Pure organic TPB single crystal for direct X-ray detection
Kai
Jiang
ab,
Lingyan
Xu
 *ab,
Rongjin
Shang
ab,
Lu
Liang
ab,
Yingming
Wang
ab,
Zhentao
Qin
ab and
Wanqi
Jie
ab
*ab,
Rongjin
Shang
ab,
Lu
Liang
ab,
Yingming
Wang
ab,
Zhentao
Qin
ab and
Wanqi
Jie
ab
aState Key Laboratory of Solidification Processing, School of Materials Science and Engineering, Northwestern Polytechnical University, Xi'an 710072, China. E-mail: xulingyan@nwpu.edu.cn
bMIIT Key Laboratory of Radiation Detection Materials and Devices, School of Materials Science and Engineering, Northwestern Polytechnical University, Xi'an 710072, China
First published on 28th March 2024
Abstract
For a long time, single crystals of the organic compound TPB (1,3,5-triphenylbenzene) have been viewed as a scintillator material for indirect X-ray detection. We here attempt to explore their potential for direct detection. A TPB single crystal with a size of up to 20 × 6 × 4 mm3 and without obvious growth steps was obtained using a solvent volatilization method. It has an orthorhombic structure with a high-quality FWHM of as low as 0.059 and preferred grown orientations along (131) and (220). AFM images clarify the two-dimensional nucleation growth mechanism. The fitted bandgap of the as-grown crystal is about 3.65 eV. TPB detectors with vertical and coplanar electrodes with a resistivity of 2.56 × 1013 Ω cm and 6.68 × 1013 Ω cm, respectively, were prepared. The mobilities for electrons (μe) and holes (μh) for the coplanar device were calculated to be 1.12 cm2 V−1 s−1 and 1.02 cm2 V−1 s−1, respectively. Under a 50 kV X-ray beam, the detection limit is as low as 0.35 μGy s−1, and the sensitivity reaches 2.72 μC Gy−1 cm−2. The TPB devices have the ability to detect 241Am@5.49 MeV α particles, showing the full-energy peak collected from the holes with an energy resolution of 39.13% at 700 V. The mobility lifetime product of the TPB devices for electrons (μτ)e and holes (μτ)h were calculated to be up to 2.24 × 10−5 cm2 V−1 and 4.04 × 10−5 cm2 V−1, respectively. The TPB detector could present a spectral response to 241Am@59.5 keV γ-rays from the holes with an energy resolution of 44.25% at 1000 V, which shows the potential for both direct and indirect detection of high-energy radiation.
Introduction
Photoelectric materials used for ionizing radiation detection can generally be divided into direct detection using semiconductors and indirect detection using scintillators. In direct detection, after ionizing radiation acts on a semiconductor, charge carriers (electron–hole pairs) are generated inside the semiconductor, and these carriers are directly received by the circuit as electrical signals.1In recent years, organic single crystals have been widely studied as photoelectric materials for ionizing radiation detectors due to their advantages of low cost, large-size production and potential in flexible optoelectronic devices.2,3 Most organic photoelectric materials are mainly composed of C, H and O. In view of this, organic photoelectric materials are considered to be equivalent to human tissue in medical treatment (Z ≈ 7). The density of organic photoelectric materials can be used to simulate the distribution of doses in the human body in the medical field.4–6
As semiconductors, organic single crystals are mainly composed of hydrogen atoms (H), so they have great advantages in converting fast neutrons into recoil protons. Organic single crystals are expected to achieve direct fast neutron detection.7–9 As scintillators, the cheap production cost and fast decay time (a few ns) of organic single crystals provide more possibilities for their application.10
Common organic single crystal semiconductors include rubrene,11 4-hydroxycyanobenzene (4HCB),12,13 1,5-dinitronaphthalene (DNN),11 1,8-naphthaleneimide (NTI),12 and TIPS-pentacene.14 Among them, 4HCB single crystals show the most outstanding performance. Zhao et al.15 successfully achieved the growth of a large-size 4HCB single crystal and measured its sensitivity to be up to 20 μC Gy−1 cm−2. Its mobility reaches 3.4 cm2 V−1 s−1. The mobility–lifetime product is 8.5 × 10−5 cm2 V−1. Xu et al.16 successfully made 4HCB into thin films, and further improved its sensitivity, reaching 93.9 μC Gy−1 cm−2. Common organic single crystal scintillators include anthracene, trans-stilbene (TSB), p-terphenyl, 9,10-diphenylanthracene (DPA), ammonium salicylate, and 1,3,5-triphenylbenzene (TPB).17,18 Among them, the performance of TSB is the most excellent.19 It has a decay time of up to 4 ns and an extremely high light yield (∼20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photons per MeV). The FOM (figure of merit) value of the PSD (pulse shape discrimination) performance for the TSB single crystal can reach 2.2.20
