Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A fluorolytic sol–gel route to access an amorphous Zr fluoride catalyst: a useful tool for C–F bond activation†
Christian
Heinekamp
 ab,
Sönke
Kneiske
b,
Ana
Guilherme Buzanich
ab,
Sönke
Kneiske
b,
Ana
Guilherme Buzanich
 a,
Mike
Ahrens
a,
Mike
Ahrens
 b,
Thomas
Braun
b,
Thomas
Braun
 *b and
Franziska
Emmerling
*b and
Franziska
Emmerling
 *ab
*ab
aDepartment Materials Chemistry, Federal Institute for Material Research and Testing, Richard-Willstätter-Straße 11, 12489 Berlin, Germany. E-mail: franziska.emmerling@bam.de
bDepartment of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Straße 2, 12489 Berlin, Germany. E-mail: thomas.braun@cms.hu-berlin.de
First published on 5th January 2024
Abstract
A route to a ZrF4 catalyst active in room temperature Friedel–Crafts and dehydrofluorination reactions was developed via a fluorolytic sol–gel route, which was followed by a postfluorination step using a stream of CHClF2. The behaviour of different Zr(IV) precursors in a sol–gel reaction with anhydrous isopropanol/HF solution was investigated. The subsequent post-fluorination step was optimised in its temperature ramp and confirmed the necessity of a fluorination of the generated xerogels to obtain catalytic activity. The process is discussed in the context of the analysis of the materials using Brunauer–Emmett–Teller analysis (BET), powder X-ray diffraction (XRD), infrared spectroscopy (IR), thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). The local structure of the amorphous catalyst was elucidated by extended X-ray absorption fine structure spectroscopy (EXAFS).
Introduction
In the past 20 years major steps were taken in heterogeneous C–F bond activation at aluminium fluorides. The nanoscopic and amorphous aluminium chlorofluoride (ACF) was established as heterogeneous catalyst.1–6 Subsequently, the Kemnitz group developed a fluorolytic sol–gel route to access metal fluorides7 such as high-surface AlF3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8,9 and MgF2
8,9 and MgF2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10–12 which possess a high surface area. Particular in aluminium-based systems, the synthetic approach led to amorphous xerogels which can be further converted into Lewis superacids by postfluorination with Freon gas such as CCl2F2 (the product was then denoted as high-surface-AlF3).8 This progress has been crucial for the development of new materials for different catalytic applications. In general, this synthetic route offers access to metal fluorides, in addition to their respective oxides, which can be applied in heterogeneous catalysis. Before, the use of fluorides had been limited due to their small surface area, which is essential in heterogeneous catalysis to achieve high activity.13,14 Following this breakthrough the research on aluminium based catalysts has also been extended to include other elements such as chromium,15–17 whereas a recent approach involved the introduction of Brønsted and Lewis acidic sites into the HS-AlF3 by using Nb doping.19 Other approaches to AlF3 bearing a high surface area consist of a plasma-fluorination of a zeolite precursor.18
10–12 which possess a high surface area. Particular in aluminium-based systems, the synthetic approach led to amorphous xerogels which can be further converted into Lewis superacids by postfluorination with Freon gas such as CCl2F2 (the product was then denoted as high-surface-AlF3).8 This progress has been crucial for the development of new materials for different catalytic applications. In general, this synthetic route offers access to metal fluorides, in addition to their respective oxides, which can be applied in heterogeneous catalysis. Before, the use of fluorides had been limited due to their small surface area, which is essential in heterogeneous catalysis to achieve high activity.13,14 Following this breakthrough the research on aluminium based catalysts has also been extended to include other elements such as chromium,15–17 whereas a recent approach involved the introduction of Brønsted and Lewis acidic sites into the HS-AlF3 by using Nb doping.19 Other approaches to AlF3 bearing a high surface area consist of a plasma-fluorination of a zeolite precursor.18
Zirconium chlorofluoride (ZCF) has initially been reported to have analogous properties to ACF, but Krahl et al. could not confirm a comparable Lewis acidity.20 ZCF has recently been reported as the first heterogeneous HF shuttle at room temperature.21 Despite this knowledge, and the fact that zirconium oxide is described as a stronger Lewis acid than other metal oxides,22 zirconium fluoride-based materials and catalysts have only recently been reported or investigated. Zirconium fluoride has been described as having Lewis-acidic properties similar to the metastable β-AlF3 phase.23–25 Furthermore, Lewis acidic ZrF4 surfaces have been synthesised and characterised by George and co-workers via atomic layer deposition.26 However, recent literature stated that ZrF4 surfaces are assumed to exhibit lower Lewis acidity and lower catalytic activity compared aluminium-based based counterparts.26,27 These results are consistent with a theoretical assessment that due to the increased size and different electronic configuration of zirconium compared to aluminium atoms a decreasing Lewis acid strength can be expected.28
In this work we present the development of an amorphous ZrF4 heterogeneous catalyst which has been tested in C–F bond activation and was thoroughly characterised. The aim is to extend the catalytic capabilities of ZrF4. Its lower Lewis acidity is thought to be advantageous to allow better substrate desorption in catalysis.
