Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fluorescent small molecule donors
Guang
Chen†
 *a,
Jing
Yu†
a,
Luling
Wu†
*a,
Jing
Yu†
a,
Luling
Wu†
 b,
Xinrui
Ji
e,
Jie
Xu
a,
Chao
Wang
b,
Xinrui
Ji
e,
Jie
Xu
a,
Chao
Wang
 a,
Siyue
Ma
a,
Qing
Miao
a,
Linlin
Wang
a,
Chen
Wang
a,
Siyue
Ma
a,
Qing
Miao
a,
Linlin
Wang
a,
Chen
Wang
 a,
Simon E.
Lewis
a,
Simon E.
Lewis
 b,
Yanfeng
Yue
b,
Yanfeng
Yue
 *f,
Zhe
Sun
*f,
Zhe
Sun
 *d,
Yuxia
Liu
*d,
Yuxia
Liu
 *a,
Bo
Tang
*a,
Bo
Tang
 *c and
Tony D.
James
*c and
Tony D.
James
 *bg
*bg
aThe Youth Innovation Team of Shaanxi Universities, Shaanxi Key Laboratory of Chemical Additives for Industry, College of Chemistry and Chemical Engineering, Shaanxi University of Science & Technology, Xi’an, 710021, China. E-mail: chenandguang@163.com; liuyuxia2008@163.com
bDepartment of Chemistry, University of Bath, Bath BA2 7AY, UK. E-mail: t.d.james@bath.ac.uk
cCollege of Chemistry, Chemical Engineering and Materials Science, Shandong Normal University, Jinan 250014, Shandong, China. E-mail: tangb@sdnu.edu.cn
dInstitute of Molecular Plus, Tianjin Key Laboratory of Molecular Optoelectronic Sciences, Tianjin University, 92 Weijin Road, Tianjin, 300072, China. E-mail: zhesun@tju.edu.cn
eDepartment of Chemical Engineering and Waterloo Institute for Nanotechnology, University of Waterloo, 200 University Avenue West, Waterloo, Ontario N2L 3G1, Canada
fDepartment of Chemistry, Delaware State University, Dover, DE 19901, USA. E-mail: yyue@desu.edu
gSchool of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, China
First published on 14th May 2024
Abstract
Small molecule donors (SMDs) play subtle roles in the signaling mechanism and disease treatments. While many excellent SMDs have been developed, dosage control, targeted delivery, spatiotemporal feedback, as well as the efficiency evaluation of small molecules are still key challenges. Accordingly, fluorescent small molecule donors (FSMDs) have emerged to meet these challenges. FSMDs enable controllable release and non-invasive real-time monitoring, providing significant advantages for drug development and clinical diagnosis. Integration of FSMDs with chemotherapeutic, photodynamic or photothermal properties can take full advantage of each mode to enhance therapeutic efficacy. Given the remarkable properties and the thriving development of FSMDs, we believe a review is needed to summarize the design, triggering strategies and tracking mechanisms of FSMDs. With this review, we compiled FSMDs for most small molecules (nitric oxide, carbon monoxide, hydrogen sulfide, sulfur dioxide, reactive oxygen species and formaldehyde), and discuss recent progress concerning their molecular design, structural classification, mechanisms of generation, triggered release, structure–activity relationships, and the fluorescence response mechanism. Firstly, from the large number of fluorescent small molecular donors available, we have organized the common structures for producing different types of small molecules, providing a general strategy for the development of FSMDs. Secondly, we have classified FSMDs in terms of the respective donor types and fluorophore structures. Thirdly, we discuss the mechanisms and factors associated with the controlled release of small molecules and the regulation of the fluorescence responses, from which universal guidelines for optical properties and structure rearrangement were established, mainly involving light-controlled, enzyme-activated, reactive oxygen species-triggered, biothiol-triggered, single-electron reduction, click chemistry, and other triggering mechanisms. Fourthly, representative applications of FSMDs for trackable release, and evaluation monitoring, as well as for visible in vivo treatment are outlined, to illustrate the potential of FSMDs in drug screening and precision medicine. Finally, we discuss the opportunities and remaining challenges for the development of FSMDs for practical and clinical applications, which we anticipate will stimulate the attention of researchers in the diverse fields of chemistry, pharmacology, chemical biology and clinical chemistry. With this review, we hope to impart new understanding thereby enabling the rapid development of the next generation of FSMDs.
Xinrui Ji a master's degree student at the Department of Chemical engineering, University of Waterloo. She joined the joint-lab of Professor Guang Chen in 2022. |
Qing Miao completed her PhD under the supervision of Prof. Marcellus Ubbink and Dr Mark Overhand in 2019 at Leiden University. Currently, she works as a junior research fellow at SUST. |
Linlin Wang is an associate professor at the College of Chemistry and Chemical Engineering, SUST. She received her PhD degree in 2019 from Nanjing University. |
Chen Wang is a professor at the College of Chemistry and Chemical Engineering, SUST. She received her PhD degree in 2016 from SUST. |
 Yanfeng Yue | Yanfeng Yue is a professor of Chemistry, Delaware State University, Dover, DE, 19901, United States. He received his PhD degree in 2008 from Peking University. |
 Yuxia Liu | Yuxia Liu received her PhD degree in 2012 from Shandong University, and now is an associate professor at SUST. |
1. Introduction
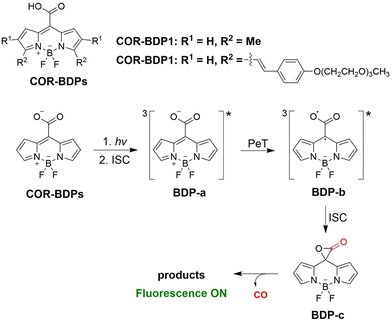
At present, nitric oxide (NO), carbon monoxide (CO), hydrogen sulfide (H2S), sulfur dioxide (SO2), reactive oxygen species (ROS), hydrogen selenide (H2Se), carbon dioxide (CO2), and formaldehyde (FA) are considered to be important gaseous signaling molecules in regulating a variety of physiological and pathological processes.1–10 For example, NO is an effective inhibitor of platelet activation and aggregation, which can block thrombosis;11–13 CO can effectively induce vasodilation in an environment of oxidative stress;14–16 administration of H2S to the cardiovascular system directly activates the ATP-sensitive potassium channel, thereby promoting a decrease in arterial blood pressure;17–20 the synergistic effect of SO2 with doxorubicin (DOX) effectively alleviates multidrug resistance in cancer chemotherapy.21,22 Clearly, SMDs represent a promising approach to eradicate serious diseases. However, for the development of such donors, a controllable dosage, targeted delivery, spatiotemporal feedback, and precise medical treatment are still key challenges. Fortunately, FSMDs can realize not only the triggered release but also the real-time monitoring of active small molecules in organisms, thereby providing remarkable advantages to meet those challenges. For example, the NO-releasing platform with ciprofloxacin loading can eradicate Pseudomonas aeruginosa biofilms in vitro and accelerate wound healing in vivo.23 Metal-free CO donors based on structurally adjustable flavonols exhibit significant anti-inflammatory efficiency.24 While ROS-responsive, self-immolative, and fluorescent H2S donors can serve as theranostic agents for myocardial infarction and other ischemic diseases.25To design FSMDs, the following basic characteristics need to be considered: (i) specific trigger: the donor can be activated by the specific environment to exclusively release the small molecule. (ii) Efficient release rate: during the rearrangement, intermediates are generated that release the small molecules. Therefore, a deep understanding of the reaction mechanisms is required. (iii) Penetration and targeting: donors need to penetrate biological barriers, and actively target regions of interest, such as the tumor microenvironment or subcellular organelles. (iv) Distinguishable fluorescence switches: during the release of small molecules, a continuous visible fluorescence response is required to enable real-time monitoring and evaluation. (v) Good biocompatibility, negligible cytotoxicity and definite by-products. Preferably, FSMDs with good biological compatibility will accelerate practical applicability. As such FSMDs contain the advantages of both SMDs and the fluorescent probes and can serve as a dynamic monitoring and supplier of active small molecules for drug screening and precision medicine.
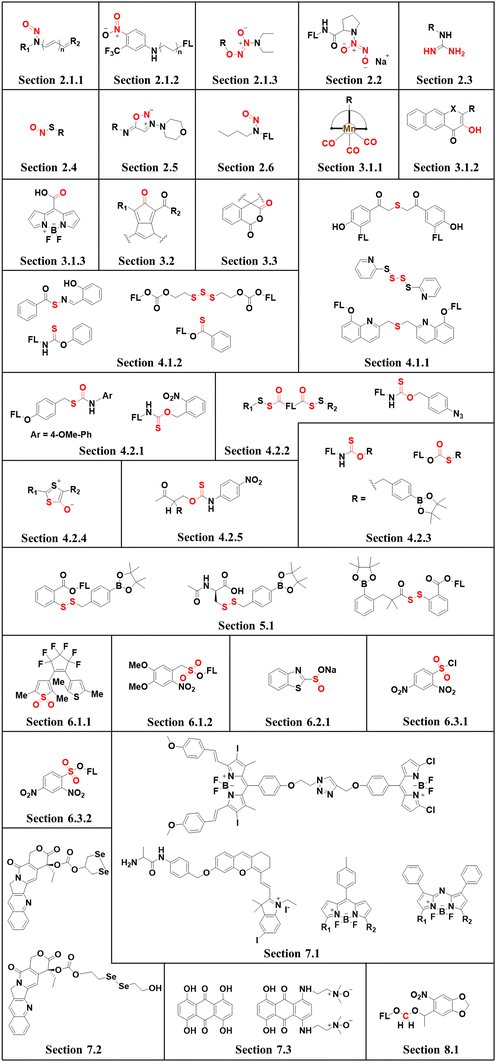
This review covers the latest developments toward FSMDs that meet the following requirements. Based on available FSMDs, we collated the common structures for generating different types of small molecules, thereby, providing some generalizations for the design of FSMDs. The central structures include the N-nitroso-type of NO donors, CO donors based on 3-hydroxyflavone derivatives, thiocarbamate-based H2S donors with self-immolation ability, SO2 donors with 2,4-dinitrobenzene sulfonic acid as the core, and H2Se donors generated by modifying GYY4137, therefore providing a general template for the design of FSMDs. The interlacing mechanisms and factors controlling the release of small molecules and the regulation of fluorescence responses are discussed, from which universal strategies are summarized, mainly including light-controlled, enzyme-activated, reactive oxygen species-triggered, biothiol-triggered, single-electron reduction, and click chemistry. We outline the feedback regulation mechanisms between small molecule release and fluorescence response. When small molecules are released, the charge transfer mechanism in the donor will lead to significant fluorescence changes (fluorescence quenching, fluorescence recovery, ratiometric or emission shift). In most cases, a fluorescence response is also a prerequisite for the release of small molecules (for example, aza-BODIPY integrates light triggering and fluorescence response). Therefore, there is a positive feedback regulation mechanism between the release of the small molecule and fluorescence response. In addition, the factors affecting the release of small molecules from FSMDs are explored: the non-planar torsional conformation of the substituent with respect to the aromatic ring, the degree of electronic delocalization between the release unit and the fluorophore framework, etc., which all influence the ability of FSMDs to release small molecules. Finally, we concentrate on the design strategies toward FSMD prodrugs and their applications for cancer treatment. Therefore, providing feasible solutions for the development of FSMDs with dose controllable, targeted release, spatiotemporal feedback, and precision medical properties, as well as pro-drug design strategies for the development of clinical drugs based on the controlled release of small molecules. Table 1 lists the simplified basic structures of FSMDs discussed in this review (the groups released are highlighted in red).
2. Nitric oxide
NO has long been recognized as a detrimental atmospheric pollutant originating from industrial processes and gasoline combustion.26 However, in 1998, research conducted by Robert F. Furchgott, Louis J. Ignarro, and Ferid Murad unveiled NO as an endogenous endothelial-derived vasodilator, earning them the Nobel Prize in Physiology or Medicine.27 Since then, intensive investigations into the biological and medical aspects of NO were initiated. NO exhibits various biological functions, involved in virtually all human physiological and pathological processes.28 It mediates vasodilation,29 confers cardioprotection,12 facilitates wound healing,30 treats osteoporosis,31 and regulates renal metabolism2 and neurogenesis.32 In addition, the concentration of NO closely correlates with cancer progression, low concentrations of NO promote cell proliferation while high concentrations of NO induce cell oxidative stress.33 As such, due to the diverse biological functions of NO, there has been significant interest in NO donors.2.1. Photo controlled NO release
 | ||
| Fig. 2 Light-controlled/one-electron reduction triggers the NO-release mechanism of N-nitroso push–pull dyes and their fluorescence changes (R = fluorophore X = alkyl, aryl, etc.). | ||
Considering the importance of N-nitroso compounds, researchers have used various fluorophores to develop FSMDs for the monitoring of NO release. The Yang group developed a series of NO donors (NOD545a–g), which was a donor that exhibited a large fluorescence response with the light triggered release of NO (Fig. 3(A)).37 Naphthalimide was selected as the fluorophore since its fluorescence quantum yield is high and its structure is easily modified. In addition, under 365 nm ultraviolet or 740 nm two-photon irradiation, NOD545a–g undergoes ICT, and the N-nitroso part transforms into an electron-rich N–H bond, thus releasing NO. At the same time, anilinyl radical by-products are produced, which are easily reduced in situ, generating 4-amino naphthalimide fluorophores (3a–g). As a result, the strong electron donor group at the 4-position contributes to strong fluorescence enhancement (up to 800-fold) and enables the real-time monitoring of NO release by spectral or microscopic methods (Fig. 3(B) and (C)).
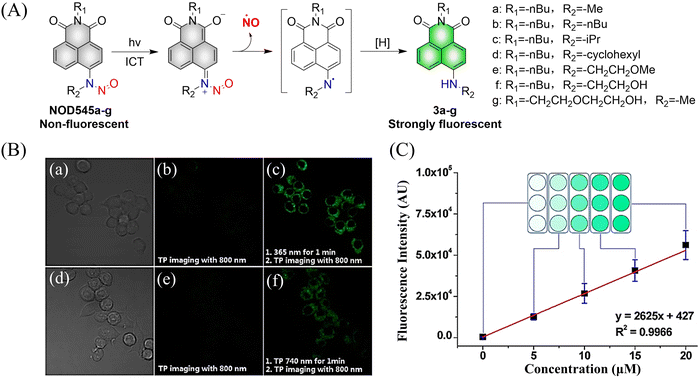 | ||
| Fig. 3 (A) The structure of NOD545a–g, the mechanism of NO release and the fluorescence changes. (B) RAW 264.7 cells incubated with NOD545f for two-photon fluorescence imaging. (C) HeLa cells incubated with different concentrations of 3f to investigate the relationship between fluorescence intensity and cell culture concentration. Parts (B) and (C) are reproduced from ref. 37 with the permission of the American Chemical Society, copyright 2016. | ||
Although the previously reported NOD545a–g donors enable monitoring of NO release, the naphthalimide fluorophores cannot realize targeted release. In order to break through this limitation, the Yang group developed the NO donors (NOD550) targeting the mitochondria using N-nitroso caged rhodamine fluorophores (single molecule localization super-resolution imaging38).39 The donor enabled the visual monitoring of mitochondrial morphology and dynamics while releasing NO. For example, under 375 nm and 532 nm light irradiation, NOD550 releases two NO molecules and one rhodamine fluorophore (Fig. 4(A)–(C)).
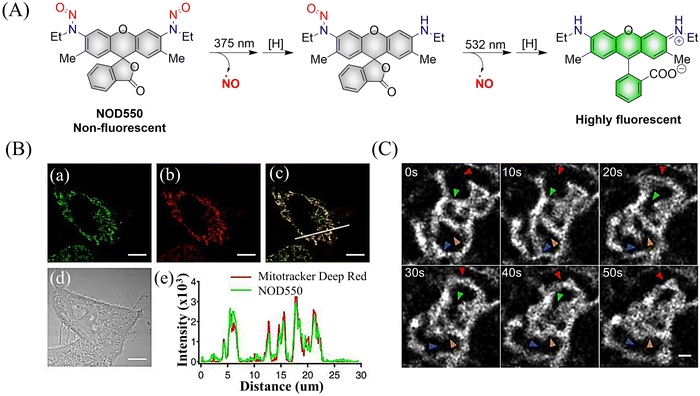 | ||
| Fig. 4 (A) The structure of NOD550, the mechanism of NO release and its fluorescence changes. (B) NOD550 and Mitotracker Deep Red were used for two-color localization imaging. (C) Super resolution monitoring of mitochondrial dynamics. Parts (B) and (C) are reproduced from ref. 39 with the permission of the American Chemical Society, copyright 2018. | ||
It was determined that NOD560 can be effectively activated by light over a wavelength range from 375–561 nm. The releases of NO simultaneously generates the rhodamine fluorophore. The resultant bright fluorescence is turned on and enables the monitoring of the NO release (Fig. 5(A)).40 In addition, NOD560 exhibits excellent characteristics, it can inhibit migration of mouse mesenchymal stem cells (MSCs) (Fig. 5(B) and (C)). The main disadvantage is that the photolysis rate is about 2300 s, and the dynamics are relatively slow.
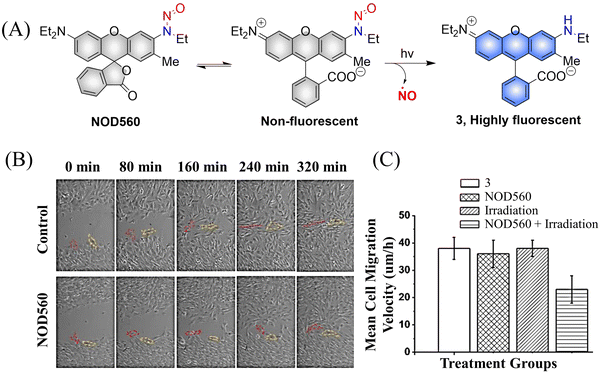 | ||
| Fig. 5 (A) The structure of NOD560, the mechanism of NO release and its fluorescence changes. (B) Migration of MSCs in wound models. (C) Average migration speed of MSCs under different conditions. Reproduced from ref. 40 with the permission of Elsevier Inc., copyright 2018. | ||
NOD565 is a water-soluble, green light triggered and light calibrated NO donor, that can effectively release NO and rhodamine fluorophores (3) under 532 nm green light irradiation (Fig. 6(A)).41 Compared with the UV triggered NO donor previously discussed, NOD565 can be triggered to release NO by a longer-wavelength of 532 nm. In addition, in vitro studies have shown that NOD565 exhibits great potential for inhibiting platelet aggregation and fungal growth (Fig. 6(B)).
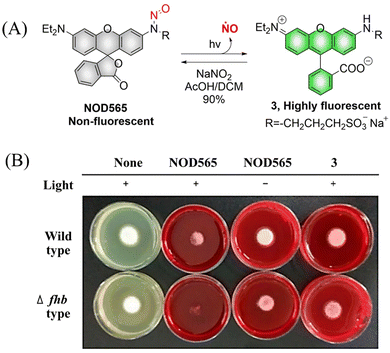 | ||
| Fig. 6 (A) The structure of NOD565, the mechanism of NO release and its fluorescence changes. (B) NOD565 inhibits fungal growth. Reproduced from ref. 41 with the permission of the American Chemical Society, copyright 2018. | ||
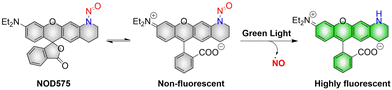
NOD575 due to its special structure (the extended six membered ring structure makes the N-nitroso and the fluorophore framework coplanar, promoting the electron delocalization of the N atom to the fluorophore framework), NOD575 can rapidly release NO under 532 nm light irradiation (Fig. 7).42 Compared with NOD560, the kinetics of NO release from NOD575 were enhanced about 20-fold.
It is well known that low concentrations of NO promote cell proliferation while high concentrations of NO induce cell oxidative stress.43–46 Therefore, it is essential to develop NO donors that can control the concentration and dose. The Zhang group introduced a polyethylene glycol (PEG) group and prepared a new type of NO donor nanoparticle (PEG-NORM) through self-assembly (Fig. 8(A)).47 PEG-NORM homolyzes upon photolysis to yield NO generating a fluorescence response, where the released NO concentration is dependent on the light intensity and duration. As such, this system can regulate biological processes more efficiently. For example, increasing the light intensity and time, the higher concentrations of NO can cause apoptosis of C. elegans germ cells and reduce the survival duration (Fig. 8(B) and (C)). Thus, the frontiers of NO biology have shifted from the elucidation of mechanisms to biomanipulation, laying the foundation for significant biological and medicinal progress for NO related systems.
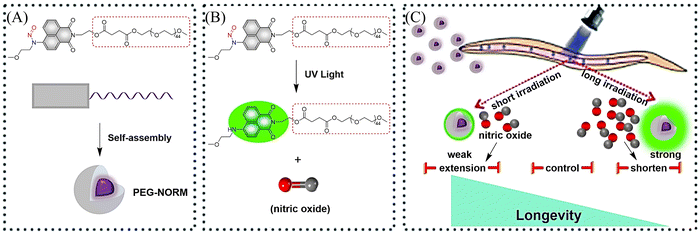 | ||
| Fig. 8 (A) Formation of PEG-NORM nanoparticles. (B) Proposed photodissociation mechanism of PEG-NORM. (C) Effect of PEG-NORM on longevity of C. elegans. Reproduced from ref. 47 with the permission of the Royal Society of Chemistry, copyright 2020. | ||
Two-photon imaging has many advantages, such as high resolution, enhanced imaging depth, and low background signals. Therefore, it is very important for the research of diseases in living animals. The Tang group have developed a two-photon NO donor (CNNO) (Fig. 9(A)).48 Under 365 nm or 800 nm two-photon irradiation, CNNO releases NO and FRET is activated due to a red-shift in the absorption spectrum of the naphthalimide receptor, which then overlaps with the emission spectrum of the coumarin donor. This system can be used to monitor NO release using two-photon ratiometric fluorescence, and the fluorescence response is not influenced by complex biological environments (Fig. 9(B)). With these advantages, the authors found that NO released by CNNO in the aorta of mice can control vasodilation.
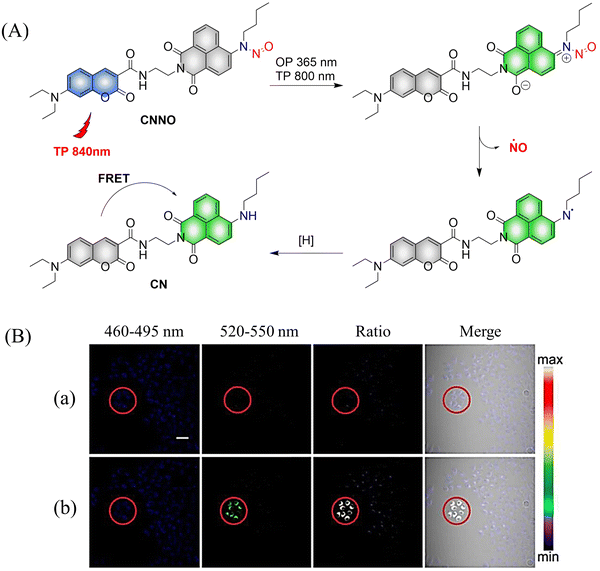 | ||
| Fig. 9 (A) The structure of CNNO, the mechanism of NO release and its fluorescence changes. (B) Two-photon fluorescence imaging of NO release by CNNO in HeLa cells. Cells were treated with 10 μM CNNO. (a) The dish was imaged without light irradiation. (b) The dish was irradiated inside the selected circle using a two-photon laser at 800 nm (20 mW) for 2 min before being imaged. Reproduced from ref. 48 with the permission of the Royal Society of Chemistry, copyright 2017. | ||
Photoacoustic (PA) tomography enables the imaging of biological tissues at centimeter depths using an NIR trigger and ultrasonic detection, providing accurate guidance for the treatment of cancer.49–54 When combined with small molecule donors, deep tissue gas therapy can be readily monitored. The Chan group reported a NO donor based on PA (photoNODs).55 Upon single-photon NIR irradiation, photoNOD-1 and photoNOD-2 release NO as well as rNOD-1 or rNOD-2, PA-active products that enable ratiometric monitoring of NO release. Significantly different PA signals enable the visual monitoring of NO release (Fig. 10(A)). In addition, photoNOD-1 can selectively influence tumor growth rates, providing a new concept for cancer treatment (Fig. 10(B)). Significantly, this design strategy could be extended to other analytes, thereby expanding the scope of synergistic NIR photorelease and PA imaging.
