Carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4 nanoparticles as a novel anode material for high energy density lithium-ion batteries†
Khadija
Kouchi
a,
Marwa
Tayoury
a,
Abdelwahed
Chari
a,
Loubna
Hdidou
a,
Zakaria
Chchiyai
b,
Khadija
El kamouny
c,
Youssef
Tamraoui
a,
Bouchaib
Manoun
ab,
Jones
Alami
a and
Mouad
Dahbi
 *a
*a
aMaterials Science, Energy, and Nano-engineering Department, Mohammed VI Polytechnic University, Lot 660-Hay Moulay Rachid, 43150, Ben Guerir, Morocco. E-mail: Mouad.dahbi@um6p.ma
bHassan First University, FST Settat, Rayonnement-Matière et Instrumentation, S3M, 26000, Settat, Morocco
cGreen Tech Institute Department, Mohammed VI Polytechnic University UM6P, Ben Guerir, Morocco
First published on 15th February 2024
Abstract
Lithium-ion batteries (LIBs) have gained considerable attention from the scientific community due to their outstanding properties, such as high energy density, low self-discharge, and environmental sustainability. Among the prominent candidates for anode materials in next-generation LIBs are the spinel ferrites, represented by the MFe2O4 series, which offer exceptional theoretical capacities, excellent reversibility, cost-effectiveness, and eco-friendliness. In the scope of this study, Ni0.5Mg0.5Fe1.7Mn0.3O4 nanoparticles were synthesized using a sol–gel synthesis method and subsequently coated with a carbon layer to further enhance their electrochemical performance. TEM images confirmed the presence of the carbon coating layer on the Ni0.5Mg0.5Fe1.7Mn0.3O4/C composite. The analysis of the measured X-ray diffraction (XRD) and Raman spectroscopy results confirmed the formation of nanocrystalline Ni0.5Mg0.5Fe1.7Mn0.3O4 before coating and amorphous carbon in the Ni0.5Mg0.5Fe1.7Mn0.3O4/C after the coating. The Ni0.5Mg0.5Fe1.7Mn0.3O4 anode material exhibited a much higher specific capacity than the traditional graphite material, with initial discharge/charge capacities of 1275 and 874 mA h g−1, respectively, at a 100 mA g−1 current density and a first coulombic efficiency of 68.54%. The long-term cycling test showed a slight capacity fading, retaining approximately 85% of its initial capacity after 75 cycles. Notably, the carbon-coating layer greatly enhanced the stability and slightly increased the capacity of the as-prepared Ni0.5Mg0.5Fe1.7Mn0.3O4. The first discharge/charge capacities of Ni0.5Mg0.5Fe1.7Mn0.3O4/C at 100 mA g−1 current density reached 1032 and 723 mA h g−1, respectively, and a first coulombic efficiency of 70.06%, with an increase of discharge/charge capacities to 826.6 and 806.2 mA h g−1, respectively, after 75 cycles (with a capacity retention of 89.7%), and a high-rate capability of 372 mA h g−1 at 2C. Additionally, a full cell was designed using a Ni0.5Mg0.5Fe1.7Mn0.3O4/C anode and an NMC811 cathode. The output voltage was about 2.8 V, with a high initial specific capacity of 755 mA h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g−1 at 0.125C, a high rate-capability of 448 mA h
g−1 at 0.125C, a high rate-capability of 448 mA h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g−1 at 2C, and a high-capacity retention of 91% after 30 cycles at 2C. The carbon coating layer on Ni0.5Mg0.5Fe1.7Mn0.3O4 nanoparticles played a crucial role in the excellent electrochemical performance, providing conducting, buffering, and protective effects.
g−1 at 2C, and a high-capacity retention of 91% after 30 cycles at 2C. The carbon coating layer on Ni0.5Mg0.5Fe1.7Mn0.3O4 nanoparticles played a crucial role in the excellent electrochemical performance, providing conducting, buffering, and protective effects.
1. Introduction
Lithium-ion batteries (LIBs) have shown great promise as an energy storage solution, finding extensive use across various domains encompassing portable devices, electric vehicles, and grid-scale energy systems.1 Their exceptional attributes, such as high energy density and prolonged cycle life, make them particularly appealing.2 LIBs consist of crucial constituents, namely the cathode, anode, electrolyte, and separator. Their effectiveness is contingent on the chemical composition of their diverse constituents and the morphology of their electrode materials.3 Hence, there is a need to develop new efficient and cost-effective electrode materials to meet the growing demand for renewable energy, portable electronic devices, electric transportation, and large-scale energy storage in smart grids.4,5 Due to its cost-effectiveness, reliable reversibility concerning lithium-ion insertion/extraction, and plentiful availability, graphite is extensively utilized as the active anode material in commercial lithium-ion batteries (LIBs). Nevertheless, graphite is hindered by its low theoretical capacity of merely 372 mA h g−1, which falls short of meeting the demands of extensive utilization in the green energy storage network.6–8 Furthermore, the utilization of graphite in battery systems gives rise to safety considerations owing to its relatively low working voltage, which is less than 0.2 V when compared to the Li/Li+ standard, and this can lead to the formation of lithium dendrites.9,10 Silicon-based materials have emerged as a promising alternative to graphite for battery systems due to their large capacities. However, significant volume changes during the lithiation–delithiation process present a major obstacle to their practical application.11To overcome the shortcomings associated with graphite and silicon-based anodes, spinel Li4Ti5O12 is another anode material that has been used for LIBs. Its discharge potential is about 1.5 V, which makes it safer than the commonly used graphite anode because the problem of lithium plating can be largely avoided.12 The Li4Ti5O12 demonstrates outstanding stability and specific capacity at high current densities. However, its theoretical capacity is limited to 175 mA h g−1.13 As a result, more research is necessary to develop new anode materials that offer improved safety, low cost, high theoretical capacity, and easy synthesis processes.
Spinel ferrite oxides, including ZnFe2O4,14 CoFe2O4,15 NiFe2O4,16 CuFe2O4,17 MgFe2O4,18etc., have gained considerable interest as potential anode materials for use in lithium-ion batteries (LIBs). This is largely because they have significantly higher theoretical capacities compared to graphite and Li4Ti5O12 anode materials.19,20
For instance, CaFe2O4, CoFe2O4, NiFe2O4, and CuFe2O4 exhibited higher specific capacities than the traditional graphite (372 mA h g−1). ZnFe2O4 can also be considered as a good anode material candidate owing to its elevated theoretical specific capacity of around 1000 mA h g−1.21
However, the main problems of spinel oxide anode materials are their low electrical conductivity and considerable volume expansion.22 To overcome these issues, carbon coating has been reported as a suitable coating material that can effectively enhance the capacity retention and cycling stability of spinel materials.17 Moreover, it participates in reducing the volume change during the discharging/charging operations and facilitates the Li-ion diffusion into the metal oxide structure.13
For example, Jin et al. have successfully prepared CuFe2O4/C hollow spheres through a hydrothermal growth method based on polymers, followed by calcination. After the 70th cycle at a current density of 100 mA cm−2, the anodic material demonstrated a specific capacity of 550 mA h g−1, which is significantly greater than the initial specific capacity of the CuFe2O4 hollow spheres (∼120 mA h g−1).23 In addition, Deng et al. reported a significant enhancement in the electrochemical properties of ZnFe2O4/C hollow spheres. They found that the ZnFe2O4 sample modified with carbon exhibited a specific capacity of 841 mA h g−1 after 30 cycles, along with a high-rate capability.24
To our knowledge, no studies have reported on the electrochemical performances of nanocrystalline Ni0.5Mg0.5Fe1.7Mn0.3O4 as anode materials for LIBs. This study details the preparation of Ni0.5Mg0.5Fe1.7Mn0.3O4 and Ni0.5Mg0.5Fe1.7Mn0.3O4/C nanoparticles, aiming to explore their potential as LIB anodes. We enhanced the cycling stability and rate capability of Ni0.5Mg0.5Fe1.7Mn0.3O4 anode through carbon coating. The nanocrystalline powders were characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and Raman spectroscopy. The anode materials’ performance for LIBs was evaluated using electrochemical tests in a lithium half-cell, including cyclic voltammetry, galvanostatic charge–discharge, and electrochemical impedance spectroscopy (EIS) tests. Additionally, the carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C anode's performance for LIBs was specifically evaluated in the full-cell configuration.
