Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Bacterial model membranes under the harsh subsurface conditions of Mars†
Attila
Tortorella
ab,
Rosario
Oliva
b,
Concetta
Giancola
 *c,
Luigi
Petraccone
*c,
Luigi
Petraccone
 *b and
Roland
Winter
*b and
Roland
Winter
 *d
*d
aScuola Superiore Meridionale (SSM), Via Mezzocannone 4, 80138, Naples, Italy
bDepartment of Chemical Sciences, University of Naples Federico II, Via Cintia 26, 80126, Naples, Italy. E-mail: luigi.petraccone@unina.it
cDepartment of Pharmacy, University of Naples Federico II, Via Domenico Montesano 49, 80131, Naples, Italy. E-mail: giancola@unina.it
dDepartment of Chemistry and Chemical Biology, Biophysical Chemistry, TU Dortmund University, Otto-Hahn-Str. 4a, 44227, Dortmund, Germany. E-mail: roland.winter@tu-dortmund.de
First published on 17th October 2023
Abstract
Biomembranes are a key component of all living systems. Most research on membranes is restricted to ambient physiological conditions. However, the influence of extreme conditions, such as the deep subsurface on Earth or extraterrestrial environments, is less well understood. The deep subsurface of Mars is thought to harbour high concentrations of chaotropic salts in brines, yet we know little about how these conditions would influence the habitability of such environments. Here, we investigated the combined effects of high concentrations of Mars-relevant salts, including sodium and magnesium perchlorate and sulphate, and high hydrostatic pressure on the stability, structure, and function of a bacterial model membrane. To this end, several biophysical techniques have been employed, including calorimetry, fluorescence and CD spectroscopy, confocal microscopy, and small-angle X-ray scattering. We demonstrate that sulphate and perchlorate salts affect the properties of the membrane differently, depending on the counterion present (Na+vs. Mg2+). We found that the perchlorates, which are believed to be abundant salts in the Martian environment, induce a more hydrated and less ordered membrane, strongly favouring the physiologically relevant fluid-like phase of the membrane even under high-pressure stress. Moreover, we show that the activity of the phospholipase A2 is strongly modulated by both high pressure and salt. Compellingly, in the presence of the chaotropic perchlorate, the enzymatic reaction proceeded at a reasonable rate even in the presence of condensing Mg2+ and at high pressure, suggesting that bacterial membranes could still persist when challenged to function in such a highly stressed Martian environment.
1. Introduction
Investigating the chemical and physical limits of life and its associated biomacromolecules allows us to assess the habitability of extreme environments.1 The biochemical machinery that enables life as we know it is based on carbon chemistry and requires the existence of water. An increasing number of organisms, the so-called extremophiles, have been found in extreme environments on Earth in recent years. They thrive in environments in which chemical and physical parameters, such as salinity, water activity and pH, temperature and pressure, are extreme.1–8 Similar to Earth, the habitability of environments on other planetary bodies and moons will be dictated by the chemical and physical environmental parameters. The subsurface represents one of the most important locations in the search for extinct and extant extraterrestrial life. Over the past years, there has been increasing evidence of periods of surface water on Mars, and it is now thought that there may exist subsurface water in the form of lakes below the poles of Mars.2,9,10 The Martian groundwater experiences hydrostatic pressures of ∼1 kbar at the base of the cryosphere if it reached a depth of 10 km.9 Perchlorate and sulphate salts are widely distributed across the Martian surface. The deep subsurface environments of Mars are expected to harbour also high concentrations of dissolved salts, yet we know little about how such highly concentrated brines shape the conditions for life. One particular ion is perchlorate, ClO4−, which is found in extreme environments also on Earth and ubiquitously on Mars, but also sulphates are widely distributed across the Martian surface.11–13 Perchlorates are also known to act as chaotropes, perturbing the structure of water and its hydrogen bonding capacity,14 and hence affect biomolecular hydration, structure, dynamics and function. Hence, to advance our understanding of the ability of such subsurface environments to support life, we need to examine the combination of strong ionic effects imposed by these salts and high hydrostatic pressures.Previous studies have demonstrated deleterious effects of destabilizing ions such as perchlorates on the activity of α-chymotrypsin and other enzymes at ambient conditions.13,14 Moreover, it has also been reported that perchlorate strongly affects the formation of protein–ligand complexes, modulating the interaction even at the level of a single binding site.15 However, the effects of these Martian salts on other biomolecular systems, such as the cellular membrane, is still largely unknown.16 Cellular membranes are essential for maintaining the internal conditions of the cells for metabolism, energy transduction and signal processing. Membranes are complex structures, consisting of many different lipids and embedded proteins, and organisms are able to adapt the properties of their membranes in response to changes in the environment by changing the lipid composition.4–6 Recently, the effect of Mars-like salts has been studied on the structure of eukaryotic model membranes.16 Here, we evaluated the impact of perchlorate and sulphate salts on a prototypical bacterial model membrane consisting of phosphatidylethanolamines (PE) and phosphatidylglycerols (PG). Employing various biophysical methods, including differential scanning calorimetry (DSC), fluorescence spectroscopy and confocal microscopy, circular dichroism (CD) spectroscopy, and small-angle X-ray scattering (SAXS), we studied the impact of the combined effect of such Mars-like brines and high hydrostatic pressure (HHP) on the structure and fluidity of the bacterial membrane. Moreover, we explored the effects of such stressors on the function of an important membrane-associated protein, phospholipase A2 (PLA2). Phospholipases are involved in regulatory processes as they interact directly with the membrane by altering both their chemical composition and physical state by hydrolysis of the sn-2 ester bond of phospholipids and take part in cell signalling and transcriptional pathways.17 The activity of PLA2 is sensitive to packing of the lipids in the cell membrane and is responsive to osmotic changes, as, for example, induced by high salt. The results demonstrate the stability of bacterial lipid membranes under particular salt and pressure conditions. Remarkably, the function of the membrane-associated protein PLA2 also remains intact under harsh conditions, such as those imposed by high magnesium perchlorate concentrations and HHP.