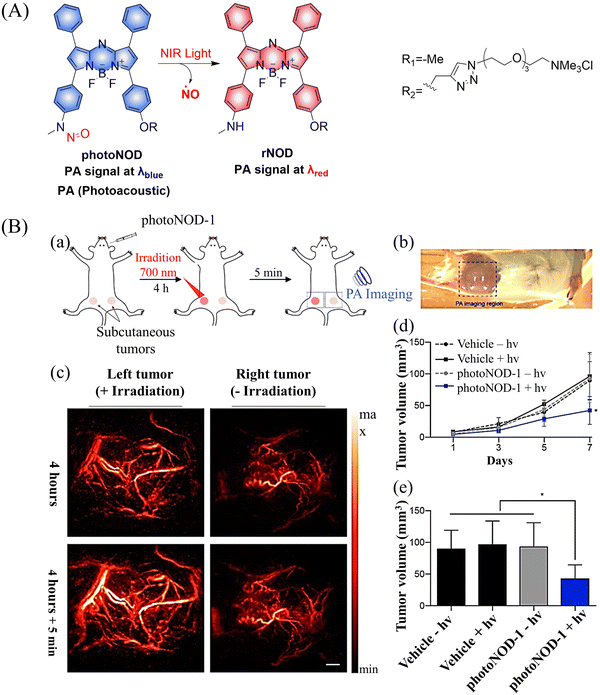 | ||
| Fig. 10 (A) The structure of photoNODs, the mechanism of NO release and its PA (photoacoustic) changes. (B) (a) Schematic illustration of photoNOD-1 administration, NO treatment, and PA monitoring. (b) Photograph of mouse imaged by a PA tomographer. Dashed line indicates region of PA imaging. (c) PA images (λPAred) acquired before/after a 5 min period with/without irradiation (λPAblue) 4 h following systemic administration of photoNOD-1 (1.2 mg kg−1, 150 μL, 20% DMSO in sterile saline). (d) and (e) Measured tumor volume under different treatment conditions. Reproduced from ref. 55 with the permission of the American Chemical Society, copyright 2018. | ||
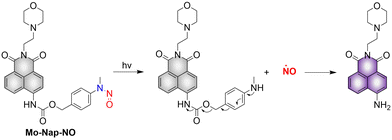
Lysosomes are important organelles, which are not only the degradation centers of cells through autophagy, but also mediate endocytosis and the transfer of extracellular substances.56,57 The Gou research group have developed a NO donor that targets lysosomes for anticancer therapy (Mo-Nap-NO).58 Mo-Nap-NO uses the N-nitroso as the NO donor, 4-amino-1,8-naphthalimide as the fluorescent moiety, and morpholine as the lysosomal targeting unit. Under 460 nm light irradiation, Mo-Nap-NO undergoes intramolecular electron transfer, releasing NO and the fluorescence is turned on (Fig. 11). In addition, the localized release of NO can rupture the lysosome, resulting in the release of cathepsin D into the cytoplasm, thus activating Caspase-3 mediated apoptosis and inhibiting the development of cancer cells.
To avoid the toxicity of ultraviolet light, Nakagawa and colleagues have developed a light controllable (λmax ≈ 500 nm) NO donor (NOBL-1), which is composed of an N-nitroso part as NO release unit and BODIPY as fluorescent dye (antenna). Moreover, the structure of NOBL-1 also contains carboxyl groups that can increase the hydrophilicity, making NOBL-1 suitable for biological applications (Fig. 12(A)).59 NOBL-1 can release NO by photoinduced electron transfer (PeT). When BODIPY is excited, single electron transfer from the N-nitrosoaminophenol to the BODIPY occurs, thus forming a stable quinone with NO release (Fig. 12(B)). Based on the visual-monitoring of NO release, the authors confirmed that vasodilation of the rat aorta could be controlled by light-induced NO release from NOBL-1 (Fig. 12(C) and (D)).
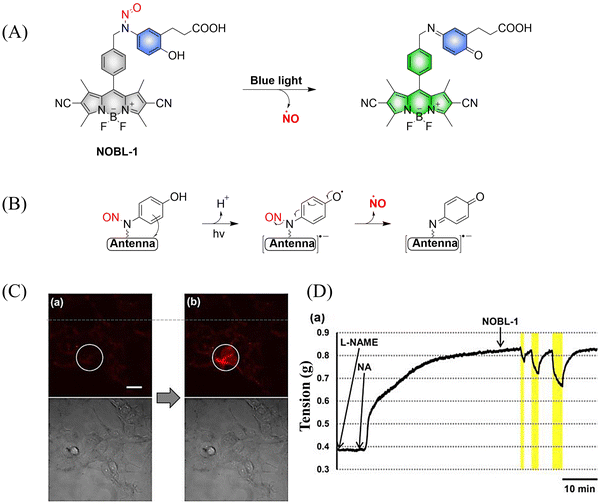 | ||
| Fig. 12 (A) and (B) The structure of NOBL-1, the mechanism of NO release and its fluorescence changes. (C) DAR-4M AM (a fluorogenic NO probe) monitored fluorescence imaging of NO released by NOBL-1 in HEK293 cells. (D) Blue-light activated NOBL-1 to release NO induced vasodilation changes in rats. Parts (C) and (D) are reproduced from ref. 59 with the permission of the American Chemical Society, copyright 2014. | ||
Subsequently, the research group designed and synthesized a new yellowish-green-light controllable (λmax ≈ 550 nm) NO donor (NO-Rosa) (Fig. 13(A)).60 Compared with the previous system this system uses longer wavelength excitation (yellowish-green-light), which displays good tissue penetration and less cytotoxicity. The structure of NO-Rosa is very similar to that of NOBL-1, except for the difference in the fluorophore part (BODIPY for NOBL-1 and rosamine for NO-Rosa), which leads to the absorption of yellowish-green-light. NO-Rosa also exhibited the advantages of time-controlled and visual-monitoring of rat aorta vasodilatation (Fig. 13(B) and (C)).
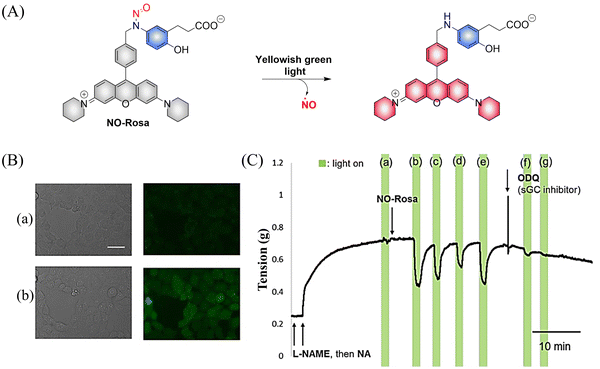 | ||
| Fig. 13 (A) The structure of NO-Rosa, the mechanism of NO release and its fluorescence changes. (B) DAR-4M AM (a fluorogenic NO probe) monitored fluorescence imaging of NO released by NO-Rosa in HEK293 cells. (C) NO-Rosa activated by yellowish-green-light to release NO and induce vasodilation changes in rats. Parts (B) and (C) are reproduced from ref. 60 with the permission of the Royal Society of Chemistry, copyright 2017. | ||
The Sortino research group reported two N-nitroso NO photo donors (NOPDs) based on nitrobenzene compounds in 2019.61 The authors used BODIPY and Rhodamine as the fluorophores, and both of them can release NO under irradiation with biocompatible green light. The systems exhibit green and orange-red fluorescence respectively, which is convenient for the localization and tracking of NO release (Fig. 14(A) and (B)).
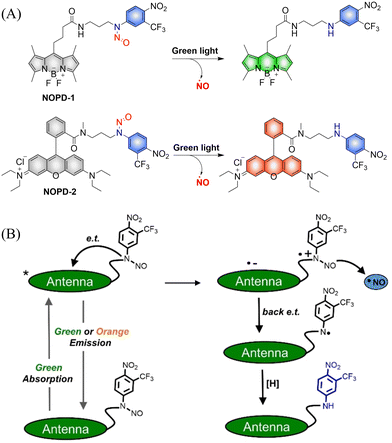 | ||
| Fig. 14 (A) The structure of NOPDs, and fluorescence changes. (B) Proposed photodissociation mechanism of NOPD-1/2. Reproduced from ref. 61 with the permission of Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, copyright 2019. | ||
To improve the fluorescence signal and quantum yield, Qian and colleagues, developed a water-soluble light-induced NO donor (CNA-NO) (Fig. 15(A)).62 In their design strategy, the introduction of cyclohexyl into CNA-NO can restrict the rotation of the benzene, decreasing the non-radiative energy loss and thereby enhancing the fluorescence signal and quantum yield of this donor. Under light irradiation of 465 nm, the fluorescence of CNA-NO increases (78-fold) and releases NO (Fig. 15(B)). The probe exhibits good biocompatibility which ensures that the donor can be used in living organisms.
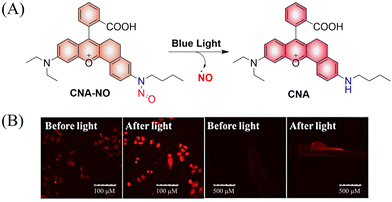 | ||
| Fig. 15 (A) The structure of CNA-NO, the mechanism of NO release and its fluorescence changes. (B) Comparison of fluorescence imaging of CNA-NO in living cells and zebrafish before and after illumination. Reproduced from ref. 62 with the permission of Elsevier B.V., copyright 2021. | ||
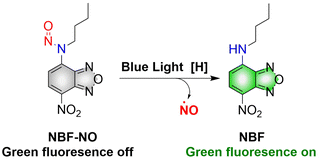
Although a large number of excellent donors have been developed, their synthesis is often complicated. Therefore, it is necessary to develop functional donors that are easy to prepare. Sortino, et al. reported a green fluorescent NO donor (NBF-NO).63 Under irradiation by 420 nm (λex = 427 nm) light, NBF-NO releases NO and a stable green fluorescent product NBF (Fig. 16). In addition, the donor can also be effectively coated by thermally responsive nano-micelles, so that the photochemical and physical properties of the probe can be protected making it suitable for use in complex biological environments.
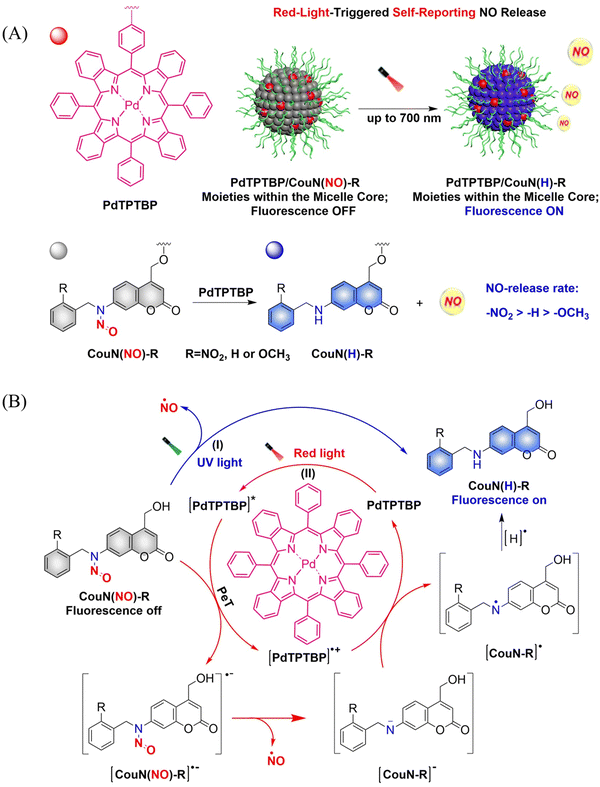
In order to achieve good imaging characteristics, a red-light mediated photocatalytic NO donor (CouN(NO)-R, R = NO2/H/OCH3) was developed by the Hu group.23 PdTPTBP/CouN(NO)-R is composed of palladium(II) tetraphenyltetrabenzoporphyrin (PdTPTBP) and N-nitrosoamine moieties (CouN(NO)-R). CouN(NO)-R provides quantification of NO concentrations (as a result of the absorbance changes caused by the quantitative conversion from CouN(NO)–NO2 to CouN(H)–NO2) and reports on NO release (via obvious fluorescence changes before and after reaction) through photoredox catalysis (Fig. 17(A)). CouN (NO) has two modes of releasing NO: (i) the donor is activated under 365 nm light irradiation (Coumarin has the maximum absorbance at 328 nm); (ii) in the presence of photosensitizer PdTPTBP, the donor is activated by 630/700 nm red-light (triplet sensitization indirectly activates receptors through a triplet–triplet energy transfer (TTET) process) (Fig. 17(B)). In addition, the authors also explored the effects of different substituents on the NO release rate. When the substituent is an electron withdrawing group, the kinetics of NO release was enhanced, that is, –NO2 > –H > –OCH3. In biomedical applications, this NO-releasing platform with ciprofloxacin loading can eradicate Pseudomonas aeruginosa biofilms in vitro and accelerate wound healing in vivo.
Photothermal therapy (PTT), as a tumor treatment strategy, has the advantages of high selectivity, spatiotemporal control, and minimal side effects. Therefore, combining PTT with gas therapy can significantly improve the effect of tumor treatment. The Liu research group have reported a near-infrared photothermal agent (S-NO) for synergetic gaseous therapy (GT) and PTT, which is composed of aryl N-nitrosamine and functionalized aza-BODIPY (antenna).64 The reason for selecting aza-BODIPY as the fluorophore group is that its conversion from electron withdrawing group (–N–NO) to donating groups (–NH) will lead to significant red-shift and enhanced near-infrared absorption. To improve the water solubility of S-NO, nano particles (S-NONPs) were prepared by assembling with the polymer DSPE-mPEG5000 (Fig. 18(A)). Under NIR-light irradiation, S-NO NPs exhibited excellent NO release capacity and significantly enhanced heat generation. This material contributed to the successful inhibition of tumor growth by synergistic GT and PTT (Fig. 18(B)). This study provides guidance for the construction of dual-effect synergistic tumor treatment platforms.
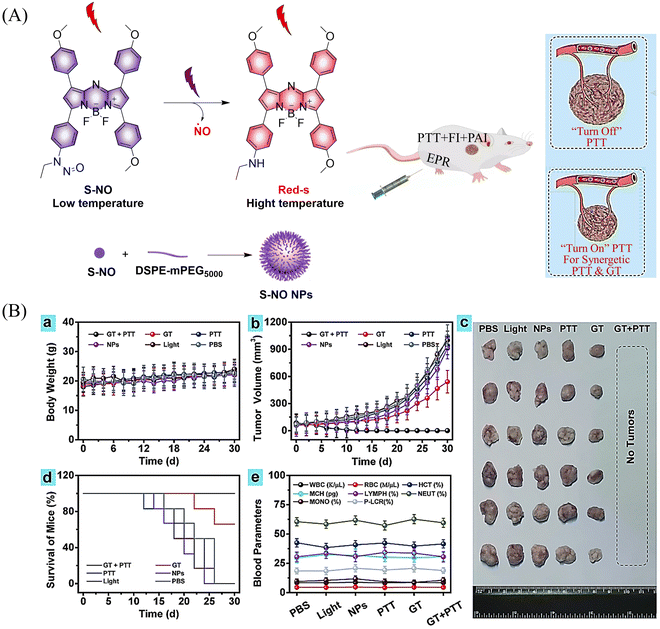 | ||
| Fig. 18 (A) The structure of S-NO, the mechanism of NO release and its fluorescence changes. (B) Assembly of S-NO NPs and synergistic tumor treatment and evaluation of GT and PTT produced by injection into mice. Reproduced from ref. 64 with the permission of Elsevier Ltd, copyright 2021. | ||
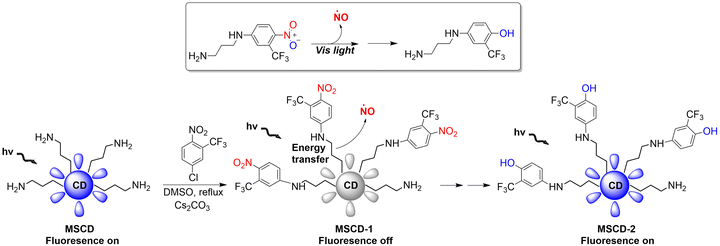
Mesoporous silica nanoparticles (SNPs) are extremely effective solid carriers (easy surface functionalization, selective endocytosis by cancer cells, and the release of loaded drugs can effectively lead to apoptosis).68–71 The Sortino research group have reported a NO donor with light-controlled multivalent silica nanoparticles as the carrier (Fig. 20).72 The nitroaniline based donor was covalently linked to the surface amino group of fluorescent carbon dots by calcination to obtain the hybrid MSCD-1. The fluorescence in MSCD-1 is quenched by effective energy transfer. However, under visible light irradiation, MSCD-1 releases NO, inhibits energy transfer, and leads to fluorescence recovery of the MSCD scaffold. The fluorescence changes before and after reaction can be used to monitor the release of NO. This provides interesting prospects for precise spatiotemporal control of NO release and real-time quantitative biomedical research.
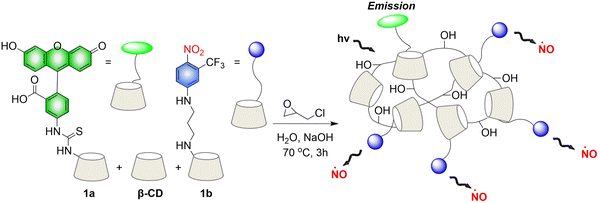
Crosslinked β-cyclodextrin polymers have been shown to be able to encapsulate visible light activated prodrug molecules in a non-covalent manner and deliver them to cancer cells.73–75 But conventional non-covalent polymer carriers do dissociate upon dilution and are weak to environmental changes. To solve these problems, in 2019, the Sortino group reported a new water-soluble β-Cyclodextrin polymer. The polymer contains covalently bound fluorescein isothiocyanate (FITC), β-cyclodextrin and NO donor unit nitroaniline, the two chromophores are activated in parallel under the induction of visible light, resulting in green fluorescence emission suitable for imaging (Fig. 21).76 The polymer can be internalized in squamous cancer cells and induces cell mortality on NO photo-decaging.
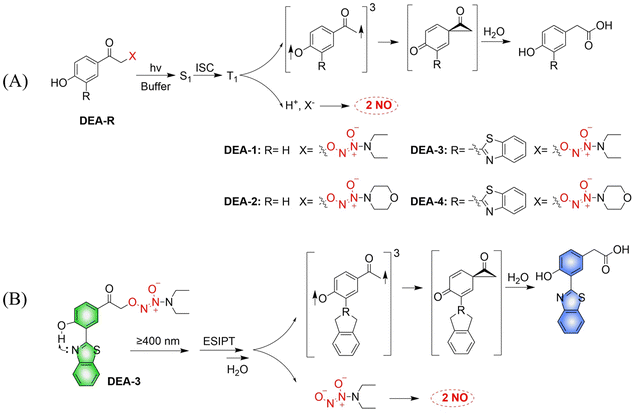
The Huang research group developed a series of NO donors (ethylenediamines).78 Since the photolysis wavelength of 4-hydroxyphenacyl is lower than 400 nm and DEA-1/2 are non-fluorescent, the author modified the system by introducing benzothiazol-2-yl in the position ortho to the hydroxyl on the benzene ring of DEA-1/2 to prepare DEA-3/4 (Fig. 23(A)). The advantages of this system are: on the one hand, DEA3/4 can undergo photolysis with irradiation of ≥410 nm visible-light; and on the other hand, excited-state intramolecular transfer (ESIPT), that is, the proton of the hydroxyl group transfers to the nitrogen atom of the benzothiazole, can help deprotonation of the hydroxyl group and accelerate the photodegradation rate of DEA3/4, thus resulting in the rapid release of NO. The DEA-3 process is accompanied by obvious fluorescence changes (from green to blue) (Fig. 23(B)).
2.2. Enzyme activated NO release
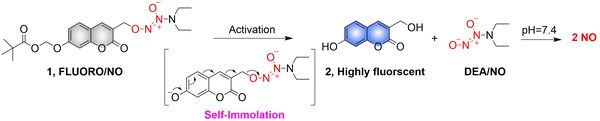
Biocatalysis can provide the precise selectivity of enzymes to achieve the release of required drugs.79–81 The Chakrapani research group developed an esterase activated N-diazeniumdiolate NO donor (FLUORO/NO).82 When the hydroxyl group of coumarin is linked with appropriate activating groups the fluorescence of the FLUORO/NO is significantly diminished. Subsequently, with the activation of esterase, FLUORO/NO undergoes a self-immolation reaction, releasing DEA, thus significantly enhancing the fluorescence of coumarin (Fig. 24).Given the prevalence of multidrug resistant bacteria, improved detection and treatment methods are urgently required. NO is a known effective antibacterial agent, but because of its gaseous nature and high reactivity, it is difficult to integrate into a stable antibacterial system. Fortunately, the emergence of multifunctional materials has solved this problem. The Reynolds group have reported a small molecule dual function NO donor (F-PROLI, bacterial indicator and antibacterial agent).83 Under UV irradiation, proline based diazeniumdiolate salts spontaneously release NO to kill bacteria. Subsequently, the bacterial enzyme prolyl aminopeptidase in Pseudomonas aeruginosa will cleave the proline group from the fluorescent aminoacridone/proline compound, resulting in a fluorescence change from blue to yellow. This material (F-PROLI) can both detect and kill Pseudomonas aeruginosa a deadly and multi-drug resistant bacterial strain (Fig. 25).
