Open Access Article
Open Access ArticleCatalytically transforming legacy phthalate esters from plastic waste into benzoic acid and benzoate plasticizers†
Simon
Windels
 and
Dirk E.
De Vos
and
Dirk E.
De Vos
 *
*
Centre for Membrane separations, Adsorption, Catalysis and Spectroscopy for sustainable solutions (cMACS), KU Leuven, Celestijnenlaan 200F, 3001 Leuven, Belgium. E-mail: dirk.devos@kuleuven.be
First published on 4th November 2024
Abstract
This study demonstrates the catalytic conversion of real plastic waste-extracted phthalate plasticizers into benzoic acid (BA), via a one-pot hydrolysis–decarboxylation process based on inexpensive Cu2O and H-beta zeolite, in water as a safe and environmentally benign solvent. As a result of the excellent product selectivity in both reactions, BA was obtained in quantitative yields (>99%). Esterification of the purified carboxylic acid resulted in the production of an alternative benzoate plasticizer, with a total process yield up to 94%.
In response to the recognized potential for endocrine disruption and reproductive toxicity of phthalate plasticizers, the European Union and North American governments have recently imposed stringent restrictions on the (re-)use of these legacy additives.1–4 In order to sustainably manage the considerable volumes of plastic waste still containing these phthalate esters, several innovative recycling methods are under development to efficiently extract the plasticizers from the plastic matrix. Such processes enable the safe reuse of the polymer material, but in turn also call for revalorisation methods to address the vast amounts of extracted legacy plasticizers.5–10 Recently, we have reported a two-stage hydrolysis–decarboxylation process capable of producing simple aromatic compounds (i.e. phthalic acid, benzoic acid and benzene) from such waste-extracted phthalate plasticizers;11 a hydrophobic H-beta zeolite, with a high Si/Al ratio of 75 is employed for the hydrolysis of the ester bonds, while a Pt-catalyst is utilized in the subsequent decarboxylation of the purified phthalic acid intermediate. While phthalic acid has few applications besides potential reuse in notorious phthalate plasticizers, the cost of the noble metal catalyst may impede a potential commercialization of the coupled revalorisation strategy. This makes the replacement of the decarboxylation-active metal with a more earth-abundant and cheap alternative a favorable advancement. In this regard, the use of Cu2O appears to be an intriguing option: Gooßen et al. reported the catalytic conversion of benzoic acids into benzene, albeit requiring expensive ligands and toxic organic solvents (i.e., quinoline and N-methyl-2-pyrrolidone).12,13 In another report, Zheng et al. documented the complete decarboxylation of aromatic acids to benzene in water. However, stoichiometric amounts of Cu2O and a very high temperature (350 °C) were required.14,15 Moreover, neither study achieved the selective production of the higher-value benzoic acid compound, which is a logical precursor for the production of safe and REACH-approved (di)benzoate plasticizers, amongst others, as potential substitutes for legacy phthalates in flexible plastics.16 In the current communication, we investigate the synergistic combination of these concepts, i.e. the selective revalorization of plastic waste-derived phthalate plasticizers into benzoic acid via a one-pot hydrolysis–decarboxylation process catalyzed in water by a H-beta zeolite and Cu2O.
In exploratory experiments, the catalytic activity of Cu2O for the decarboxylation of phthalic acid (PA) into benzoic acid (BA) and benzene (B) was assessed at a reaction temperature of 225 °C in water (Fig. 1). Given the negligible conversion of PA in the blank experiment (1% after 5 h), the decarboxylation rate observed with the Cu-catalyst is remarkable: within the initial reaction hour, 91% PA conversion was reached with merely 7 mol% of Cu. Furthermore, a comparison with the previously reported Pt-catalyzed system11 highlights a pronounced difference in product selectivity under current reaction conditions: Pt promotes the decarboxylation of both PA and of the generated BA, resulting in suboptimal BA yields (Fig. 1, Entry Pt/C). In contrast, the Cu-system demonstrates no significant conversion of BA at 225 °C even with considerably higher catalyst loadings and extended reaction times, leading to quantitative yields of BA (>99%). Another substantial difference between both catalytic systems was revealed in the assessment of the temperature dependency through Arrhenius plots (Fig. S10 in ESI†): the decarboxylation rate with Cu is much more responsive to temperature increases than that of Pt, with apparent activation energies of 95 and 35 kJ mol−1, respectively. These observations, alongside Zheng's findings of complete decarboxylation of aromatic acids to B at near-critical temperatures,14,15 emphasize the pronounced effect of the reaction temperature on the Cu-catalyzed decarboxylation in water.
Next, the integration of this Cu-catalyzed reaction in a coupled hydrolysis–decarboxylation process for legacy phthalate plasticizers was studied. In earlier work, we demonstrated that the hydrophobic H-beta 75 zeolite (Si/Al ratio of 75) is a highly efficient and recyclable catalyst for cleaving phthalate ester bonds, yielding PA along with primary alcohols and alkenes under similar reaction conditions (in water at 200 °C).11 Therefore, the effect of such side-chain fragments from phthalate hydrolysis on the decarboxylation activity of Cu2O at 200 °C was first evaluated by adding the as-produced amounts to a standard PA reaction mixture (Fig. S11 in ESI†). Considering the stability of the long-chain fragments (<5% conversion of alcohols and alkenes) and the unaffected decarboxylation outcome (rate and BA selectivity), a telescoped hydrolysis–decarboxylation approach for the revalorization of phthalate plasticizers appears feasible. To further investigate such one-pot strategy, the historically most employed plasticizer, i.e. bis(2-ethylhexyl) phthalate (DEHP), was mixed with water, Cu2O and varying amounts of H-beta 75 (0 to 150 wt%) at 200 °C for 2.5 h (Fig. 2). In the absence of the zeolite, the phthalate esters remained stable (<1% conversion), highlighting the importance of a dedicated hydrolysis catalyst, next to the Cu-catalyst in this one-pot revalorisation process. On the other hand, when H-beta 75 and Cu2O were simultaneously added to the reactor, the legacy plasticizers were rather smoothly transformed into PA and BA (DEHP conversion > 65%). The considerable amounts of the dicarboxylic acid intermediate in the reaction samples (yield PA > 20%) imply that the Cu-catalyzed decarboxylation forms the rate-determining step under current reaction conditions. By simply extending the reaction time, complete conversion of the phthalate plasticizer into BA was easily reached (>99.5% yield BA, Fig. S12 in ESI†).
 | ||
| Fig. 2 One-pot hydrolysis–decarboxylation of DEHP into PA and BA. Reaction conditions: DEHP (3 mmol), H2O (Vtot = 25 mL), Cu2O (7 mol% Cu), defined amount H-beta 75, 8 bar N2, 200 °C for 2.5 h. | ||
Interestingly, increasing the amount of H-beta 75 led to elevated PA yields, which seemingly results from an increased conversion of the phthalate plasticizer and a slightly decreased PA decarboxylation rate. Indeed, when the zeolite was added in large amounts to a standard decarboxylation of PA at 200 °C, the BA yield dropped from 78% to 39% after 2.5 h (Fig. S11 in ESI†). Apart from that, a faint blue hue was observed in the liquid samples of experiments conducted with low amounts of the zeolite, which may be indicative for copper dissolution. A control experiment demonstrated that dissolved Cu2+ is inherently active for the decarboxylation of PA, albeit less so (Fig. S11 in ESI†). Therefore, the aqueous phases of these one-pot experiments were analyzed for their metal content via inductively coupled plasma – optical emission spectrometry (ICP-OES). In accordance with the visual observations, the samples of experiments conducted with the lowest amounts of zeolite exhibited the highest levels of dissolved Cu (up to 55% of the Cu-catalyst). Furthermore, the metal content appears to decrease linearly with the amount of zeolite added to the reaction mixture, becoming negligible between 100 and 150 wt% H-beta 75 (<3% of the total Cu in solution). These results suggest that beyond its role in catalyzing the ester hydrolysis, the zeolite structure also acts as an ion exchanger, thereby sequestering catalytic metal. As a result of the relative low cation exchange capacity of the high-silica zeolite, substantial amounts of the material are required to capture all Cu, which would be essential when aiming for catalyst recycling. Therefore, subsequent experiments were conducted with 7 mol% Cu and 150 wt% of the cost-effective and recyclable zeolite structure, theoretically corresponding to 0.94 wt% Cu/H-beta 75 post reaction.
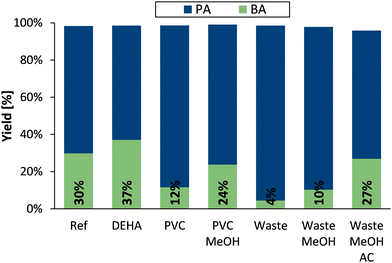
Next, a systematic evaluation was conducted on how to adapt this catalytic one-pot process from virgin phthalate esters to actual end-of-life plasticizers. As detailed elsewhere,10,11 the waste plasticizers were extracted from post-consumer PVC flooring, via a dissolution–precipitation strategy whereafter the employed solvents were again removed from the plasticizer fraction by evaporation. In addition to legacy phthalate plasticizers (75%), the crude waste extract contains other co-extracted plasticizers (20%), unprecipitated PVC (5%) and trace amounts of unspecified sulfur components. The effect of these extract contaminants on the one-pot hydrolysis–decarboxylation of virgin DEHP was first studied before actual waste trials were conducted (Fig. 3).
Given its historical use and its substantial share in the waste plasticizer mixture, bis(2-ethylhexyl) adipate (DEHA) was chosen as representative co-extracted plasticizer. In any case, virtually all PVC plasticizers are di-or polyfunctional esters; they are thus expected to behave similarly as the adipate ester.17 Therefore, a mixture of 75 mol% DEHP and 25 mol% DEHA was prepared at room temperature and subsequently applied as the plasticizer fraction in a one-pot hydrolysis–decarboxylation experiment (Fig. 3, Entry DEHA). Interestingly, an increased (relative) production of BA from DEHP was obtained, while the aliphatic plasticizer (containing equal amounts of reactive functional groups) was also converted over the same catalytic sites into successively adipic acid, pentanoic acid and butane (Fig. S4–S6 and S8 in ESI†). Considering that the total molar amount of converted PA did not increase, these results may suggest that the Cu-centers slightly prefer to be coordinated by PA, rather than by the aliphatic carboxylic acids. Alternatively, the catalyst-to-carboxylic acid ratio might slightly increase over time as a result of the rapid conversion of adipic acid. Since PVC and its plasticizers inherently exhibit high compatibility, a crude plasticizer extract will inevitably contain some polymer residues.17,18 Therefore, the effect of this impurity on the hydrolysis–decarboxylation of phthalates was investigated by employing 5 wt% PVC in DEHP (stirred at 200 rpm for 3 h in air at 100 °C) in a following one-pot experiment (Fig. 3, Entry PVC). As documented elsewhere,11 the ester hydrolysis by the hydrophobic zeolite seems largely unaffected by such levels of residual PVC in the reaction mixture. However, the presence of the polymer impurity significantly diminishes the decarboxylation activity of the Cu-catalyst. To counteract this inhibitory effect, the simulated PVC-DEHP mixture was pretreated with methanol, a well-known antisolvent for PVC (5 mL MeOH per g of plasticizer fraction, stirred at 200 rpm for 2 h at room temperature).18 The precipitated polymer was then removed from the liquid mixture by centrifugation (5000 rpm, 5 min) and the employed MeOH was evaporated (65 °C, 16 h). The catalytic outcome of the experiment conducted with the cleaned phthalate fraction confirms the effectiveness of this simple pretreatment procedure as the decarboxylation rate seems largely restored (Fig. 3, entry PVC MeOH).
Given that the presence of co-extracted plasticizers may accelerate the BA formation rate, while residual PVC tends to hinder the process, the crude plasticizers extracted from post-consumer PVC flooring were in the following one-pot experiment employed as such (Fig. 3, entry waste). Considering the robustness of the zeolite and the somewhat more polymer-sensitive Cu-catalyst, it is not entirely unexpected that this trial almost exclusively resulted in the hydrolysis of the esters (96% PA yield) with minimal decarboxylation of the formed carboxylic acids (4% BA yield). Therefore, the extracted plasticizer fraction was pretreated with MeOH (as described above). Even if visual and 1H-NMR evidence confirmed the elimination of residual PVC, the decarboxylation rate was only restored to a limited extent (Fig. 3, Entry Waste MeOH). With regard to the potential presence of additional Cu-poisons and/or oligomeric PVC chains that might be more difficult to precipitate, the waste extract was additionally treated with activated carbon. This cost effective adsorbent was added to the plasticizer-MeOH mixture (10 wt% AC relative to the extract), stirred for 6 h at room temperature and again removed by centrifugation (5000 rpm, 5 min). Following the evaporation of MeOH, the treated waste plasticizers were employed in a standard one-pot experiment, which revealed a substantially restored decarboxylation yield (Fig. 3, entry waste MeOH AC). As observed with virgin phthalate plasticizers, prolonged reaction times (24 h) resulted in the complete recovery of BA from waste phthalate plasticizers, although with a slight mass loss attributed to the pretreatment steps (mass balance 95%).
BA serves a wide range of applications, with its utilization in (di)benzoate plasticizers being particularly relevant in the context of recycling flexible PVC products.17,19 Adding such BA transformation step to our proposed hydrolysis–decarboxylation process would enable the conversion of banned phthalate plasticizers into REACH-compliant alternatives. Therefore, BA was esterified to form isononyl benzoate (IB) as a representative example of benzoate plasticizers, employing sulfuric acid as a conventional Brønsted acid/esterification catalyst (Fig. S13 in ESI†). Although the initial conversion rate at 100 °C appears to be rather independent of the exact amount of isononanol (INA) in the reaction mixture, quantitative yields of IB were only achieved with super-stoichiometric amounts of the alcohol. With the optimized reaction conditions established, this esterification reaction was subsequently coupled with the previously discussed hydrolysis–decarboxylation process (Fig. 4). Following the full conversion of the phthalate plasticizers into BA (reaction conditions as outlined above in Fig. 3 with an extended reaction time of 24 h), the carboxylic acid was isolated from the reaction mixture via a liquid–liquid extraction followed by recrystallisation (experimental details can be found in the ESI†). After drying the purified BA overnight, approximately 94% of the theoretical mass was recovered. The purified carboxylic acid was then esterified to IB (1.25 eq. INA, 5 mol% H2SO4 at 100 °C for 2.5 h), achieving an overall process yield of 89%.
