Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A pentametallic Co5L4 architecture constructed from bis-substituted phosphate-based ligands†
Jas S.
Ward‡
 * and
Paul E.
Kruger
* and
Paul E.
Kruger
 *
*
MacDiarmid Institute for Advanced Materials and Nanotechnology, School of Physical and Chemical Sciences, University of Canterbury, Christchurch, New Zealand. E-mail: james.s.ward@jyu.fi; paul.kruger@canterbury.ac.nz
First published on 17th June 2024
Abstract
A pentametallic Co 5 L 4 architecture has been constructed from the bis-substituted phosphoric acid pro-ligand P(O)(OH)(OC 6 H 4 NH 2 -3) 2 . The architecture, which is comprised of a metal centre embedded in a M 4 L 4 square, was characterised in the solid state by X-ray diffraction, confirming cobalt atoms in two different coordination geometries.
The construction of supramolecular cages through self-assembling processes is well-established and has produced a myriad of interesting architectures with various topologies,1,2 many of which are capable of accommodating guest molecules within their internal voids.3–9 This host-guest behaviour has been utilised to selectively bind guests within the cavity,10,11 catalyse reactions,12–15 extend the lifetimes of fleeting species,16–18 and even shield highly reactive species.19,20
Supramolecular squares have been reliably synthesised through the use of cis-blocked square planar Pd(II) and Pt(II) metal centres bearing 1,2-bis(diphenylphosphino)ethane (dppe) and 1,3-bis(diphenylphosphino)propane auxiliary ligands; this combination ensures a 90° ligand–metal–ligand configuration suitable for supramolecular square formation.21–23 The pre-organisation of the metal centres and their auxiliary diphosphine ligands greatly contributes to favouring discrete species over polymeric ones. Without this preorganisation, octahedrally coordinated metal nodes would generally favour the formation of 3D motifs, rather than 2D squares. The synthesis of supramolecular squares without such pre-organisation in the metal nodes is therefore less common and of interest for further studies.
Whilst phosphates ([PO4]3−) are ubiquitous in fields such as biochemistry, and simple cages derived from H3PO4 have been previously reported,24 their utilisation in the field of supramolecular chemistry is still quite rare.25 Nevertheless, examples of phosphorus-based ligands, beyond those of simply phosphine-coordinated ligands,26,27 being incorporated into supramolecular frameworks have begun to emerge in the recent years.28–31 Promisingly, applications like the selective anion extraction have also begun to be demonstrated for these phosphorus-based supramolecular complexes.32 Non-metallic supramolecular cages have also been demonstrated,33–35 which have been shown to possess improved properties over their non-supramolecular constituent parts,35 and may offer advantages to fields for which their metallic analogues are not suited, for example in pharmaceuticals, akin to boron neutron capture therapy (BNCT).36 Therefore, the investigation of multi-topic, phosphorus-based ligands with respect to their ability to construct new supramolecular architectures is of interest to a wide range of researchers across a variety of fields. With this in mind, the supramolecular potential of a ditopic phosphate-based ligand was explored.
The previously unknown phosphoric acid pro-ligand, P(O)(OH)(OC6H4NH2-3)2, was synthesised as the major product from P(O)(OC6H4NO2-3)3via a Pd/C hydrogenation in MeOH under an atmosphere of hydrogen gas. The bis-substituted P(O)(OH)(OC6H4NH2-3)2 was found to be favoured over the tris-substituted product P(O)(OC6H4NH2-3)3 with prolonged reaction times (via the opportunistic interaction of the H2O by-product of the hydrogenation reaction).37
Characterisation with NMR spectroscopy unremarkably revealed a single resonance in the 31P NMR spectrum at −10.5 ppm and four peaks in the 1H NMR spectrum at 6.35, 6.49, 6.51 and 6.88 ppm for the meta-substituted aminophenyl rings. Mass spectroscopy supported the identity of the product by observation of the [M + H]+ at m/z 281, further confirmed through an X-ray diffraction study which identified the bis-substituted phosphoric acid in its zwitterionic form (Fig. S1†). It should be noted that the bond lengths revealed that a formal single and double bond did not exist between each of the two unsubstituted oxygen atoms and the phosphorus centre, instead their crystallographic similarity indicated that the negative charge was delocalised over both terminal oxygen atoms. The phosphoric acid derivative was recognised as a potential pro-ligand for bis-bidentate self-assembly supramolecular chemistry and investigations with a suitable cobalt salt were conducted.