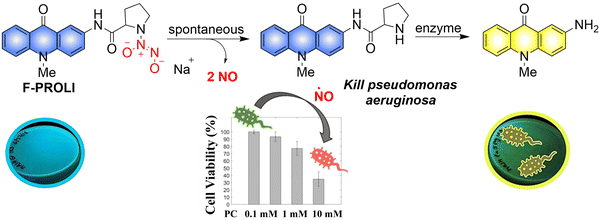 | ||
| Fig. 25 The structure of donor, the mechanism of NO release and fluorescence changes. Reproduced from ref. 83 with the permission of the Royal Society of Chemistry, copyright 2019. | ||
2.3. Reactive oxygen species triggered NO release
Arginine is a natural endogenous NO donor, which can release a large amount of NO in cells through the catalysis of nitric oxide synthase.29,84,85 The Zhang group reported fluorescent carbon nanodots based on L-Arginine (Arg-dots), to enhance the treatment of cancer with NO gas. The nanoplatform contains a hydrogen peroxide donor to increase the hydrogen peroxide concentration at the tumor site, thus overcoming the low concentration hydrogen peroxide environment that restricts NO production from arginine (Fig. 26).86 After being endocytosed by cancer cells, the Arg-dots can generate a large amount of intratumoral NO to kill cancer cells. As such, without any additional external intervention (in the context of H2O2-enriched tumor microenvironments), Arg-dots autonomously release NO at a twofold higher rate when compared to equimolar L-Arg. When combined with traditional chemotherapeutic drugs (adriamycin), Arg-dots greatly improve the treatment effect toward cancer. In addition, the size of Arg dots is very small (about 2.5 nm in diameter), thus they can effectively penetrate deep tumors and provide enhanced antitumor activity. In addition, Arg-dots can be removed by kidney filtration, thereby reducing the burden on the human body in the process of cancer treatment.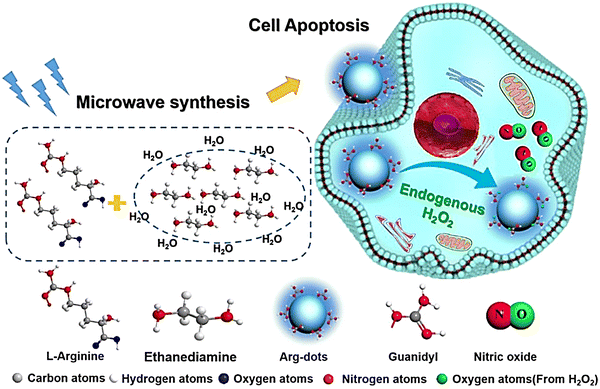 | ||
| Fig. 26 Schematic of the synthesis of Arg-dots and their NO release process in tumor cells. Reproduced from ref. 86 with the permission of Elsevier Ltd, copyright 2021. | ||
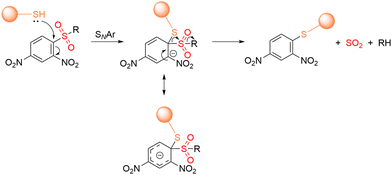
2.4. Thiol triggered NO release
It has been reported that S-NO can be cleaved to release NO under specific triggering conditions (the mechanism of NO release is similar to that of N-nitroso compounds, Fig. 2).87–90 The Yuan group recently reported a tumor-acidity and bioorthogonal chemistry-mediated in situ size transforming cluster nanosystem (PAEMA-DOX/NO(PDN)) (Fig. 27(A)).91 The system uses PAEMA and high-efficiency bioorthogonal click chemistry to form large-scale aggregates in tumor tissues which enhances the accumulation in tumor tissues and the retention of DOX and NO. Subsequently, in the acidic microenvironment of the tumor, iCPDN(PAEMA-DOX/NO(PDN)) is cleaved, and the aggregates are slowly decomposed into ultra-small nanoparticles PDN with better tumor penetration. That is, DOX (pH responsive release) and NO (GSH responsive release) are delivered to hypoxic tumor tissues (Fig. 27(B)). Such a synergistic treatment of NO and DOX may be an effective method to combat hypoxia-induced chemical tolerance and enhance anti-tumor immune response.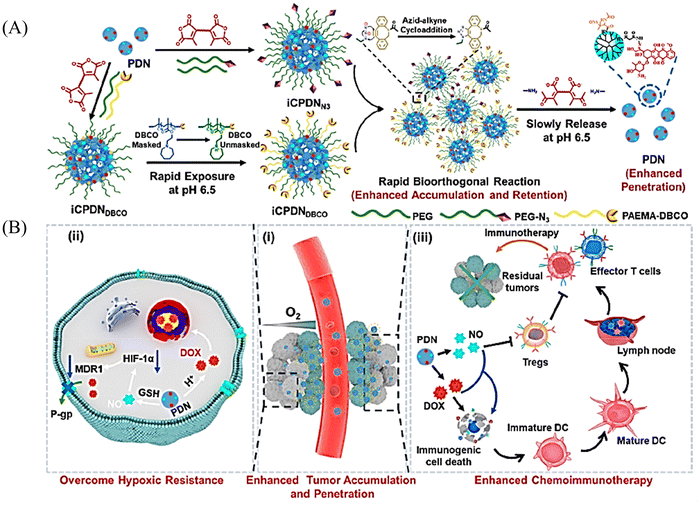 | ||
| Fig. 27 Schematic of in situ size transformation cluster nanosystem mediated by tumor acidity and bioorthogonal chemistry to overcome hypoxic resistance and enhance chemical immunotherapy. (A) Schematic diagram of preparation of iCPDNN3 and iCPDNDBCO, forming large particle size aggregates by efficient biological orthogonal click reaction under acidic environment, and then slowly releasing small particle size PDN. (B) (i) In situ size transformation cluster nanosystem enhanced tumor accumulation, retention, and penetration. (ii) NO overcomes hypoxia resistance by down-regulating HIF-1α level and enhances the chemotherapy effect of DOX. (iii) NO and DOX can induce stronger immunogenic cell death and activate anti-tumor immune responses. Reproduced from ref. 91 with the permission of the American Chemical Society, copyright 2022. | ||
2.5. Redox activated NO release
Tumor-draining lymph nodes (TDLN) contain various types of immune cells, such as natural killer cells, T cells, antigen-presenting cells and B cells, which play a key role in tumor metastasis. Therefore, lymphatic targeted anti metastasis therapy is particularly important. The Kim group developed a redox triggered NO prodrug (SISIN-1) that can target TDLE (Fig. 28(A)).92 Since albumin has the ability to enhance lymphatic drainage and endocytosis in cancer cells,93,94 AL-SISIN-1 formed by binding SISIN-1 to albumin is able to efficiently deliver NO to TDLE, thereby inhibiting tumor metastasis. The disulfide bond of SISIN-1 is reduced to a free thiol that initiates intramolecular nucleophilic attack and cleaves the carbamate linked to SIN-1, thereby releasing free SIN-1. Under physiological conditions, free SIN-1 released by SISIN-1 will spontaneously decompose and release NO. The blue shift in absorption of SISIN-1 can be used as a marker for monitoring NO release (Fig. 28(B)). This new prodrug contributes to the development of on-demand NO delivery systems for anti-metastasis and other treatments.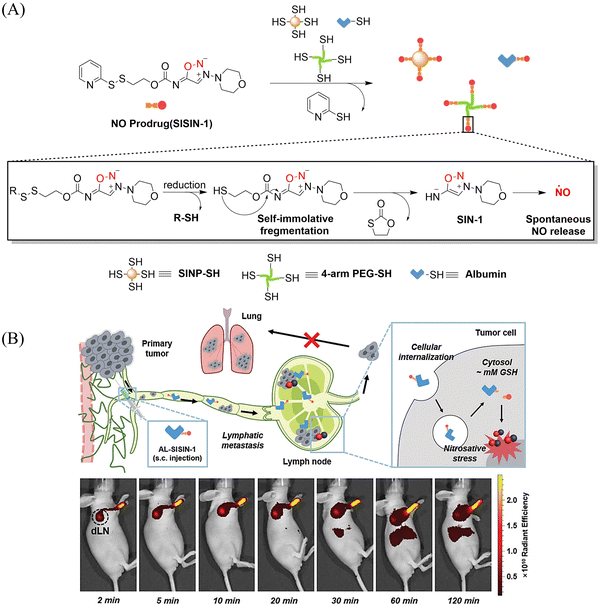 | ||
| Fig. 28 (A) The structure of SISIN-1 and mechanism of NO release (B) SISIN-1 combines with albumin to form AL-SISIN-1, which delivers drugs to tumor draining lymph nodes and inhibits cancer cell metastasis. Reproduced from ref. 92 with the permission of Wiley-VCH GmbH, copyright 2022. | ||
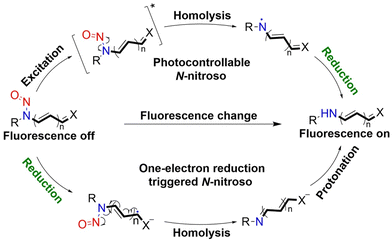
2.6. One-electron reduction NO release
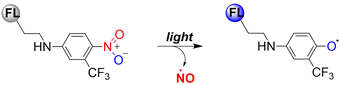
Under light, photoexcitation, N-nitroso undergoes photolysis to release NO, and its structure changes from an electron withdrawing group –NNO to electron-rich –NH. However, when the fluorophore framework is a strong electron withdrawing group, it can replace the photolysis pathway of N-nitroso, that is, to release NO through a reduction-triggered protonation cleavage pathway. The Yang research group reported a series of one-electron reduction triggered NO donors (NODf3) (Fig. 29(A)).36 NODf3 releases NO under the trigger of one-electron reduction. Since this triggering mechanism does not depend on the reduction ability of thiols, it does not affect the redox level of cells. In addition, it was found that NODf3 has good biocompatibility and efficient reduction rate and exhibits therapeutic and cytoprotective effects in oxygen and glucose deprivation (OGD) models (Fig. 29(B)).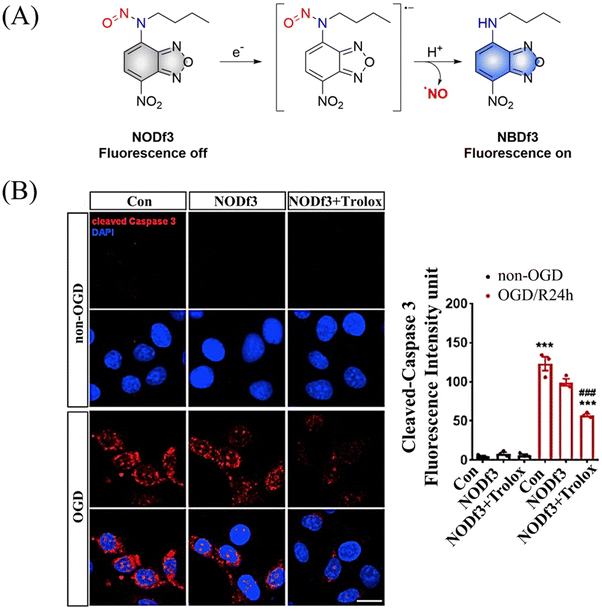 | ||
| Fig. 29 (A) The structure of NODf3, the mechanism of NO release and its fluorescence changes. (B) NODf3 combined with Trolox protects HUVECs from OGD-induced apoptosis. Reproduced from ref. 36 with the permission of Elsevier Inc., copyright 2021. | ||
3. Carbon monoxide
Carbon monoxide (CO) is a colorless, odorless inert gas that has the longest lifespan among the three main known gaseous signaling molecules (NO, CO, and H2S).95 However, despite its ability to produce acute toxicity when bound in significant quantities to hemoglobin, resulting in rapid fatality,96 CO also plays a vital role in medical treatments.16 This includes inducing vasodilation,15 mitigating acute kidney injury,97 protecting neurotransmitters,98 displaying anti-inflammatory and antioxidative properties.99 Furthermore, CO can disrupt cytochrome c oxidase in mitochondria, hindering oxidative phosphorylation, effectively inhibiting the growth and proliferation of cancer cells.10,1003.1. Photo controlled CO release
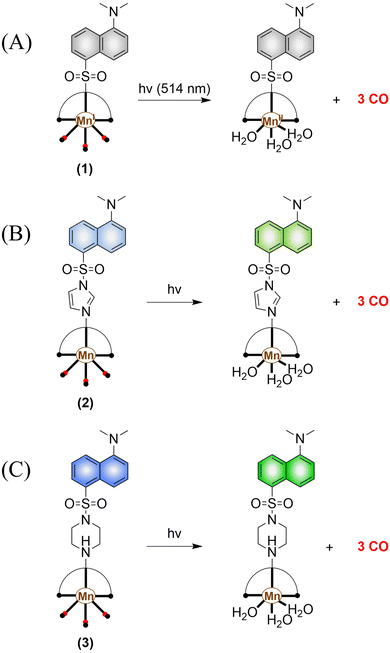 | ||
| Fig. 30 (A)–(C) The structure of Mn-carbonyl complexs, the mechanism of CO release and fluorescence changes. | ||
Since mitochondria regulate a variety of important functions in cell physiology, there is a clinical requirement for mitochondria-targeted therapy.104–108 The Liu research group have developed a multifunctional nano-platform for the mitochondria-targeted therapy of cancer (APIPB-MnCO@TPP@N,P-GQDs).109 This platform contains the covalently attached histone deacetylase inhibitors, CO donor MnBr(CO)3, and mitochondria-targeting groups (triphenylphosphine, TPP) to N,P-doped GQDs fluorescent carriers. Under 808 nm NIR light irradiation, this platform undergoes charge-transfer transitions and releases CO and generates significantly enhanced fluorescence (Fig. 31(A) and (B)). As expected, the combination of GT and histone deacetylase inhibition improves the anticancer activity.
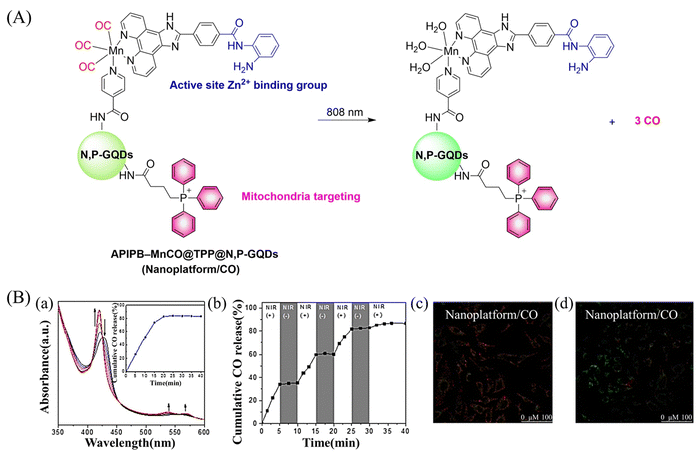 | ||
| Fig. 31 (A) The structure of APIPB–MnCO@TPP@N,P-GQDs, the mechanism of CO release and its fluorescence changes. (B) (a) The absorbance change of hemoglobin in the presence of Nanoplatform/CO irradiated by 808 nm laser (800 mW cm−2), with the inset showing the CO release profiles. (b) The NIR controllability of Nanoplatform/CO for CO release upon the on/off switching of an 808 nm laser. Fluorescence detection of mouse mitochondrial membrane potential, Nanoplatform/CO treated HeLa cells (c) under dark environment and (d) under 808 nm laser irradiation. Reproduced from ref. 109 with the permission of Elsevier Inc., copyright 2022. | ||
Other donors with excellent performance have also been reported. The Fan research group have designed nanoparticles (TTQ-MnCO NPs) with efficient NIR-II FI and NIR-II PTT/GT.110 Since weaker electron withdrawing groups are beneficial to reduce non-radiative attenuation and increase the NIR-II fluorescence intensity, the authors engineered the conjugated polymer by reducing the density of withdrawing groups (TTQ) on the main chain of the conjugated polymer. Moreover, a long alkyl side chain modified dithiophene (2TC) was introduced as an electron donor group, to improve the solubility in organic solvents. TTQ-2TC-4T and thermal responsive CO donor (Mn2(CO)10) were loaded into an amphiphilic block copolymer (PCB-b-PPG-b-PCB) to obtain TTQ-MnCO NPs (Fig. 32(A)). Under 808 nm NIR laser light, TTQ-MnCO NPs turned on the NIR-II FI. Under the irradiation of 1064 nm laser, TTQ-MnCO NPs could generate heat to trigger the effective production of toxic CO, realizing the synergistic treatment by NIR-II PTT/GT, which represents an innovative development in synergistic cancer therapy (Fig. 32(B)). This study provides a new method for optimizing the fluorescence and photothermal properties of NIR-II conjugated polymers.
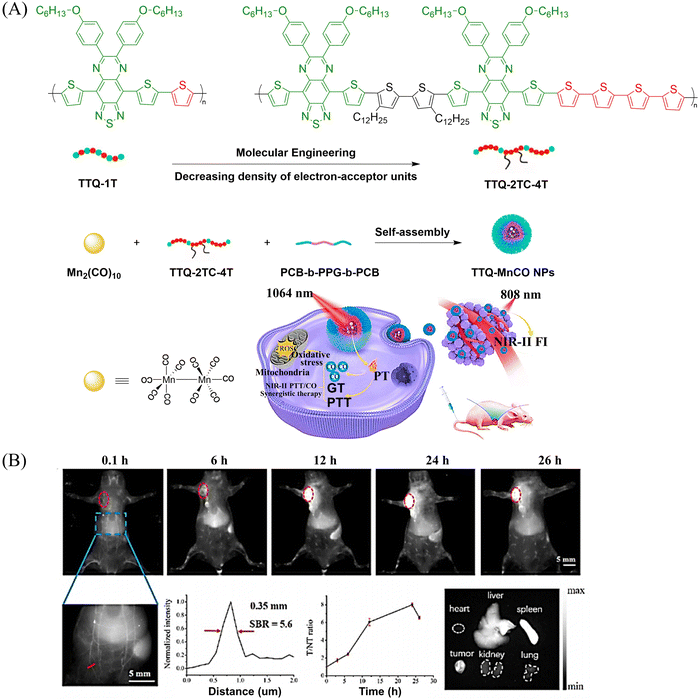 | ||
| Fig. 32 (A) Illustration of red-light-triggered self-reporting CO release from micellar nanoparticles containing TTQ-2TC-4T, PCB-b-PPG-b-PCB and CO-releasing Mn2(CO)10 moieties within the cores. (B) Infrared photothermal imaging of MCF-7 tumor mice triggered by 808 nm light and its therapeutic effect on cancer. Reproduced from ref. 110 with the permission of Elsevier Ltd, copyright 2022. | ||
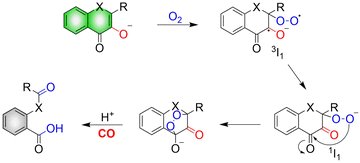
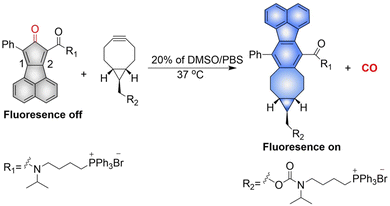
The mechanism of CO release from such donors requires: firstly, in the presence of both light and O2, the hydroxyl group of flavonols undergoes a one-electron transfer to O2, producing a triplet superoxide 3I1. Subsequently, an internal electron transfer (ICT) leads to the production of the closed shell-layer singlet oxygen 1I1. Finally, β-C is attacked by the terminal oxygen of the superoxide to form an endoperoxide to release CO (Fig. 34).114
Representative work is as follows: inspired by the work of Liu et al.,115 the Berreau group expanded the 3-hydroxy-flavonol structure by adding an acryloyl appendage to the 3-OH site (reaction 1), thus developing a fluorescent compound (SL-photoCORM) that combines thiol-detection with light-controlled CO release.119 In cells, SL-photoCORM firstly undergoes a Michael addition reaction of the acroyl group with Cys/Hcy resulting in enhanced fluorescence and monitoring of the cell redox state. The system then releases CO when triggered by visible light and O2 (Fig. 35(A) and (B)). The process follows the “AND” logic gate (Fig. 35(C)). SL-photoCORM combines the detection of redox biomarkers with the release of small molecules, realizing the dual function of a single probe.
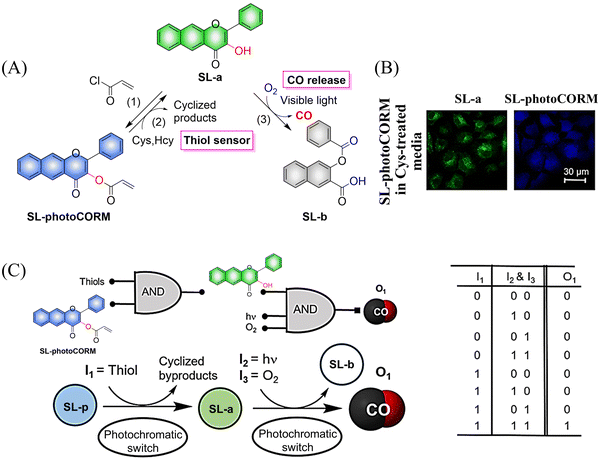 | ||
| Fig. 35 (A) The structure of SL-photoCORM (SL-p), the mechanism of CO release and its fluorescence changes. (B) SL-p in A549 cells is converted into the confocal image of SL-a under the action of mercaptan. (C) The process behave like an AND logic gate. Parts (B) and (C) are reproduced from ref. 119 with the permission of the American Chemical Society, copyright 2017. | ||
The same group reported another CO donor (MC-photoCORM) for mitochondrial-targeted release of CO (Fig. 36(A)).120 Owing to the mitochondrial targeting function of triphenylphosphine and flavonol, this donor can easily be absorbed by cells and release CO in situ (Fig. 36(B)).
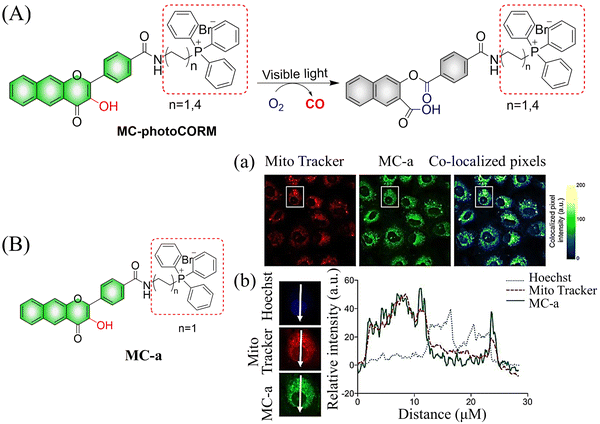 | ||
| Fig. 36 (A) The structure of MC-photoCORM, the mechanism of CO release and its fluorescence changes. (B) MC-a (n = 1) and MTR intensity distribution strongly indicates that MC-a is located in the mitochondria. Reproduced from ref. 120 with the permission of the American Chemical Society, copyright 2018. | ||
In addition to 3-hydroxyflavone, fluorescent donors based on quinolone have been widely developed for CO release. For example, the Berreau group have reported a 3-hydroxybenzo[g]quinolone framework CO donor (QA-photoCORM).121 QA-photoCORM can be tracked prior to CO release using fluorescence microscopy and produces a nontoxic byproducts following CO release. In addition, the strong noncovalent affinity of QA-photoCORM to albumin enables use of an albumin: QA-photoCORM complex for targeted CO delivery to cancer cells. The cytotoxicity IC50 value generated by this method is one of the lowest reported values for delivering CO to cancer cells through photoCORM to date (Fig. 37). QA-photoCORM complex is also the first CO delivery system to produce significant anti-inflammatory effects at nanomolar photoCORM concentrations.
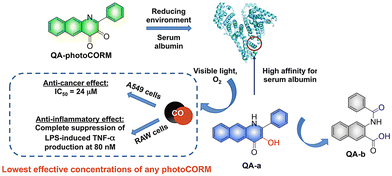 | ||
| Fig. 37 The structure of QA-photoCORM, the mechanism of CO release and its fluorescence changes. Reproduced from ref. 121 with permission of the American Chemical Society, copyright 2018. | ||
Subsequently, the Berreau group evaluated the impact of intracellular and extracellular CO production on disease treatment.123 The research indicated that the introduction of sulfonate groups to the donors changed the ability of cells to absorb the donors, and did not have any effect on the optical properties of the 3-hydroxyflavone derivatives (Fig. 38(A) and (B)). In addition, extracellular administration also generated significant anti-inflammatory efficiency (Fig. 38(C) and (D)).
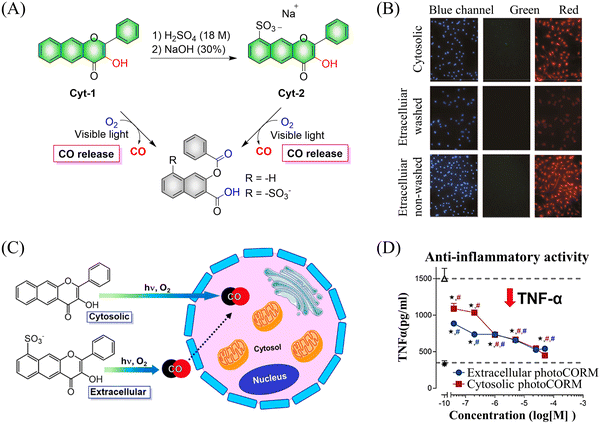 | ||
| Fig. 38 (A) The structure of cytosolic/extracellular-photoCORM, the mechanism of CO release and its fluorescence changes. (B) Fluorescence detection of CO release from Cyt-1 and Cyt-2 (50 μM) using a Nile red-based CO sensor (1-Ac) in RAW 264.7 cells. Green channel: detection of fluorescence emission by cytosolic/extracellular. Red channel: detection of CO sensor. Size of bar = 40 μm. (C) and (D) extracellular administration shows significant anti-inflammatory effect. Reproduced from ref. 123 with the permission of the American Chemical Society, copyright 2019. | ||
Using a similar mechanism, the Tang research group developed a two-photon H2O2 activated CO donor (FB).124 FB is composed of a structurally extended 3-hydroxyflavone (F) combined with a boronate ester group that specifically recognizes ROS. Under the action of ROS, FB generates F which undergoes ESIPT, subsequently F releases CO under light irradiation (Fig. 39(A)). In addition, FB also provides evidence for oxidative stress related to H2O2 after administration of angiotensin type 2. Therefore, the donor is suitable for the early warning of oxidative stress and light-controlled release of CO (Fig. 39(B)).
