 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
CO2 ultrathin film growth on a monolayer of CO2 adsorbed on the NaCl(100) surface: sticking coefficient and IR-optical signatures in the ν3 region†
Jochen
Vogt

Chemisches Institut der Universität Magdeburg, Universitätsplatz 2, Magdeburg, Germany. E-mail: jochen.vogt@ovgu.de; Fax: +49 391 6711387; Tel: +49 0391 51836
First published on 22nd July 2024
Abstract
CO2 ultrathin molecular films were grown onto a preadsorbed monolayer NaCl(100)/p(2 × 1)-CO2 at 40 K. Polarization infrared spectroscopy (PIRS) reveals that so-prepared films have better quality than directly grown films. A sticking probability of 0.74 ± 0.1 was deduced from the integrated IR absorption. The presence of the monolayer doublet in the film spectra suggests a Stranski–Krastanov film growth with locally varying film thicknesses on the surface. In the region of the ν3(12C16O2) band, fine structure was observed between the well-known transverse-optical (TO) and longitudinal optical (LO) bands. Two independent computational models were applied to analyze the nature of the observed fine structure. Both pair potential calculations in combination with a vibrational exciton model as well as plane-wave density functional theory (DFT) in combination with phonon calculations of IR intensities at the Γ-point reveal that a weak mode visible in s-polarization and p-polarization originates from a vibrational film excitation located near the substrate interface. A series of p-polarized weak bands appearing and partly disappearing upon film-growth is assigned to film stacks of unique local thickness.
1 Introduction
Thin films of CO and CO2 grown on ionic single crystal surfaces like NaCl or KBr have attracted interest in a variety of applications. In the astrophysical context, they have been used to study the infrared-optical properties of mixed, amorphous, and crystalline ice in laboratory experiments, in comparison with IR spectra from planetary or interstellar origins.1–5 Recently, the significance of vibrational pooling for isomerization processes in such films was demonstrated.6 In CO2 thin films, vibrational coupling is thought to play a crucial role in the photodesorption of CO2 from CO2 ice,7 since the excitation energy of the ν3 asymmetric stretch mode (28 kJ mol−1) comes close to the desorption energy of physisorbed CO2.The surface science approach to investigate such phenomena is based on structurally well-defined model systems, if they are available. The very similar lattice parameters of NaCl (5.64 Å at room temperature8) and cubic CO2 ice (5.62 Å at 150 K (ref. 9)) suggest that the NaCl(100) surface should be a prefered substrate to prepare and study well-defined films of CO2.
In fact, on the NaCl(100) surface below about 80 K, the first layer of carbon dioxide is a well-ordered herring-bone structure with p(2 × 1) translational symmetry.10–12 The observed characteristic doublet in the ν3 region was assigned to dipole–dipole coupling of the two translationally inequivalent molecules in this p(2 × 1) unit cell.13,14 While the surface geometry of the first layer of these is well-known from quantitative low-energy electron diffraction experiments10 (LEED), experimental information on the structure of CO2 ultrathin films of several layers thickness has been provided mainly by helium atom scattering (HAS) experiments.11 A c(2 × 2) translational symmetry was deduced for multilayer CO2 films grown on a preadsorbed monolayer on the NaCl(100) at 40 K, resembling the low-temperature crystalline structure of α-CO2 ice.9,15 Using spot analysis-LEED (SPALEED), the c(2 × 2) symmetry of CO2 multilayers has also been detected on NaCl(100) films grown epitaxially on Ge(100)12 between 78 K and 83 K. Transmission infrared spectroscopy experiments on multilayer CO2 films grown on NaCl(100) have been conducted by Berg et al.,16,17 and an elaborate analysis of the relation between IR spectrum profiles and film structure based on vibrational exciton theory has been given by these authors.17 In the region of the ν3 asymmetric stretch mode, the spectrum profiles were dominated by massive absorptions at 2344 cm−1 and 2382 cm−1, the latter being interpreted as the longitudinal optical (LO) mode of α-CO2.16 Early works by Fox and Hexter18 and Zumofen19 showed that vibrational dipole–dipole interaction in slabs of molecular crystals of α-CO or α-CO2 should show a splitting of their fundamental stretch modes into a doubly-degenerated transverse-optical (TO) and a LO mode.
It should be noted that at substrate temperatures below 35 K, CO2 grows in its amorphous phase, while above 35 K, the film structure irreversibly transforms into the cubic structure.2 In the present work, ultrathin films less than 10 monolayers thick were grown at 40 K on a preadsorbed well-defined monolayer of CO2 on the NaCl(100) surface. Polarization infrared spectroscopy (PIRS) in the ν3-region reveals that layers grown in this way have additional weak infrared features between the TO and LO bands. To the best of my knowledge, these additional peaks haven't been described in literature so far. The aim of this article is to give an interpretation of these additional features. Therefore spectrum simulations using two different computational methods are applied and support is given that the presence of these weak features correlates with the high long-range order of the so-prepared films.
The article is organized as follows: in Section 2 the experimental methods and results are described. The modeling of the thin films and the related IR spectra is presented in Section 3. In Section 4 the experimental and computational results are discussed and an interpretation of the observed spectrum profiles is given.
2 Experiment
The apparatus for surface science experiments with insulator single crystal surfaces was described in detail in previous papers.20–22 It consists of an ultrahigh vacuum chamber (UHV) coupled to a Michelson type Bruker IFS 120 HR infrared spectrometer. The design of the sample holder allows PIRS experiments in transmission geometry at off-normal incidence. The spectra were recorded using an liquid N2 cooled InSb detector at a resolution of 0.2 cm−1. The IR beam struck the sample under an angle of incidence of β = 45° with respect to the surface normal. A NaCl single crystal (Korth Kristalle, Kiel, Germany) sized 20 mm × 20 mm was cleaved in dry nitrogen atmosphere twice to obtain a slice of 3 mm thickness with two freshly prepared (100) surfaces. The sample was transferred into the UHV chamber within about 30 minutes. Under measurement conditions, the residual gas pressure was below 1 × 10−10 mbar. Residual gas analysis was possible using a quadrupole mass spectrometer. The pressure was measured using an ionization gauge. All reported pressure values are corrected for the ionization cross section of CO2. The surface quality of the bare NaCl(100) surface was checked in low-energy electron diffraction (LEED) experiments using a multichannelplate LEED optics (MCPLEED, Omicron).Carbon dioxide ultrathin films were prepared by integral dosage of CO2 (purity 99.995%) using a leak valve. In the first step a saturated monolayer CO2 was grown by exposing the NaCl(100) surface at a sample temperature of 75 K to CO2 at a partial pressure of 7 × 10−9 mbar. The formation of the saturated monolayer was checked by recording PIRS spectra in s- and in p-polarization. The topmost spectrum (black color) in Fig. 1 shows the characteristic doublet23,24 of an in-phase (ML+, 2338.6 cm−1) and an out-of-phase (ML−, 2349.1 cm−1) vibrational mode of the herringbone NaCl(100)/p(2 × 1)-CO2 monolayer phase which is stable at 75 K and pCO2 = 7 × 10−9 mbar. From these spectra, the total integrated absorbance of the saturated monolayer in the region of the doublet was determined to be As = 0.18 cm−1 in s-polarization and Ap = 0.16 cm−1 in p-polarization. These values are close to values reported for the saturated monolayer in ref. 24.