To explore the supramolecular potential of P(O)(OH)(OC6H4NH2-3)2, it was initially reacted in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
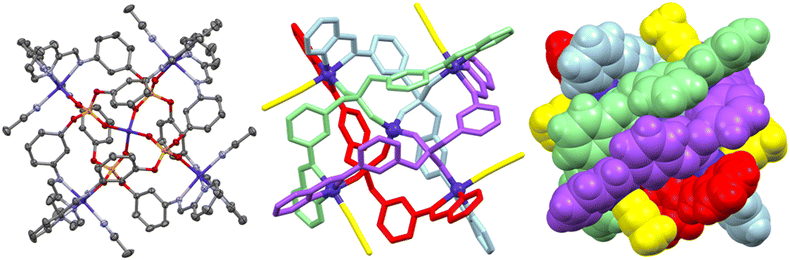
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio with 2-pyridinecarboxaldehyde and Co(BF4)2·6H2O, respectively, envisioning that the ligand (formed by metal-catalysed imine condensation of the pro-ligand) would effectively act as a linear bis-bidentate linker to form M2L3 or M4L6 species. However, when acetonitrile was used as the solvent, vapour diffusion of the crude reaction mixture (after filtration) with benzene as the anti-solvent yielded large green crystals of the supramolecular square [Co5L4(MeCN)4][BF4]6 (Fig. 1; L = bis(3-(((E)-pyridin-2-ylmethylene)amino)phenyl) phosphate). The composition of this non-traditional nano-square revealed that in addition to the Co5L4 core, each of the corner Co2+ metal centres had also acquired an acetonitrile (MeCN) molecule to satisfy their coordination sphere, which was taken from the reaction solvent used during synthesis and crystallisation of the complex.
2 ratio with 2-pyridinecarboxaldehyde and Co(BF4)2·6H2O, respectively, envisioning that the ligand (formed by metal-catalysed imine condensation of the pro-ligand) would effectively act as a linear bis-bidentate linker to form M2L3 or M4L6 species. However, when acetonitrile was used as the solvent, vapour diffusion of the crude reaction mixture (after filtration) with benzene as the anti-solvent yielded large green crystals of the supramolecular square [Co5L4(MeCN)4][BF4]6 (Fig. 1; L = bis(3-(((E)-pyridin-2-ylmethylene)amino)phenyl) phosphate). The composition of this non-traditional nano-square revealed that in addition to the Co5L4 core, each of the corner Co2+ metal centres had also acquired an acetonitrile (MeCN) molecule to satisfy their coordination sphere, which was taken from the reaction solvent used during synthesis and crystallisation of the complex.
The reaction was repeated with the correct stoichiometry of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (phosphoric acid pro-ligand
5 (phosphoric acid pro-ligand![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aldehyde
aldehyde![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co2+) to give the product in a recovered yield of 19% (Scheme 1). As a side note on the recovered yield of the desired product, a second product from reaction was isolated and found to be [Co(κ3-N{C(O)py-2}2)2][BF4] (Fig. S3†), with the [BF4]− anion confirming the presence of Co3+ rather than Co2+. The rational synthesis of this cation (with various anions) has been previously described.38–40
Co2+) to give the product in a recovered yield of 19% (Scheme 1). As a side note on the recovered yield of the desired product, a second product from reaction was isolated and found to be [Co(κ3-N{C(O)py-2}2)2][BF4] (Fig. S3†), with the [BF4]− anion confirming the presence of Co3+ rather than Co2+. The rational synthesis of this cation (with various anions) has been previously described.38–40
The paramagnetic Co2+ atoms of [Co5L4(MeCN)4][BF4]6 prevented NMR studies, however, a conclusive (R1/wR2 = 6.1/16.5%) single crystal X-ray diffraction study confirmed the complex to be a pentametallic supramolecular nano-square incorporating a fifth centrally encapsulated Co2+ centre.
