Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Design of SrTiO3-based catalysts for photocatalytic CO2 reduction†
Bíborka
Boga
ab,
Nikolaos G.
Moustakas
a,
Yunyan
Han
ac,
Haijun
Jiao
 a,
Carsten
Kreyenschulte
a,
Carsten
Kreyenschulte
 a,
Pawel
Naliwajko
a,
Thi Thanh Hoa
Duong
a,
Shuoping
Ding
a,
Pawel
Naliwajko
a,
Thi Thanh Hoa
Duong
a,
Shuoping
Ding
 a,
Anh Binh
Ngo
a,
Abdo
Hezam
d,
Tim
Peppel
a,
Anh Binh
Ngo
a,
Abdo
Hezam
d,
Tim
Peppel
 a,
Vasile-Mircea
Cristea
a,
Vasile-Mircea
Cristea
 b,
Norbert
Steinfeldt
b,
Norbert
Steinfeldt
 *a and
Jennifer
Strunk
*a and
Jennifer
Strunk
 *ad
*ad
aLeibniz Institute for Catalysis e.V. (LIKAT), Albert Einstein St., 29.A, Rostock, 18059, Germany. E-mail: Norbert.Steinfeldt@catalysis.de
bFaculty of Chemistry and Chemical Engineering, Babes-Bolyai University, Arany Janos St, 11, Cluj-Napoca, 400028, Romania
cShaanxi Key Laboratory of Phytochemistry, College of Chemistry & Chemical Engineering, Baoji University of Arts and Sciences, Baoji 721013, China
dIndustrielle Chemie und Heterogene Katalyse, Technical University of Munich (TUM), Lichtenberg St, 4, Munich, 85745, Germany. E-mail: Jennifer.Strunk@tum.de
First published on 20th May 2024
Abstract
Herein, the preparation of SrTiO3-based catalysts (i.e., NiO/support, Au-support and Au–NiO/support, where the supports were SrTiO3 and SrTiO3–SrCO3) for photocatalytic CO2 reduction considering strategic design principles is presented. The samples were comprehensively analyzed via complementary methods, such as SEM-EDX, XRD, nitrogen sorption, XPS and UV-vis (DRS), in situ EPR and in situ DRIFTS. The CO2 photoreduction activity of the samples was assessed in a high-purity gas-phase photoreactor under batch conditions. The investigations highlighted that the reaction pathway (i.e., selective H2 and C2H6 production vs. CH4) can be influenced by the modification of the electronic properties (i.e., Fermi level alignment), the interaction between Au NPs and oxygen vacancies (i.e., in situ EPR) and the enhanced charge separation in the presence of SrCO3. The participation of the structural carbonates in the reaction in association with the functionality of the components is discussed.
Introduction
The photocatalytic conversion of CO2 into valuable fuels (CH4 or higher hydrocarbons) over metal oxide-based catalysts is a smart and promising solution for overcoming the problem of increasing atmospheric CO2 concentration (425 ppm1) and energy demand.2Perovskites, also called “chameleon CO2 photocatalysts”3 are very interesting for such applications owing to multiple reasons. Among them, SrTiO3 has received considerable attention in this field, e.g., Ti-rich and Sr(OH)2-decorated SrTiO3,4 boron-doped layered polyhedron SrTiO3,5 or SnS2-decorated 3DOM-SrTiO3.6 Their impressive photocatalytic performance was mainly attributed to its advantageous band structure, more precisely its conduction band (CB) minimum edge position. Recently, studies aiming at the study of self-doped SrTiO3−δ,7 “black SrTiO3”,8 and SrTiO3 with crystalline core/amorphous shell structure (i.e., SrTiO3@SrTiO3−x)9 were conducted for photocatalytic CO2 reduction applications.
Although heterostructures formed from metal oxides possess several promising features, the coupling of metal nanoparticles with metal oxides provides additional positive aspects for both catalytic and photocatalytic applications. In the latter case, both the material/type of the metal nanoparticle, and its morphology and distribution are critical factors. Considerable importance was given to noble metal (i.e., Au, Ru, Pt, Pd) catalysts on supports in photocatalytic CO2 reduction applications.10 Among these, supported Au-catalysts received remarkable attention (i.e., Au/TiO2,11,12 Au/ZnO13) due to their enhanced visible light activity, mostly explained by the localized plasmon resonance effect of Au nanoparticles or due to Au interband transitions (hot electrons in Au/SrTiO3 systems).14 Regardless of the advantageous electronic features of SrTiO3, i.e., the optimal Schottky barrier height, in coupling with Au or Ag (i.e., in the case of Au–SrTiO3, = 1–1.5 eV15,16),17 a relatively small number of studies have focused on the study of (plasmonic) metal-SrTiO3 heterostructures (Ag/SrTiO3 (ref. 18)).
Beyond the previously discussed trivial aspects related to metal NPs (morphology and distribution), the metal/support interface must also be considered, since it governs the efficiency of interfacial electron transfer and the charge dynamics.17,19 Moreover, since not only the charge transfer but also the reaction can occur at the interface, its active sites must also be taken into account.17 The selected preparation method influences considerably the interfacial electronic structure, as for example, the quantity of the interfacial defects (Ti3+ centers), which act as electron traps, thus inhibiting the interfacial electron transfer in Au/TiO2 systems.12
In this work, the effect of NiO impregnation or/and Au-photodeposition on different SrTiO3-supports has been studied in association with the assessment of their photocatalytic CO2 reduction activity and their light-induced CO2 adsorption ability. As supports, commercial SrTiO3, commercial SrCO3, and SrTiO3–SrCO3 prepared by hydrothermal crystallization were employed. To the best of our knowledge, the combination of the aforementioned components, namely NiO, Au, SrTiO3 and SrCO3, has not been previously studied in the literature. Additional motivating points of our work are related to the fact that, despite its potential, a relatively small number of studies are dealing with SrTiO3–SrCO3 in photocatalytic CO2 reduction applications20 (it is mainly used for photooxidation of NO,21 CH4,22 and degradation of active pharmaceutical ingredients23), and that the effect of structural carbonates is still a matter of debate in photocatalytic CO2 reduction (e.g., TiO2,24 ZnO25).
Experimental
Chemicals
The chemicals for synthesis and analysis: strontium nitrate (Sr(NO3)2, Sigma Aldrich, Germany, ≥99%), titanium(IV) oxide (anatase TiO2, 10–25 nm, Iolitec, Germany, 99.5%), potassium hydroxide (KOH, Sigma Aldrich, Germany, ≥85%), nickel nitrate hexahydrate (Ni(NO3)2·6H2O, Merck, Germany, ACS), hydrogen tetrachloridoaurate(III) trihydrate (HAuCl4·3H2O, ABCR, Germany, 99.99%), ethanol (Merck, Germany, >99%), isopropanol (Merck, Germany, 99.9%) were used without any preliminary purification. The reference support materials were SrTiO3 (Iolitec, Germany, 99.99%) and SrCO3 (Merck, Germany, 99.99%).Preparation of the studied materials
Preparation of the supports
Impregnation of the support with NiO
To favor the adsorption of CO2 on the SrTiO3-based support, one promising option is the modification of the surface of a catalyst with transition metal oxides to render its surface more basic (i.e., NiO with PZC: 8–9 (ref. 26)). The impregnation of STO-SCO (HT) or STO was similar to what was previously reported in the literature.27 12.13 mg (0.066 mmoles) Ni(NO3)2·6H2O was dissolved in 30 ml distilled H2O, followed by the addition of 0.800 g support (either STO-SCO (HT) or STO). The as-prepared suspension was stirred for 1 h (500 rpm) and centrifuged, and the obtained solid was washed once with EtOH, 3 times with distilled H2O and dried in air (180 °C, 12 h). Finally, calcination of the sample was performed in a static air atmosphere at 700 °C for 4 h. The nominal content of NiO was 0.3 wt%, which was in accordance with the experimentally determined Ni2+ content (±5% relative error).Au photodeposition
The Au-photodeposition was performed according to a method described previously12,28 involving (1) pre-irradiation of 10 mL 0.594 mM ethanolic HAuCl4 solution and catalyst suspension over 10 min; (2) dropwise addition of 10 mL HAuCl4 solution over the catalyst suspension; (3) irradiation of the mixture over 15 minutes. The washing was performed with distilled H2O and centrifugation, followed by drying at 90 °C in air for 16 h. The experimentally determined Au content (error ±10%, theoretical Au content: 1 wt%) of the samples was in accordance with the expected/theoretical Au content.Remark: Since studies were already conducted to investigate the influence of Au content on the photocatalytic activity of SrTiO3-based samples (e.g., 1.1 wt% Au nanospheres over SrTiO3via precipitation–deposition,14 1 wt% Au microspheres over SrTiO3/TiO2via photoreduction29), the optimized Au content recommended by results reported in the literature was considered.
