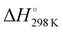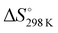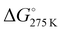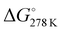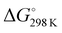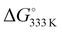Kiwi peel waste as a recyclable adsorbent to remove textile dyes from water: Direct Blue 78 removal and recovery†
Jennifer
Gubitosa
a,
Vito
Rizzi
 *a,
Paola
Fini
b,
Sergio
Nuzzo
b and
Pinalysa
Cosma
*a,
Paola
Fini
b,
Sergio
Nuzzo
b and
Pinalysa
Cosma
 ab
ab
aDipartimento di Chimica, Università degli Studi di Bari “Aldo Moro”, Via Orabona, 4-70126 Bari, Italy. E-mail: vito.rizzi@uniba.it
bConsiglio Nazionale delle Ricerche CNR-IPCF, UOS Bari, Via Orabona, 4-70126 Bari, Italy
First published on 23rd February 2024
Abstract
According to circular bioeconomy principles, the use of kiwi peels to remove Direct Blue 78 (DB) from water is investigated during this work, proposing food waste as a recyclable adsorbent substrate. Direct Blue 78 (DB) was adopted as a model pollutant, employing its visible spectrum to monitor its adsorption. The adsorption process was thus fully characterized, investigating the roles of ionic strength, pH values, adsorbent/pollutant amounts, and temperature. The thermodynamics, kinetics, and adsorption isotherms were also studied. To extend the kiwi peels’ lifetime, quite complete desorption was obtained by adopting hot water as a safe and eco-friendly strategy. Despite the relatively low kiwi peels’ maximum adsorption capacity (6 mg g−1) for DB when adsorbed in the presence of NaCl, 10 cycles of adsorption/desorption were attempted, proposing the recycling of both the dye and kiwi peels as dictated by circular economy principles. Dyeing experiments were also performed, evidencing the dye's ability to color cotton fabrics after its recycling. Finally, the removal of other textile dyes, Direct Red 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and Direct Yellow 86, was demonstrated in a mixture with DB. A preliminary investigation was performed to find the best working conditions for inducing the solid-state dye photodegradation, proposing a possible alternative for the adsorbent regeneration.
1 and Direct Yellow 86, was demonstrated in a mixture with DB. A preliminary investigation was performed to find the best working conditions for inducing the solid-state dye photodegradation, proposing a possible alternative for the adsorbent regeneration.
Introduction
Environmental disorders and pollution have become important worldwide issues for several years, and research activities in this context are still very active.1–17 Among different classes of pollutants, dyestuffs constitute an important concern because they are toxic and dangerous for human health and aquatic living systems, inducing several diseases.2,9,11–15 Currently, more than 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dye types are available on the market, and more than 7 × 105 tons are generated every year. So, their presence in water effluents is attained.18 However, dyes are not biodegradable and are very stable substances, resistant to light irradiation and heat. The degradation by using oxidizing agents cannot be considered a powerful tool due to their resistance, and, in any case, hard working conditions are needed.11,12,18 It is worth mentioning that, among textile dyes, azo dyes are extensively used and account for 70% of the total dye production.18 Due to the toxicity of amine groups in their chemical structure, azo dyes are considered hazardous compounds for the whole environment.11,12,18 Due to the large color production from industries, innovative and performant ways to remove these dyes efficiently from water should be developed, with particular attention to methods that agree with green chemistry principles, sustainability, and bioeconomy concepts.19 Several advantages arise. For this purpose, among the possible strategies used for the dye's removal (such as ion exchange, coprecipitation, filtration, reverse osmosis, Fenton oxidation, photodegradation, biodegradation, ozonation, and electrochemical reduction), the adsorption methodologies have been extensively studied for water remediation.11,12,18,20–32 Indeed, adsorption is considered a low-cost approach that should avoid the release of secondary by-products as could occur, for example, when advanced oxidation processes are used.16 Furthermore, the adsorption could be easily implemented from the laboratory to industrial scale.
000 dye types are available on the market, and more than 7 × 105 tons are generated every year. So, their presence in water effluents is attained.18 However, dyes are not biodegradable and are very stable substances, resistant to light irradiation and heat. The degradation by using oxidizing agents cannot be considered a powerful tool due to their resistance, and, in any case, hard working conditions are needed.11,12,18 It is worth mentioning that, among textile dyes, azo dyes are extensively used and account for 70% of the total dye production.18 Due to the toxicity of amine groups in their chemical structure, azo dyes are considered hazardous compounds for the whole environment.11,12,18 Due to the large color production from industries, innovative and performant ways to remove these dyes efficiently from water should be developed, with particular attention to methods that agree with green chemistry principles, sustainability, and bioeconomy concepts.19 Several advantages arise. For this purpose, among the possible strategies used for the dye's removal (such as ion exchange, coprecipitation, filtration, reverse osmosis, Fenton oxidation, photodegradation, biodegradation, ozonation, and electrochemical reduction), the adsorption methodologies have been extensively studied for water remediation.11,12,18,20–32 Indeed, adsorption is considered a low-cost approach that should avoid the release of secondary by-products as could occur, for example, when advanced oxidation processes are used.16 Furthermore, the adsorption could be easily implemented from the laboratory to industrial scale.
Due to the necessity of developing greener technologies, the innovative tendency represented by the use of wastes as adsorbent materials to remove pollutants from water has been increasing in the last few years. Particularly, the use of fruit wastes is well known, and the work of Bhatnagar et al.33 is very interesting for showing, among wastes, the use of fruit and vegetable peels for the removal of pollutants. Indeed, most fruit peels become waste without applications, with potential problems for their management. So, to face this problem, a policy is being implemented to ensure sustainable production and consumption models to achieve very important targets for the transition from linear to circular models, giving waste a new life.34 On this ground, this work proposes a sustainable management, efficient use of natural resources as dictated by bioeconomic principles, and reduction of waste through recycling to meet the requirements of the circular bioeconomy.19 An upcycling process of existing materials is thus presented, following the new eco-centric trend, valorizing the use of food wastes, particularly kiwi peels, as recyclable adsorbent materials for dye removal. Indeed, some authors of this paper recently have reported the use of this waste/adsorbent for the removal of emerging pollutants, presenting for the first time the chemical and physical features of kiwi peels.34,35 In particular, Gubitosa et al.,34,35 by the synergistic use of different techniques (such as SEM investigation, FTIR-ATR, and TG analyses), showed that the adsorbent is characterized by particular morphological features, irregular domains, and filaments on both sides of peels’ surfaces, and it is mainly constituted by lignin, cellulose, and hemicellulose. Furthermore, it was demonstrated that the main morphological and chemical features of kiwi peels were also retained after their use and reuse during the adsorption/desorption processes, enabling kiwi peels as a long-lasting adsorbent material.34,35 This work aims to widen the kiwi peels’ applicability, proposing that this waste can also be used for the anionic textile dye removal from water. Indeed, about this concern, only one work is reported in the literature, and it refers to methylene blue and rhodamine B removal.36 On the other hand, kiwi peels, to our knowledge, were employed in the past to remove Cd2+, Cr3+ and Zn2+ ions,37 nitrate,38 and oil,39 sequester Pb2+,40 and, as previously said, to remove emerging pollutants.34,35 Therefore, a deep scientific research activity should be developed to promote the sustainable management of kiwi peels’ wastes to address environmental problems related to dye pollution. Moreover, an important aspect of this paper is related to the eco-friendly kiwi peel pre-treatment, avoiding hard experimental conditions and hazardous materials, as already reported by Gubitosa et al.34,35
Furthermore, the adsorbent recycling was proposed under safe conditions, demonstrating the dyestuff reuse. More specifically, kiwi peels (i) were only washed with hot water, avoiding the use of toxic chemicals, leading to the development of an eco-design in accordance with an eco-innovation model, especially for a potential future perspective to implement the process on a large-scale; (ii) hot water was used to desorb the color, regenerating the adsorbent, dyeing cotton fibers again. In detail, to highlight the main features of the proposed adsorption process, among textile dyes, an azo one, Direct Blue 78 (DB), was selected as a model anionic compound. So, the DB adsorption process has been investigated, showing the process's mechanism, related kinetics, and thermodynamic characteristics. The DB removal was observed in the presence of an acid solution (pH 2) or salts, suggesting that, during the process, the involved interacting forces were mainly electrostatic and hydrophobic. By focusing the attention on the DB removal in the presence of salt, 10 adsorption/desorption cycles were studied, avoiding the disposal of the adsorbent as secondary hazardous waste material, recovering 90% of the DB. The adsorbent lifetime was thus extended, according to the Green Economy requests.16 The maximum adsorption capacity of kiwi peels towards DB was inferred (qmax = 6 mg g−1), and it well agrees with the values obtained by other natural adsorbents during the removal of anionic dyes.18,20–32 However, if some of the used materials showed high adsorption capacities and performances,41,42 it is worth mentioning that only a few studies26,29,30,32 discussed the adsorbent regeneration, and thus the dye desorption. In particular, concentrated NaOH solutions were proposed in these studies by proposing a long contact time and low desorption efficiency, applying only a few cycles of adsorption/desorption. In contrast, in this paper, hot water is proposed for the kiwi peel regeneration, recycling both the dye and the adsorbent for 10 cycles, which could be implemented because no change in terms of kiwi peels’ efficiency was observed. Furthermore, a dye mixture was also investigated, and kiwi peels were able to remove and desorb the mixtures of colors without any competitive effects. Additionally, focusing attention on DB, the recycled color was used again to dye cotton fibers, demonstrating its reuse. Finally, preliminary results related to the solid-state DB photodegradation after the adsorption were discussed as a potential alternative for the adsorbent regeneration by using advanced oxidation processes (AOPs). In particular, the use of TiO2 and UV light appeared as the best promising approach to obtain the DB degradation that occurred ≈75% after 8 h of irradiation time, under the proposed experimental working conditions. Novel horizons in the environmental field are thus opened, considering that this adsorbent is also able to adsorb emerging pollutants from water and desorb them in salt solutions.34,35 On the other hand, the removal of color was observed in a completely reversed situation: adsorption in salt solutions and desorption in water. So, kiwi peels, compared with other adsorbents, could be potentially considered “on-demand” adsorbents, being able to remove different classes of pollutants, according to the working conditions.
