Open Access Article
Open Access ArticleChemical strategies for antisense antibiotics
Mathijs J.
Pals†
 ,
Alexander
Lindberg†
and
Willem A.
Velema
,
Alexander
Lindberg†
and
Willem A.
Velema
 *
*
Institute for Molecules and Materials, Radboud University Nijmegen, the Netherlands. Heyendaalseweg 135, 6525 AJ Nijmegen, The Netherlands. E-mail: willem.velema@ru.nl
First published on 22nd October 2024
Abstract
Antibacterial resistance is a severe threat to modern medicine and human health. To stay ahead of constantly-evolving bacteria we need to expand our arsenal of effective antibiotics. As such, antisense therapy is an attractive approach. The programmability allows to in principle target any RNA sequence within bacteria, enabling tremendous selectivity. In this Tutorial Review we provide guidelines for devising effective antibacterial antisense agents and offer a concise perspective for future research. We will review the chemical architectures of antibacterial antisense agents with a special focus on the delivery and target selection for successful antisense design. This Tutorial Review will strive to serve as an essential guide for antibacterial antisense technology development.
Key learning points(1) How to design an antibacterial antisense oligomer.(2) The various available backbone architectures of antibacterial antisense agents. (3) Chemical modifications that can enhance performance and stability of antisense agents. (4) The chemistry behind the available delivery vehicles and how to select the best option. (5) Targetability of mRNAs and what makes an attractive target. |
1. Introduction
The alarming rise of antibiotic resistant bacteria poses a major threat to global health, with infections becoming increasingly more difficult to treat.1 Most antibiotics to date have been found between the 1940s and 1960s, generally considered the golden age of antibiotic discovery.2 In recent decades, few new classes of antibiotics have reached the clinic, and the effectiveness of the current arsenal is decreasing due to antibiotic resistance.3 There is thus a clear need to investigate alternative strategies.4 Researchers started exploring other treatment modalities that include antimicrobial peptides,5 antibodies,6 bacteriophages7 and nucleic-acid therapeutics.8Nucleic-acid therapeutics, and in particular antisense oligonucleotides (ASOs), have presented a novel approach as antibiotics, by aiming to directly modulate gene expression.9 The use of oligodeoxynucleotides to interfere with gene expression was first introduced in 1978 in the landmark papers by Zamecnik and Stephenson.10,11 By designing a 13-nucleotide (nt) oligomer complementary to the 3′ end of the Rous sarcoma virus (RSV) RNA, they were able to inhibit its replication in chicken embryo fibroblasts. First reports on bacteria came from Jayaraman and coworkers that demonstrated growth inhibition on permeable Escherichia coli (E. coli) mutants using non-ionic oligonucleotide analogues.12,13 A decade later, the first proof-of-principle was also obtained on E. coli with an intact cell wall, where mRNA translation was inhibited by short 3- to 6-nt DNA analogues complementary to the ribosome recognition consensus sequence.14 This led to the emergence of the field of antisense therapy as a new tool against infections, cancers, and genetic disorders.15 Currently, over a dozen ASO-based drugs have been approved by the Food and Drug Administration (FDA) or the European Medicines Agency (EMA), with clinical trials on their way for treatments against various genetic disorders and infectious diseases.16
ASOs attain their inhibitory effect on translation through two main mechanisms: by RNase-mediated transcript degradation or steric hindrance.17 Eukaryotic and prokaryotic cells share many ubiquitous endogenous RNases involved in the processing of various RNA substrates. RNase H is a family of endoribonucleases involved in the non-sequence-specific cleavage of RNA in RNA:DNA duplexes.18,19
Its recruitment to specific mRNA regions can be achieved through complementary DNA or DNA-like ASOs that will trigger mRNA degradation (Fig. 1, left). RNase P is another endoribonuclease involved in RNA degradation.20 It recognises specific tertiary structures found in precursor tRNAs for cleavage, independently of the sequence. Mimicking those structures by antisense molecules – referred to as external guide sequences (EGS) in this context – targeting mRNAs can therefore also trigger their cleavage and degradation. The second approach for translation inhibition relies on sterically hindering the assembly or activity of complexes required for translation.18 The most notable application involves the prevention of ribosomal binding and assembly at the gene promoters by targeting the ribosome binding site or translation start site sequences of the mRNA with high-affinity ASOs (Fig. 1, right). The dominating mechanism involved in translation inhibition is dictated by the chemical nature of the ASO (see Section 2).
Since the first proof-of-principle studies demonstrating bacterial growth inhibition using antisense agents over 40 years ago, many advances have been made. In particular, the newfound interest in nucleic-acid therapeutics sparked by the success stories of several market-approved drugs have encouraged researchers to explore the application of ASO technology towards the treatment of bacterial infections.21 This field has been systematically studying the chemical and biological requirements for an effective use of ASOs to target pathogenic bacteria over the last years. Although no antibacterial ASO has reached approval for clinical use, continued research and technological advances will aid progress towards this goal.
In this Tutorial Review, we strive to provide a guide for anyone interested in designing an effective antibacterial antisense agent. We will focus on considerations for selecting the most optimal chemical architecture, choosing the best delivery vehicle and provide guidelines for sequence target selection. In the last part, chemical advances from recent literature will be introduced to showcase potential answers to some of the obstacles facing antibacterial ASOs. This is not a comprehensive review of the large body of work on this topic, but it is meant to serve as a guide. Excellent reviews on the biological implications of antibacterial antisense agents have been published previously.8,22–27
2. Chemical architecture of the antisense oligomer: which to choose
The first studies with ASOs entailed unmodified oligonucleotides with a phosphoribose-backbone,10,11 which suffered from rapid degradation by nucleases, limiting their clinical potential.28,29 To overcome the shortcomings and improve the physical and pharmacological properties of ASOs, a wide variety of chemical modifications of the antisense backbone have been developed over the last decades.30,312.1 Early example of chemically modified antibacterial ASOs
First chemical modifications of antibacterial ASOs reported in the early 1980s involved replacement of the negatively charged phosphodiester bond with nonionic methylphosphonates (Fig. 2).32 Although, growth inhibition was demonstrated in permeable E. coli mutants, it was found that methylphosphonate oligomers exceeding four nucleotides were not taken up by wild-type E. coli, limiting their antibacterial use.12 However, later studies did show effective inhibition of translation in leukemia models with longer oligomers when delivered using liposomes,33 which may be applicable to antibacterial use as well.34 More recent studies have demonstrated the use of phosphonates for enhanced antisense stability and reduced toxicity.35,36 Since these first modifications, many improvements to the chemical structure of ASOs have been made, mostly focusing on modifying the phosphate group, the 2′OH position and the development of charge neutral ASOs (Fig. 2). | ||
| Fig. 2 Molecular structures of negatively charged and charge neutral ASO backbones. Modifications at the 2′-OH position are depicted in cyan and phosphate modifications are shown in orange. | ||
2.2 ASOs with modified phosphate groups
The so-called ‘first generation’ of chemically modified ASOs are characterized by alterations of the inter-nucleotide phosphate-linkage, including phosphorothioate-linkages (PS) (Fig. 2).37,38 In PS oligos, one of the non-bridging oxygens of the phosphate group is substituted with a sulphur. This modification results in decreased degradation by nucleases, greatly improving the in vivo half-life of the ASO.23,39 PS-based ASOs promote the degradation of the target mRNA by activation of RNase H. From a chemical perspective, PS oligos are interesting since each phosphorothioate group introduces an additional stereocenter.40 Current solid-state synthesis methods do not control the stereochemistry resulting in mixtures of diastereomers, with each isomer likely displaying different biological activity.41 Nevertheless, PS-based ASOs have been approved for clinical use.42Few examples report on the use of PS-based ASOs as antibacterials. Luo and coworkers designed a PS-based ASO that targets the mecA transcript in methicillin-resistant Staphylococcus aureus (MRSA).43mecA encodes a protein that conducts cell-wall synthesis even in the presence of β-lactam antibiotics, rendering them inactive. The ASO was delivered into bacteria using anionic liposomes and efficiently restored oxacillin susceptibility.
Although PS-ASOs provided significant improvements over non-modified oligos, they suffered from new shortcomings, such as reduced affinity for mRNA and undesired interactions with proteins.29
Recently, Vögel and coworkers found in an excellent comparative study44 that PS-based ASOs displayed significantly lower melting temperatures45 and were unable to block translation in a cell-free system. Moreover, they found that negatively charged ASOs were not efficiently delivered inside Salmonella enterica (S. enterica) using cationic cell-penetrating peptides (CPPs) (vide infra).
2.3 2′-OH modified ASOs and the gapmer approach
Many modifications of the ribose 2′-OH group have been explored including 2′-O-methyl (2′-O-Me),46 2′-O methoxyethyl (2′-O-MOE),47 2′-fluoro-arabinonucleic acid (FANA)48,49 and locked nucleic acid (LNA)50 (Fig. 2). These modifications were an improvement on the first generation in terms of binding affinity and hybridization stability. However, due to the ribose modification, most of these ASOs do no longer activate RNase H, with the exception of LNA.31,51,52 Consequently, their antisense effect is solely caused by steric blocking of the target mRNA. To overcome this limitation, so called ‘gapmers’ were developed.53 These chimeric ASOs are typically comprised of modified nucleotides at the flanking regions, providing protection against degradation and increasing affinity for the target mRNA, while the centre region contains natural nucleotides that are still recognized by nucleases.54 Several clinically approved ASOs exploit this gapmer mechanism including Mipomersen55 and Inotersen.56Ribose 2′-OH modifications have been sporadically employed for antibacterial ASOs. For example, a recent report by the group of Pelz-Stelinsky57 employed PS-linked FANA ASOs to target the ligA transcript of the bacterium Candidatus Liberibacter asiaticus (CLas). Interestingly, they observed efficient gene silencing even in in vivo models of infected insects, without the need of a delivery system of the FANA oligomers. Although few examples of antibacterial FANA ASOs have been described so far, they have been particularly effective against pathogenic Liberibacter species, without the need for additional delivery methods.58 Moreover, their capacity for “naked” uptake has also been observed in various cancer cell lines.59 However, the evidence is still limited, and the underlying mechanisms remain unelucidated. The successful uptake observed in Liberibacter species could nevertheless be due to the unique characteristics of this genera in terms of membrane composition and permeability resulting from their evolution as obligate parasites dependent on their host for the uptake of many important molecules.60
A systematic study by Vögel and coworkers showed that 2′-OH modified ASOs displayed limited antibacterial effect. 2′-O-Me, 2′-O-MOE and LNA modified ASOs conjugated to a cationic CPP (vide infra) did not display any bacterial inhibition against S. enterica, likely due to inadequate bacterial uptake.44
2.4 Charge neutral ASOs
Charge neutral ASOs are particularly interesting because their nonionic nature renders them more suitable for cellular delivery (see Section 3). While early studies were performed with alkylphosphonates,32 most recent studies employ phosphorodiamidate morpholino oligomers (PMOs)61 and peptide nucleic acids (PNAs) (Fig. 2).62 In PMOs, the nucleobase is attached on a morpholine ring instead of natural ribose (Fig. 2). These morpholine rings are connected via phosphorodiamidate-linkages resulting in a completely neutral backbone and were first described in a patent credited to Summerton and Weller.61 PNAs were first reported in 199162 and are comprised of a peptide-like N-(2-aminoethyl)glycine backbone where the nucleobase is attached via a methyl carbonyl linker (Fig. 2). Both PMOs and PNAs prevent translation of their target mRNA by steric blockage of the translation machinery. Although similar in mode-of-action and neutral of charge, there are distinct differences between PMOs and PNAs. PNAs are more flexible and have shown to bind RNA with higher affinity than PMOs. On the other hand, PMOs are better soluble in aqueous solutions than PNAs.63A landmark paper by Good and Nielsen64 described the first use of PNA as antibacterials. They targeted a β-lactamase gene in E. coli. This strategy had limited success in a wild-type strain, but readily resensitized a resistant E. coli strain with a leaky outer membrane to ampicillin. Five μM of PNA was sufficient to inhibit bacteria growth supplemented with 300 μg mL−1 ampicillin, while the control without PNA displayed no growth inhibition. In a later study,65 Good, Nielsen and coworkers showed that conjugating a CPP to a PNA targeting the essential acyl carrier protein (acpP) in wild-type E. coli, resulted in potent antibacterial effect with a minimal inhibitory concentration (MIC) – the lowest concentration that inhibits bacterial growth – of 2 μM, overcoming the initial delivery issue.