2. Results and discussion
2.1 Impact of salts and pressure on the structure and physicochemical properties of bacterial model membranes
In order to explore the effects of the Mars-relevant salts on the thermotropic properties of a bacterial model membrane, DSC measurements on multilamellar vesicles (MLVs) composed of 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE) and 1,2-dipalmitoyl-sn-glycero-3-phosphoglycerol (DPPG) 8/2 (mol mol−1) were performed. It is important to note that the DPPE/DPPG mixture was used for DSC experiments only, since it ensures a better peak resolution and a higher transition temperature with respect to the mixture formed by 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-1′-rac-glycerol (POPG) used for all other experiments. Fig. 1(A) depicts the DSC profiles of DPPE/DPPG multilamellar vesicles (MLVs) in neat buffer and in the presence of 0.5 M sulphate and perchlorate salts of sodium and magnesium. For comparison, the effect of NaCl (as well as MgCl2), the salt most important for the biochemistry on Earth, is shown. Concentration dependent DSC curves are reported in Fig. S1 (ESI†).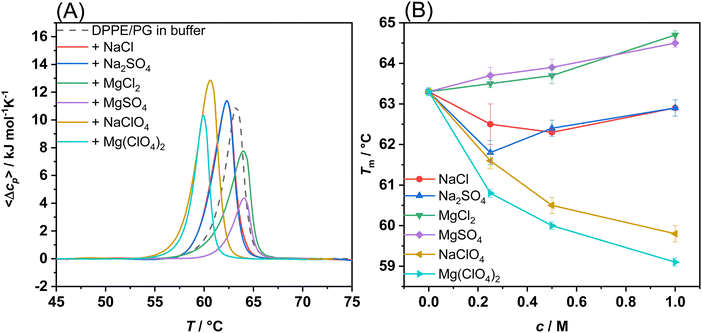 | ||
| Fig. 1 (A) DSC traces of DPPE/DPPG (8/2 mol mol−1) multilamellar vesicles dispersed in buffer and in the presence of 0.5 M of Mars-relevant salts and NaCl, as indicated in the inset. (B) Gel-to-fluid melting temperatures (Tm) of DPPE/DPPG multilamellar vesicles as a function of salt concentration, c, as inferred from the DSC thermograms reported in panel A and in Fig. S1 (ESI†). All the experiments were performed in 10 mM HEPES buffer, pH 7.4. Each experiment has been performed at least three times. | ||
The DSC profile of DPPE/DPPG vesicles in neat buffer shows a single and well-defined peak, indicating that the two lipids are miscible at the molar ratio used.18 The DSC peak is centred at 63.3 °C which is due to the phase transition of the lipid acyl chains from a lamellar ordered gel (Lβ′) phase to the liquid-crystalline fluid-like (Lα) phase.19 Thus, DSC experiments inform about the packing of the lipids’ hydrocarbon chains. Fig. 1(B) shows the melting temperatures, Tm, associated with the gel-to-fluid phase transition of the DPPE/DPPG vesicles as a function of salt concentration, c. The transition temperature of the DPPE/DPPG mixture is higher than that of the pure lipid components.18 This effect can be ascribed to the direct interaction of PEs with PGs via hydrogen bonding between neighbouring phosphate and OH groups. The presence of PEs counteracts the repulsive electrostatic interaction among PGs, leading to a higher Tm-value of the mixture. An inspection of Fig. 1(B) reveals that the presence of sodium chloride (NaCl) and sulphate (Na2SO4), even at concentrations as high as 1 M, have only a marginal effect on DPPE/DPPG vesicles, slightly decreasing the melting temperature, indicating a small perturbation of the lipid packing by these two salts. Instead, in the presence of the perchlorate salt (NaClO4), a marked decrease of Tm was observed, an effect similar to that observed on phosphatidylcholine vesicles.19 The Tm-value decreases from 63.3 °C in neat buffer to 59.8 °C in 1 M NaClO4. This result can be explained by direct interaction of perchlorate with the lipids head group (most likely to PEs) and its partial penetration into the hydrophobic chain region.20,21 Even though perchlorate possesses a negative charge, due to its large size, its surface charge density is quite low, leading to a partially hydrophobic character, in sharp contrast to smaller and more strongly hydrated anions such as Cl−.
In the presence of Mg2+ salts, a different behaviour with respect to the Na+ salts was observed. For the chloride and sulphate (MgCl2 and MgSO4), an increase of Tm (∼1.2 °C at 1 M salt concentration) was recorded, indicating stabilization of the more densely packed gel phase of the lipid bilayer. The observed increase of Tm can be ascribed to the presence of the small and highly charged 2+ cation, which interacts with the phosphate moieties of the lipids,22 in particular with the PGs’ anionic head group. Conversely, similar to the results for NaClO4, a significant decrease of Tm was detected upon addition of Mg(ClO4)2, revealing destabilization of the gel phase, which is mediated by the perchlorate anion. Interestingly, similar trends were observed for DPPC in the presence of MgSO4 and NaClO4 with the exception of Mg(ClO4)2 which abolished the gel-to-fluid transition and led to the appearance of another phase transition peak at higher temperature.16
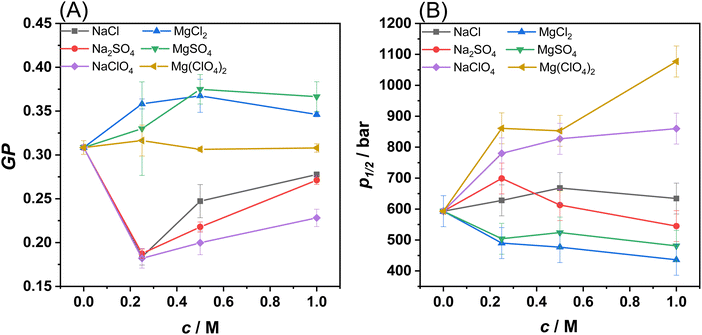
Next, to gain more quantitative information about changes in membrane fluidity upon addition of the salts, fluorescence spectra of the probe Laurdan embedded in the POPE/POPG 8/2 (mol mol−1) bilayer were recorded (see Fig. S2, ESI†). The probe Laurdan positions itself in the membrane with the fluorescent naphthalene group in close proximity of the glycerol backbone of the lipids.23 Its fluorescence spectrum is characterized by two well defined emitting states, i.e., a solvent relaxed and a non-solvent relaxed state.23,24 The predominance of the emission from one state compared to the other strongly depends on the hydration degree of the membrane which in turn depends on the lipid packing. The emission from the solvent-relaxed state, located at the red edge of the spectrum, is due to Laurdan molecules embedded in a more hydrated and less packed lipid phase, such as the fluid phase of the bilayer. Conversely, the non-solvent relaxed state (at the blue edge of the spectrum) is due to Laurdan localized in a less hydrated, more densely packed bilayer, such as in the gel phase. To describe the spectroscopic properties of Laurdan, the generalized polarization (GP) parameter can be used. In Fig. 2(A), the GP-values of Laurdan in the presence of different salts concentrations are shown. The experiments were carried out at the temperature of 35 °C, where the bilayer at neat buffer conditions is in the fluid-like phase.
