Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A multiphoton ionisation photoelectron imaging study of thiophene†
Joseph J.
Broughton
 ,
Sarbani
Patra
,
Sarbani
Patra
 ,
Michael A.
Parkes
,
Michael A.
Parkes
 ,
Graham A.
Worth
,
Graham A.
Worth
 and
Helen H.
Fielding
and
Helen H.
Fielding
 *
*
Department of Chemistry, University College London, 20 Gordon Street, London WC1H 0AJ, UK. E-mail: h.h.fielding@ucl.ac.uk
First published on 19th September 2024
Abstract
Thiophene is a prototype for the excited state photophysics that lies at the heart of many technologies within the field of organic electronics. Here, we report a multiphoton ionisation photoelectron imaging study of gas-phase thiophene using a range of photon energies to excite transitions from the ground electronic state to the first two electronically excited singlet states, from the onset of absorption to the absorption maximum. Analysis of the photoelectron spectra and angular distributions reveal features arising from direct photoionisation from the ground electronic state, and resonance-enhanced photoionisation via the electronically excited singlet states. The first two ionisation energies from the ground electronic state were confirmed to be 8.8 eV (adiabatic) and 9.6 eV (vertical). The ionisation energies from the first two electronically excited singlet states were found to be 3.7 eV (adiabatic) and 4.4 eV (vertical).
1 Introduction
Thiophene is a small, heteroaromatic molecule that is a ubiquitous building block in many photonic materials, such as organic photovoltaics1–3 and non-linear optical devices.4,5 Although there have been many attempts to elucidate both the electronic structure and the excited-state dynamics of gas-phase thiophene,6–33 its UV photoabsorption and photoionisation are still not understood fully.One of the difficulties in understanding the photoabsorption spectrum arises as a result of the first two electronically excited singlet states, S1 (ππ*) and S2 (ππ*), hereafter referred to as S1 and S2, lying very close together in energy and having similar oscillator strengths. Theoretical studies by Holland et al.26 and Prlj et al.29 have highlighted how the precise energies and ordering of these states are highly dependent on the method and the basis sets used. Although there is still not a clear consensus on the origin of structure in the UV photoabsorption of thiophene, all assignments reported in the literature do agree that the intense feature at 5.157 eV is the origin of S16,8,10,11,13,14,17,22,26,33 (Fig. 1), with the peaks below the origin being variously attributed to hot bands, combination bands, or vibronic coupling between S1 and S2.8,11,13
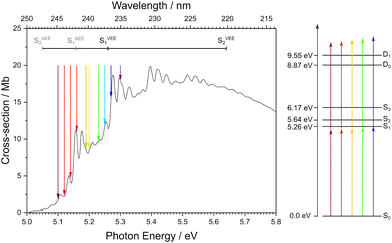 | ||
| Fig. 1 Left: Photoabsorption spectrum of thiophene recorded by Holland et al.26 Right: Schematic energy level diagram for thiophene showing the vertical excitation energies of S1, S2, and S3, and the vertical ionisation energies to D0 and D1 continua. The adiabatic excitation energy (AEE) for S1 is taken from measurements by Holland et al.,26 vertical excitation energies (VEE) for S1 and S2 are taken from magnetic circular dichroism measurements from Hakansson et al.,34 the AEE of S2 is taken from DFT/MRCI calculations by Salzmann et al.,23 the S3 VEE is from Holland et al.26 The ionisation energies D0 and D1 are taken from Weinkauf et al.22 The coloured arrows signify the different wavelengths used for one-colour measurements in the work reported here. | ||
There have been two recent vacuum ultraviolet (VUV) absorption spectroscopy studies of gas-phase thiophene.26,33 Both measurements found several progressions which were assigned to the ν6 vibrational mode, corresponding to a CH in-plane bending vibration, with an average peak spacing of ∼120 meV. This supported earlier work by Palmer et al.,17 in which the 0–0 transition was determined to be 5.157 eV, and structure below this transition was ascribed to hot bands and S1–S2 vibronic coupling.
Another way to probe electronic structure is to use photoelectron spectroscopy to determine electron binding energies, eBE = hν − eKE, where hν is the photon energy and eKE is the measured electron kinetic energy. Ionisation energies of thiophene determined from such measurements are presented in Table 1. Measuring the photoelectron angular distribution (PAD) as well as the eKE provides additional information about the molecular orbital from which the electron is removed. For a two-photon ionisation process, the angular distribution I(θ) ∝ 1 + β2P2(cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) + β4P4(cos
θ) + β4P4(cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ), where I(θ) is the probability of photoelectron emission at angle θ, defined as the angle between the laser polarisation and the velocity vector of the photoelectron, β2 and β4 are the photoelectron anisotropy parameters, and P2(cos
θ), where I(θ) is the probability of photoelectron emission at angle θ, defined as the angle between the laser polarisation and the velocity vector of the photoelectron, β2 and β4 are the photoelectron anisotropy parameters, and P2(cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) and P4(cos
θ) and P4(cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) are the second and fourth-order Legendre polynomials in cos
θ) are the second and fourth-order Legendre polynomials in cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ.35 The two limiting values of β2 are +2 and −1, corresponding to photoelectron emission predominantly parallel and perpendicular to the electric field vector of the laser, respectively. β4 represents the alignment of the excited state and, generally, positive and negative values of β4 correspond to the excited state being aligned parallel or perpendicular to the electric field vector of the laser, respectively.