Results and discussion
Direct Blue removal by kiwi peels
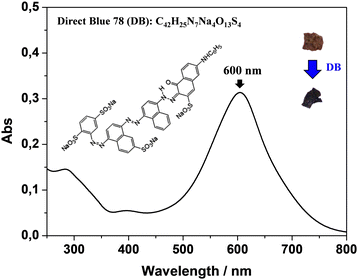
The study was carried out using visible spectroscopy as a powerful tool to rapidly monitor the amount of the anionic dye, DB, in water and consequently infer the role of parameters affecting its removal by kiwi peels’ wastes. In the visible spectrum, the DB solution showed the main maximum absorption band at λ = 600 nm (Fig. 1).It can be attributed to a π–π* transition arising from the characteristic dye chromophore, comprising azo groups (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) interacting with the adjacent aromatic moieties.11–13 This band was thus considered diagnostic to monitor the removal of color from water. As a first step, adsorption experiments were performed at room temperature by adding 50 mg of peels in distilled water at pH 6 in the presence of 13 mg L−1 of DB. The lack of DB adsorption was observed.
N–) interacting with the adjacent aromatic moieties.11–13 This band was thus considered diagnostic to monitor the removal of color from water. As a first step, adsorption experiments were performed at room temperature by adding 50 mg of peels in distilled water at pH 6 in the presence of 13 mg L−1 of DB. The lack of DB adsorption was observed.
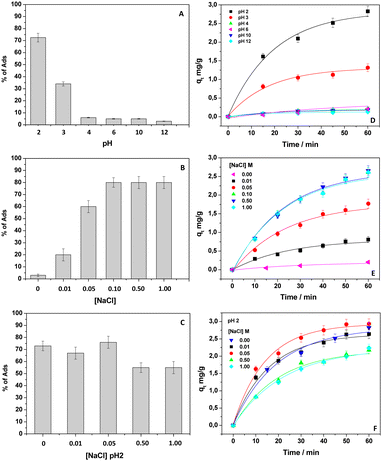
This result could be indicative of electrostatic repulsion between negatively charged DB and the kiwi peel surface.11–13,27,34 Accordingly, pHPZC of the adsorbent, experimentally calculated as reported in Fig. S1 (ESI†), occurred at around pH 4. So, it means that the adsorbent surface at pH < 4 and pH > 4 was positively and negatively charged, respectively.34 At the same time, DB's chemical features should be considered (see Fig. 1 for the DB chemical structure) to clarify the finding. The DB sulfonate groups (–SO3−) were always deprotonated, having a pKa < 2. This means that, at pH 6, DB was negatively charged, and it was repelled by the kiwi peels’ surface. On the other hand, the pKa value of the tertiary amino group is around 4, while primary and secondary amino groups start their deprotonation at pH 9.12 After these assessments, to favor the DB adsorption, it was necessary to lower the solution pH value. In these conditions, the adsorbent was positively charged, and favored the attraction of the DB sulphonate groups. Alternatively, it should be possible to perturb the charges by affecting the ionic strength of the solution containing DB and the adsorbent. So, in the first step, the effect of pH on the adsorption process was investigated by using eqn (1) for calculating the % of Ads, by adopting 60 minutes as the constant contact time. Fig. 2(A) reports the obtained results. As expected, the DB removal was high at pH < 4 and decreased with increasing pH values. Indeed, as can be evidenced in the inset of Fig. 1, the color of kiwi peels changed from dark brown to blue. The DB adsorption was practically absent in the range of pH 4–12. The finding confirmed the main presence of electrostatic interactions between the –SO3− moieties of DB and the surface of kiwi peels.12,34 On the other hand, the DB remained negatively charged at higher pH values, while the peels turned their charge surface from positive to negative, favoring the repulsion.
In the second step, for detailing the nature of the interaction, the % of Ads was calculated by changing the ionic strength of the solution with different salt concentrations, always adopting 60 minutes as the reference contact time. NaCl was chosen as the model electrolyte. Interesting results were obtained, as shown in Fig. 2(B). By maintaining the pH of the solution at 6 and increasing the NaCl concentration from 0.01 M to 0.1 M, the removal of DB greatly changed from 20% to 80%, and leveled off at this value. Indeed, when the salt was further increased to 0.5 and 1 M, the removal percentage was the same. The electrolyte presence partially neutralized the charges of the adsorbent surface, reducing its negative charge, and moderately favored the attraction between DB and kiwi peels. However, at the same time, the ions from the salt also shielded the DB charges; thus, hydrophobic interactions and the H-bonds formation, as already observed by Rizzi et al.9 in a similar work, could be also taken into account. So, the mechanism of adsorption changed according to the case: if, on the one hand, under acidic conditions of work, the coulombic interactions were mainly favored, on the other hand, by increasing the ionic strength at neutral pH, the salting-out effect should be additionally promoted in favor of hydrophobic interaction, as observed elsewhere.43,44 To better understand these findings, the adsorption experiments were performed at pH 2 and in the presence of NaCl (Fig. 2(C)). By diluting the solution containing NaCl to 0.01 and 0.05 M, the obtained adsorption percentages were quite consistent with those calculated at pH 2. Surprisingly, at higher NaCl concentrations, a significant reduction of the adsorption percentage was observed if compared with the results obtained at pH 2 and the corresponding measurement in the presence of salt at pH 6. These results suggest the competitive effect of ions that hindered the formation of electrostatic interactions observed under the acidic experimental working condition.11,12,16,34 In particular, possible shielding effects of charges mediated by cations/anions, both referring to DB functional groups and the adsorbent surface and competition effects between the pollutant and ions (in this case, anions), towards the positively charged adsorbent surface, should be considered.34 So, Na+ might shield the DB negative –SO3− groups, and Cl− could compete for the positive kiwi peel surface. At pH 2, the presence of salt played a negative role, and a balance of electrostatic and hydrophobic interactions was observed. Starting from these considerations and inferring more information about the adsorption process, the adsorption capacities were calculated according to eqn (2). In particular, the study was performed by monitoring the adsorption evolution of DB onto kiwi peels’ surface at several contact times (Fig. 2(D)–(F)).
As the pH of the DB solution decreased or the salt (NaCl) amount increased, the qt values increased, confirming the establishment of favorable interactions between DB and the adsorbent already at the beginning of the process, when a greater increase in the adsorption capacity was observed. Indeed, when the adsorption started, the DB removal appeared fast, in agreement with the presence of more functional groups available to adsorb DB.6,12,28,34 Furthermore, a weaker DB diffusion resistance should be considered at the beginning of the process. On the other hand, extending the contact time, a plateau region was reached, indicating the attainment of an equilibrium condition where the adsorption sites were occupied by DB.6,8,34 Additionally, at the end of the adsorption, the decrease in the DB concentration gradient in the solution should slow down the whole process. Moreover, Gubitosa et al.34 reported that when the increase in qt values appeared slower, the presence of repulsive forces between the free pollutant molecules in the solution and those adsorbed onto kiwi peels, should also be considered.
Since, as a whole, the qt time evolution in solutions containing DB at pH 2 and/or in the presence of salts looked similar, without important differences, the attention was focused on DB adsorption in the presence of salts, avoiding the use of acidic experimental conditions. In particular, after the evaluation of the effects of other salts on the DB adsorption onto kiwi peels, NaCl 0.5 M was selected as the reference salt concentration, and other experiments were performed to give more details about the adsorption process.
Effect of other salts on the DB adsorption onto kiwi peels
To get insight into the DB adsorption process, the nature of both anions and cations was changed by performing adsorption experiments at pH 6. In general, by considering the previous discussion, it was expected that cations should play a role in shielding the DB negative charge, acting also as competitors for the negative kiwi peel surfaces, while anions should not play an important role due to the signs of the involved charges of DB and kiwi peels. By choosing 0.5 M as the reference electrolyte concentration, the salt nature was first changed by fixing the anion (Cl−) and varying the nature of the cation. Specifically, the following series of cations were adopted: Li+, Na+, K+, Mg2+, and Ca2+, and Fig. S2A (ESI†) reports the obtained results. The qt values decreased from Li+ to K+. Therefore, by increasing the hydrated radius of cation (K+ = 2.32 Å, Na+ = 2.76 Å, and Li+ = 3.4 Å),3 the DB adsorption was favored by better neutralizing the DB charge, allowing the interaction with kiwi peels. On the other hand, by changing the cation charge from monovalent to divalent ions (Ca2+ and Mg2+), the effects tended to be similar to those observed in the presence of KCl.6,8,34 Probably, the result could be attributed to the small size of these cations along with their different affinity with –SO3− groups, if compared with monovalent ions. Subsequently, the nature of anions was changed by selecting Na+ as the cation, and Fig. S2B (ESI†) shows the results. In this case, as expected, the qt values looked like each other.Effect of DB and kiwi peel amounts on the adsorption process
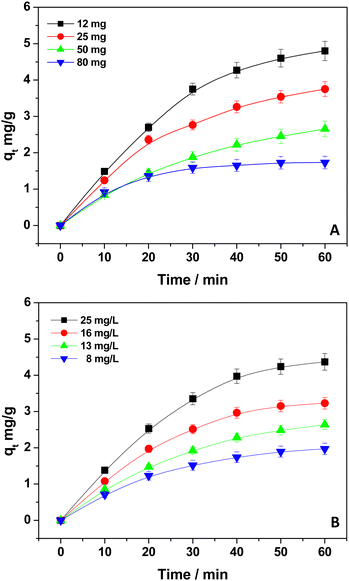
The role of active sites in the DB adsorption onto kiwi peels in the presence of NaCl 0.5 M at pH 6 was investigated by changing both the adsorbent and pollutant amounts. Fig. 3 reports the qt values calculated through eqn (2), using different quantities of dry adsorbent in contact with a 13 mg L−1 DB solution (Fig. 3(A)), and by varying the pollutant concentration, maintaining the kiwi peel amount constant at 50 mg (Fig. 3(B)).As the adsorbent quantity increased from 12 to 80 mg, the qt values decreased. However, the plateau region starting point (the condition where the equilibrium occurred) was observed later by decreasing the adsorbent amount. For example, the qt values joined the plateau region after 20 and 50 minutes when in the presence of the highest and lowest adsorbent amount, respectively. As reported by Gubitosa et al.,34 when great amounts of adsorbent were in use, the adsorption sites were not completely saturated, restituting low qt values although the DB removal was high. These results indicated that the site's availability favored the DB's adsorption onto kiwi peels’ surface. The process was especially enhanced at the beginning of the adsorption, as shown by the rapidity of achieving the plateau region.6,12,34 Instead, by reducing the adsorbent amount, the sites for DB decreased, slowing down the DB removal, and the equilibrium was reached later.
When the initial DB concentration was changed from 50 mg L−1 to 8 mg L−1, the adsorption capacities increased (Fig. 3(B)), highlighting the importance of DB's concentration gradient, which favored the pollutant transfer from the bulk's solution to the adsorbent.12,34 As described by Gubitosa et al.,34 in the presence of a high pollutant amount, the collisions with the active free sites of the adsorbent were enhanced, favoring the adsorption. As a whole, these results suggested that the DB mass transfer and its adsorption onto the adsorbent surface could have a kinetic relevance.34 Once again, it should be mentioned that the equilibrium region was obtained earlier for diluted solutions; so, under these conditions, more free active sites were available and favored the DB removal, confirming their role during the process.6,8,34
Kinetic analysis
The kinetic analysis was subsequently performed by applying both the PFO (eqn (3)) and PSO (eqn (4)) kinetic models, respectively, to the qt values reported in Fig. 3(A) and (B). The results are shown in Fig. S3 and S4 (ESI†). In particular, Fig. S3A and S4A (ESI†) report the PSO model applied to the adsorption experiments performed by changing the DB and adsorbent amounts, respectively. Fig. S3B (ESI†) and Fig. 4(B), instead, show the PFO model under the same conditions of work. The linear fitting was thus applied to infer the corresponding kinetic parameters (Tables S1 and S2, ESI†). As suggested by the literature,6,12,31,34 to choose the kinetic model suitable for describing the experimental process, the correlation coefficients R2, and the comparison between qe,exp (the experimental adsorption capacities at the equilibrium) and qe,calc (calculated adsorption capacities, obtained by applying the kinetic equations) was considered. The analysis of the output data reported in Tables S1 and S2 (ESI†) indicated that the R2 values were acceptable for both the models, with a slightly better correlation when the PSO model was applied. Interestingly, comparing qe,exp, and qe,calc, it is clear that the PFO model would be more suitable, even if the PSO well fitted the experimental data, restituting kinetic constants that changed with a trend according to the previous discussion. However, all these results highlighted the kinetic relevance of both the kinetic models during the DB adsorption process, suggesting the role of physisorption onto the kiwi surface (PSO), and the mass transfer of DB from the solution to the adsorbent surface (PFO) during the adsorption.34 So, in the first step, the DB diffused onto the adsorbent surface (external diffusion), then it was adsorbed on the surface of kiwi peels, and later, it diffused inside (internal diffusion). Consequently, the Weber–Morris model was also applied by employing eqn (5) to assess the kinetic relevance of the intraparticle diffusion. If this model is suitable for interpreting the studied system, a straight line passing through the origin should be obtained by plotting qt values versus t1/2.28,34 Once again, different amounts of adsorbent (Fig. S5A, ESI†) at constant DB concentration, and different DB concentrations in the presence of 50 mg of adsorbent (Fig. S5B, ESI†), were considered for testing the applicability of the Weber–Morris equation. As observed, the model cannot be applied in both cases since the data fitting restituted two straight lines that did not pass through the origin. So, the DB adsorption, according to the previous discussion, involved two or more kinetic steps besides the intra-particle diffusion.28 By analyzing the intraparticle diffusion results, it is possible to assess that the first straight lines described the faster DB diffusion through the boundary layer of solution to the external surface of kiwi peels. Afterward, there was the second step at the longer contact times, which described the intra-particle diffusion and adsorption as kinetic relevant steps, and at the end, the equilibrium was reached.28Thermodynamic analysis
The thermodynamic parameters related to the DB adsorption onto kiwi peels were inferred by changing the temperature values. Indeed, the adsorption process was studied at different temperatures, from 275 to 333 K, in solutions containing 50 mg of adsorbent and 13 mg L−1 of DB at pH 6 in NaCl 0.5 M. The obtained qt values are reported in Fig. 4(A).By increasing the temperature values, the DB adsorption capacities increased, evidencing the endothermic character of the process.29,34 In particular, the temperature effect was especially highlighted at the beginning of the adsorption process due to, once again, the presence of more free sites to host DB. So, by considering the obtained qt values and the amount of the not adsorbed DB at equilibrium, Keq was calculated for each temperature. Eqn (6) was thus applied to calculate the ΔG° values for each temperature, as reported in Table 1.
Negative ΔG° values were obtained, indicating the spontaneity of the process that occurred favored with the increase of the temperature.16,29,34 By using eqn (7), i.e., by plotting ln(Keq) versus 1/T (Fig. 4(B)), the  and
and  values are inferred and reported in Table 1. The high positive values of
values are inferred and reported in Table 1. The high positive values of  (+160 kJ mol−1) confirmed the endothermic character of the process. On the other hand,
(+160 kJ mol−1) confirmed the endothermic character of the process. On the other hand,  (+95 J mol−1 K) suggested, as observed in other studies, that at the adsorbent–adsorbate interface, the DB adsorption increased the randomness.16,29,34
(+95 J mol−1 K) suggested, as observed in other studies, that at the adsorbent–adsorbate interface, the DB adsorption increased the randomness.16,29,34
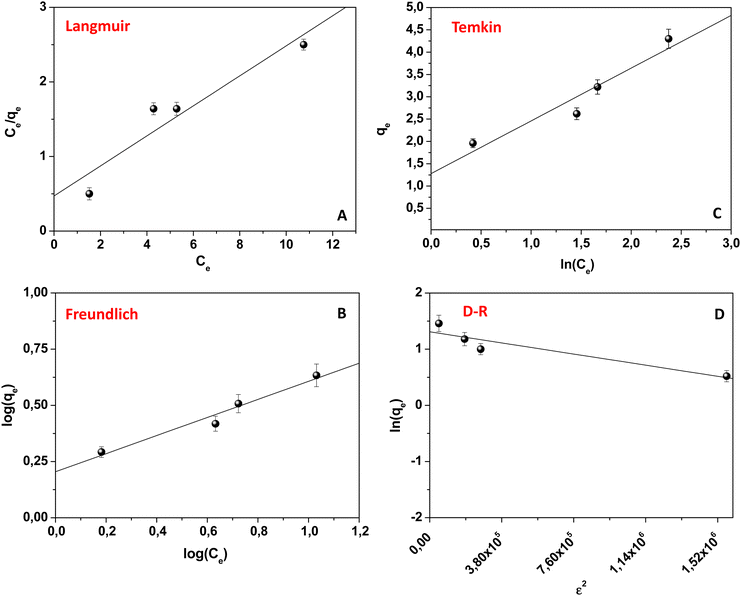
Isotherms of adsorption
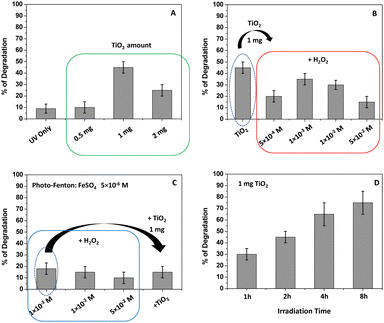
Langmuir, Freundlich, Temkin, and Dubinin–Radushkevitch (D–R) isotherm models (eqn (8)–(13)) were used to fit the experimental data.29,34Fig. 5 shows the obtained results.The correlation factor R2, arising from each linear fitting (Table 2), suggested the applicability of all the isotherm models, with a minor extent of the Langmuir one. In particular, the best fitting was observed with the Freundlich model (R2 = 0.98199). Overall, Table 2 reports the isotherm parameters calculated for each model. The results suggested that the pollutant adsorption occurred on heterogeneous surfaces, as already observed in other similar studies. Additionally, the value of the n parameter (see Table 2), derived from the Freundlich model, represents the adsorption strength, and the values of 1/n ranging from 0 to 1, as herein observed, proved that the physical DB adsorption was favored. The KL value from the Langmuir model also supported this observation since it occurred between 0 and 1; it indicated that the adsorption was favorited for the adsorbate–adsorbent system.16,29,34 Finally, the D–R model was applied, and the related parameters are reported in Table 2. The small value of E highlighted the important involvement of physical forces during the DB adsorption onto the kiwi peel surface, further confirming the previous results. Indeed, the obtained value of 1 kJ mol−1 was less than 8 kJ mol−1.16,29,34 All these findings agreed with the calculated value of 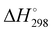 .
.
| Langmuir isotherm model | Temkin isotherm model | ||||
|---|---|---|---|---|---|
| K L (L mg−1) | Q 0 mg g−1 | R 2 | K T (L mol−1) | B 1 | R 2 |
| 0.02 | 26.00 | 0.9493 | 2.85 | 1.20 | 0.96115 |
| Freundlich isotherm model | ||
|---|---|---|
| K F (L mg−1) | 1/n | R 2 |
| 1.60 | 0.40 | 0.98199 |
| D–R isotherm model | |||
|---|---|---|---|
| K D–R (mol2 J−2) | Q 0 (mg g−1) | E (kJ mol−1) | R 2 |
| 5.0 × 10−7 | 4.00 | 1.00 | 0.9465 |
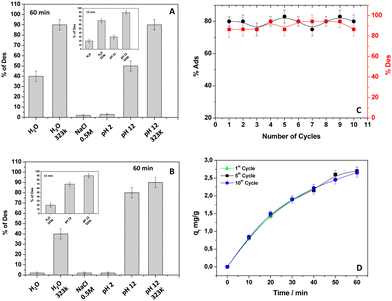
Desorption of DB and adsorbent recycling
To recycle both the adsorbent and DB, experiments of desorption were performed. The investigation occurred on kiwi peels loaded with DB adopting different experimental conditions: adsorption from an acid solution (pH 2) and from NaCl solution 0.5 M, pH 6, respectively. In detail, after the DB's adsorption (initial condition: dye concentration 13 mg and 50 mg of adsorbent), kiwi peels were left in fresh water for 15 and 60 minutes. Starting from kiwi peels loaded with DB from a solution containing NaCl, the results reported in Fig. 6(A) were observed after 60 minutes. The inset of the same figure shows the results corresponding to experiments of desorption obtained after 15 minutes of contact time. As expected, the release of DB by employing an acidic solution (pH 2) or NaCl (0.5 M) was not observed, these conditions being favorable to the dye adsorption, as discussed throughout the paper. Therefore, experimental conditions that appeared unfavorable for the DB removal will ensure the color release.Specifically, water (pH 6) and alkaline solution (pH 12) at 298 K favored the process, further improved with the increase of temperature at 323 K, as reported in Fig. 6(A). The slight differences in terms of % of desorption between water at pH 6, and pH 12 can be better appreciated in the inset of Fig. 6(A) when a short contact time of desorption was adopted. The alkaline solution slightly favored the DB desorption, and the finding can be interpreted with the negligible presence, also in this condition, of electrostatic interactions involving an ion-exchange mechanism. A similar behavior was obtained when kiwi peels loaded with DB from an acidic solution were considered (Fig. 6(B), and the inset). In this case, according to the main electrostatic nature of the interactions between DB and the adsorbent surface, the release in water at pH 6 was not favored unless at higher temperatures. On the other hand, the use of water at pH 12 ensured the DB release at room and high temperature without important differences, confirming the role of coulombic forces under acid conditions of work. On this ground, to propose kiwi peels as a recyclable adsorbent material (focusing the attention on DB adsorbed from a NaCl solution), 10 cycles of adsorption and desorption were consecutively performed, choosing hot water (323 K) at pH 6 as the best condition for the desorption. Moreover, 15 minutes were selected as the desorption contact time. Specifically, after DB adsorption from a 0.5 M NaCl solution in 60 minutes, the adsorbent was swollen in hot water for 15 minutes. The % of adsorption and desorption was thus calculated and reported in Fig. 6(C). Very interestingly, the adsorbent maintained the same efficiency without significant differences in each cycle, highlighting the great performance of the proposed material as a low-cost and eco-friendly adsorbent for water treatment. Furthermore, the adsorption capacity of kiwi peels was also calculated after the 10th cycle of adsorption/desorption, and it was compared with the results related to the first cycle. Fig. 6(D) shows the obtained results. As can be appreciated, the qt values at different adsorption times were practically superimposed, evidencing the great performance of kiwi peels. So, the runs could be extended up to 10 cycles for rendering this novel adsorbent, proposed to treat water from dyes, competitive with other more efficient materials, respecting the sustainability principles. Indeed, regarding the past literature, the use of hot water appeared eco-friendly because the desorption time was very low. For example, Singh et al.,26 about the removal of Congo Red and Direct Blue-1, reported the mycosynthesized iron nanoparticle regeneration by using a 0.08 M NaOH solution for 120 min. The obtained regenerated adsorbents were further reused, and only five cycles of regeneration were performed. The recovery of Direct Red 81 adsorbed on Argemone mexicana was achieved again by eluting the dye in NaOH solution.29 Li et al.30 reported the Direct green 6 removal and NaOH solutions were proposed for the desorption from Y(III)-chitosan-doped fly ash composite. During the study, although 5 cycles of desorption were attempted, the reported adsorption efficiencies collapsed at increasing the runs of adsorption/desorption. Noreen et al.32 showed the ZnO, MgO, and FeO high adsorption efficiencies for Direct sky Blue dye removal. However, also, in this case, the desorption was performed using 0.1–0.9 M NaOH32 for a long contact time, at least 120 minutes with low desorption %. Moreover, always for DB removal, also Rizzi et al.11,12 accounted for the regeneration of olive pomace and MCM-41 nanoparticles with NaOH. Only a few cycles of adsorption/desorption were described, and in the case of MCM-41, the adsorbent appeared disrupted after 5 runs.11,12 On the other hand, during this work, DB was recycled under safer experimental conditions, presenting a long-lasting material.
ATR-FTIR analysis
Kiwi peels are mainly composed of lignin and cellulose, exposing on their surface some functional groups: alcohols, aldehydes, ketones, carboxylic, phenolic, and ether groups. The ATR-FTIR spectrum of kiwi peels is reported in Fig. S6A (ESI†) (black line). Both the inner and outer sides of peels were investigated without great differences between them in terms of ATR-FTIR spectrum features, as already described by Gubitosa et al.34 The band at 3307 cm−1 was referred to as the O–H vibration from phenols, lignin, hemicellulose, and cellulose. The related asymmetric and symmetric C–H bond stretching of methyl and methylene groups were detected at 2840 and 2911 cm−1, respectively. At 1727 cm−1, the weak contribution of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups from hemicellulose esters or carbonyl esters of lignin was observed. The stretching and bending vibration that arose from the hydroxyl groups of cellulose was detected at 1614 cm−1. The C
O groups from hemicellulose esters or carbonyl esters of lignin was observed. The stretching and bending vibration that arose from the hydroxyl groups of cellulose was detected at 1614 cm−1. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration in the conjugated carbonyl of lignin should also be taken into account. The strong band at 1030 cm−1 was referred to as the –C–O–C– vibration from the skeleton of cellulose and hemicellulose. In the region 1100 and 1500 cm−1 other weak bands were observed and attributed to CH3, –CH2–, and C–H moieties, besides the polyphenolic aromatic ring C
O stretching vibration in the conjugated carbonyl of lignin should also be taken into account. The strong band at 1030 cm−1 was referred to as the –C–O–C– vibration from the skeleton of cellulose and hemicellulose. In the region 1100 and 1500 cm−1 other weak bands were observed and attributed to CH3, –CH2–, and C–H moieties, besides the polyphenolic aromatic ring C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibration due to the presence of cellulose, hemicellulose, and lignin. The contribution of C–O vibrations in carboxylate groups, and the stretching of esters, ethers, or phenol groups, should be taken into account. The same features were observed after the DB adsorption (red curve), from a solution at pH 6 and NaCl 0.5 M, with slight changes in band position and relative intensity. The contribution of the adsorbed DB was not significantly detected (Fig. S6B, ESI† shows the ATR-FTIR signals of DB). Conversely, focusing the attention on the typical vibration bands of kiwi peels, the signal at 1614 cm−1 shifted to a higher wavenumber, 1622 cm−1, appearing better defined, suggesting the DB coordination with cellulose OH moieties.34 Accordingly, the OH signal at 3307 cm−1 increased its relative intensity and slightly shifted, denoting the possible formation of the H-bond with DB and the adsorbent surface, besides the OH moieties vibration contribution from DB. These observations suggested the presence of H-bonds and hydrophobic interactions between DB and the kiwi peels, as previously evidenced during the discussion. Finally, after the adsorbent recycling (green line), the IR bands’ position, shape, and relative intensity appeared the same as those recorded at the beginning of the adsorption process, validating that the main adsorbent functional groups and features were not affected during the prolonged adsorption/desorption runs, as already reported by Gubitosa et al.34 during the removal/recycling of ciprofloxacin.
C vibration due to the presence of cellulose, hemicellulose, and lignin. The contribution of C–O vibrations in carboxylate groups, and the stretching of esters, ethers, or phenol groups, should be taken into account. The same features were observed after the DB adsorption (red curve), from a solution at pH 6 and NaCl 0.5 M, with slight changes in band position and relative intensity. The contribution of the adsorbed DB was not significantly detected (Fig. S6B, ESI† shows the ATR-FTIR signals of DB). Conversely, focusing the attention on the typical vibration bands of kiwi peels, the signal at 1614 cm−1 shifted to a higher wavenumber, 1622 cm−1, appearing better defined, suggesting the DB coordination with cellulose OH moieties.34 Accordingly, the OH signal at 3307 cm−1 increased its relative intensity and slightly shifted, denoting the possible formation of the H-bond with DB and the adsorbent surface, besides the OH moieties vibration contribution from DB. These observations suggested the presence of H-bonds and hydrophobic interactions between DB and the kiwi peels, as previously evidenced during the discussion. Finally, after the adsorbent recycling (green line), the IR bands’ position, shape, and relative intensity appeared the same as those recorded at the beginning of the adsorption process, validating that the main adsorbent functional groups and features were not affected during the prolonged adsorption/desorption runs, as already reported by Gubitosa et al.34 during the removal/recycling of ciprofloxacin.
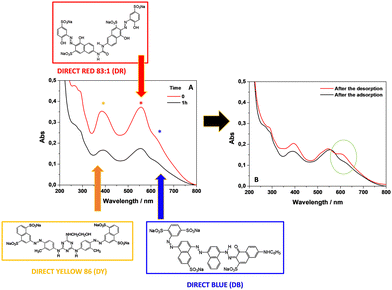
Removal of other textile dyes and their mixtures
The great performances of kiwi peels were highlighted by showing the ability to remove other textile dyes, i.e., Direct Yellow 86 (DY) and Direct Red 83:1 (DR). The experiments were performed by removing the single dyes and their mixture. Indeed, the affinity of DY and DR for kiwi peels did not appear different from the behavior exhibited by DB. Indeed, the removal of both dyes was quite complete, ≈80%, under the same condition of work proposed for the DB removal. Accordingly, the removal of the color mixture better evidenced the finding that could represent an important aspect of the real treatment of wastewater. For this purpose, a mixture of DR, DB, and DY in a 0.5 M NaCl solution was studied (Fig. 7(A)). The adsorption process was monitored through visible spectroscopy, as discussed before in the case of the DB removal. It is worth mentioning that each dye showed its typical visible absorption spectrum that contributes to the global visible absorption spectrum of the mixture, constituted by an envelope of absorption bands of a single dye. Specifically, DY contributes to its absorption showing a maximum of around 390 nm, DR at 550 nm, and DB at 600 nm (indicated with asterisks in Fig. 7(A)).13The visible spectra were collected before and after the contact with the adsorbent by selecting 60 minutes as the contact time. The high efficiency of adsorption can be appreciated in Fig. 7(A). More than 50% of each pollutant was removed from the water. Interestingly, also for the mixture of the dyes, their desorption and regeneration of kiwi peels were attained. By following the same procedure to desorb DB, in Fig. 7(B), the visible spectrum of the dye-mixture after the desorption is reported. As indicated by the green circle, the DB contribution appears greater than other dyes during the desorption, as if it was preferentially desorbed with respect to DY and DR. However, the adsorbent regeneration could be considered a success, being able to desorb the three dyes even if with different efficiencies and the absence of selectivity. These results appeared very promising for the possible scale-up of the process to treat real wastewater samples. Novel horizons in treating water polluted by dyes are thus opened: kiwi peels can be considered a low-cost and environmentally friendly adsorbent, able to adsorb different dyes and emerging pollutants without selectivity and important effects of competition, as also reported in our previous study.
Dyeing capacity experiments of dye–kiwi peels
To explore the quality of the recycled DB from kiwi peels, dyeing experiments were performed using cotton fiber. In particular, the experiments of dyeing were achieved during the kiwi peel desorption in hot water at 323 K, without further additives, adopting 60 minutes as contact time. Fig. 8 shows the obtained results.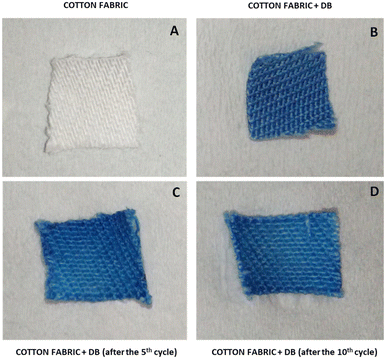 | ||
| Fig. 8 Dyeing experiments of white cotton fabrics (A) with DB prior to the adsorption onto kiwi peels (B), and after the desorption from kiwi peels used 5 times (C), and 10 times (D). | ||
Fig. 8(A) and (B) show the cotton fabrics, respectively, before and after the dying process. Interestingly, the dyeing capacity of DB was retained after its adsorption onto kiwi peels, although the adsorbent was used for several cycles. In particular, Fig. 8(C) and (D) show the cotton fabrics colored with DB released by kiwi peels after the 5th and 10th cycles of adsorption/desorption, respectively. The obtained results are very interesting because the kiwi peels could be used directly in a dying batch, releasing the dye to color fibers again, lowering the associated costs. Indeed, hot water is usually required during the dyeing process.
Preliminary information about the DB solid-state photodegradation onto kiwi peels
By adopting the same approach reported by Gubitosa et al.34 during the photodegradation of ciprofloxacin after the adsorption onto kiwi peels, an alternative process for the adsorbent recycling was herein attempted. In general, the textile dye photodegradation in solution is well known in the literature,45–47 and particularly the photoreaction of DB was studied by using AOPs, well detailing the photodegradation mechanism.46,47Therefore, during this work, a preliminary study was carried out by proposing the use of AOPs to induce, in this case, the DB solid-state photodegradation after the adsorption onto kiwi peels. The main aim was to find the best experimental conditions for obtaining the highest DB degradation and propose an alternative adsorbent regeneration strategy. Indeed, Gubitosa et al.34 demonstrated that the main kiwi peel features are also retained after the AOP treatments, not affecting the adsorption capacity of the material. On this basis, the attention was focused on the synergistic use of UV light, H2O2, UV-TiO2, UV/H2O2/TiO2, Fenton, and photo-Fenton conditions.45–50 Indeed, in these experimental conditions, the main product is the radical ˙OH:3,6,8,45–47,50 a very reactive and not selective species that favors the pollutants’ fast degradation. Furthermore, the use of TiO2, H2O2, and Fenton reagents in the presence of UV light should promote the additional formation of ˙OH. In detail, the experiments were performed by placing the kiwi peels after the adsorption in water (15 mL) at pH 2 under UV light irradiation. The choice of working at acidic pH was determined by considering that DB degradation is favored at low pH.46 Moreover, focusing the attention on the photocatalytic action of TiO2, since an important step in the photocatalytic oxidations is the adsorption of the species onto the photocatalyst surface, under acidic conditions, the TiO2 surface is positively charged, so the adsorption of negatively charged DB is furtherly facilitated.46 According to the cases reported in Fig. 9, the water solution surrounding kiwi peels was spiked with the explored oxidant's agents.
 | ||
| Fig. 9 % of DB photodegradation under different working conditions at pH 2. Effect of: UV light, TiO2 amount (A); TiO2 + H2O2 (B); photo-Fenton conditions (C); UV light irradiation time on TiO2 (D). | ||
The % of photodegraded DB was evaluated through release experiments in hot water after the performed oxidative treatments. In the first step, the degradation was attempted by placing DB-loaded kiwi peels in water and irradiating with a UV lamp for 2 h (Fig. 9(A)). Only 10% of the adsorbed DB was destroyed. After that, the loaded adsorbent was placed in a solution containing only H2O2 at different concentrations (from 5 × 10−4 M to 5 × 10−2 M), both in the dark and under UV light. The absence of any degradation was detected. The use of TiO2, suspended in water, was thus considered in different amounts by adopting, again, 2 h as irradiation time (Fig. 9(A)). The best condition was observed by placing kiwi peels in a suspension containing 1 mg of TiO2, thus obtaining ≈45% DB's degradation (Fig. 9(A)). The further increase of TiO2 amount increased the opacity of the suspension, hindering the light penetration and decreasing the hydroxyl radical formation.46 So, fixing the TiO2 amount at 1 mg, the effect of adding H2O2 was investigated. As shown in Fig. 9(B), adding H2O2 in different concentrations (ranging from 5 × 10−4 M to 5 × 10−2 M) did not improve the process, probably due to the hydroxyl radicals self-quenching in the solution surrounding kiwi peels.34 Indeed, the ˙OH quenching occurred rapidly, preventing the radicals’ migration from the bulk of the solution to the adsorbent surface where DB was located.34 Furthermore, the UV “filter” effect of H2O2 could reduce the TiO2 activation. Indeed, by increasing the H2O2 amount from 5 × 10−4 M to 5 × 10−2 M, due to the H2O2 photolysis, the effect of hydroxyl radical self-quenching was more pronounced, achieving only ≈35% of DB's degradation with 1 mg of TiO2 and H2O2 1 × 10−3 M. The latter condition was also explored by increasing the TiO2 amount from 1 mg to 2 mg, not improving the DB degradation process that remained at ≈45%. Subsequently, the photo-Fenton reagent was used (Fig. 9(C)). Experiments were performed under UV light irradiation for 2 h using different amounts of H2O2, fixing the concentration of Fe2+ at 5 × 10−6 M. Under these conditions, the DB's degradation was not high (from ≈10% to 20%), and the increase of H2O2 concentration (or [Fe2+]) did not improve the results. These findings could be explained, once again, considering that the self-quenching of hydroxyl radicals is the preferred process in solution. Indeed, by increasing the amount of H2O2, the effect was more pronounced. The same experiments were performed in the dark, without significant DB degradation. The addition of TiO2 (1 mg) was also attempted under photo-Fenton conditions, and for example, when using H2O2 1 × 10−3 M and 1 mg of TiO2, the ≈15% of DB was degraded, not enhancing the process (Fig. 9(C)).
As a general result, the discussed strategies cannot be considered useful for solid-state degradation of the dye because they do not improve the result obtained from the use of simple TiO2, which could be considered the best condition investigated. So, fixing the TiO2 amount at 1 mg, further experiments were performed by irradiating kiwi peels with UV light and increasing the irradiation time (Fig. 9(D)). The increase in irradiation time significantly improved the DB degradation observing ≈30%, 45%, 65%, and 75% after 1 h, 2 h, 4 h, and 8 h, respectively. So, the photocatalytic process could be used in synergy or as a potential alternative to the main approach discussed that proposes the DB's desorption.
In this regard, some experiments were performed to investigate if the adsorption capacity of kiwi peels changes after using UV light and TiO2, or if it remains the same as that observed previous to the treatment. In accordance with what was reported by Gubitosa et al.,34 the use of TiO2 and UV light did not affect the main chemical and morphological features of kiwi peels.
Experimental
Chemicals
The used kiwi fruits were obtained from local providers in Bari, South of Italy. Direct Blue 78 (molecular formula: C42H25N7Na4O13S4, molecular weight: 1055.91 g mol−1, CAS Registry Number: 2503-73-3) was received from Colorprint Fashion, SL, and used without further purification. All the used chemicals were purchased from Sigma Aldrich (Milan, Italy). The pollutant stock solution, 50 mg L−1, was obtained in deionized water, and it was diluted to obtain solutions having concentrations of 25, 16, 13, and 8 mg L−1. HCl and NaOH solutions were used to modify the pH of the DB solutions. NaCl, LiCl, KCl, MgCl2, CaCl2, NaBr, and NaClO4 were investigated to assess the role of ionic strength during the dye removal. All the analyses were performed in triplicate, and standard deviations were calculated and reported as error bands.Adsorbent preparation
The obtained kiwi fruits were washed well and peeled. The peels (80 mg) were placed in hot water (500 mL) under vigorous stirring for 15 minutes. If necessary, the remaining fruit pulp was mechanically removed. The process of washing was subsequently repeated three times. The obtained peels were dried in oven until reaching a constant weight and used as an adsorbent material.UV-visible absorption measurements
The Varian CARY 5 UV-Vis-NIR spectrophotometer (Varian Inc., now Agilent Technologies Inc., Santa Clara, CA, USA) was employed to collect the visible absorption spectra of DB in a range of 250–800 nm, at a 1 nm s−1 scan rate. The DB concentration was derived from the Lambert–Beer law, measuring the absorbance intensity at λ = 600 nm, and adopting a molar absorption coefficient (ε) of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 901 L mol−1 cm−1.
901 L mol−1 cm−1.
ATR-FTIR spectroscopy measurements
The ATR-FTIR spectra of kiwi peels before and after the pollutant adsorption/desorption were recorded in a 4000–400 cm−1 range using a Fourier Transform Infrared spectrometer (FTIR Spectrum Two from PerkinElmer, Waltham, MA, USA). The resolution was set at 4 cm−1, and 16 scans were obtained and summed for each acquisition.In batch equilibrium experiments
The % of DB adsorption (% of Ads) was calculated by adopting eqn (1):34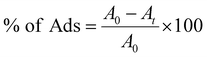 | (1) |
The adsorption capacities, qt (mg g−1), for kiwi peels were calculated by applying eqn (2) to the experimental data.2,11,13,15,34
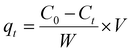 | (2) |
50 mg of adsorbent was swelled into 15 mL of water in the presence of DB having different initial concentrations (from 25 mg L−1 to 8 mg L−1) at pH 6, to assess the DB amount role during the adsorption onto the kiwi peel surface. On the other hand, the adsorbent amount role was investigated by changing the weight of peels from 12 to 80 mg, in the presence of DB 13 mg L−1, pH 6. The process was studied with constant stirring at room temperature (298 K). The solution's ionic strength was changed by using different salts after fixing the amount of the adsorbate and adsorbent (50 mg of kiwi peels, and 13 mg L−1 of DB). NaCl was adopted to infer the role of salt concentrations. The pH values, ranging from 2 to 12, and temperature values, from 275 to 333 K, were also changed during the adsorption.
Adsorption kinetics
The pseudo-first-order (PFO) and pseudo-second-order (PSO) kinetic models were applied to the experimental data. For this purpose, eqn (3) and (4) were used to describe PFO and PSO models, respectively.2,11,13,15,34| ln(qe − qt) = ln(qe) − K1 × t | (3) |
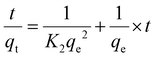 | (4) |
Eqn (5) was also used to infer the intraparticle diffusion role described by the Weber–Morris equation.34
| qt = kint × t1/2 + C | (5) |
In this case, kint represents the kinetic constant expressed in mg (g−1 min−1/2), referred to as the intra-particle diffusion rate, and C is the thickness of the boundary layer.
Thermodynamic studies
For this purpose, different temperature values ranging from 275 to 333 K were adopted. So, the free energy (ΔG°), entropy (ΔS°), and enthalpy (ΔH°) were calculated for the DB adsorption. In particular, eqn (6) was used to calculate the free energy.29,30,34ΔG° = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Keq Keq | (6) |
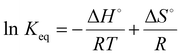 | (7) |
Adsorption isotherms
The adopted isotherms of adsorption were the following: Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich.29–32,34 The Langmuir model could be herein applied and well fit the adsorption process if all the adsorption sites are characterized by constant energy during the DB adsorption. Furthermore, a monolayer of dye can be supposed to be adsorbed on the surface of kiwi peels without strong interactions between the molecules of DB. Eqn (8) was applied to describe the Langmuir model.34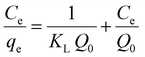 | (8) |
In eqn (8), qe (mg g−1) is the pollutant adsorbed amount at equilibrium, Ce is the correspondent equilibrium concentration in solution expressed in mg L−1, KL represents the Langmuir equilibrium constant (L mg−1), and Q0 is the adsorbent maximum adsorption capacity (mg g−1) value.34
The Freundlich isotherm, if fitted with the experimental data, should reflect the DB adsorption onto a heterogeneous surface with adsorption sites having different energies as a function of the surface coverage. In this case, the heat of adsorption should decrease exponentially during the DB removal. Eqn (9) describes the Freundlich model.34
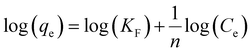 | (9) |
The Temkin model describes the influence of the adsorbate–adsorbent interactions on the adsorption energy. This isotherm suggests that the heat of adsorption should decrease linearly during the DB removal due to adsorbent/adsorbate interactions. Eqn (10) was applied to use the Temkin model.34
qe = B1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(KT) + B1 ln(KT) + B1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(Ce) ln(Ce) | (10) |
The Dubinin–Radushkevich isotherm (D–R) model was also employed by applying eqn (11). In this case, the model assumes the adsorption onto a heterogeneous surface in which a Gaussian energy distribution can be used.34
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln(Qe) − KD–R × ε2 qe = ln(Qe) − KD–R × ε2 | (11) |
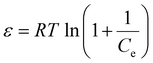 | (12) |
It is worth mentioning that this model can distinguish between physical and chemical adsorption by calculating the value of energy, E, by using eqn (13).