Material characterization
Methods
The elemental composition of the synthesized catalysts was determined by inductively coupled plasma optical emission spectroscopy (ICP-OES, Varian/Agilent 715-ES, Germany).The assessment of morpho-structural features of the catalysts was performed based on the SEM (scanning electron microscopy) micrographs recorded using a Merlin VP compact device (Zeiss, Oberkochen, Germany).
Scanning transmission electron microscopy (STEM) provided a more detailed overview on the structural features of the selected samples using a probe aberration corrected ARM200F (Jeol, Tokyo, Japan) operated at 200 kV and equipped with high angle annular dark field (HAADF) and annular bright field (ABF) detectors and a DRY SD60GV (JEOL) energy dispersive X-ray spectrometer (EDXS). Specimens were dry deposited onto a Cu grid with a holey carbon film.
An Xpert Pro diffractometer (PANalytical, the Netherlands) equipped with a CuKα1Kα2 radiation source (λ1 = 0.15406 nm, λ2 = 0.15443 nm) was used for the recording of the X-ray diffraction (XRD) patterns. The Scherrer equation was used for the calculation of the primary crystallite size.30 The Rietveld analysis was performed in the HighScore Plus software environment.
An ESCALAB 2020iXL (Thermo Fischer Scientific) spectrometer equipped with an Al Kα radiation source was used for obtaining the results related to X-ray photoelectron spectroscopy (XPS).
The nitrogen sorption data (at 77 K) were obtained using a NOVAtouch (Quantachrome Instruments). While the Brunauer–Emmett–Teller (BET) multipoint method was considered for the determination of the specific surface area, the pore volume was extracted from the Barrett–Joyner–Halenda (BJH) data. The pre-treatment of the samples was performed via heating at 350 °C under vacuum for 5 h.
The reflectance spectra in the UV-vis range (i.e., 200–800 nm) of the studied solid catalysts were recorded using a Lambda 650 spectrophotometer (Perkin Elmer). The well-known Tauc-equation was considered for the determination of the band gap energy of SrTiO3.
The paramagnetic centers of the studied SrTiO3-based samples (in our case the unpaired electron in Ti3+ centers) were detected via in situ electron paramagnetic resonance spectroscopy (in situ EPR, Bruker EMX CW-micro X-band EPR spectrometer) under UV-vis irradiation (300 W Xe-arc lamp, LOT Oriel GmbH, Germany). The X-band EPR (serial) spectra were recorded at room temperature.
The identification of surface species involved in CO2 adsorption was elucidated by in situ diffuse reflectance infrared Fourier transformation spectroscopy (in situ DRIFTS) (Nicolet Protégé spectrometer equipped with a Harrick HVC DRP-5 cell and Praying Mantis mirrors). Detailed description of the equipment can be found elsewhere.24 The desired Ar and CO2 flow rates were provided by Bronkhorst mass flow controllers (MFCs). Serial recordings were collected, with 200 scans at a resolution of 4 cm−1 per individual spectrum, and averaged in order to obtain the respective spectra. The studied catalyst was introduced into the reaction chamber, subsequently purged with Ar (31 mL min−1) for 30 min at room temperature, followed by thermal pre-treatment aiming at the removal of surface-bound H2O (heating rate: 10 K min−1 up to 400 °C, maintained at 400 °C for 60 min, natural cooling to room temperature). The CO2 adsorption experiment was performed at room temperature under an Ar–CO2 flow (29 mL min−1 Ar, 2 mL min−1 CO2) over 30 min. Prior to the CO2 adsorption experiment the as-called background spectrum was recorded, which was then subtracted from the subsequently collected spectra. The final step was purging with Ar (31 mL min−1) over 1 h. The same procedure was repeated under irradiation during the CO2 adsorption step using a Lumatec Superlite S04 lamp equipped with an optical fiber to guide the light into the chamber. Meanwhile in the first 15 min the emission was set to 320–500 nm, and during the last 15 min it was shifted to 400–700 nm with the intensity set to 25%.
A high-purity gas-phase photoreactor system was used for the assessment of the photocatalytic CO2 reduction activity of the studied SrTiO3-based samples in batch-mode. The detailed description of the experimental setup and the measurement process can be found in a recently published study from our group.31
Briefly, to ensure high-purity conditions, the photoreactor employed in this work is made of stainless steel and all the connections of the individual parts are performed using only metallic connectors and adaptors suitable for high pressure applications. No elastomeric parts were used to ensure that there are no products formed from the interaction of the equipment with CO2 or from degradation of elastomeric parts under light irradiation. To exclude the formation of C-containing products from leftover surface-bound impurities from the synthesis of the photocatalysts, extensive blank experiments were performed under a humidified He environment (0.6 vol% H2O/He) under light irradiation but in the absence of CO2. These measurements act also as a batch cleaning process: leftover carbonaceous species are gradually removed from the surface of the samples under the influence of light. Subsequently, CO2 photoreduction experiments have been initiated in the presence of CO2 (1.5 vol% CO2) and water (0.6 vol% H2O) and He.
The experiments were performed in batch mode (initial pressure 1500 mbar) and gas samples were collected periodically (every 45 min) over a total irradiation time of 6 h. A pressure-drop correction was made to account for the removed volume in between measurements. The irradiation of the samples was performed using a 200 W Hg/Xe lamp (Newport Oriel) with a light intensity of 200 mW cm−2. A water-filled IR filter was introduced in the light pathway to remove the IR region of the irradiation spectrum of the lamp and to avoid excess heat during the CO2 reduction experiments. Gas analysis was performed using a gas chromatograph (TRACERA-2010, Shimadzu) featuring a barrier discharge ionization detector (BID) and a flame ionization detector (FID).
The moles of the product, nproduct(ti) expressed in μmol, at sampling time ti were calculated following DIN SPEC91457:32
• Measured volume fraction of the product, cproduct(ti), expressed in ppm, at the current sampling time ti.
• Measured volume fractions from the previous sampling times (cproduct(tj) where tj![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t1…ti−1).
t1…ti−1).
• The pressure in the reactor, p(ti), p(tj), at sampling time ti or tj (in kPa).
• The withdrawal sample volume Vm(ti) at sampling time ti.
• Volume of the reactor, VR, expressed in L.
 | (1) |
The withdrawal sample volume Vm(ti) is calculated accounting the pressure drop in the reactor after sample withdrawal, as depicted in eqn (2):
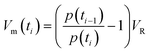 | (2) |
The products formed during the batch cleaning were subtracted from the amount of products formed during the CO2 reduction experiments for each sampling time. The normalized concentrations were obtained by dividing the concentration of the detected products by the catalyst mass and the reaction time.
To gain insight into the mechanism of CO2 reduction via SrTiO3-SrCO3-based materials, knowledge of the work function of SrTiO3 and SrCO3 and of the SrTiO3-SrCO3 composite is necessary. First-principles methods based on density functional theory (DFT) and density functional theory with the Hubbard U correction (DFT+U) were used for calculating the electronic properties of SrCO3 and SrTiO3 by using the Vienna ab initio simulation package (VASP).33–35 The projected augmented wave method (PAW)36,37 was used to describe the interaction of electrons and ions. The electron exchange and correlation energies were calculated within the generalized gradient approximation method (GGA) using the Perdew–Burke–Ernzerhof (PBE) functional.38 Geometry optimization was converged until the forces acting on the atoms were smaller than 0.03 eV Å−1, whereas the energy threshold-defining self-consistency of the electron density was set to 10−5 eV. The plane wave cut off was set to 520 eV in all simulations. The detailed methodology of the DFT calculations (i.e., density of states of bulk SrTiO3 and SrCO3) is presented in the ESI† (Fig. S32 and S33†). SrTiO3(110) and SrCO3(111) slab models were built to describe the effect of the presence of new surface atoms on the position of the Fermi level. A p(2 × 2) supercell was used to simulate the clean SrTiO3(110) surface, while a p(1 × 1) supercell was used to model the SrCO3(111) surface. All models have a five-layer-deep slab, and they were allowed to fully relax without any constrains. The simulation method used required a periodic cell. In the periodic cell, a 20 Å vacuum gap was created between the upper-most and bottom-most layers. Dipolar correction in the z direction was set for all the slab models. For SrTiO3(110), the model has 20 Sr atoms, 20 Ti atoms and 60 O atoms. For SrCO3(111), the model has 20 Sr atoms, 20 C atoms and 60 O atoms.