θ.35 The two limiting values of β2 are +2 and −1, corresponding to photoelectron emission predominantly parallel and perpendicular to the electric field vector of the laser, respectively. β4 represents the alignment of the excited state and, generally, positive and negative values of β4 correspond to the excited state being aligned parallel or perpendicular to the electric field vector of the laser, respectively.
| D0 | AIE/VIE | D1 | AIE/VIE | Method | Ref. |
|---|---|---|---|---|---|
| a Adiabatic and vertical ionisation energies not distinguished. | |||||
| 8.87 ± 0.05 | AIEa | 9.49 ± 0.1 | VIE | HeI + HEA | Eland36 |
| 8.80 ± 0.05 | AIEa | 9.44 ± 0.05 | AIEa | HeI + HEA | Baker et al.7 |
| 8.872 ± 0.015 | AIEa | 9.52 ± 0.015 | VIE | Ly-α + HEA | Derrick et al.37 |
| 8.90 ± 0.015 | VIE | 9.50 ± 0.015 | VIE | HeI + HEA | Clark et al.9 |
| 8.85 ± 0.05 | VIE | 9.49 ± 0.05 | VIE | HeI + HEA | Klasinc et al.12 |
| 8.96 ± 0.25 | VIE | 9.58 ± 0.25 | VIE | He* PIES + HEA | Kishimoto et al.38 |
| 8.8742 ± 0.0002 | AIE | — | — | ZEKE | Yang et al.39 |
| 8.87 ± 0.01 | AIE | 9.55 ± 0.01 | VIE | TRPES + TOF | Weinkauf et al.22 |
| 8.8 ± 0.1 | AIE | 9.6 ± 0.1 | VIE | MPI + VMI | This work |
Multiphoton ionisation (MPI) photoelectron spectroscopy studies of gas-phase thiophene using UV laser pulses have been reported by two groups.22,32 However, there has only been one photoelectron imaging study;32 this was a time-resolved study focussing on relaxation dynamics, and PADs were not reported. Here, we present a systematic one-colour MPI photoelectron imaging study of thiophene, employing wavelengths ranging from 243 nm, exciting just below the S0–S1 0–0 transition, to 234 nm, which corresponds to the S0–S1 VEE (Fig. 1), from which we gain further insight into the electronic structure!
2. Methods
2.1. Multiphoton photoelectron imaging
A molecular beam of thiophene was produced by passing 1 bar He carrier gas through liquid thiophene and expanding it through a 300 μm pulsed Amsterdam piezovalve40,41 operating at a repetition rate of 1 kHz and with a pulse duration of 8 μs. The molecular beam was collimated by a 3 mm skimmer before passing into the interaction region of our velocity map imaging spectrometer that has been described before.42–51 The molecular beam was intersected by femtosecond laser pulses with wavelengths in the range 234–243 nm, generated by frequency-doubling the sum frequency of the signal of the output of an optical parametric amplifier (TOPAS-Prime) pumped by a titanium: sapphire regenerative amplifier (Coherent Astrella-HE). To prevent multiphoton excitation, pulse energies were attenuated to ≈1 μJ per pulse using a variable neutral density filter. The 1/e2 pulse duration was measured as 300 fs at 243 nm and spectral full-width at half maxima were ∼1 nm (0.02 eV). Photoelectron images were recorded for 1200 s at each wavelength. Background images (without thiophene) were also recorded for 1200 s and subtracted from the photoelectron images. Photoelectron spectra were recovered from the background-subtracted data using the pBASEX image inversion algorithm.52 The energy scale was calibrated by recording 2 + 1 resonance-enhanced MPI spectra of Xe at 249.63 nm.53 The resolution was ΔE/E ≤ 3%.2.2. Ab intio calculations
The complete active space second-order perturbation theory (CASPT2)54 method with the 6-31G* basis set was used for the computation of the ground and electronically excited states of thiophene. The ground electronic state has C2v symmetry and the active space consisted of a 10-electron, 9-orbital space including all the p-orbitals of thiophene and the two pairs of σ-orbitals (σ/σ*) for both C–S bonds, which can correctly account for the two equivalent C–S bonds, either of which might undergo cleavage after photoexcitation. The same theoretical description was employed by Schnappinger et al.31 to study the relaxation of photoexcited thiophene using surface hopping including arbitrary couplings (SHARC).55 Calculations were performed using the MOLPRO 2022 program.56,57A number of other methods were also used to support the characterisation: TDDFT/B3LYP, ADC(2) and EOM-CCSD. These calculations used a 6-311+G** basis set with the QChem v5.4 program.58 The calculated excitation energies of the four singlet states matching the CASPT2 calculated states, and the first four triplet states, are listed in Table 2, along with the known singlet state experimental values. All methods agree that the singlet states are close in energy, in the range 5.7–6.5 eV. The S3 and S4 states are found to be near degenerate, with a change in order for all methods compared to the CASPT2. The triplets are spread more widely, but T1 and T2 are both predicted to lie below the S1 state. Again the character of the close-lying T3 and T4 states changes order for all methods compared to CASPT2. TDDFT also reported a singlet state at 5.982 eV and a triplet at 5.805 eV involving Rydberg orbitals.
