Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Visualizing back electron transfer in eosin Y photoredox catalysis†
Kai
Gu
a,
Wenqiao
Zhou
a and
Chunming
Liu
 *ab
*ab
aSchool of Polymer Science and Polymer Engineering, University of Akron, Akron, OH 44325, USA. E-mail: chunmingliu@uakron.edu
bDepartment of Chemistry, University of Akron, Akron, OH 44325, USA
First published on 2nd October 2024
Abstract
Back electron transfer (BET) in eosin Y (EY) photoredox catalysis was visualized via the fluorescence of single EYs. BET between the radical ion pair formed in photoinduced electron transfer (PET) induced photoblinking of single EYs under constant photoexcitation. Commonly used quenchers, alkyl bromides and a tertiary amine, were studied. BET was observed in alkyl bromides, but not in the tertiary amine. The findings helped explain the mechanism of EY-catalyzed photoinduced atom transfer radical polymerizations. The method can be applied to studying BET on photo-emissive catalysts.
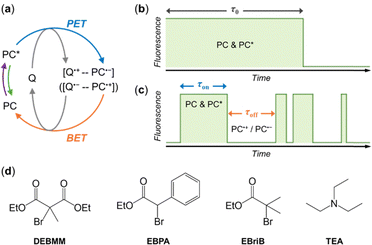
Photoredox catalysis has been widely used for organic synthesis in the past decade.1–9 The field has been growing rapidly due to the unique advantages of photoredox catalysis, e.g., mild reaction conditions, high energy efficiency, and the ease of controlling the process by light illumination. The photoredox catalyst (PC) is the essential component that absorbs light energy and initiates the photocatalytic cycle. In the electron-transfer mechanism, a radical ion pair, PC˙+–Q˙− (or PC˙−–Q˙+), is formed via photoinduced electron transfer and is expected to be embedded in a solvent cage (Fig. 1a).10–12 The radical ion pair could recombine to return to ground state molecules, which is referred to as back electron transfer (BET) or charge recombination (Fig. 1a).10–12 BET is thermodynamically favored and would make the product formation slower, because the radical ions are consumed without producing final products.13–15 In some reactions, BET could also be the key step to get the desired product. For example, in photocatalytic controlled radical polymerizations, the reversible deactivation of polymer growth is achieved through the BET between the PC radical and the propagating radical that are generated via photoinduced electron transfer (PET).16–18 Currently, BET is studied experimentally by comparing the concentration of the photoproduct after pulsed excitation of a sample with that of a reference solution that is expected to have a known BET yield.19–21 However, the direct observation of the BET process is difficult to achieve.
In this work, we demonstrate the direct visualization of BET by single-molecule fluorescence imaging. Previously, we developed the single-molecule fluorescence imaging method to study photoredox catalysis in operando, in which the redox states of individual PCs were monitored via their fluorescence signal.22 Continuous photocatalytic turnovers give rise to the reversible switching of single PC's redox states, and result in the photoblinking behavior of single PCs under continuous photoexcitation. Here, we will monitor the behavior of single PCs in the solutions of a single quencher. If BET does not occur between the quencher radical ion (Q˙+ or Q˙−) and PC radical ion (PC˙− or PC˙+), the fluorescence of individual EYs should be quenched in a single step due to the photoinduced electron transfer (Fig. 1b). If BET occurs, the PC radical ion (PC˙− or PC˙+) could return to ground state PC, and the fluorescence of the PC would be restored, giving rise to the photoblinking of PC (Fig. 1c). Besides, energy transfer between PC* and the quencher cannot change the redox state of PC, and therefore no additional photoblinking of PC should be observed if the quenching follows the energy transfer mechanism.
