Open Access Article
Open Access ArticleRigidochromism of tetranuclear Cu(I)–pyrazolate macrocycles: steric crowding with trifluoromethyl groups†
Shinaj K.
Rajagopal
 a,
Matthias
Zeller
a,
Matthias
Zeller
 a,
Sergei
Savikhin
a,
Sergei
Savikhin
 b,
Lyudmila V.
Slipchenko
b,
Lyudmila V.
Slipchenko
 a and
Alexander
Wei
a and
Alexander
Wei
 *ac
*ac
aJames and Margaret Tarpo Department of Chemistry, Purdue University, West Lafayette, Indiana 47907, USA. E-mail: alexwei@purdue.edu
bDepartment of Physics and Astronomy, Purdue University, West Lafayette, Indiana 47907, USA
cSchool of Materials Engineering, Purdue University, West Lafayette, Indiana 47907, USA
First published on 13th September 2024
Abstract
Macrocyclic Cu(I)–pyrazolate tetramers (Cu4pz4) can fold into compact structures with luminescent Cu4 cores whose emission wavelengths are sensitive to steric effects along the periphery of the macrocycle. Introducing CF3 at the C4 position of 3,5-di-tBu-pyrazolate increases steric crowding that modifies the conformational behavior of the Cu4pz4 complex, highlighted by a low-temperature martensitic transition. Variable-temperature analysis of solid-state luminescence reveal an unexpected blueshifting of emission with rising temperature.
Solid-state luminescence continues to be a fascinating subject, rejuvenated in recent years by interests in materials that can improve solid-state lighting efficiency,1 or respond to external stimuli for sensor or imaging applications.2 Among the myriad categories of luminescent materials, Cu(I) complexes have a special appeal: Copper is an earth-abundant element and has yielded a variety of photoactive materials, many of which can be made by mixing simple salts with appropriately designed organic ligands.3–6 Recent advances include Cu(I) emitters for thermally activated delayed fluorescence (TADF),7–9 circularly polarized emission from chiral Cu clusters,10,11 and efficient radioluminescence with application in X-ray imaging.12
Copper(I)–pyrazolate complexes are a class of luminescent clusters that have yielded many interesting examples of solid-state luminochromism. Trinuclear Cu(I)–pyrazolates (Cu3pz3) have been studied extensively,6,13 but attention is being paid more recently to tetranuclear Cu4pz4 species which are also highly luminescent.14–17 One important distinction is that Cu3pz3 structures are planar and prone to intermolecular stacking which strongly affects their emissive states, whereas Cu4pz4 complexes are saddle-shaped and their emission wavelengths (λem) are unaffected by neighbouring clusters.
We recently studied a series of Cu4pz4 complexes prepared from 3,5-di-tBu-pyrazole and C4-substituted derivatives, whose solid-state emissions depend primarily on electronic transitions from triplet cluster-centred (3CC) excited states.16 A remarkable feature of these macrocyclic complexes is the strong impact of the C4 substituent on λem, which is steric in nature rather than electronic. For example, a tetranuclear complex made with 3,5-di-tBu-pyrazole (Cu4(H-pz)4, 1) emits yellow light (λem 559 nm),14 whereas a complex made with 3,5-di-tBu-4-methylpyrazole (Cu4(Me-pz)4, 2) emits deep blue light (λem 457 nm).16 The C4 methyl causes the flanking tBu units to adopt bisected geometries, enabling macrocycle 2 to fold into a compact, conformationally rigid structure with the four Cu atoms compressed into a close-packed rhombus (Fig. 1, lower right). This geometry limits the excited-state contraction of the Cu4 cluster, thereby supporting deep-blue emission.16 Such long-range effects on rigidochromism motivated us to examine the influence of bulkier C4 substituents on the global conformation and photoluminescence (PL) of related Cu4pz4 species.
