Open Access Article
Open Access ArticleCd(II) and Zn(II) coordination polymer-assisted CO2/cyclohexene oxide copolymerization with a double metal cyanide catalyst†
Zi-Qing
Huang
a,
Jia-Qi
Chen
a,
Xiao-Yu
Zhang
a,
Cheng-Kai
Yuan
a,
Peng
Wang
a,
Yue
Zhao
 a,
Bao-Cheng
Zhao
*b,
Wei-Xin
Qi
*b and
Wei-Yin
Sun
a,
Bao-Cheng
Zhao
*b,
Wei-Xin
Qi
*b and
Wei-Yin
Sun
 *a
*a
aCoordination Chemistry Institute, State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing National Laboratory of Microstructures, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing 210023, China. E-mail: sunwy@nju.edu.cn
bHuaian Bud Polyurethane Science & Technology Co., Ltd., Huaian 223100, China. E-mail: zbc@budpu.com; qwx@budpu.com
First published on 14th February 2024
Abstract
It is attractive but challenging to develop a cocatalyst for CO2/cyclohexene oxide (CHO) copolymerization with a double metal cyanide (DMC) catalyst. In this study, we synthesized three coordination polymers (CPs) [Cd(3N3PY)(NDC)]·0.58H2O (1), [Zn(3N3PY)(IPA)]·3H2O (2) and [Cd(3N3PY)(NPA)]·2DMF·2H2O (3) (3N3PY = 4′-(4-(1H-1,2,4-triazol-1-yl)phenyl)-2,2′:6′,2′′-terpyridine, H2NDC = 2,6-naphthalene dicarboxylic acid, H2IPA = isophthalic acid, H2NPA = 2,2′-dinitro-4,4′-biphenyldicarboxylic acid). Crystal structural analyses show that 1–3 are different one-dimensional (1D) chains that further assemble into three-dimensional (3D) supramolecular structures by π–π and hydrogen bonding interactions. CPs 1–3 were applied as cocatalysts to assist DMC for CO2/CHO copolymerization and the efficient proportion of carbonate P–OC(O)O– in the product is 59% achieved for 2 at 80 °C in 1 MPa CO2. The proportion of carbonate F(CO2) and conversion rate of CHO (CCHO) are 63% and 93%, respectively. The mechanistic study on structure–activity relationship and chemoselectivity was performed.
Introduction
Excessive emission of carbon dioxide (CO2) is one of the main reasons for the greenhouse effect, which is hazardous to the environment and human health.1 Thus, it is meaningful to develop valid methods to consume the excessive CO2 in the atmosphere. In fact, CO2 is a kind of resource-rich, renewable and non-toxic carbon source.2 As reported previously, the ring opening reaction of epoxides with CO2 is an efficient and atom-economic way for chemically fixing CO2.3 As a result, it is attractive to create effective catalysts and explore valid methods to control the selectivity of products.4In 2020, Hu and Zhang's group synthesized an Al-salen catalyst in cooperation with an ionic liquid to convert CO2 into cyclic carbonate.5 The highest yield is 92% with a wide scope of substrates at 25 °C and 1 MPa CO2. In 2018, Wang's lab reported Al-porphyrin catalysts for copolymerization of CO2 and propylene oxide (PO).6 At 80 °C and 4 MPa CO2, the proportion of carbonate, defined as F(CO2), in the produced polymer is 53.1% in the obtained PO–CO2 polyols and the conversion rate of PO is 99%. Recently, the same group studied the copolymerization of CO2 with cyclohexene oxide (CHO) heterogeneously catalyzed by double metal cyanide (DMC) at 80 °C and 3 MPa CO2.7 As a result, the F(CO2) value is 96% and the conversion rate of CHO is 99%. In the last decades, DMC was found to be efficient for binary and ternary copolymerization with advantages of simple preparation, low synthetic cost and insensitivity to water and air.8,9 Recyclable aliphatic polycarbonate products have demands in wide applications like environmental protection, adhesives, electronic devices, biomedical materials and automobile accessories.10,11 In the reported studies, cocatalysts of DMC to enrich the function of products and optimize the reaction conditions are seldom explored.