2. Experimental
2.1. Synthesis of Ni0.5Mg0.5Fe1.7Mn0.3O4 and Ni0.5Mg0.5Fe1.7Mn0.3O4/C samples
The synthesis of the Ni0.5Mg0.5Fe1.7Mn0.3O4 spinel material was conducted utilizing the sol–gel method, as detailed in a prior study.25 A solution was prepared by dissolving Ni(NO3)2·6H2O, Mg(NO3)2·6H2O, FeCl3·6H2O, and MnCl2·4H2O in distilled water, maintaining a molar ratio of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg
Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn = 0.5
Mn = 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7
1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3. Citric acid, acting as a complexing agent, was subsequently introduced to this mixture. This combined solution was then heated to 90 °C with continuous stirring until it transformed into a viscous gel. The gel underwent a drying process at a temperature of 160 °C for a duration of 12 hours, resulting in the formation of a xerogel. Subsequently, the xerogel underwent calcination in an ambient air environment within a temperature range of 250–800 °C. Prior to each calcination phase, the sample was subjected to intermediate grindings.
0.3. Citric acid, acting as a complexing agent, was subsequently introduced to this mixture. This combined solution was then heated to 90 °C with continuous stirring until it transformed into a viscous gel. The gel underwent a drying process at a temperature of 160 °C for a duration of 12 hours, resulting in the formation of a xerogel. Subsequently, the xerogel underwent calcination in an ambient air environment within a temperature range of 250–800 °C. Prior to each calcination phase, the sample was subjected to intermediate grindings.
In this synthesis process, the reagents reacted to produce the Ni0.5Mg0.5Fe1.7Mn0.3O4 spinel oxide, as depicted by the subsequent chemical equation:
| 0.5 Ni(NO3)2·6H2O + 0.5 Mg(NO3)2·6H2O + 1.7 FeCl3·6H2O + 0.3 MnCl2·4H2O → Ni0.5Mg0.5Fe1.7Mn0.3O4 + 2.85 Cl2↑ + 2 NO2↑ + 17.4 H2O↑ |
Carbon coating was performed by mixing the as-synthesized Ni0.5Mg0.5Fe1.7Mn0.3O4 material with sucrose powder as the carbon source (Ni0.5Mg0.5Fe1.7Mn0.3O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose = 95
glucose = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, which was the percentage) using acetone solution. Subsequently, the composite underwent a calcination process at a temperature of 600 °C for a duration of 5 hours, while being exposed to an argon environment to get the Ni0.5Mg0.5Fe1.7Mn0.3O4/C sample.
5, which was the percentage) using acetone solution. Subsequently, the composite underwent a calcination process at a temperature of 600 °C for a duration of 5 hours, while being exposed to an argon environment to get the Ni0.5Mg0.5Fe1.7Mn0.3O4/C sample.
2.2. Material characterization
The sample was characterized using X-ray diffraction (XRD) analysis with a “BRUKER D8 ADVANCE” diffractometer, equipped with a Cu Kα radiation source (λ = 1.54056 Å). The diffraction data was collected within the 10 to 100 degrees (2θ) angular range, using a step size of 0.01 degrees and a count time of 10 seconds per step. To investigate the morphology and particle size of the sample, scanning electron microscopy (SEM) was performed using a JEOL JSM-IT500HRL instrument. The chemical compositions of the Ni0.5Mg0.5Fe1.7Mn0.3O4 material were examined using energy-dispersive spectroscopy (EDS). Transmission Electron Microscopy (TEM) using the TECNAI G2/FEI model was used to study the microstructure of both uncoated and carbon-coated samples and to assess the state of the carbon coating layer. Energy dispersive spectroscopy (EDS) was used to analyze the surface composition and elemental content of the carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C sample. Raman spectroscopy analysis was conducted to identify the vibrational bands present in the Ni0.5Mg0.5Fe1.7Mn0.3O4 sample, and to investigate the presence of carbon in the synthesized Ni0.5Mg0.5Fe1.7Mn0.3O4/C material. This analysis was carried out with a spectrometer (Horiba) and an excitation wavelength of 532 cm−1, using the spectral range of 100–2000 cm−1.2.3. Electrochemical measurements
The working electrodes for electrochemical tests were prepared by combining 70 wt% of the active material, 20 wt% of carbon black as a conductive agent, and 10 wt% of CarboxyMethyl Cellulose (CMC) as a binder. The resulting mixture was dispersed in double-distilled water and agitated for 6 hours to form a viscous slurry. The doctor-blade technique was employed to uniformly apply the slurry onto a copper foil current collector with a thickness of 0.1 mm, where the mass loading was 1.3–1.5 mg cm−2. The loaded copper foil was then dried overnight at 65 °C to remove the solvent (water). It was cut into disks of 11 mm diameter and left to further dry out overnight in a vacuum oven at 100 °C to eliminate all remaining solvent. The electrochemical tests of the fabricated materials were carried out using CR2032 button cells in a half-cell setup. The half-cells containing uncoated and carbon-coated materials were assembled under controlled conditions within an argon-filled glove box (Jacomex, France) at ambient temperature, while ensuring that water and oxygen levels remained below 0.5 ppm. The electrodes used in the setup comprised of a Li metal disc with an 8 mm diameter serving as the counter and reference electrodes. A Whatman separator was employed to segregate the negative and positive electrodes. The electrolyte adopted was a 2.0 M LiPF6 solution dissolved in ethylene carbonate (EC) and diethyl carbonate (DEC), mixed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 proportion. Each coin cell contained 120 μL of this electrolyte.
1 proportion. Each coin cell contained 120 μL of this electrolyte.
The galvanostatic discharge/charge and long-term cycling tests on the newly investigated uncoated and coated spinel oxide were carried out at a current density of 100 mA g−1 (100 mA g−1 = 0.125C) over the 0.01–3.00 V voltage range. Rate capability analysis was conducted at various C-rates, including 0.03C, 0.06C, 0.125C, 0.25C, 0.625C, 1.25C and 2C. Cyclic voltammetry curves of uncoated electrode Ni0.5Mg0.5Fe1.7Mn0.3O4 were obtained by varying the potential between 0.01 V and 3.0 V and utilizing a scan rate of 1 mV s−1. Electrochemical impedance spectra (EIS) were characterized over a frequency range of 100 kHz to 0.01 Hz. The electrochemical testing of the full cell was carried out at a current density of 100 mA g−1, within a voltage range of 1–4 V. For this, we used an NMC811 electrode, sourced from Argonne National Laboratory, as the positive electrode, and our synthesized carbon-coated spinel oxide Ni0.5Mg0.5Fe1.7Mn0.3O4/C as the negative electrode. The full cell, comprising NMC811 vs. Ni0.5Mg0.5Fe1.7Mn0.3O4/C, was assembled using the same procedure as for the half-cells of Ni0.5Mg0.5Fe1.7Mn0.3O4 and Ni0.5Mg0.5Fe1.7Mn0.3O4/C negative electrodes. However, in the full-cell configuration, the NMC811 electrode was used instead of the lithium metal disc. The N/P (negative/positive) mass ratio was maintained at approximately 0.84. A discharge/charge rate of 0.125C and 2C was applied, corresponding to a specific current of 100 mA g−1 and 1600 mA g−1 for the negative electrode material. All potentials were referenced to the Li/Li+ electrode. It should be noted that discharging and charging were regarded as lithiation and delithiation processes in this study. All electrochemical tests were conducted at ambient temperature utilizing a multi-channel potentiostat (MPG-2, Bio-Logic).
3. Results and discussion
The crystal structure of the Ni0.5Mg0.5Fe1.7Mn0.3O4 spinel oxide and the incorporation of carbon were characterized using X-ray diffraction (XRD) and Raman spectroscopy techniques. Fig. 1 displays the X-ray diffraction pattern acquired at ambient temperature for the uncoated Ni0.5Mg0.5Fe1.7Mn0.3O4 powder that underwent thermal treatment at a temperature of 800 °C, and carbon coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C powder. The X-ray diffraction (XRD) peaks observed in the diffraction pattern of uncoated material Ni0.5Mg0.5Fe1.7Mn0.3O4 have been successfully identified and assigned to the cubic spinel structure with Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (no. 227) as the corresponding space group.26 No additional peaks corresponding to other phases were observed in the X-ray diffraction (XRD) pattern, indicating that the Ni0.5Mg0.5Fe1.7Mn0.3O4 compound synthesized and subjected to thermal treatment at 800 °C is a pure single-phase spinel material. This outcome can be attributed to the chelation of citric acid molecules with the cations (Ni2+, Mg2+, Fe3+, and Mn2+) during the synthesis process, which effectively hinders the formation of impurities. The obtained sample of Ni0.5Mg0.5Fe1.7Mn0.3O4 exhibits a notable level of crystallinity, which is supported by the clear existence of distinct X-ray diffraction (XRD) peaks characterized by narrow full width at half maximum (FWHM) values.27 The determined crystallite size of the Ni0.5Mg0.5Fe1.7Mn0.3O4 material is 38 nm. The X-ray powder diffraction patterns of the sample Ni0.5Mg0.5Fe1.7Mn0.3O4 were analyzed using High-Score software, as depicted in Fig. 1. The analysis of the Ni0.5Mg0.5Fe1.7Mn0.3O4 combination demonstrated that all the diffraction peaks could be accurately identified as originating from the single-phase cubic spinel MgFe2O4. The space group Fd
m (no. 227) as the corresponding space group.26 No additional peaks corresponding to other phases were observed in the X-ray diffraction (XRD) pattern, indicating that the Ni0.5Mg0.5Fe1.7Mn0.3O4 compound synthesized and subjected to thermal treatment at 800 °C is a pure single-phase spinel material. This outcome can be attributed to the chelation of citric acid molecules with the cations (Ni2+, Mg2+, Fe3+, and Mn2+) during the synthesis process, which effectively hinders the formation of impurities. The obtained sample of Ni0.5Mg0.5Fe1.7Mn0.3O4 exhibits a notable level of crystallinity, which is supported by the clear existence of distinct X-ray diffraction (XRD) peaks characterized by narrow full width at half maximum (FWHM) values.27 The determined crystallite size of the Ni0.5Mg0.5Fe1.7Mn0.3O4 material is 38 nm. The X-ray powder diffraction patterns of the sample Ni0.5Mg0.5Fe1.7Mn0.3O4 were analyzed using High-Score software, as depicted in Fig. 1. The analysis of the Ni0.5Mg0.5Fe1.7Mn0.3O4 combination demonstrated that all the diffraction peaks could be accurately identified as originating from the single-phase cubic spinel MgFe2O4. The space group Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m was utilized for indexing, with reference to the standard JCPDS card (no. 88-1936) and a lattice parameter of a = 8.3827 Å. The obtained outcome was compared to the pure MgFe2O4.28
m was utilized for indexing, with reference to the standard JCPDS card (no. 88-1936) and a lattice parameter of a = 8.3827 Å. The obtained outcome was compared to the pure MgFe2O4.28
 | ||
| Fig. 1 Room temperature XRD patterns of the synthesized Ni0.5Mg0.5Fe1.7Mn0.3O4 and Ni0.5Mg0.5Fe1.7Mn0.3O4/C materials. | ||
The X-ray diffraction (XRD) pattern of the carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C composite did not reveal any diffraction peaks for carbon, indicating that the carbon produced from glucose decomposition may be amorphous.29 In addition, there is no apparent difference between the XRD patterns of uncoated Ni0.5Mg0.5Fe1.7Mn0.3O4 and carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C materials. Therefore, the XRD results confirm the formation of the Ni0.5Mg0.5Fe1.7Mn0.3O4/C composite.
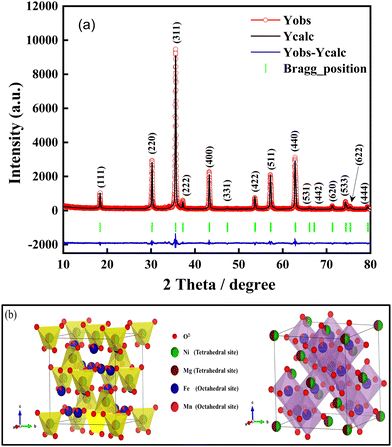
To conduct a more comprehensive examination of the structural characteristics, the X-ray diffraction (XRD) pattern of the synthesized Ni0.5Mg0.5Fe1.7Mn0.3O4 spinel ferrite was subjected to refinement using the Rietveld method. Nevertheless, the process of structural refinement was conducted using the cubic structure and the Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group. Fig. 2(a) presents the Rietveld refinement plot for the synthesized Ni0.5Mg0.5Fe1.7Mn0.3O4 spinel. The red circles represent the experimental X-ray diffraction (XRD) pattern, the black solid lines depict the theoretical XRD pattern, and the blue solid line corresponds to the difference curves. The vertical green bars indicate the positions of various Bragg reflections. Fig. 2(a) presented in this study demonstrates a strong correspondence between the observed X-ray diffraction (XRD) pattern and the calculated XRD pattern. This agreement provides evidence of the successful structural refinement achieved for the synthesized Ni0.5Mg0.5Fe1.7Mn0.3O4 spinel ferrite. Furthermore, the evaluation of the quality of structural refinement was performed by considering several R-factors, such as the weighted profile factor (Rwp), profile factor (Rp), expected factor (Rexp), and Chi-squared value (χ2). Table S1 (ESI†) summarizes the findings from the powder X-ray diffraction analysis of the produced Ni0.5Mg0.5Fe1.7Mn0.3O4 spinel oxide using Rietveld refinement. The results include structural parameters, Chi-squared factor (χ2), and various reliability R-factors. Based on the obtained R-factors values, it can be concluded that the synthesized Ni0.5Mg0.5Fe1.7Mn0.3O4 spinel oxide crystallizes in the cubic Fd
m space group. Fig. 2(a) presents the Rietveld refinement plot for the synthesized Ni0.5Mg0.5Fe1.7Mn0.3O4 spinel. The red circles represent the experimental X-ray diffraction (XRD) pattern, the black solid lines depict the theoretical XRD pattern, and the blue solid line corresponds to the difference curves. The vertical green bars indicate the positions of various Bragg reflections. Fig. 2(a) presented in this study demonstrates a strong correspondence between the observed X-ray diffraction (XRD) pattern and the calculated XRD pattern. This agreement provides evidence of the successful structural refinement achieved for the synthesized Ni0.5Mg0.5Fe1.7Mn0.3O4 spinel ferrite. Furthermore, the evaluation of the quality of structural refinement was performed by considering several R-factors, such as the weighted profile factor (Rwp), profile factor (Rp), expected factor (Rexp), and Chi-squared value (χ2). Table S1 (ESI†) summarizes the findings from the powder X-ray diffraction analysis of the produced Ni0.5Mg0.5Fe1.7Mn0.3O4 spinel oxide using Rietveld refinement. The results include structural parameters, Chi-squared factor (χ2), and various reliability R-factors. Based on the obtained R-factors values, it can be concluded that the synthesized Ni0.5Mg0.5Fe1.7Mn0.3O4 spinel oxide crystallizes in the cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structure, characterized by a lattice parameter of a = 8.36215 Å.
m structure, characterized by a lattice parameter of a = 8.36215 Å.
Fig. 2(b) illustrates the crystal structure of the Ni0.5Mg0.5Fe1.7Mn0.3O4 sample, which exhibits cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m symmetry. The analysis demonstrates that the synthesized material exhibits a crystal structure that is characterized by the connectivity of tetrahedra and octahedra sites, which are composed of oxygen anions. Based on the established framework, it becomes apparent that there are three distinct and easily discernible classifications of sites, denoted as 8a, 16d, and 32e sites. Notably, the 8a and 16d sites can be correlated with the tetrahedral and octahedral sites, respectively. The cations Ni2+ and Mg2+ are present in a ratio of 1/8 within the tetrahedral sites formed by the anions O2−. Conversely, the cations Fe3+/Mn3+ are situated in half of the octahedral sites formed by the anions O2−. Furthermore, it should be noted that all the sites in the 32e structure are currently occupied by the O2− anions. Hence, it is evident within the provided crystal structure that a substantial fraction of the tetrahedral sites (7/8) and half of the octahedral sites are vacant, thereby providing vacancies for the incorporation of lithium ions in the lacunae of the unit cell.
m symmetry. The analysis demonstrates that the synthesized material exhibits a crystal structure that is characterized by the connectivity of tetrahedra and octahedra sites, which are composed of oxygen anions. Based on the established framework, it becomes apparent that there are three distinct and easily discernible classifications of sites, denoted as 8a, 16d, and 32e sites. Notably, the 8a and 16d sites can be correlated with the tetrahedral and octahedral sites, respectively. The cations Ni2+ and Mg2+ are present in a ratio of 1/8 within the tetrahedral sites formed by the anions O2−. Conversely, the cations Fe3+/Mn3+ are situated in half of the octahedral sites formed by the anions O2−. Furthermore, it should be noted that all the sites in the 32e structure are currently occupied by the O2− anions. Hence, it is evident within the provided crystal structure that a substantial fraction of the tetrahedral sites (7/8) and half of the octahedral sites are vacant, thereby providing vacancies for the incorporation of lithium ions in the lacunae of the unit cell.