The first use of PMO as an antibiotic was published in 2003.66 Similar as PNA, unmodified PMO was able to inhibit bacterial growth of an E. coli strain with a leaky outer membrane when targeting acpP. However, downregulation of gene expression in strains with an intact outer membrane was observed only when the PMO was first conjugated to a CPP.
A more recently described charge neutral backbone is phosphoryl guanidine oligo-2′-O-methylribonucleotides (2′-OMe PGOs) developed by Stetsenko and coworkers (Fig. 2).67 They used this backbone chemistry to target the ald transcript encoding alanine dehydrogenase in Mycobacterium smegmatis (M. smegmatis).68 Remarkably, no delivery agent was required to downregulate gene expression. Moreover, 20 μM ASO significantly inhibited bacterial growth. This study is notable, since mycobacteria are notoriously challenging to penetrate with drug molecules. This however remains currently the only described use of PGO ASOs against bacteria. In different contexts, this type of ASO has failed to penetrate mammalian cell membranes without using delivery agents.69,70 The surprising success in M. smegmatis has been potentially attributed to differences in the structure of the cell envelope or experimental conditions that may have improved the uptake, and is therefore not necessarily applicable in other species.8 Nevertheless, it remains a promising modification for charge neutral ASOs and the application of 2′-OMe PGOs as antibacterial antisense therefore warrants further exploration.
Most studies to date have used PMO or PNA ASOs as antibacterial agents and these backbones have emerged as the preferred weapon of choice. Unconjugated PMOs and PNAs suffer from low affinity to plasma proteins, resulting in low target availability.71 However, efficient reduction of bacterial burden has been demonstrated in in vivo models for PMOs and PNAs.72–74
2.5 Recommendations
Current evidence suggests that PMO and PNA backbones are likely the most suitable for antibacterial applications. A recent study by Vögel and coworkers found that PMO- and PNA-CPP conjugates were the only antisense agents capable of inhibiting S. enterica growth with MIC values of 10 μM and 1.25 μM, respectively.44 LNA, 2′-O-MOE and PS oligos displayed minor growth retardation, whereas 2′-O-Me did not show any effect on bacterial growth.44 It cannot be excluded however that the other backbones would perform better with a different delivery agent.Practically, PNA is chemically more accessible since the monomers are commercially available and can be coupled together using standard peptide chemistry either manually or using an automated peptide synthesizer. Depending on the length, CPPs can be appended during the same synthesis.75
For PMO synthesis, generally more specialized phosphoramidate P(V) chemistry and equipment are required76 rendering it less accessible, though PMO sequences can be designed and purchased from commercial sources.
3. How to deliver antisense agents into bacteria?
ASOs are large and sometimes charged molecules that do not passively enter most bacterial or eukaryotic cells. Importantly, the delivery of ASOs into bacterial cells is considered more challenging than for eukaryotic cells.26 The bacterial cell envelope is a complex structure constituted of several layers, allowing it to act as an important barrier against therapeutic compounds.77 Based on its layout, a distinction can be made between Gram-negative and Gram-positive bacteria. Gram-negative bacteria possess a three-layered envelope composed of an inner phospholipid bilayer membrane, a rigid peptidoglycan cell wall and a lipid outer membrane. Gram-positive bacteria, on the other hand, lack the outer membrane, carrying instead a thicker and richer peptidoglycan cell wall. The different membrane architecture between eukaryotic and bacterial cells also affects its overall charge and resting membrane potential, resulting in differences in the interaction with charged molecules such as oligonucleotides and peptides.78 A few reports have described the use of antibacterial ASOs without the need of a delivery agent,57 but most studies show that a delivery vehicle is required for efficient antibacterial activity.22 The first aspect to consider is the chemical nature of the ASO, since this will dictate the available options.CPPs are the most commonly used delivery agents, but their performance with negatively charged ASOs has been varied, mostly limiting their use to charge neutral ASOs.44 Cationic oligomers have been described for antibacterial ASO delivery, but are also mostly limited to charge neutral ASOs. Small-molecule delivery systems are being explored and the full scope of their performance still needs to be investigated. Lastly, non-covalent systems have been applied for the delivery of antibiotic ASOs, including negatively charged ones.
3.1 Cell-penetrating peptides (CPPs)
The most widely used delivery system for ASOs are CPPs.79 CPPs are short (<30 aa) peptides that enable transport of (large) molecules over the cell wall. CPPs can be classified based on their physicochemical properties: hydrophobic, amphipathic and cationic.80The exact mechanisms of intracellular transport by CPPs has been a heated topic of debate.81 Nonetheless, three possible general pathways for internalization have been described in literature: direct penetration, endocytosis and translocation.80 In the pathway of direct penetration, the positive charge of cationic CPPs interacts with the negatively charged cell membrane. Consequently, the cell membrane is destabilized and pores are formed through which the CPPs and their cargo migrate. The endocytosis pathway is, in contrary to direct penetration, an energy-dependent pathway. In general, endocytosis can be described as an ingestion process where the cell membrane engulfs the external material followed by absorption. The translocation pathway relies on the formation of inverted micelles as a result of the CPP interacting with the cell membrane. The CPPs are trapped in these inverted micelles and migrate over the cell membrane. Although the main possible pathways of cell penetration by CPPs have been established, it is hard to elucidate the precise uptake mechanism of a certain CPP-cargo conjugate. The internalization often occurs through a combination of pathways and is influenced by a great number of factors such as the (size of the) cargo, concentration of the conjugate, cell type (eukaryotic or prokaryotic), membrane properties and temperature.81 Consequently, small experimental differences between studies of the same CPP can lead to contradictory conclusions.
Few examples of successful hydrophobic CPP-ASO conjugates exist. Aartsma-Rus and coworkers identified a 7-mer hydrophobic CPP, named ‘P4’ (Fig. 3) using a screen that successfully delivered 2′-O-methyl phosphorothioate ASOs.82 The thiol bearing ASO was coupled to a maleimide on the CPP using a Michael addition reaction.
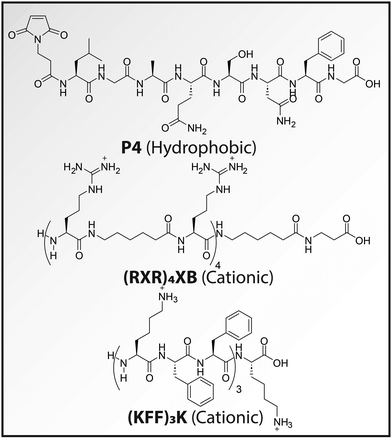 | ||
| Fig. 3 Molecular structures of highlighted CPPs. Under physiological conditions unmodified terminal amines will be positively charged as well. | ||
Amphipathic CPPs contain both hydrophilic and hydrophobic residues83 and a few successful antibacterial conjugates have been described.84 One noteworthy example of an amphipathic CPP is CADY.85 This CPP adopts a helical conformation in cell membranes, where one side contains exposed charged residues and the other consists of tryptophans.86 CADY has been applied to transport therapeutic ASOs into mammalian and bacterial cells. A recent study,84 conjugated the CADY sequence to an ASO targeting the acpP sequence in Acinetobacter baumannii (A. baumannii), which displayed growth reduction and downregulation of the targeted genes.
Typical cationic CPPs contain a relatively high number of positively charged amino acids. For example, Patenge and coworkers explored the CPP (RXR)4XB (Fig. 3) coupled to an anti-gyrA PNA that targets the transcript of the essential subunit A of DNA gyrase.87,88 In an initial screen, 10 μM of the conjugate displayed robust antibacterial effect against Streptococcus pyogenes. In a later study, they investigated the in vivo performance of several CPP-PNA conjugates, including (RXR)4XB in a Streptococcus pneumoniae infection model of Galleria mellonella larvae. They demonstrated that administration of 4 nmol of the conjugate increased survival significantly, albeit with only a few days.
Another frequently applied cationic CPP is (KFF)3K (Fig. 3), which has been successfully used for the delivery of PNAs, PMOs and negatively charged LNAs. In an early study, Vaara and Porro found that unconjugated (KFF)3K displayed a very potent synergistic effect with hydrophobic and amphipathic antibiotics.89 Rifampin showed a 1000-fold decrease in MIC value in E. coli from 10 μg mL−1 to 0.01 μg mL−1 when supplemented with 100 μg mL−1 (KFF)3K. Based on this finding, Nielsen and coworkers cleverly appended the (KFF)3K CPP to a PNA ASO targeting acpP.65 Remarkably, where in an earlier study the naked PNA was inactive against wild-type E. coli,64 the CPP conjugate displayed potent antibacterial activity, suggesting efficient bacterial uptake.65 A more recent study used (KFF)3K to internalize negatively charged antibacterial LNAs.90 An LNA targeting the mRNA encoding the essential protein FtsZ in MRSA was appended to (KFF)3K. The conjugate displayed antibacterial effect against nine MRSA strains with MIC values ranging from 1.56–12.5 μM. Interestingly, when applied to a mouse model infected with Staphylococcus aureus (S. aureus) mu50 at 3 mg kg−1, the survival rate dramatically increased from 0% to 60%, demonstrating the potential in vivo application of antibacterial ASOs. Other studies found limited bacterial uptake of LNA-(KFF)3K conjugates.44
Importantly, different bacterial species can have major differences in the structure and therefore permeability of their cell envelopes. These differences, together with abiotic factors such as temperature, are known to influence the effectiveness of particular CPPs.91,92 The choice of CPP can therefore have an important impact on the activity of the ASO.87 Generally, positively charged peptides appear to perform well on a range of bacterial species.65,87,88,90
3.2 Cationic oligomers
Although the majority of research on delivery of antibacterial ASOs focuses on CPPs, several other (non-peptide) viable carriers have been reported including cationic oligomers (Fig. 4). Polyethyleneimine (PEI) was initially used as a cationic polymer to deliver negatively charged oligomers into eukaryotic cells via encapsulation of the ASO followed by endocytosis of the complex.93 A subsequent study by Nielsen and coworkers showed that PEI enhanced the uptake of non-charged PNA-based ASOs in eukaryotic cells when conjugated via a disulfide-linkage.94 In a second study, the group of Nielsen developed cationic dendrons based on guanidinylated 2,4-diaminobutanoic acid (DAB) as effective carriers of PNA-based ASOs into eukaryotic cells as well (Fig. 4).95 These dendrons display alikeness with cationic CPPs since they contain multiple positively charged groups. Recently, the application of DAB-based cationic dendrons in the delivery of antibacterial PNA-based ASOs was demonstrated as well.96 Several dendron-PNA conjugates were synthesized that targeted the essential acpP gene of E. coli and Klebsiella pneumoniae (K. pneumoniae). The dendron-PNA conjugates showed similar in vitro antibacterial activity as compared to CPP-PNA conjugates with impressive MIC values of 0.25 μM for E. coli and 0.13 μM for K. pneumoniae. One of the dendron-PNA conjugates displayed significant in vivo activity as well against a multidrug-resistant E. coli strain in a murine peritonitis model. Moreover, the conjugate was highly stable in human and mouse serum with an impressive half-life of >24 h and well tolerated by human HepG2 cells and mice. The cytotoxicity of several conjugates with varying chain lengths of the terminal groups was assessed on HeLa cells as well. These studies demonstrated that increased chain lengths correlated with increased toxicity. | ||
| Fig. 4 Molecular structures of highlighted cationic oligomers. The DAB dendrons and vivo-morpholinos are guanidinylated at the indicated sites. | ||
Taken together, cationic DAB dendrons are a promising alternative for cationic CPPs with improved stability. However, these cationic DAB dendrons transport cargo into cells by direct penetration (similar to cationic CPPs) resulting in delivery of ASOs in both eukaryotic and prokaryotic cells, which might limit their application in terms of selectivity but could be beneficial for targeting intracellular infections.97
A more established and chemically elegant form of dendrons-vehicles are octaguanidinium-conjugates, which are applied in the in vivo delivery of PMO-based ASOs.98 In these so called “vivo-morpholinos” (Fig. 4), a branched non-peptidic structure containing eight guanidinium head groups is covalently attached to the PMO. Although numerous successful in vitro99 and animal studies,100 including virus infections models,101 of vivo-morpholinos are reported, their bacterial application remains surprisingly underexplored.