The presence of the sodium salts caused a decrease of the GP-values with respect to that observed in neat buffer. The decrease of the GP is an indication that the surface of the membrane is more hydrated and less densely packed, in agreement with the DSC results on DPPE/DPPG, where a small decrease of Tm was observed, i.e., the fluid-like phase is slightly favoured over the gel phase. Although the trend of GP-values is similar when the sodium salts concentration increased, the magnitude of the effect of perchlorate sodium salt significantly differs from the other sodium salts. Perchlorate is the sodium salt that caused the highest decrease of the GP-value. This result is consistent with the ability of perchlorate to partially penetrate the membrane and make it more hydrated and fluid compared to sodium chloride and the sulphate salts. These results are also consistent with the DSC data, showing that sodium perchlorate is better at stabilizing the membrane's fluid phase compared to the other sodium salts. Conversely, in the presence of MgCl2 and MgSO4, a small increase of GP was detected, revealing that the surface of the bilayer is less hydrated and that these two salts favour a more compact, densely packed phase, in agreement with the DSC data. Most remarkably, in the presence of Mg(ClO4)2, no significant changes in the GP-value occurred, suggesting that the lipid packing of the bilayer is not affected at all by the salt, even at 1 M concentration. Instead, from the reported DSC data on DPPE/DPPG it is clear that the perchlorate decreases the melting temperature of the lipid chains. These data can be explained considering that Mg2+ strongly interacts, through electrostatic interactions, with the phosphate groups of lipids, stabilizing the bilayer. Conversely, the perchlorate penetrates partially inside the hydrophobic core of the membrane, leading to a fluidizing effect. These two effects counterbalance each other, leading to the observed result.
Then, using the same methodology, the combined effect of salts and HHP was explored. Fig. S3 (ESI†) shows the GP-values for the different salts in the pressure range from 1 to 1400 bar. The GP vs. p plots for the POPE/POPG vesicles show the characteristic sigmoidal behaviour describing a pressure-induced liquid-to-gel phase transition.24,25 The inflection point of these curves, i.e. the transition pressure p1/2, as a function of salt concentration is shown in Fig. 2(B) for all salts. The p1/2-value for POPE/POPG vesicles in neat buffer is ∼600 bar, in agreement with the typical value of ∼20 °C bar−1 for the fluid-to-gel transition slope of phospholipids.24 In the presence of NaCl and Na2SO4, the p1/2-value remains essentially constant in the whole concentration range explored. Instead, in the presence of NaClO4, a progressive increase of p1/2 on increasing the salt concentration was observed. At 1 M NaClO4, p1/2 increased to ∼850 bar. These data are consistent with the DSC data suggesting stabilization of the fluid phase. The perchlorate anion penetrates the hydrocarbon chains, thereby hampering efficient packing of the lipid chains, which causes a drastic increase of the pressure needed to induce the gel phase. In the presence of MgCl2 and MgSO4, a small decrease of p1/2 was observed, indicating a slight destabilization of the gel phase, again, in agreement with the DSC data. When the perchlorate salt of Mg2+ is present in solution, the p1/2-value increased markedly, reaching a value of ∼1100 bar at 1 M concentration, signifying a strong stabilization effect of the fluid-like phase mediated by perchlorate. This is a remarkable result as the fluidizing effect occurred even in the presence of the Mg2+ cation, which generally promotes denser lipid packing. On the other hand, a GP-value around 0.3 in neat buffer already indicates rather efficient lipid packing.
Collectively, the reported DSC and fluorescence data clearly indicate that the sodium salts of chloride and sulphate have negligible effects on the stability of PE/PG lipid bilayers even at molar concentration. The Mg2+ salts of chloride and sulphate act to slightly stabilize the bilayer, favouring the ordered gel phase. In sharp contrast, both the perchlorate salts of Na+ and Mg2+ strongly favour the fluid-like phase through partial penetration of the anion into the hydrophobic core of the bilayer. The fluidizing effect mediated by perchlorate prevails irrespective of the nature of the corresponding cation present. Even the Mg2+ cation is not able to neutralize the fluidizing effect of the ClO4− anion.