 | ||
| Fig. 1 PIRS spectra of carbon dioxide ultrathin films grown on the NaCl(100) surface with average thickness between 1 and 3 monolayers. For the purpose of clarity the spectra are shifted vertically. For line positions and further characterization of observed infrared modes see Table 1. | ||
In order to grow additional layers of CO2, the sample was further cooled down to a temperature of 40 K while the leak-valve was closed. Then the sample was repeatedly exposed to gas-phase CO2 at a partial pressure of 7 × 10−9 mbar and defined times of exposure. Between each of such additional dosages, pairs of PIRS spectra were recorded at stopped exposure. No signs of desorption were observed under these conditions. Some of these film spectra are shown in Fig. 1 at lower coverage and, for higher coverage, in Fig. 2. For the determination of the average coverage of the CO2 film, the total integrated absorbance in the region of the ν3 asymmetric stretch mode was analyzed. The resulting total absorbances for the whole sequence of spectra are shown in Fig. 3. Apparently, a linear relation between time of exposure and integrated absorbance is observed. This justifies the calibration of the measured absorbances with the absorbances of the monolayer in order to assign an average coverage to the various film spectra and, moreover, to determine the sticking probability S of carbon dioxide molecules under the given conditions. Both sets of spectra analyzed independently are consistent with a film growth rate of r = 1.9 × 10−3 ML s−1 (1 ML = 1 monolayer = 6.28 × 1018 molecules per m2). From the comparison with the growth rate from the well-known Hertz–Knudsen equation
 | (1) |
 | ||
| Fig. 2 PIRS spectra of carbon dioxide ultrathin films grown on the NaCl(100) surface with average thickness between 3 and 7 monolayers. For the purpose of clarity the spectra are shifted vertically. For line positions and further characterization of observed infrared modes see Table 1. | ||
In the following the various observed IR absorptions shall be discussed. A list of observed peaks can be found in Table 1. A detailed inspection of the film spectra in the low-coverage regime, Fig. 1, can be summarized as follows: upon further exposure of the first layer CO2 to gas-phase CO2, there is a weakening of the doublet ML+ and ML−, while additional bands become apparent. At 2344 cm−1 a doublet develops which merges into the transverse optical modes TO-sh and TO at 2342.8 cm−1 and 2345.1 cm−1 (green spectrum in Fig. 1). In addition, the LO mode becomes visible in p-polarization.
| Mode | Experiment | Tendency | VE model | pw-DFT-D | Assignment |
|---|---|---|---|---|---|
| ML+ | 2338.6 | Weakening | 2339.4 | 2349.4 | First layer in-phase vibration |
| ML− | 2349.1 | Weakening | 2347.4 | 2356.5 | First layer out-of-phase vibration |
| TO | 2344.6 | Constantly strengthening | 2343.1 | 2357.0 | Transverse optical mode (at highest coverage) |
| TO-sh | 2342.8 | Constantly strengthening | 2344.7 | 2357.4 | Shoulder of TO mode (at highest coverage) |
| LO | 2380.2 | Constantly strengthening | 2379.8 | 2389.1 | Longitudinal optical mode (at highest coverage) |
| BL | 2335.9 | Weakening after formation | — | — | Bilayer film mode |
| SP1 | 2353.7 | Unaffected after formation | 2349.0 | 2365.9 | Film mode visible in s- and p-polarization |
| P1(5) | 2359.1 | Weakening after formation | 2357.8 | 2367.9 | Film mode (p-polarization), 5 layers |
| P1(6) | 2363.7 | Weakening after formation | 2362.6 | 2372.6 | Film mode (p-polarization), 6 layers |
| P1(7) | 2367.2 | Moderately strengthening | 2366.2 | 2376.9 | Film mode (p-polarization), 7 layers |
| P1(8) | 2369.9 | Moderately strengthening | 2369.0 | 2379.3 | Film mode (p-polarization), 8 layers |
| P1(9) | 2372.0 | — | — | — | Film mode (p-polarization), 9 layers |
| P2(8) | 2357.2 | — | 2355.9 | 2367.0 | Film mode (p-polarization), 8 layers |
Furthermore, at 2335.7 cm−1 a band denoted BL appears after additional exposure of only 2 to 4 minutes (blue spectrum), and becomes weaker again at further dosage, as the film thickness increases. This band is thus assigned to the bilayer CO2/NaCl(100), which is not further discussed in this paper, but shall be addressed in detail in further studies.
Moreover, an additional weak feature denoted SP1 is observed at 2353.7 cm−1 both in s-polarization and p-polarization. The feature does not shift in position at higher coverage (Fig. 2). Further weak absorptions are seen predominantly in p-polarisation in the high-coverage regime in Fig. 2. These absorptions are tabulated in Table 1, and are reproduced on an enlarged scale in Fig. 4 where they are denoted as peaks P1(5), P1(6), P1(7), P1(8), and P2(8). The significance of the labeling of the various peaks will become clear in the next section. Inspection of Fig. 4 reveals the following qualitative result: the peaks P1(i), i = 5, 6, 7, 8 are present in the various spectra with different strength. At the same time, the peak centers of P1(6), P1(7), and P1(8) are at the same position in the three differerent spectra, i.e. these peaks do not shift with layer thickness. While P1(5) is pronounced in the red spectrum with lowest coverage, the peak nearly disappears in the higher coverage spectra. In contrast, the feature P1(7) and P1(8) are weaker in the red spectrum and become more pronounced as coverage increases. A first interpretation of these peaks is based on their assignment to film domains with a unique number of layers coexisting on the surface. As the surface is exposed to gas-phase CO2, the abundance of a film domain with a certain number of layers first increases, and is then again expected to decrease due to the stacking of additional layers of CO2 molecules on top of it. By consequence, the peaks associated to domains with this certain number of layers will be weakened. It is the task of spectra simulations to show that a series of modes with the observed behaviour can indeed be explained in this way.