3.3 Small molecules
Besides large molecules such as CPPs and cationic polymers, smaller (bio)molecules can act as vehicles for antibacterial ASO delivery as well. For the simplicity of classification, we consider non-polymeric agents as ‘small’ molecules, although some are >1000 Da.Gryko, Trylska and coworkers explored the uptake route of vitamin B12 (B12) (Fig. 5, vitamin B12) for the delivery of antibacterial ASOs.102,103 This vitamin is an essential nutrient for the growth of most bacteria, but only a few bacterial species are able to synthesize B12. Therefore most bacteria rely on the import of vitamin B12 from the environment via active transport,104 making this biomolecule an attractive carrier of drugs. To test if vitamin B12 enhanced the uptake of ASOs, this vitamin was conjugated with PNA-based ASOs via a copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC). The ASOs targeted the mRNA of red fluorescent protein (RFP), which was encoded on a plasmid and transfected into E. coli or Salmonella typhimurium (S. typhimurium). This strategy enabled simple monitoring of gene-silencing by detection of fluorescence. The B12-PNA conjugates showed a concentration dependent reduction of RFP translation. When compared to a conjugate of the same PNA sequence with (KFF)3K, the antisense effect of the B12-PNA conjugates were slightly improved in E. coli but the opposite was observed for S. typhimurium. Nonetheless, this study indicated that B12 is a viable carrier molecule for PNA ASOs. Interestingly, in a similar study the authors showed that B12 also enhanced the uptake of negatively charged 2′-OMe based ASOs into E. coli, although not as efficiently as observed for B12-PNAs. A 50% reduction in red fluorescence was observed at concentrations of >1 μM. The antibiotic potential was assessed by constructing B12-PNA conjugates that targeted the essential acpP gene of E. coli. The conjugates displayed inhibition of bacterial growth at 5 μM, albeit not as strong as observed for the (KFF)3K-PNA control and only when using Scarlett and Turner medium.105
 | ||
| Fig. 5 Molecular structures of highlighted small molecule delivery agents. Vitamin B12 is depicted in its cyanocobalamin form. | ||
A report by Sobral Santos and coworkers106 described the use of B12-LNA conjugates for targeting the acpP mRNA in E. coli. Surprisingly, no growth inhibition was observed even at a concentration of 30 μM, whereas a CPP-PNA control targeting the same sequence displayed robust inhibitory effects. Using bacterial fractionation, they found that the B12-LNA conjugates strongly associated to E. coli, but they were mostly localized on the outer membrane and only a small fraction was found in the cytosol, explaining the lack of inhibitory effect. Taken together, B12 is a potential carrier for antibacterial ASOs but more evidence is needed to confirm sufficient and robust uptake.
Another interesting approach to enhance the delivery of ASOs is by conjugation with aminoglycoside antibiotics. This class of antibiotics consist of two or more connected aminosugars and target the bacterial ribosomes. Several studies indicated that co-administration or conjugation of ASOs to aminoglycosides enhanced the ASOs uptake in eukaryotic cells.107,108 In regard of potential antibacterial agents, the combination of an antibiotic with the ASO might result in a synergistic effect. However, there are no studies on the aminoglycoside-mediated delivery of ASOs into bacteria to date, thus the antibacterial potential remains to be determined.
Lipids directly conjugated to oligonucleotides109 could assist in their bacterial delivery. Many conjugate designs have been tested on mammalian cells,110 but few on bacteria.111 Barthélémy and coworkers incorporated two C15 lipid tails to a PS ASO through an acetal linkage with the 2′- and 3′-OH groups (Fig. 5, acetal lipid).111 The ASO was used to resensitize E. coli resistant to cefoxitin, by targeting β-lactamase encoding mRNA. A 26-fold decrease in MIC value was observed in the presence of the ASO-lipid conjugate, suggesting efficient downregulation of β-lactamase.
Recently, we and others started to explore the possibility of exploiting siderophore uptake for antibacterial ASO delivery.112,113 Siderophores are small molecule Fe3+ chelators excreted by bacteria.114 Many bacteria possess dedicated siderophore transporters. Researchers have exploited this uptake route to transport small molecule cargo into bacteria by attaching it as cargo to siderophores. This Trojan horse approach has been successfully applied to enhance the delivery of antibiotics as exemplified by the FDA-approved drug Cefidericol.115 We were interested if this same principle could be applied to ASOs as well.112 A tris-catechol siderophore mimic (Fig. 5, tris catechol) was prepared, inspired by previous work by Miller and coworkers,116 and appended to both a PNA and PMO ASO targeting acpP in E. coli. MIC values were found of 1.6 μM and 0.8 μM, respectively. Interestingly, in preliminary experiments a minimal siderophore mimic consisting of one catechol group (Fig. 5, mono catechol) was equally effective at inhibiting bacterial growth when conjugated with the anti-acpP PNA.
In a study by the Trylska lab, novel synthetic trihydroxamate peptide-siderophores (Fig. 5, trihydroxamate) were designed and conjugated with PNA-based ASOs.113 Initially, conjugates of the linear trihydroxamate siderophore and a well-established lethal 10-nt ASO targeting the acpP gene were constructed. No inhibition of E. coli growth was observed for the anti-acpP conjugate. To validate if the conjugate was taken up, they switched to a quantitative approach using a plasmid carrying the RFP gene. However, conjugates targeting RFP expression did also not result in clear antisense activity as assessed by flow cytometry. To further study the performance of the conjugates, the RFP silencing experiment was performed on Δfur E. coli mutants. Since fur represses iron uptake genes, the mutant was expected to display enhanced iron uptake. The PNA-siderophore conjugate indeed reduced RFP signal with the highest effect observed at 16 μM. Surprisingly, a control PNA conjugate only partially complementary to the RFP transcript displayed a similar effect.
3.4 Non-covalent carriers
There is a wide array of non-covalent nanocarriers available for the cellular delivery of nucleic acids ranging from lipid nanoparticles to DNA nanostructures. In particular, the success of mRNA vaccines has accelerated the research into non-covalent carriers, many of which could be applicable for delivery of ASOs into bacteria.9 In this review, we will briefly discuss selected examples that have successfully been applied to bacteria.DNA nanostructures have been used for delivering both charge neutral and negatively charged ASOs. Li and coworkers designed a DNA six-helix bundle that was loaded with a negatively charged PS ASO targeting the essential gene ftsZ in S. aureus.117 When incubated with a relatively low concentration of 0.6 μM the survival rate decreased to 57%. Interestingly, when applied to the acpP transcript in E. coli, no antibacterial effect was observed. In a similar approach employing a tetrahedral DNA nanostructure, a neutral PNA based antisense targeting the same ftsZ transcript in MRSA was employed.118 In this case, a dose-dependent growth inhibition was observed, with an inhibition rate approaching 60% at a concentration of 750 nM.
Liposomes, an early version of lipid nanoparticles, have been widely applied for the delivery of nucleic acids.119 A noteworthy example entails a study by Luo and coworkers.120 They targeted an E. coli strain resistant to fluoroquinolones using PS ASOs. The bacteria express an efflux pump encoded by the acrB gene that actively removes fluoroquinolones from the intracellular environment. The ASO was encapsulated in an anionic liposome and when applied to the resistant E. coli strain resensitized the bacteria to the fluoroquinolones ciprofloxacin and levofloxacin. An almost 50% reduction in bacterial growth was observed when 100 μg mL−1 of the ASO was employed alongside 6 μg mL−1 of ciprofloxacin, whereas PBS loaded liposomes did not affect bacterial growth.
3.5 Recommendations
Based on the available research, CPPs appear to be the preferred choice as ASO delivery agent, in particular in combination with charge neutral backbones like PNA and PMO. Nevertheless, a few potential limitations need to be mentioned. First, CPPs have been reported to be subject to enzymatic degradation, which might affect their performance. Second, CPPs have been described to be cytotoxic to varying degrees depending on the CPP, potentially limiting their in vivo applications.121,122We are optimistic about small-molecule carriers as well, that could provide certain advantages over CPPs, including bacterial selectivity and decreased toxicity. In particular the recent studies on siderophore delivery appear hopeful. Yet, these delivery modalities are still in their infancy and additional research in the years to come will be necessary to assess their full potential.
4. How to select an antibacterial antisense target?
When selecting a target for an antibacterial ASO there are two main criteria to consider. First, which transcript to select and second what sequence within this transcript to target.4.1 Transcript selection
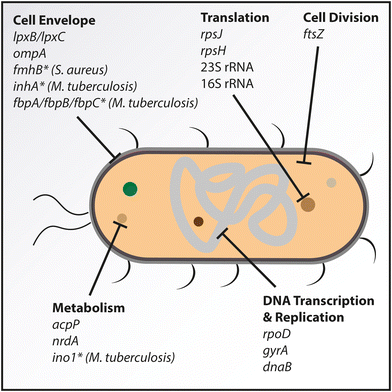
A major focus in the development of antibacterial treatments has been on genes necessary for bacterial survival and growth, encoding for example key metabolic enzymes or structural proteins.123 Many such essential genes have been identified throughout the years, revealing other important characteristics that increase their suitability as drug targets as well.124 This includes the conservation of gene sequences across various pathogenic strains within a species as well as the preferable lack of a human counterpart. Furthermore, the presence or absence of target orthologs in different bacterial species can be used to introduce selectivity in targeting. Novel antisense-based strategies have often focused on genes coding for proteins already inhibited by established antibiotics. We here review the main cell components or bacterial processes that have been targeted with examples of successful targets for each (Fig. 6). An upcoming approach is to target resistance genes with ASOs to resensitize bacteria to existing antibiotics.111,120 Though not antibacterial by themselves, the combination with traditional antibiotics might prove powerful in combatting antibiotic resistance.The UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine deacetylase (LpxC) catalyses the first step of the Raetz pathway.126 Greenberg and coworkers developed PMO-based ASOs against lpxC in Pseudomonas aeruginosa (P. aeruginosa).127 These were designed to target the Shine–Dalgarno and translation start site sequences and were conjugated at their 5′ or 3′ ends with the (RXR)4XB CPP. In vitro, the best performing PMO showed a MIC50 value of 8 μM. The PMO also reduced biofilm formation at 4 μM, which is known to complicate the treatment in patients. An in vivo infection model in mice infected intratracheally and treated with the PMO through intranasal administration showed significant reduction of the bacterial load in the lungs of the infected mice after 24 hours, but with no provided information on the survival rate. It is worth noting that the target specificity could not be confirmed due to the lack of gene expression studies on either protein or mRNA levels. Additionally, the scrambled control PMO showed a small effect in vivo, which was attributed to the potential nonspecific immune activation by the PMO or CPP molecules.