The architecture of the complex is unusual, especially when compared to conventional, edge-bridged supramolecular squares,41–47 where nano square complexes can be straightforwardly synthesised using linear ditopic ligands or linkers and metal nodes (usually with a square planar coordination sphere, though they can also be of an octahedral geometry) with two vacant cis-coordination sites, combined in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, or more aptly a 4
1 ratio, or more aptly a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 stoichiometry. However, in [Co5L4(MeCN)4][BF4]6 the four phosphate ligands are nestled, though not interwoven (Fig. 2), diagonally into one another to each bridge two opposing corner Co2+ atoms, as well as to the central Co2+ and a third Co2+ atom of an adjacent corner via the two unsubstituted terminal oxygen atoms of the phosphate ligand (upon deprotonation of the acidic pro-ligand during self-assembly). The encapsulation of the central Co2+ is particularly striking, given that the terminal oxygen substituents of the phosphate ligands are non-innocent with respect to the overall supramolecular construction, in contrast to the P = X (X = O, S, Se) groups of analogous tris-substituted phosphate-based supramolecular cages.30,31,34
4 stoichiometry. However, in [Co5L4(MeCN)4][BF4]6 the four phosphate ligands are nestled, though not interwoven (Fig. 2), diagonally into one another to each bridge two opposing corner Co2+ atoms, as well as to the central Co2+ and a third Co2+ atom of an adjacent corner via the two unsubstituted terminal oxygen atoms of the phosphate ligand (upon deprotonation of the acidic pro-ligand during self-assembly). The encapsulation of the central Co2+ is particularly striking, given that the terminal oxygen substituents of the phosphate ligands are non-innocent with respect to the overall supramolecular construction, in contrast to the P = X (X = O, S, Se) groups of analogous tris-substituted phosphate-based supramolecular cages.30,31,34
The Co–O bond lengths for the corner (2.025(4), 2.026(4) Å) and central (1.934(4), 1.938(4) Å) cobalt dications were crystallographically independent for a 6σ2 tolerance, as would be expected for octahedral and tetrahedral geometries, respectively. The differences of Co–N bond lengths for the imine (2.151(4)–2.172(4) Å) and pyridyl (2.114(4)–2.131(4) Å) nitrogen atoms were crystallographically insignificant within the complex and corresponded to the expected values for a Co(X)(Y)(L)2 coordination sphere (where L is a bis-N,N′-bidentate ligand).48
Similarly, the bond length ranges of the P–O(Co) (1.475(4)–1.492(4) Å) and P–O(R) (1.586(4)–1.606(4) Å) were crystallographically indistinguishable from the free protonated ligand P(O)2(OC6H4NH2-3)(OC6H4NH3-3) (cf. P–O 1.474(1), 1.490(1) Å; P–O(R) 1.594(1), 1.610(1) Å). However, the bond angles for the phosphate nodes showed substantial strain with values for (R)O–P–O(R) of 100.5(2) and 100.9(2)° in the supramolecular complex to accommodate the Co5L4 motif (cf. 105.47(7)° in P(O)2(OC6H4NH2-3)(OC6H4NH3-3)). To a lesser degree (though still crystallographically significant), the (Co)O–P–O(Co) bond angles of 117.1(2) and 117.5(2)° were also contracted to facilitate bridging two cobalt centres (cf. 118.89(7)° in P(O)2(OC6H4NH2-3)(OC6H4NH3-3). The Co2+ corner-corner distances were close to being square, with through space lengths of 8.018(2) and 8.128(2) Å, and the Co2+ centre-corner distances were found to be 5.672(2) and 5.699(2) Å.
The spacefill representation of [Co5L4(MeCN)4][BF4]6 (Fig. 1) demonstrates that the central Co2+ centre is completely encapsulated, suggesting that its incorporation into the supramolecular scaffold would almost certainly have to have been during assembly of the cation, and not after the Co4L4 square had been formed. This assumption supports the idea that the central Co2+ played a role in templating the [Co4L4]4+ nano-square. This motif of total encapsulation is reminiscent of other oxygen-donor, metal binding species such as crown ethers and cryptands, where [Co5L4(MeCN)4][BF4]6 can be seen as a phosphorus-based variant of these fundamental overarching families of macrocycles and molecular capsules for which Cram, Lehn, and Pedersen shared the 1987 Nobel prize in chemistry.
In conclusion, a novel pentametallic Co5L4 supramolecular architecture has been synthesised and characterised in the solid state. The supramolecular assembly involves a Co4L4 nano-square comprised of bis(substituted)phosphate ligands, which encapsulates a fifth cobalt atom through the coordination of the phosphates' terminal oxygen atoms. The construction of this unusual supramolecular nano square through the non-innocent involvement of the terminal oxygen substituents of the phosphate ligands is an interesting caveat.
The results reported herein demonstrate that the incorporation of functional groups such as phosphates into supramolecular synthons enable unexpected and non-traditional connectivities and architectures to be observed, and therefore merit further studies to fully realise their potential.
Data availability statement
The data supporting this article have been included as part of the ESI.† Crystallographic data for bis(3-aminophenyl)phosphoric acid (Pro_ligand), [Co5L4(MeCN)4][BF4]6 (Co5L4), and [C24H16CoN6O4][BF4] (Side_product) have been deposited at the Cambridge Crystallographic Data Centre (CCDC 1569646–1569648).Conflicts of interest
There are no conflicts to declare.Acknowledgements
P. E. K. gratefully acknowledges the MacDiarmid Institute for Advanced Materials and Nanotechnology, and support from the Marsden Fund Council from Government funding, managed by Royal Society Te Apārangi, and the School of Physical and Chemical Science at the University of Canterbury for additional financial and instrumental support.Notes and references
- G. Vantomme and E. W. Meijer, Science, 2019, 363, 1396–1397 CrossRef CAS PubMed.
- D. Fujita, Y. Ueda, S. Sato, N. Mizuno, T. Kumasaka and M. Fujita, Nature, 2016, 540, 563–566 CrossRef CAS PubMed.
- D. J. Cram, Nature, 1992, 356, 29–36 CrossRef CAS.
- M. Fujita, D. Oguro, M. Miyazawa, H. Oka, K. Yamaguchi and K. Ogura, Nature, 1995, 378, 469–471 CrossRef CAS.
- M. Yoshizawa, J. K. Klosterman and M. Fujita, Angew. Chem., Int. Ed., 2009, 48, 3418–3438 CrossRef CAS PubMed.
- S. J. Dalgarno, N. P. Power and J. L. Atwood, Coord. Chem. Rev., 2008, 252, 825–841 CrossRef CAS.
- M. D. Ward, Chem. Commun., 2009, 4487–4499 RSC.
- A. Ferguson, M. A. Squire, D. Siretanu, D. Mitcov, C. Mathonière, R. Clérac and P. E. Kruger, Chem. Commun., 2013, 49, 1597–1599 RSC.
- K. Byrne, M. Zubair, N. Zhu, X.-P. Zhou, D. S. Fox, H. Zhang, B. Twamley, M. J. Lennox, T. Düren and W. Schmitt, Nat. Commun., 2017, 8, 15268 CrossRef CAS PubMed.
- W. Meng, B. Breiner, K. Rissanen, J. D. Thoburn, J. K. Clegg and J. R. Nitschke, Angew. Chem., Int. Ed., 2011, 50, 3479–3483 CrossRef CAS PubMed.
- X. Zhang, D. Zhang, C. Wei, D. Wang, R. Lavendomme, S. Qi, Y. Zhu, J. Zhang, Y. Zhang, J. Wang, L. Xu, E.-Q. Gao, W. Yu, H.-B. Yang and M. He, Nat. Commun., 2024, 15, 3766 CrossRef CAS PubMed.
- M. Yoshizawa, M. Tamura and M. Fujita, Science, 2006, 312, 251–254 CrossRef CAS PubMed.
- M. D. Pluth, R. G. Bergman and K. N. Raymond, Science, 2007, 316, 85–88 CrossRef CAS PubMed.
- Y. Xue, X. Hang, J. Ding, B. Li, R. Zhu, H. Pang and Q. Xu, Coord. Chem. Rev., 2021, 430, 213656 CrossRef CAS.
- R. Ham, C. J. Nielsen, S. Pullen and J. N. H. Reek, Chem. Rev., 2023, 123, 5225–5261 CrossRef CAS PubMed.
- R. Warmuth, Angew. Chem., Int. Ed. Engl., 1997, 36, 1347–1350 CrossRef CAS.
- R. Warmuth and M. A. Marvel, Angew. Chem., Int. Ed., 2000, 39, 1117–1119 CrossRef CAS PubMed.
- S. Zarra, D. M. Wood, D. A. Roberts and J. R. Nitschke, Chem. Soc. Rev., 2015, 44, 419–432 RSC.
- P. Mal, B. Breiner, K. Rissanen and J. R. Nitschke, Science, 2009, 324, 1697–1699 CrossRef CAS PubMed.
- Y. Sun, C. Chen, J. Liu and P. J. Stang, Chem. Soc. Rev., 2020, 49, 3889–3919 RSC.
- P. J. Stang and D. H. Cao, J. Am. Chem. Soc., 1994, 116, 4981–4982 CrossRef CAS.
- P. J. Stang, D. H. Cao, S. Saito and A. M. Arif, J. Am. Chem. Soc., 1995, 117, 6273–6283 CrossRef CAS.
- S. Leininger, B. Olenyuk and P. J. Stang, Chem. Rev., 2000, 100, 853–908 CrossRef CAS.
- V. Chandrasekhar, V. Baskar, K. Gopal and J. J. Vittal, Organometallics, 2005, 24, 4926–4932 CrossRef CAS.
- P. Stricklen and J. Verkade, J. Am. Chem. Soc., 1983, 105, 2494–2495 CrossRef CAS.
- I. D. Strelnik, I. R. Dayanova, I. E. Kolesnikov, R. R. Fayzullin, I. A. Litvinov, A. I. Samigullina, T. P. Gerasimova, S. A. Katsyuba, E. I. Musina and A. A. Karasik, Inorg. Chem., 2019, 58, 1048–1057 CrossRef CAS PubMed.
- G. R. F. Orton, B. S. Pilgrim and N. R. Champness, Chem. Soc. Rev., 2021, 50, 4411–4431 RSC.
- D. Zhang, T. K. Ronson, J. Mosquera, A. Martinez, L. Guy and J. R. Nitschke, J. Am. Chem. Soc., 2017, 139, 6574–6577 CrossRef CAS PubMed.
- P. Rajasekar, S. Pandey, H. Paithankar, J. Chugh, A. Steiner and R. Boomishankar, Chem. Commun., 2018, 54, 1873–1876 RSC.
- P. W. V. Butler, P. E. Kruger and J. S. Ward, Chem. Commun., 2019, 55, 10304–10307 RSC.
- C. T. McTernan, T. K. Ronson and J. R. Nitschke, J. Am. Chem. Soc., 2019, 141, 6837–6842 CrossRef CAS PubMed.
- D. Zhang, T. K. Ronson, J. Mosquera, A. Martinez and J. R. Nitschke, Angew. Chem., Int. Ed., 2018, 57, 3717–3721 CrossRef CAS PubMed.
- P. D. Raytchev, A. Martinez, H. Gornitzka and J.-P. Dutasta, J. Am. Chem. Soc., 2011, 133, 2157–2159 CrossRef CAS PubMed.
- G. Feng, W. Liu, Y. Peng, B. Zhao, W. Huang and Y. Dai, Chem. Commun., 2016, 52, 9267–9270 RSC.
- J.-P. Dutasta and A. Martinez, ChemPlusChem, 2020, 85, 977–984 CrossRef CAS PubMed.
- K. Hu, Z. Yang, L. Zhang, L. Xie, L. Wang, H. Xu, L. Josephson, S. H. Liang and M.-R. Zhang, Coord. Chem. Rev., 2020, 405, 213139 CrossRef CAS.
- E. A. Gelder, S. D. Jackson and C. M. Lok, Chem. Commun., 2005, 522–524 RSC.
- J. M. Rowland, M. M. Olmstead and P. K. Mascharak, Inorg. Chem., 2002, 41, 2754–2760 CrossRef CAS PubMed.
- T. Kajiwara, R. Sensui, T. Noguchi, A. Kamiyama and T. Ito, Inorg. Chim. Acta, 2002, 337, 299–307 CrossRef CAS.
- R. Pandey, R. K. Gupta, P.-Z. Li, Q. Xu, A. Misra and D. S. Pandey, Org. Lett., 2012, 14, 592–595 CrossRef CAS PubMed.
- J. Manna, C. J. Kuehl, J. A. Whiteford, P. J. Stang, D. C. Muddiman, S. A. Hofstadler and R. D. Smith, J. Am. Chem. Soc., 1997, 119, 11611–11619 CrossRef CAS.
- J. A. Whiteford, P. J. Stang and S. D. Huang, Inorg. Chem., 1998, 37, 5595–5601 CrossRef CAS PubMed.
- H. Oshio, O. Tamada, H. Onodera, T. Ito, T. Ikoma and S. Tero-Kubota, Inorg. Chem., 1999, 38, 5686–5689 CrossRef CAS.
- S.-S. Sun and A. J. Lees, J. Am. Chem. Soc., 2000, 122, 8956–8967 CrossRef CAS.
- S. Ghosh and P. S. Mukherjee, Inorg. Chem., 2009, 48, 2605–2613 CrossRef CAS PubMed.
- A. G. L. Olive, K. Parkan, C. Givelet and J. Michl, J. Am. Chem. Soc., 2011, 133, 20108–20111 CrossRef CAS PubMed.
- B. K. Collins, M. Clough Mastry, A. Ehnbom, N. Bhuvanesh, M. B. Hall and J. A. Gladysz, Chem. – Eur. J., 2021, 27, 10021–10039 CrossRef CAS PubMed.
- C. S. Hawes and P. E. Kruger, Polyhedron, 2013, 52, 255–260 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis, NMR, MS, and X-ray crystallographic details. CCDC 1569646–1569648. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ce00468j |
‡ Current address: University of Jyvaskyla, Department of Chemistry, 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 014 Jyväskylä, Finland. 014 Jyväskylä, Finland. |
| This journal is © The Royal Society of Chemistry 2024 |