Owing to the open metal and bifunctional acid–base sites, coordination polymers (CPs) are considered to be desirable for catalysis by forming noncovalent interactions with organic substrates, such as ionic, cation–π, anion–π, lone pair–π, π–π stacking, etc.12,13 Furthermore, in the epoxide and CO2 atmosphere, CPs are considered to form metal carbonate species, which is derived from control experiments by separately catalysing the CHO/CO2 reaction with only heat or addition of bis(triphenylphosphine)iminium chloride (PPNCl) or tetrabutylammonium bromide (NBu4Cl) as a cocatalyst at 80 °C. Besides, the detailed kinetic studies were also conducted and analyzed.14
Based on the above-mentioned situation, in this work, we designed and synthesized three CPs with terpyridyl ligand 4′-(4-(1H-1,2,4-triazol-1-yl)phenyl)-2,2′:6′,2′′-terpyridine (3N3PY), namely [Cd(3N3PY)(NDC)]·0.58H2O (1), [Zn(3N3PY)(IPA)]·3H2O (2) and [Cd(3N3PY)(NPA)]·2DMF·2H2O (3) (H2NDC = 2,6-naphthalene dicarboxylic acid, H2IPA = isophthalic acid, H2NPA = 2,2′-dinitro-4,4′-biphenyldicarboxylic acid). They were applied as cocatalysts for CO2/CHO copolymerization with DMC. It was found that the efficient carbonate proportion was achieved with the assistance of CPs and the mechanism was also explored.
Experimental
Materials and general methods
All commercially available solvents and chemicals are of reagent grade and were used directly without further purification. DMC solvent with the formula Zn3[Co(CN)6]2·ZnCl2·solvent is a commercial product of Huaian Bud Polyurethane Science & Technology Co., Ltd. Ligand 3N3PY was prepared according to the previously reported method.15–17 Powder X-ray diffraction (PXRD) data were collected on a Bruker D8 Advance X-ray diffractometer with Cu Kα (λ = 1.5418 Å) radiation. Thermogravimetric analysis (TGA) was carried out on a Mettler-Toledo (TGA/DSC1) thermal analyzer with a heating rate of 10 °C min−1 under a N2 atmosphere. FTIR-ATR spectra were measured on a Bruker Tensor II infrared spectrophotometer equipped with a diamond ATR module in the range of 400–4000 cm−1. Elemental analyses for C, H and N were performed on an Elementar Vario MICRO elemental analyzer. 1H NMR spectra were recorded on a Bruker-DRX instrument (500 MHz).Synthesis of [Cd(3N3PY)(NDC)]·0.58H2O (1)
A mixture of 3N3PY (18.82 mg, 0.05 mmol), H2NDC (10.81 mg, 0.05 mmol) and Cd(NO3)2·4H2O (15.40 mg, 0.05 mmol) in a mixed solvent of DMF/H2O (2 mL, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 3
v = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was charged into a 10 mL glass vial, and then heated at 110 °C for 72 h under solvothermal conditions. The yield of the obtained crystals was 25% after cooling down to ambient temperature. FTIR-ATR (cm−1, Fig. S1†):
1) was charged into a 10 mL glass vial, and then heated at 110 °C for 72 h under solvothermal conditions. The yield of the obtained crystals was 25% after cooling down to ambient temperature. FTIR-ATR (cm−1, Fig. S1†):![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3658 (w),
3658 (w),![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3099 (w), 2983 (w), 2897 (w), 1662 (w), 1606 (m), 1551 (m), 1470 (w), 1400 (s), 1350 (m), 1284 (w), 1239 (w), 1143 (w), 1088 (w), 1052 (w), 1011 (w), 979 (w), 915 (w), 841 (w), 787 (s), 734 (m), 670 (m), 627 (m), 520 (w), 488 (w), 446 (m).
3099 (w), 2983 (w), 2897 (w), 1662 (w), 1606 (m), 1551 (m), 1470 (w), 1400 (s), 1350 (m), 1284 (w), 1239 (w), 1143 (w), 1088 (w), 1052 (w), 1011 (w), 979 (w), 915 (w), 841 (w), 787 (s), 734 (m), 670 (m), 627 (m), 520 (w), 488 (w), 446 (m).
Synthesis of [Zn(3N3PY)(IPA)]·3H2O (2)
Zn(NO3)2·6H2O (14.88 mg, 0.05 mmol), 3N3PY (18.82 mg, 0.05 mmol), H2IPA (8.31 mg, 0.05 mmol) and 2 mL DMF/H2O (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 3
v = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixed solvent were added into a 10 mL glass vial. Then, it was heated at 110 °C for 72 h. After cooling to room temperature, colorless crystals were obtained with a yield of 68%. FTIR-ATR (cm−1, Fig. S1†): 3483 (w), 3062 (w), 1676 (w), 1609 (s), 1554 (m), 1530 (m), 1473 (m), 1405 (m), 1337 (s), 1147 (w), 1085 (w), 1011 (w), 977 (w), 834 (w), 793 (m), 749 (m), 725 (m), 660 (w), 569 (w), 521 (w), 420 (w).