The lipid-A-disaccharide synthase (LpxB) is a glycosyltransferase catalysing the formation of the lipid A disaccharide by nucleophilic addition of lipid X and UDP-diacyl-glucosamine.125 A PNA conjugated at the 5′ end with the (KFF)3K CPP was designed against lpxB in A. baumannii,128 an important nosocomial pathogen. In vitro time-kill assays showed limited growth inhibition of the PNA-CPP at lower doses, but bactericidal activity was obtained at 35 μM. In M9 minimal media, a concentration of 2.5 μM was bactericidal, with no colony-forming units (cfu) detectable after 24 hours. In a A549 human lung epithelial cell infection assay, 5 μM PNA-CPP allowed the infected cells to reach similar viability as the uninfected controls after 30 hours. Finally, in an in vivo infection model using the greater wax moth (Galleria mellonella) caterpillar, treatment at 75 mg kg−1 reduced the mortality from 60% to 25% over a course of 100 hours. The researchers also highlighted a synergistic effect between the ASO and colistin, a last-resort medication reintroduced due to the increasing rate of antibiotic resistance.129
The group of Luo published two concomitant papers on targeting of rpoD by CPP-conjugated PNAs in a range of multidrug-resistant Gram-negative bacteria as well as in the Gram-positive MRSA.131,132 For the design of the ASO, they focused on regions with high sequence homology between species and obtained the best results by targeting the translation start site. Two different CPPs – (KFF)3K and (RXR)4XB – were conjugated to the ASO with the latter resulting in higher overall performance. They assessed the antibacterial effect on several clinical bacterial isolates including E. coli, S. enterica, Shigella flexneri (S. flexneri), A. baumannii and K. pneumoniae. The in vitro studies highlighted a species-dependent difference in performance of the antisense treatment. A bacteriostatic effect was obtained at concentrations ranging between 5–6.25 μM for A. baumannii and E. coli while the other strains required markedly higher concentrations. In a human gastric mucosal epithelial cell infection assay using E. coli, S. enterica or S. flexneri, no live bacteria could be detected after 24 hours with ASO doses as low as 5 μM, with no PNA-associated toxicity observed in the eukaryotic cells studied. Finally, in vivo mice studies with intraperitoneal injection of the bacteria followed by the PNA at 10 mg kg−1 showed an increase in survival over a week. Whilst no mice survived in the untreated groups, treatment resulted in 40% and 60% survival for the E. coli and S. flexneri infections respectively, with a significant decrease in bacterial load detected in various organs.
rpsJ is the gene encoding for the 30S ribosomal subunit protein S10 (NusE). In the same study by Greenberg and coworkers targeting lpxC in P. aeruginosa using PMOs (see Cell envelope section), they attempted to inhibit rpsJ as well.127 Although targeting rpsJ showed promising results for antisense inhibition and resulted in bactericidal activity in vitro and in vivo, it performed worse than the lpxC-targeting ASO, requiring higher concentrations to reach similar effects with a MIC50 value of 32 μM (four times higher than for lpxC). Treatment with the ASO reduced biofilm formation at concentrations of 4–8 μM, and in an in vivo mouse infection assay, they observed significant reduction of the cfu count in the lungs, albeit weaker than for the anti-lpxC treatment.
The 30S ribosomal subunit protein S8, encoded by rpsH, was also highlighted as a promising target for antisense inhibition in vitro.136 In this comparative study by Vögel and coworkers in uropathogenic E. coli, high antibacterial activity was obtained for the PNAs targeting acpP, ftsZ, rpsH and dnaB. For rpsH, a concentration of 2.5 μM resulted in both bacteriostatic and bactericidal activity, with 1–2 log units decrease in cfu mL−1 detected over 24 hours.
Targeting ftsZ in several MRSA strains with an LNA-DNA mixed ASO yielded strong bactericidal activity in vitro and in vivo.90 The ASO was designed against an internal site of the transcript and was conjugated to the (KFF)3K CPP. Depending on the bacterial strain, almost complete growth inhibition was obtained with doses between 1.56 and 6.25 μM over 18 hours, and a dose of 3.13 μM resulted in a significant decrease in cfu over 6 hours of more than 8 log cfu mL−1 units compared to the control. The ASO induced a dose-dependent decrease in mRNA and protein levels, with almost no detected transcript and protein after 18 hours at a dose of 6.25 μM. In a cell infection assay with gastric mucosa originating epithelial cells, no bacteria were detected after 24 hours with doses of 6.25 μM and higher. Finally, an in vivo mouse assay with treatment at 3 mg kg−1 resulted in 60% survival after 7 days compared to complete mortality after 2 days in the untreated control group. The strong antibacterial activity obtained in this study at low doses highlights ftsZ as one of the more successful targets investigated so far.
Riboswitches are regulatory RNA elements usually found in the 5′ UTR of mRNAs.149–153 They act as “genetic switches”, regulating gene expression on a transcriptional or translational level in response to specific ligands. A typical riboswitch contains two domains, with an aptamer domain that selectively binds the target ligand, resulting in a conformational change of a regulatory domain. This can lead to various effects in gene expression such as transcriptional repression through the formation of a terminator hairpin in the mRNA or translational activation through the release of the ribosome binding site. These highly structurally conserved elements are particularly widespread in bacteria and archaea, with no known example in humans. In several consecutive studies, Traykovska and Penchovsky have successfully targeted three important riboswitches – glucosamine-6-phosphate (glmS), thiamine pyrophsophate (TPP) and flavin mono nucleotide (FMN) – in various bacterial species using PS-ASOs coupled to a CPP, relying on the RNase H-mediated degradation of the targeted elements.145–147 These different classes of riboswitches are involved in negative feedback loops regulating the production of essential metabolites in a wide range of bacteria. All the ASOs designed resulted in bacteriostatic activity at 700 nM in the species studied. Riboswitches therefore represent a particularly interesting new class of targets due to their widespread nature in bacteria and their involvement in essential pathways. Moreover, different regions of the riboswitches often show various degrees of sequence conservation between bacterial species, potentially allowing the fine-tuning of species-selectivity of the ASOs by changing the targeted region.145,146
Bacterial small RNAs (sRNAs) are a ubiquitous type of regulatory RNAs that act post-transcriptionally to regulate gene expression.154,155 Although rarely essential, these small RNAs of 40–500 nucleotides in length often bind multiple protein or mRNA targets to modulate important physiological pathways involved in for instance virulence and stress response. Tsai and coworkers targeted for the first time the MicF sRNA, which was found to play an important role in antibiotic resistance by regulating the expression of the outer membrane porin OmpF.148 By binding to the 5′ UTR of ompF, the sRNA inhibits the translation of the porin required for the uptake of several types of antibiotics, reducing their activity. Following optimisation in a cell-free assay, they were able to reduce the MIC of several antibiotics in E. coli using a combination of two CPP-PNAs against the sRNA–mRNA interaction region. However, the observed effect was still limited, with the authors concluding that longer antisense constructs were needed to properly disrupt the interaction in bacteria. Nevertheless, targeting sRNAs could be a potential complementary approach to combat antibacterial resistance by increasing their sensitivity to antibiotics and other treatments.
4.2 Sequence selection
Various guidelines have been proposed for the optimal target site selection within a transcript for antisense inhibition in bacteria, summarized in Fig. 7. These were notably developed following systematic interrogations across the entire length of different transcripts using PNA and PMO antisense inhibitors.156,157• One of the key determinants for successful antisense inhibition is targeting of unstructured and accessible single-stranded regions of a transcript (Fig. 7A). A strong negative correlation between the inhibitory activity of the ASOs and predicted secondary structure of the targeted mRNA region was observed.156,157 Indeed, strong RNA–RNA interactions within secondary structures increase the thermodynamic cost required for ASO binding.23 Similarly, a correlation between the inhibitory activity and propensity to remain linear of the ASO itself was also highlighted in other studies.158,159
• The most successful regions for targeting appear to be the Shine–Dalgarno sequence – the bacterial ribosome-binding site – and the start codon (Fig. 7B). Although ASOs are not required to include the start anticodon, binding outside of this region was largely inefficient.156,157 This can be explained by the sensitivity of ribosome binding and translation initiation to steric hindrance. This was further confirmed in most studies mentioned in this review, although some successfully targeted internal transcript sites.
• The melting temperature and GC content of the ASOs does not seem to correlate with antibacterial activity. Interestingly, in an in vitro study in E. coli comparing the activity of CPP-conjugated PNAs against 11 essential gene targets, a strong correlation was observed between the melting temperature and the translation inhibitory activity in a cell-free translation assay (Fig. 7C).136 However, no correlation was then observed when assessing the growth inhibitory activity in the bacteria, confirming that the melting temperature is not necessarily the main factor influencing antibacterial activity.
• PMO and PNA lengths of 9 to 12 bases seemed to give optimal results both in bacterial culture and in cell-free assays (Fig. 7C). Surprisingly, this differs from results obtained in eukaryotes for which PMO lengths of less than 16 bases tend not to cause significant inhibition.160 This highlights potentially different rules and mechanisms for eukaryotic and bacterial antisense inhibition.
• Many other factors contribute to the inhibitory activity of the ASO as well, with small changes in binding position or oligonucleotide length – even of just a single nucleotide – having a great impact.161 Additionally, the structural context of the binding site also plays a crucial role in the ASO binding affinity, even in single-stranded regions.162 Ultimately, it remains challenging to accurately predict the activity of different ASOs against the same target gene, often requiring empirical trial-and-error experimentation for optimal results. It is worth noting that while a single nucleotide mismatch in the ASO in respect to the target binding site can be tolerated with sometimes only minor effects on activity, two or more mismatches almost completely abolish the inhibitory activity (Fig. 7D).64,143,159
4.3 Recommendations
Based on studies described here and elsewhere, there appears to be a selection of essential genes that render good targets in multiple bacterial strains. A comparison of the different activities and degrees of evidence obtained has highlighted acpP, ftsZ, rpoD and lpxC/lpxB as the strongest candidates so far, with acpP being the most studied.It is worth noting however that it is difficult to find an optimal metric for the direct comparison of the activity spectrum of ASOs between different studies. Although the effective concentration for bacteriostatic and bactericidal activity is a good indicator for the essentiality of a target, caution should be taken when interpreting these results, including the MIC values often provided in the studies. MIC values obtained from bacterial growth assays are known to be affected by the type of assay, the initial density of the bacterial culture, the timing of the measurements163 as well as the growth medium used.127,128 Moreover, the effective concentration of an ASO can differ significantly between strains of the same bacterial species, including different clinical isolates.90 As previously discussed, the delivery method also has an important effect on the activity, further complicating the comparison. The experimental design therefore plays an important role in the measured and reported effective concentrations, and the values should not be used on their own to directly compare the suitability of different targets.
Targeting the ribosome binding site or translation start site has given the best results in most studies presented, which would also be our recommendation when designing an antibacterial ASO. Jung et al. recently developed MASON (Make Antisense Oligomers Now), a tool to design PNA-based antibacterial ASOs for essential genes of several bacterial species.164 We envision that this type of predictive algorithms will contribute to future ASO design.
