Open Access Article
Open Access ArticleRare-earth oxychalcogenide Eu2ZnGe2OS6: a phase-matching infrared nonlinear optical material with [GeOS3] units†
Guili
Wang
ab,
Wentian
Wu
ab,
Chunxiao
Li
a and
Jiyong
Yao
 *ab
*ab
aBeijing Center for Crystal Research and Development, Key Lab of Functional Crystals and Laser Technology, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: jyao@mail.ipc.ac.cn
bCenter of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 11th November 2024
Abstract
Oxychalcogenides have been highly anticipated as nonlinear optical (NLO) crystals because of their excellent optical properties. Herein, a rare-earth oxychalcogenide Eu2ZnGe2OS6 was successfully designed and synthesized. It crystallizes in the non-centrosymmetric P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 21m space group with highly polarized mixed-anion [GeOS3] units and exhibits an indirect band gap of 2.22 eV, a moderate second harmonic generation (SHG) response (0.4 × AGS), and phase-matching properties. Additionally, Eu2ZnGe2OS6 exhibits a significant calculated birefringence of 0.173@2090 nm. This research has enriched the rarely studied rare-earth oxychalcogenide system and provided new ideas for the design of promising IR NLO crystals.
21m space group with highly polarized mixed-anion [GeOS3] units and exhibits an indirect band gap of 2.22 eV, a moderate second harmonic generation (SHG) response (0.4 × AGS), and phase-matching properties. Additionally, Eu2ZnGe2OS6 exhibits a significant calculated birefringence of 0.173@2090 nm. This research has enriched the rarely studied rare-earth oxychalcogenide system and provided new ideas for the design of promising IR NLO crystals.
Introduction
Nonlinear optical (NLO) crystals, serving as pivotal functional materials for laser wavelength frequency conversion, effectively broaden the tunable range of lasers, thereby holding substantial significance in advancing laser technology.1–7 Over the past decades, a series of oxygen-based NLO materials with excellent comprehensive performance have been discovered and commercially applied in the visible and ultraviolet (UV) regions, including KH2PO4 (KDP),8 KTiOPO4 (KTP),9 LiB3O5 (LBO),10 β-BaB2O4 (BBO),11 CsB3O5 (CBO),12 CsLiB6O10 (CLBO),13 and KBe2BO3F2 (KBBF).14,15 Comparatively, the development of infrared (IR) NLO crystals is slightly behind. In the realm of IR NLO crystals, materials like AgGaSe2 (AGSe),16 AgGaS2 (AGS),17 and ZnGeP2 (ZGP)18 that have been commercialized are characterized by their significant second-harmonic generation (SHG) coefficients, broad IR transmission ranges, and excellent phase-matching (PM) properties. Nonetheless, the limitation imposed by their relatively low laser-induced damage thresholds (LIDTs) and two-photon absorption hampers their energy conversion efficiency and constrains their practical applications.19,20Consequently, investigators have put forth several optimization strategies aimed at designing and fabricating high-performance IR NLO crystals. Among these, the strategy of incorporating mixed-anion units has proven to be effective due to its capacity to combine the advantageous properties of various compounds, such as the large band gaps and high LIDTs characteristic of oxides and halides, along with the substantial SHG coefficients associated with chalcogenides. In recent years, this strategy has been employed to design and synthesize oxyhalides, chalcohalides, and oxychalcogenides that exhibit promising IR NLO performance, including Pb17O8Cl18,21 K4ZnV5O15Cl,22 Pb4SeBr6,23 Ba4Ge3S9Cl2,24 Sr6Ge3OSe11,25 and SrGeOSe2.26 The strategic integration of these diverse anions facilitates the optimization of NLO properties, resulting in materials with enhanced photonic applications. In the case of oxychalcogenides, the mixed-anion functional units formed by the multiple combinations of the oxygen anion (O2−) and chalcogenide anions (Q = S2−, Se2−, Te2−) will generate more NLO-active building units (such as [GeOS3], [GeO3S], and [GeO2S2] units), thereby achieving excellent NLO performance.
In this work, a new rare-earth oxychalcogenide Eu2ZnGe2OS6 was successfully designed and synthesized through a spontaneous crystallization method, exhibiting balanced NLO properties. The crystal structure and optical properties of Eu2ZnGe2OS6 have been extensively studied through single crystal X-ray diffraction (XRD), IR spectrum, UV-vis-NIR diffuse reflectance spectrum, Raman spectrum, and SHG tests. Concurrently, the structure–property relationship of the title compound has been unraveled through advanced theoretical calculations employing the CASTEP package based on density functional theory (DFT).
Results and discussion
Eu2ZnGe2OS6 is isostructural to the compounds of the (A = Ca, Sr, Eu; M = Mn, Fe, Co, Zn, Cd, Hg, Ge; M′ = Ga, Ge, Sn)27–36 family and belongs to the non-centrosymmetric (NCS) tetragonal space group P
(A = Ca, Sr, Eu; M = Mn, Fe, Co, Zn, Cd, Hg, Ge; M′ = Ga, Ge, Sn)27–36 family and belongs to the non-centrosymmetric (NCS) tetragonal space group P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 21m (no. 113) with unit cell parameters of a = 9.3838(7) Å, b = 9.3838(7) Å, c = 6.1509(7) Å, and Z = 2. The crystallographic data and structure refinement for Eu2ZnGe2OS6 are illustrated in Table S1.† In its asymmetric unit, one independent Eu atom, one independent Zn atom, one independent Ge atom, one independent O atom, and two independent S atoms with full occupancy are present. The calculated bond valence sums (BVS) of Eu1, Zn1, Ge1, S1, S2, and O1 atoms are 2.101, 2.122, 4.071, −2.156, −1.968, and −1.907, respectively, which are very similar to their corresponding ideal oxidation states (Table S2†).
21m (no. 113) with unit cell parameters of a = 9.3838(7) Å, b = 9.3838(7) Å, c = 6.1509(7) Å, and Z = 2. The crystallographic data and structure refinement for Eu2ZnGe2OS6 are illustrated in Table S1.† In its asymmetric unit, one independent Eu atom, one independent Zn atom, one independent Ge atom, one independent O atom, and two independent S atoms with full occupancy are present. The calculated bond valence sums (BVS) of Eu1, Zn1, Ge1, S1, S2, and O1 atoms are 2.101, 2.122, 4.071, −2.156, −1.968, and −1.907, respectively, which are very similar to their corresponding ideal oxidation states (Table S2†).
The crystal structure of Eu2ZnGe2OS6 is presented in Fig. 1. It is characterized by innumerable two-dimensional (2D) [ZnGe2OS6]4− layers extending along the ab-plane and [EuOS7] polyhedra located between the layers (Fig. 1(a)). These 2D layers are composed of an uncountable number of [ZnS4] tetrahedra and [Ge2OS6] dimers interconnected by corner-sharing with S1 atoms, with the [Ge2OS6] dimers being formed by two [GeOS3] units sharing the common O1 atoms (Fig. 1(b)). The coordination geometry of Eu2ZnGe2OS6 is presented in Fig. S2,† and the selected bond lengths and bond angles are listed in Table S3.† Each Eu1 atom is surrounded by four S1 atoms, three S2 atoms, and one O1 atom, with an Eu–O bond length of 2.809(5) Å, which is very similar to that in EuAl2O4 [2.499(2)–3.088(14) Å].37 The Eu–S bond with a length range of 3.0265(14)–3.1027(18) Å is very reasonable concerning that reported for EuCdGeS4 (3.0106–3.1530 Å).38 Each Zn1 atom is interconnected with four S1 atoms to form a typical tetrahedron with a Zn–S bond length of 2.3245(13) Å. Reciprocally, each Ge1 atom is surrounded by two S1 atoms, one S2 atom, and one O1 atom to form a highly distorted mixed-anion [GeOS3] unit. The Ge–S bond lengths are in the range of 2.1442(18)–2.2048(13) Å, and the Ge–O bond length is 1.837(3) Å, which are very similar to the values involved in Ba3S[GeOS3]39 (Ge–S bond lengths: 2.207(3)–2.223(3) Å; Ge–O bond length: 1.755(7) Å) containing [GeOS3] units.