000 photons per MeV). The FOM (figure of merit) value of the PSD (pulse shape discrimination) performance for the TSB single crystal can reach 2.2.20
For a long time, TPB crystals have been used as an organic scintillator. Hull et al. prepared TPB single crystals with a FOM value of ∼1.6 using toluene as a solvent and the slow volatilization solvent method in 2009.20 Zaitseva et al. prepared TPB single crystals using the slow cooling method in 2011 and found that TPB also exhibits good nonlinear and scintillation properties in nonlinear optics and high-energy neutron detection applications.21 Durairaj et al. prepared TPB cylindrical single crystals with a height of 40 mm and diameter of 30 mm using tetrahydrofuran as a solvent by slow cooling solution growth in 2016.22 The cut-off wavelength of the UV-vis spectrum is ∼300 nm, and the fluorescence spectrum indicates a high-intensity emission peak at ∼360 nm.
In this paper, a TPB crystal is used as an organic semiconductor for the first time. A growth method using constant temperature volatilization is proposed to obtain high-quality and large-size TPB single crystals. Radiation detection devices based on TPB single crystals were fabricated to explore its direct detection ability.
Experimental
1,3,5-Triphenylbenzene (>99.0%), ethyl acetate (≥99.0%), ethanol (>99.8%) and dimethyl sulfoxide (>99.8%) were purchased from Aladdin. The raw materials were recrystallized at least three times to improve the purity. 0.2–1 g TPB was dissolved in a 10–50 ml mixed solution, which was then transferred to a 100 ml beaker and volatilized into the as-grown TPB crystal. Single-crystal XRD and powder XRD analyses were conducted using a BRUKER D8 QUEST (Germany) with Cu Kα1 (λ = 1.5406 Å) at a voltage of 40 kV and current of 40 mA. The scanning angle range was set as 5–50°, and the scanning rate selected was 5° min−1. The XRD rocking curve was tested using a BRUKER D8 ADVANCE (Germany). The TPB crystal surface topography was observed using a Nikon LV-100ND microscope and atomic force microscopy (AFM). UV-vis-NIR measurements were carried out using a Shimadzu UV-3150 in the wavelength range of 200–800 nm. Photocurrent–voltage (I–V) and photocurrent–time (I–t) tests were carried out using a Keithley 6517B as a stabilized bias supply. Radiation detection devices were fabricated using as-grown TPB single crystals with an Au electrode of 100 nm thickness. α particles were produced by an uncalibrated 241Am source with an energy of about 5.49 MeV, and the γ-rays were from an 241Am source with an energy of about 59.5 keV.Results and discussion
Fig. 1(a) depicts the setup for crystal growth via the solution volatilization method. An as-grown TPB single crystal with dimensions of about 20 × 6 × 4 mm3 was obtained without obvious growth steps on the surfaces, as shown in Fig. 1(b). In Fig. 1(c), the single-crystal XRD analysis of TPB shows that the as-grown crystal has an orthorhombic structure, and the spatial group is Pna21. The lattice parameters of the cell are a = 7.4657 Å, b = 19.7081 Å, c = 11.2080 Å, and α = β = γ = 90°, which agree with the reported data.23 The diffraction pattern of the TPB single crystal shows sharp and clear diffraction peaks, as shown in Fig. 1(d), wherein the (131) and (220) peaks dominated. These two orientations are preferred for TPB crystal growth. The single-crystal rocking curve of the as-grown TPB single crystal is shown in Fig. 1(e), and the full width at half maximum (FWHM) is as low as 0.059, which proves that the crystal quality is very high.As shown in Fig. 2(a), transverse and longitudinal multi-layer steps accompanied by small pits were observed on the surface topography of the TPB crystal grown using the ordinary solution method. After process optimization by reducing the volatilization temperature, the crystal surface was flat without obvious steps, as shown in Fig. 2(b). Thus, a TPB single crystal with a good surface morphology can be directly obtained through crystal growth without surface grinding or polishing treatment. The AFM topography in Fig. 2(c) shows the microscopic surface of the TPB single crystal. From the two-dimensional (2D) and three-dimensional (3D) surface topography images, it can be seen that the surface growth state of the crystal