Photofragmentation of corannulene (C20H10) and sumanene (C21H12) cations in the gas phase and their astrophysical implications†
Pavithraa
Sundararajan
 *ab,
Alessandra
Candian
*ab,
Alessandra
Candian
 c,
Jerry
Kamer
c,
Jerry
Kamer
 ab,
Harold
Linnartz‡
ab,
Harold
Linnartz‡
 ab and
Alexander G. G. M.
Tielens
bd
ab and
Alexander G. G. M.
Tielens
bd
aLaboratory for Astrophysics, Leiden University, PO Box 9513, NL-2300, RA Leiden, The Netherlands. E-mail: sundararajan@strw.leidenuniv.nl
bLeiden Observatory, Leiden University, 2300 RA Leiden, The Netherlands
cAnton Pannekoek Institute, University of Amsterdam, Science Park 904, 1098XH Amsterdam, The Netherlands
dAstronomy Department, University of Maryland, College Park, MD 20742, USA
First published on 17th June 2024
Abstract
Aromatic infrared bands (AIBs) dominate the mid-infrared spectra of many galactic and extragalactic sources. These AIBs are generally attributed to fluorescence emission from aromatic molecules. Unified efforts from experimentalists and theoreticians to assign these AIB features have recently received additional impetus with the launch of the James Webb Space Telescope (JWST) as the Mid-InfraRed Instrument (MIRI) delivers a mid-IR spectrum with greatly increased sensitivity and spectral resolution. PAHs in space can exist in either neutral or ionic form, absorb UV photons and undergo fragmentation, becoming a rich source of small hydrocarbons. This top-down mechanism of larger PAHs fragmenting into smaller species is of utmost importance in photo-dissociation regions (PDR) in space. In this work, we experimentally and theoretically investigate the photo-fragmentation pathways of two astronomically significant PAH cations – corannulene (C20H10) and sumanene (C21H12), which are structural motifs of fullerene C60, to understand their sequential fragmentation pathways. The photo-fragmentation experiments exhibit channels that are significantly different from planar PAHs. The breakdown of the carbon skeleton is found to follow different pathways for C20H10 and C21H12 because of the number and positioning of pentagon rings, yet the most abundant low mass cations produced by these two species are found to be similar. The low mass cations showcased in this work could be of interest due to their possible astronomical detections. For completeness, the qualitative photofragmentation behaviour of dicationic corannulene and sumanene has also been investigated, but the potential energy surface of these dications is beyond the scope of this paper.
1 Introduction
AIBs are discrete emission features observed in the interstellar medium (ISM), circumstellar regions, and galactic and extragalactic sources.1 The AIBs are very characteristic of the C–C and C–H vibrational modes of polycyclic aromatic hydrocarbons (PAHs) with prominent features centered in the 3.3, 6.2, 7.7, 8.6, 11.3, and 12.7 μm regions. These bands are attributed to the IR fluorescence of UV pumped PAH species that after internal conversion and intramolecular vibrational energy distribution relax through vibrational emission. PAHs can appear in the ISM either in neutral, cationic, protonated, hydrogenated, dehydrogenated or substituted (with one or more carbon atoms replaced with atoms like nitrogen, oxygen, etc.) form.2 The wavelength and relative intensity of these astronomical AIBs are found to vary with the physical environment.3 In spite of numerous theoretical and experimental investigations for several decades to deduce the emitters of AIBs, the exact form of PAHs or the group of PAHs responsible for the emission continues to puzzle astronomers. Recent identifications of small PAHs in dark clouds through their rotational spectra have confirmed the presence of this important class of molecules in space.4PAHs play an important role in the ionization and energy balance of interstellar gas.5 In addition, observations indicate that larger PAHs (with 20 to 100 carbon atoms) harbour 10–15% of the elemental carbon in the ISM. Besides PAHs, buckminsterfullerene, C60, has also been identified in the ISM through its infrared bands using the Spitzer Space Telescope,6 and this was confirmed through its electronic transitions in the far-red.7,8 It is found that, close to stars, the abundance of C60 increases rapidly while the abundance of PAHs decreases away from stars.9,10 This has been attributed to photochemical fragmentation and isomerization processes under the influence of the strong stellar ultraviolet (UV) radiation field but the details of these processes are not yet understood. A detailed study of the bright reflection nebula, NGC 7023, has provided evidence that the evolution of the profile of the mid-IR bands is related to the chemical evolution under the effect of UV photons.3
PAHs are assumed to be formed in the envelopes of evolved stars and then to be injected in the ISM.11,12 In the past, astronomical models generally postulated that the abundance of specific PAHs in the ISM was mainly controlled by their thermodynamic properties and followed the pattern of stabilomers originally identified by Stein and Fahr (1985).13 However, under the severe conditions of space where there are strong radiation fields, photochemical pathways may control the abundances of interstellar PAHs. In this scenario, it is considered that stars inject a rich variety of PAHs – formed through processes akin to those in sooting flames – into space where they are quickly weeded down into a small set of extremely stable species by the strong radiation field. PAHs in space typically absorb 108 UV photons over their lifetime (100 Myr) and undergo destruction through a photo-fragmentation process.9 The composition of the interstellar PAH family is greatly influenced by the fragmentation process initiated by UV photon absorption as highly excited PAHs can lose H atoms, CH, and C2/C2H2 groups rather than relax through IR emission. This competition between fragmentation and radiative relaxation is largely controlled by the size of PAH.10,14–17
Recent laboratory experiments demonstrate that upon photo-excitation the fragmentation of PAHs offers a new chemical paradigm of complex, interstellar molecule formation – instead of bottom–up, where reacting smaller species form larger ones, a top-down mechanism, where fragmentation products of larger precursors occur.17,18 The observed anti-correlation between PAHs and C60 abundances in astronomical environments strongly suggests that one species forms from the other and this photochemical relationship has the potential to give us direct clues on the processes that are dominant in the evolution of interstellar PAHs. The fullerenes contain fused hexagon and pentagon rings, and pentagon formation may provide the key.19 In an intense UV radiation environment, this photochemical breakdown may also contribute to the diversity of small hydrocarbon radicals commonly observed in these regions.20 Understanding the dissociation of PAHs through photochemical excitation requires dedicated laboratory studies to identify the detailed photo-fragmentation of PAHs in space.
Neutral and cationic C60 – identified through their unique IR and visible spectrum6,7 – are the largest molecular species identified in space. The presence of pentagons in a planar graphene-flake leads to bending of the molecular structure enabling closure upon itself and the formation of a 3D structure.21,22 Pentagon formation is thus a key step in the photochemical transformation of PAHs into fullerenes. Spectroscopic evidence for pentagon formation upon PAH photo-dissociation has been reported in the laboratory for small PAH species, containing up to three aromatic rings.23–25 These experiments suggest that PAHs with only hexagonal rings could be the parent molecules (Parent-PAHs) for the formation of PAHs with pentagonal ring(s) under adequate conditions. In addition, some PAHs with pentagons may be more photochemically stable than PAHs of similar size26 and therefore represent likely candidates for the interstellar molecular inventory. However, the photochemical evolution of PAHs with pentagons is largely unexplored and the generality of this conclusion is unknown. Given the presence of C60 in space, the likely astrochemical connection of C60 and PAHs in space, and the potential photochemical stability of a pentagon containing PAHs, a focused study to improve the understanding of such species is portrayed in this work.
