Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Influence of a neighbouring Cu centre on electro- and photocatalytic CO2 reduction by Fe-Mabiq
Kerstin
Rickmeyer
ab,
Matthias
Huber
ab and
Corinna R.
Hess
 *ab
*ab
aDepartment of Chemistry and Catalysis Research Center (CRC), Technical University of Munich, Garching 85748, Germany
bFaculty of Chemistry and Pharmacy, University of Regensburg, Regensburg 93053, Germany. E-mail: corinna.hess@ur.de
First published on 29th April 2024
Abstract
Correction for ‘Influence of a neighbouring Cu centre on electro- and photocatalytic CO2 reduction by Fe-Mabiq’ by Kerstin Rickmeyer et al., Chem. Commun., 2024, 60, 819–822, https://doi.org/10.1039/D3CC04777F.
In determining the amount of CO produced by our Fe–Mabiq complexes, 1 and 2 ([Fe(Mabiq)(MeCN)2]OTf and [Cu(Xantphos)Fe(Mabiq)(OTf)2], respectively), an error was made in the calculations. The amount of CO produced by the reported catalysts, and therefore the TONCO and TOFCO values, are lower than originally reported in our manuscript. The corrected values and corresponding revised statements in the manuscript are as follows:
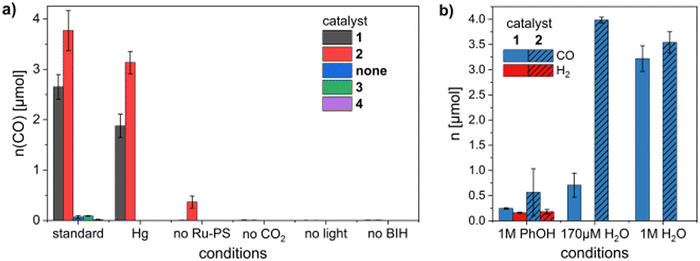
Under the reported standard photocatalysis conditions (2 μM catalyst, 200 μM Ru-PS, 170 μM PhOH, 100 mM BIH, 1 h irradiation at 455 nm) 1 and 2 produced CO as the only product in good yields, with TONs of 663 ± 61 for 1 and 942 ± 98 for 2 (the updated Fig. 2a is shown herein, and an amended Table S5 is provided in the update to the original ESI) and TOFs of 0.18 s−1 and 0.26 s−1, respectively. The values are lower than the ones reported by Chang and co-workers for photocatalytic CO2 reduction by [Fe(tpyPY2Me)] (tpyPY2Me: pentadentate polypyridyl ligand, TOF of 15 s−1).1 However, the values are still better than those reported for the most efficient Fe–porphyrin complexes under similar conditions (MeCN as solvent; TOF 0.013 s−1).2
The CO produced by 2 in the absence of the [Ru(bpy)3]2+ photosensitizer (Ru-PS) corresponds to a TON of 92 ± 30, which is one of the highest TONs reported for a self-sensitized system employing earth-abundant transition metals.1,3,4
In the investigation of photocatalytic CO2 reduction using H2O as the proton source, approximately 3 μmol CO were produced by both 1 and 2 using 1 M H2O, similar to the amounts produced at low [PhOH] (170 μM PhOH; updated Fig. 2b and Table S7 in the updated ESI). The amount of CO produced by 2 at low [H2O] (170 μM) is 3.99 μmol, whereas 1 generated 0.7 μmol CO (updated Fig. 2b, and Fig. S20 and Table S7 in the update to the ESI).
An updated Fig. 2 depicting the results described above is shown herein. Corrected CO values under all the various conditions investigated for 1 and 2 and for control studies can be found in the update to the ESI.
We note that the relative trends reported in our original manuscript all remain the same and that the overall conclusions of this work are not affected. In particular, as originally described, the Cu site of our new Cu/Fe–Mabiq complex leads to altered redox-properties, impedes Mabiq protonation and enhances photocatalytic performance. The bimetallic compound can also photocatalytically produce CO in a self-sensitized manner in good yields.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
References
- P. De La Torre, J. S. Derrick, A. Snider, P. T. Smith, M. Loipersberger, M. Head-Gordon and C. J. Chang, ACS Catal., 2022, 12, 8484–8493 CrossRef CAS.
- E. Pugliese, P. Gotico, I. Wehrung, B. Boitrel, A. Quaranta, M.-H. Ha-Thi, T. Pino, M. Sircoglou, W. Leibl, Z. Halime and A. Aukauloo, Angew. Chem., Int. Ed., 2022, 61, e202117530 CrossRef CAS PubMed.
- Z. Guo, S. Cheng, C. Cometto, E. Anxolabéhère-Mallart, S.-M. Ng, C.-C. Ko, G. Liu, L. Chen, M. Robert and T.-C. Lau, J. Am. Chem. Soc., 2016, 138, 9413–9416 CrossRef CAS PubMed.
- H. Rao, J. Bonin and M. Robert, Chem. Commun., 2017, 53, 2830–2833 RSC.
| This journal is © The Royal Society of Chemistry 2024 |