Results and discussion
Structural features and properties
The experimentally determined Au and Ni2+ contents (Auexp, Ni2+exp) agreed with the theoretical values (Autheo, Ni2+theo) based on the ICP-OES results (within an acceptable relative error range of ±10%), providing evidence about the efficiency of the impregnation and photodeposition processes (ESI,† Table S1).The reflections of cubic SrTiO3 (ICDD 00-035-0734) and orthorhombic SrCO3 (ICDD 01-084-1778) phases were revealed in all XRD patterns of the STO-SCO (HT)-based samples (Fig. 1). No additional reflections were identified in the case of the NiO/STO-SCO (HT) sample (vs. STO-SCO (HT)) given by the relatively low loading of NiO on the support.39,40 According to Sreethawong et al., the characteristic diffraction peak of NiO (at 2θ = 43.3°) can be observed for loadings higher than 5 wt%.39 Although no typical diffraction peaks of Au species (metal Au or AuxO) were expected in the recorded XRD patterns at such a low Au loading,41,42 an additional relatively broad reflection can be observed at 38.2° (2θ) in the case of Au-STO-SCO (HT) and Au-NiO/STO-SCO (HT), which corresponds to the (111) plane of the face centered cubic Au-structure.43,44 Moreover, Rietveld analysis (SrCO3 content determination) and primary crystallite size (PCS) calculation of SrTiO3 (via the Scherrer equation) were performed based on the XRD results.
Thermal annealing at 700 °C contributed to an increase of the PCS of SrTiO3 (from 19 to 23 nm in the case of the hydrothermally synthesized support, and from 28 to 32 nm in the case of the STO). (Table 1). Similar PCSs were previously observed for SrTiO3 from the hydrothermal process45 (19.3 nm) and from that after thermal annealing at 700 °C46 (23 nm).
| No. | Sample | PCS (STO) | SSA (m2g−1) | V P (cm3 g−1) | r P (nm) |
|---|---|---|---|---|---|
| Remark: PCS – primary crystallite size, SSA – specific surface area, VP – pore volume, rp – pore radius. | |||||
| 1 | STO-SCO (HT) | 19 | 52 | 0.180 | 7.040 |
| 2 | NiO/STO-SCO (HT) | 23 | 16 | 0.090 | 11.540 |
| 3 | STO | 28 | 15 | 0.770 | 13.770 |
| 4 | NiO/STO | 32 | 15 | 0.070 | 10.110 |
| 5 | SCO | 26 | 2–3 | 0.007 | 5.350 |
According to Rietveld analysis, the SCO content of the STO-SCO (HT)-based samples was 18 wt%, which was in accordance with the carbonate-content calculated based on the carbon content of the samples (EA, ∼18 wt%), indicating the crystallinity of SCO. Further XRD patterns of the studied samples are presented in the ESI† (Fig. S1–S3, respectively).
The Au-STO-SCO (HT) and Au-NiO/STO-SCO (HT) samples were analyzed via XPS. Based on the Ni 2p XP spectrum it can be concluded that mainly Ni2+ species47 (binding energy of 855.56 eV) could be identified, a small fraction of Ni3+ species48 might also be present (with a binding energy of 857.90 eV), and Ni0 was not formed (ESI,† Fig. S4). Furthermore, the Au 4f XP spectra confirmed the presence of metallic Au (Fig. S5a and b†). No considerable difference was observed in the binding energy values characterizing the electronic state of SrTiO3 (ESI,† Table S2). Furthermore, the experimental (i.e., XPS-based) and theoretical compositions of the species in the case of Au-STO-SCO (HT) and Au-NiO/STO-SCO (HT) were compared (ESI,† Tables S3 and S4).
The morphological features of the studied samples were investigated by SEM and STEM. SEM images of the selected (optimized) samples are presented in Fig. 2. Further SEM images are presented the in ESI† (Fig. S6–S12). Two morphological entities can be revealed in the case of STO-SCO (HT)-based catalysts (Fig. 2), namely nanocubes (STO) and isolated microrods (SCO). While the STO nanocubes have average particle sizes of 20–30 nm, the SCO microrods were 1.0–1.5 μm in length with a diameter of ca. 150 nm. The SEM-derived particle size of SrTiO3 agrees with the primary crystallite size from the XRD, calculated via the Scherrer equation. While the presence of Au nanospheres was revealed on the SEM images of Au-STO-SCO (HT) and Au-NiO/STO-SCO (HT) (Fig. 2b and c), the morphology of NiO could not be identified at such magnifications, therefore further analyses were performed. The EDX of Au-NiO/STO-SCO (HT) (Fig. 2e) indicated both Au and Ni on the catalyst surface in the case of Au-NiO/STO-SCO (HT). Furthermore, the EDX from SEM of Au-NiO/STO-SCO (HT) (Fig. 2e) showed the presence of potassium on the surface, which was not entirely removed during the washing step.
To have a closer look on the morphology and the contact between the components, STEM images, EDXS elemental maps and EDX spectra were recorded for the ternary composite, Au-NiO/STO-SCO (HT). As can be seen in the STEM images (Fig. S13†), especially in the medium resolution HAADF image, the support shows rather cubic shaped SrTiO3 particles which apparently have surface indentation or local pores according to the small darker areas. The high-resolution image then shows the NiO particle attached to such an SrTiO3 support particle. In the corresponding EDX spectrum (Fig. S14†) the cloudy like structure was verified to contain Ni and O, but without assigning a certain possible oxidation state. An EDXS elemental map (Fig. S15†) shows the scarcity of the NiO and the Au particles present in the sample and their lack of common localization. However, it appears locally that there are indications of a varying Sr to Ti ratio in the SrTiO3 support (Fig. S16 and S17†) which might be a minority fraction of non-stoichiometric mixed oxides or TiO2 crystallites. Further images and EDXS data (Fig. S18†) show the striking size differences in the SrTiO3 and SrCO3 parts of the Au-NiO/STO-SCO (HT) catalyst.
For an overview on the textural properties of the selected supports, the specific surface area (BET, SSA), the pore volume (BJH, VP) and the average pore size (rP) were assessed and are summarized in Table 1. The characteristics of the type IV isotherm with an H3 hysteresis loop were identified (STO-SCO (HT), NiO/STO-SCO (HT) – Fig. S19;† STO, NiO/STO – Fig. S20†), which are characteristic for micro- and mesoporous materials.49 The highest SSA can be observed for the STO-SCO (HT) sample, which may provide more CO2 adsorption sites and facilitate the involvement of the adsorbed CO2 in the subsequent surface reactions.50 As expected, the decrease of SSA was observed after sintering at 700 °C51 (i.e., from 52 m2 g−1 for STO-SCO (HT) to 16 m2 g−1 for NiO/STO-SCO (HT)), however this decrease was obvious only in the case of the hydrothermally synthesized support.
Considerable overlapping of the N2 isotherms was observed in the case of the reference samples (i.e., STO and NiO/STO, Fig. S20†), which indicates that the textural modification after the thermal treatment (at 700 °C, 4 h) of the previously studied samples may be given by the presence of carbonate (i.e., SrCO3). In addition to this, while the pore volume decreased after impregnation in all cases (Table 1, samples no. (1) and (2): from 0.18 to 0.09 cm3 g−1, samples no. (3) and (4): from 0.77 to 0.074 cm3 g−1), unexpectedly the increase of pore size was observed after impregnation and thermal treatment in the case of the carbonate containing support (i.e., STO-SCO (HT): 7.04 nm vs. NiO/STO-SCO (HT): 11.54 nm).