We monitored the behavior of single eosin Y (EY) in commonly used quenchers (Fig. 1d), including three alkyl bromides, diethyl 2-bromo-2-methylmalonate (DEBMM), ethyl α-bromophenylacetate (EBPA) and methyl α-bromoisobutyrate (MBriB), and a tertiary amine, triethylamine (TEA). EY is a commonly used photoredox catalyst that absorbs green light.6,23 Alkyl bromides are expected to quench EY via oxidative quenching, while tertiary amine is expected to quench EY via reductive quenching.23 Single-molecule imaging of EYs was carried out following the procedure we reported previously (Section S1, ESI†).22,24 Briefly, DMF was used as the solvent for all experiments, and the solutions were degassed and protected under N2 during imaging. EYs were immobilized on the glass surface and imaged using total-internal reflection fluorescence microscope. The fluorescence trajectories of individual EYs were analyzed using the MATLAB program.22,25
In DEBMM, both single-step photobleaching and photoblinking of single EYs were observed (Fig. 2a). This suggested the existence of the process that makes the EY radical ion (EY˙+) return to the ground state. Since no other reactants were present in the solution besides DEBMM, the BET between EY˙+ and the DEBMM radical anion (DEBMM˙−) should be responsible for the photoblinking of single EYs’ fluorescence (Fig. 1c). The photobleaching of EYs was accelerated by DEBMM and the photobleaching time τ0 (defined as shown in Fig. 1b) became shorter with increasing DEBMM concentration. The photobleaching rate of EY (T0−1), calculated by the inverse of the decay constant of τ0 (Fig. S1 and section S2, ESI†), was linearly correlated to the concentration of DEBMM (Fig. 2b). It was consistent with the Stern–Volmer quenching results that DEBMM effectively quenched EY.26
The percentage of photoblinking EY (Pblink) increased with DEBMM concentration (Fig. 2c). On the one hand, the increase of Pblink was due to the more efficient quenching of EY at higher DEBMM concentration, which is the prerequisite to generate photoblinking. On the other hand, the increase of Pblink also indicated that BET between EY˙+ and DEBMM˙− was efficient. The distributions of fluorescence on-time τon and off-time τoff of photoblinking EYs (as defined in Fig. 1c) were also investigated. τon followed double-exponential distribution (Fig. S2, ESI†), and τoff followed single-exponential distribution (Fig. S3, ESI†), which are consistent with our previous results.22Ton1, the faster decay constant of τon, did not show an obvious change with DEBMM concentration, indicating that the faster quenching of EY* reached saturation in the DEBMM concentration range (Fig. 2d). Ton2, the slower decay constant of τon, decreased with DEBMM concentration (Fig. 2d), which further supported that DEBMM was responsible for the quenching of EY. On the other hand, Toff, the decay constant of τoff, was independent of DEBMM concentration (Fig. 2e). Based on our interpretation, Toff−1 should represent the BET rate. Recently, it was reported that the BET rate is independent of quencher concentration.19 This result supported our hypothesis that BET between the radical ion pair (EY˙+ and DEBMM˙−) induced the photoblinking of EYs.
Next, we studied the effect of alkyl bromide structure on the behavior of single EYs. Two other alkyl bromides, EBPA and MBriB, were investigated, and gave similar results as DEBMM. Both EBPA and MBriB induced photoblinking of EYs indicating that BET occurred between EY˙+ and radical anions of EBPA and MBriB. Similarly, the photobleaching rate of EY (T0−1) by EBPA and MBriB was linearly correlated with their concentration, respectively (Fig. 3a).