 | ||
| Fig. 1 (a) Crystal structure of Eu2ZnGe2OS6 viewed from the b-axis; (b) 2D [ZnGe2OS6]4− layer viewed along the ab-plane. | ||
Fig. S1† illustrates the element distribution images and atomic percentage (%) of Eu2ZnGe2OS6. This result indicates that the elements Eu, Zn, Ge, and S are present and their atomic ratios are approximately 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, which is also consistent with the results obtained from single crystal structure determination.
6, which is also consistent with the results obtained from single crystal structure determination.
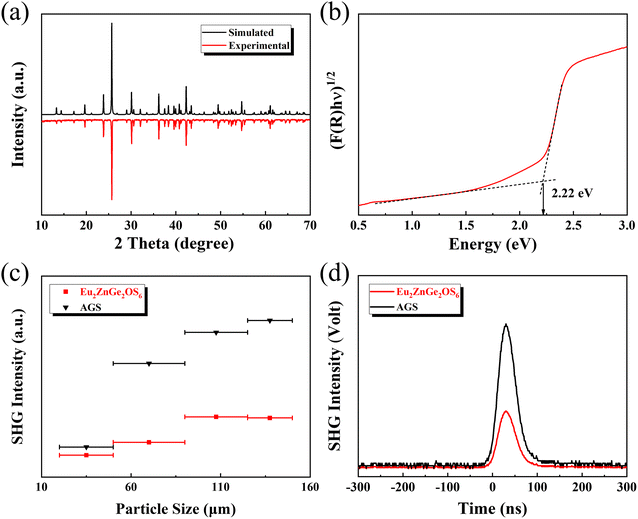
Fig. 2(a) shows the synthesized powder XRD pattern and the simulated pattern of Eu2ZnGe2OS6; the two highly similar curves imply the phase purity of the synthesized polycrystalline powder. The IR spectrum of Eu2ZnGe2OS6, as depicted in Fig. S3,† exhibits no discernible absorption peaks within the 400–4000 cm−1 range. The experimental optical band gap for Eu2ZnGe2OS6 was extrapolated to 2.22 eV through the UV-vis-NIR diffuse reflectance data, which is larger than that of commercially available IR NLO crystals AGSe (1.73 eV)40 and ZGP (2.02 eV),41 suggesting that the title compound may exhibit a larger LIDT (Fig. 2(b)). Furthermore, the Raman spectrum of a hand-picked small orange-colored blocky crystal of Eu2ZnGe2OS6 is plotted in the range of 100–500 cm−1 (Fig. S4†), from which several shifts can be observed. The most vigorous Raman shift located at 395 cm−1 is generated by the Ge–S vibration of Eu2ZnGe2OS6, from which the peaks at 303 and 451 cm−1 are also derived. The peak of lower intensity at 257 cm−1 generally originates from Zn–S vibration. In addition, the peaks below 200 cm−1 (including 163 cm−1) may be attributed to Eu–S vibrations.42–45
Considering that Eu2ZnGe2OS6 crystallizes in the NCS tetragonal space group P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 21m (no. 113), the SHG test was carried out using a laser with a wavelength of 2090 nm and AGS microcrystals as standard samples, as illustrated in Fig. 2(c). It can be seen that the SHG intensity of Eu2ZnGe2OS6 increases with increasing particle size, which indicates that Eu2ZnGe2OS6 can achieve type-I PM properties. Moreover, when the particle size is in the range of 125–150 μm, Eu2ZnGe2OS6 exhibits a moderate SHG intensity, which is about 0.4 times that of AGS (Fig. 2(d)). In the comprehensive analysis of all the compounds within the
21m (no. 113), the SHG test was carried out using a laser with a wavelength of 2090 nm and AGS microcrystals as standard samples, as illustrated in Fig. 2(c). It can be seen that the SHG intensity of Eu2ZnGe2OS6 increases with increasing particle size, which indicates that Eu2ZnGe2OS6 can achieve type-I PM properties. Moreover, when the particle size is in the range of 125–150 μm, Eu2ZnGe2OS6 exhibits a moderate SHG intensity, which is about 0.4 times that of AGS (Fig. 2(d)). In the comprehensive analysis of all the compounds within the  system, it is observed that no selenides are present. Notably, selenides are often characterized by larger SHG coefficients and superior NLO properties compared to sulfides. Consequently, for Eu2ZnGe2OS6, rational chemical substitution may emerge as a potential strategy to enhance the NLO performance, such as the substitution of S with Se, the substitution of Ge with heavier Sn, and the substitution of Zn with other heavier d10 atoms (e.g., Cd and Hg).