1) mixed solvent were added into a 10 mL glass vial. Then, it was heated at 110 °C for 72 h. After cooling to room temperature, colorless crystals were obtained with a yield of 68%. FTIR-ATR (cm−1, Fig. S1†): 3483 (w), 3062 (w), 1676 (w), 1609 (s), 1554 (m), 1530 (m), 1473 (m), 1405 (m), 1337 (s), 1147 (w), 1085 (w), 1011 (w), 977 (w), 834 (w), 793 (m), 749 (m), 725 (m), 660 (w), 569 (w), 521 (w), 420 (w).
Synthesis of [Cd(3N3PY)(NPA)]·2DMF·2H2O (3)
A mixture of Cd(NO3)2·4H2O (15.40 mg, 0.05 mmol), 3N3PY (18.82 mg, 0.05 mmol), H2NPA (16.61 mg, 0.05 mmol) and 2 mL DMF/H2O (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 3
v = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixed solvent in a 10 mL glass vial was heated at 110 °C for 72 h. After cooling to room temperature, yellow block crystals were obtained with a yield of 58%. FTIR-ATR (cm−1, Fig. S1†): 3427 (w), 3104 (w), 2923 (w), 2862 (w), 1666 (s), 1597 (s), 1526 (s), 1481 (m), 1390 (s), 1345 (s), 1279 (m), 1240 (m), 1153 (w), 1086 (m), 1007 (m), 970 (m), 916 (m), 835 (m), 785 (s), 724 (m), 669 (m), 512 (m), 482 (w), 421 (m).
1) mixed solvent in a 10 mL glass vial was heated at 110 °C for 72 h. After cooling to room temperature, yellow block crystals were obtained with a yield of 58%. FTIR-ATR (cm−1, Fig. S1†): 3427 (w), 3104 (w), 2923 (w), 2862 (w), 1666 (s), 1597 (s), 1526 (s), 1481 (m), 1390 (s), 1345 (s), 1279 (m), 1240 (m), 1153 (w), 1086 (m), 1007 (m), 970 (m), 916 (m), 835 (m), 785 (s), 724 (m), 669 (m), 512 (m), 482 (w), 421 (m).
Synthesis of methyl iodized CPs 1Me–3Me
The methyl iodized CPs were prepared with modification of the previously reported method.18 CP 1 (20 mg, 0.028 mmol)/2 (20 mg, 0.030 mmol)/3 (20 mg, 0.020 mmol), 4 mL DMF and 48 μL (0.77 mmol) methyl iodide (CH3I) were added into a glass vial. Then, the mixture was heated at 70 °C for 12 h. After cooling, the obtained product was added to 15 mL ethyl acetate and the precipitate was collected by filtration. After washing with diethyl ether and vacuum drying, the final products 1Me/2Me/3Me were obtained.Catalytic copolymerization of the CO2/CHO reaction with DMC and 1–3
5 mg DMC, 3.5 μmol activated CP obtained by heating at 230 °C in vacuum for 12 h and 0.5 mL CHO were added into a Teflon-lined stainless steel container. Then, CO2 gas was charged and pressurized at 1 MPa. The sealed reactor was heated at 80 °C using an oil bath for 12 h. After cooling to room temperature, CO2 gas was slowly released and the obtained product was determined by 1H NMR in CDCl3.Results and discussion
Crystal structure description
| Compound | 1 | 2 | 3 |
|---|---|---|---|
| a R 1 = ∑||Fo| − |Fc||/∑|Fo|. b wR2 = |∑w(|Fo|2 − |Fc|2)|/∑|w(Fo)2|1/2, where w = 1/[σ2(Fo2) + (aP)2 + bP]. P = (Fo2 + 2Fc2)/3. | |||
| Formula | C35H23.17N6O4.58Cd | C31H26N6O7Zn | C43H40N10O12Cd |
| Formula weight | 713.48 | 659.95 | 1001.25 |
| Temperature (K) | 296.15 | 193 | 193 |
| Crystal system | Monoclinic | Triclinic | Tetragonal |
