Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Isomer-specific photofragmentation of C3H3+ at the carbon K-edge†
Simon
Reinwardt
 a,
Patrick
Cieslik
a,
Patrick
Cieslik
 a,
Ticia
Buhr
a,
Ticia
Buhr
 b,
Alexander
Perry-Sassmannshausen
b,
Alexander
Perry-Sassmannshausen
 b,
Stefan
Schippers
b,
Stefan
Schippers
 b,
Alfred
Müller
b,
Alfred
Müller
 b,
Florian
Trinter
b,
Florian
Trinter
 c and
Michael
Martins
c and
Michael
Martins
 *a
*a
aInstitut für Experimentalphysik, Universität Hamburg, Luruper Chaussee 149, 22761 Hamburg, Germany. E-mail: michael.martins@uni-hamburg.de
bI. Physikalisches Institut, Justus-Liebig-Universität Gießen, Leihgesterner Weg 217, 35292 Gießen, Germany
cMolecular Physics, Fritz-Haber-Institut der Max-Planck-Gesellschaft, Faradayweg 4-6, 14195 Berlin, Germany
First published on 8th May 2024
Abstract
Individual fingerprints of different isomers of C3H3+ cations have been identified by studying photoionization, photoexcitation, and photofragmentation of C3H3+ near the carbon K-edge. The experiment was performed employing the photon-ion merged-beams technique at the photon-ion spectrometer at PETRA III (PIPE). This technique is a variant of near-edge X-ray absorption fine-structure spectroscopy, which is particularly sensitive to the 1s → π* excitation. The C3H3+ primary ions were generated by an electron cyclotron resonance ion source. C3Hn2+ product ions with n = 0, 1, 2, and 3 were observed for photon energies in the range of 279.0 eV to 295.2 eV. The experimental spectra are interpreted with the aid of theoretical calculations within the framework of time-dependent density functional theory. To this end, absorption spectra have been calculated for three different constitutional isomers of C3H3+. We find that our experimental approach offers a new possibility to study at the same time details of the electronic structure and of the geometry of molecular ions such as C3H3+.
1 Introduction
During the last decades, an increasing number of molecular species has been discovered in the interstellar medium (ISM) and in planetary atmospheres.1 These findings include a variety of carbon-containing and organic molecules ranging from small ones such as the formyl radical, HCO,2 cyclopropenylidene, c-C3H2,3 or benzene, c-C6H6,4,5 to fullerenes.Small molecules such as C3H3 are interesting candidates as precursors or intermediates for the formation of c-C6H66,7 and further to polyaromatic hydrocarbons (PAHs). The corresponding cation C3H3+ has different constitutional isomers, which cannot be distinguished with a mass spectrometer, but the constitutional isomeric form is important for further reactions. The cyclopropenyl cation, c-C3H3+, was previously detected by the Cassini mission using a mass spectrometer8 in the atmosphere of Titan, the largest moon of Saturn. The linear H2C–C–CH+ was found in the interstellar medium through radio astronomy.9 Missing information about the structure and the associated reactivity leads to significant problems in understanding subsequent ion–neutral–molecule reactions,10–12 which in turn are important to model the formation of chemical compounds in space and in the atmospheres of celestial bodies.13
c-C3H3+ is the structurally simplest aromatic molecule. Due to the high chemical importance of aromatic molecules, computer models simulating the aromaticity are steadily improving, which leads to the development of aromatic systems that are adapted to certain applications.14,15 A possible application for c-C3H3+ could be the integration into hybrid halide perovskite architectures, which may be used as a photovoltaic material.16 C3H3+ is difficult to study in isolation due to its charge. Solutions to the problem are to investigate the ion in an ion trap or a free ion beam moving in a vacuum with a kinetic energy of several keV and selecting it by mass-spectrometric techniques. In order to identify the constitutional isomers and specifically their geometry, a further examination method is required.
Since c-C3H3+ does not have a dipole moment, its detection by infrared spectroscopy is very difficult and could not be accomplished yet.7 Therefore, the dissociation of C3H3+, attached with various inert ligands has been studied by infrared spectroscopy utilizing vibrational states of molecular bonds in order to determine the C3H3+ geometrical structure.7,17–21 The neutral C3Hx (x = 0–3) species were investigated by photoelectron spectroscopy.22,23 It can be assumed that the spectroscopic properties of the molecular ions differ significantly from the properties of the corresponding neutral molecules. This has been shown, e.g., for the neutral c-C6H6 and the singly charged c-C6H6+ molecules.24 In the present study, near-edge X-ray absorption fine-structure spectroscopy (NEXAFS) at the C 1s edge is applied. NEXAFS has the advantage to be site- and element-specific. It is particularly sensitive to double bonds as well as aromaticity as shown, e.g., by Kolczewski et al.25
A powerful technique to study molecular ions with soft X-rays is the merged-beams technique, in which an ion beam is merged with a photon beam over a certain distance.26–28 Using this technique the electronic structure of the unoccupied states can be studied.29–32 Moreover, it allows one to measure fast dynamic dissociation processes, which take place on a time scale of a few femtoseconds.31
In this paper, we present the X-ray absorption spectra of a mixture of three different C3H3+ isomers at the C 1s edge by measuring the ion yield of selected photofragmentation channels using the merged-beams technique. The experimental spectra are compared to TDDFT calculations to disentangle the contributions of the three isomers.