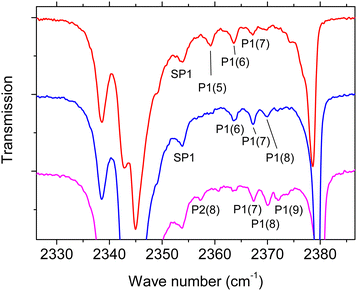 | ||
| Fig. 4 Enlarged representation of p-polarized IR spectra in the high coverage regime (Fig. 2) with fine structure features labeled SP1, P1, P2 (see also Table 1). For the purpose of clarity the spectra are shifted vertically. For line positions and further characterization of observed infrared modes see Table 1. | ||
The following observations were made in additional experiments: (A) cautious tempering of the once prepared layers at temperatures of about 60 K did not significantly change the spectra profiles. (B) At further exposure of the once prepared layers to CO2 the additional spectral features gradually become weaker.
3 Model calculations
In order to substantiate the interpretation of the observed IR absorptions, two different methods were used for the modeling of CO2 thin film spectra. Both models are based on film geometries resulting from energy minimizations at 0 K. No temperature effects are thus included. The first method is the vibrational exciton (VE) model, which is simple but intuitive. It was previously applied by Berg et al. to monolayer and multilayer CO2 thin films on the NaCl(100) surface.17 The second approach is based on state of the art phonon calculations within the framework of dispersion-corrected plane-wave density functional theory (pw-DFT-D).Matching the experimental situation, the IR absorption of polarized light in transmission geometry is considered, with ultrathin CO2 films being adsorbed both at the front side and at the back side of the NaCl single crystal. The latter is considered as a non-absorbing transparent substrate. For the index of refraction at 2350 cm−1, a value of n = 1.52 was taken from ref. 25. The IR beam strikes the front side at an angle β = 45° measured against the surface normal. It is partially reflected and transmitted through the substrate. The direction vector of the electric field, ![[small epsilon, Greek, vector]](https://www.rsc.org/images/entities/i_char_e0e7.gif) , is different at the front side and at the back side, and is calculated using Fresnel's equations.26 Explicit formulae are given in ESI† (S1), where also a sketch of the optical path of the IR beam through the substrate interfaces is given. Thus, following the treatment of Berg and Ewing,17 the model assumes a single two-layer model in which the ultrathin adsorbate films do not influence the effective electric fields at the interfaces. This approximation has been discussed in ref. 17 and analyzed in more detail in ref. 27.
, is different at the front side and at the back side, and is calculated using Fresnel's equations.26 Explicit formulae are given in ESI† (S1), where also a sketch of the optical path of the IR beam through the substrate interfaces is given. Thus, following the treatment of Berg and Ewing,17 the model assumes a single two-layer model in which the ultrathin adsorbate films do not influence the effective electric fields at the interfaces. This approximation has been discussed in ref. 17 and analyzed in more detail in ref. 27.
In general, an ordered adsorbate exhibits lower point symmetry than the NaCl(100) surface unit cell, and will thus be present in four different domain orientations with equal weights. In order to facilitate the domain averaging, it is convenient to rotate the electric field vectors accordingly instead of rotating the simulated film geometries.
3.1 Vibrational exciton model
The strength of the VE model is its conceptual simplicity, which nevertheless contains the necessary physics17,18 to understand the splittings of vibrational modes in a film of translationally inequivalent molecules coupled by dynamic dipole–dipole interaction. The implementation used in this study is essentially identical to that described in ref. 22. The approach adopts the exciton model described in ref. 17 with the special assumption of strict twodimensional periodicity.The basis of the model are the geometries of the monolayer with p(2 × 1) translational symmetry, and the multilayer films with c(2 × 2) translational symmetry. These were optimized by means of minimizing the film cohesive energy
 | (2) |
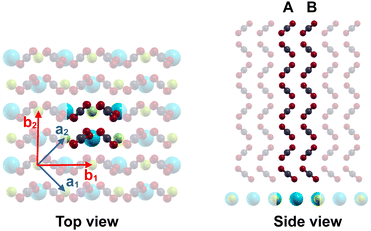
The optimized geometry of a ultrathin film comprising 8 layers and c(2 × 2) symmetry is shown in Fig. 5. Each layer contains two translationally inequivalent molecules A and B. At the interfaces, the orientation and the tilt angle of the molecules with respect to the surface normal differ somewhat from the structure in the middle of the film, where the interlayer distance is 2.67 Å. This value is slightly smaller than the bulk value of 2.79 Å for α-CO2 at 80 K, obtained by Mangan et al.15
Using the intermolecular distance
rs,s′,n,m = |![[r with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0072_20d1.gif) s − rs′ − n s − rs′ − n![[b with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0062_20d1.gif) 1 − m 1 − m![[b with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0062_20d1.gif) 2| 2| | (3) |
![[g with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0067_20d1.gif) 00 = 0 and a molecule s′ in the unit cell at
00 = 0 and a molecule s′ in the unit cell at ![[g with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0067_20d1.gif) nm indexed by the integers n and m, and the unit vector
nm indexed by the integers n and m, and the unit vector ![[v with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0076_20d1.gif) s,s′,n,m connecting their center of masses, a dipole–dipole interaction tensor18U is calculated by summation over the c(2 × 2) lattice vectors:
s,s′,n,m connecting their center of masses, a dipole–dipole interaction tensor18U is calculated by summation over the c(2 × 2) lattice vectors: | (4) |
The prime symbol in the sum denotes that s′ ≠ s for n = m = 0, and ![[e with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0065_20d1.gif) s,n,m is the unit vector pointing along the direction of the induced electric dipole moment of a molecule s in unit cell indexed by n and m, respectively. For the ν3 asymmetric stretch mode of the linear triatomic CO2, the induced dipole moment and thus
s,n,m is the unit vector pointing along the direction of the induced electric dipole moment of a molecule s in unit cell indexed by n and m, respectively. For the ν3 asymmetric stretch mode of the linear triatomic CO2, the induced dipole moment and thus ![[e with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0065_20d1.gif) s,n,m is parallel to the molecule's axis.
s,n,m is parallel to the molecule's axis.