| Space group | P21/c |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P41212 |
| a (Å) | 10.6395(3) | 8.8685(9) | 18.1361(9) |
| b (Å) | 28.9664(8) | 10.2782(11) | 18.1361(9) |
| c (Å) | 10.5656(4) | 16.752(2) | 26.421(2) |
| α (°) | 90 | 95.095(7) | 90 |
| β (°) | 114.3410(10) | 101.142(6) | 90 |
| γ (°) | 90 | 110.208(5) | 90 |
| V (Å3) | 2966.74(16) | 1385.8(3) | 8690.2(11) |
| Z | 4 | 2 | 8 |
| Dc (g cm−3) | 1.597 | 1.582 | 1.531 |
| μ (mm−1) | 0.791 | 1.153 | 3.154 |
| F(000) | 1439 | 680 | 4096 |
| Data collected | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 532 532 |
4858 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 863 863 |
| Unique reflections | 5491 | 4858 | 7974 |
| Goodness-of-fit | 1.060 | 1.041 | 1.034 |
| R 1 [I > 2σ(I)] | 0.0279 | 0.0967 | 0.0314 |
| wR2b [I > 2σ(I)] | 0.0646 | 0.2581 | 0.0795 |
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) in the triclinic crystal system, which is distinct from that of 1 (Table 1). The asymmetric unit of 2 consists of one Zn(II), one 3N3PY and one IPA2−. Zn(II) exhibits a five-coordinated environment and is surrounded by three nitrogen atoms N1, N2 and N3 from one 3N3PY and two oxygen atoms O3 and O1A [symmetry code: (x, −1 + y, z)] from two different IPA2− (Fig. 2a) with normal Zn–N and Zn–O bond distances (Table S1†). Each IPA2− coordinates with two Zn(II) atoms to form 1D chains (Fig. 2b), which are further connected with each other to form a 3D supramolecular structure (Fig. S2b†) by π–π interactions between two neighbouring 3N3PY ligands (Fig. 1c, Tables S2 and S3†) and hydrogen bonds like C(9)–H(9)⋯N(6) (2.44 Å), C(12)–H(12)⋯O(4) (2.25 Å) and C(20)–H(20)⋯O(2) (2.36 Å) (Fig. 1d and Table S4†). It is noteworthy that the 1D chain in 1 stretches as a zigzag-type with terminal 3N3PY ligands bidirectionally arranged (Fig. 1b), while in 2 the 1D chain is linear and the 3N3PY ligands arrange in one direction (Fig. 2b).
in the triclinic crystal system, which is distinct from that of 1 (Table 1). The asymmetric unit of 2 consists of one Zn(II), one 3N3PY and one IPA2−. Zn(II) exhibits a five-coordinated environment and is surrounded by three nitrogen atoms N1, N2 and N3 from one 3N3PY and two oxygen atoms O3 and O1A [symmetry code: (x, −1 + y, z)] from two different IPA2− (Fig. 2a) with normal Zn–N and Zn–O bond distances (Table S1†). Each IPA2− coordinates with two Zn(II) atoms to form 1D chains (Fig. 2b), which are further connected with each other to form a 3D supramolecular structure (Fig. S2b†) by π–π interactions between two neighbouring 3N3PY ligands (Fig. 1c, Tables S2 and S3†) and hydrogen bonds like C(9)–H(9)⋯N(6) (2.44 Å), C(12)–H(12)⋯O(4) (2.25 Å) and C(20)–H(20)⋯O(2) (2.36 Å) (Fig. 1d and Table S4†). It is noteworthy that the 1D chain in 1 stretches as a zigzag-type with terminal 3N3PY ligands bidirectionally arranged (Fig. 1b), while in 2 the 1D chain is linear and the 3N3PY ligands arrange in one direction (Fig. 2b).