The PAHs with pentagons chosen for this work are: corannulene (C20H10) and sumanene (C21H12) as shown in Fig. 1. The coordinates of the optimized geometry have been provided in Fig. S1 and S2 in the ESI.† These PAHs have been selected because they represent “pentagon-variations” on the structure of the well-studied compact PAH, coronene. Moreover, corannulene and sumanene are molecular building blocks/fragments of C60 as the pentagon is connected to five hexagons in corannulene and the pentagons in sumanene are separated from each other through (partial) rings of hexagons.27 When corannulene has ten H atoms which are all sp2 hybridized, sumanene has six H atoms that are sp2 and six more H atoms that are sp3 hybridized (Fig. 1 bottom panel). This makes the chemistry of these two molecules very different from each other. The photochemistry of the Parent-PAH, coronene as well as the fullerenes C60 and C70, has been studied in detail in the gas phase, however, the buckybowls (C20H10 and C21H12) are the least explored.28,29
 | ||
| Fig. 1 Geometry of cationic (a) corannulene, C20H10+ and (b) sumanene, C21H12+ optimized using the M062x/6-311++G(3df,2pd) method. | ||
Several spectroscopic studies have been performed on corannulene to look for its presence in the interstellar medium.30–32 In particular, the diffuse interstellar bands at 6614 and 6196 Å have been proposed to arise from the electronic states of corannulene.33 Moreover, the matrix-isolated IR spectra of hub-protonated corannulene (hub-H+C21H10+) showed close resemblance with the astronomical UIR bands,34 but gas-phase spectra are required for positive astronomical detection. Because of its non-planar structure,35 corannulene possesses a large dipole moment (2.0 Debye) and its microwave spectrum has been recorded.36 A detailed model suggests that this species could be present in the Red Rectangle nebula, yet the hunt for this molecule still continues.37 The spectroscopy of sumanene is not so well constrained. The molecule has a slightly larger bowl depth (1.11 Å) than corannulene (0.87 Å) and it is closer to the curvature of the C60 structure.27,38 The presence of three sp3-hybridized benzylic sites is a unique feature in sumanene, which makes its chemistry very different from that of corannulene.39 Sumanene is expected to be an important precursor for the formation of C60via a bottom-up mechanism.
To date, a number of laboratory experiments have been dedicated to the study of the photochemistry of smaller PAHs (C < 20).20,40–42 Experimental studies of PAHs relevant to astrochemistry have widely used a time-of-flight, photo-ionization mass spectrometry (PIMS) method, focusing on reaching internal energy at which the dissociation rate is ∼104 s−1.43
In these experiments, loss channel(s) mainly involve sequential hydrogen loss followed by the opening up of the C2H2 loss channel at slightly higher internal energies. However, different molecular dynamical timescales are showcased in the ISM. A competition takes place between fragmentation of PAHs, isomerization, and relaxation through IR emission that occurs on time scales of the order of ∼0.01–1 s−1, depending on the size of the species. This work studies in detail the photofragmentation pattern of the buckybowls, the influence of the presence of pentagon rings on the photo-dissociation pattern, and the isomerization process involved in the formation of smaller fragment cations with the aid of the mass spectroscopic data obtained in the ‘instrument for photo-dynamics of PAHs’ (i-PoP) system.
2 Experiment
The experiments presented in this paper were performed on the ‘instrument for photo-dynamics of PAHs’ (i-PoP), housed at the Laboratory for Astrophysics (LfA) at Leiden observatory. The i-PoP has been described in detail in a previous paper so only a brief description is given here.18 The setup consists of two differentially pumped chambers; a source chamber that has a commercially available quadrupole ion trap (QIT, Jordan C-1251), and a detection chamber which encompasses a reflectron time-of-flight spectrometer (Jordan D-850). The molecules used in this work are commercially available from Tokyo Chemical Industry; corannulene (C20H10, >97.0% pure), and sumanene (C21H12, 99.0% pure). The samples were evaporated in the QIT chamber using a Heat Wave Labs built oven held at around 40 °C and 70 °C for corannulene and sumanene respectively. The sample vapours were ionized by electron impact ionization at 70 eV with an electron gun (EGUN, Jordan C-950), which is integrated into the QIT chamber and the resulting cations after ionization were then guided into the ion trap through an electrostatic ion gate.A 1600 V top-top RF signal was used for these experiments on the ring electrode at an operating frequency of 1.25 MHz in the ion trap to hold ions. This enables the trap to retain masses from approx. 98 amu up to several hundred amu. In an attempt to probe a much lower mass range to look for smaller cationic fragments an RF of 1200 V was also used in some experiments which pushed the lower observable limit to 73 amu. In addition, the experiments with dications uses an RF of 1000 V for observations until reaching 61 amu. Helium buffer gas was released into the center of the ion trap up to a static pressure of 1–2 × 10−6 mbar in the QIT chamber. The PAH cations were confined to the center of the ion trap through collisions with the He buffer gas and persisted there until the cations were directed from the ion trap into the time-of-flight (TOF) detection chamber.44,45 The ions in the ion trap were irradiated with a nanosecond pulsed Quanta-Ray Nd:YAG laser (DCR2A-3235) pumping a dye laser (LIOP-TEC, Quasar2-VN) which was set to deliver a particular wavelength from 610–630 nm photons, chosen depending on the molecule to be studied. The laser was horizontally guided through the ion trap and was operated at 10 Hz to irradiate the trapped ions.
The reasoning for the choice of 610–630 nm (red light) laser radiation was to scan over the potential energy surface of corannulene and sumanene cations which was the main aim of this experiment. The choice of red light from dye laser radiation allows minimization of the multiple ionization in competition with fragmentation that are known to occur with VUV photons.46,47 For instance, using a green light for photolysis would give rise to the same set of product cations. However, there are higher chances of the cations undergoing multiple ionization, making the analysis complicated. Moreover, the breakdown analysis to deduce the steps of fragmentation would not be possible as several fragment cations are produced in higher intensities at the very beginning of the laser pulses. Hence, using longer wavelength photons in a multi-photon process allowed us to obtain more information about the fragmentation pattern of the molecules in a gentler manner to explicate additional nuances in the fragmentation patterns.
The data acquisition cycle is achieved through timing sequences controlled by a high-precision delay generator (SRS DG535), which is triggered by the Q-switch timing of the Nd:YAG laser to ensure synchronization at the start of each measurement cycle. Each operation cycle involves capturing ions and filling of the ion trap, mass isolation of the parent ion, irradiation of the ion cloud with a laser, and extraction of the ions into the TOF tube. The scan cycle begins with an empty ion trap and was initiated with the opening of the ion gate which is achieved using a normal gating procedure of applying a DC voltage to a metal lens with a circular slit in the middle as an inlet for the ions, and the ion trap fills for a duration of 2.7 s. To isolate the masses of the parent PAH ions, a 25 ms (from 2.7 s to 2.95 s) long Stored Waveform Inverse Fourier Transform (SWIFT) pulse was applied to one of the end caps of the ion trap to isolate the parent species.48 Shortly afterward a re-thermalization period was given for 0.05 s (from 2.95 s to 3.00 s). It should be noted that the intense electron impact ionization source not only produced parent PAH cations but fragments due to H/H2 loss as well. For the purposes of this work, the SWIFT pulse was applied to isolate the parent cations as well as the cations corresponding to H/H2 losses from the parent (i.e., the 247–252 amu range) and efficiently filter out other spurious signals or contaminations. After the SWIFT pulse was employed, the laser beam shutter was opened, and the ion cloud was irradiated. Typically, 40 pulses and 30 pulses from the dye laser were used for corannulene and sumanene cations, respectively. At the end of each irradiation time with the specified number of pulses, the ions were accelerated out of the ion trap and into the field-free TOF region at the end of which the ions were detected using a multichannel plate (MCP) detector and digitized using a 8-bit GaGe Cobra card. The data acquisition was carried out using LABVIEW software to obtain and calibrate the mass spectrum.
For each mass spectrum, an average of 50 scans was used and before each measurement, a normalization spectrum of 15 scans was taken with the laser under the off condition. These normalization spectra were used to normalize the peak areas of the mass spectra measured with the laser on, which resulted in errors of 5%. The number of laser pulses showcased in the experiments was 40 pulses for corannulene cations and 30 pulses for sumanene cations, in steps of 2 pulses (e.g. 0, 2, 4, 6, … 30). These numbers of pulses were chosen such that most of the parent cations would be depleted in the ion trap and converted to fragments. In each case, the total cycle duration was kept constant at 6 s and 7 s for corannulene and sumanene cations respectively to ensure that all datasets are cross comparable and the only differing parameter is pulse energy and/or number of pulses.
In short, ion gate opening → trapping ions in the ion trap → mass isolation using SWIFT → photolysis by a specified number of laser pulses in a sequence (0 or 2 or 4…) → extraction of fragment ions → recording the TOF mass spectrum, is a full cycle that repeats for each measurement. The recorded mass spectrum was calibrated from the time domain to m/z using a MATLAB code. The same software was also used to deduce the peak area of each cationic peak using a Pearson type IV fit as this gave a more reliable result for peak area compared to a Gaussian fit. The obtained peak areas were normalized using the normalized scans of mass spectrum as described above, and these normalized values of peak area were used to make the breakdown diagrams presented in this paper.