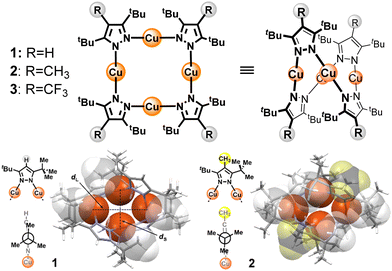 | ||
| Fig. 1 Top, Cu4pz4 complexes 1–3 (planar view and saddle-shaped structure). Bottom, top view of 1 and 2 with vdW contours for CH3 and endo-methyl units in tBu groups. The aspect ratio (dL/dS) of the Cu4 rhombus is 1.43 for 1 and 1.60 for 2, X-ray data from Ref. 16. | ||
In this paper we describe the synthesis, structure, and PL of Cu4(CF3-pz)4 (3), a tetranuclear complex prepared from 3,5-di-tBu-4-trifluoromethylpyrazole (4). The van der Waals volume of CF3 in 3 is nearly twice that of CH3 in 2 (39.2 vs. 21.0 Å3) and thus expected to maintain neighbouring tBu units in bisected conformations. However, the CF3 groups influence solid-state behaviour in unexpected ways, including a polymorphic shift at low temperature and a high-energy PL band whose intensity increases with temperature for powders and thin films.
Cu4(CF3-pz)43 can be formed in one step from compound 4, which in turn can be prepared from a 4-iodopyrazole precursor (Scheme 1). However, the insertion of a bulky CF3 between two tBu units is synthetically challenging. After exploring several different methods, we found trifluoromethyl thianthrenium triflate (CF3-TT+OTf−) developed by Ritter and coworkers to be an excellent CF3 transfer agent under Cu-mediated cross-coupling conditions,18 producing 3,5-tBu2-4-CF3-pz 4 in 88% overall yield after debenzylation (details in ESI†). Pyrazole 4 was then mixed with [Cu(CH3CN)4]BF4 in MeOH to form Cu4pz4 complex 3, which precipitated as a colourless solid in 70% yield.
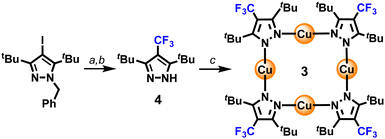 | ||
Scheme 1 Synthesis of Cu4(CF3-pz)4 (3). (a) TT(CF3)OTf (2 eq.), Cu0 (3 eq.), DMF, 60 °C. (b) 5% Pd/C (cat.), H2 (1 atm), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EtOAc:MeOH, rt. (c) [Cu(CH3CN)4]BF4 (1 eq.), Et3N (1 eq.), MeOH, rt. 1 EtOAc:MeOH, rt. (c) [Cu(CH3CN)4]BF4 (1 eq.), Et3N (1 eq.), MeOH, rt. | ||
Cu4(CF3-pz)43 produces a brilliant green luminescence in the solid state with a quantum yield of 42% and decay lifetime of 27.6 μs at 300 K, indicating room-temperature phosphorescence (Fig. S1 and Table S1, ESI†). The PL spectrum of 3 at 295 K in powder form shows a peak λem centred at 519 nm, plus a shoulder at roughly 450 nm that is amplified and broadened in thin film samples (Fig. 2a). Excitation spectra corresponding with each emission band both show a broad peak at 280 nm (Fig. S2, ESI†), a signature of the S0 → T1 transition for 3CC states.15 DFT calculations of 3 confirm that the HOMO–LUMO transition is controlled through CC orbitals (Fig. 2b). The primary role of 3CC states in Cu4pz4 emission is remarkable, given its history as a secondary, low-energy pathway in other Cu(I) clusters.3,19
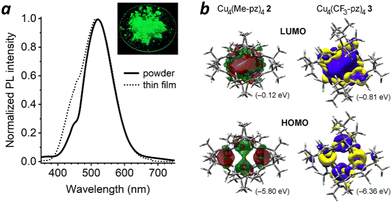 | ||
| Fig. 2 (a) PL spectra of Cu4(CF3-Pz)43 in powder and thin-film forms (λex 270 nm, peak λem 519 nm); inset, luminescent powder using 254-nm excitation. (b) DFT analysis of Cu4(Me-Pz)42 and Cu4(CF3-Pz)43 with HOMO and LUMO structures and energies; analysis of 2 is described in Ref. 16. | ||
The green luminescence of 3 is contrary to our initial expectations, as the role of steric bulk at C4 in enforcing the conformational rigidity of Cu4pz4 has been shown with smaller substituents (R = Cl, Br, and CH3), all having λem < 460 nm.16 We thus considered whether the CF3 group was sufficiently large to (i) cause distortions in the pyrazole ring by creating torsional strain between neighbouring tBu groups, and (ii) direct transannular interactions between opposing pyrazolate ligands that prevent Cu atoms from adopting a close-packed geometry.