Characterization of 1–3
IR spectra of 1–3 and ligands are shown in Fig. S1† and no vibrational bands were observed between 1680 and 1760 cm−1 for the synthesized 1–3, implying the complete deprotonation of the dicarboxylic acid to produce a dicarboxylate ligand coordinating with the metal centers in 1–3. This is in good agreement with the results of crystal structure analysis as mentioned above. The measured PXRD patterns of the as-synthesized 1–3 are consistent with the simulated results from the single crystal structure analysis (Fig. S3†), indicating the good crystallinity and phase purity of the bulky samples 1–3. As shown in Fig. S4,† thermogravimetric (TG) data reflect the thermal stability of 1–3. The weight loss of solvent molecules mainly occurred from 25 to 180 °C with 1.39% for 1 and 8.82% for 2, which are consistent with the theoretical results of 1.46% for 1 and 8.21% for 2. Further, the collapse of the 3D supramolecular framework starts from 390 °C. For 3, the weight decreased by 16.65% in the range of 25–247 °C, which is caused by the loss of DMF and water molecules with a theoretical value of 18.18%. CP 3 maintained stable at 247–308 °C and then the structure began to decompose.Catalytic copolymerization of CO2/CHO by DMC and 1–3
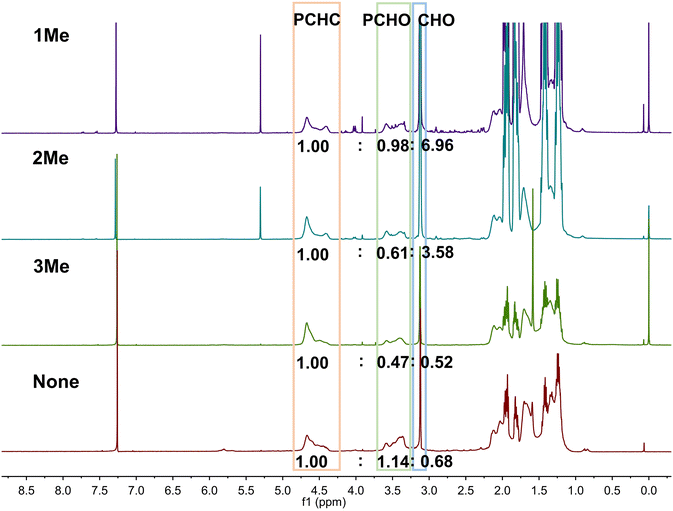
It has been proved that the ring opening reaction of epoxide with CO2 is an effective approach to the chemical fixation of CO2. Accordingly, CPs 1, 2 and 3 were utilized to cocatalyze CO2/CHO copolymerization with DMC. According to the 1H NMR data of the product, the wide signals in 3.26–3.75 and 4.32–4.80 ppm indicate that the copolymers were obtained and CO2 was fixed into the copolymers as a carbonate group. The proportion of carbonate in the obtained copolymer is recorded as the F(CO2) value and CCHO indicates the conversion rate of CHO, while P–OC(O)O– means the proportion of carbonate in the total product, and P–OC(O)O– = F(CO2) × CCHO.In this study, CPs 1, 2, and 3 with Zn(II) and Cd(II) with d10 electron configuration were applied to cocatalyze the CO2/CHO reaction with DMC at 80 °C. The F(CO2) values are 65%, 63% and 60% for CPs 1, 2 and 3, respectively, which are significantly improved comparing with the result of 47% for sole DMC without CPs (Fig. 4 and Table 2). The CCHO values are 90%, 93% and 94% for CPs 1, 2 and 3, respectively, while it was 76% in the absence of cocatalysts 1, 2, and 3. The total carbonate groups existing in the product P–OC(O)O– are 58%, 59% and 56% for CPs 1, 2 and 3, respectively, and these values are obviously improved by comparing with the result of 36% without CPs (Table 2).
| Cocatalyst | F(CO2)/% | C CHO/% | P –OC(O)O–/% |
|---|---|---|---|
| a Reaction conditions: DMC (5 mg), cocatalyst (3.5 μmol), CHO (0.5 mL) at 80 °C, 1 MPa. The results were determined by 1H NMR in CDCl3. | |||
| 1 | 65 | 90 | 58 |
| 2 | 63 | 93 | 59 |
| 3 | 60 | 94 | 56 |
| 1Me | 51 | 22 | 11 |
| 2Me | 62 | 31 | 19 |
| 3Me | 68 | 74 | 50 |
| None | 47 | 76 | 36 |
| Cd(OAc)2 | 35 | 98 | 34 |
| PhCOONa | <1 | <1 | <1 |
| 3N3PY | <1 | <1 | <1 |
Owing to the specific structure of the obtained CPs, the non-coordinated free triazole may influence the ability of adsorbing CO2 in the reaction. In order to further verify this conjecture, the triazole was methylated and the NMR spectra show the complete methylation of the triazole groups in the obtained products 1Me, 2Me and 3Me (Fig. S5–S7†). Then, they were utilized to cocatalyze the CO2/CHO copolymerization. The 1H NMR data of the products are shown in Fig. 5. After the addition of methylated CPs 1Me, 2Me and 3Me, the F(CO2) values in the produced copolymers are 51%, 62% and 68%, respectively (Table 2). Meanwhile, the calculated CCHO values are separately 22%, 31% and 74%, and the P–OC(O)O– values are 11%, 19% and 50% for CPs 1Me, 2Me and 3Me, which obviously decreased by comparing with the corresponding results of CPs 1, 2 and 3 (Table 2). The results imply the role of uncovered free triazoles. Furthermore, CCHO was gradually decreased with the sequence 3Me, 2Me and 1Me. As was reported, the methylated triazole may be able to adsorb CHO as a guest.18 As shown in Fig. S2,† the overall structure (1D chain) may be beneficial to uncovering triazole groups, which may be suitable for adsorbing CO2 during the copolymerization.22 And the carboxylate ligand of the CPs may affect the stacking arrangement of triazole groups in 3N3PY. In CP 1, ligand 3N3PY is almost perpendicular to each other, while the triazole groups in 3N3PY are presented as head-to-head in CP 2. In CP 3, the presence of nitro groups may influence the electron configuration of triazoles by d–π interactions.19
Furthermore, control experiments were carried out to test the significance of assembling the d10 metal ion(II), carboxylate ligand and terpyridine for cocatalyzing the CO2/CHO reaction and the results are summarized in Table 2. When Cd(OAc)2 was used as the cocatalyst, the F(CO2) value for the produced polymer is 35% and CCHO is 98%. However, almost no copolymerization occurred when PhCOONa or 3N3PY was added.