3 Theory
Density functional theory (DFT) was used to explore the potential energy surfaces (PES) of corannulene and sumanene cations. The Minnesota functional M06-2x which includes dispersion in combination with a triple-zeta quality basis set – 6-311++G(3df,2pd) – was chosen, including polarisation and diffuse functions which are needed to describe accurately reaction energies and barrier heights with density functional theory.49 For example, the energy difference between vinylidene (CCH2) and acetylene (C2H2) is predicted to be 1.85 eV with this method while is calculated to be 1.86 eV at the CCSD(T)/cc-pVTZ level.50 All the calculations were performed with Gaussian C.0251 and the results were visualized with the software Molden.52 Intrinsic Reaction Coordinate (IRC) calculations were performed to check that the transition states connected the intended reactants and products. The expectation value of the total spin of the system 〈S2〉 was used as a proxy in the presence of higher spin states. In the large majority of the cases the structure with the lowest spin multiplicity (e.g. doublet for the isomers of C20H10+) is also the more stable structure and that is the one we included in the PES. Special cases are indicated in the discussion. Throughout the paper, energies are zero-point corrected and expressed in eV. Due to the large number of possible photodissociation channels, the exploration of the PES with static DFT calculations is not exhaustive, yet it can shed light on some of the fragmentation behaviours seen in the experiments.4 Results and discussion
4.1 Corannulene cation (C20H10+)
Fig. 2 shows the mass spectrum of C20H10+ obtained with the i-PoP system after applying the SWIFT pulse. The m/z = 250 corresponds to the parent cation C20H10+. The less intense peaks at m/z = 251 and 252 are due to the 13C components present in the sample. It is also observed that the electron impact on gaseous C20H10+ to ionize the neutral parent species also induces H abstraction to produce C20H9+ and C20H8+.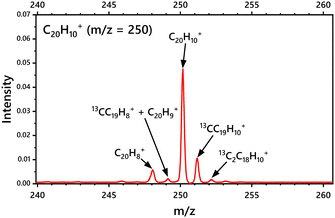 | ||
| Fig. 2 Mass spectrum of C20H10+ obtained upon electron impact using the Re-TOF instrument integrated in the i-PoP system. | ||
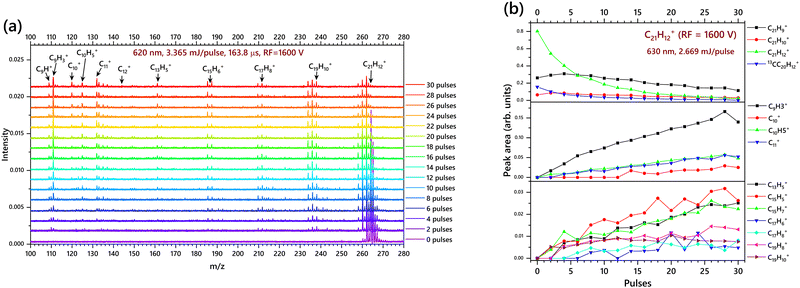
An electron energy of 70 eV and emission of 0.6 mA was used to produce a suitable amount of corannulene cations, C20H10+. Fig. 3a presents the TOF-MS of C20H10+ for the pulse energies of 2.23 mJ per pulse. The usage of red light with a pulse energy of 2.23 mJ per pulse allows to decode a number of interesting fragmentation pathways. The fragments produced upon laser irradiation shown in these figures are cationic, and their neutral counterpart fragments would not be detectable through ion trap TOF mass spectrometry. In these experiments, 50 scans were taken for each mass spectrum. Such mass spectra were obtained after 2 pulses, 4 pulses, etc. By accumulation of the fragments upon sequential laser induced fragmentation, the mass spectra are stacked to visualize the increase in fragmentation yield, as presented in Fig. 3a. The RF value used for the experiment in Fig. 3a is 1600 V which is efficient for detecting mass peaks from 98–260 m/z, which can display the CH/C2H2 loss channels in good detail.
However, unlike other medium-sized PAHs, C20H10+ loses only four H atoms before the carbon skeleton starts opening up through CH loss. In fact, the CH loss channel is one of the most dominant channels for the fragmentation of C20H10+. The CH loss fragments C19H9+, C18H8+ and C17H7+ are clearly observed in the mass spectrum:
| C20H10+(–CH) → C19H9+(–CH) → C18H8+(–CH) → C17H7+. |
We note that there could be a competition between the CH loss and C2H2 loss channel as the first C2H2 loss channel (220–228 m/z) is also getting more intense at pulse 12 and then decreases. This means that the C2H2 loss channel is possible from C20H10+ with either single or a two-step fragmentation:
| C20H10+ (–CH) → C19H9+ (–CH) → C18H8+ |
| C20H10+ (–C2H2) → C18H8+ |
After three sequential CH losses, i.e. after the parent, C20H10+, becomes C17H7+, it is not possible to lose any more CH. Fig. 3b demonstrates that after this point the C2H2 loss channel becomes dominant. The consecutive C2H2 loss can be described as:
| C17H7+(–C2H2) → C15H5+(–C2H2) → C13H3+. |
Alternately, it is also possible for C17H7+ to undergo subsequent loss of two C2 from the carbon skeleton:
| C17H7+(–C2) → C15H7+(–C2) → C13H7+. |
It is noticeable from the mass spectrum (Fig. 3a) that the cations C17H7+, C15H7+ and C13H7+ are sequentially produced after the pulses 4, 8 and 12 respectively, which demonstrates that the C2 loss is probably sequential.
Fig. 3b depicts the breakdown diagrams of the parent and product cations: the top panel shows the increase of C20H8+ upon laser irradiation up to pulse 10 and then a decrease in peak area, which could mean that this species fragments further into smaller secondary cations. Similarly in the middle panel C18H8+ and C18H6+ increases up to pulse 10 and then decreases. At this juncture (from pulse 10), the smaller cations C8H5+, C9H5+ and C11+ start to form and increase steadily. The breakdown equations predicted in this section for the origin of low mass cations (below 150 m/z) are purely based on the data obtained in the mass spectrum and the breakdown diagram. The breakdown of this corannulene cation is expected to involve several intermediate steps including H roaming, C–C bond cleavage followed by opening of the C skeleton, etc. before the formation of the observed low mass cations. The breakdown channels given below are those predicted based on the end products and do not include any intermediate or transition states. Therefore, caution must be taken in perceiving the breakdown predictions presented in this paper.
Possible breakdown mechanisms for C18H8+ could be:
| C20H10+(–C2H2) → C18H8+ → C8H5+ + C10H3, |
| C20H10+(–C2H2) → C18H8+ → C9H5+ + C9H3, |
| C20H10+(–C2H2) → C18H8+ → C11+ + C7H8. |
The C18H6+ product can be produced after two hydrogen losses followed by two consecutive CH losses, e.g.
| C20H10+(−H2/2H) → C20H8+ (–CH) |
| → C19H7+(–CH) → C18H6+. |
Or with two hydrogen losses followed by a C2H2 loss to eventually produce C8H5+, C9H5+ and C11+ with the mechanism:
| C20H10+(–H2) → C20H8+(–C2H2) → C18H6+ |
| → C8H5+ + C10H; |
| → C9H5+ + C9H; |
| → C11+ + C7H6. |
It is evident from Fig. 3b that the parent C20H10+ and its 13C component starts decreasing immediately after the laser pulses are applied; and there are few fragment cations produced right from the beginning of laser irradiation. Nevertheless, there are several fragment cations that are visible only after 6 to 10 laser pulses. This could either be because the cations visible after 6 laser pulses are initially less abundant, or, they are secondary cations (i.e. they are fragmented from the cations after H/CH/C2H2 loss of C20H10). Hence, these large cations, after CH/C2H2 losses, further fragment into smaller species upon secondary fragmentation to produce the observed low mass cations (C ≤ 11).