Methods
The samples were prepared in a MBraun glovebox and all reactions were performed in JYoung NMR tubes using conventional Schlenk techniques, if not indicated otherwise. C6D6 was dried over K-Solvona and distilled before usage, and C6D12 was purchased from Eurisotop and stored over molecular sieves. Zr(OEt)4 (99%), Zr(OtBu)4 (99%) and Zr(acac)4 (98+%) were obtained from Alfa Aeser, ABCR and ChemPur GmbH, respectively. iPrOH (99.5%, extra pure) was supplied by Carl-Roth, distilled and stored over molecular sieves. Triethylsilane and 1-fluoropentane were purchased from Sigma-Aldrich.Preparation of catalysts/materials
In a typical synthesis the Zr(IV) precursor, either Zr(OEt)4, Zr(OtBu)4 or Zr(acac)4 (10 mmol) was placed in a flask under argon. In case of Zr(acac)4 the precursor was weighed in under atmospheric conditions and placed under vacuum for 1 h in the reaction vessel before the procedure was continued. 250 mL of iPrOH were then added to the reagent. The mixture was refluxed for 1 h and allowed to cool down (Fig. 1). While the solutions including the alkoxides remained colourless the mixture obtained from Zr(acac)4 appeared to be a stable milky suspension as soon as the temperature dropped below 40 °C. Subsequently, an HF solution in iPrOH (21.5 M, 4 eq.) was added dropwise, but rapidly.The solvent was removed under vacuum, after the mixture was aged overnight under vigorous stirring. The product was stored under Ar in a glovebox. The products are designated as ZrF3–(F,OH)(OEt); ZrF3–(F,OH)(OtBu) and ZrF3–(F,OH)(acac), respectively. Note that due to the incomplete fluorination and residual organic content the xerogels should rather be considered as zirconium-alkoxo-fluorides (Zr(OR)4−xFy).
The ZrF3–(F,OH) materials were treated further in a post-fluorination step (obtained materials are denoted as ZrF4−x (post)). For this, the samples were treated under a flow of Ar and CHClF2 in a temperature programmed Ni reactor (Fig. 1). For optimisation purposes the temperature program and the ratio between Ar and CHClF2 were varied. A description of the parameters used can be found in Table 2.
X-ray diffraction
X-ray powder diffraction measurements were performed on an STOE Stadi MP diffractometer equipped with a Dectris Mythen 1 K linear silicon strip detector and Ge(111) double-crystal monochromator (Mo-K radiation) in a transmission geometry. The samples were prepared under Ar in a Glovebox.High-resolution powder X-ray diffraction patterns of ZrF3–(F,OH)(acac) were collected with Cu Kα radiation at a Bragg Brentano D8 Advanced diffractometer (Bruker AXS, Germany) equipped with a LYNXEYE XE-T detector. Samples were measured in reflection geometry in a 2θ range from 3° to 50° with a step size of 0.02° with spinning setup.
Crystal structure resolution calculations
The crystal structure resolution from powder diffraction data was performed via simulated annealing with the software EXPO2014.29Infrared spectroscopy
The IR-spectra were recorded in a glovebox at a Bruker Alpha II spectrometer equipped with a diamond ATR (attenuated total reflectance) or DRIFTS (diffuse reflectance infrared Fourier transform spectroscopy) unit (Pyroelectric DTGS detector).TGA/DSC
The TGA and DSC measurements were performed at a TGA/DSC 3+ from Mettler Toledo, Switzerland. Samples were weight in in a glovebox and sealed with the A2 closing stamp. The closed crucible was pinned in a N2 stream by the sample robot. The samples were heated from 25 to 600 °C at a rate of 10 K min−1. Afterwards the samples were cooled down to 25 °C at the same rate.N2 gas sorption measurements
Low temperature adsorption isotherms of nitrogen at 77 K were determined with an Autosorb iQ from Anton Paar. Approximately 150 mg of the samples were tempered at 150 °C for 10 h immediately before the measurement at the device. The synthesised xerogels were tempered at 100 °C for 10 h immediately before the measurement at the device. The evaluation was carried out according to the BET theory.30 The BJH method was used to determine the pore size. The pore volume was determined at P/P0 = 0.95.31EXAFS
EXAFS measurements were performed at the BAMline at BESSY-II.32 The beam was monochromatized using a double-crystal monochromator (DCM) installed at the beamline, with a resolution (ΔE/E) of about 2 × 10−4. The slits were adjusted to provide a 4 mm (H) × 1 mm (V) spot size.The measurements were performed at the Zr K-edge (17.889 keV) in transmission, as the sample preparation allowed choosing the adequate thickness for optimal absorption, establishing an edge jump factor of about 2. This was achieved by diluting the powder samples with boron nitride (BN). The excitation energy was varied from −200 eV to −20 eV below the edge in 10 eV steps, from −20 eV below the edge and 200 eV above the edge in 1 eV steps, and in the EXAFS region with a constant step in the k-space of 0.04 Å−1 until k = 16 Å. EXAFS data were processed by ATHENA and ARTEMIS.33 These GUIs programs belong to the main package IFEFFIT (v. 1.2.12). The AutoBK background subtraction procedure was used with the Rbkg parameter set to 1.0 Å and kw = 1. Afterwards all spectra were normalized to the far post-edge region, free from absorption features. Regarding the EXFAS region, with ATHENA one can plot χ(k) against R (Å) and the oscillations represent different frequencies, which correspond to the different distances for each coordination shell. Hence, Fourier transforms (FT) are necessary for the analysis process. The FT from the k-space to R-space were performed with a Hanning-type window with a range of 1.5 Å to 14 Å. By analyzing the signal in the frequency domain in ATHENA the window range was selected to exclude the noisy part of the signal.