2 Experiment
The experiment was performed using the photon-ion end-station at the synchrotron radiation source PETRA III (PIPE).33,34 The setup is a permanently installed instrument at the soft X-ray beamline P04 at DESY in Hamburg, Germany.35 It allows one to measure cross sections in a wide range of eight orders of magnitude from (2 ± 1)b36 up to several hundred Mb37 or even Gb38 with the merged-beams technique. The beamline was operated using a 400 lines mm−1 grating and an exit-slit opening of 500 μm. With this exit-slit width a photon-energy spread of 0.65 eV and a photon flux of 3 × 1013 s−1 at 280 eV photon energy were obtained. During the measurement, the photon flux was permanently monitored by a calibrated photodiode. The photon-energy scale was calibrated by a CO photoabsorption measurement at the C 1s → 2pπ* resonance. The photon-energy axis has been shifted such that the resonance lies at the literature value of (287.31 ± 0.05) eV.39The ions for the experiment were generated from butane gas in the hot plasma of a 10 GHz electron cyclotron resonance ion source.40 The pressure inside the ion source was 3 × 10−2 Pa. Due to this rather low pressure, only fragmentation products were generated from the neutral target gas in the plasma and reactive collisions or cooling of the molecular ions can be neglected. The ions were extracted by an applied voltage of 6 kV between the ion source and the puller electrode. The ion beam was focused by electrostatic lenses and a specific mass-over-charge ratio was selected by the proper setting of a dipole magnet. A specific constitutional isomer of the molecular ions could not be selected by the dipole magnet. The ion beam, which contained several energetically favorable constitutional isomers, was steered onto the axis of the photon beam by using electrostatic deflectors. The length of the mutual overlap of the photon beam with the ion beam was 1.7 m. In order to ensure a low background from ion-residual-gas collisions, the pressure in the ion-beam and photon-beam merging area was kept at 5 × 10−8 Pa. Using a second double-focusing dipole magnet with a bending radius of 0.6 m, the photoionization products were selected by a 90° deflection from the primary ion beam and other ionic products. In order to further reduce the background, for example generated from stray electrons, the photoions were deflected out of the collision plane by a 180° electrostatic deflector and then counted with a single-particle detector using a channeltron.41,42 The dark-count rate of the channeltron was 20 mHz. To improve the overlap of the merged beams, apertures on the entry side and on the exit side of the interaction area were adjusted to match the ion-beam size to the photon-beam size. The primary ion current was permanently monitored using a large Faraday cup inside the demerging magnet. A current of about 11 nA was obtained for the C3H3+ cations. The background resulting from ion-residual-gas collisions was determined by measuring the count rate without the photon beam using a photon-beam shutter. Relative cross sections were obtained by dividing the difference between the count rates with photons and without photons by the photon flux, which was recorded by a calibrated photodiode.
In our experiment, we have measured the doubly charged fragments C3Hn2+. Following a resonant C 1s excitation, the C3H3+ core-excited molecule decays via the Auger–Meitner process. This mainly produces the dication C3H32+, as the probability for a double Auger process is only on the order of 3%.43 The dication can fragment by neutral-hydrogen elimination, which produces the observed C3Hn2+ fragments. In the case of positively charged hydrogen elimination (H+ or H2+), the C3Hn photofragments will only be singly charged or even neutral and cannot be detected in our setup. Following an unlikely double ionization, a doubly charged fragment might be formed together with a charged hydrogen ion. This would result in a Coulomb repulsion and the C3Hn2+ ion would acquire a kinetic energy on the order of 0.76 eV. This small additional kinetic energy does not affect the detection efficiency for the heavy fragment. The acceptance of the detector is sufficiently large for the detection of ions with such a small additional (or reduced) kinetic energy. Thus, we conclude that practically all produced dicationic fragments were recorded in our experimental setup with equal detection probability.
3 Computations
The calculated photoabsorption spectra were computed using time-dependent density functional theory (TDDFT). The calculations were performed with the quantum-chemistry program ORCA44,45 (version 5.0.3). The first three lowest-energy conformers as determined by Cameron et al.46 were selected as possible geometries. In addition to the singlet cyclopropenyl, c-C3H3+, these are the singlet propargylium cation, [H2C–C–CH]+, and the triplet 1-propenyl cation, [H3C–C–C]+. The Becke '88 exchange and Perdew '86 correlation (BP86)47 functionals were applied together with the def2-TZVP(-f) basis set.48,49 Other local and gradient-corrected functionals such as Becke '88 exchange and Lee–Yang–Parr correlation (BLYP) or Perdew–Burke–Erzerhoff GGA (PBE) were also tried and very similar results were obtained. Hence, we decided to use the Becke '88 exchange and Perdew '86 correlation (BP86)47 functionals. In order to get a more accurate description, 10 s- and 10 p-type functions per atom according to the procedure described by Kaufmann et al.50 were added to the set of basis functions. For the calculation of the transition