Light absorption and electronic structure
To investigate the optical properties of the studied samples, the respective UV-vis diffuse reflectance spectra were recorded (Fig. 4). All the samples possess an absorption edge in the UV region (300–400 nm), highlighted in grey in Fig. 3. Considering the Tauc-plot (Fig. 3, inset) in the case of indirect semiconductors, the band gap energy (ΔEg) of SrTiO3 was determined to be 3.2 eV. The determination of the ΔEg of SrCO3 was not possible, since it cannot be resolved in the recorded range (200–800 nm).23 Moreover, considering the overlapping of the absorption edges in the recorded UV region (200–400 nm) and the low NiO loading, the determination of the NiO ΔEg is not possible based on the recorded spectra of the studied multicomponent systems (i.e., NiO/STO-SCO (HT), Au-NiO/STO-SCO (HT)). However, based on the published literature, it is known that the ΔEg of NiO is situated in the range of 3.4–4.6 eV.52,53 In the case of the Au-containing samples (i.e., Au-STO-SCO (HT), Au-NiO/STO-SCO (HT)), the plasmon resonance band is located at 540 nm, which is directly correlated to the Au NP size and shape.12,54 Further UV-vis spectra of the reference samples are presented in Fig. S21.†Unravelling the paramagnetic species under irradiation
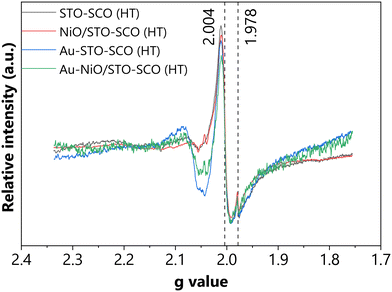
To reveal and clarify the presence of paramagnetic species under irradiation, serial recordings were performed under irradiation (ESI,† Fig. S22). The representative extracted EPR spectra of selected catalysts are presented in Fig. 4. Interestingly, in all studied samples a nearly isotropic signal was identified at g = 2.004 (g1 = g2 = g3), which was assigned to the trapped electrons in oxygen vacancies (OVs)29,41,55,56 as depicted in Fig. 4 (ESI,† Table S5). Although the presence of OVs was mainly expected after sintering (i.e., in the case of NiO/STO-SCO (HT) and Au-NiO/STO-SCO (HT)) and also after Au photodeposition on the STO-SCO (HT) support (i.e., in the case of Au-STO-SCO (HT)),12 their presence was also unexpectedly identified in the case of the bare hydrothermally synthesized support (i.e., STO-SCO (HT)). On the other hand, a relatively weak anisotropic signal was identified at 1.978, which may be assigned to the Ti3+ centers (ESI,† Table S5).55,57 In the case of the Au-containing samples (i.e., Au-STO-SCO (HT) and Au-NiO/STO-SCO (HT)), a relatively broad signal can be observed at g = 2.066, which can be correlated to the interaction between Au nanoparticles and OVs from SrTiO3.55,58In situ DRIFTS
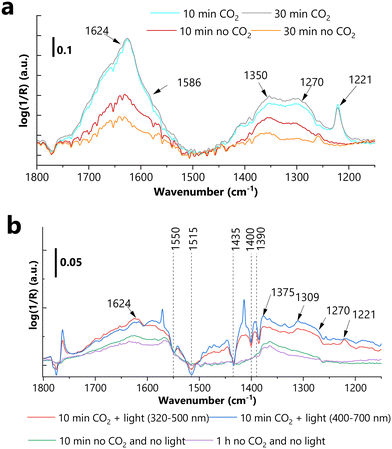
The formation of surface species after the exposure of the tested (selected) photocatalysts to CO2, and the influence of light irradiation on the CO2 adsorption, were studied using in situ DRIFTS, and the results are presented in Fig. 5 and S23–S28.† Recently, the adsorption mechanisms over metal oxides under irradiation have been studied via in situ DRIFTS.59,60 The spectral differences during CO2 adsorption in the dark vs. under irradiation may be caused by the: (1) light induced oxygen desorption and implicitly increased amount of Lewis acid sites,61 (2) different activation of adsorbed CO2, (3) different interaction of CO2 with pre-adsorbed hydroxyl groups, (4) enhanced splitting of hydroxyl groups and transfer of hydrogen to adsorbed CO2.62Since our primary intention was to unravel the visible light-induced (i.e., 500–700 nm) transformations (thus assessing the involvement of the SPR effect of Au NPs), a comparative analysis was performed (and presented in the ESI† – i.e., Tables S6–S9) based on the spectra recorded in the dark vs. under irradiation ((i) 320–400 nm or (ii) 500–700 nm).
Apart from the appearance of certain negative features (due to the spectator species, detailed discussion presented in the ESI†) under light irradiation vs. in the dark in the case of STO-SCO (HT), NiO/STO-SCO (HT) and Au-STO-SCO (HT) samples, no considerable differences were observed. For this reason, only the representative DRIFT spectra will be presented in the next section (i.e., for Au-NiO/STO-SCO (HT), Fig. 5), in addition to commenting on the influence of light on CO2 adsorption for all studied catalysts.
Under irradiation negative bands were identified at 1435, 1400 and 1390 cm−1 for all examined samples (Fig. S24, S26 and S28†), which can be directly assigned to the structural carbonates or spectator carbonate species present in the beginning of the measurement. Moreover, the Au-NiO/STO-SCO (HT) sample exhibited two additional negative features, at 1550 and 1515 cm−1 (Fig. 5). These structural carbonates or spectator carbonate species can participate in the formation of (presumably) structurally very different species. These newly formed species can be identified only in the presence of CO2, and they completely disappear when CO2 is removed. The potential assignment of the previously mentioned features is the following: νas(OCO) at 1550 cm−1,63νs(OCO) at 1390 and 1435 cm−1 (ref. 63–68) from bicarbonate species, respectively, νas(OCO) at 1515 cm−1,69νs(CO3) at 1435,63 and νs(OCO) at 1400 cm−1 (ref. 69) from monodentate carbonate.
It should be highlighted that under visible light irradiation (500–700 nm) the only observable signals can be identified at 1414 and 1560 cm−1 in Table S6.† These can be associated with the characteristic vibrational modes, i.e., νas(COH) at 1414 cm−1 (ref. 70 and 71) and νas(OCO)69 at 1560 cm−1,72 of bidentate bicarbonates. Furthermore, the previously formed bicarbonates (i.e., at 1221 and 1624 cm−1, under λ = 320–400 nm) cannot be identified in the spectra recorded under λ = 500–700 nm, which indicates their visible light-induced transformation (ESI,† Fig. S24, S26 and S28).
From the spectra presented in Fig. S23–S28,† it can be concluded that no considerable differences were observed for all four studied photocatalysts (Au-NiO/STO-SCO (HT), Au-STO-SCO (HT), NiO/STO-SCO (HT) and STO-SCO (HT)) under dark conditions. In other words, the presence of ∼0.3 wt% NiO and ∼1 wt% Au has negligible effect on CO2 adsorption in the dark. In the studied spectra the observed species were identified to be linearly adsorbed CO2, monodentate carbonate, monodentate bicarbonates and carboxylates. In addition to the previously identified species, bidentate carbonate was identified in the spectra of Au-STO-SCO (HT). Under (visible or UV-A) light irradiation though, remarkable differences were identified. One considerable difference is related to the visible-light induced transformation of monodentate bicarbonates over Au-NiO/STO-SCO (HT). Finally, regardless of the thermal treatment performed before CO2 adsorption (described in the Experimental section), the vibration feature corresponding to the COH bending (at 1222 cm−1) was always observed, which is a clear indication of the presence of hydroxyl groups on the surface for all studied samples.
Photocatalytic CO2 reduction
The products formed and their normalized concentrations (in ppm gcat−1 h−1) from the photoreduction of CO2 using SrTiO3-based catalysts under Hg–Xe light irradiation are presented in Fig. 6 (ESI,† raw data: Fig. S29–S36). Beyond the presented results, additional samples were analyzed, namely STO-SCO (SM), SCO and Au-SCO. No product formation was observed in the case of SCO and Au-SCO. The activity of STO-SCO (SM) was 17 ppm gcat−1 h−1 towards CH4 generation. Investigations of the long-term reusability and photocatalytic stability of the tested catalysts were not performed, as this work focuses on the influence of catalyst composition and the interaction between the individual sub-components, on product formation and selectivity (Fig. 6).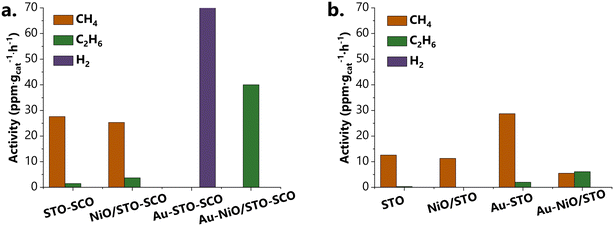 | ||
| Fig. 6 a. The activity of the STO-SCO (HT)-based samples. b. The activity of the reference series of the samples (i.e., STO-based samples). | ||
Considerably higher activity and selectivity were observed in the case of STO-SCO (HT)-supported vs. STO-supported samples. To understand the differences in activity in the case of STO- and STO-SCO-based samples, the charge transfer mechanism was studied. Conduction (CB) and valence band (VB) edges of the single components (SrTiO3, SrCO3, and NiO) were calculated using experimental results (Mott-Schottky plot, band gaps) and literature reported data (see ESI,† Fig. S37 and S38). Results show that the CB edge minimum of NiO (−3.03 V vs. NHE) is more negative than that of STO (CB: −1.00 V vs. NHE) and SCO (−0.64 V vs. NHE). Otherwise, the VB edge maximum of SCO (4.30 V vs. NHE) is more positive than that of STO (2.10 V vs. NHE) and NiO (0.37 V vs. NHE). Additionally, the electronic properties of SrTiO3 and SrCO3 were calculated using DFT. The main aspects related to the calculations will be presented shortly in the next section.