To further investigate the crystal structure of the synthesized Ni0.5Mg0.5Fe1.7Mn0.3O4 material and to confirm the presence of a carbon layer in the Ni0.5Mg0.5Fe1.7Mn0.3O4/C nanoparticles, Raman scattering spectroscopy was employed. Fig. 3 shows the Raman spectra of the uncoated Ni0.5Mg0.5Fe1.7Mn0.3O4 and carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C materials. The Raman spectra of the coated material exhibit two distinct peaks at wavenumbers of approximately 1328.21 and 1582.06 cm−1, which correspond to the typical D and G bands for carbon, respectively.30,31 The D band is associated with the A1g phonon of sp3 carbon atoms at the edge and disordered carbon, while the G band is ascribed to the in-plane vibration of sp2 carbon atoms in carbon atomic rings or long-chain carbon. The ID/IG ratio is a common evaluation value for carbon materials, indicating the degree of disorder and its association with electronic conductivity.32,33 The calculated ID/IG value of 0.97 suggests a significant degree of disorder within the carbon structure of the as-prepared Ni0.5Mg0.5Fe1.7Mn0.3O4/C composite, indicating amorphous carbon and agreeing with the XRD results.
 | ||
| Fig. 3 Raman scattering curves collected at room temperature of the uncoated Ni0.5Mg0.5Fe1.7Mn0.3O4 and carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C samples. | ||
The Raman spectra of the uncoated Ni0.5Mg0.5Fe1.7Mn0.3O4 material exhibited six peaks between 100 and 700 cm−1, which is also observed in the carbon-coated material.
It's pertinent to remember that, in the group theory analysis, the AB2O4-type spinels exhibiting cubic Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m symmetry are predicted to have five active Raman modes, which may be defined as A1g, Eg, and 3T2g.34 The Raman scattering spectra of the produced material, obtained at room temperature, are depicted in Fig. 3. The remarkable crystallinity of the Ni0.5Mg0.5Fe1.7Mn0.3O4 sample is confirmed by the Raman spectra presented in Fig. 3. A total of six distinct Raman bands were detected at the respective wavenumbers of 189, 323, 473, 523, 650, and 691 cm−1. The detected Raman bands, have distinct characteristics that align with the crystal structure of MgFe2O4, namely the cubic spinel configuration. This crystal structure is commonly associated with the Fd
m symmetry are predicted to have five active Raman modes, which may be defined as A1g, Eg, and 3T2g.34 The Raman scattering spectra of the produced material, obtained at room temperature, are depicted in Fig. 3. The remarkable crystallinity of the Ni0.5Mg0.5Fe1.7Mn0.3O4 sample is confirmed by the Raman spectra presented in Fig. 3. A total of six distinct Raman bands were detected at the respective wavenumbers of 189, 323, 473, 523, 650, and 691 cm−1. The detected Raman bands, have distinct characteristics that align with the crystal structure of MgFe2O4, namely the cubic spinel configuration. This crystal structure is commonly associated with the Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group.35 Based on prior research, it has been shown that the Raman bands detected at a wavenumber of 189 cm−1 can be attributed to the T2g(3) mode, which signifies the vibrations of the localized lattice within the octahedral sub-lattice.28 Nevertheless, it has been observed that two separate Raman bands have been found within the frequency range of 300–500 cm−1. The bands seen in this study are associated with the Eg and T2g(2) vibrational modes. Specifically, these bands are allocated to the symmetric and asymmetric bending vibrations of the A/B–O tetrahedron.23 In contrast, the three Raman peaks detected at elevated wavenumbers, namely at 523, 650, and 691 cm−1, are ascribed to the asymmetric and symmetric stretching vibrations of oxygen atoms that are linked to metal ions situated within tetrahedral sites. The vibrational modes are designated as T2g(1) for asymmetric stretching vibrations and A1g for symmetric stretching vibrations, respectively. The Raman bands observed at a frequency greater than 600 cm−1 are attributed to the vibrational modes of the A-site. The A1g mode exhibits a distinct separation into two distinct modes at about 650 and 691 cm−1, which can be attributed to the presence of various ions, specifically Mg2+ and Ni2+, within the tetrahedral site.36 This observation confirms the presence of a pure cubic spinel phase in the material that was synthesized.
m space group.35 Based on prior research, it has been shown that the Raman bands detected at a wavenumber of 189 cm−1 can be attributed to the T2g(3) mode, which signifies the vibrations of the localized lattice within the octahedral sub-lattice.28 Nevertheless, it has been observed that two separate Raman bands have been found within the frequency range of 300–500 cm−1. The bands seen in this study are associated with the Eg and T2g(2) vibrational modes. Specifically, these bands are allocated to the symmetric and asymmetric bending vibrations of the A/B–O tetrahedron.23 In contrast, the three Raman peaks detected at elevated wavenumbers, namely at 523, 650, and 691 cm−1, are ascribed to the asymmetric and symmetric stretching vibrations of oxygen atoms that are linked to metal ions situated within tetrahedral sites. The vibrational modes are designated as T2g(1) for asymmetric stretching vibrations and A1g for symmetric stretching vibrations, respectively. The Raman bands observed at a frequency greater than 600 cm−1 are attributed to the vibrational modes of the A-site. The A1g mode exhibits a distinct separation into two distinct modes at about 650 and 691 cm−1, which can be attributed to the presence of various ions, specifically Mg2+ and Ni2+, within the tetrahedral site.36 This observation confirms the presence of a pure cubic spinel phase in the material that was synthesized.
The size and morphology of particles significantly impact the material's electrochemical performance. Creating active materials at the nanoscale is a highly efficient approach to enhancing electrode kinetics. Nanoparticles provide a larger contact area between the electrode and electrolyte, shorten the pathway for Li+ ion diffusion and electron movement, and increase the number of electrochemically active sites.37
The morphology of the as-prepared Ni0.5Mg0.5Fe1.7Mn0.3O4 sample was investigated by SEM analysis, as illustrated in Fig. 4(a)–(c). The prepared material demonstrates the presence of spherical particles, with an average diameter of around 217 nm. Besides, EDS elemental mapping of Ni0.5Mg0.5Fe1.7Mn0.3O4 is illustrated in Fig. 4(d)–(h) in which the signals corresponding to O, Mg, Ni, Fe, and Mn elements are detected with well-distributed elements and concentrated on the particles without obvious element segregation. Elemental analysis of the synthesized material was conducted using EDS spectroscopy. The surface elemental composition of the Ni0.5Mg0.5Fe1.7Mn0.3O4 material was determined using EDX analysis. Fig. S1, which can be found in the ESI,† provides evidence of the presence of nickel (Ni), magnesium (Mg), iron (Fe), manganese (Mn), and oxygen (O) in the as-synthesized sample, thereby confirming its purity.
 | ||
| Fig. 4 (a)–(c) SEM images of Ni0.5Mg0.5Fe1.7Mn0.3O4 material. (d)–(h) EDS mapping images of the O, Ni, Mg, Fe, and Mn elements for Ni0.5Mg0.5Fe1.7Mn0.3O4 material. | ||
TEM analysis was used to observe the inner microstructure of the uncoated and carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4 materials. The images are presented in Fig. 5 and Fig. S2 (ESI†). Fig. S2 (ESI†) shows that there is no carbon layer on the surface of the uncoated Ni0.5Mg0.5Fe1.7Mn0.3O4 nanoparticles. However, Fig. 5(a)–(e) reveal amorphous carbon coating layers on the surface of the carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C nanoparticles. The homogeneity of various elements in the carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C powder was examined through high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) along with corresponding elemental mapping technique. The images produced by HAADF-STEM and energy-dispersive spectroscopy (EDS) mapping of Ni0.5Mg0.5Fe1.7Mn0.3O4/C, are presented in Fig. 5(f)–(l). These images reveal a consistent distribution of C, O, Ni, Mg, Fe, and Mn elements within the carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C nanoparticles.
 | ||
| Fig. 5 (a)–(e) TEM images of carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C, (f) STEM image, and (g)–(l) corresponding elemental mapping images of the Ni0.5Mg0.5Fe1.7Mn0.3O4/C sample. | ||
The electrochemical performance of Ni0.5Mg0.5Fe1.7Mn0.3O4 and Ni0.5Mg0.5Fe1.7Mn0.3O4/C were evaluated by cyclic voltammetry and galvanostatic charge/discharge experiments at various current densities. These tests were conducted at room temperature, within a potential range ranging from 0.01 V to 3.0 V. Fig. 6 illustrates the cyclic voltammetry profile of the Ni0.5Mg0.5Fe1.7Mn0.3O4 electrode within the voltage range of 0.01–3.0 V, with a scan rate of 1 mV s−1. The initial cycle of the Ni0.5Mg0.5Fe1.7Mn0.3O4 sample exhibits notable distinctions compared to subsequent cycles. This disparity can be attributed to the cathodic lithiation process during the first cycle, wherein two cathodic peaks are observed. A pronounced reduction peak is observed at a potential of 0.01 V (versus Li+/Li), which is succeeded by a subsequent reduction peak occurring at 0.74 V (versus Li+/Li). These peaks are likely a result of the incorporation of Li+ ions into the Ni0.5Mg0.5Fe1.7Mn0.3O4 material through a series of multistep reactions, these reactions could be assigned to the cations reduced to metallic status (Fe3+/Fe0, Mn3+/Mn0, and Ni2+/Ni0 without Mg2+ reduction to Mg0 due to the high bond energy of MgO38) with the formation of SEI films and the generation of amorphous Li2O (eqn (1)).36,39 However, in this case, it can be observed that the cathodic peak at 0.01 V is indicative of the distinctive behavior associated with the insertion of Li+ ions into the Ni0.5Mg0.5Fe1.7Mn0.3O4 material.40 The initial anodic peak observed at 1.8 V can be ascribed to the oxidation processes of iron (Fe) to ferric ions (Fe3+), nickel (Ni) to nickel ions (Ni2+), and manganese (Mn) to manganese ions (Mn2+), facilitated by the creation of iron(III) oxide Fe2O3 (eqn (3)), MnO (eqn (4)), and NiO (eqn (2)) phases with the decomposition of the Li2O phase.7,25 Additionally, the pronounced cathodic peak recorded at a potential of 0.01 V (vs. Li+/Li) during the initial cathodic peak could be ascribed to the formation of the solid–electrolyte interphase (SEI) layer, which arises from the decomposition of the electrolyte. This phenomenon is widely recognized for its significant contribution to the substantial reduction in capacity observed during the initial discharge cycle.41 In the subsequent four CV cycles, they have similar cathodic and anodic peaks at 0.65 V and 1.9 V, respectively, The cathodic peaks for the subsequent four cycles could be linked to the reduction of NiO, Fe2O3, and MnO to Ni, Fe, and Mn, respectively. The following anodic process might be attributed to the oxidation of metallic nickel (Ni) to divalent ions (Ni2+), metallic iron (Fe) to ferric ions (Fe3+), and metallic manganese (Mn) to divalent ions (Mn2+), indicating similar kinetics during the charge and discharge of the Ni0.5Mg0.5Fe1.7Mn0.3O4 material.
 | ||
| Fig. 6 Cyclic voltammetry profile in the voltage range of 0.01–3.0 V at a scan rate of 1 mV s−1 of the Ni0.5Mg0.5Fe1.7Mn0.3O4 electrode. | ||
Based on the literature,25,26,42,43 and the analysis that has been previously discussed, the electrochemical reactions of the Ni0.5Mg0.5Fe1.7Mn0.3O4 compound can be described as follows:
| Ni0.5Mg0.5Fe1.7Mn0.3O4 + 7.1Li+ + 7.1e− → 0.5Ni + 0.5MgO + 1.7Fe + 0.3Mn + 3.55Li2O | (1) |
| 0.5Ni + 0.5Li2O ↔ 0.5NiO + Li+ + 1e− | (2) |
| (1.7/2)Fe2O3 + 5.1Li+ + 5.1e− ↔ 1.7Fe + 2.55Li2O | (3) |
| 0.3MnO + 0.6Li+ + 0.6e− ↔ 0.3Mn + 0.3Li2O | (4) |
To improve the cycling stability of Ni0.5Mg0.5Fe1.7Mn0.3O4 material, a carbon coating process was adopted. The carbon coating was demonstrated to improve the metal oxide's electrochemical stability during long-term cycling. The carbon prevents the metal oxide particle agglomeration and maintains the structure's stability. Additionally, it participates in reducing the volume change during discharge/charge processes and facilitates the Li+ ion diffusion into the metal oxide's structure.28,44 The charge–discharge profile of both uncoated and coated anode materials is depicted in Fig. 7(a) and (b). The data were obtained by conducting experiments within the voltage range of 0.01–3 V (vs. Li/Li+) and at a current density of 100 mA g−1 for LIBs. The initial discharge and charge capacities for the uncoated and coated materials were determined to be 1275/874 and 1032/723 mA h g−1, respectively, accompanied by initial coulombic efficiencies (ICE) of 68.54% and 70.06%, respectively. Consequently, the first coulombic efficiency of the Ni0.5Mg0.5Fe1.7Mn0.3O4/C anode is slightly higher than that of the uncoated Ni0.5Mg0.5Fe1.7Mn0.3O4 anode. This improvement may be attributed to the carbon layer, which protects the electrode materials from direct contact with the liquid electrolyte.45 In addition, it is important to note that the initial capacity reduction during the first cycle is mainly due to the formation of a solid electrolyte interphase (SEI) layer on the electrode surface, accompanied by the generation of the inactive magnesium oxide phase (MgO).46 In the 75th cycle, the charge capacities of uncoated and carbon-coated anode materials were 744 mA h g−1 and 806 mA h g−1 respectively. From the long-term cycling, the coated material shows an increase in capacity after 75 cycles compared to the uncoated material which shows a decrease in capacity, as indicated in Fig. 7(c). Moreover, the carbon-coated material displayed a capacity retention of approximately 89.7%, while reaching nearly 100% coulombic efficiency in the 75th cycle. The details of the electrochemical properties of these two electrodes are shown in Table S2 (ESI†). The excellent cycle stability of the Ni0.5Mg0.5Fe1.7Mn0.3O4 material after carbon coating can be attributed to the stable solid electrolyte interphase (SEI) formed between the electrode and the electrolyte. Additionally, the improvement in the cycling stability of the coated material, compared to the uncoated sample, is a result of enhanced mechanical stability and stress resistance due to volume changes during the charge/discharge process. The coating limits the material's volume expansion by limiting its capacity; indeed, the anode did not reach its maximum capacity. Consequently, the volume change during lithiation is reduced, leading to improved stability.47 As a result, both the electrochemical performance and cycling stability of the electrode are enhanced.
To better understand the advantages of carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4 nanoparticles in lithium-based energy storage, we compared the performance of uncoated Ni0.5Mg0.5Fe1.7Mn0.3O4 and carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C rate capability in terms of Li+ insertion/extraction (Fig. 8(a)). The two electrodes were cycled at different current rates of 0.03C, 0.06C, 0.125C, 0.25C, 0.625C, 1.25C and 2C, corresponding to 25, 50, 100, 200, 500, 1000 and 1600 mA g−1. The coated electrode cell delivers average charge capacities of 720, 778, 760, 731, 690, 651, and 372 mA h g−1. At the highest current density of 1600 mA g−1, Ni0.5Mg0.5Fe1.7Mn0.3O4/C delivers 372 mA h g−1 instead of the limited capacity of 192 mA h g−1 for Ni0.5Mg0.5Fe1.7Mn0.3O4. More importantly, when the current density was returned to 25 mA g−1, a large irreversible capacity of 818 mA h g−1 was recovered, showing a strong tolerance to the rapid insertion/extraction of Li+ ions. To further evaluate the long-term cycling performance at high current density, uncoated Ni0.5Mg0.5Fe1.7Mn0.3O4, and carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C electrodes were charged and discharged for 100 cycles at 2C for comparison. As shown in Fig. 8(b), the uncoated material Ni0.5Mg0.5Fe1.7Mn0.3O4 experienced an apparent capacity fading after 50 cycles and its capacity retention reached only 18.24% after 100 cycles, while the Ni0.5Mg0.5Fe1.7Mn0.3O4/C still retained 91.17% of the initial discharge capacity after 100 cycles, reflecting the unstable structure of the uncoated Ni0.5Mg0.5Fe1.7Mn0.3O4 and the stable structure of the carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C. The details of the electrochemical properties of these two electrodes are shown in Table S2 (ESI†).