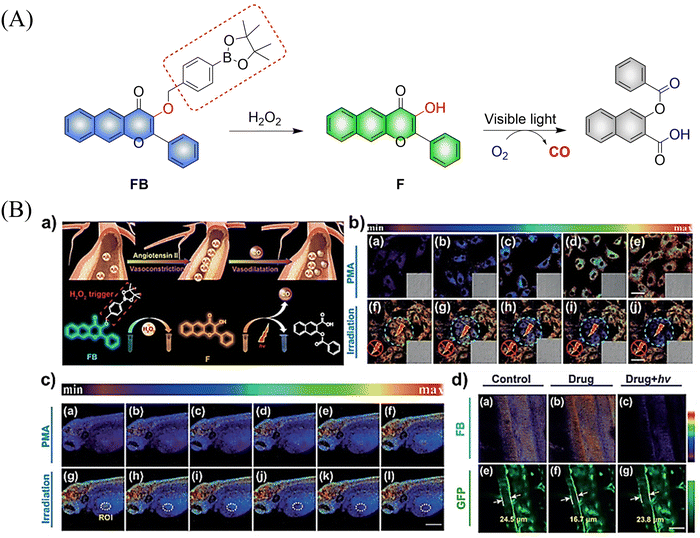 | ||
| Fig. 39 (A) The structure of FB, the mechanism of CO release and its fluorescence changes. (B) (a) The structure and sensing mechanism of FB toward H2O2-activated and CO photo-releaser. (b) The H2O2 mapping and CO releasing in vitro. (c) The H2O2 mapping and CO-release in vivo. (d) The vasodilatation effect of CO and angiotensin type 2 induced H2O2 fluctuation. Reproduced from ref. 124 with the permission of Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, copyright 2018. | ||
The Feng group have made great effort to develop CO donors.125 For example, the group reported a visible-light triggered ratiometric CO donor (Cou-Flavone) based on the coumarin-flavonol structure (Fig. 40(A)). Under visible-light irradiation, Cou-Flavone can not only release CO, but also exhibits obvious ratiometric fluorescence changes (Fig. 40(B)). Cou-Flavone can be used to monitor the release of CO by changes in the emission spectra of two fluorophores, therefore making the results more reliable and accurate.
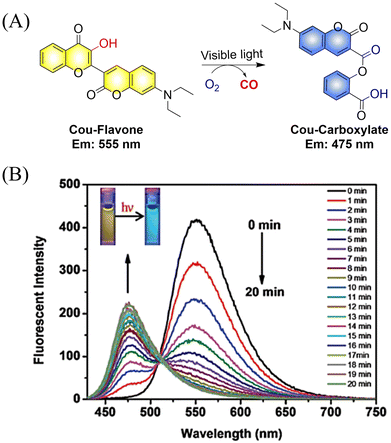 | ||
| Fig. 40 (A) The structure of Cou-Flavone, the mechanism of CO release and its fluorescence changes. (B) The capacity of Cou-Flavone for CO-release was investigated in HEPES buffer (10 mM, pH 7.4, with 10% DMSO, v/v) at room temperature under light irradiation (460 nm, 7 W cm2). Typical ratiometric fluorescence changes were observed before and after irradiation. Reproduced from ref. 125 with the permission of the Royal Society of Chemistry, copyright 2019. | ||
In addition CO exhibits excellent use and a therapeutic anti-inflammatory,126 skin trauma,127 immune imbalance128 and oxidative stress129 treatment. The group of Hu proposed a direct polymerization strategy for CO donors based on 3-hydroxyflavone (3-HF) derivatives.130 CO-releasing polymers were self-assembled into micelles (PEO-b-PFNM) using PEG, which improved the water solubility of the extended 3-HF (Fig. 41(A)). Under 410 nm visible-light irradiation, the o-nitrobenzyl group was cleaved, and the characteristics of 3-HF derivative were restored. Then in the presence of O2, PEO-b-PFNM when irradiated with 410 nm light, the 3-HF derivative released CO. The whole process exhibits fluorescence changes from blue to red and then to colorless, facilitating the monitoring of CO release in vivo and in vitro. Benefiting from these excellent properties, this donor exhibited excellent therapeutic anti-inflammatory and skin wound healing effects (Fig. 41(B)).
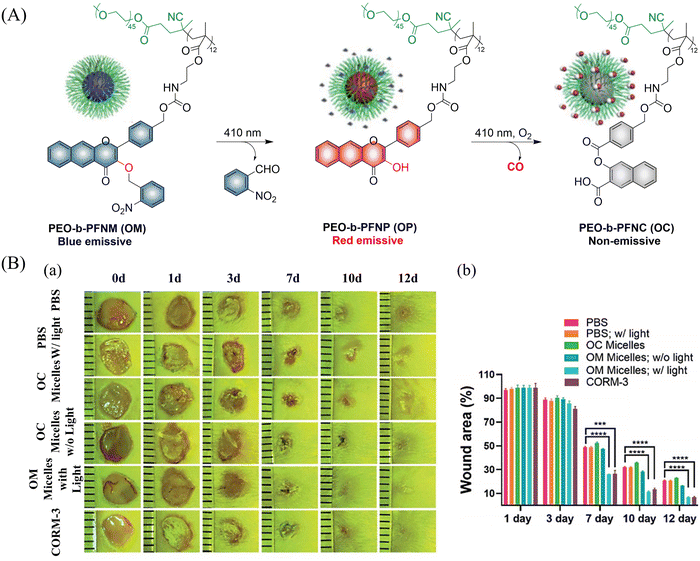 | ||
| Fig. 41 (A) The structure of PEO-b-PFNM, the mechanism of CO release and fluorescence changes. (B) Comparison of the treatment effect of PBS, OM and OC micelles on skin wounds. Reproduced from ref. 130 with the permission of the Royal Society of Chemistry, copyright 2020. | ||
Subsequently, the same group have developed metal-free CO release micelle, via a photooxidation mechanism to activate flavonol conjugated derivatives to release CO.24 Under 650 nm light excitation, tetraphenylporphyrin photosensitizer converts 3O2 into 1O2. Then, 1O2 oxidizes the conjugated derivatives of flavonol, thereby triggering the release of CO. In addition, the micelles exhibit specific antibacterial efficacy toward methicillin-resistant S. aureus (MRSA), but not Escherichia coli, so they can eradicate MRSA pathogens and accelerate the healing of MRSA infected wounds (Fig. 42).
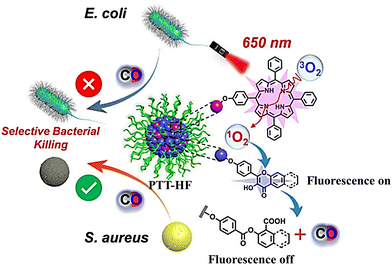 | ||
| Fig. 42 The structure of PTT-HF, the mechanism of CO release and its fluorescence changes. Reproduced from ref. 24 with the permission of Wiley-VCH GmbH, copyright 2021. | ||
In addition to single-molecule donors, two-molecule donors have also attracted great interest.131 Recently, the Hu group developed a micelle (PCON) that releases NO and CO optically (Fig. 43(A) and (B)).132 The photodegradation process was investigated using H-NOCO, which released NO under 410 nm light irradiation, and the o-nitrobenzene group was cleaved to restore the 3-HF derivative. While the fluorescence at 603 nm of compound H-NOCO was significantly enhanced under irradiation for 50 s, the fluorescence emission decreased and CO was released with further illumination. The significant change in fluorescence intensity enabled the monitoring of the release of small molecular gases in the body. In addition, the synergistic therapy of NO and CO exhibits strong antibacterial effect towards Gram-positive bacteria, and its therapeutic ability toward MRSA infection in a mouse skin trauma model was better than vancomycin (Fig. 43(C) and (D)). This donor provides a new concept for orchestrating NO and CO signaling molecules for synergistic treatment of MRSA infections.
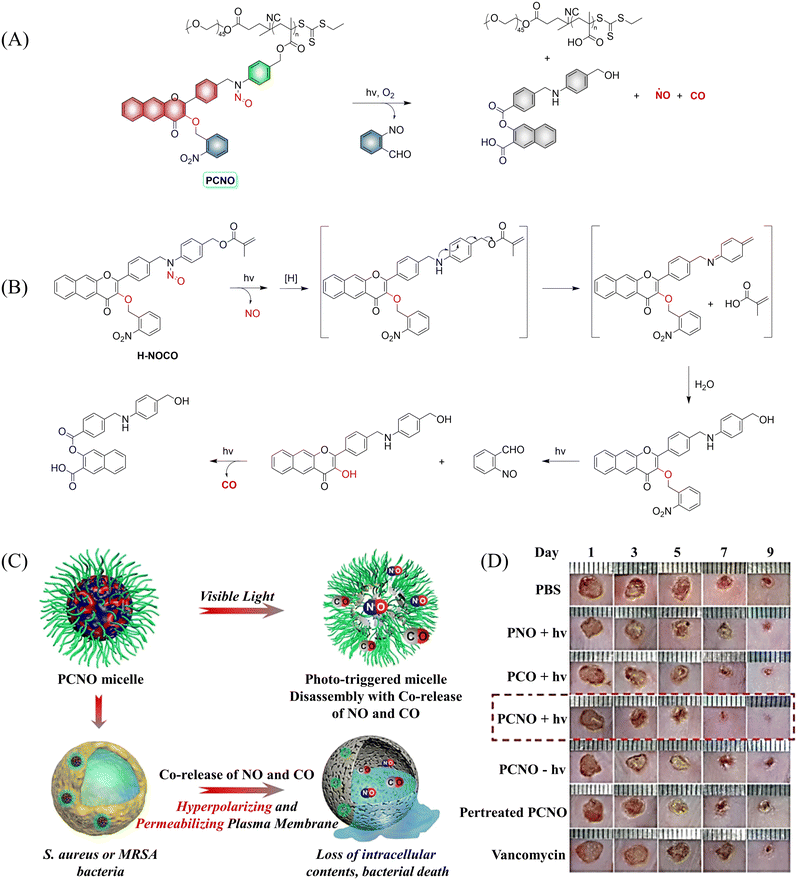 | ||
| Fig. 43 (A) The structure of PCNO, the mechanism of CO and NO release and its fluorescence changes. (B) Proposed degradation mechanism of the NO/CO-releasing H-NOCO under 410 nm light irradiation. (C) The schematic diagram of the synergistic release of NO and CO in visible-light mediated PCNO micelles can play a synergistic bactericidal role on Gram-positive bacteria through hyperpolarization and penetration of bacterial plasma membrane, leading to the loss of intracellular substances and bacterial death. (D) Evaluation of therapeutic effect on mice infected with MRSA. Parts (C) and (D) are reproduced from ref. 132 with the permission of Wiley-VCH GmbH, copyright 2021. | ||
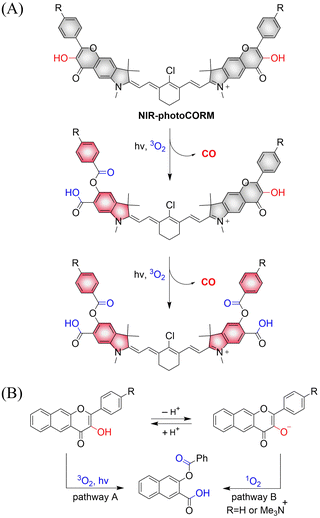
Using similar core units, Klań extended the traditional visible-light absorption of flavonol compounds and developed a photochromic CO donor (NIR-photoCORM).133 NIR-photoCORM was obtained by fusing two flavonol components that release CO with NIR cyanine dyes. NIR-photoCORM upon activation with NIR light (820 nm) liberates two molecules of CO and exhibits excellent uncaging cross-sections (Fig. 44(A)). The UV/visible absorption spectra in methanol exhibits a typical strong cyanine absorption band, the emission intensity is equivalent to that of indocyanine green, enabling CO release and tracking in vivo. With NIR-photoCORM, the authors state that light-controlled release of CO can occur through the reaction of triplet excited state of the 3-hydroxyflavone with ground state oxygen (pathway A) or through reaction of the conjugate base with singlet oxygen (pathway B) (Fig. 44(B)).
3.2. CO release using bioorthogonal click chemistry
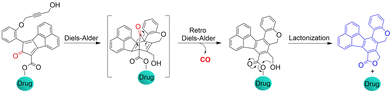
The Wang group have reported a method of bioorthogonal and controlled activation of prodrugs.135 Their system requires the enrichment of prodrugs in the mitochondria, when the concentration reaches a certain level, a click reaction is triggered which enables the localized delivery of CO (Fig. 46). Cyclopentadienones (diene in a Diels–Alder reaction) and cycloalkynes (dienophile) combined with TPP result in mitochondrial-targeting. While, incorporation of a naphthalene group to the 1, 2 position of the cyclopentadiene results in a fluorescence change after the click reaction occurs. When cyclopentadienones and alkynes are enriched in the mitochondria (mitochondrial-targeting), the Diels–Alder reaction triggers release of CO and fluorescence enhancement. This platform provides guidance on the targeted delivery of drugs to specific locations.The Wang group also reported a strategy using intramolecular inverse electron demand Diels–Alder click reactions to release CO (Fig. 47).136 Significantly, the pro-drug is activated under near-physiological conditions, and the norbornene ketone intermediate formed undergoes a self-immolation reaction to release CO. The as-formed aromatic ring can effectively release carrier drugs after isomerization, accompanied by a blue fluorescence response. This cascade reaction enables synergistic treatment of CO with other drugs, such as the combined treatment of CO and antibiotic metronidazole, resulting in enhanced antibacterial efficiency.
3.3. ONOO-triggered CO release
An increase in ONOO− levels results in ischemia-reperfusion injury,137,138 while CO is neuroprotective.139,140 Therefore, the Yang group developed a ONOO− triggered CO donor (PCOD585).141 Significantly, PCOD585 produces therapeutic CO whilst consuming ONOO−, thus realizing a multi-functional mode of action (Fig. 48(A)). PCOD585 generates ring-opened rhodamine while releasing CO (an anhydride can be produced by peroxide mediated oxidation of the 1,2-dicarbonyl, which decomposes to release CO) under ONOO− activation the fluorescence turns on enabling visual-monitoring of free-radical scavenging and CO release in vivo (Fig. 48(B)). In addition, in the process of cerebral-reperfusion injury, PCOD585 can penetrate the blood–brain barrier, reduce the infarct volume and decreased brain edema to achieve therapeutic effect. It also exhibits a protective effect on PC-12 cells injured by OGD (Fig. 48(C)). Therefore the dual action (therapeutic CO while consuming ONOO−) of PCOD585 results in a potential therapeutic for the treatment of ischemic stroke.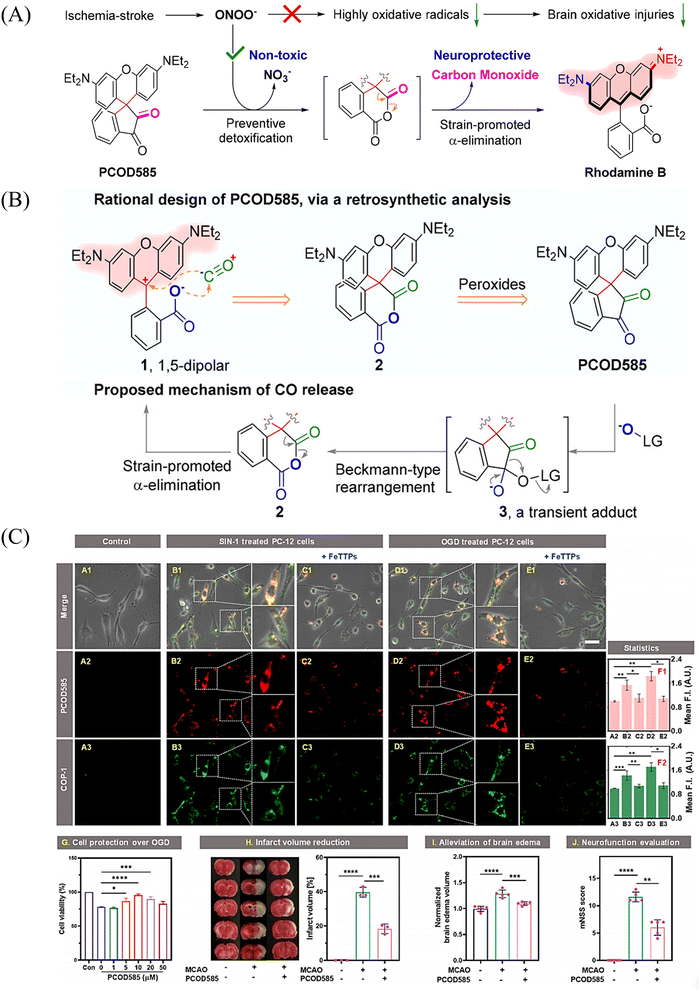 | ||
| Fig. 48 (A) and (B) Rational design of PCOD585 and its proposed mechanism of action. LG = leaving group. (C) PCOD585 was tested for in vitro applications in PC12 cells and EA.hy 926 cells. Reproduced from ref. 141 with the permission of the American Chemical Society, copyright 2022. | ||
4. Hydrogen sulfide
A number of H2S donors with fluorescence activity have been used in clinical research. Such donors combine various triggering mechanisms with self-reporting fluorescence markers to visualize and quantify H2S release. These donors can be divided into two categories in terms of the release mode: directly and indirectly produced H2S. The latter adopts a completely different strategy, which indirectly produces H2S from carbonyl sulfide (COS).4.1. Direct production of H2S donor
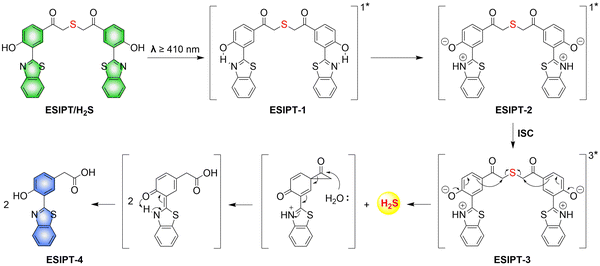
H2S is inherently toxic, and historically, research has primarily focused on its toxicity, with limited attention given to its physiological and pathological functions.142 It was not until 1996, with Abe and Kimura's discovery that mammals produce H2S gas endogenously and use it as a neuromodulator, did the understanding of the roles of H2S begin to shift.143 Further research has shown that H2S is an important gaseous signaling molecule, following NO and CO. H2S can regulate vascular remodeling,144 mediate neurotransmission,145 lower blood pressure,146 and regulate insulin release.147 In myocardial ischemia-reperfusion injury, H2S exhibits a strong cardioprotective effect.148 Additionally, H2S plays a crucial role in the treatment of stroke, various cancers, and liver diseases.149,150To solve the problems associated with UV light activation, a new H2S donor was designed (ESIPT/H2S) by the Singh group.161 Under ≥410 nm light irradiation, ESIPT/H2S is excited to its singlet state ESIPT-1, which triggers a fast proton transfer reaction from the hydroxyl to benzothiazole generating intermediate ESIPT-2. Then, intermediate ESIPT-2 undergoes effective intersystem crossing (ISC) and enters its triplet excited state ESIPT-3. Intermediate ESIPT-3 then rearranges into carboxylic acid derivative ESIPT-4 by a photo-Favorskii reaction, with the release of H2S (Fig. 50). Compared with traditional H2S donors, this donor exhibits good biocompatibility, cellular internalisation, and less cytotoxicity, which lays the foundation for effective use in biomedical applications.
The same group also developed a visible light responsive H2S nano-carrier (QuH2S-CD). The carrier is composed of fluorescent carbon dots and quinoline, and exhibits good biocompatibility, large fluorescence changes and efficient release of H2S (Fig. 51(A) and (B)).162 Under visible light irradiation, quinoline is excited into a singlet state. Subsequent carbon–sulfur bond breakage, results in formation of quinoline carbon cations and sulfide anions. Finally, H2S is generated by quenching with water. This QuH2S-CD system illustrates the importance of nano-carriers for biomedical applications.
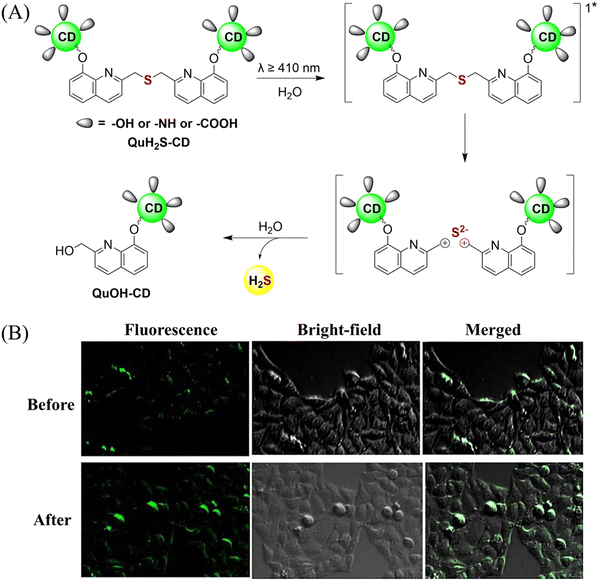 | ||
| Fig. 51 (A) Proposed Photodissociation mechanism of QuH2S-CDs. (B) The ability of cellular uptake of QuH2S-CD was observed by confocal microscope imaging. Release of H2S from QuH2S-CDs was monitored before and after irradiation for 50 min with light (λ ≥ 410 nm). Reproduced from ref. 162 with the permission of the Royal Society of Chemistry, copyright 2020. | ||
Multiple-action combined therapy is a new concept for cancer treatment.163–167 The Dai group have developed a nano-regulator for the synergistic treatment by GT and PTT (Pry-Ps@CP-PEG).168 In this nanosystem, a hydrophobic polysulfide H2S donor (Pry-Ps) was encapsulated using amphiphilic conjugated polymers (CP-PEG), resulting in Pry-Ps@CP-PEG with good biocompatibility. Under 808 nm laser irradiation, tumor-rich GSH can trigger Pry-Ps to release H2S via the cleavage of an S–S bond, resulting in mitochondrial dysfunction and a strong anti-inflammatory effect (Fig. 52(A)). The in vivo NIR-II fluorescence and efficient photothermal conversion of Pry-Ps@CP-PEG was also determined (Fig. 52(B)). In addition, H2S-regulated PTT is conducive to adaptive immune response, and has shown excellent efficacy in the treatment of skin trauma.
