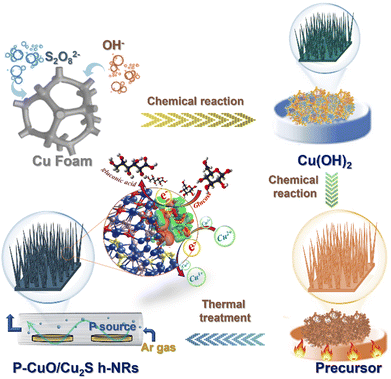
P-incorporated CuO/Cu2S heteronanorods as efficient electrocatalysts for the glucose oxidation reaction toward highly sensitive and selective glucose sensing†
L. L. D.
Thi
 *ab,
Thi H.
Ho
cd,
Tuan V.
Vu
*ab,
Thi H.
Ho
cd,
Tuan V.
Vu
 cd,
Dang L. T.
Nguyen
ab,
Minh Xuan
Tran
ab,
Sonny H.
Rhim
e and
C.-D.
Nguyen
*f
cd,
Dang L. T.
Nguyen
ab,
Minh Xuan
Tran
ab,
Sonny H.
Rhim
e and
C.-D.
Nguyen
*f
aLaboratory for Advanced Nanomaterials and Sustainable Energy Technologies, Institute for Computational Science and Artificial Intelligence, Van Lang University, Ho Chi Minh City, Vietnam. E-mail: luyen.doanthiluu@vlu.edu.vn
bFaculty of Applied Technology, School of Technology, Van Lang University, Ho Chi Minh City, Vietnam
cLaboratory for Computational Physics, Institute for Computational Science and Artificial Intelligence, Van Lang University, Ho Chi Minh City, Vietnam
dFaculty of Mechanical – Electrical and Computer Engineering, School of Technology, Van Lang University, Ho Chi Minh City, Vietnam
eDepartment of Physics and Energy Harvest Storage Research Center, University of Ulsan, Ulsan, 44610, Republic of Korea
fThe University of Danang – University of Science and Education, Danang 550000, Vietnam. E-mail: cdn@ued.udn.vn
First published on 6th December 2023
Abstract
Currently, tremendous efforts have been made to explore efficient glucose oxidation electrocatalysts for enzymeless glucose sensors to meet the urgent demands for accurate and fast detection of glucose in the fields of health care and environmental monitoring. In this work, an advanced nanostructured material based on the well-aligned CuO/Cu2S heteronanorods incorporated with P atoms is successfully synthesized on a copper substrate. The as-synthesized material shows high catalytic behavior accompanied by outstanding electrical conductivity. This, combined with the unique morphology of unstacked nanorod arrays, which endow the entire material with a greater number of exposed active sites, make the proposed material act as a highly efficient electrocatalyst for the glucose oxidation reaction. Density functional theory calculations demonstrate that P doping endows P-doped CuO/Cu2S with excellent electrical conductivity and glucose adsorption capability, significantly improving its catalytic performance. As a result, a non-enzymatic glucose sensor fabricated based on our proposed material exhibits a broad linear detection range (0.02–8.2 mM) and a low detection limit (0.95 μM) with a high sensitivity of 2.68 mA mM−1 cm−2 and excellent selectivity.
Introduction
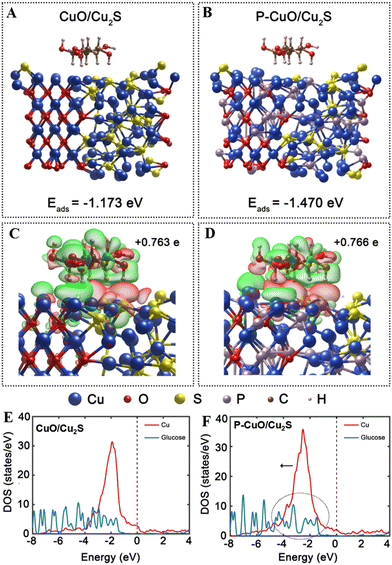
Nowadays, development of highly efficient electrocatalysts for the glucose oxidation reaction toward achieving improved sensitivity and selectivity of non-enzymatic electrochemical glucose sensors is necessary for people diagnosed with diabetes, as well as for the fields of environment, pharmaceutical, clinical diagnosis, and quality control.1–4 Transition metal-based nanostructured materials with their unique features of promising redox behavior, sufficient mechanical/chemical stability, and cost-effectiveness have been widely employed in designing and constructing glucose oxidation electrocatalysts.5,6 In addition to the mono-compounds,7,8 recent research is paying attention to the development of multiple composite-based electrocatalysts, which contain two or more transition metal-based components, owing to double advantages. The composites can fully inherit not only the advantages of individual compounds, but also their synergy. For example, Y. Gao and co-workers reported a good electrocatalyst for glucose sensing based on needle-like Co3O4–NiO