The VIEs were calculated using electron propagator theory (EPT)59–61 in Gaussian 0962 with the 6-311++G(3df,3pd) basis set, and EOM-IP-CCSD using QChem v5.4 with the 6-311+G** basis set. Dyson orbital norms (ionisation cross-sections) and β parameters (PADs) were calculated for transitions from Sn to D0 and D1 for n = 1–4 using the EOM-IP-CCSD/6-311++G** method in ezDyson.63 The calculated D0 and D1 energies are also listed in Table 2 and agree well with the experimental values of Table 1. The next two cation states are found to be much higher in energy at around 12 eV and are ignored.
3. Results and discussion
3.1. Electronic structure
The electronic configurations and CASPT2(10,9)/6-31G* calculated VEEs of the first four singlet excited states of thiophene are presented in Fig. 2, along with the electronic configurations and EPT/6-311G(3df,3pd) calculated VIEs of the first two doublet states of the thiophene cation. To a first approximation, we can use Koopmans' theorem to determine ionisation propensities. From the electronic configurations shown in Fig. 2 it is clear that the S0 state can ionise to D0 and D1, that S1 and S4 are most likely to ionise to D1, and S2 and S3 are most likely to ionise to D0. These ionisation propensities are supported by calculations of photoionisation cross-sections for one photon ionisation from S0–S4 to the D0 and D1 states of the thiophene cation (Fig. 3). | ||
| Fig. 3 One-photon photoionisation cross-sections from S0–S4 to the D0 (left) and D1 (right) states of thiophene. Dyson orbital norms were calculated using the EOM-IP-CCSD/6-311++G** method. | ||
3.2. Photoelectron spectra
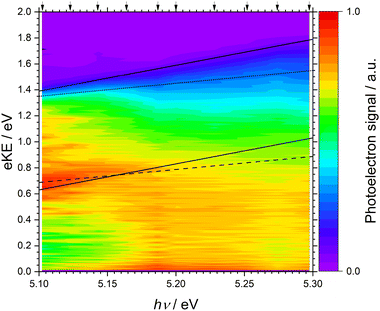
The 243–234 nm one-colour MPI photoelectron spectra of thiophene are presented as a heat map plotted as a function of eKE and photon energy (Fig. 4). The individual spectra are also plotted as a function of eKE in Fig. 5(a). Fig. 4 allows us to determine whether features in the spectra are a result of non-resonant or resonance-enhanced MPI processes. For non-resonant two-photon ionisation, electrons are emitted with eKE = 2hν − IE, where hν is the photon energy and IE is the ionisation energy. In Fig. 4, the solid black lines highlight peaks in the photoelectron spectra whose eKEs increase at twice the rate of the photon energy, and represent non-resonant ionisation from S0 to the two doublet cation states, D0 and D1. Following resonant photoexcitation of an excited electronic state Sn, with excess vibrational energy Evib = hν − AEE(Sn) where AEE(Sn) is the adiabatic excitation energy of Sn, ionisation by a second photon will result in the emission of electrons with eKE ≤ 2hν − AIE(Dm) − Evib = hν − [AIE(Dm) − AEE(Sn)], where AIE(Dm) is the adiabatic ionisation energy of the cation doublet state Dm. In Fig. 4, the dotted and dashed black lines highlight peaks in the photoelectron spectra whose eKEs increase at the same rate as the photon energy, and represent resonance-enhanced ionisation to the two doublet cation states, D0 and D1, respectively. The y-intercepts of the solid, dotted and dashed lines were obtained from plots of the MPI photoelectron spectra as functions of two- or one-photon electron binding energy, eBE = nhν − eKE, where n = 2 or 1 (Fig. 5). | ||
| Fig. 5 (a) Plot of photoelectron counts versus eKE following 1 + 1 photoionisation of thiophene for 234–243 nm. (b) Plot of photoelectron counts versus two-photon eBE following 1 + 1 photoionisation of thiophene for 234–243 nm. The solid and dashed lines show D0 = 8.8 ± 0.1 eV and D1 = 9.6 ± 0.1 eV. (c) Plot of photoelectron counts versus one-photon eBE following 1 + 1 ionisation of thiophene for 234–243 nm. The comb denotes ionisation from Sn–D0 and Sn–D1 using the S1 AEE from Holland et al.,26 the calculated S2 AEE from Salzman et al.23 and the ionisation energies from the work reported here. The solid and dashed vertical lines denote adiabatic ionisation from S1 and S2, respectively. The individual spectra were normalised to their integrated area. | ||
In Fig. 5(b), the photoelectron spectra are plotted as a function of two-photon binding energy. There are two ionisation energies that can be identified in these spectra. The small bump at 8.8 ± 0.1 eV on the rising edge of the lowest energy peak can be assigned as the S0–D0 AIE. This is in excellent agreement with the AIE obtained from PES7,9,12,22,36–38,64 and zero-kinetic energy (ZEKE) PES39 measurements, several of which determined that the AIE was equivalent to the VIE (Table 1). The peak at 9.6 ± 0.1 eV is assigned as the S0–D1 VIE, and this is also in good agreement with values reported previously.7,9,12,22,36–38,64 Note that the errors attributed to our assignments are derived from the uncertainty in the position rather than the energy resolution of our spectrometer.