It has been reported that an onium salt with an N-containing functional group is helpful for catalyzing the ring opening reaction of epoxide with CO2, and NBu4Br is the popular one for synthesizing cyclic carbonate (Fig. S8†).20–23 However, in order to fix CO2 into more valuable and stable polycarbonate, PPNCl is more suitable to alter the selectivity of the product (Fig. 6). In the tests of adding CP 1/2/3 and PPNCl, the calculated results of F(CO2) values are separately 71%, 72% and 74%, which are much better than the value of 44% in the absence of CPs (Table 3). Based on the above experimental results, it is speculated that it may result from the combination of the CPs and PPN+ to form an intermediate, which can adsorb CO2 and promote the generation of carbonate. However, as for the conversion rate of CHO, it was 81%, 58% and 49% in the presence of CPs 1, 2 and 3, respectively, and it was 89% for the sample without adding CPs. It is obvious that CP 1 presents a more efficient result for fixing CO2. Owing to similar Lewis acidic electronic effects of DMC, CPs 1–3 and PPN+, competition may occur and hinder the contact between the epoxy group in CHO and catalytic sites on DMC when PPNCl and CPs were added. The comparison with other reported systems is listed in Table S5,† and it presents a preferable result for fixing CO2 into a carboxylate polymer with the assistance of CPs at a low pressure of CO2.
| Cocatalyst | F(CO2)/% | C CHO/% | P –OC(O)O–/% |
|---|---|---|---|
| PPNCl + 1 | 71 | 81 | 58 |
| PPNCl + 2 | 72 | 58 | 42 |
| PPNCl + 3 | 74 | 49 | 36 |
| PPNCl | 44 | 89 | 39 |
Kinetic analysis of the CO2/CHO reaction catalyzed by CPs 1–3 and DMC
As shown above, CPs give superior cocatalytic performance for CO2/CHO copolymerization and 2 is used as an example for kinetic analysis characterized by 1H NMR. It is obvious that in the early stage of the reaction, the concentration of the substrate decreased linearly with time, indicating that the ring opening of CHO was a zero-order reaction. And the reaction rate constants are separately 3.19 × 10−6, 6.94 × 10−6, 1.49 × 10−5, and 1.31 × 10−4 s−1 with different concentrations of the catalyst (DMC and CP 2) at 50, 25, 12.5 and 8.3 mM (Fig. S9a–d†). Subsequently, plotting the logarithm of the observed rate coefficient vs. the logarithm of the catalyst concentration shows a linear fit between 50 and 12.5 mM, indicating a first order in the catalyst concentration (Fig. S9e†). The rate dependence on CO2 pressure was determined by measuring the observed rate coefficient over a range of CO2 pressure from 6 to 10 bar. And the obtained k values are separately 4.70, 6.51, and 3.43 × 10−5 s−1 at the pressures of 10, 8, and 6 bar (Fig. S10a–c†).The plot of the rate coefficient vs. pressure resulted in a slightly curved fit to the data, but without significant rate changes over the range of 6–10 bar, indicating the zero-order of CO2 concentration (Fig. S10d†). Therefore, the reaction proceeded in the first-order rate law: first order in the concentration of the catalyst and zero order in CO2 and single substrate CHO with the rate expression of rate = [CHO]0[CO2]0[Cat]1.Next, the pressure of CO2 was considered to be the characterization of dynamic analysis for calculating the activation energy. As shown in Fig. S11,† the calculated rate constants are 0.413, 1.22, 4.70, and 1.82 × 10−5 s−1 respectively for 313, 333, 353 and 373 K. It is obvious that the reaction rate gradually increased at 313–353 K, but decreased at 373 K, which is probably induced from the entropy reduction of the reaction and the decomposition of the generated carbonate group at high temperature. In Fig. S12a,† a plot of ln(k/T) vs. 1/T was obtained to determine the transition state enthalpy ΔH of 52.90 kJ mol−1 and ΔS of −84.32 J mol−1 K−1 according to the Eyring analysis, i.e. overall, the transition state Gibbs free energy was determined as ΔG = 82.66 kJ mol−1 (80 °C). Besides, the simple activation energy value (Ea) was also determined as 55.66 kJ mol−1 by Arrhenius methods (Fig. S12b†), which may be a preferable method to compare the efficiency of the catalyst for the CO2/CHO reaction.