nanocomposites. The proposed materials showed a desirable sensing performance, including two wide linear ranges (0.001–3.4 mM, 3.4–12.2 mM), a low detection limit of 0.81 μM, and a high sensitivity of 62 mA M−1 cm−2. Interestingly, the composites outperformed their individual components, such as Co3O4 and NiO, in terms of catalytic activity due to the synergy of Co3O4 and NiO.9 Similarly, a tremella-like NiS/CoS/NiCo2S4 hierarchical structure, as an advanced electrocatalytic material, has been developed for a non-enzymatic glucose sensor. The high performance of the proposed material for glucose oxidation could be attributed to its superior catalytic activity and good electrical conductivity.10 Among the transition metals that have been developed, copper and its compounds attracted much attention for the sensing applications, especially in the field of glucose detection, owing to their multiple oxidation states and good catalytic activities.11 Particularly, copper oxide (CuO) with a large specific surface area for forming a large number of accessible catalytic active sites, desirable catalytic properties, and decent potential for accelerating charge transfer has emerged as an interesting candidate.12–14 In addition, copper sulfide (Cu2S) with the advantage of its redox nature (Cu2+/Cu3+), which is responsible for its great capability in electron transfer, biocompatibility, and high catalytic activity, has also been considered as a promising electrocatalyst for glucose oxidation.1,15,16 Accordingly, the combination of CuO and Cu2S to form a CuO/Cu2S composite is expected to catalyze the glucose oxidation reaction well toward achieving improved performance for glucose sensing. However, the low electrical conductivity of both CuO and Cu2S is still a bottleneck for the entire CuO/Cu2S composite, and thus a further improvement of CuO and Cu2S conductivities is essential. In this regard, doping heteroatoms into CuO and Cu2S matrixes offers an ideal solution. In recent years, numerous attempts have been made to boost the conductivity of CuO and Cu2S by doping, for example Cl-doped CuO,17 S-doped Cu2O–CuO,17 W-doped CuS,18 and In3+-doped CuS.19 Amongst the dopants, phosphorus (P) has been considered an effective dopant.20,21 This is because that the lone-pair electrons in 3p orbitals and vacant 3d orbitals of the P atom can regulate the electronic structure of the host materials to boost their intrinsic properties. Nevertheless, to the best of our knowledge, incorporating P atoms into the CuO/Cu2S composite has not been studied yet in the field of glucose detection. Herein, an advanced nanostructured material based on P-doped CuO/Cu2S heteronanorods grown on copper foam (CF) (denoted as P-CuO/Cu2S h-NRs) is developed, analysed, and employed as a highly efficient electrocatalyst for glucose oxidation toward non-enzymatic glucose sening applications. The experimental results show that the catalytic performance of the P-CuO/Cu2S h-NRs catalyst for glucose sensing is better than that of its mono-counterparts: CuO nanorods (denoted as CuO NRs) and CuO/Cu2S heteronanorods without P incorporation (denoted as CuO/Cu2S h-NRs), and its parent: Cu(OH)2 nanorods (denoted as Cu(OH)2 NRs). This can be attributed to the enhanced electrical conductivity facilitated by P doping, more exposed active sites due to the unstacked nanorod structure, the full inheritance of excellent advantages of individual components CuO and Cu2S, and the synergy of the CuO/Cu2S composite. Notably, density functional theory (DFT) calculations reveal that density of states (DOS) of the P-CuO/Cu2S model extends over the Fermi level, suggesting its good charge transfer capability, which is in line with the experimental results. Also, the DFT calculations prove that the P doping endows P-CuO/Cu2S with optimal glucose adsorption capability, supported by more negative adsorption energy of P-CuO/Cu2S (−1.470 eV) than that of the pure CuO/Cu2S (−1.173 eV).Experimental