states between c-C3H3+ and [H2C–C–CH]+ in Fig. 5, the nudged elastic band with transition state (NEB-TS) optimization was used.51In the cyclic geometry, all carbon atoms are equivalent, i.e., it makes no difference which C 1s electron is excited. In order to generate the energetic degeneracy of the three C 1s orbitals for the localized C 1s core excitation, the function ‘XASLoc’ of ORCA44,45 was applied in the calculation. The calculated photoabsorption spectra were convoluted with a Voigt function consisting of a Lorentzian contribution with a full width at half maximum (FWHM) of 0.08 eV and a Gaussian contribution with a FWHM of 0.65 eV to take into account the core–hole lifetime and the experimental broadening, respectively. The calculated energies were shifted by Eshift = 15.83 eV such that the computed energy of the 1s → π* resonance of the c-C3H3+ ions agrees with the measured one. This resonance corresponds to the transition from the C 1s level to the lowest unoccupied molecular orbital (LUMO). For the triplet state a slightly different shift of Eshift = 15.6 eV was used, such that the measured peak C in Fig. 1(a) matches the computed resonance 3.3 in Fig. 1(d). The used functional gives a slightly too large energy spread of the resonant C 1s structures. For a better comparison with the experimental data we have slightly compressed the calculated spectra centered at resonance 1 of the c-C3H3+ spectrum by:
| Ecompressed = fcompression(Eabinitio + Eshift − Eres.1) + Eres.1 | (1) |
 | ||
| Fig. 1 The ionization channels C3H3+ → C3Hn2+ with n = 0–3 are shown in panel (a). In panel (b), the calculated spectrum for the cyclic isomer is shown. Since all carbon atoms are equal in this geometry, the summed spectrum for the identical atoms is provided. The theoretical resonance feature 1 is due to the excitation into the LUMO. Panel (c) shows the individual and the summed carbon spectra for the [H2C–C–CH]+ singlet geometry, which energetically lies 1.1 eV above the cyclic geometry (see Fig. 3(b)). Panel (d) displays the spectra for the [H3C–C–C]+ triplet geometry, which energetically lies 4.7 eV above the cyclic geometry [see Fig. 3(b)]. Since the three carbon atoms of the linear geometries are distinguishable, the absorption spectra for each carbon atom are shown individually in panels (c) and (d). The first digit of the resonance labels describes the atom and the second the excited orbital [see also Fig. 3]. In order to line up the theoretical with the experimental resonance structures, the theoretical energy scales in panels (b)–(d) have been adjusted as described in Section 3. | ||
4 Results
In Fig. 1(a), the sum of all recorded photon-energy-dependent ion yields is depicted. The sum has been obtained by adding the measured ion yield channels C3H3+ → C3Hn2+ with n = 0–3 shown in Fig. 2. The spectrum is dominated by the single ionization C3H3+ → C3H32+ channel. The C3H3+ → C3H22+ channel with single hydrogen loss is smaller by a factor of two compared to direct single ionization. The channels with double or triple hydrogen loss are smaller by at least one order of magnitude. The spectrum in Fig. 1(a) consists of three resonance features, A, B, and C at 282.9 eV, 285.7 eV, and 287.1 eV, respectively. Another broad structure is observed around 292 eV. Peak A at 282.9 eV photon energy has an asymmetric shape, which is an indication that it is a blend resulting from several individual resonance transitions. The main peak B at 285.7 eV photon energy exhibts an asymmetric shape. It is about a factor of three larger than peak A and partially overlaps with the smaller peak C.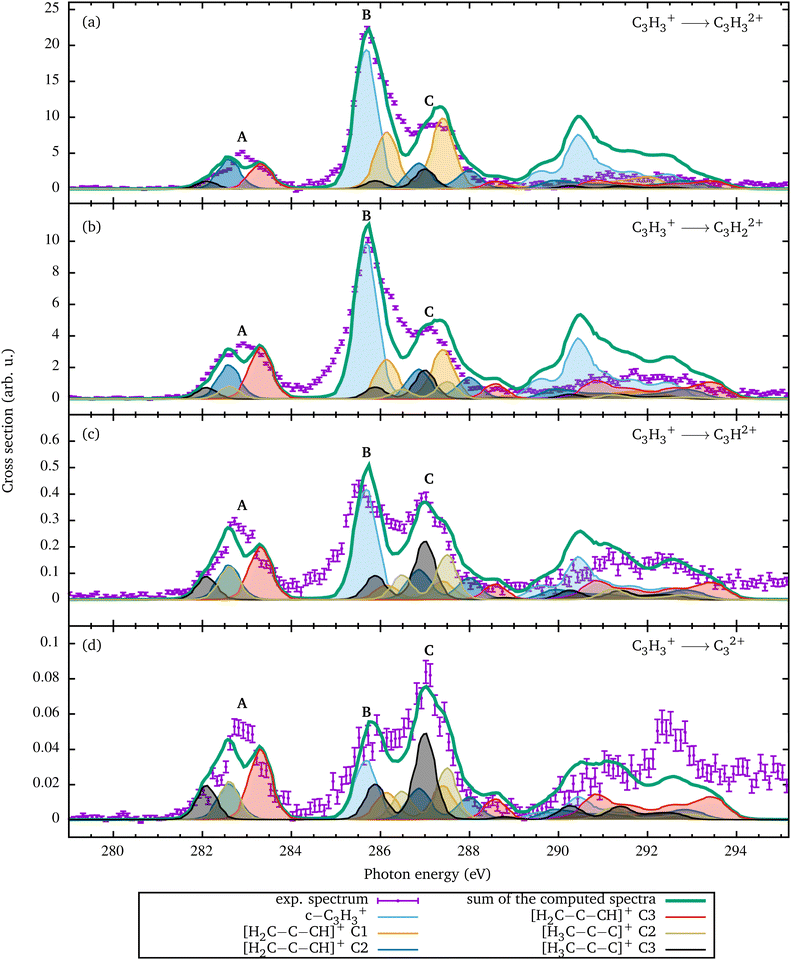 | ||
| Fig. 2 Relative cross sections for single ionization of C3H3+ [panel (a)] and for fragmentation leading to the formation of C3H22+ [panel (b)], C3H2+ [panel (c)], and C32+ [panel (d)]. Since the experimental spectra contain contributions from different geometries, these relative contributions were determined by a fit of the coefficients ai of eqn (2). The renormalized individual contributions are shown by the colored curves with shading. Their sum is the thick green curve. Since the [H3C–C–C]+ C1 conformation does not show any resonance in this energy range, it is not included in the fit. The relative contributions resulting from the fit are shown in Fig. 4. | ||
The ion yields resulting from single ionization and from the loss of up to three hydrogen atoms are shown individually in Fig. 2. These reaction channels can be described as
| C3H3+ + hν → C3Hn2+ + (3 − n)H + e−, | (2) |
To analyze the experimental spectra, the C 1s absorption spectra for the three constitutional isomers of C3H3+ were calculated using the computational methods described above. The results are depicted in Fig. 1(b)–(d). In addition, the resonant excitation energies to the singly occupied molecular orbitals (SOMOs) and the first LUMO(+k) (k = 0–2) orbitals are provided in Table 1. Since the two SOMOs of the triplet H3C–C–C+ are energetically degenerate, we do not distinguish between them. The SOMO and all depicted LUMO(+k) orbitals have a π*-like character (see Table 2). The calculated spectrum for the c-C3H3+ ion is shown in Fig. 1(b). For the [H3C–C–C]+ isomer, the triplet geometry was chosen because this state is closer by 1.5 eV to the cyclic geometry than the singlet geometry. The c-C3H3+ cation has the largest binding energy and the two isomers have lower binding energies, lower by 1.1 eV for the [H2C–C–CH]+ singlet geometry and about 4.7 eV for the [H3C–C–C]+ triplet geometry as depicted in Fig. 3(b).