C11+ could also be produced directly from the parent cation upon two or more dehydrogenations of the parent, followed by ring opening and isomerization:
| C20H10+ → C20H10−n+ → C11+ + C9H10−n. |
This is because the radical sites are necessary for the ring opening and isomerization process to take place. Similar to C11+, another carbon cation C10+ is also observed as a secondary fragment only after pulse 16 (Fig. 3a):
| C20H10+(–H2/2H) → C20H8(–C2H2) → C18H6+ |
| → C10+ + C8H6 |
| C18H8+ → C18H8−n+ → C10+ + C8H8−n. |
The other intense low mass fragments like C8H3+, C8H5+, C9H3+ and C10H5+ are observed from the beginning of laser irradiation and are observed to be primary cationic fragments from the parent cation immediately after H loss:
| C20H10+ → C20H10−n+ → C9H3+ + C11H7−n |
| C20H10+ → C20H10−n+ → C10H5+ + C10H5−n |
It is evident from the breakdown diagrams in Fig. 4b (right panel) that C7H3+ and C8H5+ are formed from the very beginning of laser irradiation, e.g.
| C20H10+ → C7H3+ + C13H7 |
| C20H10+ → C8H5+ + C12H5 |
Again, C10+ and C11+ appear to be secondary products arising from pulse 10 and pulse 6 respectively (Fig. 4b). The other cationic products are in relatively lower abundance and are also produced right after the irradiation with the first two laser pulses. This could mean that an energy of 5.3 mJ is already sufficient to break the carbon skeleton of C20H10+ into two or more fragments.
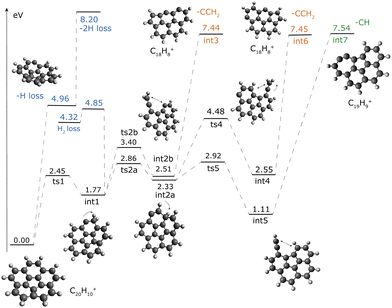
The lowest dissociation product is the dehydrogenated corannulene via H loss, that can be abstracted directly from the corannulene cation with a bond dissociation energy (BDE) of 4.96 eV or after isomerization reactions involving H roaming.§ The H roaming reactions require up to 4 eV, and each of the intermediates created (int1, Int2a and int2b, Fig. 5) can lose an H atom, all of which leads to the same final structure, i.e. singly-dehydrogenated curved corannulene (C20H9+). The BDEs for the H-shifted intermediate can be derived from the difference in energy between corannulene and singly dehydrogenated corannulene and are:
3.19 eV for int1 (loss from the aliphatic group),
2.63 eV for int2a (loss from the tertiary carbon) and
2.45 eV for int2b (loss from the vinylidene CCH2 chain).
H loss from int4 can proceed from the CCH2 chain at slightly higher BDE (3.28 eV) than for the other H-shifted isomers; this is because, in this case losing one of the aliphatic hydrogens from the chain will create a high-lying isomer of C20H9+ rather than the more stable singly-dehydrogenated corannulene structure. For int5, all the H atoms present in the molecule are aromatic (Fig. 5) and their BDE will be around 5 eV. The loss of a second H atom from the singly-dehydrogenated corannulene cation has a BDE of 3.24 eV. We also investigated the H2-loss channel from int1. The transition state is at 4.85 eV above corannulene. This behaviour and the BDEs presented in this work agree very well with previous studies on planar PAHs.55–58
Isomerization is also the first step toward the C-loss channels, which needs comparable energies of 7.4–7.5 eV (Fig. 5). Once int1 is created, the bond between two carbon atoms can be broken with a transition state (ts2b) of 3.40 eV, leading to the formation of a vinylidene group (int2b, 2.51 eV) on an almost flat molecule. From there a CCH2 unit can be released through a C–C bond dissociation with 7.44 eV of energy with respect to corannulene (4.93 eV from int2b). Interestingly, analysis of the optimized structures along the C–C dissociation curve shows that the loss of a CCH2 unit triggers the rearrangement of 2 carbon bonds in the molecule. This leads to a change in the position of the pentagon in the structure, which is now on the outside and thus leads to an almost flat structure for the C18H8+ isomer. An additional path leading to the release of a CCH2 unit goes through intermediate int2a (2.33 eV) where the H moves to the tertiary carbon atom. The breaking of a C–C bond and migration of the H atom (ts4) leads to the formation of another C20H10+ isomer with a vinylidene group (int4)¶ at 2.55 eV with respect to C20H10+. With additional 4.90 eV, int4 can lose a CCH2 unit while undergoing a carbon rearrangement similar to what was observed for CCH2 loss from int2b.
The formation of int2a can also lead to the creation of an ethynyl group (int5) through a lower transition state (ts5, 2.92 eV). Int5 is only 1.11 eV above the corannulene cation. From there, CH can be released from the molecules with 6.44 eV, leading to the formation of an almost flat isomer of C19H9+ that contains a seven-membered ring. As observed for other intermediates analysed before, the structure rearrangement (the formation of a seven-membered ring from the insertion of the remaining C atom into the six-membered ring, leading to int7) happens toward the last stages of the CH bond cleavage. Calculations show that the structure with a 7-5 membered ring is thermodynamically preferred over a 6-6 structure because the heptagon facilitates the formation of a triple bond—r = 1.23 Å—between the two dehydrogenated carbon atoms in the 7-membered ring.
 | ||
| Fig. 6 DFT optimized structures and reaction energies involved in the sequential loss of 2 CCH2 (orange solid lines), CCH2 and CH, and 3 CH loss (green dashed lines) from the corannulene cation. | ||
The bottom sequence of Fig. 5 illustrates the structures related to sequential loss of 3 CH groups from C20H10+ (green dashed arrow). Starting from C19H9+, a CH loss from the bottom right hexagon will lead to the formation of a C18H8+ isomer with two heptagons. This requires 8.15 eV of energy, just 0.7 eV higher than the BDE for the first CH loss. A third CH loss can proceed from the only hexagon left in C18H8+ with 10.28 eV, leading to the formation of a C17H7+ isomer (bottom of Fig. 6). The energy needed for this last CH loss is 2 eV higher than for the previous steps: this is likely because the ethynyl chain on C17H7+ cannot be inserted into the heptagon to create a 9-membered ring that stabilizes the structure.
Regarding the C-loss channels, the DFT calculations show that the C18H8+ peak results from CCH2 rather than acetylene loss. The investigation of the PES did not lead to any viable routes toward direct or indirect acetylene loss, even considering higher multiplicities. See the ESI† (Fig. S1) and the footnote in the previous section. The competition between the CH and CCH2 channels deduced from the experiments can be explained looking at Fig. 5, where the two channels go through int1 and have similar reaction energies. At lower internal energy the CCH2 loss happens likely from int2b, while the CH loss starts from int5. The relative importance of the two channels at different internal energies will depend on the number of possible channels (including back-reactions and further isomerization) and also on the enthalpy of formation  of the two C–H cleavage reactions, that influences the shape of the reaction rate.59 Moreover, the irradiation of corannulene cations with the dye laser produces nanosecond pulses leading to a multi-photon excitation process that eventually leads to fragmentation of the molecule involving virtual energy state(s). As the laser pulses are applied the internal energy of the molecular cation increases which leads to fragmentation following the potential energy surface. This being a non-linear photochemical process, both CH and CCH2 loss channels are produced in competition in spite of the minor energy difference of 1.5 eV (int 3 and int 7 compared to int 2a and int 5 respectively) after the H roaming and opening of the C skeleton.
of the two C–H cleavage reactions, that influences the shape of the reaction rate.59 Moreover, the irradiation of corannulene cations with the dye laser produces nanosecond pulses leading to a multi-photon excitation process that eventually leads to fragmentation of the molecule involving virtual energy state(s). As the laser pulses are applied the internal energy of the molecular cation increases which leads to fragmentation following the potential energy surface. This being a non-linear photochemical process, both CH and CCH2 loss channels are produced in competition in spite of the minor energy difference of 1.5 eV (int 3 and int 7 compared to int 2a and int 5 respectively) after the H roaming and opening of the C skeleton.