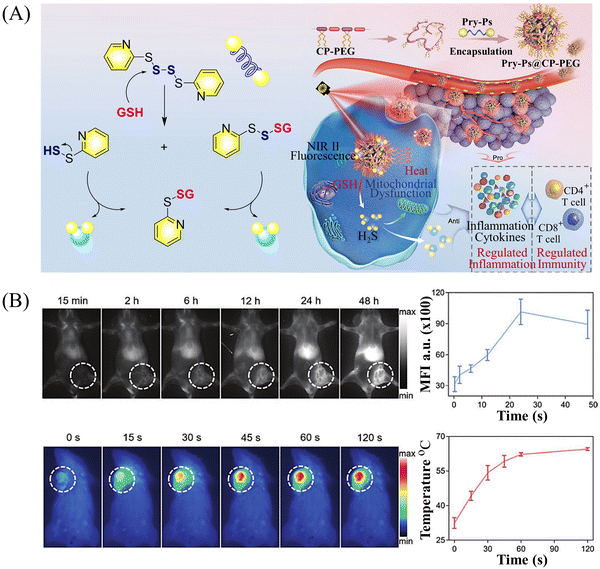 | ||
| Fig. 52 (A) The structure of Pry-Ps, the mechanism of H2S release. Schematic illustration of the NIR-II fluorescence tracked Pry-Ps@CP-PEG. (B) Pry-Ps@CP-PEG fluorescence and photothermal imaging in mice. Parts (A) and (B) are reproduced from ref. 168 with the permission of Wiley-VCH GmbH, copyright 2021. | ||
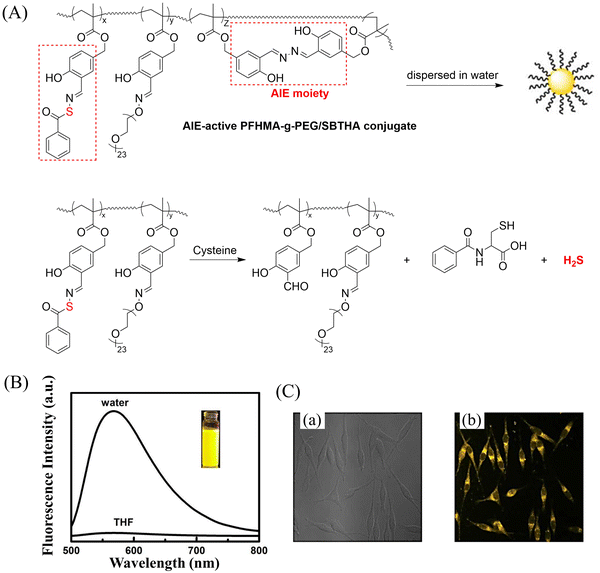 | ||
| Fig. 53 (A) The structure of AIE-active PFHMA-g-PEG/SBTHA, the mechanism of H2S release. (B) Fluorescence spectrum of AIE-active PFHMA-g-PEG/SBTHA in water or THF (λex = 390 nm, λem = 570 nm). Inset: Fluorescence image in water under 365 nm UV light. (C) CLSM images of L929 cells after incubation with 50 μg mL−1 of AIE-active PFHMA-g-PEG/SBTHA conjugate (i.e. salicylaldazine moieties). (a) Bright field, (b) excited with a 390 nm laser. Reproduced from ref. 172 with the permission of the Royal Society of Chemistry, copyright 2018. | ||
Organic trisulfide (self-immolative fluorogenic donors) triggered by bio-thiols can be used to effectively monitor intracellular H2S and lysosomal transport of H2S, laying the foundation for the development of H2S mediated disease treatment. The Bhabak group have developed a bio-thiol triggered H2S donor (UTS-1 and UTS-2), the core group of the donor comes from organic trisulfide.173 When activated by bio-thiols, UTS-1 and UTS-2 release H2S in a controlled manner (Fig. 54(A)), and generate a significant fluorescence response suitable for monitoring intracellular H2S release in real-time (Fig. 54(B)). In addition, both the donors have good compatibility with water and cell media and UTS-2 exhibits organelle specificity.
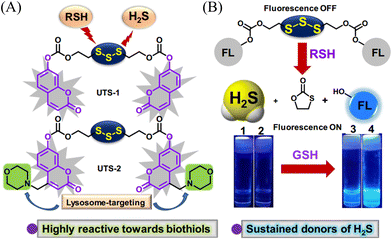 | ||
| Fig. 54 (A) The structure of UTS-1 and UTS-2. (B) The mechanism of H2S release and its fluorescence changes. Images of UTS-1 (20 μM, vial 1, 2) and UTS-1 (20 μM) with GSH (100 μM, vial 3; 200 μM, vial 4) in DMSO after 15 min. Reproduced from ref. 173 with the permission of the Royal Society of Chemistry, copyright 2020. | ||
It is well known that H2S has a protective effect for cells due to its anti-inflammatory and anti-apoptotic activity.174 Therefore, the development and research toward H2S donors is crucial to understand the potential mechanism of inflammation and optimize treatment interventions. The Zhang group developed a Cys triggered NIR fluorescent H2S donor (Pro-s).175 Because Pro-s contains a strong electron withdrawing carbon-sulfur double bond, the electron donating ability of the amino group decreases, resulting in low fluorescence output. The sulfhydryl of Cys selectively attacks the Pro-s carbon–sulfur double bond to produce intermediate Mid1. Then intramolecular cyclization occurs to form intermediate Mid 2. Intermediate Mid2 further cyclizes to produce TCOC and H2S, while TCON and N-acetylcysteine are also produced (Fig. 55(A)). Pro-s exhibits strong NIR fluorescence enhancement (70-fold), controlled H2S release (30 minutes), high H2S release efficiency (62%) and good live cell compatibility. Enabling the visual monitoring of H2S release in cells and zebrafish (Fig. 55(B)), in addition the anti-inflammatory effect of Pro-s has been confirmed in macrophages (RAW 264.7).
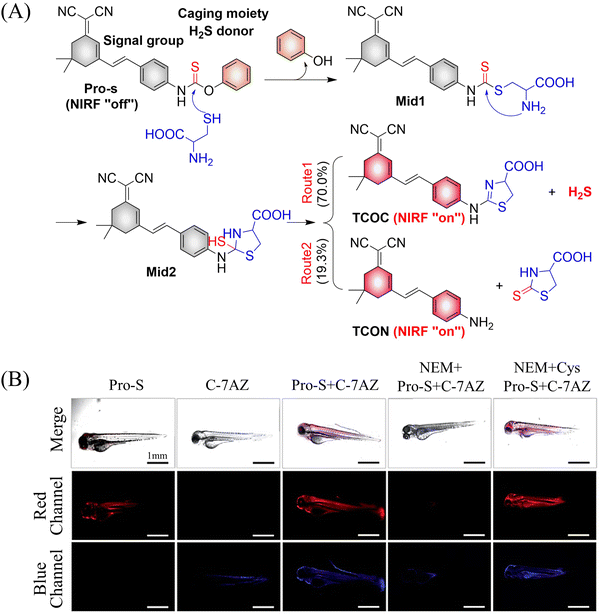 | ||
| Fig. 55 (A) The structure of Pro-s, the mechanism of H2S release and its fluorescence changes. (B) Pro-s releases H2S confocal fluorescence imaging in zebrafish. Reproduced from ref. 175 with the permission of the American Chemical Society, copyright 2021. | ||
In 2021, the Zhang group developed a Cys triggered H2S donor (HSD560).176 The fluorescence of HSD560 is quenched by the thionoester group. When the thionoester is exposed to an enriched Cys environment, HSD560 will undergo a non-enzymatic native chemical ligation (NCL) process to release H2S. In biological systems, the generated NapOH is further deprotonated to generate NapO−, thereby restoring the ICT effect (Fig. 56(A)). The generated green fluorescence enables the visual monitoring of H2S release in zebrafish (Fig. 56(B)). HSD560 could reduce the levels of NO and Prostaglandin E2, achieving anti-inflammatory effect. Compared to COS/H2S donors, the H2S release of HSD560 is non-enzymatic. Therefore, this donor may be a promising molecular tool for the delivery and physiological research of H2S in complex biological environments.
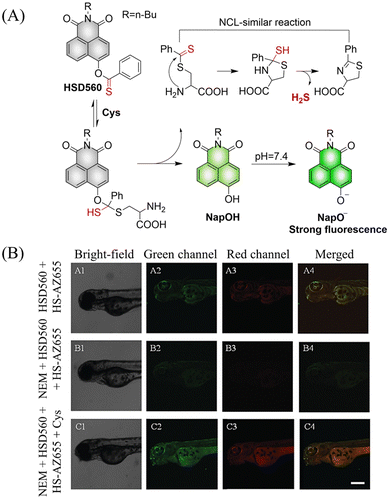 | ||
| Fig. 56 (A) The structure of HSD560, the mechanism of H2S release and its fluorescence changes. (B) HSD560 released H2S confocal fluorescence imaging in zebrafish. Reproduced from ref. 176 with the permission of the Royal Society of Chemistry, copyright 2021. | ||
4.2. Indirectly produced H2S
We now cover previously reported indirect H2S donors. Where, following a series of reactions, these donors release hydrolyzable COS, and then COS is rapidly hydrolyzed to H2S by the ubiquitous carbonic anhydrase (CA). There are two possible ways for such donors to release COS: through thiocarbamate/thiocarbonate cleavage to generate COS. The specific mechanisms are shown in Fig. 57. In addition, the donor can release COS and a fluorophore, thus providing an optical response. The strategy of using reactive intermediates to produce COS for subsequent H2S release is now a widely used method for constructing H2S donors.177–180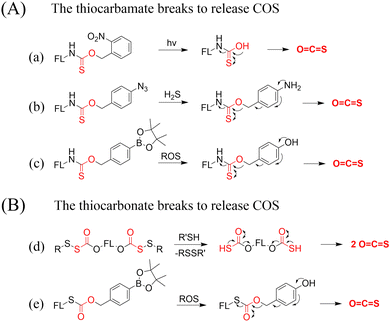 | ||
| Fig. 57 Under different triggering conditions, the thiocarbamate (A)/thiocarbonate (B) breaks to release COS. | ||
We have elucidated the biological significance of H2S by employing self-immolative thiocarbamate as COS donors with fluorescence activity. For example, o-nitrobenzyl-substituted thiocarbamate undergoes photoinduced nitro group transformation to yield 2-nitrobenzaldehyde and thiocarbamate intermediates, the thiocarbamate intermediates then decompose to generate COS and the corresponding fluorescent moieties (Fig. 57(a)).181 An azido benzene-substituted thiocarbamate, under H2S attack, undergoes 1,6-elimination to produce thiocarbamate intermediates that further decompose to release COS (azido compound serving as the H2S recognition site) (Fig. 57(b)).182 Analogously, an arylboronic acid ester-substituted thiocarbamates, under ROS conditions, also undergoes 1,6-elimination to ultimately yield COS (Fig. 57(c)).183 Examples of systems where a thiocarbonate breaks to release COS, include the example where cellular thiols (Cys and GSH) activate sulfenyl thiocarbonates through thiol-mediated disulfide reduction to release COS, which is quickly converted to H2S by CA (Fig. 57(d)).184 In addition, arylboronic acid ester-substituted thiocarbonate, under ROS treatment, can undergoes 1,6-elimination to yield COS (Fig. 57(e)).185 The highly modular approach outlined above for thiocarbamate/thiocarbonate cleavage offers a COS-based H2S donor library, which can be activated through specific stimuli to achieve H2S release that can be visualized.
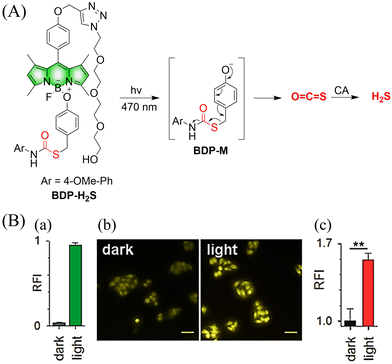 | ||
| Fig. 58 (A) Proposed decomposition mechanism of BDP-H2S. Under 470 nm light irradiation, the B–O bond of BDP-H2S is broken to produce intermediate BDP-M. Then intermediate BDP-M undergoes 1,6-elimination to generate COS, which is rapidly hydrolyzed to H2S in the presence of CA. (B) Fluorescence intensity comparison of BDP-H2S before and after light triggering (λex = 470 nm, λem = 540 nm). Reproduced from ref. 187 with the permission of the American Chemical Society, copyright 2017. | ||
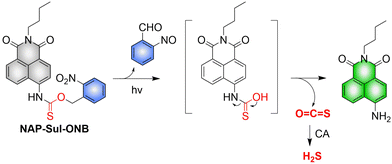
The introduction of photo-active protective group (nitrobenzyl) into a compounds structure can enable the precise activation of functional molecules by light. The Gou group developed an light-controlled H2S donor (NAP-Sul-ONB) derived from naphthalimide (Fig. 59).181 NAP-Sul-ONB was generated by connecting an amine substituted 1,8-naphthalimide and o-nitrobenzyl using a thiocarbamate. The thioamide of the amine on the 1,8-naphthalimide alters the excitation/emission wavelengths. However, once the amine is exposed, the photophysical properties are restored. In addition, under stimulation with 365 nm light, Nap-Sul-ONB can not only release H2S, but also reduces the ROS levels of phorbol-12-myristate-13-acetate treated cells and improves the survival rate of the cells.
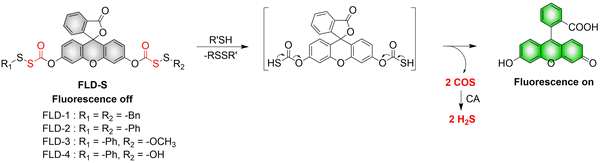 | ||
| Fig. 60 Proposed decomposition mechanism of FLD-S. Under thiol triggered, FLD-S undergoes a cascade reaction to release COS, and then COS is rapidly hydrolyzed to H2S in the presence of CA. | ||
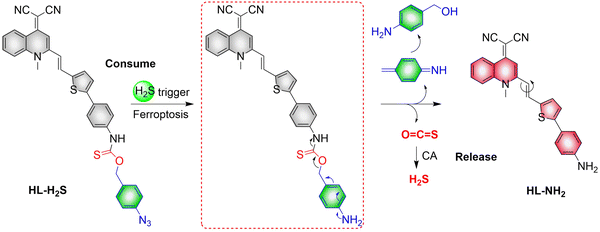
According to previous reports, cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE) can produce endogenous H2S through Cys desulfurization.188–190 Because the ferroptosis protein inhibits CBS/CSE, a reduction of H2S occurs and the redox balance in cells is broken and the process of iron cell death is aggravated. Therefore, H2S, as a representative reducing substance, can be used to monitor the ferroptosis process.191,192 As such, in 2022, a multi-functional NIR-H2S fluorescent probe (HL-H2S) was developed by the Qiang group (Fig. 61).182 In the imaging process, HL-H2S consumes H2S and undergoes 1,6-elimination to release COS, then COS is rapidly hydrolyzed to H2S under the catalysis of CA (H2S has strong reduction properties, which can reduce the electron-withdrawing azido group to an electron-donating amino group). In addition, the malonitrile structure in HL-H2S is responsive to viscosity. This strategy can maintain the redox state of cells without aggravating the process of ferroptosis. The dual action mechanism realizes the visual monitoring in high-fidelity of the ferroptosis process.
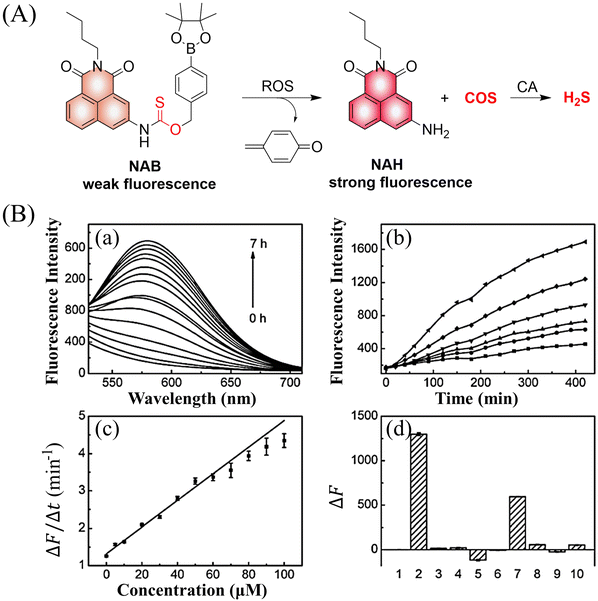 | ||
| Fig. 62 (A) Proposed decomposition mechanism of NAB. (B) (a) Fluorescence spectra of NAB (10 μM) with H2O2 (100 μM) at various times in phosphate buffer (20 mM, pH 7.4). (b) Fluorescence kinetic curves of NAB (10 μM) with different H2O2 concentrations. (c) Linear relationship between fluorescence intensity and H2O2 concentrations. (d) Fluorescence changes of NAB (10 μM) in the presence of different ROS species. (1) NAB only; (2) H2O2 (100 μM); (3) NO (100 μM); (4) ClO− (100 μM); (5) ˙OH (100 μM); (6) 1O2 (100 μM); (7) O2− (100 μM); (8) TBHP (100 μM); (9) TBO˙ (100 μM); (10) ONOO− (10 μM). (λex = 405 nm, λem = 577 nm). Reproduced from ref. 183 with the permission of the Royal Society of Chemistry, copyright 2019. | ||
The Zhang group developed a ROS triggered H2S donor (HSD545) (Fig. 63(A)).193 Triggered by H2O2, HSD545 undergoes a 1,6-elimination reaction to release COS, and then COS is rapidly hydrolyzed to H2S in the presence of CA, and a bright fluorescence response is also generated, thus realizing real-time monitoring of H2S release in vivo and in vitro (Fig. 63(B)). This donor exhibits low cytotoxicity and strong cytoprotection against ROS induced oxidative stress. Compared with previous NAB donors, changing the position of the trigger does not significantly affect the system.
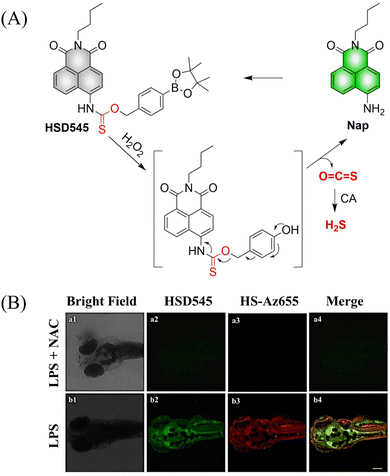 | ||
| Fig. 63 (A) Proposed decomposition mechanism of HSD545 releasing H2S. (B) Fluorescence imaging of H2S released by HSD545 in zebrafish. Reproduced from ref. 193 with the permission of Elsevier B.V., copyright 2021. | ||
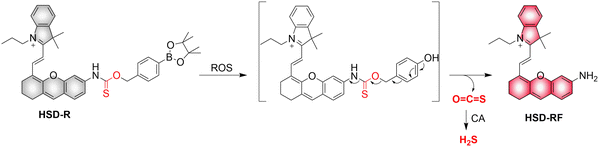
While significant advances have been made in the targeted release of H2S, a detailed understanding of the intracellular redox mechanisms under certain physiological and pathological conditions remains elusive, especially a detailed exploration of H2S levels in the pathological models of myocardial infarction. The Zhang group reported a H2S donor (HSD-R) that exhibits a fluorescence response under ROS stimulation (Fig. 64).25 HSD-R can target the mitochondria and respond to overexpressed ROS. After self-immolation the COS moiety is released, and red fluorescence is generated. During treatment, HSD-R significantly promotes the reconstruction of cardiac structure and function in a rat model of myocardial infarction and achieved cardiac protection by inhibiting proapoptotic genes (Bid, Apaf-1 and p53). As such, this ROS-responsive, self-immolative, and fluorescent H2S donor can serve as a new theranostic agent for myocardial infarction and other ischemic diseases.
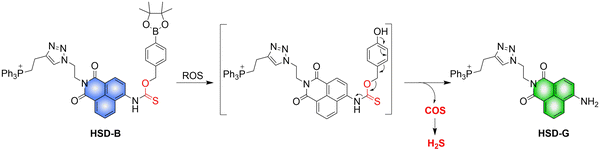
Similarly, the He group reported a new ratiometric fluorescent H2S donor (HSD-B) (Fig. 65).194 In the presence of ROS, the pinacol phenylboronate reacts and releases COS. Subsequently, COS is rapidly hydrolyzed to H2S under the catalysis of CA. At the same time, thiocarbamate substituted naphthalimide is converted to amine substituted naphthalimide HSD-G, and the fluorescence changes from blue to green. The advantages of this system are: (i) scavenging ROS and producing H2S simultaneously, realizing a dual mechanism (scavenging ROS and producing H2S simultaneously) of action in cells; (ii) obvious fluorescence changes, providing a simple monitoring method for the visualization and quantification of H2S release; (iii) by targeting the mitochondria, delivery of H2S is localized. In addition, the system exhibits a protective effect for myocardial ischemia reperfusion injury in a cell model.
The Lukesh III group linked an arylboronic acid ester to 7-hydroxy-4-methylcoumarin using a thiocarbonate alkyl ester bond to prepare a H2S donor (Zl47) (Fig. 66(A)).185 Under the stimulation of ROS, the mechanism of H2S release by Zl47 is consistent with that of similar donors. Therefore, Zl47 was suitable for cell imaging and alleviating oxidative stress in living cells and exhibits potential as a therapeutic agent for diseases related to ROS overexpression (Fig. 66(B)).
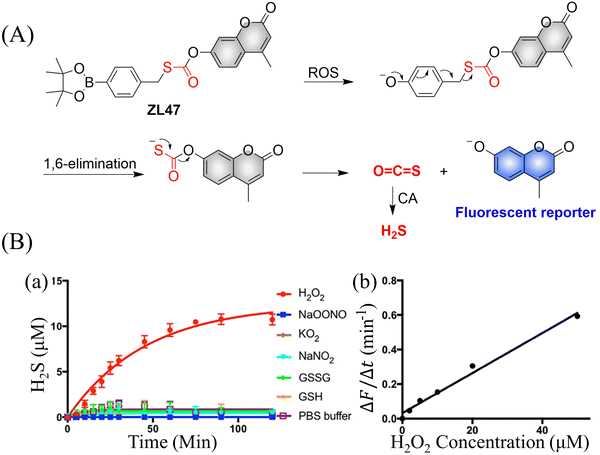 | ||
| Fig. 66 (A) Proposed decomposition mechanism of ZL47. (B) (a) Time-dependent H2S release from ZL47. (b) Linear relationship between fluorescence intensity and H2O2 (λex = 365 nm, λem = 448 nm). Reproduced from ref. 185 with the permission of Elsevier Ltd, copyright 2021. | ||
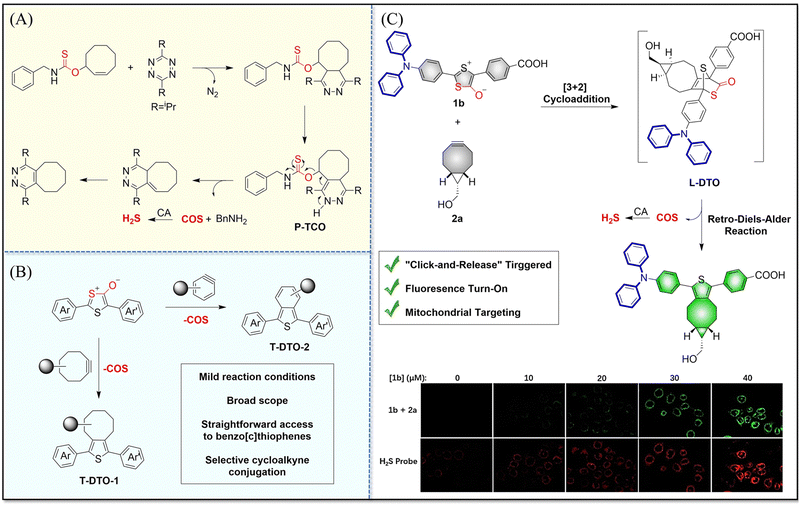 | ||
| Fig. 67 (A) and (B) P-TCO,T-DTOs structure, COS release mechanism and fluorescence changes. (C) L-DTO structure, COS release mechanism and fluorescence changes. Reproduced from ref. 201 with the permission of Wiley-VCH GmbH, copyright 2021. | ||
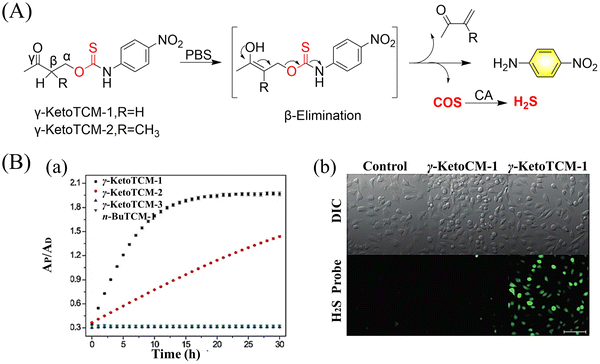 | ||
| Fig. 68 (A) γ-KetoTCM-1-2 structure, H2S release mechanism and fluorescence changes. (B) (a) Formation of p-nitroaniline (PNA) after compound activation. (b) H2S Delivery from γ-KetoTCM-1 in HeLa cells. HeLa cells were treated with a cell-trappable H2S fluorescent probe SF7-AM (5 μM) for 30 min, washed, and incubated with FBS-free DMEM only (left), with 100 μM γ-KetoCM-1 (middle), or with γ-KetoTCM-1 (right) for 2 h. Cells were then washed and imaged in PBS. Scale bar: 100 μM. Reproduced from ref. 202 with the permission of Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, copyright 2018. | ||
5. Disulfides
5.1. ROS triggered RSSR release
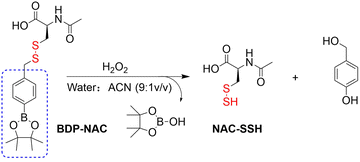
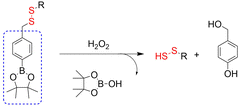
Persulfides (RSSH) are considered to be an important signaling unit in sulfur atom-mediated oxidation reduction processes. However, the direct study of persulfides is difficult. Therefore, it is necessary to develop stable RSSH donors, which can help provide a deeper understanding of RSSR. The Matson group developed a ROS triggered RSSH donor (BOP-NCA) (Fig. 69).203N-Acetylcysteine was linked to a boronic acid ester via a disulfide bond, to obtain BOP-NAC. Subsequently, action of H2O2 on BOP-NAC triggers the releases of NAC-SSH and 4-hydroxybenzyl alcohol. In addition, the as-generated NAC-SSH moiety can protect cells against oxidative stress and can help maintain redox homeostasis better than the normal H2S donors Na2S and GYY4137.Based on this design (Fig. 70) the disulfide terminal group was replaced with a fluorophore to generate the RSSR donor (BDP-fluor) (Fig. 71(A)–(C)). Intermediate BOP-1 generated under the action of H2O2 rapidly cyclizes to obtain the five membered benzodithiolone and releases 7-hydroxycoumarin enabling the visual monitoring of RSSR release.