Given a film with S inequivalent molecules with singleton wave number ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) 0 and vibrational polarizability αvib related to a fundamental vibrational mode, the eigenvalue problem
0 and vibrational polarizability αvib related to a fundamental vibrational mode, the eigenvalue problem
 | (5) |
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) k for the S exciton solutions, which are characterized by a vibrational exciton state18
k for the S exciton solutions, which are characterized by a vibrational exciton state18 | (6) |
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) 0 are given in Table 3. After solution of eqn (5) using standard linear algebra methods, the absorption stick spectrum for s-polarization and p-polarization is obtained by means of
0 are given in Table 3. After solution of eqn (5) using standard linear algebra methods, the absorption stick spectrum for s-polarization and p-polarization is obtained by means of | (7) |
F is the area of the surface unit cell. Eqn (7) sums up the contribution from the front side (i = 1) and the back side (i = 2) and performs a domain averaging using appropriate direction vectors of the electric field at the front side and at the back side, as introduced above. Eqn (7) is obtained from a textbook time-dependent perturbation theory treatment of the transition probability in analogy to gas-phase IR spectroscopy with the exception that the molecules are regularly oriented. Moreover, the factor F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β in the denominator of the prefactor accounts for the surface area irradiated by the IR beam under oblique incidence. Note also that αvib is related to the square of the dipole-moment derivative
β in the denominator of the prefactor accounts for the surface area irradiated by the IR beam under oblique incidence. Note also that αvib is related to the square of the dipole-moment derivative  via32
via32
 | (8) |
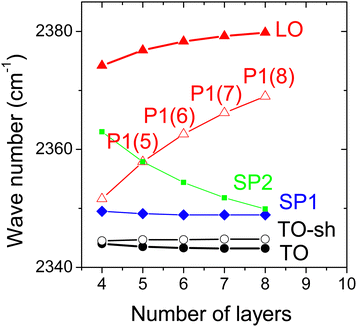 | ||
| Fig. 7 Results of vibrational exciton model for multilayer CO2 films on NaCl(100): wave numbers of the most intense modes modes dependent on film thickness. | ||
Concerning the nature of the various exciton modes, it is instructive to look at their eigenvectors, which are reproduced for some modes of an eight layer film in Table 2. For each of the 8 layers, the coefficients cs,k are tabulated for its two inequivalent molecules A and B (see also Fig. 5). Higher absolute values of cs,k correspond to a higher localization of the vibrational exciton at a certain molecule, or, accordingly, a higher vibrational displacement amplitude. While the most intense modes TO and LO have similar absolute cs,k values across the whole film, the situation is different especially for the mode SP1, which has higher absolute values in the first three layers at the bottom near the substrate interface. Similarly, the mode TO-sh appears to be located in the topmost three layers near the interface to the vacuum. Moreover, the sign of these coefficients provides information about the relative phase of the molecule's vibration. Equal phases indicate in-phase motion, while opposite signs indicate out-of-phase motion, respectively. Thus, within the vibrational exciton model, the mode TO appears to be a collective in-phase mode of all molecules within the film, while in the mode TO-sh, the two inequivalent molecules A and B within a layer are out-of-phase with each other. Similarly, molecule A and B vibrate out-of-phase also in mode SP1, while in modes P1(8) and LO, the molecules within a layer vibrate in-phase.
| Layer | TO | TO-sh | SP1 | P1(8) | LO | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mol. A | Mol. B | Mol. A | Mol. B | Mol. A | Mol. B | Mol. A | Mol. B | Mol. A | Mol. B | |
| 1 | −0.207 | −0.207 | 0.027 | −0.026 | −0.553 | 0.552 | −0.240 | −0.240 | −0.066 | −0.066 |
| 2 | −0.284 | −0.284 | 0.009 | −0.008 | −0.421 | 0.421 | 0.336 | 0.336 | 0.175 | 0.175 |
| 3 | −0.309 | −0.309 | −0.026 | 0.026 | −0.126 | 0.126 | −0.074 | −0.074 | −0.290 | −0.290 |
| 4 | −0.310 | −0.311 | −0.060 | 0.060 | −0.032 | 0.032 | −0.266 | −0.266 | 0.360 | 0.360 |
| 5 | −0.288 | −0.289 | −0.112 | 0.112 | −0.012 | 0.012 | 0.268 | 0.268 | −0.360 | −0.360 |
| 6 | −0.243 | −0.243 | −0.244 | 0.244 | −0.009 | 0.009 | 0.068 | 0.068 | 0.291 | 0.291 |
| 7 | −0.180 | −0.180 | −0.432 | 0.432 | −0.010 | 0.010 | −0.331 | −0.331 | −0.178 | −0.178 |
| 8 | −0.098 | −0.097 | −0.485 | 0.484 | −0.010 | 0.010 | 0.258 | 0.258 | 0.069 | 0.069 |
The results obtained from the VE model will be further discussed below in Section 4.