Sample preparation
Firstly, we synthesize a Cu(OH)2 NR sample via the chemical oxidation method.22 CF (size 5 cm × 5 cm) was successively sonicated with acetone, ethanol, and acetic acid for 15 min each to assure the removal of surface impurities. Then, the cleaned CF was dipped in 100 mL of the reactant solution (NaOH/(NH4)2S2O8 (weight ratio: 4/1) and deionized (DI) water) to deposit Cu(OH)2 NRs over its surface. After the surface of the CF substrate was fully wrapped with a light blue Cu(OH)2 NR layer, the product was taken out, washed with DI water, and dried for 12 h at 60 °C. Secondly, the obtained Cu(OH)2 NR sample was subject to sulfidation by dipping in 0.1 M thiourea solution at 90 °C for 5 h. Finally, a thermal treatment was conducted for the obtained composite at 300 °C for 2 h in the presence of 0.5 mg of NaH2PO2, as the P source, to form a P-CuO/Cu2S h-NR sample. The controlled samples, such as CuO NRs and CuO/Cu2S h-NRs were fabricated using the same approach, and for the details of the synthesis protocols, see the ESI.†Characterization
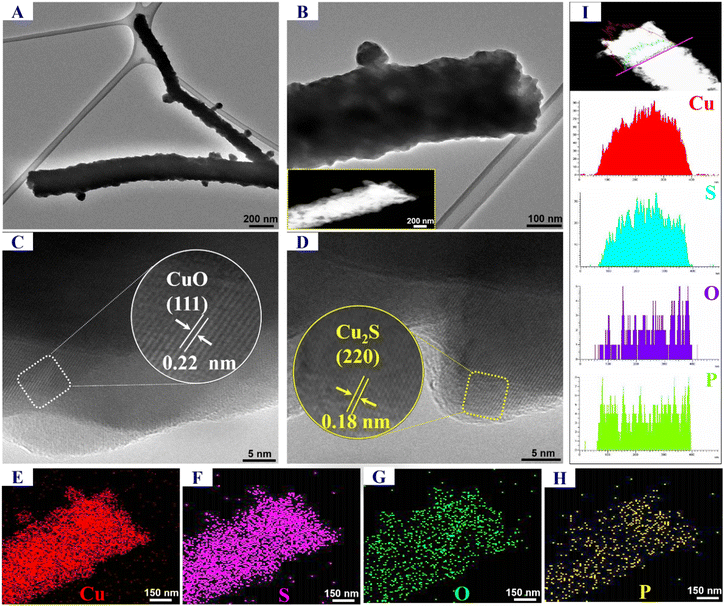
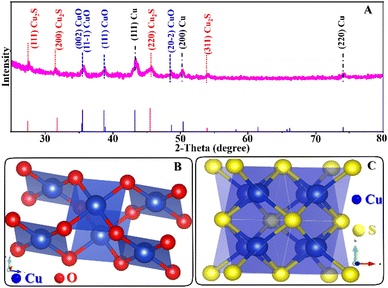
The morphological structure of catalysts was investigated by field-emission scanning electron spectroscopy (FE-SEM) using a Sigma/Carl Zeiss microscope. The microstructure data of the catalyst were obtained through transmission electron microscopy (TEM) and high-resolution TEM (HR-TEM) by using a JEOL-2100F electron microscope. Furthermore, we investigated the crystalline phase structure of catalysts through X-ray diffraction (XRD) analysis using a Benchtop X-ray powder diffractometer – a D2 phaser accompanied by Cu-Ka radiation (λ = 1.5406 Å).Catalytic activity test
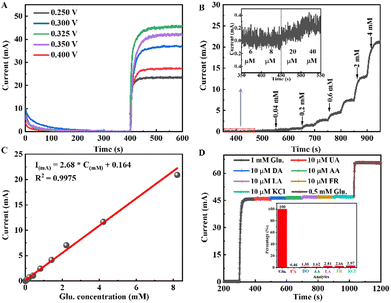
The catalytic activity test was performed on a GHI 830B electrochemical analyser (CH instruments) using a typical three-electrode configuration: Ag/AgCl, a Pt plate, and the as-synthesized material (working area: 1 cm × 1 cm) as the reference electrode, counter electrode, and working electrode, respectively, at room temperature. Cyclic voltammetric (CV) measurement was conducted at a scan rate of 50 mV s−1 in an electrolyte containing 0.1 M NaOH solution + 1 mM glucose to examine electrocatalytic properties for glucose oxidation. Electrochemical impedance spectroscopy (EIS) was also performed at open circuit potential in the frequency range from 0.01 Hz to 500 kHz in the same electrolyte solution to study charge transfer ability. Regarding the glucose detection test, chronoamperometric measurements were conducted by successive addition of various amounts of glucose to 0.1 M NaOH solution.Results and discussion
Characterization of the samples
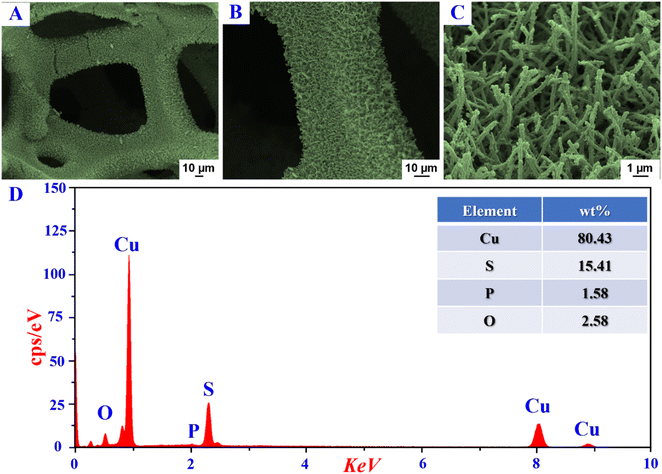
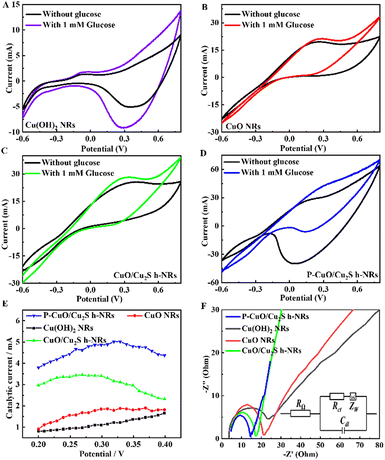
As compared with the CuO/Cu2S h-NRs, the P-CuO/Cu2S h-NRs still retained the rod-like morphology accompanied by its non-agglomeration feature even after treating under harsh conditions of phosphorization, once again confirming the non-influence of the P incorporation process on the morphological structure. Thereafter, chemical compositions of Cu(OH)2 NRs, CuO/Cu2S h-NRs, and P-CuO/Cu2S h-NRs were studied by energy dispersive X-ray spectroscopy (EDS). The EDS spectrum of Cu(OH)2 NRs (Fig. S3a, ESI†) shows the homogenous distribution of Cu and O elements, while the EDS spectrum of CuO/Cu2S h-NRs (Fig. S3b, ESI†) demonstrates the presence of Cu, O, and S elements. Regarding the P-CuO/Cu2S h-NRs (Fig. 1d), in addition to the Cu, O, and S elements, a very weak signal of the P element is observed in the EDS spectrum, as solid evidence for the successful incorporation of P atoms into the CuO/Cu2S composite. Inductively coupled plasma-optical emission spectrometry (ICP-OES) measurement was also conducted to quantify the actual content of elements in the P-CuO/Cu2S h-NRs material. The obtained results shown in Fig. S4 (ESI†) indicate that P-CuO/Cu2S h-NRs contained 82.64 wt% of Cu, 14.38 wt% of S, and 1.11 wt% of P, which are consistent with the EDS analysis results in shown in the inset of Fig. 1d.
Electrocatalytic performance
| Ic = Ig − Ib | (1) |
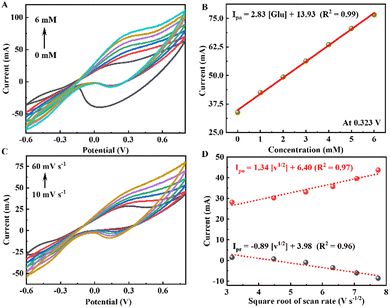
| Ipo = 2.83 × CM + 13.93 | (2) |
In this work, we carried out the CV tests on the P-CuO/Cu2S h-NRs sample at different scan rates of 10, 20, 30, 40, 50, and 60 mV s−1 in the glucose electrolyte for the purpose of kinetic analysis. The CV curves in Fig. 5c reveal the rapid rising of the redox peak current response under greater applied potentials. Based on the obtained CV results, corresponding plots of oxidation and reduction peak current vs. the square root of the scan rates are constructed and presented in Fig. 5c, showing a good linear relationship. By linear fitting the plots, the relationship between the peak current and the square root is described according to the following formulas:
| Ipo = 1.34 × v1/2 + 6.40 | (3) |
| Ipr = −0.89 × v1/2 + 3.98 | (4) |
The R2 values are also determined to be 0.97 and 0.96 for the oxidation and reduction processes, respectively. The findings indicate that the catalytic oxidation of glucose taken place on the surface of the P-CuO/Cu2S h-NRs catalyst is governed by a diffusion-controlled mechanism.46
| LOD = (k × sb)/m | (5) |
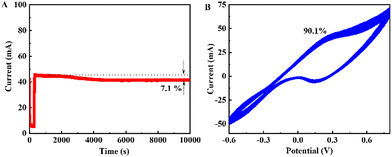
Additionally, stability and durability are some of the most important criteria for the practicability of glucose catalysts. Hence, the stability measurement of P-CuO/Cu2S h-NRs for glucose oxidation was carried out by chronoamperometry in the glucose electrolyte (1 mM glucose + 0.1 M NaOH) at the optimal working potential of 0.325 V. The chronoamperometric curve in Fig. 7a displays that the current response of the P-CuO/Cu2S h-NRs catalyst is rather stable during chronoamperometric testing. The final current response is 92.9% of the initial one, even after sustained glucose oxidation for a long time of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s. The CV measurement at a scan rate of 50 mV s−1 in the glucose electrolyte is conducted to further investigate the stability of P-CuO/Cu2S h-NRs. According to the results in Fig. 7b, the CV curve at the 100th cycle almost overlaps with the initial one, suggesting the superior stability of the proposed catalyst. To further evaluate the stability of P-CuO/Cu2S h-NRs, we determined its peak catalytic currents versus cycle frequencies, including 1, 25, 50, 75, and 100 cycles. We found as shown in Fig. S10 (ESI†) that the oxidation current response of P-CuO/Cu2S h-NRs maintains 90.1% of the initial one after 100 successive cycles. These results demonstrate that the P-CuO/Cu2S h-NR catalyst is highly stable for glucose oxidation, which is beneficial for the long-term detection of glucose. Therefore, the findings mentioned above are strong evidence for the excellent catalytic activity of P-CuO/Cu2S h-NRs for glucose oxidation, promoting its potential for application in enzymeless glucose sensing, which meets all requirements of good sensitivity, high selectivity, and superior stability for real applications.