Considering further fragmentation, Fig. 6 shows that the sequential loss of two CH units or that of one CCH2 from C20H10+ leads to a population of different C18H8+ isomers. From there only CH loss is observed, leading to C17H7+, while there is no trace of C16H6+ peaks. This is different from what is observed in the fragmentation of corannulene cation, where the two channels are in competition. Calculations of the reaction energies for CH and CCH2 loss (Fig. 6 top sequence) show that CH-loss of C18H8+ requires indeed lower energy. The presence of partly dehydrogenated sites in different rings affects the energy of this reaction, because it prevents rearrangement of the structure leading to isomers with lower energy. As mentioned above, there might be a mixture of C18H8+ isomers and they will likely not follow the same photodissociation pathway. Also, we cannot exclude that additional isomerization (e.g. H-migration) might take place on some or all of these structures. The presence of two groups of C18H8+ isomers, behaving differently, could be the reason why the experiments show that this peak increases upon increasing the laser pulses and starts decreasing on further irradiation (Fig. 3a). The isomer of C18H8+, formed by two consecutive CH losses (as shown in the bottom part of Fig. 6) is expected to undergo further fragmentation to form C11+. This behaviour could be inferred from Fig. 3b and 4b, by the fact that the intensity of the C18H8+ trace does not completely drop to zero.
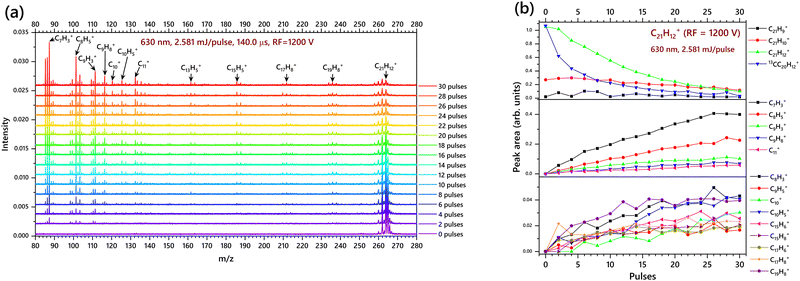
Finally, the mass spectra obtained with an RF value of 1200 V (e.g.Fig. 4a) show the presence of strong peaks corresponding to C7H3+ and C8H5+. These peaks appear very early in the fragmentation process, and it is postulated they might come directly from the parent molecule, likely after the isomerization reaction. Indeed, it has been proposed that carbon skeleton rearrangement (e.g. formation of 7-membered rings) in PAHs can lead to dissociation to form long hydrocarbon chains or carbon rings.55 Looking at Fig. 6, we note that the C17H7+ isomer at the bottom of the figure, and its parent molecule C18H8+, have a 7-membered ring with 3 H atoms. The cleavage of two carbon bonds could release a C7H3 fragment from the molecules, e.g. (without considering the charge):
| C18H8+ → C7H3+ + C11H5 |
| C17H7+ → C7H3+ + C10H4 |
In this type of experiment, it is expected that the charge of the parent molecule would remain with the largest fragment, yet a strong peak is observed for C7H3+ but not for C10H4+ or C11H5+. A possible explanation is that the large fragments mentioned above quickly break down into smaller neutral hydrocarbons in the release of C7H3+. Molecular dynamics simulations based on DFTB on PAHs have shown these multiple hydrocarbon fragmentation channels if enough energy is available.60 It could also be the case that the ionization potential of C7H3 is smaller than the remaining larger fragment. A previous study on both the cation and radical neutral version of C7H3 shows that, among the possible isomers, a three-membered carbon ring fused with the linear C4H chain is the local minimum of the PES and its ionization energy is only 1.6 eV.
A similar reasoning can be applied to the other abundant hydrocarbon, C8H5+, that can also be released from C18H8+ (Fig. 6, bottom) after consecutive cleavage of 3 C–C bonds, leaving behind a bicyclic structure made of one heptagon and one hexagon. Unfortunately, there are no studies on the properties of C8H5+ and a detailed investigation of its PES61 is beyond the scope of this paper.
4.2 Sumanene cation (C21H12+)
An electron energy of 70 eV and emission of 0.6 mA was used to produce a good amount of sumanene cations, C21H12+. Fig. 7 shows the mass spectrum of C21H12+ obtained with the i-PoP system. The m/z = 264 corresponds to the parent cation C21H12+. The moderately intense peaks at m/z = 265, 266, 267 and 268 are due to the 13C components present in the sample. The 13C isotopic abundance in the sample was estimated to be 20% by estimating their integrated band area with respect to the parent cation. It is to be noted that the natural abundance of 13C20H12 is slightly higher than that of other PAHs like corannulene or coronene. The reason behind this is still unknown as sumanene is one of the least studied PAHs to date. However, the abundance of the 13C components was reduced further to 15–17% in the photolysis experiments presented in this work by applying a narrow bandwidth SWIFT frequency. But caution must be taken in understanding the fragment cations after laser irradiation as the 13C isotopes could also contribute to them. It is also observed that the electron impact on gaseous C21H12+ to ionize the neutral parent species also induces H abstraction to produce C21H11+, C21H10+, C21H9+ and C21H8+. The mass of 263 and 261 could also arise due to the dehydrogenation of the 13C components producing 13CC20H10+ and 13CC20H8+ respectively. It is also observed that the double dehydrogenation of the sumanene cation is more favourable by comparing the peak intensity of C21H11+ and C21H10+. | ||
| Fig. 7 Mass spectrum of C21H12+ obtained upon electron impact using the Re-TOF instrument integrated into the i-PoP system. | ||
In these experiments, 50 scans were taken for each mass spectrum. Such mass spectra were obtained after 2 pulses, 4 pulses, etc. By accumulation of the fragments upon sequential laser induced fragmentation of sumanene cation, the mass spectra are stacked to visualize the increase in fragmentation yield, as presented in Fig. 8a. The RF value used in Fig. 8a is 1600 V which is efficient to detect mass peaks from 98–260 m/z. This can display the CH/C2H2 loss channels in better detail than the low mass cations. It is obvious from the mass spectrum that the C2H2 is the most dominant channel in C21H12+ fragmentation.
| C21H12+ (–C2H2) → C19H10+ (–C2H2) → C17H8+ |
| C17H10+ (–H) & (–C2H2) → C15H7+ (–C2H2) → C13H5+ |
It is to be noted that the first two C2H2 losses have an even number of H atoms and the next two C2H2 losses have an odd number of H atoms. This could be explained by the isomerization of C21H12+ after two consecutive C2H2 loss channels, as isomerization becomes the key process in the formation of such rich carbon species especially the low mass cations with m/z < 140. A carbon loss channel could also be expected here, i.e.
| C17H8+(–C2) → C15H8+ |
| C15H5+(–C2) → C13H5+. |
The C17H8+ that formed can either undergo further C2H2 loss and/or a H2 loss as shown below:
| C17H8+(–C2H2) → C15H6+ (–C2H2) → C13H4+ |
| C17H8+(–H2) → C17H6+ |
The C19H8+ cation which is observed in the mass spectrum can also be formed from the parent cation by two hydrogen losses followed by a C2H2 loss:
| C21H12+(–H2/2H) → C21H10+(–C2H2) → C19H8+. |
The C19H8+ is further found to fragment in two possible ways:
| C19H8+(–C2H2) → C17H6+ |
| C19H8+(–H2) → C19H6+. |
C10H5+ and C11+ are the second most abundant products. To get better nuances in this region experiments were performed with a lower RF value of 1200 V, as shown in Fig. 9a, where the masses below 140 m/z are intense. Here C7H3+, C8H5+ and C9H3+ are the most abundant products of fragmentation of C21H12+, which is followed by C9H8+, C10H5+ and C11+. It is evident from Fig. 9b that these low mass cations are formed from the very beginning of laser irradiation. However, the breakdown equations predicted in this section for the origin of low mass cations (below 150 m/z) are only based on the data obtained in the mass spectrum and the breakdown diagram just like the case of corannulene. The intermediate ions and transition states before the formation of the observed low mass cations are not showcased in the pathways given below.
| C21H12+ → C21H12−n + → C7H3+ + C14H9−n |
| C21H12+ → C21H12−n + → C8H5+ + C13H7−n |
| C21H12+ → C21H12−n + → C9H3+ + C12H9−n |
| C21H12+ → C21H12−n+ → C10H5+ + C11H7−n |
| C21H12+ → C21H12−n+ → C11+ + C10H12−n |
| C17H11+ → C21H12−n+ → C10+ + C7H1−n |
Since the mass visibility is more focused on the smaller cations it is clearly observed that C7H3+ and C8H5+ are the most favourable fragments of C21H12+. It is interesting to note that a laser pulse energy of 2.581 mJ per pulse is sufficient to fragment the C21H12+ to produce C7H3+ and C8H5+ just after 2 laser pulses. The other cationic fragments shown in Fig. 9b are in trace amounts; and most of them are produced at the beginning of the irradiation and keep increasing until pulse 30.
The removal of the second H atom from the pentagon where the first H was removed requires 4.25 eV, while the removal of a second hydrogen from the partly dehydrogenated hexagon requires slightly less, 4.00 eV (not in the figure). If we consider that removing a H from any of the remaining pentagons requires also around 3.95 eV (see Fig. 10), this means that there is strong competition between the different H-loss channels and the peak corresponding to C21H10+ will be made of the isomer with both the H atoms abstracted from the sp3 hybridized pentagon site. H2 can also be released from the aliphatic side with an energy barrier of 3.86 eV. As for corannulene, energetics are not enough to constrain if in the experiments we are witnessing H2 or 2H loss.
Similarities and differences between fragmentation of corannulene and sumanene cations: the initial H losses were similar for both corannulene and sumanene – more favourable even-numbered H losses, and losses up to six H atoms upon photodissociation. Corannulene cation displayed a competition between CH and C2H2 losses, whereas sumanene cation displayed only C2H2 losses. The most intense low mass cations produced by photodissociation of corannulene and sumanene were mostly similar (C7H3+, C8H5+, C9H3+, C10H5+). C11+ was observed in moderate intensity in these experiments.
The experimental and theoretical results demonstrate that peripheral H loss is more important than curvature in the photo-fragmentation process. It is evident that the amount of H loss that eventually creates two or more radical sites is crucial to determine the H roaming mechanisms. The H roaming process in corannulene and sumanene is one of the main reasons why CCH2 loss is observed in corannulene, whereas C2H2 loss is observed in sumanene.
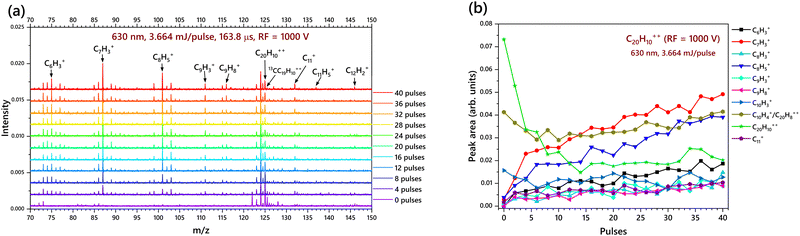
4.3 Corannulene dication (C20H10++)
The major products observed in the experiments were C6H3+, C7H3+, C8H5+, C9H3+, C9H8+ and C11+. It was not possible to observe any cations beyond 150 m/z because of the low RF value of 1000 V. The very high abundance of C6H3+ and C7H3+ suggests that C20H10++ is capable of fragmenting into more than two components, following these mechanisms:
| C20H10++ → C7H3+ + C8H5+ + C5H2 |
| C20H10++ → C6H3+ + C8H5+ + C6H2 |
| C20H10++ → C6H3+ + C7H3+ + C7H4 |
| C20H10++ → C9H3+ + C8H5+ + C3H2 |
| C20H10++ → C9H3+ + C7H3+ + C4H4 |
| C20H10++ → C9H3+ + C6H3+ + C5H4 |
| C20H10++–H2 → C20H8++ → C9H8+ + C11+ |
The breakdown equations predicted above are based on the data obtained in the mass spectrum and the breakdown diagram. The breakdown of this dication into the low mass monocations is expected to involve several intermediate steps. The equations provided do not include any intermediate or transition states, as molecular dynamics simulations alone can elucidate them. Moreover, the corannulene dications were not found to have any observable dicationic fragment (though dicationic fragments could be present in trace amounts) based on the m/z analysis. This means that the majority of the fragments are more likely to be cationic species. The structure identification, (except the structure of C7H3+ which is already known61) and the elucidation of the breakdown sequence requires sophisticated molecular dynamics calculations, which is a future scope of this research.
4.4 Sumanene dication (C21H12++)
| C21H12++ → C7H3+ + C8H5+ + C6H4 |
| C21H12++ → C6H3+ + C8H5+ + C7H4 |
| C21H12++ → C6H3+ + C7H3+ + C8H6 |
| C21H12++ → C9H3+ + C8H5+ + C4H4 |
| C21H12++ → C9H3+ + C7H3+ + C5H6 |
| C21H12++ → C9H3+ + C6H3+ + C6H6 |
The sumanene dications were not found to have any observable dicationic fragment. So, the majority of the fragments are more likely to be cationic species just like in the case of corannulene. Fig. 12b shows the breakdown diagram of the cations produced upon irradiation of C21H12++. The parent dication has the same mass as that of C11+ (m/z = 132) which cannot be resolved with the mass resolution of this TOF-MS, but is expected to be produced as one of the significant products in almost all the PAHs experimented with the i-PoP system with C < 14. Hence the trace of the peak area of the C21H12++ does not drop to zero but keeps growing after pulse 24.
5 Astrophysical implications
A clue to understanding the relationship between C60 and PAHs in space could be established by studying the molecules that could be intermediate steps leading to the formation of C60 from PAHs.17 This is where the buckybowls gain importance in astrochemistry. Theoretical calculations and molecular dynamics simulations have shown that a fully benzenoid planar PAH can undergo dehydrogenation and possess one or more radical sites, which in turn pave the way for isomerization under intense radiation fields to form pentagon rings through Stone–Wales like defects.10,13 This effect was also experimentally observed in a previous study in our laboratory where photodissociation of C66H28 produced the fullerene, C60. Such PAHs with pentagons are also suggested to be more stable under interstellar conditions.27 Alternatively, pentagons could be formed in space through CO loss from methoxy-functionalized PAHs,29 produced by photolysis of PAHs trapped in interstellar ices. In view of this, molecular dynamics of pentagon containing PAHs, such as corannulene and sumanene, becomes imperative to link the bridge between the C60 and PAHs. Moreover, these are medium-sized PAHs with C ≥ 20 that are symmetric (C5v and C3v for corannulene and sumanene respectively) and stable. Moreover, these are similar in size and structure to the fully benzenoid PAH, coronene (C24H12), which is commonly studied as a typical PAH representative in molecular astrophysics.AIBs are particularly bright in the so-called photodissociation regions (PDRs) in space. PDRs are regions in space where far-UV photons with energies between 6 and 13.6 eV penetrate molecular clouds, photo-dissociating molecules and ionizing atoms such as C and heating the gas to temperatures of 500 K. Spectroscopic observations have revealed that the characteristics of the emitting interstellar PAH family changes with location in the PDR. This has been attributed to the photochemistry and photo-isomerization produced by the penetrating far-UV photons,10,64,65 leading to the loss of functionalized groups such as CH3 and of aromatic H, and the isomerization to C60 and other fullerenes and cages. The surfaces of these PDRs also contain abundant small hydrocarbon radicals, and it has been suggested that these are the photochemically daughter products of PAHs exposed to the strong far-UV radiation field.20
This study on the photofragmentation of corannulene and sumanene demonstrates that the buckybowl cations in the photodissociation region can undergo fragmentation to produce a variety of high and low mass fragment cations, which may contribute to the rich diversity of the interstellar molecular inventory. These experiments reveal the top-down approach where large PAHs break down to form smaller hydrocarbons or carbon skeletons. Interestingly, the fragment cations like C10+, C11+, etc., produced in these experiments are expected to have ring structures and are more stable than the fragments with linear or branched structures. It is to be noted that the carbon clusters like C10+, C11+, C12+, C13+, C14+, etc., were produced predominantly for PAHs like coronene, dibenzopyrene, etc. But the buckybowl cations produce only C10+ and C11+, and only in trace amounts. This indicates the important influence of planarity on the photofragmentation of a PAH. The number of radical sites and the ring opening mechanism in the carbon skeleton are crucial in deciding the formation of such ring-structured carbon cations. The low mass cations C7H3+ and C8H5+ were produced in high abundance for both cationic and dicationic buckybowls. These small cations could be present in high abundance in the ISM. Given the intense radiation fields in the ISM, the cationic fragments C7H3+, C8H5+, C9H3+, C10H5+, C10+, C11+, etc., could potentially be the proxies to hunt for PAHs including the buckybowls (and other mid- to large-sized PAHs) in space.