Proposed catalytic mechanism
Based on the above data, the selectivity of the CO2/CHO reaction is considered. It is proposed that the double metal centers of DMC would like to act as the Lewis acid sites to activate epoxide through M–O coordination. As shown in path a (Fig. 7), if the reaction is only heated at 1 bar CO2 without a cocatalyst, the coordinated oxygen atom will connect with C+ generated at the adjacent metal in DMC and produce polycyclohexyl ether (PCHO). While, CO2 was inclined to insert into the M–O bond with the addition of ionic cocatalyst in 1 MPa CO2, because anion is known to be a good leaving group and always used as initiator.20,21 Therefore, two halide salts with different onium cations separately participated in the CO2/CHO reaction with DMC, and the chemoselectivity is obviously altered, which is supposed to be ascribed to the different electrophilic characters of the cations under the reaction conditions. Firstly, the cyclic carbonate was produced with the assistance of NBu4Cl due to its strong electrophilicity (Fig. 7, path b). In this process, the metal-activated CHO connects with CO2 and the metallic-carbonate active species perform “back-biting” to produce the five-membered ring by the SN2 pathway. And in the process of adding PPNCl with weak electrophilicity, the metallic-carbonate preferred to interact with the epoxide group activated at the adjacent metal in DMC and generated the linear polycarbonate (Fig. 7, path c).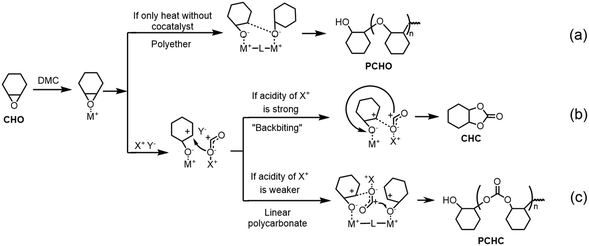 | ||
| Fig. 7 Proposed mechanism of the chemoselectivity for the CO2/CHO reaction. (a) PCHO path. (b) CHC path. (c) PCHC path. | ||
In this work, the electrophilicity of CPs 1–3 may be similar to that of PPN+ applied to fix CO2 into the metallic-carbonate intermediate and produce linear poly(cyclohexene carbonate), which is probably supported with the phenomenon of the low F(CO2) values when competitive CPs and PPNCl exist simultaneously. Besides, it is interesting that according to the superior catalytic result of CP 2, the CP with a Zn(II) ion may be more suitable for fixing CO2 than that with Cd(II) by producing metal–ligand (M–L) active sites due to the high Lewis acidity of Zn(II).24
Conclusions
In this work, three novel coordination polymers were synthesized by using Zn(II) and Cd(II) as the metal centers. The ligands were dicarboxylate and N-donor ligand 3N3PY containing a tripyridine group. Then, the obtained CPs 1–3 can assist DMC for catalyzing CO2/CHO copolymerization. The efficient carbonate proportion was obtained with the assistance of 2, in which the F(CO2) and CCHO values are separately 72% and 58%. By comparing with the results of the catalysis by the sole metal salts and ligands, the efficiency increased after assembling into CPs. Besides, the dynamic analysis of this first-order reaction was also performed and the ΔG value of the transition state was calculated to be 82.66 kJ mol−1 at 80 °C. The simple activation energy value (Ea) was 55.66 kJ mol−1. Furthermore, the mechanism was also tested. It is supposed that the uncovered triazoles in the CPs may be helpful for fixing CO2, and the chemoselectivity of the CO2/CHO reaction is probably related to the electrophilicity of cocatalysts.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (grant no. 22231006 and 22171131). This work was also supported by a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.Notes and references
- J. Liu, G. Yang, Y. Liu, D. Wu, X. Hu and Z. Zhang, Green Chem., 2019, 21, 3834–3838 RSC.