000 s. The CV measurement at a scan rate of 50 mV s−1 in the glucose electrolyte is conducted to further investigate the stability of P-CuO/Cu2S h-NRs. According to the results in Fig. 7b, the CV curve at the 100th cycle almost overlaps with the initial one, suggesting the superior stability of the proposed catalyst. To further evaluate the stability of P-CuO/Cu2S h-NRs, we determined its peak catalytic currents versus cycle frequencies, including 1, 25, 50, 75, and 100 cycles. We found as shown in Fig. S10 (ESI†) that the oxidation current response of P-CuO/Cu2S h-NRs maintains 90.1% of the initial one after 100 successive cycles. These results demonstrate that the P-CuO/Cu2S h-NR catalyst is highly stable for glucose oxidation, which is beneficial for the long-term detection of glucose. Therefore, the findings mentioned above are strong evidence for the excellent catalytic activity of P-CuO/Cu2S h-NRs for glucose oxidation, promoting its potential for application in enzymeless glucose sensing, which meets all requirements of good sensitivity, high selectivity, and superior stability for real applications.
Moreover, we calculated the differential charge density of glucose-absorbed P-CuO/Cu2S and glucose-absorbed CuO/Cu2S. The charge density map in Fig. 8c shows that after the adsorption of glucose on the surface of CuO/Cu2S, the electrons accumulate around the glucose molecules, suggesting that the adsorbed glucose can serve as a charge acceptor. After P doping, a similar phenomenon is observed from the charge density map of P-CuO/Cu2S, as seen in Fig. 8d. According to Bader charge analysis, the adsorbed glucose received +0.763 e and +0.766 e from CuO/Cu2S and P-CuO/Cu2S, respectively. In addition, Bader charge analysis was performed to find which elements in CuO/Cu2S and P-CuO/Cu2S are more likely to interact and form bonds with the glucose molecules. Table S3 (ESI†) shows the Bader charge transfer of each element to the glucose molecules in CuO/Cu2S and P-CuO/Cu2S. Cu transfers the most charge to glucose with an approximate value of 0.55 e−, implying that Cu is more likely to form bonds with the glucose molecules and plays a more critical role in glucose adsorption. To gain more understanding of the electronic structure of CuO/Cu2S before and after P-doping, the partial DOS for CuO/Cu2S and P-CuO/Cu2S models were calculated to compare and confirm their electronic properties (Fig. 8e and f). Here, only DOS of d orbitals of Cu surface atoms close to the glucose molecule are considered because P-doping only results in significant changes in the electronic structure of Cu. As shown in Fig. S11 (ESI†), the d-band center of CuO/Cu2S exhibits a downward shift below EF. In contrast, the p-band center of CuO/Cu2S is almost unchanged after P-doping, implying P-doping with changes in the electronic structure of Cu should play key roles in enhancing adsorption. In Fig. 8e and f, the downshift of Cu-d orbitals in P-CuO/Cu2S enhances the orbital overlapping between Cu and glucose, resulting in a stronger hybridization of electronic orbitals of Cu. This hybridization leads to stronger bonding and redistribution of electron density around the adsorption site. Consequently, a more favorable environment for glucose adsorption is created after P-doping. Besides, DOS P-CuO/Cu2S gets over the Fermi level and extends beyond the conduction band, indicating the good electrical conductivity for fast charge transport of P-CuO/Cu2S h-NR, which is consistent with the EIS results and recognized as one of the important reasons for the excellent glucose sensitivity of P-CuO/Cu2S h-NR.39,40 Therefore, the DFT calculations indicate that incorporating P into CuO/Cu2S boosts electron mobility for highly sensitive glucose detection and improves the glucose adsorption capability to enhance the reaction kinetics of glucose oxidation.
Conclusion
In summary, a P-CuO/Cu2S h-NR composite that contains P-doped well-aligned heterostructured CuO/Cu2S nanorods supported on three-dimensional copper foam has been successfully synthesized using a scalable approach. Benefiting from the quintessential structure of self-supporting one-dimensional nanorod arrays on the substrate without using an additive polymer binder for enlarging the contact area between the catalyst and the electrolyte/reactant molecules, promoting charge transfer to active centres that is more effective than that of other buck structures, and overcoming the negative effect of a polymer binder on catalytic performance, accompanied by the incorporation of P atoms into the CuO/Cu2S matrix, which brings a considerable improvement in the electrical conductivity of the entire material and glucose adsorption capability, the P-CuO/Cu2S h-NR catalyst shows excellent catalytic activity for glucose oxidation. Hence, P-CuO/Cu2S h-NR has desirable sensitivity, a broad linear detection range, a low LOD value, good selectivity, and extraordinary stability for glucose detection, which are favorable evidence for the great potential of our proposed catalyst for electrochemical determination of glucose in practical applications.Conflicts of interest
There are no conflicts to declare.Notes and references
- K. P. Sharma, M. Shin, G. P. Awasthi, M. B. Poudel, H. J. Kim and C. Yu, Chitosan polymer matrix-derived nanocomposite (CuS/NSC) for non-enzymatic electrochemical glucose sensor, Int. J. Biol. Macromol., 2022, 206, 708–717 CrossRef CAS PubMed.