The absorption spectrum of the interstellar medium contains a set of 500 absorption bands, the so-called diffuse interstellar bands (DIBs), in the near-UV to far-red range. These DIBs are generally attributed to molecular electronic absorptions.66,67 Indeed, five of these bands have been identified with the fullerene, C60+.7 The photochemical products of the PAHs in the experiments reported here could also contribute to the DIBs. Further laboratory studies are warranted to characterize the rotational, vibrational, and electronic spectroscopic properties of these species and to enable a search for them in space.
Finally, we note that, though single photon excitations dominate in space, multi-photon processes have a non-negligible probability to occur as some hundred million absorption events will happen over the lifetime of an interstellar PAH.9 As a consequence of these processes, the PAHs either emit IR fluorescence, fragment, or isomerize. This rich inventory of pentagon containing buckybowl cations, dications and their photo-fragmented low mass cations are important candidates to search for with the James Webb Space Telescope.
6 Conclusion
This work demonstrates that a diverse variety of fragment cations can be produced by photodissociation of a PAH cation in an ion trap. The TOF mass spectrum provides information about the various high and low mass cations that are produced by irradiation of corannulene and sumanene cations with a red light from a dye laser. Corannulene was found to lose up to six H atoms initially, where even H losses were found to be more favourable than the odd ones. This is followed by a competition between CH and CCH2 losses. The competition between the CH and CCH2 losses is evident in the comparable energy difference of 0.09 eV, as estimated from the calculated potential energy diagram (7.45 eV and 7.54 eV respectively). This means that the energy to lose CH and CCH2 can almost be considered equal considering the errors in the predicted energies.Sumanene also lost up to six H atoms which was clearly followed by only C2H2 losses. The potential energy diagram displays an energy difference of 2.71 eV between the C2H2 and CH losses (5.84 and 8.19 eV respectively for the C2H2 and CH losses) which is the reason why only C2H2 losses are observed in the case of sumanene. Interestingly, the most intense low mass cations produced by photodissociation of corannulene and sumanene cations were mostly similar (C7H3+, C8H5+, C9H3+, C10H5+). The photodissociation of corannulene and sumanene dications were also found to produce intense C7H3+, C8H5+, C9H3+, and C10H5+ cations, which indicates that the fragmentation process is irrespective of the charge state of a PAH. The high abundance of these small hydrocarbons in the experiments described in this paper is interesting and calls for similar experiments, down to low mass fragments, also for other PAH cations to understand if these species can indeed be the end point of the top-down interstellar chemistry of PAHs. In addition, experimental and theoretical characterisation of the spectroscopy of these species (e.g. C7H3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61) is fundamental to searching for them in the ISM.
61) is fundamental to searching for them in the ISM.
These results provide an insight into the evolution of PAHs with pentagon(s) in space. These experiments determine the vulnerability of pentagon-bearing PAHs under intense radiations under space conditions and will aid in identifying possible fragments of PAHs with pentagon rings that could be present in the ISM. The future scope of this work is to provide the IR characteristics of such species that can be searched for in the JWST data.
Author contributions
PS – conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, validation, visualization, writing – original draft, writing – review and editing. AC – conceptualization, formal analysis, investigation, methodology, validation, visualization, writing – original draft, writing – review and editing. JK – investigation, methodology, validation, software, writing – review and editing. HL – supervision, investigation, visualization, validation. AT – supervision, conceptualization, funding acquisition, investigation, validation, writing – review and editing.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
P. S. acknowledges the European Union and Horizon 2020 Postdoctoral funding awarded under the Marie Skłodowska-Curie action (grant number 101062984) H. L. acknowledges the Netherlands Research School for Astronomy (Nederlandse Onderzoekschool Voor Astronomie, NOVA) and the Netherlands Organisation for Scientific Research (Nederlandse Organisatie voor Wetenschappelijk Onderzoek, NWO). A. G. G. M. T. acknowledges the NWO for a Spinoza Prize (Spinozapremie). We thank SURF (https://www.surf.nl) for the support in using the National Supercomputer Snellius.References
- A. Candian, J. Zhen and A. G. Tielens, Phys. Today, 2018, 71, 38–43 CrossRef CAS.
- E. Peeters, C. Mackie, A. Candian and A. G. Tielens, Acc. Chem. Res., 2021, 54, 1921–1933 CrossRef CAS PubMed.
- C. Joblin, A. Tielens, O. Berné and P. Pilleri, EAS Publ. Ser., 2011, 46, 49–54 CrossRef.
- B. A. McGuire, R. A. Loomis, A. M. Burkhardt, K. L. K. Lee, C. N. Shingledecker, S. B. Charnley, I. R. Cooke, M. A. Cordiner, E. Herbst and S. Kalenskii, et al. , Science, 2021, 371, 1265–1269 CrossRef CAS PubMed.
- A. G. Tielens, From Gas to Stars to Dust, 1995 Search PubMed.
- J. Cami, J. Bernard-Salas, E. Peeters and S. E. Malek, Science, 2010, 329, 1180–1182 CrossRef CAS PubMed.
- E. K. Campbell, M. Holz, D. Gerlich and J. P. Maier, Nature, 2015, 523, 322–323 CrossRef CAS PubMed.
- E. K. Campbell, Mol. Phys., 2020, 118, e1797918 CrossRef.
- A. G. Tielens, The physics and chemistry of the interstellar medium, Cambridge University Press, 2005 Search PubMed.
- O. Berné and A. G. Tielens, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 401–406 CrossRef PubMed.
- M. Frenklach and E. D. Feigelson, Astrophys. J., 1989, 341, 372–384 CrossRef CAS.
- I. Cherchneff, J. R. Barker and A. G. Tielens, Astrophys. J., 1992, 401, 269–287 CrossRef CAS.
- S. E. Stein and A. Fahr, J. Phys. Chem., 1985, 89, 3714–3725 CrossRef CAS.
- O. Berné, J. Montillaud and C. Joblin, Astron. Astrophys., 2015, 577, A133 CrossRef PubMed.
- B. Croiset, A. Candian, O. Berné and A. Tielens, Astron. Astrophys., 2016, 590, A26 CrossRef.
- B. J. West, L. Lesniak and P. M. Mayer, J. Phys. Chem. A, 2019, 123, 3569–3574 CrossRef CAS PubMed.
- J. Zhen, P. Castellanos, D. M. Paardekooper, H. Linnartz and A. G. Tielens, Astrophys. J. Lett., 2014, 797, L30 CrossRef CAS.
- J. Zhen, D. Paardekooper, A. Candian, H. Linnartz and A. Tielens, Chem. Phys. Lett., 2014, 592, 211–216 CrossRef CAS.
- N. Ota, arXiv, 2017, preprint, arXiv:1704.06197 DOI:10.48550/arXiv.1704.06197.
- J. Pety, D. Teyssier, D. Fossé, M. Gerin, E. Roueff, A. Abergel, E. Habart and J. Cernicharo, Astron. Astrophys., 2005, 435, 885–899 CrossRef CAS.
- T. Chen and Y. Wang, Astron. Astrophys., 2020, 644, A146 CrossRef CAS.
- H. Kroto, Astrophys. J., 1987, 314, 352–356 CrossRef CAS.
- J. Bouwman, A. J. de Haas and J. Oomens, Chem. Commun., 2016, 52, 2636–2638 RSC.
- A. J. de Haas, J. Oomens and J. Bouwman, Phys. Chem. Chem. Phys., 2017, 19, 2974–2980 RSC.
- S. Banhatti, D. B. Rap, A. Simon, H. Leboucher, G. Wenzel, C. Joblin, B. Redlich, S. Schlemmer and S. Brünken, Phys. Chem. Chem. Phys., 2022, 24, 27343–27354 RSC.