3.2 Plane-wave DFT-D
The geometries of the monolayer as well as films with c(2 × 2) symmetry and thicknesses between four and eight layers were optimized using the quantum espresso (QE) package33,34 (version 7.1). The molecular film was stacked on a four-layer slab of NaCl(100) with a pre-optimized lattice parameter of 5.7 Å. The pseudo-potentials using Perdew–Burke–Ernzerhof-funtionals35 (PBE) and the projector augmented wave formalism36 were taken from Dal Corso's pseudopotential library.37 A cut-off energy of 41 Ry for the wavefunction and 246 Ry for the density was used in combination with a 3 × 3 × 1 Monkhorst–Pack grid. Grimme's DFT-D2 parameters38 for dispersion interaction were used.Details of the optimized geometries are given in the ESI† (S3). It is worth to mention that the interlayer distance in the middle of the film is 2.86 Å and thus somewhat larger than the experimental bulk value of 2.79 Å given above. For the optimized geometries, a phonon calculation at the Γ-point was made to determine the infrared intensities. In order to generate polarization infrared stick spectra, the QE postprocessing code for the evaluation of IR intensities from the dynamical matrix was slightly modified to print out the polarization vector ![[p with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0070_20d1.gif) k involved with a certain infrared mode k.§ Normally, the “infrared intensity” or infrared cross-section39 in units of Debye2 Å−2 amu−1 of a single mode k is computed by means of
k involved with a certain infrared mode k.§ Normally, the “infrared intensity” or infrared cross-section39 in units of Debye2 Å−2 amu−1 of a single mode k is computed by means of
I(k) = Ic(![[p with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0070_20d1.gif) k)2 k)2 | (9) |
 | (10) |
![[small epsilon, Greek, vector]](https://www.rsc.org/images/entities/i_char_e0e7.gif) i,d is the direction vector of the electric field associated with domain d for either s-polarization or p-polarization at the front side i (i = 1) or at the back side (i = 2). For explicit values of
i,d is the direction vector of the electric field associated with domain d for either s-polarization or p-polarization at the front side i (i = 1) or at the back side (i = 2). For explicit values of ![[small epsilon, Greek, vector]](https://www.rsc.org/images/entities/i_char_e0e7.gif) i,d see ESI† (S1).
i,d see ESI† (S1).
The PIRS stick spectra for the monolayer and films of various thickness are shown in Fig. 8. Explicit data of the calculated modes are tabulated in the ESI† (S3). Table S6 (ESI†) also compares calculated intensities for the monolayer modes with experimental integrated absorbances. The wave numbers of some modes are also listed in Table 1. The modes ML+, ML−, TO, and LO are blue-shifted in comparison with experimental values, mainly due to the harmonic approximation without the use of a scaling factor. However, as further discussed below, the simulated LO–TO splitting of 32.1 cm−1 for a film with 8 layers is quite close to the experimental value of 35.6 cm−1. Apart from the shoulder of the TO mode denoted TO-sh, a weak feature SP1 both in s-polarization and p-polarization changes only little between 2367 cm−1 and 2365 cm−1, as the number of layers increases. In p-polarised spectra, the wave number of feature P1 moves from 2362 cm−1 for a film with four layers up to 2379 cm−1 for a film with 8 layers. For films with 7 and 8 layers thickness, a weaker feature P2 appears in p-polarization (see Fig. 8). The vibrational displacements involved with some modes of a filme with 8 layers are shown in Fig. 9 where the scaled amplitude and direction of the displacements is indicated by green arrows. Most striking is the behaviour of mode SP1 with the largest vibrational displacements essentially in the first two layers at the interface to the NaCl substrate, in accordance to the characterization of this mode by the VE model. In the following, these results will be further discussed and compared to the experimental findings.
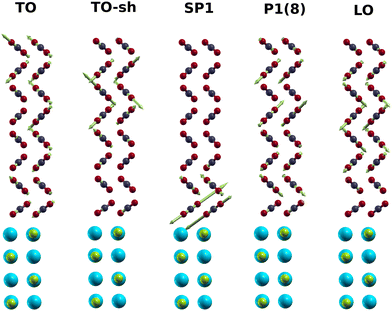 | ||
| Fig. 9 Displacement patterns of selected normal modes obtained from pw-DFT-D simulation for a CO2 film with 8 layers. Cyan and green balls represent the substrate Na+ and Cl−, respectively. Green arrows indicate the vibrational forces on atoms in a normal mode.40 The mode TO-sh is the shoulder of the TO. | ||
4 Discussion
In the HAS experiments by Lange et al.11 diffraction spots of the first layer NaCl(100)/p(2 × 1)-CO2 were visible up to an exposure of 170 L, suggesting a coexistence of the first layer with multilayer CO2 at 40 K. This excludes a layer-by-layer growth mode of CO2 despite the quite small lattice mismatch with the NaCl substrate. The present results from IR spectroscopy are consistent with this picture. As can be seen in Fig. 1, the mode ML+ assigned to the preadsorbed monolayer is still visible in the multilayer spectra, and the mode ML− stays visible as a shoulder at the high frequency side of the TO mode. In the absence of layer-by-layer growth, the coexistence of domains with different film thickness is conceivable. By consequence, the IR spectra shown in Fig. 1 should be interpreted as a superposition of spectra resulting from coexisting domains with varying number of layers. The evaluation of integrated absorbance data (Fig. 3) provides an average number between one and seven monolayers for the presented spectra. Moreover, a sticking coefficient of S = 0.74 ± 0.1 is deduced, which is a reasonable value. In a previous study a sticking probability of one was ad hoc assumed4 for CO2 ice at 80 K and below. Moreover, the present value is somewhat smaller and scarcely in line with the value of 1.0 ± 0.2 found by Weida et al.,41 and, moreover, with a value of 0.9 obtained recently from state-resolved experiments by Jansen and Juurlink.42The preparation method to grow the thin films onto a preadsorbed monolayer leads to the appearance of additional spectral features between the intense TO and LO modes. Basically two kinds of features in the ν3 spectrum in Fig. 1 and 2 are observed. Firstly, a band visible both in s-polarized and p-polarized spectra, thus denoted SP1. Secondly, a series of weak peaks only visible in p-polarized spectra. Inspection of the enlarged representation in Fig. 4 shows that the peaks P1(5) and P1(6) become weaker as the coverage increases (spectrum colored magenta), while P1(7) and P1(8) become stronger. From the Stransky–Krastanov growth mode and these findings it was concluded at the end of Section 2 that the peaks P1(i) should be assigned to film domains of a characteristic number of layers. This assumption is supported by two different computational models to simulate PIRS spectra introduced in Section 3.