It can be seen in Fig. 5(b) that the peak attributed to S0–D1 does not have any clear vibrational structure (experimental resolution ∼0.05 eV), in agreement with observations of Derrick et al. (experimental resolution ∼ 0.02 eV).37 Also, the peak broadens significantly as photon energy increases, losing the sharpness associated with non-resonant photoionisation from S0 at photon energies lower that the S0–S1 VEE. Although Rydberg states have been observed in the range 9.4–11.8 eV,26 the structured features in our 5.10–5.19 eV photoelectron spectra do not remain at constant eKE with increasing photon energy (Fig. 5(a)) so cannot be attributed to autoionising Rydberg states. Also, the 5.20–5.30 eV photoelectron spectra do not have the identical profiles expected for an autoionising Rydberg states. This leaves two possible explanations for our experimental observations. First, as the photon energy increases above 5.157 eV, S1 is excited. From Koopmans' correlations (Fig. 2) and calculation photoionisation cross-sections (Fig. 3), the S1–D1 ionisation pathway is expected to dominate over S1–D0. There may be a large geometric change between S1 and D1, which would result in a broader photoelectron spectrum. Moreover, the D1 feature shifts to higher binding energies with increasing photon energy, which is a signature of resonance-enhanced ionisation from S1 beginning to compete with, or dominate, non-resonant ionisation from S0. Second, D1 could have a short lifetime. Calculations reported by Trofimov et al. identified strong vibronic coupling between D1 and D0 and a conical intersection between them,16 which would account for a short D1 lifetime and the broad, vibrationally-unresolved photoelectron spectrum.
In Fig. 5(c), the photoelectron spectra are plotted as a function of one-photon binding energy. The comb marks Sn–Dm AIEs determined using AEEs from Holland et al.,26 and Salzmann et al.,23 and the D0 and the D1 IEs from this work. It is clear that S1–D1 is the dominant resonance-enhanced ionisation pathway as the photoelectron counts are highest around the calculated S1–D1 VIE (4.4 ± 0.1 eV). Nonetheless, there also appears to be a significant contribution from the S1–D0 ionisation channel calculated at 3.7 ± 0.1 eV. For photon energies above the S0–S1 AEE (5.157 eV), what looks like an isobestic point begins to appear. It lies at 3.7 ± 0.1 eV, which is very close to the calculated S2–D0 AIE (3.8 ± 0.1 eV) and is illustrative of an increasing contribution from the population of, and subsequent ionisation from, S2. Based on Koopmans' correlations and photoionisation calculations, the S2–D0 ionisation channel is expected to dominate over S2–D1 ionisation, although it is difficult to determine whether there is any significant contribution from S2–D1 ionisation from our spectra as they are very congested in the region where we would expect this channel to appear (4.5 ± 0.1 eV). Fig. 3 shows that the cross-sections for the dominant S2–D0 and S1–D1 ionisation channels are very similar. Population of, and ionisation from, S2 is consistent with calculations performed by Köppel et al. which reported a rapid population of S2 following photoexcitation of S1. At photon energies close to the S0–S2 VEE (5.64 eV),34 it is likely that the S2–D0 and S2–D1 channels would make a larger contribution to the measured photoelectron intensity due to the direct population of S2.
3.3. Photoelectron angular distributions
In Fig. 6(a) the measured anisotropy parameter β2 is plotted as a function of eKE and photon energy. At higher eKEs, between the solid lines marking the upper limits of direct and indirect photoionisation to D0 and D1, there is weak anisotropy with β2 ≈ −0.05. At lower eKEs, below the lines marking the upper limits of direct and indirect photoionisation to the D1 continuum, the anisotropy changes sign and becomes weakly positive, with β2 ≈ +0.05. In Fig. 6(b) the measured β4 anisotropy parameter, is plotted as a function of photon energy and eKE. At higher eKEs, β4 < 0, whereas for eKE < 0.4, β4 ≈ +0.1.The calculated one-photon β2 parameters are plotted in Fig. 7. In the energy region of interest for our experiments, β2 < 0 for one-photon photoionisation from S0, S1 and S2, to D0 and D1. The measured β2 parameter is a superposition of direct and indirect ionisation processes and although it is negative for higher eKEs, it is positive for lower eKEs. This difference between the calculated and measured patterns could be due to the β2 parameter for two-photon non-resonant ionisation from S0 having the opposite sign to our calculated one-photon β2 parameters for lower eKEs, or it could be the result of structural changes occurring on the timescale of our measurements. Interestingly, β2 > 0 for photoionisation from S3 and S4. Ionisation from S0–S2 involve removing electrons from ππ* molecular orbitals whereas ionisation from S3 and S4 involve removing electrons from πσ* molecular orbitals. (Fig. 2) This difference in orbital character could explain the difference in sign of the β2. Thus, another explanation could be that the higher lying S3 and S4 states are accessed on the timescale of the measurement, which would be consistent with ultrafast ring-opening. This is indeed found to be the case in our recent theoretical study of the photo-excited dynamics in the vibronically coupled S1–S4 states of thiophene.65
 | ||
| Fig. 7 Plots of β2 anisotropy parameters as a function of eKE following one-photon photoionisation from S0–S4 to D0 (left) and D1 (right) continua, calculated using the EOM-IP-CCSD/6-311++G** method. | ||
Thus, time-resolved photoelectron angular imaging experiments, with supporting calculations of the PADs, could prove valuable for investigating the electronic relaxation pathways following photoexcitation.
