C. N. R. Rao, ACS Nano, 2010, 4, 1539 CrossRef CAS PubMed.
- T. Jähnichen, J. Hojak, C. Bläker, C. Pasel, V. Mauer, V. Zittel, R. Denecke, D. Bathen and D. Enke, ACS Omega, 2022, 7, 33375 CrossRef PubMed.
- A. L'Hermitte, D. M. Dawson, P. Ferrer, K. Roy, G. Held, T. Tian, S. E. Ashbrook and C. Petit, J. Phys. Chem. C, 2021, 125, 27429 CrossRef.
- D. Liu, Z. Jiang, C. Zhu, K. Qian, Z. Wu and J. Xie, Dalton Trans., 2016, 45, 2505 RSC.
- J. Chen, J. Zhu, Z. Da, H. Xu, J. Yan, H. Ji, H. Shu and H. Li, Appl. Surf. Sci., 2014, 313, 1 CrossRef CAS.
- T. Sainsbury, A. Satti, P. May, Z. Wang, I. McGovern, Y. K. Gun'ko and J. Coleman, J. Am. Chem. Soc., 2012, 134, 18758 CrossRef CAS PubMed.
- C. Huang, C. Chen, X. Yie, W. Ye, J. Hu, C. Xu and X. Qiu, J. Mater. Chem. A, 2013, 1, 12191 Search PubMed.
- J. Li, Y. Huang, Z. Liu, J. Zhang, X. Liu, H. Luo, Y. Ma, X. Xu, Y. Lu, J. Lin, J. Zou and C. Tang, J. Mater. Chem. A, 2015, 3, 8185 RSC.
- Z. Li, J. Ao, Z. Wang, Z. Huang, Z. Xu, X. Wu, Z. Cheng and K. Lv, Sep. Purif. Technol., 2024, 338, 126577 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Reactivity and reusability of the catalyst. See DOI: https://doi.org/10.1039/d4cy01080a |
| This journal is © The Royal Society of Chemistry 2024 |