Compared to DEBMM, EBPA induced higher percentages of photoblinking EY at lower concentrations (Fig. 3b) and gave faster photobleaching of EY (Fig. 3a). In contrast, MBriB induced lower percentages of photoblinking EY at even higher concentrations (Fig. 3b) and gave slower photobleaching of EY (Fig. 3a). The differences could be due to the structures of DEBMM, EBPA and MBriB and the properties of their anion radicals.27 The EBPA anion radical should be more stable than the DEBMM anion radical, because the resonance structures of the EBPA anion radical could help further stabilize the negative charge. Therefore, EBPA should be more active in the photo-oxidation of EY, resulting in the faster quenching of EY. In contrast, the MBriB anion radical should be less stable than the DEBMM anion radical, because MBriB has less electron-withdrawing groups than DEBMM. Therefore, MBriB should be less active in the photo-oxidation of EY, resulting in the slower quenching of EY. Like in DEBMM, the photoblinking on-time of EY (τon) followed double-exponential distributions in both EBPA and MBriB. The faster decay constants Ton1 were nearly unaffected by EBPA and MBriB concentrations (Fig. 3c), and the slower decay constants Ton2 decreased obviously with EBPA and MBriB concentrations (Fig. 3c). The decay constants of photoblinking off-time Toff remained nearly unchanged under various EBPA and MBriB concentrations (Fig. 3d). The results suggested that BET between EY˙+ and alkyl bromide radical anions exists generally, and the BET probability could be related to the activity of alkyl bromide in PET.
In the end, we investigated the interaction between EY and tertiary amine. In the solution of TEA, the majority of EYs were photobleached in a single step. The photobleaching rate of EY (T0−1) increased with TEA concentration as expected (Fig. 4a). However, the percentage of photoblinking EY stayed at a very low level and did not increase with TEA concentration (Fig. 4b), which was different compared to the phenomena in alkyl bromides. This result indicated that there is no efficient BET between EY˙− and TEA˙+.
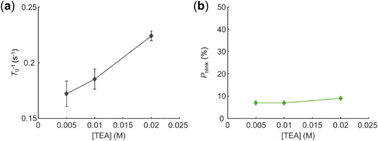 | ||
| Fig. 4 The effect of TEA concentration on (a) photobleaching rate of EY T0−1, and (b) percentage of photoblinking EY Pblink. | ||
The BET between EY˙+ and alkyl bromide radical anions could explain the mechanism of EY-catalyzed photoinduced atom transfer radical polymerization (photoATRP).28 The reaction is proposed to follow a reductive quenching mechanism, in which EY* is first reduced by tertiary amine to EY˙− and then oxidized by alkyl bromide (ATRP initiator) to EY. The alkyl bromide radical anion then generates an alkyl radical to initiate polymerization. Based on the redox potentials,23,29 EY* (either 1EY* or 3EY*) can directly reduce alkyl bromide to an alkyl bromide radical anion to initiate polymerization. However, little polymer was produced without tertiary amine.28,29 Based on our observations in this work, the reason is likely that the efficient BET between EY˙+ and alkyl bromide radical anions significantly suppressed the generation of free radicals through the photoinduced electron transfer between EY* and alkyl bromides. Furthermore, the proposed reductive quenching mechanism is also supported by our observation that the BET efficiency between EY˙− and the tertiary amine radical cation is inefficient.
In summary, we investigated the interaction of commonly used quenchers with EY photoredox catalyst by single-molecule fluorescence imaging. In all quenchers, the photobleaching of EYs was accelerated, indicating the quenchers quenched EY via an electron transfer process. In alkyl bromide quenchers, large fractions of EYs showed photoblinking behavior, suggesting the existence of BET between EY˙+ and alkyl bromide radical anions. In comparison, in tertiary amine TEA, the fraction of photoblinking EY was much lower, suggesting that there is no efficient BET between EY˙− and TEA˙+. And the mechanism of EY-catalyzed photoATRP can be explained by the difference of quenchers in BET efficiency. In future, BET between other photo-emissive photoredox catalysts and redox quenchers can be studied by the single-molecule imaging method.
The authors thank the ACS Petroleum Research Fund (65009-DNI4) and the University of Akron for providing funding support.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- D. A. Nicewicz and D. W. C. MacMillan, Science, 2008, 322, 77–80 CrossRef CAS PubMed.
- M. A. Ischay, M. E. Anzovino, J. Du and T. P. Yoon, J. Am. Chem. Soc., 2008, 130, 12886 CrossRef CAS.
- J. M. Narayanam, J. W. Tucker and C. R. Stephenson, J. Am. Chem. Soc., 2009, 131, 8756–8757 CrossRef CAS.
- D. P. Hari and B. König, Org. Lett., 2011, 13, 3852–3855 CrossRef CAS.
- C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed.
- D. P. Hari and B. Konig, Chem. Commun., 2014, 50, 6688–6699 RSC.
- D. M. Arias-Rotondo and J. K. McCusker, Chem. Soc. Rev., 2016, 45, 5803–5820 RSC.
- L. Marzo, S. K. Pagire, O. Reiser and B. Konig, Angew. Chem., Int. Ed., 2018, 57, 10034–10072 CrossRef CAS PubMed.
- Visible Light Photocatalysis in Organic Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2018.
- G. J. Kavarnos and N. J. Turro, Chem. Rev., 1986, 86, 401–449 CrossRef.
- J. Olmsted and T. J. Meyer, J. Phys. Chem., 1987, 91, 1649–1655 CrossRef.
- M. Z. Hoffman, J. Phys. Chem., 1988, 92, 3458–3464 CrossRef.
- A. I. Arkhypchuk, T. T. Tran, R. Charaf, L. Hammarström and S. Ott, Inorg. Chem., 2023, 62, 18391–18398 CrossRef PubMed.
- H. Sayre, H. H. Ripberger, E. Odella, A. Zieleniewska, D. A. Heredia, G. Rumbles, G. D. Scholes, T. A. Moore, A. L. Moore and R. R. Knowles, J. Am. Chem. Soc., 2021, 143, 13034–13043 CrossRef PubMed.
- A. Runemark and H. Sundén, J. Org. Chem., 2023, 88, 462–474 CrossRef PubMed.
- B. P. Fors and C. J. Hawker, Angew. Chem., Int. Ed., 2012, 51, 8850–8853 CrossRef CAS PubMed.
- J. C. Theriot, C. H. Lim, H. Yang, M. D. Ryan, C. B. Musgrave and G. M. Miyake, Science, 2016, 352, 1082–1086 CrossRef CAS.
- J. T. Xu, S. Shanmugam, H. T. Duong and C. Boyer, Polym. Chem., 2015, 6, 5615–5624 RSC.
- C. Wang, H. Li, T. H. Bürgin and O. S. Wenger, Nat. Chem., 2024, 16, 1151–1159 CrossRef PubMed.
- A. Ripak, S. De Kreijger, R. N. Sampaio, C. A. Vincent, E. Cauet, I. Jabin, U. K. Tambar, B. Elias and L. Troian-Gautier, Chem Catal., 2023, 3 Search PubMed.
- A. Ripak, S. De Kreijger, B. Elias and L. Troian-Gautier, STAR Protoc., 2023, 4, 102312 CrossRef.
- K. Gu, C. Yu, W. Zhou and C. Liu, J. Phys. Chem. Lett., 2024, 15, 717–724 CrossRef.
- N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075–10166 CrossRef PubMed.
- K. Gu, S. Liu and C. Liu, Langmuir, 2022, 38, 15848–15857 CrossRef PubMed.
- K. Gu and C. Liu, Chem. Biomed. Imaging, 2023, 1, 234–241 CrossRef.
- D. Fernandez Reina, A. Ruffoni, Y. S. S. Al-Faiyz, J. J. Douglas, N. S. Sheikh and D. Leonori, ACS Catal., 2017, 7, 4126–4130 CrossRef.
- W. Tang and K. Matyjaszewski, Macromolecules, 2007, 40, 1858–1863 CrossRef.
- C. Kutahya, F. S. Aykac, G. Yilmaz and Y. Yagci, Polym. Chem., 2016, 7, 6094–6098 RSC.
- C. Bian, Y. N. Zhou, J. K. Guo and Z. H. Luo, Macromolecules, 2018, 51, 2367–2376 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc04463k |
| This journal is © The Royal Society of Chemistry 2024 |