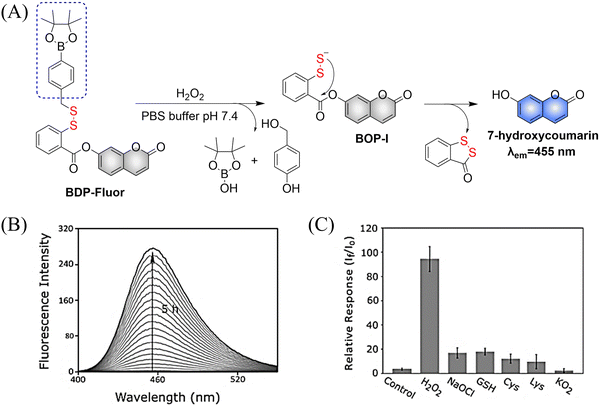 | ||
| Fig. 71 (A) The structure of BOP-fluor, the mechanism of RSSR release and its fluorescence changes. (B) Triggered by excessive H2O2, BOP fluor releases 7-hydroxycoumarin within 5 hours, resulting in a 100-fold fluorescence change. (C) The relative response of BDP fluor (3.3 μM) to each potential trigger (330 μM) or control (no trigger added). Reproduced from ref. 203 with the permission of John Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, copyright 2018. | ||
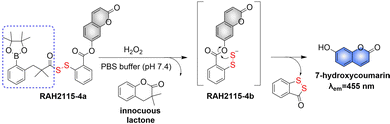
Following on from the research of Xian204 and Matson,203 the Lukesh group developed a series of highly modular RSSR fluorescent donors (RAH2115-4a) (Fig. 72).205 Here we only focus on the RAH2115-4a donor that can produce RSSR under the trigger of hydrogen peroxide. Significantly the gem-dimethyl ensures the generation of acyl disulfide intermediates by RAH2115-4a donor, the exposed sulfur atom at the end of the disulfide bond can generate RSSR by nucleophilic attack on the ester group. Firstly, the donor is triggered by H2O2 to generate a non-toxic by-product; secondly, the acyl disulfide intermediate RAH2115-4b generates a five membered cyclic benzodithiolone by a rapid cyclization reaction. This reaction results in the release of 7-hydroxycoumarin which turns on the fluorescence output, the fluorescence intensity is proportional to the concentration of RSSR, providing a visual way to monitor the release of RSSR. This highly modular design has the potential to monitor any one of the numerous diseases associated with the uncontrolled production of ROS, such as cancer, inflammation, and cardiovascular disease.
6. Sulfur dioxide
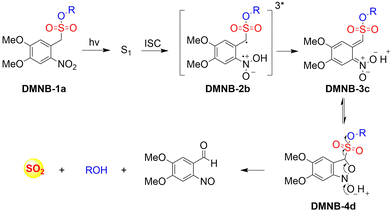
6.1. Photo controlled SO2 release
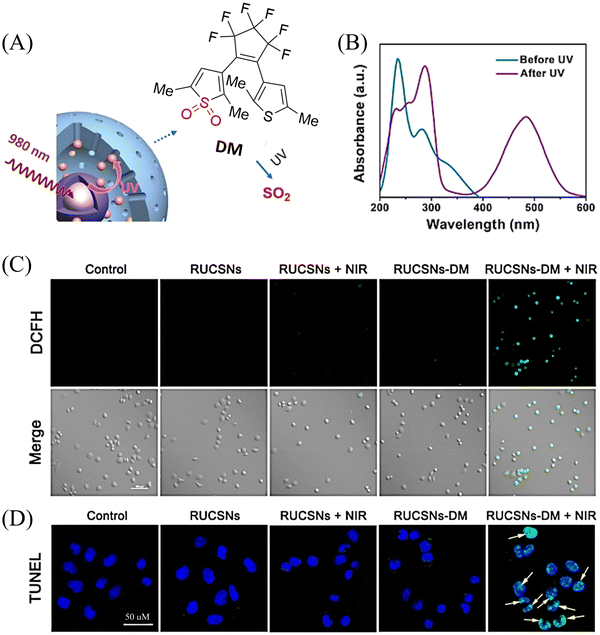 | ||
| Fig. 73 (A) Schematic illustration of NIR light-triggered SO2 generation from RUCSNs-DM. (B) UV-vis absorption spectra of DM before and after UV irradiation (365 nm). (C) Confocal imaging of intracellular ROS levels in HeLa cells after treatment with PBS (control), RUCSNs, RUCSNs + NIR (980 nm laser irradiation), RUCSNs-DM, RUCSNs-DM + NIR (980 nm laser irradiation) (D) Intracellular TUNEL staining in HeLa cells after treatment with different formulations (blue fluorescence: DAPI, green fluorescence: TUNEL). The yellow arrows indicated the overlap of blue fluorescence from DAPI and the green fluorescence from TUNEL, suggesting DNA fragmentation in the nucleus. Parts (A–D) are reproduced from ref. 208 with the permission of the American Chemical Society, copyright 2019. | ||
6.2. ROS triggered SO2 release
 | ||
| Fig. 76 (A) Formation of Au@MnO2NPs nanoparticles. (B) and (C) Proposed decomposition mechanism of BTS releasing SO2 and self-reporting GT. Fluorescein isothiocyanate (FITC) is coupled to the surface of Au by caspase-3 responsive peptide (DEVEC). The fluorescence of FITC was quenched due to the FRET effect between Au NPs and FITC. However, caspase-3 is overexpressed in apoptotic tumor cells. As such DEVEC is cleaved and FITC fluorescence is restored to achieve “self-reporting” during the therapeutic process. Reproduced from ref. 212 with the permission of Wiley-VCH GmbH, copyright 2021. | ||
6.3. Thiol triggered SO2 release
 | ||
| Fig. 78 (A) Construction of a nano platform (MON-DN@PCBMA-DOX) and its SO2-release mechanism. (B) In vivo NIR-FI of tumor-bearing mice after injection of Cy7, MON-Cy7, and MON@PCBMA-Cy7 (1 mg mL−1, 100 μL). Reproduced from ref. 215 with the permission of Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, copyright 2020. | ||
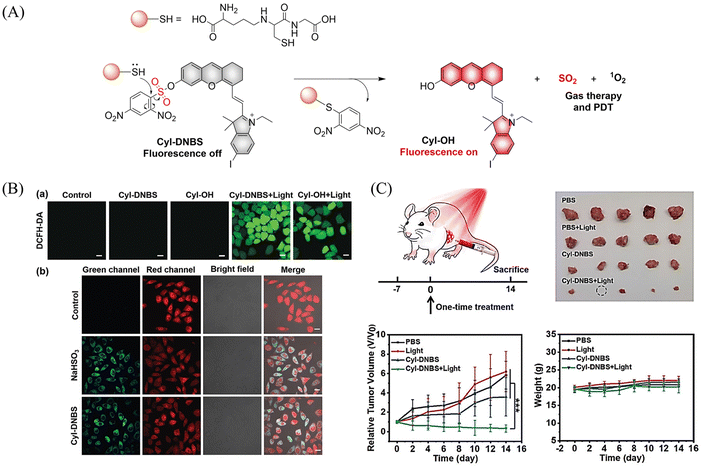 | ||
| Fig. 79 (A) The structure of Cyl-DNBS, the mechanism of SO2 release and its fluorescence change. (B) Cyl-DNBS generates ROS and SO2 fluorescence imaging in cells. (C) Effect of Cyl-DNBS on cancer mice. Parts (B) and (C) are reproduced from ref. 214 with the permission of Wiley-VCH GmbH, copyright 2021. | ||
7. Reactive oxygen species
7.1. Singlet oxygen
PDT treatment mainly depends on the 1O2 concentration, which can lead to irreversible damage of cancer tissues.216–218 The Yang group reported a smart GSH/pH synergistically activated “dual lock-and-key” supramolecular photosensitizer BIBCl-PAE NPs (Fig. 80(A)).219,220 The authors encapsulate GSH-activated photosensitizer BIBCl (first lock-and-key) into a pH-responsive diblock copolymer (PEG-PAE) to construct BIBCl-PAE NPs (second lock-and-key) for enhanced PDT therapy. In normal tissues of neutral pH and low GSH, the hydrophobic nature of BIBCl and amphiphilic polymer PEG-PAE act as “double locks” to lock the photosensitizer in a tight aggregated state, which cannot sensitize oxygen to 1O2 due to aggregation-caused quenching. While in the tumor microenvironment, BIBCl is activated by a low pH environment and high GSH concentrations. The decomposition of micelles promotes the reaction between BIBCl and GSH, releases BIBSG and realizes efficient PDT (Fig. 80(B)). In vitro and in vivo experiments confirm that BIBCl-PAE NPs are effective in targeting and inhibiting carcinoma (Fig. 80(C)). In addition, using the FRET effect of BIBSG, enhanced 1O2 production and the visual-monitoring of PDT treatment were realized. | ||
| Fig. 80 (A) The structure of BIBCl-PAE NPs, the mechanism of releasing 1O2 and its fluorescence changes. (B) and (C) Effect of PDT on tumors. Parts (B) and (C) are reproduced from ref. 219 with the permission of the Royal Society of Chemistry, copyright 2020. | ||
Aminopeptidase N, APN/CD13 (APN) is overexpressed on the surface of cancer cells. The Peng group have developed a NIR photosensitizer (APN-Cyl) for tumor imaging and photodynamic therapy, which is specifically activated by APN (Fig. 81(A)).221 APN-Cyl could be activated by APN via hydrolysis of an alanine modified group to form Cyl-OH. The hydroxyl group in Cyl-OH has an ICT effect with the hemicyanine dye, which greatly improves the near-infrared fluorescence signals. In addition, Cyl-OH can specifically target mitochondria (positive charge in the structure) and generate a significant amount of 1O2 (due to the heavy iodine atoms) under NIR irradiation, thereby improving the efficacy of PDT and inducing apoptosis of cancer cells (Fig. 81(B)).
 | ||
| Fig. 81 (A) Schematic illustration of APN-Cyl for APN imaging and cancer treatment. (B) Fluorescence imaging of endogenous APN in tumor Balb/c mice in 150 min. Reproduced from ref. 221 with the permission of Elsevier Ltd, copyright 2020. | ||
Other donors with improved functions have also been reported. The Yang group have used the aggregation and disaggregation of fluorophores and photosensitizers (Fig. 82(A)).222 Heavy atoms are added to the fluorophore to enhance the spin orbit coupling (SOC) and to improve the ISC. This reversible switching is important for the development of a controllable PDT system. Compounds BODIPY1-8 exhibit fluorescence quenching in the aggregated state, leading to the generation of ROS. Interestingly, disaggregation can restore fluorescence and prevent ROS generation (Fig. 82(B)). If the ISC process (S1 → Tn) is enhanced, the chromophore can act as a sensitizer to transfer the excitation energy of its triplet excited state to oxygen molecules to generate 1O2, thereby enhancing the PDT effect. This research provides a new strategy for designing heavy atom-free PSs and paves the way for the development of intelligent PDT systems.
 | ||
| Fig. 82 (A) The aggregated fluorophore is converted into photosensitizer (ISC on), and the fluorescence is quenched to produce ROS; the disaggregated photosensitizer is converted into a fluorophore (ISC off), and the fluorescence is restored without generating ROS. (B) BODIPY derivative structure. Reproduced from ref. 222 with the permission of the Chinese Chemical Society, copyright 2021. | ||
7.2. Superoxides
The Fang group introduced diselenated/disulfide units into camptothecin (CPT) and reported a series of seleno prodrugs CPT-Se3 and CPT-Se4 (the fluorescence of CPT was quenched by the diselenide/disulfide bond) (Fig. 83).223 Both selenium pro-drugs were activated by GSH, and the fluorescence of CPT was significantly enhanced (about 10-fold). The activation of the pro-drug is accompanied by the production of selenol intermediates, which catalyze the continuous conversion of GSH and O2 to oxidized glutathione and O2˙−. Thus, the level of ROS in cells is increased, and finally the apoptosis of cancer cells is induced. The authors confirmed that incorporating diselenide units into drugs may be a general strategy to improve drug efficacy. Furthermore, the quenching of CPT fluorescence by diselenide bonds suggests potential applications of diselenide bonds in the construction of sensors or therapeutic agents.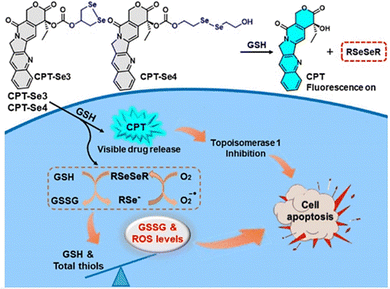 | ||
| Fig. 83 The structure of CPT-Se3 and CPT-Se4, the mechanism of CPT and O2˙− release and the fluorescence changes. Reproduced from ref. 223 with the permission of the American Chemical Society, copyright 2021. | ||
7.3. Hydroxyl radicals
Chemodynamic therapy (CDT) can occur and induce apoptosis by converting H2O2 enriched in tumors into hydroxyl radicals (˙OH) with high cytotoxicity through the Fenton reaction.224,225 The Liu group have developed a self-assembled metal–organic coordination nanoparticle (Cu-OCNP/Lap) (Fig. 84(A)).226 Cu-OCNP/Lap exhibits good photothermal effect under 1064 nm light irradiation, the corresponding local temperature rise accelerates the blood flow and provides sufficient O2 at the hypoxic tumor site. The enhanced intracellular oxygen supply effectively reinforces the β-Lap redox cycling and results in abundant intracellular H2O2 accumulation, which facilitates the Cu+ Fenton-like reaction and effectively enhances CDT efficiency. In addition, significant amounts of GSH are consumed during the decomposition of the Cu-OCNP/Lap, which alleviates the antioxidant defense of the tumor microenvironment. Due to the PeT mechanism, Cu2+ in Cu-OCNP/Lap promotes the non-radiative transitions of AQ4N and THQ, thereby improving the photothermal effect. Initially, the fluorescence of Cu-OCNP/Lap is in a quenched state. Then when the GSH reduces the Cu2+ to Cu+, the fluorescence of AQ4N rapidly recovers enabling the visual monitoring of CDT treatment (Fig. 84(B)). The development of this donor provides a new concept for enhancing CDT treatment in a hypoxic environment. | ||
| Fig. 84 Schematic illustration of (A) synthesis of Cu–OCNP/Lap and (B) its intracellular delivery and NIR-II reinforced intracellular β-Lap cyclic reaction with abundant H2O2 supply to enhance CDT. Reproduced from ref. 226 with the permission of Elsevier Ltd, copyright 2021. | ||
8. Other gaseous transmitter donors
8.1. Photo controlled HCHO release
Despite numerous donors for the release of FA, the relationship between disease and formaldehyde concentration is still a major challenge owing to a lack of quantitative FA release strategies in biological systems. In order to understand the complex biological relationship of FA, the Chan group developed a photo-activated formaldehyde donor (photoFAD-3) (Fig. 85(A)).227 PhotoFAD-3 consists of the photon cleavable o-nitrobenzyl group and silicon–xanthene fluorophore linked by an acetal. Initially the fluorescence is quenched by PeT. However, under light irradiation, the o-nitrobenzyl group in photoFAD-3 is cleaved, and the fluorescence is enhanced about 139 times when FA is released, thus realizing the visual-monitoring of FA release. Moreover, a quantitative strategy to quantify the release of intracellular FA by cell lysate calibration was developed (Fig. 85(B) and (C)). Which marks the first example where the concentration of an intracellular analyte released from photoactivated donors could be quantified. | ||
| Fig. 85 (A) Proposed decomposition mechanism of photoFAD-3 releasing FA. (B) Epifluorescence and (C) IVIS images of HEK293 cells stained with photoFAD-3 after 0, 5, 20, 60, and 180 s of photoactivation. Scale bar represents 100 μm. Parts (B) and (C) are reproduced from ref. 227 with the permission of the American Chemical Society, copyright 2020. | ||
9. Conclusions and outlook
Exhibiting visible and controllable release of functional small molecules, FSMDs have enabled drug tracking, dose control, targeted delivery, spatiotemporal feedback, and precision medicine. This review focuses on the latest research strategies and applications of FSMDs: (i) the core structural design of FSMDs for the selective release of each type of signaling molecule (NO, CO, H2S, RSSH, SO2, ROS, H2Se, CO2 and FA, etc.) has been outlined, providing guidance in choosing appropriate chemical structures. (ii) The factors influencing the release of small molecules from FSMDs, including non-planar torsional conformation, the switch of electronic delocalization, molecule rearrangement, the effect of free radicals, molecular dynamic properties, etc. have been discussed, which highlights the underlying mechanisms of small molecule release coupled with fluorescence responses for FSMDs. (iii) FSMDs-based prodrugs, as well as their integration with gaseous therapy (GT), photodynamic therapy (PDT), photothermal therapy (PTT), or chemotherapy (CT), exhibit enhanced efficiency for drug screening and cancer treatment.Although great progress in FSMDs research has been made, many challenges remain and opportunities for additional research remain.
(1) For in vivo applications. To date FSMDs have been mainly evaluated in cells, while in vivo applications remain mostly underexplored. Firstly, to address this challenge, FSMDs should be stable in a complex biological environment. We anticipate that developing suitable bioconjugates or integration with micelles should help alleviate these problems. Secondly, the effective depth of tissue penetration by the excitation and emission light are crucial for the effective use of FSMDs in vivo. Therefore, fluorophores with longer wavelengths or higher quantum yields are urgently required. Preferably, fluorophores in the NIR-II region should be used for the next generation of FSMDs.
(2) Practical efficiency. To achieve optimal properties for practical applications, the following aspects need be considered: firstly, targeted delivery and activation is essential for FSMDs. Though some classical recognition moieties have been used for the construction of FSMDs, the efficiency is dependent on the intracellular environment. Since the heterogeneous microenvironment of cells may enhance or hinder the performance of the FSMDs. Thus, introducing appropriate receptors to the molecular structure of FSMDs should be further explored. Secondly, the rapid triggering and release in situ requires an improved dynamic performance of FSMDs. So far, the dynamic evaluation of FSMDs especially in a biological environment remains limited. The rate of triggering of FSMDs, is essential since slow activation could result in inaccurate tracking. Thus, the rapid triggering of molecular rearrangement mechanisms is vital for practical applications in biological samples. Thirdly, the threshold to trigger FSMDs is essential to achieve the precise release of small molecules and real-time monitoring. Clearly, an appropriate threshold range that matches the specific biological environment should be optimized for triggering the FSMDs.
(3) Integration of multifunctionality. Cancer treatments can suffer drug resistance (chemotherapy), hypoxia (PDT), insufficient therapeutic effect (PTT), etc. Therefore, integration of FSMDs with these traditional techniques could provide a visible readout of the microenvironment, to overcome current drawbacks and improve efficiency. Small molecule intervention is of promise in the fight against cancer since it may help reverse the drug resistant microenvironment. For example, Near-infrared fluorescent probes for hydrogen sulfide: high-fidelity ferroptosis evaluation in vivo during stroke, and the synergistic effect of SO2 and DOX can effectively alleviate drug resistance in cancer chemotherapy. As such, multifunctional FSMDs could enable treatment coupled with visible evaluation, which may provide appropriate information to advance precision medicine.