presents a dense stepped shape, and the width of the steps at the same height is roughly the same. The height of the step is about 1 nm, which is in good agreement with the value measured using single-crystal XRD. It can also be inferred that the surface of the TPB crystal, whether macroscopic or microscopic, proves the step-like 2D nucleation growth mechanism. The growth solutions used were p-xylene, ethanol, etc., and the volatilization rate is very fast. It is easy to achieve the high supersaturation necessary for the 2D nucleation growth. Fig. 2(d) illustrates the relationship between the supersaturation ratio and crystal growth rate. When the supersaturation reaches the critical value β*, the solution will suddenly develop 2D nucleation and then growth will occur. Fig. 2(e) presents a schematic model of the 2D stepped nucleation growth of TPB in solution. As illustrated in Fig. 2(f), small TPB molecules first form J-dimers in a “hand-in-hand” manner in the plane direction, and then pile up in the vertical direction to form H-dimers in a “face-to-face” manner.24
Lower cut-off wavelength and higher optical transparency of the crystal are important factors for device fabrication in optical applications.25 As shown in Fig. 3(a), the TPB single crystal has a high transmittance of up to 85% in the wavelength range of 200–800 nm, which proves that there are very few internal defects in the as-grown crystal. The optical absorption edge is 340 nm. The optical band-gap width fitted using the Tauc Plot method for the TPB crystal is 3.65 eV, as shown in Fig. 3(b). Schematic diagrams of the two different structures for vertical and coplanar devices fabricated from the as-grown TPB single crystals are shown in the insets of Fig. 3(c) and (d), respectively. I–V curves for the two structures were measured, and the two resistivities were fitted to be 2.56 × 1013 Ω cm and 6.68 × 1013 Ω cm, respectively, as shown in Fig. 3(c) and (d). The TPB crystals have high resistivity, which is a significant advantage in reducing the dark current of the TPB devices. Despite the anisotropy in the crystal structure of the TPB single crystal, anisotropy in electrical conductivity of TPB device is not obvious. We selected the TPB detector with a coplanar electrode structure for the following study.
In order to reduce the ionizing radiation damage of X-ray imaging in medical applications, it is necessary to minimize the radiation exposure dose. In general, the sensitivity (S) and the signal-to-noise ratio (SNR) are calculated as follows:
 | (1) |
 | (2) |
As shown in Fig. 4(a), with increasing applied bias, the photocurrent of the TPB device with a coplanar electrode structure at different dose rates first increases linearly and then tends to saturation. As shown in Fig. 4(b), within the voltage range of 30–300 V, the photocurrent of the TPB device decreases with decreasing dose rate (337–4167 μGy s−1) of the incident X-ray radiation. The photocurrent is stable in the off states, and there is an initial burst at high dose rates, which is related to the capacitive-properties-induced relaxation phenomenon. The overall fast response also proves the excellent detection performance of TPB devices, which can sensitively capture and adjust the dose rate transient changes caused by the X-ray source.26 As shown in Fig. 4(c), when a voltage of 20 V is applied and a Cu tablet is placed at the nozzle of the X-ray source, the SNR value of the TPB device is as high as 10.4 at a dose rate of 0.35 μGy s−1, which is greater than the required value (3). The detection limit is lower than 0.35 μGy s−1, which is much lower than the minimum detection standard value (5.5 μGy s−1) required for practical medical imaging applications.27 The photocurrent density–dose rate curves of the TPB device at various applied biases are shown in the inset of Fig. 4(d). The sensitivities at the various applied biases were calculated and are shown in Fig. 4(d). The maximum value is 2.72 μC Gy−1 cm−2. As shown in Fig. 4(e), the SNR value of the TPB device shows a slow downward trend with increasing bias voltage. The overall SNR is always higher than 3, which proves that the TPB detector fabricated from the as-grown TPB crystal has excellent reliability. Fig. 4(f) shows the repeatable stability of the TPB detector. After repeating the conversion between ON and OFF for 600 seconds, there is no drift change in the photocurrent value, which proves its good stability. Additionally, the transition is very fast with no relaxation.