- T. Yang, W. Zhang, J. Wu, S. Liu and Y. Zhao, Core-shell composite of CuS nanocages and NiCo layered double hydroxide nanosheets with modulated electron structure as “two birds with one Stone” glucose oxidation electrocatalysts, Appl. Surf. Sci., 2022, 600, 154205 CrossRef CAS.
- X. Ma, J. Chen, B. Yuan, Y. Li, L. Yu and W. Zhao, Three-dimensional hollow nickel phosphate microspheres with controllable hoya-like structure for high-performance enzymeless glucose detection and supercapacitor, Appl. Surf. Sci., 2022, 588, 152928 CrossRef CAS.
- L. He, J. Su, T. You, S. Xiao, Y. Shen, P. Jiang and D. He, Mn incorporation boosted NiO nanosheets as highly efficient anode for sensitive glucose detection in beverage, LWT, 2023, 182, 114807 CrossRef CAS.
- R. Gaur, S. Shahabuddin, N. Mukherjee and P. Chandra, Recent advances in nanostructured transition metal sulfide based sensors for environmental applications, Mater. Today Proc., 2022, 62, 6945–6949 CrossRef CAS.
- M. Huang, S. Feng, C. Yang, F. Wen, D. He and P. Jiang, Construction of an MnO2 nanosheet array 3D integrated electrode for sensitive enzyme-free glucose sensing, Anal. Methods, 2021, 13, 1247–1254 RSC.
- X. Wang, M. Wang, S. Feng, D. He and P. Jiang, Controlled synthesis of flower-like cobalt phosphate microsheet arrays supported on Ni foam as a highly efficient 3D integrated anode for non-enzymatic glucose sensing, Inorg. Chem. Front., 2020, 7, 108–116 RSC.
- M. Wang, X. Wang, S. Feng, D. He and P. Jiang, Amorphous Ni-P nanoparticles anchoring on nickel foam as an efficient integrated anode for glucose sensing and oxygen evolution, Nanotechnology, 2020, 31, 455503 CrossRef CAS PubMed.
- Y. Gao, Q. Yu, Y. Du, M. Yang, L. Gao, S. Rao, Z. Yang, Q. Lan and Z. Yang, Synthesis of Co3O4–NiO nano-needles for amperometric sensing of glucose, J. Electroanal. Chem., 2019, 838, 41–47 CrossRef CAS.
- D. Li, X. Zhang, L. Pei, C. Dong, J. Shi and Y. Xu, High-performance supercapacitors and non-enzymatic electrochemical glucose sensor based on tremella-like NiS/CoS/NiCo2S4 hierarchical structure, Inorg. Chem. Commun., 2019, 110, 107581 CrossRef CAS.
- M. Waqas, L. Wu, H. Tang, C. Liu, Y. Fan, Z. Jiang, X. Wang, J. Zhong and W. Chen, Cu2O Microspheres Supported on Sulfur-Doped Carbon Nanotubes for Glucose Sensing, ACS Appl. Nano Mater., 2020, 3, 4788–4798 CrossRef CAS.
- X. Wang, C. Hu, H. Liu, G. Du, X. He and Y. Xi, Synthesis of CuO nanostructures and their application for nonenzymatic glucose sensing, Sens. Actuators, B, 2010, 144, 220–225 CrossRef CAS.
- C. Batchelor-McAuley, Y. Du, G. G. Wildgoose and R. G. Compton, The use of copper(II) oxide nanorod bundles for the non-enzymatic voltammetric sensing of carbohydrates and hydrogen peroxide, Sens. Actuators, B, 2008, 135, 230–235 CrossRef CAS.
- Z. Zhuang, X. Su, H. Yuan, Q. Sun, D. Xiao and M. M. F. Choi, An improved sensitivity non-enzymatic glucose sensor based on a CuO nanowire modified Cu electrode, Analyst, 2008, 133, 126–132 RSC.
- Y. Fu and W. Jin, Facile synthesis of core-shell CuS–Cu2S based nanocomposite for the high-performance glucose detection, Mater. Sci. Eng. C, 2019, 105, 110120 CrossRef CAS PubMed.
- S. Radhakrishnan, H.-Y. Kim and B.-S. Kim, A novel CuS microflower superstructure based sensitive and selective nonenzymatic glucose detection, Sens. Actuators, B, 2016, 233, 93–99 CrossRef CAS.
- D. P. Jaihindh, P. Anand, R.-S. Chen, W.-Y. Yu, M.-S. Wong and Y.-P. Fu, Cl-doped CuO for electrochemical hydrogen evolution reaction and tetracycline photocatalytic degradation, J. Environ. Chem. Eng., 2023, 11, 109852 CrossRef CAS.
- J. Chen, H. Pan, Y. Chen, Z. Zhou, G. Jing and X. Zhao, Efficient activation of peracetic acid for abatement of tetracycline by W-doped CuS via regulating copper redox cycling, Chem. Eng. J., 2023, 464, 142693 CrossRef CAS.