4. Conclusions
We have presented 243–234 nm multiphoton photoelectron imaging measurements of thiophene. Plots of the photoelecton spectra as a function of two-photon binding energy allowed us to identify spectral features with binding energies of 8.8 ± 0.1 eV and 9.6 ± 0.1 eV, corresponding to the S0–D0 AIE and S0–D1 VIE, respectively. These are in excellent agreement with ionisation energies determined using other methods.7,9,12,22,36–38,64 Plots of the photoelectron spectra as a function of one-photon binding energy revealed peaks around the S1–D1 VIE (4.4 ± 0.1 eV) and S2–D0 AIE (3.8 ± 0.1 eV). For spectra recorded with photon energies above the S0–S1 AEE (5.157 eV), an isobestic point was observed at hν-eKE = 3.7 ± 0.1 eV illustrating an increasing contribution from S2–D0 ionisation, compared with S1–D1 ionisation, with increasing photon energy.Plots of β2 and β4 anisotropy parameters as a function of eKE and photon energy suggest that time-resolved photoelectron imaging measurements could provide a sensitive probe of electronic relaxation following UV photoexcitation of thiophene. This work serves as a benchmark for future time-resolved studies of thiophene and more complex molecular systems containing thiophene as a building block that are ubiquitous in many photonic materials.
Author contributions
The project was conceived and supervised by H. H. F. The experimental data were recorded by J. J. B., with technical assistance from M. A. P., and processed, analysed and interpreted by J. J. B. Calculations were undertaken J. J. B., and by S. P. and M. A. P. under the guidance of G. A. W. The manuscript was written by J. J. B. and H. H. F., with contributions from S. P. and G. A. W. All authors read and approved the final version of the manuscript.Data availability
Data in this article will be made available in the UCL Research Data Repository at https://www.ucl.ac.uk/library/open-science-researchsupport/research-data-management/ucl-research-data-repository.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by EPSRC grants EP/V026690/1 and EP/L015862/1. The experiments were performed using the Ultrafast Laser Facility in the Department of Chemistry at UCL (EPSRC EP/T019182/1), with technical support from Dr Julia Davies. The authors thank Roisin Tapley for carrying out preliminary calculations of one-photon photoionisation cross-sections and β2 anisotropy calculations.Notes and references
- F. Zhang, D. Wu, Y. Xu and X. Feng, J. Mater. Chem., 2011, 21, 17590–17600 RSC.
- P. Kumaresan, S. Vegiraju, Y. Ezhumalai, S. L. Yau, C. Kim, W. H. Lee and M. C. Chen, Polymers, 2014, 6, 2645–2669 CrossRef.
- T. Taniguchi, K. Fukui, R. Asahi, Y. Urabe, A. Ikemoto, J. Nakamoto, Y. Inada, T. Yamao and S. Hotta, Synth. Met., 2017, 227, 156–162 CrossRef CAS.
- G. Kirsch, D. Prim, F. Leising and G. Mignani, J. Heterocycl. Chem., 1994, 31, 1005–1009 CrossRef CAS.
- H. L. Andriampanarivo, M. Köhler, J. L. Gejo, T. Betzwieser, B. C. Poon, P. L. Yue, S. D. Ravelomanantsoa and A. M. Braun, Photochem. Photobiol. Sci., 2015, 14, 1013–1024 CrossRef CAS PubMed.
- G. Horváth and A. I. Kiss, Spectrochim. Acta, Part A, 1967, 23, 921–924 CrossRef.
- A. D. Baker, D. Betteridge, N. R. Kemp and R. E. Kirby, Anal. Chem., 1971, 43, 375–381 CrossRef CAS PubMed.
- G. D. Lonardo, G. Galloni, A. Trombetti and C. Zauli, J. Chem. Soc., Faraday Trans., 1972, 68, 2009–2016 RSC.
- P. A. Clark, R. Gleitter and E. Heilbronner, Tetrahedron, 1973, 29, 3085–3089 CrossRef CAS.
- W. M. Flicker, O. A. Mosher and A. Kuppermann, Chem. Phys. Lett., 1976, 38, 489–492 CrossRef CAS.
- G. Varsanyi, L. Nyulaszi, T. Veszpremi and T. Narisawa, J. Chem. Soc. Perkin Trans., 1982, 761–765 RSC.