Fig. 5(a) is a schematic diagram of the principle of the α particle detection spectroscopy. α particles incident on the crystal surface (anode) ionize the organic semiconductor, producing an equal amount of electron–hole pairs. Under the influence of an applied bias, electrons recombine rapidly at the anode, and holes migrate to the cathode for signal collection. The average transit time (tf, 10–90% of full amplitude) of 1000 signals for electrons and holes in the coplanar TPB device was measured. Fig. 5(b) and (c) show the time of flight for electrons and holes as a function of the bias voltage, respectively. As the voltage increases, the drift time gradually decreases and the velocity increases. For the photo-generated carriers, the mobility μ is expressed by
 | (3) |
The full-energy peaks of the α particle detection spectrum collected from the electrons and holes were resolved at different bias voltages at room temperature, as shown in Fig. 6(a) and (b), respectively. As the bias voltage increases, the peak centroid shifts towards higher channel number, showing effective charge collection. The centroid channel is proportional to the charge collection efficiency. At high voltage, the peak centroid tends to saturation, and its behavior conforms to the Hecht plot function:28
 | (4) |
Fig. 7(a) is a schematic diagram of the principle of γ-ray detection spectroscopy. The energy of the incident γ-rays is absorbed by the outer electrons, leading to the excitation of electron–hole pairs. Under the action of a sufficiently high electric field, the electrons and holes drift to opposite poles, which in turn induces the formation of charge pulses for signal collection. Compared to α particle detection, γ-rays generally have relatively lower energy and longer penetration depth, requiring a larger device thickness to ensure adequate absorption efficiency. Fig. 6(b) and (c) show the spectral responses of the TPB devices for γ-ray detection collected from electrons at 1300 V and holes at 1000 V, respectively, with the latter exhibiting an energy resolution of up to 44.25%. It could be concluded that the TPB devices fabricated from TPB single crystals have the potential to directly detect α particles, γ-rays and other high-energy radiation.
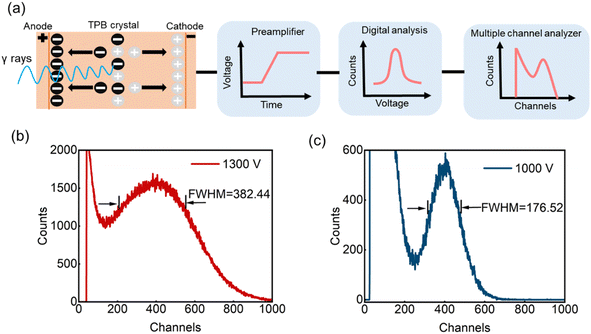 | ||
| Fig. 7 γ-Ray detection of TPB detectors. (a) Schematic diagram of the principle of γ-ray detection. Pulse height spectra collected from (b) electrons at 1300 V and (c) holes at 1000 V. | ||
Conclusions
In this work, pure organic TPB single crystals without obvious growth steps were obtained by the solution method, and their size reached 20 × 6 × 4 mm3. AFM topography images clarified the step-like two-dimensional nucleation growth mechanism. The two different structures for vertical and coplanar devices fabricated from the as-grown TPB single crystals show a high resistivity of about 2.56 × 1013 Ω cm and 6.68 × 1013 Ω cm, respectively. The TPB device has a detection limit lower than 0.35 μGy s−1, and the maximum sensitivity can reach 2.72 μC Gy−1 cm−2. The electron and hole mobilities of the TPB device with a coplanar electrode