NMR spectroscopy
Liquid NMR spectra were measured at a Bruker DPX 300 or Bruker AVANCE II 300 spectrometer at room temperature. 1H NMR chemical shifts δ were referenced to residual C6D5H (δ = 7.16 ppm) or C6D11H (δ = 1.38 ppm) respectively.19F NMR spectra were calibrated externally to CFCl3 (δ = 0 ppm).Reactivity studies using fluoropentane
15 mg of the respective material were placed in a JYoung NMR tube and triethylsilane (0.2 mmol) and fluoropentane (0.2 mmol) were added subsequently using a microsyringe. After that either C6D6 or C6D12 was added to the reaction mixture. The products were analyzed by NMR spectroscopy.Formation of ZrF4·CD3CN
The investigated sample (120 mg) was suspended in an excess of CD3CN in a Schlenk flask and stirred at 25 °C for two hours. The excess of CD3CN was removed under vacuum to obtain a light brown powder.Results and discussion
A crystalline zirconium alkoxofluoride was synthesised using a strategy for xerogel synthesis previously described for aluminium fluorides. A solution of HF in iPrOH was added in stoichiometric amounts to a solution of the metal precursor in dry isopropanol. After removal of the solvent, the obtained xerogels were exposed to a postfluorination step.34 The reaction mixtures using zirconium precursors appear as turbid gels directly after HF addition, while with aluminium alkoxides transparent gels are formed.19 The use of different zirconium alkoxides as the metal source in the isopropanol solution does not affect the appearance of the reaction mixture. However, when the acetylacetonate complex was used a slightly different observation was made. When the mixture cooled down to room temperature it became a white suspension with a comparable viscosity compared to the hot solution. Immediately after HF addition the mixture cleared and formed an optically identical gel to the alkoxides. In all cases the xerogels were obtained as white powders. Interestingly, the xerogels obtained appear as crystalline phases (Fig. 2). Regardless of the Zr source used, the same crystalline phase is formed. This is in contrast to Al based systems which form amorphous xerogels.8,19 The phases shown were compared with reported crystalline phases, but no match was not found in databases. In particular, the XRD pattern is dominated by a reflex at very low q, indicating a large unit cell size. The stability of the xerogel in air is longer than four months after which the powder diffraction pattern can be assigned to ZrF4·(H2O). | ||
| Fig. 2 Powder XRD patterns of the xerogels obtained from the respective Zr–precursor complex: (a) Zr(OEt)4; (b) Zr(OtBu)4; (c) Zr(acac)4. | ||
The similarity between the Zr-xerogels is also revealed by the IR data (Fig. 3), as almost matching spectra were observed. Besides the broad band at 3180 cm−1 which can be attributed to a [ν(O–H)], typical IR bands for the isopropyl group can be detected. The IR bands between 600–800 cm−1 can be attributed to Zr–O vibrations, by comparing with data that have previously been calculated for Zr–O clusters.35 Zr–F bands could not be detected as the measurement at this end was limited to 400 cm−1.36 The data also show bands for iPr residues, which suggests that a complete fluorination did not occur with HF.
 | ||
| Fig. 3 ATR-FTIR spectra of the xerogels obtained from the respective Zr–precursor complex: (a) Zr(OEt)4; (b) Zr(OtBu)4; (c) Zr(acac)4. | ||
Thermogravimetric analysis coupled with differential scanning calorimetry (TGA/DSC) was carried out to confirm that the organic content of all the samples was similar (Fig. 4). The TGA traces of all samples show a large weight loss of 24%, 26% and 18% respectively between 150 °C and 200 °C. While the slope of the trace for ZrF3–(F,OH)(OtBu) and ZrF3–(F,OH)(acac) is distinct, the offset point for ZrF3–(F,OH)(OEt) is less defined. It is likely that mainly bound isopropanol is lost. This weight loss is consistent with previous studies at Al fluoride compounds as usually a complete fluorination is not achieved by fluorolytic sol–gel synthesis.8,19 The differences between the samples can be explained by the stability of the complex precursors. It can be assumed that some ethoxide is still bound and not fully exchanged with isopropanol leading to the initial weight loss before 150 °C mentioned above. The tBuO group is the one most likely to be completely exchanged by isopropanol, which is indicated by the larger weight loss. The xerogel derived from the Zr(acac)4 complex ZrF3–(F,OH)(acac) shows the lowest weight loss up to a temperature of 200 °C. As acetylacetonate is a strong chelating ligand this behaviour can be expected as it is the least likely one to be exchanged with isopropanol. Therefore, it is likely that not all of the ligands were exchanged with isopropanol.37 Above 200 °C all samples show a steady decrease in weight up to about 400 °C, 325 °C and 380 °C respectively. The end of the weight loss is also accompanied by an exothermic process visible in the DSC trace. In addition to the steady weight loss ZrF3–(F,OH)(acac) shows an additional step at approximately 280 °C, which can be attributed to the removal of residual acac ligands. Overall despite the use of different precursors to generate the samples, all samples show a similar weight loss, which is also comparable to the reported weight loss for aluminium alkoxofluorides.8 Note that due to the incomplete fluorination and residual organic content the xerogels should rather be considered as zirconium-alkoxo-fluorides (Zr(OR)4−xFy) (Table 1).
 | ||
| Fig. 4 TGA (blue)/DSC (red) traces from the xerogels obtained from the respective Zr–precursor complex: (a) Zr(OEt)4; (b) Zr(OtBu)4; (c) Zr(acac)4. | ||
| Material | BET surface area/m2 g−1 | BJH pore diameter/nm |
|---|---|---|
| ZrF3–(F,OH)(OEt) | 300 | 3 |
| ZrF3–(F,OH)(OEt)200 | 34 | 34 |
| ZrF3–(F,OH)(OEt)post | 58 | 35 |
| ZrF3–(F,OH)(OtBu) | 22 | 194 |
| ZrF3–(F,OH)(OtBu)200 | 61 | 15 |
| ZrF3–(F,OH)(OtBu)post | 110 | 10 |
| ZrF3–(F,OH)(acac) | 26 | 34 |
| ZrF3–(F,OH)(acac)200 | 79 | 19 |
| ZrF3–(F,OH)(acac)post | 63 | 8 |
As mentioned above one of the main advantages of fluorolytic sol–gel synthesis is the generation of an increased surface area of the fluorides obtained. Therefore, N2 adsorption experiments were carried out (Fig. 5). Despite the comparable diffraction and spectroscopic data significantly different surface areas were found using BET. While ZrF3–(F,OH)(OEt) shows a surface area of 300 m2 g−1, ZrF3–(F,OH)(OtBu) and ZrF3–(F,OH)(acac) only show surface areas of 22 m2 g−1 and 26 m2 g−1, respectively. ZrF3–(F,OH)(OEt) does not quiet reach the values reported for the aluminium based xerogel to be 430 m2 g−1, whereas the other two precursors do not even exceed the data for crystalline metal fluorides.8
In order to get a better comparison of the newly formed materials with the AlF3 xerogels, which also loses surface area when heated without fluorinating agent, the samples were annealed under Ar atmosphere to 200 °C for 4 h. ZrF3–(F,OH)(OEt) shows the expected decrease in surface area to 34 m2 g−1. In contrast, ZrF3–(F,OH)(OtBu) and ZrF3–(F,OH)(acac) show a significant increase in surface area to 61 m2 g−1 and 79 m2 g−1 after heating, respectively. This is accompanied by the presence of a type IV hysteresis for the latter samples.38 The results indicate an organic template character which might be due to the more sterically demanding ligands, where the bound isopropoxide is removed without structural collapse.39
To investigate the formation of the unknown crystalline phase of the xerogels obtained from the sol–gel fluorination with HF, further xerogels were synthesized by using under-stoichiometric amounts of HF. Zr(acac)4 was used as the zirconium source in the sol–gel synthesis due to the easier handling. The corresponding XRD patterns were obtained by ex situ XRD measurements. The obtained data show structural changes due to the different fluorination degree in the obtained materials. The addition of one equivalent led to a distinct crystalline pattern. As two equivalents of HF are used, another diffraction pattern is observed. The use of three equivalents of HF then led to the formation of a seemingly largely amorphous material, while the reflection at low q, which was also found to be prominent for the ZrF3–(F,OH)(acac), was observed (see ESI† Fig. S3). This indicates that after addition of three equivalents of HF, already large periodic unit cells are formed, but the formation of larger crystallites is possibly hindered.
However, the experimental data and the current state of literature allow certain suggestions concerning the structure. Firstly, the powder XRD pattern indicates a large unit cell due to the first reflection at 6 nm−1. The large unit cell can include clusters as motifs. Such oxoclusters of zirconium compounds are known to some extent.40–42 A more recent example reports a Zr4 motif as a Zr-oxocation.42 Interestingly, only one example is known in literature for a Zr–F cluster, which also exhibits a four membered ring consisting of Zr coordination polyhedra with bridging F vertices (see ESI†).43 Due to this knowledge such a cluster was postulated for a crystal structure resolution. While the modelling did not provide a structure for the acac containing xerogel, cell parameters (a = 11.422 Å, b = 5.111 Å, c = 5.803 Å, β = 91.781°, volume = 338.647 Å3) for a monoclinic space group (P21) were obtained through indexing.