5. Illustrative chemical advances
Apart from the fundamental principles that underlie the design of an optimal antibacterial ASO, additional research is being conducted to further enhance the performance of future constructs. Here, we will highlight several studies that offer potential chemical strategies to improve ASO design and delivery.The main delivery agent being used currently is CPPs. Their chief shortcomings are cytotoxicity and limited stability. To overcome the latter problem, Nielsen and coworkers explored the use of D-amino acids, rather than their L-counter parts (Fig. 8A).165 First, they analysed the stability of the L (KFF)3K CPP conjugated to a PNA based ASO, by incubating it in cell-free medium obtained from an exponentially growing bacterial culture. The main degradation product contained the last three amino acid residues (FFK), still attached to the PNA (Fig. 8A). Interestingly, no PNA degradation was observed. When a similar experiment was conducted in live E. coli, the main degradation products were again the PNA with the last three amino acids residues attached to it, and PNA without any CPP appended to it. The half-life of the full CPP-PNA construct in bacterial culture was estimated to be around 1 hour. Remarkably, when the authors used the D-form of the same sequence a fully intact CPP-ASO conjugate was observed in bacterial culture. This result was explained by bacterial proteases that mostly recognize L-amino acids, leaving the D-form CPPs intact. Interestingly, the reported MIC values on wild-type E. coli MG1665 were similar for both the D- and L-form of the conjugated CPP constructs at ∼2 μM, which might be explained by truncated CPP analogues still being capable of penetrating bacteria.
 | ||
| Fig. 8 Examples of chemical innovations for antisense technology. (A) The D-amino acid counterpart of the frequently used (KFF)3K CPP is stable towards proteolytic degradation.165 (B) Schematic illustration of a circularised ASO that gets activated upon β-lactamase activity.166 | ||
Besides altering the stereochemistry, numerous other strategies167 have been developed to improve the stability of CPPs, including cyclization168 of the peptide and backbone modifications.78 Cyclic CPPs have shown to be more stable in human serum than their linear counterparts.169,170 Additionally, cyclization can improve the cell penetrating properties. For example, Patil et al.171 explored the use of a polymycin analogue, which are a class of naturally occurring antimicrobial cyclic peptides, as carrier for PNA-based ASOs. Similar to the approach for ASOs, chemical alteration of the CPP-backbone can enhance the stability of the peptide. Peptoids are an important example of peptide-backbone modification in which the side-chains are attached to the nitrogen of the amide bond instead of the α-carbon as in natural peptides. The resulting N-substituted glycines are more resistant towards proteolytic degradation.172 Moreover, a study by Rothbard and coworkers showed that peptoid-based poly-arginine CPPs outperformed the D-amino acid variants of the same sequence in the penetration of eukaryotic cells.173 However, the effect of the use of such peptidomimetic backbones on ASO delivery into bacterial cells remains to be determined. Strategies to improve CPPs can be combined to potentially lead to even greater improvements. For example, Lim and coworkers recently described the use of cyclic peptoid-based amphipathic CPPs.174 Taken together, the field of peptide engineering offers several strategies that could be applied to mitigate the stability issues of CPPs.
The group of Deiters has been examining the possibilities of temporarily and locally activating ASOs.175,176 One potential benefit could be that in its inactive form, the ASO is protected from degradation and only when necessary will be activated and become vulnerable. In a recent example, Deiters and coworkers designed an inactive cyclic PMO ASO, that can be linearized by β-lactamases to become active.166 They synthesized a β-lactam containing linker that was appended to an alkyne at the 5′ end of the PMO and to a thiol at the 3′ end with a chloroacetamide moiety, to circularize the ASO (Fig. 8B). Using a cell-free translation assay they showed that the circularized ASO did not affect expression of a luciferase gene, whereas upon treatment with β-lactamase, the luciferase signal was decreased by ∼65%, demonstrating the potential of their setup. In a non-bacterial example, they continued to assess the performance of the circular PMO ASO in zebrafish. The ASO targeted the developmental gene ntla, that when silenced results in truncated tails. β-Lactamase was expressed through injection of mRNA and only when the mRNA and ASO were both injected, was the morphant phenotype observed. This example underscores the potential for in vivo applications. Furthermore, this approach could be useful to selectively target β-lactamase expressing bacteria.
6. Outlook
In this Tutorial Review we hope to provide guidelines for the design of effective antibacterial ASOs. Although we currently have a reasonable understanding of how to develop ASOs that perform well in vitro, making the next step to routine in vivo and eventual clinical use will require considerable research efforts.First, new and better delivery agents will need to be developed. The chief method currently is CPPs, but their reported toxicity177 will likely continue to pose a hurdle for in vivo use. Small-molecule carriers have the potential to overcome some of these toxicity issues, but so far there has only been limited research conducted in this area.
Second, there have been reports of ASO-conjugate resistant mutant strains. Both Geller and coworkers and the group of Nielsen identified an inner membrane peptide transporter encoded by sbmA in E. coli involved in the uptake of CPP-conjugated ASOs.178,179 Strains carrying mutations in the gene lost their sensitivity to certain PNA and PMO conjugates in the experiments. Nevertheless, the recognition of the molecules by the transporter seemed to be depended on the CPP used, and sensitivity to the ASOs could be restored in the mutant strains by switching delivery strategy. Further research in the cellular transport mechanisms for the various antisense oligonucleotide chemistries and delivery methods is therefore needed to understand and circumvent potential resistance mechanisms. Additionally, since the activity of antisense inhibitors relies on the conservation of the target sequence, target gene mutations could lead to antimicrobial resistance. This was for example observed in a study on PMO inhibition in severe acute respiratory syndrome coronavirus (SARS-CoV).180 The advantage of antisense therapy compared to classical drugs is that the sequence of the ASOs can in principle be adapted to changes in the target sequence to circumvent these problems. ASOs can also be used in combination with antibiotics to help limit drug resistance, with studies even highlighting a synergistic antibacterial effect obtained for many such combinations.181
Lastly, it will likely be preferred to develop antibacterial ASOs that are applicable to multiple strains. This poses the challenge to identify potent target sequences that several strains have in common, as well as a delivery agent that is applicable to all the targeted strains. So far, most studies have focused on targeting only a few strains with one ASO construct. As we have seen through many of the examples discussed, the same ASO constructs can also have significantly different levels of activities between different bacterial species
Despite these challenges, we believe the use of antibacterial ASOs offer great advantages over traditional small molecule antibiotics and we are hopeful that in the near future first designs will be tested in clinical settings. We expect that alternative, non-traditional antibiotics, such as antibodies, antibacterial peptides, bacteriophages and ASOs will become increasingly important to restock the arsenal of effective antibiotics. Although each of these non-traditional antibiotics has their own specific strengths and limitations, they hold great promise to play an important role in combatting antibiotic-resistant bacteria. We believe that in particular the selectivity and sequence adaptability of ASOs will be useful for developing new antibacterials.182
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has received funding from the European Research Council under the European Union's Horizon Europe research and innovation programme under grant agreement number 101041938 (RIBOCHEM) to W. A. V. We further acknowledge support from the Dutch Research Council for NACTAR project 20812 to W. A. V.Notes and references
- C. J. Murray, K. S. Ikuta, F. Sharara, L. Swetschinski, G. R. Aguilar, A. Gray, C. Han, C. Bisignano, P. Rao, E. Wool, S. C. Johnson, A. J. Browne, M. G. Chipeta, F. Fell, S. Hackett, G. Haines-Woodhouse, B. H. K. Hamadani, E. A. P. Kumaran, B. McManigal, R. Agarwal, S. Akech, S. Albertson, J. Amuasi, J. Andrews, A. Aravkin, E. Ashley, F. Bailey, S. Baker, B. Basnyat, A. Bekker, R. Bender, A. Bethou, J. Bielicki, S. Boonkasidecha, J. Bukosia, C. Carvalheiro, C. Castañeda-Orjuela, V. Chansamouth, S. Chaurasia, S. Chiurchiù, F. Chowdhury, A. J. Cook, B. Cooper, T. R. Cressey, E. Criollo-Mora, M. Cunningham, S. Darboe, N. P. J. Day, M. D. Luca, K. Dokova, A. Dramowski, S. J. Dunachie, T. Eckmanns, D. Eibach, A. Emami, N. Feasey, N. Fisher-Pearson, K. Forrest, D. Garrett, P. Gastmeier, A. Z. Giref, R. C. Greer, V. Gupta, S. Haller, A. Haselbeck, S. I. Hay, M. Holm, S. Hopkins, K. C. Iregbu, J. Jacobs, D. Jarovsky, F. Javanmardi, M. Khorana, N. Kissoon, E. Kobeissi, T. Kostyanev, F. Krapp, R. Krumkamp, A. Kumar, H. H. Kyu, C. Lim, D. Limmathurotsakul, M. J. Loftus, M. Lunn, J. Ma, N. Mturi, T. Munera-Huertas, P. Musicha, M. M. Mussi-Pinhata, T. Nakamura, R. Nanavati, S. Nangia, P. Newton, C. Ngoun, A. Novotney, D. Nwakanma, C. W. Obiero, A. Olivas-Martinez, P. Olliaro, E. Ooko, E. Ortiz-Brizuela, A. Y. Peleg, C. Perrone, N. Plakkal, A. Ponce-de-Leon, M. Raad, T. Ramdin, A. Riddell, T. Roberts, J. V. Robotham, A. Roca, K. E. Rudd, N. Russell, J. Schnall, J. A. G. Scott, M. Shivamallappa, J. Sifuentes-Osornio, N. Steenkeste, A. J. Stewardson, T. Stoeva, N. Tasak, A. Thaiprakong, G. Thwaites, C. Turner, P. Turner, H. R. van Doorn, S. Velaphi, A. Vongpradith, H. Vu, T. Walsh, S. Waner, T. Wangrangsimakul, T. Wozniak, P. Zheng, B. Sartorius, A. D. Lopez, A. Stergachis, C. Moore, C. Dolecek and M. Naghavi, Lancet, 2022, 399, 629–655 CrossRef CAS PubMed.
- K. Lewis, Cell, 2020, 181, 29–45 CrossRef CAS PubMed.
- M. Miethke, M. Pieroni, T. Weber, M. Brönstrup, P. Hammann, L. Halby, P. B. Arimondo, P. Glaser, B. Aigle, H. B. Bode, R. Moreira, Y. Li, A. Luzhetskyy, M. H. Medema, J.-L. Pernodet, M. Stadler, J. R. Tormo, O. Genilloud, A. W. Truman, K. J. Weissman, E. Takano, S. Sabatini, E. Stegmann, H. Brötz-Oesterhelt, W. Wohlleben, M. Seemann, M. Empting, A. K. H. Hirsch, B. Loretz, C.-M. Lehr, A. Titz, J. Herrmann, T. Jaeger, S. Alt, T. Hesterkamp, M. Winterhalter, A. Schiefer, K. Pfarr, A. Hoerauf, H. Graz, M. Graz, M. Lindvall, S. Ramurthy, A. Karlén, M. van Dongen, H. Petkovic, A. Keller, F. Peyrane, S. Donadio, L. Fraisse, L. J. V. Piddock, I. H. Gilbert, H. E. Moser and R. Müller, Nat. Rev. Chem., 2021, 5, 726–749 CrossRef CAS PubMed.
- W. A. Velema, Chem. Commun., 2023, 59, 6148–6158 RSC.
- B. H. Gan, J. Gaynord, S. M. Rowe, T. Deingruber and D. R. Spring, Chem. Soc. Rev., 2021, 50, 7820–7880 RSC.
- H. Wang, D. Chen and H. Lu, Appl. Microbiol. Biotechnol., 2022, 106, 3957–3972 CrossRef CAS PubMed.
- S. Meile, J. Du, M. Dunne, S. Kilcher and M. J. Loessner, Curr. Opin. Virol., 2022, 52, 182–191 CrossRef CAS PubMed.
- L. Moreira, N. M. Guimarães, R. S. Santos, J. A. Loureiro, M. C. Pereira and N. F. Azevedo, Mol. Ther.–Nucleic Acids, 2024, 35, 102122 CrossRef CAS PubMed.
- J. A. Kulkarni, D. Witzigmann, S. B. Thomson, S. Chen, B. R. Leavitt, P. R. Cullis and R. van der Meel, Nat. Nanotechnol., 2021, 16, 630–643 CrossRef CAS PubMed.
- P. C. Zamecnik and M. L. Stephenson, Proc. Natl. Acad. Sci. U. S. A., 1978, 75, 280–284 CrossRef CAS PubMed.
- M. L. Stephenson and P. C. Zamecnik, Proc. Natl. Acad. Sci. U. S. A., 1978, 75, 285–288 CrossRef CAS PubMed.
- K. Jayaraman, K. McParland, P. Miller and P. O. P. Ts’o, Proc. Natl. Acad. Sci. U. S. A., 1981, 78, 1537–1541 CrossRef CAS PubMed.
- P. S. Miller, K. B. McParland, K. Jayaraman and P. O. P. Tso, Biochemistry, 1981, 20, 1874–1880 CrossRef CAS PubMed.
- M. A. Rahman, J. Summerton, E. Foster, K. Cunningham, E. Stirchak, D. Weller and H. W. Schaup, Antisense Res. Dev., 1991, 1, 319–327 CrossRef CAS PubMed.
- S. T. Crooke, B. F. Baker, R. M. Crooke and X. Liang, Nat. Rev. Drug Discovery, 2021, 20, 427–453 CrossRef CAS PubMed.
- D. Collotta, I. Bertocchi, E. Chiapello and M. Collino, Front. Pharmacol., 2023, 14, 1304342 CrossRef CAS PubMed.
- S. T. Crooke, X.-H. Liang, B. F. Baker and R. M. Crooke, J. Biol. Chem., 2021, 296, 100416 CrossRef CAS PubMed.
- S. T. Crooke, Nucleic Acid Ther., 2017, 27, 70–77 CrossRef CAS PubMed.
- M. Hyjek, M. Figiel and M. Nowotny, DNA Repair, 2019, 84, 102672 CrossRef CAS PubMed.
- E. W. Lundblad and S. Altman, New Biotechnol., 2010, 27, 212–221 CrossRef CAS PubMed.
- K. Dhuri, C. Bechtold, E. Quijano, H. Pham, A. Gupta, A. Vikram and R. Bahal, J. Clin. Med., 2020, 9, 2004 CrossRef CAS PubMed.
- E. K. Sully and B. L. Geller, Curr. Opin. Microbiol., 2016, 33, 47–55 CrossRef CAS PubMed.
- J. P. Hegarty and D. B. Stewart, Appl. Microbiol. Biotechnol., 2018, 102, 1055–1065 CrossRef CAS PubMed.
- U. Tsylents, I. Siekierska and J. Trylska, Eur. Biophys. J., 2023, 52, 533–544 CrossRef CAS PubMed.
- N. Angrish and G. Khare, Med. Drug Discovery, 2023, 20, 100166 CrossRef CAS.
- X.-Y. Xue, X.-G. Mao, Y. Zhou, Z. Chen, Y. Hu, Z. Hou, M.-K. Li, J.-R. Meng and X.-X. Luo, Nanomedicine, 2018, 14, 745–758 CrossRef CAS PubMed.
- S. Jani, M. S. Ramirez and M. E. Tolmasky, Biomedicines, 2021, 9, 416 CrossRef CAS PubMed.
- P. S. Eder, R. J. Devine, J. M. Dagle and J. A. Walder, Antisense Res. Dev., 1991, 1, 141–151 CrossRef CAS PubMed.
- N. Dias and C. A. Stein, Mol. Cancer Ther., 2002, 1, 347–355 CAS.
- S. Story, S. Bhaduri, S. Ganguly, R. Dakarapu, S. L. Wicks, J. Bhadra, S. Kwange and D. P. Arya, ACS Infect. Dis., 2024, 10, 971–987 CrossRef CAS PubMed.
- V. K. Sharma, R. K. Sharma and S. K. Singh, Med. Chem. Commun., 2014, 5, 1454–1471 RSC.
- P. S. Miller, Nat. Biotechnol., 1991, 9, 358–362 CrossRef CAS PubMed.
- A. M. Tari, S. D. Tucker, A. Deisseroth and G. Lopez-Berestein, Blood, 1994, 84, 601–607 CrossRef CAS PubMed.
- J. Meng, H. Wang, Z. Hou, T. Chen, J. Fu, X. Ma, G. He, X. Xue, M. Jia and X. Luo, Antimicrob. Agents Chemother., 2009, 53, 2871–2878 CrossRef CAS PubMed.
- M. T. Migawa, W. Shen, W. B. Wan, G. Vasquez, M. E. Oestergaard, A. Low, C. L. De Hoyos, R. Gupta, S. Murray, M. Tanowitz, M. Bell, J. G. Nichols, H. Gaus, X. Liang, E. E. Swayze, S. T. Crooke and P. P. Seth, Nucleic Acids Res., 2019, 47, 5465–5479 CrossRef CAS PubMed.
- K. Ota, K. Nagao, D. Hata, H. Sugiyama, Y. Segawa, R. Tokunoh, T. Seki, N. Miyamoto, Y. Sasaki and H. Ohmiya, Nat. Commun., 2023, 14, 6856 CrossRef CAS PubMed.
- H. Matzura and F. Eckstein, Eur. J. Biochem., 1968, 3, 448–452 CrossRef CAS PubMed.
- F. Eckstein and H. Gindl, FEBS Lett., 1969, 2, 262–264 CrossRef CAS PubMed.
- F. Eckstein, Nucleic Acid Ther., 2014, 24, 374–387 CrossRef CAS PubMed.
- K. W. Knouse, J. N. deGruyter, M. A. Schmidt, B. Zheng, J. C. Vantourout, C. Kingston, S. E. Mercer, I. M. Mcdonald, R. E. Olson, Y. Zhu, C. Hang, J. Zhu, C. Yuan, Q. Wang, P. Park, M. D. Eastgate and P. S. Baran, Science, 2018, 361, 1234–1238 CrossRef CAS PubMed.
- K. W. Knouse, D. T. Flood, J. C. Vantourout, M. A. Schmidt, I. M. Mcdonald, M. D. Eastgate and P. S. Baran, ACS Cent. Sci., 2021, 7, 1473–1485 CrossRef CAS PubMed.
- T. Hagenacker, C. D. Wurster, R. Günther, O. Schreiber-Katz, A. Osmanovic, S. Petri, M. Weiler, A. Ziegler, J. Kuttler, J. C. Koch, I. Schneider, G. Wunderlich, N. Schloss, H. C. Lehmann, I. Cordts, M. Deschauer, P. Lingor, C. Kamm, B. Stolte, L. Pietruck, A. Totzeck, K. Kizina, C. Mönninghoff, O. von Velsen, C. Ose, H. Reichmann, M. Forsting, A. Pechmann, J. Kirschner, A. C. Ludolph, A. Hermann and C. Kleinschnitz, Lancet Neurol., 2020, 19, 317–325 CrossRef CAS PubMed.
- J. Meng, G. He, H. Wang, M. Jia, X. Ma, F. Da, N. Wang, Z. Hou, X. Xue, M. Li, Y. Zhou and X. Luo, J. Antibiot., 2015, 68, 158–164 CrossRef CAS PubMed.
- C. Ghosh, L. Popella, V. Dhamodharan, J. Jung, J. Dietzsch, L. Barquist, C. Höbartner and J. Vogel, RNA, 2024, 30, 624–643 CAS.
- J. W. Jaroszewski, V. Clausen, J. S. Cohen and O. Dahl, Nucleic Acids Res., 1996, 24, 829–834 CrossRef CAS PubMed.
- S. Shibahara, S. Mukai, T. Nishihara, H. Inoue, E. Ohtsuka and H. Morisawa, Nucleic Acids Res., 1987, 15, 4403–4415 CrossRef CAS PubMed.
- P. Martin, Helv. Chim. Acta, 1995, 78, 486–504 CrossRef CAS.
- M. J. Damha, C. J. Wilds, A. Noronha, I. Brukner, G. Borkow, D. Arion and M. A. Parniak, J. Am. Chem. Soc., 1998, 120, 12976–12977 CrossRef CAS.
- R. El-Khoury and M. J. Damha, Acc. Chem. Res., 2021, 54, 2287–2297 CrossRef CAS PubMed.
- S. Obika, D. Nanbu, Y. Hari, K. Morio, Y. In, T. Ishida and T. Imanishi, Tetrahedron Lett., 1997, 38, 8735–8738 CrossRef CAS.
- F. Li, S. Sarkhel, C. J. Wilds, Z. Wawrzak, T. P. Prakash, M. Manoharan and M. Egli, Biochemistry, 2006, 45, 4141–4152 CrossRef CAS PubMed.
- O. Plashkevych, Q. Li and J. Chattopadhyaya, Mol. BioSyst., 2017, 13, 921–938 RSC.
- H. Inoue, Y. Hayase, S. Iwai and E. Ohtsuka, FEBS Lett., 1987, 215, 327–330 CrossRef CAS PubMed.
- K. R. Q. Lim and T. Yokota, in Gapmers: Methods and Protocols, ed. T. Yokota and R. Maruyama, Springer, US, New York, NY, 2020, pp. 3–19 Search PubMed.
- S. T. Crooke and R. S. Geary, Br. J. Clin. Pharmacol., 2013, 76, 269–276 CrossRef CAS PubMed.
- M. D. Benson, M. Waddington-Cruz, J. L. Berk, M. Polydefkis, P. J. Dyck, A. K. Wang, V. Planté-Bordeneuve, F. A. Barroso, G. Merlini, L. Obici, M. Scheinberg, T. H. Brannagan, W. J. Litchy, C. Whelan, B. M. Drachman, D. Adams, S. B. Heitner, I. Conceição, H. H. Schmidt, G. Vita, J. M. Campistol, J. Gamez, P. D. Gorevic, E. Gane, A. M. Shah, S. D. Solomon, B. P. Monia, S. G. Hughes, T. J. Kwoh, B. W. McEvoy, S. W. Jung, B. F. Baker, E. J. Ackermann, M. A. Gertz and T. Coelho, N. Engl. J. Med., 2018, 379, 22–31 CrossRef CAS PubMed.
- A. F. Sandoval-Mojica, W. B. Hunter, V. Aishwarya, S. Bonilla and K. S. Pelz-Stelinski, Sci. Rep., 2021, 11, 2760 CrossRef CAS PubMed.
- W. B. Hunter, W. R. Cooper, A. F. Sandoval-Mojica, G. McCollum, V. Aishwarya and K. S. Pelz-Stelinski, Front. Agron., 2021, 3 DOI:10.3389/fagro.2021.675247.
- N. Souleimanian, G. F. Deleavey, H. Soifer, S. Wang, K. Tiemann, M. J. Damha and C. A. Stein, Mol. Ther.–Nucleic Acids, 2012, 1, e43 CrossRef PubMed.
- S. Mishra and M. Ghanim, Int. J. Mol. Sci., 2022, 23, 4029 CrossRef PubMed.
- J. E. Summerton, D. D. Weller, US Pat., US5185444A, 1993.
- P. E. Nielsen, M. Egholm, R. H. Berg and O. Buchardt, Science, 1991, 254, 1497–1500 CrossRef CAS PubMed.
- J. E. Summerton, Int. J. Pept. Res. Ther., 2003, 10, 215–236 CrossRef CAS.
- L. Good and P. E. Nielsen, Nat. Biotechnol., 1998, 16, 355–358 CrossRef CAS PubMed.
- L. Good, S. K. Awasthi, R. Dryselius, O. Larsson and P. E. Nielsen, Nat. Biotechnol., 2001, 19, 360–364 CrossRef CAS PubMed.
- B. L. Geller, J. D. Deere, D. A. Stein, A. D. Kroeker, H. M. Moulton and P. L. Iversen, Antimicrob. Agents Chemother., 2003, 47, 3233–3239 CrossRef CAS PubMed.
- M. S. Kupryushkin, D. V. Pyshnyi and D. A. Stetsenko, Acta Nat., 2014, 6, 116–118 CrossRef CAS.
- Y. V. Skvortsova, E. G. Salina, E. A. Burakova, O. S. Bychenko, D. A. Stetsenko and T. L. Azhikina, Front. Pharmacol., 2019, 10 DOI:10.3389/fphar.2019.01049.
- M. S. Kupryushkin, A. V. Filatov, N. L. Mironova, O. A. Patutina, I. V. Chernikov, E. L. Chernolovskaya, M. A. Zenkova, D. V. Pyshnyi, D. A. Stetsenko, S. Altman and V. V. Vlassov, Mol. Ther.–Nucleic Acids, 2022, 27, 211–226 CrossRef CAS PubMed.
- A. S. Levina, M. N. Repkova, B. P. Chelobanov, E. V. Bessudnova, N. A. Mazurkova, D. A. Stetsenko and V. F. Zarytova, Mol. Biol., 2017, 51, 633–638 CrossRef CAS.
- A. Kilanowska and S. Studzińska, RSC Adv., 2020, 10, 34501–34516 RSC.
- B. L. Geller, J. Deere, L. Tilley and P. L. Iversen, J. Antimicrob. Chemother., 2005, 55, 983–988 CrossRef CAS PubMed.
- D. A. Moustafa, A. W. Wu, D. Zamora, S. M. Daly, C. R. Sturge, C. Pybus, B. L. Geller, J. B. Goldberg and D. E. Greenberg, mBio, 2021, 12 DOI:10.1128/mbio.02411-20.
- X.-X. Tan, J. K. Actor and Y. Chen, Antimicrob. Agents Chemother., 2005, 49, 3203–3207 CrossRef CAS PubMed.
- C. Li, A. J. Callahan, K. S. Phadke, B. Bellaire, C. E. Farquhar, G. Zhang, C. K. Schissel, A. J. Mijalis, N. Hartrampf, A. Loas, D. E. Verhoeven and B. L. Pentelute, ACS Cent. Sci., 2022, 8, 205–213 CrossRef CAS PubMed.
- S. Paul and M. H. Caruthers, Molecules, 2023, 28, 5380 CrossRef CAS PubMed.
- T. J. Silhavy, D. Kahne and S. Walker, Cold Spring Harbor Perspect. Biol., 2010, 2, a000414 Search PubMed.
- G. C. Kim, D. H. Cheon and Y. Lee, Biochim. Biophys. Acta, Proteins Proteomics, 2021, 1869, 140604 CrossRef CAS PubMed.
- A. Gori, G. Lodigiani, S. G. Colombarolli, G. Bergamaschi and A. Vitali, ChemMedChem, 2023, 18, e202300236 CrossRef CAS PubMed.
- H. Derakhshankhah and S. Jafari, Biomed. Pharmacother., 2018, 108, 1090–1096 CrossRef CAS PubMed.
- I. Ruseska and A. Zimmer, Beilstein J. Nanotechnol., 2020, 11, 101–123 CrossRef CAS PubMed.
- S. M. G. Jirka, H. Heemskerk, C. L. Tanganyika-de Winter, D. Muilwijk, K. H. Pang, P. C. de Visser, A. Janson, T. G. Karnaoukh, R. Vermue, P. A. C. ‘t Hoen, J. C. T. van Deutekom, B. Aguilera and A. Aartsma-Rus, Nucleic Acid Ther., 2014, 24, 25–36 CrossRef CAS PubMed.
- M. A. Maier, C. C. Esau, A. M. Siwkowski, E. V. Wancewicz, K. Albertshofer, G. A. Kinberger, N. S. Kadaba, T. Watanabe, M. Manoharan, C. F. Bennett, R. H. Griffey and E. E. Swayze, J. Med. Chem., 2006, 49, 2534–2542 CrossRef CAS PubMed.
- Z. Chen, D. Nie, Y. Hu, M. Li, Z. Hou, X. Mao, X. Luo and X. Xue, Curr. Drug Delivery, 2019, 16, 728–736 CrossRef CAS PubMed.
- L. Crombez, G. Aldrian-Herrada, K. Konate, Q. N. Nguyen, G. K. McMaster, R. Brasseur, F. Heitz and G. Divita, Mol. Ther., 2009, 17, 95–103 CrossRef CAS PubMed.
- P. Järver, T. Coursindel, S. E. Andaloussi, C. Godfrey, M. J. Wood and M. J. Gait, Mol. Ther.–Nucleic Acids, 2012, 1(6), e27 CrossRef PubMed.
- G. Barkowsky, A.-L. Lemster, R. Pappesch, A. Jacob, S. Krüger, A. Schröder, B. Kreikemeyer and N. Patenge, Mol. Ther.–Nucleic Acids, 2019, 18, 444–454 CrossRef CAS PubMed.
- G. Barkowsky, C. Abt, I. Pöhner, A. Bieda, S. Hammerschmidt, A. Jacob, B. Kreikemeyer and N. Patenge, Microbiol. Spectrum, 2022, 10, e0049722 CrossRef PubMed.
- M. Vaara and M. Porro, Antimicrob. Agents Chemother., 1996, 40, 1801–1805 CrossRef CAS PubMed.
- J. Meng, F. Da, X. Ma, N. Wang, Y. Wang, H. Zhang, M. Li, Y. Zhou, X. Xue, Z. Hou, M. Jia and X. Luo, Antimicrob. Agents Chemother., 2015, 59, 914–922 CrossRef PubMed.
- D. Toyohara, Y. Yokoi, G. Inoue, T. Muraoka and T. Mori, Front. Microbiol., 2019, 10 DOI:10.3389/fmicb.2019.02534.
- F. Zakany, I. M. Mándity, Z. Varga, G. Panyi, P. Nagy and T. Kovacs, Cells, 2023, 12, 1700 CrossRef CAS PubMed.
- O. Boussif, F. Lezoualc’h, M. A. Zanta, M. D. Mergny, D. Scherman, B. Demeneix and J. P. Behr, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 7297–7301 CrossRef CAS PubMed.
- P. R. Berthold, T. Shiraishi and P. E. Nielsen, Bioconjugate Chem., 2010, 21, 1933–1938 CrossRef CAS PubMed.
- I. M. Gabas and P. E. Nielsen, Biomacromolecules, 2020, 21, 472–483 CrossRef CAS PubMed.
- M. Iubatti, I. M. Gabas, L. M. Cavaco, E. H. Mood, E. Lim, F. Bonanno, N. Yavari, C. Brolin and P. E. Nielsen, ACS Infect. Dis., 2022, 8, 1098–1106 CrossRef CAS PubMed.
- A. Thakur, H. Mikkelsen and G. Jungersen, J. Immunol. Res., 2019, 2019, 1356540 Search PubMed.
- Y.-F. Li and P. A. Morcos, Bioconjugate Chem., 2008, 19, 1464–1470 CrossRef CAS PubMed.
- J. E. K. Hildreth, J. D. Moulton and D. J. Alcendor, Biomedicines, 2021, 9, 1018 CrossRef CAS PubMed.
- P. A. Morcos, Y. Li and S. Jiang, Biotechniques, 2008, 45, 613–623 CrossRef CAS PubMed.
- S. Lam, H. Chen, C. K. Chen, N. Min and J. J. H. Chu, Sci. Rep., 2015, 5, 12727 CrossRef CAS PubMed.
- M. Równicki, M. Wojciechowska, A. J. Wierzba, J. Czarnecki, D. Bartosik, D. Gryko and J. Trylska, Sci. Rep., 2017, 7, 7644 CrossRef PubMed.
- M. Giedyk, A. Jackowska, M. Równicki, M. Kolanowska, J. Trylska and D. Gryko, Chem. Commun., 2019, 55, 763–766 RSC.
- M. Nijland, J. M. Martínez Felices, D. J. Slotboom and C. Thangaratnarajah, in Vitamins and Hormones, ed. G. Litwack, Academic Press, 2022, vol. 119, pp. 121–148 Search PubMed.
- M. Równicki, Z. Dąbrowska, M. Wojciechowska, A. J. Wierzba, K. Maximova, D. Gryko and J. Trylska, ACS Omega, 2019, 4, 819–824 CrossRef.
- S. Pereira, R. Yao, M. Gomes, P. T. Jørgensen, J. Wengel, N. F. Azevedo and R. Sobral Santos, Antibiotics, 2021, 10, 379 CrossRef CAS PubMed.
- M. Wang, B. Wu, S. N. Shah, P. Lu and Q. Lu, Mol. Ther.–Nucleic Acids, 2019, 16, 663–674 CrossRef CAS PubMed.
- E. Riguet, S. Tripathi, B. Chaubey, J. Désiré, V. N. Pandey and J.-L. Décout, J. Med. Chem., 2004, 47, 4806–4809 CrossRef CAS PubMed.
- W. A. Velema and E. T. Kool, Org. Lett., 2018, 20, 6587–6590 CrossRef CAS PubMed.
- C. Fàbrega, A. Aviñó, N. Navarro, A. F. Jorge, S. Grijalvo and R. Eritja, Pharmaceutics, 2023, 15, 320 CrossRef PubMed.
- T. Kauss, C. Arpin, L. Bientz, P. Vinh Nguyen, B. Vialet, S. Benizri and P. Barthélémy, Sci. Rep., 2020, 10, 1054 CrossRef CAS PubMed.
- M. J. Pals, L. Wijnberg, Ç. Yildiz and W. A. Velema, Angew. Chem., Int. Ed., 2024, 63, e202402405 CrossRef CAS PubMed.
- U. Tsylents, M. Burmistrz, M. Wojciechowska, J. Stępień, P. Maj and J. Trylska, Front. Microbiol., 2024, 15 DOI:10.3389/fmicb.2024.1331021.
- J. Kramer, Ö. Özkaya and R. Kümmerli, Nat. Rev. Microbiol., 2020, 18, 152–163 CrossRef CAS PubMed.
- M. Bassetti, R. Echols, Y. Matsunaga, M. Ariyasu, Y. Doi, R. Ferrer, T. P. Lodise, T. Naas, Y. Niki, D. L. Paterson, S. Portsmouth, J. Torre-Cisneros, K. Toyoizumi, R. G. Wunderink and T. D. Nagata, Lancet Infect. Dis., 2021, 21, 226–240 CrossRef CAS PubMed.
- C. Ji, P. A. Miller and M. J. Miller, J. Am. Chem. Soc., 2012, 134, 9898–9901 CrossRef CAS PubMed.
- Q. Long, B. Jia, Y. Shi, Q. Wang, H. Yu and Z. Li, ACS Appl. Mater. Interfaces, 2021, 13, 47987–47995 CrossRef CAS PubMed.
- Y. Zhang, W. Ma, Y. Zhu, S. Shi, Q. Li, C. Mao, D. Zhao, Y. Zhan, J. Shi, W. Li, L. Wang, C. Fan and Y. Lin, Nano Lett., 2018, 18, 5652–5659 CrossRef CAS PubMed.
- R. Tenchov, R. Bird, A. E. Curtze and Q. Zhou, ACS Nano, 2021, 15, 16982–17015 CrossRef CAS PubMed.
- J. Meng, H. Bai, M. Jia, X. Ma, Z. Hou, X. Xue, Y. Zhou and X. Luo, J. Antibiot., 2012, 65, 129–134 CrossRef CAS PubMed.
- K. Saar, M. Lindgren, M. Hansen, E. Eiríksdóttir, Y. Jiang, K. Rosenthal-Aizman, M. Sassian and Ü. Langel, Anal. Biochem., 2005, 345, 55–65 CrossRef CAS PubMed.
- K. Kanekura, Y. Harada, M. Fujimoto, T. Yagi, Y. Hayamizu, K. Nagaoka and M. Kuroda, Sci. Rep., 2018, 8, 12740 CrossRef PubMed.
- E. D. Brown and G. D. Wright, Nature, 2016, 529, 336–343 CrossRef CAS PubMed.
- F. R. Fields, S. W. Lee and M. J. McConnell, Biochem. Pharmacol., 2017, 134, 74–86 CrossRef CAS PubMed.
- H. O. Bohl, K. Shi, J. K. Lee and H. Aihara, Nat. Commun., 2018, 9, 377 CrossRef PubMed.
- P. Zhou and A. W. Barb, Curr. Pharm. Biotechnol., 2008, 9, 9–15 Search PubMed.
- J. J. Howard, C. R. Sturge, D. A. Moustafa, S. M. Daly, K. R. Marshall-Batty, C. F. Felder, D. Zamora, M. Yabe-Gill, M. Labandeira-Rey, S. M. Bailey, M. Wong, J. B. Goldberg, B. L. Geller and D. E. Greenberg, Antimicrob. Agents Chemother., 2017, 61(4) DOI:10.1128/aac.01938-16.
- M. Martínez-Guitián, J. C. Vázquez-Ucha, L. Álvarez-Fraga, K. Conde-Pérez, G. Bou, M. Poza and A. Beceiro, J. Antimicrob. Chemother., 2020, 75, 51–59 CrossRef PubMed.
- L. A. Carfrae, K. Rachwalski, S. French, R. Gordzevich, L. Seidel, C. N. Tsai, M. M. Tu, C. R. MacNair, O. G. Ovchinnikova, B. R. Clarke, C. Whitfield and E. D. Brown, Nat. Microbiol., 2023, 8, 1026–1038 CrossRef CAS PubMed.
- M. S. Paget and J. D. Helmann, Genome Biol., 2003, 4, 203 CrossRef PubMed.
- H. Bai, Y. You, H. Yan, J. Meng, X. Xue, Z. Hou, Y. Zhou, X. Ma, G. Sang and X. Luo, Biomaterials, 2012, 33, 659–667 CrossRef CAS PubMed.
- H. Bai, G. Sang, Y. You, X. Xue, Y. Zhou, Z. Hou, J. Meng and X. Luo, PLoS One, 2012, 7, e29886 CrossRef CAS PubMed.
- D. N. Wilson, Nat. Rev. Microbiol., 2014, 12, 35–48 CrossRef CAS PubMed.
- S. A. Fromm, K. M. O’Connor, M. Purdy, P. R. Bhatt, G. Loughran, J. F. Atkins, A. Jomaa and S. Mattei, Nat. Commun., 2023, 14, 1095 CrossRef CAS PubMed.
- E. Roberts, A. Sethi, J. Montoya, C. R. Woese and Z. Luthey-Schulten, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 13953–13958 CrossRef CAS PubMed.
- L. Popella, J. Jung, P. T. Do, R. J. Hayward, L. Barquist and J. Vogel, Nucleic Acids Res., 2022, 50, 6435–6452 CrossRef CAS PubMed.
- M. Rawlings and J. E. Cronan, J. Biol. Chem., 1992, 267, 5751–5754 CrossRef CAS PubMed.
- D. E. Greenberg, K. R. Marshall-Batty, L. R. Brinster, K. A. Zarember, P. A. Shaw, B. L. Mellbye, P. L. Iversen, S. M. Holland and B. L. Geller, J. Infect. Dis., 2010, 201, 1822–1830 CrossRef CAS PubMed.
- B. L. Geller, K. Marshall-Batty, F. J. Schnell, M. M. McKnight, P. L. Iversen and D. E. Greenberg, J. Infect. Dis., 2013, 208, 1553–1560 CrossRef CAS PubMed.
- B. L. Geller, L. Li, F. Martinez, E. Sully, C. R. Sturge, S. M. Daly, C. Pybus and D. E. Greenberg, J. Antimicrob. Chemother., 2018, 73, 1611–1619 CrossRef CAS PubMed.
- L. D. Tilley, O. S. Hine, J. A. Kellogg, J. N. Hassinger, D. D. Weller, P. L. Iversen and B. L. Geller, Antimicrob. Agents Chemother., 2006, 50, 2789–2796 CrossRef CAS PubMed.
- B. L. Mellbye, D. D. Weller, J. N. Hassinger, M. D. Reeves, C. E. Lovejoy, P. L. Iversen and B. L. Geller, J. Antimicrob. Chemother., 2010, 65, 98–106 CrossRef CAS PubMed.
- N. Nekhotiaeva, S. K. Awasthi, P. E. Nielsen and L. Good, Mol. Ther., 2004, 10, 652–659 CrossRef CAS PubMed.
- D. W. Adams and J. Errington, Nat. Rev. Microbiol., 2009, 7, 642–653 CrossRef CAS PubMed.
- M. Traykovska and R. Penchovsky, ACS Synth. Biol., 2022, 11, 1845–1855 CrossRef CAS PubMed.
- M. Traykovska, K. B. Popova and R. Penchovsky, ACS Synth. Biol., 2021, 10, 3167–3176 CrossRef CAS PubMed.
- M. Traykovska, L. A. Otcheva and R. Penchovsky, ACS Appl. Bio Mater., 2022, 5, 4896–4902 CrossRef CAS PubMed.
- M. J. Tsai, R. A. I. Zambrano, J. L. Susas, L. Silva and M. K. Takahashi, ACS Synth. Biol., 2023, 12, 2245–2251 CrossRef CAS PubMed.
- R. R. Breaker, Mol. Cell, 2011, 43, 867–879 CrossRef CAS PubMed.
- K. Kavita and R. R. Breaker, Trends Biochem. Sci., 2023, 48, 119–141 CrossRef CAS PubMed.
- R. T. Batey, RNA, 2015, 21, 560–563 CrossRef CAS PubMed.
- A. Serganov and E. Nudler, Cell, 2013, 152, 17–24 CrossRef CAS PubMed.
- S. Crielaard, R. Maassen, T. Vosman, I. Rempkens and W. A. Velema, J. Am. Chem. Soc., 2022, 144, 10462–10470 CrossRef CAS PubMed.
- W. Li, X. Ying, Q. Lu and L. Chen, Genomics, Proteomics Bioinf., 2012, 10, 276–284 CrossRef CAS PubMed.
- E. G. H. Wagner and P. Romby, in Advances in Genetics, ed. T. Friedmann, J. C. Dunlap and S. F. Goodwin, Academic Press, 2015, vol. 90, pp. 133–208 Search PubMed.
- J. Deere, P. Iversen and B. L. Geller, Antimicrob. Agents Chemother., 2005, 49, 249–255 CrossRef CAS PubMed.
- R. Dryselius, S. K. Aswasti, G. K. Rajarao, P. E. Nielsen and L. Good, Oligonucleotides, 2003, 13, 427–433 CrossRef CAS PubMed.
- Y. Li, Z. Chen, X. Li, H. Zhang, Q. Huang, Y. Zhang and S. Xu, J. Biotechnol., 2007, 128, 726–734 CrossRef CAS PubMed.
- G. Harth, P. C. Zamecnik, J.-Y. Tang, D. Tabatadze and M. A. Horwitz, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 418–423 CrossRef CAS PubMed.
- J. Summerton, Biochim. Biophys. Acta, Gene Struct. Expression, 1999, 1489, 141–158 CrossRef CAS PubMed.
- A. Ghosal and P. E. Nielsen, Nucleic Acid Ther., 2012, 22, 323–334 CrossRef CAS PubMed.
- W. F. Lima, B. P. Monia, D. J. Ecker and S. M. Freier, Biochemistry, 1992, 31, 12055–12061 CrossRef CAS PubMed.
- J. M. Schuurmans, A. S. Nuri Hayali, B. B. Koenders and B. H. ter Kuile, J. Microbiol. Methods, 2009, 79, 44–47 CrossRef CAS PubMed.
- J. Jung, L. Popella, P. T. Do, P. Pfau, J. Vogel and L. Barquist, RNA, 2023, 29, 570–583 CrossRef CAS PubMed.
- N. Yavari, L. Goltermann and P. E. Nielsen, ACS Chem. Biol., 2021, 16, 471–479 CrossRef CAS PubMed.
- K. E. Darrah, S. Albright, R. Kumbhare, M. Tsang, J. K. Chen and A. Deiters, ACS Chem. Biol., 2023, 18, 2176–2182 CrossRef CAS PubMed.
- J. Fominaya, J. Bravo and A. Rebollo, Ther. Delivery, 2015, 6, 1171–1194 CrossRef CAS PubMed.
- S. E. Park, M. I. Sajid, K. Parang and R. K. Tiwari, Mol. Pharmaceutics, 2019, 16, 3727–3743 CrossRef CAS PubMed.
- Z. Qian, X. Xu, J. F. Amacher, D. R. Madden, E. Cormet-Boyaka and D. Pei, Angew. Chem., Int. Ed., 2015, 54, 5874–5878 CrossRef CAS PubMed.
- M. Horn, F. Reichart, S. Natividad-Tietz, D. Diaz and I. Neundorf, Chem. Commun., 2016, 52, 2261–2264 RSC.
- N. A. Patil, V. J. Thombare, R. Li, X. He, J. Lu, H. H. Yu, H. Wickremasinghe, K. Pamulapati, M. A. K. Azad, T. Velkov, K. D. Roberts and J. Li, Front. Chem., 2022, 10 DOI:10.3389/fchem.2022.843163.
- S. M. Miller, R. J. Simon, S. Ng, R. N. Zuckermann, J. M. Kerr and W. H. Moos, Bioorg. Med. Chem. Lett., 1994, 4, 2657–2662 CrossRef CAS.
- P. A. Wender, D. J. Mitchell, K. Pattabiraman, E. T. Pelkey, L. Steinman and J. B. Rothbard, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 13003–13008 CrossRef CAS PubMed.
- H.-S. Kim, Y. Lee, M. H. Shin and H.-S. Lim, Chem. Commun., 2021, 57, 6800–6803 RSC.
- W. Zhou, W. Brown, A. Bardhan, M. Delaney, A. S. Ilk, R. R. Rauen, S. I. Kahn, M. Tsang and A. Deiters, Angew. Chem., Int. Ed., 2020, 59, 8998–9003 CrossRef CAS PubMed.
- W. Brown, A. Bardhan, K. Darrah, M. Tsang and A. Deiters, J. Am. Chem. Soc., 2022, 144, 16819–16826 CrossRef CAS PubMed.
- J. Uusna, K. Langel and Ü. Langel, Methods Mol. Biol., 2015, 1324, 133–148 CrossRef PubMed.
- A. Ghosal, A. Vitali, J. E. M. Stach and P. E. Nielsen, ACS Chem. Biol., 2013, 8, 360–367 CrossRef CAS PubMed.
- S. E. Puckett, K. A. Reese, G. M. Mitev, V. Mullen, R. C. Johnson, K. R. Pomraning, B. L. Mellbye, L. D. Tilley, P. L. Iversen, M. Freitag and B. L. Geller, Antimicrob. Agents Chemother., 2012, 56, 6147–6153 CrossRef CAS PubMed.
- B. W. Neuman, D. A. Stein, A. D. Kroeker, M. J. Churchill, A. M. Kim, P. Kuhn, P. Dawson, H. M. Moulton, R. K. Bestwick, P. L. Iversen and M. J. Buchmeier, J. Virol., 2005, 79, 9665–9676 CrossRef CAS PubMed.
- R. Dryselius, N. Nekhotiaeva and L. Good, J. Antimicrob. Chemother., 2005, 56, 97–103 CrossRef CAS PubMed.
- A. N. Konwar, S. N. Hazarika, P. Bharadwaj and D. Thakur, Curr. Microbiol., 2022, 79, 330 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |