Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fluorescent chemosensors facilitate the visualization of plant health and their living environment in sustainable agriculture
Yang-Yang
Gao†
a,
Jie
He†
a,
Xiao-Hong
Li†
a,
Jian-Hong
Li
a,
Hong
Wu
a,
Ting
Wen
a,
Jun
Li
*b,
Ge-Fei
Hao
 *a and
Juyoung
Yoon
*a and
Juyoung
Yoon
 *c
*c
aState Key Laboratory of Green Pesticide, Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Center for Research and Development of Fine Chemicals, Guizhou University, Guiyang 550025, P. R. China. E-mail: gefei_hao@foxmail.com
bCollege of Chemistry, Huazhong Agricultural University, Wuhan 430070, China. E-mail: lijun1986@mail.hzau.edu.cn
cDepartment of Chemistry and Nanoscience, Ewha Womans University, Seoul 120-750, Korea. E-mail: jyoon@ewha.ac.kr
First published on 6th June 2024
Abstract
Globally, 91% of plant production encounters diverse environmental stresses that adversely affect their growth, leading to severe yield losses of 50–60%. In this case, monitoring the connection between the environment and plant health can balance population demands with environmental protection and resource distribution. Fluorescent chemosensors have shown great progress in monitoring the health and environment of plants due to their high sensitivity and biocompatibility. However, to date, no comprehensive analysis and systematic summary of fluorescent chemosensors used in monitoring the correlation between plant health and their environment have been reported. Thus, herein, we summarize the current fluorescent chemosensors ranging from their design strategies to applications in monitoring plant-environment interaction processes. First, we highlight the types of fluorescent chemosensors with design strategies to resolve the bottlenecks encountered in monitoring the health and living environment of plants. In addition, the applications of fluorescent small-molecule, nano and supramolecular chemosensors in the visualization of the health and living environment of plants are discussed. Finally, the major challenges and perspectives in this field are presented. This work will provide guidance for the design of efficient fluorescent chemosensors to monitor plant health, and then promote sustainable agricultural development.
1. Introduction
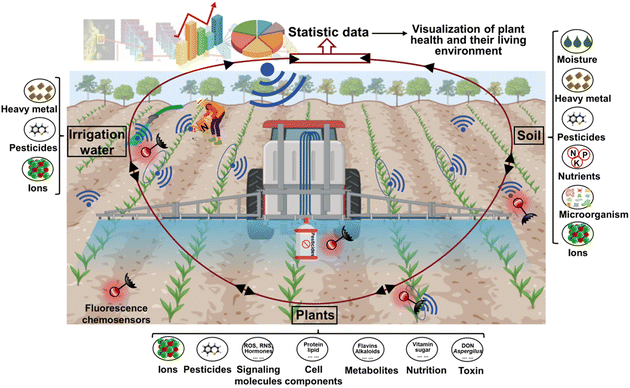
Exploring and understanding plant-environment interactions are essential components of sustainable agriculture, which is crucial for ensuring crop production and food security. Reportedly, global food production has increased by over threefold due to the advancement in sustainable agricultural practices regarding the connection between plant health and their environment during the past 50 years.1 Balancing the relationship between plants and their environment to regulate plant health can increase grain production by 33 million tons and decrease nitrogen fertilizer utilization (1.2 million tons), which is equivalent to an enhanced net worth of $12.2 billion.2 Plant health is directly associated with biodiversity, plant productivity, soil conditions, water quality, and climate change, thereby facilitating amplified ecosystem services and mitigating the dependence on extrinsic resources.3 For example, soil serves as the primary medium for plants, and consequently the presence of certain pollutants such as heavy metals in the soil may negatively impact both plant growth and human dietary safety.4 Therefore, monitoring the health and living environment of plants in sustainable agriculture is necessary for promoting the development of the economy and society.To date, many technologies have been developed to monitor the health and living environment of plants. Traditional methods including molecular technology and chromatographic detection are regularly employed. For example, DNA amplification via polymerase chain reaction (PCR) and its derivatives, namely nested PCR, quantitative PCR, digital PCR, and multiplex PCR, enzyme-linked immunosorbent assay (ELISA), and loop-mediated isothermal amplification (LAMP) are scientific testing methods for plant diseases.5,6 Also, ultra-performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS), gas chromatography–mass spectrometry (GC–MS), high performance liquid chromatography (HPLC) and other chromatographs are utilized to identify pesticide residues, soil or water contamination, and fruit nutrition.7–10 However, these procedures are laborious and time-consuming, often necessitating complex processes and proficient professionals for operation, and they fail to exhibit continuous monitoring. Consequently, due to their advantages of high sensitivity, simple operation, and spatiotemporal resolution, fluorescent chemosensors have gradually matured in exploring the connection between the health and living environment of plants.
In recent years, different types of fluorescent chemosensors including small-molecules and nano and supramolecular complexes have been used for monitoring plant health and their environment. The reasonable design of small-molecule fluorescent chemosensors using diverse chromophores such as naphthalimide, coumarin, BODIPY, rhodamine, and cyanine have been extensively applied for monitoring plant health owing to their low cost and favorable biocompatibility.11,12 Furthermore, to increase the quantum yield (QY) and recyclability, fluorescent nano-chemosensors have been designed based on quantum dots, metal–organic frameworks, covalent organic frameworks and nanoclusters and used to analyze the living environment and health of plants.13–15 The fluorescent supramolecular chemosensors formed polymer and hydrogelator systems could realize recyclability and high-specificity monitoring of the living environment of plants and food security.16–18 Consequently, fluorescence-based techniques have become one of the sustainable agriculture hotpots for monitoring plant health and their surroundings. Nevertheless, there is a lack of summary and critical reviews establishing the corresponding relationship between fluorescent chemosensors and the major issues in plant-environment interactions.
Herein, we present a comprehensive analysis on the progression and application of fluorescent chemosensors in monitoring the health and living environment of plants. The commonly used design strategies, mechanisms, and challenges associated with fluorescent chemosensors in monitoring and analyzing plant-environment interactions are introduced. Most importantly, we systematically discuss the application of small-molecule, nano and supramolecular fluorescent chemosensors in areas such as plant growth and development, abiotic and biotic stresses, soil conditions, irrigation water quality and nutrition utilization. Meanwhile, we correlate the characteristics of chemosensors with their application, and then provide insights into designing efficient and suitable sensors. Lastly, we summarize the network of fluorescent chemosensors to promote the understanding of the connection between plant health and their environment. This work will provide guidance for the future development of efficient fluorescent chemosensors, thereby realizing the real-time monitoring of the health and living environment of plants in sustainable agriculture.
2. Monitoring plant health and their living environment in sustainable agriculture
2.1 The relationship between plant health and their environment
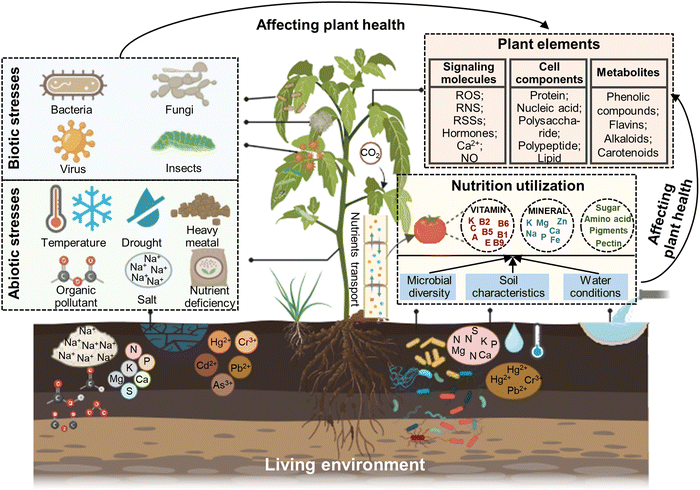
Plant health not only depends on the inherent potential of plants but also has a close relationship with environmental factors including abiotic and biotic stresses and nutrient utilization (Fig. 1).19 Abiotic and biotic stresses mainly focus on evaluating the relationship between the elements and living environment of plants.20 This is because abiotic (e.g. salinity, drought, temperature, heavy metals, and pesticides) and biotic stresses (e.g. phytoparasitic nematodes, fungi, and bacteria) are the most serious factors affecting plant growth and development. For example, drought and high temperature can cause wilting, leading to reduced photosynthesis, impaired nutrient uptake, protein denaturation, cellular damage, and ultimately plant death. Excessive levels of heavy metals can accumulate in plant tissues, disrupting cellular processes and inhibiting their growth. Biotic stresses can cause physical or tissue damage, nutrient deficiencies, and potentially mortality. During the interaction process of plants and an adverse environment, monitoring the changes in plant elements including signaling molecules (e.g. ROS, RNS, RSSs, hormones, Ca2+, and NO), cell components (e.g. protein, nucleic acid, polysaccharide, and polypeptide, lipid), metabolites (e.g. phenolic compounds, flavins, alkaloids, and carotenoids), metal ions and microenvironments (e.g. pH and pesticides) can assess plant health, and then timely strategies applied to regulate the plant's response to various environmental stresses.21,22 The efficiency of nutrient utilization is the determining factor of fruit quality, serving as the pivotal link between plant health and agricultural product supply chains. Nutrient utilization mainly depends on the diversity and equilibrium of beneficial microorganisms, nutrient sources and content, soil characteristics, water conditions, and plant health.23 Therefore, monitoring the response of plants to abiotic and biotic stresses, their living environment, and nutrition supplication and utilization is essential for understanding the interactions between plants and their environment and promoting plant health in sustainable agriculture.Currently, an array of techniques including visual assessment, PCR, immunological approaches, biochemical analysis technologies, chromatography and mass spectrometry methods, electrochemical sensors, visible light imaging, imaging spectroscopy, thermal infrared imaging and three-dimensional imaging have been used to monitor plant-environment interactions (Table 1). Visual assessment is a commonly utilized reliable strategy for evaluating plant stress phenotypes, although it has low sensitivity and is time consuming, as well as associated with subjectivity bias due to expert interpretation.24 Alternatively, PCR and immunological techniques can effectively detect plant pathogens within smaller sample sizes, with increased sensitivity and precision, but involve higher testing expenses, intricate procedures, specialized operation, non-living surveillance and potential occurrence of false positives.25 Biochemical analytical technologies are well-suited for analyzing the delicate, specific and trace analysis of plant health biomarkers in the laboratory, but their inability to conduct real-time detection and complex operation processes necessitate consideration.26 Chromatography coupled with mass spectrometry techniques (e.g. UPLC–MS/MS, LC–MS and GC–MS) facilitate the precise determination of compound identity, quantification, and separation in complex samples, featuring high sensitivity and rapid analysis, but the required instruments are costly and the detection sensitivity can occasionally be affected by substrate interference.27 Visible light imaging represents another alternative that employs digital images to capture plant phenotype at relatively lower costs, user-friendly operational pattern, and straightforward maintenance requirements, but its image quality can potentially be hindered by background factors and illumination conditions.28 Imaging spectroscopy utilizes the interaction between the solar radiation generated from samples, providing high spatial resolution over a broad spectral range. However, the high cost and the significant data storage demands for multi-spectral and hyper-spectral imaging instruments should also be considered.29 Thermal infrared imaging offers simplicity and high-resolution for quantifying the infrared radiation released by samples, though a difficulty is deciphering the infrared radiation released by samples. Meanwhile, deciphering the temperature of soil and plants beneath sparse canopies under irregular environmental conditions is a persistent challenge.30 Three-dimensional imaging can precisely quantify the electric or laser emission produced by specimens, exhibiting superior spatial resolution and robust resistance to interference, but the employed methodologies impose significant financial burden, necessitate extended scanning durations, and yield modest throughput rates.31 Thus these issues, fluorescence-based technologies can counterbalance the above-mentioned disadvantages, which have developed rapidly and now extensively applied in monitoring the health and living environment of plants to promote the development of sustainable agriculture.
| Techniques | Principles | Advantages | Disadvantages |
|---|---|---|---|
| Visual assessment | Observing plant health status | Most reliable and accurate | Low sensitivity and timeliness |
| PCR-based methods | Amplification of one or a few copies of a particular sequence of DNA | Fewer samples, timeliness, high specificity and sensitive | Expensive, complicated steps, professional operation, and false positives |
| Immunology-based methods | Interaction between antibody and antigen | Timeliness and high specificity | Non-real-time detection, and false positive |
| Biochemical technologies | Biochemical reaction between biomarkers in samples and reagents | High sensitivity and specificity, and trace analysis | Non-real-time detection, and complex operation processes |
| Chromatography and mass spectrometry | Separation, identification and quantitative analysis of compounds in complex samples | Timeliness and high-throughput | High expensive cost, and professional operation |
| Electrochemical sensing | Monitoring the changes in current or potential of sample | High sensitivity and selectivity, low equipment cost | Lack of competitiveness |
| Visible light imaging | Using digital images to mimic human perception | Low cost, ease of operation and maintenance | False positive |
| Imaging spectroscopy | Imaging the solar radiation produced from samples | High spatial resolution and wide spectral range | Expensive, and large volume of data for processing |
| Thermal infrared imaging | Measuring the emitted infrared radiation from the surface of samples | High spatial resolution, and ease of operation | Influenced by environmental temperature or conditions |
| Three dimensional imaging | Imaging the electromagnetic or laser radiation produced by samples | High three-dimensional spatial resolution, and strong ant-interference capability | High cost, long scanning times, and low throughput |
2.2 Advantages of fluorescent chemosensors
Compared with the above-mentioned technologies, fluorescence-based technology possesses advantages for visualizing plant health and their environment in some aspects, as follows:32–34 (i) for easy detection, fluorescent chemosensors emit bright and distinct signals, facilitating the detection of the presence and localization of specific components in plants or in their living environment. (ii) For specificity, fluorescent chemosensors can be designed based on the characteristics of specific targets, molecules or compounds of interest. This allows the selective identification and quantification of specific markers for implying plant health. (iii) For real-time monitoring, fluorescent chemosensors facilitate the real-time monitoring of plant health indicators. By labeling specific molecules, changes in their concentration or distribution can be observed over time. (iv) For non-destructive analysis, fluorescent chemosensors can be applied externally or systemically absorbed by plants without causing significant damage. This allows repetitive measurement over time, reducing the necessity for destructive sampling and enabling longitudinal studies. (v) For high sensitivity, fluorescent chemosensors are highly sensitive, which can often detect low concentrations of target molecules. This attribute is particularly advantageous in detecting early signs of plant stresses or nutrient deficiencies. (vi) For multiplexing capability, fluorescent chemosensors can be designed with different emission wavelengths, allowing the simultaneous detection of multiple targets within the same sample and facilitating the evaluation of various aspects of plant health. (vii) In terms of high-throughput screening, fluorescent chemosensors are employed in high-throughput screening tests, enabling the expedited examination of an extensive array of plant samples. Therefore, fluorescent chemosensors have been widely used and progressively matured in the analysis of the correlation between plant health and environment.2.3 The classification of fluorescent chemosensors
Fluorescent chemosensors are capable of monitoring plant health under abiotic and biotic stresses, various conditions of soil and water, and nutrient utilization rate. Currently, three major types of fluorescent chemosensors including small-molecule fluorescent chemosensors, fluorescent nano-chemosensors and fluorescent supramolecular chemosensors have been used in the visualization of plant health and their surroundings. Small-molecule fluorescent chemosensors are commonly used in plant imaging owing to their biocompatibility and high spatial-temporal resolution. Most fluorescent nano-chemosensors have been used to monitor the living environment of plants, which is determined by their properties of recyclability and high sensitivity. Meanwhile, fluorescent supramolecular chemosensors are often used to detect fruit quality and the living environment of plants because of their recyclability, high selectivity and strong adaptability. In the following sections, we summarize and analyze the utility of three types of fluorescent chemosensors in monitoring the health and living environment of plants.3. Applications of small-molecule fluorescent chemosensors
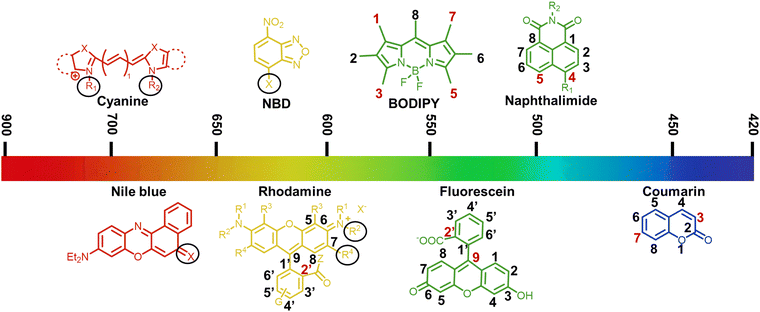
Small-molecule fluorescent chemosensors are powerful tools for visualizing plant health by changing their luminescence emission in response to specific analytes in plants under abiotic and biotic stresses. A variety of small-molecule fluorescent chemosensors based on different fluorophores such as coumarin, naphthalimide, rhodamine, fluorescein, NBD, BODIPY, Nile bule, and cyanine has been discovered and developed (Fig. 2).35 When the parent structure of the fluorophore is modified, the emission spectrum is usually in the range of 500 to 600 nm. NIR fluorescence (near-infrared, 700–900) exhibits some advantages including deeper tissue penetration to circumvent photo-bleaching and minimal phototoxicity.36 Each fluorophore has unique attributes that should be considered, which determine its suitability as a label for studying plant-environment interactions.3.1 Coumarin derivatives
Coumarin is a bi-cyclic structure consisting of a phenyl unit encircled by a pyrone ring encompassing a rigid C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, a C
C bond, a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group and six peripheral C–H sites.37 The parent coumarin molecule displays negligible or minimal fluorescence, whereas numerous coumarin derivatives with various substitutes produce considerable fluorescence in the visible light spectrum (400–500 nm). Accordingly, hundreds of coumarin dyes have been engineered due to their increased fluorescent QY, adaptability of their emission wavelengths and responsiveness to their microenvironmental polarity. Observational research has explored the relationship between the photophysical attributes of coumarin derivatives and their chemical compositions, showing that substitutions on the coumarin fragment result in blue- or red-shifted fluorescence phenomenon.38 In terms of electronic structure, the introduction of electron-withdrawing entities at positions 3 or 4, or electron-donor moieties at positions 6 or 7, induces a bathochromic shift in the emission wavelength curve (Fig. 2).39 A vast majority of fluorescent chemosensors employing coumarin as a key group in combination with other functional receptors has been designed. Particularly, the coumarin moiety positioned at the 3- and 7-positions frequently serves as the structural backbone of the fluorophore, which has been widely used to monitor the distribution and concentration of ions, hormones and amino acids in plants, as well as assessing the quality of the soil in their living environment (Table 2).40
O group and six peripheral C–H sites.37 The parent coumarin molecule displays negligible or minimal fluorescence, whereas numerous coumarin derivatives with various substitutes produce considerable fluorescence in the visible light spectrum (400–500 nm). Accordingly, hundreds of coumarin dyes have been engineered due to their increased fluorescent QY, adaptability of their emission wavelengths and responsiveness to their microenvironmental polarity. Observational research has explored the relationship between the photophysical attributes of coumarin derivatives and their chemical compositions, showing that substitutions on the coumarin fragment result in blue- or red-shifted fluorescence phenomenon.38 In terms of electronic structure, the introduction of electron-withdrawing entities at positions 3 or 4, or electron-donor moieties at positions 6 or 7, induces a bathochromic shift in the emission wavelength curve (Fig. 2).39 A vast majority of fluorescent chemosensors employing coumarin as a key group in combination with other functional receptors has been designed. Particularly, the coumarin moiety positioned at the 3- and 7-positions frequently serves as the structural backbone of the fluorophore, which has been widely used to monitor the distribution and concentration of ions, hormones and amino acids in plants, as well as assessing the quality of the soil in their living environment (Table 2).40
| Classifications | Chemosensors | λ ex/λem (nm) | Solvents | Limit of detection (LOD) | Target analytes | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Coumarin derivatives at 3-position | 1 | 425/515 | VHEPES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VEtOH = 1 VEtOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pH = 7.0 1, pH = 7.0 |
0.0260 μM | Zn2+ | Detecting exogenous Zn2+ in plant cells | 41 |
| Coumarin derivatives at 7-position | 2 | 365/460 | Vethylene glycol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VPBS = 1 VPBS = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, pH = 7.3 9, pH = 7.3 |
52.0 ppb | Ethylene | Monitoring endogenous-induced changes in ethylene biosynthesis in plant | 42 |
| Coumarin derivatives at 3- and 7-positions | 3 | 372/461 | EtOH | — | Lignin | Visualizing the cell wall lignification process by labeling lignin | 43 |
| 4 | 428/505 | VDMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VPBS = 99 VPBS = 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pH = 7.4 1, pH = 7.4 |
0.930 μM | Cysteine | Detection of endogenous cysteine activities under external stimuli in live nematode and plant | 44 | |
| 5 | 450/540–480 | Aqueous solution | — | HClO | Tacking of exogenous/endogenous HClO variations in plant cell | 45 | |
| 6 | 410/480 | VCH3OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :3VPBS = 4 :3VPBS = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, pH = 7.4 6, pH = 7.4 |
0.220 μM | CN− | Detecting CN− in flaxseed and bamboo shoots, and monitoring the viscosity of mitochondria | 46 | |
| 620/745 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VEtOH), which was comprised of up to 40% aqueous portion. It showed proficiency in discerning the extraneous Zn2+ present within plant cells, exhibiting enhanced yellow-green fluorescence intensity at 515 nm (approximately two-fold) with a limit of detection (LOD) of 0.026 μM.41 Another fluorescent coumarin derivative at the 7-position was developed by Chen et al. (2021) to monitor ethylene for evaluating the physiological state of plants (Fig. 3b). The fluorophore-tethered RhIII-based fluorogenic coumarin-ethylene chemosensor 2 exhibited superb sensitivity (LOD = 52 ppb) and instantaneous identification of ethylene in air (<2 min).
VEtOH), which was comprised of up to 40% aqueous portion. It showed proficiency in discerning the extraneous Zn2+ present within plant cells, exhibiting enhanced yellow-green fluorescence intensity at 515 nm (approximately two-fold) with a limit of detection (LOD) of 0.026 μM.41 Another fluorescent coumarin derivative at the 7-position was developed by Chen et al. (2021) to monitor ethylene for evaluating the physiological state of plants (Fig. 3b). The fluorophore-tethered RhIII-based fluorogenic coumarin-ethylene chemosensor 2 exhibited superb sensitivity (LOD = 52 ppb) and instantaneous identification of ethylene in air (<2 min).
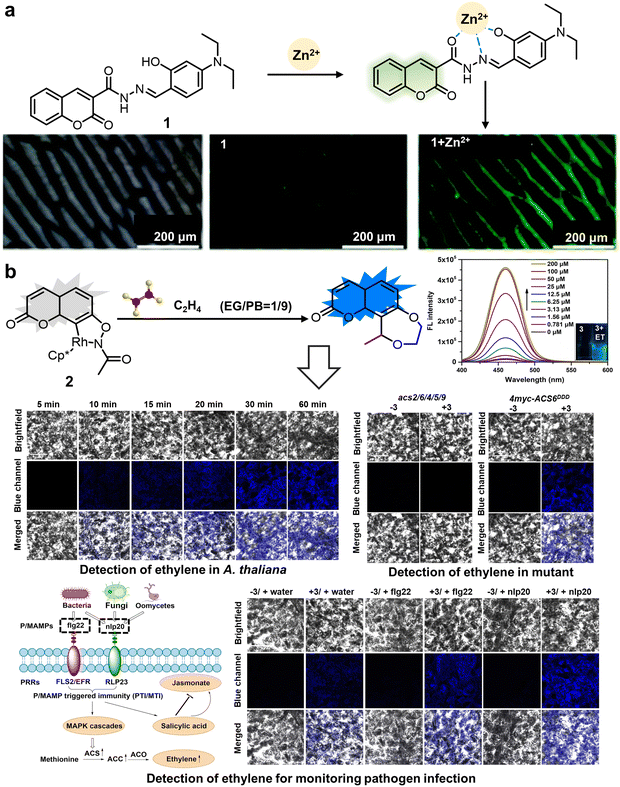 | ||
| Fig. 3 Structure and application of fluorescent chemosensors 1 and 2 based on the 3-position or 7-position of coumarin for observing ions and hormones in plants. (a) Fluorescent chemosensor 1 synthesized using coumarin-2-hydrazine carbonate and 4-(diethylamino)-2-hydroxy-benzaldehyde can be used to monitor exogenous Zn2+ in plants.41 Reproduced from ref. 41 with permission from The Royal Society of Chemistry. Copyright © 2021, The Royal Society of Chemistry. (b) Chemosensor 2 constructed utilizing the 7-position of coumarin, affording monitoring of stress-induced changes in ethylene biosynthesis and ethylene signal transduction.42 Copyright © 2021, Wiley. | ||
Upon reacting with ethylene, an associated organic activation process was verified, displacing rhodium to form a novel fluorescent entity, thereby manifesting the maximum fluorescence intensity at the mid-wavelength of 460 nm. Moreover, 2 showed superior ethylene selectivity, effectively avoiding potential interference from diverse unsaturated plant metabolite molecules. Given the pivotal role of ethylene in pathogen defense and external stress responses, this chemosensor not only offers a significant mechanism for deepening our comprehension of ethylene biosynthesis regulation and ethylene signaling transduction in plants, but also enables the monitoring of pathogen infections, which can provide guidance for preventing pathogen infection and studying the physiological process of ethylene in real time.42 Coumarin-based fluorescent derivatives located at the 3- or 7-positions are expected to improve the emission spectrum for tracking small physiological molecules within plant cells. Furthermore, it is preferable for these probes, utilizing the coumarin fluorophore, to exhibit good water solubility.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond (Fig. 4c). It sensed changes in extracellular or endogenous HClO levels inside plant cells within a short period (<100 s), causing a blue shift in fluorescence from the original green fluorescence (peak at 540 nm) to novel blue emission (peak at 480 nm). The LOD of 5 for the detection of HClO was 545 nM. 5 functioned as both a colorimetric and ratiometric chemosensor, enabling the imaging detection of HClO across diverse model systems. This highlights its broad spectrum of potential applications in the realm of plant science.45 In 2023, Pan et al. developed a dual-responsive coumarin-derived NIR fluorescent chemosensor 6 for the simultaneous quantification of mitochondrial viscosity and cyanate (CN−). This chemosensor was synthesized using coumarin by expanding its double bond and linking benzindoles to enhance its conjugated structure (Fig. 4d). The significant increase in inner mitochondrial viscosity impeded the C
N bond (Fig. 4c). It sensed changes in extracellular or endogenous HClO levels inside plant cells within a short period (<100 s), causing a blue shift in fluorescence from the original green fluorescence (peak at 540 nm) to novel blue emission (peak at 480 nm). The LOD of 5 for the detection of HClO was 545 nM. 5 functioned as both a colorimetric and ratiometric chemosensor, enabling the imaging detection of HClO across diverse model systems. This highlights its broad spectrum of potential applications in the realm of plant science.45 In 2023, Pan et al. developed a dual-responsive coumarin-derived NIR fluorescent chemosensor 6 for the simultaneous quantification of mitochondrial viscosity and cyanate (CN−). This chemosensor was synthesized using coumarin by expanding its double bond and linking benzindoles to enhance its conjugated structure (Fig. 4d). The significant increase in inner mitochondrial viscosity impeded the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond rotation and disrupted the organized conjugated system, thereby leading to a characteristic redshift of 125 nm (from 620 nm to 745 nm). Monitoring CN− levels also resulted in the enhanced excitation of the chemosensor with peaks at the wavelength of 410 nm and 620 nm, which was caused by the nucleophilic addition reaction between the chemosensors and CN−, disrupting the conjugated system and triggering a blue shift (410 nm/480 nm) at an LOD of 0.22 μM. Thus, 6 shows great promise for detecting CN− under different mitochondrial viscosities in plants.46 According to the proposed interaction mechanism, the carbonyl groups of the dicarboximide moiety with N2H4 resulted in liberated aromatic amines, which induced appreciable fluorescence shifts. These fluorescent chemosensors exhibited a large Stokes shift for fast response and high-selectivity monitoring of plant cell growth and the physiological state of mitochondria. Fluorescent chemosensors originating from the coumarin fluorophore have found extensive use in monitoring and imaging plant tissues and samples. Nonetheless, they have some limitations, such as longer emission wavelengths, elevated hydrophilicity, and minimal biological toxicity. Hence, the development and utilization of multifunctional fluorescent sensor materials, leveraging coumarin fluorophores with simple structures, versatility and high performance can facilitate the convenient detection of small molecule signals in plants.
N bond rotation and disrupted the organized conjugated system, thereby leading to a characteristic redshift of 125 nm (from 620 nm to 745 nm). Monitoring CN− levels also resulted in the enhanced excitation of the chemosensor with peaks at the wavelength of 410 nm and 620 nm, which was caused by the nucleophilic addition reaction between the chemosensors and CN−, disrupting the conjugated system and triggering a blue shift (410 nm/480 nm) at an LOD of 0.22 μM. Thus, 6 shows great promise for detecting CN− under different mitochondrial viscosities in plants.46 According to the proposed interaction mechanism, the carbonyl groups of the dicarboximide moiety with N2H4 resulted in liberated aromatic amines, which induced appreciable fluorescence shifts. These fluorescent chemosensors exhibited a large Stokes shift for fast response and high-selectivity monitoring of plant cell growth and the physiological state of mitochondria. Fluorescent chemosensors originating from the coumarin fluorophore have found extensive use in monitoring and imaging plant tissues and samples. Nonetheless, they have some limitations, such as longer emission wavelengths, elevated hydrophilicity, and minimal biological toxicity. Hence, the development and utilization of multifunctional fluorescent sensor materials, leveraging coumarin fluorophores with simple structures, versatility and high performance can facilitate the convenient detection of small molecule signals in plants.
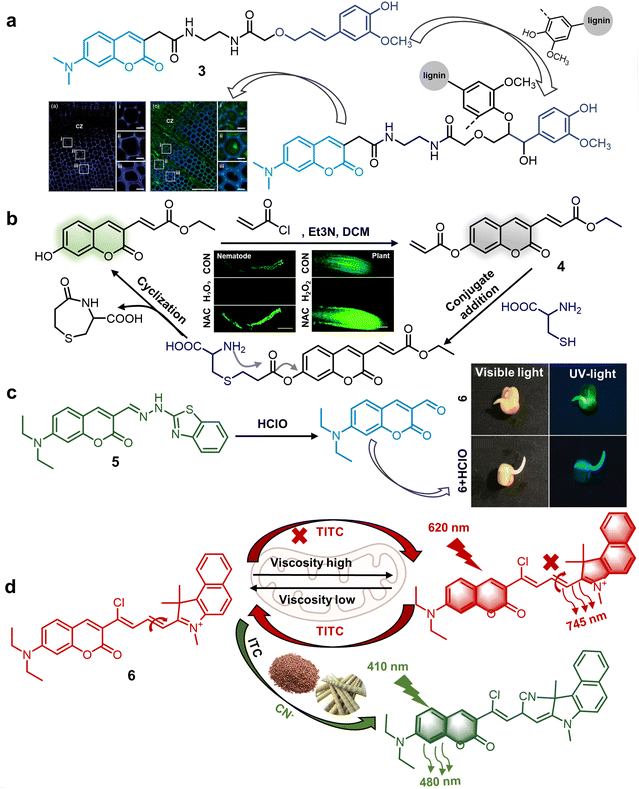 | ||
| Fig. 4 Structure and application of fluorescent chemosensors 3–6 based on the 3- and 7-positions of coumarin for monitoring the growth and components of plants. (a) Fluorescent chemosensor 3 can accurately detect the concentration of lignin to monitor plant cell wall lignification.43 Copyright © 2013, Wiley. (b) Fluorescent chemosensor 4 can monitor cysteine changes in plants and nematodes under stress-induced oxidative stress.44 Copyright © 2019, The Royal Society of Chemistry. Reproduced from ref. 44 with permission from The Royal Society of Chemistry. (c) Fluorescent chemosensor 5 can detect the concentration of HClO in plants.45 Copyright © 2021, Elsevier. (d) Fluorescent chemosensor 6 can detect CN− under different viscosity in the mitochondria in plants.46 Copyright © 2023, Elsevier. | ||
Coumarin derivatives at the 3- and 7-positions have some advantages such as high solubility, photostability and large Stokes shift, and thus have been used for monitoring the health and living environment of plants. However, coumarin fluorescent chemosensors have certain drawbacks including high synthesis cost, complex synthesis process and environmental pollution. Therefore, further studies should focus on finding some new synthesis sites, recognition sites and mechanisms of action.
3.2 Naphthalimide derivatives
The naphthalimide family represents a traditional class of D–π–A fluorophores, wherein the naphthyl nucleus functions as a π bond, R1 denotes an electron donor moiety, and the imide fragment functions as an electrophilic region.47 Naphthalimides inherently exhibit electron-deficient characteristics. Given the facile N-functionalization of the 1,8-naphthalic anhydride progenitor via the Gabriel phthalimide methodology, most of the existing reports in the literature focused on substitution reactions of non-polar alkyl substituents, leading to imide linkages, and thereby enhancing the solvent compatibility. Furthermore, there are some alternative reactive sites within the naphthalene moiety (Fig. 2),48 which can be readily modified to achieve a large Stokes shift, superior fluorescence QY and exceptional photostability. Notably, the 4-position has predominantly been subjected to manipulations due to its convenience in synthesis and diminished synthesis costs (Table 3). These chemosensors have been widely utilized for detecting ions, amino acid, pesticides, and pathogens in plants, as well as the quality of soil and water.| Classifications | Chemosensors | λ ex/λem (nm) | Solvents | Limit of detection (LOD) | Target analytes | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Naphthalimide derivatives at 4-position | 7 | 760/410, 550 | VDMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VPBS = 1 VPBS = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
5.80 nM | Formaldehyde | Investigating formaldehyde in living onion tissues | 49 |
| 8 | 405/540 | Phosphate buffer solution, pH = 7.2 | — | Plant intracellular compartments | Precise monitoring of subcellular behavior within the autophagic pathway | 50 | |
| 9 | 420/560 | VPBS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VDMSO = 8 VDMSO = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, pH = 7.4 2, pH = 7.4 |
16.3 nM | Cysteine | Detecting cysteine in plant roots, food samples and environmental water samples | 51 | |
| 10 | 440/545, 565 | Aqueous solution | — | The aggregation of anionic surfactants and bacteria | Monitoring the presence of cell membranes and bacteria | 52 | |
| 11 | 420/520 | Aqueous solution | — | Reduction products of azole in bacteria | Investigating the microbial degradation and transformation mechanism | 53 | |
| 12 | 390/510 | VCH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VHEPE = 4 VHEPE = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pH = 7.4 1, pH = 7.4 |
6.60 nM for mesotrione, 7.37 nM for tembotrione, 10.2 nM for NTBC | HPPD inhibitors | Detecting HPPD inhibitors including mesotrione, tembotrione and NTBC in soil and water | 54 | |
| 13 | 380/550 | Acetonitrile | 33.0 nM | F− | Detecting F− content in tea leaves and water | 55 | |
| 14 | 420/520 | Tris–HCl, pH = 7.01 | 2.00 nM | Hg2+ | Monitoring the concentration of Hg2+ in river water | 56 | |
| Naphthalimide derivatives at 5-position | 15 | 365/511 | Aqueous solution | 0.490 nM | 2,4-Dinitrophenylhydrazine | The detection of 2,4-dinitrophenylhydrazine in real water and soil samples | 57 |
| 16 | 440/532 | Aqueous solution | 73.0 nM | Hg2+ | Recognition of Hg2+ in actual water, soil samples | 58 |
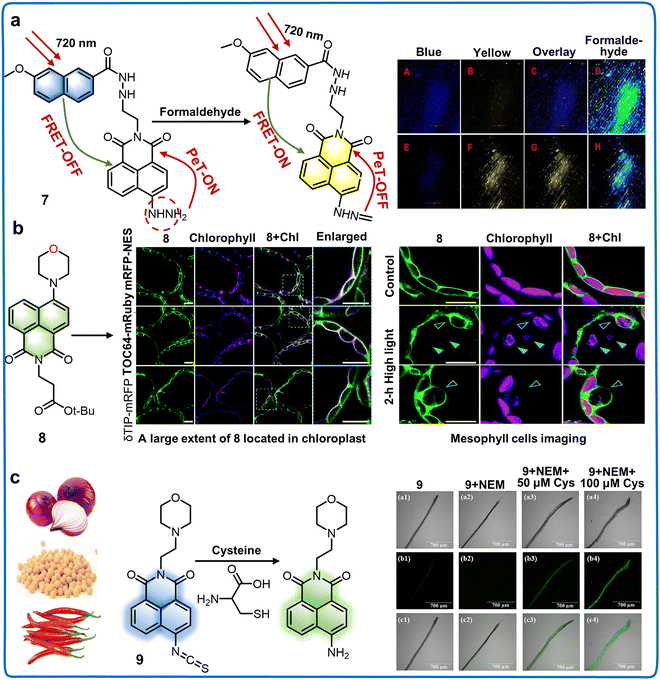 | ||
| Fig. 5 Structure and application of fluorescent chemosensors 7–9 based on the 4-position of naphthalimide to monitor the subcellular structure and amino acid concentration in plants. (a) Fluorescent chemosensor 7 can detect formaldehyde rapidly in plants.49 Copyright © 2023, Elsevier. (b) Fluorescent chemosensor 8 is capable of supervising the dynamic responses of plant organelle behaviors within an autophagic physiological process.50 Copyright © 2021, The Royal Society of Chemistry. Reproduced from ref. 50 with permission from The Royal Society of Chemistry. (c) Concentration of cysteine in plants, food and water can be detected by fluorescent chemosensor 9.51 Copyright © 2023, Elsevier. | ||
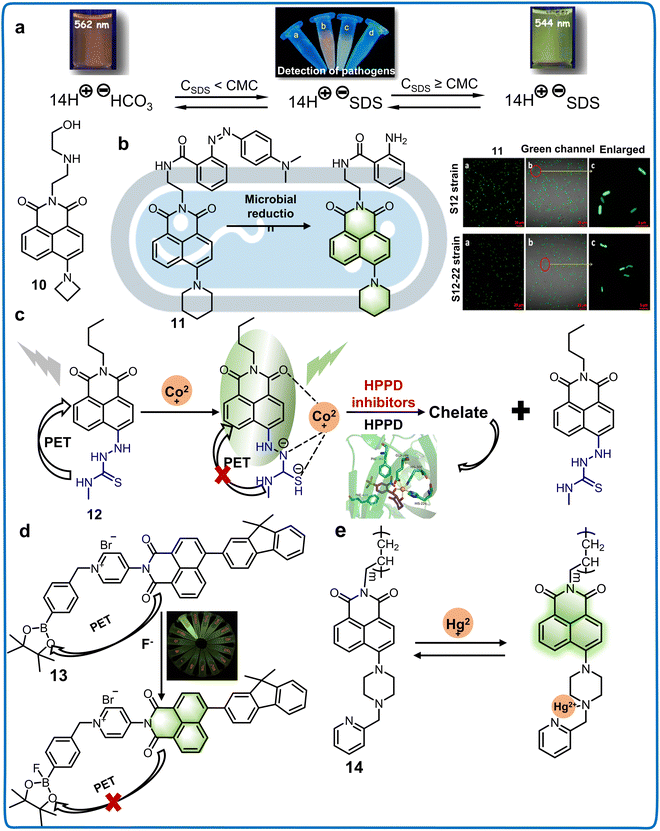 | ||
| Fig. 6 Structure and application of fluorescent chemosensors 10–14 based on 4-position of naphthalimide to detect the pathogens, pesticides and ions in the living environment of plants. (a) Fluorescent chemosensor 10 can detect cell membranes of bacteria by detecting the viscosity and polarity of the medium.52 Copyright © 2017, the American Chemical Society. (b) Fluorescent chemosensor 11 is a useful tool for understanding the degradation pathways of azo dyes in different microbial systems.53 Copyright © 2015, the American Chemical Society. (c) Fluorescent chemosensor 12 exhibits remarkable potential for precisely detecting residual levels of HPPD inhibitors in water.54 Copyright © 2021, Elsevier. (d) Fluorescent chemosensor 13 can detect F− content in foods.55 Copyright © 2022, Elsevier. (e) Fluorescent chemosensor 14 has a highly selective to response to Hg2+ in real water samples.56 Copyright © 2013, Elsevier. | ||
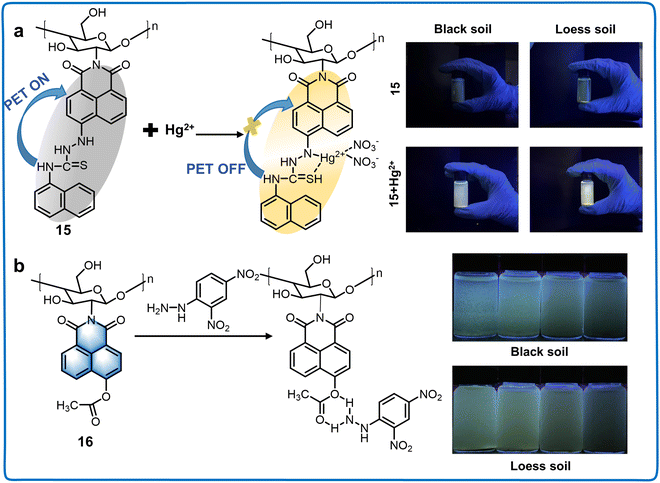 | ||
| Fig. 7 Structure and application of fluorescent chemosensors 15 and 16 based on 5-position of naphthalimide for monitoring the quality of soil in the living environment of plants. (a) Fluorescent chemosensor 15 can be applied for the quantification of Hg2+ in irrigation water.57 Copyright © 2021, Elsevier. (b) Fluorescent chemosensor 16 can monitor the concentration of DNPH in soil samples.58 Copyright © 2023, Elsevier. | ||
Currently, 4-position naphthalimide derivatives with the advantages of photostability and good two-photon properties have been applied for monitoring plant health. However, are also some disadvantages to overcome, as follows: (i) the active site of naphthalimide is the 4-position, which limits the diversity of fluorescent chemosensors. Thus, to increase their diversity, novel recognition groups and various substituent units should be investigated. (ii) The low water solubility of naphthalimide derivatives decreases their sensitivity and responsiveness. (iii) The unstable and poor heat resistance of naphthalimide derivatives limit their application scope and accuracy.
3.3 Rhodamine derivatives
Rhodamine derivatives, part of the xanthene family together with fluorescein and eosin dyes, function as fluorophores with spectra relatively unaffected by pH changes within the range of 4–10.59 Discovered in 1887, they have diverse applications in biotechnology, acting as fluorescent markers and aiding in the detection of small-molecules.60 Since 1945, rhodamine-based chemosensors, capitalizing on analyte-induced spirolactam opening, have been designed for detecting metal ions and other biologically relevant species.61 Rhodamine dyes, with moderate hydrophilicity, high absorption coefficient, elevated fluorescence QY, exceptional photostability, and visible light emission, have evolved beyond basic fluorescent labeling reagents into advanced fluorescent chemosensors.62 This section focuses on rhodamine-derived chemosensors, especially those targeting the health and living environment of plants (Table 4).63 The classical rhodamine dyes are predominantly employed in these applications, although in recent years, diverse modifications of rhodamine scaffolds have been performed for the visualization of plant components (Fig. 2).| Classifications | Chemosensors | λ ex/λem (nm) | Solvents | Limit of detection (LOD) | Target analytes | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Rhodamine Z-position derivatives | 17 | 554/578 | Vmethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 9 VH2O = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.00 nM | Salicylic acid (SA) | Detecting the concentration and distribution of SA in plant callus tissues | 64 |
| 18 | 620/695 | VH2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VEtOH = 5 VEtOH = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (HEPES, 2.0 mM, pH = 7.0) 1 (HEPES, 2.0 mM, pH = 7.0) |
0.340 μM for Hg2+ | Hg2+ and S2− | Detecting Hg2+ in plant cells | 65 | |
| 1.63 × 105 M−1 for S2− | |||||||
| 19 | 520/582 | VEtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 1 VH2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
14.2 nM | Al3+ and F− | Detection of trace Al3+ ions in water and biological systems local river and lake water/plant tissues | 66 | |
| 20 | 520/582 | VEtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 1 VH2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (HEPES, 0.5 mM, pH = 7.3) 1 (HEPES, 0.5 mM, pH = 7.3) |
0.148 μM | Fe3+ and Na4P2O7 | The detection of Fe3+ in cells, and plant tissues | 67 | |
| 21 | 500/580 | VTHF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 8 VH2O = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (HEPES, 0.01 M, pH = 7.4) 2 (HEPES, 0.01 M, pH = 7.4) |
3.00 μM | Co2+ | Detecting Co2+ in root and shoot tissues of plant | 68 | |
| Rhodamine 2′ and Z-position derivatives | 22 | 565/590 | VMeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 2 VH2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
1.00 ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nM nM |
Salicylic acid (SA) | Monitoring SA in plants and food | 69 |
| 23 | 560/700 | VEtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 1 VH2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.0770 μM for Hg2+ 0.170 μM for S2− | Hg2+ and S2− | Detecting Hg2+ in living cells, animals and plant tissues | 70 | |
| Rhodamine 2′, R2, R4-position and 2′-position derivatives | 24 | 515/555 | VAcetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VHEPES = 4 VHEPES = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pH = 7.4 1, pH = 7.4 |
0.164 ppb | As3+ | Detecting As3+ from a series of wastewater specimens | 71 |
| 25 | 470/635 | VEtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VPBS buffer = 7 VPBS buffer = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, pH = 7.4 3, pH = 7.4 |
29.0 ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nM for Cu2+ nM for Cu2+ |
Cu2+ and Hg2+ | Visualization of Hg2+ and Cu2+ in soil and plants | 72 | |
122![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nM for Hg2+ nM for Hg2+ |
|||||||
| 26 | 530/552 | Ethanol | 20.0 μM | Perillaldehyde | Detection of perillaldehyde in the leaves of plants | 73 | |
| 27 | 530, 580/640 | 30% acetonitrile aqueous solution | 26.2 nM | Ni and PPh3 | Detection of Ni and PPh3 in plant and surroundings | 74 |
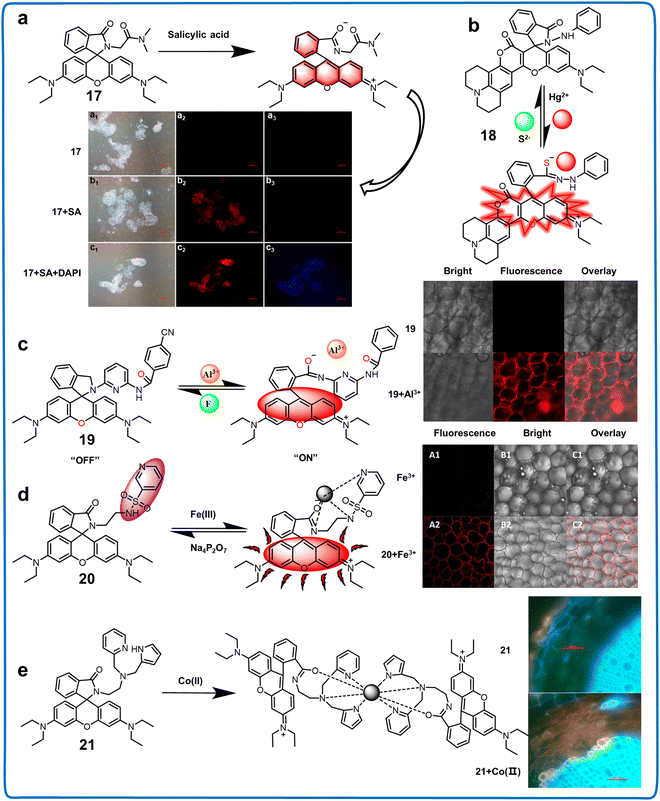 | ||
| Fig. 8 Structure, mechanism and application of fluorescent chemosensors 17–21 based on Z-position rhodamine derivatives in live cell imaging. (a) Fluorescent chemosensor 17 can monitor the distribution and concentration of SA in plant callus tissues.64 Copyright © 2020, the American Chemical Society. (b) Fluorescent chemosensor 18 can detect Hg2+ in plant cells.65 Copyright © 2022, Elsevier. (c) Fluorescent chemosensor 19 can detect trace Al3+ ions in local river/lake water samples and plant tissues.66 Copyright © 2020, Elsevier. (d) Fluorescent chemosensor 20 can monitor Fe3+ in cells, in vivo and plant tissues.67 Copyright © 2020, Elsevier. (e) Fluorescent chemosensor 21 could be utilized for the detection of Co2+ in different plant tissues.68 Copyright © 2016, Elsevier. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) μM, the test strips changed color from colorless to pink, while the fluorescence color shifted gradually from blue to pink to purplish. This advancement holds promise for plant biology studies and effectively detecting excess SA in common foods such as grapes, bananas, and cucumbers, serving as a warning system for SA-sensitive individuals (Fig. 9a).69 Another innovative design, chemosensor 23, based on thiooxo-rhodamine B, involved sulfiding rhodamine spironolactam carbonyl groups to thiocarbonyl groups (Fig. 9b).70 This fluorescent chemosensor exhibited a traditional reversible “turn-off–on” response with a uniform 1
μM, the test strips changed color from colorless to pink, while the fluorescence color shifted gradually from blue to pink to purplish. This advancement holds promise for plant biology studies and effectively detecting excess SA in common foods such as grapes, bananas, and cucumbers, serving as a warning system for SA-sensitive individuals (Fig. 9a).69 Another innovative design, chemosensor 23, based on thiooxo-rhodamine B, involved sulfiding rhodamine spironolactam carbonyl groups to thiocarbonyl groups (Fig. 9b).70 This fluorescent chemosensor exhibited a traditional reversible “turn-off–on” response with a uniform 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry. Notably, its C
1 binding stoichiometry. Notably, its C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S functional group served as a recognition group for Hg2+, achieving an LOD of 0.077 μM. Its versatility extended to detecting Hg2+ in irrigation water and plant tissues. Chemosensors utilizing this fluorophore were utilized to detect and image cations, anions, and phytohormones in plant samples. Additionally, they have the potential to expand their application in plant health monitoring by detecting metabolites, reactive oxygen species (ROS), and other pertinent compounds.
S functional group served as a recognition group for Hg2+, achieving an LOD of 0.077 μM. Its versatility extended to detecting Hg2+ in irrigation water and plant tissues. Chemosensors utilizing this fluorophore were utilized to detect and image cations, anions, and phytohormones in plant samples. Additionally, they have the potential to expand their application in plant health monitoring by detecting metabolites, reactive oxygen species (ROS), and other pertinent compounds.
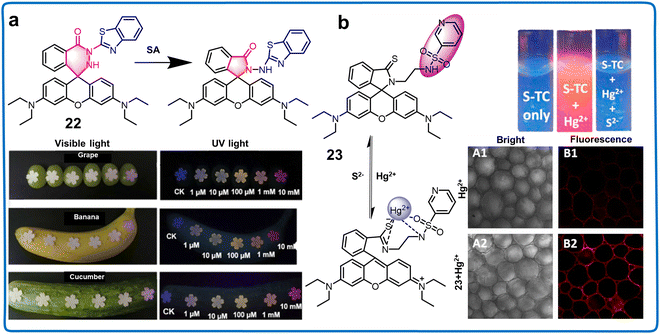 | ||
| Fig. 9 Structure, mechanism and application of fluorescent chemosensors 22 and 23 based on 2′, Z-position rhodamine derivatives in monitoring phytohormone and ions in plants. (a) Fluorescent chemosensor 22 can monitor SA in plants and food.69 Copyright © 2023, Elsevier. (b) Fluorescent chemosensor 23 can detect Hg2+ in plant tissues.70 Copyright © 2019, Elsevier. | ||
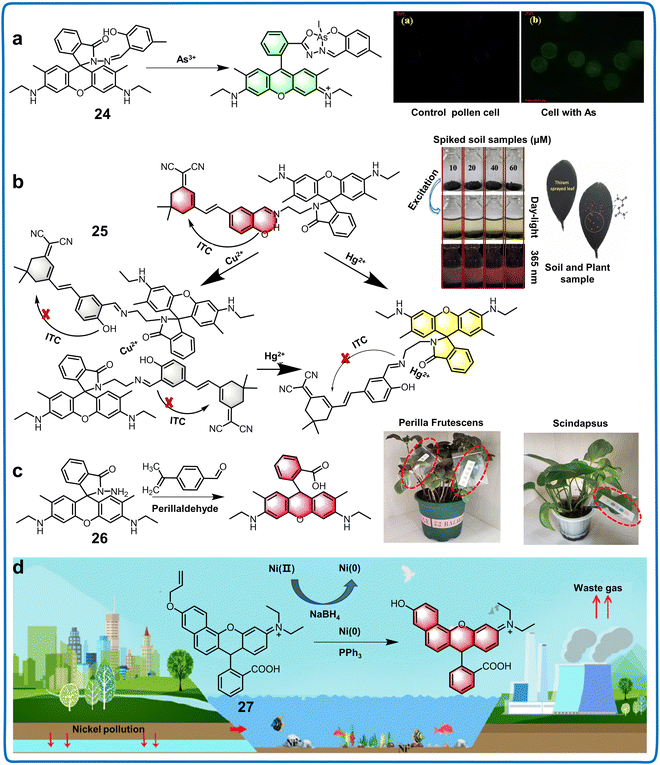 | ||
| Fig. 10 Structure, mechanism and application of fluorescent chemosensors 24–27 based on 2′, R2, R4-position and 2′-position rhodamine derivatives in soil samples and plants. (a) Fluorescent chemosensor 24 can detect As(III) in a series of water specimens.71 Copyright © 2019, the American Chemical Society. (b) Fluorescent chemosensor 25 can be used for the visualization of Hg2+ and Cu2+ in soil and plant.72 Copyright © 2023, Elsevier. (c) Fluorescent 26 can be utilized for the detection and evaluation of perillaldehyde in the solution phase and plant leaves.73 Copyright © 2022, the American Chemical Society. (d) Fluorescent chemosensor 27 can detect Ni and PPh3 in the living environment and tissues of plants.74 Copyright © 2022, Elsevier. | ||
Rhodamine derivatives have been widely used for monitoring the living environment and health of plants owing to their photostability, wide wavelength range and insensitivity to pH. However, they have some disadvantages, which should be improved including (i) poor lipophilicity and poor penetration of cells, which make it difficult for in vivo imaging and (ii) low specificity and selectivity for specific binding, which decreases their detection accuracy. Thus, some specific recognition groups should be added to improve their selectivity.
3.4 Fluorescein derivatives
Fluorescein distinguishes itself as an eminently adaptable platform for diverse fluorescent chemosensors and markers, boasting high-intensity emission peaks, substantial molar absorption coefficients, and remarkable QY in aqueous media (Table 5).75 Synthesized in 1871, fluorescein has gained significant attention and proven to be highly promising in various applications, particularly in smart sensors and bioimaging. The characteristic spirolactam structure of fluorescein enables a “close–open” transition with a “turn-on” fluorescence response in specific environments or events, making it an excellent dye for designing chemosensors.76 Fluorescent chemosensors based on fluorescein offer the advantage of modification at two distinct moieties through organic synthesis, i.e., the xanthene ring and benzoic acid moiety (benzene moiety).77 This structure enables modifications at the 2′-position, alterations to the hydroxyl group, or replacement of the oxygen atom at the 9-position (Fig. 2). Typically, most derivatives undergo modifications at these sites, enhancing the versatility of the fluorescein structure in designing functional and adaptable chemosensors (Table 5).| Classifications | Chemosensors | λ ex/λem (nm) | Solvents | Limit of detection (LOD) | Target analytes | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Fluorescein 2′-position derivatives | 28 | 365/543 | HEPES buffer (0.1 M, pH = 6.5) | 200 pmol | S-Nitrosothiols and hydrogen sulfide | Visualization of intracellular RSNO in plans seedling roots | 78 |
| Fluorescein 9-position derivatives | 29 | — | — | — | — | Assessment of stomatal dynamics in A. thaliana | 79 |
| 30 | 350, 507/650 | VH2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VTHF = 7 VTHF = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
86.7 nM | Exogenous ClO− | Monitoring of exogenous ClO− in potato sprouts and industrial effluents | 80 | |
| NBD-N derivatives | 31 | — | — | — | Auxin | Visualising the distribution of auxin in plants at cellular or subcellular levels | 81 |
| 32 | 488/500–650 | Aqueous solution | — | Monolignols | Understanding the plant cell wall lignification process | 43 | |
| 33 | 495/543 | Aqueous solution (containing 0.4% DMSO) | 19.2 nM | Hg2+ | The Hg2+ recognition in live tissues of A. thaliana | 82 | |
| 34 | 450/540 | PBS buffer (1% DMSO) | — | pH | Tracking of pH changes in mung bean sprouts | 83 | |
| NBD-O derivatives | 35 | 480, 550/625 | PBS buffer (pH = 7.4, containing 20% CH3CN, v/v) | 0.0820 μM for GSH | Cys/Hcy and GSH | Detecting Cys/Hcy and GSH in A. thaliana | 84 |
| 0.0610 μM for Cys/Hcy |
Fluorescent chemosensors based on the 2′-position of fluorescein can be used to image and monitor physiological signals in the field of plant research. Potter et al. utilized a 2′-position fluorescein derivative to exploit the thiol-dependent quenching of fluorescein isothiocyanate (FITC) in developing a sulfide-specific assay. This innovative approach employed a polydimethylsiloxane (PDMS) membrane (chemosensor 28), which is permeable to hydrogen sulfide while excluding larger charged thiols (Fig. 11a).78 They found that the formation of fluorescein dithiocarbamate (FDTC) upon reaction with sulfide could specifically interact with S-nitrosothiols (RSNO), regenerating FITC. This process acted as a specific, fluorogenic reagent capable of detecting picomolar levels of RSNO. These findings contribute to understanding the changes in plants induced by bio-thiols and peroxides.
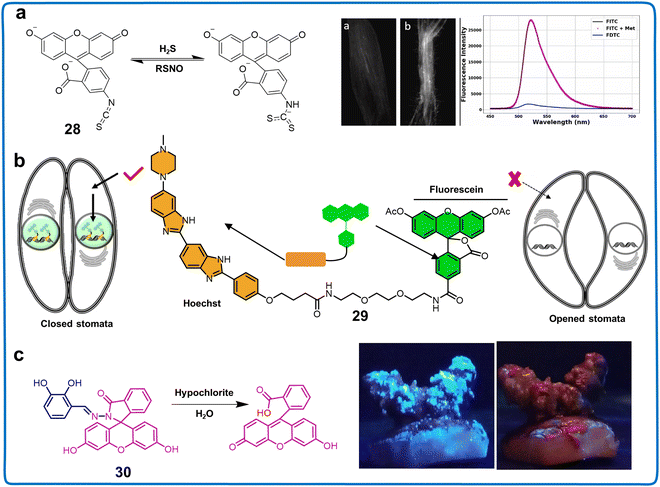 | ||
| Fig. 11 Structure, mechanism and application of fluorescent chemosensors 28–30 based on 2′-position fluorescein derivatives in imaging water samples and plants. (a) Fluorescent chemosensors 28 for the visualization of intracellular RSNO in plant seedling roots.78 Copyright © 2022, Elsevier. (b) Fluorescent chemosensors 29 is employed to assess the stomatal dynamics in A. thaliana.79 Copyright © 2020, Springer Nature. (c) Fluorescent chemosensors 30 for monitoring exogenous ClO− in potato sprouts.80 Copyright © 2023, Elsevier. | ||
Simultaneously, 9-position fluorescein derivatives have been applied in the detection of various plant components and signal changes in plants. In 2020, Takaoka et al. reported an innovative methodology involving fluorescence-imaging and utilization of fluorescein diacetate conjugated with Hoechst 33342, a well-known nuclear staining compound (chemosensor 29), facilitating the rigorous qualitative quantification of stoma dynamic responses (Fig. 11b).79 Using this method, the dynamic motion of stomata in Arabidopsis thaliana was interpreted through the straightforward surveillance of irregular changes in fluorescence intensity within the nuclear region of the stomata. The results indicated that 29 may be an alternative tool for regulating the drought response of plants or screening drought-resistant plants. Finally, a 9-position fluorescein derivative was valuable for the efficient detection of ions in food samples. Compounds functionalized with fluorescein and catechol (chemosensor 30), synthesized through a Schiff base reaction, exhibited recognition capabilities for ClO− in food items (Fig. 11c).80 Based on its excellent selectivity, high sensitivity (LOD of 36.3 nM), “fast” response time (15 s), and large Stokes shift (353 nm), 30 was employed to detect exogenous ClO− in potato sprouts. These fluorescein derivatives can be employed to detect small active molecules that are challenging to capture and to track kinetic processes in plants. The anticipated advantages of employing fluorescein in objective analyses include enhancing the capability for high-throughput examination of chemical repositories. This facilitates the development of innovative chemical chemosensors and provides insights into biosensors. The utilization of fluorescein-based chemosensors has the potential to deepen our comprehension of plant responses to environmental changes.
Fluorescein derivatives show high water solubility and QY but they also have some disadvantages, as follows: (i) their light stability is poor and their fluorescence is easily quenched after long irradiation. Further studies should focus on modifying and optimizing the structure of fluorescein derivatives to improve their stability. (ii) They are very sensitive to pH and their fluorescence intensity can change under different pH conditions, which decrease their application scope and detection accuracy.
3.5 NBD derivatives
In 1968, Ghosh and Whitehouse reported the synthesis of 4-chloro-7-nitro-2,1,3-benzoxadiazole (NBD-Cl), an ingeniously conceived fluorogenic reagent exclusively engineered for the detection of amino acids and various amines. Since its discovery, compounds featuring the NBD framework are prevalent in biochemical studies and chemical biological explorations, marking their significance in various research domains.85 The spectral range of the emission from N-monoalkyl NBD amines (NBD-NHR) extends beyond 420 nm, with their fluorescence arising from ICT transitions.86 In NBD-NHR assemblies, the amino function serves as the ICT donor, while the nitro site, a potent electron-withdrawing moiety, operates as the acceptor.87 Notably, donor–acceptor (also known as push–pull)-type dipolar fluorophores generally exhibit strong emission in organic solvents but are often less effective in aqueous media due to the hydrogen bond interactions between water and the 2-oxa-1,3-diazole moiety of NBD, leading to nonradiative dissociation channels. Consequently, NBD-NHR species in aqueous solutions tend to have lower QYs.88 However, the distinct advantages such as water solubility, eco-sensitivity, and concise dimensions of NBD-NHR fluorophores make them highly valuable, facilitating biomolecular interactions and self-assembly, especially in living systems (Table 5). The structural characteristics of the latter two compounds are commonly harnessed in the visualization of plant health (Fig. 2).NBD derivatives are some of the most used fluorophores for live plant imaging owing to their simplicity, high efficiency, high sensitivity and low detection limits in fluorescence analysis. FluorA I and II, fluorescent conjugates of 2,4-D with NBD, served as promising auxin chemosensors (31), mimicking known auxin distribution patterns in distinct developmental processes. These conjugates displayed potential for visualizing auxin distribution in plant roots and apical hooks (Fig. 12a).81 Additionally, the probe revealed the presence of fluorescent analogues in specific organelles such as the ER and endosomes. This toolkit provided high spatiotemporal resolution support for elucidating the mechanisms governing the precise, local regulation of auxin distribution in living materials. Tobimatsu et al. reported the synthesis of a range of monolignol analogs, γ-conjugated with substrates such as fluorogenic aminocoumarin and nitrobenzofuran dyes (chemosensor 32), which were examined for potential application as imaging chemosensors. They proved to be valuable for visualizing the cell wall lignification process in A. thaliana and Pinus radiata under diverse feeding conditions (Fig. 12b).43 An NBD derivative, chemosensor 33, was applied for the detection of pH, anions, cations, and biothiols in plants. 33 exhibited exclusive affinity for Hg2+ with an activation of emission wavelength at 543 nm. Its reversible fluorescence response, coupled with a low detection limit (19.2 nM) in the pH range of 6.0–7.5, positions NBDP as a prospective option for detecting Hg2+ in neutral aqueous settings. This phenomenon was attributed to the impediment of the photo-electron transfer (PET) mechanism upon complex formation with Hg2+ (Fig. 12c).82 This chemosensor with high water solubility was effectively employed for fluorescence imaging in plant tissue. Furthermore, NBDP demonstrated Hg2+ recognition ability in live tissues of A. thaliana via fluorescence imaging. NBD-NHR-based fluorescent chemosensors have been widely employed in plant detection. Yu et al. ingeniously devised a new molecular pH sensing agent, chemosensor 34 (NBD-pbz), which was formed through the strategic combination of 2-piperazin-1-yl-1,3-benzothiazole (pbz) and 7-nitro-1,2,3-benzoxadiazole (NBD) elementary units (Fig. 12d).8334 enabled the precise monitoring of pH variations spanning the range of 3.2–7.6, demonstrating a pKa value of 5.51, as observed in mung bean sprout tissues. This functionality was enabled by utilizing a fluorometric activation response together with an ICT-based operating mechanism methodology. This type of structure was versatile for detecting anions, cations, and biothiols in environmental samples. Fluorescent chemosensors based on the 2-oxa-1,3-diazole of NBD were also extensively utilized in plant detection and could sensitively detect biothiols (such as amino acids or sulfide) in plants, real environments, and food samples. For instance, Huang et al. synthesized NBD-O-1 (chemosensor 35), which was capable of detecting endogenous compounds such as Cys/Hcy and GSH in A. thaliana (Fig. 12e).84 The fluorescence emission amplitude of NBD-O-1 at 550![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm and 625
nm and 625![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm significantly increased upon exposure to Cys/Hcy with superior selectivity and LOD of 0.061 μM. The experiment demonstrated the utility of 35 in exploring the conversion and metabolic pathways of thiols in plants. NBD, known for its low molecular weight, high QY, and stability, presents numerous advantages. It has found extensive use in plant imaging to observe physiological processes within plant cells. This effort seeks to enhance our comprehension of physiological processes from the standpoint of subcellular organelles in plants, which play an important role in plant health management. Hence, the introduction of NBD derivatives can provide a new perspective to study the changes of physiological signals within the plant system.
nm significantly increased upon exposure to Cys/Hcy with superior selectivity and LOD of 0.061 μM. The experiment demonstrated the utility of 35 in exploring the conversion and metabolic pathways of thiols in plants. NBD, known for its low molecular weight, high QY, and stability, presents numerous advantages. It has found extensive use in plant imaging to observe physiological processes within plant cells. This effort seeks to enhance our comprehension of physiological processes from the standpoint of subcellular organelles in plants, which play an important role in plant health management. Hence, the introduction of NBD derivatives can provide a new perspective to study the changes of physiological signals within the plant system.
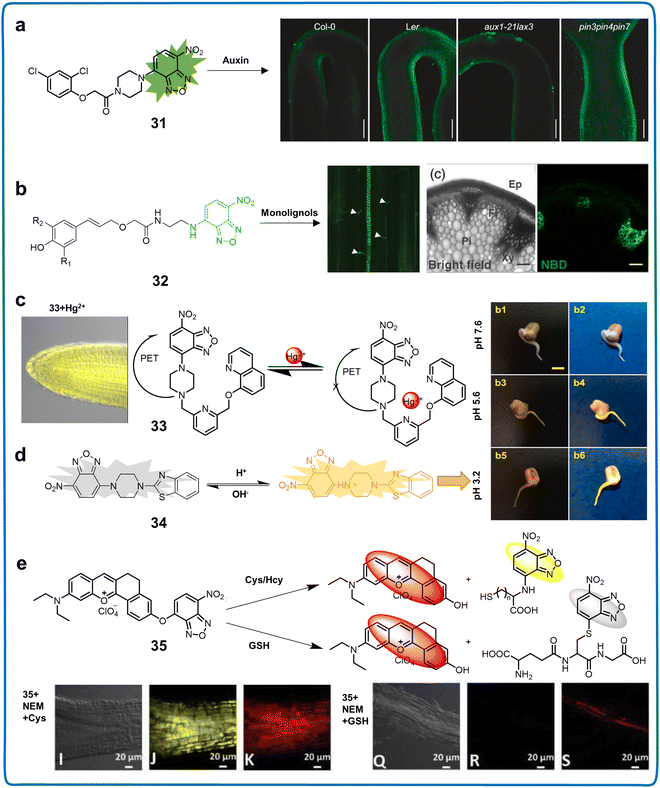 | ||
| Fig. 12 Structure, mechanism and application of fluorescent chemosensors 31–35 based on NBD-N for visualising plant growth. (a) Fluorescent chemosensor 31 for visualising auxin distribution within different plant tissues at the cellular or subcellular levels.81 Copyright © 2021, Wiley. (b) Fluorescent chemosensor 32 for understanding the intricacies of cellular wall lignification in plants.43 Copyright © 2013, Wiley. (c) Fluorescent chemosensor 33 for Hg2+ recognition in live tissues of A. thaliana.82 Copyright © 2020, Elsevier. (d) Fluorescent chemosensor 34 for tracking pH changes in mung bean sprouts.83 Copyright © 2021, Elsevier. (e) Fluorescent chemosensor 35 for detecting Cys/Hcy and GSH in A. thaliana.84 Copyright © 2019, Elsevier. | ||
The low molecular weight, high solubility, biocompatibility and relatively easy synthesis process of NBD derivatives have led to their use in plant imaging. However, they also have some disadvantages, as follows: (i) their emission spectrum is located in the range of 500–560 nm, which overlaps with some spontaneous fluorescence in biological systems, causing fluorescence interference and (ii) the relatively low structural diversity of NBD derivatives limit their application scope. Thus, more substituent or recognition groups should be added to NBD.
3.6 BODIPY derivatives
The BODIPY molecule, also known as 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene, has been attracting increasing interest due to its minimal Stokes shift, substantial absorbance, narrow absorption and emission spectra, elevated QY, insensitivity to variations in polarity and pH, and consistent chemical structure and mechanism, making it a highly favored fluorescent dye.89,90 Developed by Treibs and Kreuzer in 1968, research related to the BODIPY compound underwent a period of inactivity spanning over a decade, only regaining prominence in the late 1980s.91 In recent years, BODIPY has found extensive application in biochemistry, materials science, engineering, physics, electronics, therapeutic applications, and various other fields. The inherent adaptability of 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene-based BODIPY derivatives allow significant alterations, making them optimal candidates for use as photodynamic therapy (PDT) agents, including low dark toxicities, cellular uptake, high extinction coefficients, and low photobleaching. This versatility allows for modifications, enabling absorbance at long wavelengths.92 Acting as an acceptor, the optical properties of BODIPY can be finely tuned through facile structural modifications on its core.93 The core of BODIPY allows functionalization at its meso-, α-, β-positions and B(III) center, and thus the introduction of substituents with varying electron densities and adjusting its conjugation length with suitable spacers or π-linkers (Fig. 2). The extensive synthetic methodologies and diverse functionalization options of BODIPY make it one of the most extensively studied organic fluorophores (Table 6).| Classifications | Chemosensors | λ ex/λem (nm) | Solvents | Limit of detection (LOD) | Target analytes | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| BODIPY 3,5-position derivatives | 36 | — | Aqueous solution | 0.500 μM | Cd2+ | Detection of Cd2+ in real rice samples | 94 |
| 37 | — | — | — | Viscosity | Imaging microviscosity in key plant cell structures | 95 | |
| BODIPY 1,3,5,7-position derivatives | 38 | 640/790 | VH2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VDMF = 7 VDMF = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
2.66 × 10−4 mol L−1 | Mercury | Marking the level of Hg2+ pollution in A. thaliana | 96 |
| 39 | 495/505 | DMSO | — | Brassinosteroids (BRs) | Monitoring BRs epidermal cells of A. thaliana | 97 | |
| 40 | 505/600 | VPBS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VCH3CN = 1 VCH3CN = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pH = 7.4 1, pH = 7.4 |
11.2 nM | Cys | Detecting Cys in food samples | 98 | |
| Nile blue derivatives | 41 | 580/675 | PBS buffer (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mM, pH = 7.4) mM, pH = 7.4) |
HP-1 (0.700 μg mL−1 and 4.53 μg mL−1) | HPPD | Monitoring AtHPPD, HPPD of A. thaliana | 99 |
| HP-2 (0.800 μg mL−1 and 2.41 μg mL−1) | |||||||
| 42 | 620/675 | VEtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VPBS = 3 VPBS = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, pH = 7.4 2, pH = 7.4 |
1.80 nM | Toxic thiophenol | Determining ArSH in industrial wastewater | 100 | |
| 43 | 652/692 | PBS buffer (10 mM, pH = 7.4) | 9.50 nM | Selenol | Detecting selenol in foodstuff | 101 | |
| Cyanine derivatives | 44 | 680/790 | PBS buffer | 170 and 448 μM | NaCl | Monitoring Salt Stress of plants | 102 |
| 45 | 340, 385/440, 490 | Triton X-100/HEPES buffer pH = 6.0 | 0.540 nM | CN− | Monitoring CN− in water | 103 | |
| 46 | 510, 640/; 520–800, 650–850 | ACN | 0.0800 ppb | Phosgene | Monitoring phosgene in soil | 104 | |
| 47 | 515, 637/816 | PBS buffer (10 mM, pH = 8.0 containing 15% DMSO) | 0.240 μg mL−1 | DDVP residues | Determination of BChE and detection of DDVP residues in food samples | 105 | |
| Chemosensors with AIE property | 48 | 492/614 | — | — | Lignin | Distinguishing the meristematic zone in root tissue and primary xylem in stem tissue. | 106 |
| 49 | 300–350/675 | — | — | Plasma membranes | Imaging the morphological changes of plasma membranes | 107 |
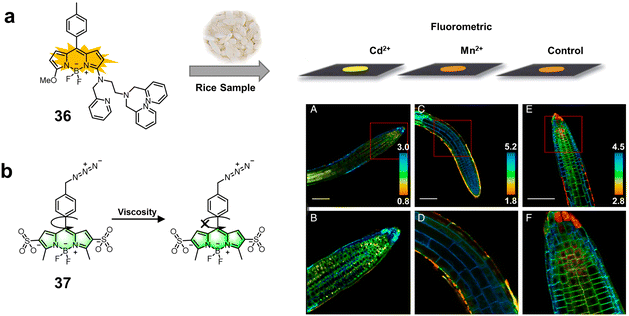 | ||
| Fig. 13 Structure and mechanism of fluorescent chemosensors 36 and 37 based on 3,5-positions derivatives of BODIPY for monitoring ions and viscosity in plant cells. (a) Fluorescent chemosensor 36 for the specific quantification of Cd2+ in real rice samples. Copyright © 2014, The Royal Society of Chemistry.94 Reproduced from ref. 94 with permission from The Royal Society of Chemistry. (b) Fluorescent chemosensor 37 for imaging microviscosity in key plant cell structures.95 Copyright © 2020, PNAS. | ||
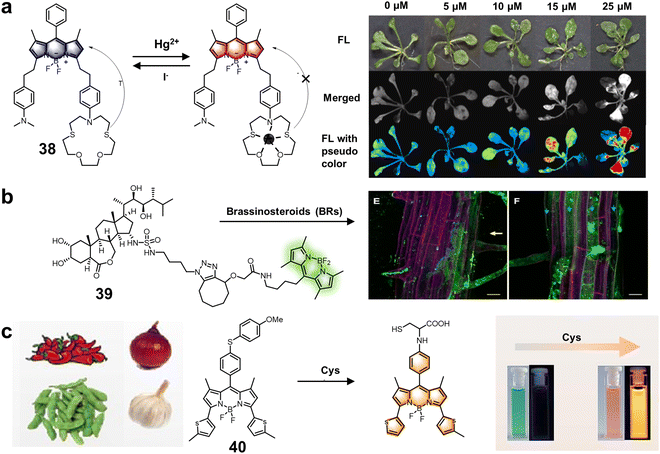 | ||
| Fig. 14 Structure and mechanism of fluorescent chemosensors 38–40 based on 1,3,5,7-position BODIPY derivatives for the evaluation of ions, hormones and amino acids in plants. (a) Fluorescent chemosensor 38 for marking the level of Hg2+ pollution in A. thaliana.96 Copyright © 2022, Elsevier. (b) Fluorescent chemosensor 39 for monitoring BR epidermal cells of A. thaliana roots.97 Copyright © 2021, MDPI. (c) Fluorescent chemosensor 40 for detecting Cys in food samples.98 Copyright © 2023, Elsevier. | ||
The advantages of BODIPY derivatives include their favorable QY, high molar extinction coefficient, and insensitivity to pH. However, they also have some disadvantages, as follows: (i) the low water solubility of BODIPY derivatives limits their application in in vivo imaging and (ii) their relatively low photostability and easy photobleaching can reduce their detection accuracy, which implies the important of timeliness of sample preparation.
3.7 Nile derivatives
Nile blue, discovered by scientist Lorrain Smith in 1908, serves to distinguish neutral fats, specifically staining fatty acids deep blue and triglycerides and steroids reddish-pink. It is comprised of oxazine sulfate (true Nile blue) and oxazone (Nile red) and is soluble in water and ethyl alcohol.108 Nile pink, its oxazone form, results from the spontaneous oxidation of Nile blue A in an aqueous solution or refluxing Nile blue A with dilute sulfuric acid (Fig. 2).109 It exhibits high solubility in neutral lipids, showing fluid characteristics at the required staining temperature. Although Nile blue is inherently hydrophobic, modifications to facilitate its water solubility are possible.110 However, its synthesized analogs evaluated thus far show fluorescence in the NIR region with a low QY in aqueous systems or in the presence of proteins (Table 6).4-Hydroxyphenylpyruvate dioxygenase (HPPD), which plays a pivotal role in the biosynthesis of vitamin E and plastoquinone in plants, has been regarded as a key herbicide target for over 30 years. Recently, it was recognized as a marker for plant health under abiotic stresses. Fu et al. introduced chemosensor 41, a tool for precise visualization and PL in in vivo studies, for the exceptionally effective identification of an HPPD-targeted inhibitor (Fig. 15a).9941 exhibited an approximately 16-fold enhancement in fluorescence levels upon exposure to HPPD, showing excellent specificity for imaging HPPD in complex environments. Importantly, it allowed the visual tracking of HPPD activity in plants spatially and temporally, overcoming the inconsistency between molecular-level HPPD-based bioevaluation and weed control efficiency.111 Zeng et al. proposed a fluorescence labelling method for HPPD, avoiding interference with normal plant growth. The innovative bioorthogonal strategy visualized HPPD in A. thaliana, integrating the evaluation of the adaptive response of plants to diverse abiotic stresses, while simultaneously monitoring the in vivo concentration and subcellular localization of HPPD in plants. Additionally, they executed a systematic molecular construction for a highly specific HPPD-reactive fluorescent chemosensor, demonstrating excellent capability for monitoring the presence of HPPD in A. thaliana and detecting dynamic fluctuations occurring under varying levels of temperature stress coupled with Cd2+ stress.112 These results indicated that this chemosensor may be an effective tool for investigating abiotic stress mechanisms associated with HPPD, detecting herbicidal compounds, evaluating enzymatic activity and elucidating uncharacterized biological functions.
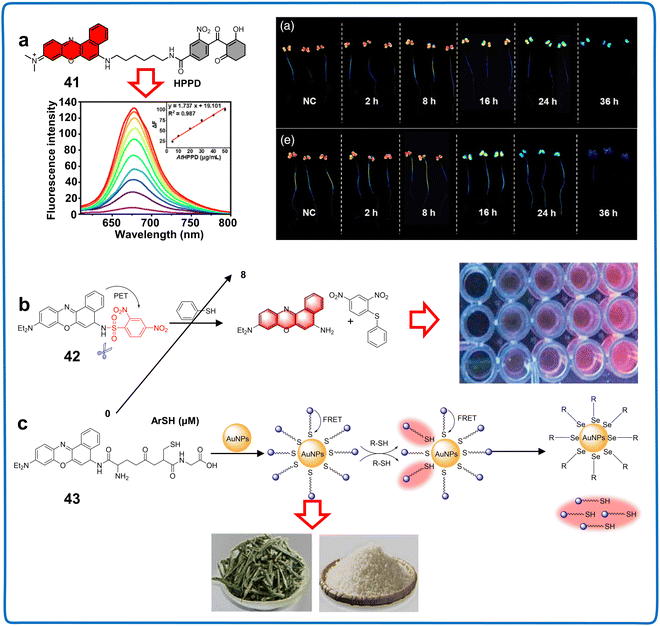 | ||
| Fig. 15 Structure, mechanism and application of fluorescent chemosensors 41–43 based on Nile blue for monitoring water quality and plant components. (a) Fluorescent chemosensor 41 for monitoring AtHPPD, HPPD in A. thaliana.99 Copyright © 2022, Wiley. (b) Fluorescent chemosensor 42 for determining ArSH in water.100 Copyright © 2019, Elsevier. (c) Fluorescent chemosensor 43 for detecting selenol in foodstuff.101 Copyright © 2020, Elsevier. | ||
The Nile blue fluorophore is extensively used in evaluating food and environmental samples. Wu et al. introduced an innovative NIR fluorescent chemosensor (42) for detecting thiophenols in environmental water samples. Through a single-step condensation process using 2,4-dinitrobenzenesulfonyl chloride in combination with Nile blue (Fig. 15b), this sensor demonstrated a remarkable chromogenic reaction and NIR fluorescent “turn-on” response against thiophenols, offering exceptional sensitivity, prompting a rapid response (12 minutes), and impressive LOD of 1.8 nM.100 Nile blue-DN effectively monitored the thiophenol levels in water with good recoveries ranging from 90% to 110%, which offered a robust strategy for facilitating highly precise measurements of the thiophenol levels in environmental water samples. Further studies should explore the integration of 42 with enzyme-responsive groups, which can increase its pesticide detection sensitivity and reduce the complex processes for enzyme activity analysis. Nile blue is also employed for detecting components in food samples. Selenocysteine (Sec), a crucial reactive selenium species, was the focus of the study by Guo et al. They reported the synthesis of a chemosensor (43) comprised of oxidized glutathione (GSH) and Nile blue ligands immobilized in gold nanoparticles (AuNPs) (Fig. 15c).101 The developed chemosensor displayed remarkable sensitivity and specificity towards Sec, facilitating the visualization of both endogenously present and externally administered Sec within plant cells via confocal fluorescence microscopy. This proposed analytical device hold substantial promise for the detection of selenol in foodstuffs such as selenium-rich rice and tea, exhibiting an impressive LOD of 9.5 nM, holding great potential for advancing the detection of selenol and unraveling its role in organisms. The results indicated that 43 can also be used as an indicator to analyze the redox balance in plants. Customizing these chemosensors for the precision targeting of distinctive biologically active compounds within plants, including reactive species, metal ions, signal-transducing molecules, and harmful substances, enables the precise and quantitative detection of these indispensable active substance.
Compared with other fluorescent chemosensors, NIR chemosensors have less background interference, strong tissue penetration and high solubility, making them more suitable for in vivo imaging. However, their rational structural modification, specific recognition groups and simple synthesis process should be explored to improve their specificity and sensitivity.
3.8 Cyanine derivatives
Cyanine (CY), a classical cyanine dye, features two nitrogen-containing heterocycles and a conjugated chain of methyl chloroform (CH)n, with an odd or even number of n.113 Manipulating the length of the poly(hypomethyl)-bridge allows the control of its absorbance and fluorescence wavelengths, resulting in higher absorbance and emission wavelengths (Fig. 2). Quinones, distinguished according to the number of carbon atoms with their carbonaceous backbone, include monomethyl (CY1, n = 0), trimethyl (CY3, n = 1), pentamethyl (CY5, n = 2), and heptamethyl (CY7, n = 3) (Table 6). CY offers several advantages for the synthesis of phloroglucinol dyes: (1) a narrow spectrum and robust signal; (2) a molar absorption coefficient surpassing that of other fluorescent dyes; (3) easy dissolution in water and low toxicity to tissues and cells, making it suitable for plant bioimaging experiments; and (4) a fluorescence band with enhanced tissue transmittance in the NIR region.114Effective methods for analyzing plant stress responses, hazardous substances, and environmental conditions are crucial. In 2022, the N-benzyloxycarbonyl-based Cy-CO2Bz (chemosensor 44) exhibited a strong fluorescence response to NaCl, serving as a sensitive indicator for discerning salt stress within live root tips and whole plants (Fig. 16a).102 NaCl induced the assembly of N-benzyloxycarbonyl Cy-CO2Bz into a J-aggregate with absorption at 890 nm. This fluorescence response implied its potential as a chemosensor for tracing salt stress in plants. The construction of J-aggregates induced by NaCl implies that it can sensitively and selectively monitor the presence of NaCl. Thus, more J-aggregates should be constructed or more functions of this system discovered for effectively monitoring other indicators to manage the health and living environment of plants. Additionally, the novel π-conjugated indolium salt fluorescent chemosensor 45 based on cyanine derivatives could detect CN− in real water samples. With an increase in the concentration of CN−, the fluorescence intensity of 45 was enhanced with an LOD of 0.54 nM. The combination 45 with gel- or paper-based indicators showed sensitivity to 1 μM of CN−, which could be observed by the naked eye, implying that this chemosensor is a useful tool for monitoring the changes in CN− in the living environment of plants (Fig. 16b). Real-time detection of chemical warfare agents (CWAs) in irrigation water and soil can mitigate their threatening effects on plant growth and development.103 A reversible, colorimetric, and fluorescent sensor for the detection of phosgene based on the cyanine scaffold (chemosensor 46) in an aqueous system was synthesized (Fig. 16c).104 The rapid dual response of 46 was constructively employed for monitoring phosgene in the environment through strip tests and soil analysis, providing up to ppb (LOD of 0.08 ppb) level detection with distinct color and fluorescence. The NIR fluorescence response of 46 indicated that it has high potential for in vivo imaging owing to its strong tissue penetration and low background interference.
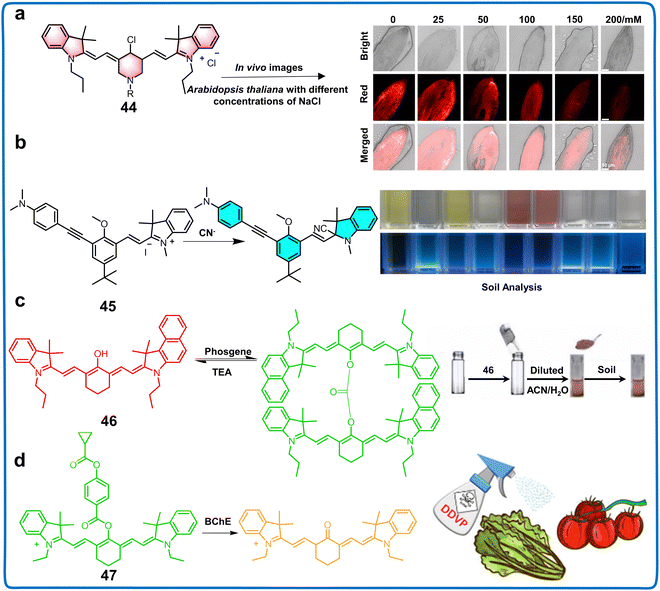 | ||
| Fig. 16 Structure, mechanism and application of fluorescent chemosensors 44–47 based on cyanine derivatives in live plant imaging, water, soil samples and food. (a) Fluorescent chemosensor 44 for monitoring salt stress of plants.102 Copyright © 2020, Wiley. (b) Fluorescent chemosensor 45 for monitoring CN− in water.103 Copyright © 2017, Elsevier. (c) Fluorescent chemosensor 46 for monitoring phosgene in soil.104 Copyright © 2022, Elsevier. (d) Fluorescent chemosensor 47 for the determination of BChE and detection of DDVP residues in food samples.105 Copyright © 2023, Elsevier. | ||
For the detection of pesticides, NIR fluorescent chemosensor 47 was developed, utilizing a cyanine scaffold to generically endorse intrinsic NIR fluorescence and circumventing interference arising from bioluminescence changes (Fig. 16d).105 An intriguing structural transformation occurs during the sensing event in this protocol, causing a reduction in conjugation. This results in a striking fluorescence change from the near-infrared (816 nm) to the red (637 nm) region, facilitating the proposed ratiometric assay. This receptor also demonstrated capability in monitoring dichlorvos (DDVP) residue in food samples with superior sensitivity and precision, showing potential as a viable substitution for detecting pesticide pollution. Collectively, the literature analysis reflects the universal utility of cyanine derivatives in detecting plant physiological stress responses, ions, and pesticides.
Cyanine derivatives have some advantages including favorable molar absorption coefficient, low background interference, high specificity and sensitivity. Alternatively, the defects of these chemosensors mainly include low photostability and aggregation quenching. Therefore, their structure should be modified or the aggregation quenching mechanism exploited to expand the application of cyanine derivatives in plants.
3.9 Chemosensors with AIE property
The phenomenon of aggregation-induced emission (AIE) arises due to the restraint of molecular rotations involving rotatable entities such as the phenyl moiety.115 The low-frequency transitions of rotor-bearing fluorophores in dilute solutions result in weak emission within their excited states.116 Conversely, upon aggregation, their rotations are obstructed by intermolecular steric interactions, thereby facilitating radiative pathways.117 Unlike traditional fluorophores that succumb to aggregation-induced quenching, AIE emits high levels of light persistently, demonstrating remarkable resilience under challenging bioimaging circumstances prevalent within plant cells.118Fluorescent chemosensors with AIE property have been used to analyze the subcellular events of plants because of their superior S/N ratios, intense fluorescence levels and fluorescent stability. In 2017, an AIE based chemosensor (48) was synthesized using diphenylimidazole-In (DPI-In) and saponin (Fig. 17a).10648 could specifically label lignin in plant roots with specific “turn-on” emission properties due to its hydrophobicity. This property was effectively utilized to monitor the composition of lignin and morphological modifications that occur during plant growth and developmental stages. Meanwhile, 48 distinguished the meristematic zone in root tissue and primary xylem in stem tissue. Compared with other commercial dyes, 48 showed greater photostability and higher sensitivity, which inspired the modification of the AIE property to manage plant health by bioimaging. In 2023, an AIE-active chemosensor (49) featuring NIR emission was developed, which was capable of four-dimensional spatiotemporal imaging of the morphological changes in plant cell plasma membranes on the subcellular level (Fig. 17b).10749 was designed based on the principles of similarity and intermiscibility, a robust imperviousness strategy, and intense electrostatic interactions, making it capable of selectively adhering to plant membranes across diverse plant cell types and plant species. 49 could quickly penetrate the cell wall and stain the cell membrane for up to 10 h and image its integrity. This makes it a highly useful tool for visually tracking plasma membrane-related events in an intuitive and real-time manner. Meanwhile, the favorable imaging performance of 49 for different plant cells indicated that it can be employed to monitor the physiological processes of plasma membranes in different plant species and cell types. We hope that more chemosensors with AIE property be designed to perform more unique and original research into plant bioluminescence imaging.
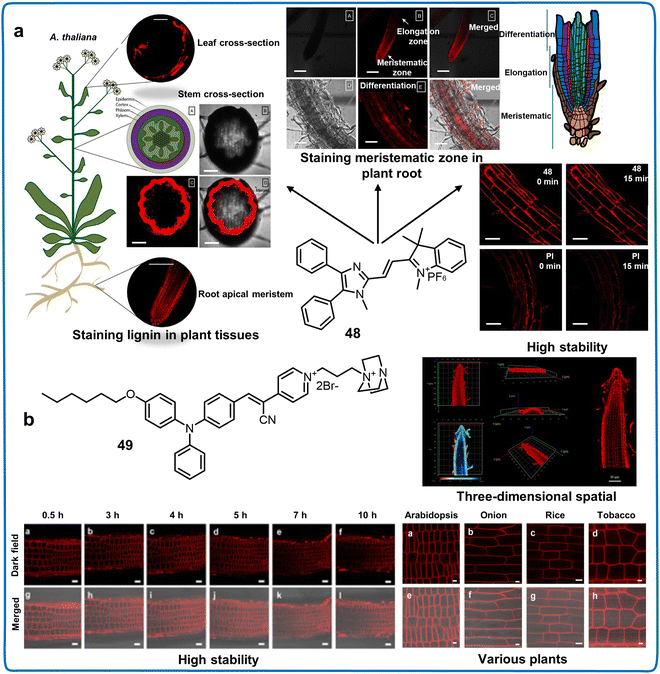 | ||
| Fig. 17 Fluorescent chemosensors with AIE property for imaging subcellular events in plants. (a) Fluorescent chemosensor 48 monitoring concentration of lignin in root and distinguishing the meristematic zone in root tissue and primary xylem in stem tissue.106 Copyright © 2017, the American Chemical Society. (b) Fluorescent chemosensor 49 monitoring the morphological and structural alterations of plasma membranes of plant.107 Copyright © 2023, The Royal Society of Chemistry. | ||
In summary, modifying the fluorophore modifiable sites in the original series of ancient dyes has revitalized this dye class, offering versatile structures, unique properties and biocompatibility, which have been widely used for imaging plants. Fluorescent chemosensors mainly developed based on the collaborative efforts between chemists and biologists have led to breakthrough progress in developing fluorescent chemosensors, particularly NIR fluorescent ones, despite ongoing challenges, as follows: (i) the majority of NIR chemosensors exhibit limited stability and low QY, making them less suitable for precise and sensitive biological imaging. (ii) Compared to chemosensors with short-wavelength emission, their NIR counterparts often have larger molecular structures, potentially compromising their water solubility. Therefore, the imperative task is to develop small molecular structure NIR dyes and chemosensors with high QY and biocompatibility. Novel heteroatom substitution strategies may represent the most viable approach for constructing these NIR dyes. (iii) To avoid interference from the plant background, it is a necessary to develop NIR-II fluorescent chemosensors or improve their QY. Equipped with potent fluorophores for investigating the intricate aspects of hazardous substances, plant physiology, adaptation mechanisms, and responses to environmental stimuli, innovative chemosensors will promote advancements in both plant science and agricultural research.
4. Applications of fluorescent nano-chemosensors
Nano-based fluorescent chemosensors exhibit great potential in monitoring the health and living environment of plants because of their enhanced surface activity, excellent recyclability, considerable surface area, robust adsorptive capability, outstanding catalytic proficiency, and substantial QY.33,119 Nanosensors are used to detect the presence of chemical species on the nanoscale, which are combined with a fluorophore to significantly enhance their fluorescence efficiency and broaden their range of applications.33 To date, various nanomaterials including quantum dots (QDs), metal organic frameworks (MOFs), covalent organic frameworks (COFs) and nanoclusters (NCs) have been used to design chemosensors to detect plant components implying plant health and environmental pollutants.13 In the following sections, we elaborate the applications of various types of nano-based fluorescent chemosensors in monitoring the health and living environment of plants.4.1 Quantum dots
Quantum dots (QDs), which are comprised of a group of nanocrystals, exhibit stable photoluminescence (PL), tunable optical properties, and good light stability. Recently, the exceptional optical properties and biocompatibility of QDs have facilitated their use as fluorescent chemosensors for the accurate identification of substances such as hormones, proteins, harmful heavy metals, anions, and pesticide residues, which can imply plant health (Table 7).120 Various types of QDs including semiconductor QDs, carbon quantum dots (CQDs), graphene quantum dots (GQDs), silicon quantum dots (SiQDs), sulfur quantum dots (S QDs), molybdenum disulfide quantum dots (MoS2 QDs), and MXene QDs are widely used in the detection of these substances.121 Due to the effectiveness of chemosensor-based QDs in monitoring the health and living environment of plants, we analyze their applications in detail (Fig. 18).| QD probe types | Chemosensors | Name | λ ex/λem (nm) | Linear range | Limit of detection (LOD) | Target analytes | Applications | Ref. |
|---|---|---|---|---|---|---|---|---|
| Semiconductor QDs | 50 | CdSe QDs | — | 1.0 × 10−6–1.0 × 10−3 M | — | γ-Aminobutyric acid | Label of gamma-aminobutyric acid in plant protoplasts | 122 |
| 51 | CdSe QDs | — | — | 27.6 μM | Cu2+ | Detection of Cu2+ in water | 123 | |
| 196 μM | Cr6+ | Detection of Cr6+ in water | ||||||
| 279 μM | Hg2+ | Detection of Hg2+ in water | ||||||
| 52 | CdSe QDs | 430/585 | — | 1.00 nM | Hg2+ | Detection of Hg2+ in water | 124 | |
| 53 | BA-QD/TGA-QD | — | 500–1000 μM | 500 μM | Glucose | Detecting the distribution and concentration of glucose in plant leaf tissue (A. thaliana) | 125 | |
| 54 | GSH-CdTe QDs | 365/575 | 20–1100 nM | 10.1 nM | Cu2+ | Detection of Cu2+ in water | 126 | |
| 55 | CdTe/ZnS | — | — | 1.00 × 10−12 M | Hg2+ | Detection of Hg2+ in water | 127 | |
| 56 | CdTe QDs | 390/530 | 0.01–1.00 μg mL−1 | 0.008 μg mL−1 | Cr(VI) | Detection of Cr(VI) in water | 128 | |
| 57 | CdTe QDs | 589/611 | 15–80 μM | 10.4 nM | CN− | Detection of CN− in cassava, lake, river water | 129 | |
| 58 | CdTe QDs | 450/520 | 0.1 nM–10 μM | 5.50 ppb | Chlorpyrifos | Monitoring the chlorpyrifos residue in apples | 130 | |
| 59 | CdTe QDs | — | 0–10−3 M | 3.40 × 10−8 M | Acetamiprid | Detection of acetamiprid in water | 131 | |
| 60 | CdTe QDs | 400/600 | 4.49–896 nM | 4.49 nM | Dichlorvos (DDVP) | Detecting the dichlorvos (DDVP) residue in apple | 132 | |
| 61 | CdS QDs | 365/505 | 1.0–90 ng mL−1 | 0.220 ng mL−1 | Cu2+ | Detection of Cu2+ in laker | 133 | |
| 8.0–30 ng mL−1 | 5.80 ng mL−1 | Hg2+ | Detection of Hg2+ in laker | |||||
| 6.0–90 ng mL−1 | 1.60 ng mL−1 | Mg2+ | Detection of Mg2+ in laker | |||||
| 62 | L-Cy-CdS QDs | 380/475 | 0.05–5 ng mL−1 | 39.0 ng mL−1 | 2,4,6-Trinitrophenol (TNP) | Detection of 2,4,6-trinitrophenol (TNP) in water | 134 | |
| 63 | CdS QDs | 390/600 | — | 49.8 μM | Glufosinate-ammonium | Detection of Glufosinate-ammonium in water | 135 | |
| 64 | CIS@ZnS QDs | 430/600 | — | 0.200 nM | Cu2+ | Detection of Cu2+ in water | 136 | |
| 5.00 nM | Hg2+ | Detection of Hg2+ in water | ||||||
| 65 | Mn:ZnS QDs | 310/450 | 0.19–0.95 μM | 0.0200 μM | Methyl parathion (MP) | Detection of methyl parathion (MP) in laker, apple | 137 | |
| 66 | ZnS:Mn2+ QDs | — | 50 nM–2.5 μM | 25.0 nM | Thiram | Detection of thiram in apple | 138 | |
| 67 | Cs3Bi2Br9:Eu3+Pe QDs | 446/620 | 5 nM–3 μM | 10.0 nM | Cu2+ | Detection of Cu2+ in water | 139 | |
| 68 | CsPbBr3@CTAB PQD | 370/436 | 5–100 μM | 30.3 nM | Pendimethalin | Monitoring the concentration of pendimethalin in potatoes and apples | 140 | |
| 69 | MIP@CsPbBr3QD | — | 50–400 ng mL−1 | 18.8 ng mL−1 | Omethoate (OMT) | Detection of omethoate (OMT) in vegetables and soil | 141 | |
| Carbon QDs | 70 | CDs | 320/388 | — | — | V. mali | Detecting the infection of V. mali in plants | 142 |
| 71 | CDs | 420/680 | — | 0.375 nM | Cu2+ | Detection of Cu2+ in onion roots | 143 | |
| 72 | CDs | 305/400 | — | 5.05 μM | Pb2+ | Detection of Pb2+ in water | 144 | |
| 73 | Fe3O4/CDs | 360/435 | 0.003–0.01 μM | 0.300 nM | Hg2+ | Detection of Hg2+ in water | 145 | |
| 74 | MAB-CDs | 280/398 | 0.5 μM–10 mM | 0.100 μM | Ni2+ | Detection of Ni2+ in water | 146 | |
| 75 | CDs | 610/670 | 0–70 μM | 15.0 nM | Cd2+ | Detection of Cd2+ in water | 147 | |
| 76 | CQDs | 340/412 | 5–100 ppb | 0.0860 ppb | Arsenite | Detection of arsenite in water | 148 | |
| 77 | CDs | 320/420 | 0.01–1.0 μg mL−1 | 3.00 ng mL−1 | Chlorpyrifos | Detecting the concentration of chlorpyrifos on cabbage leaves | 149 | |
| 78 | CDs | 423/524 | — | 0.220 μM | Paraoxon-ethyl | Monitoring the distribution of paraoxon-ethyl in cabbages | 150 | |
| 79 | CDs | 348/433 | 1.0 × 10−10–1.0 × 10−4 M | 4.80 × 10−11 M | Methyl parathion | Detecting the concentration of methyl parathion in cabbage | 151 | |
| 80 | CDs | 325/450 | — | 0.250 ng mL−1 | Diazinon | Detection of diazinon in tomatoes | 152 | |
| 81 | Tb-CDs | 360/463 | 0.5–15 μM | 50.0 nM | ppGpp | Monitoring the concentration of ppGpp in plant | 153 | |
| 82 | MnO2CD | 340/425 | 0–100 nM | 5.75 nM | Arsenic | Detection of arsenic in water and emerald plants | 154 | |
| 83 | NCDs | 370/450 | 0.01–1 μM | 4.80 nM | Hg2+ | Detection of Hg2+ in water | 155 | |
| 0.001–1 μM | 1.00 nM | Ag+ | Detection of Ag+ in water | |||||
| 84 | S-CQDs | 360/435 | 25–250 μM | 0.960 μM | Fe3+ | Detection of Fe3+ in water | 156 | |
| 85 | N-CDs | — | 11.24–241 μg L−1 | 7.45 μg L−1 | Cd2+ | Detection of Cd2+ in lake | 157 | |
| 86 | N, S-CQDs | 350/450 | — | 500 ppb | Carbaryl | Monitoring the carbaryl residue in apples | 158 | |
| 87 | N, Cl-CDs | 400/570 | 0.3–1000 μg L−1 | 30.0 ng L−1 | Organophosphorus | Detection of organophosphorus pesticides in soil | 159 | |
| 88 | N-CD-Fla | 400/502 | 1.0–2000 nM | 0.36 nM | Fenitrothion | Monitoring the concentration of fenitrothion in rice | 160 | |
| 89 | N-CDs | 420/500 | 5 pM–7 nM | 3.00 pM | Atrazine | Monitoring the concentration of atrazine in cucumbers | 161 | |
| 90 | N-CDs | — | 10–1000 μg L−1 | 3.50 μg L−1 | Methiocarb | Monitoring the concentration of methiocarb in cabbage | 162 | |
| 91 | N-CDs | 370/450 | 0–5 × 10−4 M | 1.00 × 10−7 M | p-Nitroaniline | Detection of p-nitroaniline in soil and water | 163 | |
| 92 | NCQDs@Co-MOFs@MIPs | — | 1–800 ng mL−1 | 0.350 ng mL−1 | Jasmonic acid (JA) | Monitoring the distribution and concentration changes of Jasmonic acid (JA) in plant | 164 | |
| Graphene QDs | 93 | GQDs | 320/440 | — | 1.17 μM | Hg2+ | Detection of Hg2+ in water | 165 |
| 2.46 μM | Cd2+ | Detection of Cd2+ in water | ||||||
| 2.01 μM | Pb2+ | Monitoring the concentration of Pb2+ in water | ||||||
| 94 | PEI-GQDs | 390/460 | 0–1 μM | — | Fe2+ | Detection of Fe2+ in water | 166 | |
| — | Cu2+ | Detection of Cu2+ in water | ||||||
| 95 | GQDs | 300/456 | 9.9–435.0 nM | 0.600 nM | Pb2+ | Detection of Pb2+ in water | 167 | |
| 96 | GQDs/AuNPs | 320/516 | — | 0.520 μM | CN– | Monitoring the distribution of CN− in plant | 168 | |
| 97 | B-/N-GQD | 320/425 | — | 0.00620 mg L−1 | Paraquat | Detection of paraquat in water | 169 | |
| 98 | N-GQDs | 320/423 | 0.1–17 nM | 0.041 pM | Omethoate | Detection of omethoate in cabbage leaves and water | 170 | |
| 99 | GQDs–AgNPs | 260/460 | — | 9.00 ng mL−1 | Glyphosate | Monitoring the glyphosate residue in peas | 171 | |
| 100 | GQDs/AChE/CHOx | 362/467 | 0.1–10 ppm | 0.172 ppm | Dichlorvos | Detection of dichlorvos in the lake | 172 | |
| Silicon QDs | 101 | Si QDs | 350/470 | 10–150 μM | 0.430 μM | Chlorogenic acid (CGA) | Monitoring the concentration changes of chlorogenic acid (CGA) in plant | 173 |
| 102 | S-SiQDs | 345/425 | 1–20 μM | 0.210 μM | Fe3+ | Detection of Fe3+ in water | 174 | |
| 103 | N-SiQDs | 347/440 | 0.1–4 μM | 24.0 nM | Hg2+ | Detection of Hg2+ in water | 175 | |
| 104 | Si QDs–AuNPs–CdTe QDs | — | 66–200 μM | 16.3 nM | Carbaryl | Detection of carbaryl in water, soil, and cabbage | 176 | |
| Molybdenum disulfide QDs | 105 | Eu–MoS2QDs | 310/410 | 50 nM–25 μM | 23.8 μM | ppGpp | Detection of ppGpp in plant | 177 |
| 106 | MoS2QDs–MnO2 | 360/430 | 0.33–5.00 μM | 39.0 nM | Ascorbic acid | Monitoring the distribution of ascorbic acid in hawthorn and red dates | 178 | |
| 107 | MoS2QDs | 360/440 | 3.3 ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) × ×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−7–8 × 10−3 M 10−7–8 × 10−3 M |
50.0 μM | Pb2+ | Detection of Pb2+ in water | 179 | |
| 108 | B-MoS2QDs | 335/415 | 0.005–41 μM | 1.80 nM | Hg2+ | Detection of Hg2+ in lake water | 180 | |
| 109 | β-CD-MoS2QDs | 289/400 | 0.01–18.0 ppm | 3.30 ppb | Parathion-methyl (MP) | Detection of parathion-methyl (MP) in water | 181 | |
| Sulfur QDs | 110 | SQDs | 320/431 | 5–50 μM | 4.51 μM | Co2+ | Monitoring the concentration of Co2+ in roots and stems of Salvia miltiorrhiza | 182 |
| 111 | Apt-SQDs | — | 10−15–10−7 M | 0.300 fM | Hg2+ | Detection of Hg2+ in laker, river | 183 | |
| 112 | r-SDs | 360/440 | 1.0–50.0 μmol L−1 | 0.560 μmol L−1 | Cu2+ | Detection of Cu2+ in laker | 184 | |
| 113 | RhB-SQDs | 455/580 | 0.050–0.500 μg mL−1 | 17.3 ng mL−1 | 2,4-Dichlorophenoxyac etic acid (2,4-D) | Detection of 2,4-dichlorophenoxyac etic acid (2,4-D) in water | 185 | |
| MXene QDs | 114 | Ti3C2MQDs | 350/442 | 0–1.7 μM | 5.20 nM | Mn(VII) | Detection of Mn(VII) in laker, plant leaves | 186 |
| 115 | Ti3C2QDs | 380/470 | — | 30.0 mM | Cr3+ | Detection of Cr3+ in water | 187 | |
| 116 | N-Ti3C2 MXene QDs | 330/453 | 0–2000 μM | 10.0 μM | Cu2+ | Detection of Cu2+ in water | 188 | |
| 117 | N-MQDs | 420/580 | 0–80 μM | 1.21 μM | Alizarin red (ARS) | Detection of alizarin red (ARS) in water | 189 | |
| 118 | UA@Ti3C2QDs | 360/420 | 0.01–40 μM | 9.58 nM | 2,4,6-Trinitrophenol | Detection of 2,4,6-trinitrophenol in water | 189 |
 | ||
| Fig. 18 Application of quantum dot-based fluorescent chemosensors for monitoring the living environment and elements of plants. | ||
CdSe quantum dots. Among the various semiconductor QDs, CdSe QD chemosensors have been widely used to monitor plant components and irrigation water quality. For the first time, a CdSe QD fluorescent chemosensor (50) was used to label γ-aminobutyric acid (GABA) in plants, and thus determine its distribution in plant protoplasts.122 Furthermore, its emission efficiency was improved by conjugating a ZnS shell layer to the surface of the CdSe core QD. Due to the increase in the size of the CdSe core QD, its emission peaks were red-shifted (5–20 nm). 50 penetrated and adhered to the cell membrane and specifically bind to the GABA binding pocket on the surface of plant protoplasts, dramatically reducing the fluorescence intensity of the chemosensor. This medium type of chemosensor labeling of GABA in plants provided strong evidence for the presence of the binding site of metabotropic (GABAb) at plant cell membrane (Fig. 19a).122 Meanwhile, 50 exhibited substantial biological capability in regulating Ca2+ signaling within the cell by preventing GABA from permeating the cell, which indicated that it has high potential for regulating plant development and protecting plants from stresses. A thiourea-encapsulated CdSe QD chemosensor (51) was prepared through an aqueous photochemical approach utilizing 300 nm UV light in a solution containing acetone, sodium selenosulfate, etc., which could detect the concentration of Hg2+ in water. The PL efficiency of this chemosensor decreased with an increase in temperature and pH. Among the many toxic metal ions, only Cu2+, Cd2+, and Hg2+ could significantly quench the fluorescence intensity of the chemosensor, with the LODs of 27.6 μM, 196.0 μM, and 279.2 μM, respectively (Fig. 19b).12351 could distinguish various oxidation states of metal ions such as Hg and Cr owing to the photochemically mediated electron transfer between the QDs and metal ions, which implied that 51 can analyze environmental conditions by monitoring the oxidation state of metals. The fluorescent CdSe QD (chemosensor 52) prepared using RNA via a bottom-up method could also be used to detect Hg2+ in water, whereas it has characteristics of fluorescence quenching.124 Therefore, CdSe QD chemosensors show low selectivity and fluorescence intensity in the detection of metal ions, which can be improved by eliminating the interference from the sample matrix and light scattering.
 | ||
| Fig. 19 Schematic representation of CdSe QD-based chemosensors for the visualization of plant protein level and ions in water. (a) Specific recognition and detection of GABA on the surface of plant protoplasts based on ZnS outer shell layer and CdSe inner core QD.122 Copyright © 2006, Elsevier. (b) Detection of heavy metal Hg2+ in water based on starch-capped CdSe QDs.123 Copyright © 2020, Elsevier. | ||
CdTe quantum dots. CdTe QDs, which are water-soluble sulfhydryl-capped QDs, have a narrower emission band in the NIR region than CdSe QDs, making them attractive materials for detecting plant metabolites and water quality for plant survival. A ratiometric QD-based fluorescent chemosensor (53) was recently reported to monitor the distribution and concentration of glucose in plants. 53 was designed based on CdTe/CdS QDs capped with boronic acid (BA)-coupled QDs (BA-QDs) as sensing modules. Upon the addition of glucose, it could catalyze the aggregation of 53 utilizing the covalent bonding between BA and two pairs of cis-diols from glucose. It exhibited impressive fluorescence intensity between 500 and 600 nm, which could monitor the glucose levels in individual chloroplast organelles of A. thaliana. 53 exhibited good sensitivity for glucose detection in plants, and the portable QD glucose sensing method has immense promise to be utilized for the study of photosynthesis and energy transformation in non-model plant species (Fig. 20a).125 The ability of GSH-modified CdTe QDs (chemosensor 54) to monitor Cu2+ in water was recently reported. When the excitation wavelength was 365 nm, a fluorescence emission peak appeared at 575 nm. The QY of the chemosensor markedly diminished with an increase in the concentration of Cu2+. An excellent correlation was observed between Cu2+ concentration and the fluorescence decay of 54, spanning the concentration range of 20 to 1100 nM with an LOD of 10.56 nM (Fig. 20b).126 Also, it was successfully used for the detection of Cu2+ in lake water and plants, which has great potential for monitoring food safety and water quality. Some heavy metals such as Hg2+ and Cr6+ were also detected by CdTe QD chemosensors (55 and 56).127,12855 was comprised of MSA-functionalized CdTe/ZnS core/shell (CS) QDs, which showed high selectivity for monitoring Hg2+ by electron transfer. To promote the application of 55 in the detection of water quality, a preliminary electronic apparatus should be established. The GSH capping layer of 56 can bind with Cr6+, realizing the detection of different ions by changing the crystallite dimensions of QDs. This strategy makes it easy to obtain QDs chemosensors. For instance, N-acetyl-L-Cys (NALC) CdTe QDs (chemosensor 57) could also be synergized with carbon dots (CDs) for the detection of CN−. 57 exhibited unique absorbance and emittance wavelengths at 589 nm and 611 nm, respectively. CDs and CdTe QDs showed bright blue and red colors under UV light, respectively. The fluorescence from 57 was first quenched by the introduction of Cu2+, while the fluorescence of 57 significantly recovered upon the addition of CN−. Ultra-LODs as low as 10.35 nM were achieved for CN− concentrations in cassava and water samples in the range of 0.02–10 μM and 15–80 μM, respectively (Fig. 20c).129 This ratiometric chemosensor could realize the highly sensitivity detection of CN− at the nM level and accurately determine its concentration in real samples. In addition, a CdTe QD chemosensor (58) was developed to detect the residue levels of organophosphorus pesticides in apples. The surface coordination of thiourea in the basal medium strongly inhibited the green emission of 58 through the FRET mechanism, and its fluorescence was gradually restored upon the addition of chlorpyrifos. The fluorescence of 58 was linear at concentrations in the range of 0.1 nM to 10 μM with an LOD of 0.1 nM for apples (Fig. 20d).130 Based on this type of chemosensor, it was also possible to realize the accurate detection of anilinophos and dichlorvos in water (chemosensor 59 and 60, respectively).131,132 The detection mechanism of 60 was based on the inhibition of enzymatic activity (AchE) but the stability of AchE may limit its application in practice. This phenomenon indicates that an automatic biochemical analyzer should be developed for combination with 60. CdTe QD chemosensors have been widely used in detecting water pollution but improving their detection performance remains a challenge.
 | ||
| Fig. 20 Schematic representation of CdTe quantum dot-based chemosensors for detecting ions and pesticides in plants and water. (a) Ratiometric fluorescent chemosensors based on TGA-QD and BA-QD for monitoring the glucose content in plant chloroplast organelles.125 Copyright © 2018, the American Chemical Society. (b) Detection of heavy metal Cu2+ in environmental water samples by glutathione-modified CdTe QD.126 Copyright © 2023, Elsevier. (c) Detection of cyanide anions based on synergistic interaction of N-acetyl-L-Cys-capped CdTe QDs and CDs.129 Copyright © 2019, Elsevier. (d) Detection of the organophosphorus pesticide chlorpyrifos in apples based on CdTe QD fluorescent chemosensor.130 Copyright © 2010, the American Chemical Society. | ||
CdS quantum dots. CdS QDs have unique optical and electronic properties such as high fluorescent emission and light bleaching resistance, which can be used to monitor the quality of irrigation water. For example, a yellow fluorescent CdS QD chemosensor (61) was reported for the detection of Cu2+, Hg2+, and Mg2+ in water samples. The peak values for the excitation and emission wavelengths of 61 were recorded to be 365 nm and 505 nm, respectively, and the color under UV irradiation was an intense yellow. 61 could be linearly quenched by Cu2+, Hg2+, and Mg2+ in aqueous solution with LODs of 0.22, 5.8 and 1.6 ng mL−1, respectively (Fig. 21a).133 In the presence of GSH, the fluorescence intensity of 61 increased 1.5-fold, which implied that 61 can be used to monitor the stress damage caused by ions in plants. The ability of L-Cys-coated cadmium sulfide QDs (L-Cys-CdS QDs) (chemosensor 62) with strong fluorescence was reported to detect 2,4,6-trinitrophenol (TNP) in environmental water samples. Particularly, the PL emission peak of 62 remained unaffected by a change in the excitation wavelength. The emission spectrum was strongest upon excitation at the wavelength of 365 nm. It was highly selective for TNP, and an apparent reduction in fluorescence intensity of CdS QDs was observed with an increase in TNP concentration, and its quenching effect was better under weakly acidic conditions. The linear range of TNP concentration was determined to be 0.05–5 μg mL−1 with the LOD of 39 ng mL−1 (Fig. 21b).134 Furthermore, a program for the accurate, discerning detection methodology for quantifying trace levels of TNP in water was developed. In addition, the CdS QD chemosensor (63) also enabled the accurate monitoring of dichlorvos (DDVP), paraquat, and glufosinate in environmental water samples with LODs of 0.23 mM, 1.44 μM and 49.8 μM, respectively.135 Paraquat possesses the capacity to quench the fluorescence of QDs, DDVP significantly enhances their fluorescent intensity, and glufosinate induces a blue-shift and increase in fluorescence intensity. Based on these results, we can develop software for use with a smartphone to realize the fast detection of various pesticides in the environment. Future studies should focus on improving the biocompatibility of CdS QD chemosensors to expand their imaging in plant tissues.
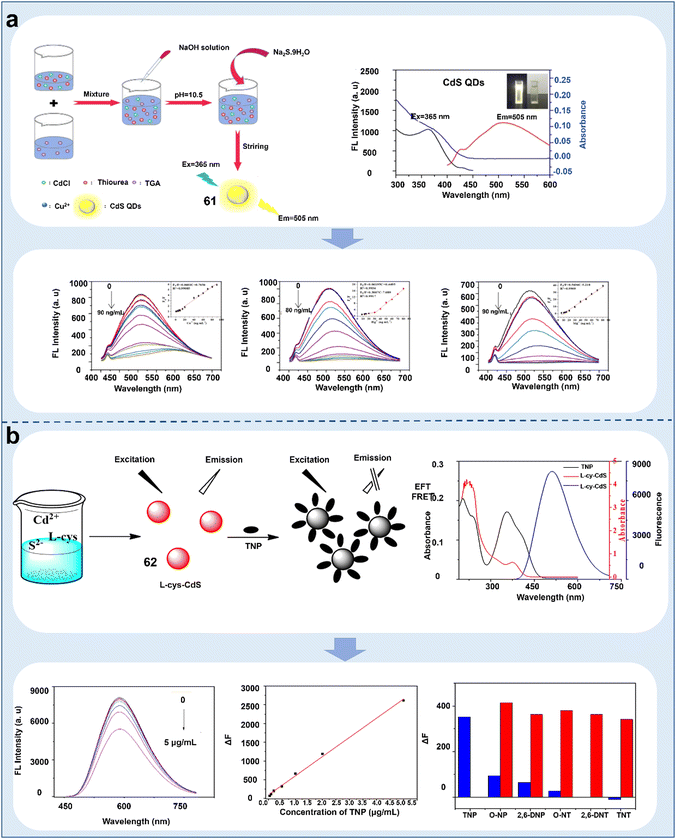 | ||
| Fig. 21 Schematic representation of CdS quantum dot-based chemosensors for monitoring irrigation water quality. (a) GSH-CdS QD chemosensor for the accurate detection of Cu2+, Hg2+, and Mg2+ in water.133 Copyright © 2022, Elsevier. (b) Detection of TNP in environmental water samples based on L-cysteine-coated cadmium sulfide quantum dot chemosensor.134 Copyright © 2019, Elsevier. | ||
ZnS quantum dots. Compared with other semiconductor quantum dots, ZnS QDs are more stable, non-toxic, and ecologically safe, and thus widely used in biosensing and toxic substance detection technologies.191 For example, CuInS2@ZnS (CIS@ZnS) (chemosensor 64) core–shell QDs were reported for the sensitive detection of heavy metals in water. 64 showed a PL excitation peak at 450 nm and emission peak at 620 nm, and the core–shell QDs exhibited blue shifts with a significant increase in emission intensity. The QY of their PL was 95%. By adding various heavy metals such as Ar3+, Cd2+, Co2+, Cu2+, Fe2+, Hg2+, and Zn2+, which are commonly found in water, to aqueous dispersions of 64, it was found that the PL of the chemosensor was quenched by the test metal ions at the nM level, such as Cu2+ and Hg2+, with an LOD of 0.22 nM in evaluating water quality (Fig. 22a).136 Besides the detection of ions, 64 could also monitor changes in cholesterol and citric acid, implying its potential as a tool to evaluate fruit quality. A proportional fluorescent chemosensor based on double-emitting Mn-doped ZnS QDs (Mn:ZnS QDs) (chemosensor 65) was demonstrated for the detection of Pb2+ and methyl parathion (MP). 65 was designed to show blue fluorescence at 400–500 nm, which was highly sensitive as a proportional fluorescent chemosensor for the detection of Pb2+. This chemosensor showed good concentration linearity between 10 nM and 60 nM for Pb2+, with an LOD of 0.5 nM. Also, 65 served as a “turn-on” fluorescent chemosensor for detecting the concentration of MP. The fluorescence response of this chemosensor showed an excellent linear response for MP in the concentration range of 19.0–450.0 μM, with LOD of 0.02 μM (Fig. 22b).137 This sensor system was employed to identify MP residues in apples and lake water. Considering the complexity of environmental samples and unknown interferences, a specific response structure or recognition moiety should be combined with 65. In addition, a ZnS QD-based chemosensor (66) also enabled the accurate detection of thiram in environmental water samples and fruit peels.138 In the presence of thiram, the Ag+ in 66 could form an Ag-thiram complex, which could suppress the phosphorescence quenching, indicating that a UV lamp can be used to easily realize pesticide detection. Therefore, compared with other semiconductor quantum dots, ZnS has lower toxicity and better stability, which has broader prospects for plant imaging in the future.
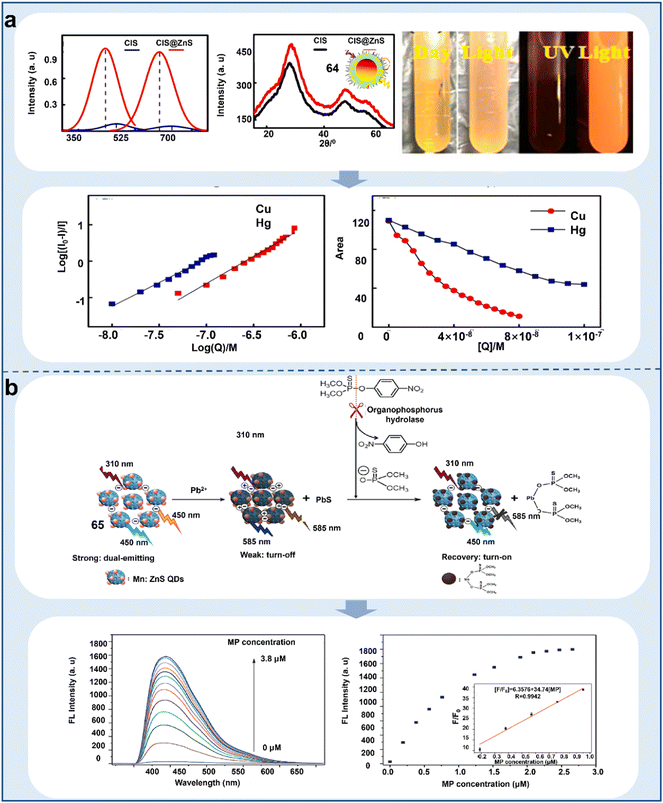 | ||
| Fig. 22 Schematic representation of ZnS quantum dot-based chemosensors for detecting ions and pesticides in water. (a) Efficient detection of copper and mercury in water quality based on CIS@ZnS QD chemosensor.136 Copyright © 2021, Elsevier. (b) Novel proportional fluorescent chemosensor based on luminescent manganese-doped ZnS QD (Mn:ZnS QD) for the detection of Pb2+ and methyl parathion (MP).137 Copyright © 2019, Elsevier. | ||
Perovskite quantum dots. Perovskite QDs are recently developed semiconductor QDs that have great potential for detecting heavy metals in soil and water. For example, the ability of Eu3+-doped lead-free Cs3Bi2Br9 Pe QDs (chemosensor 67) to detect Cu2+ in real water samples on was reported. The UV-vis absorption spectra of 67 and 67:Eu3+ showed that the absorption band edge of the bare 67 was at 389 nm. The absorption band shifted towards a higher wavelength with an increase in the concentration of Eu3+. 67:Eu3+ was characterized by high QY, non-toxicity, robust stability and multi-color emissions, thereby conferring heightened sensitivity and selectivity for detecting metal ions in environmental water. Notably, the exciton emission at 446 nm was rapidly perturbed upon the introduction of Cu2+, which has excellent detection efficiency for Cu2+ in the concentration range of 5 nM to 3 μM, with an LOD of 10 nM (Fig. 23a).139 PS-CsPbBr3@CTAB PQDs (chemosensor 68) acted as a sensor, exhibiting blue fluorescence under UV light. 68 could be used to detect dimethoate in water, and the addition of the pesticide dimethoate resulted in the fluorescence shutdown of 68. Notably, linear quenching was achieved with an increase in the concentration dimethoate in the range of 5–100 μM with an LOD of 5.0 nM. The developed method exhibited higher sensitivity compared with analytical instruments (Fig. 23b).140 In addition, the fluorescent sensor prepared by combining a molecularly imprinted polymer (MIP) with luminescent perovskite (CsPbBr3) (chemosensor 69) encapsulated in (3-aminopropyl) triethoxysilane (APTES) could sensitively detect oxidized lecithin in soil with an LOD of 18.8 ng mL−1.141 However, the low water-soluble of perovskite QDs is a challenging task, and thus focusing on improving their stability and water solubility will increase their application scope in the biological field.
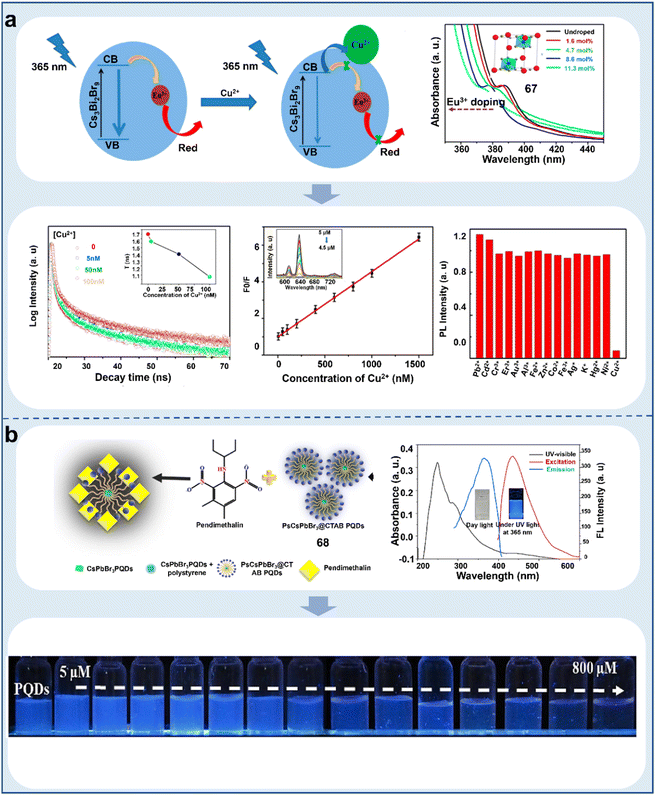 | ||
| Fig. 23 Schematic representation of chalcogenide-based quantum dot chemosensors for monitoring the concentration of ions and pesticides in water. (a) Eu3+-doped lead-free Cs3Bi2Br9 Pe QD fluorescent chemosensor for the detection of Cu2+ in real water samples.139 Copyright © 2019, the American Chemical Society. (b) Detection of the pesticide dimethoxybenzoate by the introduction of polystyrene and cetyltrimethylammonium bromide as an encapsulated assembly system prepared as CsPbBr3@CTAB PQD.140 Copyright © 2019, Elsevier. | ||
Although semiconductor QDs have been widely used to monitor pollutants in the living environment of plants, their application in plant imaging is limited, which is mainly attributed to the following factors: (i) the in vivo imaging technology of semiconductor QDs is constantly improving, but the sensitivity of fluorescence imaging for deep tissues is low; (ii) the inertness of semiconductor QDs in vivo may cause enduring toxicity to organisms; and (iii) semiconductor QDs have high surface activity, which make them easily agglomerate to form larger agglomerates.
Undoped carbon quantum dots. CQDs are mainly prepared from some natural plants and biomolecules, which are widely used in monitoring fungi, reactive oxygen species, and heavy metals in plants because of their impressive biocompatibility, negligible toxicity and remarkable stability.193 For example, fluorescent CQDs (chemosensor 70) were designed for the first time for monitoring pH changes in plant cells. 70 exhibited excitation-dependent fluorescence behavior, and its fluorescence emission intensity changed with pH. The gradual increase in fungal concentration in infected apple tissues led to a transient decrease in pH in plant cells, which caused the fluorescence intensity to increase. Consequently, the fluorescence imaging of the pathogenic fungus Valsa mali Miyabe et Yamada in apple tissues was realized (Fig. 24a).142 Meanwhile, the fluorescence of 70 in dead cells was quickly quenched because of the absence of cellular pH regulation, implying that this chemosensor can be developed into a kit for the visualization of pH regulation in plants or distinguishing live or dead cells. Accurate monitoring of Cu2+ in plants could be realized based on fluorescent R-CDs (chemosensor 71). The fluorescent carbon dots were well dispersed and water-soluble. 71 exhibited bright-red emission at 680 nm when excited at 420 nm. The red emission of 71 was found to decay upon interaction with Cu2+ in solution by incubating 71 with bean sprouts, and then transferring them to a solution of Cu2+. The LOD was 25 nM in plants. 71 was capable of overcoming the influence from the internal environment of the plant and had excellent immunity to interference, implying that this chemosensor can monitor the stress in plants in practice with nondestructive nature (Fig. 24b).143 Also, the accurate detection of Pb2+, Ni2+, Hg2+, Fe3+, Cr6+, and Cd2+ in environmental water samples could be realized based on fluorescent CQDs (chemosensor 72–75).144–147 Among them, 73 showed the lowest detection limit of 0.3 nM, which can effectively coordinate with Fe3O4/CDs by electrostatic interactions, and then quench their fluorescence signal. The results indicated that 73 can not only detect Hg2+ in the actual environment but also remove Hg2+. A CQD chemosensor (76) could detect arsenite in environmental water samples. 76 showed the strongest emission wavelength at 412 nm when excited at 340 nm and its solution appeared blue under UV light. The SH-groups of 76 could selectively interact with arsenite, which enabled the sensitive detection of arsenite in environmental water with a good response in the range of 5–100 ppb and LOD (3 s) of 0.086 ppb (Fig. 24c). However, considering that the excitation wavelength of 76 in the UV region, it is not suitable for in vivo imaging of Hg2+.148 In addition, it was reported that CDs (chemosensor 77) synthesized by burning waste paper as a carbon source could detect chlorpyrifos pesticide in vegetables. The fluorescence spectrum of 77 reached its optimum emission peak at 420 nm with an excitation wavelength of 320 nm. Its fluorescence was quenched by Fe2+ and recovered upon the introduction of chlorpyrifos. The linear concentration range extended from 0.01 to 1.0 μg mL−1, featuring an LOD of only 3 ng mL−1, which was used for imaging chlorpyrifos in vegetables (Fig. 24d). The fluorescence of 77 depended on the transformation between Fe2+ and Fe3+ by H2O2 and the production of H2O2 was inhibited by acetylcholinesterase activity, implying that this chemosensor can quantitatively detect organophosphorus pesticides and monitor enzymatic activity.149 In addition, fluorescent chemosensors (78–80) based on CDs also enabled the accurate detection of some pesticides in plants such as parathion-ethyl, cyfluthrin, glyphosate, methomyl and methyl parathion.150–152 Among them, 79 showed the lowest detection limit of 4.8 × 10−11 M owing to its detection mechanism, where methyl parathion can decrease the enzyme activity of tyrosinase. Therefore, the excellent fluorescence properties based on fluorescent CQDs enable them to be used more often as chemosensors in plants, consequently enabling an in-depth understanding of plant growth conditions and patterns.
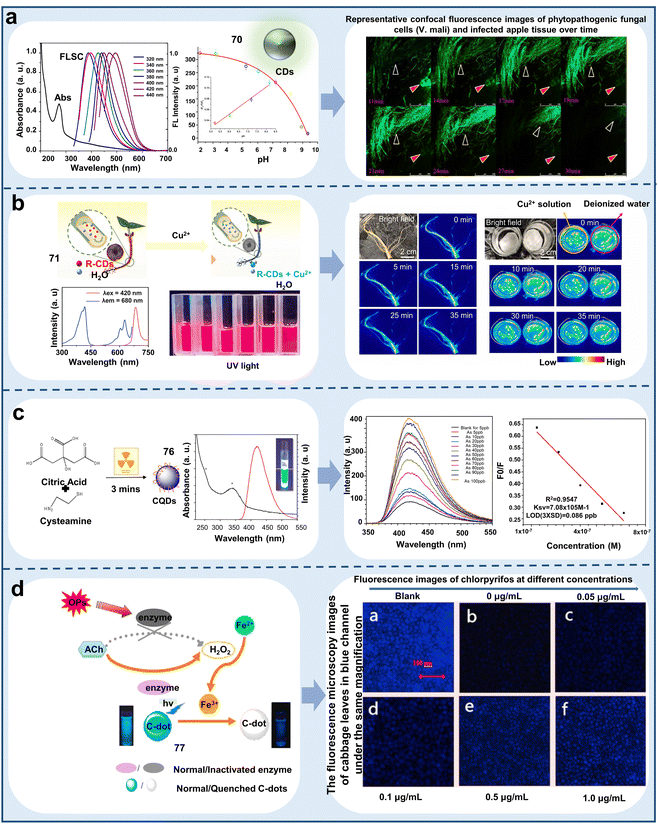 | ||
| Fig. 24 Schematic representation of the application of undoped carbon quantum dot-based chemosensors for detecting pathogens and water pollution. (a) Hydrothermal preparation of pH-sensitive fluorescent CDs for the detection of the pathogenic bacterium Valsa mali Miyabe et Yamada in apple tissues.142 Copyright © 2015, Elsevier. (b) Fluorescent sensors of red fluorescent R-CDs enable Cu2+ concentration monitoring in plant roots.143 Copyright © 2014, Elsevier. (c) Fluorescent sensor of blue fluorescent CDs enables the sensitive detection of arsenite in environmental water samples.148 Copyright © 2017, Elsevier. (d) Detection of chlorpyrifos levels in cabbage leaves by a blue fluorescent CD sensor.149 Copyright © 2018, Elsevier. | ||
Metal-doped carbon quantum dots. In recent years, doping different metal elements is an important way to enhance the physicochemical attributes of CQDs to make them more stable, and they are widely used in sensing applications. For example, Tb3+-doped CDs (CD-Tb) (chemosensor 81) showed bright-blue fluorescence emission under a 365 nm UV lamp with a relatively high QY of 54.8%. Subsequently, its sensitivity to guanosine 3′-diphosphate-5′-bisphosphate (ppGpp) was evaluated. The ppGpp molecular phosphate group was found to be able to bind to the rare-earth Tb3+, and thus effectively improved the emission intensity of 81. Paper-based sensors were developed to realize simple and intuitive ppGpp detection. A linear relationship emerged in a broad concentration range of 25 to 750 μM (Fig. 25a). Among the tested amino acids, 81 could selectivity detect ppGpp owing to the production of antenna and coordination binding.153 This scheme confirmed the great potential of rare earth ion-modified CDs as a novel proportional FL chromophore. An MnO2-doped carbon QD chemosensor (MnO2CD) (82) was prepared by a hydrothermal methodology based on the combination of citric acid and KMnO4. This chemosensor exhibited good photostability with no discernible alteration in its PL spectra, even in the presence of elevated NaCl concentrations up to 2.5 M. No change in its PL peak position was observed with a change in pH conditions. Importantly, 82 enabled the first visual identification of arsenic-containing compounds in rice and green plants. The luminescence signal of 82 was mainly presented in the vascular system of the roots, and As3+ was linearly correlated in the concentration range of 0–0.991 μM. The LOD of arsenite in the chlorotic plants reached 9.2 μM (Fig. 25b).154 Thus, 82 showed good translocation properties in lateral and green rice plants, demonstrating its potential for detecting the deposition of arsenic within plant root structures. The temperature-sensitive fluorescent properties of the chemosensor of MnO2CD establish its potential for use as a nano-temperature meter in physiological environments. Therefore, the introduction of metal ions increases the detection range of CQDs.
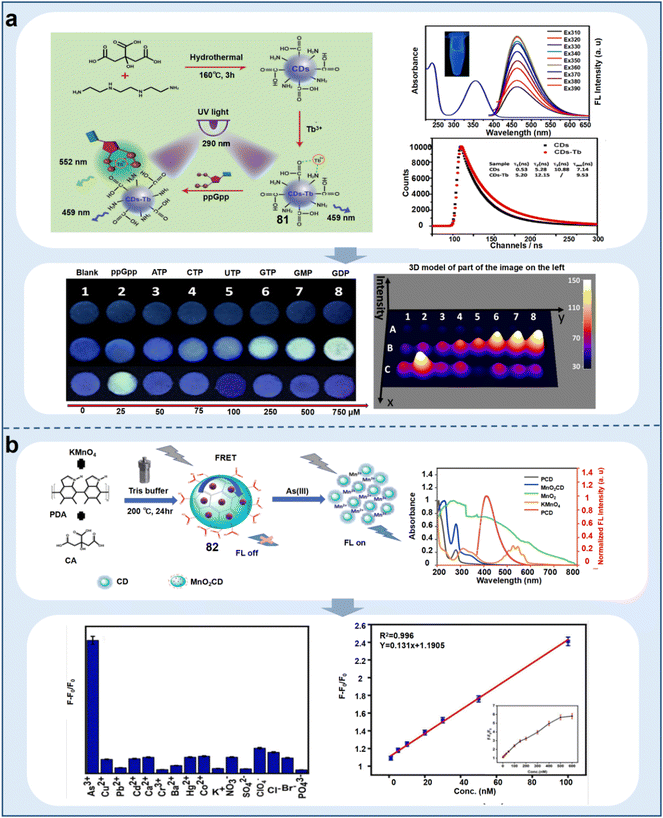 | ||
| Fig. 25 Schematic representation of metal-doped carbon quantum dot-based chemosensors for monitoring the living environment of plants. (a) Terbium-modified fluorescent CD chemosensor for the detection of ppGpp.153 Copyright © 2018, the American Chemical Society. (b) Detection of As(III) in environmental water samples by MnO2 CD fluorescent chemosensor synthesized by hydrothermal decomposition.154 Copyright © 2022, Elsevier. | ||
Nonmetal-doped carbon quantum dots. Compared with metal element doping, nonmetal-doped CQD chemosensors with low toxicity, good biocompatibility, and high-intensity PL have been used in biosensing and environmental monitoring. For example, nitrogen-doped carbon QDs (NCDs) (chemosensor 83) prepared using uronic acid were reported to be capable of detecting Hg2+ and Ag+ in water. 83 exhibited a light transparent color under daylight conditions and a bright-blue color under 365 nm excitation. The fluorescence intensity of 83 could be linearly quenched in the presence of Hg2+ and Ag+ in water with LODs of 4.8 nM and 1 nM, respectively (Fig. 26a). Furthermore, 83 could be recovered by EDTA solution, indicating that this chemosensor may be a potential tool for sensing Hg2+/Ag+ in the real environment because of its recyclability.155 NCD chemosensors enabled accurate detection of Fe3+, Pb2+, Cr2+, and Cu2+ in water (chemosensors 84 and 85).156,157 Among them, 85 is an electrochemical sensor that could sensitively detect Pb2+ and Cr2+ owing to its electroactive surface and oxygen-functional groups. CQDs based on N, S co-doping (N, S-CQDs) (chemosensor 86) also accurately detected carbaryl in apples. 86 achieved a QY of 12.5% with a PL lifetime of 2.8 ns, and carbaryl could quench its fluorescence in the presence of acetylcholinesterase. The linear concentration range was 6.3 × 10−9 g L−1 to 6.3 × 10−4 g L−1, with the lowest detection limit of 5.4 × 10−9 g L−1 because of its dual enzyme response mechanism (AChE and ChOx enzymes) (Fig. 26b).158 N, S-CQD chemosensors also enabled the fluorescence detection of paraoxon, p-fenitrothion, dithiopyr, aflatoxin, methimazole, and fenitrothion in soil, water or vegetable samples (chemosensors 87–91).159–163 Among them, 88 was been proven to detect fenitrothion, dinoseb, dithianon, and their analogues, which was attributed to the electron transition of C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in flavonoids. Additionally, fluorescent NCDs, metal–organic frameworks, and molecularly imprinted polymers, in conjunction with a proportional fluorescent sensor NCQD@Co-MOFs@MIPs (chemosensor 92), were proposed for the accurate quantification of the jasmonic acid (JA) content in plants. This groundbreaking study represented the first instance integrating fluorescent carbon dots with these composites. Co-MOF and MIPs acted synergistically in trapping the JA molecule, improving the sensitivity and selectivity for monitoring the distribution of JA. Upon the incorporation of JA in 92, it formed a chemical interaction with the NCQDs via PET, leading to changes in the fluorescence signal of 92. The LOD reached 0.35 ng mL−1, offering new insights for developing low-cost and highly sensitive methods for monitoring phytohormones.
N in flavonoids. Additionally, fluorescent NCDs, metal–organic frameworks, and molecularly imprinted polymers, in conjunction with a proportional fluorescent sensor NCQD@Co-MOFs@MIPs (chemosensor 92), were proposed for the accurate quantification of the jasmonic acid (JA) content in plants. This groundbreaking study represented the first instance integrating fluorescent carbon dots with these composites. Co-MOF and MIPs acted synergistically in trapping the JA molecule, improving the sensitivity and selectivity for monitoring the distribution of JA. Upon the incorporation of JA in 92, it formed a chemical interaction with the NCQDs via PET, leading to changes in the fluorescence signal of 92. The LOD reached 0.35 ng mL−1, offering new insights for developing low-cost and highly sensitive methods for monitoring phytohormones.
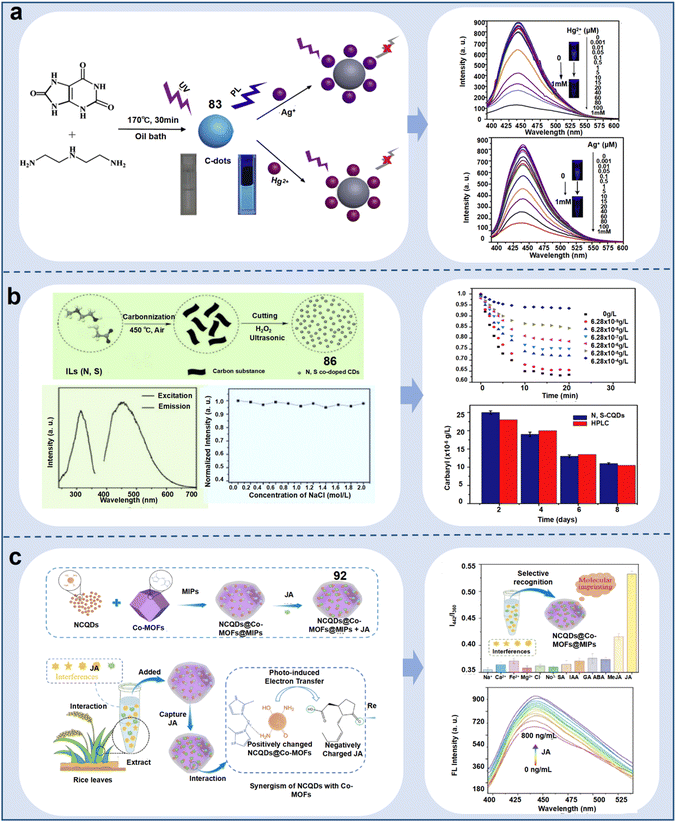 | ||
| Fig. 26 Schematic representation of non-metal doped carbon quantum dot-based chemosensors for monitoring ions in water, pesticides and hormones in plants. (a) Fluorescent chemosensors with N-doped carbon dots for the detection of Hg2+ and Ag+ in water samples.155 Copyright © 2017, Elsevier. (b) Fluorescent chemosensors of N, S co-doped carbon quantum dots were used for the detection of the pesticide carbaryl in fruit samples.158 Copyright © 2016, Elsevier. (c) Detection of phytohormone jasmonic acid based on NCQD@Co-MOFs@MIP ratiometric fluorescent chemosensor.164 Copyright © 2023, Elsevier. | ||
Implying that 92 is useful for monitoring the response of plants to biotic and abiotic stresses (Fig. 26c).164 Therefore, the combination of non-metallic doped quantum dot chemosensors and smartphones and composites is an important approach to improve the detection efficiency and practical applications.
Compared with semiconductor QDs, CQDs have some advantages such as low cytotoxicity, high biocompatibility, and fluorescence stability, but CQDs also have some disadvantages, as follows: (i) the fluorescence wavelength of CQDs is 400–520 nm, and most of them require UV excitation. This short-wavelength has low penetration depth in biological tissues and easily causes photodamage to biological tissue. (ii) Short-wavelength can cause strong autofluorescence in biological tissues, resulting in serious fluorescence background, which interferes with the analysis of fluorescence signals.
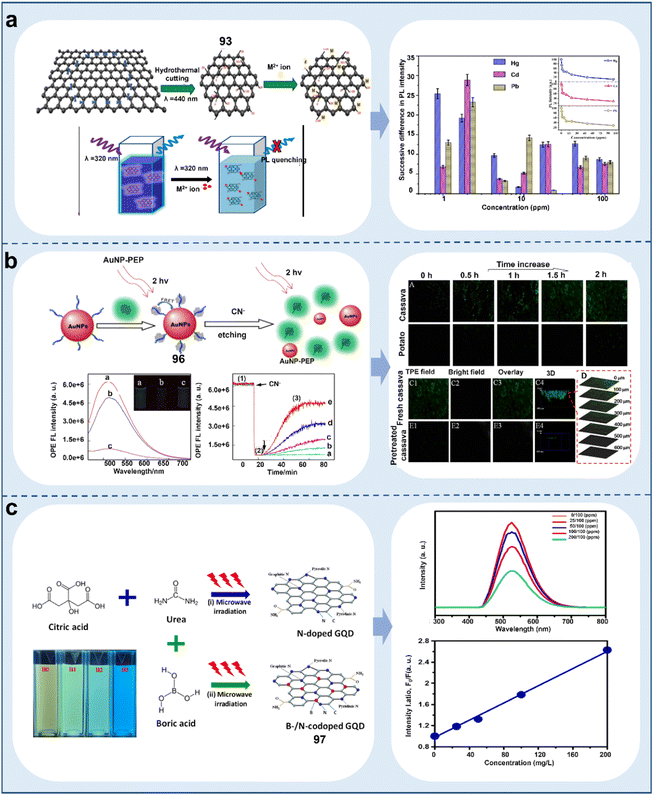 | ||
| Fig. 27 Schematic representation of graphene-based quantum dot chemosensors for detecting water pollution. (a) Hydrophilic graphene quantum dot chemosensor for the detection of heavy metals Hg2+, Cd2+, and Pb2+ in water.165 Copyright © 2021, Elsevier. (b) Novel dual-photo-excitation nanofluorescent sensor with GQD/AuNP conjugates for the detection of CN− in plants.168 Copyright © 2015, the American Chemical Society. (c) Microwave synthesis of boron and nitrogen co-doped graphene quantum dot fluorescent chemosensors for the detection of paraquat in aqueous media.169 Copyright © 2023, Elsevier. | ||
GQDs show strong fluorescence characteristics, high solubility, excellent biocompatibility, low toxicity and photostability, but they also have some disadvantages, as follows: (i) the structure of GQDs is too small, and then not enough QY can be produced by chemosensors; (ii) the redox reaction and coordination of recognition parts cause GQDs to have weak anti-interference and poor specificity; and (iii) the synthesis process of GQDs is complex and requires many solvents that can increase their synthesis cost and toxicity.
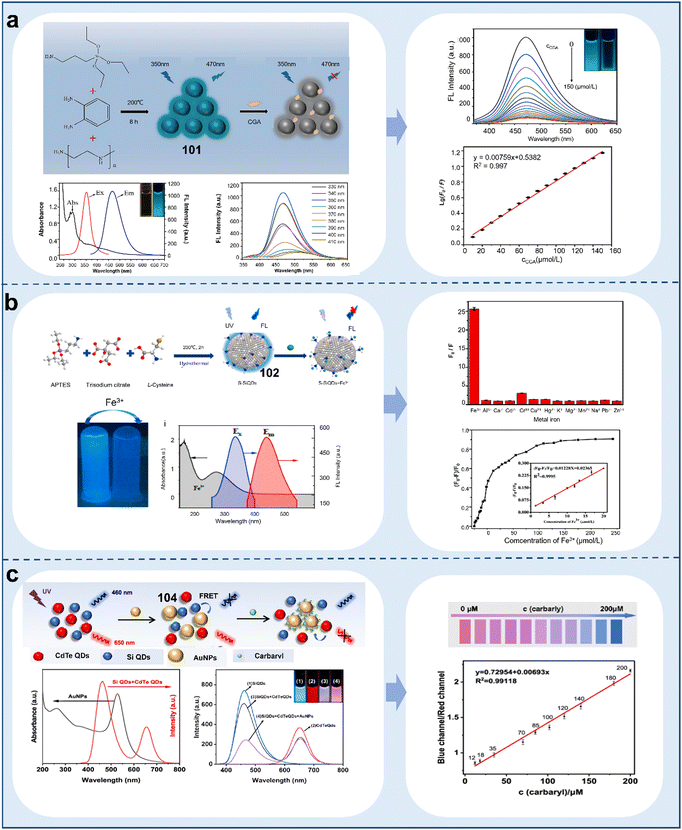 | ||
| Fig. 28 Schematic representation of silicon quantum dot-based chemosensors for monitoring the living environment and quality of plants. (a) Cyanoluminescent Si quantum dot fluorescent chemosensor for the detection of chlorogenic acid.173 Copyright © 2023, Elsevier. (b) Detection of Fe2+ in water by S-doped Si quantum dot chemosensor.174 Copyright © 2020, Elsevier. (c) Detection of carbaryl in vegetables by a fluorescent sensor with amino-modified gold nanoparticles, blue-emitting silicon quantum dots and red-emitting cadmium telluride quantum dots.176 Copyright © 2023, the American Chemical Society. | ||
Upon the addition of Hg2+, the fluorescence intensity of 103 decreased rapidly, which could be recovered by coordination with glutathione. The linear range for the detection of glutathione and Hg2+ was 0.1–5 μM and 0.1–4 μM, with LODs of 55 nM and 24 nM, respectively. The results indicated that the chemosensor can monitor plant health under ion stresses. In addition, sensing centers based on Si QDs, gold nanoparticles (AuNPs) and CdTe QDs (chemosensor 104) detected carbaryl in lake water and in apples. The blue Si QDs showed an emission spectrum at 460 nm, whereas the red-light emitting CdTe QDs showed an emission peak at 650 nm. Carbaryl exhibited a tendency to adsorb on AuNPs, eliciting a gradual increase in the fluorescence emission intensity of the Si QDs, while simultaneously inducing a notable reduction in the fluorescence emission intensity of 104. The LOD of carbaryl in lake water and vegetable samples containing carbaryl was 3.3 nM in the concentration range of 80–200 μM. Importantly, it could be detected in real-time by a smartphone sensing platform (Fig. 28c).176 Therefore, the proposed sensing scheme enables new proportional fluorescence detection with satisfactory specificity and sensitivity, paving the way for implementing smart phone-centric pesticide detection platforms.
Si QDs have some advantages such as high QY, abundant silicon source, low toxicity and environmentally friendly nature, but they also have disadvantages, as follows: (i) the low water solubility of Si QDs limit their application in biological imaging and decrease their sensitivity and (ii) the poor stability and easy coacervation of Si QDs can affect their fluorescence efficiency, which can be improved by modifying their surface.
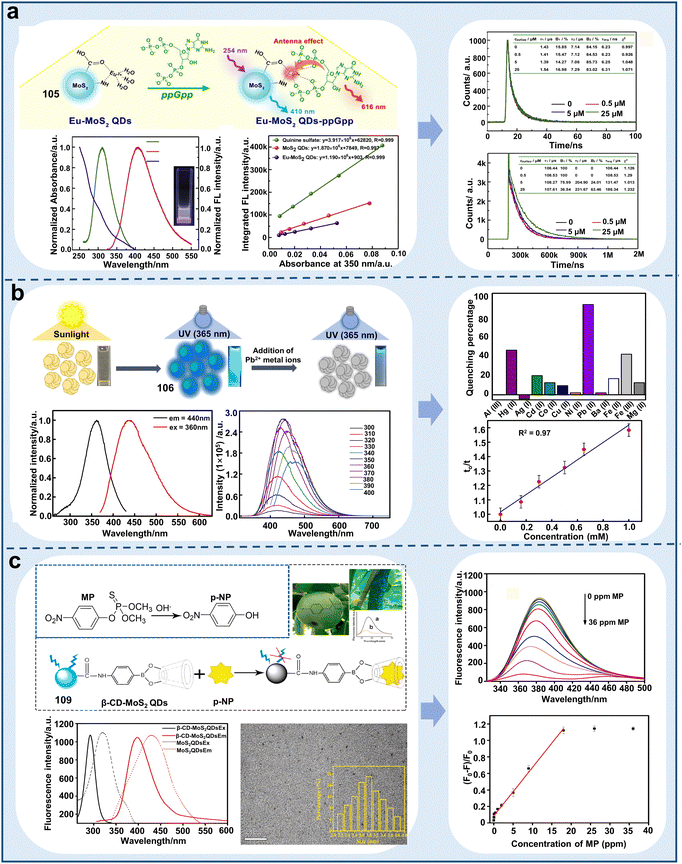 | ||
| Fig. 29 Schematic representation of molybdenum disulfide-based quantum dot chemosensors for detecting ions and pesticides in plants or the environment. (a) Eu-doped MoS2 quantum dot fluorescent chemosensor developed for paper sensor detection of ppGpp.177 Copyright © 2020, Elsevier. (b) Functionalized molybdenum disulfide quantum dot fluorescent chemosensor for the detection of Pb2+ in water to determine its quality.179 Copyright © 2020, Elsevier. (c) Detection of p-methyl parathion in fruits by β-cyclodextrin-functionalized molybdenum disulfide quantum dot fluorescent chemosensor.181 Copyright © 2021, Elsevier. | ||
MoS2 QDs have been widely used to detect ions and pesticides in the living environment of plants owing to their high QY, biocompatibility, and low toxicity, but their applications are limited due to the following facts: (i) most MoS2 QD-based biosensors are turn-off fluorescence sensors, and their fluorescence signal is susceptible to the detection system, which may lead to incorrect detection results and (ii) the processes for the synthesis of MoS2 QDs are complex, which lead to a low yield, size various, and difficult removal of organic solvents.
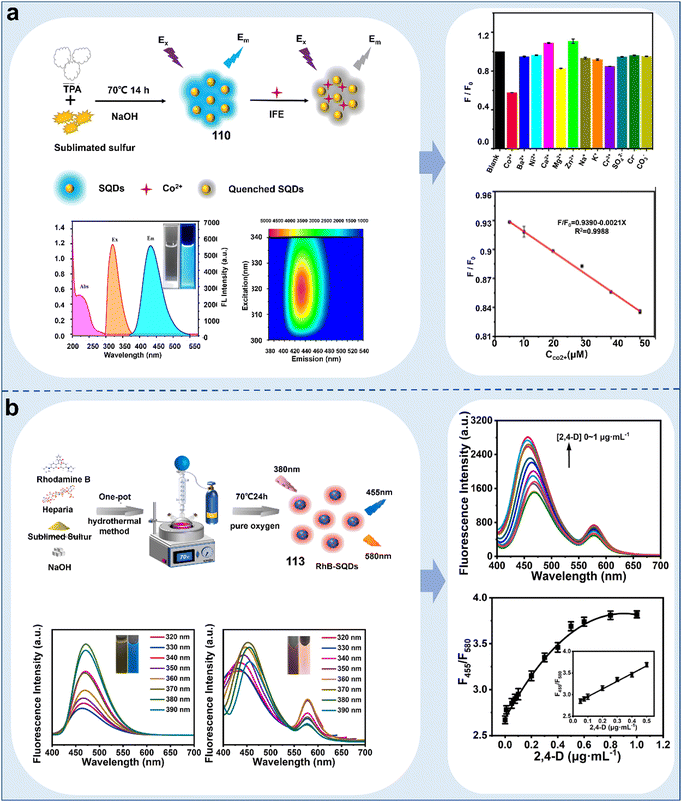 | ||
| Fig. 30 Schematic representation of sulfur-based quantum dot chemosensors for detecting ions and pesticides in plants and water. (a) Detection of Co2+ in water by a sulfur quantum dot fluorescent chemosensor.182 Copyright © 2023, Elsevier. (b) Novel dual-emission sulfur quantum dot fluorescent chemosensor enables the accurate detection of 2,4-dichlorophenoxyacetic acid.185 Copyright © 2023, Elsevier. | ||
S QDs have been widely used to image ions in plants owing to their high fluorescence intensity, low toxicity and photostability, while they also have some disadvantages, as follows: (i) the temperature instability of S QDs implies that they can be developed as a temperature sensor and (ii) the excitation wavelength of S QDs is mainly in the blue region, which limits their application in biological imaging.
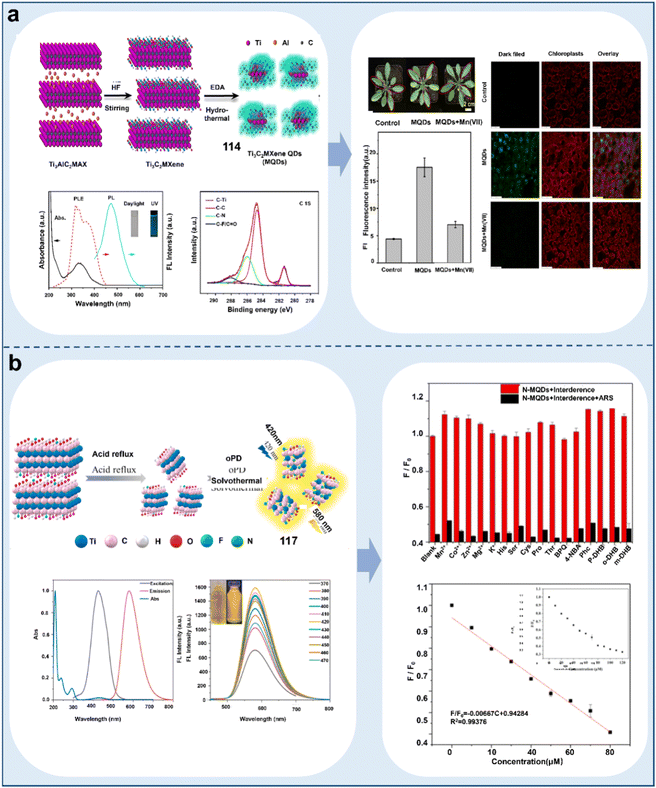 | ||
| Fig. 31 Schematic diagram of the application of MXene quantum dot-based chemosensors for the detection of ions in water and plants. (a) Accurate detection of Mn(VII) in water and plants based on MQD fluorescent chemosensors.186 Copyright © 2023, Elsevier. (b) Solvothermal synthesis of N-MQD fluorescent chemosensors for the detection of ARS in real water samples.198 Copyright © 2021, Elsevier. | ||
We summarized the application of MXene QD chemosensors in detecting the factors affecting or reflecting plant health, which showed good detection efficiency in these areas.199 However, there are some disadvantages that need to be improved, as follows: (i) almost all MXene QD chemosensors showed UV or blue excitation light, which can reduce the application of these chemosensors for detecting plant hormones, proteins, or enzymes; (ii) the synthesis strategies for MXene QDs chemosensors should be improved such as fluorine-free or high efficiency and stability of catalyst, which can reduce the toxicity of MXene QD chemosensors and increase their application scope; and (iii) combining MXene QD chemosensors with smart devices to improve the detection and removal efficiency, which will be the future development direction.
4.2 Metal–organic frameworks (MOFs)
MOFs are a class of coordination polymers self-assembled with metal centers (clusters) and organic ligands through coordination bonds. Due to their superiorities of adjustable metal ion species, diverse functional sites and clear structure, MOFs have emerged as exciting sensing and detection materials.200,201 A distinctive approach for the fabrication and design of luminescent MOFs (LMOFs) involves utilizing the porous nature of the MOF host to encapsulate diverse luminescent guests (LG), leading to the formation of a composite system known as “LG@MOF”.202 LG are mainly derived from metallic constituents, organic linkers and encapsulated guest entities (e.g., lanthanide ions, organic dyes and QDs) within MOFs.203 Besides, organic ligands with versatile reactive functional groups such as free pyridyl, imidazole, hydroxyl, and carboxyl groups, have been skillfully immobilized on the pore surfaces of fluorescent MOFs. This strategy aims to amplify the fluorescence sensing ability of MOFs by facilitating a wide array of host–guest interactions.204 Based on the differences in guest molecules, the main types of LG-MOFs include organic dyes@MOF, metal ions@MOF, metal nanoclusters@MOF, quantum dots@MOF and perovskites@MOF.204–207 Thus far, LMOF-based detection platforms have been developed to evaluate the living environment of plants (Fig. 32). Table 8 lists some classic examples of various developed MOF-based fluorescent chemosensors applied in this field.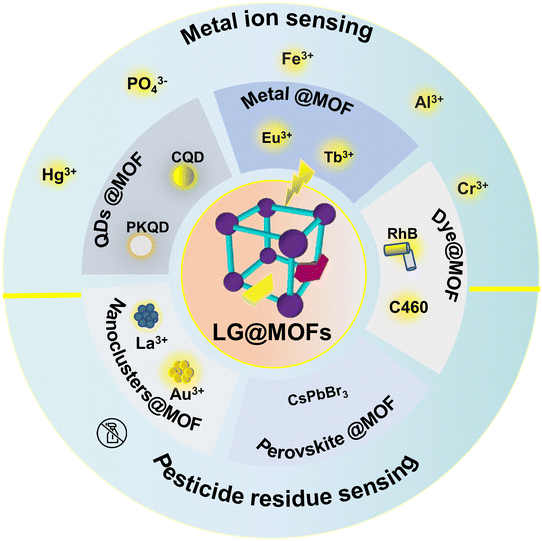 | ||
| Fig. 32 Classification and synthesis of LG@MOF-based chemosensors and their applications in monitoring the living environment of plants. | ||
| Nanofluorescence sensors | Chemosensors | Name | Linear range | Limit of detection (LOD) | Target analytes | Applications | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Metal ions@MOF | Lanthanide irons | 119 | Eu–MOF | — | 0.280 μM for Fe3+, 0.150 μM for Cr2O7 | Fe3+, Fe2+, Cr2O72− | Detection of Fe3+, Fe2+, and Cr2O72− in water | 208 |
| 120 | Eu–MOF | 0–110 and 0–90 μM | 0.120 μM for Fe3+, 0.360 μM for Al3+ | Fe3+, Al3+ | Monitoring the concentration of Fe3+, and Al3+ water | 209 | ||
| 121 | Eu–MOF | 0.02–200 μM | 2.60 nM | Hg2+ | Detection of Hg2+ in water | 210 | ||
| 122 | Eu–MOF | — | 10−5 mol L−1 | PO43− | Detection of PO43− in water | 211 | ||
| 123 | Eu–MOF | — | 0.170 μM | Pesticide DCNA | Monitoring the concentration of pesticide DCNA in water | 212 | ||
| 124 | Tb-MOF | 0.06–4 and 4–40 μg mL−1 | 18.0 ng mL−1 | Carbendazim (CBZ) | Detection of CBZ in apples and tea | 213 | ||
| 125 | Tb-MOF | 0–80 μg L−1 | 0.271 μg L−1 | Thiabendazole (TBZ) | Detection of TBZ in orange samples | 214 | ||
| 126 | Tb-MOF | 10–1000 ng mL−1 | 3.41 ng mL−1 | Chlorpyrifos | Detecting CPF in fruit juice | 214 | ||
| 127 | Eu, Tb-MOF | — | — | Flavonoids, stilbenes, anthraquinones | Monitoring the concentration changes of saponins, flavonoids, stilbenes, anthraquinones in plant | 215 | ||
| 128 | Tb-MOF | — | 4.50 ppm | PO43− | Detection of PO43− in water | 216 | ||
| 129 | Tb-MOF | 10−6 to 10−3 M | 0.100 ppm | PO43− | Detection of PO43− in water | 217 | ||
| 130 | Ce-MOF | 0.02–30 μg mL−1 | 0.00620 μg mL−1 | Glyphosate | Detection of glyphosate in water | 218 | ||
| 131 | Al-MOF | — | 102 nM | Pd2+ | Detection of Pd2+ in water | 219 | ||
| 132 | Zn/Fe-MOF | 0.001–100 mg L−1 | 0.340 μg L−1 | Paraquat (PQ) | Monitoring the content of PQ in lettuce, cabbage and agriculture irrigation water | 220 | ||
| 133 | UiO-66(Zr MOF) | 0–200 μM for Cr2O72−, | 0.160 μM for Cr2O72−, 0.170 μM for CrO42− | Cr3+ (Cr2O72−/CrO42−) | Detecting the concentration of Cr3+ in food | 221 | ||
| 0–220 μM for CrO42− | ||||||||
| 134 | Mn/Fe-MOF | 10−9–10−7 M | 0.850 nM or 0.290 ppb | Glyphosate | Detection of glyphosate in water | 222 | ||
| Quantum dots@MOF | 135 | Cu-MOFs@CDs | 0.0307–0.769 μmol L−1 | 3.67 nmol L−1 | Thiophanate-methyl (TM) | Microcystin-LR | 223 | |
| 136 | MOF-5@CH3NH3PbBr3 CDs | — | — | Al3+, Bi3+, Co2+, Cu2+, Fe3+, Cd2+ | Monitoring various ions in water | 224 | ||
| 137 | MOF-derived In2O3 hollow tubulars @Ti3C2 QDs | 0.5–4 × 105 pmol L−1 | 0.169 pmol L−1 | Photoelectrochemical (PEC) | Detection of PEC in water | 225 | ||
| 138 | ZIF-8@ZnS QDs | 0–80 μM | 0.890 μM | Chlorpyrifos | Detection of CPF in water | 226 | ||
| 139 | ZIF-67@ZnSQDs | 3–500 nM | 0.960 nM | Cu2+ | Detection of Cu2+ in water and soil | 227 | ||
| Organic dyes@MOFs | 140 | RhB@Zr-MOF | — | 1.60 μM for Fe3+, 0.200 μM for nitenpyram | Fe3+, nitenpyram | Detecting the content of Fe3+ and nitenpyram in water | 228 | |
| 141 | RhB@HNU-48 | — | 0.590 ppb | Alachlor | Monitoring the concentration of alachlor in paddy water and soil, polished rice and cucumber | 229 | ||
| 142 | RhB@Zr-MOF | — | 6.27 for Cr2O72−, 5.26 ppb for CrO42− | Cr2O72−, CrO42− | Detection of Cr2O72− and CrO42− in water | 230 | ||
| 143 | Doxorubicin@AzoMOF | 0–5 μg mL−1 | 2.96 ng mL−1 | Dufulin | Detection of dufulin in water | 231 | ||
| 144 | Curcumin@MOF-5 | — | 3.10 μM for A-curcumin@MOF-5, 2.84 μM for B-curcumin@MOF-5 | Al3+ | Monitoring the concentration of Al3+ in lettuce extract and water sample | 232 | ||
| Metal clusters@MOFs | 145 | AuNCs@ZIF-8 | 3–30 nM | 1.00 nM | Hg2+ | Detection of Hg2+ in food | 233 | |
| 146 | AuNCs@Zr-MOF | — | 193 pM | Hg2+ | Detection of Hg2+ in water | 234 | ||
| 147 | AuNCs@ZIF-8 | 0.75 μg L−1–100 mg L−1 | 0.300 μg L−1 | OPs | Detection of OPs in water | 235 | ||
| 148 | AgNCs@Zr-MOF | 0.010–0.5 μg mL−1 | 1.80 μg L−1 | Hg2+ | Detection of OPs in water | 236 | ||
| 149 | CuNCs@MOFs UiO-66-(COOH)2 | 1–30 μM | 178 nM | Cu2+ | Detection of Cu2+ in water | 237 | ||
| 150 | LaNCs@MOFs | 16.6–167 μM | 16.6 μM | Fe3+ | Detection of Fe3+ in water | 238 | ||
| Perovskite@MOFs | 151 | Eu3+@CsPbBr3 nanocrystal | 0–1 μM | 0.116 nM | Hg2+ | Detection of Hg2+ in water | 239 | |
Eu3+@MOF-based fluorescent chemosensors. Among the metal ions, Eu3+ ions are most commonly used to dope MOFs or as atomic centers for the construction of single emission or double emission fluorescent chemosensors to detect harmful anions and cations in the living environment of plants.240 For instance, Xu et al. introduced Eu3+ in the MIL124 MOF to develop a highly selective and sensitive single-emission chemosensor (Eu3+@MIL124, 119) for the detection of Fe2+, Fe3+, and Cr2O72−via the exceptional fluorescent quenching exhibited by Eu3+ and MOF compared to other metal ions.208 However, although the single luminescence based on MOFs is easily measured,204 their intensity is often interrupted by the concentration and environment of chemosensors. In this case, the incorporation of two or more emitting sites resulting from the dual emission or bimetallic properties of Ln metal ions and organic ligands can effectively avoid false responses, thereby improving the S/N ratio.240 For example, Xia et al. successfully developed a novel dual-emission MOF incorporating Eu3+ ions and 2-hydroxyterephthalic acid (H2BDC-OH) in a UiO-66-type MOF material (chemosensor 120).209120 displayed dual emission peaks at 450 and 614 nm and exhibited good anti-interference characteristics for Fe3+ and Al3+ in the complex aquatic environment. Besides, 120 exhibited remarkable selectivity and sensitivity towards Fe3+ and Al3+, and their detection linear range was 0–110 and 0–90 μM, respectively (Fig. 33a). Additionally, Hao et al. successfully synthesized a dual-emission Ln-MOF material (Eu-Ca-MOF) by incorporating Eu3+ ions in the channels of a Ca-MOF parent framework (chemosensor 121).210121 exhibited excellent PL properties, in tandem with precise capacity to detect trace levels of Hg2+ in water, achieving an LOD of 2.6 nM (Fig. 33b). Besides, a novel Eu-based three-dimensional framework was obtained based on the 1,3,5-benzenetribenzoate (H3BTB) ligand with high C3 symmetry (chemosensor 122).211122 demonstrated remarkable thermostability and water stability for the detection of PO43− ions among various colorless anions. This work represents a pioneering example of regenerable MOF-based luminescent chemosensors for detecting PO43−. Besides monitoring harmful ions, Eu–MOF-based chemosensors have also been used for detecting pesticide residues. For example, Qin et al. designed ultrathin fluorescent Eu3+@Zn-MOF nanosheets (Eu3+@Zn-MOF-NS, chemosensor 123) to rapidly detect the residues of organic pesticides.211 In the synthesis of 123, 2,6-dichloro-4-nitroaniline (DCNA) was employed, utilizing Zn2+ as the metal node and pyridine and 1,4-benzenedicarboxylate (H2BDC) (Py) as the organic ligands. 123 offered a unique combination of benefits of both MOFs and 2D nanomaterials simultaneously. Notably, its LOD and Ksv were 0.17 μM (35 ppb) and 3.2 × 105 M−1 in water, respectively. 123 could potentially be applied for the rapid, in situ, and visualized detection of DCNA residues on fresh vegetables (Fig. 33c). The intense PL exhibited by 123 could be significantly quenched by DCNA in water, without any interference from other amino acids, small organic molecules, pesticides, or commonly encountered anions and ions. Interestingly, the as-obtained Eu3+@Zn-MOF-NS probe in this work is promising for the visual, rapid, and in situ detection of DCNA residues on fresh vegetables. This work provides meaningful information on using Eu3+-based MOF probes for the effective detection of pesticide residues in practical applications. However, although Eu3+-based sensors have been applied in environment monitoring, related studies on bioimaging in plants are still lacking.
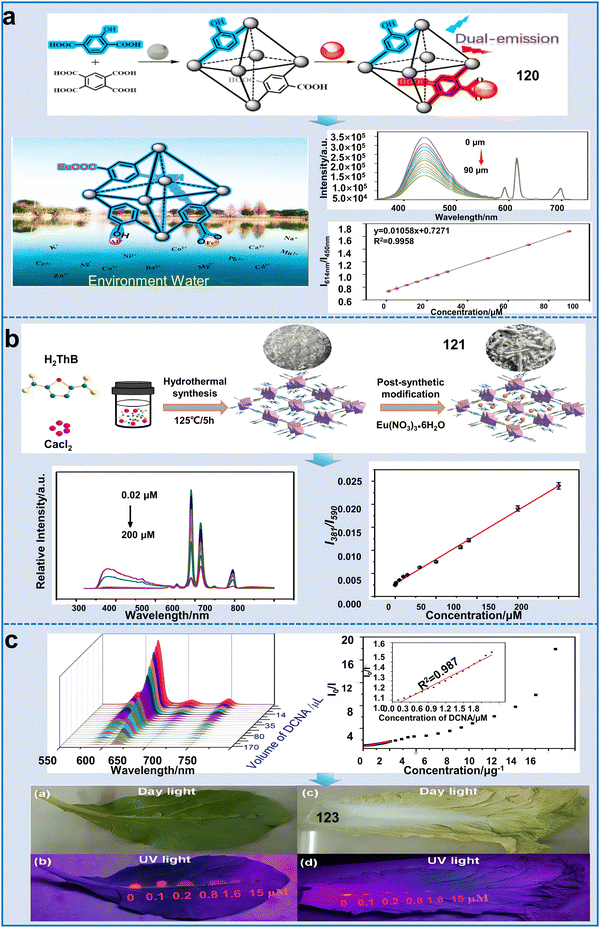 | ||
| Fig. 33 Applications of Eu3+-based sensors for monitoring the living environment of plants. (a) Synthesis of a highly water-stable dual-emission Eu3+-loaded MOF (EuUCH) and its detection of Fe3+ and Al3+ metal ions.210 Copyright © 2022, Elsevier. (b) Synthesis of Eu-Ca-MOF fluorescence sensor and its detection sensitivity for Hg2+ in water.211 Copyright © 2022, Elsevier. (c) Detection sensitivity of Eu3+@Zn-MOF for pesticide and its sensing effect in actual samples.211 Copyright © 2022, the American Chemical Society. | ||
Tb3+@MOF-based fluorescent chemosensors. In addition to the incorporation of Eu3+ ions mentioned earlier, other commonly used Ln ions such as Tb3+ have been extensively employed in the construction of LMOFs to detect natural metabolites, metal ions and pesticide residues. For example, Wang et al. successfully fabricated a fluorescent nanosheet of a two-dimensional terbium-based MOF (2D Tb-MOF) for the detection of carbendazim (CBZ) (chemosensor 124).213 The 2D Tb-MOF nanosheets, synthesized with 5-borono-1,3-benzenedicarboxylic acid (BBDC) and Tb3+ ions as the precursors, demonstrated outstanding optical features. After the addition of CBZ, the fluorescence of the Tb-MOF nanosheets was quenched due to dynamic quenching and the inner filter effect (IFE). 124 offered two linear ranges of 0.06–4 μg mL−1 and 4–40 μg mL−1 with an LOD of 17.95 ng mL−1 (Fig. 34a). Furthermore, the developed sensing platform was effectively utilized to detect CBZ in apples and tea samples, yielding satisfactory results. However, in the presence of other coexisting substances such as NH4+, glucose and fipronil, the reaction between Tb-MOF and CBZ was slightly hindered. Another study constructed a highly selective luminescent sensor (chemosensor 125) for monitoring thiabendazole (TBZ) in oranges based on a Tb3+-functionalized Zr-MOF (Tb3+@1). 125 with high selectivity exhibited a good linear range of 0–80 μM, rapid response time (less than 1 min) and LOD of 0.271 μM. Additionally, this sensor presented good recoveries from 98.41% to 104.48% when used for detecting TBZ in real orange samples and this process only took 35 min (Fig. 33b). Coupling antibodies or aptamers with Tb-MOFs was helpful for the detection selectivity and sensitivity by providing specific recognition sites to match the target analyte molecules.214 Another mesoporous molecular imprinted polymer (mMIP) layer imprinted Tb-MOF (MOF-76) fluorescent chemosensor (126) was constructed for the analysis of chlorpyrifos in fruit juice and the environment.214126 contained specific sites for the adsorption of chlorpyrifos, ensuring the selectivity of the designed chemosensor. It exhibited a linear response in the concentration range of 10–1000 ng mL−1 for chlorpyrifos, with an LOD of 3.41 ng mL−1. These studies provide useful analytical tools for the rapid and sensitive detection of CPF in the living environment of plants.
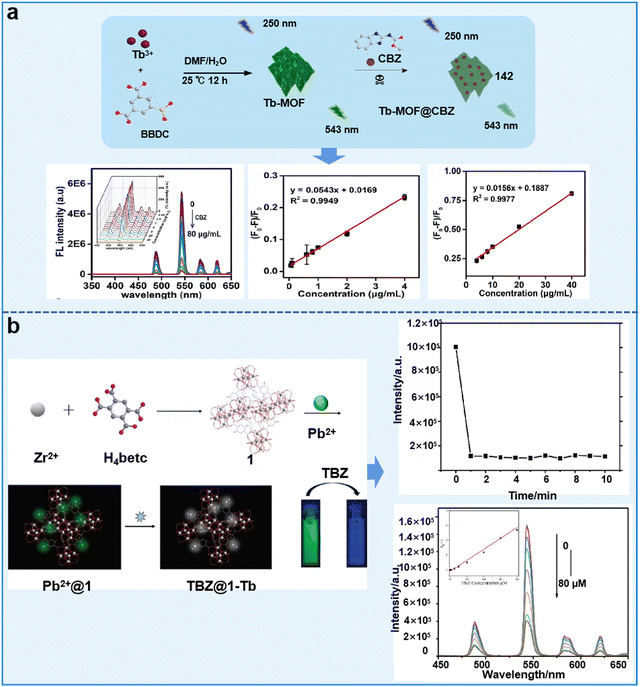 | ||
| Fig. 34 Examples of Tb-MOF chemosensors for sensing pesticide residues. (a) Synthesis process of Tb-MOF@CBZ and its sensing effect and sensitivity for CBZ.213 Copyright © 2023, Elsevier. (b) Synthesis process of Tb3+-functionalized Zr-MOF and its sensing mechanism and sensitivity for TBZ.214 Copyright © 2021, Elsevier. | ||
Besides the detection of pesticides, Tb-MOF chemosensors have also been used to monitor the distribution and concentration of inorganic anions and plant metabolites. For example, Yin et al. designed a fluorescent chemosensor (127) that incorporated Eu3+ and Tb3+ ions within an MOF. 127 enabled the rapid identification of various natural compounds, including 7 flavonoids, 12 saponins, 4 anthraquinones and 3 stilbenes.215127 possessed powerful identification and analysis ability, and the detection accuracy of each type of compound was up to 100% (Fig. 35a). Another study developed a Tb-MOF-based fluorescent chemosensor (128) for the detection of PO43−.216128 was demonstrated to be an exceptional luminescent chemosensor with a low detection limit, rapid response time, and an extensive detection range. Its versatile capabilities enabled the recognition of anions in water or other highly polar solvents, providing exciting prospects for various applications. Ji et al. encapsulated the Tb3+ cation into a 3D microporous MOF to construct a fluorescent-functionalized Tb@Zn-MOF (chemosensor 129).217129 showed both excellent chemical stability and the characteristic emission of Tb3+. 129 emerged as an advantageous photoluminescent reagent demonstrating exceptional specificity and precision for the quantitative analysis of PO43−. It presented a swift response time of only 10 s with a noteworthy LOD of 0.1 ppm. This investigation served as the pioneering prototype of a competent excitable fluorescent sensor to quantitatively assess the concentration of PO43− in simulated biological systems and detecting its presence in aquatic environments with a broad concentration range of 10−6 to 10−3 M (Fig. 35b). This sensor offers a new approach for identifying inorganic anions, which is highly significant in both physiological and ecological settings. It is evident that metal ion@LMOF sensors with high efficiency and sensibility for the detection of phosphorus anions in the living environment of plants are still lacking.
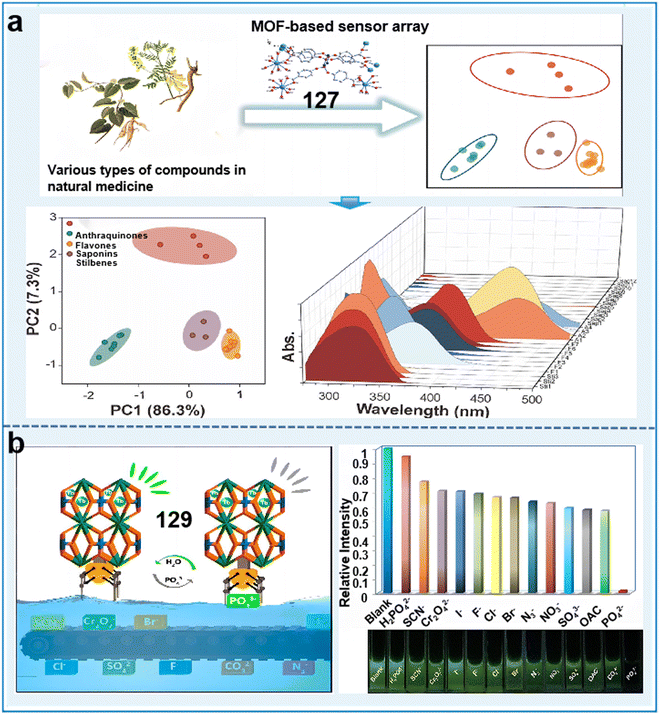 | ||
| Fig. 35 Examples of Tb-MOF chemosensors for sensing ions and plant metabolites. (a) Schematic diagram of Ln-MOF-based sensor arrays for the identification and detection of natural compounds.215 Copyright © 2021, the American Chemical Society. (b) Sensing sensitivity of Tb-MOF chemosensor for inorganic anions.217 Copyright © 2018, the American Chemical Society. | ||
Other metal ion@MOF-based fluorescent chemosensors. In addition to Eu3+ and Tb3+, some other metal ions have also been doped in MOFs to construct fluorescent chemosensors. For example, a study prepared a nanocomposite LMOF, incorporating UiO-67 nanoparticles within the pores of carbonaceous materials isolated from a novel Ce-MOF (Ce-PC) for the fluorescent sensing of glyphosate (chemosensor 130).218 The prevalent oxygen-bearing hydroxyl moiety (M–O–H) present in UiO-67/Ce-PC was capable of recognizing the phosphoryl moieties (–PO3H2) in glyphosate via ligand substitution and exhibited specificity toward glyphosate (Fig. 36a). Findings revealed that glyphosate could be reliably detected in cereal specimens in the concentration range of 0.02 to 30 μg mL−1 with an LOD of 0.0062 μg mL−1. These findings represent a promising approach for developing MOF-derived nanocomposite materials for the precise trace analysis of pollutants in the living environment of plants. Chakraborty et al. introduced an innovative ultrafast Al(III)-MOF chemosensor (131) for the detection of Pd2+.219131 demonstrated the lowest response time (30 s) and a remarkable low LOD of 102 nM, which is the shortest response time for MOF-based Pd2+ sensing thus far. Compared to the previously reported palladium sensors based on MOFs, 131 exhibited excellent recyclability, maintaining consistent effectiveness and selectivity for Pd2+ detection over five cycles in real water with the same efficiency (Fig. 36b). The quick response, sensitivity, and selectivity of this sensor make it an excellent choice for the selective detection of Pd2+ ions in water. More importantly, we believe that this work based on the alkyne–π interaction concept will inspire studies in diverse areas, particularly the development of various functionalized MOF-based sensors for detecting other hazardous heavy metal ions. Besides single metal doping, bimetallic-doped MOF-based sensors are promising to improve the sensing signal and sensitivity. For instance, Wu et al. developed an innovative electrochemical method for the detection of paraquat through the use of a modified glassy carbon electrode. The modification involved the incorporation of a Zn/Fe dual-metal ZIF-generated nanoporous carbon composite (Zn/Fe-ZIF-NPC) with Ni hexacyanoferrate nanoparticles (NiHCF-NPs) on the electrode substrate (chemosensor 132).220 NiHCF-NPs functioned as the dedicated signal chemosensor, whereas Zn/Fe-ZIF-NPC promoted swift electron transfer and powerfully amplified the discernible signal originating from NiHCF-NPs. Consequently, the modified electrode exhibited a linear concentration range of 0.001 to 100 mg L−1 and with an impressive LOD of 0.34 μg L−1. These outcomes provide strong evidence supporting the high reliability and application of the proposed methodology for discerning PQ contamination in actual vegetables and agricultural irrigation water. Besides the above-mentioned chemosensors, some other chemosensors based on metal-MOF could also detect anions, cations, and pesticides in the environment (chemosensors 133 and 134).221,222
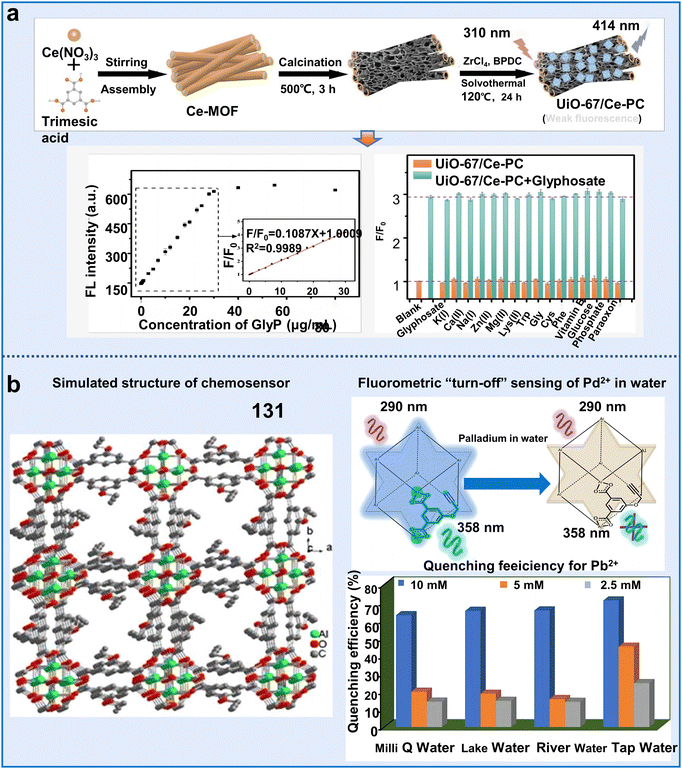 | ||
| Fig. 36 Examples of Tb-MOF chemosensors for sensing ions and plant metabolites. (a) Schematic diagram of Ln-MOF-based sensor arrays for the identification and detection of natural compounds.218 Copyright © 2021, the American Chemical Society. (b) Sensing sensitivity of Tb-MOF chemosensor for inorganic anions.219 Copyright © 2018, the American Chemical Society. | ||
In summary, these results offer some available strategies for future studies to develop more effective and sensitive fluorescent chemosensors based on metal ion MOFs for monitoring the living environmental conditions of plants. However, although considerable results have been achieved, there are still some concerns in their actual application, as follows: (i) the use of Ln ions for constructing LMOFs is currently limited to a narrow range of options, and the Earth's supply of Ln ions is also restricted. These factors present obstacles to the widespread application of Ln-based MOFs.204 (ii) The efficiency of exciting Ln ions through the excitation light is limited due to the forbidden nature of the 4f–4f electronic transition, and the generation of higher external quantum efficiency is hindered for Ln-ions. (iii) Most of the studies on metal ion@MOF sensors mainly focus on detecting pollutants and bio-small molecules in the living environment of plants, but there is still a lack of research on biological detection, such as plant hormones. (iv) To ensure successful sensing in water or other sensing media, it is crucial to enhance the stability of LnMOF-based sensors, particularly their water stability. This improvement is necessary to prevent structural breakdown or degradation before the sensing process is completed.240
In recent years, carbon dots (CDs) have attracted significant research interest in the field of sensing due to their remarkable attributes including low toxicity, tunability and robust fluorescence emission stability.244 The abundant functional groups on the surface of CDs facilitate their direct chemical coordination with MOFs.223 These phenomena indicate that integrating CDs with MOFs is a promising and efficient strategy for the detection of harmful substances in plant surroundings. For example, a simple and efficient ratiometric fluorescence chemosensor, CDs@Cu-MOF (135), was constructed for the detection of pesticide thiophanate-methyl (TM). This chemosensor was formed by coordinating the –COO– groups present on the surface of CDs with Cu2+ ions of Cu-MOF.223135 demonstrated excellent selectivity and accuracy for detecting TM in food samples, which was achieved through the interplay of π stacking and Cu2+ chelation between TM and Cu-MOFs and exhibited a remarkable LOD of 3.67 nmol L−1. Therefore, CD-based MOF sensors present a novel and effective strategy for pesticide detection, offering potential applications in monitoring plant health.
Hybrid organic–inorganic halide perovskites, such as CH3NH3PbX3 (X = Cl, Br, and I), have attracted significant interest in recent years. However, the practical use of CH3NH3PbX3 is limited due to the intrinsic susceptibility of its organic cation CH3NH3+. Under diverse environmental conditions such as UV light, moisture, high temperatures, and oxygen this cation degrades rapidly.224 Accordingly, integrating CH3NH3PbX3 QDs in MOFs can significantly improve their stability, and perovskites QD-based MOF fluorescent chemosensors have been successfully implemented to detect pesticides and heavy metal ions in the environment. Zhang et al. developed an innovative methodology for enhancing the stability of CH3NH3PbX3 perovskite QDs by encapsulating them within MOF-5 microcrystals. This was achieved by adding PbBr2 and CH3NH3PbX3 precursors in a stepwise manner to create a stable CH3NH3PbX3@MOF-5 composite (chemosensor 136).224136 demonstrated significantly enhanced water resistance and thermal stability compared to CH3NH3PbX3 QDs, which was useful for detecting various metal ions in water including Al3+, Bi3+, Co2+, Cu2+, Fe3+, and Cd2+ (Fig. 37a). However, although this work improves the relative stability of perovskite QDs, the exploration of the fundamental properties, operating principles, and prospective applications of CH3NH3PbX3 QDs encapsulated in MOFs remains a limited and challenging area of research.
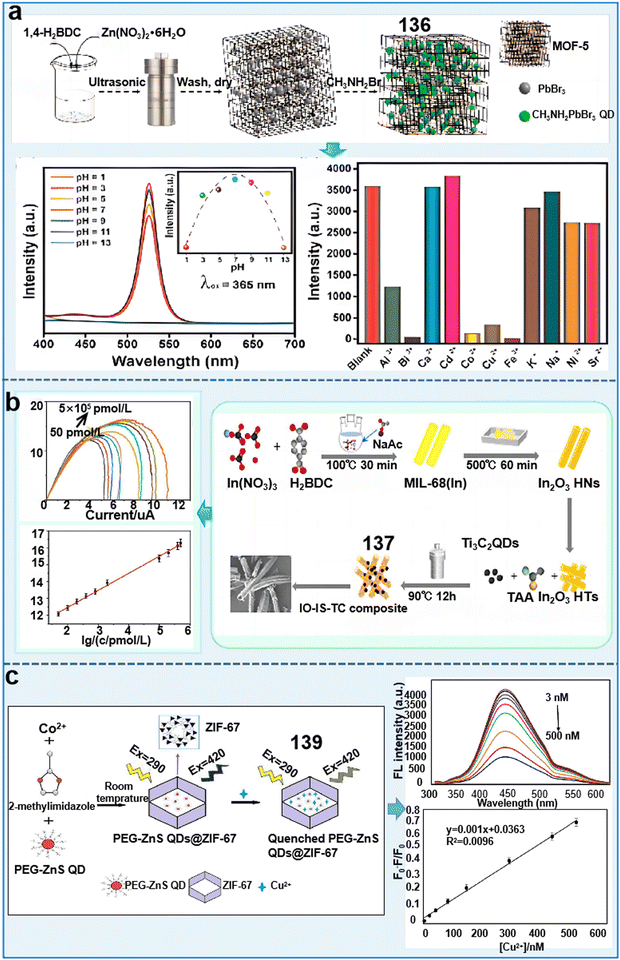 | ||
| Fig. 37 Examples of quantum dot@MOF-based fluorescent sensors for monitoring the living environment of plants. (a) Emission intensities and selectivities of CH3NH3PbBr3@MOF-5 composite for sensing various ions.224 Copyright © 2018, Elsevier. (b) Synthesis process of Ti3C2 QD@MOF chemosensor and its detection sensitivity for PEC.225 Copyright © 2022, ACS Appl. Mater. Interfaces. (c) Synthesis process of ZnSQDs@ZIF-67 and the detection sensitivity for CPF.227 Copyright © 2019, Elsevier. | ||
In addition to CQDs, Ti3C2 QDs are also excellent candidates for constructing fluorescent chemosensors due to their inherent hydrophilicity and conductivity originating from 2D MXene. Moreover, they offer advantages such as abundant active sites, enhanced redox activity, and excellent stability. Studies indicated that microcystin-LR (MC-LR) can inhibit the growth and reproduction of bacteria, aquatic animals, and some phytoplankton in water bodies, and thus the effective detection of MC-LR is very important. Yan et al. developed a self-powered aptasensing platform for the detection of MC-LR, which utilized a dual-mode approach combining photoelectrochemical (PEC) and photofuel cell (PC) techniques (chemosensor 137).225 The platform utilized an In2O3–In2S3–Ti3C2 (IO–IS–TC) composite assembled on the base of MOF-derived In2O3 hollow tubulars, with Ti3C2 QDs serving as a photoelectrode matrix to enhance the PEC performance. Aptamer-based recognition was employed for the efficient capture of MC-LR molecules, enabling highly sensitive detection. The PC aptasensor exhibited a detection range of 5–50 × 105 pmol L−1 and LOD of 17.4 pmol L−1, while the PEC aptasensor achieved a detection range of 0.5–4 × 105 pmol L−1 and LOD of 0.169 pmol L−1 (Fig. 37b). This work established a foundation for the formulation of photoelectrode-centric PFC applications, significantly enhancing the scope for the establishment of highly sensitive PEC environmental analytical methodologies.
Phosphorescence, with its longer lifetime compared to fluorescence, is a relatively uncommon luminescent phenomenon. This unique characteristic allows the examination signal to successfully counteract the interference of the matrix autofluorescence and disseminated light.226 ZnS QDs have attracted substantial interest and research emphasis with favorable phosphorescent emission characteristics, which are widely used as phosphorescent chemosensors for detecting pollutants in the living environment of plants. For example, a pioneering phosphorescent sensor was engineered utilizing a technologically advanced core–shell structure, Mn:ZnS QD@ZIF-8@MIP (chemosensor 138), for the highly sensitive and selective detection of CPF.226 Under the optimal operating parameters, 138 displayed the rapid detection of CPF within 5 min. It also exhibited a consistent, linear correlation over a concentration scope extending from 0 to 80 μM. Besides, the recoveries of CPF ranged from 92% to 105% in water samples with an LOD of 0.89 μM. This sensor combined the benefits of phosphorescent emission and molecular imprinting, significantly reducing the potential interferences from competing substances, scattered light, and background fluorescence. This innovation lays the foundation for the sensitive and selective detection of water pollutants using molecularly imprinted phosphorescent sensors. Another study prepared a novel and highly fluorescent MOF-based host–guest hybrid system (ZnSQD@ZIF-67) for the detection of Cu2+ by incorporating polyethylene glycol (PEG)-coated ZnS QDs in ZIF-67 (chemosensor 139).227 The PEG-ZnS QD@ZIF-67 nanohybrids combined the accumulation effect of ZIF-67 and ZnS QDs showed a highly selective and sensitive fluorescence response to specifically detect Cu2+. This allowed the detection of Cu2+, spanning a broad concentration range of 3–500 nM with an LOD of 0.96 nM (Fig. 37c). Compared to fluorescent sensors based on QDs without MOFs, nanohybrid 139 exhibited a significant enhancement in fluorescent QY. Therefore, ZnS QD-based sensors have shown substantial potential towards the comprehensive evaluation of the health and surroundings of plants.
However, although QDs have attracted significant attention due to their robust photobleaching stability, broad excitation range, and enhanced photostability, there are still some challenges that need to be addressed, as follows: (i) occasionally, it becomes imperative to modify QDs to enhance their stability and luminescent QY when coated within MOFs. Concurrently, the inclusion of an encapsulating agent during modification can efficaciously counteract the aggregation of QDs, although it may introduce complexities in the operational process. (ii) Inorganic semiconducting QDs routinely incorporate hazardous substances such as cadmium and lead, which can pose toxicity concerns when utilized for biological detection. (iii) Studies on QDs@MOF fluorescence chemosensors for biological detection are still lacking.
Rhodamine B (RhB) is the most widely used fluorescent dye for constructing luminescent chemosensors to detect anions, cations and pesticide residues in the environment. For instance, a novel RhB@Zr-MOF composite exhibiting dual-emission capabilities was developed (chemosensor 140).228 This unique construct was attributed to the interaction between the luminescent host Zr-MOF and the fluorescent guest RhB, enabling it to specifically recognize Fe3+ and nitenpyram. The introduction of RhB greatly improved the thermal stability, recyclability and low LOD (Fe3+ was 1.6 μM and nitenpyram was 0.2 μM) for sensing various analytes. Li et al. successfully designed a dual-emission fluorescent sensor by introducing RhB in the framework of HNU-48 (named RhB@HNU-48, chemosensor 141).229 The addition of RhB led to a significant improvement in the sensing sensitivity of alachlor, achieving an ultra-LOD of 0.59 ppb. This enhancement was due to the host–guest interactions, which influenced the excitation and emission spectra of the composite (Fig. 38a). This work was the first report on the use of a dual-emission fluorescence probe for the detection of alachlor, and the detection capability of RhB@HNU-48 is higher than that of most sensors reported thus far. Furthermore, the practical application of this sensor was confirmed through its sensing performance in river and soil samples. This study created a logic gate using the sensing ability of RhB@HNU-48 to assess alachlor residue safety levels. Importantly, it presented a promising strategy for developing tailored sensors for agricultural residues. Li et al. developed an orange-yellow fluorescence sensor assembled by in situ-encapsulating rhodamine B (RhB) in a zirconium-biquinoline-based MOF (chemosensor 142).230 This approach effectively prevented the aggregation fluorescence quenching (ACQ) effect of RhB molecules within the RhB@Zr-MOF structure. The efficient electron transfer in RhB@Zr-MOF significantly enhanced its sensitivity in detecting Cr2O72− and CrO42− ions, achieving ultra-LODs of 6.27 and 5.26 ppb, respectively. Compared to the pristine Zr-MOF, the sensitivity of RhB@Zr-MOF increased by approximately 6 and 9 times for Cr2O72− and CrO42− ions, respectively (Fig. 38b). The aggregation fluorescence quenching (ACQ) effect of RhB molecules was effectively overcome. Importantly, RhB@Zr-MOF exhibited a rapid fluorescence quenching response toward Cr(VI) ions with high selectivity, sensitivity, and anti-interference resistance. This research offers a new approach for modulating the fluorescent detection sensitivity and photochemical removal efficiency for heavy metal ions and dyes. In addition to RhB, some other fluorescence dyes including coumarin, curcumin and doxorubicin have also attracted attention from researchers. The inclusion of 7-diethylamino-4-methyl coumarin (C460) within the pores of MOFs effectively mitigated the aggregation-induced quenching of dyes, without altering the original structure of the MOFs. For example, Liu et al. designed a dye-encapsulated zoterephthalate MOF-structured fluorescent chemosensor (143), specifically engineered for precise dufulin recognition.231143 exhibited excellent sensitivity and linearity for the detection of dufulin and demonstrated a robust concentration-response relationship in the range of 0 to 5 μg mL−1 with an LOD of 2.96 ng mL−1 (Fig. 38c).
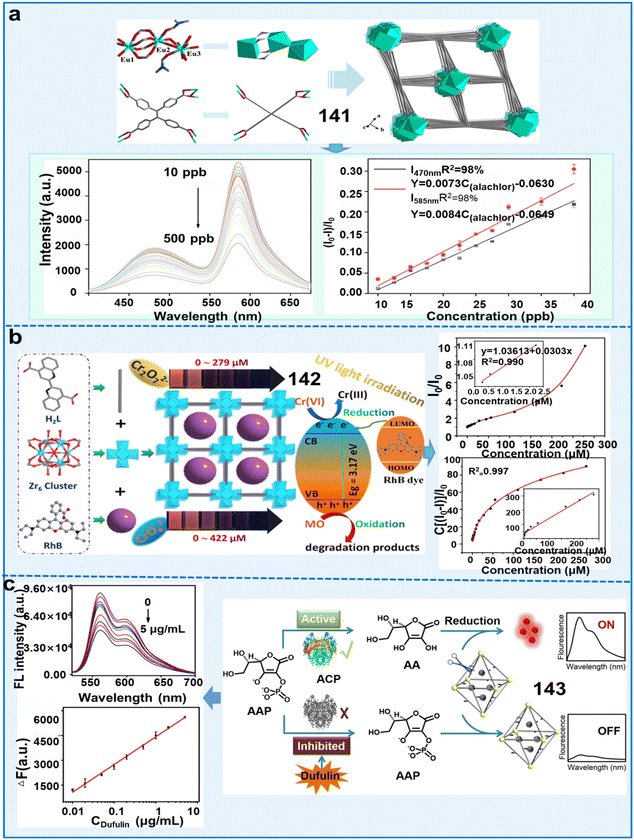 | ||
| Fig. 38 Examples of organic dye@MOF-based fluorescent sensors for monitoring the living environment of plants. (a) Process for the synthesis of RhB@HNU-48 chemosensor and its detection sensitivity for alachlor.229 Copyright © 2022, ACS Appl. Mater. Interfaces. (b) Detection mechanism of RhB@Zr-MOF composites for sensing ions of Cr2O72− and CrO42−.230 Copyright © 2022, ACS Appl. Mater. Interfaces. (c) Process for the synthesis of DMOF chemosensor and its detection sensitivity for dufulin.231 Copyright © 2022, Elsevier. | ||
Importantly, 143 was successfully employed for examining the content of dufulin in cucumbers, polished rice, soil, and water. This investigation clearly demonstrated the practicality and untapped potential of MOF-derived nanosensors for detecting pesticides in the environment and food. Additionally, this work also opens up an avenue for the design of enzyme-based probes for pesticide sensing in environmental assessment and food safety. Zhong et al. synthesized a curcumin@MOF-5 composite via two methods (chemosensor 144),232 which showed highly selective fluorescence enhancement responses to Al3+ with LODs of 3.10 μM and 2.84 μM for A-curcumin@MOF-5 and B-curcumin@MOF-5, respectively.
In conclusion, multiple fluorescent dyes have been used for the fluorescence detection recently; however, some limitations hinder their applications, as follows: (i) organic dyes frequently exhibit aggregation behavior, leading to a significant reduction in their luminescent intensity; (ii) the parameters including pH, temperature, and other substances modulate the fluorescent stability of organic dyes; and (iii) although introducing them in MOFs can improve their performance, studies on the detection of substances related to plant health are still lacking.
Among the above-mentioned metal nanoclusters, gold nanoclusters (AuNCs) have attracted tremendous interest owing to their distinctive properties and high application value in biosensing. For example, ZIF-8 was utilized to enwrap both CDs and gold nanoclusters (CDs/AuNCs@ZIF-8), subsequently producing an aggregate for the ratiometric fluorescence determination of Hg2+ (chemosensor 145).233145 enabled the highly sensitive detection of Hg2+, where the fluorescence intensity ratio (I640/I440) exhibited a distinct decreasing trend with an increase in the concentration of Hg2+ in the concentration range of 3 to 30 nM, achieving an LOD of 1 nM. In another study, AuNCs were synthesized within a Zr-MOF (AuNCs@UiO-66) for monitoring the concentration of Hg2+ in water (chemosensor 146).234 This nanocomposite exhibited exceptional sensitivity for detecting Hg2+ in real water samples, achieving an LOD of 193 pM. Compared with the above-mentioned chemosensor, this composite showed higher detection sensitivity. 146 with stable and high fluorescence could be easily fabricated using a simple method. Its properties make it valuable not only for detecting various metal ions but also for applications in the field of biology. Additionally, Cai et al. successfully constructed a dual-signal chemosensor of AuNCs@ZIF-8 for the detection of organophosphorus pesticides (chemosensor 147).235147 exhibited strong fluorescence with a fluorescence lifetime and QY of 6.83 μs and 4.63%, respectively. The MOF carrier restricted the molecular mobility of AuNCs, leading to the manifestation of the AIE effect. As a result, the composite exhibited a robust fluorescence signal, characterized by a fluorescence lifetime of 6.83 μs and QY of 4.63%. In addition, an innovative smartphone application (app) was designed to ensure real-time monitoring of pesticide contamination. The gray values of the solution were determined using a smart phone app, subsequently yielding calculated recoveries of OPs in the range of 85.2% to 113.8% (Fig. 39a). Compared to conventional single-signal biosensors, this chemosensor exhibited higher sensitivity and reliability. The reasons for this improvement are as follows: (1) encapsulating AuNCs with an AIE effect into ZIF-8 restricts their molecular movement and amplifies their fluorescence signal. (2) By inhibiting the enzymatic hydrolysis product, H2O2, using the decomposition effect of H2O2 on ZIF-8 and the peroxidase mimic activity of AuNCs, a dual signal detection is achieved through fluorescence and color changes. Furthermore, a colorimetric test paper was developed for the visual detection of OPs. Additionally, the gray values of the solution were obtained using a smartphone app for the on-site quantitative detection of OPs with an LOD of 0.4 μg L−1.
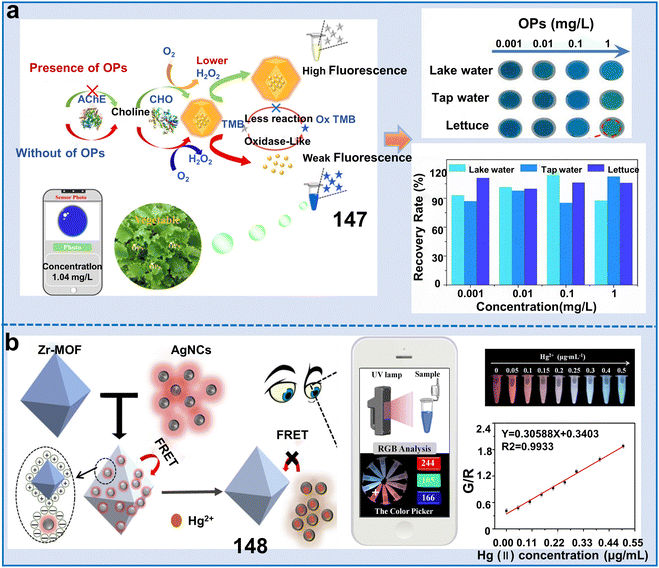 | ||
| Fig. 39 Application of metal nanocluster@COF-based sensors for monitoring the living environment of plants. (a) Schematic diagram of the synthesis of AuNCs@ZIF-8 and the mechanism for the detection of OPs.235 Copyright © 2022, ACS Appl. Mater. Interfaces. (b) Schematic diagram of the synthesis of Zr-MOF sensor and its mechanism for the detection of Hg2+.236 Copyright © 2022, Elsevier. | ||
This method exhibited higher sensitivity and reliability compared to traditional single-signal biosensors. Besides AuCNs, AgNCs, CuNCs and LaNCs have also gained attention for fluorescence imaging. For instance, a dual-emission ratiometric fluorescence chemosensor (148) based on Zr-MOF and AgNCs was constructed for the accurate and intuitive detection of Hg2+.236 Upon exposure to Hg2+, the AgNCs in the chemosensor underwent aggregation, causing a decrease in fluorescence. In contrast, the fluorescence intensity exhibited by Zr-MOFs demonstrated a slight increase because of the diminished FRET interaction between AgNCs and Zr-MOFs. This sensor demonstrated excellent sensitivity in the concentration range of 0.010 to 0.5 μg mL−1, achieving an LOD of 1.8 μg L−1 (Fig. 39b). This simple, fast, and highly sensitive fluorescence probe provides a promising approach for assessing the safety of food contaminated with Hg2+. Another study synthesized a new dual-emission fluorescent sensor (chemosensor 149) CuNCs@Tb@UiO-66-(COOH)2 for the detection of Cu2+.237 The fluorescence ratio and Cu2+ concentration showed a good linear relationship when fitted in the range of 1–30 μM, with an LOD of 178 nM. This study offers a novel approach to design multifunctional ratiometric fluorescent sensors using a one-pot method with MOFs as support materials. Abdelhamid et al. synthesized a series of new isostructural lanthanide-based MOFs based on the trimolecular linker of 2,4,6-tri-p-carboxyphenyl pyridine (H3L2), together with La3+ as their select metal cluster (chemosensor 150).238 The fluorescence emission intensity exhibited a linear decrease in the concentration range of Fe3+ from 16.6 to 167 μM, and the Stern–Volmer analysis further verified linearity and revealed an LOD of 16.6 μM. This study introduced a novel platform with potential applications in biosensing. Therefore, NC-based fluorescent sensors possess great efficiency and performance for the detection of metal ions and pesticide residues, but only a few types of metal ions has been reported for designing fluorescent sensors currently. Moreover, further endeavors are necessary to enhance the sensitivity and selectivity of materials for improved performance in future applications.
Recently, only one study reported the function of perovskite@MOFs for the identification of substantial heavy metal ions. Subsequently, this research disclosed an innovative methodology that was pioneering in incorporating bisected inorganic perovskite nanocrystalline components in lanthanide MOFs, which constructed a ratiometric fluorescent chemosensor (151) for the determination of Hg2+ in aquatic medium (Fig. 40).239151, consisting of CsPbBr3@Eu-BTC, exhibited exceptional stability and fluorescence output in water. When exposed to Hg2+, the fluorescence of CsPbBr3 emitting at 520 nm was instantaneously depleted via an electronic transfer mechanism. However, the red emission from Eu-BTC peaking at 616 nm remained unaffected. Meantime, this color change was easily observed by the naked eye, providing a visual indication of the existence of Hg2+. The CsPbBr3@Eu-BTC fluorescent chemosensor could rapidly, sensitively and selectively detect Hg2+ and exhibited a linear concentration range of 0 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) μM and impressive LOD of 0.116 nM. Therefore, introducing perovskites in MOFs effectively improves the analytical proficiency of MOF-based sensors, but studies on the design and application of perovskite-based MOF fluorescent sensors are still lacking.
μM and impressive LOD of 0.116 nM. Therefore, introducing perovskites in MOFs effectively improves the analytical proficiency of MOF-based sensors, but studies on the design and application of perovskite-based MOF fluorescent sensors are still lacking.
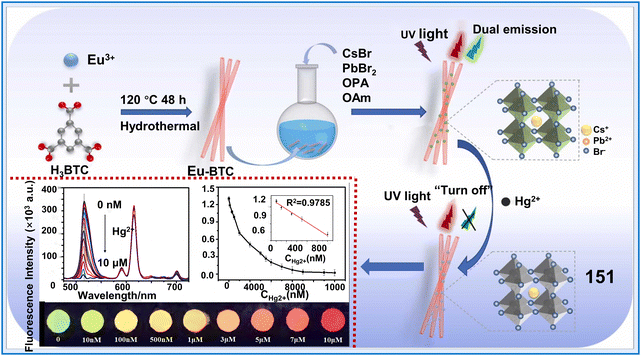 | ||
| Fig. 40 Application of CsPbBr3@Eu-BTC for the visualization of Hg2+.239 Copyright © 2022, Elsevier. | ||
In summary, the application of perovskite-containing MOF materials for fluorescence sensing is still somewhat restricted owing to a few key challenges, as follows: (i) the strategies for combining and incorporating perovskite nanocrystals with luminescent MOFs are still complex and not fully understood and (ii) the primary obstacle hindering the practical sensing applications of perovskite@MOF fluorescent sensors in water is their poor fluorescence and water stability.
4.3 Covalent organic frameworks (COFs)
COFs have become attractive materials for fluorescence sensing due to their high fluorescence QY, extendable framework, and excellent chemostability and thermostability.246 The luminescence properties of COFs are determined by the presence of abundant π-conjugation structures within their skeleton, which are created through a broad array of chemical bindings and affinities resulting from the unique monomer constituents of the material. The diverse functional groups in COFs as chromophores also have potential to promote their luminous efficiency.247 Nevertheless, the poor intrinsic conductivity and weak electrocatalysis of most COFs obviously limit their application. Thus, to amplify the sensitivity of COF-based materials, various desirable modifications have been implemented, including metal encapsulation, incorporation of carbon-based nanomaterials, and the fabrication of COF composites with other porous nanostructures.248 According to previous reports, COF-based fluorescent sensors have made great achievements in detecting pesticide residues, anions and cations in the living environment of plants (Fig. 41). In the following parts, we elaborate the applications of functional COF-based fluorescent chemosensors including various linkage types of COFs and COF-based composites in the detection of plant-related aspects (Table 9). | ||
| Fig. 41 Classification of functional COF-based sensors and their application in the detection of pesticide residues, metal ions and plant disease monitoring. | ||
| Classifications | Chemosensors | Name | Response range | Limit of detection (LOD) | Target analytes | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Special linkage types of COFs | 152 | IC-COF | — | 0.610 μM | PO43− | Detection of PO43− in water | 249 |
| 153 | TFPPy-TPh-COF | 0.01–1000 ng mL−1 | 2.44 pg mL−1 | Malathion | Monitoring the concentration of malathion in pakchoi, lettuce, and apples | 250 | |
| 154 | Triazine-COF | 0.1–10 μM | 96.0 nM | Glyphosate | Detecting the distribution of glyphosate in water and soil | 251 | |
| 155 | Bth-Dma COF | 0–100 μM | 0.170 μM | Fe3+ | Monitoring the concentration of Fe3+ in water | 252 | |
| 156 | sp2-carbon-linked COF | 0–150 μM | 1.43 μM | Cu2+ | Monitoring the concentration of Cu2+ in water | 253 | |
| 157 | Bpy-sp2c-COF | 0–20 nM | Hg2+: 2.42 nM, Ni2+: 2.73 nM, Cu2+: 1.47 nM, Co+: 2.20 nM | Hg2+, Ni2+, Cu2+, Co+ | Detection of various ions including Hg2+, Ni2+, Cu2+, and Co+ in water | 254 | |
| COF-based composite fluorescent sensors | 158 | AuNPs@COF | 10–800 ng mL−1 | 0.610 ng mL−1 | OPs | Monitoring the concentration of OPs in food and water | 255 |
| 159 | MnO2@COF | — | 0.0632 and 0.108 ng mL−1 by two treatments | Chlorpyrifos | Detecting the residue of chlorpyrifos in water and vegetables | 256 | |
| 160 | CDs@TDCOFs@PNIPAM | 5–400 μg L−1 | 4.88 μg L−1 | Pyrethroids | Monitoring the pyrethroids concentration in cabbage, cauliflower, and apple | 257 | |
| 161 | CDs@COF | 10–100 μg L−1 | 0.750 μg L−1 | Hg2+ | Detection of Hg2+ in water | 258 | |
| 162 | Dye/MOFs@COFs | 0.1–1 mg L−1 | — | VOCs | Monitoring the VOCs from wheat scab | 259 |
Imine-type COFs, known as “Schiff bases”, are synthesized through an esterification response between aldehydes and amines.248 This reaction leads to the formation of the Schiff base functionality (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), which is characteristic of imine linkages in the COF structure. This chemical union imparts noticeably high stability and robustness to the ensuing framework, presenting imine-type COFs with potential usage in the detection of pesticides and metal ions.
N–), which is characteristic of imine linkages in the COF structure. This chemical union imparts noticeably high stability and robustness to the ensuing framework, presenting imine-type COFs with potential usage in the detection of pesticides and metal ions.
For example, a luminescence sensor was engineered by fabricating a novel imine-bonded conjugated COF (IC-COF) through the reaction between 2,4,6-tris(4-formylphenoxy)-1,3,5-triazine and 1,5-diaminonaphthalene (chemosensor 152). 152 demonstrated remarkable selectivity towards PO43− and an LOD of 0.61 μM (Fig. 42).249 Furthermore, the exceptional recycling capability of IC-COF display substantial potential for reutilization without a discernible degradation in performance (5.2% decrease post 5 recycling cycles). The persistent issue with imine-based COFs is the propensity for nonradiative transition in response to π–π stacking interactions, giving rise to the aggregation-caused quenching (ACQ) effect. This triggered a reduction or complete cessation of COF luminescence emission.250 Thus, to solve this problem, Song et al. synthesized an electrochemical luminescent material with properties to mitigate the ACQ event. Subsequently, they synthesized an imine-linked COF (TFPPy-TPh-COF) with high electrochemiluminescence (ECL) emission and the capability of eliminating the ACQ effect and further constructed an ECL sensor for the detection of malathion (chemosensor 153). The efficient quenching of ECL was achieved by electrochemiluminescence resonance energy transfer (ERET) from the excited state of TFPPy-TPh-COF to zeolite imidazolate framework-8 (ZIF-8) and the steric hindrance of ZIF-8. 153 exhibited an extremely low LOD of 2.44 pg mL−1 together with a broad dynamic concentration range of 0.01 to 1000 ng mL−1. It could be effectively employed for the detection of other OPs (e.g., paraoxon, methidathion, and chlorpyrifos) and utilized for the measurement of malathion in apples, lettuce, and pakchoi. This research offers a new opportunity for the synthesis of ECL materials with high performance and the development of new ECL sensors for the detection of various pesticides.
 | ||
| Fig. 42 Precipitation methodology for IC-COF and the influence of phosphate ions on its fluorescence properties.249 Copyright © 2023, the American Chemical Society. | ||
Besides, triazine-type and hydrazone-linked COFs possess several remarkable properties such as chemical stability, good thermal stability, and high nitrogen content. These properties make them promising for various applications, particularly fluorescence detection in the study of plant-environment interactions. For example, a study constructed a triazine-COF-derived electrochemical chemosensor (154), which was designed to facilitate specific non-covalent interactions with glyphosate (GLY) for detecting GLY.251154 exhibited a wide linear operation range of 0.1 μM to 10 μM of glyphosate and demonstrated an LOD of 96 nM and a limit of quantification (LOQ) of 320 nM. This work shows significant potential for further improvements and commercialization as a versatile disposable sensing platform specifically designed for the detection of glyphosate in water and fruits. Chen et al. designed and successfully synthesized two stable crystalline hydrazone-linked COFs containing functional O,N,O′-chelating sites by Schiff-base condensation reactions (chemosensor 155) (Fig. 43).252 The as-synthesized Bth-Dma COF exhibited strong fluorescence in both the solid state and aqueous suspension and demonstrated exceptional sensitivity for detecting Fe3+ in water with an LOD of 0.17 μM. The recognition process can be attributed to the coordination interaction with Fe3+. This groundbreaking study introduced the first instance of utilizing a robust luminescent COF material with pre-designed O,N,O′-chelating sites for the detection of Fe3+. This research may lay the foundation for developing luminescent COF sensors with targeted binding sites to detect specific metal ions. Therefore, triazine-type and hydrazone-linked COFs have shown great efficiency for the detection of ions and pesticides in the environment, but related studies on their applications in the monitoring of the health and environment of plants are still lacking.
 | ||
| Fig. 43 Schematic illustration of the designed synthesis of Bth-Dha and Bth-Dma COFs, as well as their functional O,N,O′-chelating sites.252 Copyright © 2019, the American Chemical Society. | ||
Additionally, COFs with sp2-carbon chains exhibited commendable photoelectric attributes that make them effective in the fields of luminescent material production and chemical detection systems. Notable, they have been harnessed for plant habitat monitoring purposes also. For instance, Yan et al. constructed an AIE-active unit-containing and sp2-carbon-linked COF (chemosensor 156).253 The conjugated backbone of sp2-TPE-COF, interlinked by olefinic bonds, functioned as a highly informative sensor for detecting metal ions. 156 significantly improved the dispersion of COFs and finely modulated their sensitivity towards Cu2+, resulting in a remarkably increased Stern–Volmer constant (Ksv) value of 15.15 × 104 m−1 (Fig. 44a). The present investigation underscored the considerable promise inherent in employing surfactant-modulated, extensively π-conjugated COFs for sensor technology applications. This work can open up opportunities for creating more fully conjugated COFs to detect a wider range of water-soluble toxic analytes. Zhu et al. reported a fully π-conjugated COF with dual binding sites, Bpy-sp2c-COF, which demonstrated commendably swift fluorescence recognition and substantially amplified adsorption tendencies toward divalent heavy metal ions (chemosensor 157) (Fig. 44b).254 XPS analysis confirmed the concurrent coordination between metal ions and both cyano and bipyridine groups in Bpy-sp2c-COF, leading to its efficient fluorescence recognition and adsorption capabilities for Cu2+, Hg2+, Ni2+ and Co2+. The fully π-conjugated COF, demonstrating dual ligand binding sites, offered a promising and innovative method for formulating sensors possessing exceptional attributes. At present, exploration into robustly π-conjugated COFs for fluorescent sensing and contaminant remediation remain scarce, primarily owing to their intricate synthetic procedures, which typically result in relatively modest success rates. Thus, further efforts should be focused on sp2 c-COFs and their versatile applications.
 | ||
| Fig. 44 Application of sp2-carbon-linked COFs for monitoring the living environment of plants. (a) Synthesis of sp2-TPE-COF, copper ion detection, and its electron transfer process during copper ion sensing with sp2-TPE-COF.253 Copyright © 2022 Wiley-VCH GmbH. (b) Schematic illustration of Bpy-sp2 c-COF for the detection and removal of divalent heavy metal ions.254 Copyright © 2022, Elsevier. | ||
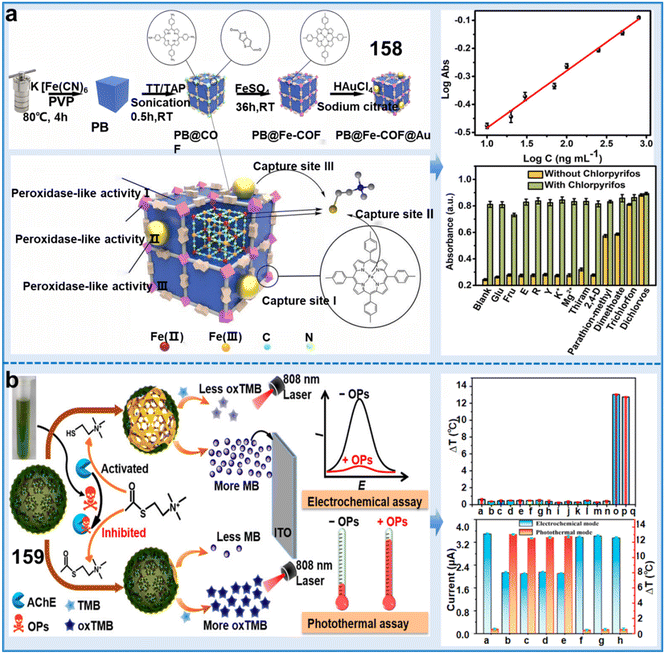 | ||
| Fig. 45 Fluorescence sensors based on metal and metal oxide NP composites for the detection of OPs in the environment. (a) Synthesis of PB@Fe-COF@Au composite and its diagrammatic sketch of triplet peroxidase-like active sites and multiple capture sites.255 Copyright © 2023, the American Chemical Society. (b) Synthesis of the COF/MB@MnO2 composite and its detection mechanism, selectivity and anti-interference capacity of OPs (from a to p: blank, Fe2+, Fe3+, Cu2+, Pb2+, Cd2+, Hg2+, As5+, glucose, methionine, arginine, glycine, citric acid, thiamine nitrate, chlorpyrifos, and a mixture of all the above-mentioned substances, respectively).256 Copyright © 2023, the American Chemical Society. | ||
Besides, metal oxide MnO2 NPs have also been introduced in COFs to improve their catalytic efficiency due to their expansive surface area, substantial adsorptive potential and superior biocompatibility. For example, Wen et al. developed a cutting-edge COF/methylene blue@MnO2 (COF/MB@MnO2) composite, which demonstrated superior signal amplification, a unique binding entity, and robust oxidase-mimicking activity (chemosensor 159).256 In the absence of OPs, the MnO2 coating on the surface could detect thiocholine (TCh) due to the enzymatic breakdown of acetylthiocholine (ATCh), catalyzed by acetylcholinesterase (AChE). The inhibitory activity of OPs against AChE and their impact on the generation of TCh provided the basis for the accurate detection of chlorpyrifos. The detection could be achieved through complementary electrochemical and photothermal measurements, with LODs of 0.0632 ng mL−1 and 0.108 ng mL−1, respectively. Moreover, this sensor demonstrated exceptional selectivity and robust anti-interference capability for the precise detection of chlorpyrifos in diverse environmental and biological samples, effectively distinguishing it from interfering substances (Fig. 45b). This study offered a dual-mode detection method for OPs that is convenient and suitable for on-site testing in various scenarios. Additionally, it broadened the practical utilization range of COF-derived multifunctional composites for application in multimodal sensing systems. Therefore, introducing metal oxides into MOFs is another effective strategy to improve the detection efficiency.
COFs employed together with carbon nanomaterials can avoid the lack of electron conductivity. However, only a few studies reported the combination of CDs in COFs to detect pesticide residues and heavy metal ions in the environment. CDs, as quasi-spherical fluorescent nanoparticles based on carbon, possess excellent chemical stability, abundant low-cost sources, and strong resistance. These attributes make them highly promising for selective detection applications. However, the nanoscale size of CDs leads to intrinsic self-aggregation, resulting in the quenching of AIE.257 Therefore, to prevent self-aggregation, a viable and intelligent approach is to encapsulate CDs within the channels or pores of COFs. For example, Zhang et al. created a fluorescent composite called CDs@TDCOFs@PNIPAM by encapsulating CDs within 1,3,5-tris(4-formylphenyl)benzene (TFB)/2,5-dihydroxyterephthalohydrazide grafted with thermally responsive poly(N-isopropylacrylamide) (PNIPAM) (CDs@TDCOFs@PNIPAM, chemosensor 160) (Fig. 46).257160 demonstrated a temperature-reactive “on/off” detection module for pyrethroids through target-catalyzed electron exchange. The model manifested an expansive linear measurement range of 5 to 400 μg L−1 with an LOD of 0.69 μg L−1. Concurrently, CDs@TDCOFs@PNIPAM, equipped with red, green, and blue (RGB) fluorescent emissions, was combined with a smartphone-assisted apparatus, facilitating the visually discernible and astute quantification analysis of pyrethroids, exhibiting an LOD of 4.875 μg L−1 (Fig. 46). Another study designed and synthesized an innovative composite of imine-bonded COF doped with CDs for the analytical monitoring of Hg2+ in environmental water (chemosensor 161), which exhibited superior sensitivity and discriminative fluorescence response to low concentrations of Hg2+ in water.258 The analytical threshold of the sensor was in the range of 10 to 100 μg L−1, presenting an LOD of 0.75 μg L−1. This cost-effective and simple-to-construct fluorescent COF offers promising prospects for the precise detection and efficient removal of diverse pollutants present in irrigation water.
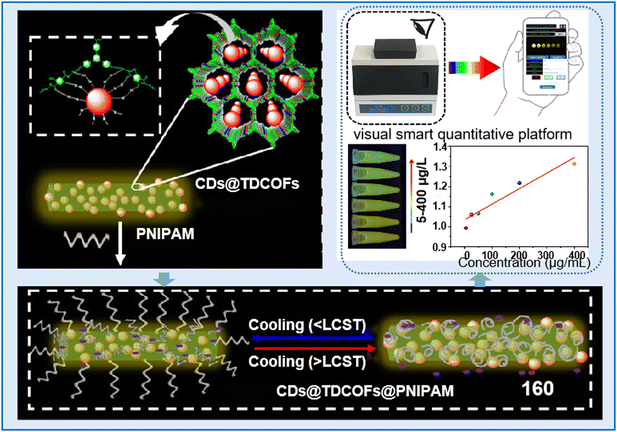 | ||
| Fig. 46 Process for the synthesis of CD@TDCOF@PNIPAM composite and CD@TDCOF@PNIPAM-based smartphone readout analysis of LCY in real samples.257 Copyright © 2022, the American Chemical Society. | ||
MOFs are traditionally known as a family of structurally aligned substances affiliated with COFs, exhibiting comparable essential characteristics to COFs such as high surface area, tunable accessibility and extended crystalline structure. COFs possess strong covalent linkages, which endow them with the exceptional thermal and chemical stability vital for chemical detection under harsh conditions.261 Integrating MOFs with COFs presents a promising strategy to elevate the performance and expand the application scope of COFs.260 Currently, only one study reported an MOF@COF composite as a chemical fluorescence sensor to detect the substances related to plant health. Furthermore, the practical application of colorimetric sensor arrays still face challenges, including environmental humidity interference and insufficient sensitivity towards minute traces of target volatile organic compounds (VOCs).259 Thus, to address these challenges, Wang et al. developed a solution by creating dye/MOF@COF core–shell composites via the application of hydrophobic COF overlayers on top of MOFs loaded with dyes (chemosensor 162).259 The composite-based sensor, benefiting from the uniform dispersion of dyes across the porous MOF structure, exhibited enhanced sensitivity at sub-ppm levels for the detection of VOCs compared to conventional dye sensors. The developed dye/UiO@TPB-DVA-COF composites exhibited excellent sensing capabilities for the differentiation and detection of eight plant VOCs.
This array facilitated the precise and timely detection of wheat scab, even as early as one day after inoculation (Fig. 47). This study suggested that MOF@COF composites offer a versatile platform for constructing colorimetric sensing arrays exhibiting exceptional humidity sensing capabilities and robustness. These arrays hold significant promise for practical application scenarios, particularly in monitoring the health of plants.
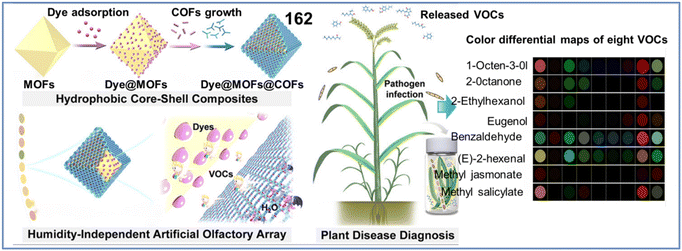 | ||
| Fig. 47 Schematic diagram of the synthesis of dye/MOF@COF core–shell composite and the detection of the released VOCs by pathogens for the diagnosis of diseases in plants.259 Copyright © 2022, the American Chemical Society. | ||
In summary, although considerable achievements have been made in the development of COF-based sensors, the synthesis of COFs and their composite counterparts encounters significant hurdles, as follows: (i) the exorbitant expense associated with precursor materials, together with the ecological risks posed by these substances and the organic solvents utilized throughout the process pose significant obstacles in their large-scale industrial production; (ii) enhancing the operational duration and performance of COF-modified electrodes is crucial, and there is a pressing need to develop materials that offer high loading efficiency and excellent conductivity; (iii) further innovation is required in the methods and means of functionalizing COFs; and (iv) their application range is still limited, especially in the field of monitoring plant health. Thus, designing COFs with better biocompatibility and adaptability is imperative to further expand the application scope of electrochemical sensors.
4.4 Nanoclusters
Nanoclusters (NCs), typically consisting of a single or multi-metal core with a diameter of less than 2–3 nm, have attracted significant attention due to their broad Stokes shifts, very intense fluorescence bands, good biocompatibility and strong PL.262 Additionally, NCs are known for their simple preparation and low toxicity, making them a preferred choice compared to other fluorescent materials such as toxic QDs and organic dyes.263 Nanoclusters can be categorized into single metallic clusters and bimetallic clusters based on the composition of species within their cores.262 Thus far, these NC-based fluorescent sensors have been widely used for monitoring the living environment of plants (Fig. 48). Here, we introduce the applications of single NCs, bimetallic NCs and NC-based fluorescent chemosensors in the above-mentioned aspects in detail (Table 10).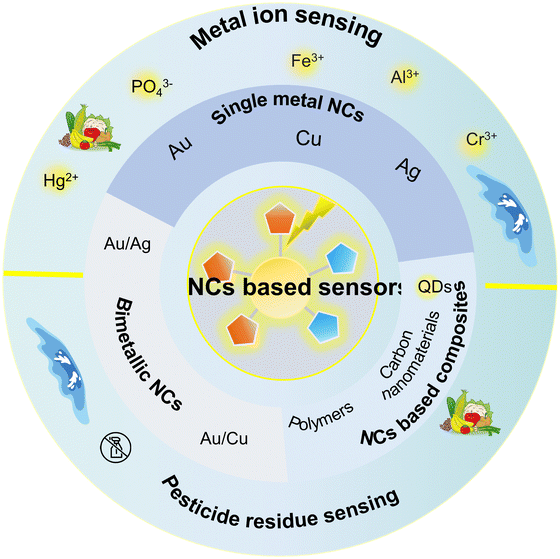 | ||
| Fig. 48 Classification and applications of NC-based sensors in plant-related environment monitoring. | ||
| Classifications | Name | Chemosensors | Response range | Limit of detection (LOD) | Target analytes | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Single NC-based sensors | Au NCs | 163 | 0.05–20 ng mL−1 | 0.0150 ng mL−1 | Methidathion | Detecting the methidathion residue in foods | 205 |
| 164 | 10–2000 ng mL−1 | 2.05 ng mL−1 | OPs | Monitoring the concentration of OPs in vegetables | 264 | ||
| 165 | Ag+: 0.050–1.00 μM | 17.0 nM for Ag+ | Ag+, Fe3+, Fe2+, Mn2+, Sn2+, Pb2+, and Hg2+ | Detection of metal ions in water | 265 | ||
| Fe3+: 0.100–25.0 μM | |||||||
| Sn2+: 0.100–10.0 μM | |||||||
| 166 | — | 3.20 μM | Fe2+ | Detection of Fe2+ in water | 266 | ||
| Cu NCs | 167 | — | 1.50 μM | Pb2+, Fe3+, Cu2+, Cd2+, Cr3+, Co2+, Ni2+, Zn2+, Ag+, Fe2+, Hg2+, and Al3+ | Detection of 12 metal ions in water | 267 | |
| Ag NCs | 168 | 0–10 ppm | 0.400 mg L−1 | Cu2+ | Monitoring the concentration of Cu2+ in foods | 268 | |
| 0–3.0 μM | 21.0 nM | Glyphosate | Detecting the glyphosate residue in lettuce and A. thaliana | 269 | |||
| Bimetallic based sensors | Au/Cu NCs | 169 | 50 nM–1 mM | 10.0 nM | Cr3+ | Detection of Cr3+ in water | 270 |
| 170 | Two linear range of 5.0 × 10−7–7.0 × 10−6 M and 7.0 × 10−6–1.0 × 10−4 M | 0.100 μM | Fe3+ | Detection of Fe3+ in water | 271 | ||
| 171 | Hg2+: 10–100 μM | 2.45 nM for Hg2+, | Hg2+ and Cu2+ | Monitoring the distribution of Hg2+ and Cu2+ in water and food | 272 | ||
| Cu2+: 0–50 μM | 1.92 nM for Cu2+ | ||||||
| NC-based composite-based sensors | CQDs@CuNCs | 172 | 10 to 500 nM for thiram, 5 to 100 nM for paraquat | 7.49 nM for thiram, 3.03 nM for paraquat | Thiram and paraquat | Detecting the occurrence and concentration of thiram and paraquat in water | 268 |
| N-GQDs | 173 | Cu2+: 8 × 10−8 mol L−1 × 10−6 mol L−1 | 4.12 × 10−9 mol L−1 for Cu2+, | Cu2+ and Cd2+ | Monitoring the concentration changes of Cu2+ and Cd2+ in food | 273 | |
| Cd2+: 1 × 10−6 mol L−1 × 10−5 mol L−1 | 9.43 × 10−7 mol L−1 for Cd2+ | ||||||
| Blue-emitting CDs | 174 | Cu2+: 0–600 nM | 5.00 nM for Cu2+, | Cu2+ and Hg2+ | Monitoring Cu2+ and Hg2+ in water | 274 | |
| Hg2+: 0–2000 nM | 7.00 nM for Hg2+ | ||||||
| WS2-QDs | 175 | 0.3 to 3 μM | 0.120 μM | Cu2+ | Detection of Cu2+ in water | 275 | |
| AuNC@nanogel | 176 | 0.625 to 125 ng mL−1 | 0.590 ng mL−1 | Chlorpyrifos | Monitoring the concentration of chlorpyrifos in food | 276 | |
| BiNCs@AB | 177 | 3.0 ng L−1 to 1.0 mg L−1 | 1.00 ng L−1 | Pb2+ | Detection of Pb2+ in water | 277 |
Gold nanoclusters (AuNCs), an exclusive subset of metallic NCs, demonstrate a marked Stokes shift, adjustable emission response, and commendable biosafety, which are emerging as a cutting-edge subclass of fluorescent materials for developing high-performance sensors.278 Fluorescent AuNCs have been successfully employed for the identification of various pollutants, including pesticide residues and metal ions in the living environment of plants. For example, Li et al. synthesized hydrolysate-response disulfide bond-functionalized gold nanoclusters (S-S-AuNCs, chemosensor 163).205 Remarkably, S-S-AuNCs exhibited a distinct response to low pH values and thiol compounds, enabling their application as highly efficient sensors for organophosphates (OPs). This sensing mechanism relied on the AChE-facilitated hydrolysis of acetylthiocholine in thiocholine and CH3COOH, with OPs inhibiting the activity of AChE (Fig. 49a). In addition, S-S-AuNCs were implemented for the meticulous examination of the distribution, residue and metabolization of methidathion within pakchoi. Another study also developed a novel dual-signal fluorescent sensor for detecting OPs in vegetables based on GSH-synthesized AuNCs (GSH-AuNCs, chemosensor 164).264164 is based on the catalytic activities of acetylcholinesterase (AChE) and choline oxidase (ChOx), which convert acetylcholine (ACh) into choline and generate hydrogen peroxide (H2O2). This process leads to increased cyan fluorescence due to aggregation. Besides a mobile application called “RGB Color Picker” was employed to visually detect organophosphates (OPs) by recognizing the RGB values of fluorescent colors associated with different OP concentrations. This sensor exhibited a linear dynamic range of 10–2000 ng mL−1 with a detection limit of 2.05 ng mL−1. This sensor demonstrated the ability to detect a wide range of organophosphates (OPs), but it lacked specificity in distinguishing individual types of OPs.
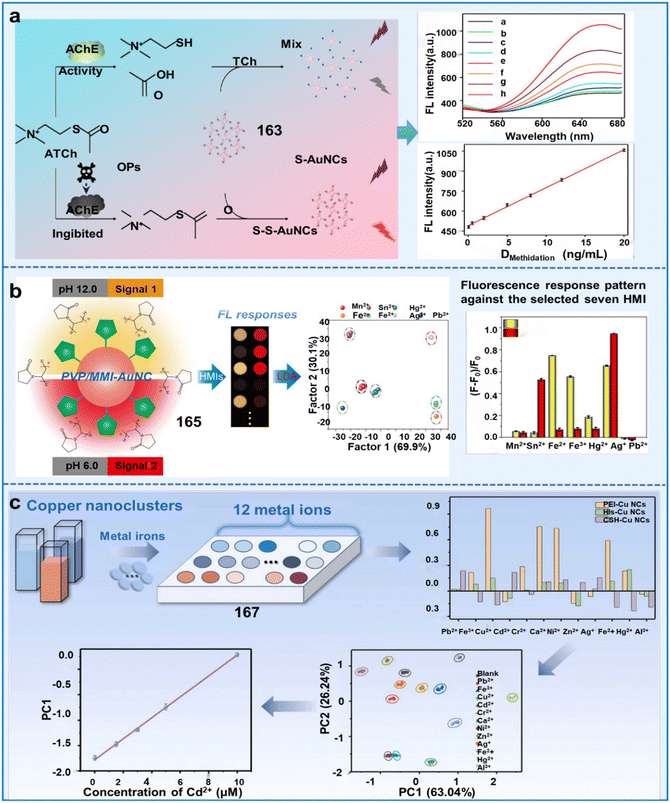 | ||
| Fig. 49 Single-metal NCs based fluorescent sensors used for monitoring the living environment of plants. (a) Sensing mechanism and application of S-S-AuNCs for detecting OPs.205 Copyright © 2021, the American Chemical Society. (b) Schematic illustration of the CuNC-based fluorescent sensor array for detecting different metal ions.265 Copyright © 2022, Elsevier. (c) Schematic representation of the PVP/MMI-AuNC-based fluorescent sensing system for identifying various HMIs.267 Copyright © 2021, Elsevier. | ||
Besides the detection of pesticides, several studies reported that AuNCs can also detect metal ions in the environment. For instance, Zhang et al. developed a simple fluorescent chemosensor (165) using a single AuNC chemosensor for the rapid discrimination of heavy metal ions (HMIs).265165 was synthesized by chemically reducing the gold precursor, HAuCl4, using 2-mercapto-1-methylimidazole (MMI) in the presence of polyvinylpyrrolidone (PVP). The resulting PVP/MMI-AuNC exhibited significant responsiveness towards diverse metal ions. This resulted in a distinctive fluorescent response at both wavelengths of 512 nm and 700 nm (Fig. 49b). The observed changes in fluorescence could serve as a unique fingerprint, enabling the accurate discrimination of metal ions. By utilizing this newly developed PVP/MMI-AuNC-based fluorescent sensor array as our primary receptor, we could detect and unequivocally identify numerous heavy metals such as Ag+, Fe3+, Fe2+, Mn2+, Sn2+, Pb2+, and Hg2+. Hada et al. achieved the successful synthesis of photoluminescent His-AuNCs, enabling the quantification of hazardous levels of Fe2+ in low-volume samples (chemosensor 166).266 Importantly, it exhibited high selectivity and accuracy for detecting Fe2+, achieving an LOD of 3.2 μM. Finally, the realized transportable sensor was ascertained for its functionality in authentically assessing the Fe2+ levels in water samples that had been spiked with 35 μM Fe2+. This portable sensing platform could monitor the concentration of heavy metals in water samples. Hence, AuNC-based sensors exhibit excellent selectivity and accuracy for detecting ions and pesticides in the environment and can monitor their toxicity level in the real environment.
In addition to AuNCs, CuNCs and AgNCs also have very intense fluorescence signals, which have been applied in the field of fluorescence detection.279 For example, Xu et al. developed the first CuNC-based fluorescent sensor array (chemosensor 167) to identify 12 metal ions (Pb2+, Fe3+, Cu2+, Cd2+, Cr3+, Co2+, Ni2+, Zn2+, Ag+, Fe2+, Hg2+, and Al3+) and various organic compounds, such as humic substances, lipids, fatty acids, amino acids, and lignans.267 These 12 metal ions could be detected at a concentration limit of 1.5 μM. Furthermore, the developed method allowed for the quantification of metal ions even at a low concentration of 0.83 μM (Zn2+) (Fig. 49c). This constitutes the pioneering fluorescent sensor array constructed with CuNCs for the sensitive detection of various metal ions in water. The array was also employed to distinguish seawater and riverine samples from various regions, showcasing its potential for water quality evaluation. Chen et al. accomplished the development of a dual-emission nanocomposite using His-AgNCs for the detection of Cu2+ (chemosensor 168).268 It showed high detection sensitivity, good selectivity and anti-interference, and LOD of 0.4 ppm. In addition, this work designed a polymer film to load a dual-emission nanochemosensor, which meant that the visual detection of Cu2+ is possible. This work indicates the potential application prospects of dual-emission fluorescent nanochemosensors in the analysis of the living environment of plants.269
Au-based bimetallic NCs are widely utilized for the detection of metal ions in soil and irrigation water. For example, Nie et al. prepared highly luminescent Cu/Au bimetallic nanoclusters (BNCs) by employing GSH for not only the reduction process but also as an enveloping shield, thus circumventing the aggregation of the synthesized nanoclusters (chemosensor 169).270 When excited at 380 nm, Cu/Au BNCs showed an emission peak at 450 nm. Selective quenching of Cu/Au BNCs by Cr3+ facilitated the implementation of an analytical approach benefiting from their broad linear dynamic range of 50 nM to 1 mM and superior sensitivity (LOD = 10 nM, S/N = 3). Shojaeifard et al. utilized the remarkable metallophilicity and PL properties of Au and Cu clusters to design a chemosensor based on bimetallic gold-copper nanoclusters (PA-AuCu bi-MNCs, 170).271 The fluorescence intensity of PA-AuCu bi-MNCs was selectively quenched in the presence of Fe3+, resulting in the hindrance of their emission. This work demonstrated an LOD of 0.1 μM and highlighted the independence from most interferences, making it an important feature of the study. Therefore, compared to single NCs, Au-based bimetallic NCs show high selectivity and anti-interference.
Additionally, using other materials for the immobilization of bimetallic NCs has gained great attention due to their improved fluorescence performance for the trace identification of metal ions and pesticides in food and water. For example, Wu et al. synthesized a ratiometric fluorescent system (chemosensor 171) by integrating AuAgNCs within electrospun cellulose acetate nanofibrous membranes (ENM). This strategy enabled the facile, rapid and visual detection of both Cu2+ and Hg2+ in Petri dishes under diverse conditions.272 It was successfully utilized for detecting amino acid L-histidine (His) sequentially, a pivotal stem that integrated His analysis with a smartphone platform for quantitative on-site monitoring. This sensor exhibited exceptional performance in detecting traces of Hg2+ and Cu2+, with LODs of 12.36 nM and 25.90 nM, respectively. The high selectivity of the AuAg-ENM system further enhanced its capability for monitoring and controlling the safety standard of Hg2+ and Cu2+ in multiple applications (Fig. 50). This developed system was validated using real environmental water samples, demonstrating its practicality for on-site detection using a smartphone with convenient operation.
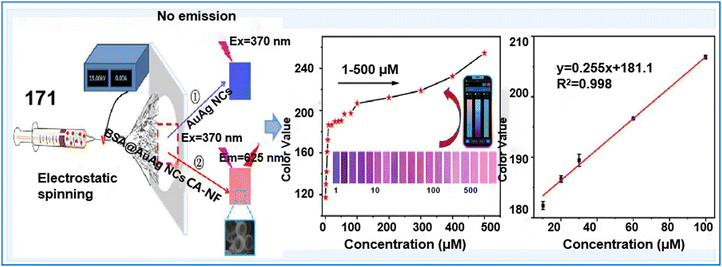 | ||
| Fig. 50 Schematic illustration of the fabrication of AuAg-ENM and its sensing process.272 Copyright © 2023, Elsevier. | ||
QDs have been introduced in NC sensors to improve their detection efficiency and sensitivity through their exceptional optical attributes, robust photochemical stability, prolonged fluorescence lifetime, low toxicity, and stable emission. For example, a ratiometric fluorescent sensing system (chemosensor 172) was constructed by leveraging red-emitted CuNC complexes and blue-radiating N-CQDs to effectively assess the concentrations of thiram and paraquat with LODs of 7.49 nM and 3.03 nM, respectively (Fig. 51a).269 At the same time, the ratiometric fluorescence probe achieved satisfactory recovery results, ranging from 82% to 114%, for actual food measurements. More interestingly, thiram and paraquat could be swiftly and visually detected via a smartphone-enabled colorimetric evaluation, offering an efficient methodology for the real-time monitoring of pesticide residues in fruits. Another study constructed a ratiometric fluorescence nanomixture of GQDs-AuNCs specifically for the quantitative analysis of Cu2+ and Cd2+ in fruits (chemosensor 173).273 Under the optimal conditions, the fluorescence intensity ratio (I565/I403) of the GQD-AuNC system showed a linear relationship with the concentration of Cu2+ or Cd2+ in the range of 8 × 10−8 M–6 × 10−6 M and 1 × 10−6 M–4 × 10−5 M, respectively. Also, its detection limits were 4.12 × 10−9 M and 9.43 × 10−7 M, respectively. When Cu2+ and Cd2+ were present, the paper-based visual sensor exhibited detectable fluorescent color changes, enabling rapid on-site detection. The findings suggest that the ratiometric fluorescence sensor holds great significance in food safety and biological analysis. Babaee et al. designed an innovative dual-emissive ratiometric nanohybrid chemosensor comprised of red-emitting (Ag/Au)@insulin NCs and blue-emitting CDs for the sensitive and selective ratiometric determination of Hg2+ and Cu2+ (chemosensor 174).274 The LODs for the determination of Cu2+ and Hg2+ were estimated to be 5 nM and 7 nM, respectively. Interestingly, the selectivity of this probe towards these two ions could be easily controlled by adjusting the pH of the probe solution without requiring any chelating agents. This unique feature allowed the selective detection of Cu2+ and Cd2+ ions by simply modifying the pH of the probe solution without the need for any additional reagents. Another study introduced a ratiometric fluorescent nanosensor (NCs/QDs, chemosensor 175) for the detection of Cu2+ ions by combining the stable optical properties of AuAgNCs and WS2-QDs.275 The addition of WS2-QDs could avoid the interference from Pb2+ and Cd2+ in the process of detecting Cu2+ due to their advantages of simple synthesis and stable luminescence properties. This sensor exhibits significant potential for detecting Cu2+ in river samples, offering great application potential in practical environmental monitoring. Therefore, QD-based NCs can achieve the fast and visual detection of ions and pesticides in the environment.
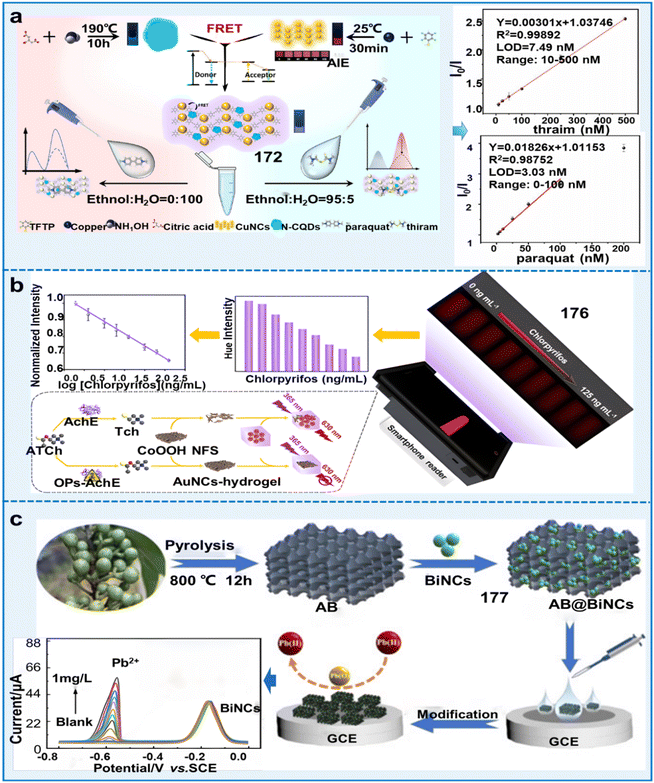 | ||
| Fig. 51 Applications of NC-based composite-based fluorescent sensors in monitoring the living environment of plants. (a) Schematic representation of the ratiometric fluorescent sensor for the detection of pesticides using N-CQDs@CuNCs.268 Copyright © 2023, Elsevier. (b) Schematic illustration of the portable fluorescent hydrogel test kit for the on-site quantitation of chlorpyrifos.276 Copyright © 2021, the American Chemical Society. (c) Process for the fabrication of the BiNC@AB composite sensor for the sensing of Pb2+.277 Copyright © 2021 The Authors. Published by the American Chemical Society. | ||
Hydrogels, as stable 3D networks with environmentally friendly properties, have gained considerable interest for immobilization and encapsulation purposes in sensor technology. Hydrogels, serving as water-holding networks, offer nanometer-scale porous structures that enable the passage of small molecules by providing physical encapsulation to trap nanoparticles.276 The effective integration of the excellent performance of hydrogels with NCs can greatly enhance the accuracy and stability of sensors. For example, Wang et al. developed a portable handheld platform integrated with smartphones for the on-site detection of OPs (chemosensor 176).276 Cobalt oxyhydroxide nanoflakes (CoOOH NFs) could effectively suppress the fluorescence originating from AuNC hydrogel systems through the FRET effect (Fig. 51b). By converting the fluorescent colors into digital information by using self-made auxiliary devices and smartphones, the on-site monitoring of the concentration of chlorpyrifos was achieved with an LOD of 0.59 ng mL−1. This sensor presented good selectivity, anti-interference, high sensitivity and capability due to the encapsulation of the hydrogel into specific AuNC signatures in practical analysis, offering huge potential for on-site applications.
Besides, a highly porous activated biochar (AB), exhibiting superior electrical conductivity, extensive surface area, and robust chemical stability, was selected as a support material to assist NCs in enhancing their excellent conductivity and electrocatalytic efficiencies. As demonstrated by Zou et al., a ratiometric electrochemical chemosensor (177) was constructed using the BiNC@AB-modified electrode to analyze Pb2+.277 BiNCs were capable of not only forming an alloy with Pb2+ to enhance the affinity of Pb2+ towards the electrode interface, but also generating reference signals crucial in the precise and accurate process of electrochemical detection using a ratiometric approach (Fig. 51c). The prepared sensor exhibited an excellent electrocatalytic performance towards Pb2+, with a satisfactory linear range and detection limit, which was attributed to the synergistic effect of these advantages. Therefore, AB as the support material can greatly improve the detection efficiency of NCs, but related studies are still limited.
In conclusion, despite the significant progress in the development of nanoclusters, many hurdles persist when advancing and endorsing the practical utilization of COFs for electrochemical analysis. (i) Reports regarding the utilization of metal nanoclusters as sensors for detecting Na+, K+, and Ca2+ ions are rare, mainly because of the suboptimal binding affinity displayed by the core of metal nanoclusters towards these ions; (ii) controlling the size regulation of nanoclusters remains challenging with the aim to enhance their photochemical attributes such as quantum efficiency and mitigate the complexities associated with post-synthesis purification tasks;280 (iii) the incomplete understanding of the internal assembly process involved in metal nanocluster synthesis, thereby exacerbating the intricacy associated with enhancing their photochemical attributes; (iv) primary utilization of nanoclusters typically occurs within water samples, as opposed to plant samples where further detection utilities still exist and demand exploration in this field.
5. Applications of fluorescent supramolecular chemosensors
To provide accurate information for managing plant health, fluorescent supramolecular chemosensors with the advantages of recyclability, high sensitivity, selectivity and adaptability are suitable for a wide range of on-site environments.281 To date, there are two types of fluorescent supramolecular materials, polymers and hydrogels, which are excellent tools for monitoring the health and living environment of plants (pesticides, ions and plant components). In the following section, we summarize and analyze the application of fluorescent supramolecular chemosensors in these aspects.5.1 Polymer systems
Fluorescent polymers are specific due to the properties of their fluorophores such as photovoltaic properties and non-covalent bonding that stimulate reactivity and reversibility.282 The non-covalent linkages affect the luminous characteristics of chromophores given that they have the ability to control the aggregation states and energy transfer through assembly-disassembly processes.283 Utilizing a variety of structures, polymer sensors have been widely used for various analytes, such as pesticides, metals, and toxins (Table 11). In the following sections, we discuss the application of conjugated fluorescent polymers, organometallic polymers, and molecularly imprinted polymers for monitoring the above-mentioned analytes (Fig. 52).| Classifications | Chemosensors | Name | λ ex/λem (nm) | Solvents | Limit of detection (LOD) | Target analytes | Applications | Ref. |
|---|---|---|---|---|---|---|---|---|
| Conjugated fluorescent polymers | 178 | PAN-Cs | 365/467–500 | Aqueous solutions | 0.350 μM | Trifluralin, isopropalin, glyphosate, fenitrothion, imidacloprid, and cyfluothrin | Detecting six different pesticides in water | 284 |
| 179 | PAN-Ls | 365/479–490 | Ethanol | 0.100 mM | Fenitrothion, trifluralin and glyphosate | Detection for three pesticides in water | 285 | |
| 180 | FCPNPs–MnO2 nanosheets | 470/536 | Phosphate buffer (PB, 10 mM, pH = 8.5) | 1.12 pg mL−1 | Carbaryl | Detecting Carbaryl by fluorescence quenching | 286 | |
| 181 | Su-TPE/PrS | 320/470 | Tris-buffer (20 mM, pH = 8.2) | 500 μM | Paraoxon methyl | Detection of paraoxon methyl in farmland or water | 287 | |
| 182 | UCNPs–GNPs | 980/660 | Acetone-phosphate buffer (0.3 mmol L−1, pH = 6.5) | 1.42 nM | Malathion | Detection of malathion in water and tea powder | 288 | |
| 183 | rGO-TPP | 414/649, 717 | VDMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Vmethanol = 1 Vmethanol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
27.7 fM | Cd2+ | Monitoring the concentration of Cd2+ in water | 289 | |
| Organometallic polymers | 184 | — | 293/617 | Methanol | 0.800 μM | Fipronil | Monitoring fipronil in water | 290 |
| 185 | — | 377/590, 615 | Hexane | — | Triethyl phosphate | Detecting triethyl phosphate in solution and gas states | 291 | |
| 186 | CFP-Sm-CeO2- | 310/350, 615 | Tris–HCl buffer solution (5 mmol L−1, pH = 8.0) | 1.00 μM | Methyl parathion | Detection of methyl paraoxon in Chinese herbal plants (Poria cocos and semen coicis) | 292 | |
| 187 | Cd-CBCD | 390/467 | Dimethylacetamide | 145 ppb | 2,6-Dichloro-4-nitroaniline (DCN) | Detecting DCN in water | 293 | |
| 188 | LCP 1 | 345/405 | THF | 1.15 μM | DCN | Detection of DCN in water | 294 | |
| Aqueous solution | 3.83 μM for Fe2+, 2.33 μM for Hg2+, 1.97 μM for Fe3+ | Fe2+ | Detecting Fe3+. Fe2+, Hg2+, and Fe3+ in river water | |||||
| Hg2+ | ||||||||
| Fe3+ | ||||||||
| 189 | — | 324/462 | Aqueous solution | 0.360 μM | DCN | Detection of the pesticide DCN in water | 295 | |
| 0.590 μM | Fe3+ | Detection of the Fe3+ in water | ||||||
| 190 | — | 365/484 | Aqueous solution | 1.02 ppm | Fe3+ | Detection of Fe3+ in river water samples | 296 | |
| Molecularly/ion imprinted polymers | 191 | Microfluidic paper chip (paper@QDs@NBD@MIPs) | 395/528, 640 | NR | 90.0 nM | 2,4-Dichlorophenoxyacetic acid (2,4-D) | Detecting 2,4-D in soybean sprout and lake water | 297 |
| 192 | Molecularly imprinted membrane chromatography strip | 450–470/525 | NR | 20.0 μg mL−1 | Triazophos | Detecting the concentration of triazophos in water | 298 | |
| 193 | AgNPs-MIP films sensor | 365/420 | Na-phosphate buffer (20 mM, pH = 6.0, containing 10% acetonitrile) | 0.300 ng mL−1 | Aflatoxin B1 | Detecting the distribution and concentration of AFB1 in maize flour | 299 | |
| 194 | ARS-MIP | 469/560 | Phosphate buffer (20 mM, pH = 8.5) | 0.100–100 μM | Cu2+ | Detecting Cu2+ in water. | 300 | |
| 195 | CQDs@Cu-IIP | 343/448 | Aqueous solution | 0.0160 mg L−1 | Cu2+ | Detecting Cu2+ in river water | 301 | |
| 0.0280 mg L−1 | ||||||||
| 196 | Cu(II)-FIIS | 438/493 | Double-distilled water | 0.140 μM | Cu2+ | Detecting Cu2+ in river water | 302 | |
| 197 | Pb(II)-IIP | 395/430 | Pure water | 2.10 μg L−1 | Pb2+ | Detecting Pb2+ in different water samples | 303 | |
| Other special fluorescent polymers | 198 | FD@ALB | 370/505, 575 | PBS (1 mM, pH = 7.4) | 0.570 μM | Chlorpyrifos | Detection the concentration of CPF in food, water, and soil | 304 |
| 199 | HN-Chitosan-3 | 440/555 | Deionized water containing 0.1 M hydrochloric acid | 1.66 μM | HCHO | Detecting HCHO in water samples | 305 |
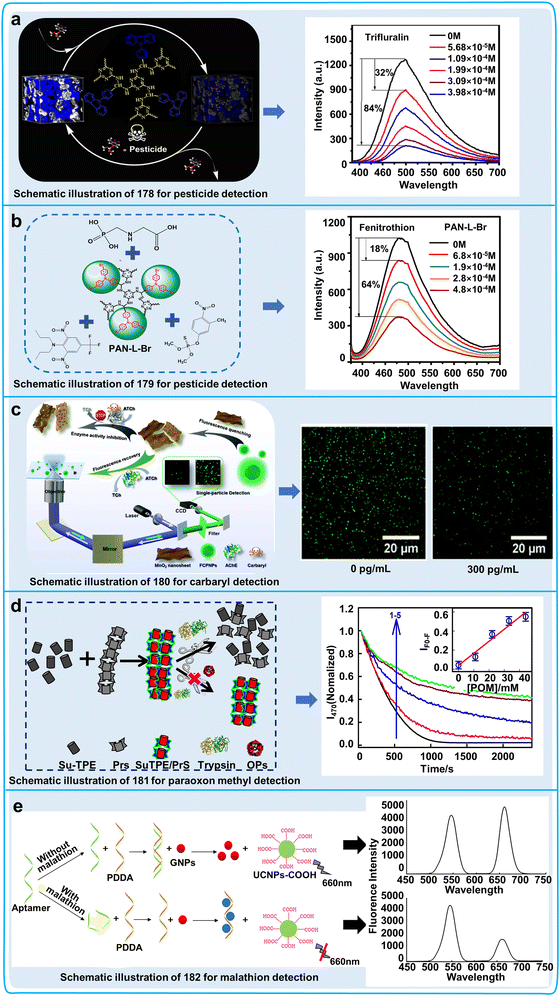 | ||
| Fig. 53 Detection process, data graphs and practical application diagrams of conjugated fluorescent polymers for the detection of pesticides. (a) Detection mechanism and spectra of different concentrations of pesticides (trifluralin as an example) in water by 178.284 Copyright © 2020, the American Chemical Society. (b) Schematic and spectra of 179 for the detection of different concentrations of pesticides (fenitrothion as an example).285 Copyright © 2018, the American Chemical Society. (c) Schematic representation of the detection of the pesticide carbaryl by 180 and fluorescence images of FCPNPs detecting different concentrations of carbaryl. Reproduced from ref. 286 with permission from The Royal Society of Chemistry.286 Copyright © 2019, The Royal Society of Chemistry. (d) Schematic representation of the 181 assay for methyl paraoxon and a plot of data on the inhibition of trypsin activity as measured by the fluorescence decay of the 181 complex at different concentrations of methyl paraoxon.287 Copyright © 2021, Elsevier. (e) Schematic and fluorescence spectra of malathion detection by 182.288 Copyright © 2020, Elsevier. | ||
To realize the detection of pesticide residues in water resources, farmland and agricultural products, researchers have developed a variety of pesticide sensors. One paper reported a fluorescent sensor combining fluorescent conjugated polymer nanoparticles (FCPNPs) (chemosensor 180) with manganese oxide (MnO2) nanosheets, and then the fluorescence of 180 could be quenched by the MnO2 nanosheets. AChE could induce the disruption of MnO2 nanosheets, and then result in the recovery of the fluorescence of FCPNPs. To restrict the recovery of the fluorescence signals, we could limit the destruction of MnO2 nanosheets through the inhibition of AChE activity via carbaryl (Fig. 53c). 180 showed good linear concentration responses in the range of 5–300 pg mL−1 with an LOD of 1.02 μU mL−1. The “off–on” property increased the sensitivity and accuracy of 180 for monitoring the effect of pesticides on AchE activity.286 Researchers also developed a polymerization-induced emission (AIE)-based sensor (181) assembled by electrostatic interaction between a cationic polyelectrolyte protamine (PrS) and tetra-anionic sulphonyl derivative of tetraphenylethylene (Su-TPE). The supramolecular complex disintegrated as a result of the enzyme degradation of PrS in the presence of trypsin, and the enzymatic activity of trypsin was significantly inhibited by the pesticide methyl paraoxon, thus restoring the fluorescent signals of the Su-TPE/PrS polymers (Fig. 53d). 181 showed a good linear concentration range of 0 nM to 16 nM with an LOD of 0.22 nM.287 The results implied that the use of 181 was an effective method for the quantitative detection of organophosphorus in agricultural fields or water resources by monitoring enzymatic activity. In another conjugated polymer (chemosensor 182) for the detection of pesticides assembled by electrostatic interactions, to produce FRET, negatively charged upconversion fluorescent nanoparticles (UCNPs) were combined with cationic conjugated polymer-encapsulated gold nanoparticles (GNPs), and the fluorescence intensity decreased linearly with an increase in malathion concentration from 0.01 to 1 μM with an LOD of 1.42 nM (Fig. 53e). However, the fluorescence quenching of 182 may increase false positives in the detection of pesticides, which implies that the polymerization mechanism of 182 should be improved.288 These studies have successfully designed fluorescent sensors for monitoring a variety of pesticides, and further studies should focus on developing novel detection kits for measuring enzymatic activity or pesticide concentration.
In addition to detecting pesticides, conjugated fluorescent polymer sensors were designed to detect heavy metal ions in water. One study employed noncovalent-coupled 5,10,15,20-tetraphenylporphyrin-reduced graphene oxide (rGO-TPP) to create a sensor (chemosensor 183), which could detect Cd2+ with high sensitivity and without interference. The detection behavior of 183 was examined for 20 significant metal ions, and only Cd2+ was found to enhance the non-fluorescent rGO-TPP sensor to resume fluorescence. Cd2+-induced fluorescence turn-on of the analyte was observed in the response range from micromolar to femtomolar, and the system was found to be the most sensitive Cd2+ sensor to date at the lowest experimentally detectable concentration of 3.86 fM (Fig. 54). Ultimately, rGO-TPP was also created as a solid-phase filter paper sensor that may be employed in on-site Cd2+ monitoring applications.289 In terms of convenience and applicability, the rGO-TPP filter paper sensor is an effective tool for detecting metal ions in practice, but its application scope should be improved.
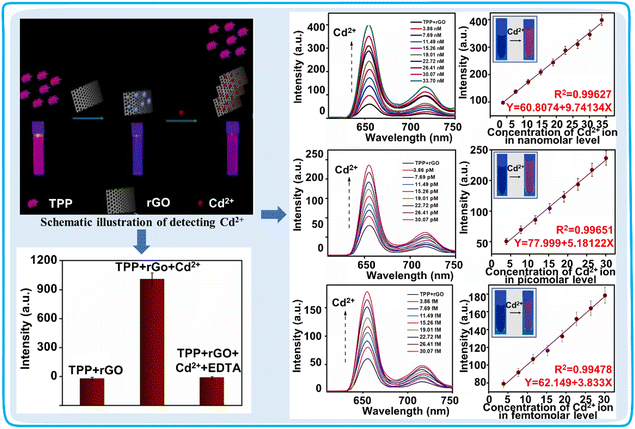 | ||
| Fig. 54 Schematic of the detection of Cd2+ by the 183 sensor, statistical plot of the competitive interaction in the presence of different molecules and quantitative fluorescence detection spectra of Cd2+ concentration (nanomolar, picomolar and femtomolar concentrations) by 183.289 Copyright © 2023, Elsevier. | ||
Currently, conjugated fluorescent polymers are commonly used to identify pesticides and ions in the living environment of plants. To improve the application scope of these sensors, some challenges should be solved. (i) We should focus on the development of “off–on” conjugated fluorescent polymers that can improve the detection accuracy and sensitivity. (ii) Further studies should focus on the detection of more stable conjugated fluorescent polymers or combined with a smartphone for increasing their application in a variety of practical environments. (iii) The complex synthesis process and difficult purification of conjugated fluorescent polymers limit their wide application.
 | ||
| Fig. 55 Detection process, data graphs and practical application diagrams of organometallic fluorescent polymers for the detection of pesticides. (a) Spectrograms and Stern–Volmer plots of 184 for the detection of different concentrations of fipronil in methanol solutions. Reproduced from ref. 290 with permission from The Royal Society of Chemistry.290 Copyright © 2018, The Royal Society of Chemistry. (b) Fluorescence images and spectrograms of 185 thin film test triethyl phosphate (solution and gas states).291 Reproduced from ref. 291 with permission from The Royal Society of Chemistry. Copyright © 2010, The Royal Society of Chemistry. (c) Schematic diagram, fluorescence spectrogram and linear range plot of the fluorescence detection system 186 for the detection of methyl paraoxon (MP).292 Copyright © 2023, BioMed Central Ltd. | ||
In addition, studies have shown that metal coordination polymers can detect heavy metals and trace chemicals that affect plant health. The carbazole-functionalized metal–organic polymer [Cd3(CBCD)2(DMA)4(H2O)2]-10DMA (Cd-CBCD, chemosensor 187) was developed as a highly efficient new fluorescent sensor for the detection of 2,6-dichloro-4-nitroaniline (DCN) by fluorescence quenching. 187 featured an LOD of 145 ppb together with a high quenching effect coefficient. 187 showed biofunction for the removal or detection of chemicals in water systems, implying it can be used as a scavenger of environmental pollutants (Fig. 56a).293 In addition to carbazole-functionalized metal–organic polymers for the identification of DCN, other metal coordination polymers can also detect DCN. For example, a new Ag(I) luminescent coordination polymer, LCP1 (chemosensor 188), was developed with multi-substance recognition capability. It was found that 188 could specifically identify DCN, organosolvents and heavy metal ions by static quenching (Fig. 56b). The fluorescence quenching of 188 was ascribed to the FRET mechanism.294 In another example, a novel cadmium-based metal organic fluorescent polymer (chemosensor 189) was developed, which showed fluorescence turn-off behavior in water towards the pesticide DCN (Fig. 56c), with quenching efficiencies of up to 94.45%.295 Interestingly, chemosensor 189 was also designed as an optical signal quenching fluorescent sensor for monitoring metal ions. Among fifteen metal cations, only Fe3+ could induce the quenching of the fluorescence emission signal of 189 in water (Fig. 56c). The fluorescence of Fe3+@189 was recovered after the addition of ascorbic acid. This chemosensor showed an “on–off–on” mechanism for the recognition of DCN, Fe3+ and ascorbic acid, implying that the complex mechanism may reduce the detection accuracy of 189.295 The final example of metal ion recognition was a water-stable uranyl coordination polymer (chemosensor 190), which enabled the feasible and effective detection of Fe3+ in real river water samples (Fig. 56d). 190 was a 3D supramolecular framework that was formed by π–π and hydrogen bond interactions. This chemosensor has bifunctions for identifying and detecting Fe3+ and pesticides.296 If metal coordination polymers have dual detection function, that can be widely used to detect or remove pesticides and metal ions in water sources.
 | ||
| Fig. 56 Detection process and data graphs of organometallic fluorescent polymers for the detection of 2,6-dichloro-4-nitroaniline (DCN) or metal ions. (a) Fluorescence spectra and Stern–Volmer plots of different concentrations of DCN detected by 187. Reproduced from ref. 293 with permission from The Royal Society of Chemistry.293 Copyright © 2019, The Royal Society of Chemistry. (b) Fluorescence images and titration spectra of 188 for the detection of different concentrations of DCN, Hg2+, Fe2+, and Fe3+.294 Reproduced from ref. 294 with permission from The Royal Society of Chemistry. Copyright © 2020, The Royal Society of Chemistry. (c) Titration spectra of 189 detecting different concentrations of Fe3+ and DCN.295 Copyright © 2020, Elsevier. (d) Fluorescence images and titration spectra of different concentrations of Fe3+ detected by 190.296 Copyright © 2019, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
A well-known advantage of using organometallic polymers is their high sensitivity. However, their main disadvantage is that many metals and even heavy metals are toxic and can have a negative effect on plant health, and also there is radiation from radioactive metals. The raw materials for the preparation of organometallic polymers, the prepared organometallic polymers and the products after the use of organometallic polymers may all have a certain degree of toxicity.
Therefore, it is necessary to choose less toxic metals as the raw materials for the preparation of organometallic polymers, which is also a direction that needs to be the focus of research.
 | ||
| Fig. 57 Extraction mechanism and data tracing of molecularly imprinted polymers employed in pesticides, metal ions and toxin detection. (a) Procedure for fabricating 191 and its fluorescence spectra and images for the detection of 2,4-D. Reproduced from ref. 297 with permission from The Royal Society of Chemistry.297 Copyright © 2020, The Royal Society of Chemistry. (b) Structure of test strips of 192, competitive fluorescence detection process for triazophos, images and calibration curves of test strips for the determination of distinct concentrations of triazophos.298 Reproduced from ref. 298 with permission from The Royal Society of Chemistry. Copyright © 2020, The Royal Society of Chemistry. (c) Fluorescence spectra of AFB1 detected by silver-doped and non-silver-doped 193. Reproduced from ref. 299 with permission from The Royal Society of Chemistry.299 Copyright © 2022, The Royal Society of Chemistry. (d) Synthesis process of 194-imprinted polymer particles and their principle and fluorescence spectra for the detection of Cu2+.300 Reproduced from ref. 300 with permission from The Royal Society of Chemistry. Copyright © 2022, The Royal Society of Chemistry. | ||
By imprinting and polymerizing template ions in the imprinting process, ion-imprinted polymers are unique polymers that have the ability to selectively adsorb the template ions. In the following research examples, a batch of ion-imprinted polymers for the selective detection of metal ions should be prepared based on various heavy metal ions as effective tool for the analysis and removal of contaminants in the living environment of plant. For instance, citric acid was used as a carbon source for the synthesis of CQDs via the hydrothermal method, and then an amidation procedure. The produced CQDs were attached as fluorescent groups on the surface of SBA-15 mesoporous molecular sieves. Then, the fluorescent sensor CQDs@Cu-IIP (chemosensor 195) was prepared via the surface blotting method, and Cu2+ could effectively quench the fluorescence of CQDs@Cu-IIP. Thus, CQDs@Cu-IIP was used as an ion-imprinted polymer for the detection of Cu2+ in river water (Fig. 58a).301 In addition to other IIPs for the detection of Cu2+, a type of quick and easy fluorescent ion-imprinted sensor (FIIS) for Cu2+ detection was made by creating a fluorescent polymeric ligand. 4-(2-Aminomethyl)pyridinyl-N-allylnaphthalimide was employed as a fluorescent functional monomer, a layer of Cu(II) ion-imprinted polymer was coated on the surface of a PVDF membrane with a surface-functionalized FIIS (chemosensor 196). The fluorescence emission intensity of 196 decreased with an increase in the incorporation of copper(II) ions (Fig. 58b).302 The Cu2+ concentration in river water samples was effectively determined using 196, which possessed a high degree of selectivity and sensitive recognition capacity for Cu2+. This study indicated that 1,8-naphthalimide can serve as versatile building block, implying that other fluorophores such as coumarin and rhodamine can also have the same function. In another ion-imprinted polymer, anthracene-coupled styrene motifs and 5-amino-8-hydroxyquinoline served as significant chemical units in a precipitation copolymerization process to create a novel Pb(II)-fluorescent IIP (chemosensor 197). 197 demonstrated a remarkably selective fluorescence switching property for Pb2+ and high fluorescence enhancement upon the binding of Pb2+ (Fig. 58c), which proved its successful quantitative detection of Pb2+ in environmental water. Compared with normal fluorescent chemosensors, the imprinted polymer showed more selective properties for detecting ions in the environment.303 Ion imprinted polymers are similar to molecularly imprinted polymers, which they have high specificity for the analytes used as templates.
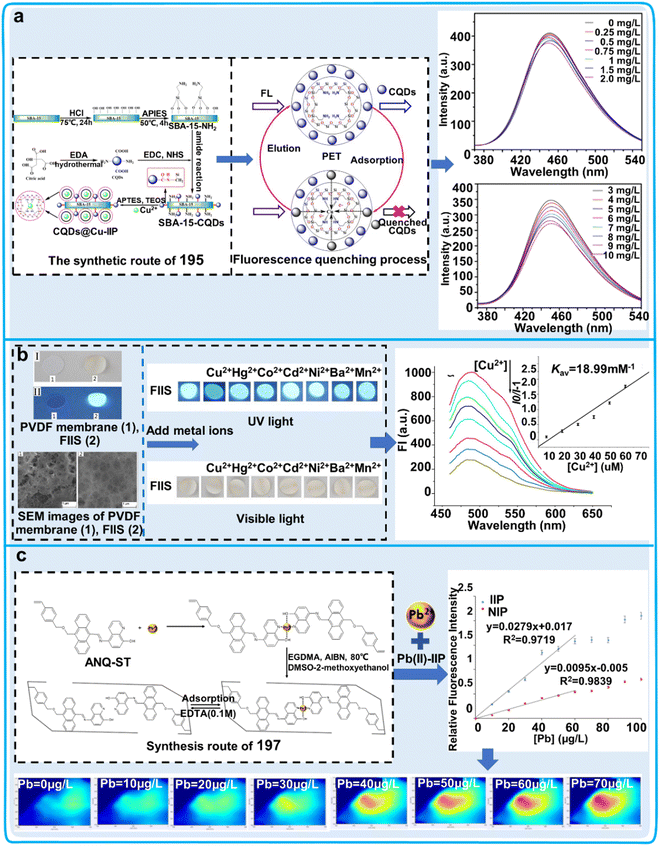 | ||
| Fig. 58 Detection process and data graphs of ion imprinted polymers for the detection of metal ions. (a) Synthesis route of 195 and its quenching process and fluorescence spectrum for detecting Cu2+.301 Copyright © 2021, Multidisciplinary Digital Publishing Institute. (b) Images of 196 testing different metal ions under visible and UV light, and fluorescence spectra of 196 detecting Cu2+.302 Copyright © 2021, Elsevier. (c) Synthesis route of 197, and its fluorescence curves and images for the detection of Pb2+.303 Copyright © 2020, Elsevier. | ||
Overall, MIPs/IIPs have become popular as pesticide sensors due to their extremely high selectivity for target analytes and their durability in a variety of environments. However, they still have certain drawbacks, as follows: (i) the preparation of MIPs/IIPs is still difficult and how to prepare these two types of imprinted polymers at lower cost and higher yields should be investigated. (ii) The specific recognition ability caused by the template may be lost after preparation and optimizing the preparation process of MIPs/IIPs will be an important condition to promote their application.
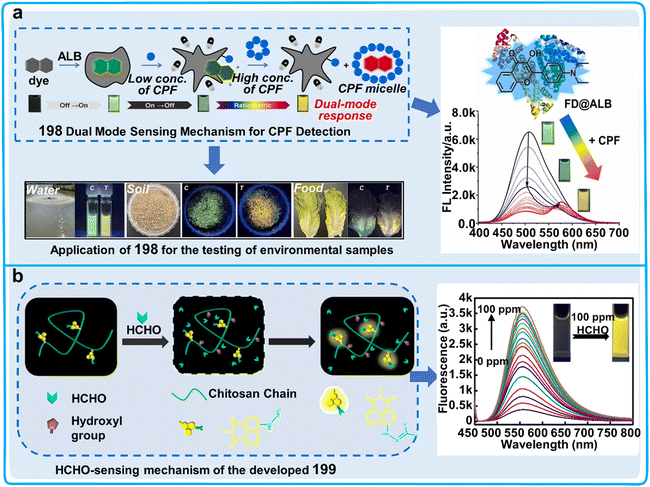 | ||
| Fig. 59 Detection process, data graphs and practical application diagrams of other special fluorescent polymers for the detection of pesticides and toxins. (a) Dual-mode sensing mechanism of 198 for CPF and its titration spectrum for CPF and application to detect CPF in samples.304 Copyright © 2023, Elsevier. (b) Sensing mechanism and titration spectra of HCHO by 199.305 Copyright © 2018, the American Chemical Society. | ||
In summary, the advantages of using these combinatorial systems include a variety of detection response modes and adaptability to a wide range of field environments. Therefore, there is great potential for the progression of novel combinatorial patterns and superior-performing fluorescent polymers.
5.2 Hydrogelator systems
The self-assembling network structures present in supramolecular hydrogels are developed by the non-covalent connections in hydrogelators.306 Supramolecular fluorogenic hydrogels generate fluorescence with an autonomic assembly procedure to generate potent nanoscale architectures for visualization.307 In comparison to other types of chemosensor imaging, fluorescent supramolecular hydrogels have the advantages of versatility, biocompatibility, accessibility, low damage and high stability, and have been used in the fields of bioimaging and environmental monitoring (Table 12).307,308 In the following section we introduce the applications of supramolecular fluorescent hydrogels in monitoring the living environment of plants, including detecting hazardous heavy metals in their surrounding environment and pesticide residues in fruits (Fig. 60).| Chemosensor | Name | λ ex/λem (nm) | Solvents | Limit of detection (LOD) | Target analytes | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| 200 | NDG | 300/470 | VDMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 3.3 VH2O = 3.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.7 6.7 |
5.33 nM | Fe3+ | Detecting Fe3+ in water via the form of fluorescence quenching | 309 |
| 201 | 2-QF | 317/377 | Aqueous solutions | 0.145 μM | Zn2+ | Detecting Zn2+ in water via the form of fluorescence enhancement | 310 |
| 202 | FHCP | 365/501 | Aqueous solutions | 8.00 nM | Hg2+ | Detecting Hg2+ in plants and water via the form of fluorescence color change | 311 |
| 203 | GQDs/Enz/Gels | 360/465 | Phosphate buffer solution (pH = 8) | 26.1 nM | Dichlorvos | Detection the concentration of dichlorvos in plants via the form of fluorescence enhancement | 312 |
| 204 | Cyclo-WW+Zn(II) | 450/550 | Aqueous solution | 2.90 μg L−1 | Lambda cyhalothrin | Detected Lambda cyhalothrin in plants via the form of fluorescence quenching | 313 |
There are some examples of the preparation of fluorescent supramolecular hydrogels that have been instrumental for assessing heavy metals in environmental water samples. A novel bifunctional AIE hydrogel (NDG, chemosensor 200) was established, which was ingeniously devised through a straightforward host–guest interaction. This involved a 1-naphthaleneacetic hydrazide amino-derivatized tripodal hydrazide, acting as the molecular host (NTS), and a tri-(pyridine-4-yl)-functionalized trimesoyl amide functioning as the guest (DTB) (Fig. 61a).309 Researchers delved into the cation-responsive attributes by incorporating and disseminating various metal ions in water into 200 and only the presence of Fe3+ caused the quenching of the fluorescence of this sensor. Moreover, with an increase in the Fe3+ dosage (0–0.45 equiv.), the emission intensity corresponding to the fluorescent response of 200 showed an impressive degree of linearity along the titration curve, and the fluorescence emission band decreased at 470 nm with an LOD of 5.33 × 10−9 M. Meanwhile, 200 exhibited bifunction for the removal and detection of metal ions and this chemosensor could be applied to selectively identify Fe3+ in real water samples. Another example involved the development of an expedient gelator (chemosensor 201), which was prepared using a phenylalanine derivative augmented with a quinoline ring harbouring a single N atom (3-phenyl-2-[(quinolin-2-ylmethyl)-amino]-propionic acid, namely, 2-QF) (Fig. 61b).310 The impact of diverse metallic ions on the photoluminescence of 201 was assessed, which revealed that the visible fluorescence response to Zn2+ of 201 was perceivable to the naked eye under UV radiation with a wavelength of 365 nm. Notably, the fluorescence intensity of 201 was substantially augmented by approximately 230-fold upon incorporating Zn2+, whereas the systems of 201-Cd or 201-Pb exhibited only a marginal increase in fluorescence intensity. These findings suggested that 201 exhibits potential as a superior sensor for the detection of Zn2+. 201 showed favorable membrane permeability, indicating that it can be applied for detecting metal ions in the environment and plants. Meanwhile, supramolecular fluorescent hydrogels have been used to detect heavy metal contaminants in plants. It was reported that fluorescent hydrogel-coated sensing gloves were created for monitoring the Hg2+ content in plants based on chemosensor 202 coated with a flexible paper/textile film (Fig. 61c).311
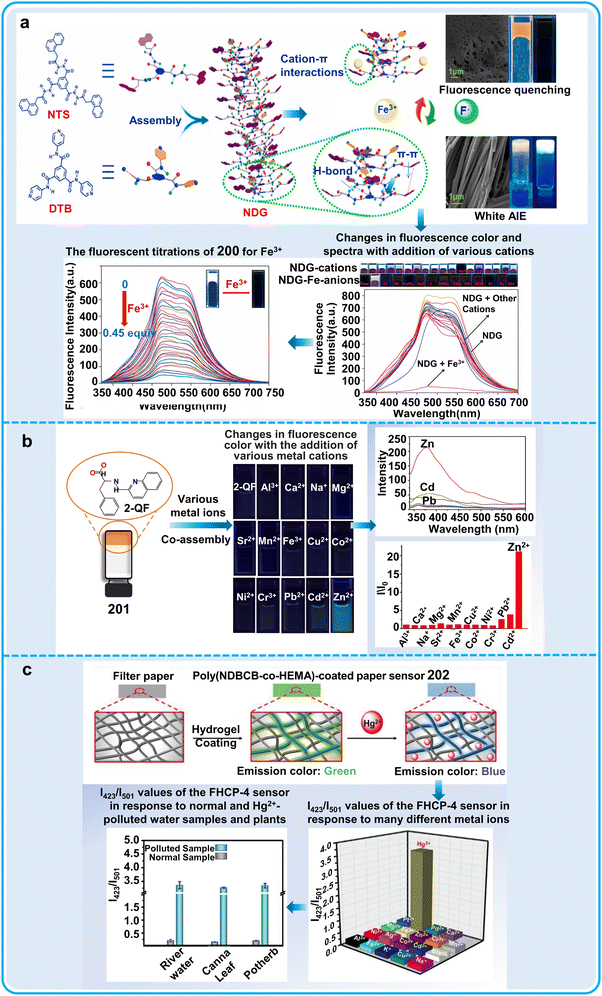 | ||
| Fig. 61 Detection process, data graphs and practical application diagrams of supramolecular fluorescent hydrogels system for detecting ions in plant surroundings. (a) Construction of 200 and the possible mechanisms, as well as the fluorescence effect and data statistics about the sensing of Fe3+ and other cations.309 Copyright © 2020, Elsevier. (b) Chemical structure of 201 and the fluorescence effect and data statistics of Zn2+ and other metal ions.310 Copyright © 2020, Elsevier. (c) Preparation of the fluorescent hydrogel-coated paper (FHCP, 202) for Hg2+ detection in water and food samples.311 Copyright © 2020, WILEY-VCH Verlag GmbH. | ||
The results showed color changes from green to blue with an increase in the concentration of Hg2+. 202 showed remarkable analytical selectivity for Hg2+ with an LOD of 2.61 × 10−8 M. 202 was used to test Hg2+ in potherb and Canna leaf, and the measured results showed good consistency. Supramolecular hydrogels are not the only potential candidates for the evolution of a new class of digital apparatus, but also aid in the synthesis of smart supramolecular polymer sensors with superior fluorescent attributes. If widely used, supramolecular hydrogels can discover and help remove heavy metal ions in water samples and foods.
In addition to detecting heavy metals, researchers have developed supramolecular fluorescent hydrogels for detecting trace amounts of pesticides in agricultural products and the environment. It was reported that researchers synthesized new fluorescent hybrid materials comprised of enzymatic entities affixed to an L-phenylalanine-derived bis(urea) supramolecular hydrogel platform coupled with graphene quantum dots (GQDs/Enz/Gels, chemosensor 203) for the qualitative analysis of organophosphates (Fig. 62a).312 AChE induces the degradation of acetylcholine (ACh), yielding choline. Subsequently, choline is incessantly oxidized by choline oxidase (ChOx), leading to the production of H2O2 and culminating in photochemical suppression of the integrated gel assemblies. Upon exposure to UV radiation at 365 nm, a substantial enhancement in visible fluorescence image intensity was observed for 203 impregnated with dichlorvos as the analyte concentration increased from 1 × 10−6 to 1 × 10−3 M. The anti-cholinergic potency of dichlorvos against AChE demonstrated a linear response in the range of 1.25 × 10−8 to 1.25 × 10−4 M, indicating that the low molecular weight hybrid hydrogels can also have high sensitivity and stability for detecting pesticides. Considering that AChE and ChOx can be combined in 203 as a signaling unit, this chemosensor can be developed as an enzymatic activity detection kit. Another visual platform (chemosensor 204) was constructed using a cyclo-ditryptophan (cyclo-WW) + Zn(II)-based photoluminescent hydrogel, which showed good ability to detect lambda cyhalothrin (LC) (Fig. 62b).313 In the process of oxidative PET, post formation of a coordination complex between the surface group of 204 and LC, this complex triggered non-radiative emission upon relaxation from the excited electronic state into the ground state. Under these conditions, 204 acted as an electron donor, providing electrons to the electron acceptor LC. When subjected to LC, the intensity of the yellow luminescence emitted by 204 progressively diminished with an increase in the concentration of LC. 204 exhibited outstanding precision and robust stability when assessing pesticides in actual samples, which can provide guidance for developing polypeptide-based biosensors. The above-mentioned studies developed a miniature hybrid hydrogel confined to a glass slide, or developed a smartphone-assisted sample-to-answer analyzer, which can be used as a rapid, convenient, and high-throughput sensor for the detection of pesticides.
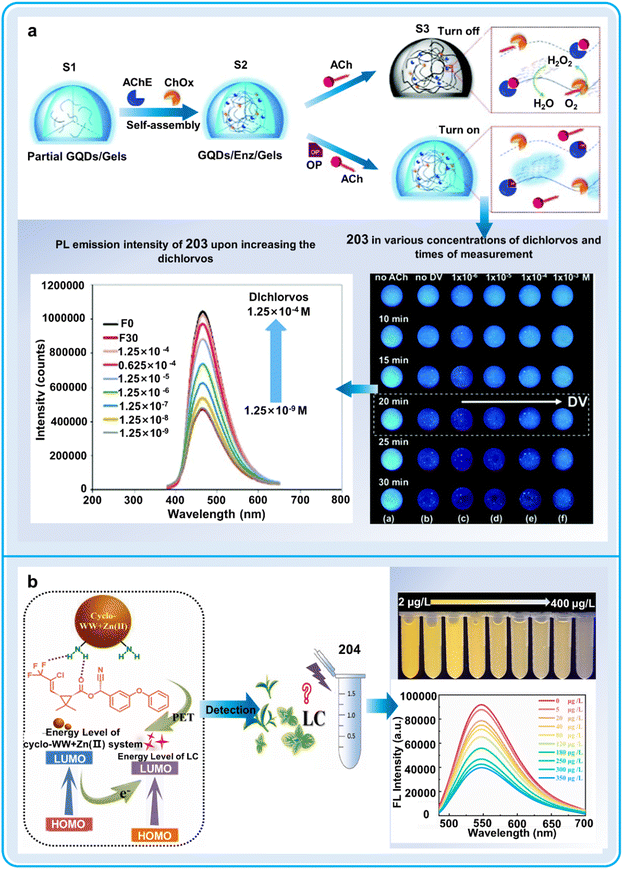 | ||
| Fig. 62 Detection process, data graphs and practical application diagrams of supramolecular fluorescent hydrogel system for the detection of pesticides in the living environment of plants. (a) Proposed mechanism of hybrid 203 for organophosphate pesticide detection and the fluorescence effect and data statistics after adding dichlorvos.312 Reproduced from ref. 312 with permission from The Royal Society of Chemistry. Copyright © 2021, The Royal Society of Chemistry. (b) Schematic illustration of the sensing mechanism and the fluorescence effect of 204 and data statistics after adding lambda cyhalothrin.313 Copyright © 2022, Elsevier. | ||
In conclusion, the environmental friendliness and high sensitivity of supramolecular fluorescent hydrogels make these chemosensors suitable for detecting heavy metals in the environment and pesticide residues in plants and food. However, there are also some challenges that need to be considered, as follows: (i) modification of the backbone structure frequently perturbs the stability of the hydrogel networks and (ii) the leakage of fluorescent materials combined with the accumulation of chromophores may induce a reduction in optimum emission intensity and potential quenching of fluorescence. Therefore, improving the stability of supramolecular fluorescent hydrogels will be an important aspect to promote their practical application.
6. Analysis of fluorescent chemosensor data
6.1 Techniques for fluorescent chemosensor data analysis
The processes for dealing with the data obtained from fluorescent chemosensors mainly involve two steps, image capture and image processing. High-quality and resolution fluorescence images can maintain the integrity, accuracy and repeatability of the results from microscopy. High precision analytical techniques and statistically rigorous quantification methods are frequently required to gain enhanced comprehension of the intricate biological mechanisms for governing plant health and monitoring the living environment of plants. In the following section, we describe these two steps in detail.For image capture, fluorescence microscopy, including fluorescence microscopy, confocal laser scanning microscopy (CLSM), two-photon fluorescence microscopy, fluorescence lifetime imaging microscopy (FLIM), stimulated emission depletion (STED), single molecule localization microscopy (SMLM), structured illumination microscopy (SIM), and total internal reflection fluorescent microscope (TIRFM) are frequently used (Fig. 63a). (1) Fluorescence microscopy can only visualize pre-sliced specimens and does not have the potential of optical slicing. In the case of three-dimensional samples thicker than 2 μm, the whole sample fluoresces, causing defocused emissions, obscuring the focal plane detail and reducing the image contrast. However, it also has some advantages such as the utilization of a mercury arc lamp or xenon lamp to offer a comprehensive spectral range spanning the UV, visible and NIR bands.314 (2) CLSM represents an alternative technique for recovering thin fluorescent sections through optical isolation of fluorescence emission within the imaged plane, facilitating the visualization of plant cells in thick sections at high resolution. However, despite point-scan imaging, the imaging speed of CLSM is slow due to the limited laser excitation, narrow frequency band, and high intensity laser radiation.315 (3) Two-photon fluorescence microscopy allows non-destructive examination of biological specimens in three-dimensions with submicron precision. Excitation of two-photon fluorophores originates from dual photon absorption simultaneously.
This distinctive emission mechanism possesses several advantages such as diminished sample photodegradation, reduced photoblinking, improved penetrative depth, and producing superior contrasting images.316 (4) FLIM, a microscopy strategy dependent on the nanosecond fluorescence lifetime of molecules, has gained immense popularity due to its superior sensitivity to the environment and variations in the structure of molecules. This technology shows advantageous characteristics such as identifying spectrally overlapping fluorophores by the fluorescence lifetime, detecting fluctuations within fluorophore molecular environments, measuring quenching dynamics, and executing self-referenced assessment. However, FLIM analysis needs technical proficiency and has high computational costs, extensive photon usage for fluorescence lifetime determination, and susceptibility to multiple factors ranging from molecular interactions/binding activities to environmental conditions (temperature, viscosity and pH).317 (5) In the case of SIM, a collection of complex patterns of intensity is utilized to illuminate the targeted object, thereby facilitating the reconstruction of an accurate image of the subject material. Its advantages include straightforward sample processing, swift imaging rate, expansive imaging field, and widespread adoption in biology. However, SIM displays low tissue penetration depth, restricting its application in single-cell imaging due to the substantial scattering caused by its low excitation power density.318 (6) SMLM is an imaging technique capable of overcoming the diffraction obstacle to yield super-resolution images. The random switching between on and off states is followed by the collection of substantial photons to computationally simulate and merge the locations of all individually activated fluorophores to generate a super-resolution image. However, this technology is associated with some challenges such as complex sample processing procedures, fluorophore photobleaching, and substantial computational requirements.319 (7) STED is a novel enhancement of confocal microscopy, which is regarded as the primary far-field super-resolution imaging technology applicable to cell observation. Its proficiency in cell imaging and three-dimensional chromatographic capacity have attracted considerable attention in biological research. Nevertheless, these advantages are counterbalanced considering its potential cellular phototoxicity, photobleaching, and advanced technical proficiency.320 (8) TIRFM is complex optical technology, enabling the activation of fluorophores within an exceptionally thin axial zone, and then producing a satisfactory S/N ratio. By exploiting the inherent characteristics of an evanescent wave or field confined to a specific specimen vicinity immediately adjacent to the interface between two media possessing different refractive indices, it facilitates the comprehensive observation of the entire wide field rather than utilizing point-by-point scanning. The fundamental limitation of TIRFM as an innovative detection tool in biochemical and chemical research is its inability to utilize selective fluorescent chemosensors specifically.321 In short, each of the above-mentioned eight primary techniques has advantages and limitations, indicating that we should select the optimal equipment depending on the sample under investigation and the associated scientific requirements.
The traditional methodologies for processing microscopic images mainly include morphological differentiation, image fusion, edge detection, classification, region growing, and feature extraction. These techniques typically necessitate the expertise of computational experts, who are scarce within plant science and necessitate complex computational processes. Additionally, traditional fluorescence microscopy images consistently exhibit relatively diminished resolution and inferior S/N ratio owing to the restraints imposed by imaging systems and the restrictions of diffraction. Consequently, conventional image processing methodologies are often insufficient for efficient data interpretation.322 Deep learning (DL), a novel approach representing data learning, is a significant segment of artificial intelligence (Fig. 63b).323 For microscopic image analysis, scientists are progressively incorporating DL into various complex challenges such as cellular components, subcellular structures, tissue architectures, and the diversity of environment. Specially, DL in images processing involves four main steps including image classification, image segmentation, target tracking and super-resolution reconstruction.324 (1) Classification. Establishing classification tools capable of distinguishing diverse pollutants within the living environment of plants, specific cell stages or subcellular structures can provide important insights into plant health. This may enable farmers or chemical engineers to adopt effective strategies for managing plant health. Presently, convolution neural network (CNN), including ResNet, AlexNet, and LeNet, are prevalent in classification procedures. Typically, initial DL-based classification exhibits higher accuracy and greater efficiency compared to its conventional counterparts. (2) Segmentation. Establishing boundaries around regions of interest is an indispensable phase in microscopic image analysis, facilitating focus on data-rich areas of significance. Intracellular compartment segmentation supplies quantifiable data regarding cell structure and functionality. Dl-Based segmentation methodologies are categorized into two types, instance-level segmentation and semantic-level segmentation, depending on the incorporation of a fully interconnected layer. Prioritized semantic-level and instance-level segmentation algorithms originate from the U-Net and R-CNN models, respectively. (3) Tracking. Assessing the viscosity of cells, infection of pathogens or distribution of pollutants within the living environment of plants is fundamental for discerning significant plant health signals. The networks employed for localization predominantly utilize the methodologies of recurrent neural networks (RNNs). With advancements in long short-term memory (LSTM), issues related to gradient explosions and gradient vanishing have been effectively managed. Consequently, data such as the prevalence of environmental pollutants, early plant health diagnosis, or the interaction between plant-environment interactions can be more accurately recorded. (4) Reconstruction. Image reconstruction involves generating a two-dimensional or three-dimensional visualization from fragmented or incomplete data. The discipline of microscopy image interpretation includes techniques such as super-resolution reconstruction, denoising, and three-dimensional reconstruction to generate high-quality and comprehensive images. DL-based image restoration techniques can facilitate the rapid capture of photosensitive specimens and promote high-quality and long-term dynamic observation of plants. Methods integrating GANs have been employed to facilitate super-resolution imaging across various microscope platforms, enabling the conversion of diffraction-limiting signals into super-resolution images. Furthermore, recent developments in augmented intelligent microscopy and proficiency in deep learning technologies are beneficial for managing plant-environment interactions in sustainable agriculture.
6.2 Networking of fluorescent chemosensor data
The spatial and temporal complexity of the health and surrounding ecosystems of plants pose substantial challenges to accurately enforce plant health management. Despite the extensive reliance on remote sensing techniques or sophisticated mass spectrometry chromatography tandem methods, they are limited by the spatial and temporal resolution, labor intensive and resource consuming. As illustrated in Fig. 64, fluorescent chemosensors can be effectively employed to monitor the soil environment, the quality of irrigation water and plant health in a timely manner and accurately. Firstly, fluorescent chemosensors can detect moisture, pesticides, nutrients, heavy metals, microorganism diversity and ions to evaluate the soil environment. Soil functioning as a living ecosystem can offer various services such as food and fibrous yield, water quality and supply, managing pest and disease, modulating the atmospheric composition, climate regulation and biodiversity conservation. Wholesome soil produces robust plants, significantly contributing to plant proliferation and maturity. Any pollution of the soil with detrimental substances such as inorganic pollutants and organic toxins can disrupt plant assimilation and metabolic activities, resulting in impaired plant growth and developmental disorders. Meanwhile, soil organic matter functions as an indispensable nutrient source for plants, providing crucial materials for maintaining soil texture, regulating pH, and storing nutrients. Insufficient soil organic matter can hinder plant growth and potentially lead to nutritional deficiencies. Secondly, fluorescent chemosensors facilitate the continuous monitoring of irrigation water such as heavy metals, pesticides, and ions, which have a direct impact on plant growth. Irrigation is crucial in addressing food insecurity and water scarcity issues. High-quality irrigation water can provide sufficient water together with nutrients that can promote plant physiological metabolism, facilitate robust root systems evolution, and enhance crop vitality. In contrast, low-quality irrigation water can decrease plant nutrient assimilation capabilities, facilitating the transfer of damaging pathogens and pests, thereby increasing plant disease incidence. For example, heavy metals in irrigation water are found to be assimilated by plants, and subsequently accumulated across multiple plant tissues, which not only influence plant root growth and above-ground biomass production but also pose potential health risks to humans. Thirdly, fluorescent chemosensors offer an invaluable tool to evaluate plant health by evaluating the nutrient uptake dynamics, signaling molecule synthesis, cell components, metabolites, and food quality. For example, hormones serve as critical signaling molecules and are essential for governing numerous plant physiological processes, such as growth, metabolism, organogenesis, reproduction and stress resistance, which significantly affect plant growth and survival. A comprehensive array of metabolically diverse molecules with diverging structural and functional attributes, serving a myriad of roles in plant health, by modulating plant resilience amidst persistently fluctuating environmental variables together with both non-living stressors and biological aggressions. Therefore, the use of fluorescent chemosensors provides real-time data and a feasible solution to improve our capability to manage plant health.7. Conclusions and outlook
This work provides a comparatively comprehensive summary and analysis of studies during the last decade towards the design of fluorescence chemosensors including small-molecule, nano- and supramolecular-chemosensors in regulating plant health. Chemosensors can provide a better understanding of the plant health status under abiotic and biotic stresses, and different nutrient utilization, as well as their living environments. We described the structural features of these chemosensors, their fluorescence characteristics, response mechanism and the intrinsic multifunction. This fluorescent technique will contribute to the enhanced comprehension of the complex interdependence among the environment, human behavior and plant health.When initiating the detailed design processes for a state-of-the-art fluorescent chemosensor to monitor the health and surroundings of plants, it is vital to establish a connection between the fluorescence features of chemosensors and the requirements of plant health regulation. Therefore, future studies should focus on the following aspects: (1) increased emphasis must be placed on developing two-photon and NIR-II fluorescent probes. It is universally acknowledged plant imaging presents difficulties distinct from other model systems because of its robust endogenous auto-fluorescence attributed to chlorophyll in the wavelength range of 400–800 nm. The NIR laser utilized by two-photon and NIR-II fluorescent chemosensors could not only avoid the autofluorescence within plant tissues, but also achieve superior imaging resolution compared to traditional fluorophores. (2) To enhance comprehension of the distribution, translocation and metabolism of signaling molecules under biotic or abiotic stresses, fluorescent chemosensors should be endowed with sub-organelle targeting potential including mitochondria and plasma membrane.
By pinpointing their location at the subcellular level, the elucidation of signaling molecules involved in plant health can be enhanced. (3) Fluorescent nano-chemosensors constructed from QD, COF and MOF materials have made great progress in detecting agricultural pollutants with good recyclability. Future endeavors should concentrate on minimizing the nano dimensions of the aforementioned materials for improving the functionality within plant tissues such as molecular imaging, pesticide release, and labeling signaling molecules. (4) Presently, existing detection methods for plant health are primarily concentrated on individual plant tissues, and thus implementing comprehensive field testing utilizing portable smart devices poses a novel hurdle in future investigations. (5) Further studies should devise super-resolved fluorescent chemosensors to determine the subcellular distribution and transportation mechanism of HPPD in plants to monitor the plant health condition under abiotic stress. Meanwhile, it will serve as an useful tool for in vivo and in vitro inhibitor screening. The inhibitors can compete with the binding sites of proteins with fluorescent chemosensors. When the binding affinity of inhibitors with proteins surpasses their affinity towards fluorescent chemosensors, they may be regarded as highly effective inhibitors. (6) AI should be applied in processing fluorescence images by improving image quality, extracting relevant information and analyzing data. Compared with the vision of professionals, AI technologies have some advantages such as improving image quality with reducing noise, correcting illumination, enhancing contrast, precise analysis by segmentation images, identification of interesting objects, tracking dynamics of biological processes by analyzing temporal changes in images, and comprehensive data analysis by integrating fluorescence images data with other types of data including genomic or proteomic data.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (32125033, 21806048). J. Y. thanks to the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (no. 2022R1A2C3005420 and 2022M3E5F3080873).Notes and references
- J. Pretty, Science, 2018, 362, eaav0294 CrossRef PubMed.
- Z. L. Cui, H. Y. Zhang, X. P. Chen, C. C. Zhang, W. Q. Ma, C. D. Huang, W. F. Zhang, G. H. Mi, Y. X. Miao, X. L. Li, Q. Gao, J. C. Yang, Z. H. Wang, Y. L. Ye, S. W. Guo, J. W. Lu, J. L. Huang, S. H. Lv, Y. X. Sun, Y. Y. Liu, X. L. Peng, J. Ren, S. Q. Li, X. P. Deng, X. J. Shi, Q. Zhang, Z. P. Yang, L. Tang, C. Z. Wei, L. L. Jia, J. W. Zhang, M. R. He, Y. A. Tong, Q. Y. Tang, X. H. Zhong, Z. H. Liu, N. Cao, C. L. Kou, H. Ying, Y. L. Yin, X. Q. Jiao, Q. S. Zhang, M. S. Fan, R. F. Jiang, F. S. Zhang and Z. X. Dou, Nature, 2018, 555, 363–366 CrossRef CAS PubMed.
- A. Muñoz-Sáez and L. L. R. Renwick, Nature, 2022, 609, 32 CrossRef PubMed.
- P. K. Rai, S. S. Lee, M. Zhang, Y. F. Tsang and K. H. Kim, Environ. Int., 2019, 125, 365–385 CrossRef CAS PubMed.
- F. Martinelli, R. Scalenghe, S. Davino, S. Panno, G. Scuderi, P. Ruisi, P. Villa, D. Stroppiana, M. Boschetti, L. R. Goulart, C. E. Davis and A. M. Dandekar, Agron. Sustainable Dev., 2015, 35, 1–25 CrossRef.
- Y. Fang and R. P. Ramasamy, Biosensors, 2015, 5, 537–561 CrossRef CAS PubMed.
- P. Li, Y. Y. Chen, Q. R. Xie, Y. Z. Xu, Z. Li, Y. Li, Z. B. Yin, X. H. Zhu, H. H. Xu and X. Z. Wu, Adv. Agrochem, 2023, 2, 340–348 CrossRef CAS.
- Y. L. Wu, L. J. Han, X. M. Wu, W. N. Jiang, H. Liao, Z. Xu and C. P. Pan, Adv. Agrochem, 2022, 1, 113–124 CrossRef.
- E. M. Hamilton, S. D. Young, E. H. Bailey, O. S. Humphrey and M. J. Watts, Environ. Sci. Technol., 2021, 55, 2422–2429 CrossRef CAS PubMed.
- A. M. Mustafa, S. Angeloni, D. Abouelenein, L. Acquaticci, J. B. Xiao, G. Sagratini, F. Maggi, S. Vittori and G. Caprioli, Food Chem., 2022, 367, 130743 CrossRef CAS PubMed.
- X. H. Li, X. H. Gao, W. Shi and H. M. Ma, Chem. Rev., 2014, 114, 590–659 CrossRef CAS PubMed.
- X. G. Liu, Adv. Agrochem, 2023, 2, 1–2 CrossRef CAS.
- Y. B. Huang, J. Zhai, L. H. Liu, Z. Y. Shang, X. Zhang, H. Huang, B. X. Shen and G. X. Chen, Anal. Chim. Acta, 2022, 1215, 339974 CrossRef CAS PubMed.
- S. Luo, R. C. Peng, Y. Wang, X. J. Liu, J. L. Ren, W. Li, Y. Xiong, S. L. Yi and Q. Wen, Anal. Bioanal. Chem., 2023, 415, 4849–4859 CrossRef CAS PubMed.
- M. Wang, K. Su, J. Cao, Y. X. She, A. M. Abd El-Aty, A. Hacimüftüoglu, J. Wang, M. M. Yan, S. H. Hong, S. B. Lao and Y. L. Wang, Talanta, 2019, 192, 295–300 CrossRef CAS PubMed.
- X. B. Shi, W. Wei, Z. D. Fu, W. L. Gao, C. Y. Zhang, Q. Zhao, F. M. Dene and X. Y. Lu, Talanta, 2019, 194, 809–821 CrossRef CAS PubMed.
- J. Wang, D. Q. Li, Y. X. Ye, Y. Qiu, J. W. Liu, L. Huang, B. Liang and B. L. Chen, Adv. Mater., 2021, 33, 2008020 CrossRef CAS PubMed.
- W. S. Zhang, H. D. Zhong, P. P. Zhao, A. G. Shen, H. B. Li and X. H. Liu, Food Control, 2022, 133, 108591 CrossRef.
- Z. Z. Gong, L. M. Xiong, H. Z. Shi, S. H. Yang, L. R. Herrera-Estrella, G. H. Xu, D. Y. Chao, J. R. Li, P. Y. Wang, F. Qin, J. J. Li, Y. L. Ding, Y. T. Shi, Y. Wang, Y. Q. Yang, Y. Guo and J. K. Zhu, Sci. China: Life Sci., 2020, 63, 635–674 CrossRef PubMed.
- A. Anzano, G. Bonanomi, S. Mazzoleni and V. Lanzotti, Phytochem. Rev., 2022, 21, 503–524 CrossRef CAS.
- P. Coatsworth, L. Gonzalez-Macia, A. S. P. Collins, T. Bozkurt and F. Gueder, Nat. Rev. Chem., 2023, 7, 7–25 CrossRef PubMed.
- H. Zhang, J. Zhu, Z. Gong and J. K. Zhu, Nat. Rev. Genet., 2022, 23, 104–119 CrossRef CAS PubMed.
- M. Weih, K. Hamnér and F. Pourazari, Plant Soil, 2018, 430, 7–21 CrossRef CAS.
- A. Singh, S. Jones, B. Ganapathysubramanian, S. Sarkar, D. Mueller, K. Sandhu and K. Nagasubramanian, Trends Plant Sci., 2021, 26, 53–69 CrossRef CAS PubMed.
- M. Ray, A. Ray, S. Dash, A. Mishra, K. G. Achary, S. Nayak and S. Singh, Biosens. Bioelectron., 2017, 87, 708–723 CrossRef CAS PubMed.
- J. M. Roper, J. F. Garcia and H. Tsutsui, ACS Omega, 2021, 6, 5101–5107 CrossRef CAS PubMed.
- L. Y. Wang, Y. L. Zou, H. Y. Kaw, G. Wang, H. Z. Sun, L. Cai, C. Y. Li, L. Y. Meng and D. H. Li, Plant Methods, 2020, 16, 1–17 CrossRef PubMed.
- L. Li, Q. Zhang and D. F. Huang, Sensors, 2014, 14, 20078–20111 CrossRef PubMed.
- B. A. Boughton, D. Thinagaran, D. Sarabia, A. Bacic and U. Roessner, Phytochem. Rev., 2016, 15, 445–488 CrossRef CAS PubMed.
- T. Wen, J. H. Li, Q. Wang, Y. Y. Gao, G. F. Hao and B. A. Song, Sci. Total Environ., 2023, 899, 165626 CrossRef CAS PubMed.
- S. Paulus, Plant Methods, 2019, 15, 1–13 CrossRef PubMed.
- D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Chem. Soc. Rev., 2017, 46, 7105–7123 RSC.
- J. Krämer, R. Kang, L. M. Grimm, L. De Cola, P. Picchetti and F. Biedermann, Chem. Rev., 2022, 122, 3459–3636 CrossRef PubMed.
- Y. Pang, M. Lu, H. Rha, W. Yang, A. Sharma, Y. Sun and J. S. Kim, Sci. China: Chem., 2023, 66, 1–14 Search PubMed.
- J. L. Yin, L. Huang, L. L. Wu, J. F. Li, T. D. James and W. Y. Lin, Chem. Soc. Rev., 2021, 50, 12098–12150 RSC.
- J. Du, K. Chen, Z. Yu, Y. Qiao, J. Liu, Q. Zhai, Z. Hu, S. G. Yang, J. Li and H. Teng, Adv. Agrochem., 2022, 1, 162–173 CrossRef.
- D. X. Cao, Z. Q. Liu, P. Verwilst, S. Koo, P. Jangjili, J. S. Kim and W. Y. Lin, Chem. Rev., 2019, 119, 10403–10519 CrossRef CAS PubMed.
- A. Dorlars, C. W. Schellhammer and J. Schroeder, Angew. Chem., Int. Ed. Engl., 1975, 14, 665–679 CrossRef.
- D. Lancet and I. Pecht, Biochemistry, 1977, 16, 5150–5157 CrossRef CAS PubMed.
- H. Zhan, Y. Wang, Z. Li, Z. Tang, J. Tian and X. Fei, J. Phys. Chem. A, 2021, 125, 2866–2875 CrossRef CAS PubMed.
- Y. Y. Zhang, Y. L. Zhao, Y. N. Wu, A. Y. Zhou, Q. L. Qu, X. L. Zhang, B. Song, K. M. Liu, R. H. Xiong and C. B. Huang, Mater. Chem. Front., 2021, 5, 4981–4988 RSC.
- Y. L. Chen, W. Yan, D. J. Guo, Y. Li, J. Li, H. Liu, L. R. Wei, N. Yu, B. Wang, Y. Zheng, M. F. Jing, J. Zhao and Y. H. Ye, Angew. Chem., Int. Ed., 2021, 60, 21934–21942 CrossRef CAS PubMed.
- Y. Tobimatsu, A. Wagner, L. Donaldson, P. Mitra, C. Niculaes, O. Dima, J. I. Kim, N. Anderson, D. Loque, W. Boerjan, C. Chapple and J. Ralph, Plant J., 2013, 76, 357–366 CrossRef CAS PubMed.
- K. Chen, M. Zhang, Y. Qi, J. Fan, X. Ma, H. L. Zhu and Y. Qian, Analyst, 2019, 144, 2320–2326 RSC.
- Y. L. Wang, F. Ding, X. S. Sun, S. J. Chen, H. R. Huang and H. Chen, Talanta, 2021, 234, 122655 CrossRef CAS PubMed.
- W. Pan, L. L. Han, X. Q. Cao, S. L. Shen, X. H. Pang and Y. Zhu, Food Chem., 2023, 407, 135163 CrossRef CAS PubMed.
- H. Q. Dong, T. B. Wei, X. Q. Ma, Q. Y. Yang, Y. F. Zhang, Y. J. Sun, B. B. Shi, H. Yao, Y. M. Zhang and Q. Lin, J. Mater. Chem. C, 2020, 8, 13501–13529 RSC.
- P. Gopikrishna, N. Meher and P. K. Iyer, ACS Appl. Mater. Interfaces, 2018, 10, 12081–12111 CrossRef CAS PubMed.
- G. Q. Yuan, H. Y. Ding, L. P. Peng, L. Y. Zhou and Q. L. Lin, Food Chem., 2020, 331, 127221 CrossRef CAS PubMed.
- S. Kusano, S. Nakamura, M. Izumi and S. Hagihara, Chem. Commun., 2022, 58, 1685–1688 RSC.
- Y. K. Zhang, C. Xu, H. Sun, J. D. Ai, M. G. Ren and F. G. Kong, Spectrochim. Acta, Part A, 2024, 304, 123345 CrossRef CAS PubMed.
- L. Wang, G. Wang, C. D. Shang, R. Kang and Y. Fang, ACS Appl. Mater. Interfaces, 2017, 9, 35419–35426 CrossRef CAS PubMed.
- F. Liu, M. Y. Xu, X. J. Chen, Y. G. Yang, H. J. Wang and G. P. Sun, Environ. Sci. Technol., 2015, 49, 11356–11362 CrossRef CAS PubMed.
- L. Li, S. Gao, L. Yang, Y. L. Liu, P. Li, F. Ye and Y. Fu, J. Hazard. Mater., 2021, 404, 124015 CrossRef CAS PubMed.
- Y. P. Zhang, Y. Qu, Y. Q. Zhang, Y. Gao and L. Wang, Chem. Eng. J., 2022, 448, 137631 CrossRef CAS.
- X.-F. Wu, Q.-J. Ma, X.-J. Wei, Y.-M. Hou and X. Zhu, Sens. Actuators, B, 2013, 183, 565–573 CrossRef CAS.
- Z. Y. Meng, J. Yin, F. Zhao, M. X. Li, Y. Zhang, Y. Y. Liang, Z. L. Wang and Y. Q. Yang, Eur. Polym. J., 2021, 158, 110705 CrossRef CAS.
- Z. Y. Meng, X. Y. Li, Y. Y. Liang, Y. X. Gu, X. Xu, Z. L. Wang, Y. Q. Yang and S. F. Wang, Int. J. Biol. Macromol., 2023, 247, 125807 CrossRef CAS PubMed.
- J. B. Grimm, A. N. Tkachuk, L. Q. Xie, H. Choi, B. Mohar, N. Falco, K. Schaefer, R. Patel, Q. S. Zheng, Z. Liu, J. Lippincott-Schwartz, T. A. Brown and L. D. Lavis, Nat. Methods, 2020, 17, 815–821 CrossRef CAS PubMed.
- C. J. Liu and C. N. Scott, Dyes Pigm., 2021, 196, 109792 CrossRef CAS.
- Z. X. Han, X. B. Zhang, L. Zhuo, Y. J. Gong, X. Y. Wu, J. Zhen, C. M. He, L. X. Jian, Z. Jing, G. L. Shen and R. Q. Yu, Anal. Chem., 2010, 82, 3108–3113 CrossRef CAS PubMed.
- F. T. Suo, X. W. Chen, H. X. Fang, Q. Y. Gong, C. M. Yu, N. D. Yang, S. Li, Q. Wu, L. Li and W. Huang, Dyes Pigm., 2019, 170, 107639 CrossRef CAS.
- F. Deng and Z. C. Xu, Chin. Chem. Lett., 2019, 30, 1667–1681 CrossRef CAS.
- L. L. Yang, S. Y. Zou, Y. H. Fu, W. Li, X. P. Wen, P. Y. Wang, Z. C. Wang, G. P. Ouyang, Z. Li and S. Yang, J. Agric. Food Chem., 2020, 68, 4285–4291 CrossRef CAS PubMed.
- L. Y. Wu, X. Wang, J. Huang, H. Y. Wei and C. Kan, J. Photochem. Photobiol., A, 2022, 429, 113936 CrossRef CAS.
- C. Kan, X. T. Shao, L. Y. Wu, Y. Zhang, X. F. Bao and J. Zhu, J. Photochem. Photobiol., A, 2020, 398, 112618 CrossRef CAS.
- C. Kan, F. Song, X. T. Shao, L. Y. Wu and J. Zhu, J. Photochem. Photobiol., A, 2020, 390, 112306 CrossRef CAS.
- B. Biswal, D. Mallick, M. Thirunavoukkarasu, R. Mohanty and B. Bag, Sens. Actuators, B, 2016, 232, 410–419 CrossRef CAS.
- W. J. Ma, S. Tan, P. Yang, A. L. Tang, L. L. Yang, J. Y. Chen, S. T. Liu, M. H. Ge, X. Zhou and S. Yang, Sens. Actuators, B, 2023, 390, 133948 CrossRef CAS.
- F. Song, C. Yang, X. T. Shao, L. Du, J. Zhu and C. Kan, Dyes Pigm., 2019, 165, 444–450 CrossRef CAS.
- S. Paul, S. Bhuyan, S. K. Mukhopadhyay, N. C. Murmu and P. Banerjee, ACS Sustainable Chem. Eng., 2019, 7, 13687–13697 CrossRef CAS.
- S. Erdemir, M. Oguz and S. Malkondu, J. Hazard. Mater., 2023, 452, 131278 CrossRef CAS PubMed.
- J. Y. Zhao, K. Liu, R. T. Wang, T. H. Liu, Z. F. Wu, L. P. Ding and Y. Fang, ACS Appl. Mater. Interfaces, 2022, 14, 53323–53330 CrossRef CAS PubMed.
- X. Wang, S. Y. Cheng, C. Y. Liu, Y. Zhang, M. J. Su, X. D. Rong, H. C. Zhu, M. H. Yu, W. L. Sheng and B. C. Zhu, Sci. Total Environ., 2022, 840, 156445 CrossRef CAS PubMed.
- F. Y. Yan, K. Q. Fan, Z. J. Bai, R. Q. Zhang, F. L. Zu, J. X. Xu and X. Li, TrAC, Trends Anal. Chem., 2017, 97, 15–35 CrossRef CAS.
- S. Keerthana, B. Sam, L. George, Y. N. Sudhakar and A. Varghese, J. Fluoresc., 2021, 31, 1251–1276 CrossRef PubMed.
- T. S. Wang, N. Zhang, W. Bar and Y. Y. Bao, Polym. Chem., 2020, 11, 3095–3114 RSC.
- M. N. Potter, J. R. Green and B. Mutus, Talanta, 2022, 237, 122981 CrossRef CAS PubMed.
- Y. Takaoka, S. Miyagawa, A. Nakamura, S. Egoshi, S. Tsukiji and M. Ueda, Sci. Rep., 2020, 10, 5333 CrossRef CAS PubMed.
- M. A. Assiri, M. T. Waseem, A. Hamad, M. Imran, U. Farooq and S. A. Shahzad, Spectrochim. Acta, Part A, 2023, 290, 122298 CrossRef CAS PubMed.
- B. Parízkova, A. Zukauskaite, T. Vain, P. Grones, S. Raggi, M. F. Kubes, M. Kieffer, S. M. Doyle, M. Strnad, S. Kepinski, R. Napier, K. Dolezal, S. Robert and O. Novák, New Phytol., 2021, 230, 535–549 CrossRef PubMed.