| Geometry | Resonance | Energy (eV) | Excited orbital |
|---|---|---|---|
| c-C3H3+ | 1 | 285.6 | LUMO |
| 2 | 290.5 | σ* | |
| [H2C–C–CH]+ | 1.1 | 281.7 | LUMO |
| 1.2 | 286.0 | LUMO+1 | |
| 1.3 | 287.5 | LUMO+2 | |
| 2.1 | 282.7 | LUMO | |
| 2.2 | 287.0 | LUMO+1 | |
| 2.3 | 288.1 | LUMO+2 | |
| 3.1 | 283.4 | LUMO | |
| 3.2 | 287.4 | LUMO+1 | |
| 3.3 | 288.7 | LUMO+2 | |
| [H3C–C–C]+ | 1.1 | 280.7 | SOMO |
| 1.2 | 289.0 | σ* | |
| 2.1 | 282.5 | SOMO | |
| 2.2 | 286.4 | LUMO | |
| 2.3 | 287.4 | β LUMO | |
| 3.1 | 282.0 | SOMO | |
| 3.2 | 285.8 | LUMO | |
| 3.3 | 286.9 | β LUMO | |
The equivalence of the carbon atoms in the cyclic geometry of the C3H3+ ion results in almost identical transitions for the three C atoms in the c-C3H3+ ion [Fig. 3(a)]. Yet, the calculations show a small difference in the C 1s orbital energies. This small difference is due to the antisymmetric C 1s based wave function of the three identical carbon atoms in c-C3H3+ and results in a small splitting of the three corresponding C 1s energy levels. One C 1s eigenstate has a 60 meV higher binding energy than the other. This is equivalent to the gerade–ungerade splitting of the N2 1s energy levels.52 This difference has a negligible effect on the calculated spectrum as can be seen in the stick spectrum at the resonance labeled with 1 in Fig. 1(b). The low-energy C 1s based state is due to a  state with no node and two degenerate 1e′ states with one node. The LUMO state has an e′′ symmetry. Hence, the excitation from the
state with no node and two degenerate 1e′ states with one node. The LUMO state has an e′′ symmetry. Hence, the excitation from the  to the e′′ state is much weaker because the molecular orbitals do not have the same symmetry. In addition to the main resonance, there is a much smaller contribution to peak 1, which is associated with this small difference. With this, the main peak B in Fig. 1(a) can be assigned to an excitation of a C 1s electron to the c-C3H3+ π* LUMO. The group of resonances around 290 eV can be assigned to σ* core-excited energy levels.
to the e′′ state is much weaker because the molecular orbitals do not have the same symmetry. In addition to the main resonance, there is a much smaller contribution to peak 1, which is associated with this small difference. With this, the main peak B in Fig. 1(a) can be assigned to an excitation of a C 1s electron to the c-C3H3+ π* LUMO. The group of resonances around 290 eV can be assigned to σ* core-excited energy levels.
Fig. 1(c) shows the spectrum for the linear isomer [H2C–C–CH]+ whose binding energy is smaller by 1.1 eV than that of the c-C3H3+ isomer. In contrast to the cyclic geometry, the three C 1s orbitals have substantially different binding energies. This results in site-specific C 1s excitation energies as depicted in Fig. 3(a). The TDDFT calculation permits us to disentangle the contributions by each of the three C 1s orbitals. The transitions are designated as X.Y with X being the number of the carbon atom and Y the excited LUMO–(Y−1) orbital.
In principle, there are 9 possible transitions for [H2C–C–CH]+ in the experimental energy range. Fig. 1(c) clearly shows that the present experimental resolving power permits us to discriminate between the different site-specific resonance positions. The TDDFT calculation shows that transitions 1.1 and 3.2 are extremely weak. Therefore, they are missing, and the two resonances near 283 eV in Fig. 3(a) can be assigned to an excitation of a C 1s electron originating from the C atoms 2 and 3 into the LUMO. The excitations from the carbon atom 1 appear at higher photon energies of 285.9 eV and 287.4 eV, which correspond to excitations into the LUMO+1 and LUMO+2 states, respectively.
Fig. 1(d) depicts the spectrum for the constitutional isomer [H3C–C–C]+. Again 9 transitions are possible [see Fig. 3(a)]. For the carbon atom C1, only the 1.1 excitation into the SOMO is visible. The excitations to the LUMOs cannot be seen in the calculated spectrum, since the associated cross sections are too small. Resonance 1.2 is most likely a resonance structure from excitations into σ*-like non-bonding states with a much larger width and therefore not visible. The C2 and C3 atoms show very similar spectra, which are shifted with respect to the C1 spectrum according to the differences in the binding energies of the C 1s orbitals.