- B. L. Klasinc, A. Sabljic, G. Kluge, J. Rieger, K. Marx Universitat Leipzig, S. Chemie and G. D. Republic, J. Chem. Soc., Perkin Trans. 2, 1982, 539–543 RSC.
- E. J. Beiting, K. J. Zeringue and R. E. Stickel, Spectrochim. Acta, Part A, 1985, 41, 1413–1418 CrossRef.
- L. Nyulászi and T. Veszprémi, J. Mol. Struct., 1986, 140, 253–259 CrossRef.
- F. Negri and M. Z. Zgierski, J. Chem. Phys., 1994, 100, 2571–2587 CrossRef CAS.
- A. B. Trofimov, H. Köppel and J. Schirmer, J. Chem. Phys., 1998, 109, 1025–1040 CrossRef CAS.
- M. H. Palmer, I. C. Walker and M. F. Guest, Chem. Phys., 1999, 241, 275–296 CrossRef CAS.
- F. Qi, O. Sorkhabi, A. H. Rizvi, A. G. Suits, C. Sciences, V. Di, E. Orlando and L. Berkeley, J. Phys. Chem. A, 1999, 103, 8351–8358 CrossRef CAS.
- J. Wan, M. Hada, M. Ehara and H. Nakatsuji, J. Chem. Phys., 2001, 114, 842–850 CrossRef CAS.
- E. E. Rennie, D. M. Holland, D. A. Shaw, C. A. Johnson and J. E. Parker, Chem. Phys., 2004, 306, 295–308 CrossRef CAS.
- H. Köppel, E. V. Gromov and A. B. Trofimov, Chem. Phys., 2004, 304, 35–49 CrossRef.
- R. Weinkauf, L. Lehr, E. W. Schlag, S. Salzmann and C. M. Marian, Phys. Chem. Chem. Phys., 2008, 10, 393–404 RSC.
- S. Salzmann, M. Kleinschmidt, J. Tatchen, R. Weinkauf and C. M. Marian, Phys. Chem. Chem. Phys., 2008, 10, 380–392 RSC.
- G. Cui and W. Fang, J. Phys. Chem. A, 2011, 115, 11544–11550 CrossRef CAS PubMed.
- M. Stenrup, Chem. Phys., 2012, 397, 18–25 CrossRef CAS.
- D. M. Holland, A. B. Trofimov, E. A. Seddon, E. V. Gromov, T. Korona, N. de Oliveira, L. E. Archer, D. Joyeux and L. Nahon, Phys. Chem. Chem. Phys., 2014, 16, 21629–21644 RSC.
- D. Fazzi, M. Barbatti and W. Thiel, Phys. Chem. Chem. Phys., 2015, 17, 7787–7799 RSC.
- A. Prlj, B. F. Curchod and C. Corminboeuf, Phys. Chem. Chem. Phys., 2015, 17, 14719–14730 RSC.
- A. Prlj, B. F. Curchod, A. Fabrizio, L. Floryan and C. Corminboeuf, J. Phys. Chem. Lett., 2015, 6, 13–21 CrossRef CAS.
- P. Kölle, T. Schnappinger and R. D. Vivie-Riedle, Phys. Chem. Chem. Phys., 2016, 18, 7903–7915 RSC.
- T. Schnappinger, P. Kölle, M. Marazzi, A. Monari, L. González and R. D. Vivie-Riedle, Phys. Chem. Chem. Phys., 2017, 19, 25662–25670 RSC.
- O. Schalk, M. A. Larsen, A. B. Skov, M. B. Liisberg, T. Geng, T. I. Sølling and R. D. Thomas, J. Phys. Chem. A, 2018, 122, 8809–8818 CrossRef CAS PubMed.
- D. B. Jones, M. Mendes, P. Limão-Vieira, F. Ferreira da Silva, N. C. Jones, S. V. Hoffmann and M. J. Brunger, J. Chem. Phys., 2019, 150, 064303 CrossRef CAS PubMed.
- R. Hakansson, B. Nordén and E. W. Thulstrup, Chem. Phys. Lett., 1977, 50, 306–308 CrossRef.
- K. L. Reid, Annu. Rev. Phys. Chem., 2003, 54, 397–424 CrossRef CAS PubMed.
- J. H. Eland, Int. J. Mass Spectrom. Ion Phys., 1969, 2, 471–484 CrossRef CAS.
- P. Derrick, L. Åsbrink, O. Edqvist, B.-O. Jonsson and E. Lindholm, Int. J. Mass Spectrom. Ion Phys., 1971, 6, 177–190 CrossRef CAS.
- N. Kishimoto, H. Yamakado and K. Ohno, J. Phys. Chem., 1996, 100, 8204–8211 CrossRef CAS.
- J. Yang, J. Li and Y. Mo, J. Chem. Phys., 2006, 125, 174313–174317 CrossRef PubMed.
- D. Irimia, D. Dobrikov, R. Kortekaas, H. Voet, D. A. Van Den Ende, W. A. Groen and M. H. Janssen, Rev. Sci. Instrum., 2009, 80, 1–6 CrossRef PubMed.