2.2 The impact of Mars-like salts on the mesophase structure and topology of lipid vesicles
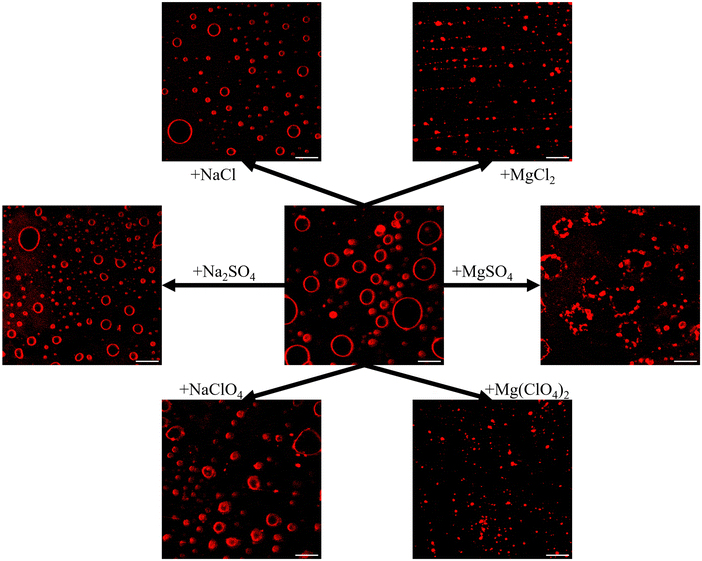
To gain insights into the effect of the Mars-relevant salts on the morphology of the lipid vesicles, confocal fluorescence microscopy measurements were carried out. Giant unilamellar vesicles (GUVs) composed of POPE/POPG 8/2 mol mol−1 containing the fluorescent lipid probe N-Rh-DHPE were used. Fig. 3 depicts GUVs of POPE/POPG before and after the addition of the salts at a concentration of 0.5 M.After the application of the electroformation protocol, spherical μm-sized GUVs were obtained, where the contour of GUVs is clearly visible due to the presence of rhodamine-labeled PE.
After the addition of NaCl and Na2SO4, no significant changes in the GUVs’ morphology were observed, i.e., their spherical shape was preserved. The GUVs average size and size distribution were obtained by analysing multiple images (Fig. S4 and S5, ESI†). Vesicle sizes were found to be heterogenous with size distributions being narrower for Mg2+ salts than for Na+ salts (Fig. S5, ESI†). The average area of the vesicles was 25.9 and 26.6 μm2 for the NaCl and Na2SO4 salt, respectively, with a maximum of 297 and 358 μm2. Instead, the presence of NaClO4 lead to marked changes of the vesicles’ shape, looking fluffy and deformed. As perchlorate is penetrating the membrane, the bending modulus (elasticity) of the bilayer obviously decreased,26 which is in agreement with the calorimetric and spectroscopic data reported above, indicating a less densely packed membrane. Interestingly, the GUVs dimensions were not really different from the other sodium salts, with an average area of 41.6 μm2. Further, the GUVs average size was smaller in the presence of Na+ salts compared to the buffer (64.3 μm2). On the contrary, all the Mg2+ salts seemed to affect the morphology of the GUVs drastically. The presence of these salts led to a remarkable shrinkage of the vesicles, reducing the average area to 5.6, 10.0 and 4.8 μm2 for MgCl2, MgSO4 and Mg(ClO4)2 respectively, and the maximum area to less than 27 μm2, which is due to osmotic stress and clustering of smaller vesicles in particular for MgSO4. Most likely, association of vesicles is favoured by Mg2+ coordination in the lipid headgroup region, which decreases the electrostatic repulsion of the negatively charged liposomes. Moreover, similar results were also obtained for DOPC in the presence of magnesium salts,16 suggesting that the shrinking of vesicles occurs regardless of the membrane charge. However, it is important to note that the bilayer integrity is preserved (as also inferred from calorimetric and spectroscopic data). As bacteria have evolved mechanisms that allow them to adopt to severe changes in osmolarity, e.g., by uptake or biosynthesis of compatible osmolytes, the salt-induced osmotic stress might not cause a serious problem.
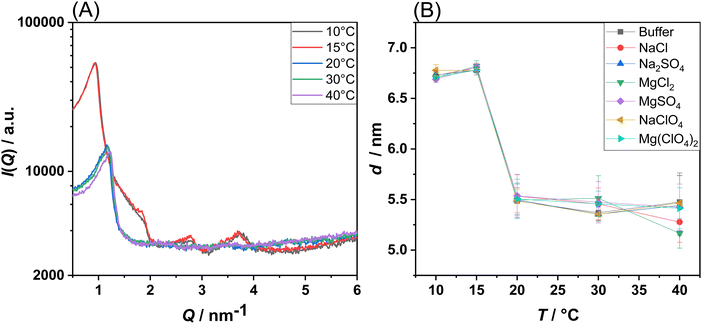
In the end, temperature-dependent small-angle X-ray scattering (SAXS) experiments were carried out on corresponding multilamellar vesicles to reveal changes in the lamellarity of the MLVs and to check if the bilayer structure is still preserved at high salt concentrations, or if phase transitions to other lipid structures (e.g., hexagonal, cubic or micellar lipid phases) are induced. As an example, Fig. 4(A) shows the SAXS patterns of POPE/POPG MLVs at selected temperatures under neat buffer conditions. Fig. S6 (ESI†) shows the diffractograms obtained in the presence of the salts. SAXS data allow for the determination of lamellar distance constants of MLVs by the appearance of Bragg-peaks in the elastic scattering curve, I(Q), as described in the Methods section. Higher-order Bragg reflections are observed for the lower temperatures only, as the multi-lamellarity is largely lost at higher temperatures due to increasing thermally induced fluctuations.