5 Discussion
From the comparison of the experimental with the calculated spectra of the three C3H3+ isomers, it is obvious that the experimental spectra cannot originate from just a single isomer. Due to the rather high temperature of the plasma in the electron cyclotron resonance ion source, C3H3+ cations with different geometries were likely produced simultaneously. After passing the approximately 10 m distance from the source region to the merged-beams interaction region within a flight time of 58 μs, the ions were in the electronic ground states of the respective isomers. Vibrationally excited states of the C3H3+ ions can survive the flight time for this distance and, thus, the ions in the interaction region were not necessarily in the vibrational ground state.The high intensity of the main peak B in Fig. 1(a) is reproduced by the calculation. From the calculation it follows that due to the almost degenerate C 1s electrons in c-C3H3+, the excitation into the LUMO, which is associated with the very prominent resonance labeled 1 at 285.6 eV, is 2–3 times larger than the excitation features from [H2C–C–CH]+ in Fig. 1(c) and [H3C–C–C]+ in Fig. 1(d).
To disentangle the contributions of the three isomers in the experimental spectra, the calculated isomer spectra have been superimposed using a linear combination, i.e.,
 | (3) |
The relative contributions ai of the different spectra were determined by fitting the weighted sum of the individual theoretical cross sections to the experimental data in the energy range from 281 to 288 eV. This energy range has been chosen since it covers all 1s → π* excitation features. It can be assumed that the fragmentation of the molecules at the 1s → π* excitations is less likely than the fragmentation at the 1s → 3p* excitations, which are energetically above 288 eV. Since the [H3C–C–C]+ C1 spectrum only shows the small 1.1 resonance in this region and 1.2 is considered as a broad resonance structure with excitation to σ* orbitals, which usually have non-bonding energy surfaces, the spectrum was not taken into account in the fit. The [H3C–C–C]+ C2 and C3 spectra were combined in the analysis due to their similarity. Above 288 eV, the relative ion yield is significantly smaller than below. As already mentioned, the measured relative ion yield in the energy range above 288 eV significantly increases with increasing hydrogen loss. For example, for the channel C3H3+ → C32+ in Fig. 2(d), the measured ion yield in the range above 288 eV is higher than the fitted calculated cross section.
In order to make statements about the compositions of the different photoion channels, the coefficients ai from the fit for each individual fragmentation channel are normalized such that the sum of the normalized coefficients amounts to one in each fragmentation channel. These normalized coefficients are visualized in Fig. 4 and detailed in Table 3. The c-C3H3+ isomer is more stable against H loss following a C 1s → π* excitation than the linear isomers, which can be related to the rather stable aromatic state. Due to the binding character of the core-excited π* state in c-C3H3+ also vibrational states can be excited. This is also suggested by the asymmetry of the main resonance B and is similar to what was observed earlier for benzene.25
 | ||
| Fig. 4 Relative contributions of the different constitutional isomers to the observed fragmentation channels as resulting from the fit shown in Fig. 2. The error bars show the uncertainties from the fits. Potential uncertainties from the TDDFT calculations were not taken into account. | ||
| Geometry | C3H32+ | C3H22+ | C3H2+ | C32+ |
|---|---|---|---|---|
| c-C3H3+ | 0.44(2) | 0.42(3) | 0.32(3) | 0.16(3) |
| [H2C–C–CH]+ C1 | 0.26(3) | 0.15(5) | 0.06(6) | 0.11(5) |
| [H2C–C–CH]+ C2 | 0.15(4) | 0.15(6) | 0.16(7) | 0.16(6) |
| [H2C–C–CH]+ C3 | 0.10(2) | 0.17(3) | 0.18(3) | 0.23(3) |
| [H3C–C–C]+ C2 | 0.05(2) | 0.06(3) | 0.13(3) | 0.18(3) |
| [H3C–C–C]+ C3 | 0.003(4) | 0.05(5) | 0.15(7) | 0.16(6) |
For the linear [H3C–C–C]+ triplet geometry with the lowest electronic ground-state binding energy the trend is opposite. Following an excitation of C2 or C3 the relative cross section of [H3C–C–C]+ increases with the number of H atoms lost. The relative contributions from C3H2+ and C32+ are significantly larger than the relative contributions from C3H32+ and C3H22+. This trend can also be seen in the changing of resonance C in Fig. 2. The C1 contribution of the linear [H3C–C–C]+ isomer corresponds to a CH3 methyl group. An excitation into the π* orbitals between C2 and C3 is unlikely. This can be clearly seen from the calculated LUMO orbital, which is very similar to the β LUMO orbital. The notation β orbital was chosen for the corresponding β spin function, which is a down electron eigenfunction. The LUMO orbital shows an electron density located at the C1 atom. It can be assumed that the generated charge is transferred to the methyl group, which finally leads to the fragmentation.
The second linear isomer [H2C–C–CH]+ shows an increasing probability for H atom losses following a C 1s excitation at atoms C2 or C3 compared to the [H3C–C–C]+ isomer. However, for C1 the distribution is somewhat different. Here, ionization without fragmentation is slightly favored over ionization with fragmentation, and the H loss channels have identical probabilities within the error bars.
The increase in H losses can be seen already from the resonance A in Fig. 2(b). The resonance shape changes in comparison to the resonance A in Fig. 2(a). Obviously, the two C3H3+ linear isomers are significantly less stable against H losses subsequent to a 1s → π* excitation than the cyclic isomer.
From the coefficients ai in eqn (2), the portions of the three isomer fragments in the ion beam in the merged-beams region can be estimated as (43 ± 2)% c-C3H3+, (49 ± 11)% [H2C–C–CH]+, and (8 ± 7)% [H3C–C–C]+. In a rough approximation, these values represent the composition of the ion beam from the electron cyclotron resonance ion source. This approximation is limited to the measured ionization and fragmentation channels. The ratios might change slightly when all possible channels were analyzed. From this composition and the total energies of the three isomers (see Fig. 3), a plasma temperature T of about 31 kK (Ethermal = 3/2kBT = 4 eV) is obtained via the Maxwell–Boltzmann distribution, which is in very good agreement with the conditions for the production of different iron ions with the same source.54 However, the molecules should cool down rather quickly, which can be estimated by assuming that cooling proceeds by the emission of black-body radiation. With a c-C3H3+ radius of R ≅ 1.66 Å the surface of the molecule is estimated as A = 4πR2. The temperature change dT during a short time dt is given by
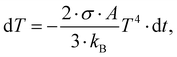 | (4) |
To analyze whether the metastable, linear [CH2–C–CH]+ isomer can decay into the c-C3H3+ isomer, we have also calculated the energy barrier between the two singlet states in Fig. 5, the nudged elastic band with transition state (NEB-TS) optimization was used.51 A change of the electron spin is unlikely, hence the transition from the [H3C–C–C]+ triplet state was not included. The result of the calculation is depicted in Fig. 5. The energy barrier of 2.28 eV suggests that within the electron cyclotron resonance ion source both isomers are produced and right after leaving the ion source a transition between the two isomers is no longer possible. The prop-2-en-1-yl-3-ylidine cation ([CH2–CH–C]+), which is formed during the transition, has a very low energy barrier of approximately 8 meV to the c-C3H3+ isomer. Due to this very low barrier, the ion can easily relax into the c-C3H3+ isomer. Liu et al. have calculated a similar energy barrier of 2.15 eV for the pathway from [H2C–C–CH]+ to c-C3H3+via [H2C–CH–C]+.55 The small difference to our value shows that the chosen basis set as well as the functional describes the C3H3+ isomers equally well as the approach of Liu et al. They report a second transition path with a higher barrier of 3.85 eV,55 which is not relevant for our results.
 | ||
| Fig. 5 The minimum energy pathway from the [H2C–C–CH]+ to the c-C3H3+ isomer calculated with the NEB-TS method.51 The height of the energy barrier between the two states is 2.28 eV. The transition proceeds via the prop-2-en-1-yl-3-ylidine cation. | ||
6 Conclusions and outlook
Using the photon-ion merged-beams technique, inner-shell excitation in the soft X-ray range was applied for analyzing the photoionization and photofragmentation of C3H3+ hydrocarbon ions. Employing TDDFT, the cross sections for different carbon atoms of the three constitutional isomers were calculated. With the help of the calculated absorption spectra, the measured NEXAFS spectra could be analyzed and the relative contributions of the different carbon atoms to the observed spectra could be determined. The cyclic c-C3H3+ isomer undergoes considerably less fragmentation than the two linear isomers. The linear [H2C–C–CH]+ isomer, which lies energetically 1.1 eV above the cyclic ground state, manifests itself in the spectra with enhanced hydrogen elimination. Additionally, we find indications for an 8% contribution of the isomer [CH3–C–C]+ in a triplet state, which lies 4.7 eV above the cyclic singlet ground state.Using NEXAFS spectroscopy, the different constitutional isomers can be distinguished very well. The formation of the isomers strongly depends on the plasma temperature, or, more precisely, on the electron energy. Marimuthu et al. describe a beam of C3H3+ ions consisting of 81% c-C3H3+ and 19% [H2C–C–CH]+.20 This distribution would correspond to a temperature of 5.9 kK (0.76 eV). At this temperature, the fraction of the linear triplet [H3C–C–C]+ isomer would be 0.02%. In our measurements, the plasma of the electron cyclotron resonance ion source had a temperature of 31 kK (4 eV). At this source temperature, the two singlet isomers were present in almost equal proportions in the ion beam and the linear triplet contribution is estimated to be ≈8%.
The ratio between the cyclic and linear C3H3+ isomers can be important for reactivity and chemical reactions, e.g., in space and planetary atmospheres, where larger molecular aggregates are formed such as benzene, C6H6. Due to the generally exothermic formation process of molecular ions, such as C3H3+ by collisions in the planetary ionospheres, they may have a thermal energy in the range up to a few electron volts shortly after the collision process, and the different isomers can be created. The ground-state cyclic isomer, as the smallest aromatic molecule, is more stable and has a lower reactivity, because a reaction will typically be associated with a ring opening. This process is not required for the linear isomers and a step-wise growth by an addition reaction, e.g., with methane, might be favored. Clearly, the relative contributions of the different isomers in space are important for the probability of producing larger molecules. Using NEXAFS spectroscopy, we can distinguish these isomers from each other, which is a challenge in infrared spectroscopy due to the vanishing dipole moment of c-C3H3+.9
From our results we are encouraged to anticipate that different ion sources can be optimized for the production of a specific constitutional isomer mixture. This would allow for experiments on ion–neutral–gas reactions of the different isomers to understand the role of the isomer in planetary atmospheres. Time-resolved NEXAFS spectroscopy at the C 1s edge will be the ideal tool to investigate the dynamics of the isomerization process as depicted in Fig. 5.
Author contributions
S. R. and M. M. conceived the beam time. S. R., P. C., T. B., A. P.-S., S. S., A. M., F. T., and M. M. carried out the experiments. S. R. performed the data analysis and the calculations using ORCA. S. R. and M. M. interpreted the results. S. R. wrote the manuscript with input from all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge DESY (Hamburg, Germany), a member of the Helmholtz Association HGF, for the provision of experimental facilities. Parts of this research were carried out at PETRA III and we would like to thank M. Hoesch, J. Buck, F. Scholz, K. Bagschik, and J. Seltmann for assistance in using beamline P04. Beamtime was allocated for proposal I-20191440. This project was funded by the Bundesministerium für Bildung und Forschung (BMBF, Federal Ministry of Education and Research) funding scheme (Projects no. 05K10RG1, no. 05K10GUB, no. 05K16RG1, no. 05K16GUC, no. 05K19GU4, and no. 15K22GUC), and by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation – Projects no. 245652604, no. 389115454, and no. 201267377). F. T. acknowledges funding by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project 509471550, Emmy Noether Programme.Notes and references
- B. A. McGuire, Astrophys. J., Suppl. Ser., 2022, 259, 30 CrossRef CAS.
- L. E. Snyder, M. S. Schenewerk and J. M. Hollis, Astrophys. J., 1985, 298, 360 CrossRef CAS.
- C. A. Nixon, A. E. Thelen, M. A. Cordiner, Z. Kisiel, S. B. Charnley, E. M. Molter, J. Serigano, P. G. J. Irwin, N. A. Teanby and Y.-J. Kuan, Astron. J., 2020, 160, 205 CrossRef CAS.
- V. Vuitton, R. V. Yelle and J. Cui, J. Geophys. Res., 2008, 113, E05007 CrossRef.
- J. H. Waite, Jr., D. T. Young, T. E. Cravens, A. J. Coates, F. J. Crary, B. Magee and J. Westlake, Science, 2007, 316, 870–875 CrossRef PubMed.
- V. Vuitton, R. V. Yelle and P. Lavvas, Philos. Trans. R. Soc., A, 2008, 367, 729–741 CrossRef PubMed.
- D. Zhao, K. D. Doney and H. Linnartz, Astrophys. J., 2014, 791, L28 CrossRef.
- T. E. Cravens, I. P. Robertson, J. H. Waite, Jr., R. V. Yelle, W. T. Kasprzak, C. N. Keller, S. A. Ledvina, H. B. Niemann, J. G. Luhmann, R. L. McNutt, W.-H. Ip, V. De La Haye, I. Mueller-Wodarg, J.-E. Wahlund, V. G. Anicich and V. Vuitton, Geophys. Res. Lett., 2006, 33, L07105 CrossRef.
- W. G. D. P. Silva, J. Cernicharo, S. Schlemmer, N. Marcelino, J.-C. Loison, M. Agúndez, D. Gupta, V. Wakelam, S. Thorwirth, C. Cabezas, B. Tercero, J. L. Doménech, R. Fuentetaja, W.-J. Kim, P. de Vicente and O. Asvany, Astron. Astrophys., 2023, 676, L1 CrossRef CAS.
- D. Gerlich and S. Horning, Chem. Rev., 1992, 92, 1509–1539 CrossRef CAS.
- J. S. Brodbelt, Mass Spectrom. Rev., 1997, 16, 91–110 CrossRef CAS.
- R. Wester, Phys. Chem. Chem. Phys., 2014, 16, 396–405 RSC.
- D. J. Hollenbach and A. G. G. M. Tielens, Rev. Mod. Phys., 1999, 71, 173 CrossRef CAS.
- A. Stanger, Chem. Commun., 2009, 1939–1947 RSC.
- Aromaticity: Modern Computational Methods and Applications, ed. I. Fernandez, Elsevier, 2021 Search PubMed.
- T. W. Kasel, A. T. Murray and C. H. Hendon, J. Phys. Chem. C, 2018, 122, 2041–2045 CrossRef CAS.
- O. Dopfer, D. Roth and J. P. Maier, J. Am. Chem. Soc., 2002, 124, 494–502 CrossRef CAS PubMed.
- O. Dopfer, D. Roth and J. P. Maier, Int. J. Mass Spectrom., 2002, 218, 281–297 CrossRef CAS.
- D. Roth and O. Dopfer, Phys. Chem. Chem. Phys., 2002, 4, 4855–4865 RSC.
- A. N. Marimuthu, D. Sundelin, S. Thorwirth, B. Redlich, W. D. Geppert and S. Brünken, J. Mol. Spectrosc., 2020, 374, 111377 CrossRef CAS.
- W. G. D. P. Silva, D. Gupta, E. Plaar, J. L. Doménech, S. Schlemmer and O. Asvany, Mol. Phys., 2023, e2296613 CrossRef.
- G. A. Garcia, B. Gans, J. Krüger, F. Holzmeier, A. Röder, A. Lopes, C. Fittschen, C. Alcaraz and J.-C. Loison, Phys. Chem. Chem. Phys., 2018, 20, 8707–8718 RSC.
- H. Gao, Z. Lu, L. Yang, J. Zhou and C. Y. Ng, J. Chem. Phys., 2012, 137, 161101 CrossRef PubMed.
- M. Epshtein, V. Scutelnic, Z. Yang, T. Xue, M. L. Vidal, A. I. Krylov, S. Coriani and S. R. Leone, J. Phys. Chem. A, 2020, 124, 9524–9531 CrossRef CAS PubMed.
- C. Kolczewski, R. Püttner, M. Martins, A. S. Schlachter, G. Snell, M. M. Sant’Anna, K. Hermann and G. Kaindl, J. Chem. Phys., 2006, 124, 034302 CrossRef CAS PubMed.
- I. C. Lyon, B. Peart, J. B. West and K. Dolder, J. Phys. B: At. Mol. Phys., 1986, 19, 4137 CrossRef CAS.
- H. Kjeldsen, J. Phys. B: At., Mol. Opt. Phys., 2006, 39, R325 CrossRef CAS.
- S. Schippers, A. L. D. Kilcoyne, R. A. Phaneuf and A. Müller, Contemp. Phys., 2016, 57, 215–229 CrossRef CAS.
- J.-P. Mosnier, E. T. Kennedy, P. van Kampen, D. Cubaynes, S. Guilbaud, N. Sisourat, A. Puglisi, S. Carniato and J.-M. Bizau, Phys. Rev. A, 2016, 93, 061401(R) CrossRef.
- S. Carniato, J.-M. Bizau, D. Cubaynes, E. T. Kennedy, S. Guilbaud, E. Sokell, B. McLaughlin and J.-P. Mosnier, Atoms, 2020, 8, 67 CrossRef CAS.