To understand the carbon coating effect in more detail, electrochemical impedance spectroscopy (EIS) tests were performed before and after 100 cycles at a high current rate of 2C (100 mA g−1 = 0.125C). These tests were performed in the frequency range of 100 kHz to 0.01 Hz using cells with uncoated Ni0.5Mg0.5Fe1.7Mn0.3O4 and carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C electrodes (Fig. 8(c) and (d)). From Fig. 8(c) and (d), the observed impedance spectra showed a semicircle in the high-frequency region, corresponding to the charge transfer resistance due to the transport of lithium ions across the electrode/electrolyte interface, and an inclined straight line in the low-frequency region, corresponding to the Warburg impedance due to the diffusion of Li+ ions into the electrode materials.48Fig. 8(c) and (d) show the equivalent circuit model, consisting of the contact resistance (Rs), surface film resistance (Rf), charge transfer resistance (Rct) and Warburg impedance (W4); the fitting parameters are summarized in Table S3 (ESI†).49 The Rtotal (where Rtotal = Rs + Rf + Rct) can be closely monitored to explore the origin of the electrochemical properties of LiB cells in practice. A small Rtotal indicates improved cycling performance and rate capability of LiB. From the equivalent circuit model, the fitted Rct parameter for cells before cycling are 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 646 and 10
646 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 237 Ω for uncoated Ni0.5Mg0.5Fe1.7Mn0.3O4 and carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C electrodes, respectively. The lower Rct value of the carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C electrode suggests a higher charge diffusion efficiency in this novel nanostructure.50 Meanwhile, Rct decreased to 2145 and 400 Ω after 100 cycles at a 2C rate for uncoated Ni0.5Mg0.5Fe1.7Mn0.3O4 and carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C electrodes, respectively. It is observed that the Rct values after 100 cycles were lower than those of the cell before cycling, indicating a lower charge transfer resistance, which suggests an improvement in electron transport during the repeated lithiation and delithiation processes. For Rtotal, the results indicate that the carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C electrode has a significantly lower total resistance after cycling compared to the uncoated Ni0.5Mg0.5Fe1.7Mn0.3O4 and carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C electrodes before cycling, indicating improved stability and rate capability performance. Thus, the EIS result shows that the Ni0.5Mg0.5Fe1.7Mn0.3O4/C composite electrode has higher electrical conductivity compared to the uncoated Ni0.5Mg0.5Fe1.7Mn0.3O4 material, resulting in stable and higher reversible capacity.
237 Ω for uncoated Ni0.5Mg0.5Fe1.7Mn0.3O4 and carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C electrodes, respectively. The lower Rct value of the carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C electrode suggests a higher charge diffusion efficiency in this novel nanostructure.50 Meanwhile, Rct decreased to 2145 and 400 Ω after 100 cycles at a 2C rate for uncoated Ni0.5Mg0.5Fe1.7Mn0.3O4 and carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C electrodes, respectively. It is observed that the Rct values after 100 cycles were lower than those of the cell before cycling, indicating a lower charge transfer resistance, which suggests an improvement in electron transport during the repeated lithiation and delithiation processes. For Rtotal, the results indicate that the carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C electrode has a significantly lower total resistance after cycling compared to the uncoated Ni0.5Mg0.5Fe1.7Mn0.3O4 and carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C electrodes before cycling, indicating improved stability and rate capability performance. Thus, the EIS result shows that the Ni0.5Mg0.5Fe1.7Mn0.3O4/C composite electrode has higher electrical conductivity compared to the uncoated Ni0.5Mg0.5Fe1.7Mn0.3O4 material, resulting in stable and higher reversible capacity.
The Ni0.5Mg0.5Fe1.7Mn0.3O4/C carbon-coated electrode exhibits excellent cycling performance and rate capability compared to the uncoated Ni0.5Mg0.5Fe1.7Mn0.3O4 electrode, which is attributed to the protective carbon coating layer which protects the Ni0.5Mg0.5Fe1.7Mn0.3O4 nanoparticles from direct contact with the electrolyte, thereby maintaining the structural integrity of the nanoparticles during the lithiation–delithiation process. Furthermore, the carbon layers not only mitigate the volume expansion of Ni0.5Mg0.5Fe1.7Mn0.3O4 nanoparticles but also maintain stable electronic conductivity during the lithiation–delithiation process impedance text ref. 51.
To evaluate the performance of the carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C material in a complete battery system, a full cell was designed using an NMC811 cathode (Fig. 9(a) and (b)). The full cell's output voltage was 2.8 V at 0.125C current rate, within the voltage window of 1–4 V, matching the voltage difference between the NMC811 cathode and Ni0.5Mg0.5Fe1.7Mn0.3O4/C anode. Furthermore, in Fig. 9(a), the charge/discharge cycles of the full cell at a 0.125C current rate are illustrated within the voltage range of 1–4 V. The full cell exhibited a capacity of 755 mA h g−1 with a coulombic efficiency of 65% during the first cycle, based on the mass of the Ni0.5Mg0.5Fe1.7Mn0.3O4/C anode. Subsequently, after 4 cycles, the capacity decreased to 633 mA h g−1, with an increase in the coulombic efficiency to 97%. The cycling performance of the full cell was investigated at 2C within the voltage window of 1–4 V (Fig. 9(b)). After 30 cycles, a capacity of 448 mA h g−1 was achieved with a capacity retention of 91% and a coulombic efficiency of 98%. The low capacity of the Ni0.5Mg0.5Fe1.7Mn0.3O4/C electrode in the full cell is mainly due to its low first coulombic efficiency, which did not exceed 65%. The low first coulombic efficiency of the Ni0.5Mg0.5Fe1.7Mn0.3O4/C anode may be attributed to various factors, such as the inadequate balance between the positive and negative electrodes, electrolyte decomposition during cycling, and other factors.
Fig. 10 shows the reversible capacity, the average potential, and the estimated energy density of anode materials for lithium full cells. In this study, we demonstrate that the Ni0.5Mg0.5Fe1.7Mn0.3O4/C composite material displays a reversible capacity above 800 mA h g−1. This value is notably 2–3 times greater than the reversible capacity observed in the graphite anode material,35 and also shows a high specific capacity compared to the spinel Li4Ti5O12,52 TiO2,53 and Li3VO4,54 anode materials. Furthermore, the energy density of spinel oxide Ni0.5Mg0.5Fe1.7Mn0.3O4/C Li-ion cell is calculated to be 360 Wh kg−1 based on the capacity and the average potential of positive and negative electrodes with a relatively high operating voltage of 1.1 V versus Li/Li+ compared to the traditional graphite.
4. Conclusion
In summary, Ni0.5Mg0.5Fe1.7Mn0.3O4 spinel ferrite oxide nanoparticles were prepared using a simple and efficient sol–gel synthesis method and then coated with carbon. Analysis using X-ray diffraction and Raman spectroscopy confirmed that the synthesized materials have a single cubic spinel phase with the space group Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, and also confirmed the presence of an amorphous carbon layer on Ni0.5Mg0.5Fe1.7Mn0.3O4/C nanoparticles. Scanning electron microscopy analysis highlighted the existence of spherical nanoparticles in the synthesized substance, with a particle size of 217 nm. Further confirmation of the existence of the carbon layer was obtained through TEM analysis. The Ni0.5Mg0.5Fe1.7Mn0.3O4 electrode was developed as an anode for lithium-ion batteries and demonstrated an initial specific capacity of over 874 mA h g−1, as well as a capacity retention of approximately 85% after 75 cycles. Carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C nanoparticles exhibited excellent cycling stability as an anode for LIBs, with a capacity retention of approximately 89.7%. Furthermore, the carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C showed enhanced capacity, stability, and rate capability over extended cycling tests, underscoring the value of the carbon coating in improving the material's electrochemical performance. The combination of Ni0.5Mg0.5Fe1.7Mn0.3O4/C material in a full-cell configuration with an NMC811 cathode demonstrated promising results, including high specific capacity, and stability after 30 cycles at 2C. These findings suggest that this material could serve as a promising candidate for next-generation anode materials in lithium-ion batteries.
m, and also confirmed the presence of an amorphous carbon layer on Ni0.5Mg0.5Fe1.7Mn0.3O4/C nanoparticles. Scanning electron microscopy analysis highlighted the existence of spherical nanoparticles in the synthesized substance, with a particle size of 217 nm. Further confirmation of the existence of the carbon layer was obtained through TEM analysis. The Ni0.5Mg0.5Fe1.7Mn0.3O4 electrode was developed as an anode for lithium-ion batteries and demonstrated an initial specific capacity of over 874 mA h g−1, as well as a capacity retention of approximately 85% after 75 cycles. Carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C nanoparticles exhibited excellent cycling stability as an anode for LIBs, with a capacity retention of approximately 89.7%. Furthermore, the carbon-coated Ni0.5Mg0.5Fe1.7Mn0.3O4/C showed enhanced capacity, stability, and rate capability over extended cycling tests, underscoring the value of the carbon coating in improving the material's electrochemical performance. The combination of Ni0.5Mg0.5Fe1.7Mn0.3O4/C material in a full-cell configuration with an NMC811 cathode demonstrated promising results, including high specific capacity, and stability after 30 cycles at 2C. These findings suggest that this material could serve as a promising candidate for next-generation anode materials in lithium-ion batteries.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank Office Chérifien des Phosphates (OCP S. A.), and Mohammed VI Polytechnic University for their invaluable financial support. Additionally, special thanks are due to the Argonne National Laboratory for providing the NMC811 electrodes utilized in this study.References
- D. Darbar, M. V. Reddy, S. Sundarrajan, R. Pattabiraman, S. Ramakrishna and B. V. R. Chowdari, Anodic electrochemical performances of MgCo2O4 synthesized by oxalate decomposition method and electrospinning technique for Li-ion battery application, Mater. Res. Bull., 2016, 73, 369–376 CrossRef CAS.
- S. Yuvaraj, R. K. Selvan and Y. S. Lee, RSC Adv., 2016, 6, 21448–21474 RSC.
- M. T. Jeena, T. Bok, S. H. Kim, S. Perk, J. Y. Kim, S. Park and J. H. Ryu, A siloxane-incorporated copolymer as an in situ cross-linkable binder for high performance silicon anodes in Li-ion batteries, Nanoscale, 2016, 8, 9245–9253 RSC.
- F. Cheng, J. Liang, Z. Tao and J. Chen, Functional materials for rechargeable batteries, Adv. Mater., 2011, 23, 1695–1715 CrossRef CAS PubMed.
- F. Cheng, Z. Tao, J. Liang and J. Chen, Template-directed materials for rechargeable lithium-ion batteries, Chem. Mater., 2008, 20, 667–681 CrossRef CAS.
- J. Asenbauer, T. Eisenmann, M. Kuenzel, A. Kazzazi, Z. Chen and D. Bresser, Sustainable Energy Fuels, 2020, 4, 5387–5416 RSC.
- J. Liu, R. Wang, X. Zhong, K. Yan, Y. Li and Z. Xu, Li and Na storage behaviours of MgFe2O4 nanoparticles as anode materials for lithium ion and sodium ion batteries, Int. J. Electrochem. Sci., 2019, 14, 1725–1732 CrossRef CAS.
- Z. H. Li, T. P. Zhao, X. Y. Zhan, D. S. Gao, Q. Z. Xiao and G. T. Lei, High capacity three-dimensional ordered macroporous CoFe2O4 as anode material for lithium ion batteries, Electrochim. Acta, 2010, 55, 4594–4598 CrossRef CAS.
- N. Schweikert, H. Hahn and S. Indris, Cycling behaviour of Li/Li4Ti5O12 cells studied by electrochemical impedance spectroscopy, Phys. Chem. Chem. Phys., 2011, 13, 6234–6240 RSC.
- J. Lim, E. Choi, V. Mathew, D. Kim, D. Ahn, J. Gim, S.-H. Kang and J. Kim, Enhanced High-Rate Performance of Li[sub 4]Ti[sub 5]O[sub 12] Nanoparticles for Rechargeable Li-Ion Batteries, J. Electrochem. Soc., 2011, 158, A275 CrossRef CAS.
- J. Wang, D. Kober, G. Shao, J. D. Epping, O. Görke, S. Li, A. Gurlo and M. F. Bekheet, Stable anodes for lithium-ion batteries based on tin-containing silicon oxycarbonitride ceramic nanocomposites, Mater. Today Energy, 2022 DOI:10.1016/j.mtener.2022.100989.
- G. N. Zhu, H. J. Liu, J. H. Zhuang, C. X. Wang, Y. G. Wang and Y. Y. Xia, Carbon-coated nano-sized Li4Ti5O12 nanoporous micro-sphere as anode material for high-rate lithium-ion batteries, Energy Environ. Sci., 2011, 4, 4016–4022 RSC.
- Y. Pan, Y. Zhang, X. Wei, C. Yuan, J. Yin, D. Cao and G. Wang, MgFe2O4 nanoparticles as anode materials for lithium-ion batteries, Electrochim. Acta, 2013, 109, 89–94 CrossRef CAS.
- J. G. Kim, Y. Noh, Y. Kim, S. Lee and W. B. Kim, Formation of ordered macroporous ZnFe2O4 anode materials for highly reversible lithium storage, Chem. Eng. J., 2019, 372, 363–372 CrossRef CAS.
- S. Mitra, P. S. Veluri, A. Chakraborthy and R. K. Petla, Electrochemical Properties of Spinel Cobalt Ferrite Nanoparticles with Sodium Alginate as Interactive Binder, ChemElectroChem, 2014, 1, 1068–1074 CrossRef CAS.
- M. Mujahid, R. Ullah Khan, M. Mumtaz, Mubasher, S. A. Soomro and S. Ullah, NiFe2O4 nanoparticles/MWCNTs nanohybrid as anode material for lithium-ion battery, Ceram. Int., 2019, 45, 8486–8493 CrossRef CAS.
- Z. Xing, Z. Ju, J. Yang, H. Xu and Y. Qian, One-step solid state reaction to selectively fabricate cubic and tetragonal CuFe2O4 anode material for high power lithium ion batteries, Electrochim. Acta, 2013, 102, 51–57 CrossRef CAS.
- D. Narsimulu, B. N. Rao, M. Venkateswarlu, E. S. Srinadhu and N. Satyanarayana, Electrical and electrochemical studies of nanocrystalline mesoporous MgFe2O4 as anode material for lithium battery applications, Ceram. Int., 2016, 42, 16789–16797 CrossRef CAS.
- F. Wang, Y. Liu, Y. Zhao, Y. Wang, Z. Wang, W. Zhang and F. Ren, Facile synthesis of two-dimensional porous MgCo2O4 nanosheets as anode for lithium-ion batteries, Appl. Sci., 2018, 8(1), 22 CrossRef.
- K. Kouchi, M. Tayoury, A. Chari, Z. Chchiyai, L. Hdidou, Y. Tamraoui, J. Alami, B. Manoun and M. Dahbi, in 2021 9th International Renewable and Sustainable Energy Conference (IRSEC), IEEE, 2021, pp. 1–5.
- T. Li, X. Li, Z. Wang, H. Guo and Y. Li, A novel NiCo2O4 anode morphology for lithium-ion batteries, J. Mater. Chem. A, 2015, 3, 11970–11975 RSC.
- C. T. Cherian, J. Sundaramurthy, M. V. Reddy, P. Suresh Kumar, K. Mani, D. Pliszka, C. H. Sow, S. Ramakrishna and B. V. R. Chowdari, Morphologically robust NiFe2O4 nanofibers as high capacity Li-Ion battery anode material, ACS Appl. Mater. Interfaces, 2013, 5, 9957–9963 CrossRef CAS PubMed.
- S. J. Rajoba, R. D. Kale, S. B. Kulkarni, V. G. Parale, R. Patil, H. Olin, H. H. Park, R. P. Dhavale and M. Phadatare, Synthesis and electrochemical performance of mesoporous NiMn2O4 nanoparticles as an anode for lithium-ion battery, J. Compos. Sci., 2021, 5(3), 69 CrossRef CAS.
- K. Cai, S. hua Luo, J. Cong, K. Li, S. xue Yan, P. qing Hou, Y. Song, Q. Wang, Y. Zhang, X. Liu, X. Lei, W. Mu and J. Gao, Sol–gel synthesis of nano block-like ZnMn2O4 using citric acid complexing agent and electrochemical performance as anode for lithium-ion batteries, J. Alloys Compd., 2022, 909, 164882 CrossRef CAS.
- Z. Chchiyai, L. Hdidou, M. Tayoury, A. Chari, Y. Tamraoui, J. Alami, M. Dahbi and B. Manoun, Synthesis and electrochemical properties of Mn-doped porous Mg0.9Zn0.1Fe2−xMnxO4 (0 ≤ x ≤ 1.25) spinel oxides as anode materials for lithium-ion batteries, J. Alloys Compd., 2023, 935, 167997 CrossRef CAS.
- N. Huo, Y. Yin, W. Liu, J. Zhang, Y. Ding, Q. Wang, Z. Shi and S. Yang, Facile synthesis of MgFe2O4/C composites as anode materials for lithium-ion batteries with excellent cycling and rate performance, New J. Chem., 2016, 40, 7068–7074 RSC.
- L. Luo, D. Li, J. Zang, C. Chen, J. Zhu, H. Qiao, Y. Cai, K. Lu, X. Zhang and Q. Wei, Carbon-Coated Magnesium Ferrite Nanofibers for Lithium-Ion Battery Anodes with Enhanced Cycling Performance, Energy Technol., 2017, 5, 1364–1372 CrossRef CAS.
- Y. Yu, M. Li, Q. Li, J. Zhang, M. Sun, W. Qi and J. Li, Core–shell MgFe2O4@C nano-composites derived via thermal decomposition-reduction dual strategy for superior lithium storage, J. Alloys Compd., 2020, 834, 155207 CrossRef CAS.
- J. Mao, X. Hou, X. Wang, G. He, Z. Shao and S. Hu, Corncob-shaped ZnFe2O4/C nanostructures for improved anode rate and cycle performance in lithium-ion batteries, RSC Adv., 2015, 5, 31807–31814 RSC.
- M. Zhang, X. Yang, X. Kan, X. Wang, L. Ma and M. Jia, Carbon-encapsulated CoFe2O4/graphene nanocomposite as high performance anode for lithium ion batteries, Electrochim. Acta, 2013, 112, 727–734 CrossRef CAS.
- S. Li, B. Wang, J. Liu and M. Yu, In situ one-step synthesis of CoFe2O4/graphene nanocomposites as high-performance anode for lithium-ion batteries, Electrochim. Acta, 2014, 129, 33–39 CrossRef CAS.
- H. Xia, D. Zhu, Y. Fu and X. Wang, CoFe2O4-graphene nanocomposite as a high-capacity anode material for lithium-ion batteries, Electrochim. Acta, 2012, 83, 166–174 CrossRef CAS.
- L. Luo, D. Li, J. Zang, C. Chen, J. Zhu, H. Qiao, Y. Cai, K. Lu, X. Zhang and Q. Wei, Carbon-Coated Magnesium Ferrite Nanofibers for Lithium-Ion Battery Anodes with Enhanced Cycling Performance, Energy Technol., 2017, 5, 1364–1372 CrossRef CAS.
- W. B. White and B. A. DeAngelis, Interpretation of the vibrational spectra of spinels, Spectrochim. Acta, Part A, 1967, 23, 985–995 CrossRef CAS.
- J. Chandradass, A. H. Jadhav, K. H. Kim and H. Kim, Influence of processing methodology on the structural and magnetic behavior of MgFe2O4 nanopowders, J. Alloys Compd., 2012, 517, 164–169 CrossRef CAS.
- C. Murugesan and G. Chandrasekaran, Structural and Magnetic Properties of Mn1−xZnxFe2O4 Ferrite Nanoparticles, J. Supercond. Novel Magn., 2016, 29, 2887–2897 CrossRef CAS.
- J. Zhang, J. Qiao, K. Sun and Z. Wang, Balancing particle properties for practical lithium-ion batteries, Particuology, 2022, 61, 18–29 CrossRef CAS.
- X. Wang, G. Zhai and H. Wang, Facile synthesis of MgCo2O4 nanowires as binder-free flexible anode materials for high-performance Li-ion batteries, J. Nanopart. Res., 2015, 17, 339 CrossRef.
- M. Islam, G. Ali, M. G. Jeong, W. Choi, K. Y. Chung and H. G. Jung, Study on the Electrochemical Reaction Mechanism of NiFe2O4 as a High-Performance Anode for Li-Ion Batteries, ACS Appl. Mater. Interfaces, 2017, 9, 14833–14843 CrossRef CAS PubMed.
- Y. Liu, N. Zhang, C. Yu, L. Jiao and J. Chen, MnFe2O4@C Nanofibers as High-Performance Anode for Sodium-Ion Batteries, Nano Lett., 2016, 16, 3321–3328 CrossRef CAS PubMed.
- C. Yue, Z. Liu, W. J. Chang, W. Il Park and T. Song, Hollow C nanobox: An efficient Ge anode supporting structure applied to high-performance Li ion batteries, Electrochim. Acta, 2018, 290, 236–243 CrossRef CAS.
- G. Huang, F. Zhang, L. Zhang, X. Du, J. Wang and L. Wang, Hierarchical NiFe2O4/Fe2O3 nanotubes derived from metal organic frameworks for superior lithium ion battery anodes, J. Mater. Chem. A, 2014, 2, 8048–8053 RSC.
- J. G. Kim, Y. Noh, Y. Kim, S. Lee and W. B. Kim, Formation of ordered macroporous ZnFe2O4 anode materials for highly reversible lithium storage, Chem. Eng. J., 2019, 372, 363–372 CrossRef CAS.
- Y. Luo, C. Chen, L. Chen, M. Zhang and T. Wang, 3D reticular pomegranate-like CoMn2O4/C for ultrahigh rate lithium-ion storage with re-oxidation of manganese, Electrochim. Acta, 2017, 241, 244–251 CrossRef CAS.
- L. Yao, X. Hou, S. Hu, J. Wang, M. Li, C. Su, M. O. Tade, Z. Shao and X. Liu, Green synthesis of mesoporous ZnFe2O4/C composite microspheres as superior anode materials for lithium-ion batteries, J. Power Sources, 2014, 258, 305–313 CrossRef CAS.
- F. Luo, D. Ma, Y. Li, H. Mi, P. Zhang and S. Luo, Hollow Co3S4/C anchored on nitrogen-doped carbon nanofibers as a free-standing anode for high-performance Li-ion batteries, Electrochim. Acta, 2019, 299, 173–181 CrossRef CAS.
- N. Huo, Y. Yin, W. Liu, J. Zhang, Y. Ding, Q. Wang, Z. Shi and S. Yang, Facile synthesis of MgFe2O4/C composites as anode materials for lithium-ion batteries with excellent cycling and rate performance, New J. Chem., 2016, 40, 7068–7074 RSC.
- L. Luo, D. Li, J. Zang, C. Chen, J. Zhu, H. Qiao, Y. Cai, K. Lu, X. Zhang and Q. Wei, Carbon-Coated Magnesium Ferrite Nanofibers for Lithium-Ion Battery Anodes with Enhanced Cycling Performance, Energy Technol., 2017, 5, 1364–1372 CrossRef CAS.
- D. Narsimulu, B. N. Rao, N. Satyanarayana and E. S. Srinadhu, High Capacity Electrospun MgFe2O4–C Composite Nanofibers as an Anode Material for Lithium Ion Batteries, ChemistrySelect, 2018, 3, 8010–8017 CrossRef CAS.
- J. Wang, G. Yang, L. Wang, W. Yan and W. Wei, C@CoFe2O4 fiber-in-tube mesoporous nanostructure: Formation mechanism and high electrochemical performance as an anode for lithium-ion batteries, J. Alloys Compd., 2017, 693, 110–117 CrossRef CAS.
- L. Lin and Q. Pan, ZnFe2O4@C/graphene nanocomposite as excellent anode materials for lithium batteries, J. Mater. Chem. A, 2015, 3, 1724–1729 RSC.
- F. Liu, B. Bai, L. Cheng and C. Xu, Rapid synthesis of Li4Ti5O12 as lithium-ion battery anode by reactive flash sintering, J. Am. Ceram. Soc., 2022, 105, 419–427 CrossRef CAS.
- N. El Halya, K. Elouardi, A. Chari, A. El Bouari, J. Alami and M. Dahbi, Chapter TiO2 Based Nanomaterials and Their Application as Anode for Rechargeable Lithium-Ion Batteries, Titanium Dioxide - Advances and Applications, 2021 Search PubMed.
- E. Elmaataouy, A. Chari, M. Tayoury, J. Alami and M. Dahbi, in 2021 9th International Renewable and Sustainable Energy Conference (IRSEC), IEEE, 2021, pp. 1–4.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cp00182f |
| This journal is © the Owner Societies 2024 |