Xerogels obtained by the fluorolytic sol–gel process can be further fluorinated by a post-fluorination process on using freon gases.8,44 CHClF2 (R22) was used for the gas phase fluorination of ZrF3–(F,OH)(OEt). To test the catalytic activity of the obtained materials after the post-fluorination, i.e. Friedel–Crafts reactions and dehydrofluorination reactions (Scheme 1) were performed. Both transformations have been studied previously and the activity relates to the presence of Lewis-acidic centres for activation.45–48 In both reactions 1-fluoropentane was transformed in the presence of stoichiometric amounts of triethyl silane. In the case of the Friedel–Crafts reaction, deuterated benzene was used as a solvent acting as reagent at the same time. Regarding the dehydrofluorination reaction deuterated cyclohexane was used which is not active for Friedel–Crafts reactions.
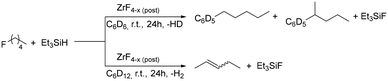 | ||
| Scheme 1 Reaction scheme of Friedel–Crafts-reaction (in C6D6) and dehydrofluorination (in C6D12) reactivity tests at room temperature. | ||
Although both reactivities are reported in literature, it is uncommon for heterogeneous catalysts to perform at room temperature.45–48
Mechanistically, in both cases the silane might adsorb to the Lewis acidic centres on the surface of the catalyst forming a silylium ion species, which then activates the C–F bond of the fluoroalkane to yield a carbenium-like species. The silane then acts as hydrogen source in both the dehydrofluorination and the Friedel–Crafts reaction.45 Hence, dihydrogen and HD are formed, respectively. Activity of both reactions could not be observed, if the xerogel was used. In the following section both reactivities are discussed in parallel.
The postfluorination which was performed in a nickel gas flow reactor (Fig. 1) using argon as carrier gas and R22 as fluorinating agent, was optimised systematically. Conditions regarding the R22 content (20%) and contact time (1.2 s) used for the postfluorination for the formation of HS-AlF3 did not succeed for the zirconium based xerogel, leading to a slight crystalline material that does not show any catalytic activity (Table 2, entry 1). An increase of the amount of R22 in the gas stream affected the reaction mixture in two ways. Firstly, the concentration of the fluorinating agent increases. Secondly, the contact time of the gas stream with the material is reduced as the carrier gas flow is kept constant. This can lead to a contradictory situation that the reaction time is not sufficient, although more fluorinating agent is available. This effect might be the reason for lower activity of the material in catalysis when the R22 content (total fraction in the Ar/R22-gas stream) was increased to 43% (Table 2, entry 3) compared to the 33% content (Table 2, entry 2). Thus, entry 2 clearly shows significantly higher yield in both, the dehydrofluorination reaction of fluoropentane and in the Friedel–Crafts alkylation, with an additional absolute yield difference of 43%, whereas entry 3 shows equivalent but lower yields for both reactions. Interestingly, if the total postfluorination time is kept constant but the temperature is varied the “selectivity” observed for entry 2 changed to higher yields for the Friedel–Crafts alkylation (Table 2, entries 4, 6 and 7). It was observed that if the temperature regime at 240 °C is reduced or omitted, only low yields in the dehydrofluorination reaction at room temperature were found, whereas Friedel–Crafts alkylations stay above 10% (Table 2, entries 6 and 7). When the temperature program is altered to include higher temperatures or a prolonged heating at 240 °C, no activity was observed anymore.