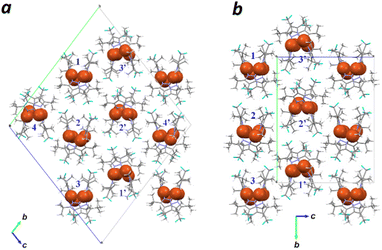
X-ray analysis of crystals grown from a toluene/CH2Cl2 solution of 3 confirms that the Cu4(CF3-pz)4 macrocycle adopts a saddle-shaped conformation (Fig. 3a). Analysis at 150 K yields a triclinic unit cell containing two sets of four independent structures, each with a slightly different conformation but otherwise adopting the same folded geometry (Fig. 3b and c). However, whereas the Cu4 core of Cu4(Me-pz)42 is a planar, close-packed rhombus with a large aspect ratio (dL/dS = 1.60; Fig. 1),16 the Cu atoms of 3 form nonplanar quadrangles with low aspect ratios (1.03–1.16).
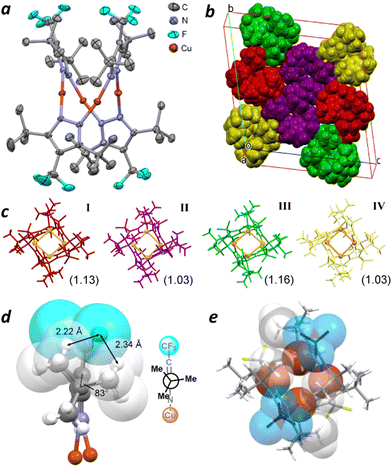 | ||
| Fig. 3 (a) X-ray crystal structure of 3 (Conformer I) at 150 K; thermal ellipsoids drawn at the 50% probability level with H atoms removed for clarity; see Fig. S5 (ESI†) for other conformers. (b) and (c) Triclinic unit cell of Cu4(CF3-Pz)43 at 150 K with conformers I–IV; Cu4 quadrangle drawn in orange with dL/dS in parentheses. (d) Edge view of CF3-pz showing select F⋯H distances and dihedral angle of protruding CH3. (e) Top view of 3 (conformer I) with vdW contours for CF3 groups and endo-methyl units in tBu groups. | ||
A close inspection of the pyrazolate ligands in 3 reveals that the CF3 is tightly wedged against adjacent tBu groups with nearest-neighbour F⋯H distances of 2.15–2.35 Å, much shorter than the sum of their vdW radii (2.6–2.7 Å).20 Torsional strain is reduced by (i) bending tBu groups out of plane by up to 10° and (ii) rotating their methyl units 12–24° away from their ideal bisected conformations (ϕ = 60°), with one unit projected nearly normal to the pyrazole ring (Fig. 3d). These distortions reflect the sizable allylic strain imposed on the tBu units by CF3.
t Bu methyl groups that project inward (endo) perturb the conformation of the Cu4pz4 macrocycle. To reduce transannular steric interactions, the pyrazolate ligands twist so that each face is positioned directly across an opposing tBu unit, resulting in the interdigitation of endo methyls (Fig. 3e). The twisting of pyrazolate rings causes the Cu4 quadrangles to buckle with bend angles of 27.4–32.9° (Fig. 3a and Fig. S8, Table S2, ESI†), and creates a sizable gap in the Cu4 core of 3 with dS values of 3.65–3.90 Å. In comparison, the Cu4 rhombus of 2 has a bend angle of 0° with dS of 3.05 Å (Fig. 1 and Table S2, ESI†).