structure were fitted to be 1.02 cm2 V−1 s−1 and 1.12 cm2 V−1 s−1, respectively. The mobility lifetime products for electrons and holes were fitted to be 2.24 × 10−5 cm2 V−1 and 4.04 × 10−5 cm2 V−1, respectively. The energy resolutions of the photo-peaks collected from electrons and holes were calculated to be 41.2% and 39.13%, respectively. The spectral response of the TPB device for γ-ray detection collected from holes had an energy resolution of up to 44.25% at 1000 V, exhibiting the ability to directly detect 241Am@59.5 keV γ-rays. Overall, this work has demonstrated that TPB crystals are the first pure organic single crystals feasible for both the direct and indirect detection of high-energy radiation. TPB single crystals have the advantages of low cost, easy growth to a large size, and high detection performance, among others. Moreover, these results and analyses will significantly advance the development of organic single-crystal materials and their applications in various fields in the future. To further enhance the detection performance of TPB devices, the possibility of incorporating doping or substituting functional groups could be explored for optimization of crystal growth and detector fabrication. This could lead to improved sensitivity, efficiency and overall performance of TPB detectors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Key Research and Development Program of China (2023YFF0719501, 2023YFE0206300), the National Natural Science Foundation of China (No. 52372015), the Natural Science Basic Research Program of Shaanxi (No. 2022JQ-411), the Heavy Ion Research Facility in Lanzhou (HIRFL), the Key Research and Development Program of Shaanxi (No. 2023-LL-QY-32), and the ND Basic Research Funds (G2022WD).References
- H. Wei and J. Huang, Nat. Commun., 2019, 10, 1066 CrossRef PubMed.
- H. Ling, S. Liu, Z. Zheng and F. Yan, Small Methods, 2018, 2, 1800070 CrossRef.
- Y. Wang, L. Sun, C. Wang, F. Yang, X. Ren, X. Zhang, H. Dong and W. Hu, Chem. Soc. Rev., 2019, 48, 1492–1530 RSC.
- M. Bruzzi, M. Bucciolini, F. Nava, S. Pini and S. Russo, Nucl. Instrum. Methods Phys. Res., Sect. A, 2002, 485, 172–177 CrossRef CAS.
- A. Intaniwet, J. L. Keddie, M. Shkunov and P. J. Sellin, Org. Electron., 2011, 12, 1903–1908 CrossRef CAS.
- L. Basiricò, A. Ciavatti, I. Fratelli, D. Dreossi, G. Tromba, S. Lai, P. Cosseddu, A. Bonfiglio, F. Mariotti, C. Dalla Val, V. Bellucci, J. E. Anthony and B. Fraboni, Front. Phys., 2020, 8, 13 CrossRef.
- A. Pietropaolo, M. Angelone, R. Bedogni, N. Colonna, A. J. Hurd, A. Khaplanov, F. Murtas, M. Pillon, F. Piscitelli, E. M. Schooneveld and K. Zeitelhack, Phys. Rep., 2020, 875, 1–65 CrossRef CAS.
- J. R. Dunning, G. B. Pegram, G. A. Fink and D. P. Mitchell, Phys. Rev., 1935, 48, 265–280 CrossRef CAS.
- S. V. Budakovsky, N. Z. Galunov, N. L. Karavaeva, J. K. Kim, Y. K. Kim, O. A. Tarasenko and E. V. Martynenko, IEEE Trans. Nucl. Sci., 2007, 54, 2734–2740 CAS.
- A. Metcalfe, G. R. Fern, P. R. Hobson, T. Ireland, A. Salimian, J. Silver, D. R. Smith, G. Lefeuvre and R. Saenger, Nucl. Instrum. Methods Phys. Res., Sect. A, 2017, 845, 128–131 CrossRef CAS.