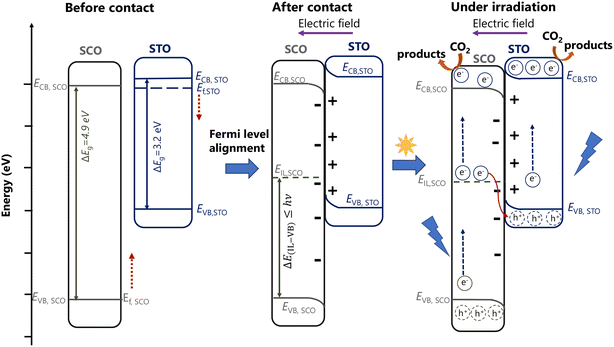 | ||
| Fig. 7 Scheme of the charge transfer in the case of SrTiO3-SrCO3via accounting the formation of the internal electric field – rebuilt based on the work written by Han and coworkers74 (CB – conduction band, IL – intermediate level, SCO – SrCO3, STO – SrTiO3, VB – valence band); remarks: (i) – the conduction and valence band positions of STO and SCO were taken from the literature20,21 and (ii) the red arrows in the left side (“before contact”) indicate the Fermi level alignment of each component. | ||
(1.1) Several scientific studies (Li and coworkers20 and Jin and coworkers21) report that the efficient charge separation is given by the functionality of SrCO3 as an electron trap (Fig. S39†). This approach assumes the accumulation of electrons in the CB of SrCO3, and the localization of holes at the VB of SrTiO3. However, based on this charge transfer scheme, it is highly possible that CO2 reduction would take place over SrCO3. At the same time, it must be highlighted that this approach does not account for the band bending and the formation of an electric field. One can explain the validity of this approach by the fact that since SrCO3 is an insulator, no charge transfer would take place (given by the large work function difference between SrTiO3 and SrCO3), and subsequently, there would be no band bending or generation of an interfacial electric field.
(1.2) With the building of a semiconductor–insulator (SrTiO3-SrCO3) heterojunction a Fermi level alignment between SrTiO3 and SrCO3 might occur resulting in the formation of a built-in electric field which points from SrTiO3 to SrCO3 as suggested by Han74 (Fig. 7). Considering the direction of the built-in electric field, the transfer of the electrons from the CB of SrTiO3 to the CB of SrCO3 is not possible. However, during the heterojunction formation, generation of OVs and surface doping of SrCO3 might occur leading to the formation of an intermediate electronic level (IL) over the SrCO3 near the interphase for which |EVB − EIL| = ΔE(VB–IL) ≤ hv. At such circumstance, under irradiation a translocation of electrons from the VB of SrCO3 to its IL and from there to the CB of SrCO3 would be possible. Electrons from the IL of SrCO3 might also be transferred to the VB of SrTiO3, where they can recombine with holes from the VB of SrTiO3 which will suppress the charge carrier recombination between 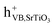 and
and  .
.
To investigate the possibility of a built-in electric field formation in the case of SrTiO3-SrCO3 (which would be the result of the Fermi level alignment after contact between SrTiO3 and SrCO3, as depicted in Fig. 7) the work function (and implicitly the Fermi level) of the pure (i.e., SrTiO3, SrCO3) and doped materials (C-doped SrTiO3, Ti-doped SrCO3) was calculated via computational methods. As already described in the Methods section, the SrTiO3(110) and SrCO3(111) slab models were built. As to SrTiO3(110), two different terminations (SrO- and TiO-) were considered. To study the interaction between SrTiO3(110) and SrCO3(111), both terminations of SrTiO3(110) were doped by a C atom, i.e., one surface Ti atom was replaced by a C atom. As to SrCO3(111), one surface C atom was replaced by a Ti atom. Then, the density of states of all the surfaces was calculated. Furthermore, the work function (Φ) was calculated based on Φ = Evac − Ef (Evac is the vacuum potential and Ef is the Fermi energy). The models and density of states are shown in the ESI† (Fig. S42–S46) and the work functions are listed in Table 2. Based on the computational results listed in Table 2, the termination of SrTiO3 (either SrO- or TiO-terminated, cases 1.1 and 1.2) influences significantly the absolute value of the work function (i.e., 5.7 vs. 2.1 eV). Similar observations were reported by Jacobs and coworkers75 based on their computational investigations. Most studies in the literature consider the electronic properties of TiO-terminated SrTiO3(110), which define a difference of work function of 3.6 eV (and implicitly a difference in the Fermi level) between SrTiO3 and SrCO3. This also implies that the termination of SrTiO3 (SrO- and TiO-termination) in contact with SrCO3 is a decisive factor. The computational results support the hypotheses related to the direction of the Fermi level shift (Table 2) after the interaction between the two components (as marked in Fig. 7). While the work function increases in the case of C-doped SrTiO3(110) (for both terminations), the work function of Ti-doped SrCO3(111) decreases. The Fermi level shift in both components leads to the generation of an internal electric field, which agrees with the results presented by Han and coworkers.74 The computational results are presented in detail in the ESI† (Fig. S42–S46).
| Case | Surface | Φ (eV) | Modified surface | Φ (eV) | ΔΦ (eV) |
|---|---|---|---|---|---|
| 1. SrTiO3 | 1.1. SrO-terminated SrTiO3(110) | 5.7 | C-doped SrO-terminated SrTiO3(110) | 6.1 | 0.4 |
| 1.2. TiO-terminated SrTiO3(110) | 2.1 | C-doped TiO-terminated SrTiO3(110) | 2.3 | 0.2 | |
| 2. SrCO3 | SrCO3(111) | 5.7 | Ti-doped SrCO3(111) | 5.3 | −0.4 |
The methane formation rate during batch cleaning (gas mixture: He + H2O) over STO-SCO (HT) catalysts was higher when compared to that of STO (Fig. S29 and S33†) which indicates the participation of SrCO3 in CH4 formation. Here, CH4 might be formed by the reaction of adsorbed carbon species and photogenerated charge carriers. Because no methane formation was observed when using SCO, it is assumed that the higher activity of STO-SCO (HT) compared to STO is mainly caused by a larger number of photocatalytic active species. However, the direct participation of the SrCO3 phase in product formation over STO-SCO (HT) cannot be excluded completely. To exclude the influence of the structural peculiarities of SrCO3 and SrTiO3 when it comes to the hydrothermally synthesized and commercially available samples (different morphology), the activity of STO and of STO-SCO (SM) in CO2 reduction was also compared (i.e., 12 vs. 17 ppm gcat−1 h−1 STO vs. STO-SCO (SM)). Again, the presence of SCO (in STO-SCO (SM)) leads to higher activity.
Enhanced CH4 production was observed over Au-STO vs. STO samples (Fig. 6b), which can be correlated with the involvement of Au NPs as an electron donor.15 In contrast to this, over Au-STO-SCO (HT) the formation of carbon-based products in the presence of CO2 was similar to that for batch cleaning and H2 was the main product in CO2 reduction over this catalyst. Hydrogen generation over Au during photocatalytic CO2 reduction was already reported by Pougin et al. using Au@TiO2 catalysts.81 Hydrogen formation is both kinetically and thermodynamically a more facile reaction (vs. CO2-to-CH4 or CO2-to-CO).82–84 One reason for the various reaction products obtained over Au-STO and Au-STO-SCO (HT) might be the differences between the Fermi level of the corresponding support and the Fermi level of Au. For Au-STO-SCO (HT), the Fermi level of the support is assumed to be lower than that of Au (Ef,aligned (SrTiO3-SrCO3) < Ef(Au). After alignment, electrons from the STO-SCO (HT) support will be transferred to Au where H2 formation will occur under irradiation, In addition to this, SrCO3 may contribute to the spatial isolation of the sites for H+ and electrons in the presence of CO2 and H2O41,84 in the case of Au-STO-SCO (HT) vs. (Au-STO), which leads to the formation of H2. For Au-STO, the electron transfer is expected in the opposite direction as shown in Fig. S41.† The transferred electrons react on the SrTiO3 surface with activated CO2 and protons to form mainly CH4.