- S. P. Ekern, A. G. Marshall, J. Szczepanski and M. Vala, J. Phys. Chem. A, 1998, 102, 3498–3504 CrossRef CAS.
- G. NarahariáSastry, et al. , J. Chem. Soc., Perkin Trans. 2, 1993, 1867–1871 Search PubMed.
- J. Oomens, B. G. Sartakov, A. Tielens, G. Meijer and G. von Helden, Astrophys. J., 2001, 560, L99 CrossRef CAS.
- J. Zhen, P. Castellanos, H. Linnartz and A. G. Tielens, Mol. Astrophys., 2016, 5, 1–8 CrossRef.
- M. Gatchell, P. Martini, F. Laimer, M. Goulart, F. Calvo and P. Scheier, Faraday Discuss., 2019, 217, 276–289 RSC.
- G. Rouillé, C. Jäger, M. Steglich, F. Huisken, T. Henning, G. Theumer, I. Bauer and H.-J. Knölker, ChemPhysChem, 2008, 9, 2085–2091 CrossRef PubMed.
- C. Pérez, A. L. Steber, A. M. Rijs, B. Temelso, G. C. Shields, J. C. Lopez, Z. Kisiel and M. Schnell, Phys. Chem. Chem. Phys., 2017, 19, 14214–14223 RSC.
- L. Bernstein, R. Shroll, G. Galazutdinov and Y. Beletsky, Astophys. J., 2018, 859, 174 CrossRef.
- P. Sundararajan, M. Tsuge, M. Baba and Y.-P. Lee, ACS Earth Space Chem., 2018, 2, 1001–1010 CrossRef CAS.
- I. Weber, M. Tsuge, P. Sundararajan, M. Baba, H. Sakurai and Y.-P. Lee, J. Phys. Chem. A, 2022, 126, 5283–5293 CrossRef CAS PubMed.
- F. J. Lovas, R. J. McMahon, J.-U. Grabow, M. Schnell, J. Mack, L. T. Scott and R. L. Kuczkowski, J. Am. Chem., 2005, 127, 4345–4349 CrossRef CAS PubMed.
- P. Pilleri, D. Herberth, T. Giesen, M. Gerin, C. Joblin, G. Mulas, G. Malloci, J.-U. Grabow, S. Brünken and L. Surin, et al. , Mon. Not. R. Astron. Soc., 2009, 397, 1053–1060 CrossRef CAS.
- R. G. Lawton and W. E. Barth, J. Am. Chem., 1971, 93, 1730–1745 CrossRef CAS.
- T. Amaya and T. Hirao, Chem. Rec., 2015, 15, 310–321 CrossRef CAS PubMed.
- C. Lifshitz, Int. Rev. Phys. Chem., 1997, 16, 113–139 Search PubMed.
- I. Garkusha, J. Fulara, P. J. Sarre and J. P. Maier, J. Phys. Chem. A, 2011, 115, 10972–10978 CrossRef CAS PubMed.
- C. Joblin, L. D'Hendecourt, A. Leger and D. Defourneau, The First Symposium on the Infrared Cirrus and Diffuse Interstellar Clouds, 1994, p. 291.
- H. Jochims, H. Baumgärtel and S. Leach, Astrophys. J., 1999, 512, 500 CrossRef CAS.
- N. A. Sassin, S. C. Everhart, J. I. Cline and K. M. Ervin, J. Chem. Phys., 2008, 128, 234305 CrossRef PubMed.
- K. Gulyuz, C. N. Stedwell, D. Wang and N. C. Polfer, Rev. Sci. Instrum., 2011, 82, 054101 CrossRef PubMed.
- J. Zhen, S. R. Castillo, C. Joblin, G. Mulas, H. Sabbah, A. Giuliani, L. Nahon, S. Martin, J.-P. Champeaux and P. M. Mayer, Astrophys. J., 2016, 822, 113 CrossRef PubMed.
- G. Wenzel, C. Joblin, A. Giuliani, S. R. Castillo, G. Mulas, M. Ji, H. Sabbah, S. Quiroga, D. Peña and L. Nahon, Astron. Astrophys., 2020, 641, A98 CrossRef CAS PubMed.
- V. M. Doroshenko and R. J. Cotter, Rapid Commun. Mass Spectrom., 1996, 10, 65–73 CrossRef CAS PubMed.
- B. J. Lynch, Y. Zhao and D. G. Truhlar, J. Phys. Chem. A, 2003, 107, 1384–1388 CrossRef CAS.
- N.-y Chang, M.-y Shen and C.-h Yu, J. Chem. Phys., 1997, 106, 3237–3242 CrossRef CAS.
- M. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb and J. Cheeseman, et al., Gaussian 16, Rev. C.01, Gaussian Inc., Wallingford, CT, 2016 Search PubMed.
- G. Schaftenaar and J. H. Noordik, J. Comput.-Aided Mol. Des., 2000, 14, 123–134 CrossRef CAS PubMed.
- H. R. Hrodmarsson, J. Bouwman, A. G. Tielens and H. Linnartz, Int. J. Mass Spectrom., 2022, 476, 116834 CrossRef CAS.
- R. E. March and J. F. Todd, Quadrupole ion trap mass spectrometry, John Wiley & Sons, 2005 Search PubMed.
- S. Panchagnula, J. Kamer, A. Candian, H. Hrodmarsson, H. V. J. Linnartz, J. Bouwman and A. G. G. M. Tielens, Phys. Chem. Chem. Phys., 2024 10.1039/d4cp01301h.
- P. Castellanos, A. Candian, J. Zhen, H. Linnartz and A. Tielens, Astron. Astrophys., 2018, 616, A166 CrossRef.
- G. Trinquier, A. Simon, M. Rapacioli and F. X. Gadéa, Mol. Astrophys., 2017, 7, 37–59 CrossRef.
- B. West, F. Useli-Bacchitta, H. Sabbah, V. Blanchet, A. Bodi, P. M. Mayer and C. Joblin, J. Phys. Chem. A, 2014, 118, 7824–7831 CrossRef CAS PubMed.
- T. Baer and P. M. Mayer, J. Am. Soc. Mass Spectrom., 1997, 8, 103–115 CrossRef CAS.
- A. Simon, M. Rapacioli, G. Rouaut, G. Trinquier and F. X. Gadéa, Philos. Trans. R. Soc., 2017, 375, 20160195 CrossRef PubMed.
- A. Chakraborty, J. Fulara, R. Dietsche and J. P. Maier, Phys. Chem. Chem. Phys., 2014, 16, 7023–7030 RSC.
- B. West, S. R. Castillo, A. Sit, S. Mohamad, B. Lowe, C. Joblin, A. Bodi and P. M. Mayer, Phys. Chem. Chem. Phys., 2018, 20, 7195–7205 RSC.
- J. Szczepanski, J. Banisaukas, M. Vala, S. Hirata and W. R. Wiley, J. Phys. Chem. A, 2002, 106, 6935–6940 CrossRef CAS.
- C. Joblin, A. Tielens, L. Allamandola and T. Geballe, Astrophys. J., 1996, 458, 610 CrossRef CAS PubMed.
- R. Chown, A. Sidhu, E. Peeters, A. G. Tielens, J. Cami, O. Berné, E. Habart, F. Alarcón, A. Canin and I. Schroetter, et al. , Astron. Astrophys., 2024, 685, A75 CrossRef.
- A. G. Tielens and T. P. Snow, The diffuse interstellar bands, Springer Science & Business Media, 2012, vol. 202 Search PubMed.
- N. Cox and J. Cami, Proc. Int. Astron. Union., 2013, 9, 412–415 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cp01247j |
| ‡ Prof. Harold Linnartz sadly passed away on 31st December 2023. |
| § In the rest of this paper we use the term H roaming and H migration interchangeably. |
| ¶ Optimising int4 with quadruplet multiplicity leads to an alternative structure with a –CH–CH group at 4.24 eV with respect to the corannulene (1.69 eV higher than int4 in the doublet spin state (not in the figure). From there we found a transition state leading to the loss of C2H2 with a barrier of 6.36 eV. However, the IRC calculation was inconclusive and the products (C18H8+ + C2H2) lie at 7 eV, higher than the transition state, making this path unlikely. |
| This journal is © the Owner Societies 2024 |