- M. Martins, S. Reinwardt, J. O. Schunck, J. Schwarz, K. Baev, A. Müller, T. Buhr, A. Perry-Sassmannshausen, S. Klumpp and S. Schippers, J. Phys. Chem. Lett., 2021, 12, 1390–1395 CrossRef CAS PubMed.
- S. Schippers, P.-M. Hillenbrand, A. Perry-Sassmannshausen, T. Buhr, S. Fuchs, S. Reinwardt, F. Trinter, A. Müller and M. Martins, ChemPhysChem, 2023, 24, e202300061 CrossRef CAS PubMed.
- S. Schippers, S. Ricz, T. Buhr, A. Borovik, Jr., J. Hellhund, K. Holste, K. Huber, H.-J. Schäfer, D. Schury, S. Klumpp, K. Mertens, M. Martins, R. Flesch, G. Ulrich, E. Rühl, T. Jahnke, J. Lower, D. Metz, L. P. H. Schmidt, M. Schöffler, J. B. Williams, L. Glaser, F. Scholz, J. Seltmann, J. Viefhaus, A. Dorn, A. Wolf, J. Ullrich and A. Müller, J. Phys. B: At., Mol. Opt. Phys., 2014, 47, 115602 CrossRef.
- S. Schippers, A. Borovik, Jr., T. Buhr, J. Hellhund, K. Holste, A. L. D. Kilcoyne, S. Klumpp, M. Martins, A. Müller, S. Ricz and S. Fritzsche, J. Phys. B: At., Mol. Opt. Phys., 2015, 48, 144003 CrossRef.
- J. Viefhaus, F. Scholz, S. Deinert, L. Glaser, M. Ilchen, J. Seltmann, P. Walter and F. Siewert, Nucl. Instrum. Methods Phys. Res., Sect. A, 2013, 710, 151–154 CrossRef CAS.
- A. Müller, M. Martins, A. Borovik, Jr., T. Buhr, A. Perry-Sassmannshausen, S. Reinwardt, F. Trinter, S. Schippers, S. Fritzsche and A. S. Kheifets, Phys. Rev. A, 2021, 104, 033105 CrossRef.
- A. Müller, A. Borovik, Jr., S. Bari, T. Buhr, K. Holste, M. Martins, A. Perry-Saßmannshausen, R. A. Phaneuf, S. Reinwardt, S. Ricz, K. Schubert and S. Schippers, Phys. Rev. Lett., 2018, 120, 133202 CrossRef PubMed.
- A. Müller, E. Lindroth, S. Bari, A. Borovik, Jr., P.-M. Hillenbrand, K. Holste, P. Indelicato, A. L. D. Kilcoyne, S. Klumpp, M. Martins, J. Viefhaus, P. Wilhelm and S. Schippers, Phys. Rev. A, 2018, 98, 033416 CrossRef.
- A. P. Hitchcock and C. E. Brion, J. Electron Spectrosc. Relat. Phenom., 1980, 18, 1–21 CrossRef CAS.
- M. Schlapp, R. Trassl, E. Salzborn, R. W. McCullough, T. K. McLaughlin and H. B. Gilbody, Nucl. Instrum. Methods Phys. Res., Sect. B, 1995, 98, 525–527 CrossRef CAS.
- J. Fricke, A. Müller and E. Salzborn, Nucl. Instrum. Methods, 1980, 175, 379–384 CrossRef CAS.
- K. Rinn, A. Müller, H. Eichenauer and E. Salzborn, Rev. Sci. Instrum., 1982, 53, 829–837 CrossRef CAS.
- A. Müller, A. Borovik, Jr., T. Buhr, J. Hellhund, K. Holste, A. L. D. Kilcoyne, S. Klumpp, M. Martins, S. Ricz, J. Viefhaus and S. Schippers, Phys. Rev. A, 2018, 97, 013409 CrossRef.
- F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73–78 CAS.
- F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2018, 8, e1327 Search PubMed.
- A. Cameron, J. Leszczynski, M. C. Zerner and B. Weiner, J. Phys. Chem., 1989, 93, 139–144 CrossRef CAS.
- J. P. Perdew, Phys. Rev. B, 1986, 33, 8822(R) CrossRef PubMed.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- F. Weigend, Phys. Chem. Chem. Phys., 2006, 8, 1057–1065 RSC.
- K. Kaufmann, W. Baumeister and M. Jungen, J. Phys. B: At., Mol. Opt. Phys., 1989, 22, 2223 CrossRef CAS.
- V. Ásgeirsson, B. O. Birgisson, R. Bjornsson, U. Becker, F. Neese, C. Riplinger and H. Jónsson, J. Chem. Theory Comput., 2021, 17, 4929–4945 CrossRef PubMed.
- U. Hergenhahn, O. Kugeler, A. Rüdel, E. E. Rennie and A. M. Bradshaw, J. Phys. Chem. A, 2001, 105, 5704–5708 CrossRef CAS.
- G. Knizia, J. Chem. Theory Comput., 2013, 9, 4834–4843 CrossRef CAS PubMed.
- S. Schippers, R. Beerwerth, S. Bari, T. Buhr, K. Holste, A. L. D. Kilcoyne, A. Perry-Sassmannshausen, R. A. Phaneuf, S. Reinwardt, D. W. Savin, K. Schubert, S. Fritzsche, M. Martins and A. Müller, Astrophys. J., 2021, 908, 52 CrossRef CAS.
- G.-x. Liu, Z.-s. Li, Y.-h. Ding, Q. Fu, X.-r. Huang, C.-c. Sun and A.-c. Tang, J. Phys. Chem. A, 2002, 106, 10415–10422 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cp00370e |
| This journal is © the Owner Societies 2024 |