| Entry | Precursor | Temperature gradient | R22 contenta/% | Contact time/s | Yield pent-2-eneb/% | Yield pentylbenzeneb/% | |||
|---|---|---|---|---|---|---|---|---|---|
| 150 °C | 200 °C | 240 °C | 300 °C | ||||||
| a The portion of R22 in the overall Ar/R22 gas flow. b Calculated by the conversion of 1-fluoropentane into the respective products by integration of the 1H-NMR signals after 24 h. c Adapted conditions from HS-AlF3 postfluorination. | |||||||||
| 1c | ZrF3–(F,OH)(OEt) | 1 h | 2 h | 2 h | — | 20 | 1.2 | 0 | 0 |
| 2 | ZrF3–(F,OH)(OEt) | 1 h | 2 h | 2 h | — | 33 | 1 | 82 | 39 |
| 3 | ZrF3–(F,OH)(OEt) | 1 h | 2 h | 2 h | — | 43 | 0.85 | 11 | 11 |
| 4 | ZrF3–(F,OH)(OEt) | 1 h | 3 h | 1 h | — | 33 | 1 | 2 | 9 |
| 5 | ZrF3–(F,OH)(OEt) | 1 h | 1 h | 3 h | — | 33 | 1 | 0 | 0 |
| 6 | ZrF3–(F,OH)(OEt) | 1 h | 4 h | — | — | 33 | 1 | 0.5 | 18 |
| 7 | ZrF3–(F,OH)(OEt) | 5 h | — | — | — | 33 | 1 | 2 | 11 |
| 8 | ZrF3–(F,OH)(OEt) | 1 h | 2 h | 3 h | — | 33 | 1 | 0 | 0 |
| 9 | ZrF3–(F,OH)(OEt) | 1 h | 2 h | — | 2 h | 33 | 1 | 0 | 0 |
| 10 | ZrF3–(F,OH)(OtBu) | 1 h | 2 h | 2 h | — | 33 | 1 | 25 | 14 |
| 11 | ZrF3–(F,OH)(acac) | 1 h | 2 h | 2 h | — | 33 | 1 | 65 | 25 |
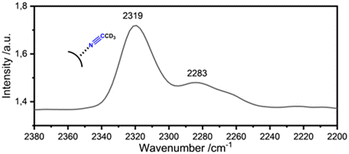
Using the optimized conditions shown in entry 2 for ZrF3–(F,OH)(OEt), the reactivity for the xerogels ZrF3–(F,OH)(OtBu) and ZrF3–(F,OH)(acac) was also studied (entries 10 and 11). Overall the catalytic activity of the material that require Lewis acidic sites.48 For the qualitative characterisation these Lewis acidic sites49 diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) was performed at a CD3CN adduct ZCF·CD3CN (Table 2 entry 11; Fig. 6) The adsorption of the CD3CN on the surface, explicitly on the acidic Lewis sites, causes a blue shift of the CN vibrational band at 2258 cm−1. The extent of the observed blue shift indicates the strength of the available sites. In the DRIFTS spectrum of the post-fluorinated material described in entry 11 (Table 2) two vibrational bands can be observed after the adsorption of CD3CN. The band at 2283 cm−1 (Δν = 25 cm−1), corresponding to very weak Lewis acidic centres. However, a band at 2319 cm−1 (Δν = 61 cm−1) can be attributed to medium to strong Lewis acidic sites.
Despite the reactivity difference for the ZrF3–(F,OH)(acac) based catalyst, the yield drop observed when the ZrF3–(F,OH)(OtBu) based material was used is more significant. This change cannot be directly attributed to structural differences as all catalysts are amorphous and knowledge about the local structure of the active site is very limited. This is supported by the IR measurements of the materials after postfluorination obtained by the conversions in the entries 10 and 11 which exhibit bands for residual organics whereas the material produced in entry 2 shows no bands in that region (see ESI†). Despite these minor IR bands visible for the Zr(acac)4 and Zr(OtBu)4 based samples, all three materials display a very low mass loss of about 3% in TGA measurements, which indicates a very high fluorination degree. The influence of the choice of precursor on the surface area has already been mentioned. The postfluorinated xerogels ZrF3–(F,OH)(OEt)post, ZrF3–(F,OH)(OtBu)post and ZrF3–(F,OH)(acac)post which exhibit surface areas according to BET of 58 m2 g−1, 110 m2 g−1 and 63 m2 g−1, respectively. All BET curves display a slight hysteresis loop according to isotherm IV (Fig. 5).50 The reason for the significantly higher surface area of ZrF3–(F,OH)(OtBu)post is unclear.
Extended X-ray absorption fine structure spectroscopy (EXAFS) was performed on the Zr-K-edge (Fig. 7) for the best performing catalyst related to the conversion shown in Table 2 entry 2. The elemental specificity of EXAFS allowed the local coordination sphere at the Zr centres to be probed. Starting from the eightfold coordination in β-ZrF4 (ref. 24 and 51) the coordination sphere was modelled. Surprisingly, the model was able to fit up to a radial distance of more than 4 Å which begins to describe the second coordination sphere. Typically, one would expect information about the first 3 Å in such system. This means that although the material is amorphous it consists on average of similar extended local environments around the Zr centres as β-ZrF4.
 | ||
| Fig. 7 EXAFS data obtained from the Zr K-edge. Magnitude of χ from Table 2 entry 2 ZrF4−x (post) using a fitting model of β-ZrF4 (R-factor = 0.026). | ||
Conclusions
We have shown that active Lewis acidic zirconium fluoride catalysts are accessible via a fluorolytic sol–gel route. It has been revealed that the surface area of the resulting product can be influenced by the Zr source used. More importantly the use of the air-stable precursor compound Zr(acac)4 was found to be a reasonable starting material for the sol–gel process, avoiding the need for air sensitive alkoxides. The synthesised catalysts obtained by postfluorination of the xerogels are active in Friedel–Crafts and dehydrofluorination reactions at room temperature. The development of defluorination processes play an important role concerning the evolving scarcity of fluorspar, the source of HF, which needs to be addressed.52Author contributions
Christian Heinekamp and Sönke Kneiske performed the synthesis and analysis (NMR IR, TGA XRD). Christian Heinekamp drafted the first version of the manuscript. Ana Guilherme Buzanich performed the EXAFS measurements. The EXAFS data were jointly analysed by Christian Heinekamp and Ana Guilherme Buzanich. Structural resolution was performed by Christian Heinekamp and Franziska Emmerling. Thomas Braun, Mike Ahrens and Franziska Emmerling supervised the work and contributed to manuscript writing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge financial support from the CRC 1349 “Fluorine Specific Interactions” funded by the German Research Foundation (project number 387284271). The authors thank Minh Bui for BET experiments. EXAFS experiments were performed at the BAMline at the BESSY-II storage ring (Helmholtz Center Berlin). We thank the Helmholtz-Zentrum Berlin für Materialien und Energie for the allocation of synchrotron radiation beamtime. We are thankful to Stephanos Karafiludis for helpful discussions.Notes and references
- T. Krahl, R. Stosser, E. Kemnitz, G. Scholz, M. Feist, G. Silly and J. Y. Buzare, Inorg. Chem., 2003, 42, 6474–6483 CrossRef CAS PubMed.