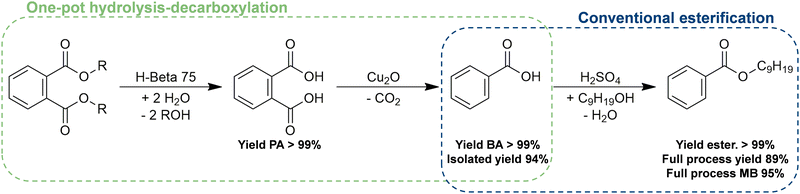 | ||
| Fig. 4 From legacy phthalate plasticizers to benzoate plasticizers: one-pot hydrolysis–decarboxylation followed by esterification of the isolated BA. | ||
This project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement no. 821366, and from FWO (SBO project S001819N). The authors also thank A. L. for performing the ICP-OES measurements and Fraunhofer IVV for the delivery of extracted plasticizers from post-consumer PVC flooring waste.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.References
- European Chemicals Agency, Annex XVII to REACH – Conditions of restriction: Entry 51, 2021, https://echa.europa.eu/substances-restricted-under-reach, accessed January, 2024.
- United States Consumer Product Safety Commission, Phthalates Business Guidance & Small Entity Compliance Guide, 2019, https://www.cpsc.gov/Business-Manufacturing/Business-Education/Business-Guidance/Phthalates-Information, accessed January, 2024.
- P. M. Lorz, F. K. Towae, W. Enke, R. Jäckh, N. Bhargava and W. Hillesheim, Phthalic Acid and Derivatives in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Germany, 2007, 27, 131–180 Search PubMed.
- K. J. Groh, T. Backhaus, B. Carney-Almroth, B. Geueke, P. A. Inostroza, A. Lennquist, H. A. Leslie, M. Maffini, D. Slunge, L. Trasande, A. M. Warhurst and J. Muncke, Sci. Total Environ, 2019, 651, 3253–3268 CrossRef CAS.
- U. Arena and F. Ardolino, Resour. Consrv. Recycl., 2022, 183, 106379 CrossRef CAS.
- Recycling plastics – The CreaSolv® Process, 2023, https://www.ivv.fraunhofer.de/en/recycling-environment/recycling-plasticscreasolv.html, accessed January, 2024.
- S. Ügdüler, K. M. Van Geem, M. Roosen, E. I. P. Delbeke and S. De Meester, Waste Manage., 2020, 104, 148–182 CrossRef.
- S. Wagner and M. Schlummer, Resour. Consrv. Recycl., 2020, 158, 104800 CrossRef.
- K. Pivnenko, M. K. Eriksen, J. A. Martín-Fernández, E. Eriksson and T. F. Astrup, Waste Manage., 2016, 54, 44–52 CrossRef CAS.
- S. Windels, T. Diefenhardt, N. Jain, C. Marquez, S. Bals, M. Schlummer and D. E. De Vos, Green Chem., 2022, 24, 754–766 RSC.
- S. Windels, N. Seynaeve, W. Stuyck and D. E. De Vos, Front. Chem. Eng., 2024, 6, 1463638 CrossRef.
- L. J. Gooßen, W. R. Thiel, N. Rodríguez, C. Linder and B. Melzer, Adv. Synth. Catal., 2007, 349, 2241–2246 CrossRef.
- L. J. Gooßen, F. Manjolinho, B. A. Khan and N. Rodríguez, J. Org. Chem., 2009, 74, 2620–2623 CrossRef.
- Q. Zheng, M. Morimoto and T. Takanohashi, Green Chem., 2015, 17, 791–794 RSC.
- Q. Zheng, M. Morimoto and T. Takanohashi, Fuel, 2016, 182, 437–445 CrossRef CAS.
- T. Maki and K. Takeda, Benzoic Acid and Derivatives in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Germany, 2000, 5, 329 – 342 Search PubMed.
- M. Bocqué, C. Voirin, V. Lapinte, S. Caillol and J.-J. Robin, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 11–33 CrossRef.
- G. Grause, S. Hirahashi, H. Toyoda, T. Kameda and T. Yoshioka, J. Mater. Cycles Waste Manage., 2017, 19, 612–622 CrossRef CAS.
- D. F. Cadogan and C. J. Howick, Plasticizers in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Germany, 2000, 27, 600–618 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc05158k |
| This journal is © The Royal Society of Chemistry 2024 |