For all salt conditions, a lamellar lipid phase was recorded, both in the gel and in the fluid-like state. This is an interesting result, since in a previous work the induction of a bicontinuous inverse cubic lipid phase was suspected in DPPC liposomes in the presence of high concentrations of Mg(ClO4)2.16 In the present case, for a prototypical bacterial PE/PG membrane, the presence of such phase was not observed. This highlights the possibility that the bacterial membrane could still exist in the lamellar phase under such harsh Martian salt conditions, a prerequisite for the development of bacterial-based life.27Fig. 4(B) shows the temperature dependence of the lamellar d-spacing in the absence and presence of the Mars-relevant salts. For POPE/POPG vesicles in the gel phase, a value of d = 6.75 nm was obtained, which decreases to ∼5.5 nm in the fluid phase, a typical value for such bilayers in their fluid-like state.28 The decrease of d can be explained by the much smaller length of the acyl chains in their disordered fluid state and a decrease of the interlamellar water layer. Interestingly, it seems that the lamellar repeat distance is not significantly affected by the presence of the salts.
2.3 Effects of Mars-like salts and pressure on a model enzymatic reaction
Finally, to demonstrate how the presence of the Mars-relevant salts in combination with high hydrostatic pressure can modulate biologically relevant reactions, the kinetics of the hydrolysis of phospholipids operated by the bee venom phospholipase A2 (PLA2) was monitored.17,29 PLA2 is an esterase which is able to catalyse the hydrolysis of membrane glycerophospholipids at the sn-2 position leading to the formation of a free fatty acid and lysophospholipid (which contains only one hydrophobic chain) (Fig. 5(A)).30,31 Before carrying out these experiments, it was important to verify if the presence of the salts used alters the conformation and stability of the protein, which will, in turn, affect the activity of the enzyme. To this end, CD spectra of the protein were acquired in the far-UV range (200–260 nm), which provides information about the secondary structure elements of the protein.32 Fig. S7 (ESI†) shows the CD spectra of PLA2 in neat buffer and in the presence of the sodium and magnesium salts at 0.5 M concentration. The CD spectrum of PLA2 in neat buffer shows two minima at around 208 and 230 nm, which indicate that the protein adopts mainly an α-helical structure, in accordance to literature data.17,30 The intensity of the minimum at 230 nm is lower with respect to that at 208 nm, revealing the presence of other secondary structure elements, β-sheets and loops. Upon addition of the Mars-relevant salts, no significant changes were observed, i.e., the integrity of the protein's structure seems to be preserved at high salt. As pressure dependent activity studies are carried out as well, also the pressure resistance of the enzyme must be confirmed. In fact, some of us showed in an earlier work, employing high-pressure FTIR spectroscopy, that the enzyme structure is stable at pressures below 4 kbar.17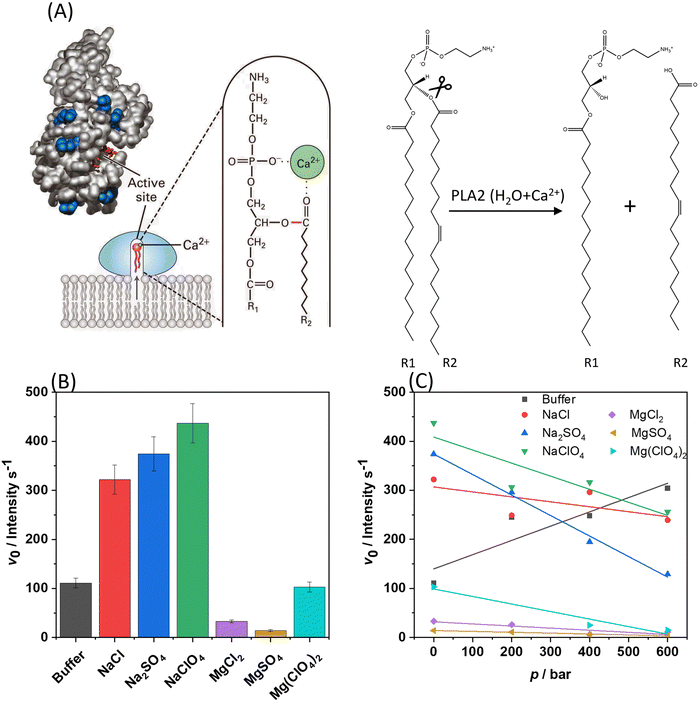 | ||
| Fig. 5 (A) Left: Interfacial binding and mechanism of PLA2 action. A small conformational change in phospholipase A2 induced by binding to the water–lipid interface opens a hydrophobic channel and fixes the protein to the phospholipid heads. The Ca2+-containing active site is buried in a channel enriched with hydrophobic amino acids facilitating protrusion of a phospholipid from the bilayer to the catalytic site. Right: Hydrolysis of the sn-2 ester of phospholipid yields a free fatty acyl chain (R2), leaving a lysophospholipid molecule behind (R1).29 (B) Initial velocity (v0) plots for the hydrolysis reaction (fluorescence intensity per s), at 1 bar and 35 °C, carried out by PLA2 on DBPC embedded in POPE/POPG vesicles in neat buffer and in the presence of 0.5 M of the Mars-relevant salts, as indicated in the plot. (C) v0-values for the same reaction in the presence of 0.5 M salts as a function of pressure. For Mg(ClO4)2, the v0-value at 200 bar was discarded due to its large error bar. All the experiments were performed in 10 mM HEPES buffer, pH 7.4 each experiment has been performed at least twice. | ||
Once the structural stability of the enzyme was assessed, the kinetics of the hydrolysis of phospholipids mediated by PLA2 was measured (Fig. 5(B)). To this end, liposomes composed of POPE/POPG with 1% mol of the fluorescence probe DBPC was used. In this probe, the fluorescent molecule BODIPY is covalently attached to the hydrocarbon chain in position sn-1. The hydrocarbon chain at position sn-2 contains the molecule DABCYL, which acts as nearby quencher of the fluorescence emission of BODIPY. Once PLA2 has hydrolysed the ester bond at position sn-2, the fatty acid carrying DABCYL is released, which is accompanied by an increase of the fluorescence intensity of the BODIPY, reporting the kinetics of the reaction. To quantitatively evaluate the kinetics of the hydrolysis reaction, the slope of the linear portion of the fluorescence intensity vs. time plot was determined, i.e., the initial velocity v0 of the enzyme reaction. Figs. S8 and S9 (ESI†) show examples of the kinetic plots of the hydrolysis reaction. Fig. 5(C) shows the v0-values obtained for all salts at 1 bar and 25 °C. In these experiments, the concentration of the enzyme was fixed at 0.01 μM, the total lipid concentration was 150 μM, and the salt concentration was 0.5 M.