The computational models yield similar results for the film modes in the ν3 region, although there are differences in the absolute position of the IR bands. With the vibrational polarizabilities and singleton-frequencies given in Table 3, the vibrational exciton approach reproduces quite well the Davydov-splitting in the p(2 × 1) monolayer phase, and the TO–LO-splittings of the film modes (35.6 cm−1 experimentally versus 36.1 cm−1 calculated, see Table 1). The DFT approach has no extra optical parameters and no scaling factor was applied to compensate for the missing anharmonicity in the model. Thus the calculated band positions appear basically blue-shifted by about 10 cm−1. Nevertheless, the calculated TO–LO-splitting of 32.1 cm−1 is close to the experiment. It can thus be noted that both computational models reproduce the observed characteristic splittings of the films in a satisfactory manner. It is worth to mention that both models reproduce the slight blue shift of the LO peak with increasing thickness seen in Fig. 2. In the following, the discussion focuses on the most intense of these additional spectral features seen in the experiment. The peak labeled SP1 is visible both in s-polarized and p-polarized film spectra, and its wavenumber near 2354 cm−1 (see Table 1, Fig. 1 and 2) does not change substantially as the film thickness increases. The counterpart in the simulated spectra are film modes at 2349 cm−1 (VE approach) and between 2366 cm−1 and 2369 cm−1 (DFT-model). In both simulations, SP1 is the strongest feature visible both in s-polarization and p-polarization. And, moreover, within both models the feature does not change substantially its position as the number of layers increases (see Fig. 6–8 and 10). As described above, further analysis of the simulation results suggest that this mode is a vibrational excitation of the molecules in the layers close to the NaCl substrate, and this might in part explain the only minor change in wave number with increasing coverage. Indeed, in terminology of surface phonon lattice dynamics, one could characterize SP1 as a optical localized mode43 at the interface.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) 0 (cm−1)
0 (cm−1) |
α vib (Å3) | |
|---|---|---|
| CO2-p(2 × 1)/NaCl(100) | 2344.8 | 0.48 |
| CO2 multilayers | 2349.8 | 0.30 |
 | ||
| Fig. 10 Results of pw-DFT-D for multilayer CO2 films on NaCl(100): wave numbers of the most intense modes modes. | ||
In both models, the strongest peak P1 largely visible in p-polarization has a considerable increase in wave number as the thickness of the film increases between 4 and 8 layers (see Fig. 7 and 10). This indeed justifies the assignment to the series of peaks denoted P1(i) in the experiments (see Table 1). Visibility in p-polarization is related to an induced total vibrational dipole moment nearly perpendicular to the surface plane. The displacement pattern for the pw-DFT-D model depicted for this mode in Fig. 9 and the eigenvector coefficients cs,k from vibrational exciton theory in Table 2 reveal an in-phase vibration of the molecules within a layer, and strongly alternating vibrational amplitudes of the molecules in different layers. This behaviour resembles to some extent to a nodal pattern of excitation of the entire film stack. In spite of the similarities in the two models, however, there are also some differences concerning the amplitude and phase of the molecules across the film stack. So, unlike in the case of mode SP1, a further more concrete interpretation of the nature of the mode P1 seems not possible.
There are also some differences between the two models concerning the very weak modes. While the vibrational exciton ansatz predicts a further series of peaks SP2 visible in s-polarization and p-polarization (cf.Fig. 6), this feature was not observed in the experiment (cf.Fig. 2), and is also not markedly present in the pw-DFT-D stick spectra. It is conceivable, that within the vibrational exciton approach, dynamic dipole–dipole coupling is to some extent overestimated, since the interlayer distance from the cohesive energy minimization was about 0.1 Å smaller than the respective bulk value, while the interlayer distances from pw-DFT-D exceed the bulk value by about 0.1 Å. Note that in the VE model, this structural property affects the dipole–dipole sum U in eqn (4)via the intermolecular distance r between two molecules. Within the pw-DFT-D model, on the other hand, an extra feature P2 visible in p-polarization is predicted for film thicknesses of 7 and 8 layers, respectively (see Fig. 8). In the enlarged version of p-polarized experimental spectra shown in Fig. 4, an albeit weak peak at 2357.2 cm−1 was tentatively assigned as P2(8).
Next, the conditions for the appearance of the additional weak peaks in experimental spectra shall be discussed. Both computational models assume strict two-dimensional periodicity of a slab of CO2 molecules, which on a real surface, is only met in an approximate fashion, due to truncation effects, and various forms of static and dynamical disorder. It seems that long-range order within a film domain within a sufficiently large number of 2D unit cells is a precondition for the appearance of the observed additional spectral features. From the widths of diffraction peaks observed in the HAS experiments,11 Lange et al. estimated an extension of ordered CO2 film domains of about 250 Å, corresponding to about 2000 c(2 × 2) unit cells. In the present study, CO2 films were grown under similar conditions on a preadsorbed monolayer at the same temperature and similar growth rates. If a similar film quality would thus be presumed for the films prepared in the present work, an eight layer film with this extension would contain about 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 CO2 molecules. In the detailed IR spectroscopic study by Berg et al.,17 multilayer films with an estimated thickness of about 4 monolayers were prepared at 65 K at a ten times larger growth rate.¶ The spectrum of the so prepared films only shows the monolayer doublet and the TO and LO peaks. Moreover, Berg et al. conducted a vibrational exciton spectrum simulation in this study.17 It involved a four layer finite film cluster of 1800 molecules, thus extending over 225 unit cells. Their simulated spectrum did not exhibit additional pronounced spectral features. From gas-phase clusters it is well-known that shape and size can crucially affect the vibrational spectrum.44 A further hint is the observation mentioned at the end of Section 2 that the additional peaks gradually become weaker with increasing exposure. It is conceivable that film domains become more fragmented and domain sizes have the tendency to decrease if the film thickness increases. So it seems that the presence of additional spectral features as those observed in this work is related to large film domains with long-range order, and ultrathin films with coverages up to about 10 monolayers. It is also worth to mention that in experiments with multilayer adsorption on other substrates (CaF2(111), KBr(100), KCl(100)) this kind of size-dependent fine structure was not observed so far.
000 CO2 molecules. In the detailed IR spectroscopic study by Berg et al.,17 multilayer films with an estimated thickness of about 4 monolayers were prepared at 65 K at a ten times larger growth rate.¶ The spectrum of the so prepared films only shows the monolayer doublet and the TO and LO peaks. Moreover, Berg et al. conducted a vibrational exciton spectrum simulation in this study.17 It involved a four layer finite film cluster of 1800 molecules, thus extending over 225 unit cells. Their simulated spectrum did not exhibit additional pronounced spectral features. From gas-phase clusters it is well-known that shape and size can crucially affect the vibrational spectrum.44 A further hint is the observation mentioned at the end of Section 2 that the additional peaks gradually become weaker with increasing exposure. It is conceivable that film domains become more fragmented and domain sizes have the tendency to decrease if the film thickness increases. So it seems that the presence of additional spectral features as those observed in this work is related to large film domains with long-range order, and ultrathin films with coverages up to about 10 monolayers. It is also worth to mention that in experiments with multilayer adsorption on other substrates (CaF2(111), KBr(100), KCl(100)) this kind of size-dependent fine structure was not observed so far.
Finally, possible alternative mechansim causing line splittings in the CO2 films shall be briefly discussed. Mode interactions due to Fermi resonances in carbon dioxide do not fall in the region of the ν3 assymmetric stretch mode,45 why these can be ruled out. Moreover, combination bands of internal modes and external modes have been ruled out previously17 as a source of splittings, since for physisorbed molecules like CO2 such combination bands are expected to be weak and diffuse.17 Finally, the presence of molecules in different energetically inequivalent sites could lead to splittings. While in systems like CO chemisorbed on Cu(211) or Cu(111) the IR spectra of CO show distinct splittings due to different red-shifts,46 such effects are much less pronounced in the case of weakly interacting physisorbed molecules, and are thus unlikely to explain the oberved splittings. Vibrational dipole–dipole coupling thus appears to be the dominant mechanism explaining the film spectra of CO2 in the physisorbed state.
Possible applications of the observed spectral features between the TO and LO modes might involve experiments with the possibility to vibrationally excite an ultrathin film e.g. specifically at its interfaces. Vibrational energy transfer in a CO2 molecular crystal has been considered by Falk.2 Only recently, Ioppolo et al. studied the possibility of reorientation and desorption from amorphous and crystalline CO2 ice by tunable free-electron laser irradiation at various energies near the ν3 mode.47 While a frequency dependent restructuring was not clearly observed in crystalline ice,47 such experiments could show quite different results with ultrathin films on NaCl(100) if the excitation energy would match the various additional modes found in this study.
Moreover, the observed fine structure related to long range ordered film structure is an argument to use NaCl as a preferred substrate to grow high quality CO2 films. The only slightly higher effort to grow CO2 on a preadsorbed monolayer has the benefit to prepare well-defined films, and the presence of the weak features between the TO and LO modes is a verification of their quality.
As noted in Section 2, future work shall investigate in detail the formation of the bilayer and the possible reorientation effects involved if the second and the third layer starts to form.
5 Conclusions
Multilayer CO2 films were grown at low adsorption rates on a preadsorbed monolayer NaCl(100)/p(2 × 1)-CO2 at 40 K. Polarization IR spectroscopy experiments revealed a size-dependent fine structure in the ν3 asymmetric stretch region. In agreement with a previous helium diffraction study,11 a Stranski–Krastanov growth of CO2 is deduced, and a sticking coefficient of 0.74 ± 0.1 was determined based on the integrated absorbance of the IR spectra. Between the TO and LO peaks an additional absorption at 2354 cm−1 was detected independent of coverage both in s-polarization and p-polarization. Moreover, a series of weak peaks appearing exclusively in p-polarized spectra were assigned to film domains with a unique number of layers. Two kinds of spectra simulations based on the vibrational exciton approach and on plane-wave density functional theory were used to calculate stick spectra of the monolayer and films between 4 and 8 monolayers. According to these models, the additional feature in s-polarization and p-polarization can be assigned to a vibrational excitation of the molecules in the layers located near the interface to the NaCl substrate. Moreover, both models support an additional mode visible in p-polarization with a marked increase of wave number with the number of layers. Assuming the coexistence of film domains with varying number of layers in the experiment, this mode explains the series of features occurring in p-polarized spectra.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
I am grateful to the University of Magdeburg and its facilities for financial and technical support of the ongoing projects.Notes and references
- I. R. Cooke, E. C. Fayolle and K. I. Öberg, Astrophys. J., 2016, 832, 5 CrossRef.
- M. Falk, J. Chem. Phys., 1987, 86, 560–564 CrossRef CAS.
- R. M. Escribano, G. M. M. Caro, G. A. Cruz-Diaz, Y. Rodríguez-Lazcano and B. Maté, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 12899–12904 CrossRef CAS PubMed.
- K. Isokoski, C. A. Poteet and H. Linnartz, Astron. Astrophys., 2013, 555, A85 CrossRef.
- S. A. Sandford and L. J. Allamandola, Astrophys. J., 1990, 355, 357 CrossRef CAS PubMed.
- J. A. Lau, A. Choudhury, C. Li, D. Schwarzer, V. B. Verma and A. M. Wodtke, Science, 2020, 367, 175–178 CrossRef CAS PubMed.
- C. Yuan, J. Yates and T. John, J. Chem. Phys., 2013, 138, 154303 CrossRef PubMed.
- Y. Li, Y. Li, C. Li, X. Zhang, F. Zeng, H. Lin, Z. Su and C. Mahadevan, J. Alloys Compd., 2020, 849, 156592 CrossRef CAS.
- A. Simon and K. Peters, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1980, 36, 2750–2751 CrossRef.
- J. Vogt and H. Weiss, J. Chem. Phys., 2003, 119, 1105–1114 CrossRef CAS.
- G. Lange, D. Schmicker, J. P. Toennies, R. Vollmer and H. Weiss, J. Chem. Phys., 1995, 103, 2308–2319 CrossRef CAS.
- J. Schimmelpfennig, S. Fölsch and M. Henzler, Surf. Sci., 1991, 250, 198–206 CrossRef CAS.
- J. Heidberg, E. Kampshoff, O. Schönekäs, H. Stein and H. Weiss, Ber. Bunsenges. Phys. Chem., 1990, 94, 112–118 CrossRef CAS.
- J. Heidberg, E. Kampshoff, R. Kühnemuth and O. Schönekäs, Surf. Sci., 1992, 269–270, 120–127 CrossRef CAS.
- T. Mangan, C. Salzmann, J. Plane and B. Murray, Icarus, 2017, 294, 201–208 CrossRef CAS.
- O. Berg and G. E. Ewing, Surf. Sci., 1989, 220, 207–229 CrossRef CAS.
- O. Berg, R. Disselkamp and G. E. Ewing, Surf. Sci., 1992, 277, 8–20 CrossRef CAS.
- D. Fox and R. M. Hexter, J. Chem. Phys., 1964, 41, 1125–1139 CrossRef CAS.
- G. Zumofen, J. Chem. Phys., 1978, 68, 3747–3759 CrossRef CAS.
- A. G. Cabello-Cartagena, J. Vogt and H. Weiss, J. Chem. Phys., 2010, 132, 074706 CrossRef CAS PubMed.
- J. Vogt and H. Weiss, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 125415 CrossRef.
- F. Traeger, M. Hadnadjev, J. Vogt and H. Weiss, J. Phys. Chem. A, 2011, 115, 6986–6996 CrossRef CAS PubMed.
- J. Heidberg, M. Hustedt, E. Kampshoff and V. Rozenbaum, Surf. Sci., 1999, 427–428, 431–438 CrossRef CAS.
- J. Heidberg, E. Kampshoff, R. Kühnemuth, O. Schönekäs, H. Stein and H. Weiss, Surf. Sci., 1990, 226, L43–L47 CrossRef CAS.
- E. D. Palik, Handbook of Optical Constants of Solids, Academic Press, Orlando, Florida, 1985 Search PubMed.
- V. Tolstoy, I. Chernishova and V. Skryshevsky, Handbook of Infrared Spectroscopy of Ultrathin Films, Wiley, Hoboken, New Jersey, 2003 Search PubMed.
- H. H. Richardson, H.-C. Chang, C. Noda and G. E. Ewing, Surf. Sci., 1989, 216, 93–104 CrossRef CAS.
- R. Bukowski, J. Sadlej, B. Jeziorski, P. Jankowski, K. Szalewicz, S. A. Kucharski, H. L. Williams and B. M. Rice, J. Chem. Phys., 1999, 110, 3785–3803 CrossRef CAS.
- J. E. Lennard-Jones and B. M. Dent, Trans. Faraday Soc., 1928, 24, 92–108 RSC.
- Z. Zhang and Z. Duan, J. Chem. Phys., 2005, 122, 214507 CrossRef PubMed.
- D. E. Smith and L. X. Dang, J. Chem. Phys., 1994, 100, 3757–3766 CrossRef CAS.
- D. Rinaldi, M. Ruiz-Lopez, M. Costa and J. Rivail, Chem. Phys. Lett., 1986, 128, 177–181 CrossRef CAS.
- P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G. L. Chiarotti, M. Cococcioni, I. Dabo, A. D. Corso, S. de Gironcoli, S. Fabris, G. Fratesi, R. Gebauer, U. Gerstmann, C. Gougoussis, A. Kokalj, M. Lazzeri, L. Martin-Samos, N. Marzari, F. Mauri, R. Mazzarello, S. Paolini, A. Pasquarello, L. Paulatto, C. Sbraccia, S. Scandolo, G. Sclauzero, A. P. Seitsonen, A. Smogunov, P. Umari and R. M. Wentzcovitch, J. Phys.: Condens. Matter, 2009, 21, 395502 CrossRef PubMed.
- P. Giannozzi, O. Andreussi, T. Brumme, O. Bunau, M. B. Nardelli, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, M. Cococcioni, N. Colonna, I. Carnimeo, A. D. Corso, S. de Gironcoli, P. Delugas, R. A. DiStasio, A. Ferretti, A. Floris, G. Fratesi, G. Fugallo, R. Gebauer, U. Gerstmann, F. Giustino, T. Gorni, J. Jia, M. Kawamura, H.-Y. Ko, A. Kokalj, E. Küçükbenli, M. Lazzeri, M. Marsili, N. Marzari, F. Mauri, N. L. Nguyen, H.-V. Nguyen, A. O. de-la Roza, L. Paulatto, S. Poncé, D. Rocca, R. Sabatini, B. Santra, M. Schlipf, A. P. Seitsonen, A. Smogunov, I. Timrov, T. Thonhauser, P. Umari, N. Vast, X. Wu and S. Baroni, J. Phys.: Condens. Matter, 2017, 29, 465901 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed.
- A. Dal Corso, Comput. Mater. Sci., 2014, 95, 337–350 CrossRef CAS.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed.
- P. Giannozzi and S. Baroni, in Density-Functional Perturbation Theory, Handbook of Materials Modeling: Methods, ed. S. Yip, Springer, Netherlands, Dordrecht, 2005, pp. 195–214 Search PubMed.
- A. Kokalj, J. Mol. Graphics Modell., 1999, 17, 176–179 CrossRef CAS PubMed.
- M. J. Weida, J. M. Sperhac and D. J. Nesbitt, J. Chem. Phys., 1996, 105, 749–766 CrossRef CAS.
- C. Jansen and L. B. F. Juurlink, Front. Chem., 2023, 11 DOI:10.3389/fchem.2023.1250711.
- G. Benedek, M. Bernasconi, D. Campi, J. P. Toennies and M. J. Verstraete, in Surface Phonons: Theoretical Methods and Results, ed. M. Rocca, T. S. Rahman and L. Vattuone, Springer International Publishing, Cham, 2020, pp. 737–782 Search PubMed.
- R. Signorell, J. Chem. Phys., 2003, 118, 2707–2715 CrossRef CAS.
- C. W. McCluskey and D. S. Stoker, Raman observations of quantum interference in the ν1/2ν2Fermi dyad region of carbon dioxide, 2006, https://arxiv.org/abs/physics/0601182.
- D. Zhang, V. Virchenko, C. Jansen, J. M. Bakker, J. Meyer, A. W. Kleyn, I. M. N. Groot, O. T. Berg and L. B. F. Juurlink, J. Phys. Chem. C, 2023, 127, 24158–24167 CrossRef CAS PubMed.
- S. Ioppolo, J. A. Noble, A. Traspas Muiña, H. M. Cuppen, S. Coussan and B. Redlich, J. Mol. Spectrosc., 2022, 385, 111601 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cp02311k |
| ‡ Accordingly, the second most intense of the weaker peaks visible only in p-polarisation would be denoted P2(i) and so on. |
§ The vector ![[p with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0070_20d1.gif) is obtained by matrix multiplication of the effective charge tensor and the eigen displacements involved with a certain mode. is obtained by matrix multiplication of the effective charge tensor and the eigen displacements involved with a certain mode. |
| ¶ This is estimated from the CO2 partial pressure of 8 × 10−8 mbar reported in ref. 17. |
| This journal is © the Owner Societies 2024 |


![[a with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0061_20d1.gif)