- B. Fraboni, A. Ciavatti, L. Basiricò and A. Fraleoni-Morgera, Faraday Discuss., 2014, 174, 219–234 RSC.
- B. Fraboni, A. Ciavatti, F. Merlo, L. Pasquini, A. Cavallini, A. Quaranta, A. Bonfiglio and A. Fraleoni-Morgera, Adv. Mater., 2012, 24, 2289–2293 CrossRef CAS PubMed.
- A. Ciavatti, E. Capria, A. Fraleoni-Morgera, G. Tromba, D. Dreossi, P. J. Sellin, P. Cosseddu, A. Bonfiglio and B. Fraboni, Adv. Mater., 2015, 27, 7213–7220 CrossRef CAS PubMed.
- G. Pipan, M. Bogar, A. Ciavatti, L. Basiricò, T. Cramer, B. Fraboni and A. Fraleoni-Morgera, Adv. Mater. Interfaces, 2018, 5, 1700925 CrossRef.
- D. Zhao, M. Xu, B. Xiao, B. Zhang, L. Yan, G. Zeng, A. Dubois, P. Sellin, W. Jie and Y. Xu, J. Mater. Chem. A, 2020, 8, 5217–5226 RSC.
- M. Xu, M. Zhu, D. Zhao, S. Chen, S. Liu, Q. Zhang, P. Yuan, B. Zhang, P. Sellin, W. Jie and Y. Xu, J. Mater. Sci. Technol., 2023, 135, 46–53 CrossRef CAS.
- S. Kalainathan, N. Durairaj and R. Kumar, Int. J. Soc. Mater. Eng. Resour., 2018, 23, 64–67 CrossRef CAS.
- N. Durairaj, S. Kalainathan and S. M. Babu, in Photonic Crystal and Its Applications for Next Generation Systems, ed. S. S. Dhanabalan, A. Thirumurugan, R. Raju, S.-K. Kamaraj and S. Thirumaran, Springer Nature Singapore, Singapore, 2023, pp. 71–90 Search PubMed.
- M. Koshimizu, Jpn. J. Appl. Phys., 2023, 62, 010503 CrossRef CAS.
- G. Hull, N. P. Zaitseva, N. J. Cherepy, J. R. Newby, W. Stoeffl and S. A. Payne, IEEE Trans. Nucl. Sci., 2009, 56, 899–903 CAS.
- N. Zaitseva, L. Carman, A. Glenn, J. Newby, M. Faust, S. Hamel, N. Cherepy and S. Payne, J. Cryst. Growth, 2011, 314, 163–170 CrossRef CAS.
- N. Durairaj, S. Kalainathan and M. V. Krishnaiah, Mater. Chem. Phys., 2016, 181, 529–537 CrossRef CAS.
- K. Lonsdale, Nature, 1934, 133, 67–67 CrossRef CAS.
- M. Hara, T. Takeshita, H. Kurata and T. Kimura, J. Fluoresc., 2023, 33, 1559–1563 CrossRef CAS PubMed.
- A. Krishna, N. Vijayan, C. Bagdia, K. Thukral, S. Sonia, D. Haranath, K. K. Maurya and G. Bhagavannarayana, CrystEngComm, 2016, 18, 4844–4850 RSC.
- Q. Sun, X. Yan, S. Chen, J. Yuan, J. Li, Q. Luo, T. Jiang, Z. Gao, H. Wang, M. Yuan, D. Ji, F. Yang, X. Ren, X. Zhang and W. Hu, Mater. Today, 2023, 66, 105–113 CrossRef CAS.
- I. Clairand, J.-M. Bordy, J. Daures, J. Debroas, M. Denoziere, L. Donadille, M. Ginjaume, C. Itie, C. Koukorava, S. Krim, A.-L. Lebacq, P. Martin, L. Struelens, M. Sans-Merce, M. Tosic and F. Vanhavere, Radiat. Prot. Dosim., 2011, 144, 453–458 CrossRef CAS PubMed.
- J. Kočka, C. E. Nebel and C. D. Abel, Philos. Mag. B, 1991, 63, 221–246 Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |






