Open Access Article
Open Access ArticleInsights on adsorption of pyocyanin in montmorillonite using molecular dynamics simulation†
Bidemi
Fashina
 *ab,
Youjun
Deng
*a,
Tahir
Cagin
c and
Randall
Cygan
*ab,
Youjun
Deng
*a,
Tahir
Cagin
c and
Randall
Cygan
 a
a
aDepartment of Soil and Crop Sciences, Texas A&M University, College Station, TX 77843-2474, USA. E-mail: btfashi@sandia.gov
bGeochemistry Department, Sandia National Laboratories, P. O. Box 5800-0754, Albuquerque, New Mexico 87123, USA
cMaterials Science and Engineering, Chemical Engineering, Texas A&M University, TX 77843-2474, USA
First published on 18th March 2024
Abstract
Pyocyanin is an important virulence factor in the resistance of Pseudomonas aeruginosa to antibiotics. Pyocyanin is a planar three ring aromatic molecule that occurs as zwitterionic (PYO) or protonated species (PYOH+). Our earlier studies have shown that montmorillonite, through adsorption and transformation, can inactivate both PYO and PYOH+ in the interlayer space. The objective of this study was to elucidate the interaction mechanisms between montmorillonite and the adsorbed pyocyanin and to characterize the structure of the pyocyanin–montmorillonite complex via molecular dynamics (MD) simulations. The MD simulations were performed for the complexes of hydrated Na-montmorillonite (HM) with (i) neutral pyocyanin (HMP) and (ii) protonated pyocyanin (HMPH); and dehydrated Na-montmorillonite (DM) with (iii) neutral pyocyanin (DMP) and (iv) protonated pyocyanin (DMPH). The simulations indicated that in dry conditions, both PYO and PYOH+ were well-ordered in the midplane of the interlayer of montmorillonite, with the three aromatic rings almost parallel to the basal surface and sandwiched in-between basal surface-adsorbed Na+ planes. In humid conditions, the pyocyanin and Na+ were solvated in the interlayer space and the pyocyanin was less ordered compared to dehydrated models. Ion–dipole interaction (Na–O) was the dominant interaction for the dehydrated complexes DMPH and DMP but the interaction was stronger in the latter. The Na–O ion–dipole interaction remained the dominant interaction in hydrated HMP while in HMPH, water outcompeted PYOH+ for Na+ resulting in water–Na interaction being the dominant interaction. These results revealed the arrangement of the two species of pyocyanin in the interlayer spaces of montmorillonite and the mechanism of interaction between the pyocyanin and montmorillonite.
1. Introduction
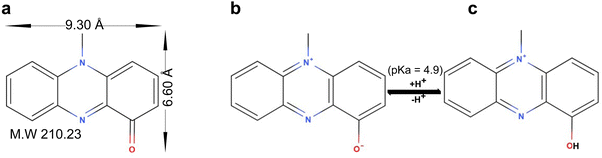
Pyocyanin (C13H10N2O) is one of the redox-active tricyclic phenazines produced as a virulence factor by the drug-resistant Pseudomonas aeruginosa through a series of complex gene-mediated steps.1,2 Pyocyanin has a pKa of 4.9 and exists as protonated species (PYOH+) at pH < pKa or neutral zwitterionic species (PYO) at pH > pKa (Fig. 1). Pyocyanin facilitates the recalcitrance and resistance of P. aeruginosa to antibiotics by killing and inhibiting the growth of competing microbes,3,4 suppression of host immune system,5 and formation of biofilms.6–8 In view of the importance of pyocyanin in the pathogenicity of P. aeruginosa, the inactivation/deactivation of the molecule or the modulation of pyocyanin production was proposed as an alternative approach to disarming the pathogen.9,10 Indeed, the inactivation of virulence factors (including pyocyanin) by adsorption has been shown to attenuate the cytotoxicity of the pathogen by neutralizing virulence factors, and transcriptionally suppress virulence gene expression.11,12Our earlier study showed that clay minerals (Na-montmorillonite and sepiolite) adsorbed pyocyanin and probably transformed pyocyanin in the interlayer of the montmorillonite and in the tunnel of sepiolite.13 For Na-montmorillonite, at pH < pKa, the mechanism of adsorption is by ion-exchange of interlayer cation by PYOH+. At pH > pKa, there are two types of adsorption mechanism which was mainly dictated by the type of interlayer cations.14 Mechanism I – protonation of a fraction of the neutral pyocyanin (i.e., xPYO + (x − y)H+ → yPYO + (x − y)PYOH+) followed by incomplete ion-exchange of PYOH+ for interlayer cation while mechanism II – the diffusion and the adsorption of the zwitterionic PYO in interlayer of montmorillonite with little to no protonation. Mechanism I was mostly observed in alkali and alkali earth-montmorillonite while mechanism II was dominant on Cu2+- and Mn2+-montmorillonite.14
These studies demonstrated that clay minerals can combat P. aeruginosa infections by pyocyanin adsorption and also elucidate the influence of smectite properties (type of interlayer cation, layer charge density, and source of layer charge) and the speciation of pyocyanin on the adsorption of pyocyanin.13,14 Yet, little is known about the details of pyocyanin–montmorillonite bonding/interaction at the molecular level. More specifically, the arrangement of pyocyanin in the interlayer of montmorillonite and the interaction between pyocyanin and exchangeable cations, water molecules, and the siloxane surfaces of the mineral. An understanding of the interaction is important in the selection and improvement (modification) of smectites as pyocyanin adsorbent/degrader and in the design of functional nanomaterials to serve similar purposes.
As a result of the complexity of clay minerals, experimental approach is often insufficient to convincingly elucidate the dynamics and bonding mechanism between adsorbed organics and smectites components – especially involving basal surfaces, interlayer cations and solvation water. On the other hand, molecular simulation approaches – molecular dynamics (MD) and density functional theory (DFT) – when guided and constrained by experimental data can aid the understanding of the energy, structure, and dynamics of hydrated and dehydrated smectite and organic–smectite systems.
With respect to organic–smectite complexes, MD simulations have been an important tool in understanding the structure, dynamics, interactions, and interfacial energetics of these complex systems.15–20 Using MD simulation, this present study investigated the interactions of pyocyanin with the cations and water molecules in the interlayer space of montmorillonite to provide a molecular scale insight into the interaction mechanism in dehydrated and hydrated montmorillonite–pyocyanin systems. The specific objectives of the current study were to characterize the structures of pyocyanin–montmorillonite complexes and investigate the interaction mechanisms in pyocyanin–montmorillonite complexes.
2. Methods
Initial structures of the montmorillonite and pyocyanin were constructed with BIOVIA Materials Studio (MS) 2018; all the MD simulations were performed with the LAMMPS code; structure output files were visualized in OVITO and MS; and post analysis was either computed on the fly in LAMMPS or using in-house Python scripts.21–232.1 Pristine dehydrated and hydrated montmorillonite
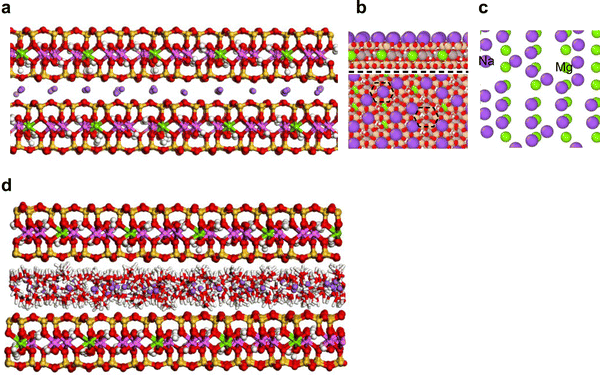
A unit cell of montmorillonite with layer charge of −1e was built from an orthogonal unit cell of pyrophyllite.24 The charge deficit was balanced with one Na+ placed in a 3.153 Å slab (i.e., interlayer space) built above the siloxane surface. The unit cell (NaSi8Al3MgO20(OH)4) has cell parameters of 5.160, 8.966, and 12.50 Å, along the a, b, and c-axis, respectively. The model was expanded (16a × 10b × 4c) to create an 82.56 × 89.66 × 50 Å supercell. This represents the dehydrated Na-montmorillonite (DM, Fig. 2(a)). To construct hydrated Na-montmorillonite, 4800 water molecules were dispersed in the interlayer of DM (i.e., 1200 H2O/interlayer). The H2O/Na+, H2O/(unit cell) and water content in the supercell were of 7.5, 7.5 and ∼180 mg H2O gclay−1, respectively. The model was referred to as HM (Fig. 2(b)). This large amount of water was considered because the forcefield employed in this study renders the smectite structure metastable for smectite structures of this large size, high charge density, and low hydration. To circumvent this issue we added enough water to the interlayer to hydrate all cations thus discouraging electrostatic attraction between interlayer cations and the basal surface.2.2 Arrangement of pyocyanin in the interlayer of montmorillonite
The X-ray diffractograms of pyocyanin–montmorillonite complexes have shown that only a single layer of pyocyanin can be adsorbed in the interlayer of montmorillonite13,14 but the arrangement of pyocyanin was not clear. To determine the most thermodynamically favourable arrangement of pyocyanin in the interlayer of montmorillonite, three possible arrangements (tail-to-head, head-to-head, and random) were investigated using a 2 × 5 × 2 montmorilonite supercell (see Fig. S1, ESI†). Results indicated there was no significant difference in the potential energy or crystallographic parameters for the three arrangements (see Table S1, ESI†). Consequently, the random arrangement was adopted.2.3 Dehydrated and hydrated PYOH+–montmorillonite
In accordance with the results from X-ray energy dispersive spectroscopy,13,14 half of the interlayer Na+ in DM was randomly replaced with PYOH+ to represent the cation exchange mechanism (mechanism I) of pyocyanin adsorption. This structure represented the dehydrated protonated PYOH+–Na-montmorillonite complex and denoted as DMPH (Fig. 2(c)). Then, each interlayer of DMPH was saturated with (i) 4000 water molecules (i.e., 1000 water/interlayer) to represent the hydrated PYOH+–Na-montmorillonite complexes (HMPH, see Fig. 2(d)).2.4 Dehydrated and hydrated PYO–montmorillonite
To depict mechanism II, the PYOH+ molecules in both DMPH and HMPH were deprotonated followed by the addition of Na+ to maintain a neutral model. The resulting structures correspond to the dehydrated and hydrated PYO–montmorillonite and denoted as DMP and HMP, respectively.2.5 Forcefields
The forcefield parameters for water and pyocyanin were taken from the flexible SPC water model and consistent valence forcefield (CVFF), respectively.25,26 The forcefield parameters for Na-montmorillonite were taken from Clayff27,28 with slight modification to the parameter for Na+ and bridging oxygen.29 This modification was necessary to avoid the unrealistic attraction of Na+ deep into the ditrigonal cavity of dehydrated montmorillonite as observed in previous simulation studies.30–32 See Tables S2–S4 (ESI†) and Fig. 3 for a summary of the assigned forcefield parameters for the non-bonded and bonded terms in Na-montmorillonite and pyocyanin structure.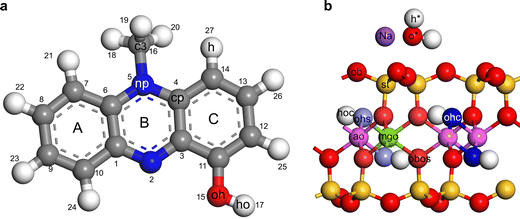 | ||
| Fig. 3 Forcefield assignment for (a) protonated pyocyanin and (b) Na-montmorillonite. For neutral pyocyanin the ho was deleted while the oh was reassigned as o′ (see Tables S2–S4 for a summary of the assigned forcefield parameters, ESI†). | ||
2.6 Simulation
Using the LAMMPS software package, MD simulations were performed with 3D periodic boundary conditions. The energy of the non-bonded component was computed according to eqn (S1.2)–(S1.4) (ESI†). The distance and energy interaction parameters for the non-bonded atoms was computed using the Lorentz–Berthelot combination rules.33 A 10.0 Å real-space cut-off was used for short-range interactions, and the particle–particle particle–mesh algorithm (PPPM) was used to compute long-range interactions at an accuracy of 1.0 × 10−4.34Using a time step of 1 fs, MD simulations were sequentially performed using the microcanonical NVE ensemble (constant number of atoms N, constant volume V, and constant energy E), the canonical NVT ensemble (constant temperature T), and the isothermal–isobaric NPT ensemble (constant pressure P). Temperature and pressure were controlled using a Nosé–Hoover thermostat and barostat.35
As indicated in the MD simulation protocols (Fig. 4), an energy minimization was first performed for the models using conjugate gradient algorithm36 followed by MD equilibration (NVE) of the models at 300 K for 1 ns. Two NVT runs were each conducted at 300 K for 2 ns while damping (relaxing) the temperature at 100 timestep. The first NVT (NVT1) was performed to maintain a stable temperature prior to the second NVT run (NVT2). Following the NVT ensemble simulation, two NPT runs were each performed at 1 atm and 300 K for 2 ns. The first NPT (NPT1) was performed to equilibrate the system while the second NPT (NPT2) was used to compute thermodynamic properties, trajectories, d-spacings every 100 fs and radial distribution functions (RDF) as an average of every 100 fs. Atomic density profiles (ADP) were computed from the atomic trajectories.