system, it is observed that no selenides are present. Notably, selenides are often characterized by larger SHG coefficients and superior NLO properties compared to sulfides. Consequently, for Eu2ZnGe2OS6, rational chemical substitution may emerge as a potential strategy to enhance the NLO performance, such as the substitution of S with Se, the substitution of Ge with heavier Sn, and the substitution of Zn with other heavier d10 atoms (e.g., Cd and Hg).
As depicted in Fig. 3(a), Eu2ZnGe2OS6 exhibits an indirect band gap of 1.81 eV, with the conduction band (CB) minimum situated at the Γ point and the valence band (VB) maximum situated at the M point. This theoretical band gap is slightly lower than the experimentally determined value of 2.22 eV, which can be attributed to the limitations of the local density approximation (LDA) function when employed in DFT calculations. The LDA function often underestimates the band gap due to its inability to accurately account for the exchange–correlation potential, particularly the discontinuity in the derivative of the exchange–correlation energy concerning the electron density.46–48 The analysis of the total and partial density of states (TDOS and PDOS) as depicted in Fig. 3(b) reveals that the S 3p orbitals are the predominant contributors to the VB maximum, with a minor incorporation of Eu 4f and 5d orbitals. Similarly, the CB minimum is largely influenced by the S 3p and Ge 4s orbitals, complemented by contributions from the Zn 4s orbitals. This suggests that the optical characteristics of Eu2ZnGe2OS6 are predominantly governed by the [ZnS4] tetrahedron and [GeOS3] groups. Moreover, the intermixing of Eu 4f orbitals could potentially introduce extra electronic transition pathways, which may subsequently influence the optical properties of Eu2ZnGe2OS6. Birefringence (Δn), the difference in refractive index at a specific wavelength, is a characteristic unique to anisotropic crystal systems. It is a pivotal property for functional optical crystals, as it significantly influences angular PM and the ability to adjust the polarization of light. Achieving PM is not possible if the nonlinear optical material's birefringence is too low; thus, a moderate birefringence is necessary for a substantial SHG response.49–52 The birefringence of Eu2ZnGe2OS6 has been calculated to be 0.173 at 2090 nm, a value substantial enough to facilitate PM behavior. This finding is also consistent with the results obtained from the powder SHG tests. Additionally, the dipole moment (DM) analysis of the [GeOS3] unit and the [ZnS4] tetrahedron was conducted utilizing the bond valence method.53 It is evident that the DM of the [GeOS3] unit substantially exceeds that of the [ZnS4] tetrahedron, as detailed in Table S4.† This significant difference in DM suggests that the [GeOS3] unit plays a predominant role in the substantial birefringence observed in Eu2ZnGe2OS6.
 | ||
| Fig. 3 The calculated results of Eu2ZnGe2OS6: (a) electronic band structure, (b) DOS, and (c) birefringence. | ||
Conclusion
In conclusion, a new rare-earth oxychalcogenide Eu2ZnGe2OS6 was successfully designed and synthesized, which crystallizes in the NCS P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 21m space group, featuring highly polarized mixed-anion [GeOS3] units. The title compound exhibits an experimentally determined indirect band gap of 2.22 eV, a modest SHG response (0.4 × AGS), and PM properties. Rational chemical substitution strategies are anticipated to enhance the NLO properties further. This study enriches the rarely studied rare-earth oxychalcogenide system and offers new insights for the design of promising oxychalcogenide IR NLO crystals.
21m space group, featuring highly polarized mixed-anion [GeOS3] units. The title compound exhibits an experimentally determined indirect band gap of 2.22 eV, a modest SHG response (0.4 × AGS), and PM properties. Rational chemical substitution strategies are anticipated to enhance the NLO properties further. This study enriches the rarely studied rare-earth oxychalcogenide system and offers new insights for the design of promising oxychalcogenide IR NLO crystals.
Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for Eu2ZnGe2OS6 has been deposited at the CCDC under 2390087.Author contributions
G. L. Wang prepared the samples, designed and conducted the experiments, and wrote the original draft of the manuscript; W. T. Wu performed theoretical calculations; C. X. Li helped with powder SHG tests. J. Y. Yao reviewed and edited the manuscript. All authors reviewed and approved the final form of the submitted manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Thanks for the support of the National Natural Science Foundation of China (no. 22175190).References
- D. Pestov, X. Wang, G. O. Ariunbold, R. K. Murawski, V. A. Sautenkov, A. Dogariu, A. V. Sokolov and M. O. Scully, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 422–427 CrossRef CAS PubMed.
- V. A. Serebryakov, É. V. Boĭko, N. N. Petrishchev and A. V. Yan, J. Opt. Technol., 2010, 77, 6–17 CrossRef CAS.
- A. Abudurusuli, J. Li and S. Pan, Dalton Trans., 2021, 50, 3155–3160 RSC.
- M. Sun, W. Xing, S. K. Chen, C. Li, W. Liu, M.-H. Lee and J. Yao, Chem. Mater., 2023, 35, 7218–7228 CrossRef CAS.
- C. Li, X. Meng, Z. Li and J. Yao, Coord. Chem. Rev., 2022, 453, 214328 CrossRef CAS.
- I. Chung and M. G. Kanatzidis, Chem. Mater., 2014, 26, 849–869 CrossRef CAS.
- W. Huang, L. Gan, H. Li, Y. Ma and T. Zhai, CrystEngComm, 2016, 18, 3968–3984 RSC.
- D. Eimerl, Ferroelectrics, 1987, 72, 95–139 CrossRef CAS.
- F. C. Zumsteg, J. D. Bierlein and T. E. Gier, J. Appl. Phys., 2008, 47, 4980–4985 CrossRef.
- C. Chen, Y. Wu, A. Jiang, B. Wu, G. You, R. Li and S. Lin, J. Opt. Soc. Am. B, 1989, 6, 616–621 CrossRef CAS.
- D. N. Nikogosyan, Appl. Phys. A: Mater. Sci. Process., 1991, 52, 359–368 CrossRef.
- Y. Wu, T. Sasaki, S. Nakai, A. Yokotani, H. Tang and C. Chen, Appl. Phys. Lett., 1993, 62, 2614–2615 CrossRef CAS.
- Y. Mori, I. Kuroda, S. Nakajima, T. Sasaki and S. Nakai, Appl. Phys. Lett., 1995, 67, 1818–1820 CrossRef CAS.
- Y. Xia, C. Chen, D. Tang and B. Wu, Adv. Mater., 1995, 7, 79–81 CrossRef CAS.
- Y. Sang, D. Yu, M. Avdeev, R. Piltz, N. Sharma, N. Ye, H. Liu and J. Wang, CrystEngComm, 2012, 14, 6079–6084 RSC.
- G. Boyd, H. Kasper, J. McFee and F. Storz, IEEE J. Quantum Electron., 1972, 8, 900–908 CrossRef CAS.
- D. S. Chemla, P. J. Kupecek, D. S. Robertson and R. C. Smith, Opt. Commun., 1971, 3, 29–31 CrossRef CAS.
- G. D. Boyd, E. Buehler and F. G. Storz, Appl. Phys. Lett., 1971, 18, 301–304 CrossRef CAS.
- A. H. Akiko Harasaki and K. K. Kiyoshi Kato, Jpn. J. Appl. Phys., 1997, 36, 700 CrossRef.
- G. C. Catella, L. R. Shiozawa, J. R. Hietanen, R. C. Eckardt, R. K. Route, R. S. Feigelson, D. G. Cooper and C. L. Marquardt, Appl. Opt., 1993, 32, 3948–3951 CrossRef CAS PubMed.
- H. Zhang, M. Zhang, S. Pan, X. Dong, Z. Yang, X. Hou, Z. Wang, K. B. Chang and K. R. Poeppelmeier, J. Am. Chem. Soc., 2015, 137, 8360–8363 CrossRef CAS PubMed.
- W. Li, L. Geng, C. Meng, S. Ma, B. Zhu, Y. Lan and Q. Wu, Adv. Opt. Mater., 2024, 12, 2302560 CrossRef CAS.
- J. Wang, H. Wu, H. Yu, Z. Hu, J. Wang and Y. Wu, Adv. Opt. Mater., 2022, 10, 2102673 CrossRef CAS.
- P.-F. Liu, Y.-Y. Li, Y.-J. Zheng, J.-S. Yu, R.-H. Duan, H. Chen, H. Lin, L. Chen and L.-M. Wu, Dalton Trans., 2017, 46, 2715–2721 RSC.
- L. T. Menezes, E. Gage, A. Assoud, M. Liang, P. S. Halasyamani and H. Kleinke, Chem. Mater., 2023, 35, 3033–3040 CrossRef CAS.
- M.-Y. Ran, Z. Ma, H. Chen, B. Li, X.-T. Wu, H. Lin and Q.-L. Zhu, Chem. Mater., 2020, 32, 5890–5896 CrossRef CAS.
- M.-Y. Ran, S.-H. Zhou, X.-T. Wu, H. Lin and Q.-L. Zhu, Mater. Today Phys., 2024, 44, 101442 CrossRef CAS.
- R. Wang, F. Liang, X. Liu, Y. Xiao, Q. Liu, X. Zhang, L.-M. Wu, L. Chen and F. Huang, ACS Appl. Mater. Interfaces, 2022, 14, 23645–23652 CrossRef CAS PubMed.
- T. Endo, Y. Doi, M. Wakeshima, K. Suzuki, Y. Matsuo, K. Tezuka, T. Ohtsuki, Y. J. Shan and Y. Hinatsu, Inorg. Chem., 2017, 56, 2459–2466 CrossRef CAS PubMed.
- M.-Y. Ran, S.-H. Zhou, B. Li, W. Wei, X.-T. Wu, H. Lin and Q.-L. Zhu, Chem. Mater., 2022, 34, 3853–3861 CrossRef CAS.
- H.-D. Yang, S.-H. Zhou, M.-Y. Ran, X.-T. Wu, H. Lin and Q.-L. Zhu, Inorg. Chem. Front., 2023, 10, 2030–2038 RSC.
- N. Zhang, Q.-T. Xu, Z.-H. Shi, M. Yang and S.-P. Guo, Inorg. Chem., 2022, 61, 17002–17006 CrossRef CAS PubMed.
- X. Tian, X. Zhang, Y. Xiao, X. Wu, B. Zhang, D. Yang and K. Wu, RSC Adv., 2022, 12, 16296–16300 RSC.
- Y. Cheng, H. Wu, H. Yu, Z. Hu, J. Wang and Y. Wu, Chem. Sci., 2022, 13, 5305–5310 RSC.
- N. Zhang, X. Huang, W.-D. Yao, Y. Chen, Z.-R. Pan, B. Li, W. Liu and S.-P. Guo, Inorg. Chem., 2023, 62, 16299–16303 CrossRef CAS PubMed.
- G. Wang, C. Li, M.-H. Lee and J. Yao, Inorg. Chem., 2024, 63, 10288–10295 CrossRef CAS PubMed.
- K. Tezuka, Y. Tokuhara, M. Wakeshima, Y. J. Shan, H. Imoto and Y. Hinatsu, Inorg. Chem., 2013, 52, 12972–12979 CrossRef CAS PubMed.
- W. Xing, N. Wang, Y. Guo, Z. Li, J. Tang, K. Kang, W. Yin, Z. Lin, J. Yao and B. Kang, Dalton Trans., 2019, 48, 17620–17625 RSC.
- S. Cui, H. Wu, Z. Hu, J. Wang, Y. Wu and H. Yu, Adv. Sci., 2023, 10, 2204755 CrossRef CAS PubMed.
- G. A. Babu, R. S. Raja, P. Ramasamy and N. Karunagaran, AIP Conf. Proc., 2012, 1447, 1045–1046 CrossRef CAS.
- X. Zhao, S. Zhu, B. Zhao, B. Chen, Z. He, R. Wang, H. Yang, Y. Sun and J. Cheng, J. Cryst. Growth, 2008, 311, 190–193 CrossRef CAS.
- K. Wu, X. Su, Z. Yang and S. Pan, Dalton Trans., 2015, 44, 19856–19864 RSC.
- H. Wang, X. Pan, W. Zhao, Y. Chu and J. Li, Inorg. Chem. Front., 2023, 10, 6253–6261 RSC.
- H. Ben Yahia, K. Motohashi, S. Mori, A. Sakuda and A. Hayashi, J. Alloys Compd., 2023, 960, 170600 CrossRef CAS.
- C. Power, E. Moreno, E. Quintero, D. Rivero, M. Quintero, C. Rincón, J. A. Henao and M. A. Macías, Phys. Status Solidi B, 2016, 253, 335–339 CrossRef CAS.
- J. P. Perdew, Int. J. Quantum Chem., 1986, 30, 451 CrossRef.
- D. Koller, F. Tran and P. Blaha, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 195134 CrossRef.
- R. W. Godby, M. Schlüter and L. J. Sham, Phys. Rev. B: Condens. Matter Mater. Phys., 1987, 36, 6497–6500 CrossRef CAS PubMed.
- X. Chen, Y. Li, J. Luo and S. Zhao, Chin. J. Struct. Chem., 2023, 42, 100044 CrossRef CAS.
- Y. Li, Q. Wu, Z. Lin, Y. Liu, Y. Zhou, X. Chen, M. Li, M. Hong, J. Luo and S. Zhao, Fundam. Res., 2023, 3, 974–978 CrossRef CAS PubMed.
- T. Ouyang, Y. Shen and S. Zhao, Chin. J. Struct. Chem., 2023, 42, 100024 CrossRef CAS.
- Y. Zhao, L. Zhu, Y. Li, X. Kuang, J. Luo and S. Zhao, Mater. Chem. Front., 2023, 7, 3986–3993 RSC.
- P. A. Maggard, T. S. Nault, C. L. Stern and K. R. Poeppelmeier, J. Solid State Chem., 2003, 175, 27–33 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2390087. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ce01051e |
| This journal is © The Royal Society of Chemistry 2024 |