- T. Krahl and E. Kemnitz, Angew. Chem., Int. Ed., 2004, 43, 6653–6656 CrossRef CAS PubMed.
- M. C. Kervarec, T. Braun, M. Ahrens and E. Kemnitz, Beilstein J. Org. Chem., 2020, 16, 2623–2635 CrossRef CAS PubMed.
- M.-C. Kervarec, C. P. Marshall, T. Braun and E. Kemnitz, J. Fluorine Chem., 2019, 221, 61–65 CrossRef CAS.
- B. Calvo, J. Wuttke, T. Braun and E. Kemnitz, ChemCatChem, 2016, 8, 1945–1950 CrossRef CAS.
- G. Meißner, K. Kretschmar, T. Braun and E. Kemnitz, Angew. Chem., 2017, 129, 16556–16559 CrossRef.
- E. Kemnitz and J. Noack, Dalton Trans., 2015, 44, 19411–19431 RSC.
- E. Kemnitz, U. Gross, S. Rudiger and C. S. Shekar, Angew. Chem., Int. Ed., 2003, 42, 4251–4254 CrossRef CAS PubMed.
- E. Kemnitz, U. Groß, S. Rüdiger and C. S. Shekar, Angew. Chem., 2003, 115, 4383–4386 CrossRef.
- A. Dimitrov, S. Wuttke, S. Troyanov and E. Kemnitz, Angew. Chem., Int. Ed., 2008, 47, 190–192 CrossRef CAS PubMed.
- S. Wuttke, S. M. Coman, G. Scholz, H. Kirmse, A. Vimont, M. Daturi, S. L. M. Schroeder and E. Kemnitz, Chem. – Eur. J., 2008, 14, 11488–11499 CrossRef CAS PubMed.
- J. Krishna Murthy, U. Groß, S. Rüdiger, E. Kemnitz and J. M. Winfield, J. Solid State Chem., 2006, 179, 739–746 CrossRef CAS.
- M. Boudart, in Handbook of heterogeneous catalysis, Wiley-VCH, Weinheim, 2nd edn, 2008 Search PubMed.
- G. Busca, Heterogeneous catalytic materials: solid state chemistry, surface chemistry and catalytic behaviour, Elsevier, Amsterdam Heidelberg, 1st edn, 2014 Search PubMed.
- G. Tavčar and T. Skapin, J. Fluorine Chem., 2019, 222–223, 81–89 CrossRef.
- I. K. Murwani, K. Scheurell and E. Kemnitz, Catal. Commun., 2008, 10, 227–231 CrossRef CAS.
- W. Mao, Y. Bai, Z. Jia, Y. Qin, B. Wang, W. Zhang, J. Lu and E. Kemnitz, Dalton Trans., 2022, 51, 935–945 RSC.
- J. L. Delattre, P. J. Chupas, C. P. Grey and A. M. Stacy, J. Am. Chem. Soc., 2001, 123, 5364–5365 CrossRef CAS PubMed.
- C. P. Marshall, G. Scholz, T. Braun and E. Kemnitz, Dalton Trans., 2019, 48, 6834–6845 RSC.
- T. Krahl and E. Kemnitz, J. Fluorine Chem., 2006, 127, 663–678 CrossRef CAS.
- C. Heinekamp, A. G. Buzanich, M. Ahrens, T. Braun and F. Emmerling, Chem. Commun., 2023, 59, 11224–11227 RSC.
- C. Nicollet, C. Toparli, G. F. Harrington, T. Defferriere, B. Yildiz and H. L. Tuller, Nat. Catal., 2020, 3, 913–920 CrossRef CAS.
- C. L. Bailey, A. Wander, S. Mukhopadhyay, B. G. Searle and N. M. Harrison, Phys. Chem. Chem. Phys., 2008, 10, 2918–2924 RSC.
- S. L. Benjamin, W. Levason, D. Pugh, G. Reid and W. Zhang, Dalton Trans., 2012, 41, 12548–12557 RSC.