Remarkably, compared to the reaction rate (v0) in neat buffer solution, the addition of all sodium salts led to a significant increase of the kinetics of the reaction. The general trend seems to follow the order NaCl ≤ Na2SO4 ≤ NaClO4. However, the differences among the three sodium salts are quite small. Generally, the kinetics of the hydrolysis reaction is affected by the lipid composition, charge density, fluidity, bending modulus, and therefore also the phase state of the lipid bilayer.33,34 As two water molecules are involved in the mechanism of hydrolysis of PLA2, the level of hydration is important as well.35,36 As inferred from the data reported above, the POPE/POPG bilayer is more hydrated and the lipid chains are less densely packed with respect to neat buffer conditions in the presence of sodium salts, an effect that is particularly pronounced in the presence of NaClO4. Thus, the enhancement of the reaction rate could be ascribed to the fluidization of the bilayer imposed by the salts, which is accompanied with an increase of the level of hydration of the lipid headgroup area, as inferred from the Laurdan experiments.
Conversely, in the presence of MgCl2 and MgSO4 similar values of v0 were determined, which are significantly smaller compared to the values obtained at neat buffer conditions, indicating that the divalent cation is responsible for the strong reduction of the hydrolysis rate. The presence of MgCl2 and MgSO4 induces a more densely packed and less hydrated bilayer (as inferred from the Laurdan data reported in Fig. 2), which is expected to decrease the reaction rate as the surface of the membrane becomes less accessible to the protein and, further, there is less water present to sustain the reaction. Furthermore, it cannot be excluded that the strong coordination of the negatively charged lipid headgroups by Mg2+ play an additional role in reducing the PLA2's affinity for the membrane by lowering the membrane negative surface density. Indeed, it was reported that the presence of negatively charged lipids enhances the rate of the reaction,37 favouring the binding of the enzyme to the membrane, which is the initial step of its action mechanism. Finally, it is important to highlight that, even if our CD experiments did not detect any conformational change of PLA2 imposed by Mg2+ salts, small local conformational changes (e.g., in the active site) and/or subtle changes in the tertiary structure of the enzyme cannot be excluded a priori which could contribute to the observed decrease of the reaction rate. Compellingly, in the presence of Mg(ClO4)2, the reaction rate is comparable to that obtained in neat buffer conditions, which is in line with the calorimetric and spectroscopic data showing that Mg(ClO4)2 favours a more hydrated and less packed bilayer. In other words, the presence of the chaotropic perchlorate anion outweighs the condensing effect of Mg2+ and restores the reaction rate observed under neat buffer conditions. Of note, previous studies12,13,38 showed that the effect of both NaClO4 and Mg(ClO4)2 on other enzyme-substrate systems was always to decrease the reaction rate. Our results point toward the opposite direction, showing that the effect of salt-membrane interaction is beneficial for the PLA2 enzymatic activity. These findings are consistent with the observation that some halophilic organisms can adapt and thrive in the presence of Martian relevant salts.39–41
Next, the same experiments were also performed as a function of pressure at conditions where the bilayer is still in its biologically relevant fluid-like state (p < 600 bar). The results for v0(p) are depicted in Fig. 5(C). In this low pressure range, no conformational changes of the enzyme take place.17 Under neat buffer conditions, the hydrolysis rate increases with increasing pressure in the POPE/POPG membrane. The pressure dependence of the initial velocity depends both on the binding volume (ΔVb) and the activation volume (ΔV#) of the reaction, and, according to Le Châtelier's principle, pressure will always favour the state that occupies the overall smallest possible volume.42,43 ΔVb is the partial molar volume difference between the complex formed by enzyme and substrate and the uncomplexed state (the sum of the volume of the enzyme and the substrate). The activation volume, ΔV#, is the partial molar volume difference between the activated enzyme–substrate complex (ES#) and the enzyme-substrate complex in the ground state (ES). It can be expected that these parameters depend not only on a given enzyme–substrate system, but also on the composition of the bilayer, on lipid packing, headgroup charge and hydration. It was found that in the presence of all sodium salts, the reaction rate decreased significantly with increasing pressure. However, it is important to note that these values are still comparable to those obtained in neat buffer at ambient pressure. In other words, the reaction still takes place at such harsh salt conditions at high pressure, with reduced rate, however. The reduced v0 at HHP conditions can be rationalized invoking the increase in the lipid packing imposed by pressure. In fact, high-pressure 2H NMR studies showed that the lipid chain length of fluid phospholipid membranes increases by about 1.1 Å kbar−1 only, whereas the lipid chain cross-sectional area decreases markedly by about 10 Å2 kbar−1.42,44 The lateral self-diffusion constant of fluid lipid vesicles decreases by about 25% upon a pressure increase of 1 kbar and more drastically when entering the pressure-induced gel phases.42,44 Remarkably, the presence of NaClO4 with its fluidization properties on the membrane is able to partially counteract this effect, leading to a reaction rate comparable to that observed in buffer.
The presence of the chloride and sulphate salts of Mg2+ caused a marked decrease of the reaction rate, which decreased even further with increasing pressure, which is due to the combined action of pressure and Mg2+, both favouring a more compact and stiffer state of the bilayer. The v0-value in the presence of NaClO4 is higher than that in Mg(ClO4)2. For NaClO4, the anion has the major role in the modulation of the kinetics. Instead, in the presence of Mg(ClO4)2, the perturbating effect of the anion is balanced by the cation binding to the head groups which promotes a higher degree of lipid order. This effect is further enhanced by pressure, leading to a slower kinetics in the presence of magnesium perchlorate compared to the sodium one.