2.7 Radial distribution function (RDF)
The radial distribution function, g(r), is a description of the changes in density as a function of distance from a reference point. The RDF is estimated as the probability of finding an atom A from at a distance r from an atom B and can be calculated:33 | (1) |
2.8 Atomic density profiles (ADP)
The ADP along the z-axis (i.e., 00l) was computed as the distance between an atom and a reference atom (basal oxygen “ob” in this case). The coordinates were extracted from the trajectory files of the second NPT run of the simulation and 400 bins were used to compute histograms. The computed densities were normalized to densities per layer by stipulating a maximum|cut-off|value for the distance between an atom and the reference atom (ob, see Fig. 3(b)).3. Results and discussion
3.1 Basal spacings
| Unit cell parameters | ||||||
|---|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | c′ (Å) | α, β, γ | ||
| Note: the a, b, and c dimensions are per unit cell; c′ = experimentally observed dimension in vacuum (*), at 200 °C (†) or at 65% relative humidity (**) conditions. The † and ** are from Fashina and Deng13,14 and * is under atmosphere of nitrogen (see Fig. S2, ESI). | ||||||
| Start | 5.16 | 8.97 | 12.5 | 90 | ||
| DM | 5.18 ± 0.02 | 9.00 ± 0.02 | 9.82 ± 0.02 | 9.73* | 9.75† | 90 |
| HM | 5.18 ± 0.02 | 8.98 ± 0.02 | 14.14 ± 0.05 | 12.70** | 90 | |
| DMPH | 5.19 ± 0.02 | 9.00 ± 0.02 | 13.02 ± 0.03 | 12.54* | 12.48† | 90 |
| HMPH | 5.19 ± 0.02 | 8.98 ± 0.02 | 16.21 ± 0.06 | 12.70** | 90 | |
| DMP | 5.19 ± 0.02 | 9.01 ± 0.02 | 13.34 ± 0.06 | 12.54* | 12.48† | 90 |
| HMP | 5.19 ± 0.02 | 8.98 ± 0.02 | 16.38 ± 0.06 | 12.70** | 90 | |
3.2 Atomic density profile – structure and arrangement
 | ||
| Fig. 8 Atomic density profile of (a) dehydrated PYOH+–Na-montmorillonite, (b) hydrated PYOH+–Na-montmorillonite, (c) dehydrated PYO–Na-montmorillonite, and (d) hydrated PYO–Na-montmorillonite. | ||
Unlike the structure of DM, most of the Na+ in DMPH were adsorbed closer to the clay surface (Fig. 8(a)). This strong adsorption suggests (i) strong repulsion between charged PYOH+ and Na+ or (ii) that the inorganic cation has a strong preference for the clay surface when PYOH+ is adsorbed. The former is supported by the fact that the protonated end of PYOH+ always points away from adsorbed Na+ and towards the edge of the ditrigonal cavity unoccupied by Na+ (Fig. 7(c)). This arrangement exposed both oh and N to interact with Na+ (i.e. ion–dipole interactions).
The o* and h* each has two peaks (a sharp and a broad peak) on opposite sides of the interlayer indicating two layers of water solvating two layers of Na+. The 1L was the dominant with peaks at 2.65 Å (o*) and 1.63 Å (h*) from the basal surface while the 2L has peaks at 1.93 Å (o*) and 2.81 Å (h*) from the basal ob. The 2L of water has the H-atoms pointed towards the basal surface and allows water to mainly interact with the (i) basal surface of the clay (i.e., ob–h*) by H-bonding and (ii) with the interlayer Na+ ions (i.e., o*–Na) by ion–dipole. The 1L of water has the H-atoms pointed inwards towards the interlayer allowing water to interact mainly with PYOH+ (o*–ho) or form a network of H-bonding (o*–h*) with the 2L of water.
The oh/ho have two peaks on opposite sides of the interlayer and are at similar distance to the basal surface as o* and h* (Table S4, ESI† and Fig. 8(b)). The splitting of the oh and ho is indicative of two possible orientations/configurations of the oh/ho atoms on the single layer of PYOH+. The first configuration has the oh and ho at 2.52 and 1.63 Å from the basal ob, respectively. The second configuration of PYOH+ has the oh and ho at 1.93 and 2.81 Å from the basal surface, respectively. As evident by the weak small peak, the first configuration is the dominant.