Abbreviations
| AIE | Aggregation induced emission |
| APN | Aminopeptidase N, APN/CD13 |
| aza-BODIPY | Aza-boron-dipyrromethene |
| BTS | Benzothiazole sulfinate |
| CA | Carbonic anhydrase |
| CBS | Cystathionine-β-synthase |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| CORMs | Carbon monoxide-releasing molecules |
| COS | Carbonyl sulfide |
| CPT | Camptothecin |
| CSE | Cystathionine-γ-lyase |
| CT | Chemotherapy |
| DM | 1-(2,5-Dimethylthien-1,1-dioxide-3-yl)-2-(2,5-dimethylthien-3-yl)-hexafluorocyclopentene |
| DNBS | 2,4-Dinitrobenzenesulfonate |
| DOX | Doxorubicin |
| ESIPT | Excited-state intramolecular transfer |
| FA | Formaldehyde |
| FAEE | Ferulic acid ethyl ester |
| FHMA | 3-Formyl-4-hydroxybenzyl methacrylate |
| FITC | Fluorescein isothiocyanate |
| FRET | Fluorescence resonance energy transfer |
| FSMDs | Fluorescent small molecule donors |
| GSH | Glutathione |
| GT | Gaseous therapy |
| Hcy | Homocysteine |
| H2O2 | Hydrogen peroxide |
| H2S | Hydrogen sulfide |
| H2Se | Hydrogen selenide |
| IC50 | Half maximal inhibitory concentration |
| ICG | Indocyanine green |
| ICT | Intramolecular charge transfer |
| ISC | Intersystem crossing |
| MSCs | Mesenchymal stem cells |
| MRSA | Methicillin-resistant S. aureus |
| NCL | Native chemical ligation |
| NIR | Near infrared |
| NIR-II | Near-infrared-II |
| NO | Nitric oxide |
| NOPD | NO photo donors |
| ˙OH | Hydroxyl radicals |
| OGD | Oxygen and glucose deprivation |
| OP | One-photon |
| PA | Photoacoustic tomography |
| PDT | Photodynamic therapy |
| PEG | Poly-ethylene glycol |
| PeT | Photoinduced electron transfer |
| pH | Scale used to specify the acidity or basicity of an aqueous solution |
| PTT | Photothermal therapy |
| ROS | Reactive oxygen species |
| SBTHA | s-Benzoyl thiohydroxylamine |
| SOC | Spin orbit coupling |
| SO2 | Sulfur dioxide |
| TCO | Trans-cyclooctene |
| SMDs | Small molecule donors |
| TDLN | Tumor-draining lymph node |
| TICT | Twisted intramolecular charge transfer |
| TP | Two-photon |
| UV | Ultraviolet |
| 2TC | Bithiophene |
Author contributions
Guang Chen, Jing Yu, Jie Xu, Chao Wang, Siyue Ma, Qing Miao, Linlin Wang, Chen Wang, and Zhe Sun wrote and edited the original proposal and draft. Luling Wu and Simon E. Lewis contributed to the scientific illustrations and editing of the manuscript. Xinrui Ji, Yanfeng Yue, Yuxia Liu created the outline and optimized the contents for the review paper. Bo Tang and Tony D. James conceived the topic and revised the manuscript. All authors contributed to the final checking of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22174090); Long-term Project of high-level talents innovation in Shaanxi Province (G. C.); the Natural Science Basic Research Program of Shaanxi Province (2022JM-089). S. E. L. Thanks EPSRC (EP/W036193/1) and BBSRC (BB/X003426/1) for funding. LW thanks the University of Bath for supporting his postdoctoral work in the UK. TDJ wishes to thank the University of Bath and the Open Research Fund of the School of Chemistry and Chemical Engineering, Henan Normal University (2020ZD01) for support. BT wishes to thank the National Natural Science Foundation of China (92253304, 21874085), Natural Science Foundation of Shandong Province of China (ZR2019JQ06), Local Science and Technology Development Fund Guided by the Central Government (YDZX20203700002568), and the Major Science and Technology Innovation Project of Shandong Province (2021ZDSYS09).References
- C. Szabo, Nat. Rev. Drug Discovery, 2016, 15, 185–203 CrossRef CAS PubMed.
- M. Carlstrom, Nat. Rev. Nephrol., 2021, 17, 575–590 CrossRef PubMed.
- S. J. Forrester, D. S. Kikuchi, M. S. Hernandes, Q. Xu and K. K. Griendling, Circ. Res., 2018, 122, 877–902 CrossRef CAS PubMed.
- Y. Kou, H. Zhao, D. Cui, H. Han and Z. Tong, Ageing Res. Rev., 2022, 73, 101512 CrossRef CAS PubMed.
- Z. Li, D. J. Polhemus and D. J. Lefer, Circ. Res., 2018, 123, 590–600 CrossRef CAS PubMed.
- R. Liu, Y. Peng, L. Lu, S. Peng, T. Chen and M. Zhan, J. Nanobiotechnol., 2021, 19, 443 CrossRef CAS PubMed.
- X. Pan, X. Song, C. Wang, T. Cheng, D. Luan, K. Xu and B. Tang, Theranostics, 2019, 9, 1794–1808 CrossRef CAS PubMed.
- Y. Song, J. Song, Z. Zhu, H. Peng, X. Ding, F. Yang, K. Li, X. Yu, G. Yang, Y. Tao, D. Bu, C. Tang, Y. Huang, J. Du and H. Jin, Redox Biol., 2021, 48, 102192 CrossRef CAS PubMed.
- L. Wang, X. Xie, B. Ke, W. Huang, X. Jiang and G. He, J. Adv. Res., 2022, 38, 261–274 CrossRef CAS PubMed.
- Y. Zhou, W. Yu, J. Cao and H. Gao, Biomaterials, 2020, 255, 120193 CrossRef CAS PubMed.
- H. Qiu, P. Qi, J. Liu, Y. Yang, X. Tan, Y. Xiao, M. F. Maitz, N. Huang and Z. Yang, Biomaterials, 2019, 207, 10–22 CrossRef CAS PubMed.
- D. Zhu, J. Hou, M. Qian, D. Jin, T. Hao, Y. Pan, H. Wang, S. Wu, S. Liu, F. Wang, L. Wu, Y. Zhong, Z. Yang, Y. Che, J. Shen, D. Kong, M. Yin and Q. Zhao, Nat. Commun., 2021, 12, 4501 CrossRef CAS PubMed.
- J. Mendhi, I. Prasadam, S. Subramaniam, L. Bai, W. Gao, J. Batra, R. Crawford, Y. Yang and Y. Xiao, Appl. Mater. Today, 2021, 22, 100919 CrossRef.
- B. E. Mann and R. Motterlini, Chem. Commun., 2007, 4197–4208 RSC.
- A. Kobayashi, K. Ishikawa, H. Matsumoto, S. Kimura, Y. Kamiyama and Y. Maruyama, Hypertension, 2007, 50, 1040–1048 CrossRef CAS PubMed.
- R. Motterlini and L. E. Otterbein, Nat. Rev. Drug Discovery, 2010, 9, 728–743 CrossRef CAS PubMed.
- W. Zhao, J. Zhang, Y. Lu and R. Wang, EMBO J., 2001, 20, 6008–6016 CrossRef CAS PubMed.
- X. Zhu, Y. He, Z. Liu, Z. Zhu, Y. He, J. Qiu, D. Liu, M. Mo, P. Wang, X. Tian and P. Xu, Appl. Mater. Today, 2020, 19, 100559 CrossRef.
- S. Mani, H. Li, A. Untereiner, L. Wu, G. Yang, R. C. Austin, J. G. Dickhout, S. Lhotak, Q. H. Meng and R. Wang, Circulation, 2013, 127, 2523–2534 CrossRef CAS PubMed.
- C. Szabo, Nat. Rev. Drug Discovery, 2007, 6, 917–935 CrossRef CAS PubMed.
- W. Shen, W. Liu, H. Yang, P. Zhang, C. Xiao and X. Chen, Biomaterials, 2018, 178, 706–719 CrossRef CAS PubMed.
- L. An, P. Zhang, W. Shen, X. Yi, W. Yin, R. Jiang and C. Xiao, Bioact. Mater., 2021, 6, 1365–1374 CAS.
- Z. Shen, S. Zheng, S. Xiao, R. Shen, S. Liu and J. Hu, Angew. Chem., Int. Ed., 2021, 60, 20452–20460 CrossRef CAS PubMed.
- J. Cheng, G. Gan, Z. Shen, L. Gao, G. Zhang and J. Hu, Angew. Chem., Int. Ed., 2021, 60, 13513–13520 CrossRef CAS PubMed.
- M. Yao, Y. Lu, L. Shi, Y. Huang, Q. Zhang, J. Tan, P. Hu, J. Zhang, G. Luo and N. Zhang, Bioact. Mater., 2022, 9, 168–182 CAS.
- C. W. Spicer, Atmospheric Environ., 1977, 11, 1089–1095 CrossRef CAS.
- J. R. Lancaster, Biochem. Pharmacol., 2020, 176, 113793 CrossRef CAS PubMed.
- A. W. Carpenter and M. H. Schoenfisch, Chem. Soc. Rev., 2012, 41, 3742 RSC.
- T. Liu, M. Zhang, G. T. Mukosera, D. Borchardt, Q. Li, T. E. Tipple, A. S. Ishtiaq Ahmed, G. G. Power and A. B. Blood, Redox Biol., 2019, 26, 101238 CrossRef CAS PubMed.
- X. Wan, S. Liu, X. Xin, P. Li, J. Dou, X. Han, I.-K. Kang, J. Yuan, B. Chi and J. Shen, Chem. Eng. J., 2020, 400, 125964 CrossRef CAS.
- S. A. Jamal and C. J. Hamilton, Curr. Osteoporos. Rep., 2012, 10, 86–92 CrossRef PubMed.
- A. I. Santos, A. S. Lourenço, S. Simão, D. Marques da Silva, D. F. Santos, A. P. Onofre de Carvalho, A. C. Pereira, A. Izquierdo-Álvarez, E. Ramos, E. Morato, A. Marina, A. Martínez-Ruiz and I. M. Araújo, Redox Biol., 2020, 32, 101457 CrossRef CAS PubMed.
- A. B. Seabra and N. Durán, Eur. J. Pharmacol., 2018, 826, 158–168 CrossRef CAS PubMed.
- K. Piech, T. Bally, A. Sikora and A. Marcinek, J. Am. Chem. Soc., 2007, 129, 3211–3217 CrossRef CAS PubMed.
- T. A. Grover, J. A. Ramseyer and L. H. Piette, Free Radical Biol. Med., 1987, 3, 27–32 CrossRef CAS PubMed.
- X. Xu, M. Sun, X. Luo, Z. Zhang, L. Su, L. Cui, Z. Zhu, X. Lu, R. Wang, F. Han, X. Qian and Y. Yang, Free Radical Biol. Med., 2021, 164, 13–19 CrossRef CAS PubMed.
- Z. Zhang, J. Wu, Z. Shang, C. Wang, J. Cheng, X. Qian, Y. Xiao, Z. Xu and Y. Yang, Anal. Chem., 2016, 88, 7274–7280 CrossRef CAS PubMed.
- Z. Shen, S. Zheng, S. Xiao, R. Shen, S. Liu and J. Hu, Angew. Chem., Int. Ed., 2021, 60, 20452–20460 CrossRef CAS PubMed.
- H. He, Z. Ye, Y. Xiao, W. Yang, X. Qian and Y. Yang, Anal. Chem., 2018, 90, 2164–2169 CrossRef CAS PubMed.
- H. He, Y. Liu, Z. Zhou, C. Guo, H. Y. Wang, Z. Wang, X. Wang, Z. Zhang, F. G. Wu, H. Wang, D. Chen, D. Yang, X. Liang, J. Chen, S. Zhou, X. Liang, X. Qian and Y. Yang, Free Radical Biol. Med., 2018, 123, 1–7 CrossRef CAS PubMed.
- H. He, Y. Xia, Y. Qi, H. Y. Wang, Z. Wang, J. Bao, Z. Zhang, F. G. Wu, H. Wang, D. Chen, D. Yang, X. Liang, J. Chen, S. Zhou, X. Liang, X. Qian and Y. Yang, Bioconjugate Chem., 2018, 29, 1194–1198 CrossRef CAS PubMed.
- H. He, T. He, Z. Zhang, X. Xu, H. Yang, X. Qian and Y. Yang, Chin. Chem. Lett., 2018, 29, 1497–1499 CrossRef CAS.
- D. D. Thomas, L. A. Ridnour, J. S. Isenberg, W. Flores-Santana, C. H. Switzer, S. Donzelli, P. Hussain, C. Vecoli, N. Paolocci, S. Ambs, C. A. Colton, C. C. Harris, D. D. Roberts and D. A. Wink, Free Radical Biol. Med., 2008, 45, 18–31 CrossRef CAS PubMed.
- D. Fukumura, S. Kashiwagi and R. K. Jain, Nat. Rev. Cancer, 2006, 6, 521–534 CrossRef CAS PubMed.
- S. Mocellin, V. Bronte and D. Nitti, Med. Res. Rev., 2007, 27, 317–352 CrossRef CAS PubMed.
- Y. Liu, X. Wang, J. Li, J. Tang, B. Li, Y. Zhang, N. Gu and F. Yang, Adv. Mater., 2021, 33, 2101701 CrossRef CAS PubMed.
- D. Jiang, L. Cheng, Y. Xue, C. Chen, C. Wang, G. Yang, A. Xu, Y. Yang, Y. Gao and W. Zhang, Chem. Sci., 2020, 11, 8785–8792 RSC.
- X. Xie, J. Fan, M. Liang, Y. Li, X. Jiao, X. Wang and B. Tang, Chem. Commun., 2017, 53, 11941–11944 RSC.
- O. Liba and A. de la Zerda, Nat. Biomed. Eng., 2017, 1, 0075 CrossRef CAS.
- L. V. Wang and J. Yao, Nat. Methods, 2016, 13, 627–638 CrossRef CAS PubMed.
- M. J. Waldner, F. Knieling, C. Egger, S. Morscher, J. Claussen, M. Vetter, C. Kielisch, S. Fischer, L. Pfeifer, A. Hagel, R. S. Goertz, D. Wildner, R. Atreya, D. Strobel and M. F. Neurath, Gastroenterology, 2016, 151, 238–240 CrossRef PubMed.
- J. Ouyang, L. Sun, Z. Zeng, C. Zeng, F. Zeng and S. Wu, Angew. Chem., Int. Ed., 2020, 59, 10111–10121 CrossRef CAS PubMed.
- L. Yu, S. A. Abbas Abedi, J. Lee, Y. Xu, S. Son, W. Chi, M. Li, X. Liu, J. H. Park and J. S. Kim, Angew. Chem., Int. Ed., 2023, 62, e202307797 CrossRef CAS PubMed.
- Z. Mao, H. Rha, J. Kim, X. You, F. Zhang, W. Tao and J. S. Kim, Adv. Sci., 2023, 10, 2301177 CrossRef CAS PubMed.
- E. Y. Zhou, H. J. Knox, C. J. Reinhardt, G. Partipilo, M. J. Nilges and J. Chan, J. Am. Chem. Soc., 2018, 140, 11686–11697 CrossRef CAS PubMed.
- L. K. K. Holland, I. Ø. Nielsen, K. Maeda and M. Jäättelä, Cell, 2020, 181, 748 CrossRef CAS PubMed.
- H. Xu and D. Ren, Annu. Rev. Physiol., 2015, 77, 57–80 CrossRef CAS PubMed.
- W. Hua, J. Zhao, X. Wang, S. Pei and S. Gou, Analyst, 2019, 144, 6681–6688 RSC.
- N. Ieda, Y. Hotta, N. Miyata, K. Kimura and H. Nakagawa, J. Am. Chem. Soc., 2014, 136, 7085–7091 CrossRef CAS PubMed.
- H. Okuno, N. Ieda, Y. Hotta, M. Kawaguchi, K. Kimura and H. Nakagawa, Org. Biomol. Chem., 2017, 15, 2791–2796 RSC.
- C. Parisi, M. Failla, A. Fraix, B. Rolando, E. Gianquinto, F. Spyrakis, E. Gazzano, C. Riganti, L. Lazzarato, R. Fruttero, A. Gasco and S. Sortino, Chem. – Eur. J., 2019, 25, 11080–11084 CrossRef CAS PubMed.
- R. Shen and Y. Qian, J. Lumin., 2021, 232, 117872 CrossRef CAS.
- C. Parisi, M. Seggio, A. Fraix and S. Sortino, ChemPhotoChem, 2020, 4, 742–748 CrossRef CAS.
- Y. Xu, S. Wang, Z. Chen, R. Hu, Y. Zhao, K. Wang, J. Qu and L. Liu, Biomaterials, 2021, 276, 121017 CrossRef CAS PubMed.
- T. Suzuki, O. Nagae, Y. Kato, H. Nakagawa, K. Fukuhara and N. Miyata, J. Am. Chem. Soc., 2005, 127, 11720–11726 CrossRef CAS PubMed.
- E. B. Caruso, S. Petralia, S. Conoci, S. Giuffrida and S. Sortino, J. Am. Chem. Soc., 2007, 129, 480–481 CrossRef CAS PubMed.
- K. Hishikawa, H. Nakagawa, T. Furuta, K. Fukuhara, H. Tsumoto, T. Suzuki and N. Miyata, J. Am. Chem. Soc., 2009, 131, 7488–7489 CrossRef CAS PubMed.
- K. K. Cotí, M. E. Belowich, M. Liong, M. W. Ambrogio, Y. A. Lau, H. A. Khatib, J. I. Zink, N. M. Khashab and J. F. Stoddart, Nanoscale, 2009, 1, 16–39 RSC.
- N. Ž. Knežević and J. O. Durand, ChemPlusChem, 2015, 80, 26–36 CrossRef.
- R. K. Kankala, Y. H. Han, J. Na, C. H. Lee, Z. Sun, S. B. Wang, T. Kimura, Y. S. Ok, Y. Yamauchi, A. Z. Chen and K. C. Wu, Adv. Mater., 2020, 32, 1907035 CrossRef CAS PubMed.
- A. García-Fernández, F. Sancenón and R. Martínez-Máñez, Adv. Drug Delivery Rev., 2021, 177, 113953 CrossRef PubMed.
- D. Afonso, S. Valetti, A. Fraix, C. Bascetta, S. Petralia, S. Conoci, A. Feiler and S. Sortino, Nanoscale, 2017, 9, 13404–13408 RSC.
- F. Trotta, F. Caldera, R. Cavalli, A. Mele, C. Punta, L. Melone, F. Castiglione, B. Rossi, M. Ferro, V. Crupi, D. Majolino, V. Venuti and D. Scalarone, Beilstein J. Org. Chem., 2014, 10, 2586–2593 CrossRef PubMed.
- E. Deniz, N. Kandoth, A. Fraix, V. Cardile, A. C. E. Graziano, D. Lo Furno, R. Gref, F. M. Raymo and S. Sortino, Chem. – Eur. J., 2012, 18, 15782–15787 CrossRef CAS PubMed.
- Z. Liu, L. Ye, J. Xi, J. Wang and Z. G. Feng, Prog. Polym. Sci., 2021, 118, 101408 CrossRef CAS.
- M. Malanga, M. Seggio, V. Kirejev, A. Fraix, I. Di Bari, E. Fenyvesi, M. B. Ericson and S. Sortino, Biomater. Sci., 2019, 7, 2272–2276 RSC.
- J. A. Hrabie and L. K. Keefer, Chem. Rev., 2002, 102, 1135–1154 CrossRef CAS PubMed.
- X. Luo, J. Wu, T. Lv, Y. Lai, H. Zhang, J. J. Lu, Y. Zhang and Z. Huang, Org. Chem. Front., 2017, 4, 2445–2449 RSC.
- H. Li, Q. Li, W. Hou, J. Zhang, C. Yu, D. Zeng, G. Liu and F. Li, ACS Appl. Mater. Interfaces, 2019, 11, 43581–43587 CrossRef CAS PubMed.
- Z. Wang, Y. Lu, K. Qin, Y. Wu, Y. Tian, J. Wang, J. Zhang, J. Hou, Y. Cui and K. Wang, J. Controlled Release, 2015, 210, 179–188 CrossRef CAS PubMed.
- F. Yu, X. Shang, Y. Zhu, H. Lou, Y. Liu, T. Meng, Y. Hong, H. Yuan and F. J. B. Hu, Biomaterials, 2021, 275, 120927 CrossRef CAS PubMed.
- G. Ravikumar, M. Bagheri, D. K. Saini and H. Chakrapani, ChemBioChem, 2017, 18, 1529–1534 CrossRef CAS PubMed.
- H. A. J. Hibbard and M. M. Reynolds, J. Mater. Chem. B, 2019, 7, 2009–2018 RSC.
- A. Farrell and D. Blake, Ann. Rheum. Dis., 1996, 55, 7–20 CrossRef CAS PubMed.
- C. Baylis, Nat. Clin. Pract. Nephrol., 2006, 2, 209–220 CAS.
- X. Liu, Y. Liu, A. S. Thakor, B. D. Kevadiya, J. Cheng, M. Chen, Y. Li, Q. Xu, Q. Wu, Y. Wu and G. Zhang, Acta Biomater., 2021, 136, 485–494 CrossRef CAS PubMed.
- M. D. Bartberger, J. D. Mannion, S. C. Powell, J. S. Stamler, K. N. Houk and E. J. Toone, J. Am. Chem. Soc., 2001, 123, 8868–8869 CrossRef CAS PubMed.
- T. Yang, A. N. Zelikin and R. Chandrawati, Small, 2020, 16, 1907635 CrossRef CAS PubMed.
- B. Li, P. Ji, S. Y. Peng, P. Pan, D. W. Zheng, C. X. Li, Y. X. Sun and X. Z. Zhang, Adv. Mater., 2020, 32, 2000376 CrossRef CAS PubMed.
- R. Kumar, H. Massoumi, M. K. Chug and E. J. Brisbois, ACS Appl. Mater. Interfaces, 2021, 13, 25813–25824 CrossRef CAS PubMed.
- K. Wang, M. Jiang, J. Zhou, Y. Liu, Q. Zong and Y. Yuan, ACS Nano, 2022, 16, 721–735 CrossRef CAS PubMed.
- T. Kim, J. Suh, J. Kim and W. J. Kim, Adv. Sci., 2022, 9, 2101935 CrossRef CAS PubMed.
- E. N. Hoogenboezem and C. L. Duvall, Adv. Drug Delivery Rev., 2018, 130, 73–89 CrossRef CAS PubMed.
- H. Schueffl, S. Theiner, G. Hermann, J. Mayr, P. Fronik, D. Groza, S. van Schonhooven, L. Galvez, N. S. Sommerfeld and A. Schintlmeister, Chem. Sci., 2021, 12, 12587–12599 RSC.
- G. Farrugia and J. H. Szurszewski, Gastroenterology, 2014, 147, 303–313 CrossRef CAS PubMed.
- J. Ashcroft, E. Fraser, S. Krishnamoorthy and S. Westwood-Ruttledge, BMJ, 2019, 365, l2299 CrossRef PubMed.
- X. Yang, M. de Caestecker, L. E. Otterbein and B. Wang, Med. Res. Rev., 2019, 40, 1147–1177 CrossRef PubMed.
- Y. K. Choi, T. Maki, E. T. Mandeville, S.-H. Koh, K. Hayakawa, K. Arai, Y.-M. Kim, M. J. Whalen, C. Xing, X. Wang, K.-W. Kim and E. H. Lo, Nat. Med., 2016, 22, 1335–1341 CrossRef CAS PubMed.
- S. Nagao, K. Taguchi, H. Sakai, K. Yamasaki, H. Watanabe, M. Otagiri and T. Maruyama, Int. J. Nanomed., 2016, 11, 5611–5620 CrossRef CAS PubMed.
- B. Wegiel, D. Gallo, E. Csizmadia, C. Harris, J. Belcher, G. M. Vercellotti, N. Penacho, P. Seth, V. Sukhatme, A. Ahmed, P. P. Pandolfi, L. Helczynski, A. Bjartell, J. L. Persson and L. E. Otterbein, Cancer Res., 2013, 73, 7009–7021 CrossRef CAS PubMed.
- G. Upendar Reddy, J. Axthelm, P. Hoffmann, N. Taye, S. Gläser, H. Görls, S. L. Hopkins, W. Plass, U. Neugebauer, S. Bonnet and A. Schiller, J. Am. Chem. Soc., 2017, 139, 4991–4994 CrossRef PubMed.
- J. Jimenez, I. Chakraborty, A. Dominguez, J. Martinez-Gonzalez, W. M. C. Sameera and P. K. Mascharak, Inorg. Chem., 2018, 57, 1766–1773 CrossRef CAS PubMed.
- J. Jimenez, M. N. Pinto, J. Martinez-Gonzalez and P. K. Mascharak, Inorg. Chim. Acta, 2019, 485, 112–117 CrossRef CAS.
- X. Guo, N. Yang, W. Ji, H. Zhang, X. Dong, Z. Zhou, L. Li, H. M. Shen, S. Q. Yao and W. Huang, Adv. Mater., 2021, 33, 2007778 CrossRef CAS PubMed.
- H. Singh, D. Sareen, J. M. George, V. Bhardwaj, S. Rha, S. J. Lee, S. Sharma, A. Sharma and J. S. Kim, Coord. Chem. Rev., 2022, 452, 214283 CrossRef CAS.
- X. Luo, X. Gong, L. Su, H. Lin, Z. Yang, X. Yan and J. Gao, Angew. Chem., Int. Ed., 2021, 60, 1403–1410 CrossRef CAS PubMed.
- M. Park, K. Sunwoo, Y.-J. Kim, M. Won, Y. Xu, J. Kim, Z. Pu, M. Li, J. Y. Kim and J. H. Seo, J. Am. Chem. Soc., 2023, 145, 4647–4658 CrossRef CAS PubMed.
- P. Liu, F. Ren, S. Son, M. S. Ji, P. Li, Z. Cai, J. Shi, Y. Liu, Y. Dong and J. S. Kim, Biomaterials, 2022, 283, 121409 CrossRef CAS PubMed.
- Q. Tang, Y. T. Yu, H. L. Zhang, Y. Wang, J. Liu, S. P. Yang and J. G. Liu, J. Inorg. Biochem., 2022, 226, 111656 CrossRef CAS PubMed.
- P. Sun, X. Jiang, B. Sun, H. Wang, J. Li, Q. Fan and W. Huang, Biomaterials, 2022, 280, 121319 CrossRef CAS PubMed.
- X. Zhao, X. Li, S. Liang, X. Dong and Z. Zhang, RSC Adv., 2021, 11, 28851–28862 RSC.
- S. N. Anderson, J. M. Richards, H. J. Esquer, A. D. Benninghoff, A. M. Arif and L. M. Berreau, ChemistryOpen, 2015, 4, 590–594 CrossRef CAS PubMed.
- M. Popova, T. Soboleva, A. M. Arif and L. M. Berreau, RSC Adv., 2017, 7, 21997–22007 RSC.
- T. Soboleva and L. M. Berreau, Molecules, 2019, 24, 1252 CrossRef PubMed.
- B. Liu, J. Wang, G. Zhang, R. Bai and Y. Pang, ACS Appl. Mater. Interfaces, 2014, 6, 4402–4407 CrossRef CAS PubMed.
- L. Mikaliunaite and D. B. Green, Anal. Methods, 2021, 13, 2915–2925 RSC.
- M. Popova, T. Soboleva, A. D. Benninghoff and L. M. Berreau, ACS Omega, 2020, 5, 10021–10033 CrossRef CAS PubMed.
- T. Soboleva, A. D. Benninghoff and L. M. Berreau, ChemPlusChem, 2017, 82, 1408–1412 CrossRef CAS PubMed.
- L. S. Lazarus, H. J. Esquer, A. D. Benninghoff and L. M. Berreau, J. Am. Chem. Soc., 2017, 139, 9435–9438 CrossRef PubMed.
- L. S. Lazarus, H. J. Esquer, S. N. Anderson, L. M. Berreau and A. D. Benninghoff, ACS Chem. Biol., 2018, 13, 2220–2228 CrossRef PubMed.
- M. Popova, L. S. Lazarus, S. Ayad, A. D. Benninghoff and L. M. Berreau, J. Am. Chem. Soc., 2018, 140, 9721–9729 CrossRef CAS PubMed.
- L. S. Lazarus, A. D. Benninghoff and L. M. Berreau, Acc. Chem. Res., 2020, 53, 2273–2285 CrossRef CAS PubMed.
- L. S. Lazarus, C. R. Simons, A. Arcidiacono, A. D. Benninghoff and L. M. Berreau, J. Med. Chem., 2019, 62, 9990–9995 CrossRef PubMed.
- Y. Li, Y. Shu, M. Liang, X. Xie, X. Jiao, X. Wang and B. Tang, Angew. Chem., Int. Ed., 2018, 57, 12415–12419 CrossRef CAS PubMed.
- W. Feng, S. Feng and G. Feng, Chem. Commun., 2019, 55, 8987–8990 RSC.
- L. Cao, X. Lin, X. Liu, M. Wu, S. Liu, T. Wang, D. Mao and B. Liu, Nano Lett., 2023, 23, 9769–9777 CrossRef CAS PubMed.
- M. Correa-Costa, D. Gallo, E. Csizmadia, E. Gomperts, J. L. Lieberum, C. J. Hauser, X. Ji, B. Wang, N. O. S. Câmara, S. C. Robson and L. E. Otterbein, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, E2302–E2310 CrossRef CAS PubMed.
- X. She, X. Gao, K. Wang, H. Yang, K. Ma, B. Cui and Z. Xi, J. Occup. Health, 2021, 63, e12235 CrossRef CAS PubMed.
- X. Zhang, Z. Yuan, J. Wu, Y. He, G. Lu, D. Zhang, Y. Zhao, R. Wu, Y. Lv, K. Cai and S. He, ACS Nano, 2023, 17, 21116–21133 CrossRef PubMed.
- J. Cheng, B. Zheng, S. Cheng, G. Zhang and J. Hu, Chem. Sci., 2020, 11, 4499–4507 RSC.
- J. H. Kim, J. M. Park, E. Jung, J. Lee, J. Han, Y.-J. Kim, J. Y. Kim, J. H. Seo and J. S. Kim, Biomaterials, 2022, 289, 121781 CrossRef CAS PubMed.
- L. Gao, J. Cheng, Z. Shen, G. Zhang, S. Liu and J. Hu, Angew. Chem., Int. Ed., 2022, 61, e202112782 CrossRef CAS PubMed.
- L. Stackova, M. Russo, L. Muchova, V. Orel, L. Vitek, P. Stacko and P. Klan, Chem. – Eur. J., 2020, 26, 13184–13190 CrossRef CAS PubMed.
- E. Palao, T. Slanina, L. Muchova, T. Solomek, L. Vitek and P. Klan, J. Am. Chem. Soc., 2016, 138, 126–133 CrossRef CAS PubMed.
- Y. Zheng, X. Ji, B. Yu, K. Ji, D. Gallo, E. Csizmadia, M. Zhu, M. R. Choudhury, L. K. C. De La Cruz, V. Chittavong, Z. Pan, Z. Yuan, L. E. Otterbein and B. Wang, Nat. Chem., 2018, 10, 787–794 CrossRef CAS PubMed.
- L. K. C. De La Cruz, S. L. Benoit, Z. Pan, B. Yu, R. J. Maier, X. Ji and B. Wang, Org. Lett., 2018, 20, 897–900 CrossRef CAS PubMed.
- W. Yasmin, K. D. Strynadka and R. Schulz, Cardiovasc. Res., 1997, 33, 422–432 CrossRef CAS PubMed.
- P. Wang, L. Yu, J. Gong, J. Xiong, S. Zi, H. Xie, F. Zhang, Z. Mao, Z. Liu and J. S. Kim, Angew. Chem., Int. Ed., 2022, 61, e202206894 CrossRef CAS PubMed.
- F. Ulbrich, C. Hagmann, H. Buerkle, C. C. Romao, N. Schallner, U. Goebel and J. Biermann, J. Neuroinflammation, 2017, 14, 130 CrossRef PubMed.
- J. D. Seixas, A. Mukhopadhyay, T. Santos-Silva, L. E. Otterbein, D. J. Gallo, S. S. Rodrigues, B. H. Guerreiro, A. M. Goncalves, N. Penacho, A. R. Marques, A. C. Coelho, P. M. Reis, M. J. Romao and C. C. Romao, Dalton Trans., 2013, 42, 5985–5998 RSC.
- L. Xing, B. Wang, J. Li, X. Guo, X. Lu, X. Chen, H. Sun, Z. Sun, X. Luo, S. Qi, X. Qian and Y. Yang, J. Am. Chem. Soc., 2022, 144, 2114–2119 CrossRef CAS PubMed.
- A. G. Buret, T. Allain, J.-P. Motta and J. L. Wallace, Antioxid. Redox Signaling, 2022, 36, 211–219 CrossRef CAS PubMed.
- C. Szabo, Biochem. Pharmacol., 2018, 149, 5–19 CrossRef CAS PubMed.
- B. Lu, X. Han, A. Zhao, D. Luo, M. F. Maitz, H. Wang, P. Yang and N. Huang, Bioact. Mater., 2021, 6, 1040–1050 CAS.
- A. Salvi, P. Bankhele, J. M. Jamil, M. Kulkarni-Chitnis, Y. F. Njie-Mbye, S. E. Ohia and C. A. Opere, Neurochem. Res., 2016, 41, 1020–1028 CrossRef CAS PubMed.
- H. van Goor, J. C. van den Born, J.-L. Hillebrands and J. A. Joles, Curr. Opin. Nephrol. Hypertens., 2016, 25, 107–113 CrossRef CAS PubMed.
- H. Zhang, Y. Huang, S. Chen, C. Tang, G. Wang, J. Du and H. Jin, J. Adv. Res., 2021, 27, 19–30 CrossRef CAS PubMed.
- J. Kang, Z. Li, C. L. Organ, C.-M. Park, C.-T. Yang, A. Pacheco, D. Wang, D. J. Lefer and M. Xian, J. Am. Chem. Soc., 2016, 138, 6336–6339 CrossRef CAS PubMed.
- H. Liu, Y. Wang, Y. Xiao, Z. Hua, J. Cheng and J. Jia, Transl. Stroke Res., 2016, 7, 209–219 CrossRef CAS PubMed.
- E. E. Ngowi, A. Afzal, M. Sarfraz, S. Khattak, S. U. Zaman, N. H. Khan, T. Li, Q. Y. Jiang, X. Zhang, S. F. Duan, X. Y. Ji and D. D. Wu, Int. J. Biol. Sci., 2021, 17, 73–88 CrossRef CAS PubMed.
- H. Nakagawa, Chem. Rec., 2018, 18, 1708–1716 CrossRef CAS PubMed.
- P. Klan, T. Solomek, C. G. Bochet, A. Blanc, R. Givens, M. Rubina, V. Popik, A. Kostikov and J. Wirz, Chem. Rev., 2013, 113, 119–191 CrossRef CAS PubMed.
- M. M. Dcona, K. Mitra and M. C. T. Hartman, RSC Med. Chem., 2020, 11, 982–1002 RSC.
- M. D. Pluth, Y. Zhao and M. M. Cerda, Meth. Enzymol., 2020, 641, 149–164 CrossRef CAS PubMed.
- M. Dan, S. Yu, Y. Li, S. Wei, J. Xiang and Y. Zhou, J. Photochem. Photobiol., C, 2020, 42, 100339 CrossRef CAS.
- H. Xiong, Y. Xu, B. Kim, H. Rha, B. Zhang, M. Li, G.-F. Yang and J. S. Kim, Chem, 2023, 9, 29–64 CAS.
- N. O. Devarie-Baez, P. E. Bagdon, B. Peng, Y. Zhao, C. M. Park and M. Xian, Org. Lett., 2013, 15, 2786–2789 CrossRef CAS PubMed.
- M. Voronkov, L. Shagun, L. Ermolyuk and L. Timokhina, J. Sulphur Chem., 2004, 25, 131–153 CrossRef CAS.
- N. Fukushima, N. Ieda, K. Sasakura, T. Nagano, K. Hanaoka, T. Suzuki, N. Miyata and H. Nakagawa, Chem. Commun., 2014, 50, 587–589 RSC.
- N. Fukushima, N. Ieda, M. Kawaguchi, K. Sasakura, T. Nagano, K. Hanaoka, N. Miyata and H. Nakagawa, Bioorg. Med. Chem. Lett., 2015, 25, 175–178 CrossRef CAS PubMed.
- Y. Venkatesh, J. Das, A. Chaudhuri, A. Karmakar, T. K. Maiti and N. D. P. Singh, Chem. Commun., 2018, 54, 3106–3109 RSC.
- M. Bera, S. Maji, A. Paul, B. K. Sahoo, T. K. Maiti and N. D. P. Singh, J. Mater. Chem. B, 2020, 8, 1026–1032 RSC.
- S. B. Thakar, P. N. Ghorpade, B. Shaker, J. Lee and D. Na, Environ. Chem. Lett., 2021, 19, 2981–2993 CrossRef CAS.
- X. Li, J. F. Lovell, J. Yoon and X. Chen, Nat. Rev. Clin. Oncol., 2020, 17, 657–674 CrossRef PubMed.
- M. Zhang, X. Liu, Q. Luo, Q. Wang, L. Zhao, G. Deng, R. Ge, L. Zhang, J. Hu and J. Lu, Chem. Eng. J., 2020, 389, 124450 CrossRef CAS.
- A. Zhang, S. Pan, Y. Zhang, J. Chang, J. Cheng, Z. Huang, T. Li, C. Zhang, J. M. de la Fuentea, Q. Zhang and D. Cui, Theranostics, 2019, 9, 3443–3458 CrossRef CAS PubMed.
- L. H. Liu, W. X. Qiu, B. Li, C. Zhang, L. F. Sun, S. S. Wan, L. Rong and X. Z. Zhang, Adv. Funct. Mater., 2016, 26, 6257–6269 CrossRef CAS.
- J. Li, L. Xie, B. Li, C. Yin, G. Wang, W. Sang, W. Li, H. Tian, Z. Zhang, X. Zhang, Q. Fan and Y. Dai, Adv. Mater., 2021, 33, 2008481 CrossRef CAS PubMed.
- X. Zhao, L. Liu, T. An, M. Xian, J. A. Luckanagul, Z. Su, Y. Lin and Q. Wang, Acta Biomater., 2020, 104, 85–94 CrossRef CAS PubMed.
- J. C. Foster, R. J. Carrazzone, N. J. Spear, S. C. Radzinski, K. J. Arrington and J. B. Matson, Macromolecules, 2019, 52, 1104–1111 CrossRef CAS PubMed.
- X. Han, L. Wang, Y. Shang, X. Liu, J. Yuan and J. Shen, J. Mater. Chem. B, 2022, 10, 8672–8683 RSC.
- L. Lin, H. Qin, J. Huang, H. Liang, D. Quan and J. Lu, Polym. Chem., 2018, 9, 2942–2950 RSC.
- S. K. Mahato, D. Bhattacherjee and K. P. Bhabak, Chem. Commun., 2020, 56, 7769–7772 RSC.
- J. L. Wallace, R. W. Blackler, M. V. Chan, G. J. Da Silva, W. Elsheikh, K. L. Flannigan, I. Gamaniek, A. Manko, L. Wang, J. P. Motta and A. G. Buret, Antioxid. Redox Signaling, 2015, 22, 398–410 CrossRef CAS PubMed.
- X. Zhao, L. Ning, X. Zhou, Z. Song, J. Zhang, F. Guan and X. F. Yang, Anal. Chem., 2021, 93, 4894–4901 CrossRef CAS PubMed.
- L. Li, Z. Lin, Y. Cheng, Y. Tang and Z. Zhang, Analyst, 2021, 146, 7374–7378 RSC.
- C. M. Levinn, M. M. Cerda and M. D. Pluth, Acc. Chem. Res., 2019, 52, 2723–2731 CrossRef CAS PubMed.
- Y. Zhao, H. A. Henthorn and M. D. Pluth, J. Am. Chem. Soc., 2017, 139, 16365–16376 CrossRef CAS PubMed.
- Y. Zhao, A. K. Steiger and M. D. Pluth, Chem. Commun., 2018, 54, 4951–4954 RSC.
- Y. Zhao, A. K. Steiger and M. D. Pluth, J. Am. Chem. Soc., 2019, 141, 13610–13618 CrossRef CAS PubMed.
- W. Hua, J. Zhao and S. Gou, Analyst, 2020, 145, 3878–3884 RSC.
- T. Liang, T. Qiang, L. Ren, F. Cheng, B. Wang, M. Li, W. Hu and T. D. James, Chem. Sci., 2022, 13, 2992–3001 RSC.
- Y. Hu, X. Li, Y. Fang, W. Shi, X. Li, W. Chen, M. Xian and H. Ma, Chem. Sci., 2019, 10, 7690–7694 RSC.
- Y. Zhao, M. M. Cerda and M. D. Pluth, Chem. Sci., 2019, 10, 1873–1878 RSC.
- C. Zhu, S. I. Suarez and J. C. Lukesh, Tetrahedron Lett., 2021, 69, 152944 CrossRef CAS.
- G. Shim, S. Ko, D. Kim, Q. V. Le, G. T. Park, J. Lee, T. Kwon, H. G. Choi, Y. B. Kim and Y. K. Oh, J. Controlled Release, 2017, 267, 67–79 CrossRef CAS PubMed.
- A. K. Sharma, M. Nair, P. Chauhan, K. Gupta, D. K. Saini and H. Chakrapani, Org. Lett., 2017, 19, 4822–4825 CrossRef CAS PubMed.
- M. D. Hartle and M. D. Pluth, Chem. Soc. Rev., 2016, 45, 6108–6117 RSC.
- Y. Zhao, T. D. Biggs and M. Xian, Chem. Commun., 2014, 50, 11788–11805 RSC.
- T. Chiku, D. Padovani, W. Zhu, S. Singh, V. Vitvitsky and R. Banerjee, J. Biol. Chem., 2009, 284, 11601–11612 CrossRef CAS PubMed.
- J. Zhang, H. Shan, L. Tao and M. Zhang, J. Mol. Neurosci., 2020, 70, 2020–2030 CrossRef CAS PubMed.
- H. Wei, Y. Yu, G. Wu, Y. Wang, S. Duan, J. Han, W. Cheng, C. Li, X. Tian and X. Zhang, Sens. Actuators, B, 2022, 350, 130862 CrossRef CAS.
- Y. Chen, Z. Hu, M. Yang, J. Gao, J. Luo, H. Li and Z. Yuan, Sens. Actuators, B, 2022, 362, 131742 CrossRef CAS.
- N. Zhang, P. Hu, Y. Wang, Q. Tang, Q. Zheng, Z. Wang and Y. He, ACS Sens., 2020, 5, 319–326 CrossRef CAS PubMed.
- N. K. Devaraj, ACS Cent. Sci., 2018, 4, 952–959 CrossRef CAS PubMed.
- X. Fan, J. Li and P. R. Chen, Natl. Sci. Rev., 2017, 4, 300–302 CrossRef CAS.
- Kenry and B. Liu, Trends Chem., 2019, 1, 763–778 CrossRef CAS.
- Y. Li and H. Fu, ChemistryOpen, 2020, 9, 835–853 CrossRef CAS PubMed.
- A. K. Steiger, Y. Yang, M. Royzen and M. D. Pluth, Chem. Commun., 2017, 53, 1378–1380 RSC.
- R. A. Kumar, M. R. Pattanayak, E. Yen-Pon, J. Eliyan, K. Porte, S. Bernard, M. Riomet, P. Thuery, D. Audisio and F. Taran, Angew. Chem., Int. Ed., 2019, 58, 14544–14548 CrossRef CAS PubMed.
- Y. Chen, R. Zhao, C. Tang, C. Zhang, W. Xu, L. Wu, Y. Wang, D. Ye and Y. Liang, Angew. Chem., Int. Ed., 2022, 134, e202112734 CrossRef.
- Y. Zhao, A. K. Steiger and M. D. Pluth, Angew. Chem., Int. Ed., 2018, 57, 13101–13105 CrossRef CAS PubMed.
- C. R. Powell, K. M. Dillon, Y. Wang, R. J. Carrazzone and J. B. Matson, Angew. Chem., Int. Ed., 2018, 57, 6324–6328 CrossRef CAS PubMed.
- W. Chen, C. Liu, B. Peng, Y. Zhao, A. Pacheco and M. Xian, Chem. Sci., 2013, 4, 2892–2896 RSC.
- R. A. Hankins, S. I. Suarez, M. A. Kalk, N. M. Green, M. N. Harty and J. C. Lukesh, Angew. Chem., Int. Ed., 2020, 59, 22238–22245 CrossRef CAS PubMed.
- W. Wang and B. Wang, Front. Chem., 2018, 6, 559–573 CrossRef CAS PubMed.
- E. M. El-Labbad, X. Ji, K. A. Abouzid and B. Wang, Curr. Top. Med. Chem., 2021, 21, 2870–2881 CrossRef CAS PubMed.
- S. Li, R. Liu, X. Jiang, Y. Qiu, X. Song, G. Huang, N. Fu, L. Lin, J. Song, X. Chen and H. Yang, ACS Nano, 2019, 13, 2103–2113 CAS.
- R. Kodama, K. Sumaru, K. Morishita, T. Kanamori, K. Hyodo, T. Kamitanaka, M. Morimoto, S. Yokojima, S. Nakamura and K. Uchida, Chem. Commun., 2015, 51, 1736–1738 RSC.
- K. Hofman, N. W. Liu and G. Manolikakes, Chem. – Eur. J., 2018, 24, 11852–11863 CrossRef CAS PubMed.
- Y. Venkatesh, K. S. Kiran, S. S. Shah, A. Chaudhuri, S. Dey and N. D. P. Singh, Org. Biomol. Chem., 2019, 17, 2640–2645 RSC.
- L. Yue, K. Yang, J. Li, Q. Cheng and R. Wang, Small, 2021, 17, 2102286 CrossRef CAS PubMed.
- M. Y. Lucero and J. Chan, Nat. Chem., 2021, 13, 1248–1256 CrossRef CAS PubMed.
- R. Wang, X. Xia, Y. Yang, X. Rong, T. Liu, Z. Su, X. Zeng, J. Du, J. Fan, W. Sun and X. Peng, Adv. Healthcare Mater., 2022, 11, 2102017 CrossRef CAS PubMed.
- X. Yao, S. Ma, S. Peng, G. Zhou, R. Xie, Q. Jiang, S. Guo, Q. He and W. Yang, Adv. Healthcare Mater., 2020, 9, 1901582 CrossRef CAS PubMed.
- Z. Liu, T. Cao, Y. Xue, M. Li, M. Wu, J. W. Engle, Q. He, W. Cai, M. Lan and W. Zhang, Angew. Chem., Int. Ed., 2020, 59, 3711–3717 CrossRef CAS PubMed.
- W. Piao, K. Hanaoka, T. Fujisawa, S. Takeuchi, T. Komatsu, T. Ueno, T. Terai, T. Tahara, T. Nagano and Y. Urano, J. Am. Chem. Soc., 2017, 139, 13713–13719 CrossRef CAS PubMed.
- H. S. Jung, S. Koo, M. Won, S. An, H. Park, J. L. Sessler, J. Han and J. S. Kim, Chem. Sci., 2023, 14, 1808–1819 RSC.
- K. X. Teng, L. Y. Niu, Y. F. Kang and Q. Z. Yang, Chem. Sci., 2020, 11, 9703–9711 RSC.
- K. X. Teng, L. Y. Niu, Y. F. Kang and Q. Z. Yang, Chem. Sci., 2021, 12, 488–489 RSC.
- X. Zhou, H. Li, C. Shi, F. Xu, Z. Zhang, Q. Yao, H. Ma, W. Sun, K. Shao, J. Du, S. Long, J. Fan, J. Wang and X. Peng, Biomaterials, 2020, 253, 120089 CrossRef CAS PubMed.
- Y. F. Kang, W. K. Chen, K. X. Teng, L. Y. Wang, X. C. Xu, L. Y. Niu, G. Cui and Q. Z. Yang, CCS Chem., 2022, 4, 3516–3528 CrossRef CAS.
- J. Zhao, Z. Wang, M. Zhong, Q. Xu, X. Li, B. Chang and J. Fang, J. Med. Chem., 2021, 64, 17979–17991 CrossRef CAS PubMed.
- G. Yang, D. Wang, S. Z. F. Phua, A. K. Bindra, C. Qian, R. Zhang, L. Cheng, G. Liu, H. Wu, Z. Liu and Y. Zhao, ACS Nano, 2022, 16, 2319–2329 CrossRef CAS PubMed.
- L. Zhao, Z. Li, J. Wei, Y. Xiao, Y. She, Q. Su, T. Zhao, J. Li and J. Shao, Chem. Eng. J., 2022, 430, 133057 CrossRef CAS.
- Y. He, S. Guo, Y. Zhang, Y. Liu and H. Ju, Biomaterials, 2021, 275, 120962 CrossRef CAS PubMed.
- L. P. Smaga, N. W. Pino, G. E. Ibarra, V. Krishnamurthy and J. Chan, J. Am. Chem. Soc., 2020, 142, 680–684 CrossRef CAS PubMed.
Footnote |
| † These authors contribute equally. |
| This journal is © The Royal Society of Chemistry 2024 |