Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Not antiaromaticity gain, but increased asynchronicity enhances the Diels–Alder reactivity of tropone
Eveline H.
Tiekink
 ,
Pascal
Vermeeren
,
Pascal
Vermeeren
 * and
Trevor A.
Hamlin
* and
Trevor A.
Hamlin
 *
*
Department of Theoretical Chemistry, Amsterdam Institute of Molecular and Life Sciences (AIMMS), Amsterdam Center for Multiscale Modeling (ACMM), Vrije Universiteit Amsterdam, De Boelelaan 1108, 1081 HZ Amsterdam, The Netherlands. E-mail: p.vermeeren@vu.nl; t.a.hamlin@vu.nl
First published on 12th April 2024
Abstract
Correction for ‘Not antiaromaticity gain, but increased asynchronicity enhances the Diels–Alder reactivity of tropone’ by Eveline H. Tiekink et al., Chem. Commun., 2023, 59, 3703–3706, https://doi.org/10.1039/D3CC00512G.
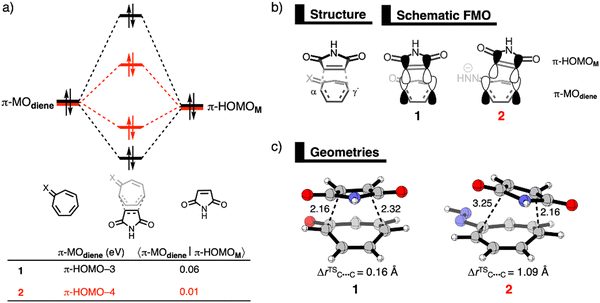
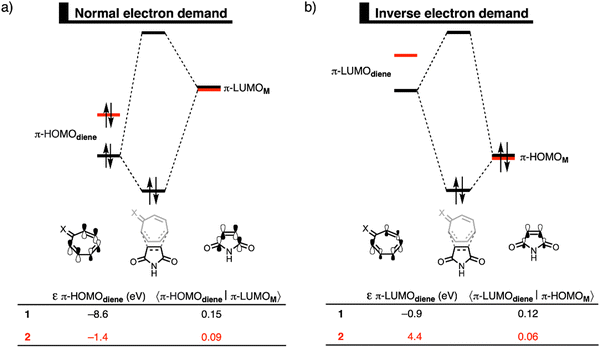
In the original paper, the reactivity of tropone and its neutral and deprotonated hydrazone analogs was studied using the generalized gradient approximation functional BP86-D3(BJ) for geometries and the range-separated hybrid functional ωB97X-D for energies. The authors have since identified a technical issue in the implementation of the ωB97X-D functional in the ADF software package, specifically the dispersion correction. Therefore, they have recomputed all energetic values (the optimized geometries remain the same as in the original manuscript due to using BP86) with ZORA-ωB97X-D4/TZ2P and prepared new tables (Tables 1 and 2) and figures (Fig. 1 and 2) from these results.
The conclusions are congruent with the original ZORA-ωB97X-D/TZ2P results, while the absolute energy values have changed, and the relative energy of all stationary points are stabilized. The updated results are reported below.
All analyses are performed at the ZORA-ωB97X-D4/TZ2P1 level of theory on the geometries optimized by ZORA-BP86-D3(BJ)/TZ2P. The results included in this correction act as an update to those previously given in the original publication. Please see the original publication for further details related to the methodology. The associated electronic supplementary information has also been updated with additional data for the calculations reported here.
Results
| Diene | ΔE | ΔEstrain | ΔEint | ΔVelstat | ΔEPauli | ΔEoi | ΔEdisp |
|---|---|---|---|---|---|---|---|
| a Computed at consistent TS-like geometries (CM⋯Cdiene = 2.160 Å) at ZORA-ωB97X-D4/TZ2P//ZORA-BP86-D3(BJ)/TZ2P. | |||||||
| 1 | 19.9 | 33.8 | −13.9 | −49.8 | 94.1 | −56.7 | −1.6 |
| 2 | −6.9 | 21.4 | −28.3 | −52.8 | 79.9 | −53.9 | −1.6 |
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
References
- (a) J.-D. Chai and M. Head-Gordon, J. Chem. Phys., 2008, 128, 084106 CrossRef PubMed; (b) E. Caldeweyher, S. Ehlert, A. Hansen, H. Neugebauer, S. Spicher, C. Bannwarth and S. Grimme, J. Chem. Phys., 2019, 150, 154122 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2024 |