The λem peak at 519 nm for 3 (Fig. 2a) is in accord with other luminescent Cu4pz4 complexes with nonplanar Cu4 cores.14–16 We have noted previously that Cu atom mobility promotes excited-state contraction and can induce a redshift in Cu4pz4 emission.16 In the case of complex 3, the presence of several conformers in the unit cell at 150 K indicates that the Cu4pz4 macrocycle can adopt multiple low-energy structures, with DFT calculations of conformers I–IV suggesting ΔΔH0 <1 kcal mol−1 (Table S5, ESI†). Although all Cu4pz4 conformations are stabilized by the interdigitation of tBu groups, time-dependent (TD) DFT analysis of their ground (S0) and first excited triplet (T1) states reveals very similar degrees of excited-state contraction by the Cu4 core (Fig. S9 and S10, ESI†), confirming the importance of Cu-atom close packing in the rigidochromism of Cu4pz4 complexes.16
Gradual warming of 3 between 150 and 200 K induces a martensitic transition from a triclinic (P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) to monoclinic lattice (P21/c; Fig. 4). X-ray analysis at 200 K shows a single conformer with some rotational disorder in the CF3 and tBu groups (Fig. S6, ESI†). The distance between Cu4 centroids along the [01−1] direction at 150 K (13.45 Å) decreases by 1.1% in the [010] direction at 200 K and the separation of lattice planes along [001] increases by 4%, along with minor changes in Euler angles (Table 1). The Cu4 quadrangle at 200 K has a fixed aspect ratio of 1.07 and bend angle of 28°, and a macrocyclic conformation similar to those of I–IV (RMS deviations of 0.05–0.19 Å). Further analysis of the static disorder at 200 K suggests that CF3 reorientation drives the librational exchange of its neighbouring tBu groups (Fig. S7, ESI†). In addition to the low-temperature polymorphic shift, reversible phase transitions were recorded by differential scanning calorimetry at 170 and 200 K (Fig. S11, ESI†).
) to monoclinic lattice (P21/c; Fig. 4). X-ray analysis at 200 K shows a single conformer with some rotational disorder in the CF3 and tBu groups (Fig. S6, ESI†). The distance between Cu4 centroids along the [01−1] direction at 150 K (13.45 Å) decreases by 1.1% in the [010] direction at 200 K and the separation of lattice planes along [001] increases by 4%, along with minor changes in Euler angles (Table 1). The Cu4 quadrangle at 200 K has a fixed aspect ratio of 1.07 and bend angle of 28°, and a macrocyclic conformation similar to those of I–IV (RMS deviations of 0.05–0.19 Å). Further analysis of the static disorder at 200 K suggests that CF3 reorientation drives the librational exchange of its neighbouring tBu groups (Fig. S7, ESI†). In addition to the low-temperature polymorphic shift, reversible phase transitions were recorded by differential scanning calorimetry at 170 and 200 K (Fig. S11, ESI†).
To determine whether low-temperature phase transitions might influence solid-state emission, variable-temperature PL studies were performed on powder and thin-film samples of 3 (Fig. 5). Both samples produce strong and well-defined emission bands at 77 K (λem 532–537 nm); minor peaks in the violet region (400–415 nm) are also observed. The main PL band broadens and blueshifts upon warming to 300 K (513–518 nm), accompanied by a notable increase of a secondary PL band in the blue region (420–480 nm).