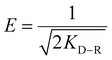 | (13) |
If the output value of E is in the range of 8–16 kJ mol−1, the chemisorption should be considered during the adsorption process; conversely, for E < 8 kJ mol−1, the physisorption should be more important.28,34
In batch desorption experiments
Desorption experiments were performed by using different conditions of work. However, hot distilled water at 323 K was proposed (adopted volume of 15 mL) to perform 10 cycles of DB adsorption and desorption. Visible absorption spectroscopy was used to infer the amount of desorbed DB.Kiwi peel point of zero charge determination
The pH at which the kiwi peel point of zero charge occurred (pHPZC) was calculated by following the procedure adopted by Gubitosa et al.34 In detail, 30 mL of a 5.0 × 10−2 M NaCl solution was prepared after changing the pH values from 2 to 12 (pHi) by using HCl and NaOH. So, the pH of solutions was measured before (pHi) and after (pHF) swelling 50 mg of the kiwi peels for 48 h, under continuous stirring. The pHPZC value was obtained by plotting pHiversus pHi and pHiversus pHF; the intersection of these lines represents the pHPZC value.Photodegradation of Direct Blue 78
The solid-state photodegradation of DB was obtained after its removal from water using AOPs. A UV lamp (UV fluorescent lamp, Spectroline, Model CNF 280C/FE, λ 254 nm, light flux 0.2 mW cm−2; USA) was employed to irradiate the adsorbent placed in water (15 mL) by adopting several conditions of work detailed in the paper. The irradiation was performed on solutions under continuous stirring. Commercial Evonik Aeroxide TiO2 P25 (formerly Degussa P25), FeSO4 1 × 10−3 M, and commercial H2O2 (30%) were used during the experiments.Conclusions
This work proposes kiwi peels’ wastes as a resource for the textile dye removal from wastewater. DB was adopted as a model textile dye, evidencing the main chemical and physical features of the whole adsorption process. The removal occurred both under acidic working conditions and/or in the presence of salts at neutral pH values. If during the former experimental conditions, the presence of an electrostatic interaction between DB and the adsorbent was important, in the latter one, the contribution of hydrophobic interactions and the presence of H-bonds predominated. In particular, focusing first on the pollutant removal in the absence of salts, it occurred high at pH values 2 and 3 and decreased by increasing the pH values. The DB removal was blocked up to pH 4. The results agree with the DB's pKa < 2 for SO3− moieties and the PZC of the adsorbent (pHPZC = 4). At pH 2, the –SO3− groups of DB were deprotonated and negatively charged; on the other hand, the kiwi peel surface was positive. The attraction predominates. After pH 4, the kiwi peels acquired a net negative charge, and the DB was still negative, so the repulsion occurred. When the DB adsorption was carried out in the presence of salt, i.e., NaCl, the process was salt-amount dependent, and by increasing the concentration of NaCl from 0.01 M to 1 M, the DB removal results were enhanced. The presence of a hydrophobic interaction was confirmed by FTIR-ATR analysis, which also evidenced the contribution of H-bonds. To avoid hard working conditions, the main focus was on the adsorption process in the presence of electrolytes. As suggested by the thermodynamic parameters, the process was spontaneous (ΔG° < 0), and endothermic (ΔH° > 0), showing an increase in entropy during the process. The isotherms and the kinetics were studied, and the adsorption process was well described by all the proposed isotherm models, showing a very good correlation with the Freundlich equation. The pseudo-first and second-order kinetic models described DB adsorption as a multi-step process where both the inner and external DB diffusion acquire kinetic relevance. Accordingly, it was observed that the kiwi peel adsorption capacities increased by increasing the adsorbent and pollutant amount, suggesting the important role of free active sites on the peel surface and DB diffusion during the adsorption. Finally, using eco-friendly approaches, pollutant desorption was investigated for favoring the recycling of both DB and kiwi peels. For this purpose, hot water was selected, and 10 cycles of adsorption/desorption were performed, increasing the adsorbent lifetime, according to Green Chemistry and Circular Economy principles. Furthermore, besides the recycling of the adsorbent, DB recycling was also demonstrated, proposing qualitatively cotton fabric dyeing by using the desorbed DB. So, kiwi peels appeared as a powerful candidate as potential adsorbents to clean wastewater containing textile dyes. Indeed, adsorption experiments were also performed in the presence of dye mixtures, i.e., DB, DR, and DY, evidencing the possibility of removing the pollutants even in this case. Finally, preliminary experiments related to the solid-state DB photodegradation were attempted as an alternative to its recycling, by exploiting AOPs. More specifically, the use of TiO2 and UV light occurred as the best working condition to obtain the highest DB degradation that was time-dependent. The adsorption capacity of kiwi peels was again studied after the UV light treatment, resulting in not being affected by the photocatalytic treatment. The opportunity to adopt this alternative strategy for adsorbent regeneration was thus achieved and proposed.Author contributions
Vito Rizzi: conceptualization, investigation, validation, methodology, writing – review, and editing; Jennifer Gubitosa: conceptualization, validation, investigation, writing – original draft preparation; Paola Fini: investigation, validation, data curation; Sergio Nuzzo: visualization, imaging elaboration; Pinalysa Cosma: formal analysis, resources, project administration, writing – review and editing, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the following projects: “Research for Innovation (REFIN) per l’individuazione dei progetti di ricerca” – PUGLIA FESR-FSE 2014/2020, Project title: “Incontro tra Ricerca & Impresa per lo Sviluppo Sostenibile del territorio (IRISS): valorizzazione di scarti alimentari per la rimozione di contaminati emergenti dalle acque”; “Dottorati di ricerca in Puglia XXXIII, XXXIV, XXXV ciclo, POR PUGLIA FESR-FSE 2014/2020”; Horizon Europe Seeds, Project title: “Gestione sostenibile di scarti Agroalimentari come fonte Innovativa di biomateriali multifunzionali per la salute umana e l’Ambiente (G.A.I.A)”.Notes and references
- I. B. Obinna and Enyoh Ebere, A review: Water pollution by heavy metal and organic pollutants: Brief review of sources, effects and progress on remediation with aquatic plants, Anal. Methods Environ. Chem. J., 2019, 2(3), 5–38 CrossRef CAS.
- V. Rizzi, J. Gubitosa, P. Fini, S. Nuzzo and P. Cosma, Amino-grafted mesoporous MCM-41 and SBA-15 recyclable adsorbents: Desert-rose-petals-like SBA-15 type as the most efficient to remove azo textile dyes and their mixture from water, Sustainable Mater. Technol., 2020, 26, e00231 CrossRef CAS.
- V. Rizzi, J. Gubitosa, P. Fini, R. Romita, A. Agostiano, S. Nuzzo and P. Cosma, Commercial bentonite clay as low-cost and recyclable “natural” adsorbent for the Carbendazim removal/recover from water: Overview on the adsorption process and preliminary photodegradation considerations, Colloids Surf., A, 2020, 602, 125060 CrossRef CAS.
- V. Rizzi, J. Gubitosa, P. Fini, A. Petrella, R. Romita, A. Agostiano and P. Cosma, A “classic” material for capture and detoxification of emergent contaminants for water purification: The case of tetracycline, Environ. Technol. Innovation, 2020, 19, 100812 CrossRef.
- V. Rizzi, J. Gubitosa, P. Fini, R. Romita, S. Nuzzo and P. Cosma, Chitosan Biopolymer from Crab Shell as Recyclable Film to Remove/Recover in Batch Ketoprofen from Water: Understanding the Factors Affecting the Adsorption Process, Materials, 2019, 12, 3810 CrossRef CAS PubMed.
- V. Rizzi, F. Romanazzi, J. Gubitosa, P. Fini, R. Romita, A. Agostiano, A. Petrella and P. Cosma, Chitosan Film as Eco-Friendly and Recyclable Bio-Adsorbent to Remove/Recover Diclofenac, Ketoprofen, and their Mixture from Wastewater, Biomolecules, 2019, 9, 571 CrossRef CAS PubMed.
- R. Romita, V. Rizzi, P. Semeraro, J. Gubitosa, J. A. Gabaldón, M. I. F. Gorbe, V. M. G. López, P. Cosma and P. Fini, Operational parameters affecting the atrazine removal from water by using cyclodextrin based polymers as efficient adsorbents for cleaner technologies, Environ. Technol. Innovation, 2019, 16, 100454 CrossRef.
- V. Rizzi, D. Lacalamita, J. Gubitosa, P. Fini, A. Petrella, R. Romita, A. Agostiano, J. A. J. A. Gabaldón, M. I. M. I. Fortea Gorbe, T. Gómez-Morte and P. Cosma, Removal of tetracycline from polluted water by chitosan-olive pomace adsorbing films, Sci. Total Environ., 2019, 693, 133620 CrossRef CAS PubMed.
- V. Rizzi, C. Mongiovì, P. Fini, A. Petrella, P. Semeraro and P. Cosma, Operational parameters affecting the removal and recycling of direct blue industrial dye from wastewater using bleached oil mill waste as alternative adsorbent material, Int. J. Environ. Agric. Biotechnol., 2017, 2, 1560–1572 Search PubMed.
- A. Petrella, D. Spasiano, V. Rizzi, P. Cosma, M. Race and N. De Vietro, Thermodynamic and kinetic investigation of heavy metals sorption in packed bed columns by recycled lignocellulosic materials from olive oil production, Chem. Eng. Commun., 2019, 206, 1715–1730 CrossRef CAS.
- V. Rizzi, E. A. Prasetyanto, P. Chen, J. Gubitosa, P. Fini, A. Agostiano, L. De Cola and P. Cosma, Amino grafted MCM-41 as highly efficient and reversible ecofriendly adsorbent material for the Direct Blue removal from wastewater, J. Mol. Liq., 2019, 273, 435–446 CrossRef CAS.
- V. Rizzi, F. Fiorini, G. Lamanna, J. Gubitosa, E. A. Prasetyanto, P. Fini, F. Fanelli, A. Nacci, L. De Cola and P. Cosma, Polyamidoamine-Based Hydrogel for Removal of Blue and Red Dyes from Wastewater, Adv. Sustainable Syst., 2018, 2, 1700146 CrossRef.
- V. Rizzi, A. Longo, T. Placido, P. Fini, J. Gubitosa, T. Sibillano, C. Giannini, P. Semeraro, E. Franco, M. Ferrandiz and P. Cosma, A comprehensive investigation of dye–chitosan blended films for green chemistry applications, J. Appl. Polym. Sci., 2017, 135, 45945 CrossRef.
- V. Rizzi, F. D’Agostino, J. Gubitosa, P. Fini, A. Petrella, A. Agostiano, P. Semeraro and P. Cosma, An Alternative Use of Olive Pomace as a Wide-Ranging Bioremediation Strategy to Adsorb and Recover Disperse Orange and Disperse Red Industrial Dyes from Wastewater, Separations, 2017, 4(4), 29 CrossRef.
- V. Rizzi, F. D’Agostino, P. Fini, P. Semeraro and P. Cosma, An interesting environmental friendly cleanup: The excellent potential of olive pomace for disperse blue adsorption/desorption from wastewater, Dyes Pigm., 2017, 140, 480–490 CrossRef CAS.
- V. Rizzi, J. Gubitosa, R. Signorile, P. Fini, C. Cecone, A. Matencio, F. Trotta and P. Cosma, Cyclodextrin nanosponges as adsorbent material to remove hazardous pollutants from water: The case of ciprofloxacin, Chem. Eng. J., 2021, 411, 128514 CrossRef CAS.
- V. Rizzi, J. Gubitosa, P. Fini, R. Romita, S. Nuzzo, J. A. Gabaldón, M. I. F. Gorbe, T. Gómez-Morte and P. Cosma, Chitosan film as recyclable adsorbent membrane to remove/recover hazardous pharmaceutical pollutants from water: the case of the emerging pollutant Furosemide, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2021, 56, 145–156 CAS.
- A. Murcia-Salvador, J. A. Pellicer, M. I. Fortea, V. M. Gómez-López, M. I. Rodríguez-López, E. Núñez-Delicado and J. A. Gabaldón, Polymer, 2019, 11 Search PubMed.
- D. D’Amato and J. Korhonen, Integrating the green economy, circular economy and bioeconomy in a strategic sustainability framework, Ecol. Econ., 2021, 188, 107143 CrossRef.
- M. Li, D. Gao, S. Cui, Y. Shi and N. Liu, Fabrication of Fe3O4/ZIF-67 composite for removal of direct blue 80 from water, Water Environ. Res., 2020, 92, 740–748 CrossRef CAS PubMed.
- M. H. Marzbali, A. Mir, M. Pazoki, R. Pourjamshidian and M. Tabeshnia, Removal of direct yellow 12 from aqueous solution by adsorption onto spirulina algae as a high-efficiency adsorbent, J. Environ. Chem. Eng., 2017, 5, 1946–1956 CrossRef CAS.
- A. Bhatnagar, E. Kumar, A. K. Minocha, B.-H. Jeon, H. Song and Y.-C. Seo, Removal of Anionic Dyes from Water using Citrus limonum (Lemon) Peel: Equilibrium Studies and Kinetic Modeling, Sep. Sci. Technol., 2009, 44, 316–334 CrossRef CAS.
- V. S. Munagapati, V. Yarramuthi, Y. Kim, K. M. Lee and D. S. Kim, Removal of anionic dyes (Reactive Black 5 and Congo Red) from aqueous solutions using Banana Peel Powder as an adsorbent, Ecotoxicol. Environ. Saf., 2018, 148, 601–607 CrossRef CAS PubMed.
- D. Sun, X. Zhang, Y. Wu and X. Liu, Adsorption of anionic dyes from aqueous solution on fly ash, J. Hazard. Mater., 2010, 181, 335–342 CrossRef CAS PubMed.
- R. Jiang, G. Yu, P. Ndagijimana, Y. Wang, F. You, Z. Xing and Y. Wang, Effective adsorption of Direct Red 23 by sludge biochar-based adsorbent: adsorption kinetics, thermodynamics and mechanisms study, Water Sci. Technol., 2021, 83, 2424–2436 CrossRef CAS PubMed.
- G. Singh, V. Kumar and S. K. Dwivedi, Comparative Investigation of Congo Red and Direct Blue-1 Adsorption on Mycosynthesized Iron Nanoparticle, J. Clust. Sci., 2022, 33, 1889–1905 CrossRef CAS.
- T. El Malah, H. F. Nour, E. K. Radwan, R. E. Abdel Mageid, T. A. Khattab and M. A. Olson, A bipyridinium-based polyhydrazone adsorbent that exhibits ultrahigh adsorption capacity for the anionic azo dye, direct blue 71, Chem. Eng. J., 2021, 409, 128195 CrossRef CAS.
- A. Alhujaily, H. Yu, X. Zhang and F. Ma, Adsorptive removal of anionic dyes from aqueous solutions using spent mushroom waste, Appl. Water Sci., 2020, 10, 183 CrossRef CAS.
- S. Khamparia and D. Jaspal, Adsorptive removal of Direct Red 81 dye from aqueous solution onto Argemone mexicana, Sustainable Environ. Res., 2016, 26, 117–123 CrossRef CAS.
- B. Li and Z. Ren, Superior Adsorption of Direct Dye from Aqueous Solution by Y(III)-Chitosan-Doped Fly Ash Composite as Low-Cost Adsorbent, J. Polym. Environ., 2020, 28, 1811–1821 CrossRef CAS.
- E. A. Alabbad, Efficacy assessment of natural zeolite containing wastewater on the adsorption behaviour of Direct Yellow 50 from; equilibrium, kinetics and thermodynamic studies, Arabian J. Chem., 2021, 14, 103041 CrossRef CAS.
- S. Noreen, U. Khalid, S. M. Ibrahim, T. Javed, A. Ghani, S. Naz and M. Iqbal, ZnO, MgO and FeO adsorption efficiencies for direct sky Blue dye: Equilibrium, kinetics and thermodynamics studies, J. Mater. Res. Technol., 2020, 9, 5881–5893 CrossRef CAS.
- A. Bhatnagar, M. Sillanpää and A. Witek-Krowiak, Agricultural waste peels as versatile biomass for water purification – A review, Chem. Eng. J., 2015, 270, 244–271 CrossRef CAS.
- J. Gubitosa, V. Rizzi, D. Cignolo, P. Fini, F. Fanelli and P. Cosma, From agricultural wastes to a resource: Kiwi Peels, as long-lasting, recyclable adsorbent, to remove emerging pollutants from water. The case of Ciprofloxacin removal, Sustainable Chem. Pharm., 2022, 29, 100749 CrossRef CAS.
- J. Gubitosa, V. Rizzi, P. Fini, S. Nuzzo and P. Cosma, Process., 2022, 10 Search PubMed.
- S. Alkan, A. rıza Kul and A. Yagiz, Adsorption of textile dyes from aqueous solutions by using kiwifruit (Ordu) peel, Fresenius Environ. Bull., 2017, 26, 7045–7053 CAS.
- K. M. Al-Qahtani, Water purification using different waste fruit cortexes for the removal of heavy metals, J. Taibah Univ. Sci., 2016, 10, 700–708 CrossRef.
- L. A. Mokif, N. A. Abdulhusain and S. O. H. AL-Mamoori, The Possibility of Using the kiwi Peels as an Adsorbent for Removing Nitrate from Water, J. Univ. Babylon Eng. Sci., 2018, 26, 192–197 Search PubMed.
- A. Saleh Jafer and A. A. Hassan, J. Phys.: Conf. Ser., 2019, 1294, 072013 CrossRef.
- M. Rahimnejad, K. Pirzadeh, I. Mahdavi and S. M. Peyghambarzadeh, Pb(II) removal from aqueous solution by adsorption on activated carbon from kiwi peel, Environ. Eng. Manag. J., 2018, 17, 1293–1300 CrossRef CAS.
- J. Wang, Y. Zhou, X. Liu, Q. Liu, M. Hao, S. Wang, Z. Chen, H. Yang and X. Wang, Design and application of metal–organic framework membranes for gas and liquid separations, Sep. Purif. Technol., 2024, 329, 125178 CrossRef CAS.
- S. Dhaka, R. Kumar, A. Deep, M. B. Kurade, S. W. Ji and B. H. Jeon, Metal–organic frameworks (MOFs) for the removal of emerging contaminants from aquatic environments, Coord. Chem. Rev., 2019, 380, 330–352 CrossRef CAS.
- S. Endo, A. Pfennigsdorff and K. U. Gos, Salting-Out Effect in Aqueous NaCl Solutions: Trends with Size and Polarity of Solute Molecules, Environ. Sci. Technol., 2012, 46, 1496–1503 CrossRef CAS PubMed.
- S. M. Burkinshaw, The role of inorganic electrolyte (salt) in cellulosic fibre dyeing: Part 2 theories of how inorganic electrolyte promotes dye uptake, Color. Technol., 2021, 137, 547–586 CAS.
- A. Serrano-Martínez, M. T. Mercader-Ros, I. Martínez-Alcalá, C. Lucas-Abellán, J. A. Gabaldón and V. M. Gómez-López, Degradation and toxicity evaluation of azo dye Direct red 83:1 by an advanced oxidation process driven by pulsed light, J. Water Process Eng., 2020, 37, 101530 CrossRef.
- Y. Song, J. Li and B. Bai, TiO2-Assisted Photodegradation of Direct Blue 78 in Aqueous Solution in Sunlight, Water, Air, Soil Pollut., 2010, 213, 311–317 CrossRef CAS.
- D. Marković, B. Jokić, Z. Šaponjić, B. Potkonjak, P. Jovančić and M. Radetić, Photocatalytic Degradation of Dye C.I. Direct Blue 78 Using TiO2 Nanoparticles Immobilized on Recycled Wool-Based Nonwoven Material, Clean: Soil, Air, Water, 2013, 41, 1002–1009 Search PubMed.
- V. Rizzi, I. Losito, A. Ventrella, P. Fini, A. Fraix, S. Sortino, A. Agostiano, F. Longobardi and P. Cosma, Rose Bengal-photosensitized oxidation of 4-thiothymidine in aqueous medium: evidence for the reaction of the nucleoside with singlet state oxygen, Phys. Chem. Chem. Phys., 2015, 17, 26307–26319 RSC.
- V. Rizzi, I. Losito, R. Abbattista, P. Fini, A. Agostiano, T. R. I. Cataldi and P. Cosma, Potential of 4-thiothymidine as a molecular probe for H2O2 in systems related to PhotoDynamic therapy: A structuristic and mechanistic insight by UV-visible and FTIR-ATR spectroscopies and by ElectroSpray ionization mass spectrometry, J. Mol. Liq., 2018, 264, 398–409 CrossRef CAS.
- V. Rizzi, P. Cosma, R. Abbattista, P. Fini, A. Agostiano, T. R. I. Cataldi and I. Losito, Reactivity of 4-thiothymidine with Fenton reagent investigated by UV-visible spectroscopy and electrospray ionization mass spectrometry, J. Mass Spectrom., 2019, 54, 389–401 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cp00174e |
| This journal is © the Owner Societies 2024 |