- D. Irimia, R. Kortekaas and M. H. Janssen, Phys. Chem. Chem. Phys., 2009, 11, 3958–3966 RSC.
- G. A. Worth, R. E. Carley and H. H. Fielding, Chem. Phys., 2007, 338, 220–227 CrossRef CAS.
- D. S. Parker, R. S. Minns, T. J. Penfold, G. A. Worth and H. H. Fielding, Chem. Phys. Lett., 2009, 469, 43–47 CrossRef CAS.
- A. D. Nunn, R. S. Minns, R. Spesyvtsev, M. J. Bearpark, M. A. Robb and H. H. Fielding, Phys. Chem. Chem. Phys., 2010, 12, 15751–15759 RSC.
- R. S. Minns, D. S. Parker, T. J. Penfold, G. A. Worth and H. H. Fielding, Phys. Chem. Chem. Phys., 2010, 12, 15607–15615 RSC.
- R. Spesyvtsev, O. M. Kirkby, M. Vacher and H. H. Fielding, Phys. Chem. Chem. Phys., 2012, 14, 9942–9947 RSC.
- R. Spesyvtsev, O. M. Kirkby and H. H. Fielding, Faraday Discuss., 2012, 157, 165–179 RSC.
- O. M. Kirkby, M. Sala, G. Balerdi, R. De Nalda, L. Bañares, S. Guérin and H. H. Fielding, Phys. Chem. Chem. Phys., 2015, 17, 16270–16276 RSC.
- S. P. Neville, O. M. Kirkby, N. Kaltsoyannis, G. A. Worth and H. H. Fielding, Nat. Commun., 2016, 7, 11357 CrossRef CAS PubMed.
- O. M. Kirkby, M. A. Parkes, S. P. Neville, G. A. Worth and H. H. Fielding, Chem. Phys. Lett., 2017, 683, 179–185 CrossRef CAS.
- J. W. Riley, B. Wang, J. L. Woodhouse, M. Assmann, G. A. Worth and H. H. Fielding, J. Phys. Chem. Lett., 2018, 9, 678–682 CrossRef CAS PubMed.
- G. A. Garcia, L. Nahon and I. Powis, Rev. Sci. Instrum., 2004, 75, 4989–4996 CrossRef CAS.
- S. J. Bajic, R. N. Compton, X. Tang and P. Lambropoulos, Phys. Rev. A, 1991, 44, 2102–2112 CrossRef CAS PubMed.
- P. Celani and H.-J. Werner, J. Chem. Phys., 2000, 112, 5546–5557 CrossRef CAS.
- M. Richter, P. Marquetand, J. González-Vázquez, I. Sola and L. González, J. Chem. Theory Comput., 2011, 7, 1253–1258 CrossRef CAS PubMed.
- H.-J. Werner, P. J. Knowles, G. Knizia, F. R. Manby and M. Schütz, WIREs Comput. Mol. Sci., 2012, 2, 242–253 CrossRef CAS.
- H.-J. Werner, P. J. Knowles, P. Celani, W. Györffy, A. Hesselmann, D. Kats, G. Knizia, A. Köhn, T. Korona, D. Kreplin, R. Lindh, Q. Ma, F. R. Manby, A. Mitrushenkov, G. Rauhut, M. Schütz, K. R. Shamasundar, T. B. Adler, R. D. Amos, S. J. Bennie, A. Bernhardsson, A. Berning, J. A. Black, P. J. Bygrave, R. Cimiraglia, D. L. Cooper, D. Coughtrie, M. J. O. Deegan, A. J. Dobbyn, K. Doll, M. Dornbach, F. Eckert, S. Erfort, E. Goll, C. Hampel, G. Hetzer, J. G. Hill, M. Hodges, T. Hrenar, G. Jansen, C. Köppl, C. Kollmar, S. J. R. Lee, Y. Liu, A. W. Lloyd, R. A. Mata, A. J. May, B. Mussard, S. J. McNicholas, W. Meyer, T. F. Miller III, M. E. Mura, A. Nicklass, D. P. O'Neill, P. Palmieri, D. Peng, K. A. Peterson, K. Pflüger, R. Pitzer, I. Polyak, M. Reiher, J. O. Richardson, J. B. Robinson, B. Schröder, M. Schwilk, T. Shiozaki, M. Sibaev, H. Stoll, A. J. Stone, R. Tarroni, T. Thorsteinsson, J. Toulouse, M. Wang, M. Welborn and B. Ziegler, MOLPRO, 2022.1, a package of ab initio programs, 2022, see https://www.molpro.net/.