- A. N. Romanov, E. V. Haula, Z. T. Fattakhova, A. A. Veber, V. B. Tsvetkov, D. M. Zhigunov, V. N. Korchak and V. B. Sulimov, Opt. Mater., 2011, 34, 155–158 CrossRef CAS.
- Y. Lee, H. X. Sun, M. J. Young and S. M. George, Chem. Mater., 2016, 28, 2022–2032 CrossRef CAS.
- X. Wu, Y. Zeng, Z.-T. Jiang, Y. Zhu, L. Xie and Y. Xia, Org. Lett., 2022, 24, 8429–8434 CrossRef CAS PubMed.
- D. P. N. Satchell and R. S. Satchell, Q. Rev., Chem. Soc., 1971, 25, 171–199 RSC.
- A. Altomare, C. Cuocci, C. Giacovazzo, A. Moliterni, R. Rizzi, N. Corriero and A. Falcicchio, J. Appl. Crystallogr., 2013, 46, 1231–1235 CrossRef CAS.
- S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60, 309–319 CrossRef CAS.
- E. P. Barrett, L. G. Joyner and P. P. Halenda, J. Am. Chem. Soc., 1951, 73, 373–380 CrossRef CAS.
- A. Guilherme Buzanich, M. Radtke, K. V. Yusenko, T. M. Stawski, A. Kulow, C. T. Cakir, B. Röder, C. Naese, R. Britzke, M. Sintschuk and F. Emmerling, J. Chem. Phys., 2023, 158, 244202 CrossRef CAS PubMed.
- B. Ravel and M. Newville, J. Synchrotron Radiat., 2005, 12, 537–541 CrossRef CAS PubMed.
- C. P. Marshall, T. Braun and E. Kemnitz, Catal. Sci. Technol., 2018, 8, 3151–3159 RSC.
- R. Jin, Y. Zhang, S. Huang, P. Wang and P. Tian, Chin. J. Chem., 2011, 29, 13–20 CrossRef CAS.
- V. Ya. Kavun, A. B. Slobodyuk, E. I. Voit, S. L. Sinebryukhov, E. B. Merkulov and V. K. Goncharuk, J. Struct. Chem., 2010, 51, 862–868 CrossRef CAS.
- D. Gibson, Coord. Chem. Rev., 1969, 4, 225–240 CrossRef CAS.
- Characterization of porous solids and powders: surface area, pore size, and density, Springer, Dordrecht, Boston, 2006 Search PubMed.
- K. J. C. van Bommel, A. Friggeri and S. Shinkai, Angew. Chem., Int. Ed., 2003, 42, 980–999 CrossRef CAS PubMed.
- F. R. Kogler, M. Jupa, M. Puchberger and U. Schubert, J. Mater. Chem., 2004, 14, 3133–3138 RSC.
- J. Napal, B. Artetxe, G. Beobide, O. Castillo, A. Luque, J. Pascual-Colino, S. Pérez-Yáñez and M. Perfecto-Irigaray, Inorg. Chem. Front., 2022, 9, 935–940 RSC.
- J. A. Sommers, D. C. Hutchison, N. P. Martin, K. Kozma, D. A. Keszler and M. Nyman, J. Am. Chem. Soc., 2019, 141, 16894–16902 CrossRef CAS PubMed.
- C. R. Ross, B. L. Paulsen, R. M. Nielson and S. C. Abrahams, Acta Crystallogr., Sect. B: Struct. Sci., 1998, 54, 417–423 CrossRef.
- E. Kemnitz, A. Hess, G. Rother and S. Troyanov, J. Catal., 1996, 159, 332–339 CrossRef CAS.
- M. Ahrens, G. Scholz, T. Braun and E. Kemnitz, Angew. Chem., Int. Ed., 2013, 52, 5328–5332 CrossRef CAS PubMed.
- G. Meißner, D. Dirican, C. Jäger, T. Braun and E. Kemnitz, Catal. Sci. Technol., 2017, 7, 3348–3354 RSC.
- C. P. Marshall, G. Scholz, T. Braun and E. Kemnitz, Catal. Sci. Technol., 2020, 10, 391–402 RSC.
- M. Bui, K. Hoffmann, T. Braun, S. Riedel, C. Heinekamp, K. Scheurell, G. Scholz, T. Stawski and F. Emmerling, ChemCatChem, 2023, e202300350 CrossRef CAS.
- P. O. Scokart and P. G. Rouxhet, J. Colloid Interface Sci., 1982, 86, 96–104 CrossRef CAS.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87, 1051–1069 CrossRef CAS.
- C. Legein, F. Fayon, C. Martineau, M. Body, J.-Y. Buzaré, D. Massiot, E. Durand, A. Tressaud, A. Demourgues, O. Péron and B. Boulard, Inorg. Chem., 2006, 45, 10636–10641 CrossRef CAS PubMed.
- S. Bobba, S. Carrara, J. Huisman, F. Mathieux and C. Pavel, Critical raw materials for strategic technologies and sectors in the EU—a foresight study, 2020 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cy01439h |
| This journal is © The Royal Society of Chemistry 2024 |