- J. H. Wang, Y. M. Liu, Z. M. Dong, J. B. Chao, H. Wang, Y. Wang and S. M. Shuang, J. Hazard. Mater., 2020, 382, 121056 CrossRef CAS PubMed.
- Q. H. Yu, F. Ding, J. L. Shen and X. J. He, Talanta, 2021, 228, 122218 CrossRef CAS PubMed.
- Y. F. Huang, Y. B. Zhang, F. G. Huo, Y. M. Liu and C. X. Yin, Sens. Actuators, B, 2019, 301, 127123 CrossRef CAS.
- C. Y. Jiang, H. J. Huang, X. Y. Kang, L. Yang, Z. Xi, H. Y. Sun, M. D. Pluth and L. Yi, Chem. Soc. Rev., 2021, 50, 7436–7495 RSC.
- Q. Li, L. J. Chai, G. P. Dong, X. M. Zhang and L. P. Du, Front. Mol. Biosci., 2021, 8, 666605 CrossRef CAS PubMed.
- S. Lee, M. Jen, T. Jang, G. Lee and Y. Pang, Sci. Rep., 2022, 12, 6557 CrossRef CAS PubMed.
- T. Ueno and T. Nagano, Nat. Methods, 2011, 8, 642–645 CrossRef CAS PubMed.
- M. Benstead, G. H. Mehl and R. W. Boyle, Tetrahedron, 2011, 67, 3573–3601 CrossRef CAS.
- H. Wen, Q. H. Wu, C. N. Li, T. T. Sun and Z. G. Xie, ACS Appl. Nano Mater., 2022, 5, 1500–1507 CrossRef CAS.
- H. Lu and Z. Shen, Front. Chem., 2020, 8, 290 CrossRef PubMed.
- A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess, Chem. Soc. Rev., 2013, 42, 77–88 RSC.
- M. Poddar and R. Misra, Coord. Chem. Rev., 2020, 421, 213462 CrossRef CAS.
- Y. Zhang, H. Li, L. Y. Niu, Q. Z. Yang, Y. F. Guan and L. Feng, Analyst, 2014, 139, 3146–3153 RSC.
- L. Michels, V. Gorelova, Y. Harnvanichvech, J. W. Borst, B. Albada, D. Weijers and J. Sprakel, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 18110–18118 CrossRef CAS PubMed.
- W. J. Chen, Y. H. Guan, Q. Chen, J. Ren, Y. Xie and J. Yin, Dyes Pigm., 2022, 200, 110134 CrossRef CAS.
- A. Starodubtseva, T. Kalachova, O. Iakovenko, V. Stoudková, V. Zhabinskii, V. Khripach, E. Ruelland, J. Martinec, L. Burketová and V. Kravets, Int. J. Mol. Sci., 2021, 22, 3599 CrossRef CAS PubMed.
- X. K. Yang, J. M. Wang, Z. L. Zhang, B. Zhang, X. L. Du, J. Zhang and J. H. Wang, Food Chem., 2023, 416, 135730 CrossRef CAS PubMed.
- Y. X. Fu, Z. Y. Zhang, W. Y. Z. Guo, Y. J. Dai, Z. Y. Wang, W. C. Yang and G. F. Yang, Pest Manage. Sci., 2022, 78, 4947–4955 CrossRef CAS PubMed.
- J. J. Wu, D. D. Su, C. Q. Qin, W. Li, J. Rodrigues, R. L. Sheng and L. T. Zeng, Talanta, 2019, 201, 111–118 CrossRef CAS PubMed.
- Y. D. Guo, Y. Luo, N. Wang, M. G. Tang, J. C. Xiao, S. W. Chen and J. Y. Wang, Talanta, 2020, 212, 120583 CrossRef CAS PubMed.
- X. X. Ma, Y. R. Huang, W. J. Chen, J. Liu, S. H. Liu, J. Yin and G. F. Yang, Angew. Chem., Int. Ed., 2023, 62, e202216109 CrossRef CAS PubMed.
- A. Promchat, P. Rashatasakhon and M. Sukwattanasinitt, J. Hazard. Mater., 2017, 329, 255–261 CrossRef CAS PubMed.
- N. Vijay, S. P. Wu and S. Velmathi, Sens. Actuators, B, 2022, 371, 132567 CrossRef CAS.
- W. Yuan, C. Y. Wan, J. J. Zhang, Q. S. Li, P. Zhang, K. Zheng, Q. Zhang and C. F. Ding, Spectrochim. Acta, Part A, 2023, 297, 122719 CrossRef CAS PubMed.
- A. Nicol, K. Wang, R. T. K. Kwok, Z. G. Song, N. Li and B. Z. Tang, ACS Appl. Mater. Interfaces, 2019, 11, 22028 CrossRef CAS PubMed.
- J. Q. Zuo, E. A. Zhu, W. J. Yin, C. Y. Yao, J. J. Liao, X. N. Ping, Y. Q. Zhu, X. T. Cai, Y. C. Rao, H. Feng, K. W. Zhang and Z. S. Qian, Chem. Sci., 2023, 14, 2139–2148 RSC.
- V. Martinez and M. Henary, Chem. – Eur. J., 2016, 22, 13764–13782 CrossRef CAS PubMed.
- H. Tajalli, A. G. Gilani, M. S. Zakerhamidi and P. Tajalli, Dyes Pigm., 2008, 78, 15–24 CrossRef CAS.
- G. S. Alemán-Nava, S. P. Cuellar-Bermudez, M. Cuaresma, R. Bosma, K. Muylaert, B. E. Ritmann and R. Parra, J. Microbiol. Methods, 2016, 128, 74–79 CrossRef PubMed.
- X. Y. Zeng, X. X. Ma, J. Dong, B. Li, S. H. Liu, J. Yin and G. F. Yang, Angew. Chem., Int. Ed., 2023, 135, e202312618 CrossRef.
- X. Zeng, Y. Huang, J. Dong, X. Ma, J.-X. Nan, W. Chen, H.-Y. Lin, W.-C. Yang, X. Liu, J. Yin and G.-F. Yang, Adv. Agrochem., 2022, 1, 73–84 CrossRef.
- H. A. Shindy, Dyes Pigm., 2017, 145, 505–513 CrossRef CAS.
- L. Li, X. G. Dong, J. R. Li and J. Wei, Dyes Pigm., 2020, 183, 108756 CrossRef CAS.
- Y. N. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332–4353 RSC.
- Q. Peng, Y. Yi, Z. Shuai and J. Shao, J. Am. Chem. Soc., 2007, 129, 9333–9339 CrossRef CAS PubMed.
- M. Wang, G. X. Zhang, D. Q. Zhang, D. B. Zhu and B. Tang, J. Mater. Chem., 2010, 20, 1858–1867 RSC.
- D. Ding, K. Li, B. Liu and B. Z. Tang, Acc. Chem. Res., 2013, 46, 2441–2453 CrossRef CAS PubMed.
- L. Burratti, E. Ciotta, F. De Matteis and P. Prosposito, Nanomaterials, 2021, 11, 276 CrossRef CAS PubMed.
- J. M. Wang, J. Z. Jiang, F. Y. Li, J. Zou, K. Xiang, H. T. Wang, Y. J. Li and X. Li, Green Chem., 2023, 25, 32–58 RSC.
- G. Gao, Y. W. Jiang, W. Sun and F. G. Wu, Chin. Chem. Lett., 2018, 29, 1475–1485 CrossRef CAS.
- G. Yu, J. Liang, Z. He and M. Sun, Chem. Biol., 2006, 13, 723–731 CrossRef CAS PubMed.
- A. Singh, A. Guleria, S. Neogy and M. C. Rath, Arabian J. Chem., 2020, 13, 3149–3158 CrossRef CAS.
- A. Kumar and K. Gupta, J. Mater. Chem. A, 2017, 5, 6146–6163 RSC.
- J. M. Li, H. H. Wu, I. Santana, M. Fahlgren and J. P. Giraldo, ACS Appl. Mater. Interfaces, 2018, 10, 28279–28289 CrossRef CAS PubMed.
- Z. K. Hu, W. J. Long, T. K. Liu, Y. T. Guan, G. H. Lei, Y. X. Suo, M. G. Jia, J. L. He, H. Y. Chen, Y. B. She and H. Y. Fu, Spectrochim. Acta, Part A, 2023, 294, 122517 CrossRef CAS PubMed.
- D. Saika, P. Dutta, N. Sen Sarma and N. C. Adhikary, Sens. Actuators, B, 2016, 230, 149–156 CrossRef.
- L. Zhang, C. Xu and B. Li, Microchim. Acta, 2009, 166, 61–68 CrossRef CAS.
- J. Wang, D. Q. Li, Y. Qiu, X. Y. Liu, X. Zhang, L. Huang, H. M. Wen and J. Hu, Sens. Actuators, B, 2019, 301, 126984 CrossRef CAS.
- K. Zhang, Q. Mei, G. Guan, B. Liu, S. Wang and Z. Zhang, Anal. Chem., 2010, 82, 9579–9586 CrossRef CAS PubMed.
- F. Qu, X. Zhou, J. Xu, H. Li and G. Xie, Talanta, 2009, 78, 1359–1363 CrossRef CAS PubMed.
- X. W. Meng, J. F. Wei, X. L. Ren, J. Ren and F. Q. Tang, Biosens. Bioelectron., 2013, 47, 402–407 CrossRef CAS PubMed.
- K. He, X. Yu, L. Qin and Y. Wu, Food Chem., 2022, 390, 133116 CrossRef CAS PubMed.
- H. Wang, C. Chen, Y. Liu, Y. Wu, Y. Yuan and Q. Zhou, Talanta, 2019, 198, 242–248 CrossRef CAS PubMed.
- S. Y. Chen, S. N. Yun, Y. J. Liu, R. J. Yu, Q. Tu, J. Y. Wang and M. S. Yuan, Analyst, 2022, 147, 3258–3265 RSC.
- I. A. Mir, S. Kumar, M. A. Bhat, Y. L. Xie, A. A. Wani and L. Zhu, Colloids Surf., A, 2021, 626, 127090 CrossRef CAS.
- F. Zhang, Y. Liu, P. Ma, S. Tao, Y. Sun, X. Wang and D. Song, Talanta, 2019, 204, 13–19 CrossRef CAS PubMed.
- C. Zhang, K. Zhang, T. T. Zhao, B. H. Liu, Z. Y. Wang and Z. P. Zhang, Sens. Actuators, B, 2017, 252, 1083–1088 CrossRef CAS.
- N. Ding, D. L. Zhou, G. C. Pan, W. Xu, X. Chen, D. Y. Li, X. H. Zhang, J. Y. Zhu, Y. A. Ji and H. W. Song, ACS Sustainable Chem. Eng., 2019, 7, 8397–8404 CrossRef CAS.
- N. V. Ghinaiya, T. J. Park and S. K. Kailasa, J. Photochem. Photobiol., A, 2023, 444, 114980 CrossRef CAS.
- S. Huang, M. Guo, J. Tan, Y. Geng, J. Wu, Y. Tang, C. Su, C. C. Lin and Y. Liang, ACS Appl. Mater. Interfaces, 2018, 10, 39056–39063 CrossRef CAS PubMed.
- X. Z. Jin, X. B. Sun, G. Chen, L. X. Ding, Y. H. Li, Z. K. Liu, Z. J. Wang, W. Pan, C. H. Hu and J. P. Wang, Carbon, 2015, 81, 388–395 CrossRef CAS.
- J. Lin, X. Huang, E. Kou, W. Cai, H. Zhang, X. Zhang, Y. Liu, W. Li, Y. Zheng and B. Lei, Biosens. Bioelectron., 2023, 219, 114848 CrossRef CAS PubMed.
- S. S. Wee, Y. H. Ng and S. M. Ng, Talanta, 2013, 116, 71–76 CrossRef CAS PubMed.
- R. Y. Xie, Y. Y. Qu, M. Y. Tang, J. Q. Zhao, S. Chua, T. T. Li, F. Zhang, A. E. H. Wheatley and F. Chai, Food Chem., 2021, 364, 130366 CrossRef CAS PubMed.
- J. Plácido, S. Bustamante-López, K. E. Meissner, D. E. Kelly and S. L. Kelly, Sci. Total Environ., 2019, 656, 531–539 CrossRef PubMed.
- P. Keerthana, A. K. Das, M. Bharath, M. Ghosh and A. Varghese, J. Environ. Chem. Eng., 2023, 11, 109325 CrossRef CAS.
- D. Pooja, S. Saini, A. Thakur, B. Kumar, S. Tyagi and M. K. Nayak, J. Hazard. Mater., 2017, 328, 117–126 CrossRef CAS PubMed.
- B. X. Lin, Y. Yan, M. L. Guo, Y. J. Cao, Y. Yu, T. Y. Zhang, Y. Huang and D. Wu, Food Chem., 2018, 245, 1176–1182 CrossRef CAS PubMed.
- M. M. F. Chang, I. R. Ginjom and S. M. Ng, Sens. Actuators, B, 2017, 242, 1050–1056 CrossRef CAS.
- J. Hou, J. Dong, H. Zhu, X. Teng, S. Ai and M. Mang, Biosens. Bioelectron., 2015, 68, 20–26 CrossRef CAS PubMed.
- F. A. Tafreshi, Z. Fatahi, S. F. Ghasemi, A. Taherian and N. Esfandiari, PLoS One, 2020, 15, e0230646 CrossRef PubMed.
- B. B. Chen, M. L. Liu, L. Zhan, C. M. Li and C. Z. Huang, Anal. Chem., 2018, 90, 4003–4009 CrossRef CAS PubMed.
- L. Behera, D. Pati, B. B. Sahu and S. Mohapatra, Colloids Surf., A, 2022, 653, 130002 CrossRef CAS.
- G. J. Ren, Q. Zhang, S. Li, S. S. Fu, F. Chai, C. G. Wang and F. Y. Qu, Sens. Actuators, B, 2017, 243, 244–253 CrossRef CAS.
- G. Yang, X. Wan, Y.-K. Su, X. Zeng and J. Tang, J. Mater. Chem. A, 2016, 4, 12841–12849 RSC.
- L. Li, D. Liu, A. P. Shi and T. Y. You, Sens. Actuators, B, 2018, 255, 1762–1770 CrossRef CAS.
- H. Li, C. Sun, R. Vijayaraghavan, F. Zhou, X. Zhang and D. R. MacFarlane, Carbon, 2016, 104, 33–39 CrossRef CAS.
- M. Yang, M. Liu, Z. Wu, Y. He, Y. Ge, G. Song and J. Zhou, Microchim. Acta, 2019, 186, 585 CrossRef PubMed.
- W. K. Li, J. T. Feng and Z. Q. Ma, Carbon, 2020, 161, 685–693 CrossRef CAS.
- S. Mohapatra, M. K. Bera and R. K. Das, Sens. Actuators, B, 2018, 263, 459–468 CrossRef CAS.
- S. Patel, K. Shrivas, D. Sinha, I. Karbhal, T. K. Patle, Monisha and Tikeshwari, Spectrochim. Acta, Part A, 2023, 299, 122824 CrossRef CAS PubMed.
- H. Yuan, D. Li, Y. Liu, X. Xu and C. Xiong, Analyst, 2015, 140, 1428–1431 RSC.
- Y. Q. Shi, D. D. Kong, W. T. Li, Y. Z. Wei, X. Wei, F. F. Qu, Y. H. Zhang, P. C. Nie, X. P. Feng and Y. He, Anal. Chim. Acta, 2023, 1244, 340844 CrossRef CAS PubMed.
- T. Anusuya, V. Kumar and V. Kumar, Chemosphere, 2021, 282, 131019 CrossRef CAS PubMed.
- L. Wang, W. Li, B. Wu, Z. Li, S. Wang, Y. Liu, D. Pan and M. Wu, Chem. Eng. J., 2016, 300, 75–82 CrossRef CAS.
- Z. S. Qian, X. Y. Shan, L. J. Chai, J. R. Chen and H. Peng, Biosens. Bioelectron., 2015, 68, 225–231 CrossRef CAS PubMed.
- L. L. Wang, J. Zheng, S. Yang, C. C. Wu, C. H. Liu, Y. Xiao, Y. H. Li, Z. H. Qing and R. H. Yang, ACS Appl. Mater. Interfaces, 2015, 7, 19509–19515 CrossRef CAS PubMed.
- C.-T. Hsieh, P.-Y. Sung, Y. A. Gandomi, K. S. Khoo and J.-K. Chang, Chemosphere, 2023, 318, 137926 CrossRef CAS PubMed.
- C. P. Zhang, B. X. Lin, Y. J. Cao, M. L. Guo and Y. Yu, J. Agric. Food Chem., 2017, 65, 3065–3073 CrossRef CAS PubMed.
- J. Jiménez-López, E. J. Llorent-Martínez, P. Ortega-Barrales and A. Ruiz-Medina, Talanta, 2020, 207, 120344 CrossRef PubMed.
- C. Sahub, T. Tuntulani, T. Nhujak and B. Tomapatanaget, Sens. Actuators, B, 2018, 258, 88–97 CrossRef CAS.
- Y. Liu, L. Cao, M. Zan, J. Peng, P. Wang, X. Pang, Y. Zhang, L. Li, W.-F. Dong and Q. Mei, Talanta, 2021, 233, 122465 CrossRef CAS PubMed.
- X. Zhang, C. Li, S. Zhao, H. Pang, Y. Han, X. Luo, W. Tang and Z. Li, Opt. Mater., 2020, 110, 110461 CrossRef CAS.
- S. Shen, B. Huang, X. Guo and H. Wang, J. Mater. Chem. B, 2019, 7, 7033–7041 RSC.
- Z. Zhang, Q. R. Zhang, L. F. Li, D. Lin and C. L. Jiang, ACS Sustainable Chem. Eng., 2023, 11, 4998–5006 CrossRef CAS.
- M. C. Rong, J. C. Ye, B. Y. Chen, Y. Z. Wen, X. Z. Deng and Z. Q. Liu, Sens. Actuators, B, 2020, 309, 127807 CrossRef CAS.
- Y. L. Xu, X. Y. Niu, H. L. Chen, S. G. Zhao and X. G. Chen, Chin. Chem. Lett., 2017, 28, 338–344 CrossRef CAS.
- P. Sharma and M. S. Mehata, Mater. Res. Bull., 2020, 131, 110978 CrossRef CAS.
- L. Zhang, Z.-W. Wang, S.-J. Xiao, D. Peng, J.-Q. Chen, R.-P. Liang, J. Jiang and J.-D. Qiu, ACS Appl. Nano Mater., 2018, 1, 1484–1491 CrossRef CAS.
- Y. E. Shi, P. Zhang, D. Q. Yang and Z. G. Wang, Chem. Commun., 2020, 56, 10982–10988 RSC.
- X. Wang, W. Guo, X. Wang, Q. Hua, F. Tang, X. Li, F. Luan, Z. Zhang, C. Tian, X. Zhuang and L. Zhao, Arabian J. Chem., 2022, 15, 104080 CrossRef CAS.
- H. Q. Zhang, Y. F. Li and F. Gan, Sens. Actuators, B, 2023, 374, 132817 CrossRef CAS.
- X. Y. Li, C. Chen, F. F. Xu, Z. G. Liang, G. H. Xu, F. D. Wei, J. Yang, Q. Hu, J. J. Zou and Y. Cen, Talanta, 2023, 260, 124639 CrossRef CAS PubMed.
- J. Sun, B. S. Shengping Zhang, M. Alomar, A. S. Alqarni, M. S. Najla Alotaibi, M. S. Badriah Alshahrani, A. A. Alghamdi, Z. Kou, W. Shen, Y. Chen and J. Zhang, Chem. Rec., 2023, 23, e202200268 CrossRef CAS PubMed.
- J. Gu, X. Lu, G. Li, B. Shan, J. Liu, Y. Qu, H. Ye, K. Xi and H. Wu, Chem. Eng. J., 2023, 467, 143445 CrossRef CAS.
- S. Zhang, Z. J. Qi and Y. Li, Ceram. Int., 2022, 48, 21118–21124 CrossRef CAS.
- Y. Feng, F. Zhou, Q. Deng and C. Peng, Ceram. Int., 2020, 46, 8320–8327 CrossRef CAS.
- X. Wang, X. Zhang, C. Haiyan and Y. Huang, J. Mater. Chem. B, 2020, 8, 10837–10844 RSC.
- F. P. G. de Arquer, D. V. Talapin, V. I. Klimov, Y. Arakawa, M. Bayer and E. H. Sargent, Science, 2021, 373, eaaz8541 CrossRef PubMed.
- R. Chen and D. J. Lockwood, J. Electrochem. Soc., 2002, 149, S69 CrossRef CAS.
- M. Zulfajri, H. N. Abdelhamid, S. Sudewi, S. Dayalan, A. Rasool, A. Habib and G. G. Huang, Biosensors, 2020, 10, 68 CrossRef CAS PubMed.
- D. Xu, Q. Lin and H.-T. Chang, Small Methods, 2020, 4, 1900387 CrossRef CAS.
- X. R. Li, H. Ma, M. Deng, A. Iqbal, X. Y. Liu, B. Li, W. S. Liu, J. P. Li and W. W. Qin, J. Mater. Chem. C, 2017, 5, 2149–2152 RSC.
- I. Song, C. Park and H. C. Choi, RSC Adv., 2015, 5, 7495–7514 RSC.
- Z. Li, X. Yang, Y. Zhou, A. Huang, Y. Sun, Z. Duan, S. Yang, C. Liao, Y. Liu and X. Wen, Microchem. J., 2023, 193, 109135 CrossRef CAS.
- J. Gu, X. Lu, G. Li, B. Shan, J. Liu, Y. Qu, H. Ye, K. Xi and H. Wu, Chem. Eng. J., 2023, 467, 143445 CrossRef CAS.
- F. Yan, J. Sun, Y. Zang, Z. Sun, H. Zhang, J. Xu and X. Wang, Dyes Pigm., 2021, 195, 109720 CrossRef CAS.
- C. Guan, X. Yue, J. Fan and Q. Xiang, Chin. J. Catal., 2022, 43, 2484–2499 CrossRef CAS.
- R. Li, T. T. Chen and X. L. Pan, ACS Nano, 2021, 15, 3808–3848 CrossRef CAS PubMed.
- S. Y. Wu, H. Min, W. Shi and P. Cheng, Adv. Mater., 2020, 32, 1805871 CrossRef CAS PubMed.
- X. Xu, M. Ma, T. Sun, X. Zhao and L. Zhang, Biosensors, 2023, 13, 435 CrossRef CAS PubMed.
- D. Zhao, S. Yu, W. J. Jiang, Z. H. Cai, D. L. Li, Y. L. Liu and Z. Z. Chen, Molecules, 2022, 27, 2226 CrossRef CAS PubMed.
- Y. Shu, Q. Y. Ye, T. Dai, Q. Xu and X. Y. Hu, ACS Sens., 2021, 6, 641–658 CrossRef CAS PubMed.
- Q. Li, J. H. Wu, Q. T. Yang, H. Y. Li and F. Li, Anal. Chem., 2021, 93, 7362–7368 CrossRef CAS PubMed.
- W. X. Chen, J. S. Fang, Y. W. Zhang, G. L. Chen, S. Zhao, C. Zhang, R. Xu, J. H. Bao, Y. M. Zhou and X. Xiang, Nanoscale, 2018, 10, 4463–4474 RSC.
- J. Yang, C. J. Ren, M. Liu, W. W. Li, D. J. Gao, H. D. Li and Z. L. Ning, Molecules, 2023, 28, 4919 CrossRef CAS PubMed.
- X. Y. Xu and B. Yan, ACS Appl. Mater. Interfaces, 2015, 7, 721–729 CrossRef CAS PubMed.
- Y. F. Xia, G. M. Bao, X. X. Peng, X. Y. Wu, H. F. Lu, Y. F. Zhong, W. Li, J. X. He, S. Y. Liu, Q. Fan, S. H. Li, W. Xiao and H. Q. Yuan, Anal. Chim. Acta, 2022, 1221, 340115 CrossRef CAS PubMed.
- N. W. H. Guo, L. P. Peng, Y. Chen, Y. S. Liu, C. L. Li, H. Zhang and W. Yang, Talanta, 2022, 250, 123710 CrossRef PubMed.
- H. Xu, C. S. Cao and B. Zhao, Chem. Commun., 2015, 51, 10280–10283 RSC.
- G. X. Qin, Y. Q. Kong, T. J. Gan and Y. H. Ni, Inorg. Chem., 2022, 61, 8966–8975 CrossRef CAS PubMed.
- T. Wang, L. Zhang, J. Y. Zhang, G. X. Guo, X. H. Jiang, Z. W. Zhang and S. Q. Li, Food Chem., 2023, 416, 135853 CrossRef CAS PubMed.
- H. Eskandari, M. Amirzehni, J. Hassanzadeh and B. Vahid, Microchim. Acta, 2020, 187, 673 CrossRef CAS PubMed.
- K. P. Yin, S. Q. Wu, H. Zheng, L. Gao, J. F. Liu, C. L. Yang, L. W. Qi and J. J. Peng, Langmuir, 2021, 37, 5321–5328 CrossRef CAS PubMed.
- S. L. Zhao, J. N. Xiao, T. X. Zheng, M. Y. Liu, H. N. Wu and Z. L. Liu, ACS Omega, 2019, 4, 16378–16384 CrossRef CAS PubMed.
- G. F. Ji, X. C. Gao, T. X. Zheng, W. H. Guan, H. T. Liu and Z. L. Liu, Inorg. Chem., 2018, 57, 10525–10532 CrossRef CAS PubMed.
- Y. Qiang, W. X. Yang, X. S. Zhang, X. L. Luo, W. Z. Tang, T. L. Yue and Z. H. Li, Microchim. Acta, 2022, 189, 130 CrossRef CAS PubMed.
- P. Chakraborty, A. Rana, S. Mukherjee and S. Biswas, Inorg. Chem., 2023, 62, 802–809 CrossRef CAS PubMed.
- Q. Wu, H. Tao, Y. Wu, X. Wang, Q. Shi and D. Xiang, Foods, 2022, 11, 2405 CrossRef CAS PubMed.
- P. Janjani, U. Bhardwaj, R. Gupta and H. S. Kushwaha, Anal. Chim. Acta, 2022, 1202, 339676 CrossRef CAS PubMed.
- Y. Q. Zhang, J. X. Liu, X. H. Wu, W. Q. Tao and Z. Li, Anal. Chim. Acta, 2020, 1131, 68–79 CrossRef CAS PubMed.
- Y. Yu, G. L. Huang, X. L. Luo, W. M. Lin, Y. Han, J. H. Huang and Z. H. Li, Microchim. Acta, 2022, 189, 325 CrossRef CAS PubMed.
- D. W. Zhang, Y. Xu, Q. L. Liu and Z. G. Xia, Inorg. Chem., 2018, 57, 4613–4619 CrossRef CAS PubMed.
- T. Yan, H. L. Ding, R. Feng, R. F. Yuan, Y. X. Zhao, M. Sun, L. G. Yan and Q. Wei, ACS Appl. Mater. Interfaces, 2022, 14, 25308–25316 CrossRef CAS PubMed.
- M. X. Fan, T. T. Gan, G. F. Yin, F. B. B. Cheng and N. J. Zhao, RSC Adv., 2021, 11, 27845–27854 RSC.
- F. Asadi, S. N. Azizi and M. J. Chaichi, Mater. Sci. Eng., C, 2019, 105, 110058 CrossRef CAS PubMed.
- L. Yang, Y. L. Liu, C. G. Liu, Y. Fu and F. Ye, RSC Adv., 2020, 10, 19149–19156 RSC.
- M. L. Li, Y. Gao, W. T. Yang, C. W. Zhang, Y. Fang, C. Wang, S. Y. Song and Q. H. Pan, Inorg. Chem., 2022, 61, 9801–9807 CrossRef CAS PubMed.
- Q. Li, D. Li, Z. Q. Wu, K. Shi, T. H. Liu, H. Y. Yin, X. B. Cai, Z. L. Fan, W. Zhu and D. X. Xue, Inorg. Chem., 2022, 61, 15213–15224 CrossRef CAS PubMed.
- J. T. Liu, L. Y. Ye, Y. Y. Mo and H. Yang, Food Chem., 2022, 370, 131034 CrossRef CAS PubMed.
- T. Y. Zhong, D. L. Li, C. Li, Z. Zhang and G. Wang, Anal. Methods, 2022, 14, 2714–2722 RSC.
- M. L. Guo, J. T. Chi, Y. J. Li, G. I. N. Waterhouse, S. Y. Ai, J. Y. Hou and X. Y. Li, Microchim. Acta, 2020, 187, 534 CrossRef CAS PubMed.
- S. Govindaraju, P. Puthiaraj, M. H. Lee and K. Yun, ACS Omega, 2018, 3, 12052–12059 CrossRef CAS PubMed.
- Y. Cai, H. S. Zhu, W. C. Zhou, Z. Y. Qiu, C. C. Chen, A. R. Qileng, K. S. Li and Y. J. Liu, Anal. Chem., 2021, 93, 7275–7282 CrossRef CAS PubMed.
- W. T. Li, X. A. Zhang, X. T. Hu, Y. Q. Shi, W. Xin, N. N. Liang, T. T. Shen, J. B. Xiao, M. Daglia, X. B. Zou and J. Y. Shi, Anal. Chim. Acta, 2022, 1226, 340153 CrossRef CAS PubMed.
- P. T. Liu, R. S. Hao, W. L. Sun, Z. Y. Lin and T. F. Jing, Luminescence, 2022, 37, 1793–1799 CrossRef CAS PubMed.
- H. N. Abdelhamid, A. Bermejo-Gómez, B. Martín-Matute and X. D. Zou, Microchim. Acta, 2017, 184, 3363–3371 CrossRef CAS PubMed.
- Y. Shu, Q. Y. Ye, T. Dai, J. Guan, Z. P. Ji, Q. Xu and X. Y. Hu, J. Hazard. Mater., 2022, 430, 128360 CrossRef CAS PubMed.
- Y. Q. Sun, Y. Cheng and X. B. Yin, Anal. Chem., 2021, 93, 3559–3566 CrossRef CAS PubMed.
- M. Gutiérrez, Y. Zhang and J. C. Tan, Chem. Rev., 2022, 122, 10438–10483 CrossRef PubMed.
- Z. K. Zhang, L. Zhang, P. Han and Q. J. Liu, Microchim. Acta, 2022, 189, 438 CrossRef CAS PubMed.
- A. Biranje, N. Azmi, A. Tiwari and A. Chaskar, J. Fluoresc., 2021, 31, 1241–1250 CrossRef CAS PubMed.
- B. K. John, T. Abraham and B. Mathew, J. Fluoresc., 2022, 32, 449–471 CrossRef CAS PubMed.
- R. Yousefi, S. Asgari, A. B. Dehkordi, G. M. Ziarani, A. Badiei, F. Mohajer, R. S. Varma and S. Iravani, Environ. Res., 2023, 226, 115664 CrossRef CAS PubMed.
- R. Xue, Y. S. Liu, S. L. Huang and G. Y. Yang, ACS Sens., 2023, 8, 2124–2148 CrossRef CAS PubMed.
- J. X. Fan, J. Li, W. F. Zhou, H. X. Gao, R. H. Lu and H. C. Guo, Luminescence, 2023, 38, 1729–1737 CrossRef CAS PubMed.
- R. Paz, H. Viltres, N. K. Gupta, V. Phung, S. Srinivasan, A. R. Rajabzadeh and C. Leyva, Chemosphere, 2023, 342, 140145 CrossRef CAS PubMed.
- M. Afshari, M. Dinari, H. Farrokhpour and F. Zamora, ACS Appl. Mater. Interfaces, 2022, 14, 22398–22406 CrossRef CAS PubMed.
- L. L. Song, Q. Zhang, L. Min, X. Y. Guo, W. Q. Gao, L. Cui and C. Y. Zhang, Talanta, 2024, 266, 124964 CrossRef CAS PubMed.
- S. Knežević, N. T. Jovanović, F. Vlahović, V. Ajdačić, V. Costache, J. Vidić, I. Opsenica and D. Stanković, Chemosphere, 2023, 341, 139930 CrossRef PubMed.
- G. Chen, H. H. Lan, S. L. Cai, B. Sun, X. L. Li, Z. H. He, S. R. Zheng, J. Fan, Y. Liu and W. G. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 12830–12837 CrossRef CAS PubMed.
- Z. F. Yan, L. Fang, Z. G. He, H. Xie, B. B. Liu, B. Guo and Y. W. Yao, Small, 2022, 18, 2200388 CrossRef CAS PubMed.
- B. Zhu, L. Zhu, S. Deng, Y. Wan, F. Qin, H. Han and J. Luo, J. Hazard. Mater., 2023, 459, 132081 CrossRef CAS PubMed.
- J. W. Li, M. C. Gao, X. Y. Xia, Y. Cen, F. D. Wei, J. Yang, L. Wang, Q. Hu and G. H. Xu, ACS Appl. Mater. Interfaces, 2023, 15, 6473–6485 CrossRef CAS PubMed.
- S. H. Wen, H. Y. Zhang, S. Yu, J. P. Ma, J. J. Zhu and Y. Z. Zhou, Anal. Chem., 2023, 95, 14914–14924 CrossRef CAS PubMed.
- Y. Zhang, X. C. Zhu, M. J. Li, H. L. Liu and B. G. Sun, J. Agric. Food Chem., 2022, 70, 6059–6071 CrossRef CAS PubMed.
- S. L. Qin, X. D. He, F. L. Jin, Y. Wang, H. T. Chu, S. Han, Y. Y. Sun and L. D. Gao, RSC Adv., 2022, 12, 18784–18793 RSC.
- X. Wang, Y. X. Wang, H. Qi, Y. Chen, W. Guo, H. Y. Yu, H. Y. Chen and Y. B. Ying, ACS Nano, 2022, 16, 14297–14307 CrossRef CAS PubMed.
- X. L. Zhang, G. L. Li, D. Wu, B. Zhang, N. Hu, H. L. Wang, J. H. Liu and Y. N. Wu, Biosens. Bioelectron., 2019, 145, 111699 CrossRef CAS PubMed.
- Z. Meng and K. A. Mirica, Chem. Soc. Rev., 2021, 50, 13498–13558 RSC.
- A. Aparna, H. Sreehari, A. Chandran, K. P. Anjali, A. M. Alex, P. Anuvinda, G. B. Gouthami, N. P. Pillai, N. Parvathy, S. Sadanandan and A. Saritha, Talanta, 2022, 239, 123134 CrossRef CAS PubMed.
- G. Panthi and M. Park, J. Hazard. Mater., 2022, 424, 127565 CrossRef CAS PubMed.
- X. Y. Wang, H. J. Yu, Q. H. Li, Y. H. Tian, X. L. Gao, W. Q. Zhang, Z. C. Sun, Y. T. Mou, X. Sun, Y. M. Guo and F. L. Li, Food Chem., 2024, 431, 137067 CrossRef CAS PubMed.
- X. P. Zhang, K. Y. Huang, S. B. He, H. P. Peng, X. H. Xia, W. Chen and H. H. Deng, J. Hazard. Mater., 2021, 405, 124259 CrossRef CAS PubMed.
- A. M. Hada, M. Zetes, M. Focsan, S. Astilean and A. M. Craciun, Int. J. Mol. Sci., 2022, 23, 12410 CrossRef CAS PubMed.
- J. M. Xu, H. M. Zhou, Y. X. Zhang, Y. Zhao, H. Yuan, X. X. He, Y. Wu and S. Zhang, J. Hazard. Mater., 2022, 428, 128158 CrossRef CAS PubMed.
- M. Chen, W. Ao, J. N. Bai, P. J. Li, W. Wei, S. J. Pang and X. D. Yang, Nanotechnology, 2022, 33, 345501 CrossRef CAS PubMed.
- L. Chen, J. Lu, M. Luo, H. Yu, X. J. Chen, J. G. Deng, X. T. Hou, E. W. Hao, J. C. Wei and P. Li, Food Chem., 2022, 379, 132139 CrossRef CAS PubMed.
- F. R. Nie, L. Ga, J. Ai and Y. Wang, RSC Adv., 2018, 8, 13708–13713 RSC.
- Z. Shojaeifard, N. Heidari and B. Hemmateenejad, Spectrochim. Acta, Part A, 2019, 209, 202–208 CrossRef CAS PubMed.
- H. B. Wu, R. Y. Xie, Y. Q. Hao, J. Y. Pang, H. Gao, F. Y. Qu, M. M. Tian, C. H. Guo, B. D. Mao and F. Chai, Food Chem., 2023, 418, 135961 CrossRef CAS PubMed.
- X. Gao, Z. Y. Ma, M. J. Sun, X. Y. Liu, K. L. Zhong, L. J. Tang, X. P. Li and J. R. Li, Food Chem., 2022, 369, 130964 CrossRef CAS PubMed.
- E. Babaee, A. Barati, M. B. Gholivand, A. Taherpour, N. Zolfaghar and M. Shamsipur, J. Hazard. Mater., 2019, 367, 437–446 CrossRef CAS PubMed.
- Z. Y. Wang, R. Liu, Z. F. Fu, X. Yi, Y. J. Hu, C. H. Liu, D. Pan and Z. Y. Wu, Anal. Methods, 2023, 15, 2505–2511 RSC.
- T. H. Wang, L. N. Zhang and H. Xin, Front. Chem., 2022, 10, 855281 CrossRef CAS PubMed.
- J. Zou, Q. Yu, Y. S. Gao, S. X. Chen, X. G. Huang, D. N. Hu, S. W. Liu and L. M. Lu, ACS Omega, 2022, 7, 1132–1138 CrossRef CAS PubMed.
- I. M. Khan, S. Niazi, L. Yue, Y. Zhang, I. Pasha, M. K. I. Khan, W. Akhtar, A. Mohsin, M. F. J. Chughati and Z. P. Wang, Talanta, 2022, 241, 123228 CrossRef CAS PubMed.
- J. Tang, H. H. Shi, G. Y. Ma, L. P. Luo and Z. H. Tang, Front. Bioeng. Biotechnol., 2020, 8, 1019 CrossRef PubMed.
- G. Z. Ou, J. Zhao, P. Chen, C. J. Xiong, F. Dong, B. Li and X. J. Feng, Anal. Bioanal. Chem., 2018, 410, 2485–2498 CrossRef CAS PubMed.
- T. L. Mako, J. M. Racicot and M. Levine, Chem. Rev., 2019, 119, 322–477 CrossRef CAS PubMed.
- A. Alvarez, J. M. Costa-Fernández, R. Pereiro, A. Sanz-Medel and A. Salinas-Castillo, TrAC, Trends Anal. Chem., 2011, 30, 1513–1525 CrossRef CAS.
- Y. Deng, M. Wang, Y. Zhuang, S. Liu, W. Huang and Q. Zhao, Light: Sci. Appl., 2021, 10, 76 CrossRef CAS PubMed.
- B. Zhang, B. Li and Z. G. Wang, ACS Sens., 2020, 5, 162–170 CrossRef CAS PubMed.
- B. Zhang, J. Yan, Y. Q. Shang and Z. G. Wang, Macromolecules, 2018, 51, 1769–1776 CrossRef CAS.
- Y. M. Han, Z. J. Ye, F. Y. Wang, T. Y. Chen, L. Wei, L. X. Chen and L. H. Xiao, Nanoscale, 2019, 11, 14793–14801 RSC.
- J. Kaur, J. N. Malegaonkar, S. V. Bhosale and P. K. Singh, J. Mol. Liq., 2021, 333, 115980 CrossRef CAS.
- Q. S. Chen, R. Sheng, P. Y. Wang, Q. Ouyang, A. C. Wang, S. Ali, M. Zareef and M. M. Hassan, Spectrochim. Acta, Part A, 2020, 241, 118654 CrossRef CAS PubMed.
- A. A. Kumaran, R. Gopal, C. P. Jijil, D. Joshy, N. K. Hijas, S. B. A. Rajukrishnan and R. N. Kizhakayil, J. Environ. Chem. Eng., 2023, 11, 109918 CrossRef CAS.
- S. L. Yang, J. N. Lu, S. J. Zhang, C. X. Zhang and Q. L. Wang, Analyst, 2018, 143, 4901–4906 RSC.
- J. Kumpfer, J. Jin and S. Rowan, J. Mater. Chem., 2010, 20, 145–151 RSC.
- J. C. Wei, Y. Xue, J. Y. Dong, S. P. Wang, H. Hu, H. Gao, P. Li and Y. T. Wang, Chin. Med., 2020, 15, 22 CrossRef CAS PubMed.
- N. Xu, Q. H. Zhang and G. A. Zhang, Dalton Trans., 2019, 48, 2683–2691 RSC.
- J. X. Ma, Y. Wang, G. C. Liu, N. Xu and X. L. Wang, RSC Adv., 2020, 10, 44712–44718 RSC.
- D. D. Feng, J. Tang, J. Yang, X. H. Ma, C. Z. Fan and X. Q. Wang, J. Mol. Struct., 2020, 1221, 128841 CrossRef CAS.
- L. Wang, W. Xu, W. Y. Li, M. Xie and Y. Q. Zheng, Chem. – Asian J., 2019, 14, 4246–4254 CrossRef CAS PubMed.
- Z. Zhang, X. Ma, B. W. Li, J. Zhao, J. Qi, G. Y. Hao, J. H. Rong and X. B. Yang, Analyst, 2020, 145, 963–974 RSC.
- Y. H. He, S. H. Hong, M. Wang, J. Wang, A. M. Abd El-Aty, J. Wang, A. Hacimuftuoglu, M. Khan and Y. X. She, New J. Chem., 2020, 44, 6026–6036 RSC.
- T. Sergeyeva, D. Yarynka, V. Lytvyn, P. Demydov, A. Lopatynskyi, Y. Stepanenko, O. Brovko, A. Pinchuk and V. Chegel, Analyst, 2022, 147, 1135–1143 RSC.
- X. T. Xue, M. Zhang, H. Y. Gong and L. Ye, J. Mater. Chem. B, 2022, 10, 6698–6706 RSC.
- Z. M. Wang, C. Zhou, S. W. Wu and C. Y. Sun, Polymers, 2021, 13, 1376 CrossRef CAS PubMed.
- Z. F. Xu, P. H. Deng, J. H. Li and S. P. Tang, Sens. Actuators, B, 2018, 255, 2095–2104 CrossRef CAS.
- W. René, V. Lenoble, M. Chioukh and C. Branger, Sens. Actuators, B, 2020, 319, 128252 CrossRef.
- M. Y. Zhang, Z. H. Chen, X. H. Liu, C. Song, C. H. Zeng, T. Y. Z. Lv, Z. Y. Xu, X. Q. Chen, L. Wang, B. Liu and X. J. Peng, J. Hazard. Mater., 2023, 452, 131177 CrossRef CAS PubMed.
- P. Li, D. Zhang, Y. C. Zhang, W. Lu, W. Q. Wang and T. Chen, ACS Sens., 2018, 3, 2394–2401 CrossRef CAS PubMed.
- J. Hoque, N. Sangaj and S. Varghese, Macromol. Biosci., 2019, 19, 1800259 CrossRef PubMed.
- N. Mehwish, X. Q. Dou, Y. Zhao and C. L. Feng, Mater. Horiz., 2019, 6, 14–44 RSC.
- R. J. Dong, Y. Pang, Y. Su and X. Y. Zhu, Biomater. Sci., 2015, 3, 937–954 RSC.
- Q. Zhao, X. Y. Dai, H. Yao, Y. M. Zhang, W. J. Qu, Q. Lin and T. B. Wei, Dyes Pigm., 2021, 184, 108875 CrossRef CAS.
- H. X. Wang, C. W. Wei, X. J. Wang, H. F. Xiang, X. Z. Yang, G. L. Wu and Y. W. Lin, Spectrochim. Acta, Part A, 2021, 250, 119378 CrossRef CAS PubMed.
- D. Zhang, Y. C. Zhang, W. Lu, X. X. Le, P. Li, L. Huang, J. W. Zhang, J. T. Yang, M. J. Serpe, D. D. Chen and T. Chen, Adv. Mater. Technol., 2019, 4, 1800201 CrossRef.
- C. Sahub, J. L. Andrews, J. P. Smith, M. A. M. Arif, B. Tomapatanaget and J. W. Steed, Mater. Chem. Front., 2021, 5, 6850–6859 RSC.
- X. C. Zhu, Y. Zhang, L. X. Han, H. L. Liu and B. G. Sun, Biosens. Bioelectron., 2022, 210, 114265 CrossRef CAS PubMed.
- J. W. Lichtman and J.-A. Conchello, Nat. Methods, 2005, 2, 910–919 CrossRef CAS PubMed.
- N. Sharif, S. Khoshnoudi-Nia and S. M. Jafari, in Characterization of Nanoencapsulated Food Ingredients, ed. S. M. Jafari, Academic Press, 2020, vol. 4, pp. 131–158 Search PubMed.
- P. T. C. So, C. Y. Dong, B. R. Masters and K. M. Berland, Annu. Rev. Biomed. Eng., 2000, 2, 399–429 CrossRef CAS PubMed.
- R. Datta, T. M. Heaster, J. T. Sharick, A. A. Gillette and M. C. Skala, J. Biomed. Opt., 2020, 25, 1–43 Search PubMed.
- X. Chen, S. Zhong, Y. Hou, R. Cao, W. Wang, D. Li, Q. Dai, D. Kim and P. Xi, Light: Sci. Appl., 2023, 12, 172 CrossRef CAS PubMed.
- I. M. Khater, I. R. Nabi and G. Hamarneh, Patterns, 2020, 1, 100038 CrossRef CAS PubMed.
- G. Vicidomini, P. Bianchini and A. Diaspro, Nat. Methods, 2018, 15, 173–182 CrossRef CAS PubMed.
- K. N. Fish, Curr. Protoc. Cytom., 2009, 50, 12–18 Search PubMed.
- R. B. Hegde, K. Prasad, H. Hebbar and B. M. K. Singh, Probl. Biocybern. Biomed. Eng., 2019, 39, 382–392 CrossRef.
- E. Moen, D. Bannon, T. Kudo, W. Graf, M. Covert and D. Van Valen, Nat. Methods, 2019, 16, 1233–1246 CrossRef CAS PubMed.
- Z. C. Liu, L. H. Jin, J. C. Chen, Q. Y. Fang, S. Ablameyko, Z. Z. Yin and Y. K. Xu, Comput. Biol. Med., 2021, 134, 104523 CrossRef PubMed.
Footnote |
| † Equal contribution. |
| This journal is © The Royal Society of Chemistry 2024 |