The dominant configuration allows the proton to interact (i) with basal ob (i.e., ho–ob) via inner sphere complexation or electrostatic interaction and (ii) with both configurations of water (i.e., ho–o*) via H-bonding. The oxygen (i.e., oh) of the dominant configuration can also interact with the interlayer Na+ (i.e., oh–Na) through ion–dipole at distance of 1.35 and 0.67 Å for the farthest and nearest Na from the basal ob, respectively. The second and weaker oh/ho configuration interacts (i) with water (i.e., ho–o* and oh–h*) through H-bonding at a distance of 0.88 Å and, (ii) interlayer Na+ (i.e., oh–Na) through ion–dipole of length 1.94 and 1.26 Å for the farthest and nearest Na from the basal ob, respectively. This suggests that the dominant oh/ho configuration allows stronger interaction with the basal surface and interlayer components while the second oh/ho configuration is mostly solvated—water bridged.
The split two N peaks on each side of the basal surface represents the two types of N in tilted pyrazine ring of PYOH+ rather than different configurations of the pyrazine ring. This statement is corroborated by the c (i.e., c3) peak which showed no splitting on each side of the interlayer. If indeed the split N peaks represented two different configurations, the c3 should show similar split. The distance of both N and c3 peak from basal ob suggest that the N peaks at 3.87 and 3.19 Å correspond to methyl substituted N (atom 5 on Fig. 3(a)) and unsubstituted N (atom 2 on Fig. 3(a)), respectively. The unsubstituted N of the pyrazine ring is about 0.40 Å from the h* suggesting interaction with water. Similar to this MD study, experimental results indicate that H-bonded phenazine–water and pyrazine–water complexes were possible through the unsubstituted N in the pyrazine ring.40,41 Along the z-axis, the two Na+ and two N peaks were at the same distance from the basal surface. We propose that the Na+ farthest from the basal surface could interact with the unsubstituted N. Such ion–dipole interaction might contribute to the stabilization of the PYOH+–montmorillonite complex.
The C (c3) peak was 2.65 Å from the basal surface. Factoring the C–H bond, the length of the methyl H from the surface can be estimated to be about 1.75 Å. This is similar to the 2.0 Å estimated for the DMPH. Hence, as in under dry conditions, PYOH+ could be interacting with the basal surfaces through the methyl group (i.e., H-bonding).
In relation to Na+, the main configuration of o* was 1.07 and 0.84 Å from the two Na+ peaks indicating that both Na was well-solvated. The pyrazine ring is almost at the same height as the solvated Na+ which suggest that the solvation of the Na might disfavour the formation of direct Na–N ion–dipole in HMPH.
The o′, N, and C (i.e., c3) are at ∼3.37 Å from the basal surface indicating that the rings of PYO remained planar in the midplane and parallel to the basal surface (Fig. 8(c) and Table S4, ESI†). Unlike in DMPH, the o′ atoms in PYO remained midplane because there is no proton being attracted to the basal surfaces. Similar to DMPH, the molecules of PYO are well-ordered midplane in the interlayer and the angle of tilt of the pyrazine ring (ring B, Fig. 3(a)) is estimated to be less than 15°. The C (i.e., c3) did not split (in contrast to DMPH) but was at a farther distance from the basal surfaces compared to the DMPH. Yet, a c3 distance of 3.37 Å from the basal surface means that the H atoms of the methyl group are at about 2.50 Å from the basal surface. This suggests H-bonding at a slightly longer distance compared to the 2.00 Å observed for DMPH.
Like what was observed in DMPH, most of the Na+ in DMP were adsorbed on the surface at 1.26 Å from the clay surface (Fig. 8(c) and Table S4, ESI†). This strong adsorption suggests that the earlier observed surface adsorption in DMPH might not be entirely due to the repulsion between the proton of PYOH+ and Na+. Rather, in dry conditions, Na+ ions have a higher affinity to be attracted to the basal surfaces of smectite. This arrangement exposes both o′ and N to Na+ at distance of 2.11 Å each. This suggests Na+–O and Na+–N ion–dipole interactions. The former being at longer distance in DMP compared to DMPH while the latter is at same distance in both DMPH and DMP.
The dominant peak of o′ was at 4.87 Å from the basal ob and another small peak at 2.95 Å from the basal ob (Fig. 8(d)). The dominant peak was right in the middle of the interlayer suggesting that most PYO molecules were adsorbed in the midplane. The major o′ peaks were 1.07 and 1.90 Å from both Na+ suggesting an ion–dipole interaction. Hence, the ion–dipole interaction in DMP is shorter (0.54 and 1.20 Å) than in HMP (1.07 and 1.90 Å). The major o′ peak is about 2.20 Å from the major h* peak implying the possibility of H-bonding.
The C (i.e., c3) peak was 2.94 Å from the basal ob. The distance of the H-methyl from the surface ob can be estimated as 1.94 Å which is the same as the value observed for HMPH but lower than DMP. Hence, interaction between the H-methyl and the basal ob is more favourable under humid conditions.
3.3 Interlayer bonding between adsorbed pyocyanin and montmorillonite
Radial distribution functions, derived from the equilibrated MD simulations, help to better assess the relative strengths of atom–atom interactions.4. Conclusion
This experiment-guided molecular simulation study investigated the interaction mechanism and the structure of protonated (PYOH+) and zwitterionic (PYO) pyocyanin in the interlayer of Na-montmorillonite in dry and humid conditions. Large-scale molecular dynamics simulations and accurate interatomic potentials were used to determine stable structures and configurations of pyocyanin species, water molecules, and counterions within the interlayer gallery of montmorillonite. With limited experimental and spectroscopic data for organic-clay systems, the molecular simulations provide key insights into the nature of atomic interactions in the interlayer of smectite minerals. Based on the equilibrated MD simulations, the following conclusions were reached:(1) In dry condition, PYOH+ and PYO are well-ordered, oriented subparallel to the basal surface and situated between basal surface-adsorbed Na+.
(2) In humid conditions, Na+, PYOH+ and PYO are solvated near the midplane of the interlayer gallery and the pyocyanin molecules are less orderly arranged compared to MD results for the dry DMPH and DMP systems.
(3) The dominant interlayer bonding in DMPH is mainly a 2.7 Å ion–dipole between Na+ and the –OH of PYOH+ and, to a lesser degree, ion–dipole interaction between Na+ and N at a distance of about 3 Å. In DMP, these two interactions were also observed but occur at shorter distances of 2.30 Å (Na–OH) and 2.80 Å (Na–N). Hence, similar interaction mechanisms dominated the interaction of montmorillonite and pyocyanin in dry conditions irrespective of the speciation of pyocyanin.
(4) The dominant interlayer bonding in HMPH is between the Na+ and water. This bonding is at least an order of magnitude greater than the interaction between –OH of PYOH+ and water which suggests that PYOH+ cannot outcompete water for adsorption sites. Water bridged Na+ and PYOH interaction were also observed. In HMP, the dominant pair coordination was ion–dipole Na+–o′ at 2.35 Å and, to a lesser degree, H-bonding between water and PYO. The Na+–o′ coordination is more common compared to the coordination of water to Na+. Therefore, unlike PYOH+, the PYO molecules can outcompete water for the Na+ in the interlayer. There is on-going spectroscopic work to verify these mechanisms.
Overall, this molecular dynamics simulation study improved the understanding of the arrangement of pyocyanin species in the interlayer of montmorillonite and, provided insights into the main mechanisms of interaction in pyocyanin–montmorillonite complexes.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the provision of supercomputing facilities and software by the Texas A&M University High Performance Research Computing (HPRC). The research was financially supported by the Department of Soil and Crop Sciences, Texas A&M University and the Joe and Martha Dixon Endowment. Thank you to Diane Uwacu for reviewing the codes used for post-processing of the MD results.References
- D. V. Mavrodi, R. F. Bonsall, S. M. Delaney, M. J. Soule, G. Phillips and L. S. Thomashow, Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1, J. Bacteriol., 2001, 183, 6454–6465 CrossRef CAS PubMed.
- K. J. Reszka, Y. O’Malley, M. L. McCormick, G. M. Denning and B. E. Britigan, Oxidation of pyocyanin, a cytotoxic product from Pseudomonas aeruginosa, by microperoxidase 11 and hydrogen peroxide, Free Radical Biol. Med., 2004, 36, 1448–1459 CrossRef CAS PubMed.
- K. L. Tomlin, O. P. Coll and H. Ceri, Interspecies biofilms of Pseudomonas aeruginosa and Burkholderia cepacia, Can. J. Microbiol., 2001, 47, 949–954 CrossRef CAS PubMed.
- A. Price-Whelan, L. E. P. Dietrich and D. K. Newman, Nat. Chem. Biol., 2006, 2, 71–78 CrossRef CAS PubMed.
- J. B. Lyczak, C. L. Cannon and G. B. Pier, Clin. Microbiol. Rev., 2002, 15, 194–222 CrossRef CAS PubMed.
- T. Das and M. Manefield, Pyocyanin Promotes Extracellular DNA Release in Pseudomonas aeruginosa, PLoS One, 2012 DOI:10.1371/journal.pone.0046718.
- T. Das, A. I. Ibugo, W. Klare and M. Manefield, Microbial Biofilms – Importance and Applications, InTech, 2016 Search PubMed.
- P. K. Taylor, A. T. Y. Yeung and R. E. W. Hancock, Antibiotic resistance in Pseudomonas aeruginosa biofilms: Towards the development of novel anti-biofilm therapies, J. Biotechnol., 2014, 191, 121–130 CrossRef CAS PubMed.
- K. C. Costa, M. Bergkessel, S. Saunders, J. Korlach and D. K. Newman, Enzymatic degradation of phenazines can generate energy and protect sensitive organisms from toxicity, mBio, 2015, 6(6), 10–1128 CrossRef PubMed.
- J. Jabłońska, A. Augustyniak, K. Dubrowska and R. Rakoczy, The two faces of pyocyanin–why and how to steer its production?, World J. Microbiol. Biotechnol., 2023, 39, 103 CrossRef PubMed.
- H. Hirakawa, A. Kimura, A. Takita, S. Chihara, K. Tanimoto and H. Tomita, Adsorption of extracellular proteases and pyocyanin produced by Pseudomonas aeruginosa using a macroporous magnesium oxide-templated carbon decreases cytotoxicity, Curr. Res. Microb. Sci., 2022, 3, 100160 CAS.
- D. Wang, W. Johnston, S. Ching, B. Fashina and Y. Deng, in Poultry Science Asociation 2022 Latin America Scientific Conference, Parana, 2022.
- B. Fashina and Y. Deng, Smectite, sepiolite, and palygorskite for inactivation of pyocyanin, a biotoxin produced by drug-resistant Pseudomonas aeruginosa, Microporous Mesoporous Mater., 2022, 331, 111668 CrossRef CAS.
- B. Fashina and Y. Deng, The effect of layer charge origin and density, type of interlayer cations, and occupancy of the octahedral sheet of smectites on the adsorption of pyocyanin, Appl. Clay Sci., 2023, 237, 106884 CrossRef CAS.
- J. A. Greathouse and R. T. Cygan, Water structure and aqueous uranyl(VI) adsorption equilibria onto external surfaces of beidellite, montmorillonite, and pyrophyllite: Results from molecular simulations, Environ. Sci. Technol., 2006, 40, 3865–3871 CrossRef CAS PubMed.
- V. Aggarwal, H. Li and B. J. Teppen, Triazine adsorption by saponite and beidellite clay minerals, Environ. Toxicol. Chem., 2006, 25, 392–399 CrossRef CAS PubMed.
- Q. Zhou, W. Shen, J. Zhu, R. Zhu, H. He, J. Zhou and P. Yuan, Structure and dynamic properties of water saturated CTMA-montmorillonite: molecular dynamics simulations, Appl. Clay Sci., 2014, 97–98, 62–71 CrossRef CAS.
- M. Maruthi Sena and M. Krishnan, Role of Cations in Adsorption of Supercritical Carbon Dioxide at Smectite Mineral-Water Interfaces: Molecular Dynamics and Adaptive Biasing Force Simulation Studies, J. Phys. Chem. C, 2019, 123, 1170–1184 CrossRef CAS.
- M. Szczerba, Y. Deng and M. Kowalik-Hyla, Molecular Modeling to Predict the Optimal Mineralogy of Smectites as Binders of Aflatoxin, Clays Clay Miner., 2022, 70(6), 824–836 CrossRef CAS.
- T. A. Ho, C. F. Jove-Colon and Y. Wang, Low hydrogen solubility in clay interlayers limits gas loss in hydrogen geological storage, Sustainable Energy Fuels, 2023, 7(14), 3232–3238 RSC.
- D. S. Biovia, Material Studio, v. 08, San Diego Dassault Syst, 2018 Search PubMed.
- S. Plimpton, Fast parallel algorithms for short-range molecular dynamics, J. Comput. Phys., 1995, 117, 1–19 CrossRef CAS.
- A. Stukowski, Visualization and analysis of atomistic simulation data with OVITO-the Open Visualization Tool, Model. Simul. Mater. Sci. Eng., 2009, 18, 15012 CrossRef.
- J. H. Lee and S. Guggenheim, Single crystal X-ray refinement of pyrophyllite-1 Tc, Am. Mineral., 1981, 66, 350–357 CAS.
- P. Dauber-Osguthorpe, V. A. Roberts, D. J. Osguthorpe, J. Wolff, M. Genest and A. T. Hagler, Structure and energetics of ligand binding to proteins: Escherichia coli dihydrofolate reductase-trimethoprim, a drug-receptor system, Proteins: Struct., Funct., Bioinf., 1988, 4, 31–47 CrossRef CAS PubMed.
- O. Teleman, B. Jönsson and S. Engström, A molecular dynamics simulation of a water model with intramolecular degrees of freedom, Mol. Phys., 1987, 60, 193–203 CrossRef CAS.
- R. T. Cygan, J.-J. Liang and A. G. Kalinichev, Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field, J. Phys. Chem. B, 2004, 108, 1255–1266 CrossRef CAS.
- R. T. Cygan, J. A. Greathouse and A. G. Kalinichev, Advances in Clayff Molecular Simulation of Layered and Nanoporous Materials and Their Aqueous Interfaces, J. Phys. Chem. C, 2021, 125, 17573–17589 CrossRef CAS.
- T. A. Ho, L. J. Criscenti and J. A. Greathouse, Revealing Transition States during the Hydration of Clay Minerals, J. Phys. Chem. Lett., 2019, 10, 3704–3709 CrossRef CAS PubMed.
- L. N. Lammers, I. C. Bourg, M. Okumura, K. Kolluri, G. Sposito and M. Machida, Molecular dynamics simulations of cesium adsorption on illite nanoparticles, J. Colloid Interface Sci., 2017, 490, 608–620 CrossRef CAS PubMed.
- S. L. Teich-McGoldrick, J. A. Greathouse, C. F. Jové-Colón and R. T. Cygan, Swelling Properties of Montmorillonite and Beidellite Clay Minerals from Molecular Simulation: Comparison of Temperature, Interlayer Cation, and Charge Location Effects, J. Phys. Chem. C, 2015, 119, 20880–20891 CrossRef CAS.
- J. A. Greathouse, D. B. Hart, G. M. Bowers, R. J. Kirkpatrick and R. T. Cygan, Molecular Simulation of Structure and Diffusion at Smectite-Water Interfaces: Using Expanded Clay Interlayers as Model Nanopores, J. Phys. Chem. C, 2015, 119, 17126–17136 CrossRef CAS.
- M. P. Allen and D. J. Tildesley, Computer simulation of liquids, Oxford University Press, 2017 Search PubMed.
- R. W. Hockney and J. W. Eastwood, Computer Simulation Using Particles, 2021 DOI:10.1887/0852743920.
- W. Shinoda, M. Shiga and M. Mikami, Rapid estimation of elastic constants by molecular dynamics simulation under constant stress, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 134103 CrossRef.
- W. Ford, Krylov Subspace Methods, Numer. Linear Algebra Appl., 2015, 491–532 Search PubMed.
- Z. Malek, V. Balek, D. Garfinkel-Shweky and S. Yariv, The study of the dehydration and dehydroxylation of smectites by emanation thermal analysis, J. Therm. Anal., 1997, 48, 83–92 CrossRef CAS.
- L. Zhang, X. Lu, X. Liu, J. Zhou and H. Zhou, Hydration and mobility of interlayer ions of (Nax, Cay)-montmorillonite: A molecular dynamics study, J. Phys. Chem. C, 2014, 118, 29811–29821 CrossRef CAS.
- R. J. Graves and H. W. White, Study of the adsorption of acridine and phenazine on aluminum oxide using tunnelling spectroscopy, Spectrochim. Acta, Part A, 1987, 43, 107–118 CrossRef.
- S. D. Choudhury and S. Basu, Interaction of phenazine with water and DNA bases, Spectrochim. Acta, Part A, 2005, 62, 736–739 CrossRef PubMed.
- C. Marzzacco, Electronic spectra of the various hydrogen-bonded species of pyrazine in a mixed hydroxylic solvent, J. Am. Chem. Soc., 1973, 95, 1774–1777 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cp05762c |
| This journal is © the Owner Societies 2024 |