3. Conclusions
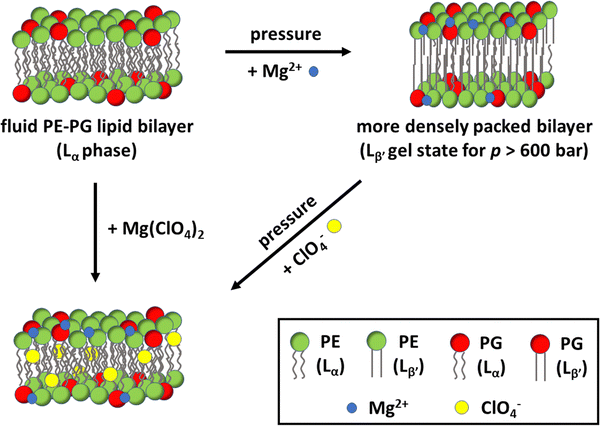
In this work, the effect of Mars-relevant salts on the microscopic, mesoscopic and functional properties of bilayers composed of the bacterial lipids PE and PG were explored. The two used lipids are the most abundant ones found in bacteria such as E. coli and, thus, the vesicles composed of PE/PG 8/2 (mol mol−1) represent a good model of the inner bacterial membrane. The multi-techniques approach employed allowed us to disentangle the effect of Mars-relevant brines on the structure, lipid packing, hydration, and the morphological properties of the lipid vesicles. Mimicking subsurface conditions on Mars, measurements were also performed at high-pressure conditions. In combination with high perchlorate and sulphate salt concentrations, these conditions represent the harsh environment expected to be encountered in the subsurface brines of Mars. To reveal the effect of such harsh conditions on an important membrane-associated enzyme reaction, we also measured the hydrolysis rate of phospholipase A2 at such conditions.Our data clearly demonstrate that the chloride, sulphate, and perchlorate salts affect the properties of the lipid bilayer differently, being dependent also on the kind of the counterion present (Na+vs. Mg2+), which dictates the overall perturbation imposed on the membrane. It was found that the perchlorate salts, which are believed to be the most abundant salts in the Martian environment, induce a more hydrated and less ordered membranes, strongly favouring the physiologically relevant fluid-like phase of the bilayer even under high-pressure stress. Indeed, a fluid membrane is required to support all biochemical reactions that are fundamental to life.45 Moreover, the lamellar structure of the bacterial model membrane persists at these high salt conditions, which seems to be add odds with an eukaryotic plasma model membrane.16 In the end, we showed that the activity of the enzyme PLA2 is strongly modulated by both HHP and the salts, the effect of the salts strongly depending on the type of anion and cation, however. Compellingly, in the presence of the chaotropic perchlorate anion, the enzymatic reaction proceeded at a reasonable rate even in the presence of the condensing Mg2+ cations and at high pressure (Fig. 6). The results presented here thus support the idea that a functional, fluid, and lamellar bilayer involving bacterial lipids may persist in such highly stressed Martian subsurface environments, and that bacterial life is possible in such harsh conditions.
Conflicts of interest
There are no conflicts of interest.Acknowledgements
The authors acknowledge funding from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy – EXC 2033 – project number 390677874-RESOLV. R. O. is grateful to the Italian MUR for being granted research associated position (PON R&I 2014-2020, CUP: E65F21003250003).References
- A. A. Yayanos, Proc. Natl. Acad. Sci. U. S. A., 1986, 83, 9542–9546 CrossRef CAS.
- C. S. Cockell, Astrobiology: Understanding Life in the Universe, John Wiley & Sons, 2020 Search PubMed.
- N. Merino, H. S. Aronson, D. P. Bojanova, J. Feyhl-Buska, M. L. Wong, S. Zhang and D. Giovannelli, Front. Microbiol., 2019, 10 DOI:10.3389/fmicb.2019.00780.
- I. Daniel, P. Oger and R. Winter, Chem. Soc. Rev., 2006, 35, 858–875 RSC.
- P. M. Oger and M. Jebbar, Res. Microbiol., 2010, 161, 799–809 CrossRef.
- F. Meersman, I. Daniel, D. H. Bartlett, R. Winter, R. Hazael and P. F. McMillan, Rev. Mineral. Geochem., 2013, 75, 607–648 CrossRef CAS.
- H. Wang, Y. Zhang, D. H. Bartlett and X. Xiao, Microb. Ecol., 2021, 81, 617–629 CrossRef CAS.
- J.-M. Knop, S. Mukherjee, M. W. Jaworek, S. Kriegler, M. Manisegaran, Z. Fetahaj, L. Ostermeier, R. Oliva, S. Gault, C. S. Cockell and R. Winter, Chem. Rev., 2023, 123, 73–104 CrossRef CAS.
- S. M. Clifford, J. Lasue, E. Heggy, J. Boisson, P. McGovern and M. D. Max, J. Geophys. Res.: Planets, 2010, 115 DOI:10.1029/2009JE003462.
- R. Orosei, S. E. Lauro, E. Pettinelli, A. Cicchetti, M. Coradini, B. Cosciotti, F. Di Paolo, E. Flamini, E. Mattei, M. Pajola, F. Soldovieri, M. Cartacci, F. Cassenti, A. Frigeri, S. Giuppi, R. Martufi, A. Masdea, G. Mitri, C. Nenna, R. Noschese, M. Restano and R. Seu, Science, 2018, 361, 490–493 CrossRef CAS PubMed.
- M. H. Hecht, S. P. Kounaves, R. C. Quinn, S. J. West, S. M. M. Young, D. W. Ming, D. C. Catling, B. C. Clark, W. V. Boynton, J. Hoffman, L. P. DeFlores, K. Gospodinova, J. Kapit and P. H. Smith, Science, 2009, 325, 64–67 CrossRef CAS PubMed.