- E. Epifanovsky, A. T. B. Gilbert, X. Feng, J. Lee, Y. Mao, N. Mardirossian, P. Pokhilko, A. F. White, M. P. Coons, A. L. Dempwolff, Z. Gan, D. Hait, P. R. Horn, L. D. Jacobson, I. Kaliman, J. Kussmann, A. W. Lange, K. U. Lao, D. S. Levine, J. Liu, S. C. McKenzie, A. F. Morrison, K. D. Nanda, F. Plasser, D. R. Rehn, M. L. Vidal, Z.-Q. You, Y. Zhu, B. Alam, B. J. Albrecht, A. Aldossary, E. Alguire, J. H. Andersen, V. Athavale, D. Barton, K. Begam, A. Behn, N. Bellonzi, Y. A. Bernard, E. J. Berquist, H. G. A. Burton, A. Carreras, K. Carter-Fenk, R. Chakraborty, A. D. Chien, K. D. Closser, V. Cofer-Shabica, S. Dasgupta, M. de Wergifosse, J. Deng, M. Diedenhofen, H. Do, S. Ehlert, P.-T. Fang, S. Fatehi, Q. Feng, T. Friedhoff, J. Gayvert, Q. Ge, G. Gidofalvi, M. Goldey, J. Gomes, C. E. González-Espinoza, S. Gulania, A. O. Gunina, M. W. D. Hanson-Heine, P. H. P. Harbach, A. Hauser, M. F. Herbst, M. Hernández Vera, M. Hodecker, Z. C. Holden, S. Houck, X. Huang, K. Hui, B. C. Huynh, M. Ivanov, A. Jász, H. Ji, H. Jiang, B. Kaduk, S. Kähler, K. Khistyaev, J. Kim, G. Kis, P. Klunzinger, Z. Koczor-Benda, J. H. Koh, D. Kosenkov, L. Koulias, T. Kowalczyk, C. M. Krauter, K. Kue, A. Kunitsa, T. Kus, I. Ladjánszki, A. Landau, K. V. Lawler, D. Lefrancois, S. Lehtola, R. R. Li, Y.-P. Li, J. Liang, M. Liebenthal, H.-H. Lin, Y.-S. Lin, F. Liu, K.-Y. Liu, M. Loipersberger, A. Luenser, A. Manjanath, P. Manohar, E. Mansoor, S. F. Manzer, S.-P. Mao, A. V. Marenich, T. Markovich, S. Mason, S. A. Maurer, P. F. McLaughlin, M. F. S. J. Menger, J.-M. Mewes, S. A. Mewes, P. Morgante, J. W. Mullinax, K. J. Oosterbaan, G. Paran, A. C. Paul, S. K. Paul, F. Pavo, Z. Pei, S. Prager, E. I. Proynov, A. Rák, E. Ramos-Cordoba, B. Rana, A. E. Rask, A. Rettig, R. M. Richard, F. Rob, E. Rossomme, T. Scheele, M. Scheurer, M. Schneider, N. Sergueev, S. M. Sharada, W. Skomorowski, D. W. Small, C. J. Stein, Y.-C. Su, E. J. Sundstrom, Z. Tao, J. Thirman, G. J. Tornai, T. Tsuchimochi, N. M. Tubman, S. P. Veccham, O. Vydrov, J. Wenzel, J. Witte, A. Yamada, K. Yao, S. Yeganeh, S. R. Yost, A. Zech, I. Y. Zhang, X. Zhang, Y. Zhang, D. Zuev, A. Aspuru-Guzik, A. T. Bell, N. A. Besley, K. B. Bravaya, B. R. Brooks, D. Casanova, J.-D. Chai, S. Coriani, C. J. Cramer, G. Cserey, A. E. DePrince, R. A. DiStasio, A. Dreuw, B. D. Dunietz, T. R. Furlani, W. A. Goddard, S. Hammes-Schiffer, T. Head-Gordon, W. J. Hehre, C.-P. Hsu, T.-C. Jagau, Y. Jung, A. Klamt, J. Kong, D. S. Lambrecht, W. Liang, N. J. Mayhall, C. W. McCurdy, J. B. Neaton, C. Ochsenfeld, J. A. Parkhill, R. Peverati, V. A. Rassolov, Y. Shao, L. V. Slipchenko, T. Stauch, R. P. Steele, J. E. Subotnik, A. J. W. Thom, A. Tkatchenko, D. G. Truhlar, T. Van Voorhis, T. A. Wesolowski, K. B. Whaley, H. L. Woodcock, P. M. Zimmerman, S. Faraji, P. M. W. Gill, M. Head-Gordon, J. M. Herbert and A. I. Krylov, J. Chem. Phys., 2021, 155, 084801 CrossRef CAS PubMed.
- J. V. Ortiz, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2013, 3, 123–142 CAS.
- H. H. Corzo and J. V. Ortiz, Adv. Quantum Chem., 2017, 74, 267–298 CrossRef CAS.
- J. V. Ortiz, J. Chem. Phys., 2020, 153, 1–28 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. MontgomeryJr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian09 Revision E.01.
- S. Gozem and A. I. Krylov, WIREs Comput. Mol. Sci., 2022, 12, e1546 CrossRef CAS.
- J. W. Rabalais, L. O. Werme, T. Bergmark, L. Karlsson and K. Siegbahn, Int. J. Mass Spectrom. Ion Phys., 1972, 9, 185–196 CrossRef CAS.
- M. A. Parkes and G. A. Worth, J. Chem. Phys., 2024, 161, 114305 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cp02504k |
| This journal is © the Owner Societies 2024 |