- K. Vinotha, B. Jayasutha, M. J. Abel and K. Vinoth, In3+-doped CuS thin films: physicochemical characteristics and photocatalytic property, J. Mater. Sci. Mater. Electron., 2022, 33, 22862–22882 CrossRef CAS.
- Z. Xiao, Y. Wang, Y.-C. Huang, Z. Wei, C.-L. Dong, J. Ma, S. Shen, Y. Li and S. Wang, Filling the oxygen vacancies in Co3O4 with phosphorus: an ultra-efficient electrocatalyst for overall water splitting, Energy Environ. Sci., 2017, 10, 2563–2569 RSC.
- W. Yao, C. Tian, C. Yang, J. Xu, Y. Meng, I. Manke, N. Chen, Z. Wu, L. Zhan, Y. Wang and R. Chen, P-Doped NiTe2 with Te-Vacancies in Lithium–Sulfur Batteries Prevents Shuttling and Promotes Polysulfide Conversion, Adv. Mater., 2022, 34, 2106370 CrossRef CAS PubMed.
- S. Lv, J. Li, B. Zhang, Y. Shi, X. Liu and T. Wang, In-situ growth of hierarchical CuO@Cu3P heterostructures with transferable active centers on copper foam substrates as bifunctional electrocatalysts for overall water splitting in alkaline media, Int. J. Hydrogen Energy, 2022, 47, 9593–9605 CrossRef CAS.
- H.-C. Chen, Y.-C. Yeh and M.-H. Yen, Synthesis of Au or Ag/Cu2O/aluminum doped zinc oxide nanorods hybrid electrode for high sensitive non-enzymatic glucose sensor: Mechanism investigation of formation and surface plasmon resonance, Mater. Chem. Phys., 2022, 282, 125924 CrossRef CAS.
- M. Kuznowicz, T. Rębiś, A. Jędrzak, G. Nowaczyk, M. Szybowicz and T. Jesionowski, Glucose determination using amperometric non-enzymatic sensor based on electroactive poly(caffeic acid)@MWCNT decorated with CuO nanoparticles, Microchim. Acta, 2022, 189, 159 CrossRef CAS PubMed.
- Z. Li, J. Wu, L. Liao, X. He, B. Huang, S. Zhang, Y. Wei, S. Wang and W. Zhou, Surface engineering of hematite nanorods photoanode towards optimized photoelectrochemical water splitting, J. Colloid Interface Sci., 2022, 626, 879–888 CrossRef CAS PubMed.
- Y. Zhai, Y. Ji, G. Wang, Y. Zhu, H. Liu, Z. Zhong and F. Su, Controllable wet synthesis of multicomponent copper-based catalysts for Rochow reaction, RSC Adv., 2015, 5, 73011–73019 RSC.
- A. Singh, R. Manivannan and S. Noyel Victoria, Simple one-pot sonochemical synthesis of copper sulphide nanoparticles for solar cell applications, Arab. J. Chem., 2019, 12, 2439–2447 CrossRef CAS.
- R. D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- M. Xiao, D. Han, X. Yang, N. Tsona Tchinda, L. Du, Y. Guo, Y. Wei, X. Yu and M. Ge, Ni-doping-induced oxygen vacancy in Pt-CeO2 catalyst for toluene oxidation: Enhanced catalytic activity, water-resistance, and SO2-tolerance, Appl. Catal., B, 2023, 323, 122173 CrossRef CAS.
- Y. Qin, F. Fang, Z. Xie, H. Lin, K. Zhang, X. Yu and K. Chang, La,Al-Codoped SrTiO3 as a Photocatalyst in Overall Water Splitting: Significant Surface Engineering Effects on Defect Engineering, ACS Catal., 2021, 11, 11429–11439 CrossRef CAS.
- G. Wei, Z. Xu, X. Zhao, S. Wang, F. Kong and C. An, N-doped CoSe2 nanomeshes as highly-efficient bifunctional electrocatalysts for water splitting, J. Alloys Compd., 2022, 893, 162328 CrossRef CAS.
- C. Zhou, L. Xu, J. Song, R. Xing, S. Xu, D. Liu and H. Song, Ultrasensitive non-enzymatic glucose sensor based on three-dimensional network of ZnO-CuO hierarchical nanocomposites by electrospinning, Sci. Rep., 2014, 4, 7382 CrossRef CAS PubMed.
- M. Cao, H. Wang, P. Kannan, S. Ji, X. Wang, Q. Zhao, V. Linkov and R. Wang, Highly efficient non-enzymatic glucose sensor based on CuxS hollow nanospheres, Appl. Surf. Sci., 2019, 492, 407–416 CrossRef CAS.
- N. Le Minh Tri, D. Q. Trung, D. Van Thuan, N. T. Dieu Cam, T. Al Tahtamouni, T.-D. Pham, D. S. Duc, M. H. Thanh Tung, H. Van Ha, N. H. Anh Thu and H. T. Trang, The advanced photocatalytic performance of V doped CuWO4 for water splitting to produce hydrogen, Int. J. Hydrogen Energy, 2020, 45, 18186–18194 CrossRef CAS.
- Z. Han, G. Li, X. Zeng, Y. Zhu, N. Li, J. Zhang, W. Zhao and Q. Jiao, Electrochemical construction of Cu@NF frameworks and synthesis of self-supported microflower Cu2S@NF as bifunctional catalysts for overall water splitting, Int. J. Hydrogen Energy, 2022, 47, 15695–15705 CrossRef CAS.
- S. Huang, S. Ma, L. Liu, Z. Jin, P. Gao, K. Peng, Y. Jiang, A. Naseri, Z. Hu and J. Zhang, P-doped Co3S4/NiS2 heterostructures embedded in N-doped carbon nanoboxes: Synergistical electronic structure regulation for overall water splitting, J. Colloid Interface Sci., 2023, 652, 369–379 CrossRef CAS PubMed.
- S. Masudy-Panah, R. Siavash Moakhar, C. S. Chua, H. R. Tan, T. I. Wong, D. Chi and G. K. Dalapati, Nanocrystal Engineering of Sputter-Grown CuO Photocathode for Visible-Light-Driven Electrochemical Water Splitting, ACS Appl. Mater. Interfaces, 2016, 8, 1206–1213 CrossRef CAS PubMed.
- Y. Dang, G. Wang, G. Su, Z. Lu, Y. Wang, T. Liu, X. Pu, X. Wang, C. Wu, C. Song, Q. Zhao, H. Rao and M. Sun, Rational Construction of a Ni/CoMoO4 Heterostructure with Strong Ni–O–Co Bonds for Improving Multifunctional Nanozyme Activity, ACS Nano, 2022, 16, 4536–4550 CrossRef CAS PubMed.
- J. Wang, M. M. Rahman, C. Ge and J.-J. Lee, Electrodeposition of Cu2S nanoparticles on fluorine-doped tin oxide for efficient counter electrode of quantum-dot-sensitized solar cells, J. Ind. Eng. Chem., 2018, 62, 185–191 CrossRef CAS.
- T. Wang, X. Zhang, X. Zhu, Q. Liu, S. Lu, A. M. Asiri, Y. Luo and X. Sun, Hierarchical CuO@ZnCo LDH heterostructured nanowire arrays toward enhanced water oxidation electrocatalysis, Nanoscale, 2020, 12, 5359–5362 RSC.
- C. Liu, D. Jia, Q. Hao, X. Zheng, Y. Li, C. Tang, H. Liu, J. Zhang and X. Zheng, P-Doped Iron–Nickel Sulfide Nanosheet Arrays for Highly Efficient Overall Water Splitting, ACS Appl. Mater. Interfaces, 2019, 11, 27667–27676 CrossRef CAS PubMed.
- H. Lei, M. Chen, Z. Liang, C. Liu, W. Zhang and R. Cao, Ni2 P hollow microspheres for electrocatalytic oxygen evolution and reduction reactions, Catal. Sci. Technol., 2018, 8, 2289–2293 RSC.
- H. Sim, J.-H. Kim, S.-K. Lee, M.-J. Song, D.-H. Yoon, D.-S. Lim and S.-I. Hong, High-sensitivity non-enzymatic glucose biosensor based on Cu(OH)2 nanoflower electrode covered with boron-doped nanocrystalline diamond layer, Thin Solid Films, 2012, 520, 7219–7223 CrossRef CAS.
- L.-C. Jiang and W.-D. Zhang, A highly sensitive nonenzymatic glucose sensor based on CuO nanoparticles-modified carbon nanotube electrode, Biosens. Bioelectron., 2010, 25, 1402–1407 CrossRef CAS PubMed.
- K. Samdhyan, P. Chand and H. Anand, Effective doping of phosphorus in copper sulfide for high performance energy storage devices, J. Alloys Compd., 2023, 936, 168322 CrossRef CAS.
- X. Mei, Q. Wei, H. Long, Z. Yu, Z. Deng, L. Meng, J. Wang, J. Luo, C.-T. Lin, L. Ma, K. Zheng and N. Hu, Long-term stability of Au nanoparticle-anchored porous boron-doped diamond hybrid electrode for enhanced dopamine detection, Electrochim. Acta, 2018, 271, 84–91 CrossRef CAS.
- A. Saljooqi, T. Shamspur and A. Mostafavi, The MWCNT-Ag-PT GCE Electrochemical Sensor Functionalized With dsDNA for Mitoxantrone Sensing in Biological Media, IEEE Sens. J., 2019, 19, 4364–4368 CAS.
- D. Geng, X. Bo and L. Guo, Ni-doped molybdenum disulfide nanoparticles anchored on reduced graphene oxide as novel electroactive material for a non-enzymatic glucose sensor, Sens. Actuators, B, 2017, 244, 131–141 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cp04095j |
| This journal is © the Owner Societies 2024 |