- X. B. Lu and D. J. Darensbourg, Chem. Soc. Rev., 2012, 41, 1462–1484 RSC.
- G. Trott, J. A. Garden and C. K. Williams, Chem. Sci., 2019, 10, 5851–5852 RSC.
- C. Zhuo, H. Cao, H. You, S. Liu, X. Wang and F. Wang, ACS Macro Lett., 2022, 11, 941–947 CrossRef CAS PubMed.
- J. Liu, G. Yang, Y. Liu, D. Zhang, X. Hu and Z. Zhang, Green Chem., 2020, 22, 4509–4515 RSC.
- C. Zhuo, H. Cao, X. Wang, S. Liu and X. Wang, Chin. Chem. Lett., 2023, 34, 108011 CrossRef CAS.
- W. Mo, C. Zhuo, S. Liu, X. Wang and F. Wang, Polym. Chem., 2023, 14, 152–160 RSC.
- I. Kim, M. J. Yi, K. J. Lee, D. W. Park, B. U. Kim and C. S. Ha, Catal. Today, 2006, 111, 292–296 CrossRef CAS.
- A. Peeters, P. Valvekens, R. Ameloot, G. Sankar, C. E. A. Kirschhock and D. E. De Vos, ACS Catal., 2013, 3, 597–607 CrossRef CAS.
- P. Wei, G. A. Bhat and D. J. Darensbourg, Angew. Chem., Int. Ed., 2023, e202307507 CAS.
- S. Z. Islam, D. S. Sholl, J. A. Steckel and R. L. Thompson, Process Saf. Prog., 2023, 42, 371–376 CrossRef CAS.
- Y. S. Kang, Y. Lu, K. Chen, Y. Zhao, P. Wang and W. Y. Sun, Coord. Chem. Rev., 2019, 378, 262–280 CrossRef CAS.
- A. V. Gurbanov, F. I. Guseinov, M. Fátima and C. Guedes da Silva, Coord. Chem. Rev., 2019, 387, 32–46 CrossRef.
- P. K. Saini, C. Romaina and C. K. Williams, Chem. Commun., 2014, 50, 4164–4167 RSC.
- P. Wang, Z. Li, G. C. Lv, H. P. Zhou, C. Hou, W. Y. Sun and Y. P. Tian, Inorg. Chem. Commun., 2012, 18, 87–91 CrossRef CAS.
- P. Wang, L. Luo, T. Okamura, H. P. Zhou, W. Y. Sun and Y. P. Tian, Polyhedron, 2012, 44, 18–27 CrossRef CAS.
- P. Wang, T. Okamura, H. P. Zhou, W. Y. Sun and Y. P. Tian, Chin. Chem. Lett., 2013, 24, 20–22 CrossRef CAS.
- Y. L. Liu, Z. H. Huang, X. X. Tan, Z. Q. Wang and X. Zhang, Chem. Commun., 2013, 49, 5766–5768 RSC.
- M. Kurihara and H. Nishihara, Coord. Chem. Rev., 2002, 226, 125–135 CrossRef CAS.
- Z. Su, M. S. Chen, J. Fan, M. Chen, S. S. Chen, L. Luo and W. Y. Sun, CrystEngComm, 2010, 12, 2040–2043 RSC.
- B. W. Jiang, J. Liu, W. J. Xiong, M. L. Weng, J. G. An, Y. W. Fan, L. Z. Zheng, G. Q. Yang and Z. B. Zhang, Chem. Eng. J., 2023, 476, 146873 CrossRef CAS.
- D. Zhao, X. H. Liu, Z. Z. Shi, C. D. Zhu, Y. Zhao, P. Wang and W. Y. Sun, Dalton Trans., 2016, 45, 14184 RSC.
- C. J. Zhang, S. Q. Wu, S. Boopathi, X. H. Zhang, X. Hong, Y. Gnanou and X. S. Feng, ACS Sustainable Chem. Eng., 2020, 8, 13056–13063 CrossRef CAS.
- F. Guo, R. X. Li, S. Z. Yang, X. Y. Zhang, H. J. Yu, J. J. Urban and W. Y. Sun, Angew. Chem., Int. Ed., 2023, 62, e202216232 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2314992–2314994. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ce01289a |
| This journal is © The Royal Society of Chemistry 2024 |