- V. J. Laye and S. DasSarma, Astrobiology, 2018, 18, 412–418 CrossRef CAS PubMed.
- S. Gault, M. W. Jaworek, R. Winter and C. S. Cockell, Commun. Biol., 2020, 3, 1–9 CrossRef.
- S. Lenton, N. H. Rhys, J. J. Towey, A. K. Soper and L. Dougan, Nat. Commun., 2017, 8, 919 CrossRef.
- N. Jahmidi-Azizi, R. Oliva, S. Gault, C. S. Cockell and R. Winter, Biology, 2021, 10, 687 CrossRef CAS.
- S. Kriegler, M. Herzog, R. Oliva, S. Gault, C. S. Cockell and R. Winter, Phys. Chem. Chem. Phys., 2021, 23, 14212–14223 RSC.
- S. Suladze, S. Cinar, B. Sperlich and R. Winter, J. Am. Chem. Soc., 2015, 137, 12588–12596 CrossRef CAS.
- P. Garidel and A. Blume, Eur. Biophys. J., 2000, 28, 629–638 CrossRef CAS.
- R. Gaglione, G. Smaldone, A. Cesaro, M. Rumolo, M. De Luca, R. Di Girolamo, L. Petraccone, P. Del Vecchio, R. Oliva, E. Notomista, E. Pedone and A. Arciello, Pharmaceuticals, 2021, 14, 631 CrossRef CAS PubMed.
- J. R. Rydall and P. M. Macdonald, Biochemistry, 1992, 31, 1092–1099 CrossRef CAS.
- G. L. Jendrasiak, R. Smith and A. A. Ribeiro, Biochim. Biophys. Acta, Biomembr., 1993, 1145, 25–32 CrossRef CAS.
- M. Fragata, F. Bellemare and E. K. Nénonéné, J. Phys. Chem. B, 1997, 101, 1916–1921 CrossRef CAS.
- T. Parasassi, G. De Stasio, A. d’Ubaldo and E. Gratton, Biophys. J., 1990, 57, 1179–1186 CrossRef CAS PubMed.
- B. Gironi, R. Oliva, L. Petraccone, M. Paolantoni, A. Morresi, P. Del Vecchio and P. Sassi, Biochim. Biophys. Acta, Biomembr., 2019, 1861, 183052 CrossRef CAS PubMed.
- R. Winter and C. Jeworrek, Soft Matter, 2009, 5, 3157–3173 RSC.
- R. Bartucci, S. Belsito and L. Sportelli, Chem. Phys. Lipids, 1996, 79, 171–180 CrossRef CAS.
- X. Cheng and J. C. Smith, Chem. Rev., 2019, 119, 5849–5880 CrossRef CAS.
- W. Patrick Williams, B. A. Cunningham, D. H. Wolfe, G. E. Derbyshire, G. R. Mant and W. Bras, Biochim. Biophys. Acta, Biomembr., 1996, 1284, 86–96 CrossRef.
- J. Castro-Amorim, A. Novo De Oliveira, S. L. Da Silva, A. M. Soares, A. K. Mukherjee, M. J. Ramos and P. A. Fernandes, J. Med. Chem., 2023, 66, 5364–5376 CrossRef CAS.
- J. E. Burke and E. A. Dennis, Cardiovasc. Drugs Ther., 2009, 23, 49–59 CrossRef CAS.
- R. H. Schaloske and E. A. Dennis, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2006, 1761, 1246–1259 CrossRef CAS.
- S. M. Kelly, T. J. Jess and N. C. Price, Biochim. Biophys. Acta, Proteins Proteomics, 2005, 1751, 119–139 CrossRef CAS PubMed.
- W. R. Burack and R. L. Biltonen, Chem. Phys. Lipids, 1994, 73, 209–222 CrossRef CAS.
- J. C. Wilschut, J. Regts, H. Westenberg and G. Scherphof, Biochim. Biophys. Acta, Biomembr., 1978, 508, 185–196 CrossRef CAS PubMed.
- B.-Z. Yu, J. Rogers, G. R. Nicol, K. H. Theopold, K. Seshadri, S. Vishweshwara and M. K. Jain, Biochemistry, 1998, 37, 12576–12587 CrossRef CAS.
- H. M. Verheij, J. J. Volwerk, E. H. J. M. Jansen, W. C. Puyk, B. W. Dijkstra, J. Drenth and G. H. De Haas, Biochemistry, 1980, 19, 743–750 CrossRef CAS.
- A. S. Alekseeva, P. E. Volynsky and I. A. Boldyrev, Biochem. (Moscow), Suppl. Ser., 2021, 15, 329–333 CrossRef CAS.
- J. C. Warren and S. G. Cheatum, Biochemistry, 1966, 5, 1702–1707 CrossRef CAS PubMed.
- A. F. A. Soudi, O. Farhat, F. Chen, B. C. Clark and M. A. Schneegurt, Int. J. Astrobiol., 2017, 16, 229–235 CrossRef.
- L. García-Descalzo, M. Á. Lezcano, D. Carrizo and A. G. Fairén, Front. Astron. Space Sci., 2023, 14 DOI:10.3389/fmicb.2023.1197797.
- R. M. Cesur, I. M. Ansari, F. Chen, B. C. Clark and M. A. Schneegurt, Astrobiology, 2022, 22, 104–115 CrossRef CAS PubMed.
- R. Oliva and R. Winter, J. Phys. Chem. Lett., 2022, 13, 12099–12115 CrossRef CAS.
- R. Oliva, S. Banerjee, H. Cinar and R. Winter, Chem. Commun., 2020, 56, 395–398 RSC.
- R. Winter, Annu. Rev. Biophys., 2019, 48, 441–463 CrossRef CAS PubMed.
- W. Stillwell, An Introduction to Biological Membranes, Elsevier, 2016, pp. 3–15 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cp03911k |
| This journal is © the Owner Societies 2024 |