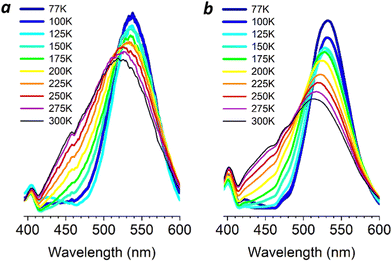 | ||
| Fig. 5 Variable-temperature PL spectra of 3 in the solid state. (a) Powder in borosilicate glass tube (λex 300 nm); (b) thin film on quartz (λex 270 nm). | ||
The higher energy PL band is curious and may be related to the lowering of excited-state energies by the strongly electronegative CF3 group (Fig. 2). Variable-temperature analysis of PL lifetimes indicates a modest decrease in τ at 450 nm and no changes at 520 or 532 nm with rising temperature, ruling out the possibility of TADF (Fig. S5, ESI†). We postulate that complex 3 in these samples adopts numerous conformations at domain interfaces or in amorphous regions. The distribution of states can increase from several conformers below 150 K to a multitude of conformations above 200 K, with an increasing number of close-packed Cu4 clusters that support blue emission.16 While the distributions are under thermodynamic control, the solid-state conformations are kinetically stable on the microsecond timescale and support varying degrees of rigidochromism based on their ground-state structures.
In conclusion, introducing CF3 between two tBu groups on a trisubstituted pyrazole generates steric crowding that impacts the conformational and luminescence behavior of the Cu4pz4 macrocycle. Whereas C4-CH3 units drive neighbouring tBu groups into bisected rotamers that result in a compact Cu4pz4 conformation with nearly close-packed Cu atoms,16 C4-CF3 units distort local geometries that produce competing steric effects and a gap in the Cu4 core. Overall we find that the rigidochromism of Cu4pz4 is best reinforced by C4 substituents of intermediate size, to support conformations that minimize excited-state reorganization of the Cu4 cluster.
S. K. R.: synthesis, PL studies, DFT analysis; M. Z.: x-ray crystallography; S. S.: PL training; L. V. S.: DFT supervision; A. W.: research design. S. K. R. and A. W. wrote the manuscript.
We thank the US National Science Foundation (CHE-2102639, CHE-2204206), Dept. of Energy (DE-SC0018239), NIH (P30-CA023168), and Yuichiro Watanabe for discussions. Research was also partly supported through computational resources provided by Information Technology at Purdue University.
Data availability
Additional data supporting this article have been included as part of the ESI.† Crystallographic X-ray data for compound 3 at 150 and 200 K has been deposited as CIF files into the Cambridge Structural Database (CCDC 2370077, 2370073†) and can be downloaded from https://www.ccdc.cam.ac.uk/data_request/cif. DFT and TD-DFT output files (Gaussian16) will be made available upon request. Figures for X-ray structures were generated using publicly available data (Mercury 2024.1.0, Build 401958) from the Cambridge Crystallographic Data Centre (CCDC) at https://www.ccdc.cam.ac.uk/solutions/software/free-mercury/.Conflicts of interest
There are no conflicts to declare.References
- G. Hong, X. Gan, C. Leonhardt, Z. Zhang, J. Seibert, J. M. Busch and S. Bräse, Adv. Mater., 2021, 33, 2005630 CrossRef CAS PubMed.
- P. Bamfield and M. Hutchings, Chromic Phenomena – Technological Applications of Colour, Chemistry Royal Society of Chemistry RSC, 2018 Search PubMed.
- (a) P. C. Ford, E. Cariati and J. Bourassa, Chem. Rev., 1999, 99, 3625–3648 CrossRef CAS PubMed; (b) E. Cariati, E. Lucenti, C. Botta, U. Giovanella, D. Marinotto and S. Righetto, Coord. Chem. Rev., 2016, 306, 566–614 CrossRef CAS.
- M. A. Halcrow, Dalton Trans., 2009, 2059–2073 RSC.
- (a) L. P. Ravaro, K. P. S. Zanoni and A. S. S. de Camargo, Energy Rep., 2020, 6, 37–45 CrossRef; (b) V. K.-M. Au, Energy Fuels, 2021, 35, 18982–18999 CrossRef CAS.
- J. Zheng, Z. Lu, K. Wu, G.-H. Ning and D. Li, Chem. Rev., 2020, 120, 9675–9742 CrossRef CAS PubMed.
- R. Hamze, J. L. Peltier, D. Sylvinson, M. Jung, J. Cardenas, R. Haiges, M. Soleilhavoup, R. Jazzar, P. I. Djurovich, G. Bertrand and M. E. Thompson, Science, 2019, 363, 601–606 CrossRef CAS PubMed.
- H.-J. Wang, Y. Liu, B. Yu, S.-Q. Song, Y.-X. Zheng, K. Liu, P. Chen, H. Wang, J. Jiang and T.-Y. Li, Angew. Chem., Int. Ed., 2023, 62, e202217195 CrossRef CAS PubMed.
- H.-J. Wang, Y. Liu, B. Yu, S.-Q. Song, Y.-X. Zheng, K. Liu, P. Chen, H. Wang, J. Jiang and T.-Y. Li, Angew. Chem., Int. Ed., 2023, 62, e202217195 CrossRef CAS PubMed.
- C. Dutta, S. Maniappan and J. Kumar, Chem. Sci., 2023, 14, 5593–5601 RSC.
- X.-H. Ma, Y. Si, J.-H. Hu, X.-Y. Dong, G. Xie, F. Pan, Y.-L. Wei, S.-Q. Zang and Y. Zhao, J. Am. Chem. Soc., 2023, 145, 25874–25886 CrossRef CAS PubMed.
- Y. Wang, W. Zhao, Y. Guo, W. Hu, C. Peng, L. Li, Y. Wei, Z. Wu, W. Xu, X. Li, Y. D. Suh, X. Liu and W. Huang, Light: Sci. Appl., 2023, 12, 155 CrossRef CAS PubMed.
- (a) I. Boldog, E. B. Rusanov, J. Sieler, S. Blaurock and K. V. Domasevitch, Chem. Commun., 2003, 740–741 RSC; (b) M. A. Rawashdeh-Omary, Comments Inorg. Chem., 2012, 33, 88–101 CrossRef; (c) A. A. Titov, O. A. Filippov, L. M. Epstein, N. V. Belkova and E. S. Shubina, Inorg. Chim. Acta, 2018, 470, 22–35 CrossRef CAS; (d) A. A. Titov, V. A. Larionov, A. F. Smol’yakov, M. I. Godovikova, E. M. Titova, V. I. Maleev and E. S. Shubina, Chem. Commun., 2019, 55, 290–293 RSC.
- K. Fujisawa, Y. Ishikawa, Y. Miyashita and K.-I. Okamoto, Inorg. Chim. Acta, 2010, 363, 2977–2989 CrossRef CAS.
- H. V. R. Dias, H. V. K. Diyabalanage, M. M. Ghimire, J. M. Hudson, D. Parasar, C. S. Palehepitiya Gamage, S. Li and M. A. Omary, Dalton Trans., 2019, 48, 14979–14983 RSC.
- Y. Watanabe, B. M. Washer, M. Zeller, S. Savikhin, L. Slipchenko and A. Wei, J. Am. Chem. Soc., 2022, 144, 10186–10192 CrossRef CAS PubMed.
- R. A. Smith, R. Kulmaczewski and M. A. Halcrow, Inorg. Chem., 2023, 62, 9300–9305 CrossRef CAS PubMed.
- H. Jia, A. P. Häring, F. Berger, L. Zhang and T. Ritter, J. Am. Chem. Soc., 2021, 143, 7623–7628 CrossRef CAS PubMed.
- M. Xie, C. Han, Q. Liang, J. Zhang, G. Xie and H. Xu, Sci. Adv., 2019, 5, eaav9857 CrossRef CAS PubMed.
- A. Bondi, J. Phys. Chem., 1964, 68, 441–451 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis details and chemical characterization, photophysical and x-ray crystallographic data, computational DFT and TD-DFT analysis. CCDC 2370073 and 2370077. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc04259j |
| This journal is © The Royal Society of Chemistry 2024 |