Conclusions
In summary, the individual and simultaneous influence of SrCO3, NiO and Au on the photocatalytic CO2 reduction activity of SrTiO3-based materials was studied. Based on experimental and computational investigations the following conclusions can be drawn:• The presence of SrCO3 has a positive effect on the photocatalytic activity of SrTiO3 in CO2 reduction. It is assumed that by using a SrTiO3-SrCO3 heterojunction a higher number of reactive species are available for the reaction under irradiation compared to pure SrTiO3.
• The low amount of NiO (0.3 wt%) (on either supports: SrTiO3, SrTiO3-SrCO3) has only a minor effect on the activity and product formation.
• CO2 reduction products of Au containing samples are affected by the support. In the case of Au-SrTiO3 mainly CH4 was formed, whereas for Au-SrTiO3-SrCO3 H2 was the main product. This difference in product formation might be explained by considering Fermi level alignment.
• The simultaneous presence of Au and NiO on the SrTiO3-SrCO3 surface supports the dimerization of C1 intermediates.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Bíborka Boga gratefully acknowledges the Innovation Fellowship for Establishment of (Inter)national Collaborations provided by Leibniz Institute for Catalysis (LIKAT) for the 2022–2023 academic year. Moreover, Bíborka Boga would like to thank the scholarship provided by Márton Áron Szakkollégium funded by the Hungarian Ministry of Foreign Affairs and Trade during her doctoral studies, and the Alumni Fellowship provided by the German Federal Environmental Foundation (Deutsche Bundesstiftung Umwelt, 01.12.2023-29.02.2024). Special thanks for the Analytical Department of LIKAT, more precisely for Dr. Henrik Lund (XRD), Felix Lorenz (BET), Anja Simmula (ICP-OES) and Sandra Leiminger (C, H content), Dr. Stephan Bartling (XPS). Moreover, the authors would like to express their gratitude to Dr. Armin Springer (SEM, University of Rostock) and Dr. Jabor Rabeah (EPR, LIKAT). Furthermore, Bíborka Boga thanks Dr. Sebastian Cisneros (LIKAT) for the fruitful discussion regarding to the EPR interpretations.References
- NASA, Global Climate Change, https://climate.nasa.gov/vital-signs/carbon-dioxide/ (last accessed on 05.02.2024) Search PubMed.
- World Energy Outlook 2022, 2022, pp. 233–236, https://iea.blob.core.windows.net/assets/7e42db90-d8ea-459d-be1e-1256acd11330/WorldEnergyOutlook2022.pdf Search PubMed.
- Y.-F. Xu, M. Lee, Y. Jun and G. A. Ozin, Perovskite, the chameleon CO2 photocatalyst, Cell Rep. Phys. Sci., 2021, 2(1), 100300 CrossRef CAS.
- C. Luo, J. Zhao, Y. Li, W. Zhao, Y. Zeng and C. Wang, Photocatalytic CO2 reduction over SrTiO3: Correlation between surface structure and activity, Appl. Surf. Sci., 2018, 447, 627–635 CrossRef CAS.
- J. Shan, F. Raziq, M. Humayun, W. Zhou, Y. Qu, G. Wang and Y. Li, Improved charge separation and surface activation via boron-doped layered polyhedron SrTiO3 for co-catalyst free photocatalytic CO2 conversion, Appl. Catal., B, 2017, 219, 10–17 CrossRef CAS.
- W. He, X. Wu, Y. Li, J. Xiong, Z. Tang, Y. Wei, Z. Zhao, X. Zhang and J. Liu, Z-scheme heterojunction of SnS2-decorated 3DOM-SrTiO3 for selectively photocatalytic CO2 reduction into CH4, Chin. Chem. Lett., 2020, 31(10), 2774–2778 CrossRef CAS.
- K. Xie, N. Umezawa, N. Zhang, P. Reunchan, Y. Zhang and J. Ye, Self-doped SrTiO3− δ photocatalyst with enhanced activity for artificial photosynthesis under visible light, Energy Environ. Sci., 2011, 4(10), 4211–4219 RSC.
- W. Zhao, W. Zhao, G. Zhu, T. Lin, F. Xu and F. Huang, Black strontium titanate nanocrystals of enhanced solar absorption for photocatalysis, CrystEngComm, 2015, 17(39), 7528–7534 RSC.
- H. Tan, Z. Zhao, W.-b. Zhu, E. N. Coker, B. Li, M. Zheng, W. Yu, H. Fan and Z. Sun, Oxygen vacancy enhanced photocatalytic activity of pervoskite SrTiO3, ACS Appl. Mater. Interfaces, 2014, 6(21), 19184–19190 CrossRef CAS PubMed.
- N. G. Moustakas and J. Strunk, Photocatalytic CO2 Reduction on TiO2-Based Materials under Controlled Reaction Conditions: Systematic Insights from a Literature Study, Chem. – Eur. J., 2018, 24(49), 12739–12746 CrossRef CAS PubMed.
- M. Dilla, A. Pougin and J. Strunk, Evaluation of the plasmonic effect of Au and Ag on Ti-based photocatalysts in the reduction of CO2 to CH4, J. Energy Chem., 2017, 26(2), 277–283 CrossRef.
- J. B. Priebe, J. R. Radnik, A. J. Lennox, M.-M. Pohl, M. Karnahl, D. Hollmann, K. Grabow, U. Bentrup, H. Junge and M. Beller, Solar hydrogen production by plasmonic Au–TiO2 catalysts: impact of synthesis protocol and TiO2 phase on charge transfer efficiency and H2 evolution rates, ACS Catal., 2015, 5(4), 2137–2148 CrossRef CAS.
- C. Wang, O. Ranasingha, S. Natesakhawat, P. R. Ohodnicki, M. Andio, J. P. Lewis and C. Matranga, Visible light plasmonic heating of Au–ZnO for the catalytic reduction of CO2, Nanoscale, 2013, 5(15), 6968–6974 RSC.
- L. Liu, P. Li, B. Adisak, S. Ouyang, N. Umezawa, J. Ye, R. Kodiyath, T. Tanabe, G. V. Ramesh and S. Ueda, Gold photosensitized SrTiO3 for visible-light water oxidation induced by Au interband transitions, J. Mater. Chem. A, 2014, 2(25), 9875–9882 RSC.
- Y. Ham, T. Minegishi, T. Hisatomi and K. Domen, A SrTiO3 photoanode prepared by the particle transfer method for oxygen evolution from water with high quantum efficiencies, Chem. Commun., 2016, 52(28), 5011–5014 RSC.
- F. Horikiri, T. Ichikawa, K. Sato, K. Yashiro, T. Kawada and J. Mizusaki, The Barrier Formation Mechanism on SrTiO3 for High-Temperature Photo-Electronic Devices, ECS Trans., 2009, 16(51), 451 CrossRef CAS.
- W. Guo, J. Huang and W. D. Wei, Plasmonic Metal/Semiconductor Heterostructures, in Plasmonic Catalysis: From Fundamentals to Applications, ed. P. H. C. Camargo and E. Cortés, Wiley-VCH, Weinheim, Germany, 2021, pp. 295–322 Search PubMed.
- K. Shao, Y. Wang, M. Iqbal, L. Lin, K. Wang, X. Zhang, M. He and T. He, Modification of Ag nanoparticles on the surface of SrTiO3 particles and resultant influence on photoreduction of CO2, Appl. Surf. Sci., 2018, 434, 717–724 CrossRef CAS.
- L. Collado, A. Reynal, F. Fresno, M. Barawi, C. Escudero, V. Perez-Dieste, J. M. Coronado, D. P. Serrano, J. R. Durrant and V. A. de la Peña O'Shea, Unravelling the effect of charge dynamics at the plasmonic metal/semiconductor interface for CO2 photoreduction, Nat. Commun., 2018, 9(1), 4986 CrossRef PubMed.
- Z. Li, P. Zheng, W. Zhang, S. Gong, L. Zhu, J. Xu, F. Rao, X. Xie and G. Zhu, Constructing SrCO3/SrTiO3 nanocomposites with highly selective photocatalytic CO2-to-CO reduction, Colloids Surf., A, 2022, 129686 CrossRef CAS.
- S. Jin, G. Dong, J. Luo, F. Ma and C. Wang, Improved photocatalytic NO removal activity of SrTiO3 by using SrCO3 as a new co-catalyst, Appl. Catal., B, 2018, 227, 24–34 CrossRef CAS.
- X. Pan, X. Chen and Z. Yi, Photocatalytic oxidation of methane over SrCO3 decorated SrTiO3 nanocatalysts via a synergistic effect, Phys. Chem. Chem. Phys., 2016, 18(46), 31400–31409 RSC.
- B. Boga, N. Steinfeldt, N. G. Moustakas, T. Peppel, H. Lund, J. Rabeah, Z. Pap, V.-M. Cristea and J. Strunk, Role of SrCO3 on Photocatalytic Performance of SrTiO3-SrCO3 Composites, Catalysts, 2022, 12(9), 978 CrossRef CAS.
- A. Pougin, M. Dilla and J. Strunk, Identification and exclusion of intermediates of photocatalytic CO2 reduction on TiO2 under conditions of highest purity, Phys. Chem. Chem. Phys., 2016, 18(16), 10809–10817 RSC.
- C. Xin, M. Hu, K. Wang and X. Wang, Significant enhancement of photocatalytic reduction of CO2 with H2O over ZnO by the formation of basic zinc carbonate, Langmuir, 2017, 33(27), 6667–6676 CrossRef CAS PubMed.
- T. Mahmood, M. T. Saddique, A. Naeem, P. Westerhoff, S. Mustafa and A. Alum, Comparison of different methods for the point of zero charge determination of NiO, Ind. Eng. Chem. Res., 2011, 50(17), 10017–10023 CrossRef CAS.
- K. Domen, S. Naito, T. Onishi, K. Tamaru and M. Soma, Study of the photocatalytic decomposition of water vapor over a nickel (II) oxide-strontium titanate (SrTiO3) catalyst, J. Phys. Chem., 1982, 86(18), 3657–3661 CrossRef CAS.
- J. F. Fernando, M. P. Shortell, C. J. Noble, J. R. Harmer, E. A. Jaatinen and E. R. Waclawik, Controlling Au photodeposition on large ZnO nanoparticles, ACS Appl. Mater. Interfaces, 2016, 8(22), 14271–14283 CrossRef CAS PubMed.
- S. Han, L. Yu, H. Zhang, Z. Chu, X. Chen, H. Xi and J. Long, Gold plasmon-enhanced solar hydrogen production over SrTiO3/TiO2 heterostructures, ChemCatChem, 2019, 11(24), 6203–6207 CrossRef CAS.
- A. Monshi, M. R. Foroughi and M. R. Monshi, Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD, World J. Nano Sci. Eng., 2012, 2(3), 154–160 CrossRef.
- N. G. Moustakas, M. Klahn, B. T. Mei, A. Pougin, M. Dilla, T. Peppel, S. Ristig and J. Strunk, A high-purity gas-solid photoreactor for reliable and reproducible photocatalytic CO2 reduction measurements, HardwareX, 2023, e00448 CrossRef PubMed.
- DIN SPEC 91457 Photocatalysis - Determination of product formation in CO2 reduction, https://www.din.de/en/wdc-beuth:din21:370373675 (last accessed on 14.12.2023).
- G. Kresse and J. Hafner, First-principles study of the adsorption of atomic H on Ni (111), (100) and (110), Surf. Sci., 2000, 459(3), 287–302 CrossRef CAS.
- G. Kresse and J. Furthmüller, Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set, Comput. Mater. Sci., 1996, 6(1), 15–50 CrossRef CAS.
- G. Kresse and J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54(16), 11169–11186 CrossRef CAS PubMed.
- P. E. Blöchl, Projector augmented-wave method, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50(24), 17953–17979 CrossRef PubMed.
- G. Kresse and D. Joubert, From ultrasoft pseudopotentials to the projector augmented-wave method, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59(3), 1758–1775 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77(18), 3865–3868 CrossRef CAS PubMed.
- T. Sreethawong, Y. Suzuki and S. Yoshikawa, Photocatalytic evolution of hydrogen over mesoporous TiO2 supported NiO photocatalyst prepared by single-step sol–gel process with surfactant template, Int. J. Hydrogen Energy, 2005, 30(10), 1053–1062 CrossRef CAS.
- R. Vinoth, P. Karthik, K. Devan, B. Neppolian and M. Ashokkumar, TiO2–NiO p–n nanocomposite with enhanced sonophotocatalytic activity under diffused sunlight, Ultrason. Sonochem., 2017, 35, 655–663 CrossRef CAS PubMed.
- S. Cai, J. Chen, Q. Li and H. Jia, Enhanced photocatalytic CO2 reduction with photothermal effect by cooperative effect of oxygen vacancy and Au cocatalyst, ACS Appl. Mater. Interfaces, 2021, 13(12), 14221–14229 CrossRef CAS PubMed.
- A. I. Rabee, D. Zhao, S. Cisneros, C. R. Kreyenschulte, V. Kondratenko, S. Bartling, C. Kubis, E. V. Kondratenko, A. Brückner and J. Rabeah, Role of interfacial oxygen vacancies in low-loaded Au-based catalysts for the low-temperature reverse water gas shift reaction, Appl. Catal., B, 2023, 321, 122083 CrossRef CAS.
- H. Sun, J. He, J. Wang, S.-Y. Zhang, C. Liu, T. Sritharan, S. Mhaisalkar, M.-Y. Han, D. Wang and H. Chen, Investigating the multiple roles of polyvinylpyrrolidone for a general methodology of oxide encapsulation, J. Am. Chem. Soc., 2013, 135(24), 9099–9110 CrossRef CAS PubMed.
- B.-Y. Wang, Y.-S. Hsiao, P.-C. Wei, Y.-T. Liu, C.-C. Chu and V. K. Hsiao, Visible Light-Induced Photocatalyst with Au/TiO2 Nanocomposites Fabricated through Pulsed Laser-Induced Photolysis, Catalysts, 2022, 12(5), 564 CrossRef CAS.
- M. Yadav, T. Gyulavári, J. Kiss, K. B. Ábrahámné, A. Efremova, Á. Szamosvölgyi, Z. Pap, A. Sápi, Á. Kukovecz and Z. Kónya, Noble metal nanoparticles and nanodiamond modified strontium titanate photocatalysts for room temperature CO production from direct hydrogenation of CO2, J. CO2 Util., 2023, 78, 102621 CrossRef CAS.
- T. Klaytae, P. Panthong and S. Thountom, Preparation of nanocrystalline SrTiO3 powder by sol–gel combustion method, Ceram. Int., 2013, 39, S405–S408 CrossRef CAS.
- S. Gerhold, M. Riva, Z. Wang, R. Bliem, M. Wagner, J. Osiecki, K. Schulte, M. Schmid and U. Diebold, Nickel-oxide-modified SrTiO3 (110)-(4× 1) surfaces and their interaction with water, J. Phys. Chem. C, 2015, 119(35), 20481–20487 CrossRef CAS PubMed.
- Y. Chen, J. Kang, B. Chen, B. Gao, L. Liu, X. Liu, Y. Wang, L. Wu, H. Yu and J. Wang, Microscopic mechanism for unipolar resistive switching behaviour of nickel oxides, J. Phys. D: Appl. Phys., 2012, 45(6), 065303 CrossRef.
- R. Bardestani, G. S. Patience and S. Kaliaguine, Experimental methods in chemical engineering: specific surface area and pore size distribution measurements—BET, BJH, and DFT, Can. J. Chem. Eng., 2019, 97(11), 2781–2791 CrossRef CAS.
- J. Di, C. Chen, C. Zhu, P. Song, J. Xiong, M. Ji, J. Zhou, Q. Fu, M. Xu and W. Hao, Bismuth vacancy-tuned bismuth oxybromide ultrathin nanosheets toward photocatalytic CO2 reduction, ACS Appl. Mater. Interfaces, 2019, 11(34), 30786–30792 CrossRef CAS PubMed.
- S. Ding, T. Dong, T. Peppel, N. Steinfeldt, J. Hu and J. Strunk, Construction of amorphous SiO2 modified β-Bi2O3 porous hierarchical microspheres for photocatalytic antibiotics degradation, J. Colloid Interface Sci., 2022, 607, 1717–1729 CrossRef CAS PubMed.
- H. Sato, T. Minami, S. Takata and T. Yamada, Transparent conducting p-type NiO thin films prepared by magnetron sputtering, Thin Solid Films, 1993, 236(1–2), 27–31 CrossRef CAS.
- M. C. Toroker, D. K. Kanan, N. Alidoust, L. Y. Isseroff, P. Liao and E. A. Carter, First principles scheme to evaluate band edge positions in potential transition metal oxide photocatalysts and photoelectrodes, Phys. Chem. Chem. Phys., 2011, 13(37), 16644–16654 RSC.
- S. Linic, P. Christopher and D. B. Ingram, Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy, Nat. Mater., 2011, 10(12), 911–921 CrossRef CAS PubMed.
- J. B. Priebe, M. Karnahl, H. Junge, M. Beller, D. Hollmann and A. Brückner, Water reduction with visible light: synergy between optical transitions and electron transfer in Au-TiO2 catalysts visualized by in situ EPR spectroscopy, Angew. Chem., Int. Ed., 2013, 52(43), 11420–11424 CrossRef CAS PubMed.
- Z. Zhang, X. Wang, J. Long, Q. Gu, Z. Ding and X. Fu, Nitrogen-doped titanium dioxide visible light photocatalyst: spectroscopic identification of photoactive centers, J. Catal., 2010, 276(2), 201–214 CrossRef CAS.
- M. Okumura, J. M. Coronado, J. Soria, M. Haruta and J. C. Conesa, EPR study of CO and O2 interaction with supported Au catalysts, J. Catal., 2001, 203(1), 168–174 CrossRef CAS.
- P. Claus, A. Brückner, C. Mohr and H. Hofmeister, Supported gold nanoparticles from quantum dot to mesoscopic size scale: Effect of electronic and structural properties on catalytic hydrogenation of conjugated functional groups, J. Am. Chem. Soc., 2000, 122(46), 11430–11439 CrossRef CAS.
- J. C. Wu and C.-W. Huang, In situ DRIFTS study of photocatalytic CO2 reduction under UV irradiation, Front. Chem. Eng. China, 2010, 4, 120–126 CrossRef CAS.
- C. Mu, C. Lv, X. Meng, J. Sun, Z. Tong and K. Huang, In situ characterization techniques applied in photocatalysis: a review, Adv. Mater. Interfaces, 2023, 10(3), 2201842 CrossRef.
- P. Naliwajko, T. Peppel and J. Strunk, Thermal and light induced infrared blackening of ZnO revisited: Rediscovery of fundamental scientific knowledge, React. Kinet., Mech. Catal., 2022, 135(5), 2291–2305 CrossRef CAS.
- X. Chang, T. Wang and J. Gong, CO2 photo-reduction: insights into CO2 activation and reaction on surfaces of photocatalysts, Energy Environ. Sci., 2016, 9(7), 2177–2196 RSC.
- K. Bhattacharyya, A. Danon, K. B. Vijayan, K. A. Gray, P. C. Stair and E. Weitz, Role of the surface lewis acid and base sites in the adsorption of CO2 on titania nanotubes and platinized titania nanotubes: an in situ FT-IR study, J. Phys. Chem. C, 2013, 117(24), 12661–12678 CrossRef CAS.
- G. Martra, Lewis acid and base sites at the surface of microcrystalline TiO2 anatase: relationships between surface morphology and chemical behaviour, Appl. Catal., A, 2000, 200(1–2), 275–285 CrossRef CAS.
- W. Su, J. Zhang, Z. Feng, T. Chen, P. Ying and C. Li, Surface phases of TiO2 nanoparticles studied by UV Raman spectroscopy and FT-IR spectroscopy, J. Phys. Chem. C, 2008, 112(20), 7710–7716 CrossRef CAS.
- K. K. Bando, K. Sayama, H. Kusama, K. Okabe and H. Arakawa, In-situ FT-IR study on CO2 hydrogenation over Cu catalysts supported on SiO2, Al2O3, and TiO2, Appl. Catal., A, 1997, 165(1–2), 391–409 CrossRef CAS.
- L.-F. Liao, C.-F. Lien, D.-L. Shieh, M.-T. Chen and J.-L. Lin, FTIR study of adsorption and photoassisted oxygen isotopic exchange of carbon monoxide, carbon dioxide, carbonate, and formate on TiO2, J. Phys. Chem. B, 2002, 106(43), 11240–11245 CrossRef CAS.
- J. Rasko and F. Solymosi, Infrared spectroscopic study of the photoinduced activation of CO2 on TiO2 and Rh/TiO2 catalysts, J. Phys. Chem., 1994, 98(29), 7147–7152 CrossRef CAS.
- J. Baltrusaitis, J. Schuttlefield, E. Zeitler and V. H. Grassian, Carbon dioxide adsorption on oxide nanoparticle surfaces, Chem. Eng. J., 2011, 170(2–3), 471–481 CrossRef CAS.
- L. Mino, G. Spoto and A. M. Ferrari, CO2 capture by TiO2 anatase surfaces: a combined DFT and FTIR study, J. Phys. Chem. C, 2014, 118(43), 25016–25026 CrossRef CAS.
- D. Bernitt, K. Hartman and I. Hisatsune, Infrared spectra of isotopic bicarbonate monomer ions, J. Chem. Phys., 1965, 42(10), 3553–3558 CrossRef CAS.
- C.-C. Yang, Y.-H. Yu, B. van der Linden, J. C. Wu and G. Mul, Artificial photosynthesis over crystalline TiO2-based catalysts: fact or fiction?, J. Am. Chem. Soc., 2010, 132(24), 8398–8406 CrossRef CAS PubMed.
- K. Li, S. Zhang, Q. Tan, X. Wu, Y. Li, Q. Li, J. Fan and K. Lv, Insulator in photocatalysis: Essential roles and activation strategies, Chem. Eng. J., 2021, 426, 130772 CrossRef CAS.
- S. Han, X. Li, Y. Tan, Y. Huang, Z. Wu, M. Wang, W. Ho and S.-C. Lee, In-situ self-sacrificed fabrication of insulator-based SrTiO3/SrCO3 heterojunction interface for gaseous HCHO and NO photocatalytic degradation, Appl. Surf. Sci., 2023, 612, 155806 CrossRef CAS.
- R. Jacobs, J. Booske and D. Morgan, Understanding and controlling the work function of perovskite oxides using density functional theory, Adv. Funct. Mater., 2016, 26(30), 5471–5482 CrossRef CAS.
- K. H. Zhang, R. Wu, F. Tang, W. Li, F. E. Oropeza, L. Qiao, V. K. Lazarov, Y. Du, D. J. Payne and J. L. MacManus-Driscoll, Electronic structure and band alignment at the NiO and SrTiO3 p–n heterojunctions, ACS Appl. Mater. Interfaces, 2017, 9(31), 26549–26555 CrossRef CAS PubMed.
- K. Domen, A. Kudo and T. Onishi, Mechanism of photocatalytic decomposition of water into H2 and O2 over NiO-SrTiO3, J. Catal., 1986, 102(1), 92–98 CrossRef CAS.
- S. A. Rawool, M. R. Pai, A. M. Banerjee, A. Arya, R. Ningthoujam, R. Tewari, R. Rao, B. Chalke, P. Ayyub and A. Tripathi, pn Heterojunctions in NiO: TiO2 composites with type-II band alignment assisting sunlight driven photocatalytic H2 generation, Appl. Catal., B, 2018, 221, 443–458 CrossRef CAS.
- B. Sun, G. Zhou, T. Gao, H. Zhang and H. Yu, NiO nanosheet/TiO2 nanorod-constructed p–n heterostructures for improved photocatalytic activity, Appl. Surf. Sci., 2016, 364, 322–331 CrossRef CAS.
- A. Hezam, T. Peppel and J. Strunk, Pathways towards a systematic development of Z scheme photocatalysts for CO2 reduction, Curr. Opin. Green Sustainable Chem., 2023, 100789 CrossRef CAS.
- A. Pougin, G. Dodekatos, M. Dilla, H. Tüysüz and J. Strunk, Au@ TiO2 core–shell composites for the photocatalytic reduction of CO2, Chem. – Eur. J., 2018, 24(47), 12416–12425 CrossRef CAS PubMed.
- H. Shen, T. Peppel, J. Strunk and Z. Sun, Photocatalytic reduction of CO2 by metal-free-based materials: recent advances and future perspective, Sol. RRL, 2020, 4(8), 1900546 CrossRef CAS.
- K. Li, X. An, K. H. Park, M. Khraisheh and J. Tang, A critical review of CO2 photoconversion: Catalysts and reactors, Catal. Today, 2014, 224, 3–12 CrossRef CAS.
- K. Li, B. Peng and T. Peng, Recent advances in heterogeneous photocatalytic CO2 conversion to solar fuels, ACS Catal., 2016, 6(11), 7485–7527 CrossRef CAS.
- L. Liao, G. Xie, X. Xie and N. Zhang, Advances in Modulating the Activity and Selectivity of Photocatalytic CO2 Reduction to Multicarbon Products, J. Phys. Chem. C, 2023, 127(6), 2766–2781 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cy00313f |
| This journal is © The Royal Society of Chemistry 2024 |